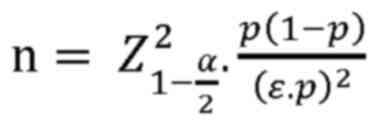Introduction
Nasopharyngeal carcinoma (NPC) is the most prevalent
type of head and neck cancer globally, accounting for ~120,434 new
cases and 73,482 deaths annually (1). The highest age-standardized incidence
rates (ASIRs) are observed in East and Southeast Asia, including
Singapore, the Maldives and Indonesia (ASIRs ~7); Malaysia and
Vietnam (~6); and China (~3) (2).
In Vietnam, NPC ranks among the top 10 cancers, with 5,613 new
cases (3.1% of all cancers) and 3,453 deaths (2.9%) annually. The
current 5-year incidence rate is 16,007 individuals (3).
Radiotherapy is the main treatment for NPC. While
early-stage cases may be effectively managed with radiotherapy
alone, intermediate- and late-stage diagnoses often require
concurrent radiotherapy and chemotherapy (2). A main concern during treatment is
malnutrition, driven by both the disease and its treatment side
effects. Radiation therapy, in particular, is associated with high
malnutrition rates, with hypoproteinemia-related symptoms including
weight loss, lower limb edema and cachexia (4,5).
Malnutrition weakens the immune system, prolongs the period of
hospitalization, aggravates the side-effects of radiotherapy,
interrupts treatment schedules, and worsens the prognosis and
quality of life (QOL) of patients (6,7).
Malnutrition often begins early in the course of
treatment and worsens over time (8). Studies have reported that 20.2% of
patients lose >10% of their body weight during chemoradiotherapy
(9), and malnutrition prevalence
can rise from 16.8% pre-treatment to 91.2% post-treatment (10). Wei et al (11) reported a severe malnutrition rate
of 80.7% during radiotherapy, while Zhuang et al (12) found that 69.0% of patients were
malnourished upon the completion of treatment.
Can Tho Oncology Hospital (Can Tho, Vietnam), a
grade I facility serving as a specialized center in the Mekong
Delta region of Vietnam, is the only hospital in Can Tho City
equipped with a radiotherapy machine. Of note, >100 patients
with NPC from across the region are treated annually at this
hospital. However, nutritional neglect during treatment often leads
to delayed and prolonged periods of hospitalization.
To address this issue, a nutritional assessment
program has been initiated to detect malnutrition early and guide
timely interventions to improve the overall QOL of patients. The
present study aimed to assess the nutritional status and QOL of
patients with NPC at Can Tho Oncology Hospital in 2024, using the
Patient-Generated Subjective Global Assessment (PG-SGA) and the
European Organisation for Research and Treatment of Cancer Quality
of Life Questionnaire (EORTC QLQ-C30). The PG-SGA is used to assess
nutritional status, helping to identify malnutrition early for
timely intervention. The EORTC QLQ-C30 evaluates the QOL, guiding
improvements in patient care and overall well-being.
Patients and methods
Study population
The present study included patients with NPC treated
at Can Tho Oncology Hospital from February, 1 to December, 31,
2024. The eligibility criteria included an age ≥18 years and a
histopathological diagnosis of primary NPC. Patients with severe
dementia or mental disorders precluding cooperation were excluded.
All patients were fully informed and voluntarily consented to
participate in the study. Ethics approval was first obtained from
Can Tho Oncology Hospital on February 1, 2024 according to Decision
no. 02/HĐĐĐ-BVUBCT (Cantho, Vietnam). The present study was also
then approved by the Ethics Committee of Hanoi Medical University
(Hanoi, Vietnam) under Decision no. NCS2024/ GCN-HMUIRB, dated May
15, 2024. It is understood that the study team provided the
potential participants with study-related information on the
contents and objectives of the study and sought their consent to
participate by signing the consent form.
Study design
The present study was a descriptive cross-sectional
study using convenience sampling. The sampling technique used was
non-probability sampling. As regards sample size and sample
selection, the sample size was calculated according to the formula
for calculating the estimated sample size of 1 mean with the
population sample size, as follows:
where ‘α’ represents the type 1 error, α=0.05;
=1.96 (equivalent to 95% confidence
level); and ‘ε’ represents the relative estimation error, ε=0.1.
According to the study by Wei et al (11), 80.7% of patients wth NPC received
chemoradiotherapy. Substituting into the formula, the sample size
was n=92. In fact, the present study collected data from 129
patients.
The present study investigated patients diagnosed
with NPC who received various treatment modalities, including
chemotherapy (specifically cisplatin, carboplatin, gemcitabine, or
5-fluorouracil), radiotherapy alone, or a combination of
chemoradiotherapy (utilizing cisplatin and 5-fluorouracil). Medical
records provided the number, location, stage and size of primary
tumors; the type of the most recent primary tumor; the number and
types of treatments; and the start and end dates of each
treatment.
