Introduction
Vitamin D3 (VD3) is produced by the photochemical
conversion of 7-dehydrocholesterol, which is synthesized from
acetyl-CoA produced in the tricarboxylic acid cycle by the
ultraviolet radiation of B energy in the epidermis (1-4).
Conversely, VD3 of food origin is absorbed in the small intestine
with other dietary fats; however, the percentage of VD3 from
dietary sources in the body is low, mostly due to homeostatic
synthesis (5). VD3 is hydroxylated
in the liver to 25-hydroxy vitamin D3 [25(OH)D], which leaks into
the blood and is hydroxylated in the kidneys, and 25(OH)D then
becomes 1,25(OH)2D as active VD3 (3,6).
Active VD3 is also generated by the 25-hydroxyvitamin D-1 alpha
hydroxylase, mitochondrial, which is encoded by the mitochondrial
cytochrome P450 family 27 subfamily B member 1 (CYP27B1)
gene; therefore, cells expressing CYP27B1 can produce active
VD3(7). The active form of VD3
binds to the vitamin D receptor (VDR), a known nuclear receptor,
and binds upstream of specific gene sequences in the genomic DNA as
a transcriptional regulator to control the transcription of
downstream genes (8-10).
The oral administration of VD3 supplements has been
shown to increase pregnancy rates (11). Although this supplementation was
originally considered to affect fertilized eggs, VD3
supplementation has been reported to increase homeobox A10
(HOXA10), an indicator of endometrial embryonic receptivity
(12). HOXA10 functions as a
regulator of endometrial development and decidualization (13) and as a transcriptional regulator of
CYP27B1 (14). Human
endometrial decidualization is caused by elevated blood
progesterone levels following ovulation (15). In the secretory phase, normal
progesterone delivery to the endometrium causes the decidualization
of endometrial stromal cells (EnSCs) via the progesterone receptor
(PGR). First, the upregulation of heart and neural crest
derivatives-expressed transcript 2 (HAND2) and forkhead box
O1 (FOXO1) (whose proteins are pivotal transcription factors
that promote the decidualization of human EnSCs as an upstream of
progesterone signaling) (16),
occurs during the decidualization of EnSCs (17,18).
Subsequently, insulin-like growth factor binding protein 1
(IGFBP1), prolactin (PRL), interleukin (IL)15)
and other genes are initiated during their transcriptions in
decidual EnSCs by HAND2 and FOXO1(15). Translated and secreted PRL
regulates extravillous trophoblast (EVT) growth and invasion and,
in concert with IL-15, is involved in the functions of
uterine-specific natural killer (uNK) cells. uNK cells, in concert
with EnSCs, promote spiral artery remodeling, which further
promotes endometrial decidualization (16). In addition, uNK cells play a
critical role in immune tolerance, which is essential for embryonic
receptivity (19). Moreover,
IGFBP1 promotes the migration of embryo-derived EVTs, contributing
to placentation (16).
Abnormalities in EnSC decidualization are known to cause
preeclampsia, miscarriage implantation and fetal growth failures,
as well as placenta accreta (20),
EnSC decidualization is critical for the normal development of the
fetus in utero.
The involvement of VD3 in endometrial function,
i.e., embryo implantation via decidualization, has been suggested;
however, the mechanisms involved remain unclear. Therefore, the
present study examined the effects of VD3 on endometrial function,
particularly in EnSC decidualization, using a human EnSC line.
Materials and methods
Culture of the EnSC KCO2-44D cell line
and human choriocarcinoma BeWO cell line
Human EnSC KC02-44D cells (cat. no. SC-6000)
(CVCL_E224) (21) and human
choriocarcinoma BeWO cells (cat. no. JCRB9111) (RRID: CVCL_0044)
(22) (which has been used as an
EVT model) (23) were obtained
from the American Type Culture Collection (ATCC) and the JCRB cell
bank (Osaka, Japan), respectively. The KC02-44D and BeWO cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with phenol
red (Life Technologies; Thermo Fisher Scientific, Inc.) containing
100 unit/ml penicillin (Nacalai Tesque, Inc.), 100 µg/ml
streptomycin (Nacalai Tesque, Inc.), 10 mM HEPES (pH 7.4) (Life
Technologies; Thermo Fisher Scientific, Inc.) and 10% fetal bovine
serum (FBS, Global Life Sciences Technologies Japan K.K.; Cytiva)
and Ham's F12 (Life Technologies; Thermo Fisher Scientific, Inc.)
containing 100 U/ml penicillin (Nacalai Tesque, Inc.), 100 µg/ml
streptomycin (Nacalai Tesque, Inc.), 10 mM HEPES (pH 7.4) (Life
Technologies; Thermo Fisher Scientific, Inc.) and 15% FBS (Global
Life Sciences Technologies Japan K.K.; Cytiva) at 37˚C and 5%
CO2.
