Introduction
Sarcopenia is the loss of muscle strength and muscle
mass, which can be caused by various factors, including ageing,
inflammation, malnutrition and chronic disease (1,2).
Sarcopenia is observed in approximately 30% of patients with
chronic liver disease and in approximately 40% of patients with
liver cirrhosis (3). Sarcopenia is
associated with an increased risk of falls (4) and muscle cramps (5). Sarcopenia is also a risk factor for
hepatic encephalopathy (6),
decompensated liver cirrhosis (7)
and hepatocellular carcinoma (8).
In addition, Hanai et al reported that sarcopenia impairs
the prognosis of patients with liver cirrhosis (9).
One of causative factor for sarcopenia is carnitine
deficiency and carnitine deficiency is frequently observed in
patients with liver cirrhosis (10). Carnitine transports long-chain
fatty acids into the mitochondria, leading to the production of
energy through fatty acid oxidation. Carnitine deficiency is
involved in the development of sarcopenia in patients with liver
cirrhosis (10). On the other
hand, L-carnitine treatment has been reported to suppress the loss
of skeletal muscle mass in patients with liver cirrhosis (10). Furthermore, in cirrhotic patients,
L-carnitine has been reported to improve hyperammonemia, which is
associated with the loss of skeletal muscle mass (11). In addition, it has been
demonstrated that L-carnitine treatment prevents the loss of
skeletal muscle mass (12). Thus,
L-carnitine treatment is beneficial for the prevention of
sarcopenia in patients with liver cirrhosis. However, the
mechanisms through which L-carnitine treatment affects muscle mass
remain unclear.
Recently, skeletal muscle has been shown to secrete
cytokines and proteoglycan peptides named ‘myokines’ by muscle
contraction (13). Myokines
perform various biological functions through the activation of
receptors on the liver, bone, fat, brain and heart, as well as in
muscle (14). Myostatin, irisin
and decorin are myokines regulating muscle mass (15-17).
In particular, serum levels of myostatin are known to be
significantly elevated in patients with liver cirrhosis compared
with healthy controls (18). In
addition, higher serum myostatin levels have been shown to be
associated with hyperammonemia and muscle atrophy in patients with
liver cirrhosis (19,20). Moreover, high serum myostatin
levels are associated with a high mortality rate of patients with
liver cirrhosis (19,20). However, alterations in the serum
levels of myokines following L-carnitine treatment in patients with
chronic liver disease have yet to be determined, at least to the
best of our knowledge. The aim of the present study was to
investigate alterations in the serum levels of myokines following
L-carnitine treatment in patients with chronic liver disease.
Patients and methods
Study design
The present study was a retrospective observational
study that aimed to investigate alterations in the serum levels of
myokines following L-carnitine treatment in patients with chronic
liver disease.
Ethics
The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki as reflected in the prior
approval granted by the Institutional Review Board of Kurume
University. An opt-out approach was used to obtain informed consent
from the patients, and personal information was protected during
data collection. None of the patients were institutionalized.
Patients
From July, 2017 to January, 2018, 7 patients were
enrolled who met following the following inclusion and exclusion
criteria. The inclusion criteria were patients with liver cirrhosis
who: i) Were ≥20 years of age; ii) treatment with L-carnitine for 3
months; and iii) had undergone biochemical examination. The
exclusion criteria were patients with: i) Hepatocellular carcinoma;
and ii) severe heart, pulmonary, renal, or brain failure.
For stratification analysis, patients were
classified into the low FIB-4 index (n=3) or high FIB-4 index
groups (n=4) according to the cut-off value of >3.25 of the
FIB-4 index. The cut-off value of the FIB-4 index for advanced
fibrosis was based on a previous study (21).
Laboratory determinations
Venous blood samples were drawn before and 12 weeks
after treatment of L-carnitine. Red blood cell count, hemoglobin,
white blood cell count, lymphocytes, platelet count, prothrombin
activity and serum levels of aspartate aminotransferase, alanine
aminotransferase, lactate dehydrogenase, alkaline phosphatase,
gamma-glutamyl transpeptidase, total protein, albumin, total
bilirubin, total cholesterol, triglycerides, α-fetoprotein,
des-γ-carboxy prothrombin, blood urea nitrogen, creatinine, sodium,
creatine kinase (CK) and ammonia were measured using standard
clinical methods as previously described (9,11).
The Child-Pugh score and FIB-4 index were calculated as previously
described (22). The estimated
glomerular filtration rate (eGFR) was also calculated as previously
described (23).
Evaluation of myokines
In the present study, 3 myokines, namely as
myostatin, irisin and decorin were evaluated. The serum levels of
myostatin, irisin and decorin were evaluated by enzyme-linked
immunosorbent assay (ELISA) (Irisin, recombinant-ELISA kit,
EK-067-29, Phoenix Pharmaceuticals Inc.; Decorin, Human Decorin
ELISA, ab99998, Abcam; Myostatin, GDF-8/Myostatin Quantikine ELISA
kit, DGDF80, Research and Diagnostic Systems Inc.).
