Introduction
Medicinal plants have attained a commanding role in
the global health care system as sources of various phytochemicals,
several of which possess potent antioxidant properties. Such
products cover a large part of the global market, exceeding $100
billion annually and this coverage is expected to reach $550
billion by 2030 with a compound annual growth rate of 18.9%
(1). The World Health Organization
(WHO) has reported that 80% of the Earth's population relies on
traditional medicine for their primary health care needs, and a
main part of this therapy involves the use of plant extracts and
their active compounds (2).
Specifically, for the past 3,000 years, the therapeutic principles
of the active compounds of medicinal herbs have established their
importance in health practices in traditional medicine in China,
India and Africa, which has been ascertained as such by Western
standards. In consonance with the definition adopted by the WHO,
traditional medicine refers to ‘the sum total of the knowledge,
skill, and practices based on the theories, beliefs, and
experiences indigenous to different cultures, whether explicable or
not, used in the maintenance of health as well as in the
prevention, diagnosis, improvement or treatment of physical and
mental illness’ (3). During the
period between 1950-1970, ~100 plant-based drugs were introduced to
the US drug market, including vincristine, a plant alkaloid,
composing a chemotherapy medication used as a treatment for several
types of cancer (4).
Natural products offer a plethora of advantages to
the drug development process compared to conventional synthetic
compounds. To begin with, natural products can be found in high
abundance in nature, allowing scientists to yield almost endless
quantities of these. Notably, natural products have a higher
structural complexity and scaffold diversity than typical synthetic
small-molecule libraries (5).
Moreover, due to their structural diversity and optimization via
co-evolution in biological systems, natural products have increased
their probability to interact with proteins, an important
characteristic lending them potent chemopreventive properties
(6). On the other hand, despite
their rapid action, synthetic drugs are often associated with
adverse effects that negatively affect the human body in the
long-term (7).
Synthetic antioxidants were the food industries
first candidates used to counteract the potential adverse effects
of various food products on the health of consumers (8,9).
However, questions involving their nutritional value and potential
toxic side-effects, rapidly led to emerging concerns with respect
to human safety (10,11). At the same time, herb-derived
secondary metabolites, that are commonly associated with notable
biological characteristics, such as antioxidant, antimicrobial and
antimutagenic activities, have been given precedence in the use of
natural antioxidants. Of note, natural antioxidants are capable of
exerting these beneficial properties at micromolar concentrations
either via the direct scavenging of free radicals or through the
induction of hormetic mechanisms (12). Therefore, the consequent reduction
of oxidative modifications, and the prevention of mutagenesis,
carcinogenesis and aging, constitute the robust argument of using
plant-derived antioxidants against the synthetic ones (13).
Several methodologies have been developed for the
purpose of evaluating the antioxidant capacity of crude natural
extracts or pure isolated chemical compounds that are derived from
natural sources (14,15). Within this context, 47 natural
extracts derived from routinely used medicinal or edible herbs from
the Epirus region in Greece were screened in terms of their
antioxidant properties. On that note, the total phenolic content of
herb decoctions, as well as their antioxidant, reducing and
antigenotoxic activities were evaluated using a series of in
vitro cell-free assays. Subsequently, non-cytotoxic
concentrations of the four most potent herb decoction extracts were
used to treat EA.hy 926 endothelial cells in order to examine their
effects on the intracellular redox status by measuring the reduced
glutathione (GSH) and reactive oxygen species (ROS) levels. Thus,
the proposed study, will allow us to identify herbs that possess
promising antioxidant capacity in order to be introduced in
follow-up studies that will use in vivo models of oxidative
stress-mediated diseases.
Materials and methods
Chemicals, reagents and cell culture
medium
All the herbs were derived from local producers in
the Epirus region of Greece. To determine the total phenolic
content, Folin-Ciocalteu reagent and gallic acid were purchased
from Sigma-Aldrich; Merck KGaA. For the appraisal of the
antiradical and reducing activities of the herb decoction extracts,
2,2'-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS),
horseradish peroxidase (HRP), hydrogen peroxide
(H2O2) solution 30%, methanol (MeOH),
1,1-diphenyl-2-picrylhydrazyl (DPPH•), ferric chloride,
2-deoxyribose, nicotinamide adenine dinucleotide (NADH), nitroblue
tetrazolium (NBT) and phenazine methosulfate (PMS) were obtained
from Sigma-Aldrich; Merck KGaA. Furthermore, trichloroacetic acid
(TCA) and 2-thiobarbituric acid (TBA) were obtained from Merck
KGaA. To estimate the potential antigenotoxic properties,
pBluescript (SK+) plasmid DNA was purchased from Stratagene;
Agilent Technologies, Inc. and 2,2'-azobis(2-amidinopropane)
dihydrochloride (AAPH) from Sigma-Aldrich; Merck KGaA. With respect
to the tested cell line, EA.hy926 endothelial cells were donated by
Professor George Koukoulis (University of Thessaly, Larissa,
Greece). For cell cultures, Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS)
and trypsin-EDTA solution 0.25% were purchased from Gibco; Thermo
Fischer Scientific, Inc. The TACS XTT Cell Proliferation assay kit
was purchased from R&D Systems, Inc. Finally, to determine the
intracellular GSH and ROS levels, mercury orange and
2,7-dichlorofluorescein diacetate (DCF-DA) were purchased from
Sigma-Aldrich; Merck KGaA. All solvents were of analytical
grade.
Herb decoctions
To prepare herb decoction extracts, 2 g of dry herb
leaves were added to 200 ml tap water, followed by boiling for 3
min. Subsequently, the boiled samples were allowed to stand for 5
min. The resulting decoction was filtered, followed by
lyophilization of the total filtrate. The yield of the products
following extraction is presented Table SI. The lyophilized product was
used to prepare the final decoction in which the polyphenolic
content and bioactivity were evaluated.
Total phenolic content (TPC)
The TPC of the samples was determined using
Folin-Ciocalteu reagent. Briefly, 1 ml dH2O, 100 µl
Folin-Ciocalteu reagent and 20 µl of each sample were added to test
tubes and the mixture was incubated for 3 min at 25˚C under dark
conditions. Subsequently, 280 µl of a sodium carbonate solution
(25% w/v) and 600 µl dH2O were added, followed by
incubation for 1 h at 25˚C under ambient conditions in the dark,
and the absorbance was then determined at 765 nm using a
spectrophotometer (Hitachi, U-1900 UV/VIS, Hitachi
High-Technologies Corporation). A test tube containing
Folin-Ciocalteu reagent and dH2O was used as a blank.
The phenolic content was determined using a standard curve of
gallic acid (0, 50, 150, 250 and 500 µg/ml), and the results are
expressed as mg of gallic acid per g of dry sample.
DPPH• radical scavenging
assay
The radical scavenging capacity (RSC) of the tested
herb decoctions was evaluated using a slightly modified method of
the DPPH• assay (16),
as previously described (17).
Briefly, 50 µl of the tested samples at various concentrations was
mixed with 900 µl of methanol (MeOH), and subsequently 50 µl of a
freshly prepared methanolic solution of
2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) (2 mΜ) was
added. The samples were incubated for 20 min in the dark at room
temperature, and the absorbance was then determined at 517 nm using
a spectrophotometer (Hitachi, U-1900 UV/VIS, Hitachi
High-Technologies Corporation). Furthermore, MeOH was used as a
blank and the free radical solution alone in MeOH was used as a
control. The percentage RSC of the tested samples was calculated
using the following equation:
%RCS=[(ODcontrol-ODsample)/ODcontrol]
x100, where ODcontrol and ODsample refer to
the absorbance values of the control and the tested sample,
respectively. To compare the radical scavenging efficiency of the
different herb decoctions, an IC50 (half maximal
inhibitory concentration) value was estimated.
ABTS•+ radical scavenging
assay
The ABTS•+ RSC of the tested samples was
determined as previously described by Cano (18), with some minor modifications
(17,19). Briefly, 500 µl ABTS (1 mM), 50 µl
H2O2 (30 µM), 50 µl HRP (6 µM) in PBS (50 mM,
pH 7.5) and 400 µl dH2O were mixed together, vortexed
and incubated for 45 min in the dark at room temperature.
Subsequently, 50 µl of the tested samples were added, and the
absorbance was monitored spectrophotometrically at 730 nm nm using
a spectrophotometer (Hitachi, U-1900 UV/VIS, Hitachi
High-Technologies Corporation). For each experiment, the mixture
without HRP was used as a blank, while the mixture without the
tested sample was used as a control. The percentage RSC was
determined using the same equation as the one described above for
the DPPH• assay. Finally, an IC50 value was
estimated to compare the RSC of the different herb decoctions.
Superoxide radical scavenging
assay
The superoxide anion radical scavenging ability of
the herb decoctions was assessed using the method of Gülçin et
al (20) with some
modifications (19). The system of
PMS, NADH and NBT was used for the generation of superoxide
radicals. Briefly, 125 µl NBT (300 µM), 125 µl NADH (468 µM) and 50
µl of the tested samples at various concentrations were added to a
test tube containing 625 µl Tris-HCl buffer (16 mM, pH 8.0). The
reaction began following the addition of 125 µl of PMS (60 µM) to
the mixture. A vigorous vortex followed, as well as a 5-min
incubation at room temperature. Finally, the absorbance was
measured spectrophotometrically at using a spectrophotometer
(Hitachi, U-1900 UV/VIS, Hitachi High-Technologies Corporation). In
each experiment, a sample without PMS and the tested sample was
used as a blank, while a sample without the sample was used as a
control. The superoxide anion RSC of the tested samples was
calculated using the equation described above. Eventually, an
IC50 value was estimated to compare the radical
scavenging efficiency of the different herb decoctions.
