Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune
disorder in which pancreatic β-cells are destroyed, leading to low
insulin production and chronic hyperglycemia. Although T1DM only
accounts for 5-10% of all cases of diabetes, it still places a
heavy burden on healthcare systems because of its progressive
course and various complications such as diabetic ketoacidosis,
retinopathy, nephropathy, neuropathy and cardiovascular disease
(1). Achieving tight glycemic
control remains challenging because intensive insulin therapy is
limited by hypoglycemia risk and glycemic variability despite
frequent monitoring (2). Achieving
tight glycemic control remains challenging, and patients with T1DM
are susceptible to microvascular and macrovascular complications
(such as retinopathy, nephropathy, neuropathy and cardiovascular
disease) (3,4). As the global incidence rate of T1DM
continues to rise, ~8.4 million individuals were living with T1DM
in 2021, with 510,000 incident cases and 175,000 deaths annually
(5).
Dyslipidemia, usually characterized by an increase
in the low-density lipoprotein (LDL) cholesterol and a decrease in
the high-density lipoprotein (HDL) cholesterol, increases the
cardiovascular risk in T1DM (6-8).
These lipid changes are also associated with poor insulin signaling
and oxidative stress (9,10). Therefore, treatments that normalize
the lipid profile may help reduce the overall burden of T1DM.
Leucine (Leu), a branched-chain amino acid, is known to support
muscle health, glucose control and insulin sensitivity (11). Mechanistically, Leu activates the
mammalian target of rapamycin (mTOR) pathway and stimulates AMPK
signaling (e.g., via the SIRT1-AMPK axis), which promotes
fatty-acid oxidation and glucose uptake (12,13).
Leu supplementation has been shown to improve lipid handling and
relieve dyslipidemia in several animal and human studies (14-16).
However, its exact effect on T1DM-associated lipid changes,
especially on HDL and LDL, is yet to be elucidated.
Therefore, to fill the aforementioned gap in the
knowledge, the present study used a metabolomics approach with a
mouse model of T1DM. The present study investigated whether Leu
supplementation could adjust the HDL/LDL levels and the associated
downstream metabolic pathways. Male C57BL/6J mice were placed into
four groups, namely Normal, Normal with Leu supplementation
(Normal-Leu), T1DM and T1DM with Leu supplementation (T1DM-Leu).
T1DM was induced using streptozotocin (STZ) administered
intraperitoneally at 50 mg/kg/day for 5 consecutive days, and Leu
(1.5%) was provided in drinking water for 6 weeks. Plasma samples
were then used in liquid chromatography-tandem mass spectrometry
(LC-MS/MS) for metabolomic profiling and biochemical assays of
plasma triglyceride (TG), total cholesterol (TC), high-density
lipoprotein cholesterol (HDL-C) and low-density lipoprotein
cholesterol (LDL-C).
Materials and methods
Materials
Leu and STZ were purchased from Sigma-Aldrich; Merck
KGaA. Isoflurane, acetonitrile and methanol were obtained from
Shanghai Aladdin Biochemical Technology Co., Ltd. Assay kits for
plasma glucose [Glucose (GOD-POD) Assay Kit], triglycerides
[Triglyceride (GPO-PAP) Assay Kit], total cholesterol [Total
Cholesterol (CHOD-PAP) Assay Kit], HDL-C [Direct HDL-C Assay Kit],
LDL-C [Direct LDL-C Assay Kit], and whole-blood glycated hemoglobin
(HbA1c) [Boronate-affinity HbA1c Assay Kit] were from Beijing
Solarbio Science & Technology Co., Ltd., and were used
according to the manufacturer's instructions.
Animals
A total of 40 male C57BL/6J mice (8 weeks old; 20-24
g at study start) were used. Mice were obtained from Shanghai SLAC
Laboratory Animal Co., Ltd. and acclimatized for 1 week. Animals
were randomly assigned to four groups (n=10/group): Normal,
Normal-Leu, T1DM and T1DM-Leu. All procedures conformed to the
Guide for the Care and Use of Laboratory Animals (8th edition)
(17) and the ARRIVE guidelines
2.0(18), and were approved by the
IACUC of Xiamen University (approval no. 20210305042). Mice were
housed in an SPF facility under 22±2˚C, 50±10% relative humidity, a
12:12 h light/dark cycle, with ad libitum access to standard
chow and water; 4-5 mice per cage with corncob bedding and
environmental enrichment. Anesthesia was induced using 4-5%
isoflurane in 1 l/min oxygen via an induction chamber and
maintained at 1.5-2% using a nose cone during blood collection.
Euthanasia was carried out using ≥5% isoflurane to for ≥3 min.
Death was confirmed by the absence of respiration, heartbeat and
corneal reflex. Every effort was made to minimize the discomfort to
the animals. Humane endpoints were predefined as ≥20% body-weight
loss, severe dehydration/ketonuria with prostration, persistent
hypothermia/recumbency or inability to eat/drink; animals reaching
any endpoint would be euthanized by isoflurane overdose. No animals
reached a humane endpoint prior to planned study termination.