Statistical analysis
Data are expressed as the median (interquartile
range), range, or number. Changes in variables before and after
treatment of L-carnitine were analyzed by Wilcoxon signed rank
tests. The correlation between the FIB-4 index and changes in serum
myostatin level was analyzed by Spearman's rank correlation
analysis. The level of statistical significance was set at
P<0.05.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median age was 66
years, and the ratio of female to male was 6:1. The median body
mass index was 24.7 kg/m2. Child-Pugh class B was
observed in 71.4% of patients. The median level of blood ammonia
and FIB-4 index were 79.5 µg/dl and 5.35, respectively. The median
level of serum CK was 94.5 U/l.
 | Table IPatient characteristics and
differences in variables before and after L-carnitine
treatment. |
Table I
Patient characteristics and
differences in variables before and after L-carnitine
treatment.
| | | L-carnitine
treatment |
|---|
| | | Before | After | |
|---|
| Variable | Reference value | Median (IQR) | Range (min-max) | Median (IQR) | Range (min-max) | P-value |
|---|
| No. of patients | N/A | 7 | N/A | N/A | N/A | |
| Age (years) | N/A | 66 (39-75) | 31-75 | N/A | N/A | |
| Sex
(female/male) | N/A | 6/1 | N/A | N/A | N/A | |
| Body mass index
(kg/m2) | 18.5-24.9 | 24.7
(21.7-31.7) | 18.9-34.2 | N/A | N/A | |
| Child-Pugh class
(A/B/C) | N/A | 14.3/71.4/14.3%
(1/5/1) | N/A | | | |
| FIB-4 index | <1.45 | 5.35
(2.48-6.91) | 0.93-11.04 | | | |
| Performance
status | 0-4 | 1 (0-2) | 0-3 | N/A | N/A | |
|
HCV/NASH/ALD/PBC/Wilson | N/A | 2/1/1/2/1 | N/A | N/A | N/A | |
| Biochemical
examinations | | | | | | |
|
Red blood
cell count (x104/µl) | 435-555 | 410 (295-474) | 257-506 | 380 (287-462) | 246-471 | 0.85 |
|
Hemoglobin
(g/dl) | 13.7-16.8 | 13.4
(9.5-13.9) | 7.3-13.9 | 12.6
(9.5-13.8) | 9.1-14.8 | 0.94 |
|
White blood
cell count (/µl) | 3,300-8,600 | 4,850
(2,775-8,075) | 2,100-8,900 | 4,200
(1,900-8,600) | 1,800-13,900 | 0.58 |
|
Platelet
count (x103/mm3) | 3.3-8.6 | 10.1
