Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN)
is a rare and highly aggressive hematological malignancy that
originates from precursor plasmacytoid dendritic cells (pDCs)
(1). BPDCN is characterized by
unique clinical and pathological features, most commonly presenting
with cutaneous involvement, including nodules, plaques or tumors.
Varying degrees of bone marrow (BM) involvement, lymphadenopathy,
splenomegaly and/or cytopenias have been reported (2). Being a rare condition, BPDCN is often
misdiagnosed as other hematological or dermatological conditions
(3), leading to delayed treatment
initiation and a poor prognosis (4). Before December 2018, the management
of BPDCN primarily relied on intensive chemotherapy with regimens
used to treat acute myeloid leukemia (AML) or acute lymphoblastic
leukemia (ALL). However, treatment responses were transient, and
the overall survival (OS) rate was poor (5). Novel targeted therapies, such as
tagraxofusp and SL-401, have been recently introduced to improve
treatment outcomes in BPDCN. Tagraxofusp, a CD123-targeting
monoclonal antibody, shows promise as frontline therapy; however,
its clinical applications are limited due to severe adverse
effects, such as capillary leak syndrome (CLS) and hepatotoxicity
(6). Given that BPDCN cells
frequently overexpress the antiapoptotic protein B-cell lymphoma-2
(BCL-2), BCL-2 inhibitors, including venetoclax, in combination
with hypomethylating agents (HMAs) such as azacitidine have emerged
as a potential therapeutic strategy, especially for elderly and
relapsed/refractory patients (7).
The current study reports a case of BPDCN that
presented with distinct clinical manifestations and showed a good
treatment response. The aim of the present case study is to
highlight the clinical variability of BPDCN, the role of targeted
therapy in its management and the need for further research to
optimize treatment strategies.
Case report
Case. A 51-year-old man was admitted to
Beijing Luhe Hospital Affiliated to Capital Medical University
(Beijing, China) in July 2024 due to bilateral shoulder and lower
limb pain that had persisted for 1 month, and a fever that had been
present for 1 week. The patient initially developed spontaneous
pain in both shoulders in June 2024, which resolved with
cephalosporin therapy administered at an external hospital for 6
days in the same month. The patient had sudden lower left limb pain
progressing to intermittent lumbodorsal and bilateral lower limb
pain. Symptomatic improvement was achieved with mecobalamin and
vitamin B1 at an external hospital. Right lower limb pain worsened
in July 2024, and 2 days later, a high fever (39˚C) with chills
necessitated hospital admission after self-administered
antipyretics. The patient's past medical history included a chronic
hepatitis B carrier status for 30 years and a left upper lobectomy
with appendectomy for bronchiectasis in 2001.
A physical examination revealed generalized pallor
of the skin and mucosa, a 0.5x0.5-cm purple rash (which had existed
for several years without change) on the right medial malleolus, a
10-cm surgical scar on the right thorax, absence of palpable
superficial lymphadenopathy, negative sternal tenderness, and no
lymphadenopathy or hepatosplenomegaly. A BM aspirate smear
demonstrated disrupted trilineage hematopoiesis accompanied by
focal stromal fibrosis and scattered hematopoietic cells. BM
aspiration showed hypercellularity, with 62.5% of abnormal cells
characterized by large size and basophilic vacuolated cytoplasm
(Fig. 1A). Some cells also
exhibited a tailing phenomenon (Fig.
1B) and were negative for peroxidase staining.
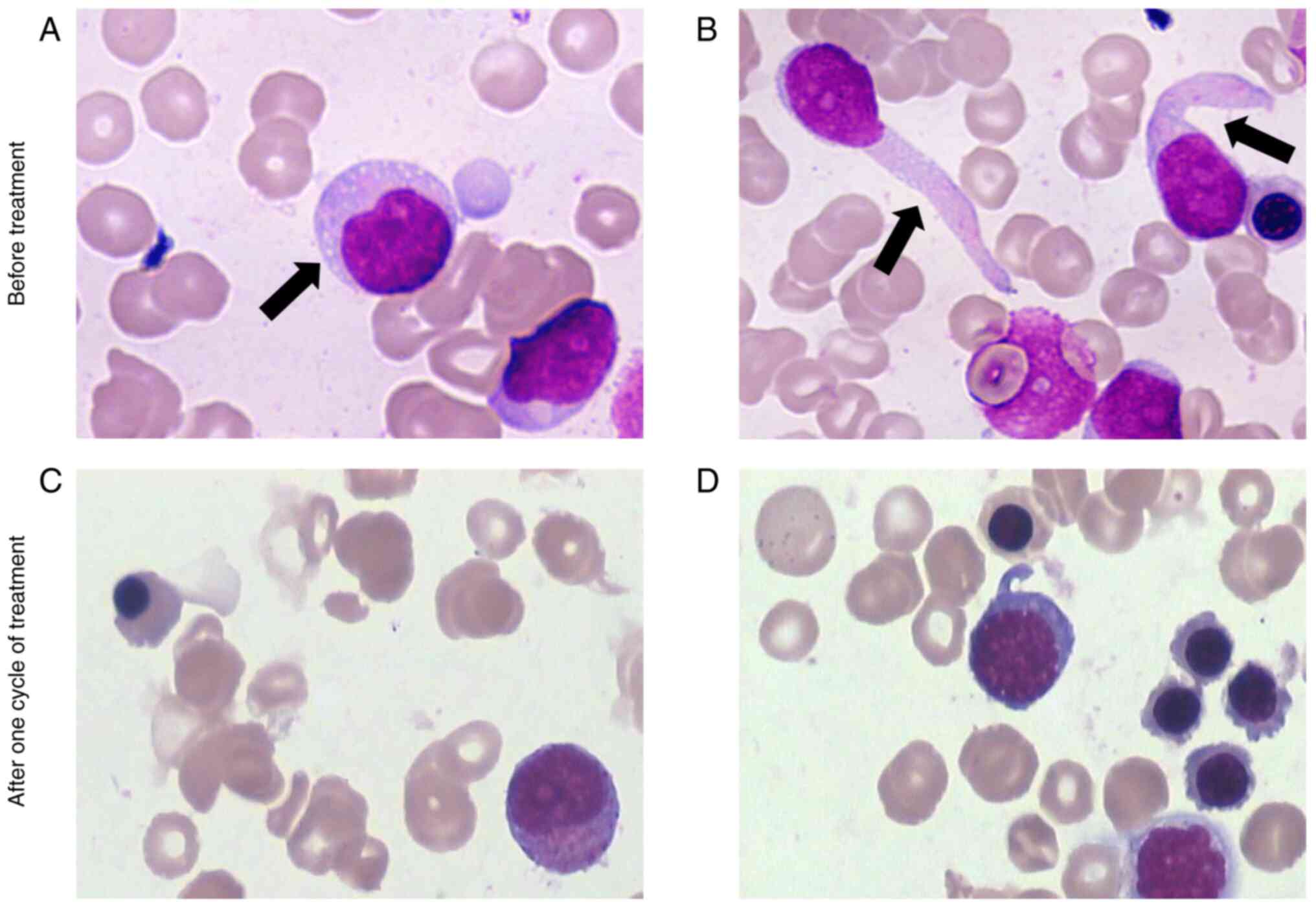 | Figure 1Wright-Giemsa-stained BM aspirate
smear images (A and B) before treatment and (C and D) after one
cycle of treatment, with a magnification of x1,000). In the
pre-treatment BM aspirate smear, the cytoplasm is basophilic, with
occasional (A) vacuoles (arrow) and (B) tail-like protrusions
(arrow). (C) After one cycle of treatment, mature erythrocytes,
promyelocytes, myelocytes and other hematopoietic cells were