Induction of T1DM
STZ (50 mg/kg/day) was freshly dissolved in cold 0.1
M sodium citrate buffer (pH 4.5) at 5 mg/ml. A volume of 10 ml/kg
(0.20-0.25 ml per 20-25 g mouse) was administered intraperitoneally
once daily for five consecutive days (17); control groups received equal-volume
citrate buffer i.p. Additionally, the Normal-Leu and T1DM-Leu
groups received 1.5% Leu in their drinking water, while the Normal
and T1DM groups received drinking water without additions (18). Diabetes was confirmed 7 days after
the last STZ injection when fasting blood glucose ≥12 mmol/l was
recorded from tail-vein blood (~5 µl) using a handheld glucometer
after a 6 h fast (water ad libitum); one sample per mouse
was taken for confirmation.
Plasma preparation
On week 6 following STZ-induced T1DM, mice were
anesthetized with isoflurane and euthanized. Blood (0.8-1.0 ml per
mouse) was collected by cardiac puncture into pre-chilled K2-EDTA
microtubes and the entire volume was centrifuged at 2,000 x g for
10 min at 4˚C to obtain plasma, which was stored at -20˚C. Plasma
from six randomly selected mice per group was used for LC-MS/MS,
and plasma from all animals was used for biochemical assays.
Metabolomics analysis
Plasma samples (100 µl) were mixed with 400 µl
methanol, vortexed and sonicated to precipitate proteins. After
centrifugation (15,000 x g for 10 min at 4˚C), the supernatants
were dried under nitrogen (35˚C; nebuliser pressure 15 psi),
reconstituted in 100 µl acetonitrile and centrifuged again at
15,000 x g for 10 min at 4˚C. A 2 µl aliquot was injected into a
UPLC-QTOF-MS system (Waters Xevo G2-XS QTOF with an ACQUITY BEH C18
1.8 µm 2.1x50 mm column; Waters Corporation), using electrospray
ionization in positive mode. The mobile phase consisted of 0.01%
formic acid in water (solvent A) or acetonitrile (solvent B) with a
flow rate of 0.4 ml/min. The gradient elution used was 0-1 min with
10% solvent B, 1-7.5 min with 10-65% solvent B, 7.5-10.5 min with
65-100% solvent B and 10.5-12 min with 100-10% solvent B.
Leucine-enkephalin (m/z 556.2771) was infused for lock-mass
calibration. A pooled quality control (QC) sample was prepared by
combining 10 µl aliquots of each extract. Five QC injections were
used at the start to condition the system; one QC was injected
every eight study samples; and three QC injections were run at the
end. All injections were randomized and interleaved with blank
samples.
Raw data were processed using Progenesis QI (version
2.4; Nonlinear Dynamics; Waters Corporation). After alignment to
the third QC injection, ion features were extracted, normalized
with the total ion current and corrected for drift using QC-based
locally estimated scatterplot smoothing. Features with a relative
SD of >30% in QCs or present in <80% of QC samples were
excluded. All detected ion features have been deposited in the
MassIVE repository (accession no. MSV000099368). Multivariate and
pathway analyses were conducted using MetaboAnalyst version 5.0
(https://www.metaboanalyst.ca). Principal
component (PC) analysis was used for visualization. For metabolite
set enrichment analysis (MSEA), differential ions were first
selected using the volcano plot criteria (a fold change of more
than two; P<0.05) and mapped to pathways in the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp). Pathways with a P-value of
<0.05 and more than or equal to two matched metabolites were
considered significant. Metabolite annotation was based on accurate
m/z and MS/MS spectrum matches against the Human Metabolome
Database (https://hmdb.ca).
Biochemical measurements
Plasma glucose, TG, total cholesterol, HDL and LDL
were quantified in plasma samples, whereas HbA1c was determined
from whole blood (erythrocyte hemolysates), using the respective
Beijing Solarbio Science & Technology Co., Ltd. kits according
to the manufacturer's protocols.
Statistical analysis
Analyses were carried out using SPSS version 16.0
(SPSS, Inc.). One-way ANOVA followed by Tukey's post hoc test was
used for multiple-group comparisons. Data are presented as mean ±
SD or as box-and-whisker plots (median, interquartile range and
10-90th percentiles) as specified in the figure legends. P<0.05
was considered to indicate a statistically significant difference.
Biochemical outcomes (glucose, TG, total cholesterol, HDL, LDL and
HbA1c) were measured in 10 mice per group (biological replicates);
each assay was run in duplicate and the mean was used for
statistics. Untargeted metabolomics used plasma from 6 mice per
group.
Results
Metabolite profiling and pathway
analysis
Untargeted LC-MS/MS detected 1,692 ion features in
the plasma of mice. After excluding peaks with a QC relative SD
>30% in QCs (Fig. 1A) and
applying an interquartile range filter, 888 features remained for
statistical evaluation. One-way ANOVA followed by Tukey's post hoc
test identified ions with significant differences among the four
groups (FDR-adjusted P<0.05; full list provided in the public
MassIVE dataset) (Fig. 1A). PC
analysis further revealed a clear metabolic separation between the
Normal, Normal-Leu, T1DM and T1DM-Leu group clusters. Where PC1 and
PC2 accounted for 38.2 and 19.9% of the total variance,
respectively (Fig. 1B).