Reducing power assay
The reducing power capacity was determined according
to the method described in the study by Yen and Duh (21) with minor modifications (19). Briefly, 50 µl of the tested samples
at different concentrations were mixed with 200 µl of phosphate
buffer (0.2 M, pH 6.6) and 250 µl of potassium ferricyanide (1%
w/v) in dH2O. The reaction mixture was placed in a dry
bath incubator at 50˚C for 20 min. The samples were then placed on
ice for an additional 5 min. Subsequently, 250 µl of TCA (10%) were
added, and the samples were centrifuged at 900 x g for 10 min at
25˚C. Subsequently, 700 µl of the supernatant were transferred to
new test tubes and 250 µl dH2O and 50 µl ferric chloride
(0.1%) in dH2O were added. The mixtures were incubated
at room temperature for 10 min. Finally, the absorbance was
determined spectrophotometrically at 700 nm using a
spectrophotometer (Hitachi, U-1900 UV/VIS, Hitachi
High-Technologies Corporation). An AU0.5 value was
extrapolated using graph-plotted absorbance against the sample
concentration, indicating the sample concentration that causes an
absorbance of 0.5.
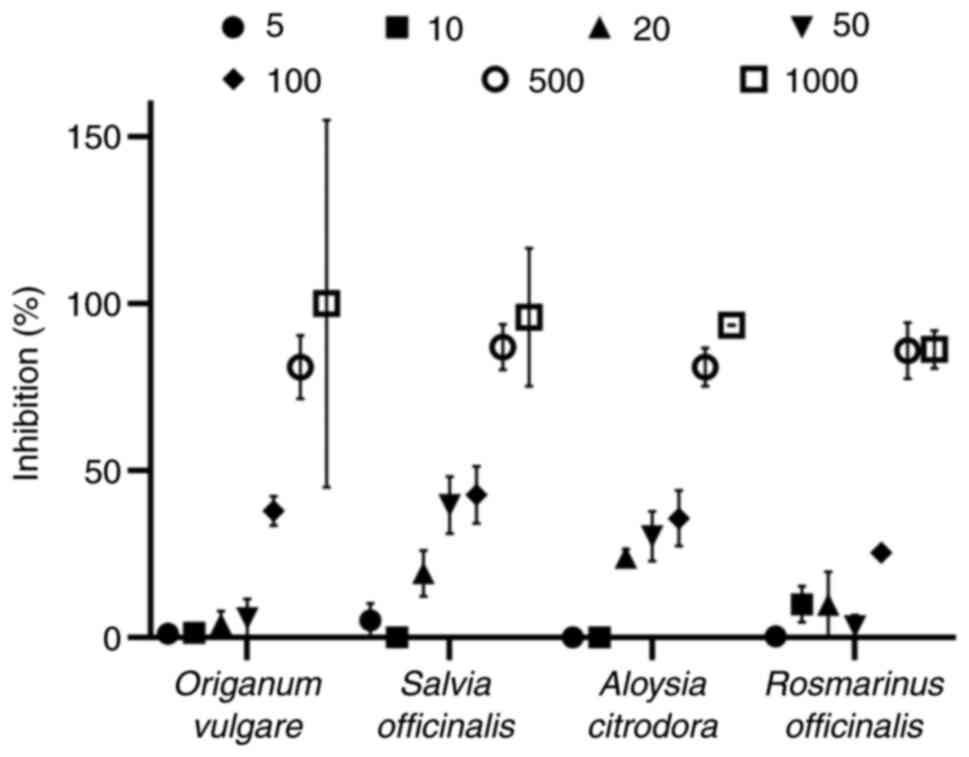
Peroxyl radical-induced DNA plasmid
strand cleavage
The assay was performed using a procedure previously
described (22) with some
modifications as reported by Priftis et al (23). Peroxyl radicals (ROO•)
were generated via the thermal decomposition of AAPH. The reaction
mixture (10 µl) containing 1 µg pBluescript (SK+) plasmid DNA, 2.5
mM AAPH in PBS and the tested samples at various concentrations was
incubated in the dark for 45 min at 37˚C. It should be noted that a
negative control consisting of plasmid DNA and PBS, and a positive
control containing plasmid DNA, PBS and AAPH were also used.
Subsequently, 3 µl loading buffer (bromophenol blue 0.25% + 30%
glycerol) were added to terminate the reaction, and the samples
were loaded on a 0.8% (w/v) agarose gel. The samples ran at 80 V
for 55 min. Ethidium bromide (10 mg/ml) was used as intercalating
dye. The acquisition of images was achieved using a MultiImage
Light Cabinet (Alpha Innotech Corporation). Finally, the Alpha View
suite was used to analyze the UV exposed gels. The percentage
inhibition of peroxyl radicals by the tested herb decoctions was
estimated through the following equation: %
Inhibition=[(S-So)/(Scontrol-So)]
x100, where S represents the percentage of the supercoiled plasmid
DNA in the tested samples, whereas So refers to the
percentage of the supercoiled plasmid DNA in the positive control.
Additionally, Scontrol represents the percentage of the
supercoiled DNA in the negative control. An IC50 value
was determined to compare the efficacy of different herb decoctions
against the peroxyl radical-induced DNA damage.
Cells and cell culture
Following the cell-free based assays and the
characterization of the antioxidant properties of the tested herb
decoctions, the four most potent herb decoction extracts were
assessed for their cytotoxic and intracellular antioxidant
properties in the EA.hy926 cell line. EA.hy926 is a stable human
endothelial cell line derived by hybridizing human umbilical vein
endothelial cells, namely human umbilical vein endothelial cells
(HUVECs), with the A549 human lung carcinoma cells. The endothelial
cells were cultured in 25 cm2 tissue culture flasks and
incubated for 24 h at 37˚C in 5% CO2 and 80-95% humidity
to reach ~70-80% confluency. The cell culture medium used was DMEM
containing 1 g/l D-glucose, 4 mM L-glutamine and supplemented with
10% (v/v) FBS, 100 units/ml penicillin and 100 units/ml
streptomycin. A morphology examination at high and low culture
densities was conducted using a microscope (Kern, OCL251, KERN
& SOHN GmbH; data not shown) to authenticate the state of
cells, through their phenotypic characteristics. According to the
international guidelines on good cell culture practice (24), the cell line used was checked for
mycoplasma using PCR and it was mycoplasma-free.
XTT cell viability assay
Cell viability was assessed using the XTT assay kit
(R&D Systems, Inc.). Briefly, 104 cells were seeded
into a 96-well plate with their respective complete medium.
Following a 24-h incubation, the cells were treated with increasing
concentrations of the Epirus herb decoctions in serum-free medium
for an additional 24 h. Subsequently, 50 µl of the XTT test
solution were prepared by mixing 50 µl XTT-labeling reagent with 1
µl XTT activator, and 50 µl of the XTT test solution were added to
each well. Following a 4-h incubation, the optical density was
measured at 450 and 630 nm (reference wavelength) using a
microplate reader (Bio-Tek ELx800; Bio-Tek Instruments, Inc.). Cell
cultures in serum-free medium were used as a negative control.
Moreover, the absorbance of every tested sample concentration alone
in serum-free medium and XTT test solution was also measured at 450
nm using a plate reader (EL808; BioTek Instruments, Inc.). The
absorbance values that were obtained in wells that contained only
herb decoctions extracts were subtracted from the ones that
acquired from wells that contained the respective extract
concentration and seeded cells. Data were calculated as follows:
Cell viability (% of
control)=(ODsample/ODcontrol) x100, where
ODcontrol and ODsample indicate the optical
density of the negative control and the test compounds,
respectively. All experiments were carried out in duplicate and at
least on two separate occasions.
Flow cytometric analysis of GSH and
ROS levels
The endothelial cells were seeded in 25
cm2 culture flasks for GSH and ROS determination and
incubated for 24 h at 37˚C in 5% CO2 and 80-95% humidity
to reach about 70-80% confluency. The culture medium was then
removed and replaced with serum-free medium containing the herb
decoction extracts tested at different concentrations. Following a
24-h incubation, the cells were trypsinized, collected and washed
twice following consecutive centrifugations at 300 x g for 10 min
at 5˚C. After each centrifugation the supernatant was discarded,
and the cellular pellet was resuspended in PBS. After the second
wash the cellular pellet (106 cells/ml) was ready for
intracellular staining for determinations of GSH and ROS levels
using flow cytometry with mercury orange and DCF-DA, respectively.
The fluorescent mercury orange binds directly to GSH, whereas
DCF-DA is deacetylated by esterases within the cells, and is
further converted to fluorescent DCF by the oxidative action of
ROS. A 400 µM stock solution of mercury orange was created in
acetone and stored at 4˚C, while a fresh 400 µM stock solution of
DCF-DA was prepared in methanol. To assess the GSH and ROS levels,
the cells (106 cells/ml) were resuspended in PBS and
incubated in the presence of mercury orange (40 µΜ) or DCF-DA (10
µΜ) in the dark at 37˚C for 30 min. Following incubation, the cells
were washed with PBS to remove the excess dye, centrifuged (300 x
g, 10 min, 4˚C) and resuspended in PBS. The cells were then
submitted to flow cytometric analysis using a FACSCalibur flow
cytometer (BD Biosciences) with excitation and emission length at
488 and 530 nm for ROS, and at 488 and 580 nm for GSH. Forward
angle and right-angle light scattering representable of the cells
size and cell internal complexity, respectively, were measured.
Analyses were performed on 10,000 cells per sample, at a flow rate
of 1,000 events/sec, and fluorescence intensities were measured on
a logarithmic scale. Data were analyzed using BD Cell Quest
software 6.0 (BD Biosciences). Each experiment was repeated at
least three times.