Decidualization and VD3 treatment of
KC02-44D cells
The KC02-44D cells were seeded in 24-well plates
(Corning, Inc.) until reaching confluency (0.4x106 cells
per well) and then stimulated as described below. As phenol red is
an estrogen-like agonist, phenol red-free DMEM (Life Technologies;
Thermo Fisher Scientific, Inc.) containing 10% charcoal-stripped
(CS)-FBS (activated charcoal was used to adsorb and remove other
hormones in the serum), 10 mM HEPES (pH 7.4) (Life Technologies;
Thermo Fisher Scientific, Inc.), 100 unit/ml penicillin (Nacalai
Tesque, Inc.), 100 µg/ml streptomycin (Nacalai Tesque, Inc.) and 1%
GlutaMAX (Life Technologies; Thermo Fisher Scientific, Inc.) was
used as the control medium. The control group was cultured in the
aforementioned medium; the VD3-treated group was cultured in the
aforementioned medium with 10 nM VD3 (25-hydroxy vitamin D3, Cayman
Chemical Co.); the decidualization-treated group was cultured in
the aforementioned medium with 10-8 M estradiol
(MilliporeSigma), 10-6 M medroxyprogesterone acetate
(MPA; MilliporeSigma), an analog of progesterone and 0.5 mM
8-Bromo-cAMP (MilliporeSigma), a cell-permeable analog of cAMP that
activates cyclic-AMP-dependent protein kinase and promotes
decidualization; the decidualization + VD3 treatment group was
cultured in the aforementioned medium with 0.5 mM 8-Bromo-cAMP,
10-8 M estradiol, 10-6 M MPA and 10 nM VD3.
These stimuli were performed in triplicate, and samples were
ultimately prepared for 8-9 wells per group.
Extraction of total RNA, and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the KC02-44D cells
(0.4x106 cells) cultured for 6 days with
Sepasol®-RNA I Super G (Nacalai Tesque, Inc.). ReverTra
Ace® qPCR RT Master Mix with gDNA Remover (Toyobo Co.)
was used for the reverse transcription of total RNA into cDNA. qPCR
was conducted with cDNA, Thunderbird SYBR Next qPCR mix (Toyobo
Co.), and primers using a Light Cycler96 (Roche Diagnostics). For
PCR, following pre-incubation (95˚C, 30 sec), 45 cycles of two-step
amplification (95˚C, 5 sec; 60˚C, 30 sec) were conducted, followed
by a melting reaction to confirm the primer specificity. The gene
names and primer sequences used are listed in Table I. A Primer3Plus web interface was
used for primer design (24). As a
housekeeping gene, hypoxanthine phosphoribosyltransferase 1
(HPRT1) was used and the relative expression levels were
calculated from the threshold cycle (Cq) values of each gene from
each sample using the 2-ΔΔCq method (25).
 | Table ISequences of the primers used in the
present study. |
Table I
Sequences of the primers used in the
present study.
| Gene symbol | Definition | Position | Sequence |
|---|
| HPRT1 | Hypoxanthine | 895F |
5'-CTAGTTCTGTGGCCATCTGCTTAG-3' |
| |
phosphoribosyltransferase 1 | 1034R |
5'-GGGAACTGATAGTCTATAGGCTCATAGTG-3' |
| VDR | Vitamin D
receptor | 695F |
5'-TGACCTGGTCAGTTACAGCATC-3' |
| | | 829R |
5'-TTGGAGCGCAACATGATGAC-3' |
| CYP27B1 | Cytochrome P450
family | 594F |
5'-TGGCGGGGGAATTTTACAAG-3' |
| | 27 subfamily B
member 1 | 740R |
5'-TCAACAGCGTGGACACAAAC-3' |
| ESR1 | Estrogen receptor
1 | 1514F |
5'-TGCTGGCTACATCATCTCGGT-3' |
| | | 1665R |
5'-GACTCGGTGGATATGGTCCTTC-3' |
| ESR2 | Estrogen receptor
2 | 617F |
5'-CTAACTTGGAAGGTGGGCCTG-3' |
| | | 767R |
5'-AGCGATCTTGCTTCACACCA-3' |
| PGR | Progesterone
receptor | 2484F |
5'-CCTTTGGAAGGGCTACGAAGT-3' |
| | | 2593R |
5'-GAGCTCGACACAACTCCTTTTTG-3' |
| PRL | Prolactin | 374F |
5'-ATTCGATAAACGGTATACCCATGGC-3' |
| | | 623R |
5'-TTGCTCCTCAATCTCTACAGCTTTG-3' |
| IGFBP1 | Insulin-like growth
factor | 636F |
5'-CTATGATGGCTCGAAGGCTC-3' |
| | binding protein
1 | 791R |
5'-TTCTTGTTGCAGTTTGGCAG-3' |
| IL15 | Interleukin 15 | 165F |
5'-GTTCACCCCAGTTGCAAAGT-3' |
| | | 351R |
5'-CCTCCAGTTCCTCACATTC-3' |
| HAND2 | Heart and Neural
Crest | 1479F |
5'-AGAGGAAGAAGGAGCTGAACGA-3' |
| | Derivatives
expressed 2 | 1552R |
5'-CGTCCGGCCTTTGGTTTT-3' |
| FOXO1 | Forkhead box
protein O1 | 2879F |
5'-TGTTTTCTGCGGAACTGACG-3' |
| | | 2970R |
5'-TTCTGTGGCAACGTGAACAG-3' |
| HOXA10 | Homeobox A10 | 963F |
5'-GATTCCCTGGGCAATTCCAAAG-3' |
| | | 1083R |
5'-ACAGAAACTCCTTCTCCAGCTC-3' |
Cell invasion assay
Until reaching 85-90% confluency, the KC02-44D cells
were cultured in the bottom part of 24-well plates (Corning, Inc.).