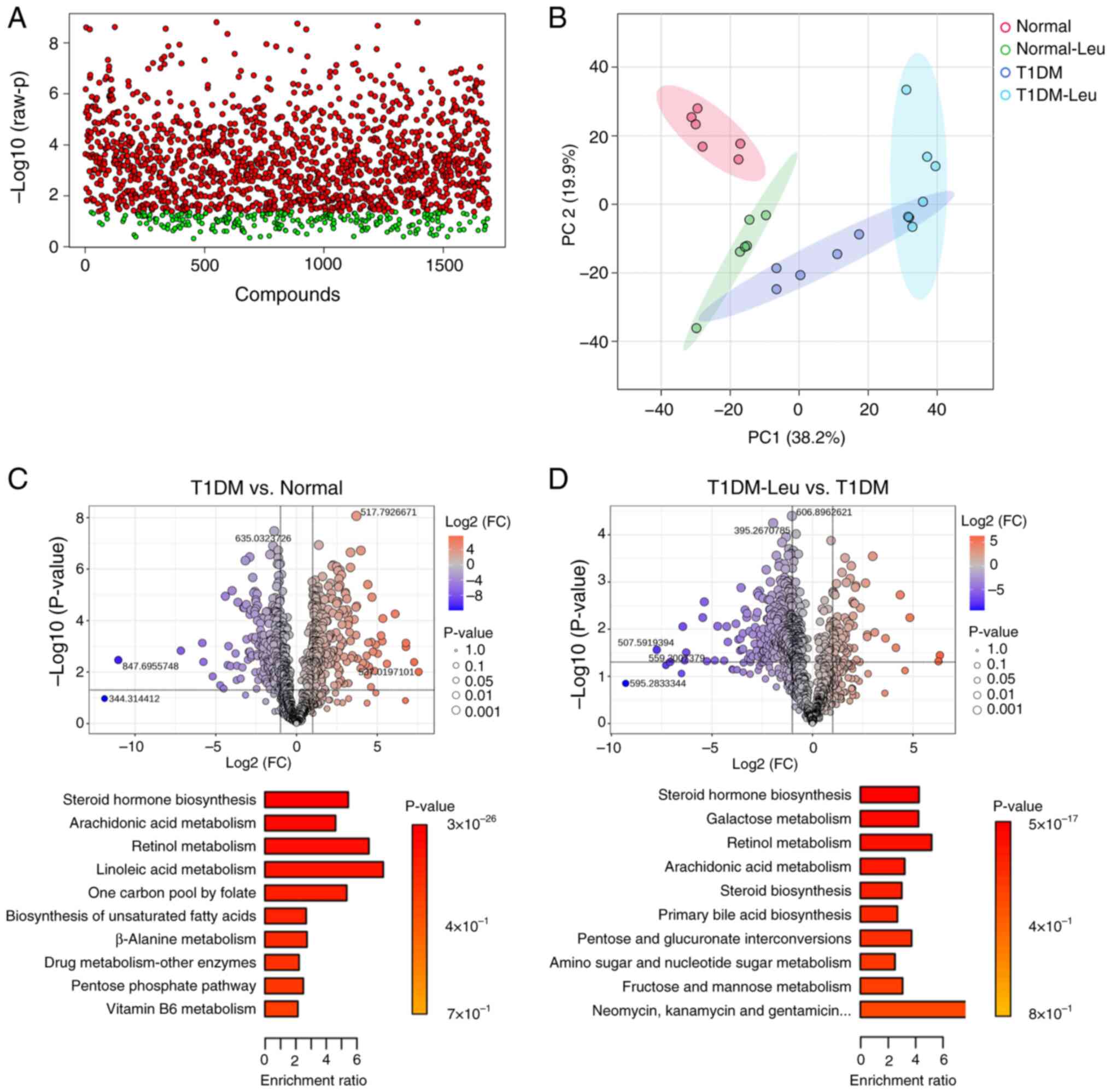 | Figure 1Metabolomic overview of plasma
samples. (A) One-way ANOVA of 1,692 detected ion features after
quality-control filtering (relative SD >30%), followed by
Tukey's post hoc test. Red dots represent ions with P-values of
<0.05; green dots represent non-significant ions. (B) PC
analysis scores plot illustrating the separation of the Normal,
Normal-Leu, T1DM and T1DM-Leu groups. PC1 and PC2 explain 38.2 and
19.9% of the variance, respectively. (C) The top panel shows a
volcano plot of the differential features in the T1DM vs. Normal
group comparison (FC, >2; P<0.05). The bottom panel shows the
KEGG MSEA of the top 10 pathways altered in the mice in the T1DM
group compared with those in the Normal group. (D) The top panel
shows a volcano plot of the differential features in the T1DM-Leu
vs. T1DM group comparison (FC, >2; P<0.05). The bottom panel
shows the KEGG MSEA of the top 10 pathways modulated by Leu
supplementation in mice with T1DM. Leu, leucine; Normal-Leu, Normal
with 1.5% Leu supplementation; T1DM, type 1 diabetes mellitus;
T1DM-Leu, T1DM with 1.5% Leu supplementation; PC, principal
component; FC, fold-change; KEGG, Kyoto Encyclopedia of Genes and
Genomes; MSEA, metabolite-set enrichment analysis. |
Pairwise analyses were then carried out. In the T1DM
vs. Normal comparison, 227 features were higher in T1DM