(7.7-23.4) | 7.0-28.1 | 24.7
(7.9-24.7) | 6.8-25.7 | 0.47 |
|
ALT
(IU/l) | 10-30 | 35 (24-114) | 12-135 | 41 (20-131) | 16-225 | 0.63 |
|
GGT
(IU/l) | 13-64 | 37 (12-70) | 12-125 | 50 (14-86) | 14-124 | 0.27 |
|
Total
protein (g/dl) | 6.6-8.1 | 6.77
(6.27-6.90) | 4.42-7.22 | 7.23
(49.00-7.58) | 4.38-8.18 | 0.48 |
|
Albumin
(g/dl) | 4.1-5.1 | 2.95
(2.65-3.99) | 2.10-4.28 | 2.83
(2.22-4.27) | 2.08-4.44 | 0.67 |
|
Prothrombin
activity (%) | 80-120 | 71 (65-92) | 59-104 | 65 (62-100) | 53-115 | 0.30 |
|
Ammonia
(µg/dl) | 12-66 | 79.5
(60.0-106.8) | 48.0-133.0 | 120.5
(57-160.5) | 39-162 | 0.60 |
|
Total
bilirubin (mg/dl) | 0.40-1.20 | 1.09
(0.73-1.40) | 0.70-11.54 | 1.21
(0.68-1.93) | 0.45-17.47 | 0.36 |
|
Total
cholesterol (mg/dl) | 142-219 | 144 (127-170) | 103-242 | 124 (104-162) | 94-243 | 0.10 |
|
Triglyceride
(mg/dl) | 40-149 | 115.5
(45.0-258.0) | 45.0-258.0 | 95.5
(39.8-208.8) | 36-217 | 0.34 |
|
AFP
(ng/ml) | ≤7.0 | 5.0
(0.2-144.3) | 0.95-40.425 | 6.15
(1.6-2539) | 3.4-734.5 | 0.37 |
|
Des-γ-carboxy
prothrombin (mAU/ml) | <40 | 20.0
(8.0-57.5) | 13.5-44.8 | 79.3
(9.0-290.0) | 17.25-167.8 | 0.35 |
|
BUN
(mg/dl) | 8.0-20.0 | 11.8
(8.2-15.1) | 1.0-23.6 | 12.2
(9.1-17.9) | 0.7-24.8 | 0.21 |
|
Creatinine
(mg/dl) | 0.65-1.07 | 0.50
(0.46-0.63) | 0.43-0.65 | 0.62
(0.55-0.75) | 0.55-0.82 | 0.06 |
|
eGFR
(ml/min/1.73 m2) | >90.0 | 97.1
(72.1-111.5) | 66.8-126.3 | 76.1
(66.4-90.3) | 51.2-112.1 | 0.07 |
|
Creatine
kinase (U/l) | 59-248 | 94.5
(60.0-117.5) | 45-131 | 123 (101-153) | 71-205 | 0.03 |
Alterations in biochemical
examinations before and after L-carnitine treatment
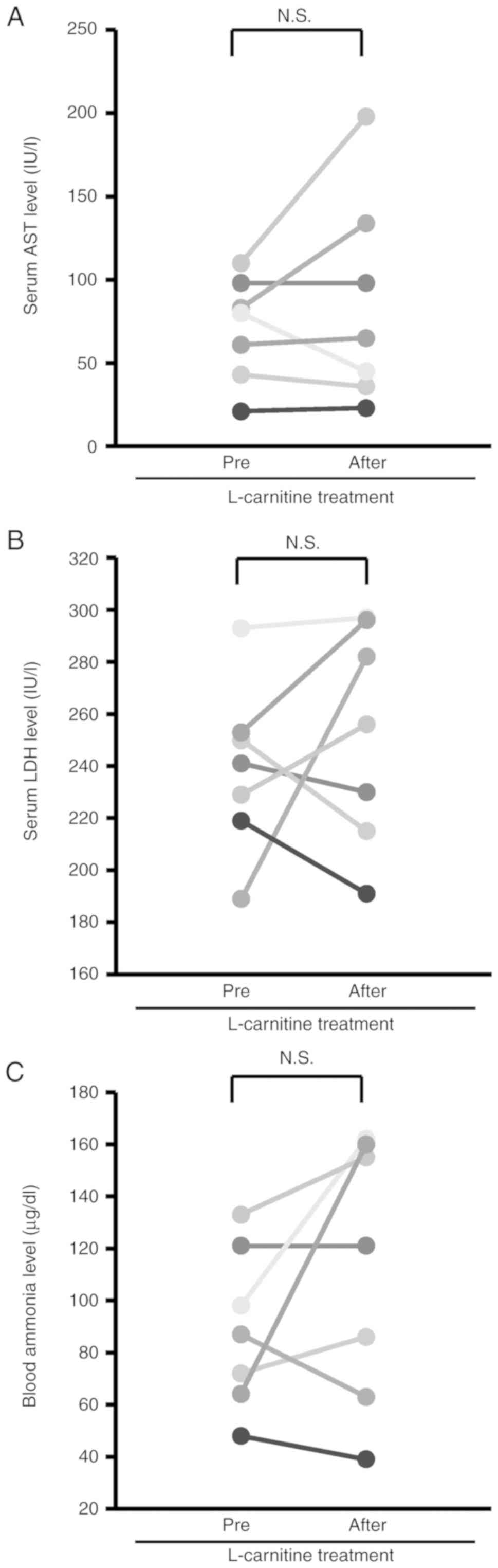
Alterations in biochemical examinations before and
after L-carnitine treatment are summarized in Table I. No significant differences were
observed in the serum levels of aspartate aminotransferase, and
lactate dehydrogenase before and after L-carnitine treatment
(Fig. 1A and B). In addition, no significant
differences were observed in the blood ammonia level before and
after L-carnitine treatment (Fig.
