Introduction
Primary effusion lymphoma (PEL) is a unique form of
non-Hodgkin’s lymphoma that mainly occurs in severely
immunocompromised HIV-positive patients (1,2). PEL
is etiologically related to human herpes virus-8 (HHV-8) and
usually presents as lymphomatous body cavity effusion (pleural,
pericardial and peritoneal) in the absence of a solid tumor mass.
Among AIDS-related lymphomas, PEL generally has an extremely
aggressive clinical course with a median survival ranging from 2 to
6 months (3). Its prognosis
remains poor even after the advent of highly active anti retroviral
treatment (HAART) (2). PEL is well
known to have strong chemotherapy resistance, and most PEL patients
do not respond to combination chemotherapy, such as CHOP. Thus, the
optimal treatment for PEL has not yet been defined, and novel
effective agents are expected.
Radiotherapy, including total body irradiation, is a
crucial treatment option for hematological malignancies, in
particular malignant lymphoma (4,5). The
treatment of lymphoma depends on numerous factors, including stage,
grade, age, and performance status. Since the total irradiation
dose for patients is limited, radiotherapy has been used to treat
bulky masses, extranodal sites and low-grade stage IA patients.
Here, we examined the radiation sensitivity of PEL
cell lines. PEL cells were sensitive to γ-irradiation as compared
with other hematological malignant cells. Using an in vivo
immunodeficient mice model bearing the PEL cell line, we also
demonstrated the inhibition of tumor cell growth by total body
irradiation. Our findings suggest that radiotherapy can be
successfully applied for the treatment of this
chemotherapy-resistant malignant lymphoma.
Materials and methods
Cell lines and radiation exposure
device
The human PEL cell lines BCBL-1 (obtained through
the AIDS Research and Reference Reagent Program, Division of AIDS,
NIAID, NIH) (6), BC-1 (7) and BC-3 (8) (purchased from the American Type
Culture Collection, Manassas, VA), and the non-PEL human leukemic
cell lines Raji, Jurkat and K562 (obtained from RIKEN Cell Bank,
Tsukuba, Japan) were maintained in RPMI1640 supplemented with 10%
heat-inactivated fetal calf serum, penicillin (100 U/ml) and
streptomycin (100 μg/ml) in a humidified incubator at 37°C and 5%
CO2. The cells and mice were irradiated at a dose rate
of 0.9 Gy/min to a total dose of 1–10 Gy using a 137Cs
source (Gammacell 40 Exactor; MDS Nordion Inc., Ottawa,
Canada).
MTT assay
The antiproliferative effects of γ-irradiation on
PEL and non-PEL leukemic cell lines were measured by the
tetrazolium dye methylthiotetrazole (MTT) method (Sigma, St.
Louis, MO). Briefly, 2×104 cells were incubated in
triplicate in a 96-well microculture plate in the presence of
various doses of ionized irradiation in a final volume of 0.1 ml
for 48 h at 37°C. Subsequently, MTT (0.5 mg/ml final concentration)
was added to each well. After 4 h of additional incubation, 100 μl
of a solution containing 10% SDS plus 0.01 N HCl was added to
dissolve the crystal. Absorption values at 595 nm were determined
with an automatic ELISA plate reader (Multiskan; Thermo Electron
Vantaa, Finland). Values were normalized to untreated (control)
samples.
DNA fragmentation assay
To characterize the cell death pattern, DNA ladder
assays were performed as previously described (9). Briefly, 1×106 cells were
lysed in 100 μl of 10 mM Tris-HCl buffer (pH 7.4) containing 10 mM
EDTA and 0.5% Triton X. After centrifugation for 5 min at 15,000
rpm, supernatant samples were treated with RNase A (Sigma) and
Proteinase K (Wako Pure Chemical, Osaka, Japan). Subsequently, 20
μl of 5 M NaCl and 120 μl isopropanol were added, and the sample
was maintained at −20°C for 6 h. Following centrifugation for 15
min at 15,000 rpm, the pellets were dissolved in 20 μl of TE buffer
(10 mM Tris-HCl and 1 mM EDTA) as loading samples. To assess the
DNA fragmentation pattern, samples were loaded onto 1.5% agarose
gel and electrophoresis was performed.
Flow cytometric analysis of DNA
fragmentation
PEL cells were incubated in hypotonic lysing buffer
(0.1% sodium citrate, 0.1% Triton X, 0.1% RNase A and 50 μg/ml
propidium iodide (PI) at 4°C for 4 h (10). DNA content in each cell was
analyzed on an LSR II flow cytometer (BD Biosciences, San Jose,
CA). Data were analyzed using FlowJo software (Tree Star, San
Carlos, CA).
Annexin V assay
Apoptosis was quantified using the Annexin V-PE
Apoptosis Detection kit I (BD Biosciences). Briefly, after
γ-irradiation, cells were harvested, washed, and then incubated
with Annexin V-PE and 7-AAD for 15 min in the dark, before being
analyzed on an LSR II cytometer.