The control, decidualization-treated and decidualization + VD3
treatment groups were stimulated for 6 days as described above, and
three wells were prepared for each group. After 6 days, the insert
in the BioCoat Matrigel Invasion Chamber (Corning, Inc.) was
hydrated, and 50,000 BeWO cells were incubated at 37˚C and 5%
CO2 for 24 h. After 24 h, the BeWO cells that had
infiltrated the bottom of the filter were stained using Diff-Quick
(Sysmex Corporation), and the number of stained cells was counted
using an inverted microscope (Eclipse Ts2-FL, Nikon Corporation)
and MicroStudio software (version x64, 1.5.18608.20210313, Wraymer,
Inc,). Finally, the infiltration frequency per unit area was
calculated.
Statistical analysis
After confirming the normality of each group by
performing the Shapiro-Wilk test on the data obtained for each
group, a two-tailed Welch's unpaired t-test was used to estimate
the difference between the means of the two groups. The Bonferroni
correction was then performed to avoid a type 1 error according to
multiple testing. The IBM SPSS Statistics software (version 29.0;
IBM Corp., Inc.) was used for statistical analyses. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Reactivity of the KC02-44D cell line
against VD3
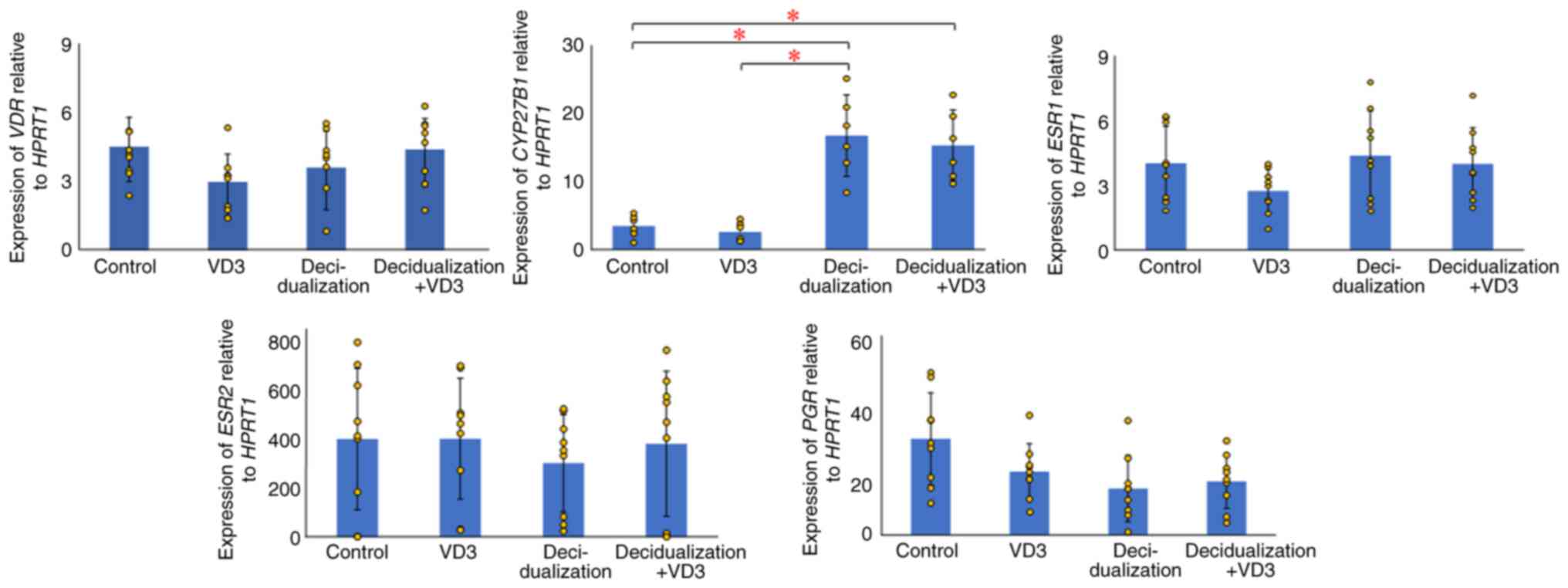
The present study examined the expression of
VDR, whose protein affects cellular function by binding to
active VD3 in KC02-44D cells. Although VD3 expression was
found in KC02-44D cells, no significant differences were observed
among the VD3-(P=0.398) and decidualization-treated groups (P=0.366
and 0.641) compared with the control group (Fig. 1). CYP27B1 expression was
also examined; the protein converts VD3 to its active form, and it
was found that CYP27B1 expression was significantly elevated
in the decidualization-treated groups compared with the control
group (P=0.014 and 0.009) (Fig.