Statistical analyses
For in vitro cell-free based assays, an
IC50 or AU0.5 value for each tested sample
was estimated. Each experiment was conducted in triplicate and on
two separate occasions. As regards the cell culture experiments,
duplicates of the cell replicate and two separate occasions were
used. Data were analyzed using one-way ANOVA followed by Dunnett's
tests for multiple pairwise comparisons, using the statistical
package SPSS (version 21.0; SPSS, Inc.). All data are presented as
the mean ± SEM and a value of P<0.05 was considered to indicate
a statistically significant difference.
Results
In vitro cell-free measurements for
the assessment of the antioxidant, reducing and antigenotoxic
capacity of the herb decoction extracts TPC
Initially, the TPC of all the herb decoction
extracts, that were supplied to us by Epirus local producers, was
determined. According to the results, the highest polyphenolic
content was observed in the sage extract (Salvia
officinalis; code 30). Furthermore, a high phenolic content
(>0.8 mg gallic acid/ml) was found in decoction extracts derived
from perforate St. John's wort (Hypericum perforatum; codes
4 and 19), rosemary (Rosmarinus officinalis; code 45),
spearmint (Mentha spicata, code 28), hawthorn (Crataegus
monogyna, code 23), garden thyme (Thymus vulgaris; code
40), ironwort (Sideritis scardica; code 2), lemon beebrush
(Aloysia citrodora; code 18) and pennyroyal (Mentha
pulegium, code 14) (Table
I).
 | Table ITotal phenolic content,
IC50 and AU0.5 values of Epirus herb
decoction extracts evaluated using in vitro cell-free based
assays. |
Table I
Total phenolic content,
IC50 and AU0.5 values of Epirus herb
decoction extracts evaluated using in vitro cell-free based
assays.
| Code | Herb | Common name | TPC (mg gallic
acid/ml) | DPPH•
IC50 (µg/ml) | ABTS•+
IC50 (µg/ml) | Superoxide
IC50 (µg/ml) | Reducing power
AU0.5 (µg/ml) | Plasmid relaxation
assay IC50 (µg/ml) |
|---|
| 1 | Matricaria
chamomilla | Chamomile | 0.51 | 97.10±2.97 | 45.09±5.73 | 43±1.42 | 46±5.49 | 154±6.78 |
| 2 | Sideritis
scardica | Ironwort | 0.85 | 33.23±0.67 | 25.16±0.57 | 26±2.45 | 14±1.69 | 112±9.24 |
| 3 | Mentha
piperita | Peppermint | 0.53 | 50.29±3.28 | 37.24±0.90 | 45±3.78 | 32±1.42 | 125±8.75 |
| 4 | Hypericum
perforatum | Perforate St.
John's wort | 1 | 16.11±3.49 | 12.47±0.70 | 32±5.21 | 11±0.78 | 138±4.59 |
| 5 | Ocimum
basilicum | Basil | 0.69 | 26.31±1.31 | 22.92±0.17 | 11.5±1.01 | 22±1.57 | 163±9.36 |
| 6 | Matricaria
chamomilla | Chamomile | 0.29 | 94.29±9.63 | 64.97±1.15 | 45.5±2.47 | 36±2.59 | 158±4.97 |
| 7 | Mentha
piperita | Peppermint | 0.69 | 16.30±1.27 | 11.29±0.26 | 28±2.35 | 7±0.24 | 157±8.52 |
| 8 | Melissa
officinalis | Lemon balm | 0.72 | 20.64±1.78 | 8.33±0.51 | 28±1.89 | 8.3±0.36 | 79±5.47 |
| 9 | Laurus
nobilis | Bay laurel | 0.51 | 72.85±8.22 | 27.21±0.47 | 45±1.93 | 7±0.58 | 174±9.63 |
| 10 | Tilia
europea | Linden | 0.72 | 23.07±0.26 | 14.23±0.64 | 28±2.41 | 18±0.49 | 140±2.89 |
| 11 | Mentha
piperita | Peppermint | 0.67 | 28.11±0.63 | 23.63±0.08 | 37.5±1.58 | 15±2.14 | 87±3.56 |
| 12 | Sideritis
scardica | Ironwort | 0.79 | 36.55±0.11 | 33.56±0.67 | 37±2.63 | 18±2.39 | 156±8.54 |
| 13 | Salvia
officinalis | Sage | 0.44 | 26.68±1.22 | 19.07±0.09 | 6.5±0.25 | 8±0.35 | 54±4.51 |
| 14 | Mentha
pulegium | Pennyroyal | 0.82 | 27.53±1.24 | 14.32±0.53 | 29±1.28 | 12.5±1.54 | 65±5.62 |
| 15 | Ocimum
basilicum | Basil | 0.52 | 42.88±2.36 | 27.53±8.82 | 23.5±2.54 | 125±7.98 | >200 |
| 16 | Aloysia
citrodora | Lemon beebrush | 0.64 | 23.68±1.12 | 28.3±0.16 | 27±3.56 | 9±0.86 | 50±2.78 |
| 17 | Achillea
millefolium | Yarrow | 0.28 | 50.50±3.50 | 35.81±7.34 | 26.5±3.41 | 45±3.97 | 135±5.41 |
| 18 | Aloysia
citrodora | Lemon Beebrush | 0.83 | 10.25±0.25 | 8.29±1.13 | 49±2.39 | 3.5±0.13 | 26±2.14 |
| 19 | Hypericum
perforatum | Perforate St.
John's wort | 0.83 | 27.89±0.43 | 13.78±0.67 | 63±5.78 | 13.5±0.89 | 36±1.36 |
| 20 | Sideritis
scardica | Ironwort | 0.48 | 90.75±0.26 | 36.96±0.52 | 32.5±5.02 | 30±4.07 | 83±3.65 |
| 21 | Melissa
officinalis | Lemon Balm | 0.62 | 17.75±3.7 | 8.95±0.30 | 9±0.98 | 7±0.69 | 40±2.89 |
| 22 | Melissa
officinalis | Lemon balm | 0.69 | 18.21±0.05 | 9.06±0.62 | 23±1.25 | 7.2±0.78 | 32±3.45 |
| 23 | Crataegus
monogyna | Hawthorn | 0.89 | 20.72±1.83 | 14.05±0.23 | 59±4.13 | 25±2.19 | 42±2.56 |
| 24 | Salvia
officinalis | Sage | 0.76 | 23.26±1.89 | 20.96±2.63 | 37.5±4.52 | 7.5±0.67 | 35±3.74 |
| 25 | Sideritis
scardica | Ironwort | 0.44 | 80.99±0.16 | 49.58±0.04 | 48±6.02 | 10±0.92 | 156±8.59 |
| 26 | Sideritis
scardica (Organic farming) | Ironwort | 0.64 | 39.36±0.15 | 32.23±0.39 | 59±5.49 | 26±1.28 | 145±9.63 |
| 27 | Salvia
officinalis | Sage | 0.51 | 27.37±1.05 | 16.73±0.12 | 20.5±1.43 | 30±4.59 | 50±1.49 |
| 28 | Mentha
spicata | Spearmint | 0.92 | 29.35±0.52 | 20.71±1.83 | 24±0.97 | 10.5±2.30 | 53±2.15 |
| 29 | Sideritis
scardica | Ironwort | 0.52 | 88.22±10.73 | 38.05±2.05 | 25±1.49 | 59±5.62 | 145±7.46 |
| 30 | Salvia
officinalis | Sage | 1.04 | 20.44±0.01 | 14.73±0.46 | 20.5±1.34 | 6.1±0.34 | 56±3.54 |
| 31 | Tilia
europea | Linden | 0.41 | 33.46±2.26 | 23.74±1.16 | 20.5±2.96 | 12.5±0.57 | 45±2.57 |
| 32 | Rosmarinus
officinalis | Rosemary | 0.66 | 11.63±4.32 | 12.27±0.38 | 14.5±1.09 | 7.5±0.48 | 25±1.27 |
| 33 | Rosmarinus
officinalis | Rosemary | 0.45 | 17.14±1.10 | 14.17±0.19 | 27±2.56 | 9.7±0.92 | 36±2.37 |
| 34 | Origanum
vulgare (Organic farming) | Oregano | 0.68 | 14.11±0.93 | 8.88 ±0.40 | 20.5±1.74 | 9.5±0.81 | 45±2.18 |
| 35 | Origanum
vulgare | Oregano | 0.78 | 16.77±0.66 | 11.53±0.20 | 20.5±1.85 | 11±0.74 | 49±4.37 |
| 36 | Origanum
vulgare | Oregano | 0.52 | 21.86±1.76 | 11.63±0.14 | 43±2.94 | 12±0.93 | 55±5.04 |
| 37 | Origanum
vulgare | Oregano | 0.39 | 24.09±1.36 | 14.72±0.88 | 23.5±2.36 | 9±0.53 | 93±2.05 |
| 38 | Satureja
montana | Winter savory | 0.61 | 25.49±4.66 | 20.03±2.29 | 30±1.86 | 24±2.19 | 84±6.54 |
| 39 | Origanum
vulgare | Oregano | 0.62 | 20.99±1.10 | 11.77±0.22 | 15±2.45 | 12±1.24 | 65±4.83 |
| 40 | Thymus
vulgaris | Garden thyme | 0.87 | 21.52±0.89 | 23.68±0.84 | 15.5±1.75 | 4±0.02 | 67±4.09 |
| 41 | Origanum
vulgare | Oregano | 0.62 | 22.25±0.01 | 13.72±0.11 | 18±1.27 | 9.5±0.35 | 54±2.51 |
| 42 | Thymus
vulgaris | Garden thyme | 0.59 | 41.68±0.07 | 30.76±5.04 | 34±1.93 | 6.5±0.56 | 56±3.64 |
| 43 | Origanum
vulgare | Oregano | 0.73 | 17.43±3.39 | 13.19±0.21 | 38±2.54 | 10±0.84 | 70±5.06 |
| 44 | Origanum
vulgare | Oregano | 0.77 | 16.81±0.30 | 12.22±0.06 | 16.5±1.41 | 5.8±0.86 | 78±4.23 |
| 45 | Rosmarinus
officinalis | Rosemary | 0.93 | 18.96±0.59 | 15.36±1.15 | 19±1.23 | 10.5±1.07 | 82±5.82 |
| 46 | Origanum
vulgare | Oregano | 0.51 | 6.60±1.50 | 7.85±0.56 | 12±1.07 | 7.5±0.41 | 35±3.06 |
| 47 | Origanum
vulgare | Oregano | 0.67 | 18.74±4.96 | 14.22±0.24 | 12±1.17 | 9.4±0.45 | 46±4.09 |
Determination of IC50
values of extracts in DPPH•, ABTS•+ and
superoxide radical scavenging assays
According to the DPPH• assay, the
decoction extract that derived from oregano (Origanum
vulgare; code 46) displayed the highest scavenging activity
(6.6 µg/ml) (Table I). Following
that, several other extracts were found to scavenge half of the
DPPH radical at concentrations <20 µg/ml. Among these, lemon
beebrush (Aloysia citrodora; code 18), perforate St. John's
wort (Hypericum perforatum; code 4), and rosemary
(Rosmarinus officinalis; code 45) were also rich in phenol
content, as described above. Of note, all the rosemary extracts
that were tested, derived from three different producers (code 32,
33 and 45), exhibited an IC50 value <20 µg/ml,
whereas all 10 different types of oregano [nine non-biologically
cultivated (codes 35, 36, 37, 39, 41, 43, 44, 46 and 47) and one
biologically cultivated (code 34)] exhibited an IC50
value <25 µg/ml (Table I).