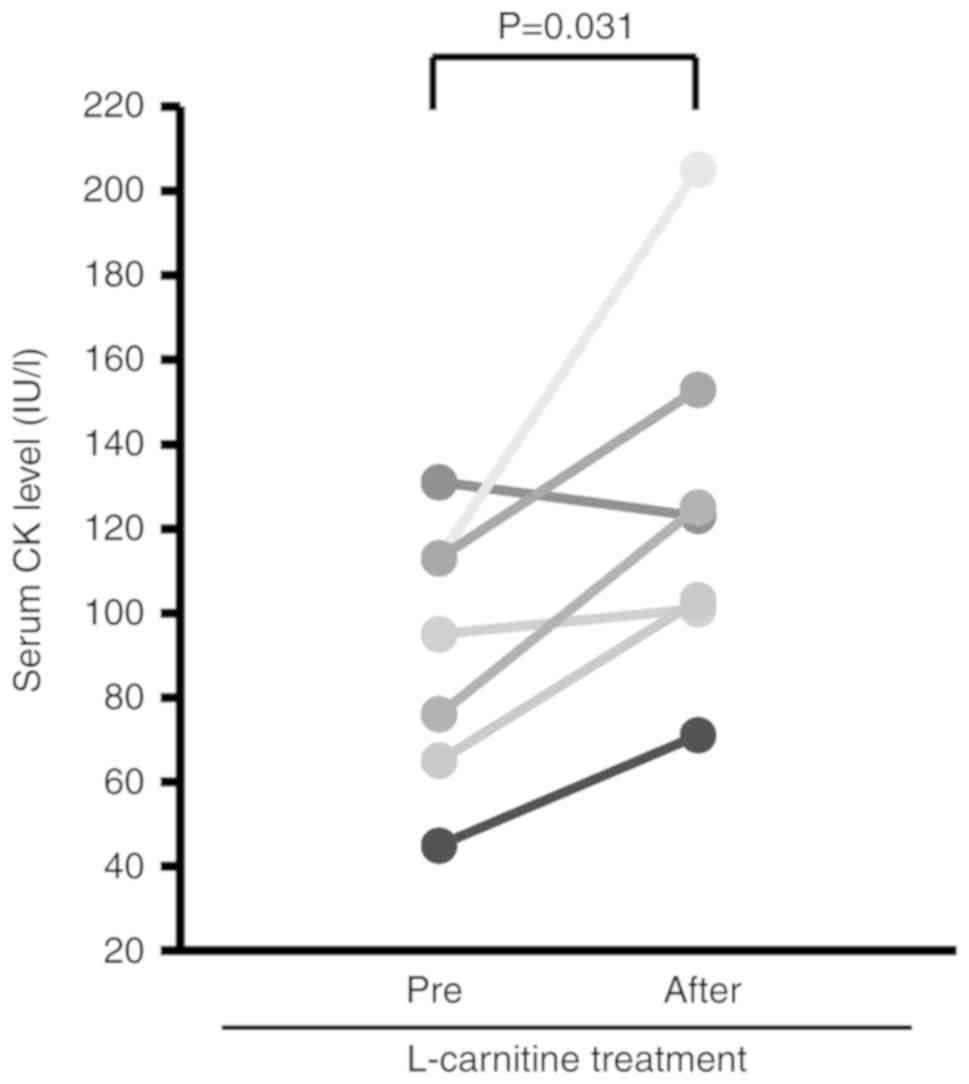
1C). The serum CK level was significantly increased after
L-carnitine treatment (Fig.
2).
Alterations in serum levels of
myostatin, irisin, and decorin after L-carnitine treatment
Alterations in the serum levels of, irisin, decorin
and myostatin after L-carnitine treatment were investigated. No
significant differences were observed in the serum levels of
irisin, decorin and myostatin before and after L-carnitine
treatment (Table II).
 | Table IIChanges in serum levels of myokines
before and after L-carnitine treatment. |
Table II
Changes in serum levels of myokines
before and after L-carnitine treatment.
| | L-carnitine
treatment | |
|---|
| | Before | After | |
|---|
| Myokine | Median (IQR) | Range
(min-max) | Median (IQR) | Range
(min-max) | P-value |
|---|
| Irisin (ng/ml) | 5.87
(5.23-6.71) | 4.41-9.43 | 5.18
(4.77-7.52) | 4.58-9.49 | 0.61 |
| Decorin
(pg/ml) | 7479
(6031-15037) | 2977-26523 | 9642
(5756-14080) | 2454-32901 | 0.41 |
| Myostatin
(pg/ml) | 68.56
(49.42-86.87) | 49.15-98.87 | 68.55
(41.73-86.87) | 38.94-96.90 | 0.86 |
