Introduction
In contemporary societies with aging populations,
the potential of extracting ursolic acid (UA) from apple peels to
utilize biomass, support sustainable resource management and
promote health is attracting attention (1,2).
Such efforts provide a promising novel approach for treating
frailty (3). Typically, apples are
peeled from their pulp, and the peels are often discarded as waste.
However, the peels are a valuable source of reusable components,
particularly UA, a natural triterpene compound and one of the most
prevalent components in apple peels. UA has a C-30 chemical
structure derived from isoprenoid units (4), exhibits low toxicity and demonstrates
anti-inflammatory effects. These effects result from the inhibition
of the inflammatory cytokines, cyclooxygenase and inducible nitric
oxide synthase (5). Additional
effects include the activation of p53, promoting the production of
reactive oxygen species, inhibiting cell proliferation (6) and exhibiting anticancer action
(6). Moreover, UA inhibits
α-glucosidase and α-amylase enzymes (7), inhibits myostatin, a key regulator of
skeletal muscle, and prevents muscle atrophy by downregulating
muscle atrophy-related genes [such as atrogin-1, muscle ring-finger
protein-1 (MuRF1)] (8).
Notably, UA has the ability to activate muscle stem
cells and myoblasts, which are involved in maintaining and
regenerating muscle health. Thus, it is considered very promising
for preventing and improving frailty characterized by muscle
weakness, physical debilitation (9), and loss of physical strength
(10). However, the clinical
applications of UA are limited due to its poor solubility (11). Improving solubility could pave the
way for expanding its applications in nutritional therapies.
γ-cyclodextrin (γ-CD) is an oligosaccharide
consisting of a glucose molecule ring joined by α-1,4 linkages and
composed of eight glucose units. CDs form inclusion complexes when
one molecule is internalized by another guest molecule. This
property is expected to improve drug stability, solubilization and
sustained release (12-15).
Metal-organic frameworks (MOFs) are porous materials composed of
organic ligands and metals joined through coordination bonds and
are characterized by very high pore volumes and surface areas
compared to activated carbon and zeolites (16,17).
Among these, the most notable, γ-CD-MOF, can be prepared using
bioelectrolyte salts [potassium hydroxide (KOH)], alcohol (ethanol)
and γ-CD (16). Previous studies
have suggested that CD-MOF can encapsulate drugs within the MOF
cavity, not only enhancing the water solubility and stability of
the drug, but possibly also improving the efficiency of drug
delivery through the biomembrane (18,19).
Consequently, CD-MOFs are promising and innovative materials for
drug delivery systems. In summary, CD-MOF, a combination of γ-CD
and MOF, can markedly improve drug properties and is expected to be
widely used in drug development and biological applications.
The present study focused on UA and the findings
presented herein demonstrate that its solubility can be improved by
forming inclusion complexes, such as UA/γ-CD and
UA/CD-MOF-1(20). The UA inclusion
complex has the potential to activate muscle stem cells, which play
crucial roles in maintaining muscle health and facilitating muscle
regeneration. This suggests that it may be useful in preventing or
mitigating frailty, a condition characterized by muscle weakness
and reduced physical strength. Furthermore, investigating the
effects of UA with enhanced solubility will likely contribute to
the development of a wide range of pharmaceutical formulations.
The present study used UA/γ-CD and UA/CD-MOF-1
inclusion complexes to improve the handling and availability of UA
and explore its potential for pharmaceutical applications. Myotube
cell proliferation was evaluated using C2C12 cells derived from
mouse skeletal muscles.
Materials and methods
Cells and culture conditions
C2C12 cells (cat. no. RCB0987) were purchased from
RIKEN BioResource Center. They were cultured in Dulbecco's modified
Eagle's medium (DMEM) (high glucose), purchased from FUJIFILM Wako
Pure Chemical Corporation. The DMEM was supplemented with 10% fetal
bovine serum (FBS) containing 1% penicillin (100 U/ml) and 1%
streptomycin (100 µg/ml). Penicillin-streptomycin solution (100X)
was purchased from FUJIFILM Wako Pure Chemical Corporation. Trypsin
was purchased from Nacalai Tesque, Inc. FBS was purchased from
BioWest. The cells were incubated at 37˚C and 5% CO2/95%
air. The supernatant was then removed, the cells was washed well
with 4 ml phosphate-buffered saline (PBS), 1 ml of 0.05% trypsin
was added and incubated for a further 5 min at 37˚C. Following
trypsinization, the cells were collected into 15-ml tubes (AS ONE
Corporation), diluted 10-fold with 9 ml PBS, and centrifuged at 400
x g for 5 min at 20˚C. The supernatant was discarded, and the cell
pellet was resuspended in 1 ml of 10% FBS-DMEM. The cells were
counted using a blood cell counter, seeded at a density of
6.0x105 cells per dish, and incubated for 3 days at 37˚C
in 100-mm culture dishes (SARSTEDT, Nümbrecht, Germany). C2C12
myoblast differentiation into myotubes was induced by switching to
high-glucose DMEM containing 2% horse serum (HS-DMEM) after the
cells reached confluency (>90%). The cell culture medium was
replaced every two days. HS was purchased from MilliporeSigma.