observed, with no BPDCN cells identified. (D) The remaining fields
showed hematopoietic cells consistent with those in (C), and no
BPDCN cells were identified. BM, bone marrow. |
Immunophenotyping using flow cytometry (FCM)
demonstrated that the abnormal cells were positive for terminal
deoxynucleotidyl transferase, human leukocyte antigen-DR, CD56,
CD304, CD123 (dim), CD4 (dim), CD99, CD2, CD71 (dim), and CD38 and
negative for CD34, CD303, myeloperoxidase, CD117, CD13, CD33, CD64,
CD10, CD11b, CD11c, CD15, CD16, CD14, CD9, CD7, c/mCD3, CD5, CD8,
CD19, CD20, CD22, cCD79a, CD94, CD161, CD81, CD30, cytokeratin,
CD61, CD41, CD41a, CD42a, CD42b and Ki67 (Fig. 2). Myeloid hematological
neoplasm-related gene variation testing identified ASXL2 p.Q612X
(38.28%) and ETV6 c.1253+1G>T (47.32%) mutations, with negative
leukemia fusion genes and a normal male karyotype [46,XY(20)]. Laboratory examinations
demonstrated pancytopenia: Neutrophils, 1.20x109/l
(normal range, 2.00-7.50x109/l); hemoglobin, 82.00 g/l
(normal range, 120.00-160.00 g/l for adult males); and platelets,
44.00x109/l (normal range,
100.00-300.00x109/l). Peripheral blood (PB) contained 8%
blasts. Biochemical analyses revealed increases in lactate
dehydrogenase (518 U/l; normal range, 109-245 U/l), C-reactive
protein (64.52 mg/l; normal range, 0-10 mg/l), β2-microglobulin
(3.12 mg/l; normal range, 1.00-3.00 mg/l) and alkaline phosphatase
(191 U/l; normal range, 40-150 U/l). Coagulation studies indicated
elevated D-dimer levels [8.51 mg/l (fibrinogen equivalent units);
normal range, 0-0.55 mg/l (fibrinogen equivalent units)], whereas
cytokine profiling revealed increased interleukin (IL)-6, IL-8 and
IL-10 levels. Findings from positron emission tomography-computed
tomography (CT) demonstrated diffuse hypermetabolic BM (SUVmax 4.9)
and multiple metabolically active lymphadenopathies. CT of the
chest revealed post-left lobectomy changes with bilateral
supraclavicular and axillary lymphadenopathy. Based on the
aforementioned symptoms and outcomes, the patient was diagnosed
with BPDCN.Cycle one of VA therapy (100 mg/m2
azacitidine on days 1-7 + 200 mg venetoclax daily on days 1-21) was
initiated for the patient in July 2024 (day 3 post-admission).
Meanwhile, 300 mg posaconazole was administered orally on a daily
basis for 2 weeks per cycle. A BM aspirate was performed in August
2024 (day 14 after starting treatment). No BPDCN cells were
identified on the BM aspirate smear (Fig. 1C and D). Flow cytometry demonstrated 0%
aberrant cells in the BM, and the previously positive
BPDCN-associated markers were undetectable (Fig. 2). These findings confirmed CR with
minimal residual disease (MRD) negativity [there is currently no
international consensus on the criteria for defining MRD negativity
in BPDCN. In Beijing Luhe Hospital Affiliated to Capital Medical
University, MRD negativity (detected by flow cytometry) is defined
as BPDCN-associated aberrant immunophenotypic cells in BM
<0.01%].
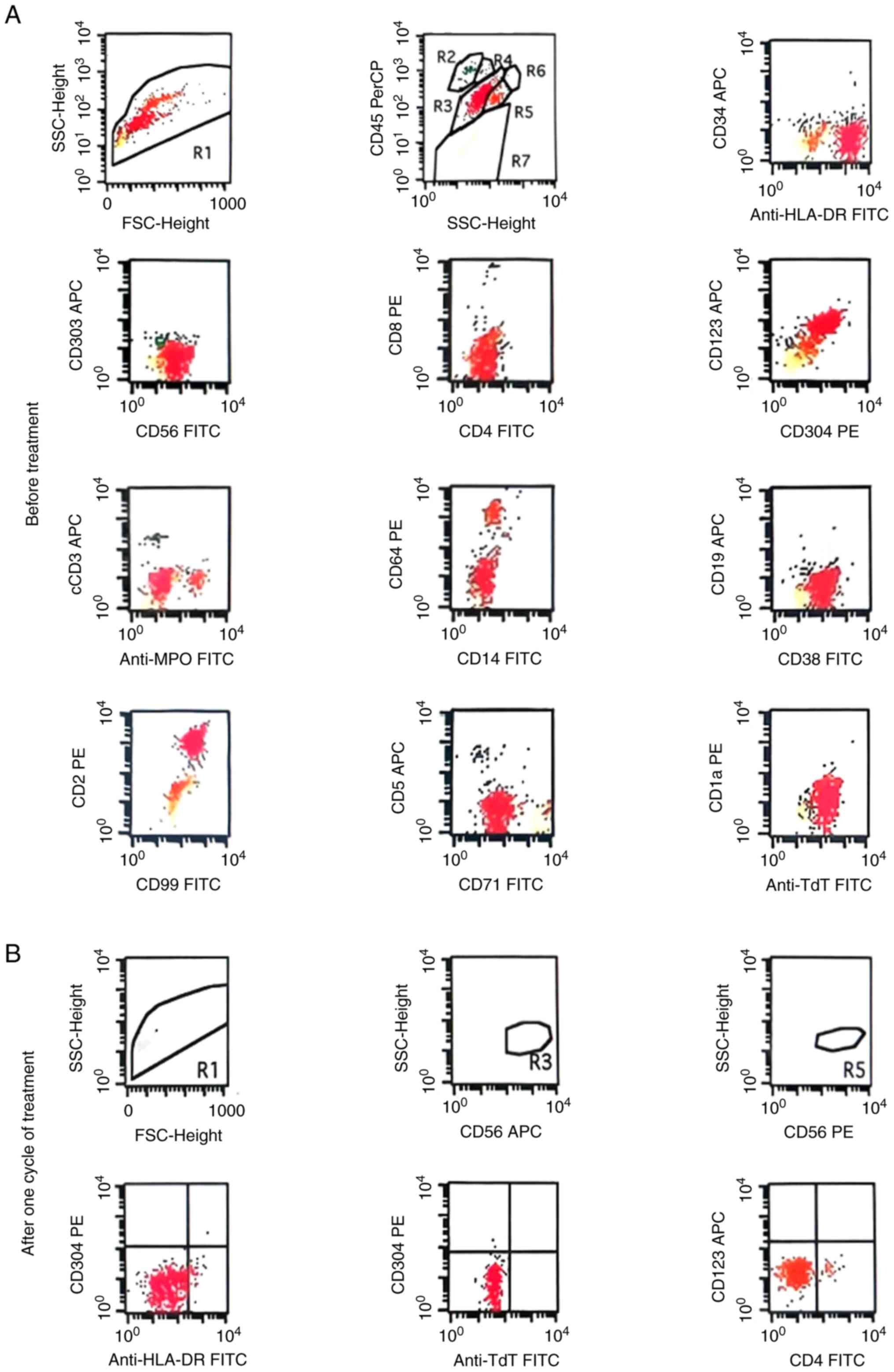 | Figure 2Flow cytometry prior to treatment
indicating that the R3 population consists of abnormal cells,
accounting for ~75.07% of nucleated cells. (A) This abnormal cell
population showed 100% positive expression of CD4 (dim), CD56,
CD123 (dim), CD304, TdT, HLA-DR, CD99, CD2, CD71 (dim) and CD38,
while being negative for CD303, CD3, CD14, CD19, CD34 and MPO. (B)
After one treatment cycle, the proportion of abnormal cells in the
bone marrow decreased to 0.00%, and the positive expression rates
of CD4, CD56, CD123 and CD304 were all 0%. (This figure only
displays the major relevant immunophenotypes and does not present
the complete detection results of all immunophenotypes). FSC,
forward scatter; SSC, side scatter; TdT, terminal deoxynucleotidyl
transferase; HLA-DR, human leukocyte antigen-DR; APC,
allophycocyanin; FITC, fluorescein isothiocyanate; PE,
phycoerythrin. |
Three cycles were completed without the occurrence
of severe complications. After completing initial treatment at Luhe
Hospital Affiliated to Capital Medical University, the patient was
subsequently managed in an external hospital, so further follow-up
details could not be obtained.