(upregulated) and 186 were lower in T1DM (downregulated) relative
to Normal (Fig. 1C). KEGG-based
MSEA (using a criteria of P<0.05 and more than or equal to two
hits) pointed to marked disturbances in steroid-hormone
biosynthesis, arachidonic-acid metabolism, retinol metabolism,
linoleic-acid metabolism and a number of lipid-related pathways
(Fig. 1C). Compared with mice in
the untreated T1DM group, Leu supplementation in the T1DM-Leu group
resulted in an increase in 75 features and a decrease in 251
features (Fig. 1D). Enrichment
analysis indicated that Leu modulated steroid-hormone biosynthesis,
arachidonic-acid metabolism, galactose metabolism,
pentose-and-glucuronate interconversions and primary bile-acid
biosynthesis (Fig. 1D). In
summary, these results indicated that Leu treatment may partially
reverse the metabolic disruptions of untreated T1DM, especially
within pathways associated with lipid and bile-acid metabolism.
Key metabolites affected by Leu
supplementation
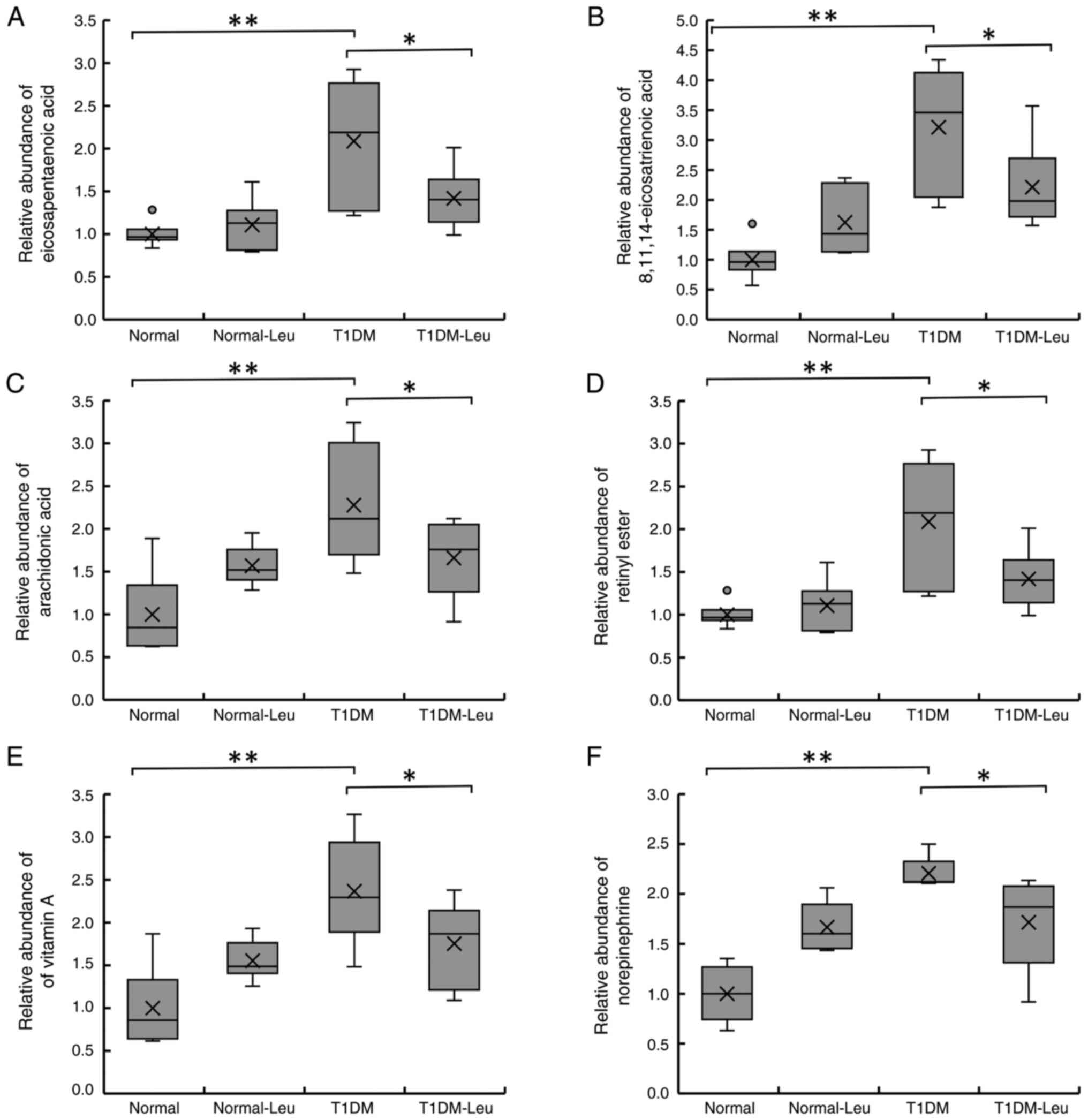
Fig. 2 indicates
that eicosapentaenoic acid, 8,11,14-eicosatrienoic acid,
arachidonic acid, retinyl ester, vitamin A and norepinephrine
levels were significantly higher in the T1DM group compared with
the Normal control group, and Leu supplementation (T1DM-Leu)
reduced these metabolites toward control levels. After 6 weeks of
