Introduction
Ischemic stroke, accounting for roughly 87% of all
strokes, is the most prevalent type of cerebrovascular accident. It
primarily results from a disruption in cerebral blood flow
(1). Among them, progressive
cerebral infarction (PCI) constitutes between 26 and 43% (2) of all cerebral infarction cases and is
associated with elevated mortality and disability rates. The
pathogenesis of PCI is complex (3-6),
involving thrombus expansion and regeneration, reduced cerebral
perfusion, cerebral edema and failure to promptly establish
collateral circulation within the ischemic region (7), thereby resulting in cerebral tissue
hypoxia and subsequently presenting symptoms of neurological
dysfunction. Currently, there is no accepted definition of
progressive ischemic stroke (PIS). In both domestic and
international studies and reports, the relevant concepts
predominantly comprise early neurological deterioration,
progressive neurological deficits, stroke progression and early
recurrent ischemic stroke. The difference lies in the time window
and the assessment method, and there is a significant variation in
treatment options. Currently, treatment mainly includes specific
and fundamental approaches. As discussed in this article, the
combination of tirofiban with oral antiplatelet drugs is a specific
treatment.
Glycoprotein IIb/IIIa, a key receptor found on the
surface of platelets, plays an essential role in regulating
platelet aggregation. Glycoprotein IIb/IIIa inhibitors, a category
of highly selective platelet antagonists, bind specifically to the
glycoprotein IIb/IIIa receptors on platelet surfaces. These
inhibitors effectively and reversibly impede fibrin attachment to
the aforementioned receptors through this binding interaction, thus
preventing platelet aggregation (8). Experimental data from stroke animals
treated with glycoprotein IIb/IIIa inhibitors indicated that, even
when administered later, the volume of the infarct may still be
reduced (9).
Tirofiban is a reversible non-peptide platelet GP
IIb/IIIa receptor antagonist. It directly inhibits the GP IIb/IIIa
receptor, blocking fibrinogen crosslinking (10). Tirofiban acts rapidly, with
immediate effect upon intravenous administration, making it
suitable for rapid anti-thrombotic action in acute situations.
Conversely, aspirin and clopidogrel are primarily used for
long-term prevention. Therefore, Tirofiban provides a rapid,
controllable and reversible antiplatelet effect in acute thrombotic
events through its unique GP IIb/IIIa targeting mechanism,
complementing the role of aspirin and clopidogrel.
Meanwhile, Tirofiban is highly selective and can
reversibly prevent platelet aggregation. Among them, tirofiban
reduces the probability of thrombotic events during percutaneous
coronary intervention (11).
Certain studies have demonstrated that tirofiban can enhance
neurological function 90 days after acute ischemic stroke (12). Additionally, it has been indicated
that intravenous tirofiban markedly improved the clinical outcomes
of patients with PIS (13).
However, it has also been associated with a higher occurrence of
fatal intracranial hemorrhage (ICH) (14). Therefore, it is essential to study
both the therapeutic effect of tirofiban in patients with
progressive stroke and its associated safety profile.
The medical literature persists with controversy
regarding the impact of tirofiban on progressive stroke. The extant
evidence is predominantly derived from observational,
non-randomized or retrospective studies, with a paucity of
randomized controlled trials (RCTs). Therefore, a systematic review
of the included RCTs and retrospective and cohort studies was
conducted to assess their safety and efficacy, providing reliable
evidence.
Patients and methods
Registration
This meta-analysis was reported in accordance with
the Preferred Reporting Items for Systematic reviews and
Meta-Analyses (PRISMA) guidelines (15). It has been registered at PROSPERO
(https://www.crd.york.ac.uk/PROSPERO/)
under the ID no. CRD42025633357.
Search strategy
The relevant literature was searched using the
following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/), Web
of Science (https://www.webofscience.com/), China National
Knowledge Infrastructure (CNKI; https://www.cnki.net/), WanFang Data (https://www.wanfangdata.com.cn/), China Science
and Technology Journal Database (VIP; http://www.cqvip.com/) and Sinomed (http://www.sinomed.ac.cn/). The objective was to
identify RCTs and cohort studies, comparing tirofiban combined with
oral antiplatelet drugs to oral antiplatelet drugs alone in
treating patients with PIS. The comprehensive search strategy
relied on the following search terms: (‘PIS’, ‘progressive cerebral
infarction’ or ‘progressive stroke’ or ‘progressive cerebral
thrombosis’ or ‘early neurological deterioration’) and
(‘tirofiban’). Furthermore, the reference lists of eligible studies
and related reviews were manually screened to identify other
potentially relevant literature for inclusion. The search was
conducted on May 5th, 2025, covering literature published up to May
5th, 2025 without any language restrictions, and strictly followed
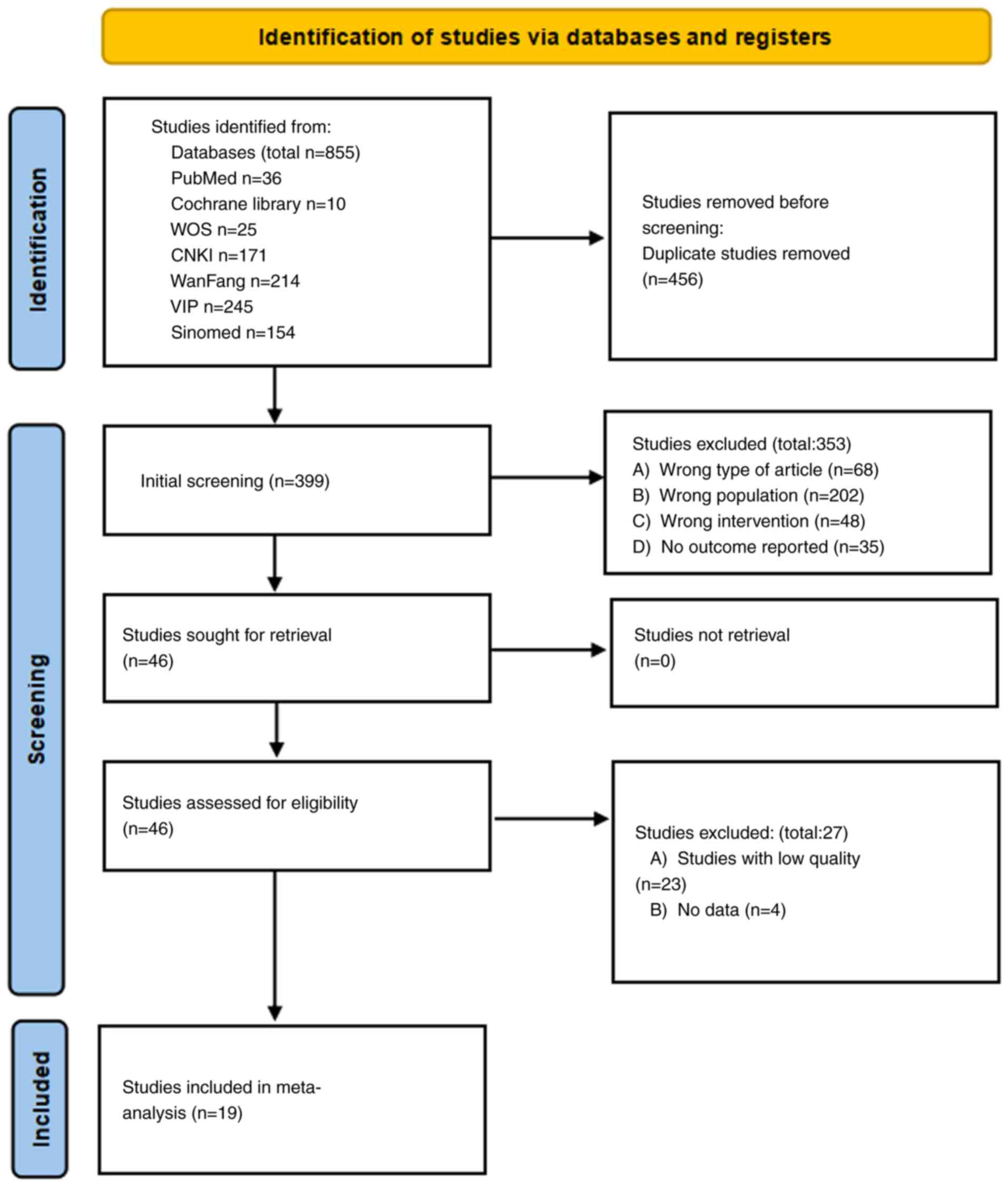
the PRISMA 2020 guidelines. The PRISMA flowchart (Fig. 1) details the study selection
process.
Inclusion and exclusion criteria
The study's inclusion criteria were defined as
follows: First, the study type was confined to prospective or
retrospective cohort studies and RCTs. Observational studies were
included despite their inherent risk of bias (e.g., selection bias,
unmeasured confounding). The Newcastle-Ottawa Scale (NOS) was used
to assess their quality, although residual bias might persist
(16). These studies were designed
to explore the application of tirofiban in combination with oral
antiplatelet drugs in treating patients diagnosed with PIS,
irrespective of whether blinding procedures were incorporated.
Secondly, PIS is defined as an increase of ≥2 points from baseline
in the National Institutes of Health Stroke Scale (NIHSS) score
(17) within 72 h of stroke onset.
The included studies used different definitions, but all met the
core criterion of neurological deterioration within 72 h. The
subjects of this study were carefully selected. The subjects were
patients with PIS who had a persistent decline in neurological
function after onset, an NIHSS score of no less than 2 and an onset
time within 72 h. Thirdly, concerning the intervention measures,
the control group was subjected to conventional treatment along
with oral antiplatelet drug therapy. By contrast, the experimental
group was further administered tirofiban in addition to the control
group's treatment protocol. Lastly, the outcome measures were
bifurcated into efficacy and safety measures. The efficacy measures
encompassed the NIHSS score, modified Rankin scale (mRS) score
(18), activities of daily living
(ADL) scores (19), Barthel index
(BI) scale scores (19), platelet
aggregation rate (PAgR) and platelet adhesion rate (PAdR) (20,21).
The safety measures comprised the incidence of intracranial
hemorrhage, hemorrhage in other systems, mortality rate and serious
adverse events. The exclusion criteria were single-arm trials,
animal studies, conference abstracts, case reports and any reports
from which data cannot be extracted based on published articles.