Materials and methods
BM aspirate smears. BM aspirate smears were
prepared by placing drops of aspirated marrow onto glass slides and
air-drying. Smears were stained with a Wright-Giemsa Composite
Stain Kit (cat. no. BA-4017; Changde Bickman Biotechnology Co.,
Ltd.) according to the manufacturer's instructions: The stain was
applied to fully cover each smear and staining was performed at
25˚C for 1-2 min. After staining, excess stain was rinsed off with
distilled water, and slides were air-dried again. Cellular
morphology was examined by light microscopy (Olympus CX43; Olympus
Corporation).
FCM. Sample preparation and staining.
Ice-cold acetic acid (190 µl) was mixed with 10 µl BM aspirate, and
cells were then counted using a hemocytometer. This step was
performed to estimate the total cell number, determine the
appropriate antibody panels and total staining volume, and set up
control tubes. For surface immunostaining, the required antibodies
were added to the specimen, gently mixed and incubated in the dark
for 15 min. Subsequently, 3 ml lysing solution was added and gently
mixed to lyse erythrocytes, and incubated for another 10 min. An
appropriate volume of phosphate-buffered saline (PBS) was added,
and the mixture was centrifuged at 362 x g for 5 min. The
supernatant was discarded, and the pellet was washed once with PBS.
After gentle mixing, the mixture was centrifuged again at 362 x g
for 5 min, and the supernatant was discarded. Finally, the pellet
was resuspended in 300 µl PBS, gently mixed and subjected to flow
cytometric acquisition. For intracellular antibody staining, the
specimen was first incubated with surface antibodies in the dark
for 15 min. Next, 100 µl of fixative was added, gently mixed and
incubated in the dark for 5 min. Next, 3 ml of lysing solution was
added, gently mixed and incubated in the dark for 10 min to lyse
residual erythrocytes. The mixture was centrifuged at 362 x g for 5
min, and the supernatant was discarded. A total of 50 µl of
permeabilization reagent was added to the pellet, gently mixed and
incubated in the dark for 3 min. Intracellular antibodies were then
added, gently mixed and incubated in the dark for 15 min. An
appropriate volume of PBS was added for washing, and the mixture
was centrifuged at 362 x g for 5 min; the supernatant was
discarded. Finally, the cells were resuspended in 300 µl PBS, mixed
thoroughly and analyzed by flow cytometry. All experimental steps
were performed at room temperature (25˚C).
Data analysis. FCM files acquired on the
instrument were imported into the analysis software. Cell
populations were first gated using a two-parameter dot plot
reflecting forward scatter and side scatter to exclude cell debris.
A second two-parameter dot plot was then used to discriminate
singlets from cell aggregates, thus excluding aggregated cells.
Subsequently, a dot plot of CD45 fluorescence intensity vs. side
scatter was generated to gate CD45-positive nucleated cells. Within
the gated nucleated cell compartment, a series of dot plots were
used to evaluate the expression of myeloid and lymphoid markers.
These marker expressions were further compared against normal
reference profiles to screen for aberrant cell populations. The
proportions of each cell population and the intensity of marker
expression were calculated and recorded. Dot plots and statistical
data were exported, and these results were integrated with
morphological review findings to generate the final bone marrow
immunophenotyping report.
Reagents. The reagents used included lysing
solution for FCM (cat. no. 349202), BD IntraSure Kit RUO
(containing fixative and permeabilization reagents; cat. no.
641776) and BD FACSFlow Sheath Fluid (cat. no. 342003) (all BD
Biosciences). The fluorescently labeled antibodies used were as
follows: CD1a (cat. no. 560945), CD2 (cat. no. A07744), CD3 (cat.
no. 555335), CD4 (cat. no. 340133), CD5 (cat. no. 665001), CD8
(cat. no. A07757), CD13 (cat. no. 557454), CD14 (cat. no. 665753),
CD19 (cat. no. 652804), CD25 (cat. no. 560503), CD26 (cat. no.
340426), CD33 (cat. no. 664937), CD34 (cat. no. 652837), CD38 (cat.
no. A07778), CD45RA (cat. no. 663496), CD45RO (cat. no. 340438),
CD56 (cat. no. 347747), CD64 (cat. no. 652830), CD71 (cat. no.
665339), CD94 (cat. no. 559876), CD117 (cat. no. 664936), CD123
(cat. no. 560087), human leukocyte antigen-DR (cat. no. 665745),
Myeloperoxidase (cat. no. 665337) (all BD Biosciences), CD7 (cat.
no. 007-103-3), CD99 (cat. no. 099-101-3), CD304 (cat. no.
304-102-3), terminal deoxynucleotidyl transferase (cat. no.
815-101-3) (Suzhou Sizhengbai Biotechnology Co., Ltd.) and CD303
(cat. no. 354206; BioLegend, Inc.).
Instrumentation and software. Flow cytometric
acquisition was performed using a BD FACSCalibur flow cytometer
(cat. no. E97600149; BD Biosciences). Data analysis was performed
on a computer equipped with macOS 10.1, using BD CellQuest Pro (for
both data acquisition and analysis) and BD FACSComp (for instrument
calibration).