treatment with 1.5% Leu, there was a significant decrease in each
of these metabolites in the mice in the T1DM-Leu group. Across all
six metabolites (eicosapentaenoic acid, 8,11,14-eicosatrienoic
acid, arachidonic acid, retinyl ester, vitamin A and
norepinephrine), levels in the T1DM group were higher than those in
the Normal group (P<0.01), whereas leucine supplementation
lowered these levels in the T1DM-Leu group compared with T1DM
(one-way ANOVA with Tukey's post hoc test; P<0.05). In most
cases, T1DM-Leu values were intermediate between T1DM and
Normal/Normal-Leu, and Leu alone did not differ from Normal. These
changes indicated that Leu may potentially oppose the
lipid-inflammatory and retinoid disturbances associated with
early-stage T1DM.
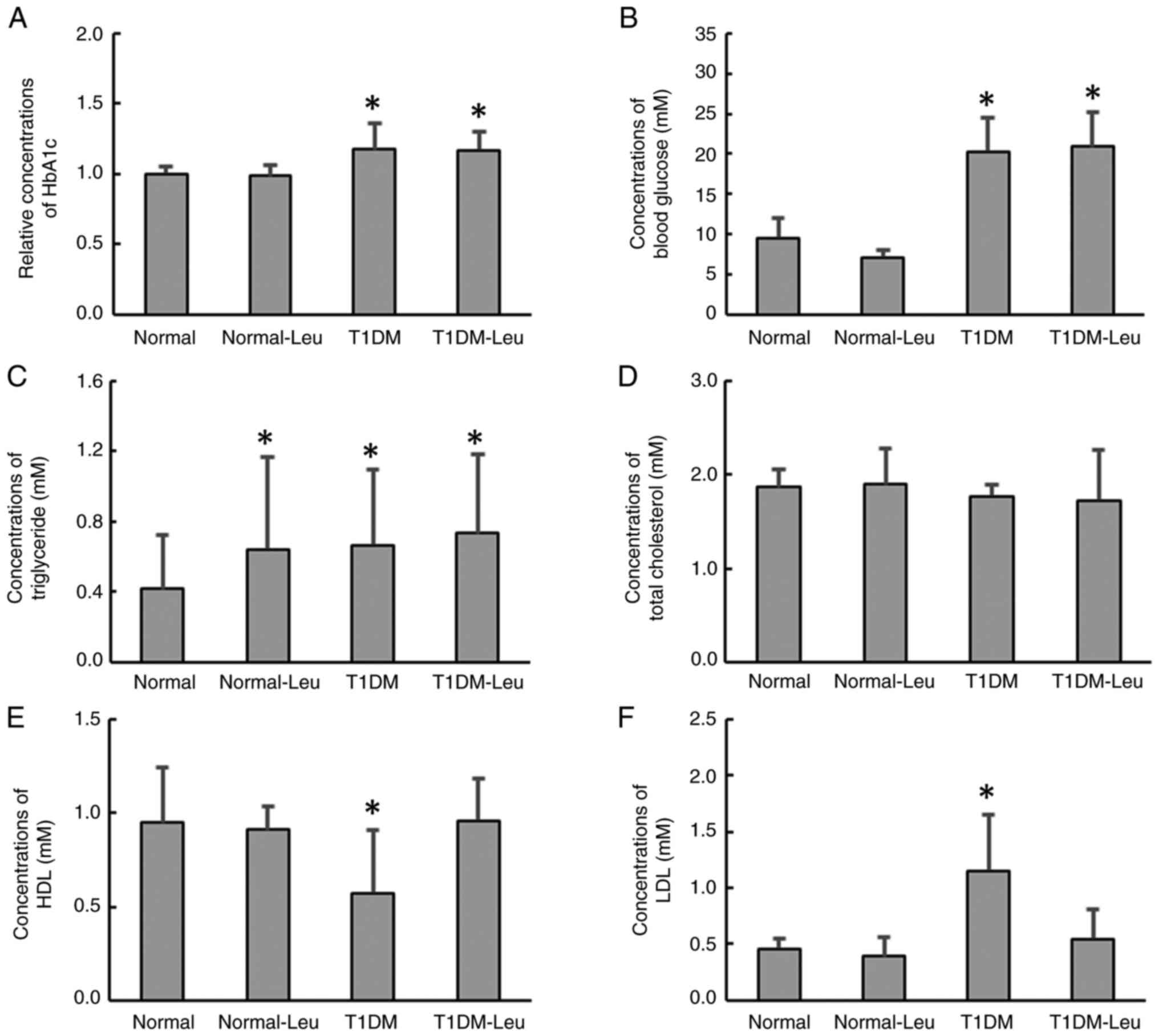
Biochemical analyses
HbA1c and fasting blood glucose were significantly
higher in the T1DM group compared with the Normal group. In the
T1DM-Leu group, both indices remained elevated and were not
significantly different from the T1DM group (Fig. 3A and B). TG levels were significantly higher
than in the Normal group in all three intervention groups; however,
there were no significant differences among the intervention groups
(Fig. 3C). There were no
significant differences in the total cholesterol levels between the
Normal and the treatment groups (Fig.