Also excluded were studies focusing on non-PIS during the entire
surgical process, those where the intervention did not involve the
combination of tirofiban with oral antiplatelet drugs and studies
in which each group had fewer than 25 participants.
Data extraction
A total of two authors (YY and YFJ) conducted the
literature screening independently and then cross-verified the
results. In the event of any disagreement, they consulted a third
researcher until a consensus was ultimately reached. The data
retrieved from the literature comprised the first author's name,
publication year, study type, sample size, therapeutic outcome and
safety outcome. The efficacy outcomes were as follows: i) 3-month
mRS score of 0-2, ii) NIHSS score, iii) ADL score, iv) PAgR, v)
PAdR and vi) effective rate. The following safety outcomes were
evaluated: i) Symptomatic ICH (sICH), ii) other systemic
hemorrhage, iii) mortality rate and iv) serious adverse events.
Quality assessment
The quality of RCTs was appraised using the Cochrane
Risk of Bias Tool (22). For
observational studies, quality was assessed using the NOS, which
assesses three domains: Participant selection (0 to 4 points),
inter-group comparability (0 to 2 points) and outcome assessment (0
to 3 points), with a maximum score of 9. It is important to note
that no NOS score threshold was pre-set as an inclusion criterion;
all observational studies that met the initial inclusion criteria
(e.g., study design, population, interventions) were included in
the meta-analysis, regardless of their potential NOS score. After
assessing quality, it was found that the six included observational
studies all had NOS scores between 7 and 9.
Statistical analysis
The data analysis was conducted with Stata software
(version 17.0; StataCorp. LP). The random-effects model was
employed for categorical variables to calculate the pooled
estimates of odds ratios (ORs), while the mean differences were
computed for continuous variables. Heterogeneity was evaluated via
the Cochran Q test and the I² test. When I² was <50% or P>0.1
(indicating low heterogeneity), the fixed-effects model was
utilized. Continuous data were evaluated using the standardized
mean difference (SMD) and its 95% CI, whereas binary data were
analyzed with the OR and 95% confidence interval. P<0.05 was
regarded as statistically significant. Publication bias was
evaluated by visually examining the data and calculating the
P-value with Egger's test and Begg's test. If publication risk of
bias was detected, the nonparametric trim-and-fill method was
further used to adjust for the potential effect of missing studies.
Additionally, subgroup analyses were performed to explore the
potential sources of heterogeneity across studies. Based on
clinical relevance, subgroup analyses were pre-defined according to
the antiplatelet treatment regimens (monotherapy and dual therapy),
but comparisons between subgroups should be considered exploratory
analyses. To explore the potential source of heterogeneity,
subgroup analyses were further performed based on the duration of
tirofiban administration (short course vs. long course). A short
course was defined as tirofiban infusion ≤48 h and a long course as
>48 h, according to the median duration reported in included
studies.
Sensitivity analyses
To further validate the robustness of the primary
study results and to account for possible biases in the mixed study
design, sensitivity analyses were performed for only 13 RCTs. The
statistical methods were consistent with the main analysis (using
Stata 17.0 software; ORs were used for categorical outcomes and
SMDs for continuous outcomes).
Results
Literature search results
A total of 855 relevant entries were identified
through database searches. After eliminating 456 redundant
articles, excluding 353 studies upon reading the titles, abstracts
and perusing the full texts, 46 full texts remained for detailed
evaluation. Among these, 27 articles were further excluded (23 due
to low quality and 4 due to unavailable data). Eventually, 19
articles were included (10,23-40),
of which 12 were published in Chinese and 7 in English. Fig. 1 illustrates the detailed screening
process.
Characteristics of included
studies
A total of 19 studies were included in the
meta-analysis. All articles were published between 2020 and 2024. A
total of 3,667 patients were included, among which 1,859 were in
the experimental group and 1,808 were in the control group.
Table I presents the key
characteristics of the individual studies included in the
analysis.
 | Table IBaseline characteristics of the
included studies. |
Table I
Baseline characteristics of the
included studies.
| Author/s, year | Year | Design | Sample | T | C | Safety outcome | Efficacy
outcome | Measures | Maintenance of 0.1
µg/(kg min) | Treatment
course | (Refs.) |
|---|
| Zi et al,
2023 | 2023 | RCT | 1177 | 606 | 571 | ①③④ | 90mRS (0-2) | 1 | for 48 h | SC | (10) |
| Han et al,
2022 | 2022 | RCT | 357 | 177 | 180 | ①②③ | 90mRS (0-2) | 1 | for 48 h | SC | (23) |
| Zhao et al,
2024 | 2024 | RCT | 384 | 196 | 188 | ①③④ | 90mRS (0-2) | 1 | for 71.5 h | LC | (24) |
| Chen et al,
2024 | 2024 | RCT | 70 | 35 | 35 | ②④ | Evaluation of
therapeutic efficacy, NIHSS, ADL | 2 | for 72 h | LC | (25) |
| Sun et al,
2022 | 2022 | RCT | 127 | 68 | 59 | NA | PAdR, PAgR | 1 | for 24 h | SC | (26) |
| Liu et al,
2024 | 2024 | RCT | 80 | 40 | 40 | ②④ | Evaluation of
therapeutic efficacy, NHISS | 2 | for 48 h | SC | (27) |
| Li et al,
2024 | 2024 | RCT | 108 | 54 | 54 | ②④ | mRS, NHISS,
ADL | 1 | for 48 h | SC | (28) |
| Li et al,
2024 | 2024 | RCT | 80 | 40 | 40 | ② | Evaluation of
therapeutic efficacy | 2 | for 48 h | SC | (29) |
| Luo et al,
2020 | 2020 | RCT | 90 | 45 | 45 | NA | NHISS, BI, PAdR,
PAgR | 2 | for 2 w | LC | (30) |
| Xiao et al,
2024 | 2024 | RCT | 66 | 33 | 33 | ②④ | Evaluation of
therapeutic efficacy, NHISS, ADL, PAdR, PAgR | 1 | for 2 w | LC | (31) |
| Duan et al,
2024 | 2024 | RCT | 120 | 60 | 60 | ①② | Evaluation of
therapeutic efficacy, NHISS, BI, PAdR, PAgR | 2 | for 48 h | SC | (32) |
| Bian et al,
2024 | 2024 | RCT | 317 | 167 | 150 | ①② | NHISS, BI, PAdR,
PAgR | 2 | for 48 h | SC | (33) |
| Liao et al,
2020 | 2020 | RCT | 106 | 53 | 53 | ①② | Evaluation of
therapeutic efficacy, mRS, NHISS, PAdR, PAgR | 2 | for 108 h | LC | (34) |
| Du et al,
2022 | 2022 | CS | 150 | 75 | 75 | ①② | Evaluation of
therapeutic efficacy, mRS, NHISS, BI, PAdR, PAgR | 2 | for 72 h | LC | (35) |
| Wang et al,
2020 | 2020 | CS | 108 | 54 | 54 | ①②③ | mRS | 2 | for 24 h | SC | (36) |
| Zhang et al,
2023 | 2023 | RS | 75 | 34 | 41 | ① | 90mRS (0-2),
NHISS | 1 | for 48 h | SC | (37) |
| Zhang et al,
2021 | 2021 | CS | 104 | 52 | 52 | ①②③ | Evaluation of
therapeutic efficacy, mRS, NHISS | 2 | for 24 h | SC | (38) |
| Ma et al,
2020 | 2020 | CS | 93 | 46 | 47 | ①② | mRS | 2 | for 24 h | SC | (39) |
| Jiang et al,
2020 | 2020 | CS | 55 | 24 | 31 | ①② | mRS, NHISS | 2 | for 24 h | SC | (40) |
Quality assessment
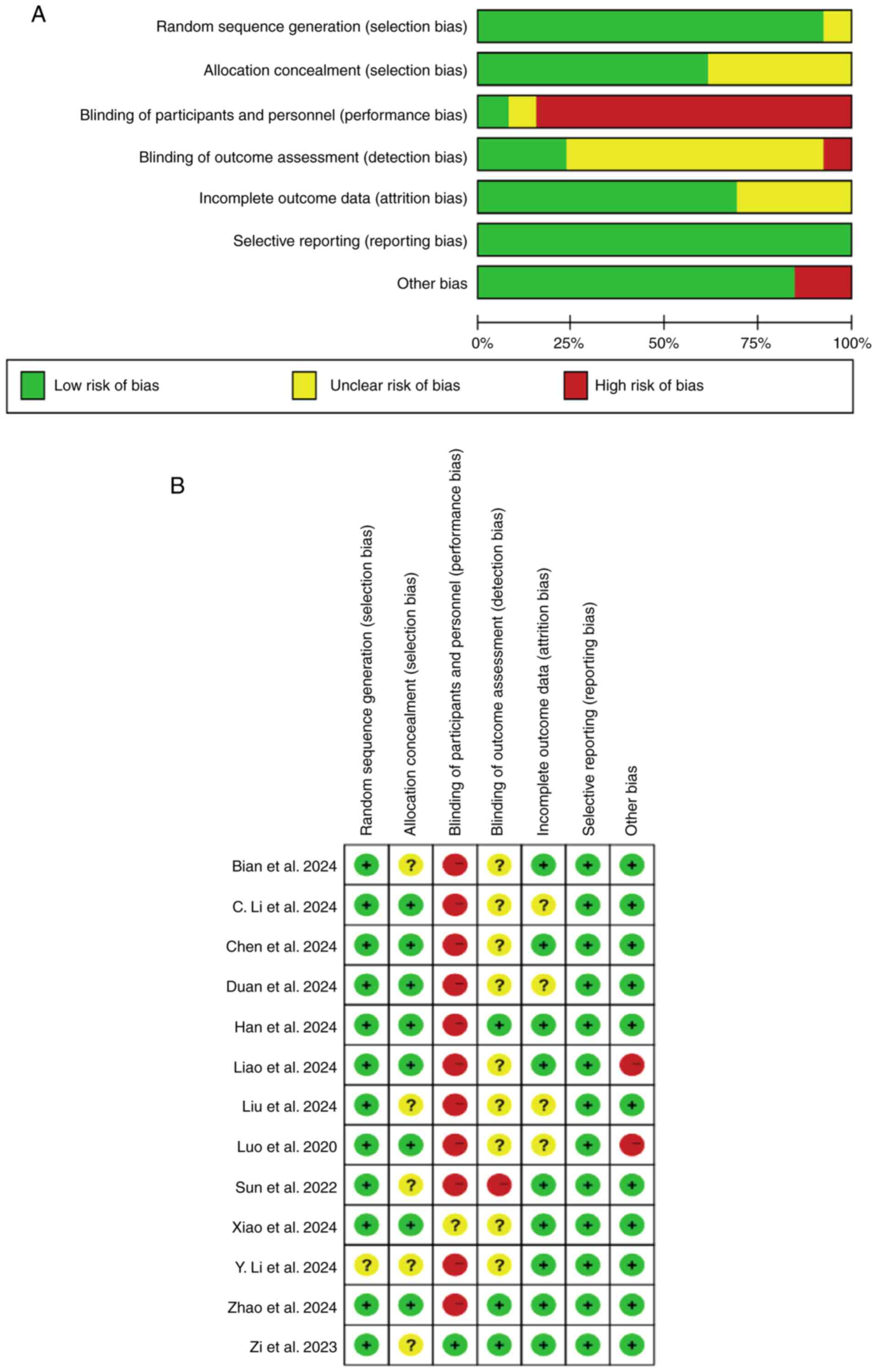
Two authors (YY and YFJ) assessed the quality of 13
RCTs using the Cochrane risk of bias tool. All studies were
randomised, with one study not describing a specific method of
randomisation and 12 studies having specified it. All RCTs were at
low risk of attrition bias. In addition, none of the 13 studies
selectively reported their findings and only four studies had
incomplete outcome measures. Regarding blinding bias of
participants and investigators, 12 of the 13 RCTs had high or
unclear risk for blinding and five studies were inadequately
allocated for concealment (selection bias). Risk of bias maps were
generated using Review Manager 5.3 (https://tech.cochrane.org/revman) and the specific
results are shown in Fig. 2. The
NOS scale was used to assess six observational studies (see
Table SI for full assessment
details). No study was excluded based on the NOS score; all six
studies met the initial inclusion criteria (e.g., focusing on PIS
and comparing ticagrelor combined with oral antiplatelet agents vs.