Alterations in serum levels of irisin,
decorin and myostatin after L-carnitine treatment in the high FIB-4
index group
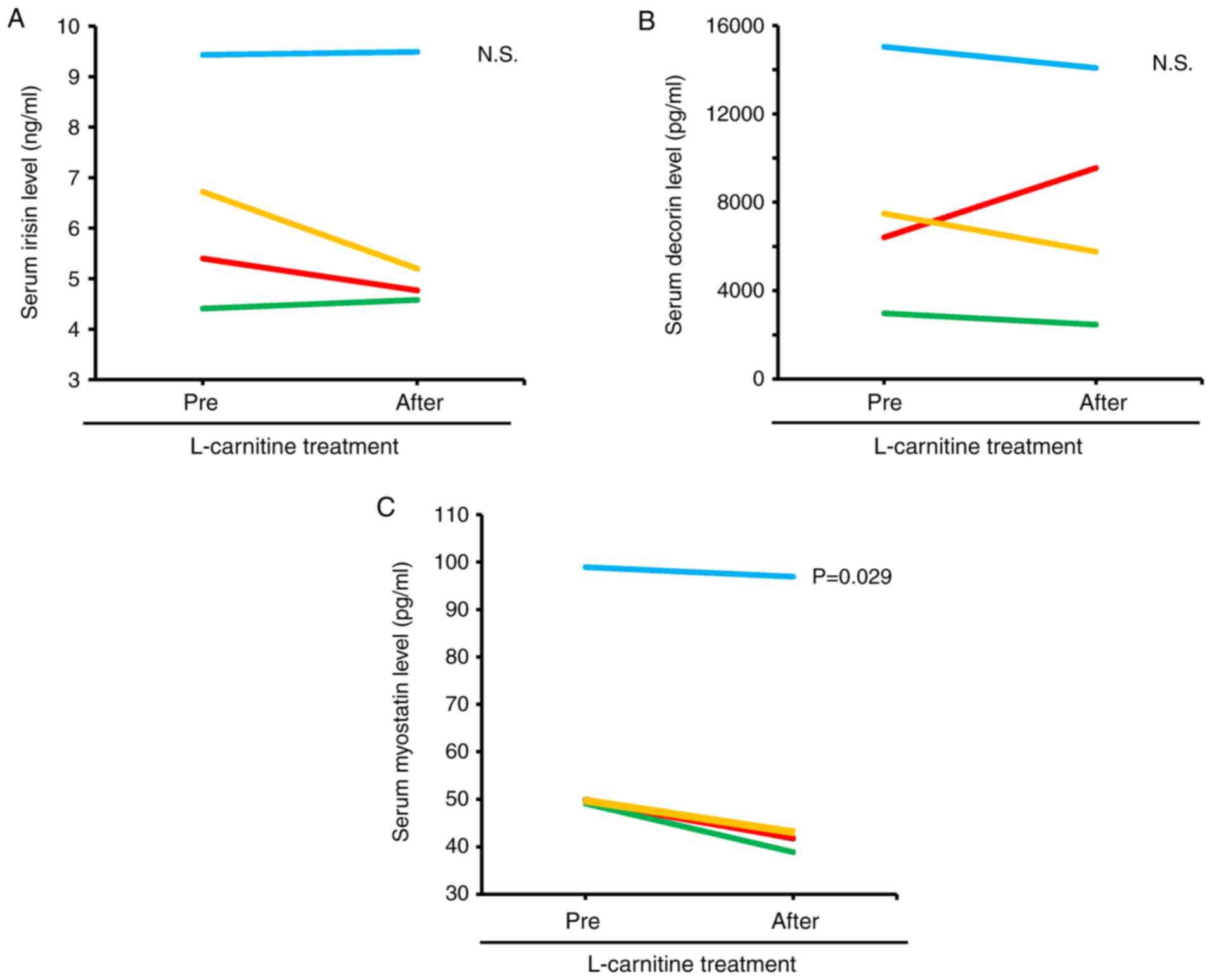
In the stratification analysis according to the
FIB-4 index, patients were classified into the low FIB-4 or high
FIB-4 groups based on the cut off value of >3.25 of the FIB-4
index, which corresponds to the F4 stage. No significant
differences were observed in the serum levels of irisin and decorin
before and after L-carnitine treatment in the high FIB-4 group
(Fig. 3A and B). In the high FIB-4 group, the serum
level of myostatin was significantly decreased after L-carnitine
treatment (Fig. 3C).
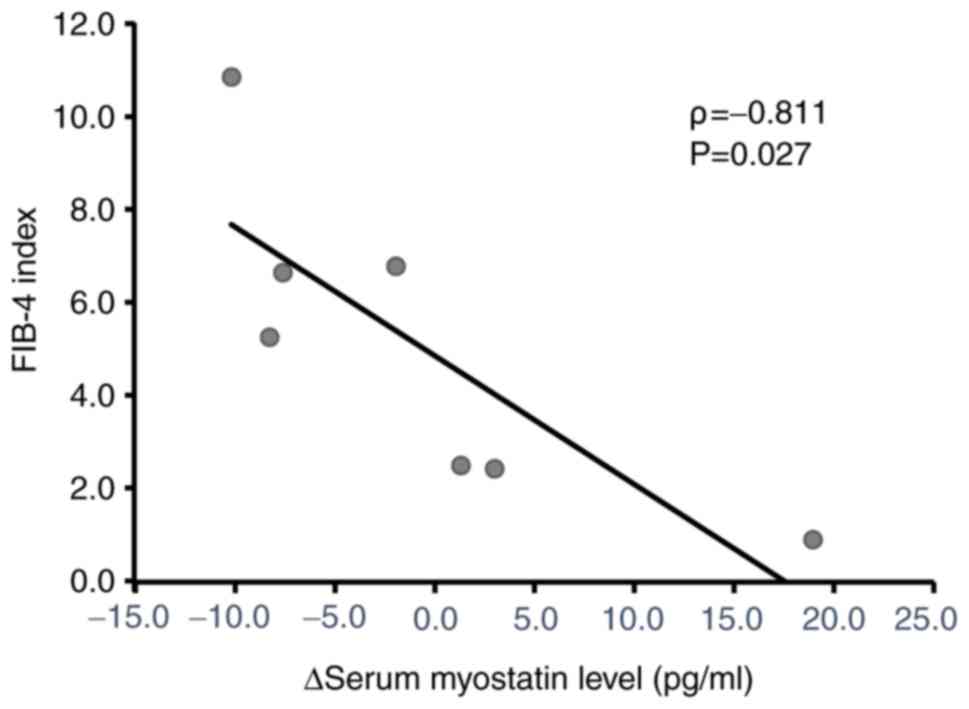
Correlation between the FIB-4 index
and changes in the serum myostatin level after L-carnitine
treatment
The correlation between the FIB-4 index and changes
in the serum myostatin level was analyzed by Spearman's rank
correlation analysis. A significant negative correlation was
observed between the FIB-4 index and changes in the serum myostatin
level (Fig. 4).