3D). HDL levels were significantly reduced in the T1DM group
compared with the Normal group; however, in the T1DM-Leu group, the
HDL levels were similar to the levels in the Normal group (Fig. 3E). By contrast, LDL levels were
significantly increased in the T1DM group compared with the Normal
group, whereas the LDL levels in the T1DM-Leu group were similar to
the levels in the Normal and Normal-Leu groups (Fig. 3F).
Discussion
The findings of the present study suggested that Leu
supplementation may mitigate the metabolic disturbances in T1DM,
particularly in lipid metabolism. In the plasma metabolomics data,
eicosapentaenoic acid, 8,11,14-eicosatrienoic acid, arachidonic
acid, retinyl ester, vitamin A and norepinephrine were lower in
T1DM-Leu than in T1DM mice. Taken together with the
pathway-enrichment results (MetaboAnalyst/KEGG), these findings
suggest a partial amelioration of lipid-related pathways, including
arachidonic-acid metabolism (KEGG map00590), linoleic-acid
metabolism (map00591), and biosynthesis of unsaturated fatty acids
(map01040), as well as retinol metabolism (map00830).
Altered levels of arachidonic acid and associated
lipid metabolites are implicated in inflammation and insulin
resistance, and their dysregulation contributes to diabetic
complications such as cardiovascular disease and nephropathy
(19,20). Reduced levels of retinyl ester and
vitamin A are observed in mice with diabetes and may exacerbate
oxidative stress-for example, mitochondrial ROS overproduction,
NADPH oxidase/AGE-RAGE-driven lipid peroxidation- and weaken
antioxidant defenses such as superoxide dismutase, catalase and
glutathione peroxidase (21).
Therefore, the Leu-mediated reduction of these metabolites in the
mice with T1DM may potentially represent a protective metabolic
shift. Additionally, norepinephrine dysregulation is associated
with an increase in the risk of cardiovascular issues in patients
with diabetes (22). This suggests
that the reduction of the norepinephrine levels in the mice with
T1DM following Leu supplementation may be of clinical
importance.
The results of the present study revealed that Leu
supplementation also helped restore the HDL and LDL levels in mice
with T1DM, which potentially suggested a favorable effect on
lipoprotein profiles. HDL-cholesterol is well known to have
protective cardiovascular effects by promoting reverse cholesterol
transport, reducing inflammation and improving endothelial
function. However, elevated LDL-cholesterol levels accelerates
atherogenesis, which notably increases the risk of cardiovascular
complications in diabetes (23).
Therefore, restoring the balance of HDL and LDL with Leu
supplementation may potentially offer cardiovascular benefits in
the management of T1DM. The findings of the present study align
with those of previous studies that demonstrate beneficial effects
of Leu on glucose and lipid metabolism (18,24,25).
Mechanistically, Leu may benefit T1DM via the activation of mTOR
and AMPK, which are pathways that regulate metabolic processes
(26-31).
For example, AMPK activation inhibits lipogenesis, promotes fatty
acid oxidation and improves lipid homeostasis, processes that are
often impaired in diabetes (32).
Furthermore, mTOR activation by Leu modulates lipid metabolism
through the regulation of lipid synthesis genes and mitochondrial
function (12). These previously
identified mechanisms align with the findings of the present study,
which indicated that Leu supplementation corrected T1DM-induced
metabolic dysregulation of lipid pathways. In addition, enrichment
analysis showed shifts in galactose metabolism and primary
bile-acid biosynthesis, which suggested that Leu may also influence
carbohydrate metabolism and bile-acid signaling in mice with T1DM,
both of which can influence lipid homeostasis. Therefore, these
secondary pathways may warrant further follow-up experiments in the
future.