Myeloid hematological neoplasm-related gene
variation testing. Genomic DNA was extracted from all samples
and fragmented to ~200 bp by restriction enzyme digestion.
Pre-libraries were generated by end repair, adapter ligation and
pre-amplification. Target enrichment was performed using Roche
targeted capture (Roche Diagnostics GmbH) for a panel of 138 genes
implicated in myeloid hematological neoplasms. Libraries were
sequenced on an Illumina Inc., instrument with 2x150 bp paired-end
reads, yielding an average on-target coverage of 1,500 times.
Sequence variants were detected via Sentieon software; public
databases (ClinVar, http://www.ncbi.nlm.nih.gov/clinvar/; COSMIC,
https://cancer.sanger.ac.uk) were used
to filter germline variants, and the final report included somatic
single-nucleotide variants and short insertions/deletions.
Discussion
The diagnosis of BPDCN poses significant challenges
and is frequently misdiagnosed as other hematological or
dermatological malignancies (8). A
systematic review of case reports related to BPDCN published
between January 2020 and October 2024, identified 68 patients from
57 reports retrieved through PubMed (https://pubmed.ncbi.nlm.nih.gov/) (9-65).
Eligible cases were defined as newly diagnosed patients with BPDCN
whose diagnosis was confirmed by flow cytometry in accordance with
the 2022 World Health Organization classification of hematopoietic
and lymphoid tumors (66).
Exclusion criteria included recurrent or refractory BPDCN,
duplicate reports and insufficient diagnostic information. Analysis
of these cases revealed that BPDCN most commonly involved the skin,
BM, PB and lymph nodes (Table I).
The highly variable clinical manifestations, ranging from localized
cutaneous lesions to systemic involvement, pose substantial
difficulties in the early and accurate diagnosis of the
disease.
 | Table IClinical manifestation of 68 cases
extracted from 57 studies related to blastic plasmacytoid dendritic
cell neoplasm (covering case reports published between January 2020
and October 2024) (9-65). |
Table I
Clinical manifestation of 68 cases
extracted from 57 studies related to blastic plasmacytoid dendritic
cell neoplasm (covering case reports published between January 2020
and October 2024) (9-65).
| Main lesion
region | Percentage of cases
(n/total n) |
|---|
| Skin | 88.24 (60/68) |
| BM | 83.82 (57/68) |
| PB | 66.18 (45/68) |
| Lymph node | 47.06 (32/68) |
| Spleen | 33.82 (23/68) |
| Liver | 13.24 (9/68) |
| CNS | 10.29 (7/68) |
| Lung | 7.35 (5/68) |
| Nose | 2.94 (2/68) |
| Breast | 2.94 (2/68) |
The patient in the current case report initially
presented with pain and fever as clinical symptoms, with the lesion
site originating in the BM. Laboratory tests revealed 75.07%
abnormal cells in the BM and only 8% blast cells in the PB. To the
best of our knowledge, no study has directly clarified the
mechanism of BPDCN BM-PB tumor-burden difference. We hypothesize
two plausible explanations. First, BPDCN originates from BM clonal
hematopoiesis (CH), and CH clones can be preserved via autologous
stem cell transplantation and undergo cross-lineage evolution
(67). This may help explain why
BPDCN cells tend to proliferate and persist in the BM. Second,
multiple studies have confirmed the involvement of the CXCR4/CXCL12
axis as the core mechanism for the retention of various tumor cells
by the BM (68-70).
Given that normal pDC precursors (from which BPDCN cells originate)
highly express the chemokine receptor CXCR4(71), we speculate that this may also lead
to the retention of BPDCN cells in the BM and their reduced release
into the PB.
According to the Fifth Edition of the World Health
Organization Classification of Tumors of Hematopoietic and Lymphoid
Tissues in 2022(66), the
diagnosis of BPDCN relies primarily on immunophenotypic criteria,
including expected positivity for CD123*, TCF4*, TCL1*, CD303,
CD304*, CD4 and CD56, and negativity for CD3, CD14, CD19, CD34,
lysozyme and myeloperoxidase. The immunophenotypic diagnostic
criteria are defined as follows: i) In addition to CD4 and/or CD56
positivity, there is the co-expression of CD123 and at least one
additional pDC marker (indicated as * in the aforementioned list);
ii) when there is an absence of CD4 and CD56 expression but yet
there is positivity for any three pDC markers along with the
concurrent negativity for all anticipated negative markers
(66). Pathological biopsy remains
a cornerstone for its definitive diagnosis (72). Therefore, clinicians should
integrate clinical symptoms with the timely selection of
appropriate diagnostic modalities such as immunophenotypic
analysis, skin biopsy and BM aspiration to optimize the diagnostic
workflow for BPDCN.
The role of hematopoietic stem cell transplantation
(HSCT) in treating BPDCN has been firmly established. Chemotherapy
alone cannot sustain long-term remission in BPDCN; allogeneic HSCT
(allo-HSCT) is key for durable remission. Notably, patients with
BPDCN undergoing allo-HSCT during first CR (CR1) have a 1-year
disease-free survival (DFS) rate of 80%, which is significantly
higher than the <50% 1-year DFS rate in those treated with
chemotherapy alone (73). A
single-center study has reported that among patients with BPDCN who
received allo-HSCT in CR1, the 3-year OS rate was >60%; by
contrast, the 3-year OS rate was >40% for patients who were not
in CR1(74).
However, a number of patients are ineligible for
HSCT due to their poor physical condition. Moreover, they require
induction therapy to achieve remission before transplantation. Some
targeted drugs are currently under active research, development and
clinical applications, boasting broad prospects. These drugs
include CD123-targeted agents, such as tagraxofusp, IMGN632 and
SL-401(7). Among them, tagraxofusp
is the only drug approved by the US Food and Drug Administration
(FDA) for the treatment of BPDCN (75). A clinical study has demonstrated
its CR rate of 72% in treatment-naive patients (7). However, its use is contingent on
strict patient-selection criteria (for example, patients with
reduced ejection fraction, hyperbilirubinemia or hypoalbuminemia
are ineligible) and it carries a risk of severe CLS (76).
Chemotherapy regimens tailored to treat myeloid
leukemia, lymphoid leukemia or lymphoma are also commonly adopted
to treat BPDCN. These regimens are viable options for first-line
therapy (77). Studies have shown
that the outcomes of ALL-oriented and lymphoma-oriented regimens
[for example, hyperfractionated cyclophosphamide + vincristine +
doxorubicin + dexamethasone (hyper-CVAD); and cyclophosphamide +
doxorubicin + vincristine + prednisone] are better than those of
AML-oriented regimens (for example, ifosfamide + carboplatin +
etoposide) (78-80).
A retrospective study that recruited 100 patients with BPDCN
demonstrated that first-line hyper-CVAD-based therapy achieved a CR
rate of 80%, which was significantly higher than that of the
CD123-targeted agent SL-401 and other regimens. This intensive
chemotherapy regimen is suitable for younger patients with good
performance status and is particularly indicated for individuals at
high risk of central nervous system involvement (81).
