Introduction
Metabolic syndrome (MS) is a collection of disorders
that includes abdominal obesity, dyslipidemia, hypertension and
hypertriglyceridemia, which combined predict type 2 diabetes and
cardiovascular disease (1).
Authors previously proposed non-traditional components of MS, such
as subclinical inflammation, microalbuminuria and more recently,
the non-alcoholic fatty liver disease (NAFLD) which also predicts
cardiometabolic risk (2,3).
NAFLD describes a clinical pathologic condition
characterized by a significant deposit of fat in the liver
parenchyma and spectrum disorders ranging from a simple steatosis
to more severe forms that include a non-alcoholic steatohepatitis
(NASH) which may progress to fibrosis and hepatic cirrhosis
(4).
The histological characteristics of NAFLD are very
similar to those described for the alcoholic liver disease
(5). Thus, both are responsible
for the metabolic imbalance that exceeds the ability of the liver
to adapt to injury (6). This
disease is often associated with obesity (7), diabetes mellitus type 2 (8,9),
dyslipidemia (10) and
hypertension (11). Each of these
abnormalities carries a risk of cardiovascular disease and together
defines the syndrome of insulin resistance (SIR) or MS (11). The syndrome of insulin resistance
and oxidative stress play a crucial role in the pathogenesis of
NAFLD and progression from impaired glucose tolerance to diabetes.
Predictors of the progression to fibrosis in patients with NASH,
such as age (>45 years), obesity (BMI >30 kg/m2),
cirrhosis (ASAT/ALAT >1) and diabetes are associated with an
increase in insulin resistance (IR) (12,13).
Even though it is believed that NASH is a multifactorial disease,
IR appears to be the main factor responsible for the progression of
a simple steatosis to steatohepatitis (14).
Obese and overweight individuals with NAFLD present
with high levels of markers of liver disease such as
aspartate-aminotrasferase (ASAT), alanine-aminotransferase (ALAT)
and γ-glutamyl transferase (GGT) (15).
It should be considered that the adipocytes not only
store energy but also respond to metabolic signals by releasing
free fatty acids, secreting hormones and cytokines which exert a
local (adipose tissue), central (nerve tissue) and peripheral
effect (organs such as liver, muscles, pancreas) (16). Cytokines are substances released
from adipose tissue, with a significant impact on the general
homeostasis of the body, eating habits and insulin sensitivity
(17). Besides high levels of free
fatty acids being closely associated with IR, they appear to be
cytotoxic and may lead to peroxisomal oxidation generating hydrogen
peroxide as a source of oxidative stress (18–20).
The main adipocytokines are leptin, adiponectin and
resistin, while other molecules that include tumor necrosis factor
α (TNF-α), interleukin 6 (IL-6), plasminogen activator inhibitor 1
(PAI-1), complement proteins, proteins of the renin-angiotensin
system also act as adipocytokines (21–23).
In contrast, studies showed that IL-6 and TNF-α are
significant co-stimulators of C-reactive protein (CRP) in the
liver. Consequently, we proposed that due to its easy
accessibility, the measurement of the high-sensitivity CRP
(hs-CRP), may be used in conjunction with the measurement of liver
enzymes as an adjunct to the diagnosis and evaluation of NAFLD and
its relationship with MS (1).
Patients and methods
Patients
Patients with previous informed consent were
selected from the Research Program for Cardiovascular Disease Risk
Factors from the Universidad de Talca. Patients comprised a total
of 510 adults between 40–65 years of age, and were classified into
those who had MS (n=264) and those without MS (n=246), according to
adult treatment panel III (ATP III) criteria (23). Blood pressure, waist index, height
and weight were measured in all patients, and a blood sample after
fasting was extracted for biochemical tests such as glucose, lipid
profile, uricemia, liver enzymes and hs-PCR.
Methods
Blood pressure, height and weight were measured
according to the World Health Organization recommendations
(24,25). The venous blood samples from each
patient were extracted after a 12-h fast. The biochemical
characterization such as glucose, uric acid, lipid profile [total
cholesterol, high density lipoprotein (HDL) cholesterol,
triglycerides] was measured enzymatically. hs-PCR was conducted
using the immunochemical method and the ASAT, ALAT and GGT enzymes
were measured using the kinetic-spectrophotometric methodology. For
all determinations, reagents from Roche Laboratories (Mannheim,
Germany) were used with a Hitachi 711 auto analyzer. Human serum
Biosystems (Barcelona, Spain) was used as a control.