Materials and reagents
The PBS components were as follows: NaCl, 137 mM;
Na2HPO4·12H2O, 8.1 mM; KCl: 2.68
mM; and KH2PO4, 1.47 mM. 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was
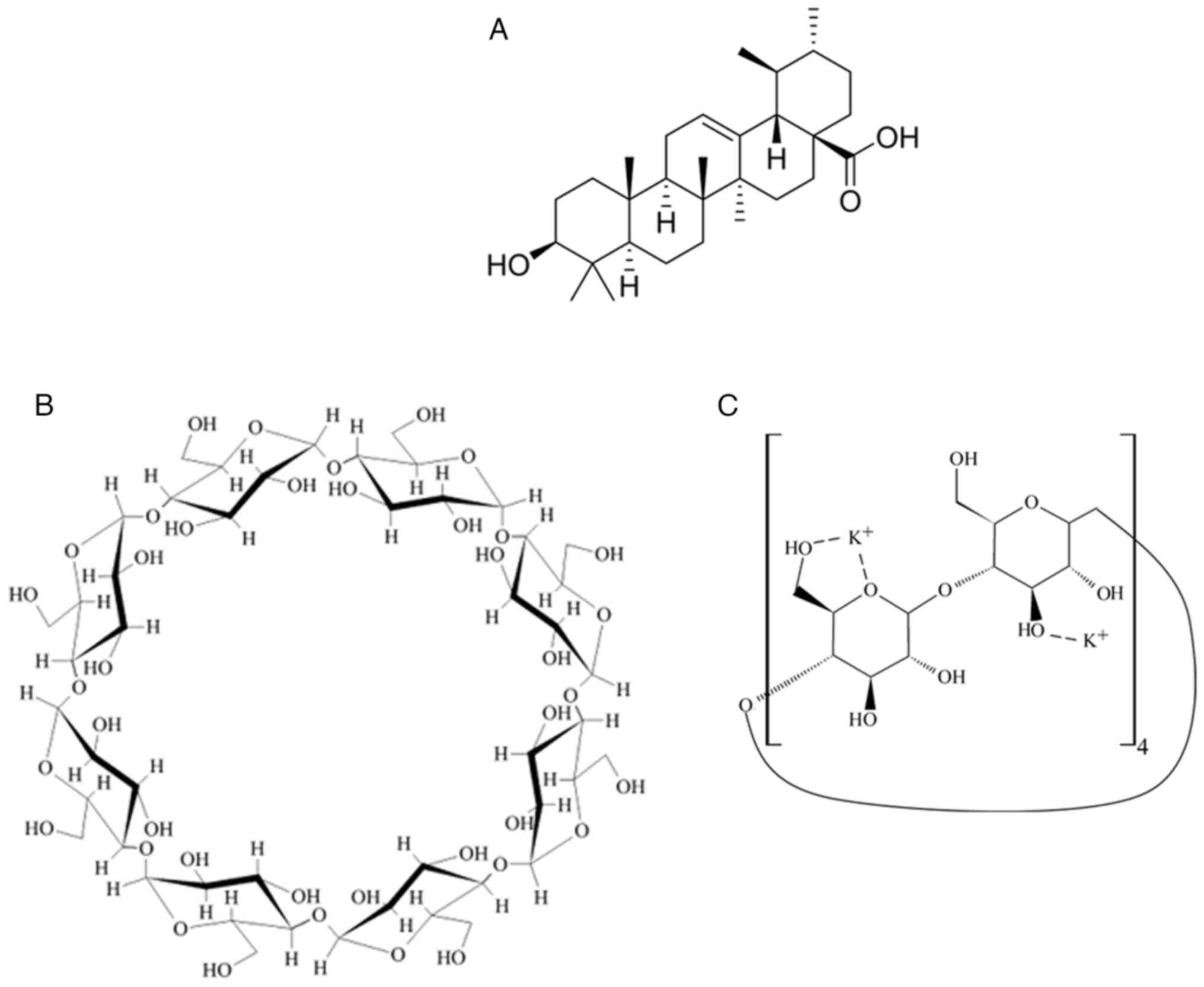
purchased from Tokyo Chemical Industry Co. γ-CD (lot 801005) was
supplied by CycloChem Bio Co., Ltd. (chemical structure illustrated
in Fig. 1). UA (lot VH5LE-HL) was
purchased from Tokyo Chemical Industry Co. May-Grünwald Giemsa
stain solution and Giemsa stain solution were purchased from
Nacalai Tesque, Inc. All other reagents were purchased from
FUJIFILM Wako Pure Chemical Corporation.
Sample preparation of UA/γ-CD and
UA/CD-MOF-1
CD-MOF-1 was prepared as described in the study by
Sarabia-Vallejo et al (15). Briefly, γ-CD (1 mmol) and KOH (8
mmol) were dissolved in distilled water (~20 ml) and allowed to
stand for 1 week at room temperature with ethanol (50 ml) for vapor
diffusion. The precipitated crystals were filtered, washed with
ethanol, and air-dried at room temperature. The CD-MOF-1 was stored
with desiccant beads at room temperature in a desiccator. To
prepare three-dimensional mixed granulates (3DGM), a method
previously reported by the authors' laboratory was used (20). As previously described, the 3DGM
(UA/γ-CD=1/1 molar ratio) and 3DGM (UA/CD-MOF-1=1/1 molar ratio)
were prepared by grinding in a three-dimension ball mill with a Φ5
mm 200 g ball for 60 min.
Preparation of UA, UA/γ-CD and
UA/CD-MOF-1 cell-test solutions
UA was dissolved in dimethyl sulfoxide (DMSO) and
diluted to a final concentration of 2.5-40 µM. UA/γ-CD and γ-CD
were dissolved in 10% FBS-DMEM or 2% HS-DMEM (high glucose).