Nutritional assessments and interventions are not
standard practice for NPC in a research location. Nutritional
therapy, encompassing both enteral and parenteral nutrition, was
administered to patients at risk of malnutrition based on physician
discretion rather than nutritionists. The records only indicated
whether patients received nutritional therapy, without specifying
the types of therapies prescribed or their adequacy for addressing
malnutrition.
Assessment of nutritional status
Data were collected through structured
questionnaires covering demographics, the PG-SGA-based nutritional
assessment tool, and anthropometric and biochemical measurements.
Body weight (kg) and height (cm) were recorded, and percentage
weight loss was calculated as follows:
The PG-SGA evaluates weight loss history, dietary
intake, gastrointestinal symptoms, functional status and physical
signs (e.g., fat/muscle wasting, edema) (13,14).
Scoring thresholds were as follows: 0-1, well-nourished; 2-3,
moderate or suspected malnutrition, warranting patient and family
education; 4-8, severe malnutrition requiring intervention; and ≥9,
critical malnutrition necessitating urgent nutritional support.
Assessment of QOL
QOL was assessed using the EORTC QLQ-C30, a
well-validated cancer-specific tool (15), and has been validated in Vietnamese
cancer populations (16). The
questionnaire comprises 30 items covering five functional domains
(physical, role, emotional, cognitive, and social), symptom scales
(e.g., fatigue, pain) and a global health/QOL scale (17,18).
Scores were calculated and linearly transformed (range 0-100), with
higher functional/global scores indicating an improved QOL and
higher symptom scores indicating worse symptoms (16).
Data collection
Self-report questionnaires collected demographic
data (age, sex, education level, etc.) and disease-related
information (cancer stage, treatment, etc.). All eligible patients
who consented were asked to complete the self-report questionnaire,
PG-SGA and QLQ-C30 upon their initial admission to the inpatient
chemotherapy and radiotherapy department. The investigators
assisted patients who had difficulty answering the questions,
ensuring that all questions were fully answered within 10-15 min.
Clinical staff completed the clinician-assessed PG-SGA section
immediately upon receiving the questionnaires from the
patients.
Statistical analysis
Data were entered using Epidata 3.1 and analyzed
using SPSS 22 software (IBM Corp.). Descriptive statistics were
reported as frequencies, percentages, and the mean ± standard
deviation (SD). Differences in age groups, sex, education levels,
cancer stages, treatment types (chemotherapy, radiotherapy,
chemoradiotherapy, or others), and nutritional status (malnourished
vs. well-nourished patients) were analyzed using the Chi-squared
test. However, in the case that >20% of the cells had expected
frequencies <5, Fisher's exact test was applied instead. The
Mann-Whitney U test was used to compare PG-SGA scores and QOL
scores due to non-normal distribution. The association between
malnutrition and QOL was analyzed using linear regression analyses.
In linear regression analyses using the enter method, the PG-SGA
score, sex (male vs. female), age (years), feeding route (oral vs.
tube) and disease stage (I-IV) were included as predictors. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
The present study included 129 patients with NPC
with a mean age of 52.4±12.5 years; 75.2% of the patients were
<60 years of age. The male-to-female ratio was 2.91:1, and
approximately half of the patients had a primary school education.
The majority of the patients were diagnosed at stage III-IV disease
(82.9%) and were undergoing chemotherapy (60.5%). Severe
malnutrition (PG-SGA ≥9) was observed in 74.4% of the patients,
with a mean PG-SGA score of 15.98±9.3. Patients experienced an
average weight loss of 2.9 kg in 1 month, and 31.8% of the patients
lost >5% of their body weight (Table I).
 | Table IDemographic and clinical
characteristics of the patients with NPC (n=129). |
Table I
Demographic and clinical
characteristics of the patients with NPC (n=129).
| Characteristic | Count, n (%) |
|---|
| Age, years | |
|
<60 | 97 (75.2) |
|
≥60 | 32 (24.8) |
|
Mean ±
SD | 52.4±12.5 |
| Sex | |
|
Male | 96 (74.4) |
|
Female | 33 (25,6) |
| Education
level | |
|
Illiteracy | 8 (6.2) |
|
Primary
school | 56 (43.4) |
|
Middle
school | 42 (32.6) |
|
High
school | 17 (13.2) |
|
Post-high
school | 6 (4.7) |
| Stage | |
|
I-II | 22 (17.1) |
|
III-IV | 107 (82.9) |
| Treatment | |
|
Chemotherapy | 78 (60.5) |
|
Radiotherary | 11 (8.5) |
|
Chemo-radiotherary | 28 (21.7) |
|
Other | 12 (9.3) |
| Feeding route | |
|
Oral
feeding | 112 (78.7) |
|
Tube
feeding | 17 (21.3) |
| PG-SGA | |
|
≤1 | 2 (1.6) |
|
2-3 | 3 (2.3) |
|
4-8 | 28 (21.7) |
|
≥9 | 96 (74.4) |
| Weight loss in 1
month | |
|
<5% | 88 (68.2) |
|
≥5% | 41 (31.8) |
The anthropometric and biochemical characteristics
of the patients are presented in Table II. The mean weight and height of
the patients were 55.2±10.3 kg and 161.3±7.7 cm, respectively (body
mass index, 21.1±3.3 kg/m²). The mean PG-SGA score was 15.98±9.3.