Statistical analysis
Since measured variables were not normally
distributed, median and interquartile ranges (IQR = 75th
percentile-25th percentile) were used for description. The
non-parametric Mann-Whitney U test was used to compare the
variables of MS for men and women. To evaluate the effect of hs-CRP
(log-transformed) in the liver enzymes as response variables, a
multivariate regression analysis was used, controlled by MS, age
and gender. A 0.05 level was considered to be significant.
Results
We studied 510 adults of whom 264, with an average
age of 52.3±6.5 years, presented MS and a significantly higher BMI
than adults without MS (31.2, 26.5, respectively) (p<0.001). The
general characteristics of patients studied are shown in Table I, and it can be seen that there are
significantly higher levels of liver enzymes in women with MS with
respect to those without the syndrome, while in males these enzymes
show no significant differences. In relation to hs-PCR, it is
observed that both men and women with MS have significantly higher
levels than those without MS (p<0.001, males and females,
respectively).
 | Table I.Patient data. |
Table I.
Patient data.
| Variable | Men
| Women
|
|---|
| Non-MS, n=75 Median
(IQR) | MS, n=80 Median
(IQR) | p-valuea | Non-MS, n=171 Median
(IQR) | MS, n=184 Median
(IQR) | p-valuea |
|---|
| Age | 48.0 (13.0) | 52.0 (12.0) | 0.006 | 48.0 (10.0) | 52.0 (10.0) | <0.001 |
| Glycemia (mg/dl) | 90.0 (11.0) | 102.0 (31.0) | <0.001 | 87.0 (12.0) | 99.0 (23.0) | <0.001 |
| hs-PCR (mg/l) | 1.1 (1.8) | 2.1 (3.2) | <0.001 | 1.5 (2.9) | 3.6 (5.0) | <0.001 |
| ALAT (U/l) | 7.8 (5.6) | 8.5 (8.7) | 0.344 | 5.4 (4.5) | 6.9 (5.1) | <0.001 |
| ASAT (U/l) | 17.2 (7.5) | 17.0 (7.9) | 0.409 | 13.4 (5.3) | 16.5 (8.4) | <0.001 |
| GGT (U/l) | 24.0 (23.0) | 33.0 (35.0) | 0.003 | 17.0 (14.0) | 24.0 (28.0) | <0.001 |
| Uric acid
(mg/dl) | 5.0 (1.2) | 6.1 (1.6) | <0.001 | 3.9 (1.3) | 4.4 (1.5) | <0.001 |
| Cholesterol
(mg/dl) | 199.0 (47.0) | 191.5 (51.0) | 0.720 | 202.0 (50.0) | 209.0 (54.0) | 0.226 |
| LDL-C (mg/dl) | 120.0 (40.0) | 111.0 (34.0) | 0.099 | 116.5 (39.0) | 122.0 (41.0) | 0.250 |
| Triglycerides
(mg/dl) | 128.0 (74.0) | 185.0 (111) | <0.001 | 110.5 (59.0) | 168.5 (80.0) | <0.001 |
| Waist (cm) | 93.0 (9.0) | 103.0 (13.0) | <0.001 | 84.0 (13.0) | 96.5 (16.0) | <0.001 |
| HDL-C (mg/dl) | 46.0 (17.0) | 39.0 (11.0) | <0.001 | 59.0 (18.0) | 46.0 (13.0) | <0.001 |
| BMI
kg/m2 | 26.9 (3.8) | 30.3 (5.1) | <0.001 | 26.3 (5.6) | 31.8 (7.3) | <0.001 |
| Pressure S.
(mmHg) | 122.0 (19.0) | 143.0 (22.0) | <0.001 | 117.0 (19.0) | 135.0 (23.0) | <0.001 |
| Pressure D.
(mmHg) | 76.0 (13.0) | 89.0 (14.0) | <0.001 | 73.0 (11.0) | 84.0 (13.0) | <0.001 |
Results of the multivariate linear regression on the
ASAT and GGT enzymes showed that the enzyme levels are
significantly higher among patients with MS than those who do not
present it (p<0.01, p<0.001, respectively).