UA/CD-MOF-1 and CD-MOF-1 were dissolved in 10% FBS-DMEM or 2%
HS-DMEM (high glucose) containing 20 mM HEPES. Each sample was
diluted with added medium to reach a final concentration of 2.5-40
µM. Herein, the ‘control’ group refers to the control configured
vehicle (CCV: UA at 0 µM).
Cell viability under cell-growth
conditions
Cell viability was evaluated using an MTT assay. The
C2C12 cells were prepared at 1.0x105 cells/ml and seeded
at 50 µl in 96-well plates (flat bottom) (AS ONE Corporation), and
incubated in 10% FBS-DMEM for 24 h at 37˚C. Subsequently, without
removing the supernatant, 50 µl of each sample were diluted in 10%
FBS-DMEM to twice the desired concentration, bringing the total
volume to 100 µl; each sample was then adjusted to the desired
concentration. The cells were incubated for 48 h at 37˚C, following
which, the medium was removed. MTT reagent (MTT dissolved in PBS 5
mg/ml) was diluted 10-fold in 10% FBS-DMEM, 100 µl/well was added,
and the cells were incubated for 3 h at 37˚C. Following this, 200
µl/well of PBS were added and the cells were washed with PBS.
Subsequently, the supernatant was removed, 200 µl/well of PBS were
added, the cells were washed with PBS, 100 µl/well of 2-propanol
containing 0.029% hydrochloric acid (as solubilizing solution) was
added, and the cells were allowed to stand at room temperature for
30 min under light-shielded conditions. Absorbance was measured at
a wavelength of 570 nm and a reference wavelength of 650 nm using a
Spectra MaxM2 microplate reader (Molecular Devices, LLC). Cell
viability was calculated by comparison with 0 µM (UA, UA/γ-CD, or
UA/CD-MOF-1).
MTT cell viability assay under
differentiation conditions
Cell viability was calculated using MTT assay. C2C12
cells were seeded into 96-well plates at 4.0x104
cells/well and incubated at at 37˚C for 48 h after cell confluency
(>90%) was confirmed. UA, UA/γ-CD, γ-CD, UA/CD-MOF-1 and
CD-MOF-1 were added in 2% HS-DMEM (high glucose) diluted to the
desired concentration, and the medium was changed every 2 days for
6 days to induce differentiation. The supernatant was removed
following incubation at 37˚C. MTT reagent was then diluted 10-fold
in 2% HS-DMEM (high glucose) and 100 µl/well were added to the
plates followed by 3 h of incubation at 37˚C. Subsequently, 200
µl/well PBS were added, the cells were washed with PBS, 100 µl/well
of solubilizing solution (2-propanol containing 0.029% hydrochloric
acid) was added, and the cells were allowed to stand for 30 min
under light-shielded, room-temperature conditions. The absorbance
was measured using a Spectra MaxM2 microplate reader at a
wavelength of 570 nm and a reference wavelength of 650 nm. Cell
viability was calculated by comparison with 0 µM (UA, UA/γ-CD, or
UA/CD-MOF-1).
Measurement of C2C12 cell myotube
diameters under differentiation conditions
The C2C12 cells were stained with May-Grünwald
Giemsa stain, and the myotube diameters were measured. A total of
3-15 passages were completed as the cells were less likely to
differentiate as the number of passages increased. The C2C12 cells
were prepared at 5.0x105 cells/ml and seeded at 500 µl
in 24-well plates. After confirming that the cells were confluent,
the medium was changed to 2% HS-DMEM, and differentiation was
induced by changing the medium every 2 days for 6 days. Following
the induction of differentiation, DMSO, UA, UA/γ-CD, and
UA/CD-MOF-1 were diluted in 2% HS-DMEM and 500 µl/well were added.
Of note, 1 h of incubation was followed by incubation with
dexamethasone (DEX) (FUJIFILM Wako Pure Chemical Corporation) for a
further 24 h at 37˚C, and May-Grünwald Giemsa staining was
performed. The cells were fixed with methanol (500 µl/well) for 5
min and air-dried for 10 min at room temperature. May-Grünwald
stain solution/phosphate buffer (1/3) was added, and the cells were
stained for 5 min at room temperature. The cells were then washed
twice with Milli-Q (MilliporeSigma) water (300 µl/well). The cells
were then stained with Giemsa stain solution/Milli-Q water (1/10)
solution for 20 min at room temperature and washed three times with
Milli-Q water. Microscopic observation (CKX53, Olympus Corporation)
of the stained cells was performed with a random-field selection of
stained cells from the wells, and the diameter of myotube cells
with at least three cell nuclei was measured. At least 50 myotubes
were analyzed under each condition.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). The analysis of MTT assay and measurement of myotube
diameters were performed using one-way ANOVA with Dunnett's test
for multiple comparisons. Comparisons between experimental groups
were also assessed using one-way ANOVA followed by Tukey's test.
Values of P<0.05 or P<0.01l were considered to indicate
statistically significant or highly statistically significant
differences, respectively. Data were analyzed using Statcel - the
Useful Addin Forms on Excel, 4th edition.