Discussion
The present study investigated the association
between L-carnitine and the serum levels of myostatin, irisin and
decorin in patients with chronic liver disease. However, no
significant differences in the serum levels of myostatin, irisin
and decorin were observed before and after L-carnitine treatment in
patients with chronic liver disease. A a stratification analysis
was also performed according to the FIB-4 index and it was found
that the serum myostatin levels were decreased after L-carnitine
treatment in patients with a high FIB-4 index. Thus, L-carnitine
treatment may suppress the serum myostatin level in patients with
liver cirrhosis.
Blood ammonia levels did differ significantly after
L-carnitine treatment in the present study. L-carnitine is known to
enhance ammonia detoxification in the urea cycle by increasing
N-acetylglutamate, leading to a decrease in blood ammonia
levels in patients with liver cirrhosis (24). Abbasnezhad et al performed a
meta-analysis on 799 patients from 9 randomized clinical trials and
demonstrated that L-carnitine treatment significantly reduced the
blood ammonia level (25). Thus,
the results of the present study differ from those of that previous
study. Although the reason for this discrepancy remains unclear,
the small sample size may be a possible reason. Another possible
reason may be renal dysfunction. The kidneys are involved in
ammonia metabolism by removing a significant amount of ammonia in
urine in cirrhotic patients with hyperammonemia (26). The meta-analysis by Abbasnezhad
et al demonstrated an improvement of renal function by
L-carnitine treatment (25).
However, in the present study, the tendency of an increase in the
serum creatinine level and a decrease in eGFR was observed during
the study period. Thus, an impairment of removing ammonia in the
urine is a possible reason for the blood ammonia level exhibiting
no change in the present study.
The serum CK level was significantly increased by
L-carnitine treatment in the present study. However, the serum
aspartate aminotransferase and lactate dehydrogenase levels were
not significantly increased by L-carnitine treatment, indicating
that the increase in the serum CK level was not due to muscle
damage. L-carnitine is known to improve fatigue and muscle cramps
(27,28), which can result in an increase in
physical activity, and the serum CK level is known as a maker for
physical activity (29). In
addition, CK is an enzyme, which catalyzes the reversible
phosphorylation of creatine to phosphocreatine and of adenosine
diphosphate (ADP) to adenosine triphosphate (ATP) (30). The expression of CK is known to be
increased during muscle hypertrophy in order to adapt to energy
demands (30). Although the
present study did not evaluate the effect of L-carnitine on muscle
mass, L-carnitine has been reported to cause muscle hypertrophy
(10,11). Thus, an increase in the serum CK
level may be a response to an increase in physical activity and/or
muscle hypertrophy in the present study.
L-carnitine has been reported to cause muscle
hypertrophy even in patients with no improvement of hyperammonemia
(10). The present study examined
the effects L-carnitine treatment on serum myokine levels, which
are associated with muscle hypertrophy. It was found that the serum
level of myostatin, but not that of irisin and decorin, was
decreased 12 weeks after L-carnitine treatment in patients with a
high FIB-4 index. In addition, there was a significant negative
correlation between the FIB-4 index and the changes in the
myostatin level after L-carnitine treatment. Since myostatin is a
negative regulator of muscle hypertrophy (17), L-carnitine may suppress myostatin,
leading to muscle hypertrophy. The mechanisms responsible for the
L-carnitine-induced suppression of myostatin remain unclear; the
expression of myostatin is regulated by AMP-activated protein
kinase (AMPK) (31). L-carnitine
transports long-chain fatty acids from the cytosol to the
mitochondrial matrix, leading to an increase in ATP production
through β-oxidation (32). An
increase in intracellular ATP level is known to downregulate AMPK
(33). Thus, L-carnitine may
suppress myostatin expression via the suppression of AMPK.