The multivariate analysis on the ASAT and GGT
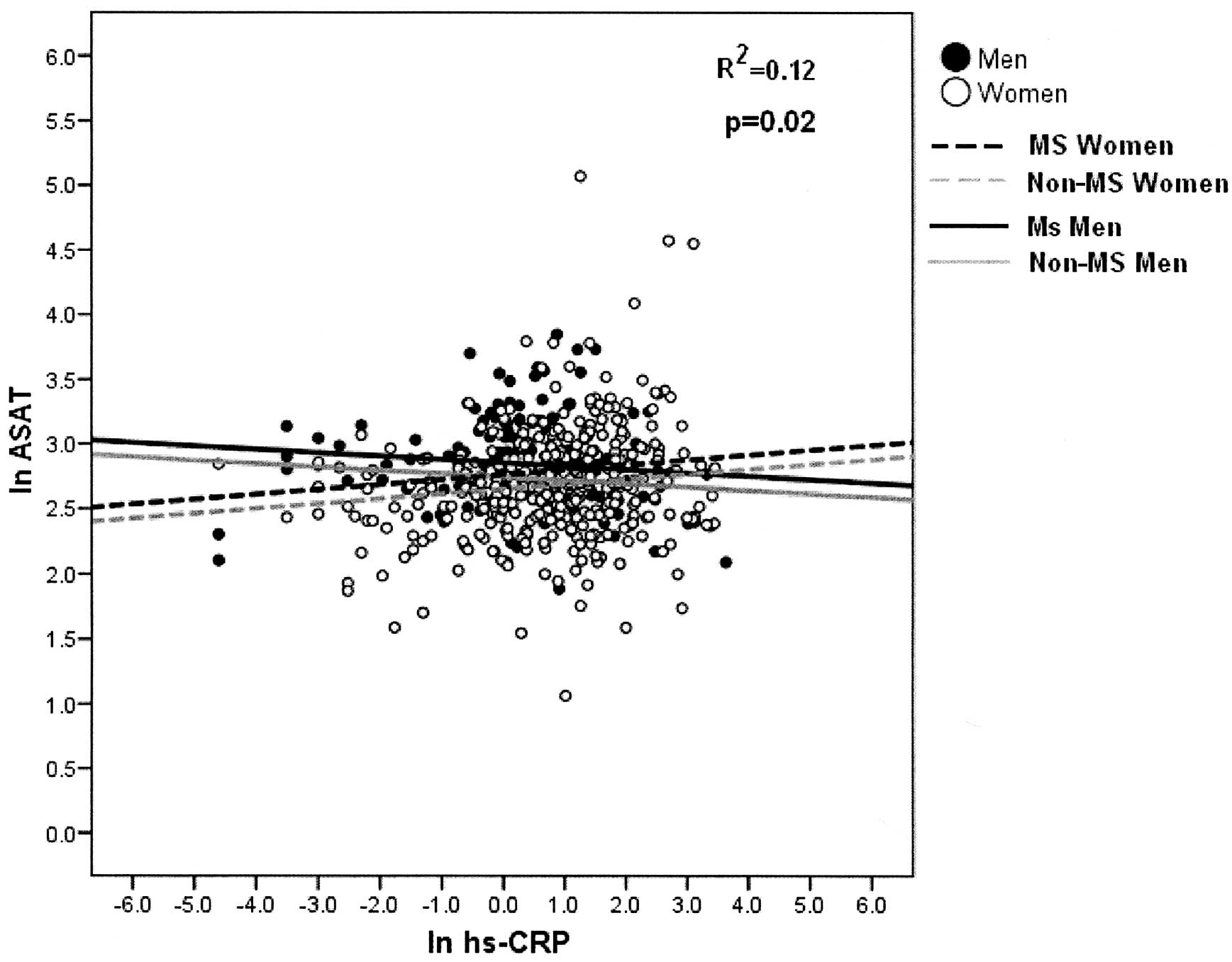
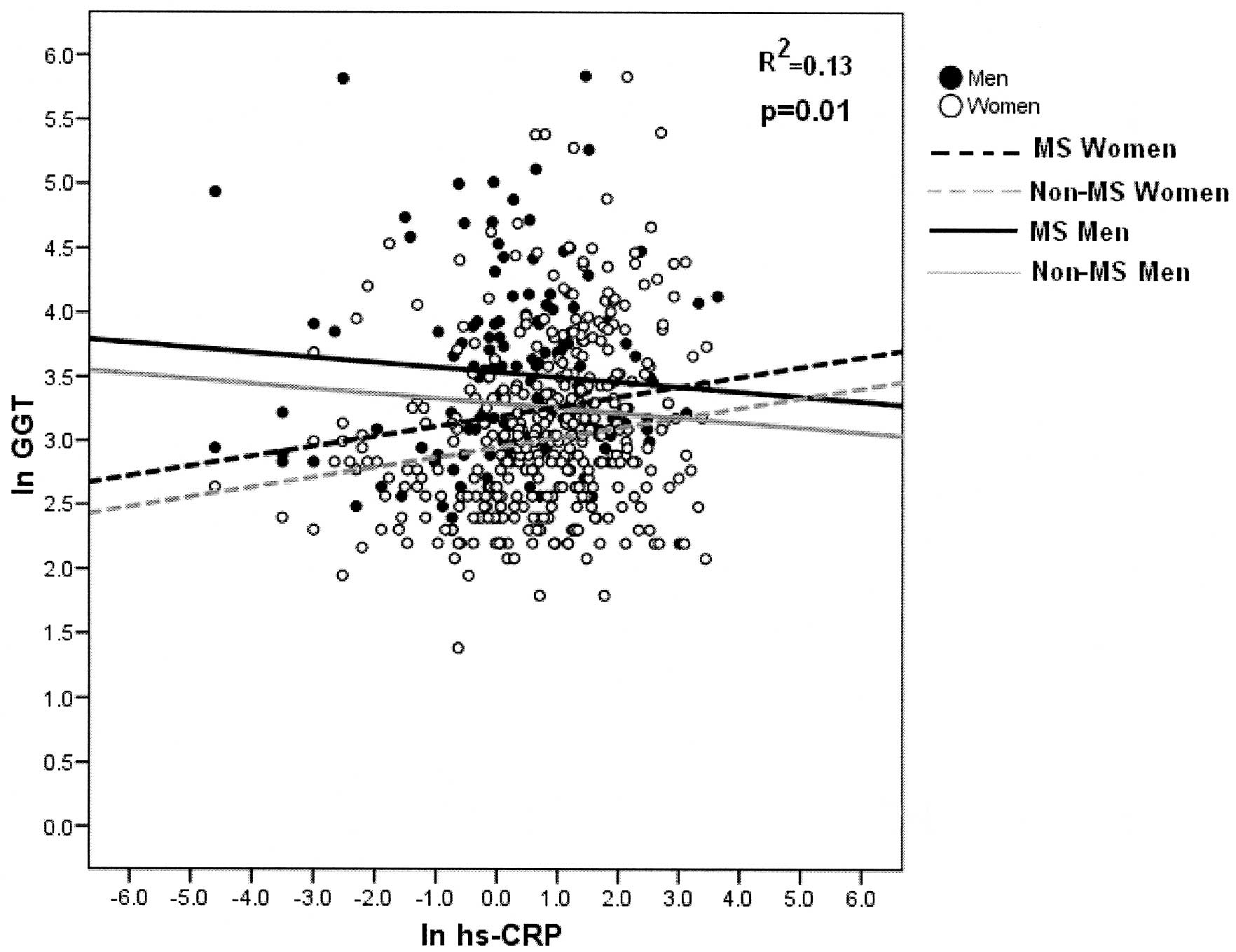
enzymes shows that there is a significant linear regression with
hs-CRP, and the slope is modified by gender; positive in women and
negative in men (p<0.013, p<0.014, respectively). There are
also significant differences between MS and non-MS subjects
(p<0.001, p<0.0001, respectively). The adjusted determination
coefficient was 8.3 and 12.5% for each model (Figs. 1 and 2).