Xenograft and radiotherapy mouse
model
Balb/c Rag-2 deficient (Rag-2−/−) mice
and Balb/c Jak3-deficient (Jak3−/ −) mice were
established by crossing Rag-2−/ − mice (11) or Jak3−/ − mice (12) with the Balb/c strain for 10
generations, respectively. Balb/c Rag-2/Jak3 double deficient
(Rag-2−/ −Jak3−/ −) mice were established by
crossing Balb/c Rag-2−/ − mice and Balb/c Jak3−/
− mice, and were housed and monitored in our animal research
facility according to institutional guidelines. Experimental
procedures and protocols were approved by the Institutional Animal
Care and Use Committee of Kumamoto University. In a subcutaneous
xenograft mouse model, 8- to 10-week-old Rag-2−/
−Jak3−/ − mice were subcutaneously inoculated with
5×106 BCBL-1 cells suspended in 200 μl PBS. In an
intraperitoneal xenograft mouse model, Rag-2−/
−Jak3−/ − mice were intraperitoneally inoculated
with 5×106 BC-3 cells suspended in 200 μl PBS. Seven
days after the xenotransplantation of PEL cells, the recipient mice
were irradiated (4 Gy × 2), and the bone marrow cells from
Rag-2−/ −Jak3−/ − mice were transplanted into
irradiated mice. Tumor burden was evaluated by measuring the tumor
mass body weight, or ascites. Tumor growth was monitored by
measuring maximal and minimal diameters with calipers every week,