1). This indicates that EnSCs locally produce active VD3 during
decidualization, suggesting the need for active VD3 in
decidualization and the regulation of VDR target gene expression in
EnSCs.
Effects of VD3 on the decidualization
of EnSCs
The present study examined the changes due to the
effects of VD3 by adding 100 µM inactive VD3 to decidualized
KC02-44D cells using RT-qPCR. The results revealed no significant
differences in either VDR (P=0.281) or CYP27B1 (P=0.478)
between the decidualization and decidualization + VD3 treatment
groups (Fig. 1). Similar to
VDR, no significant differences were found in the nuclear
receptors, such as estrogen receptor 1 (ESR1) in the
VD3-(P=0.075) and decidualization-treated groups (P=0.692 and
0.975), ESR2 in the VD3-(P=0.986) and
decidualization-treated groups (P=0.415 and 0.894), and PGR
in the VD3-(P=0.091) and decidualization-treated groups (P=0.019
and 0.032), compared with the control.
The PRL levels were not significantly altered
in the VD3-treated group compared with the control group (P=0.371);
however, a significant increase was found between the
decidualization-treated (P=0.000007) and decidualization + VD3
treatment groups (P=0.000001), and the control, as well as between
the decidualization-treated group and VD3-treated group
(P=0.000007) or decidualization + VD3 treatment groups (P=0.003)
(Fig. 2). IGFBP1 expression
was significantly elevated in the decidualization (P=0.00001) and
decidualization + VD3 treatment groups (P=0.000001; P<0.05)
compared with the control, although there was no significant
difference between the VD3-treated group and the control group
(P=0.075) (Fig. 2). There were no
significant differences in IGFBP1 expression between the
decidualization and decidualization + VD3 treatment groups
(P=0.282) (Fig. 2). The results
also revealed that the expression of IL15 was significantly
increased in the decidualization (P=0.001) and decidualization +
VD3 treatment groups (P=0.001) compared with the control, although
there was no significant difference between the VD3-treated group
and the control group (P=0.489; P<0.05) (Fig. 2). There were no significant
differences in the expression of IL15 between the
decidualization and decidualization + VD3 groups (P=0.564).
HAND2 expression was significantly increased in the
decidualization-treated (P=0.005) and decidualization + VD3
treatment groups (P=0.0002) compared with the control, although
there was no significant difference between the VD3-treated and
control group (P=0.032) (Fig. 2).
By contrast, the addition of VD3 during decidualization
significantly increased HAND2 expression compared with the
decidualization group (P=0.004) (Fig.
2). FOXO1 expression was significantly elevated in the
decidualization (P=7.69541E-05) and decidualization + VD3 treatment
groups (P=1.76083E-05) compared with the control, although there
was no significant difference in the VD3-treated group compared
with the control group (P=0.538) (Fig.
2). There were no significant differences in FOXO1
expression between the decidualization and decidualization + VD3
treatment groups (P=0.659). As regards HOXA10, there was no
significant difference in HOXA10 expression between the
control and VD3-treated groups (P=0.607); however, HOXA10
expression was significantly upregulated in the
decidualization-treated (P=0.003) and decidualization + VD3
treatment groups compared with the control (P=0.001) (Fig. 2). There were no significant
differences in HOXA10 expression between the decidualization
and decidualization + VD3 treatment groups (P=0.873).
