Introduction
Cutaneous metastasis as the initial manifestation of
visceral malignancy is a rare but clinically significant event,
occurring in ~0.8% of patients with solid tumors (1). Among solid organ malignancies, lung
carcinoma metastasized to the skin is associated with a poor
prognosis. The clinical presentation is often non-specific, with
skin nodules or plaques that may be mistaken for benign
dermatologic conditions such as dermatofibromas or inflammatory
lesions (2). This diagnostic
challenge is further confounded by imaging findings, as various
benign entities can mimic malignancy even with advanced imaging
techniques (3). Therefore,
knowledge of false-positive nuclear imaging findings in benign skin
and soft tissue conditions is crucial to avoid misdiagnosis.
Aneurysmal dermatofibroma (ADF), a rare variant of
dermatofibroma, is notable for its histologic overlap with
malignant skin tumors and its potential to mimic metastasis on
imaging (4). This mimicry
highlights the importance of careful histopathologic evaluation and
the integration of clinical and radiologic data when evaluating
patients with suspected cutaneous metastasis.
The aim of this work is to highlight the diagnostic
challenge posed by a rare neoplasm presenting as possible cutaneous
metastasis in a patient with lung carcinoma. The present study
outlines the multidisciplinary approach, integrating clinical,
radiologic and pathologic findings to facilitate accurate diagnosis
and optimal patient management.
Case report
A 71-year-old male with a prior diagnosis of
non-small cell lung cancer (NSCLC) presented to Seoul St. Mary's
Hospital (The Catholic University of Korea, Seoul, Republic of
Korea) in March 2024, with a 3-month history of a painless, slowly
growing cutaneous nodule located on the right chest wall. The
patient had a significant smoking history of 50 pack-years and no
history of other malignancies or chronic diseases, such as
hypertension or diabetes. Histologic examination of the lung cancer
revealed squamous cell carcinoma, clinically staged as cT1cN3M1c,
stage IVB. The patient had undergone video-assisted thoracoscopic
surgery, including lobectomy and mediastinal lymph node dissection,
followed by adjuvant chemotherapy consisting of vinorelbine (22.5
mg/m2) and cisplatin (60 mg/m2) administered
at 75% of the standard dose for three cycles. On physical
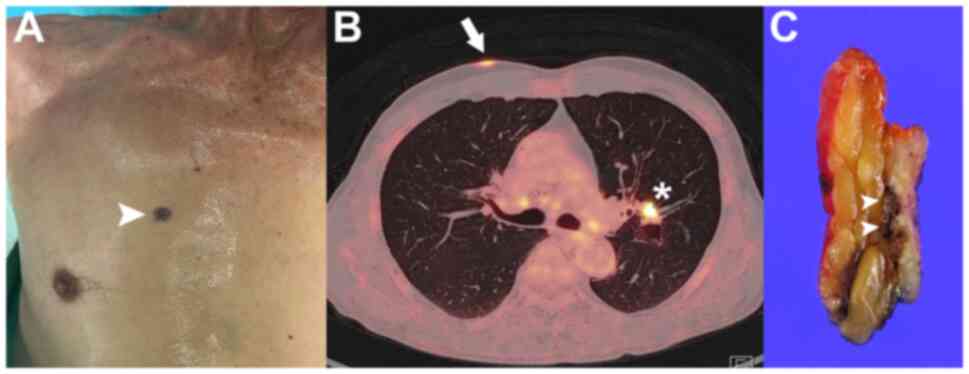
examination, a two-centimeter, non-ulcerated, blackish nodule with
intermittent contact bleeding was observed at the upper right chest
(Fig. 1A). Satellite lesions were
not identified. The lesion initially raised no suspicion until
positron emission tomography-CT (PET-CT) demonstrated
hypermetabolic activity (maximum standardized uptake volume, 6.9;
Fig. 1B), although the primary
lung lesion remained present on imaging. The cutaneous lesion was
subsequently identified during a focused physical examination that
followed the PET-CT scan, prompted by the imaging studies. The
hypermetabolic activity detected by PET-CT raised suspicion of
cutaneous metastasis, prompting surgical excision of the skin
lesion.
No other distant metastases were identified at the
time of the initial evaluation. Given that cutaneous metastasis of
lung cancer was suspected, an excisional biopsy was performed for
pathologic diagnosis. Wide local excision with 5-mm safety margins
was carried out (Fig. 1C).