oral antiplatelet agents alone) and were included in the analysis.
Their NOS scores ranged from 7 to 8 (with a median of 8),
demonstrating high scores in selection (all ≥3/4) and comparability
(all ≥1/2), indicating a low risk of bias.
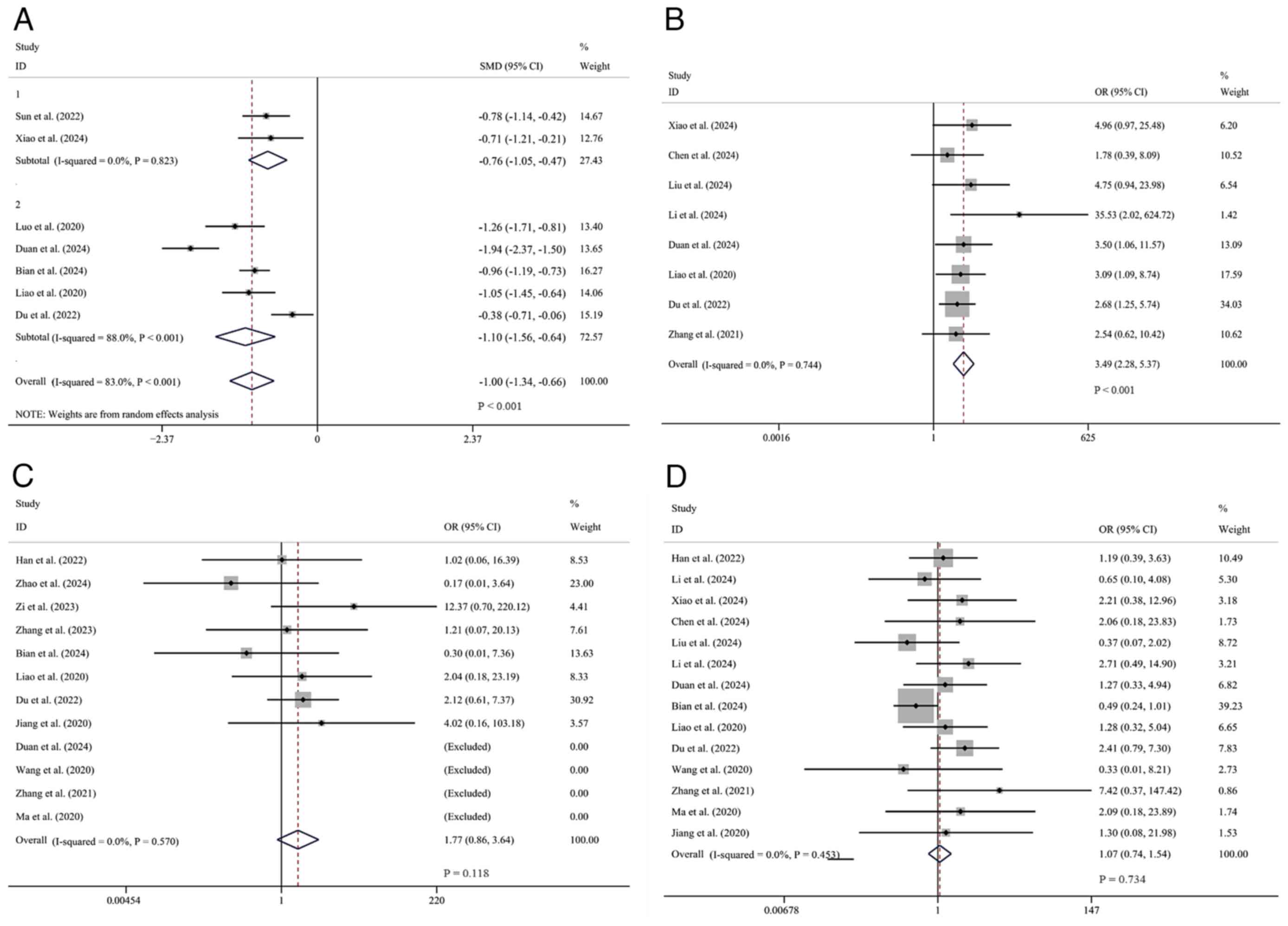
Efficacy outcomes. mRS score (0-2
within 3 months)
A total of 4 studies (10,23,24,37)
reporting on the occurrence of mRS scores ranging from 0 to 2
within 3 months were comprehensively analyzed. Data were obtained
from 2,032 patients, among whom 1,028 cases received tirofiban in
combination with oral antiplatelet drugs, while 1,004 cases were
treated only with oral antiplatelet drugs. Heterogeneity analysis
showed I²=0% and P>0.1 (low heterogeneity), so the fixed-effects
model was used. The pooled OR showed the experimental group had a
higher 3-month mRS 0-2 rate [OR=1.31, 95% CI (1.10, 1.58), P=0.003]
(Fig. 3A).
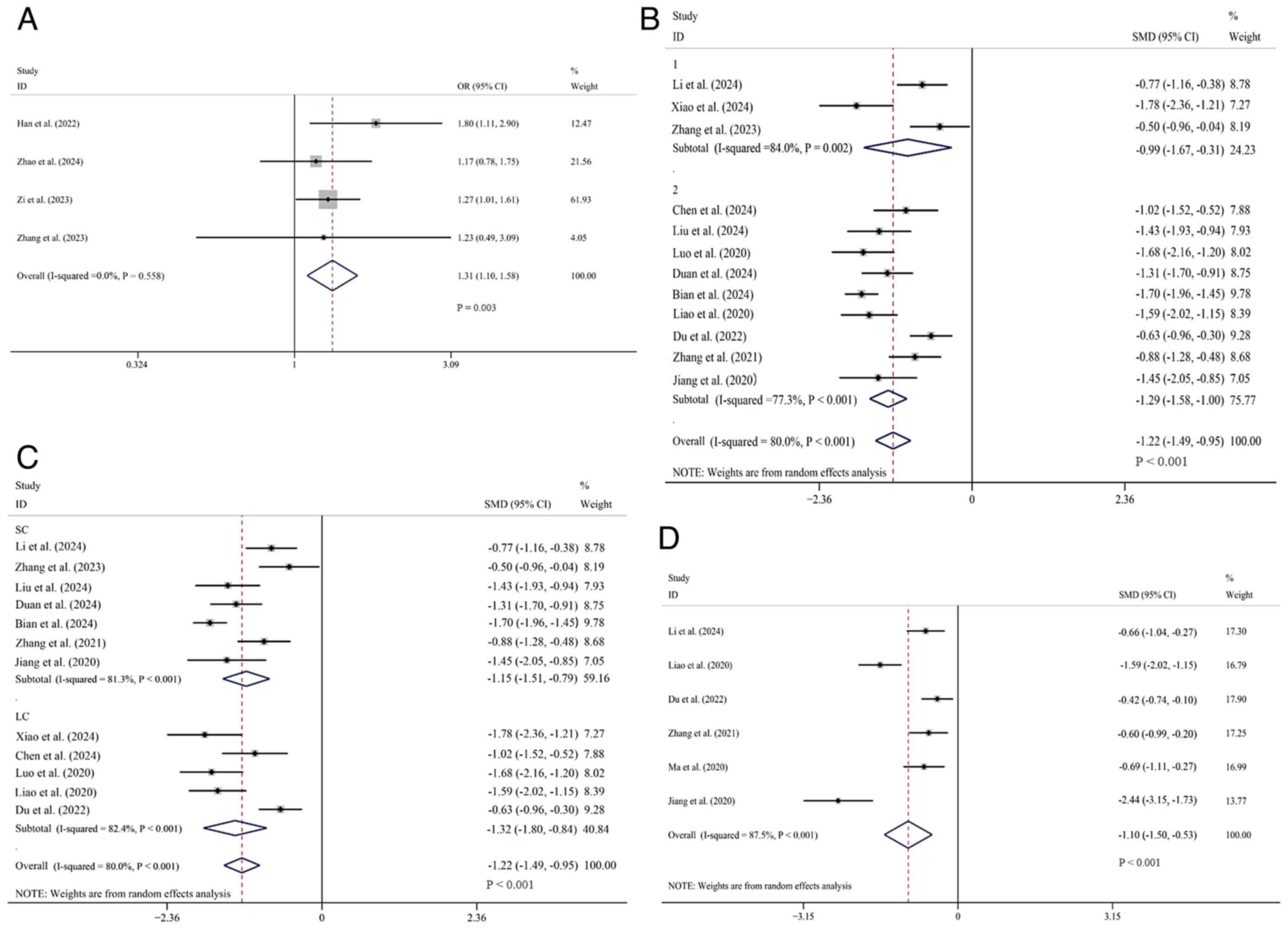 | Figure 3Forest plots comparing tirofiban vs.
non-tirofiban therapy. (A) Proportion of patients with mRS scores
0-2 at 3 months. (B) Subgroup analysis of NIHSS scores. (C) NIHSS
score subgroup analysis stratified by tirofiban treatment duration.