Patients lost an average of 2.9 kg in 1 month and 7.6 kg over a
period of 6 months. The mean white blood cell and lymphocyte counts
were 8.8x109/l and 1.7x109/l, respectively.
The mean hemoglobin level was 115.8 g/l.
 | Table IIAnthropometric and biochemical
characteristics of nutrition. |
Table II
Anthropometric and biochemical
characteristics of nutrition.
| Parameter | Mean ± SD |
|---|
| Weight (kg) | 55.2±10.3 |
| Height (cm) | 161.3±7.7 |
| BMI
(kg/m2) | 21.1±3.3 |
| PG-SGA | 15.98±9.3 |
| Weight loss in 1
month (kg) | 2.9±5.3 |
| Weight loss in 6
months (kg) | 7.6±8.4 |
| WBC count,
109/l | 8.8±9.3 |
| Lymphocyte count,
109/l | 1.7±2.7 |
| Hemoglobin,
g/l | 115.8±19.4 |
No significant differences were found in age, sex,
treatments, education, or tumor stage (I/II vs. III/IV) between the
malnourished and well-nourished patients (P>0.05). However, a
significant association was found between the nutritional status
(PG-SGA) and feeding route (P<0.05), with all patients with NPC
using feeding tubes being malnourished. The analysis of the
biochemical nutrition characteristics (white blood cell count,
lymphocyte count and hemoglobin) did not reveal any no significant
associations between these factors, nutritional status and QOL
(P>0.05) (Table III).
 | Table IIINutritional status according to
PG-SGA and some related factors. |
Table III
Nutritional status according to
PG-SGA and some related factors.
| Characteristic | Well-nourished
patients (n=33), (%) | Malnourished
patients (n=96), (%) | P-value |
|---|
| Age, years | | | |
|
<60 | 28 (28.9) | 69 (71.1) | 0.17a |
|
≥60 | 5 (15.6) | 27 (84.4) | |
| Sex | | | |
|
Male | 27 (28.1) | 69 (71.9) | 0.36a |
|
Female | 6 (18.2) | 27 (81.8) | |
| Education
level | | | |
|
Illiteracy | 0 (0) | 8(100) | 0.21a |
|
Primary
school | 14(25) | 42(75) | |
|
Middle
school | 13(31) | 29(69) | |
|
High
school | 3 (17.6) | 14 (82.4) | |
|
Post-high
school | 3(50) | 3(50) | |
| Stage | | | |
|
I-II | 6 (27.3) | 16 (72.7) | 0.999a |
|
III-IV | 27 (25.2) | 80 (74.8) | |
| Treatment | | | |
|
Chemotherapy | 26 (33.3) | 52 (66.7) | 0.09b |
|
Radiotherary | 1 (9.1) | 10 (90.9) | |
|
Chemo-radiotherary | 5 (17.9) | 23 (82.1) | |
|
Other | 1 (8.3) | 11 (91.7) | |
| Feeding route | | | |
|
Oral
feeding | 33 (29.5) | 79 (70.5) |
0.01b |
|
Tube
feeding | 0 (0) | 17(100) | |
| Weight loss in 1
month | | | |
|
<5% | 32 (36.4) | 56 (63.6) |
0.001b |
|
≥5% | 1 (2.4) | 40 (97.6) | |
| WBC count,
109/l | | | |
|
<4 | 4 (21.1) | 15 (78.9) | 0.78b |
|
≥4 | 29 (26.4) | 81 (73.6) | |
| Lymphocyte count,
109/l | | | |
|
<1 | 11 (21.2) | 41 (78.8) | 0.41a |
|
≥1 | 22 (28.6) | 55 (71.4) | |
| Hemoglobin,
g/l | | | |
|
<120 | 16 (21.6) | 58 (78.4) | 0.31a |
|
≥120 | 17 (30.9) | 38 (69.1) | |
The univariate association between nutritional
status (PG-SGA scores) and QOL is presented in Table IV. The average scores for general
health, physical, role-based, social, cognitive and emotional
functioning decreased with malnutrition. Conversely, the average
score of fatigue, nausea/vomiting, pain, appetite loss and
constipation increased in the malnourished patients vs. the
well-nourished patients (P<0.05).