and tumor size was estimated with the formula: tumor size
(mm3) = length (mm) × width2 (mm) × 0.4, as
described previously (13,14). For assessment of disease-free
survival, Kaplan-Meier analysis was performed and P-values were
determined by 2-tailed analysis with log-rank tests.
Statistical analysis
Assays were performed in triplicate and expressed as
the mean ± SD. The statistical significance of differences observed
between experimental groups was determined using the Student’s
t-test. P-values <0.05 were considered significant.
Results
Inhibitory effect of ionized irradiation
on the proliferation of PEL cell lines
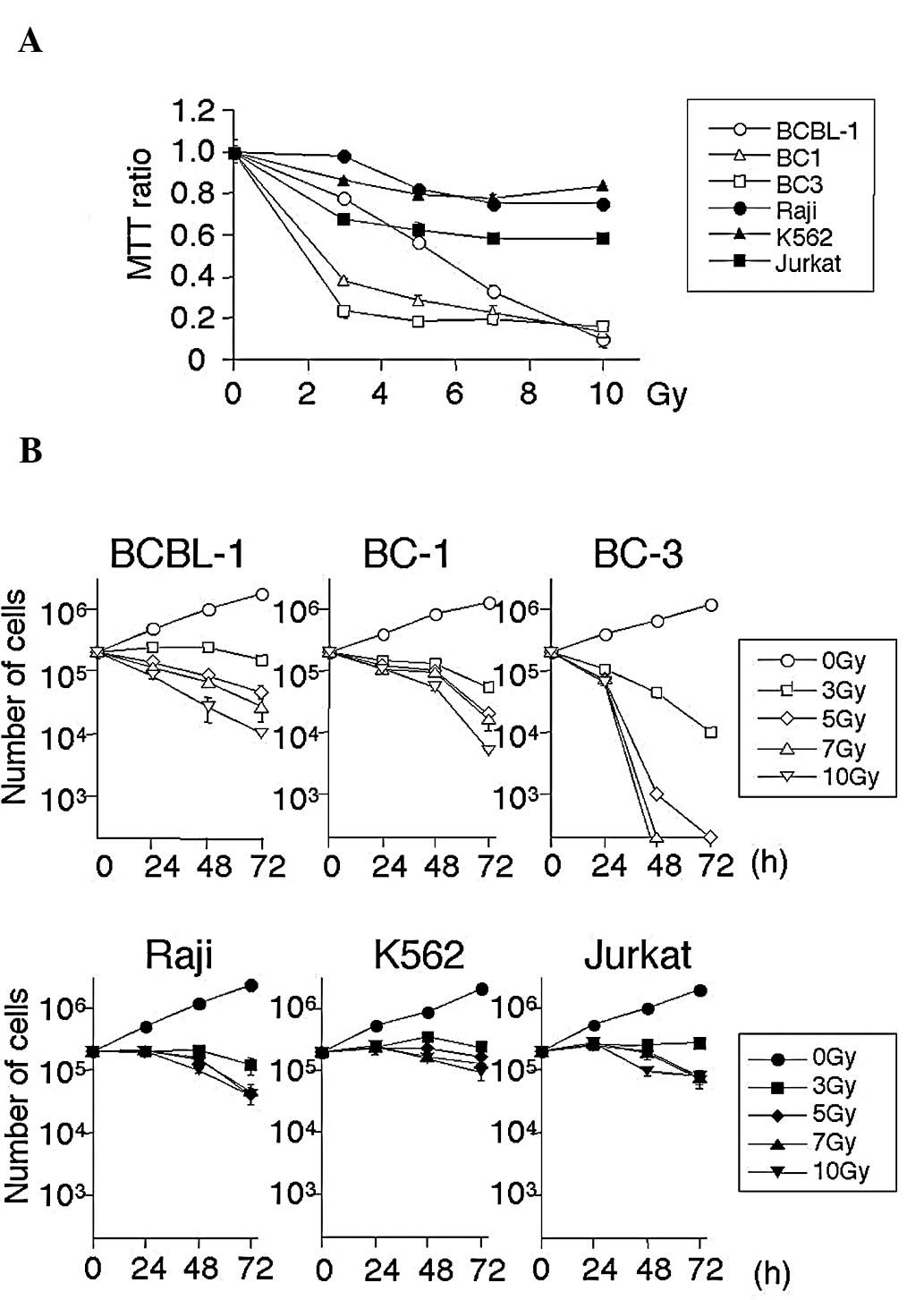
Initially, the radiosensitivity of PEL cells was
examined. Three PEL cell lines (BCBL-1, BC-1 and BC-3) and three
non-PEL cell lines (Raji, B lymphoid; Jurkat, T lymphoid; and K562,
erythroleukemia) were cultured after irradiation (0, 3, 5, 7 and 10
Gy) for 3 days, and proliferation was analyzed by the MTT assays.
As shown in Fig. 1, irradiation
inhibited cell growth in a dose-dependent manner in all PEL and
non-PEL cell lines. PEL cell lines were more sensitive than non-PEL
cell lines to radiotherapy. The effects of irradiation were then
confirmed by traditional cell count. PEL and non-PEL cell lines
were cultured after irradiation (0, 3, 5, 7 and 10 Gy) for 3 days,