However, not all metabolites investigated in the
present study exhibited identical degrees of modulation. For
example, after Leu supplementation, the levels of arachidonic acid,
8,11,14-eicosatrienoic acid (20:3 n-6) and eicosapentaenoic acid
(20:5 n-3) were significantly reduced in T1DM-Leu vs. T1DM mice,
although the magnitude of change varied across metabolites,
indicating a possible selective modulation of inflammatory and
lipid pathways. Furthermore, vitamin A levels were partially, but
significantly, restored. These differential responses may reflect
distinct underlying regulatory mechanisms or pathway-specific
sensitivities to Leu intervention.
In the present study, although supplementary Leu did
not significantly lower blood glucose or HbA1c levels compared with
those in the T1DM-only group, the selective improvement in lipid
parameters suggested the potential utility of Leu in addressing
dyslipidemia in T1DM. These results align with previous findings in
which Leu supplementation is associated with improved lipid
metabolism and cardiovascular health (7,15,33-35).
However, there were limitations of the present
study. For example, the present study only focused on early-stage
T1DM without examining advanced disease settings. Additionally, the
present study did not fully elucidate the molecular mechanisms
through which Leu exerted its effects. Therefore, future studies
are needed for a more comprehensive evaluation. Finally, the safety
and potential adverse effects of chronic Leu use are yet to be
assessed. Overall, the findings of the present study supported the
hypothesis that Leu supplementation may offer therapeutic benefits
in T1DM by correcting imbalances in key lipids (for example,
arachidonic acid, 8,11,14-eicosatrienoic acid, eicosapentaenoic
acid, retinyl ester/vitamin A and HDL/LDL) and by modulating
metabolic pathways highlighted by our analysis, including
arachidonic/linoleic-acid and retinol metabolism, steroid-hormone
biosynthesis, galactose metabolism and primary bile-acid
biosynthesis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Fujian Province, China (grant no. 2022D024) and the
Xiamen Municipal Health Commission Guiding Project (grant no.
3502Z20244ZD1151).
Availability of data and materials
The data generated in the present study may be found
in the MassIVE database under accession number MSV000099368 or at
the following URL: https://doi.org/10.25345/C5MG7G83D.
Authors' contributions
JY contributed to the conception and design of the
present study. DT, CF, GX, TY and RZ contributed to the data
collection, analysis and interpretation of results. DT and CF
confirm the authenticity of all the raw data generated and analyzed
in this study. JY prepared the draft of the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal procedures were performed in accordance
with the guidelines and regulations approved by the Institutional
Animal Care and Use Committee of Xiamen University (Xiamen, China;
approval no. 20210305042).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atkinson MA, Eisenbarth GS and Michels AW:
Type 1 diabetes. Lancet. 383:69–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maahs DM, West NA, Lawrence JM and
Mayer-Davis EJ: Epidemiology of type 1 diabetes. Endocrinol Metab
Clin North Am. 39:481–497. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Ferranti SD, de Boer IH, Fonseca V, Fox
CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ,
et al: Type 1 diabetes mellitus and cardiovascular disease: A
scientific statement from the American heart association and
American diabetes association. Diabetes Care. 37:2843–2863.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gregory GA, Robinson TIG, Linklater SE,
Wang F, Colagiuri S, de Beaufort C and Donaghue KC: International
Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults
Special Interest Group. Magliano DJ, Maniam J, et al: Global
incidence, prevalence, and mortality of type 1 diabetes in 2021
with projection to 2040: A modelling study. Lancet Diabetes
Endocrinol. 10:741–760. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tell S, Nadeau KJ and Eckel RH: Lipid
management for cardiovascular risk reduction in type 1 diabetes.
Curr Opin Endocrinol Diabetes Obes. 27:207–214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang X, Pei J, Zheng K and Hu X:
High-density lipoprotein cholesterol levels are associated with
major adverse cardiovascular events in male but not female patients
with hypertension. Clin Cardiol. 44:723–730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sniderman AD, Williams K, Contois JH,
Monroe HM, McQueen MJ, de Graaf J and Furberg CD: A meta-analysis
of low-density lipoprotein cholesterol, non-high-density
lipoprotein cholesterol, and apolipoprotein B as markers of
cardiovascular risk. Circ Cardiovasc Qual Outcomes. 4:337–345.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu X and Xiao B: TDAG51 attenuates
impaired lipid metabolism and insulin resistance in gestational
diabetes mellitus through SREBP-1/ANGPTL8 pathway. Balkan Med J.
40:175–181. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marques CL, Beretta MV, Prates RE, de
Almeida JC and da Costa Rodrigues T: Body adiposity markers and
insulin resistance in patients with type 1 diabetes. Arch
Endocrinol Metab. 67:401–407. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mero A: Leucine supplementation and
intensive training. Sports Med. 27:347–358. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Horman S, Browne G, Krause U, Patel J,
Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C and Rider M:
Activation of AMP-activated protein kinase leads to the
phosphorylation of elongation factor 2 and an inhibition of protein
synthesis. Curr Biol. 12:1419–1423. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y, Guo K, LeBlanc RE, Loh D,
Schwartz GJ and Yu YH: Increasing dietary leucine intake reduces
diet-induced obesity and improves glucose and cholesterol
metabolism in mice via multimechanisms. Diabetes. 56:1647–1654.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang L, Li F, Guo Q, Duan Y, Wang W,
Zhong Y, Yang Y and Yin Y: Leucine supplementation: A novel
strategy for modulating lipid metabolism and energy homeostasis.