Unexpectedly, histopathologic evaluation revealed features
consistent with ADF. Histopathologic examination revealed a
well-circumscribed dermal lesion composed of spindle-like cells
arranged in a storiform pattern, with multiple large, blood-filled
pseudovascular spaces characteristic of ADF (Fig. 2A and B). Formalin-fixed (10% neutral-buffered
formalin; room temperature; 24 h), paraffin-embedded tissue
sections were cut to 4 µm thickness, deparaffinized in xylene and
rehydrated through a decreasing series of ethanol solutions (100,
95 and 70%; each for 3 min). The sections were stained with
hematoxylin (at room temperature for 5 min) to visualize cell
nuclei, followed by counterstaining with eosin (at room temperature
for 2 min) to visualize the cytoplasm and extracellular matrix.
After staining, the slides were dehydrated, cleared and mounted for
microscopic examination under a light microscope. The overlying
epidermis was intact, and no significant cytologic atypia or
increased mitotic figures were identified. Immunohistochemical
staining was performed at the pathology laboratory of the Catholic
University of Korea (Seoul, Korea; part of Seoul St. Mary's
Hospital) using a BenchMark ULTRA autostainer (Roche Tissue
Diagnostics). Formalin-fixed (10% neutral-buffered formalin; room
temperature; 24 h), paraffin-embedded tissue sections (4 µm thick)
were deparaffinized in xylene and rehydrated with a descending
ethanol series (100, 95 and 70%). Heat-induced epitope retrieval
was carried out using Cell Conditioner 1 (pH 8.0; Roche Tissue
Diagnostics) at 95˚C for 36 min. Endogenous peroxidase was blocked
with a hydrogen peroxide solution (ready to use; Roche Tissue
Diagnostics) at room temperature for 4 min. After blocking with a
commercially available antibody diluent (ready to use; applied
undiluted according to the manufacturer's instructions; Roche
Tissue Diagnostics) at room temperature (20-25˚C) for 10 min, the
tissue sections were incubated with the following primary
antibodies at room temperature for 32 min (BenchMark ULTRA standard
protocol): Anti-CD34 (clone QBEnd/10; ready to use; cat. no.
MA1-10202; Dako; Agilent Technologies, Inc.), anti-HHV-8 (clone
13B10; ready to use; cat. no. 359A-14; Cell Marque; Merck KGaA) and
anti-Ki-67 (clone 30-9; ready to use; cat. no. 790-4286; Roche
Tissue Diagnostics). The ultraView Universal DAB Detection Kit
(ready to use; cat. no. 760-500; Roche Tissue Diagnostics) was used
as a secondary detection reagent. This system utilizes an
HRP-labeled multimer antibody complex that does not require further
dilution. Incubation with the secondary HRP-conjugate was performed
for 8 min at room temperature, as per the manufacturer's standard
protocol. Hematoxylin counterstaining (Roche Tissue Diagnostics;
room temperature; 12 min) and bluing reagent incubation (Roche
Tissue Diagnostics; room temperature; 4 min) were applied
sequentially. Slides were examined under a light microscope. The
Ki-67 proliferation index was assessed manually by a
board-certified pathologist. The pathologist selected a
representative high-proliferation ‘hot spot’, defined as an area
containing a high density of positively stained nuclei. Within this
selected region, >1,000 tumor cells were visually evaluated
under a light microscope, and the proportion of Ki-67-positive
nuclei among total tumor cells was estimated to derive the
proliferation index. All staining procedures and evaluations were
conducted by a board-certified pathologist according to the
institution's standard protocols. CD34 immunohistochemistry showed
staining limited to the endothelial lining of vascular spaces,
while the spindle-like cells remained negative (Fig. 2C). HHV-8 immunostaining was
negative (Fig. 2D), excluding
Kaposi sarcoma. The Ki-67 proliferation index was low at 5%
(Fig. 2E). These findings
confirmed the diagnosis of ADF.