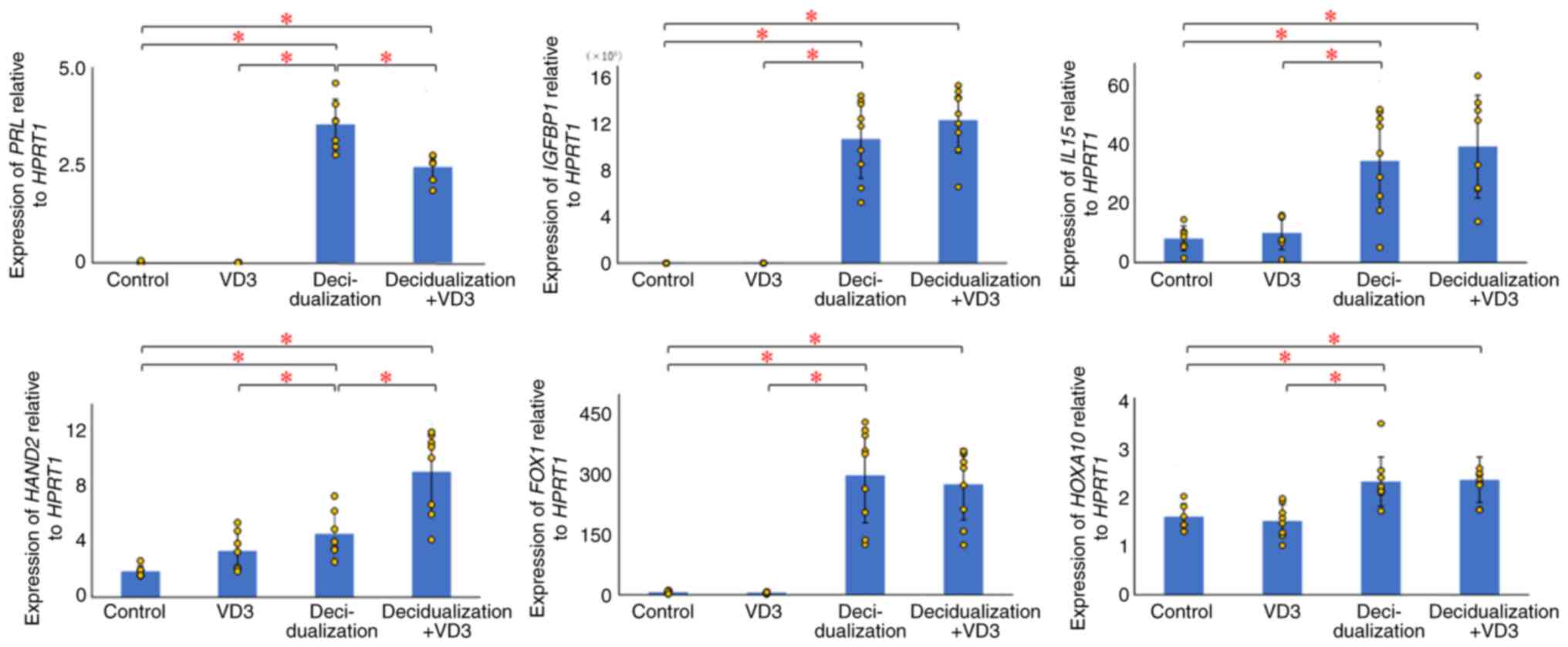 | Figure 2Effects of VD3 on decidualization
markers in KC02-44D cells. The values for each group are presented
as bars (mean) and error bars (standard deviation). The significant
upregulation of PRL, IGFBP1, IL15,
HAND2, FOXO1 and HOXA10 was observed in the
decidualized KC02-44D cells. Additional VD3 stimulation affected
PRL and HAND2 in the decidualized KC02-44D cells.
*P<0.05, indicates significant differences between
groups using Welch's t-test with the Bonferroni correction. VD3,
vitamin D3; HPRT1, hypoxanthine phosphoribosyltransferase 1;
PRL, prolactin; IGFBP1, insulin-like growth
factor-binding protein 1; IL15, interleukin 15;
HAND2, heart and neural crest derivatives expressed 2;
FOXO1, forkhead box protein O1; HOXA10, homeobox
A10. |
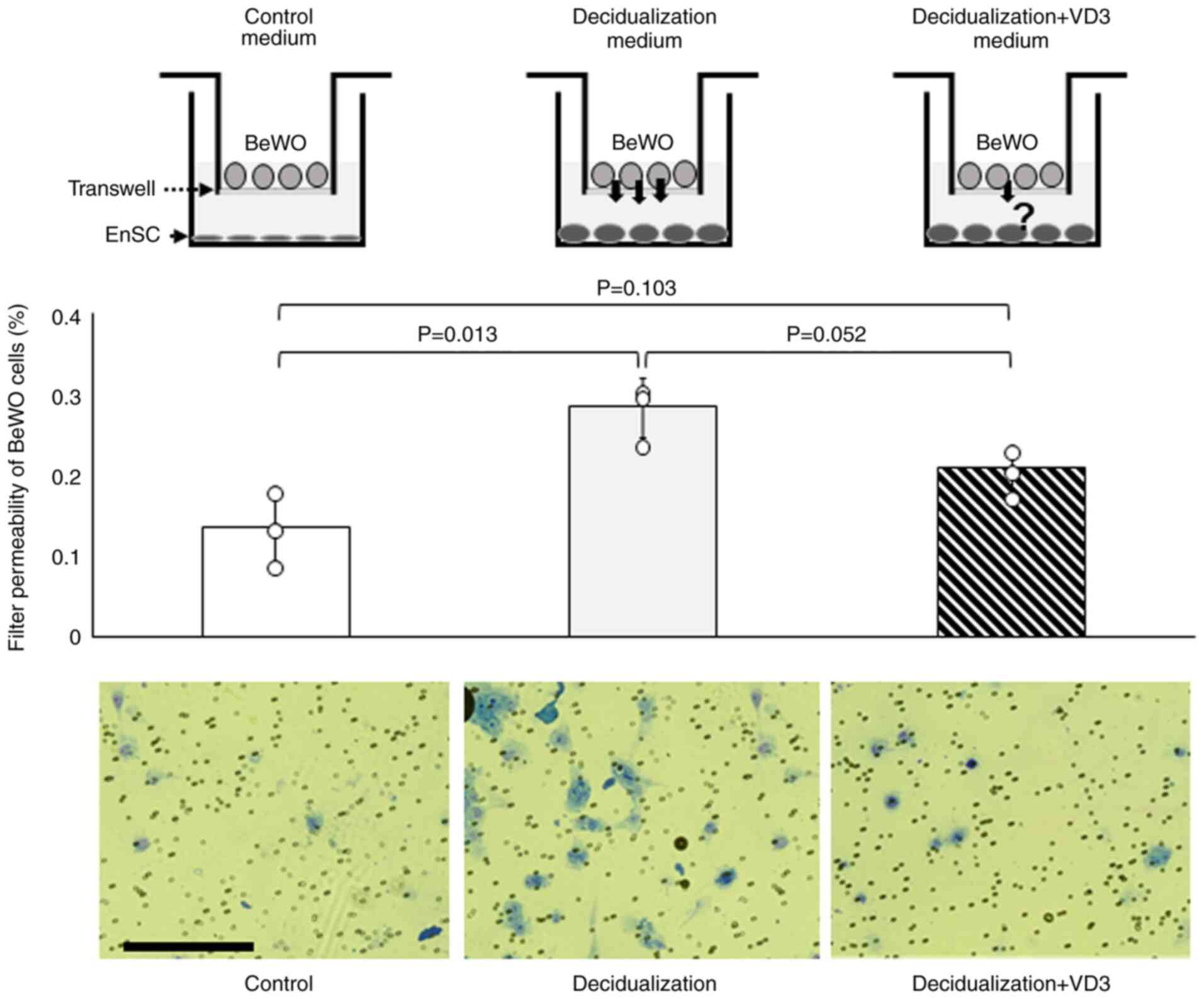
VD3 decreases the invasive capacity of
EVTs
Following implantation, placentation occurs as the
EVTs invade the decidua of the endometrium. The present study then
performed an invasion assay to examine the effects of VD3 on the
invasive ability of the human choriocarcinoma cell line, BeWO, with
or without decidualization and VD3. The results revealed that the
invasive ability of BeWO cells was significantly increased in
decidualization-conditioned medium with KC02-44D cells compared to
that in the control medium (P=0.013) (Fig. 3), whereas no difference was
observed in the decidualization + VD3-added medium compared with
the control medium (P=0.103) (Fig.
3).
Discussion
In the present study, an increase in HOXA10
expression and a subsequent increase in CYP27B1 (7) expression during decidualization in
KC02-44D cells, an EnSC line, were observed. The activation of VD3
by the CYP27B1 enzyme is considered to facilitate the translocation