BPDCN cells exhibit the characteristic of high BCL2
expression, and their survival is directly dependent on the BCL2
pathway. This finding provides a clear biological basis for the use
of the BCL2 inhibitor venetoclax in treating BPDCN (82). The venetoclax-HMA regimen, which is
a combination of venetoclax with HMAs (such as azacitidine and
decitabine), has emerged as a highly promising therapeutic strategy
for BPDCN. The regimen is particularly suitable for patients who
cannot tolerate intensive chemotherapy, those ineligible for HSCT
and those with relapsed or refractory disease following prior
treatment (83,84). A retrospective study of 10 elderly
or frail patients with BPDCN treated with VA showed that 60% of
patients achieved CR, and 2 patients were successfully bridged to
allo-HSCT (84). Another study
reported that although two elderly patients with BPDCN with
multiple relapsed and refractory disease (following prior lines of
treatment) did not meet the ‘formal response criteria’ (namely, CR)
after VA therapy, 1 patient experienced a 50% reduction in BM
blasts, and both patients showed improvement in skin lesions
(83).
In the present study, VA therapy was chosen for the
patient, who had poor general condition at treatment onset
(intermittent fever for 1 week, maximum temperature of 39˚C,
lethargy and generalized anemic appearance) and was intolerant of
intensive chemotherapy or CD123-targeted agents. Hematological
toxicity is a major concern with venetoclax-based regimens
(82). Common grade 3/4 adverse
events include febrile neutropenia, leukopenia and anemia, whereas
infectious complications are primarily pneumonia and sepsis
(85). These toxic effects require
close monitoring and supportive care to ensure patients' tolerance
to treatment. Other therapeutic options include combination
regimens involving pralatrexate, enasidenib and bortezomib
(76,80).
Notably, the dosage of venetoclax used to treat
BPDCN in one previous study ranged from 400-800 mg per day
(83). However, in clinical
practice, drug interactions are a crucial factor impacting the
formulation of treatment regimens. In the present case study,
venetoclax dosage adjustment was necessary as the patient's
neutropenia required posaconazole intervention (a potent CYP3A4
inhibitor) as antifungal prophylaxis. The FDA recommendation of
reducing venetoclax dosage to 70 mg when co-administered with
posaconazole is included in the package insert (86). A previous study demonstrated that
reducing the venetoclax dosage to 50 mg in patients with AML did
not compromise treatment efficacy when used in combination with
potent CYP3A4 inhibitors (86).
Another study that enrolled 43 patients with relapsed and
refractory AML and related myeloid malignancies used venetoclax in
combination therapy and found that 3 patients who received higher
than the recommended dosages of venetoclax still achieved a
clinical response (83).
Nevertheless, due to differences in disease pathogenesis, tumor
classification and other aspects between BPDCN and AML, these
dosage regimens cannot be directly applied to BPDCN treatment.
Therefore, based on empirical treatment, 200 mg/day of venetoclax
was administered to the current patient. Further large-scale
randomized controlled trials are warranted to determine the optimal
dosage regimen of VA when used in combination with CYP3A4
inhibitors to treat BPDCN.
Overall, VA therapy demonstrated favorable efficacy
in treating BPDCN in the present patient, achieving rapid remission
while maintaining good tolerability. However, the lack of long-term
follow-up data limits a comprehensive assessment of treatment
durability. Nonetheless, this case report provides a clinical
reference for selecting therapeutic options for patients with BPDCN
who are unable to tolerate intensive chemotherapy or CD123-targeted
agents. Moreover, when the patient received the VA therapy in
combination with posaconazole, the venetoclax dose was adjusted
empirically and was not reduced to the FDA-recommended dose range.
Nevertheless, the patient achieved a favorable clinical response
and no adverse events were observed. This finding suggests that the
dose-reduction thresholds for venetoclax when co-administered with
CYP3A4 inhibitors in the treatment of BPDCN still need validation
in prospective clinical studies with larger sample sizes. Given the
rarity of BPDCN, the ongoing collection of real-world data and
additional well-designed clinical trials is imperative to optimize
treatment paradigms, refine treatment strategies and improve
patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Two-in-One Project of
the High-level ‘Double Ten’ Science and Technology Innovation
Cultivation Special Project in Tongzhou District, Beijing (grant
no. KJ2023SS007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XC and HZ designed the study, advised on patient
treatment and analyzed patient data. YL was responsible for
collecting clinical, imaging and pathological data of the patient
and was responsible for the conception, design and content of the
manuscript. XC and HZ confirm the authenticity of all the raw data.
YL and XC wrote the original draft. HZ reviewed and edited the
original draft. YL revised the manuscript. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study, which includes this case as part
of a project evaluating the efficacy of venetoclax combined with
azacitidine in treating hematological diseases, was approved by the
Ethics Committee of the Affiliated Beijing Luhe Hospital of Capital
Medical University (approval no. 2024-LHKY-087-02) and was
conducted in accordance with the guidelines of the Declaration of
Helsinki.
Patient consent for publication
Patient written consent was obtained for publication
of images and data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jain A and Sweet K: Blastic plasmacytoid
dendritic cell neoplasm. J Natl Compr Canc Netw. 21:515–521.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Garnache-Ottou F, Vidal C, Biichlé S,
Renosi F, Poret E, Pagadoy M, Desmarets M, Roggy A, Seilles E,
Soret L, et al: How should we diagnose and treat blastic
plasmacytoid dendritic cell neoplasm patients? Blood Adv.
3:4238–4251. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wilson NR, Konopleva M, Khoury JD and
Pemmaraju N: Novel therapeutic approaches in blastic plasmacytoid
dendritic cell neoplasm (BPDCN): Era of targeted therapy. Clin
Lymphoma Myeloma Leuk. 21:734–740. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adimora IJ, Wilson NR and Pemmaraju N:
Blastic plasmacytoid dendritic cell neoplasm (BPDCN): A promising
future in the era of targeted therapeutics. Cancer. 128:3019–3026.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pollyea DA, Altman JK, Assi R, Bixby D,
Fathi AT, Foran JM, Gojo I, Hall AC, Jonas BA, Kishtagari A, et al:
Acute myeloid leukemia, version 3.2023, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 21:503–513.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Economides MP, McCue D, Lane AA and
Pemmaraju N: Tagraxofusp, the first CD123-targeted therapy and
first targeted treatment for blastic plasmacytoid dendritic cell
neoplasm. Expert Rev Clin Pharmacol. 12:941–946. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pemmaraju N, Kantarjian H, Sweet K, Wang
E, Senapati J, Wilson NR, Konopleva M, Frankel AE, Gupta V, Mesa R,
et al: North American blastic plasmacytoid dendritic cell neoplasm
consortium: Position on standards of care and areas of need. Blood.
141:567–578. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morrison GM, Hopkins A, Knapp C, Kulkarni
R and Scopetta JP: Blastic plasmacytoid dendritic cell neoplasm
presenting as violaceous forehead plaque. Dermatol Online J 28:
10.5070/D328458522., 2022.
|
|
10
|
Azad F, Zhang J, Miranda CJ and Gravina M:
Venetoclax and azacitidine in the treatment of blastic plasmacytoid
dendritic cell neoplasm refractory to conventional therapy. Cureus.