In the ABTS•+ assay, the extract derived
from oregano (Origanum vulgare; code 46) again exerted the
highest scavenging activity (7.85 µg/ml; Table I). Similarly, all extracts that
were derived from oregano [nine non-biologically cultivated (codes
35, 36, 37, 39, 41, 43, 44, 46 and 47) and one biologically
cultivated (code 34)], exhibited potent scavenging activities
against ABTS•+ with IC50 values <15 µg/ml.
Even though the lemon balm (Melissa officinalis) extracts
were not that rich in phenol content, they displayed high
ABTS•+ scavenging activity (code 8, 8.33 µg/ml; code 21,
8.95 µg/ml; code 22; 9.06 µg/ml). On the contrary, the decoction
extract derived from lemon beebrush (Aloysia citrodora; code
18) exhibited a high ABTS•+ scavenging activity (8.29
µg/ml) following its ability to scavenge DPPH• and its
high phenol content.
In the superoxide assay, the extract that was
derived from sage (Salvia officinalis; code 13) displayed
the highest efficacy (6.5 µg/ml), albeit its polyphenol content was
one of the lowest detected (0.44 mg GA/ml) (Table I). Even though the sage extracts
from different producers had a high polyphenolic content, their
efficacy to scavenge superoxide anion was diminished. Similarly,
lemon balm extract (Melissa officinalis; code 21) that
exhibited the second higher efficacy against superoxide anion
radicals (9 µg/ml), was the one with the lowest polyphenol content
among the three lemon balm extracts tested. Subsequently, basil
(Ocimum basilicum, 11.5 µg/ml) extract (code 5) and two of
the oregano (Origanum vulgare; 12 µg/ml in both of them)
extracts (codes 46 and 47) exhibited a potent efficacy to scavenge
superoxide anion radicals. Of note, oregano with code 46 exhibited
the highest activity against DPPH• and
ABTS•+, as described above.
Determination of reducing power
capacity
The extract that exhibited the highest reducing
power capacity was the one derived from lemon beebrush (Aloysia
citrodora; code 18, 3.5 µg/ml) (Table I). The same extract displayed a
high polyphenol content and was also one of the most potent as
regards the DPPH• and ABTS•+ scavenging
activity. Subsequently, an increased reducing power was exerted by
both garden thyme (Thymus vulgaris; 4 and 6.5 µg/ml, codes
40 and 42, respectively). More specifically, the garden thyme
extract with code 40 was one of the highly enriched in polyphenol
extracts among the ones we tested. All extracts that derived from
oregano had robust reducing power capacity ranging from 5.8 up to
12 µg/ml. Furthermore, three out of the four sage extracts and all
three lemon balm (Melissa officinalis; codes 8, 21 and 22)
extracts exhibited an almost similar reducing power capacity (6.1-8
and 7-8.3 µg/ml, respectively). Other extracts that sporadically
exhibited a potent reducing power capacity were extracts derived
from peppermint (Mentha piperita; code 7, 7 µg/ml) and bay
laurel (Laurus nobilis; code 9, 7 µg/ml).
Antigenotoxic activity of herb
decoction extracts via plasmid relaxation assay
The plasmid relaxation assay revealed that rosemary
(Rosmarinus officinalis; code 32) and lemon beebrush
(Aloysia citrodora; code 18) extracts from the same producer
had the most potent antigenotoxic activity of 25 and 26 µg/ml,
respectively (Table I). Similarly,
another rosemary extract (code 33) also exhibited a high activity
(36 µg/ml) to protect plasmid DNA. Oregano (Origanum
vulgare; code 46) extract, that displayed the highest efficacy
against DPPH• and ABTS•+ and one of the
highest activities against superoxide anion radical, was also
highly efficacious (35 µg/ml) to protect nucleic acids from
single-strand breaks. Finally, two extracts derived from lemon balm
(Melissa officinalis; codes 22 and 21, 32 and 40 µg/ml,
respectively), perforate St. John's wort (Hypericum
perforatum; code 19, 36 µg/ml) and sage (Salvia
officinalis; code 24, 35 µg/ml) also exhibited a highly notable
antigenotoxic activity.
In vitro cell-based measurements for
the assessment of the herb decoction extracts antioxidant
activity
Four of the extracts that displayed the highest
cell-free antioxidant capacity in the methods tested were screened
using the EA.hy926 cells for cytotoxicity and antioxidant-related
parameters. More specifically, oregano (Origanum vulgare;
code 46), sage (Salvia officinalis; code 13), lemon beebrush
(Aloysia citrodora; code 18) and rosemary (Rosmarinus
officinalis; code 32) extracts were the ones that were selected
for more elaborate analysis for the determination of their in
vitro cell-based antioxidant ability.
XTT cell proliferation assay
Initially, the authors wished to examine whether
these four extracts exerted any cytotoxic effects. For this
purpose, XTT cell proliferation assay was performed using EA.hy926
cells. The Origanum vulgare decoction extract exhibited an
IC50 value of 191.81 µg/ml with 50 µg/ml being almost
non-cytotoxic (Fig. 1). The
Salvia officinalis decoction extract exerted a lower
IC50 value (154 µg/ml) with 10 µg/ml to be the highest
concentration that did not present any cytotoxic manifestation.
Similarly, 10 µg/ml of the Aloysia citrodora decoction
extract did not inhibit EA.hy926 cell proliferation, with the
IC50 value determined at 228 µg/ml (data not shown).
Finally, Rosmarinus officinalis decoction extract had an
IC50 value of 285.35 µg/ml (data not shown), rendering
it as the less cytotoxic among the four more efficacious
extracts.