(D) mRS scores. mRS, modified Rankin Scale; NHISS, National
Institutes of Health Stroke Scale; ADL, Activities of Daily Living;
1, oral single antiplatelet drug; 2, oral dual antiplatelet drugs;
LC, long course of treatment; SC, short course of treatment; SMD,
standard mean deviation; CI, confidence intervals; OR, odds
ratio. |
NHISS score. A total of 19 studies comparing
the NHISS scores after treatment between the two groups were
comprehensively analyzed. Among these, the data of 3 studies
(23,29,39)
did not follow a normal distribution and the specific arithmetic
means and standard deviations were not provided explicitly in 4
studies (10,24,26,36).
For the remaining 12 studies (25,27,28,30-35,37,38,40),
1,341 patients were included, with 672 placed in the experimental
group and 669 in the control group. I²=80% and P<0.1 (high
heterogeneity), so the random-effects model was used. The result
demonstrated that the experimental group had a significantly lower
NHISS score compared to the control group [SMD=-1.22, 95% CI
(-1.49, -0.95), P<0.01]. Subgroup analysis was subsequently
conducted based on different intervention measures, using tirofiban
combined with oral single antiplatelet drugs or oral dual
antiplatelet drugs. In the oral single antiplatelet drug subgroup,
three studies involving 249 patients were incorporated, with 121 in
the experimental group and 128 in the control group (28,31,37).
Overall, the NHISS score of the experimental group was
significantly lower than that of the control group [SMD=-0.99, 95%
CI (-1.67, -0.31), P=0.004]. A total of nine studies, comprising
1,092 patients, were included in the oral dual antiplatelet drug
subgroup (25,27,30,32-35,38,40).
The experimental group comprised 551 participants, while the
control group included 541 individuals. The analysis revealed that
the NHISS score for the experimental group was significantly lower
than that for the control group [SMD=-1.29, 95% CI (-1.58, -1.00),
P<0.01]. The difference in the NHISS score between the
experimental and control groups was more noticeable in the
tirofiban combined with oral dual antiplatelet drug subgroup than
in the subgroup using oral single antiplatelet drugs (Fig. 3B).
At the same time, an additional subgroup analysis
included 12 studies for which data were available. In seven studies
(n=859) in the short-course subgroup (27,28,32,33,37,38,40),
NIHSS scores were significantly lower in the experimental group
compared with the control group [SMD=-1.15, 95% CI (-1.51, -0.79),
P<0.001]. The heterogeneity in this subgroup was high (I²=81.3%,
P<0.001). In five studies (n=482) in the long-duration subgroup
(25,30,31,34,35),
NIHSS scores were also significantly lower in the trial group
[SMD=-1.32, 95% CI (-1.80, -0.84), P<0.001], with similarly high
heterogeneity (I²=82.4%, P<0.001). The overall effect remained
significant [SMD=-1.22, 95% CI (-1.49, -0.95), P<0.001] under
the random-effects model, with considerable heterogeneity observed
across all studies (I²=80.0%, P<0.001). This suggests that
tirofiban improved neurological function regardless of the duration
of treatment, but there was no reduction in heterogeneity in either
subgroup (Fig. 3C).
mRS score. A total of 6 studies (28,34,35,38-40)
comparing the mRS scores after treatment were comprehensively
analyzed. Data were retrieved from these studies, which involved
616 patients. Among them, 304 cases were included in the
experimental group and 312 cases in the control group.
Heterogeneity was significant (I2=87.5%, P<0.1), and
a random-effects model was used. Overall, it was demonstrated that
the mRS score of the experimental group was markedly lower than
that of the control group [SMD=-1.10, 95% CI (-1.50, -0.53),
P<0.01] (Fig. 3D). Notably, a
higher mRS score indicates more severe symptoms.
ADL score. A total of 3 studies (25,28,31)
comparing ADL scores were comprehensively analyzed. Data were
obtainable from these studies. There were 244 patients in total,
with 122 in the experimental group and 122 in the control group.
The heterogeneity was significant (I²=78.9%, P<0.1) and a
random-effects model was employed. Overall, the experimental group
was shown to be superior to the control group in ADL scores
[SMD=1.19, 95% CI (0.58, 1.80), P<0.01] (Fig. 4A).
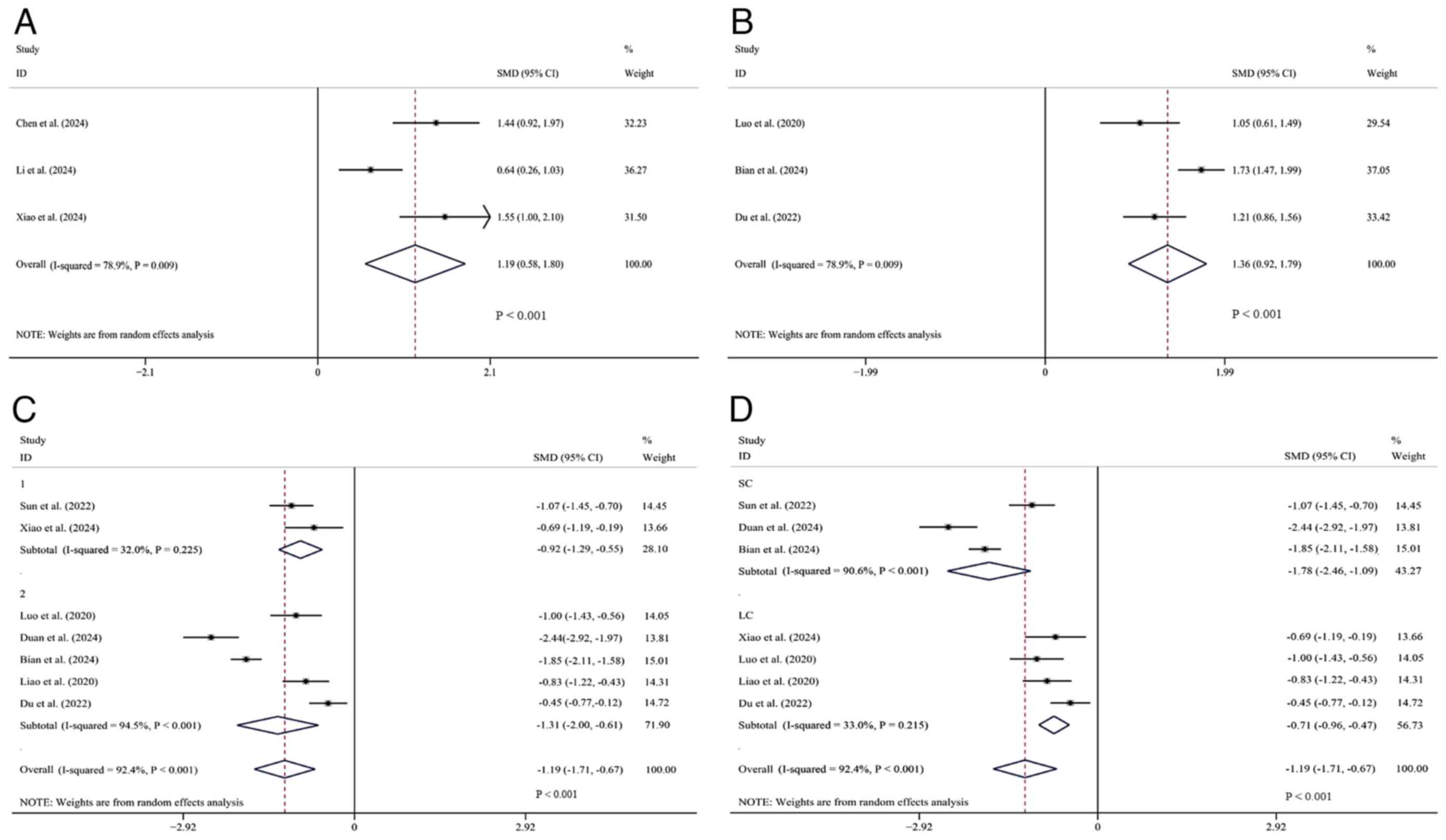 | Figure 4Forest plots comparing tirofiban
therapy vs. non-tirofiban therapy for (A) ADL score, (B) BI score,
(C) subgroup analysis of PAgR, and (D) subgroup analysis of PAgR
based on tirofiban treatment duration. ADL, Activities of Daily
Living; BI, Barthel Index; PAgR, platelet aggregation rate; 1, oral
single antiplatelet drug; 2, oral dual antiplatelet drugs; LC, long
course of treatment; SC, short course of treatment; SMD, standard
mean deviation; CI, confidence intervals. |
BI index. A total of 3 studies (30,33,35)
that reported on the BI index were included. The data retrievable
from these studies involved a total of 557 patients, with 287 in
the experimental group and 270 in the control group. A
random-effects model was used due to significant heterogeneity
(I²=78.9%, P<0.1). Overall, the SMD was significantly >0,
which indicated that the BI index of the experimental group was
superior to that of the control group [SMD=1.36, 95% Cl (0.92,
1.79), P<0.01] (Fig. 4B).
PAgR. A total of 7 studies (26,30-35)
involving the measurement of PAgR were comprehensively analyzed.