14(e33109)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma YQ, Sun Z, Li Y-M and Xu H: Blastic
plasmacytoid dendritic cell neoplasm: Two case reports. World J
Clin Oncol. 15:1207–1214. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tao LL, Wen HT, Wang ZY, Cheng J and Zhao
L: Azacitidine maintenance therapy for blastic plasmacytoid
dendritic cell neoplasm allograft: A case report. World J Clin
Cases. 12:136–141. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Alwan A, Khalid F, Vyas C, Sirpal V and
Bader H: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) in an
elderly female: A rare rash. J Community Hosp Intern Med Perspect.
13:79–81. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alam H, Saeed N and Rashid A:
Indispensable role of immunophenotyping in diagnosing leukemic
phase of blastic plasmacytoid dendritic cell neoplasm without
cutaneous manifestation. Leuk Res Rep. 17(100317)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Albiol N, Novelli S, Mozos A, Pratcorona
M, Martino R and Sierra J: Venetoclax in relapsed/refractory
blastic plasmacytoid dendritic cell neoplasm with central nervous
system involvement: A case report and review of the literature. J
Med Case Rep. 15(326)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aran BM, Duran J, Whittemore D and Gru AA:
A CD56- immunoblastoid variant of blastic plasmacytoid dendritic
cell neoplasm. J Cutan Pathol. 51:40–44. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar PA, Ombada M, Pemmasani G, Tambe A,
Banki K and Gentile T: Liposomal daunorubicin and cytarabine, a
potential therapy for blastic plasmacytoid dendritic cell neoplasm.
J Investig Med High Impact Case Rep.
10(23247096221127114)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brett VE, Menguy S, Arcourt A, Bidet A,
Lechevalier A, Leguay T, Klein E, Garnache-Ottou F and Vial JP: An
unusual case of cytoplasmic CD3 expressing BPDCN supporting the
T-lineage origin of plasmacytoid dendritic cells. Cytometry B Clin
Cytom. 102:175–177. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cai JW, Li MY, Wang WH, Shi HQ, Yang YH
and Chen JJ: Blastic plasmacytoid dendritic cell neoplasm in
Jinhua, China: Two case reports. World J Clin Cases. 12:5263–5270.
2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Castaño-Bonilla T, Mata R, Láinez-González
D, Gonzalo R, Castañón S, Pinta FJDdl, Blas C, López-Lorenzo JL and
Alonso-Domínguez JM: Spontaneous remission of blastic plasmacytoid
dendritic cell neoplasm: A case report. Medicina (Kaunas).
60(807)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chan G, Akintorin S, Luu M and Harter N:
Blastic plasmacytoid dendritic cell neoplasm with cutaneous
presentation: A case series in children. Pediatr Dermatol.
38:883–886. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen J, Zhang X, Ma L, Gao Y, Fu Z and Liu
M: 18F-FDG PET/CT findings in a patient with blastic
plasmacytoid dendritic cell neoplasm and post-transplant
lymphoproliferative disorder after hematopoietic stem cell
transplantation: a case report. Front Med (Lausanne).
10(1258310)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cianga VA, Dănăilă CD, Antohe I, Oană R,
Mențel M, Ivanov I, Dragoș L and Dăscălescu AS: A very rare
case of FLT3-D835 positive blastic plasmacytoid dendritic cell
neoplasm. Arch Clin Cases. 7:57–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dang X, Zhou D, Meng L and Bi L: Blastic
plasmacytoid dendritic cell neoplasm with genetic mutations in
multiple epigenetic modifiers: A case report. J Int Med Res.
49(300060520982667)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dhakal P, Sy M, Sutamtewagul G, Mou E, Yu
N and Pemmaraju N: Overcoming Tagraxofusp-Erzs monotherapy
resistance in blastic plasmacytoid dendritic cell neoplasm (BPDCN)
in a real-world clinical setting. J Immunother Precis Oncol.
7:205–209. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ding Y, Yang J and Lindsey K: Cytologic
features of blastic plasmacytoid dendritic cell neoplasm involving
liver: A case report and literature review. Diagn Cytopathol.
49:E80–E83. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Edjtemaei R, Ghanadan A and Ameli F:
Blastic plasmacytoid dendritic cell neoplasm of skin, a rare
dermatohematologic malignancy-A case report. Clin Case Rep.
12(e9398)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
El Hussein S, Yabe M, Wang W, Pemmaraju N,
Loghavi S, Jelloul FZ, Fang H, Medeiros LJ, Burack WR, Evans AG, et
al: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) arising in
the setting of polycythemia vera (PV): An illustration of the
emerging role of flow cytometry analysis in monitoring progression
of myeloproliferative neoplasms. EJHaem. 3:954–957. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Fakhfakh Y, Aribia NB, Mnif S, Sannenna H,
Mnif H and Louati N: Blastic plasmacytoid dendritic cell neoplasm:
A diagnosis not to be missed, about three case reports. Tunis Med.
100:647–651. 2022.PubMed/NCBI
|
|
30
|
Fay CJ, Iriarte C, Moslehi D, Sheets AR
and LeBoeuf NR: Blastic plasmacytoid dendritic cell neoplasm
mimicking dermatomyositis. JAAD Case Rep. 39:70–73. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fei F, Liedtke M and Silva O: Case report:
Mature plasmacytoid dendritic cell proliferation associated with a
lymphoid neoplasm. Front Oncol. 12(903113)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Florescu AM, Sørensen ALT, Nielsen HV,
Tolnai D, Sjö LD, Larsen KL and Al-Karagholi MAM: Blastic
plasmacytoid dendritic cell neoplasm and cerebral toxoplasmosis: A
case report. BMC Neurol. 22(233)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gulati R, Abu-Salah A, Salous T and
Nassiri M: Relapse of tagraxofusp treated blastic plasmacytoid
dendritic cell neoplasm with loss of CD123 expression. J Hematop.
15:35–39. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guo JH, Zhang HW, Wang L, Bai W and Wang
JF: Blastic plasmacytoid dendritic cell neoplasm with skin and bone
marrow involvement: Report of three cases. World J Clin Oncol.
9:10293–10299. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hoffmann E, Böke S, De-Colle C, Lengerke
C, Niyazi KM and Gani C: Ulcerating skin lesions from blastic
plasmacytoid dendritic cell neoplasm responding to low-dose
radiotherapy-a case report and literature review. Strahlenther
Onkol. 200:908–915. 2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu X, Ediriwickrema A, Saleem A, Tan B,
Pemmaraju N and Mannis GN: CD38 and BCL2 expression guides
treatment with daratumumab and venetoclax in tagraxofusp-refractory
blastic plasmacytoid dendritic cell neoplasm (BPDCN) featuring
dynamic loss of CD123. Leuk Res. 139(107479)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jhajj B, Henrie R, El-Khalidy Y and Razavi
HM: A case of acute myeloid leukemia mimicking blastic plasmacytoid
dendritic cell neoplasm: Utility of the proposed upcoming WHO-5
diagnostic criteria. Case Rep Hematol. 2023(5014728)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jiang Y, Li Q, Yuan T, Jiang Y and Deng Q:
Case Report of Anti-CD123 chimeric antigen receptor T-cell therapy
followed by radiotherapy for a recurrence of blastic plasmacytoid
dendritic cell neoplasm after allogeneic hematopoietic stem cell
transplantation. Onco Targets Ther. 13:3425–3430. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Khan F, Hashmi F, Ghahramanyan N, Baloyan
E, Tamamyan G, Konopleva M, Pemmaraju M and Voskanyan A: Diagnosing
and treating blastic plasmacytoid dendritic cell neoplasm in a
resource-limited setting. Oncology (Williston Park). 38:104–106.