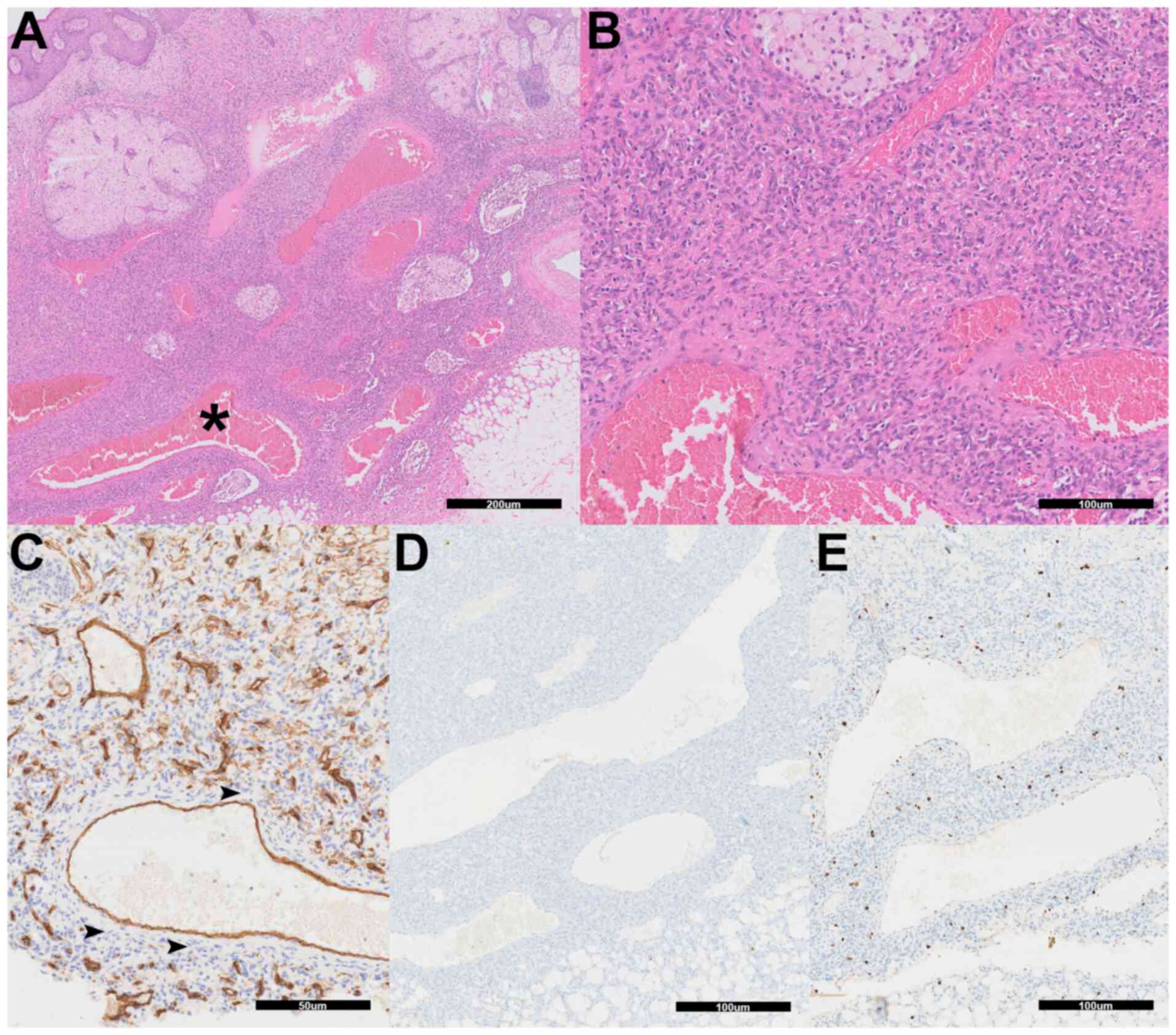 | Figure 2Histopathologic and
immunohistochemical features of aneurysmal dermatofibroma. (A) The
specimen demonstrates a dermal-based spindle-like cell
proliferation with multiple blood-filled pseudovascular spaces
(asterisk) characteristic of aneurysmal dermatofibroma (H&E;
magnification, x40; scale bar, 200 µm). (B) Higher magnification
shows the storiform arrangement of spindle-like cells surrounding
the aneurysmal spaces (H&E; magnification, x200; scale bar, 100
µm). (C) CD34 immunohistochemistry reveals positive staining
limited to endothelial cells lining vascular structures, while the
spindle cell component remains negative (arrowheads; scale bar, 50
µm). (D) Human herpesvirus-8 immunohistochemistry is negative
throughout the lesion, excluding Kaposi sarcoma. (E) Ki-67
immunohistochemistry demonstrates low proliferative activity with
scarce positive nuclei (magnification, x100; scale bars, 100 µm).
H&E, hematoxylin and eosin. |
With this definitive diagnosis, the patient was
ruled out for cutaneous metastasis, allowing for accurate tumor
staging and appropriate surgical management.