Results and Discussion
Cell viability under proliferative
condition, examined using MTT assay
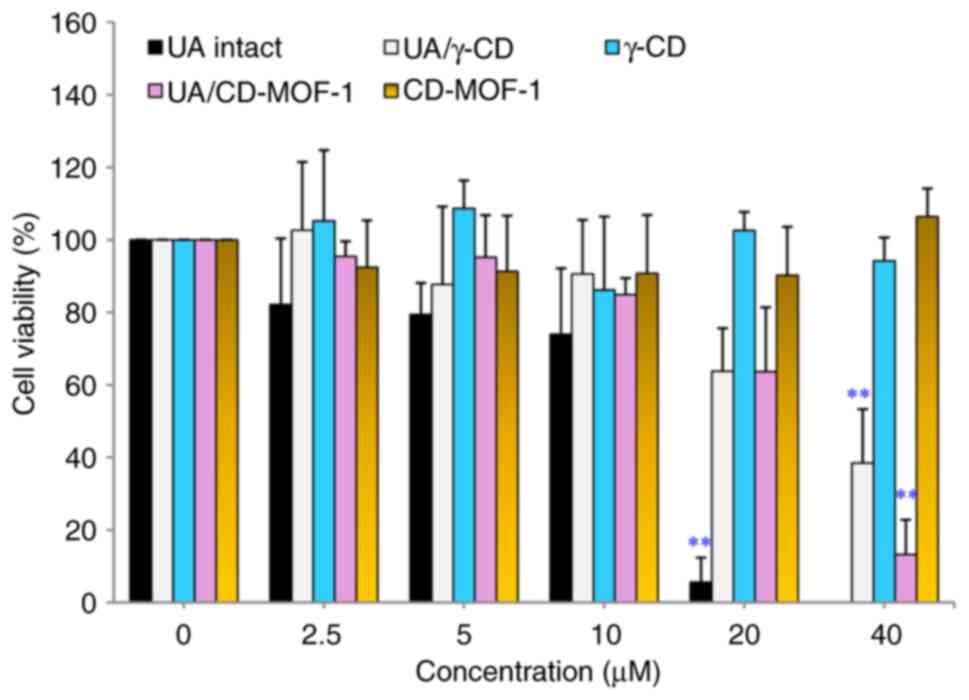
The results of cell viability assay with intact UA,
UA/γ-CD, γ-CD, UA/CD-MOF-1 and CD-MOF-1 under growth conditions
using the MTT assay test are presented in Fig. 2. For intact UA, the results
revealed that the concentrations of 0-10 µM had no effect on cell
viability; however, a significant difference was observed between 0
and 20 µM, confirming cytotoxicity. For UA/γ-CD, cell viability was
not affected at 0-20 µM; however, a significant difference was
observed between 0 and 40 µM, confirming cytotoxicity. For
UA/CD-MOF-1, cell viability was not affected at 0-20 µM; however, a
significant difference was observed between 0 and 40 µM, confirming
cytotoxicity. No effect on cell viability was observed for γ-CD and
CD-MOF-1, as no significant difference was observed at 0-40 µM. The
cell viability assay revealed that UA/γ-CD was not cytotoxic to the
C2C12 cells under proliferative conditions at concentrations up to
and higher than those of intact UA, suggesting that the cytotoxic
effect of UA was reduced. The cytotoxic effects of UA alone were
comparable with those of the UACD-MOF-1 inclusion product. This
finding may be related to the difference in the inclusion modes of
UA/γ-CD and UA/CD-MOF-1.
Cell viability under differentiation
conditions
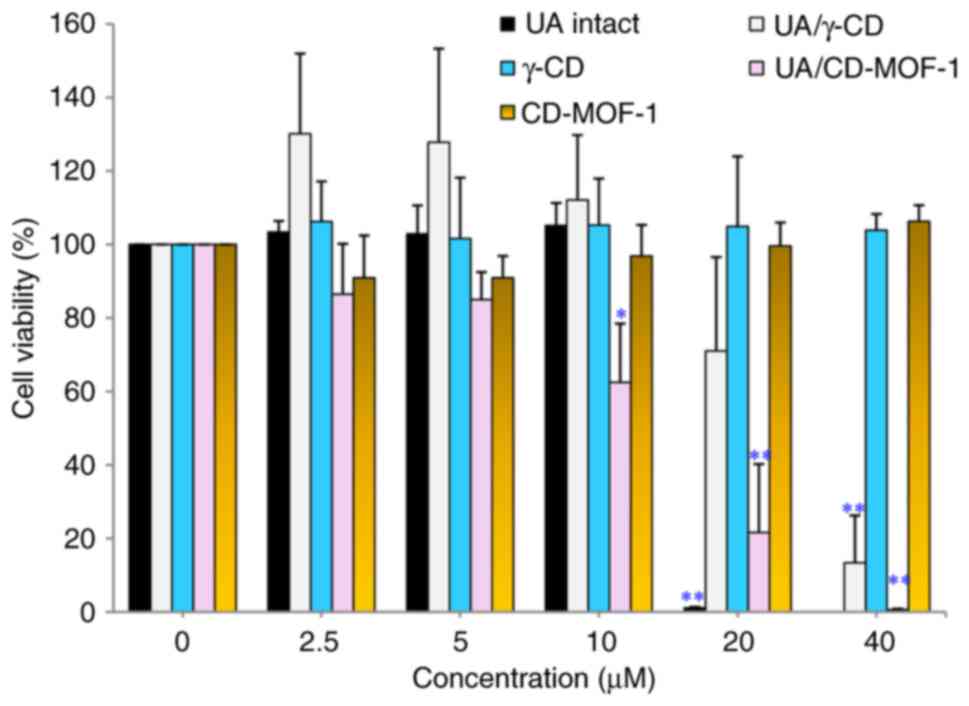
The results of cell viability assay with intact UA,
UA/γ-CD, γ-CD, UA/CD-MOF-1 and CD-MOF-1 under differentiation
conditions are illustrated in Fig.