Data can be obtained from these studies, with 976 patients in
total, 501 in the experimental groups and 475 in the control
groups. Significant heterogeneity was observed (I²=92.4%,
P<0.001) and a random-effects model was applied. It shows that
the PAgR in the experimental group was significantly lower compared
with that in the control group [SMD=-1.19, 95% CI (-1.71, -0.67),
P<0.01]. Further subgroup analysis was based on different
intervention measures. In the single antiplatelet drug subgroup,
two studies included 193 patients (101 in experimental group and 92
in control group) (26,31), and the PAgR of the experimental
group was lower [SMD=-0.92, 95% CI (-1.29, -0.55), P<0.01]. In
the oral dual antiplatelet drug subgroup, 5 studies included 783
patients (400 in experimental group and 383 in control group)
(30,32-35),
and the PAgR of the experimental group was lower [SMD=-1.31, 95% CI
(-2.00, -0.61), P<0.01]. Obviously, in terms of the PAgR
results, the experimental group had a more significant decrease in
PAgR than the control group in the subgroup using a combination of
tirofiban and oral dual antiplatelet drugs (Fig. 4C).
Another subgroup analysis included 7 studies with
available data. In the short-term subgroup with 3 studies (n=564)
(26,32,33),
the PAgT in the experimental group was significantly reduced
compared to the control group [SMD=-1.78, 95% CI (-2.46, -1.09),
P<0.001]. This short-term subgroup exhibited substantial
heterogeneity (I²=90.6%, P<0.001). In the long-term subgroup
with 4 studies (n=412) (30,31,34,35),
the experimental group also showed a significant reduction in PAgR
[SMD=-0.71, 95% CI (-0.96, -0.47), P<0.001]. In contrast, this
long-term subgroup showed low heterogeneity (I²=33.0%, P=0.215).
The overall effect was significant [SMD=-1.19, 95% CI (-1.71,
-0.67), P<0.001] under the random-effects model. However,
heterogeneity was observed across all studies (I²=92.4%,
P<0.001). This indicates that the inhibitory effect of tirofiban
on platelet aggregation was more pronounced in the short-term
subgroup, while the heterogeneity in the long-term subgroup
significantly decreased (Fig.
4D).
PAdR. Data were obtainable from 7 studies
(26,30-35),
encompassing a total of 976 patients, among whom 501 were allocated
to the experimental group and 475 to the control group. A
random-effects model was used owing to significant heterogeneity
(I²=83.0%, P<0.1). Overall, it was demonstrated that the
experimental group had a lower PAdR compared to the control group
[SMD=-1.00, 95% CI (-1.34, -0.66), P<0.01]. Subgroup analysis
was carried out based on distinct intervention modalities,
specifically, tirofiban in combination with oral single
antiplatelet or oral dual antiplatelet drugs. In the oral single
antiplatelet drug subgroup, two studies (26,31)
were incorporated, comprising 193 patients in total. Of these, 101
participants were allocated to the experimental group and 92 to the
control group. It was ascertained that the experimental group had a
lower PAdR than the control group [SMD=-0.76, 95% CI (-1.05,
-0.47), P<0.01]. In the oral dual antiplatelet drug subgroup,
five studies (30,32-35)
were included, involving 783 patients, with 400 in the experimental
group and 383 in the control group. The results indicated that the
experimental group had a lower PAdR than the control group
[SMD=-1.10, 95% CI (-1.56, -0.64); P<0.01]. Evidently, for the
PAdR results, the experimental group in the subgroup using
tirofiban and oral dual antiplatelet drugs had a significantly more
significant PAdR reduction than the control group. Furthermore,
this reduction was more pronounced than the subgroup using oral
single antiplatelet drugs (Fig.
5A).
Effective rate. A total of 8 studies
(25,27,29,31,32,34,35,38)
regarding the determination of the effective rate were
comprehensively analyzed. Data were obtainable from these studies,
encompassing 776 patients, among whom 388 were in the experimental
group and 388 in the control group. Heterogeneity was negligible
(I²=0.0%, P=0.744), and thus a fixed-effects model was used.
Overall, it was found that the experimental group had an advantage
over the control group [OR=3.49, 95% CI (2.28, 5.37), P<0.01]
(Fig. 5B).
Safety outcomes. Symptomatic
intracerebral hemorrhage
A total of 12 studies (10,23,24,32-40)
focusing on sICH were comprehensively analyzed. Data were
obtainable from these studies, including a total of 3,086 patients,
with 1,574 in the experimental group and 1,512 in the control
group. No significant heterogeneity was found (I²=0.0%, P>0.1),
and a fixed-effects model was used. Overall, no significant
difference was found in the incidence of intracranial hemorrhage
between the two groups [OR=1.77, 95% CI (0.86, 3.64), P=0.118]
(Fig. 5C).
Other intracranial hemorrhage. A total of 14
studies (23,25,27-29,31-36,38-40)
concerning other intracranial hemorrhages were comprehensively
analyzed. Data were obtained from these studies, comprising 1,835
patients, 920 in the experimental groups and 915 in the control
groups. No significant heterogeneity was detected (I²=0.0%,
P>0.1), and a fixed-effects model was employed. Overall, no
significant difference in the incidence of cerebral hemorrhage was
observed between the two groups [OR=1.07, 95% CI (0.74, 1.54),
P=0.734] (Fig. 5D).
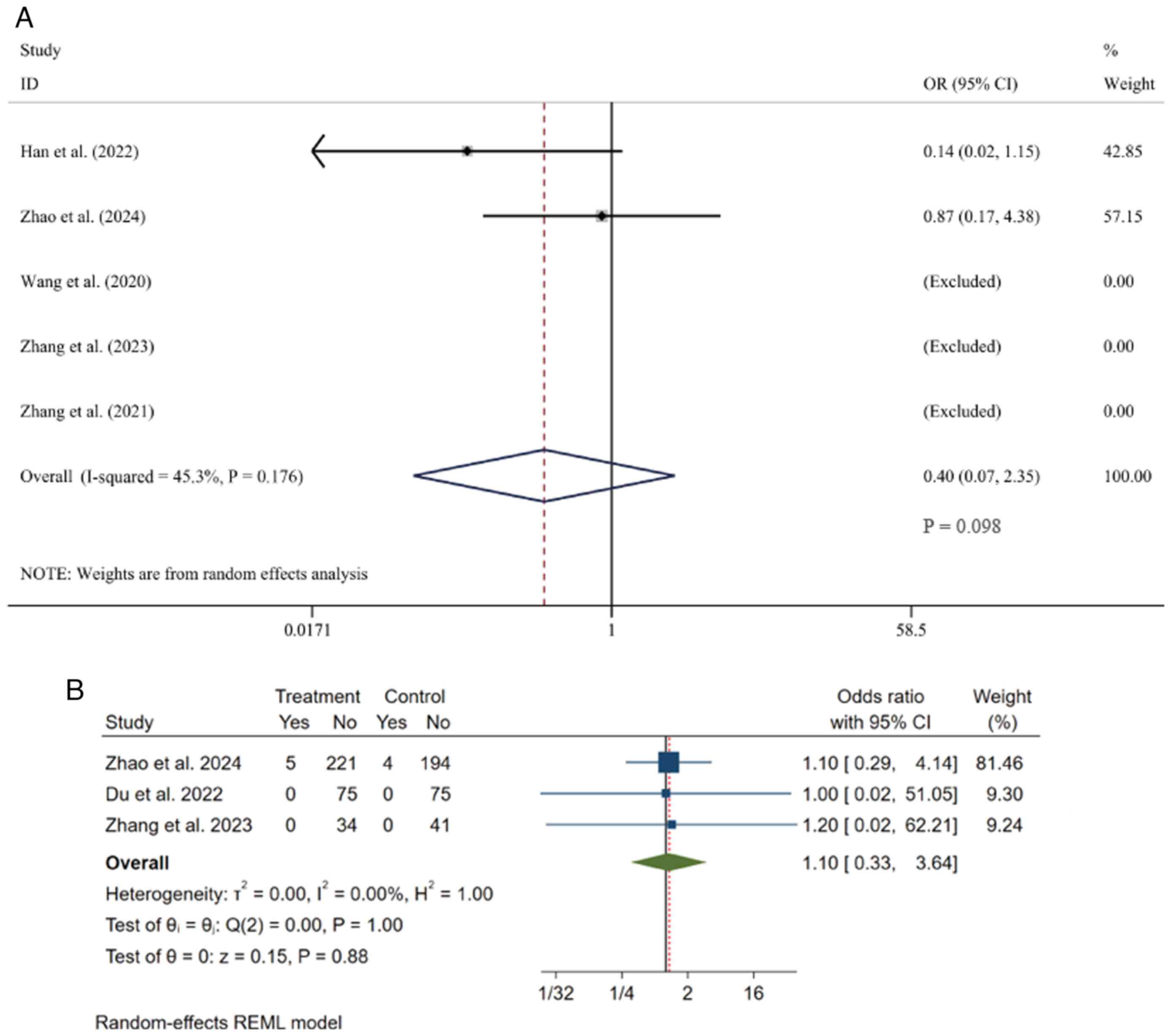
Mortality rate. A total of 5 studies
(23,24,36-38)
regarding the mortality rate were comprehensively analyzed. Data
were obtainable from these studies, which included 1,068 patients,
among whom 543 were in the experimental group and 525 in the
control group. Given the presence of moderate heterogeneity
(I²=45.3%), a random-effects model was used for the meta-analysis.
Overall, the two groups showed no statistically significant
difference in mortality rates [OR=0.40, 95% CI (0.07, 2.35),
P=0.098] (Fig. 6A).
Serious adverse events. A total of 3 studies
(24,35,37),
involving 649 patients (335 in the experimental group and 314 in
the control group), were included in the meta-analysis of serious
adverse events. Under the random-effects model, the analysis showed
no heterogeneity among the studies (I²=0%, P=1.00), indicating that
pooling their results was appropriate. The pooled analysis
demonstrated that there was no statistically significant difference
in the incidence of serious adverse events between the two groups
[OR=1.10, 95% CI (0.33, 3.64), P=0.88 for effect] (Fig. 6B).
Sensitivity analyses
To assess the robustness of the results of the
analyses of the present study for possible bias due to study design
heterogeneity, pre-specified sensitivity analyses were performed,
limited to RCTs. Functional recovery (90mRS 0-2) showed the same
effect size (OR=1.31), there was a 95% CI overlap (1.10-1.58 vs.
1.06-1.59) and the I² increased from 0 to 2.6%. The NIHSS showed a
15% increase in effect size (SMD=-1.22 to -1.41) and the I²
decreased from 80 to 67%. The safety profile remained stable, with
no substantial change in the risk of sICH (OR=1.773 vs. 1.524) or
mortality (OR=0.40). It is important to note that secondary
outcomes including mRS, ADL and BI indices remained consistent in
the direction of the effect, although only RCTs had wider CIs. When
limited to RCT evidence, the persistence of treatment effects
confirms the reliability of the primary conclusions of the present
study (Table SII). The robustness
of the main study results was verified. At the same time, future
RCTs using standardized regimens (e.g., unified tirofiban dosing
regimens) may reduce residual heterogeneity.