2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Khouloud M, Nader S, Ahlem B, Ines S,
Montacer H and Rayhan C: A rare case of Blastic plasmacytoid
dendritic cell neoplasm with gynecologic presentation. Leuk Res
Rep. 21(100462)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Koerber RM, Held SAE, Vonnahme M, Feldmann
G, Wenzel J, Gütgemann I, Brossart P and Heine A: Blastic
plasmacytoid dendritic-cell neoplasia: A challenging case report. J
Cancer Res Clin Oncol. 148:743–748. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kolerova A, Sergeeva I, Krinitsyna J,
Pronkina N, Sizikova S, Filimonov P and Kryuchkova I: Blastic
plasmacytoid dendritic cell neoplasm: Case report and literature
overview. Indian J Dermatol. 65:217–221. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kosasih HJ, Healey G, Brennan MS,
Bjelosevic S, Sadras T, Jalud FB, Ibnat T, Ng AP, Mayoh C, Mao J,
et al: A novel MYB::PAIP1 oncogenic fusion in pediatric blastic
plasmacytoid dendritic cell neoplasm (BPDCN) is dependent on BCL2
expression and is sensitive to venetoclax. Hemasphere.
8(e1)2024.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Lee HJ, Park HM, Ki SY, Choi YD, Yun SJ
and Lim HS: Blastic plasmacytoid dendritic cell neoplasm of the
breast: A case report and review of the literature. Medicine
(Baltimore). 100(e25699)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu F, Qi F, Zhang J, Tan Y and Zhang X:
Blastic plasmacytoid dendritic cell neoplasm with lung involvement
and cytopenia: A case report and a literature review. Clin Cosmet
Investig Dermatol. 16:2211–2216. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mirgh S, Sharma A, Folbs B, Khushooc V,
Kapoorc J, Tejwanic N, Ahmedc R, Agrawald N, Choudharye PS, Mehta P
and Bhurani D: Daratumumab-based therapy after prior
Azacytidine-Venetoclax in an octagenerian female with BPDCN
(blastic plasmacytoid dendritic cell neoplasm)-a new perspective.
Leuk Lymphoma. 62:3039–3042. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nagate Y, Nakaya A, Kamimura R, Hirose Y,
Nojima S, Fujita J, Kiyohara E and Shibayama H: Venetoclax combined
with azacytidine can be a first-line treatment option for elderly
blastic plasmacytoid dendritic cell neoplasm. Intern Med.
62:2547–2551. 2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nasiri A, Lami A, Alhumaidi A, Madkhali A,
Althaqib A, Aljarwan N and Alkharras R: Blastic plasmacytoid
dendritic cell neoplasm: A case report. Cureus.
15(e37016)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nguyen K, Korsing S, Mansour Y and Meier
K: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) : A rare
hematologic neoplasm with frequent cutaneous involvement.
Dermatologie (Heidelb). 74:787–792. 2023.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
50
|
Phusuphitchayanan P, Vejjabhinanta V,
Takpradit C, Sudtikoonaseth P, Chairatchaneeboon M, Kiatvichukul T
and Sukpanichnant S: A rare case of blastic plasmacytoid dendritic
cell neoplasm in a child mimicking lymphoma/leukemia cutis.
Dermatopathology (Basel). 9:321–326. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Razzeto A, Garala P, Amoozgar B, Daliparty
VM, Rehman F and Razzeto M: Blastic Plasmacytoid dendritic cell
neoplasm without cutaneous manifestation: A case report. Am J Case
Rep. 22(e932887)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sagou K, Ito M, Kawamura Y, Ukai S, Goto
M, Fukushima N, Ozeki K, Fukuyama R and Kohno A: Severe tumor lysis
syndrome during the induction therapy for the treatment of blastic
plasmacytoid dendritic cell neoplasm arising from
myelodysplastic/myeloproliferative neoplasms. Clin Case Rep.
9:878–882. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sasaki Y, Murai S, Shiozawa E, Yamochi T
and Hattori N: Blastic plasmacytoid dendritic cell neoplasm in
long-term complete remission after venetoclax monotherapy. Cureus.
16(e52446)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shenjere P, Chasty R, Chaturvedi A, Dennis
MW, Ong A, Wiseman DH and Menasce LP: E-Cadherin expression in
blastic plasmacytoid dendritic cell neoplasms: An unrecognized
finding and potential diagnostic pitfall. Int J Surg Pathol.
29:289–293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shi J, Xu N, Niu Y, Jia SX, Yang CM and
Fang MY: Blastic plasmacytoid dendritic cell tumor treated with DVT
regimen: a case report and literature review. Zhonghua Xue Ye Xue
Za Zhi. 45:86–89. 2024.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
56
|
Sibai J, Chen R, Nabhani IA, Perusini MA
and Sibai H: Foot gangrene following Tagraxofusp treatment for
blastic plasmacytoid dendritic cell neoplasm: Case report. EJHaem.
3:1374–1376. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Suárez A, Soler N, Calderon A, Martinez B
and Piña M: Pediatric blastic plasmacytoid dendritic cell neoplasm,
clinical features and immunophenotype: A case report. Cureus.
15(e34549)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Suzuki A, Abe S, Koyama K, Suzuki S, Nagao
M, Kobayashi M, Nomura J, Tsutsumi T, Takeda T, Oka Y, et al:
Spontaneous regression of blastic plasmacytoid dendritic cell
neoplasm following sepsis by Serratia marcescens: A case
report and literature review. Intern Med. 60:927–933.
2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Suzuki H, Takeshita M, Hirai R, Tanimura A
and Miwa A: Blastic plasmacytoid dendritic cell neoplasm developed
in chronic myeloid leukemia in molecular remission during a
four-year treatment-free interval after six years of dasatinib
treatment. Cureus. 16(e61944)2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tirado CA, Reinartz J, Lapp K, Zhao D,
Nguyen AM and Stieglbauer K: Cytogenetic findings in a case of
blastic plasmacytoid dendritic cell neoplasm (BPDCN). J Assoc Genet
Technol. 46:5–13. 2020.PubMed/NCBI
|
|
61
|
Tong J, Aksenov S, Siegel BM, Wei L and
Rodgers WH: A rare case of blastic plasmacytoid dendritic cell
neoplasm occurred in postchemotherapy of breast cancer. Case Rep
Hematol. 2023(7573037)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang X, Guo J, Liu Y, Zheng N, Xu S, Wu L,
Yuan R, Xue L and Li J: Venetoclax combined with azacitidine in
blastic plasmacytoid dendritic cell neoplasm: A case report and
comprehensive review on the current and future treatment. Front Med
(Lausanne). 11(1425833)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yoshioka K, Kurokawa R, Amemiya S,
Koyamaab H, Matsudab K, Hondab A, Kurokawab M, Shinozaki-Ushikuc A
and Abe O: Rapidly progressing blastic plasmacytoid dendritic cell
neoplasm causing diffuse skin thickening: A case report with
sequential computed tomography examinations. Radiol Case Rep.