After surgical removal of ADF, the patient was
monitored every 3 months at the outpatient clinic through clinical
examination. No evidence of recurrence or new lesions was observed
during 1 year of follow-up. The patient continues to be managed
conservatively with regular clinical evaluations. The patient's
overall condition remained stable and there were no complications
related to the disease in March 2025.
Discussion
This case draws attention to the diagnostic
uncertainty that can arise when evaluating new skin lesions in
patients with a known diagnosis of cancer. Although skin metastases
from lung cancer are not common, their presence usually signals
advanced disease and often requires a change of the treatment plan.
In this case, the clinical and imaging findings initially pointed
toward metastatic disease, particularly given the patient's recent
diagnosis of NSCLC and the increased uptake of the lesion seen on
PET-CT.
Importantly, the patient was diagnosed with stage
IVB (cT1cN3M1c) squamous cell carcinoma of the lung and had
undergone prior surgical resection and adjuvant chemotherapy. This
advanced-stage lung cancer background added complexity to the
clinical suspicion of metastasis, making differentiation from
benign mimics crucial to avoid unnecessary alterations in
treatment.
Fluorodeoxyglucose (FDG) uptake in the lesion
resulted in a false-positive PET-CT scan interpretation, suggesting
metastasis. Benign tumors with FDG uptake are rare, and the
reported cases in the literature are predominantly of tumors other
than dermatofibroma (5,6). To the best of our knowledge,
dermatofibroma showing FDG uptake has only been reported in one
previous case (7).
ADF is a rare subtype, accounting for <2% of all
dermatofibromas, and 0.8% of all solid tumors (8). Recent studies emphasize the
histopathologic overlap between ADF and malignancies, necessitating
rigorous immunohistochemical evaluation to avoid misdiagnosis
(3,9). Recent large series and reviews
emphasize the importance of thorough histopathologic and
immunohistochemical evaluation to avoid misdiagnosis and
unnecessary aggressive treatment, particularly in oncology patients
presenting with new or atypical skin lesions (3,10).
On gross morphology, the lesion of the present case
appeared as a two-centimeter, non-ulcerated, blackish nodule on the
upper right chest, with intermittent contact bleeding. The
differential diagnosis for this lesion includes cutaneous
metastasis, angiosarcoma, Kaposi sarcoma, spindle cell hemangioma,
malignant melanoma and other benign or malignant spindle cell
neoplasms such as ADF (11).
ADF can closely resemble malignant tumors both in
radiologic and histological aspects (12). In this patient, the lesion's
metabolic activity on PET-CT was within the range seen in
malignancies, which raised high suspicion for cutaneous metastasis
of the lung lesion or other primary malignancy. The presence of
blood-filled spaces and spindle-like cells may be mistaken for
angiosarcoma or metastatic carcinoma (13). This is particularly the case if
tissue samples are limited due to partial skin and soft tissue
excision or punch biopsy performed in the outpatient setting for
diagnostic evaluation of potential malignancy (14); however, in the present study, a
full excisional biopsy with clear margins was performed at the
initial surgery. To rule out other primary malignancies,
differential diagnosis with immunohistochemical staining was made.
The absence of CD34 immunoreactivity ruled out dermatofibrosarcoma
protuberance and negativity for HHV-8 expression ruled out Kaposi
sarcoma, along with a low proliferation index (15,16).
These findings finalized the diagnosis of ADF (3). This outcome prevented the patient
from being incorrectly classified as having metastatic disease or a
double primary malignancy, which would have affected the overall
treatment plan.
The main limitation of this report is that it
describes a single patient case; therefore, it is not possible to
generalize the findings to a broader population. Further research
with a larger cohort study is necessary to validate these findings
and support conclusions.
This case shows that a high FDG uptake lesion in a
cancer patient may not represent metastasis. Benign tumors and
tumor-like conditions are often incidentally detected on FDG PET/CT
during workup and should be differentiated from metastasis.
Over-reliance on imaging or clinical impression, without tissue
confirmation, can lead to inappropriate changes in treatment plans.
Careful evaluation, including histopathology and appropriate
immunostains, remains essential when the diagnosis is
uncertain.
In conclusion, this case emphasizes the critical
importance of histopathologic evaluation in distinguishing benign
lesions from true metastatic disease. To avoid misdiagnosis due to
the tendency of ADF to mimic malignancy in cancer patients,
multidisciplinary integration of imaging, histopathology and
immunohistochemistry is essential.
Acknowledgements
Not applicable.