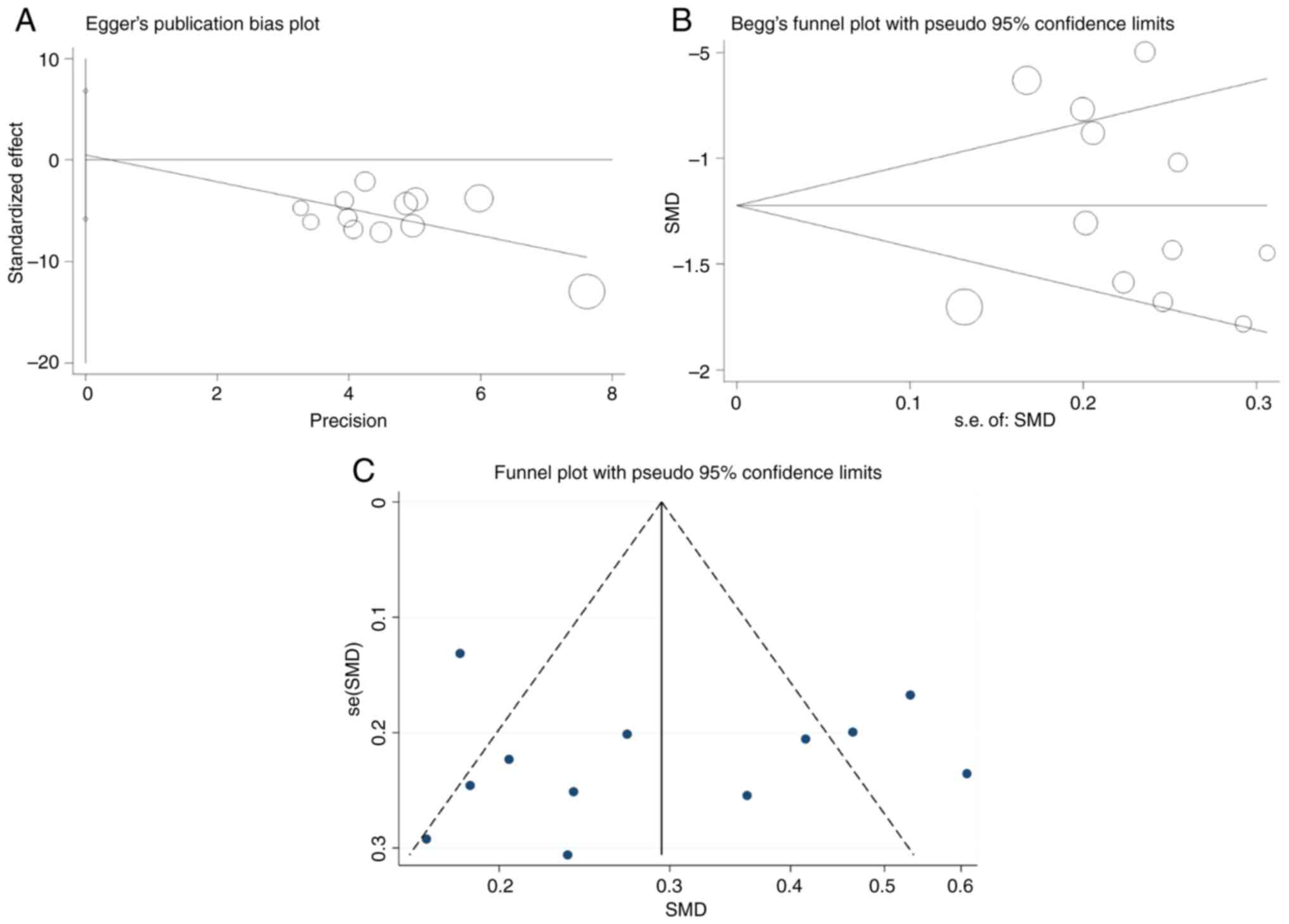
Publication bias
Publication bias was assessed using Egger's test and
Begg's test, which showed no significant evidence of publication
bias (P=0.861) (Fig. 7A), which
was also confirmed by Begg's test (P=0.373) (Fig. 7B), indicating that the pooled
effect size was not significantly affected by publication bias.
Visual examination of the funnel plot (Fig. 7C) revealed a slight asymmetry, but
in combination with statistical tests, this asymmetry is more
likely to be due to random variation in a small-sample study than
to systematic bias. Overall, the study was at low risk of
publication bias and the reliability of the results was somewhat
supported.
Impact of methodological biases on
outcomes
Inadequate allocation concealment and unblinding can
lead to bias in assessing treatment effects. However, there are
three pieces of evidence supporting the reliability of the primary
conclusion: First, the objective safety outcomes remain unaffected;
they rely on imaging or laboratory confirmation, and are less
susceptible to detection bias. Second, the results from different
study designs are consistent: The forest plots (Fig. 3) show that the effect direction of
all efficacy outcomes (e.g., NIHSS, mRS, ADL) is consistent in both
RCTs and observational studies. Third, the sensitivity analysis
confirms that excluding studies with a high risk of bias did not
result in substantial changes to the effect estimates [e.g., after
removing three non-blinded RCTs, the mean difference in NHISS
changed from -1.22 to -1.09, as indicated by the sensitivity
analysis (data not shown)]. Therefore, although the extent of
functional improvement may be moderately overestimated, the
treatment effect shows a positive trend, with statistical
significance and safety thoroughly validated.
Discussion
The present meta-analysis aimed to confirm the
safety and effectiveness of combining tirofiban with oral
antiplatelet drugs for treating patients with PIS. A total of 3,667
patients from 19 articles were incorporated in the analysis. This
combination can improve the patients' neurological function, as
indicated by the NIHSS, ADL and mRS scores. The experimental group,
receiving tirofiban injection, had a significantly better
neurological improvement effect and comparable safety. In addition,
this analysis also showed that tirofiban has a more substantial
effect to inhibit thrombosis formation.
Prior to this, meta-analyses have indicated that
tirofiban may enhance neurological functional outcomes and lower
the 3-month mortality rate of patients with acute ischemic stroke
treated with endovascular methods (41,42).
Furthermore, tirofiban has been shown to have the effects of
improving prognoses and reducing patient mortality in those
undergoing mechanical thrombectomy (43) or those treated with a combination
of tirofiban and intravenous thrombolysis for acute ischemic stroke
(44). However, for PIS, no
meta-analysis to date has evaluated the efficacy of combining
tirofiban with oral antiplatelet agents. During the treatment of
PIS, this combination therapy represents a promising research
direction.
Atherosclerosis is an important pathological basis
affecting cerebrovascular and cardiovascular diseases. Platelet
activation is the key step, thus becoming an important potential
target for antiplatelet therapy (45). At present, the commonly utilized
antiplatelet drugs in the clinical setting encompass cyclooxygenase
inhibitors represented by aspirin, P2Y12 receptor antagonists
represented by clopidogrel and glycoprotein (GP)IIb/IIIa receptor
antagonists represented by tirofiban, among others. Aspirin has
emerged as the gold standard for antiplatelet treatment (46-48).
Tirofiban is a new type of antiplatelet aggregation drug that
reduces thrombosis by inhibiting the formation of fibrinogen (the
bridge between adjacent platelets) (49,50).
By contrast, compared to tirofiban, aspirin and clopidogrel have
limited ability to affect other molecular pathways, particularly
GPIIb/IIIa, as the receptor activation state is hardly influenced.
As a result, they may be less effective in suppressing platelet
aggregation and thrombosis formation (51). Therefore, based on the analysis
results, aspirin, clopidogrel, or their combination was selected as
the control group for antiplatelet aggregation treatment. The total
effective rate of patients who received tirofiban injections was
higher than of those who did not. Furthermore, the improvement in
platelet aggregation rate and adhesion rate was also better in the
injection group than in the non-injection group.
Among them, antiplatelet aggregation is key to
treating progressive stroke (52).
In actual clinical practice, dual anti-platelet therapy is only
practical for ~70% of patients. For ~30% of patients, symptoms
continue to progress even after administering loading doses. The
possible reasons for this are as follows: Firstly, both aspirin and
clopidogrel act on the intermediate pathways of platelet
aggregation and their mechanisms of action do not fully cover all
the signaling pathways that trigger platelet aggregation. Secondly,
certain patients have clopidogrel resistance and the oral
absorption of this drug is relatively slow, thus failing to achieve
the desired effect (53,54). By contrast, tirofiban disrupts the
normal function of the platelet GPIIb/IIIa receptor, which
represents an effective mechanism for inhibiting thrombosis
formation. Additionally, tirofiban can reduce the release of
serotonin from platelets, thereby alleviating microcirculatory
vasospasm from a hemodynamic perspective (55).
In addition, tirofiban may inhibit the formation of
micro-thrombosis in capillaries. Experiments have shown that
tirofiban also exerts additional beneficial effects by modulating
cerebral microcirculation. As a GPIIb/IIIa inhibitor, it inhibits
the formation of micro-thrombosis by blocking the ultimate common
pathway of platelet aggregation, reducing the adhesion and
aggregation of platelets at the site of microvascular injury
(56,57). Experimental and clinical evidence
suggests that tirofiban reduces the release of the platelet-derived
vasoconstrictor serotonin (serotonin). Tirofiban, as a platelet
glycoprotein class IIb receptor antagonist, relieves
micro-vasospasm by inhibiting platelet glycoprotein receptors,
specifically binding to fibrinogen and blocking them, preventing
platelet aggregation, thereby reducing the release of serotonin
(58). Furthermore, tirofiban has
the characteristics of rapid onset and short half-life, with
platelet function recovering in ~50% of patients within 4 h after
drug discontinuation.