There are several limitations to the present study.
First, this was a retrospective pilot study with a small sample
size, which conducted in a single center. Second, the etiology and
severity of liver cirrhosis vary, suggesting the heterogeneity of
the enrolled subjects. Third, no information was available for
dietary habits, as L-carnitine is an abundant nutrient found in red
meat (34). Thus, a multi-center
randomized controlled trial with information on dietary habits is
required to prove the effects of L-carnitine on the serum myostatin
level.
In conclusion, the present study investigated an
association between L-carnitine treatment and the serum levels of
myostatin, irisin and decorin in patients with chronic liver
disease. No significant differences were observed in the serum
levels of these 3 myokines. However, it was found that the serum
level of myostatin, but not that of irisin and decorin, was
decreased 12 weeks after L-carnitine treatment in patients with a
high FIB-4 index. Thus, L-carnitine may downregulate myostatin,
leading to improved muscle atrophy in patients with liver
cirrhosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the JSPS
Grant-in-Aid for Scientific Research (C) JP20K08395.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DN and TK participated in the study conception and
design, data acquisition and interpretation, and manuscript
drafting. TTs, SY, KS and RH participated in data acquisition and
interpretation. TTs, SY, KS, HK and TTo participated in the
analysis and interpretation of data. HK and TTo participated in
revising the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki, as reflected in the
prior approval given by the institutional review board of Kurume
University. An opt-out approach was used to obtain informed consent
from patients, and personal information was protected during data
collection. None of the patients were institutionalized.
Patient consent for publication
Not applicable.
Competing interests
TK discloses honoraria (lecture fees) from
Mitsubishi Tanabe Pharma Corporation, MSD K.K, and Otsuka
Pharmaceutical Co., Ltd. The other authors disclose no competing
interests.
References
|
1
|
Reiss J, Iglseder B, Alzner R, Mayr-Pirker
B, Pirich C, Kässmann H, Kreutzer M, Dovjak P and Reiter R:
Consequences of applying the new EWGSOP2 guideline instead of the
former EWGSOP guideline for sarcopenia case finding in older
patients. Age Ageing. 48:719–724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the asian working group for
sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan Society of Hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schneider DA and Trence DL: Possible role
of nutrition in prevention of sarcopenia and falls. Endocr Pract.
25:1184–1190. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sawada Y, Kawaratani H, Kubo T, Fujinaga
Y, Furukawa M, Saikawa S, Sato S, Takaya H, Kaji k, Shimozato N, et
al: Effect of furosemide on muscle cramps in patients with liver
cirrhosis. J Gastroenterol Hepatol. 35:76–81. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang KV, Chen JD, Wu WT, Huang KC, Lin HY
and Han DS: Is sarcopenia associated with hepatic encephalopathy in
liver cirrhosis? A systematic review and meta-analysis. J Formos
Med Assoc. 118:833–842. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Praktiknjo M, Clees C, Pigliacelli A,
Fischer S, Jansen C, Lehmann J, Pohlmann A, Lattanzi B, Krabbe VK,
Strassburg CP, et al: Sarcopenia is associated with development of
acute-on-chronic liver failure in decompensated liver cirrhosis
receiving transjugular intrahepatic portosystemic shunt. Clin
Transl Gastroenterol. 10(e00025)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kamachi S, Mizuta T, Otsuka T, Nakashita
S, Ide Y, Miyoshi A, Kitahara K, Eguchi Y, Ozaki I and Anzai K:
Sarcopenia is a risk factor for the recurrence of hepatocellular
carcinoma after curative treatment. Hepatol Res. 46:201–208.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hanai T, Shiraki M, Nishimura K, Ohnishi
S, Imai K, Suetsugu A, Takai K, Shimizu M and Moriwaki H:
Sarcopenia impairs prognosis of patients with liver cirrhosis.
Nutrition. 31:193–199. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohara M, Ogawa K, Suda G, Kimura M,
Maehara O, Shimazaki T, Suzuki K, Nakamura A, Umemura M, Izumi T,
et al: L-Carnitine suppresses loss of skeletal muscle mass in
patients with liver cirrhosis. Hepatol Commun. 2:906–918.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hiramatsu A, Aikata H, Uchikawa S, Ohya K,
Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, Honda F, et al:
Levocarnitine use is associated with improvement in sarcopenia in
patients with liver cirrhosis. Hepatol Commun. 3:348–355.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hiraoka A, Kiguchi D, Ninomiya T, Hirooka
M, Abe M, Matsuura B, Hiasa Y and Michitaka K: Can L-carnitine
supplementation and exercise improve muscle complications in
patients with liver cirrhosis who receive branched-chain amino acid
supplementation? Eur J Gastroenterol Hepatol. 31:878–884.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gomarasca M, Banfi G and Lombardi G:
Myokines: The endocrine coupling of skeletal muscle and bone. Adv
Clin Chem. 94:155–218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Delezie J and Handschin C: Endocrine
crosstalk between skeletal muscle and the brain. Front Neurol.