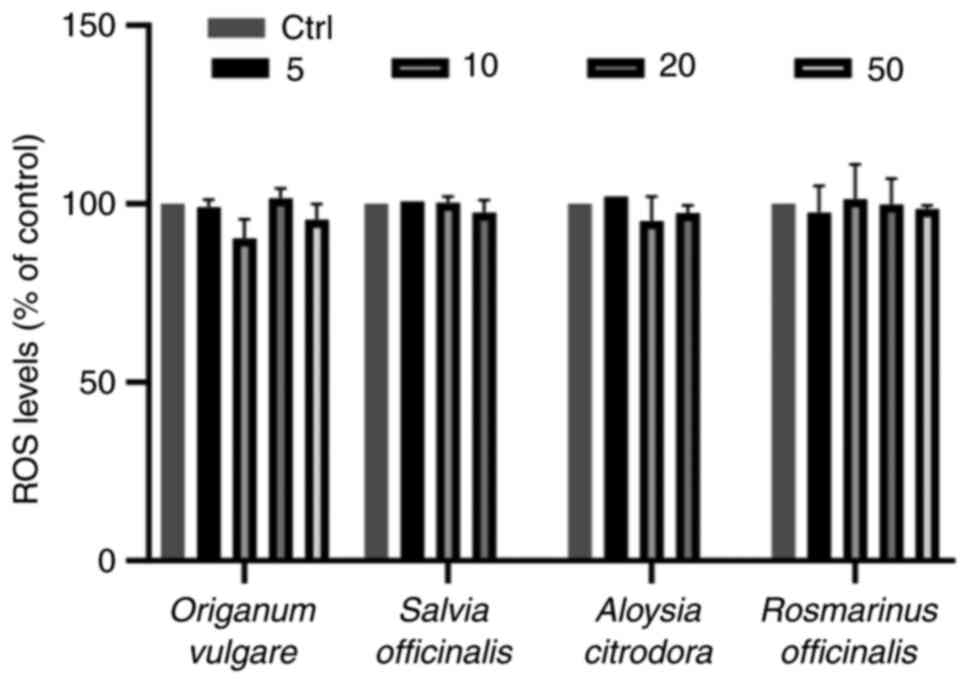
Determination of intracellular GSH and
ROS levels
Subsequently, the present study examined whether
sublethal concentrations of the herb decoction extracts were able
to alter the intracellular levels of GSH and ROS, since they both
play crucial roles in physiology, particularly in cells with a
cancerous profile. The sublethal concentrations of all four herbs
decoction extracts were unable to affect the ROS levels as compared
with the control group (Figs. 2
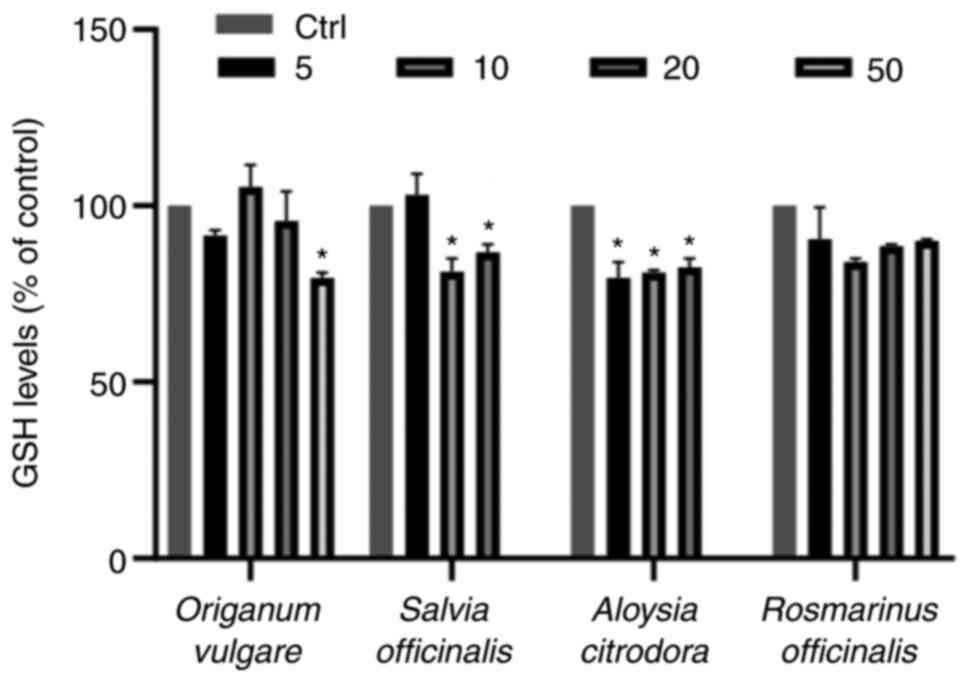
and S1). On the contrary, three
herb decoction extracts were able to decrease the GSH levels in the
already intracellular distorted cancerous physiology in comparison
with the control group. More specifically, 50 µg/ml oregano
(Origanum vulgare) were able to significantly decrease the
GSH levels as compared with the untreated cells (Figs. 3 and S2). Additionally, 10 and 20 µg/ml of
sage (Salvia officinalis) decoction extract diminished GSH
levels in EA.hy926 cells. The same effect was evident in treatments
with lemon beebrush decoction extracts (Aloysia citrodora),
in which all concentrations tested reduced the intracellular GSH
levels significantly. On the contrary, all concentrations tested
from rosemary (Rosmarinus officinalis) decoction extract did
not affect the GSH levels.
Discussion
The present study aimed to determine the
redox-related properties of well-known and routinely used herb
decoctions derived from Epirus region, Greece, predominantly for
their extensive use in everyday life, their integral part in human
diet, and eventually for their potential exploitation as
chemopreventive agents. The results suggest the potent antioxidant
activity of Epirus medicinal and aromatic herbs. Moreover, the lack
of cytotoxicity and the alterations induced in the GSH/ROS
equilibrium represent promising paraphernalia for activity,
strengthening the logic of using herbal decoctions as a prevention
strategy against oxidative stress related diseases which currently
stand out as the dominant threats of human health (25).
The range of the TPC in the tested decoctions was
from 0.44 mg gallic acid/ml for the Sideritis scardica
extract to 1.04 mg gallic acid/ml for the Salvia
officinalis. These levels differ compared to those in previous
studies (26,27), a discrepancy that may be attributed
to the different extraction protocols, solvents, different
microenvironment and cultivation processes used. Phenolic acids are
a subclass of phenolic compounds, widely spread throughout the
plant kingdom. In the present study, considerable variation was
detected in phenolic compounds content among the different herb
species. The high level of diversity and complexity of the natural
mixtures of phenolic compounds that are present in herb decoctions
render difficult to characterize every compound, elucidate its
structure, and attribute its activity. Of note, further studies are
required to identify the major groups and important aglycones of
the phenolic compounds, allowing us to associate their presence
with their enhanced activity like we have done in our previous
study (28). Nonetheless, several
medical herbs have been studied and to some extent their phenolic
chemistry is known (29).
The potent antioxidant potential that the
polyphenolic compounds of the herb decoctions possess is a
manifestation that has been already reported (30). The chemical structure and type of
the compounds, the level of substrate oxidation and the conditions
of the oxidation process, constitute parameters that affect their
activity (31). These compounds
consist of a hydroxyl group and play a major role in the
antioxidant capacity because of their ability to release hydrogen
and to form stable radical intermediates. Moreover, the mechanism
of their action mainly comprises neutralization of free radicals,
enzyme induction and chelation of metal ions.
The experiments performed in the present study
clearly indicated that the extracts of Origanum vulgare, Salvia
officinalis, Rosmarinus officinalis and Aloysia
citrodora possessed a potent antioxidant potential and may be
stronger radical scavengers than the other tested Epirus herbs. In
particular, it was found that Origanum vulgare exhibited an
enhanced antioxidant potential in the DPPH• and
ABTS•+ assays. Given the fact that the aforementioned
assays use both organic and water-based solvents, they allow for
the evaluation if the antioxidant effect of both lipophilic and
hydrophilic polyphenols (32,33).
Even though the oregano (Origanum vulgare) decoction extract
exhibited a potent scavenging ability against superoxide radical,
the highest efficiency was achieved by the sage (Salvia
officinalis) decoction extract. Flavonoids contained in this
herb extract have also been previously reported for their
effectiveness against superoxide anions (34), that have been proven to harm
cellular components (35),
predominantly lipids, as they are involved in initiation of the
lipid peroxidation process (36).
Lemon beebrush (Aloysia citrodora) exhibitd the highest
reducing power capacity among herbs tested, serving as a
significant indicator of its potential antioxidant activity.
Similarly, it was found to be one of the most efficacious in
response to scavenge DPPH• and ABTS•+
generating radicals. The reducing capacity composes a distinct
mechanism by which antioxidants exert their activity together with
chain initiation, decomposition of peroxides, reducing capacity and
radical scavenging (37). A
previous study also suggested that the ability of plant-derived
decoctions to act as reducing agents and free radical scavengers or
as quenchers of singlet oxygen formation was probably attributed to
their potent antioxidant effectiveness in vitro (38). In consonance, some authors have
ascertained the fact that phenolic compounds are able to chelate
metal ions and report that intracellular binding of iron is
responsible for the protection offered by flavonoids against
H2O2-induced DNA damage (39). DNA damage, as defined by strand
breakage in response to oxidative stress, was most effectively
inhibited with the rosemary (Rosmarinus officinalis)
decoction extract.
The results obtained herein correspond with those of
other studies examining the antioxidant properties of the medicinal
and aromatic herbs Origanum vulgare, Aloysia citrodora,
Salvia officinalis and Rosmarinus officinalis. Taken
together, their beneficial properties have been basically
attributed to their major chemical compounds, such as carvacrol,
thymol, diterpenes and carnosol (40). In particular, Origanum
vulgare extract exhibits antioxidant and antibacterial
activities, mostly attributed to its carvacrol and thymol content
(41,42). Furthermore, the antioxidant
potential of Aloysia citrodora was evaluated in several
scientific studies that have demonstrated the strong activity of
this plant (43,44). Additionally, decoctions derived
from organ (shoots and hairy roots) and undifferentiated (cell and
callus) cultures of Salvia officinalis, as well as from
shoots and roots of in vitro regenerated plants, as well its
chemical components, were evaluated for their antioxidant
properties using several in vitro models (45,46).
Moreover, it has been demonstrated that Rosmarinus
officinalis essential oil, apart from exhibiting free radical
scavenging activity determined by DPPH• assay, exerts
its hepatoprotective effects through the activation of
physiological defense mechanisms. The beneficial effects of this
plant herb have been attributed to its main chemical constituents,
including diterpenes, carnosol and carnosic acid, as well as to its
essential oil components (47).
In the global literature, there is a constant debate
as to the plant herb biologically active substances that can affect
the activity and metabolism of cells. Cell-free methodologies are
able to provide valuable preamble data concerning their efficacy;
however, cell-based in vitro experiments are also used to
minimize the mechanistic limitations of protocols using cell-free
systems. Using cell lines integrates a spectrum of protective
mechanisms represented by a shield of important cellular molecules
against oxidants toxic effects that cell-free methodologies do not
contain and examine. Hence, in the present study, the four
decoction extracts that exhibited the most potent antioxidant
activity in cell-free methodologies (Origanum vulgare, Salvia
officinalis, Aloysia citrodora and Rosmarinus
officinalis) were examined for their redox-related properties
using human endothelial EA.hy926 cells.