Although previous studies have reported an increased
risk of intracranial hemorrhage with tirofiban in patients with
acute ischemic stroke (14,36),
the present analysis focused specifically on patients with PIS and
did not find an increased risk of hemorrhage. This difference may
stem from the following key differences: First, the dosing regimen
used in the included studies of this meta-analysis was lower than
that commonly reported in neurointerventional settings [e.g., a
lower-dose regimen of 0.4 µg/kg/min for loading and 0.1 µg/kg/min
for maintenance in our cohort vs. a higher loading dose of 0.4
µg/kg/min for 30 min followed by 0.1 µg/kg/min maintenance, as used
in some prior stroke trials (14,36)], and it is established that higher
loading doses may increase the risk of bleeding (59). The cohort did not receive any
concomitant thrombolytic therapy, which is associated with an
increased risk of bleeding when synergistic with GPIIb/IIIa
inhibitors. Therefore, differences in patient selection and dosing
regimens collectively reduce the risk of bleeding in this study
cohort, resulting in inconsistent results from previous studies.
The present meta-analysis clarifies that tirofiban effectively
prevents platelet aggregation and thrombosis formation without
elevating the risk of bleeding events, and there was no statistical
difference in the safety outcomes.
Meanwhile, large RCTs, such as the CHANCE and POINT
trials, have consistently indicated that combining clopidogrel with
aspirin is more effective than aspirin alone in reducing the risk
of early neurological deterioration (60,61).
Furthermore, there is no difference in safety (62). When treating patients with acute
mild to moderate stroke, dual antiplatelet therapy may be a better
option than aspirin alone. In addition, this meta-analysis also
conducted a subgroup analysis. The experimental group receiving
tirofiban with oral dual antiplatelet drugs showed a significantly
greater reduction in NIHSS score, PAgR and PAdR than those
receiving tirofiban with oral single antiplatelet drugs.
However, this finding should be regarded as a
hypothesis rather than conclusive evidence. Given the lack of
direct comparisons of these treatment regimens in RCTs, the
observed advantages may be influenced by confounding factors such
as baseline severity of stroke or concomitant medication, and
therefore should be interpreted with caution. Future studies should
specifically assess the safety and efficacy of dual antiplatelet
therapy combined with tirofiban for the treatment of PIS before
clinical recommendations are made. Until dedicated RCTs confirm the
benefit-risk profile, we advise against the routine clinical
adoption of this approach.
For sources of heterogeneity, subgroup analyses
based on the course of tirofiban revealed different patterns of
heterogeneity. Both short and long courses showed significant
improvement in NIHSS scores, but high heterogeneity remained highly
heterogeneous, suggesting that factors other than the course of
treatment (e.g., baseline severity, concomitant medications, etc.)
may contribute to this variability. For PAgT, heterogeneity was
significantly reduced in the long-course subgroup, suggesting that
a longer course may stabilize inhibition of platelet aggregation
and may be more consistent in the regulation of platelet function
over time. However, the effect size of a shorter course was larger,
which may reflect a more rapid antiplatelet response in the acute
setting. These findings suggest that duration of treatment is a
partial source of PAgT heterogeneity, but not of NIHSS
heterogeneity, highlighting the complexity of prognostic
factors.
Of note, the present study had certain limitations.
First, incomplete data precluded subgroup analyses by stroke
subtype, which may mask other sources of heterogeneity. Although
the length of treatment explained the heterogeneity related to PAgR
to a certain extent, there was still unexplained heterogeneity in
NIHSS scores, suggesting that other influencing factors need to be
further explored in future studies to supplement and improve.
Second, with regard to safety outcomes, there was a lack of
standardized definitions of sICH in the other included studies.
While 18 trials (94.7%) used trial-specific criteria, only one
study adopted the European Cooperative Acute Stroke Study III
definition (24). This can lead to
aggregate estimates that may underestimate the true security risk.
Nonetheless, there is clinical consistency in all definitions of
sICH, which include two core elements of radiographic confirmation
of intracerebral hemorrhage and neurological deterioration. Studies
with broader criteria may have included more borderline cases,
while studies with more stringent definitions may have counted
fewer events. Similarly, although the definition of mortality is
relatively consistent (all-cause mortality during a given follow-up
period), subtle differences in the length of follow-up between
studies (e.g., 30- vs. 90-day mortality) may have affected the
pooled results. These inconsistencies limit the accuracy of the
present safety conclusions and highlight the need for broadly
recognized standards for future studies. Third, the trials included
in the present study consist of RCTs and non-RCTs. The level of
evidence is relatively low. Furthermore, although updates and
analyses were made to include recent RCTs, the pooled sample size
was still insufficient. Heterogeneity is mainly due to differences
in antiplatelet regimens and dosing strategies. However, the
direction of the effect of each trial suggests the potential of
tirofiban in a particular subgroup. At the same time, more
large-scale, multi-center clinical trials are needed in the future.
Furthermore, the generalizability of the present findings may be
limited by geographical bias, as the included studies were
predominantly conducted in a single region (China). In addition,
although the NIHSS deterioration threshold varied (2-4 points
gain), all studies met the set core criteria for deterioration of
neurological function at 72 h. This agreement improves
comparability, but heterogeneity related to thresholds still needs
to be taken into account when interpreting the NIHSS. Finally, the
included RCTs had a high risk of blinding and selection bias, or
were unclear in terms of bias, which may have exaggerated estimates
of functional outcomes.
Although all observational studies scored NOS ≥7
points, this reflects incidental retrieval rather than intentional
selection. Should there be any studies with lower NOS scores in the
future, they can be included in an updated meta-analysis to
validate the generalizability of the findings of the present
analysis.
In conclusion, the present meta-analysis showed that
in PIS, tirofiban injection combined with aspirin and clopidogrel
showed the potential to improve neurological deficits, inhibit
platelet aggregation and improve clinical efficacy in patients and
had a good safety profile, but it needs to be validated by further
large-sample, multi-center, prospective RCTs of antiplatelet
strategies before widespread clinical use.
Supplementary Material
Quality assessment of observational
studies using the Newcastle-Ottawa Scale.
Sensitivity analysis: RCT-only vs.
original analysis.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81904173), the Natural Science
Foundation of Hunan Province (grant nos. 2023JJ30362 and
2025JJ40099), the Graduate Innovation Project of Hunan University
of Traditional Chinese Medicine (grant no. 2024CX132) and the Hunan
Furong Plan High-level Health Talents Project in 2024.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YY conceived and designed the study, searched and
screened the literature, extracted data, applied software and
prepared the first draft. THD extracted data and applied software.
YFJ supplemented data and revised the manuscript. YY and YFJ
checked and confirmed the authenticity of the raw data. YCL and JWG
selected the research topic, designed and planned the study and
reviewed the manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Philipps J, Thomalla G, Glahn J, Schwarze
M and Röther J: Treatment of progressive stroke with
tirofiban-experience in 35 patients. Cerebrovasc Dis. 28:435–438.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegler JE, Boehme AK, Albright KC, George
AJ, Monlezun DJ, Beasley TM and Martin-Schild S: A proposal for the
classification of etiologies of neurologic deterioration after
acute ischemic stroke. J Stroke Cerebrovasc Dis. 22:e549–e556.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tada Y, Uno M, Matsubara S, Suzue A,
Shimada K, Morita N, Harada M and Nagahiro S: Reversibility of
ischemic findings on 3-T T2*-weighted imaging after emergency
superficial temporal artery-middle cerebral artery anastomosis in
patients with progressive ischemic stroke -two case reports. Neurol
Med Chir (Tokyo). 50:1006–1011. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Derflinger S, Fiebach JB, Böttger S,
Haberl RL and Audebert HJ: The progressive course of neurological
symptoms in anterior choroidal artery infarcts. Int J Stroke.
10:134–137. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Eriksson M, Stecksén A, Glader EL,
Norrving B, Appelros P, Hulter Åsberg K, Stegmayr B, Terént A and
Asplund K: Riks-Stroke Collaboration. Discarding heparins as
treatment for progressive stroke in Sweden 2001 to 2008. Stroke.
41:2552–2558. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang C, Zhao S, Zang Y, Gu F, Mao S, Feng
S, Hu L and Zhang C: The efficacy and safety of
Dl-3n-butylphthalide on progressive cerebral infarction: A
randomized controlled STROBE study. Medicine (Baltimore).
96(e7257)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fullard JF: The role of the platelet
glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr Pharm
Des. 10:1567–1576. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choudhri TF, Hoh BL, Zerwes HG,
Prestigiacomo CJ, Kim SC, Connolly ES Jr, Kottirsch G and Pinsky
DJ: Reduced microvascular thrombosis and improved outcome in acute
murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet
aggregation. J Clin Invest. 102:1301–1310. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zi W, Song J, Kong W, Huang J, Guo C, He
W, Yu Y, Zhang B, Geng W, Tan X, et al: Tirofiban for stroke
without large or medium-sized vessel occlusion. N Engl J Med.
388:2025–2036. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Van't Hof AW, Ten Berg J, Heestermans T,
Dill T, Funck RC, van Werkum W, Dambrink JH, Suryapranata H, van
Houwelingen G, Ottervanger JP, et al: Prehospital initiation of
tirofiban in patients with ST-elevation myocardial infarction
undergoing primary angioplasty (on-TIME 2): A multicentre,
double-blind, randomised controlled trial. Lancet. 372:537–546.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu C, Sun C, Wang L, Lian Y, Xie N, Huang
S, Zhao W, Ren M, Wu D, Ding J, et al: Low-dose tirofiban treatment
improves neurological deterioration outcome after intravenous
thrombolysis. Stroke. 50:3481–3487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Du Y, Li Y, Duan Z, Ma C, Wang H, Liu R,
Li S and Lian Y: The efficacy and safety of intravenous tirofiban
in the treatment of acute ischemic stroke patients with early
neurological deterioration. J Clin Pharm Ther. 47:2350–2359.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kellert L, Hametner C, Rohde S, Bendszus
M, Hacke W, Ringleb P and Stampfl S: Endovascular stroke therapy.
Stroke. 44:1453–1455. 2013.
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: an updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: Guidelines for the early management of patients
with acute ischemic stroke: 2019 update to the 2018 guidelines for
the early management of acute ischemic stroke: A guideline for
healthcare professionals from the American heart
association/American stroke association. Stroke. 50:e344–e418.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Quinn TJ, Lees KR, Hardemark HG, Dawson J
and Walters MR: Initial experience of a digital training resource
for modified Rankin scale assessment in clinical trials. Stroke.
38:2257–2261. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mahoney FI and Barthel DW: Functional
evaluation: The barthel index. Md State Med J. 14:61–65.