and cell numbers were counted by trypan blue staining. As shown in
Fig. 1B, the number of PEL cells
decreased in a dose- and time-dependent manner. In contrast,
non-PEL cells survived, indicating that PEL cells are more
sensitive to irradiation.
Induction of apoptosis in PEL cells by
γ-irradiation
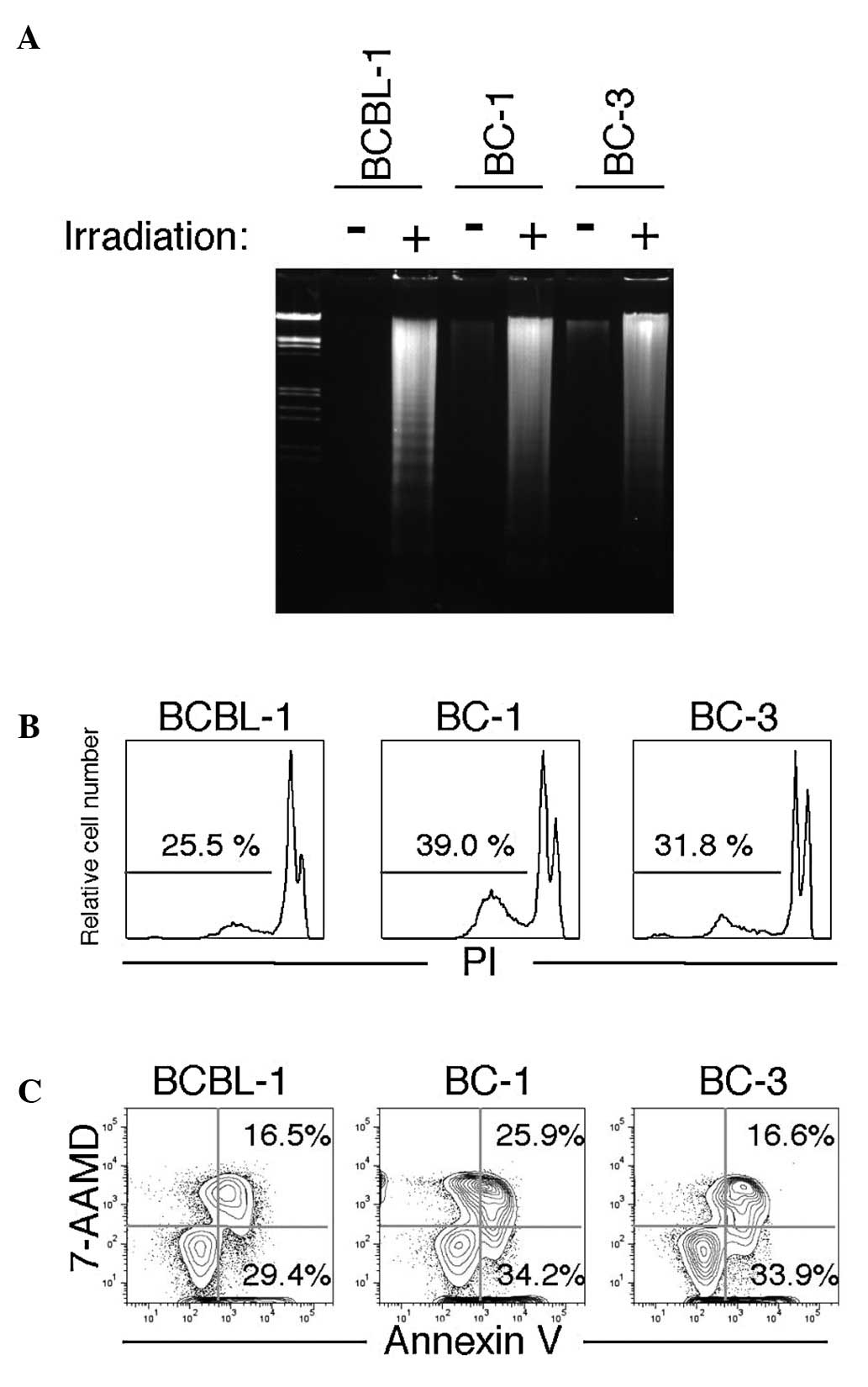
To determine whether growth inhibition by ionized
irradiation was attributable to apoptosis in PEL cells, DNA
fragmentation analysis (ladder formation and detection of the
sub-G1 fraction by flow cytometry) was performed 48 h after ionized
irradiation. As shown in Fig. 2A,
irradiation caused DNA fragmentation, which is a characteristic of
apoptotic cell death (9). In
addition, sub-G1 populations (the apoptotic fraction) were detected
in all three PEL cell lines (Fig.
2B).
An Annexin V binding assay was also performed for
further confirmation of irradiation-induced apoptosis in PEL cells.
The annexin positive 7-AAD negative fraction represents the early
phase of apoptosis, whereas the Annexin positive 7-AAD positive
fraction represents the late phase of apoptosis and necrosis.
(15). As shown in Fig. 2C, irradiation induced apoptosis in
PEL cell lines. These results suggest that growth inhibition by
irradiation occurs via the induction of apoptosis.
In vivo effects of irradiation on severe