3. For UA intact, cell viability was unaffected at 0-10 µM,
although a significant difference was observed between 0 and 20 µM,
confirming cytotoxicity. For UA/γ-CD, cell viability was not
affected at 0-20 µM; however, a significant difference was observed
between 0 and 40 µM, confirming cytotoxicity. For UA/CD-MOF-1, no
effect on cell viability was observed at 0-5 µM, although a
significant difference was observed between 0 and 10-40 µM,
confirming cytotoxicity. For γ-CD and CD-MOF-1, no significant
difference was observed between 0 and 40 µM, demonstrating no
effect on cell viability. The cell viability assay revealed that
UA/γ-CD (40 µM) was not cytotoxic to C2C12 cells under
differentiated conditions up to or at higher concentrations than
intact UA (20 µM), suggesting that the inclusion of UA into γ-CD
reduced the cytotoxicity of UA. Nevertheless, the cytotoxic effects
of UA under differentiation conditions were not reduced by the
inclusion of UA in CD-MOF-1. These findings suggest that the
exposure of UA/CD-MOF-1 complexes to unincorporated UA under
differentiation conditions may affect the cytotoxicity of the
complexes. In other words, the mode of inclusion of UA with γ-CD or
CD-MOF-1 under both proliferative and differentiation conditions
may affect cytotoxicity. Based on these results, the authors
decided to use a UA concentration of 5 µM for the C2C12 cell
myotube study to match the concentration at which cytotoxicity was
not observed with UA/γ-CD and UA/CD-MOF-1. UA is toxic to normal
cells at high concentrations. On the other hand, the results of MTT
assay revealed that the UA/γCD complex was less cytotoxic than UA
alone. The myotube diameter measurement results suggested that the
addition of UA, UA/γ-CD, or UA/CD-MOF-1 may alleviate the
DEX-induced atrophy. However, the UA/CD-MOF-1 is different from the
UA/γCD inclusion mode, suggesting that even if UA is included in
CD-MOF-1, the amount of UA released may not be sufficient to
suppress the toxicity to cells.
Measurement of myotube diameters in
differentiated C2C12 cells
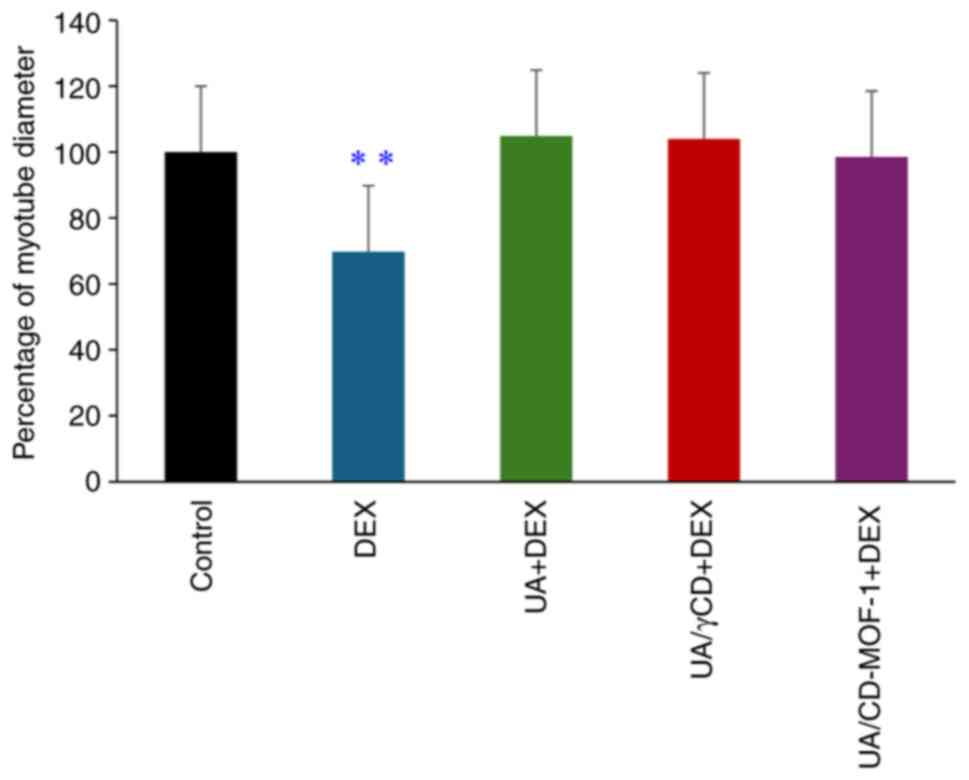
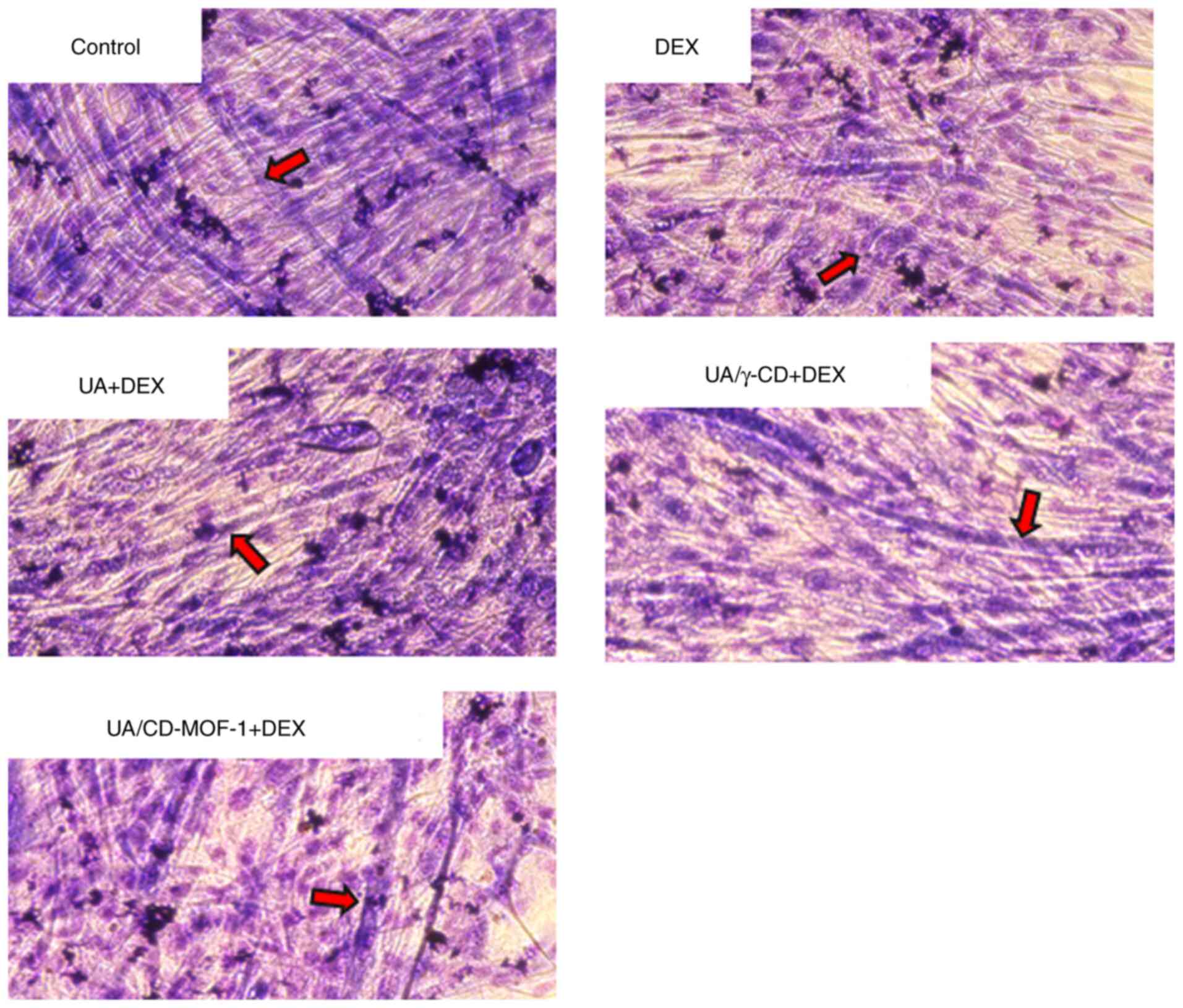
The results of the myotube diameter measurements are
shown in Fig. 4. Images of C2C12
cells stained with May-Grünwald Giemsa are shown in Fig. 5. The fusion of nuclei, forming