of VDR into the nucleus and cause changes in its target gene
expression during decidualization. Indeed, the observed
upregulation of HAND2 and downregulation of PRL upon
the addition of VD3 during decidualization suggests that these
genes may be transcriptionally regulated, either directly or
indirectly, by the VD3-VDR complex. The VD3-VDR complex may also be
involved in EVT invasiveness via PRL by VD3, as observed in
the invasion assay herein.
HAND2 is a master regulator that acts upstream of
progesterone signaling and promotes the establishment of pregnancy
as a key to decidualization (15,26).
The addition of VD3 during decidualization significantly increased
HAND2 expression, suggesting that the VDR activated by VD3
binding cooperates with the PGR to regulate HAND2
transcription, an essential function for decidualization. The
authors manually searched for the VDR binding motif
[-AGGGTCA-GAGTTC(-GTTGGT-AGAGAGGG)] (27) in the 2k-basepairs upstream region
of HAND2 gene (ACC no. NC_000004.12; Homo sapiens
chromosome 4, GRCh38.p14 Primary Assembly, from 173524091 to
173530229, 2024/04/15 version). Consequently, a VDR binding
candidate motif (GGGTCA) was found at position-562/-556 from the
transcription start site, as well as another candidate VDR-binding
motif (GAGTTC) at -1493/-1488. As a limitation, changes in HAND2
protein levels were not evaluated in the present study, as the
antibodies used in a previous study by the authors (goat dHAND
antibody (M-19), cat. no. sc-9409; Santa Cruz Biotechnology, Inc.,
Dallas, TX) (18) are no longer
available, and no other suitable antibodies have been found since
then. Additionally, only a candidate binding sequence was found,
and further functional analysis are thus necessary to confirm the
details of the regulation of HAND2 expression by the VDR.