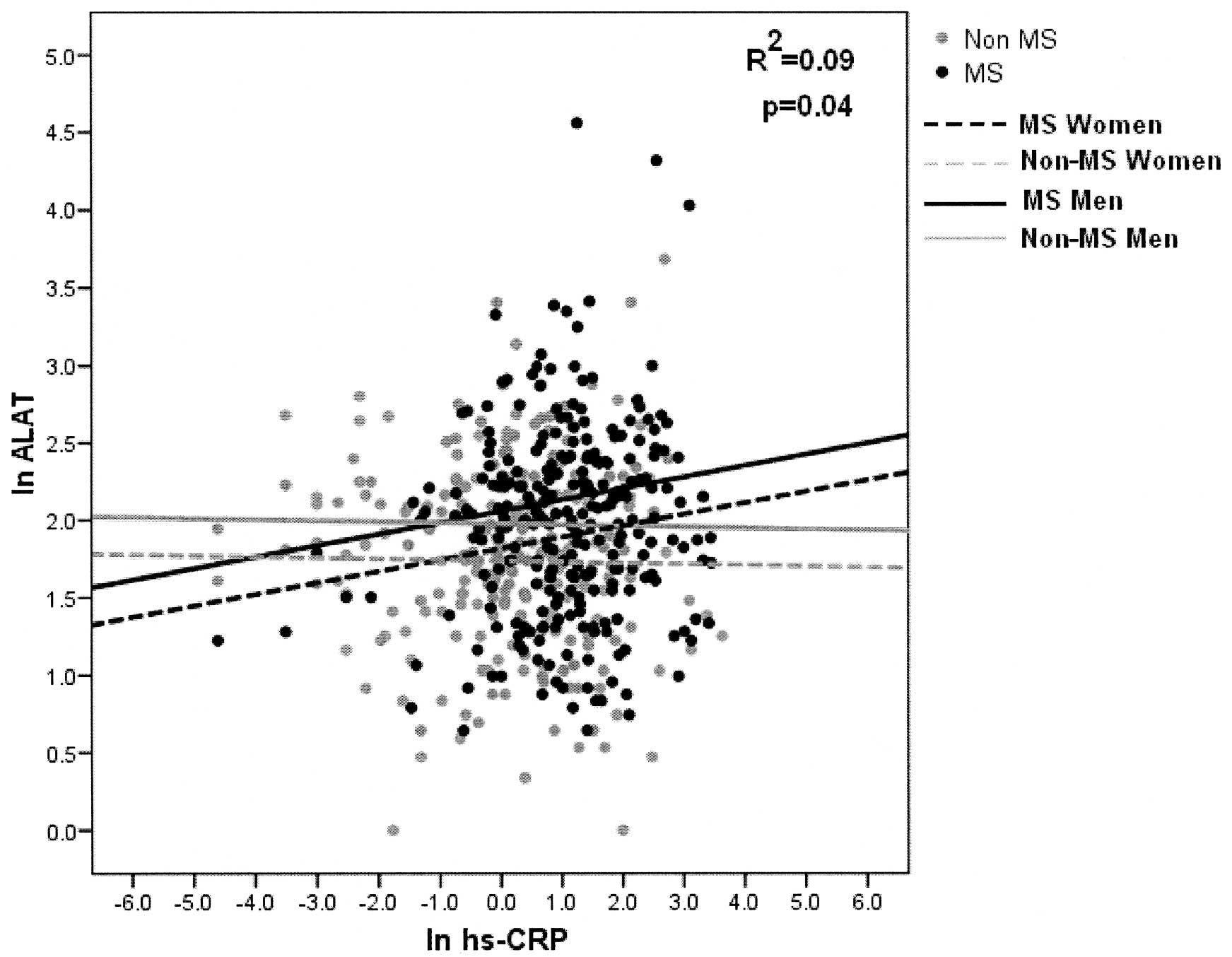
With regard to the linear relationship between the
ALAT enzyme and hs-CRP, it can be seen that the slope increases
significantly in patients with MS, while there is a negative
relationship with non-MS subjects (p=0.045) (Fig. 3). Finally, there is a statistically
significant difference between men and women. Men have higher ALAT
levels than women (p<0.0001). The adjusted determination
coefficient was 8.5% for the ALAT enzyme model (Fig. 3).
Increased levels of hs-CRP variably affect men and
women, increasing ASAT and GGT enzyme levels in women and
decreasing them in the case of men (p=0.02 and p=0.013,
respectively) (Figs. 1 and
2).
With regard to the ALAT enzyme, a significant
increase was observed in patients with MS, in accordance with the
increase in the levels of hs-PCR (p=0.048) (Fig. 3). A statistically significant
difference in the levels of this enzyme between men and women was
also noted (p<0.001).
Discussion
The present study investigated the relationship of
the levels of liver enzymes ALAT, ASAT and GGT in the presence of
MS, and the levels of hs-PCR. The most relevant results were: a) we
found higher levels of liver enzymes and hs-PCR in patients with MS
compared to those without MS and b) from the multivariate analysis
linear regression it was observed in women with and without MS that
increased levels of hs-CRP induce an increased level of ASAT and
GGT, exhibiting significantly higher levels of these enzymes in
women with MS.