tubular structures, indicated that the C2C12 cells underwent
differentiation. Compared with the control group (without UA
addition), thinner myotubes were observed in the DEX group, while
the UA + DEX, UA/γ-CD and UA/CD-MOF-1 groups did not exhibit such
thinning. A significant reduction in myotube diameter was observed
in the DEX group when compared with that in the control group
(Fig. 4). This indicated that DEX
induced atrophy in C2C12 cells, as reflected by the reduced myotube
diameter. Moreover, no significant differences were observed
between the control group and the UA + DEX, UA/γ-CD + DEX, or
UA/CD-MOF-1 + DEX groups (Fig. 4).
It has been previously demonstrated that DEX and cortisol suppress
the production of IL-6 in RAW264.9 murine macrophages, human
monocytes, endothelial cells and fibroblasts (21). By contrast, UA has been shown to
inhibit muscle degradation pathways by suppressing the
ubiquitin-proteasome system and the expression of atrogenes, such
as atrogin-1 and MuRF1(22).
Additionally, UA increased Akt activity in skeletal muscles,
leading to muscle hypertrophy. Furthermore, UA enhanced exercise
capacity and reduced the resting heart rate (23). In the present study, the absence of
changes in myotube diameter in the UA + DEX, UA/γ-CD + DEX, and
UA/CD-MOF-1 + DEX groups when compared with the control suggests
that UA-containing samples ameliorated DEX-induced atrophy. This
indicates that UA contributed to the maintenance of myotube
diameter, even when encapsulated in γ-CD or CD-MOF-1 inclusion
complexes, thereby confirming its beneficial effects on myotube
growth. In C2C12 cells, the activation of the IGF-1/PI3K/Akt
pathway promotes muscle protein synthesis and suppresses muscle
atrophy (24). UA has also been
reported to increase IGF-1 expression, activate the PI3K/Akt
pathway and promote Akt phosphorylation (9,25).
Consequently, the present study confirmed that UA not only improved
solubility by forming a complex with γCD or CD-MOF-1, but also
improved muscle atrophy; thus, it was hypothesized that the
functional groups of the UA structure that are not encapsulated in
the CD cavity affect C2C12 cells. It already has been reported that
the inclusion of UA with γ-CD and CD-MOF-1 improves the solubility
and stability of UA and reduces its cytotoxicity (26,27).