The high energy demand of cancer cells, and
concomitantly, their intense metabolic rates lead to abundant ROS
production in the cellular environment, derived primarily from the
mitochondria and the endoplasmic reticulum. Albeit the continuous
and elevated ROS levels can result in the death of normal cells,
through the induction of oxidative stress, the high rate of ROS
generation in cancer cells is compensated by the equally high
activation of the respective antioxidant mechanisms (48). Considering that the nuclear
transcriptional factor, nuclear factor erythroid 2-related factor 2
(NRF2), enhances cell survival under oxidative stress conditions,
its overactivation enables cancer cells to take advantage over the
normal ones (49). In the case
that the elevated levels of ROS cannot be alleviated, the cancer
cells are vulnerable to cell death mediated by oxidative stress
(48). In this context, strategies
related to intracellular ROS generation or target endogenous
antioxidant mechanisms have been tested as potential anticancer
therapies (50,51). As regards the polyphenol activity,
it is known that these molecules exert a biphasic effect; at low
concentrations, they act as antioxidants, whereas at high
concentrations, they promote elevated oxidation that results in
cytotoxicity (52).
To address the above, the present study evaluated
the cytotoxicity exerted by the four most potent decoction extracts
in order to determine the effects of non-cytotoxic concentrations
of these on the intracellular GSH and ROS levels. The results from
XTT assay revealed that Origanum vulgare and Rosmarinus
officinalis decoctions exhibited significant cytotoxicity
>100 µg/ml, whereas the cytotoxic threshold for Aloysia
citrodora and Salvia officinalis decoctions extracts was
>50 µg/ml.
The assessment of the effects of the extracts on
the antioxidant capacity of endothelial cells was based on the
measurement of the GSH and ROS levels using flow cytometry. The
regulation of intracellular GSH levels following extract treatment
is crucial, since GSH is considered a significant endogenous
antioxidant molecule in cells (53). GSH can directly scavenge free
radicals by donating one hydrogen atom from its sulfhydryl group or
is used as substrate by antioxidant enzymes (53). For endothelial cells in particular,
GSH is important not only as an antioxidant, but also as a crucial
regulator of cell signaling (54,55).
Endothelial cells as part of the inflammatory tumor
microenvironment play a critical role in inflammatory processes,
since the secretion of endothelial mitogens and chemotactic factors
driven by endothelial cells, stimulates their proliferation and
angiogenesis (56). Endothelial
cells release growth and survival factors (such as IL-6) to protect
tumor cells (57). Consequently,
the dependence of tumor growth and expansion to new blood vessels
formed by proliferating endothelial cells warrants investigation.
The latter implies the need for the examination of strategies
targeting the functions of tumor endothelial cells as key players
in angiogenic processes (58).
Therefore, the assessment of the mechanisms through which medicinal
herbs affect molecular pathways that regulate the GSH and ROS
levels in the EA.hy926 cell line may be of utmost importance.
In the present study, the results revealed that
Origanum vulgare decoction extract significantly decreased
the GSH levels at 50 µg/ml compared with the control. It has been
previously described that carvacrol and thymol are the components
considered responsible for the antioxidant activity of the
essential oil of oregano (59,60).
It was reported that carvacrol increases ROS and depletes GSH
levels in two distinct human cell lines. In line with the above
results, carvacrol has been reported to induce ROS levels in V79
cells (61) and to reduce the
levels of antioxidant enzymes catalase (CAT) and superoxide
dismutase (SOD) in HL-60 (human acute promyelocytic leukemia cells)
and Jurkat (human T lymphocyte cells) cells (62). It is possible that their antitumor
activity does not rely on the increase of intracellular ROS levels,
but on the elevation of the difference between the GSH and ROS
levels (63), rendering cells
vulnerable to the already increased ROS levels due to their
cancerous phenotype (64).
In the present study, Salvia officinalis
decoction extract was also found to decrease the GSH levels at
concentrations of 10 and 20 µg/ml compared with the control.
Salvia officinalis may exert its cytotoxic effect in a
similar manner to oregano. A previous study that investigated the
effect of Salvia chloroleuca reported that was able to
induce the apoptosis of MCF-7 human breast cells through a
ROS-mediated pathway (65). The
results of the present study and the previous one (65) are contradictory to data from
previous literature that reported that HepG2 cells pre-treated with
the Salvia officinalis extract formed less oxidant-induced
DNA lesions (66). Although Kozics
et al (66) proposed that
the observed DNA-protective activity could be explained by both the
elevation of glutathione peroxidase (GPx) activity in the
pre-treated cells, as well as to its well documented in
vitro antioxidant activity, their finding of an elevated GPx
activity may justify the decrease levels of GSH found in the
present study. Previously, Salvia officinalis was reported
to decrease peripheral inflammation that may support blood brain
barrier function and cerebral blood flow, contributing to
longer-term benefits towards cognitive health in older adults
(67).
In the present study, the Aloysia citrodora
decoction extract also decreased the GSH levels in the EA.hy926
cell line at all concentrations tested (5, 10 and 20 µg/ml) in
comparison with the control. Notably, Aloysia citrodora
decoction extract has been previously linked to an increase in
glutathione reductase (GR) levels accompanied by lower levels of
malondialdehyde and protein carbonyls, as proposed by a
double-blind study using human subjects (68). Furthermore, Fitsiou et al
(69) reported potent anticancer
and antimicrobial properties accompanied by a weak direct
antioxidant activity, as shown by comet assay in Jurkat cells.
Another study demonstrated results similar with to the data
presented herein, attributing lemon beebrush leaf infusion as a
source of compounds with significant free radical scavenger ability
and antigenotoxic activity (70).
Even though Rosmarinus officinalis decoction
extract exhibited a potent antioxidant capacity, the present study
failed to detect any changes in GSH and ROS levels. As
aforementioned, it has been demonstrated that the depletion of
endogenous GSH levels is considered to increase the efficacy of
therapeutic interventions (71,72).
Furthermore, it has been shown that Rosmarinus officinalis
contains rosmarinic acid, and that its administration in a
xenograft tumor model was able to suppress tumor growth (73). Furthermore, rosmarinic acid can
damage murine melanoma cells through a double-axis effect, namely
the possible protection of healthy cells (increased GSH) and the
concomitant damage of cancer cells (depletion of GSH) (74).
The increase of the ‘spare capacity’ between the
GSH and ROS levels in the cancer endothelial cell line, as proposed
by the results of the present study may be part of a further
disruption of their redox status compared to normal cells. This
phenomenon may compose a critical step for the selection of
appropriate chemopreventive strategies based on appropriate
configurations of the redox potential of cancer cells. Chinese
herbs have been associated with the metabolic reprogramming of
cancer cells, enabling their experimental use as therapeutic
compounds against metabolism-related diseases (75).
Αn uncertainty that the present study generates
lies in the obvious discrepancy between results in decoctions
examined in the authors' laboratory and originating from the same
plant type, but have been provided by different producers. This
could relate to the fact that herb biological properties are
dependent on differences in the exact geographical location and
cultivation micro-environment conditions. Previously, Karydas et
al (76) reported that even
different land areas can modulate antioxidant potential and
polyphenolic content. More elaborately, the different land areas
can be further fragmented into different habitats and the specific
microclimate conditions that include altitude, soil composition,
temperature variation, and watering during the day or night hours.
Furthermore, even though the in vitro cell-free
methodologies rely on the ability of the extract to scavenge the
generating radical, small differences in the methodology mechanisms
can justify the differentiation of the efficacies that each extract
exhibits. This has been frequently reported in studies examining a
series of protocol schemes (77-79).
Therefore, it is necessary not to rely on a single test or even the
analyzed parameter (80). These
fluctuations in efficacies have not only been observed among
experimental protocols applied, but also between herbs that were
derived from different producers. Nevertheless, it is clear that
certain decoction extracts (e.g., Origanum vulgare) have
exhibited almost an constant antioxidant efficacy.
In conclusion, the results of the present study
support the promising role of the tested decoctions as a source of
antioxidant active compounds in follow-up in vivo studies,
since they possess the ability to interfere with or modify the
redox state of cells. Nevertheless, antioxidant protection involves
a variety of factors, such as the concentration of antioxidant
compounds, the synergetic effect that they may possess and how they
can modulate the different branches of cellular oxidative status.
Therefore, the scientific community needs to remain alert and
acknowledge the aforementioned limitations that do not allow us to
reach a solid outcome, which is also dependent on the methodology
used to examine the extracts. Likewise, it is reasonable that the
outcome in in vitro applications may differ from that in
vivo due to advanced levels of complexity of the biological
system. The need for further research focusing on the effects of
medicinal and aromatic herbs in vivo is critical to
corroborate the beneficial effects proposed by the present study.
Additionally, the cytotoxicity and bioactivity of the samples
examined appears to be dependent on various factors, such as a
plant's geographical location and cultivation process, parts of the
plant used for decoction preparation and the extraction protocol
(solvent, temperature, time, etc.). However, clinical trials and
primary prevention studies using high doses of such herbs in humans
did not yield the expected beneficial outcome (81). Conclusively, the generating trend
of the use of herbs in order to exert beneficial effects on human
health and well-being requires further exploration. The setting of
prerequisites for the investigation of the interrelation between
particular herb harvests and cultivation conditions may lead to new
dimensions and complexities that the scientific community needs to
focus their interest and shed light on. Finally, the assessment of
the polyphenol content in the decoction extracts that possess a
higher efficacy and the identification of those molecules that may
exert significant biological effects is of utmost importance. The
aforementioned should be followed with mechanistic in vitro
and in vivo experimental models that will elucidate the
molecular mechanisms induced by the compounds.
Supplementary Material
Scatter plots and histograms from flow
cytometric analysis for the determination of ROS levels in the
EA.hy926 cell line. Note that the control plots apply to all
extracts. ROS, reactive oxygen species.
Scatter plots and histograms from flow
cytometric analysis for the determination of GSH levels in the
EA.hy926 cell line. Note that the control plots apply to all
extracts. GSH, glutathione.
Yield of all the products following
extraction.
Acknowledgements
Not applicable.