immunodeficient mice inoculated with a PEL cell line
Since the above results suggested the efficacy of
radiotherapy for the treatment of PEL patients, we next examined
the in vivo effects of irradiation in an immunodeficient
mice model. Severe immunodeficient Rag-2−/ −Jak3−/
− mice were inoculated subcutaneously with 5×106
BCBL-1 cells. Seven days after the xenotransplantation of the
BCBL-1 cells, the recipient mice were irradiated (4 Gy × 2). Total
bone marrow cells from Rag-2−/ −Jak3−/− mice
(1×107/mouse) were transplanted into the irradiated
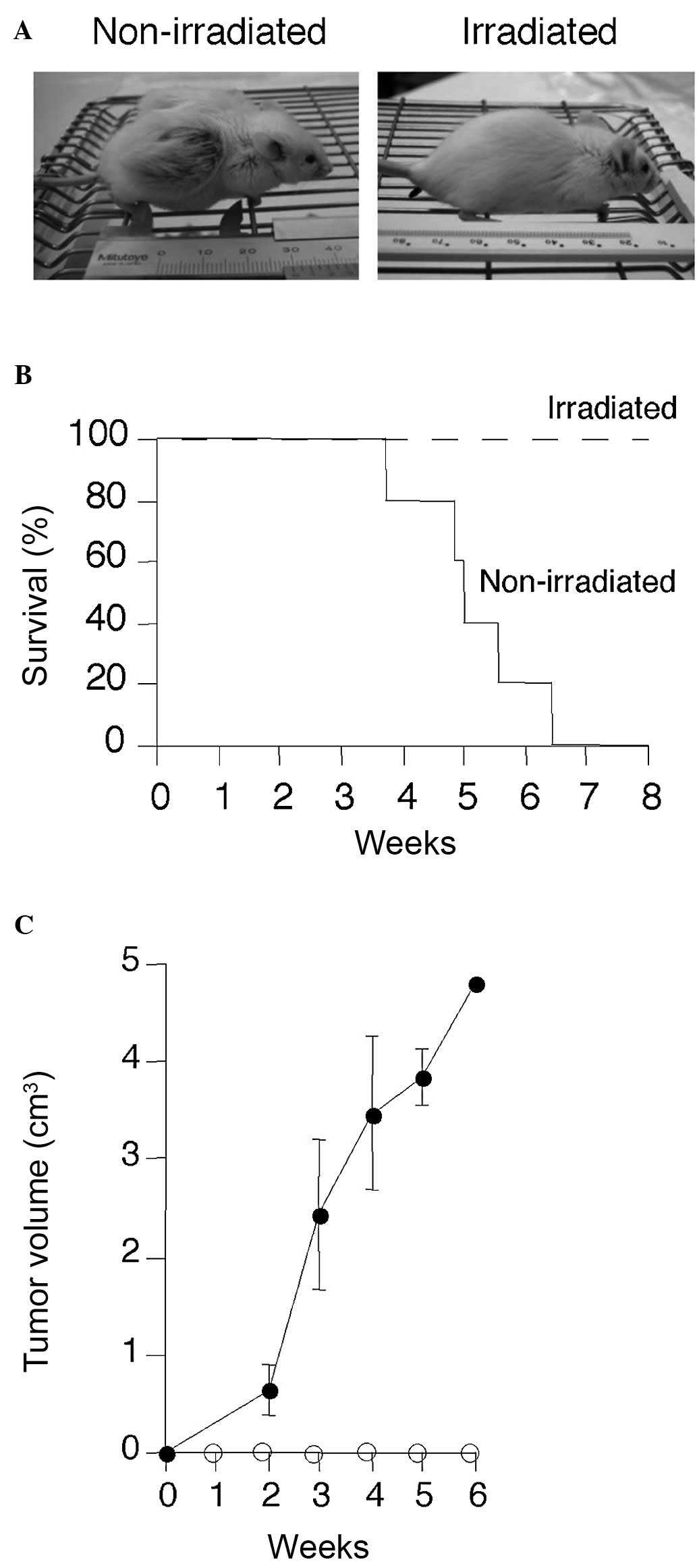
mice. Non-irradiated mice efficiently developed large subcutaneous
tumors (Fig. 3A), exhibited
clinical signs of near-death, such as piloerection, weight loss and
cachexia, and succumbed within 3–6 weeks of transplantation
(Fig. 3B and C). On the other
hand, irradiated mice did not develop tumors and survived for more
than 3 months after transplantation without developing tumors.
Since the formation of massive effusion is the
nature of PEL, Rag-2/Jak3 double-deficient mice were inoculated
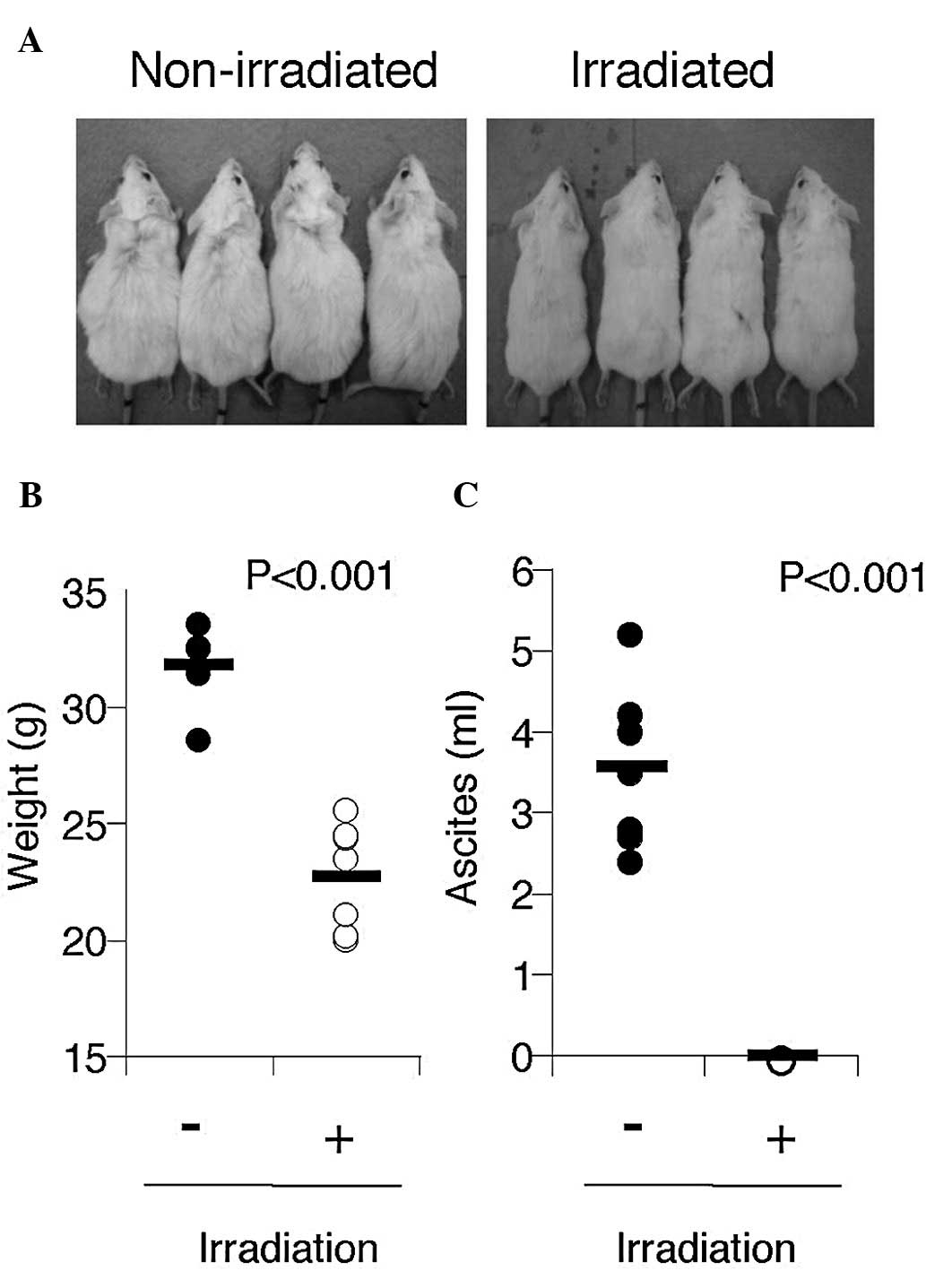
intraperitoneally with 5×106 BC-3 cells. BC-3 produced
massive ascites within 6 weeks of inoculation (3.5±1.0 ml, n=7)
(Fig. 4A) and body weight was
significantly increased in mice (Fig.