9(698)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Colaianni G, Mongelli T, Cuscito C,
Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G,
Mori G, et al: Irisin prevents and restores bone loss and muscle
atrophy in hind-limb suspended mice. Sci Rep.
7(2811)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanzleiter T, Rath M, Görgens SW, Jensen
J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A and
Eckardt K: The myokine decorin is regulated by contraction and
involved in muscle hypertrophy. Biochem Biophys Res Commun.
450:1089–1094. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rodriguez J, Vernus B, Chelh I,
Cassar-Malek I, Gabillard JC, Hadj Sassi A, Seiliez I, Picard B and
Bonnieu A: Myostatin and the skeletal muscle atrophy and
hypertrophy signaling pathways. Cell Mol Life Sci. 71:4361–4371.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Garcia PS, Cabbabe A, Kambadur R, Nicholas
G and Csete M: Brief-reports: Elevated myostatin levels in patients
with liver disease: A potential contributor to skeletal muscle
wasting. Anesth Analg. 111:707–709. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nishikawa H, Enomoto H, Ishii A, Iwata Y,
Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, et
al: Elevated serum myostatin level is associated with worse
survival in patients with liver cirrhosis. J Cachexia Sarcopenia
Muscle. 8:915–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Skladany L, Koller T, Molcan P, Vnencakova
J, Zilincan M, Jancekova D and Kukla M: Prognostic usefulness of
serum myostatin in advanced chronic liver disease: Its relation to
gender and correlation with inflammatory status. J Physiol
Pharmacol. 70:357–368. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vallet-Pichard A, Mallet V and Pol S:
FIB-4: A simple, inexpensive and accurate marker of fibrosis in
HCV-infected patients. Hepatology. 44:769–770. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A,
Collaborators developing the Japanese equation for estimated GFR
, et al: Revised equations for estimated GFR from serum
creatinine in Japan. Am J Kidney Dis. 53:982–992. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shiraki M, Shimizu M, Moriwaki H, Okita K
and Koike K: Carnitine dynamics and their effects on hyperammonemia
in cirrhotic Japanese patients. Hepatol Res. 47:321–327.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abbasnezhad A, Choghakhori R, Kashkooli S,
Alipour M, Asbaghi O and Mohammadi R: Effect of L-carnitine on
liver enzymes and biochemical factors in hepatic encephalopathy: A
systematic review and meta-analysis. J Gastroenterol Hepatol.
34:2062–2070. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frederick RT: Current concepts in the
pathophysiology and management of hepatic encephalopathy.
Gastroenterol Hepatol (N Y). 7:222–233. 2011.PubMed/NCBI
|
|
27
|
Matsui H, Einama T, Shichi S, Kanazawa R,
Shibuya K, Suzuki T, Matsuzawa F, Hashimoto T, Homma S, Yamamoto J,
et al: L-Carnitine supplementation reduces the general fatigue of
cancer patients during chemotherapy. Mol Clin Oncol. 8:413–416.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakanishi H, Kurosaki M, Tsuchiya K,
Nakakuki N, Takada H, Matsuda S, Gondo K, Asano Y, Hattori N,
Tamaki N, et al: L-carnitine reduces muscle cramps in patients with
cirrhosis. Clin Gastroenterol Hepatol. 13:1540–1543.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schneider CM, Dennehy CA, Rodearmel SJ and
Hayward JR: Effects of physical activity on creatine phosphokinase
and the isoenzyme creatine kinase-MB. Ann Emerg Med. 25:520–524.
1995.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baird MF, Graham SM, Baker JS and
Bickerstaff GF: Creatine-kinase- and exercise-related muscle damage
implications for muscle performance and recovery. J Nutr Metab.
2012(960363)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Das AK, Yang QY, Fu X, Liang JF, Duarte
MS, Zhu MJ, Trobridge GD and Du M: AMP-activated protein kinase
stimulates myostatin expression in C2C12 cells. Biochem Biophys Res
Commun. 427:36–40. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gnoni A, Longo S, Gnoni GV and Giudetti
AM: Carnitine in human muscle bioenergetics: Can carnitine
supplementation improve physical exercise? Molecules.
25(182)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Stella C, Beckwith-Hall B, Cloarec O,
Holmes E, Lindon JC, Powell J, van der Ouderaa F, Bingham S, Cross
AJ and Nicholson JK: Susceptibility of human metabolic phenotypes
to dietary modulation. J Proteome Res. 5:2780–2788. 2006.PubMed/NCBI View Article : Google Scholar
|