Inclusion complexes of UA and γ-cyclodextrin were prepared and
their structural properties and cytotoxicity in C2C12 cells were
evaluated in a previous study. The results demonstrated that the
inclusion complexation improved the solubility of UA and reduced
its cytotoxicity (11). An
inclusion complex of UA with CD-MOF-1 has been identified, and
potassium has been implicated as a ligand for CD-MOF-1. It has been
shown that CD-MOF-1 exhibits no toxicity or effect on cell
viability up to 100 µM (28).
However, since potassium ions generally play a crucial role in
cellular physiology, changes in potassium concentrations may affect
cell viability and function in the MTT study. Therefore, it is
considered that future studies are required to determine how the
combination of UA and potassium affects cytotoxicity. These
findings suggest that UA exerts beneficial effects on muscle growth
and function, potentially contributing to the maintenance of muscle
health and prevention of frailty.
The present study aimed to confirm the effects of
UA/γ-CD and UA/CD-MOF-1 complexes on myotubes in C2C12 cells as an
initial step and limitation. Therefore, further investigations of
muscle atrophy improvement through gene expression and in
vivo studies using UA/γ-CD and UA/CD-MOF-1 complexes are
warranted. In addition, elucidating the biochemical mechanisms
underpinning this process remains a challenge for future research.
The findings of the present study provide fundamental insighs into
the safety of UA/γCD and UA/CD-MOF-1 complexes prepared in the
authors' laboratory, as well as their effects on mesenchymal stem
cells. Moving forward, the authors aim to conduct in vivo
studies using UA/γCD and UA/CD-MOF-1 in the future, with the aim of
establishing a foundation to enhance the translational nature of
the study.
In conclusion, the present study demonstrated that
UA/γ-CD reduced the cytotoxic effects of UA based on the results of
cell viability assay under proliferative and differentiation
conditions. By contrast, UA/CD-MOF-1 did not reduce the cytotoxic
effects of UA. The results of the C2C12 cell myotube diameter
measurements confirmed that the addition of UA, UA/γ-CD and
UA/CD-MOF-1 reduced the atrophy induced by DEX. Moreover, the same
muscle atrophy ameliorating effect of UA was confirmed in the
experiments using UA/γ-CD and UA/CD-MOF-1 inclusion complexes with
improved solubility. UA activated muscle stem cells and myoblasts,
which are involved in the maintenance of muscle health and
regeneration. It also prevented or ameliorated frailty, the problem
of muscle weakness, and the loss of physical strength (23). These findings have expanded the
potential for the clinical utilization of UA; it is hoped that
these findings may lead to the further applications of UA in
pharmaceutical formulations. In the future, it will be necessary to
elucidate the mechanisms of muscle atrophy amelioration by gene
expression studies, as well as to conduct in vivo studies
for wider UA applications.
Acknowledgements
The authors are grateful to Cyclo Chem Bio Co., Ltd.
for providing the cyclodextrin samples. The authors would also like
to thank the Laboratory of Nutri-Pharmacotherapeutics Management,
Josai University, for their research support while taking measures
against COVID-19 (SARS-CoV-2) infection.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RK, TT and YIn conceived the study and designed the
experiments. RK, MI, TT and YIn designed the study and drafted the
manuscript. RK, MI and TT performed the experiments. RK, MI, TT,
YIs, DN, KT and YIn participated in data acquisition, analysis, and
interpretation. RK, TT, YIs, DN, KT and YIn provided resources,
reviewed, and edited the manuscript, and supervised the study. RK,
TT and YI confirm the authenticity of all the raw data. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the present study received
γ-cyclodextrin from CycloChem Co., Ltd.; however, the company was
not involved in the study design, collection, analysis,
interpretation of data, in the writing of this article, or the
decision to submit it for publication.
References
|
1
|
Li H, Liu Y, Guo S, Shi M, Qin S and Zeng
C: Extraction of ursolic acid from apple peel with hydrophobic deep
eutectic solvents: Comparison between response surface methodology
and artificial neural networks. Foods. 9(310)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yamaguchi H, Noshita T, Kidachi Y, Umetsu
H, Hayashi M, Komiyama K, Funayama S and Ryoyama K: Isolation of
ursolic acid from apple peels and its specific efficacy as a potent
antitumor agent. J Health Sci. 54:654–660. 2008.
|
|
3
|
Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko
KS, Kwak HB and Han J: Ursolic acid in health and disease. J
Physiol Pharmacol. 25:235–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Murakami A, Kuki W, Takahashi Y, Yonei H,
Nakamura Y, Ohto Y, Ohigashi H and Koshimizu K: Auraptene, a citrus
coumarin, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced
tumor promotion in ICR mouse skin, possibly through suppression of
superoxide generation in leukocytes. Jpn J Cancer Res. 88:443–452.
1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Silalahi J: Anticancer and health
protective properties of citrus fruit components. Asia Pac J Clin
Nutr. 11:79–84. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang YS and Ho SC: Polymethoxy flavones
are responsible for the anti-inflammatory activity of citrus fruit
peel. Food Chem. 119:868–873. 2010.
|
|
7
|
Tripoli E, Guardia ML, Giammanco S, Majo
DD and Giammanco M: Citrus flavonoids: Molecular structure,
biological activity and nutritional properties: A review. Food
Chem. 104:466–479. 2007.
|
|
8
|
Nakajima VM, Macedo GA and Macedo JA:
Citrus bioactive phenolics: Role in the obesity treatment. LWT-Food
Sci Technol. 59:1205–1212. 2014.
|
|
9
|
Castillero E, Alamdari N, Lecker SH and
Hasselgren PO: Suppression of atrogin-1 and MuRF1 prevents
dexamethasone-induced atrophy of cultured myotubes. Metabolism.