Nutrients. 12(1299)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bishop CA, Schulze MB, Klaus S and
Weitkunat K: The branched-chain amino acids valine and leucine have
differential effects on hepatic lipid metabolism. FASEB J.
34:9727–9739. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chaudhry ZZ, Morris DL, Moss DR, Sims EK,
Chiong Y, Kono T and Evans-Molina C: Streptozotocin is equally
diabetogenic whether administered to fed or fasted mice. Lab Anim.
47:257–265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiao J, Han SF, Zhang W, Xu JY, Tong X,
Yin XB, Yuan LX and Qin LQ: Chronic leucine supplementation
improves lipid metabolism in C57BL/6J mice fed with a
high-fat/cholesterol diet. Food Nutr Res. 60(31304)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang T, Fu X, Chen Q, Patra JK, Wang D,
Wang Z and Gai Z: Arachidonic acid metabolism and kidney
inflammation. Int J Mol Sci. 20(3683)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sears B and Perry M: The role of fatty
acids in insulin resistance. Lipids Health Dis.
14(121)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brun PJ, Yang KJZ, Lee SA, Yuen JJ and
Blaner WS: Retinoids: Potent regulators of metabolism. Biofactors.
39:151–163. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tentolouris N, Argyrakopoulou G and
Katsilambros N: Perturbed autonomic nervous system function in
metabolic syndrome. Neuromolecular Med. 10:169–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vergès B: Pathophysiology of diabetic
dyslipidaemia: Where are we? Diabetologia. 58:886–899.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rivera ME, Lyon ES, Johnson MA and Vaughan
RA: Leucine increases mitochondrial metabolism and lipid content
without altering insulin signaling in myotubes. Biochimie.
168:124–133. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu L, Li F, Bruckbauer A, Cao Q, Cui X, Wu
R, Shi H, Xue B and Zemel MB: Interaction between leucine and
phosphodiesterase 5 inhibition in modulating insulin sensitivity
and lipid metabolism. Diabetes Metab Syndr Obes. 8:227–239.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Holowaty MNH, Lees MJ, Abou Sawan S,
Paulussen KJM, Jäger R, Purpura M, Paluska SA, Burd NA, Hodson N
and Moore DR: Leucine ingestion promotes mTOR translocation to the
periphery and enhances total and peripheral RPS6 phosphorylation in
human skeletal muscle. Amino Acids. 55:253–261. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Azzout-Marniche D: New insight into the
understanding of muscle glycolysis: Sestrins, key pivotal proteins
integrating glucose and leucine to control mTOR activation. J Nutr.
153:915–916. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cui C, Wu C, Wang J, Zheng X, Ma Z, Zhu P,
Guan W, Zhang S and Chen F: Leucine supplementation during late
gestation globally alters placental metabolism and nutrient
transport via modulation of the PI3K/AKT/mTOR signaling pathway in
sows. Food Funct. 13:2083–2097. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang MM, Guo HX, Huang YY, Liu WB, Wang X,
Xiao K, Xiong W, Hua HK, Li XF and Jiang GZ: Dietary leucine
supplementation improves muscle fiber growth and development by
activating AMPK/Sirt1 pathway in blunt snout bream (megalobrama
amblycephala). Aquac Nutr. 2022(7285851)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Martin SB, Reiche WS, Fifelski NA, Schultz
AJ, Stanford SJ, Martin AA, Nack DL, Radlwimmer B, Boyer MP and
Ananieva EA: Leucine and branched-chain amino acid metabolism
contribute to the growth of bone sarcomas by regulating AMPK and
mTORC1 signaling. Biochem J. 477:1579–1599. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen X, Xiang L, Jia G, Liu G, Zhao H and
Huang Z: Leucine regulates slow-twitch muscle fibers expression and
mitochondrial function by Sirt1/AMPK signaling in porcine skeletal
muscle satellite cells. Anim Sci J. 90:255–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yun SY, Rim JH, Kang H, Lee SG and Lim JB:
Associations of LDL cholesterol, non-HDL cholesterol, and
apolipoprotein B with cardiovascular disease occurrence in adults:
Korean genome and epidemiology study. Ann Lab Med. 43:237–243.
2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lynch CJ and Adams SH: Branched-chain
amino acids in metabolic signalling and insulin resistance. Nat Rev
Endocrinol. 10:723–736. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McCormack SE, Shaham O, McCarthy MA, Deik
AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK and
Fleischman A: Circulating branched-chain amino acid concentrations
are associated with obesity and future insulin resistance in
children and adolescents. Pediatr Obes. 8:52–61. 2013.PubMed/NCBI View Article : Google Scholar
|