The finding of elevated liver enzymes in MS patients
is somewhat expected as they have higher levels of obesity that
determine, to a large extent, this alteration in liver enzymes.
However, the pathogenic significance of these relations requires
in-depth investigation. Several studies showed a relationship
between high levels of ASAT, ALAT and GGT with impaired glucose
tolerance and diabetes (26–29),
although there is no clarity on the possible consequences of this
interaction.
It was previously shown that adipocytokines act in
hepatocytes and Küppfer cells initiating liver fibrosis. Peripheral
IR in patients with NASH leads to an increase in the transport of
fatty acids from adipose tissue to the liver. Thus, the fat
metabolism routes are overloaded in the hepatocytes, which along
with the increase of oxidative stress and/or mitochondrial
dysfunction cause an increase in the adipocytokines, which begin a
vicious cycle ending with the development of NASH (13,18,30).
Assuming that oxidative stress plays a critical role
in the pathogenesis of NAFLD (3),
as well as in the progression of the alteration of fasting glucose
to diabetes, it is noteworthy that Nannipieri et al
(29) found that GGT was an
independent predictor of progression to glucose intolerance or
diabetes. Similarly, we noted that the positive relationship
between hs-PCR and levels of liver enzymes in patients with MS is
important in identifying a subpopulation that possibly has more
inflammatory and oxidative activity, which would have a higher risk
of progressing to diabetes and/or towards NASH. Other studies that
support this idea are those that have linked higher levels of GGT
with the development of various diseases, including diabetes,
Alzheimer's and atherosclerosis (15,31).
The evidence supporting this hypothesis is based on previous
studies indicating that GGT is an oxidative stress marker because
of its importance in the transport of glutathione in cells
(32).
Our study also showed significant gender differences
(Table I and in Figs. 1 and 2). Although there are higher levels of
hs-PCR in individuals with MS in relation to those without MS, it
is interesting that the levels of women are superior to men with
and without MS.
In light of the results that allow us to establish
the relationships detailed above, we believe that future monitoring
of these or other groups of patients with similar characteristics,
with the aim of identifying groups most at risk, is
significant.
Acknowledgements
Supported by the Research Program for
Cardiovascular Disease Risk Factors (PIFRECV) Universidad de Talca,
Chile and Roche Chile.
References
|
1.
|
Hanley AJ, Williams K, Festa A,
Wagenknecht LE, D'Agostino RB Jr and Haffner SM: Liver markers and
development of the metabolic syndrome: the insulin resistance
atherosclerosis study. Diabetes. 54:3140–3147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mulhall BP, Ong JP and Younossi ZM:
Non-alcoholic fatty liver disease: an overview. J Gastroenterol
Hepatol. 17:1136–1143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Angulo P and Lindor KD: Non-alcoholic
fatty liver disease. J Gastroenterol Hepatol. 17:S186–S190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Grant LM and Lisker-Melman M: Nonalcoholic
fatty liver disease. Ann Hepatol. 3:93–99. 2004.PubMed/NCBI
|
|
5.
|
Diehl AM, Goodman Z and Ishak KG:
Alcohollike liver disease in nonalcoholics. A clinical and
histologic comparison with alcohol-induced liver injury.
Gastroenterology. 95:1056–1062. 1988.PubMed/NCBI
|
|
6.
|
Bellentani S, Saccoccio G, Masutti F, et
al: Prevalence of and risk factors for hepatic steatosis in
Northern Italy. Ann Intern Med. 132:112–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Akbar DH and Kawther AH: Nonalcoholic
fatty liver disease in Saudi type 2 diabetic subjects attending a
medical outpatient clinic: prevalence and general characteristics.
Diabetes Care. 26:3351–3352. 2003. View Article : Google Scholar
|
|
8.
|
Gupte P, Amarapurkar D, Agal S, et al:
Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J
Gastroenterol Hepatol. 19:854–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Assy N, Kaita K, Mymin D, Levy C, Rosser B
and Minuk G: Fatty infiltration of liver in hyperlipidemic
patients. Dig Dis Sci. 45:1929–1934. 2000. View Article : Google Scholar
|
|
10.
|
Donati G, Stagni B, Piscaglia F, et al:
Increased prevalence of fatty liver in arterial hypertensive
patients with normal liver enzymes: role of insulin resistance.