62:1495–1502. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ebert SM, Dyle MC, Bullard SA, Dierdorff
JM, Murry DJ, Fox DK, Bongers KS, Lira VA, Meyerholz DK, Talley JJ
and Adams CM: Identification and small molecule inhibition of an
activating transcription factor 4 (ATF4)-dependent pathway to
age-related skeletal muscle weakness and atrophy. J Biol Chem.
290:25497–25511. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alfei S, Marengo B, Valenti GE and Zuccari
G: Considerable improvement of ursolic acid water solubility by its
encapsulation in dendrimer nanoparticles. Nanomaterials.
11(2196)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mazurek AH and Szeleszczuk Ł: Current
status of quantum chemical studies of cyclodextrin host-guest
complexes. Molecules. 27(3874)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kfoury M, Landy D and Fourmentin S:
Characterization of cyclodextrin/volatile inclusion complexes: A
review. Molecules. 23(1204)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Loftsson T, Sigurdsson HH and Jansook P:
Anomalous properties of cyclodextrins and their complexes in
aqueous solutions. Materials (Basel). 16(2223)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sarabia-Vallejo Á, Caja MDM, Olives AI,
Martín MA and Menéndez JC: Cyclodextrin inclusion complexes for
improved drug bioavailability and activity: Synthetic and
analytical aspects. Pharmaceutics. 15(2345)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel HA, Islamoglu T, Liu Z, Nalluri SKM,
Samanta A, Anamimoghadam O, Malliakas CD, Farha OK and Stoddart JF:
Noninvasive substitution of K+ sites in cyclodextrin metal organic
frameworks by Li+ ions. J Am Chem Soc. 139:11020–11023.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Benedetto G, Cleary BM, Morrell CT, Durbin
C, Brinks AL, Tietjen J and Mirica KA: CD-MOF-1 for CO2
uptake: Remote and hybrid green chemistry synthesis of a framework
material with environmentally conscious applications. J Chem Educ.
100:1289–1295. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nanri A, Yoshida M, Ishida Y, Nakata D,
Terao K, Arce FJ, See GL, Tanikawa T and Inoue Y: Preparation and
characterization of a hybrid complex of cyclodextrin-based
metal-organic frameworks-1 and ascorbic acid derivatives. Materials
(Basel). 14(7309)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang N, Wei L, Teng Y, Yu P, Xiang C and
Liu J: Cyclodextrin-based metal-organic frameworks transforming
drug delivery. Eur J Med Chem. 274(116546)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Inoue Y, Motoda A, Tanikawa T, Takao K,
Arce F Jr, See GL, Ishida Y, Nakata D and Terao K: Inclusion
complexes of ursolic acid with cyclodextrin-based metal-organic
framework-1 enhance its solubility. J Drug Deliv Sci Technol.
89(104986)2023.
|
|
21
|
Waage A, Slupphaug G and Shalaby R:
Glucocorticoids inhibit the production of IL6 from monocytes,
endothelial cells and fibroblasts. Eur J Immunol. 20:2439–2443.
1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu R, Chen JA, Xu J, Cao J, Wang Y, Thomas
SS and Hu Z: Suppression of muscle wasting by the plant-derived
compound ursolic acid in a model of chronic kidney disease. J
Cachexia Sarcopenia Muscle. 8:327–341. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kunkel SD, Elmore CJ, Bongers KS, Ebert
SM, Fox DK, Dyle MC, Bullard SA and Adams CM: Ursolic acid
increases skeletal muscle and brown fat and decreases diet-induced
obesity, glucose intolerance and fatty liver disease. PLoS One.
7(e39332)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rommel C, Bodin SC, Clarke BA, Rossman R,
Nunez L, Stitt TN, Yancopoulos GD and Glass DJ: Mediation of
IGF-1-induced skeletal myotube hypertrophy by PI3K/Akt/mTOR and
PI3K/Akt/GSK3 pathways. Nat Cell Biol. 3:1009–1013. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ebert SM, Nicolas CS, Schreiber P, Lopez
JG, Taylor AT, Judge AR, Judge SM, Rasmussen BB, Talley JJ, Rème CA
and Adams CM: Ursolic acid induces beneficial changes in skeletal
muscle mRNA expression and increases exercise participation and
performance in dogs with age-related muscle atrophy. Animals
(Basel). 14(186)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Song S, Gao K, Niu R, Yi W, Zhang J, Gao
C, Yang B and Liao X: Binding behavior, water solubility and in
vitro cytotoxicity of inclusion complexes between ursolic acid and
amino-appended β-cyclodextrins. J Mol Liquids.
296(111993)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zong W and Mei BS: The Preparation and
characterization of inclusion complex of ursolic acid with
γ-Cyclodextrin. Adv Mater Res. 403-408:712–716. 2011.
|
|
28
|
Hartlieb KJ, Ferris DP, Holcroft JM,
Kandela I, Stern CL, Nassar MS, Botros YY and Stoddart JF:
Encapsulation of ibuprofen in CD-MOF and related bioavailability
studies. Mol Pharm. 14:1831–1839. 2017.PubMed/NCBI View Article : Google Scholar
|