Furthermore, the epigenetic changes in the HAND2 promoter
region need to be determined, since the VDR-binding sequence in the
vicinity of the HAND2 promoter region may become a
euchromatin region due to decidualization, and gene expression may
be actively underway.
HAND2 is known to be an upregulator of PRL
expression (28), which is
inconsistent with the present results showing HAND2
upregulation but PRL downregulation. Additionally, given
that no VDR-binding candidate motif was found in the PRL
promoter region, it may be necessary to consider other factors
regulated by the VDR in the regulation of PRL expression
during decidualization.
PRL is an indicator of EnSC decidualization,
and the action of PRL in the endometrial microenvironment
stimulates EVT functions, prevents the rejection of embryos,
regulates the survival of uNK cells and facilitates angiogenesis
(16). Elevated blood levels of
PRL inhibit the secretion of gonadotropin-releasing hormone from
the hypothalamus and luteinizing hormone from the pituitary gland
and suppress ESR1 expression in the pituitary gland, causing
hypogonadotropic hypogonadism with amenorrhea (29). In the ovary, elevated blood levels
of PRL cause anovulation (30),
suppress follicle maturation and lead to inadequate corpus luteum
formation, with decreased luteinizing hormone receptor affinity in
the corpus luteum and concomitant decreased progesterone production
and secretion (30). In the
uterus, hyperprolactinemia has been implicated in
hyperproliferative myoma (31), as
well as endometriosis and consequent infertility (30). Taken together, the findings
presented herein suggest that VD3 may prevent endometriosis and
uterine fibroids owing to excess PRL in the endometrial
microenvironment by decreasing PRL expression.
The present study found that VD3 regulates
HAND2 expression, the master regulator of decidualization,
and PRL, which is critical for the uterine microenvironment
in decidualization. In light of the effects on PRL in the
present study, further research is required to decide the optimal
timing of VD3 supplementation. By contrast, in patients with
cellular tumor antigen p53-positive gastrointestinal cancers,
vitamin D supplementation has been shown to reduce the risk of
recurrence/mortality (32). In
addition, nutritional approaches, including VD3 for the management
of gynecological cancers molecularly classified by polymerase
epsilon and cellular tumor antigen p53, particularly endometrial
and ovarian cancers (33), may
become useful.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Takeda Science
Foundation (2018) and the Yamaguchi Endocrine Research Foundation
(2024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST conceptualized the study and was involved in the
study methodology. ST also provided the methodology, research
environment and reagents, etc., supervised the study, and was also
involved in project administration and in funding acquisition. NY,
KT and ST were involved in data validation and data curation, as
well as in the writing, review and editing of the manuscript and in
the preparation of the figures. NY, KT and AT were involved in the
formal analysis and in the investigative aspects of the study. NY
and ST were involved in the writing and preparation of the original
draft of the manuscript. NY and ST confirm the authenticity of all
the raw data. All authors have read and agreed to the published
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bär M, Domaschke D, Meye A, Lehmann B and
Meurer M: Wavelength-dependent induction of CYP24A1-mRNA after
UVB-triggered calcitriol synthesis in cultured human keratinocytes.
J Invest Dermatol. 127:206–213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bikle DD, Nemanic MK, Gee E and Elias P:
1,25-Dihydroxyvitamin D3 production by human keratinocytes.
Kinetics and regulation. J Clin Invest. 78:557–566. 1986.PubMed/NCBI View Article : Google Scholar
|
|
3
|
DeLuca HF: Overview of general physiologic
features and functions of vitamin D. Am J Clin Nutr. 80 (Suppl
6):1689S–1696S. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Holick MF and Clark MB: The
photobiogenesis and metabolism of vitamin D. Fed Proc.
37:2567–2574. 1978.PubMed/NCBI
|
|
5
|
Haddad JG, Matsuoka LY, Hollis BW, Hu YZ
and Wortsman J: Human plasma transport of vitamin D after its
endogenous synthesis. J Clin Invest. 91:2552–2555. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dennis C, Dillon J, Cohen DJ, Halquist MS,
Pearcy AC, Schwartz Z and Boyan BD: Local production of active
vitamin D3 metabolites in breast cancer cells by CYP24A1
and CYP27B1. J Steroid Biochem Mol Biol. 232(106331)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jeon SM and Shin EA: Exploring vitamin D
metabolism and function in cancer. Exp Mol Med. 50:1–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pike JW and Meyer MB: The vitamin D
receptor: New paradigms for the regulation of gene expression by
1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am.