4B), indicating that this was a clinically relevant PEL
model.
The irradiated mice appeared to be healthy, with the
same body weight as non-tumor inoculated mice, and did not have
ascites (Fig. 4B). The body weight
of non-irradiated mice was significantly increased by massive
ascites compared with that of irradiated mice (31.7±1.6 g vs.
22.8±2.3 g, n=7, P<0.001).
These data indicate that γ-irradiation significantly
inhibits the growth and infiltration of PEL cells in
vivo.
Discussion
In the present study, we investigated the direct
effects of γ-irradiation on PEL cells in vitro and in
vivo. PEL cells were relatively radiosensitive compared with
other hematological malignant cells in vitro, and total body
irradiation with bone marrow transplantation rescued PEL-inoculated
Rag-2/Jak3 double-deficient mice, preventing the formation of
tumors and effusions. Thus, we suggest that γ-irradiation is a
promising candidate for chemotherapy-resistant PEL.
PEL is a rare high-grade B-cell malignancy
associated with herpes virus HHV-8 infection, and is mainly
observed during the course of HIV infection. The outcome of PEL
after polychemotherapy, such as CHOP, has generally been poor, even
since the advent of HAART. The potential for methotrexate to
diffuse in serous cavities suggests the use of high-dose
methotrexate in association with a CHOP-derived regimen (16); however, it has been reported that
patients with serous effusion might be at increased risk of
toxicity following high-dose methotrexate chemotherapy (17). In addition, there are reports in
which even intensive treatment regimens were unsuccessful (18). Thus, the optimal treatment for PEL
has not yet been defined. HHV-8 contains a homologue of cellular
FLIP protein called vFLIP that has the ability to activate the
NF-κB pathway (19), which is
known to protect against apoptosis induced by diverse stimuli
(19–21). The NF-κB pathway is known to induce
the expression of a number of anti-apoptotic genes, such as Bcl-xL,
A1, cIAP1, cIAP2, XIAP and IEX-1 (22). In addition, it has been
demonstrated that the inhibition of NF-κB induces the apoptosis of
PEL cells (23,24). vFLIP-mediated NF-κB activation is
necessary for the survival of PEL cells, and may cause them to
become chemoresistant. Thus, the NF-κB pathway represents an
appropriate target for the molecular therapy of PEL, and several
NF-κB inhibitors are already potential candidates (24–26).
One of these candidates, the proteasome inhibitor bortezomib, has
been shown to inhibit NF-κB activity and induce the apoptosis of
PEL cell lines in vitro (27,28);
however, bortezomib failed to control the progression of PEL in a
clinical trial (29), indicating
that preclinical anti-tumor activity does not necessarily translate
directly into activity in patients, and that preclinical studies
using animal models are required to determine the actual advantage
of NF-κB inhibitors in PEL (29).
Malignant lymphomas are characterized by a high
degree of radioresponsiveness. Consequently, radiotherapy is an
important modality in controlling these malignancies (30). However, as most lymphomas are
systemic diseases that are chemotherapy sensitive, use of
radiotherapy has been limited to localized lymphomas. Recently, it
was reported that chemotherapy-refractory HIV-associated PEL
patients achieved remission and survived for more than 12 months
(31). Our findings also suggest
that PEL is sensitive to radiation treatment (Fig. 1). In addition, it has been shown
that the in vitro radiosensitivity of tumor cells correlates
with the response to therapeutic irradiation (32). Thus, radiotherapy should be
considered as part of the treatment recommendation for patients
with chemotherapy-refractory PEL.