Funding
Funding: The present study was partly funded by the project
entitled ‘BioActHerb: A cloud-based platform for the bioactivity of
herbs in Epirus Region’, co-financed by the European Union and
Greek national funds through the Operational Program for Research
and Innovation Smart specialization Strategy (RIS3) of Ipeiros
(Project Code: HP1AB-0028215).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZS, IDK, PV and FT analyzed and interpreted the
data regarding the antioxidant activity of the herbs. ZS, IDK and
PV were major contributors to the writing of the manuscript. KA, NG
and DK, were involved in the design and conception of the study,
and also confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DK is an Editor of the journal, but had no personal
involvement in the reviewing process, or any influence in terms of
adjudicating on the final decision, for this article. The other
authors declare that they have no competing interests.
References
|
1
|
GlobeNewswire by notified: Global
Medicinal Herbs Market Size, Trends, Company Profiles, Growth Rate,
Revenue, Demand and Forecast. GlobeNewswire, Inc., 2021. https://www.globenewswire.com/en/news-release/2021/02/16/2176036/0/en/Herbal-Medicine-Market-Global-Sales-Are-Expected-To-Reach-US-550-Billion-by-2030-as-stated-by-insightSLICE.html.
|
|
2
|
Joshi B, Sah GP, Basnet BB, Bhatt MR,
Sharma D, Subedi K, Pandey J and Malla R: Phytochemical extraction
and antimicrobial properties of different medicinal plants:
Ocimum sanctum (Tulsi), Eugenia caryophyllata
(Clove), Achyranthes bidentata (Datiwan) and Azadirachta
indica (Neem). J Microbiol Antimicrob. 3:1–7. 2011.
|
|
3
|
World Health Organization (WHO): WHO
Traditional Medicine Strategy: 2014-2023. WHO, Geneva, 2013.
https://apps.who.int/iris/handle/10665/92455.
|
|
4
|
Garg AK, Faheem M and Singh S: Role of
medicinal plant in human health disease. Asian J Plant Sci Res.
11:19–21. 2021.
|
|
5
|
Galloway WRJD, Isidro-Llobet A and Spring
DR: Diversity-oriented synthesis as a tool for the discovery of
novel biologically active small molecules. Nat Commun.
1(80)2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hopkins AL, Mason JS and Overington JP:
Can we rationally design promiscuous drugs? Curr Opin Struct Biol.
16:127–136. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ekor M: The growing use of herbal
medicines: Issues relating to adverse reactions and challenges in
monitoring safety. Front Pharmacol. 4(177)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sindhi V, Gupta V, Sharma K, Bhatnagar S,
Kumari R and Dhaka N: Potential applications of antioxidants-A
review. J Pharm Res. 7:828–835. 2013.
|
|
9
|
Kebede M and Admassu S: Application of
antioxidants in food processing industry: Options to improve the
extraction yields and market value of natural products. Adv Food
Technol Nutr Sci Open J. 5:38–49. 2019.
|
|
10
|
Taghvaei M and Jafari S: Application and
stability of natural antioxidants in edible oils in order to
substitute synthetic additives. J Food Sci Technol. 52:1272–1282.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Augustyniak A, Bartosz G, Cipak A, Duburs
G, Horáková L, Luczaj W, Majekova M, Odysseos AD, Rackova L,
Skrzydlewska E, et al: Natural and synthetic antioxidants: An
updated overview. Free Radic Res. 44:1216–1262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mattson MP: Dietary factors, hormesis and
health. Ageing Res Rev. 7:43–48. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mossa ATH and Nawwar GAM: Free radical
scavenging and antiacetylcholinesterase activities of Origanum
majorana L. essential oil. Hum Exp Toxicol. 30:1501–1513.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Veskoukis A, Kerasioti E, Priftis A, Kouka
P, Spanidis Y, Makri S and Kouretas D: A battery of translational
biomarkers for the assessment of the in vitro and in vivo
antioxidant action of plant polyphenolic compounds: The biomarker
issue. Curr Opin Toxicol. 13:99–109. 2019.
|
|
15
|
Kyriazis I, Skaperda Z, Tekos F, Makri S,
Vardakas P, Vassi E, Patouna A, Terizi K, Angelakis C and Kouretas
D: Methodology for the biofunctional assessment of honey (Review).
Int J Funct Nutr. 2:2634–7989. 2021.
|
|
16
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
LWT-Food Sci Technol. 28:25–30. 1995.
|
|
17
|
Kouka P, Priftis A, Stagos D, Angelis A,
Stathopoulos P, Xinos N, Skaltsounis AL, Mamoulakis C, Tsatsakis
AM, Spandidos DA and Kouretas D: Assessment of the antioxidant
activity of an olive oil total polyphenolic fraction and
hydroxytyrosol from a Greek Olea europea variety in endothelial
cells and myoblasts. Int J Mol Med. 40:703–712. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cano A: An end-point method for estimation
of the total antioxidant activity in plant material. Phytochem
Anal. 9:196–202. 1998.
|
|
19
|
Vardakas P, Skaperda Z, Tekos F, Trompeta
AF, Tsatsakis A, Charitidis CA and Kouretas D: An integrated
approach for assessing the in vitro and in vivo redox-related
effects of nanomaterials. Environ Res. 197(111083)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gülçin I, Küfrevioǧlu ÖI, Oktay M and
Büyükokuroǧlu ME: Antioxidant, antimicrobial, antiulcer and
analgesic activities of nettle (Urtica dioica L.). J
Ethnopharmacol. 90:205–215. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yen GC and Duh PD: Antioxidative
properties of methanolic extracts from peanut hulls. J Am Oil Chem
Soc. 70:383–386. 1993.
|
|
22
|
Hu C, Zhang Y and Kitts DD: Evaluation of
antioxidant and prooxidant activities of bamboo phyllostachys nigra
var. Henonis Leaf Extract in vitro. J Agric Food Chem.
48:3170–3176. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Priftis A, Stagos D, Konstantinopoulos K,
Tsitsimpikou C, Spandidos DA, Tsatsakis AM, Tzatzarakis MN and
Kouretas D: Comparison of antioxidant activity between green and
roasted coffee beans using molecular methods. Mol Med Rep.
12:7293–7302. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bal-Price A and Coecke S: Guidance on good
cell culture practice (GCCP). Neuromethods. 56:1–25. 2011.
|
|
25
|
Tiwari AK: Imbalance in antioxidant
defence and human diseases: Multiple approach of natural
antioxidants therapy. Curr Sci. 81:1179–1187. 2001.
|
|
26
|
Lakka A, Bozinou E, Makris DP and Lalas
SI: Evaluation of pulsed electric field polyphenol extraction from
vitis vinifera, sideritis scardica and crocus sativus.
ChemEngineering. 5(25)2021.
|
|
27
|
Khiya Z, Oualcadi Y, Gamar A, Berrekhis F,
Zair T and Hilali FEL: Correlation of total polyphenolic content
with antioxidant activity of hydromethanolic extract and their
fractions of the Salvia officinalis leaves from different
regions of Morocco. J Chem. 2021(8585313)2021.
|
|
28
|
Tekos F, Makri S, Skaperda ZV, Patouna A,
Terizi K, Kyriazis ID, Kotseridis Y, Mikropoulou EV, Papaefstathiou
G, Halabalaki M and Kouretas D: Assessment of antioxidant and
antimutagenic properties of red and white wine extracts in vitro.
Metabolites. 11(436)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cai Y, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carneiro de Siqueira K, Garcia LF, Lobón
GS, Thomaza DV, Moreno EKG, de Carvalho MF, Rocha ML, dos Santos
WTP and de Souza Gil E: Antioxidant activity evaluation of dried
herbal extracts: An electroanalytical approach. Rev Bras Farmacogn.
28:325–332. 2018.
|
|
31
|
Santos-Sánchez NF, Salas-Coronado R,
Villanueva-Cañongo C and Beatriz HC: Antioxidant Compounds and
Their Antioxidant Mechanism. IntechOpen, London, 2019. https://www.intechopen.com/chapters/66259. Accessed
March 22, 2019.
|
|
32
|
Zegarac JP, Zulj LV, Stipčević T and
Martinez S: Electrochemical determination of antioxidant capacity
of fruit tea infusions. Food Chem. 121:820–825. 2010.
|
|
33
|
Oliveira-Neto JR, Rezende SG, de Fátima
Reis C, Benjamin SR, Rocha ML and de Souza Gil E: Electrochemical
behavior and determination of major phenolic antioxidants in
selected coffee samples. Food Chem. 190:506–512. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Robak J and Gryglewski RJ: Flavonoids are
scavengers of superoxide anions. Biochem Pharmacol. 37:837–841.
1988.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Korycka-Dahl M and Richardson T:
Photogeneration of superoxide anion in serum of bovine milk and in
model systems containing riboflavin and amino acids. J Dairy Sci.
61:400–407. 1978.
|
|
36
|
Ighodaro OM and Akinloye OA: First line
defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alexandria J Med. 54:287–293. 2018.
|
|
37
|
Yildirim A, Mavi A, Oktay M, Kara AA,
Algur OF and Bilaloglu V: Comparison of antioxidant and
antimicrobial activities of tilia (Tilia argentea Desf ex
DC), sage (Salvia triloba L.), and black tea
(Camellia sinensis) extracts. J Agric Food Chem.
48:5030–5034. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Komaki A, Hoseini F, Shahidi S and
Baharlouei N: Study of the effect of extract of Thymus
vulgaris on anxiety in male rats. J Tradit Complement Med.
6:257–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Melidou M, Riganakos K and Galaris D:
Protection against nuclear DNA damage offered by flavonoids in
cells exposed to hydrogen peroxide: The role of iron chelation.