39:255–269. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vanhevel J, Verlinden L, Doms S, Wildiers
H and Verstuyf A: The role of vitamin D in breast cancer risk and
progression. Endocr Relat Cancer. 29:R33–R55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chu J, Gallos I, Tobias A, Robinson L,
Kirkman-Brown J, Dhillon-Smith R, Harb H, Eapen A, Rajkhowa M and
Coomarasamy A: Vitamin D and assisted reproductive treatment
outcome: A prospective cohort study. Reprod Health.
16(106)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kuroshli Z, Novin MG, Nazarian H,
Abdollahifar MA, Zademodarres S, Pirani M, Jahvani FA, Fathabady FF
and Mofarahe ZS: The efficacy of vitamin D supplement in the
expression and protein levels of endometrial decidualization
factors in women with recurrent implantation failure. Reprod Sci.
31:675–686. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ekanayake DL, Małopolska MM, Schwarz T,
Tuz R and Bartlewski PM: The roles and expression of HOXA/Hoxa10
gene: A prospective marker of mammalian female fertility? Reprod
Biol. 22(100647)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eun Kwon H and Taylor HS: The role of HOX
genes in human implantation. Ann N Y Acad Sci. 1034:1–18.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Murata H, Tanaka S and Okada H: Immune
tolerance of the human decidua. J Clin Med. 10(351)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Okada H, Tsuzuki T and Murata H:
Decidualization of the human endometrium. Reprod Med Biol.
17:220–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gellersen B and Brosens JJ: Cyclic
decidualization of the human endometrium in reproductive health and
failure. Endocr Rev. 35:851–905. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Murata H, Tanaka S, Tsuzuki-Nakao T, Kido
T, Kakita-Kobayashi M, Kida N, Hisamatsu Y, Tsubokura H, Hashimoto
Y, Kitada M and Okada H: The transcription factor HAND2
up-regulates transcription of the IL15 gene in human endometrial
stromal cells. J Biol Chem. 295:9596–9605. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dey SK, Lim H, Das SK, Reese J, Paria BC,
Daikoku T and Wang H: Molecular cues to implantation. Endocr Rev.
25:341–373. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cha J, Sun X and Dey SK: Mechanisms of
implantation: Strategies for successful pregnancy. Nat Med.
18:1754–1767. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Yuhki M, Kajitani T, Mizuno T, Aoki Y and
Maruyama T: Establishment of an immortalized human endometrial
stromal cell line with functional responses to ovarian stimuli.
Reprod Biol Endocrinol. 9(104)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hsu TC and Kellogg DS Jr: Primary
cultivation and continuous propagation in vitro of tissues from
small biopsy specimens. J Natl Cancer Inst. 25:221–235.
1960.PubMed/NCBI
|
|
23
|
Deryabin PI and Borodkina AV: Stromal cell
senescence contributes to impaired endometrial decidualization and
defective interaction with trophoblast cells. Hum Reprod.
37:1505–1524. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Untergasser A, Cutcutache I, Koressaar T,
Ye J, Faircloth BC, Remm M and Rozen SG: Primer3-new capabilities
and interfaces. Nucleic Acids Res. 40(e115)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke
PS, Yamagishi H, Srivastava D, Bagchi MK and Bagchi IC: The
antiproliferative action of progesterone in uterine epithelium is
mediated by Hand2. Science. 331:912–916. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mutchie TR, Yu OB, Di Milo ES and Arnold
LA: Alternative binding sites at the vitamin D receptor and their
ligands. Mol Cell Endocrinol. 485:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shindoh H, Okada H, Tsuzuki T, Nishigaki A
and Kanzaki H: Requirement of heart and neural crest
derivatives-expressed transcript 2 during decidualization of human
endometrial stromal cells in vitro. Fertil Steril.
101:1781–1790.e1-e5. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khattab S, Yu CH and Shah S: Prolactinoma
and adenomyosis-more than meets the eye: A case report. AACE Clin
Case Rep. 10:20–23. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Esmaeilzadeh S, Mirabi P, Basirat Z,
Zeinalzadeh M and Khafri S: Association between endometriosis and
hyperprolactinemia in infertile women. Iran J Reprod Med.
13:155–160. 2015.PubMed/NCBI
|
|
31
|
Mirabi P, Alamolhoda SH,
Golsorkhtabaramiri M, Namdari M and Esmaeilzadeh S: Prolactin
concentration in various stages of endometriosis in infertile
women. JBRA Assist Reprod. 23:225–229. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kanno K, Akutsu T, Ohdaira H, Suzuki Y and
Urashima M: Effect of vitamin D supplements on relapse or death in
a p53-immunoreactive subgroup with digestive tract cancer: Post hoc
analysis of the AMATERASU randomized clinical trial. JAMA Netw
Open. 6(e2328886)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Di Donato V, Giannini A and Bogani G:
Recent advances in endometrial cancer management. J Clin Med.
12(2241)2023.PubMed/NCBI View Article : Google Scholar
|