In vivo experiments showed that non-treated
mice subcutaneously xenografted with PEL developed large tumors,
while peritoneally xenografted mice gained body weight and effusion
in the peritoneal cavity (Figs. 3
and 4). On the other hand, the
irradiated groups did not have either effusions or tumors for 12
weeks, indicating that irradiation is capable of rescuing
PEL-xenografted mice. Animal models of human malignancies have been
applied to study the nature of cancer stem cells and to assess the
therapeutic effects of novel therapeutic strategies against
malignant neoplasms (33,34). In particular, the recent
introduction of severe immunodeficient mice has enabled us to
develop mice mimicking hematologic malignancies (26,35).
In this study, we used PEL-xenografted Rag2/Jak3 double-deficient
mice resembling the diffuse nature of human PEL, which is quite
useful to assess the therapeutic efficacy of γ-irradiation in mice
in a hematological malignancy model.
In summary, the present study demonstrated that
γ-irradiation is quite effective for the treatment of PEL both
in vitro and in vivo. Our study shows the usefulness
of radiotherapy for the treatment of chemotherapy-resistant PEL
patients. Radiotherapy should therefore be considered for the
treatment of chemotherapy-resistant PEL patients.
Acknowledgements
We thank Ms. I. Suzu for technical
assistance and Ms. Y. Endo and Ms. K. Tokunaga for secretarial
assistance. This work was supported in part by a Health and Labour
Sciences Research Grants from the Ministry of Health, Labour and
Welfare of Japan (H19-AIDS-003), by a Grant-in-Aid for Scientific
Research (C) from the Japan Society for the Promotion of Science
(JSPS) and by the Global COE Program ‘Global Education and Research
Center Aiming at the Control of AIDS’ from the Ministry of
Education, Culture, Sports, Science and Technology (MEXT) of
Japan.
References
|
1.
|
Nador RG, Cesarman E, Chadburn A, et al:
Primary effusion lymphoma: a distinct clinicopathologic entity
associated with the Kaposi’s sarcoma-associated herpes virus.
Blood. 88:645–656. 1996.
|
|
2.
|
Chen YB, Rahemtullah A and Hochberg E:
Primary effusion lymphoma. Oncologist. 12:569–576. 2007.
|
|
3.
|
Cesarman E, Chang Y, Moore PS, et al:
Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in
AIDS-related body-cavity-based lymphomas. N Engl J Med.
332:1186–1191. 1995.
|
|
4.
|
Lee CK: Evolving role of radiation therapy
for hematologic malignancies. Hematol Oncol Clin North Am.
20:471–503. 2006.
|
|
5.
|
Gustavsson A, Osterman B and
Cavallin-Stahl E: A systematic overview of radiation therapy
effects in non-Hodgkin’s lymphoma. Acta Oncol. 42:605–619.
2003.
|
|
6.
|
Renne R, Zhong W, Herndier B, et al: Lytic
growth of Kaposi’s sarcoma-associated herpesvirus (human
herpesvirus 8) in culture. Nat Med. 2:342–346. 1996.
|
|
7.
|
Cesarman E, Moore PS, Rao PH, et al: In
vitro establishment and characterization of two acquired
immunodeficiency syndrome-related lymphoma cell lines (BC-1 and
BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like
(KSHV) DNA sequences. Blood. 86:2708–2714. 1995.
|
|
8.
|
Arvanitakis L, Mesri EA, Nador RG, et al:
Establishment and characterization of a primary effusion (body
cavity-based) lymphoma cell line (BC-3) harboring kaposi’s
sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of
Epstein-Barr virus. Blood. 88:2648–2654. 1996.
|
|
9.
|
Sellins KS and Cohen JJ: Gene induction by
gamma-irradiation leads to DNA fragmentation in lymphocytes. J
Immunol. 139:3199–3206. 1987.
|
|
10.
|
Nicoletti I, Migliorati G, Pagliacci MC,
et al: A rapid and simple method for measuring thymocyte apoptosis
by propidium iodide staining and flow cytometry. J Immunol Methods.
139:271–279. 1991.
|
|
11.
|
Shinkai Y, Rathbun G, Lam KP, et al:
RAG-2-deficient mice lack mature lymphocytes owing to inability to
initiate V(D)J rearrangement. Cell. 68:855–867. 1992.
|
|
12.
|
Park SY, Saijo K, Takahashi T, et al:
Developmental defects of lymphoid cells in Jak3 kinase-deficient
mice. Immunity. 3:771–782. 1995.
|
|
13.
|
Attia MA and Weiss DW: Immunology of
spontaneous mammary carcinomas in mice. V. Acquired tumor
resistance and enhancement in strain A mice infected with mammary
tumor virus. Cancer Res. 26:1787–1800. 1966.
|
|
14.
|
Harada H, Suzu S, Ito T, et al: Selective
expansion and engraftment of human CD16+ NK cells in NOD/SCID mice.