Gut. 53:1020–1023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Reaven GM: Banting lecture 1988. Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Neuschwander-Tetri BA and Caldwell SH:
Nonalcoholic steatohepatitis: summary of an AASLD Single Topic
Conference. Hepatology. 37:1202–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Preuss HG: Effects of glucose/insulin
perturbations on aging and chronic disorders of aging: the
evidence. J Am Coll Nutr. 16:397–403. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Balaban YH, Sumer H, Simsek H, Us D and
Tatar G: Metabolic syndrome, non-alcoholic steatohepatitis (NASH),
and hepatocyte growth factor (HGF). Ann Hepatol. 5:109–114.
2006.PubMed/NCBI
|
|
15.
|
Lee DH, Ha MH, Kim JH, et al:
Gamma-glutamyltransferase and diabetes – a 4 year follow-up study.
Diabetologia. 46:359–364. 2003.
|
|
16.
|
Czaja MJ: Liver injury in the setting of
steatosis: crosstalk between adipokine and cytokine. Hepatology.
40:19–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Matsuzawa Y, Funahashi T and Nakamura T:
Molecular mechanism of metabolic syndrome X: contribution of
adipocytokines adipocyte-derived bioactive substances. Ann NY Acad
Sci. 892:146–154. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Day CP and James OF: Steatohepatitis: a
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998.
|
|
19.
|
Gentile CL and Pagliassotti MJ: The role
of fatty acids in the development and progression of nonalcoholic
fatty liver disease. J Nutr Biochem. 19:567–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mehta K, van Thiel DH, Shah N and Mobarhan
S: Non-alcoholic fatty liver disease: pathogenesis and the role of
antioxidants. Nutr Rev. 60:289–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fain JN, Madan AK, Hiler ML, Cheema P and
Bahouth SW: Comparison of the release of adipokines by adipose
tissue, adipose tissue matrix, and adipocytes from visceral and
subcutaneous abdominal adipose tissues of obese humans.
Endocrinology. 145:2273–2282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kershaw EE and Flier JS: Adipose tissue as
an endocrine organ. J Clin Endocrinol Metab. 89:2548–2556. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Executive summary of the third report of
the National Cholesterol Education Program (NCEP) expert panel on
detection, evaluation, and treatment of high blood cholesterol in
adults (Adult Treatment Panel III). Jama. 285:2486–2497. 2001.
View Article : Google Scholar
|
|
24.
|
Clinical guidelines on the identification,
evaluation, and treatment of overweight and obesity in adults - the
evidence report. National Institutes of Health. Obes Res. 6(Suppl
2): S51–S209. 1998.PubMed/NCBI
|
|
25.
|
The sixth report of the Joint National
Committee on prevention, detection, evaluation, and treatment of
high blood pressure. Arch Intern Med. 157:2413–2446. 1997.
View Article : Google Scholar
|
|
26.
|
Marchesini G, Avagnina S, Barantani EG, et
al: Aminotransferase and gamma-glutamyltranspeptidase levels in
obesity are associated with insulin resistance and the metabolic
syndrome. J Endocrinol Invest. 28:333–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
James OF and Day CP: Non-alcoholic
steatohepatitis (NASH): a disease of emerging identity and
importance. J Hepatol. 29:495–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lee YS, Kek BL, Poh LK, Saw SM and Loke
KY: Association of raised liver transaminases with physical
inactivity, increased waist-hip ratio, and other metabolic
morbidities in severely obese children. J Pediatr Gastroenterol
Nutr. 47:172–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Nannipieri M, Gonzales C, Baldi S, et al:
Liver enzymes, the metabolic syndrome, and incident diabetes: the
Mexico City diabetes study. Diabetes Care. 28:1757–1762. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Medina J, Fernandez-Salazar LI,
Garcia-Buey L and Moreno-Otero R: Approach to the pathogenesis and
treatment of non-alcoholic steatohepatitis. Diabetes Care.
27:2057–2066. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yavuz BB, Yavuz B, Halil M, et al: Serum
elevated gamma glutamyltransferase levels may be a marker for
oxidative stress in Alzheimer's disease. Int Psychogeriatr.
20:815–823. 2008.PubMed/NCBI
|
|
32.
|
Karp DR, Shimooku K and Lipsky PE:
Expression of gamma-glutamyl transpeptidase protects ramos B cells
from oxidation-induced cell death. J Biol Chem. 276:3798–3804.
2001. View Article : Google Scholar : PubMed/NCBI
|