Funding
Funding: The present study received financial support from the
Catholic Medical Center Research Foundation in the program year of
2024 (grant no. 5-2024-B0001-00104).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JYC was involved in the conceptualization and
investigation (including conducting experiments, and collecting
clinical data and specimens), and wrote the original draft. HP
performed data curation and formal analysis (application of
statistical, computational and other quantitative techniques to
analyze and interpret study data). JC participated in
conceptualization, supervision, and review and editing of the
manuscript. JYC, HP and JC confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board (IRB) of The Catholic University of Korea (Seoul, Korea; IRB
no. 2025-1442-0001). The study was performed in accordance with the
principles of the Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case details and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakhri S, Zemni I, Ayadi MA, Naija L,
Boujelbene N and Ben Dhiab T: Cutaneous metastasis as a first
presentation of lung carcinoma: A case series. J Med Case Rep.
17(315)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Munekata Y, Kitamura S, Yanagi T, Shimano
M and Ujiie H: Dermoscopic features of aneurysmal dermatofibroma: A
case report and review of the literature. J Dermatol. 49:e169–e170.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Orzan OA, Dorobanțu AM, Gurău CD, Ali S,
Mihai MM, Popa LG, Giurcăneanu C, Tudose I and Bălăceanu B:
Challenging patterns of atypical dermatofibromas and promising
diagnostic tools for differential diagnosis of malignant lesions.
Diagnostics (Basel). 13(671)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alves JV, Matos DM, Barreiros HF and
Bártolo EA: Variants of dermatofibroma-a histopathological study.
An Bras Dermatol. 89:472–477. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Choi YY, Kim JY and Yang SO: PET/CT in
benign and malignant musculoskeletal tumors and tumor-like
conditions. Semin Musculoskelet Radiol. 18:133–148. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Metser U and Even-Sapir E: Increased
(18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions
found on whole-body positron emission tomography/computed
tomography (PET/CT): Accumulated data from four years of experience
with PET/CT. Semin Nucl Med. 37:206–222. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Demir MK, Ozdemir H, Gençhallaç H, Altaner
S and Kartal O: Dermatofibroma mimicking malignancy on integrated
F-18 fluorodeoxyglucose PET-CT. Diagn Interv Radiol. 15:61–63.
2009.PubMed/NCBI
|
|
8
|
Luzar B and Calonje E: Cutaneous
fibrohistiocytic tumours-an update. Histopathology. 56:148–165.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Erdil DI, Leblebici C, Erdil D, Manav V,
Erdemir VA and Aksu AEK: Dermatofibroma: Clinicopathological
analysis of 239 cases. An Bras Dermatol. 100:150–155.
2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zaballos P, Álvarez-Salafranca M,
Llambrich A, Malvehy J, Taberner R, Medina C, Argenziano G, Thomas
L, Pizarro Á, Del Pozo LJ, et al: Dermoscopy of
haemosiderotic/aneurysmal dermatofibroma: A morphological study of
110 cases. J Eur Acad Dermatol Venereol. 37:317–327.
2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morariu SH, Suciu M, Vartolomei MD, Badea
MA and Cotoi OS: Aneurysmal dermatofibroma mimicking both clinical
and dermoscopic malignant melanoma and Kaposi's sarcoma. Rom J
Morphol Embryo. 55 (3 Suppl):S1221–S1224. 2014.PubMed/NCBI
|
|
12
|
Kaddu S, McMenamin ME and Fletcher CD:
Atypical fibrous histiocytoma of the skin-clinicopathologic
analysis of 59 cases with evidence of infrequent metastasis. Am J
Surg Pathol. 26:35–46. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lewis JR and James S: Spindle cell
lesions—neoplastic or non-neoplastic? Spindle cell carcinoma and
other atypical spindle cell lesions of the head and neck. Head and
neck pathology. 2:103–110. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pickett: Shave and punch biopsy for skin
lesions. American family physician. 84:995–1002. 2011.PubMed/NCBI
|
|
15
|
Khamdan F, Brailsford C, Dirr MA, Sagut P,
Nietert PJ and Elston D: Dermatofibroma versus dermatofibrosarcoma
protuberans: A nuclear morphology study. Am J Dermatopathol.
45:631–634. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alexanian S, Said J, Lones M and Pullarkat
ST: KSHV/HHV8-negative effusion-based lymphoma, a distinct entity
associated with fluid overload states. Am J Surg Pathol.
37:241–249. 2013.PubMed/NCBI View Article : Google Scholar
|