Eur J Immunol. 35:3599–3609. 2005.
|
|
15.
|
Vermes I, Haanen C, Steffens-Nakken H, et
al: A novel assay for apoptosis. Flow cytometric detection of
phosphatidylserine expression on early apoptotic cells using
fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995.
|
|
16.
|
Boulanger E, Daniel MT, Agbalika F, et al:
Combined chemotherapy including high-dose methotrexate in
KSHV/HHV8-associated primary effusion lymphoma. Am J Hematol.
73:143–148. 2003.
|
|
17.
|
Pauley JL, Panetta JC, Schmidt J, et al:
Late-onset delayed excretion of methotrexate. Cancer Chemother
Pharmacol. 54:146–152. 2004.
|
|
18.
|
Waddington TW and Aboulafia DM: Failure to
eradicate AIDS-associated primary effusion lymphoma with high-dose
chemotherapy and autologous stem cell reinfusion: case report and
literature review. AIDS Patient Care STDS. 18:67–73. 2004.
|
|
19.
|
Liu L, Eby MT, Rathore N, et al: The human
herpes virus 8-encoded viral FLICE inhibitory protein physically
associates with and persistently activates the Ikappa B kinase
complex. J Biol Chem. 277:13745–13751. 2002.
|
|
20.
|
Chaudhary PM, Jasmin A, Eby MT, et al:
Modulation of the NF-kappa B pathway by virally encoded death
effector domains-containing proteins. Oncogene. 18:5738–5746.
1999.
|
|
21.
|
Sun Q, Matta H and Chaudhary PM: The human
herpes virus 8-encoded viral FLICE inhibitory protein protects
against growth factor withdrawal-induced apoptosis via NF-kappa B
activation. Blood. 101:1956–1961. 2003.
|
|
22.
|
Mayo MW and Baldwin AS: The transcription
factor NF-kappaB: control of oncogenesis and cancer therapy
resistance. Biochim Biophys Acta. 1470:M55–M62. 2000.
|
|
23.
|
Keller SA, Schattner EJ and Cesarman E:
Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary
effusion lymphoma cells. Blood. 96:2537–2542. 2000.
|
|
24.
|
Dabaghmanesh N, Matsubara A, Miyake A, et
al: Transient inhibition of NF-kappaB by DHMEQ induces cell death
of primary effusion lymphoma without HHV-8 reactivation. Cancer
Sci. 100:737–746. 2009.
|
|
25.
|
Keller SA, Hernandez-Hopkins D, Vider J,
et al: NF-kappaB is essential for the progression of KSHV- and
EBV-infected lymphomas in vivo. Blood. 107:3295–3302.
2006.
|
|
26.
|
Takahashi-Makise N, Suzu S, Hiyoshi M, et
al: Biscoclaurine alkaloid cepharanthine inhibits the growth of
primary effusion lymphoma in vitro and in vivo and
induces apoptosis via suppression of the NF-kappaB pathway. Int J
Cancer. 125:1464–1472. 2009.
|
|
27.
|
McConkey D, Nawrocki ST and Andtbacka R:
Velcade displays promising activity in primary effusion lymphoma
cells. Cancer Biol Ther. 4:491–492. 2005.
|
|
28.
|
An J, Sun Y, Fisher M, et al: Antitumor
effects of bortezomib (PS-341) on primary effusion lymphomas.
Leukemia. 18:1699–1704. 2004.
|
|
29.
|
Boulanger E, Meignin V and Oksenhendler E:
Bortezomib (PS-341) in patients with human herpesvirus 8-associated
primary effusion lymphoma. Br J Haematol. 141:559–561. 2008.
|
|
30.
|
Gospodarowicz M: Radiotherapy in
non-Hodgkin lymphomas. Ann Oncol. 19(Suppl 4): iv47–iv50. 2008.
|
|
31.
|
Cassoni A, Ali U, Cave J, et al: Remission
after radiotherapy for a patient with chemotherapy-refractory
HIV-associated primary effusion lymphoma. J Clin Oncol.
26:5297–5299. 2008.
|
|
32.
|
Dubray B, Breton C, Delic J, et al: In
vitro radiation-induced apoptosis and early response to
low-dose radiotherapy in non-Hodgkin’s lymphomas. Radiother Oncol.
46:185–191. 1998.
|
|
33.
|
Bankert RB, Hess SD and Egilmez NK: SCID
mouse models to study human cancer pathogenesis and approaches to
therapy: potential, limitations, and future directions. Front
Biosci. 7:c44–c62. 2002.
|
|
34.
|
Bankert RB, Egilmez NK and Hess SD:
Human-SCID mouse chimeric models for the evaluation of anti-cancer
therapies. Trends Immunol. 22:386–393. 2001.
|
|
35.
|
Shultz LD, Ishikawa F and Greiner DL:
Humanized mice in translational biomedical research. Nat Rev
Immunol. 7:118–130. 2007.
|