Free Radic Biol Med. 39:1591–1600. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ngo SNT, Williams DB and Head RJ: Rosemary
and cancer prevention: Preclinical perspectives. Crit Rev Food Sci
Nutr. 51:946–954. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hrnčič MK, Cör D, Simonovska J, Knez Ž,
Kavrakovski Z and Rafajlovska V: Extraction techniques and
analytical methods for characterization of active compounds in
origanum species. Molecules. 25(4735)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Coccimiglio J, Alipour M, Jiang ZH,
Gottardo C and Suntres Z: Antioxidant, antibacterial, and cytotoxic
activities of the ethanolic Origanum vulgare extract and its
major constituents. Oxid Med Cell Longev.
2016(1404505)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bilia AR, Giomi M, Innocenti M, Gallori S
and Vincieri FF: HPLC-DAD-ESI-MS analysis of the constituents of
aqueous preparations of verbena and lemon verbena and evaluation of
the antioxidant activity. J Pharm Biomed Anal. 46:463–470.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Casanova E, García-Mina JM and Calvo MI:
Antioxidant and antifungal activity of Verbena officinalis L.
leaves. Plant Foods Hum Nutr. 63:93–97. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Grzegorczyk I, Matkowski A and Wysokińska
H: Antioxidant activity of extracts from in vitro cultures of
Salvia officinalis L. Food Chem. 104:536–541. 2007.
|
|
46
|
Miura K, Kikuzaki H and Nakatani N:
Antioxidant activity of chemical components from sage (Salvia
officinalis L.) and Thyme (Thymus vulgaris L.) measured
by the oil stability index method. J Agric Food Chem. 50:1845–1851.
2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rašković A, Milanović I, Pavlović N,
Ćebović T, Vukmirović S and Mikov M: Antioxidant activity of
rosemary (Rosmarinus officinalis L.) essential oil and its
hepatoprotective potential. BMC Complement Altern Med.
14(225)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Panieri E, Buha A, Telkoparan-Akillilar P,
Cevik D, Kouretas D, Veskoukis A, Skaperda Z, Tsatsakis A, Wallace
D, Suzen S and Saso L: Potential applications of NRF2 modulators in
cancer therapy. Antioxidants (Basel). 9(193)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kang SW, Lee S and Lee EK: ROS and energy
metabolism in cancer cells: Alliance for fast growth. Arch
Pharmacal Res. 38:338–345. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Skaperda Z, Tekos F, Makri S, Angelakis C,
Vassi E, Vardakas P, Patouna A, Terizi K, Kyriazi D and Kouretas D:
A novel combined bioactivity/chemoactivity holistic approach for
the evaluation of dietary supplements. Food Chem Toxicol.
152(112159)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mileo A and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016(6475624)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Aquilano K, Baldelli S and Ciriolo MR:
Glutathione: New roles in redox signaling for an old antioxidant.
Front Pharmacol. 5(196)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Elliott SJ and Koliwad SK: Redox control
of ion channel activity in vascular endothelial cells by
glutathione. Microcirculation. 4:341–347. 1997.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Espinosa-Díez C, Miguel V, Vallejo S,
Sánchez FJ, Sandoval E, Blanco E, Cannata P, Peiró C,
Sánchez-Ferrer CF and Lamas S: Role of glutathione biosynthesis in
endothelial dysfunction and fibrosis. Redox Biol. 14:88–99.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Folkman J and Camphausen K: What does
radiotherapy do to endothelial cells? Science. 293:227–228.
2001.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rak JW, St Croix BD and Kerbel RS:
Consequences of angiogenesis for tumor progression, metastasis and
cancer therapy. Anticancer Drugs. 6:3–18. 1995.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bussolati B, Deregibus MC and Camussi G:
Characterization of molecular and functional alterations of tumor
endothelial cells to design anti-angiogenic strategies. Curr Vasc
Pharmacol. 8:220–232. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vardar-Unlü G, Candan F, Sökmen A,
Daferera D, Polissiou M, Sökmen M, Dönmez E and Tepe B:
Antimicrobial and antioxidant activity of the essential oil and
methanol extracts of Thymus pectinatus Fisch. et Mey. Var.
pectinatus (Lamiaceae). J Agric Food Chem. 51:63–67.
2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bozin B, Mimica-Dukic N, Simin N and
Anackov G: Characterization of the volatile composition of
essential oils of some lamiaceae spices and the antimicrobial and
antioxidant activities of the entire oils. J Agric Food Chem.
54:1822–1828. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ündeğer Ü, Başaran A, Degen GH and Başaran
N: Antioxidant activities of major thyme ingredients and lack of
(oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells
at low levels of carvacrol and thymol. Food Chem Toxicol.
47:2037–2043. 2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bhakkiyalakshmi E, Suganya N, Sireesh D,
Krishnamurthi K, Saravana Devi S, Rajaguru P and Ramkumar KM:
Carvacrol induces mitochondria-mediated apoptosis in HL-60
promyelocytic and Jurkat T lymphoma cells. Eur J Pharmacol.
772:92–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Spyridopoulou K, Fitsiou E, Bouloukosta E,
Tiptiri-Kourpeti A, Vamvakias M, Oreopoulou A, Papavassilopoulou E,
Pappa A and Chlichlia K: Extraction, chemical composition, and
anticancer potential of Origanum onites L. essential oil.
Molecules. 24(2612)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liou G and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Tayarani-Najaran Z, Asili J, Aioubi E and
Emami SA: Growth inhibition and apoptosis induction of salvia
chloroleuca on MCF-7 breast cancer cell line. Iran J Pharm Res.
12:789–799. 2013.PubMed/NCBI
|
|
66
|
Kozics K, Klusová V, Srančíková A, Mučaji
P, Slameňová D, Hunáková L, Kusznierewicz B and Horváthová E:
Effects of Salvia officinalis and Thymus vulgaris on
oxidant-induced DNA damage and antioxidant status in HepG2 cells.
Food Chem. 141:2198–2206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Scholey AB, Tildesley NTJ, Ballard CG,
Wesnes KA, Tasker A, Perry EK and Kennedy DO: An extract of Salvia
(sage) with anticholinesterase properties improves memory and
attention in healthy older volunteers. Psychopharmacology (Berl).
198:127–139. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Carrera-Quintanar L, Funes L, Viudes E,
Tur J, Micol V, Roche E and Pons A: Antioxidant effect of lemon
verbena extracts in lymphocytes of university students performing
aerobic training program. Scand J Med Sci Sports. 22:454–461.
2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Fitsiou E, Mitropoulou G, Spyridopoulou K,
Vamvakias M, Bardouki H, Galanis A, Chlichlia K, Kourkoutas Y,
Panayiotidis MΙ and Pappa A: Chemical composition and evaluation of
the biological properties of the essential oil of the dietary
phytochemical lippia citriodora. Molecules. 23(123)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Malekirad A, Hosseini N and Bayrami M:
Benefit of lemon verbena in healthy subjects; Targeting diseases
associated with oxidative StRESS. Asian J Anim Vet Adv. 6:953–957.
2011.
|
|
71
|
Tagde A, Singh H, Kang MH and Reynolds CP:
The glutathione synthesis inhibitor buthionine sulfoximine
synergistically enhanced melphalan activity against preclinical
models of multiple myeloma. Blood Cancer J. 4(e229)2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rodman SN, Spence JM, Ronnfeldt TJ, Zhu Y,
Solst SR, O'Neill RA, Allen BG, Guan X, Spitz DR and Fath MA:
Enhancement of radiation response in breast cancer stem cells by
inhibition of thioredoxin- and glutathione-dependent metabolism.
Radiat Res. 186:385–395. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Petiwala SM, Berhe S, Li G, Puthenveetil
AG, Rahman O, Nonn L and Johnson JJ: Rosemary (Rosmarinus
officinalis) extract modulates CHOP/GADD153 to promote androgen
receptor degradation and decreases xenograft tumor growth. PLoS
One. 9(e89772)2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Olivares A, Alcaraz-Saura M, Achel DG and
Alcaraz M: Effect of rosmarinic acid and ionizing radiation on
glutathione in melanoma B16F10 cells: A translational opportunity.
Antioxidants (Basel). 9(1291)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhong Z, Qiang WW, Tan W, Zhang H, Wang S,
Wang C, Qiang W and Wang Y: Chinese herbs interfering with cancer
reprogramming metabolism. Evid Based Complement Alternat Med.
2016(9282813)2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Karydas C, Iatrou M, Kouretas D, Patouna
A, Iatrou G, Lazos N, Gewehr S, Tseni X, Tekos F, Zartaloudis Z, et
al: Prediction of antioxidant activity of cherry fruits from UAS
multispectral imagery using machine learning. Antioxidants (Basel).
9(156)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Dorman HJD, Koşar M, Kahlos K, Holm Y and
Hiltunen R: Antioxidant properties and composition of aqueous
extracts from Mentha species, hybrids, varieties, and
cultivars. J Agric Food Chem. 51:4563–4569. 2003.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mantle D, Eddeb F and Pickering AT:
Comparison of relative antioxidant activities of British medicinal
plant species in vitro. J Ethnopharmacol. 72:47–51. 2000.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Matkowski A and Piotrowska M: Antioxidant
and free radical scavenging activities of some medicinal plants
from the Lamiaceae. Fitoterapia. 77:346–353. 2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Aruoma O: Methodological considerations
for characterizing potential antioxidant actions of bioactive
components in plant foods. Mutat Res. 523-524:9–20. 2003.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fatima N and Nayeem N: Toxic Effects as a
Result of Herbal Medicine Intake. IntechOpen, London, 2016.
https://www.intechopen.com/chapters/51762. Accessed
October 26, 2016.
|