1965.PubMed/NCBI
|
|
20
|
Born GV: Aggregation of blood platelets by
adenosine diphosphate and its reversal. Nature. 194:927–929.
1962.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Salzman EW: Measurement of platelet
adhesiveness. A simple in vitro technique demonstrating an
abnormality in von Willebrand's disease. J Lab Clin Med.
62:724–735. 1963.PubMed/NCBI
|
|
22
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han B, Ma T, Liu Z, Wu Y, Tan W, Sun S, Li
X, Shao C, Tang D and Sun J: Efficacy and safety of tirofiban in
clinical patients with acute ischemic stroke. Front Neurol.
12(785836)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao W, Li S, Li C, Wu C, Wang J, Xing L,
Wan Y, Qin J, Xu Y, Wang R, et al: Effects of tirofiban on
neurological deterioration in patients with acute ischemic stroke.
JAMA Neurol. 81:594–602. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ruiqing C, Yuqing D and Pengcheng L:
Effect of tirofiban and dual antiplatelet therapy in patients with
acute progressive cerebral infarction with hyperthrombolytic time
window. J Clin Exper Med. 23:1013–1017. 2024.(In Chinese).
|
|
26
|
Lusheng S and Jiang X: Effects of
clopidogrel combined with tirofiban on inflammatory indexes and
platelet function in progressive stroke. Special Health. 63–64.
2022.(In Chinese).
|
|
27
|
Jingjing L, Lianping L, Yakun G and Jing
P: Observation on the clinical efficacy of tirofiban in the
treatment of acute progressive cerebral infarction. Drug
Evaluation. 21:117–120. 2024.(In Chinese).
|
|
28
|
Caixia L, Xiaokai W and Xiaoli L: Effect
of tirofiban combined with ticagrelor on neurological function and
recurrence in patients with acute progressive cerebral infarction.
Henan Medical Research. 1086–1090. 2024.(In Chinese).
|
|
29
|
Yanxiao L, Qing C, Huanhuan W, Aixia S and
Qian X: Analysis of the clinical efficacy and safety of tirofiban
injection combined with aspirin and clopidogrel in patients with
acute progressive cerebral infarction. Chin J Med. 902–905.
2024.(In Chinese).
|
|
30
|
Xiaochen L, Yongjiang L and Rongying W:
The effect of tirofiban on matrix metalloproteinase levels in
patients with acute progressive cerebral infarction. Chin J
Clinicians. 48:680–683. 2020.(In Chinese).
|
|
31
|
Zhihua X, Yunming T and Hong L: Efficacy
of tirofiban combined with clopidogrel bisulfate in the treatment
of progressive ischemic stroke and its impact on neurological
function. Chin J Clin Rational Drug Use. 17:2024.(In Chinese).
|
|
32
|
Shujuan D: The effect of tirofiban on
acute progressive cerebral infarction beyond the thrombolytic time
window: A title for a research paper. Clin Med. 44:105–107.
2024.(In Chinese).
|
|
33
|
Hui B, Huiyun N and Yan L: Analysis of
safety of tirofiban for patients with acute progressive cerebral
infarction. Chin J Modern Drug Appl. 18:66–68. 2024.(In
Chinese).
|
|
34
|
Zhongzheng L, Zheng B, Fangkun Z, Rong Y
and Xiaotan W: Effects of tirofiban on neurological function and
platelet function in patients with progressive cerebral infarction.
J Brain Nervous System Dis. 433–436. 2020.(In Chinese).
|
|
35
|
Du N, Wang LX, Liu YL, Yin XL, Zhao JS and
Yang L: Effect of tirofiban in treating patients with progressive
ischemic stroke. Eur Rev Med Pharmacol Sci. 26:2098–2105.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Li X, Liu C, Huang S, Liang C and
Zhang M: Effects of oral antiplatelet agents and tirofiban on
functional outcomes of patients with non-disabling minor acute
ischemic stroke. J Stroke Cerebrovasc Dis.
29(104829)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang ZM, Lin ZH and Zhu GL: Tirofiban in
the treatment of cancer-associated ischemic stroke. Eur Rev Med
Pharmacol Sci. 27:3590–3596. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang H, Lin F, Zhao Y, Chang W, Liu H,
Yin J and Li L: Assessing the efficacy and safety of tirofiban in
combination with dual-antiplatelet therapy in progressive ischemic
stroke patients. J Cardiovasc Pharmacol. 78:448–452.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhengfei M, Ping Z, Gang Z and Yongxing S:
Therapeutic effect of tirofiban on patients with acute
non-intracranial large vessel occlusive progressive cerebral
infarction and its safety. Chin J Cardiovasc Rehab Med. 29:665–668.
2020.(In Chinese).
|
|
40
|
Wenzhou J: Clinical efficacy and safety of
tirofiban in the treatment of acute progressive cerebral
infarction: A study. Chin J Clinicians. 48:1433–1435. 2020.(In
Chinese).
|
|
41
|
Tang L, Tang X and Yang Q: The application
of tirofiban in the endovascular treatment of acute ischemic
stroke: A meta-analysis. Cerebrovasc Dis. 50:121–131.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu WZ, Lin HA, Hou SK, Bai CH and Lin SF:
Efficacy and safety of tirofiban in patients with acute ischemic
stroke treated with endovascular thrombectomy: A frequentist and
Bayesian meta-analysis. Vascul Pharmacol.
153(107244)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu C, Yang X, Liu M, Wang J and Li G:
Meta-analysis of the efficacy and safety of tirofiban in patients
with acute ischaemic stroke undergoing mechanical thrombectomy.
Clin Neurol Neurosurg. 228(107702)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi H, Hou MM, Ren G, He ZF, Liu XL, Li XY
and Sun B: Tirofiban for acute ischemic stroke patients receiving
intravenous thrombolysis: A systematic review and meta-analysis.
Cerebrovasc Dis. 52:587–596. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Niu J, Ding Y, Zhai T, Ju F, Lu T, Xue T,
Yin D, Fang D, Chen H and Zhao G: The efficacy and safety of
tirofiban for patients with acute ischemic stroke: A protocol for
systematic review and a meta-analysis. Medicine (Baltimore).
98(e14673)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Behari S, Singh S and Bhaisora KS:
Ischemic stroke associated with ankylosing spondylitis: An integral
part of disease spectrum, or a natural consequence of progressive
infirmity? Acta Neurochir (Wien). 160:959–961. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao G, Lin F, Wang Z, Shao X, Gong Y,
Zhang S, Cui Y, Yang D, Lei H, Cheng Z, et al: Dual antiplatelet
therapy after intravenous thrombolysis for acute minor ischemic
stroke. Eur Neurol. 82:93–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yagudina RI, Kulikov AY, Krylov VA,
Solovieva EY and Fedin AI: Pharmacoeconomic analysis of the
neuroprotective medicines in the treatment of ischemic stroke. Zh
Nevrol Psikhiatr Im S S Korsakova. 119:60–68. 2019.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
49
|
Gruber P, Hlavica M, Berberat J, Victor
Ineichen B, Diepers M, Nedeltchev K, Kahles T and Remonda L: Acute
administration of tirofiban versus aspirin in emergent carotid
artery stenting. Interv Neuroradiol. 25:219–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang M, Huo X, Miao Z and Wang Y: Platelet
glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute
ischemic stroke. Drugs. 79:515–529. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Huo X, Yang M, Ma N, Gao F, Mo D, Li X,
Wang A, Wang Y and Miao Z: Safety and efficacy of tirofiban during
mechanical thrombectomy for stroke patients with preceding
intravenous thrombolysis. Clin Interv Aging. 15:1241–1248.
2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Field TS and Benavente OR: Current status
of antiplatelet agents to prevent stroke. Curr Neurol Neurosci Rep.
11:6–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Feng L, Liu J, Liu Y, Chen J, Su C, Lv C
and Wei Y: Tirofiban combined with urokinase selective
intra-arterial thrombolysis for the treatment of middle cerebral
artery occlusion. Exp Ther Med. 11:1011–1016. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Seo JH, Jeong HW, Kim ST and Kim EG:
Adjuvant tirofiban injection through deployed solitaire stent as a
rescue technique after failed mechanical thrombectomy in acute
stroke. Neurointervention. 10:22–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Weimar C, Mieck T, Buchthal J, Ehrenfeld
CE, Schmid E and Diener HC: German Stroke Study Collaboration.
Neurologic worsening during the acute phase of ischemic stroke.
Arch Neurol. 62:393–397. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zanaty M, Osorno-Cruz C, Byer S, Roa JA,
Limaye K, Ishii D, Nakagawa D, Torner J, Yongjun L,
Ortega-Gutiérrez S, et al: Tirofiban protocol protects against
delayed cerebral ischemia: A case-series study. Neurosurgery.
87:E552–E556. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yates YJ, Farias CL, Kazmier FR, Puckett
CL and Concannon MJ: The effect of tirofiban on microvascular
thrombosis: Crush model. Plast Reconstr Surg. 116:205–208.
2005.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mingming Z and Qizhi F: Observation on the
clinical efficacy of tirofiban combined with antiplatelet drugs in
the treatment of transient ischemic attack. J Clin Rational Drug
Use. 13:31–32. 2020.(In Chinese).
|
|
59
|
Siebler M, Hennerici MG, Schneider D, von
Reutern GM, Seitz RJ, Röther J, Witte OW, Hamann G, Junghans U,
Villringer A and Fiebach JB: Safety of tirofiban in acute ischemic
stroke: The SaTIS trial. Stroke. 42:2388–2392. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang Y, Wang Y, Zhao X, Liu L, Wang D,
Wang C, Wang C, Li H, Meng X, Cui L, et al: Clopidogrel with
aspirin in acute minor stroke or transient ischemic attack. N Engl
J Med. 369:11–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Johnston SC, Easton JD, Farrant M, Barsan
W, Conwit RA, Elm JJ, Kim AS, Lindblad AS and Palesch YY:
Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA.
N Engl J Med. 379:215–225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen HS, Cui Y, Wang XH, Ma YT, Han J,
Duan YJ, Lu J, Shen LY, Liang Y, Wang WZ, et al: Clopidogrel plus
aspirin vs aspirin alone in patients with acute mild to moderate
stroke: The ATAMIS randomized clinical trial. JAMA Neurol.
81:450–460. 2024.PubMed/NCBI View Article : Google Scholar
|