16(29292933)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang L, Wang Y, Lu M, Shen M and Duan Z:
Patients with blastic plasmacytoid dendritic cell neoplasm in
pregnancy: A rare case report. Medicine (Baltimore).
101(e30622)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang X, Hsi ED, Crane GM and Cheng YW:
Biallelic TET2 mutations and canonical ASXL1 mutations are frequent
and cooccur in blastic plasmacytoid dendritic cell neoplasm
(BPDCN): An institutional experience and review of literature.
EJHaem. 4:236–240. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Khoury JD, Solary E, Abla O, Akkari Y,
Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et
al: The 5th edition of the world health organization classification
of haematolymphoid tumours: Myeloid and histiocytic/dendritic
neoplasms. Leukemia. 36:1703–1719. 2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Denker S, Künstner A, Schwarting J, Witte
HM, Bernard V, Stölting S, Lohneis P, Kusch K, Bubnoff Nv, Merz H,
et al: Clonal evolution and blastic plasmacytoid dendritic cell
neoplasm: malignancies of divergent hematopoietic lineages emerging
from a common founding clone. Leukemia. 38:1858–1861.
2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt
MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ and
DiPersio JF: Chemosensitization of acute myeloid leukemia (AML)
following mobilization by the CXCR4 antagonist AMD3100. Blood.
113:6206–6214. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Crees ZD, Rettig MP and DiPersio JF:
Innovations in hematopoietic stem-cell mobilization: A review of
the novel CXCR4 inhibitor motixafortide. Ther Adv Hematol.
14(20406207231174304)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhao R, Liu J, Li Z, Zhang W, Wang F and
Zhang B: Recent advances in CXCL12/CXCR4 antagonists and nano-based
drug delivery systems for cancer therapy. Pharmaceutics.
14(1541)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hosoba S, Harris WA, Lin KL and Waller EK:
Chemokine and lymph node homing receptor expression on pDC vary by
graft source. Oncoimmunology. 3(e958957)2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sweet K: Blastic plasmacytoid dendritic
cell neoplasm: Diagnosis, manifestations, and treatment. Curr Opin
Hematol. 27:103–107. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Deotare U, Yee KWL, Le LW, Porwit A,
Tierens A, Musani R, Barth D, Torlakovic E, Schimmer A, Schuh AC,
et al: Blastic plasmacytoid dendritic cell neoplasm with leukemic
presentation: 10-Color flow cytometry diagnosis and HyperCVAD
therapy. Am J Hematol. 91:283–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Bashir Q, Milton DR, Popat UR, Kebriaei P,
Hosing C, Khouri IF, Rezvani K, Nieto Y, Oran B, Srour SA, et al:
Allogeneic hematopoietic cell transplantation for patients with
blastic plasmacytoid dendritic cell neoplasm (BPDCN). Bone Marrow
Transplant. 57:51–56. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Jen EY, Gao X, Li L, Zhuang L, Simoson NE,
Aryal B, Wang R, Przepiorka D, Shen YL, Leong R, et al: FDA
approval summary: Tagraxofusp-erzs For treatment of blastic
plasmacytoid dendritic cell neoplasm. Clin Cancer Res. 26:532–536.
2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Shimony S, Luskin MR, Gangat N, LeBoeuf
NR, Feraco AM and Lane AA: Blastic plasmacytoid dendritic cell
neoplasm (BPDCN): 2025 Update on diagnosis, pathophysiology, risk
assessment, and management. Am J Hematol. 100:1408–1422.
2025.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yun S, Chan O, Kerr D, Vincelette ND,
Idrees A, Mo Q, Sweet K, Lancet JE, Kharfan-Dabaja MA, Zhang L and
Sokol L: Survival outcomes in blastic plasmacytoid dendritic cell
neoplasm by first-line treatment and stem cell transplant. Blood
Adv. 4:3435–3442. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Pagano L, Valentini CG, Pulsoni A, Fisogni
S, Carluccio P, Mannelli F, Lunghi M, Pica G, Onida F, Cattaneo C,
et al: Blastic plasmacytoid dendritic cell neoplasm with leukemic
presentation: an Italian multicenter study. Haematologica.
98:239–246. 2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Laribi K, Baugier De Materre A, Sobh M,
Cerroni L, Giovanna Valentini C, Aoki T, Suzuki R, Takeuchi K,
Frankel AE, Cota C, et al: Blastic plasmacytoid dendritic cell
neoplasms: Results of an international survey on 398 adult
patients. Blood Adv. 4:4838–4848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Taylor J, Haddadin M, Upadhyay VA, Grussie
E, Mehta-Shah N, Brunner AM, Jr AM, Lovitch SB, Dogan A, Fathi AT,
et al: Multicenter analysis of outcomes in blastic plasmacytoid
dendritic cell neoplasm offers a pretargeted therapy benchmark.
Blood. 134:678–687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Pemmaraju N, Wilson NR, Garcia-Manero G,
Sasaki K, Khoury JD, Jain N, Borthakur G, Ravandi F, Daver N, Kadia
T, et al: Characteristics and outcomes of patients with blastic
plasmacytoid dendritic cell neoplasm treated with frontline HCVAD.
Blood Adv. 6:3027–3035. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Montero J, Stephansky J, Cai T, Griffin
GK, Cabal-Hierro L, Togami K, Hogdal LJ, Galinsky I, Morgan EA,
Aster JC, et al: Blastic plasmacytoid dendritic cell neoplasm is
dependent on BCL2 and sensitive to venetoclax. Cancer Discov.
7:156–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
DiNardo CD, Rausch CR, Benton C, Kadia T,
Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, et al:
Clinical experience with the BCL2-inhibitor venetoclax in
combination therapy for relapsed and refractory acute myeloid
leukemia and related myeloid malignancies. Am J Hematol.
93:401–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gangat N, Konopleva M, Patnaik MM, Jabbour
E, DiNardo C, Kadia T, Tefferi A and Pemmaraju N: Venetoclax and
hypomethylating agents in older/unfit patients with blastic
plasmacytoid dendritic cell neoplasm. Am J Hematol. 97:E62–E67.
2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Diebold K, Parker D, Worth S,
Espinoza-Gutarra M, Vachhani P, Bachiashvili K, Rangaraju S, Mohty
R, Bhatia and Jamy O: Outcomes with venetoclax 50 mg,
hypomethylating agents, and voriconazole or posaconazole in acute
myeloid leukemia. EJHaem. 6(e70049)2025.PubMed/NCBI View Article : Google Scholar
|
