 | Table IVAssociation between nutritional
status according to PG-SGA and quality of life. |
Table IV
Association between nutritional
status according to PG-SGA and quality of life.
| | Well-nourished
patients (n=33) | Malnourished
patients (n=96) | |
|---|
| Characteristic | Median | IQR | Median | IQR |
P-valuea |
|---|
| Global quality of
life | 77.8 | 50.0; 75.0 | 62.1 | 66.7; 95.8 | 0.001 |
| Functioning
scales | | | | | |
|
Physical | 97.2 | 80.0; 100 | 82.2 | 100; 100 | 0.001 |
|
Role | 91.9 | 50.0; 100 | 68.9 | 91.7; 100 | 0.001 |
|
Emotional | 87.6 | 66.7; 91.7 | 78.2 | 75.0; 100 | 0.009 |
|
Cognitive | 97.5 | 83.3; 100 | 86.8 | 100; 100 | 0.001 |
|
Social | 86.4 | 33.3; 100 | 63.7 | 66.7; 100 | 0.001 |
| Symptom
scale/items | | | | | |
|
Fatigue | 5.7 | 11.1; 44.4 | 31.4 | 0.0; 5.6 | 0.001 |
|
Nausea and
vomiting | 1.0 | 0.0; 33.3 | 16.7 | 0.0; 0.0 | 0.001 |
|
Pain | 8.1 | 0.0; 0.0 | 31.6 | 0.0; 0.50 | 0.001 |
|
Dyspnea | 2.0 | 0.0; 0.0 | 2.8 | 0.0; 0.0 | 0.500 |
|
Sleep
disturbance | 20.2 | 0.0; 33.3 | 28.8 | 0.0; 33.3 | 0.200 |
|
Appetite
loss | 9.1 | 33.3; 33.3 | 37.5 | 0.0; 16.7 | 0.001 |
|
Constipation | 1.0 | 0.0; 0.0 | 9.8 | 0.0; 0.0 | 0.023 |
|
Diarrhea | 2.0 | 0.0; 0.0 | 3.1 | 0.0; 0.0 | 0.401 |
|
Financial
impact | 28.3 | 0.0; 50.0 | 39.2 | 0.0; 66.7 | 0.178 |
To investigate the factors influencing the QOL of
patients, a multivariate linear regression analysis was conducted.
Independent variables included demographic characteristics,
clinical features and nutritional status assessments that exhibited
statistically significant associations in prior analyses (e.g.,
feeding route, age, disease stage, percentage weight loss over 1
month and PG-SGA score). All QOL scale scores were used as
dependent variables. The PG-SGA score significantly predicted
overall QOL, explaining 39% of the variance (adjusted R²=0.39,
F=15.95, P=0.001). The PG-SGA score also significantly affected
different areas of QOL, including physical (37%), role (32%),
emotional (17%), cognitive (17%), social (31%), fatigue (56%),
nausea/vomiting (29%) and appetite loss (53%). Furthermore, the
PG-SGA score and age significantly predicted pain symptoms (40%
variance, adjusted R²=0.40, F=42.24, P=0.001), while PG-SGA score,
age and feeding route predicted constipation symptoms (31%
variance, adjusted R²=0.31, F=18.84, P=0.001) (see Table III for detailed data) (Table V).
 | Table VResults of multivariate linear
regression analysis (enter) to predict scores on EORTC QLQ-C30
scales. |
Table V
Results of multivariate linear
regression analysis (enter) to predict scores on EORTC QLQ-C30
scales.
| | Unstandardized
coefficients | |
|---|
| Dependent
variable | Independent
variable | R2 | Adjusted
R2 | F | P-value | b | SE b | t | P-value |
|---|
| Global quality of
life | Model | 0.63 | 0.39 | 15.95 | 0.01 | | | | |
| | PG-SGA | | | | 0.01 | -1.18 | 0.24 | -4.98 | 0.01 |
| Physical | Model | 0.61 | 0.37 | 14.66 | 0.01 | | | | |
| | PG-SGA | | | | | -1.31 | 0.28 | -4.64 | 0.01 |
| Role | Model | 0.56 | 0.32 | 11.32 | 0.01 | | | | |
| | PG-SGA | | | | 0.01 | -1.96 | 0.40 | -4.96 | 0.01 |
| Emotional | Model | 0.41 | 0.17 | 4.93 | 0.01 | | | | 0.01 |
| | PG-SGA | | | | | -0.96 | 0.28 | -3.38 | 0.01 |
| Cognitive | Model | 0.41 | 0.17 | 5.09 | 0.01 | | | | |
| | PG-SGA | | | | | -1.01 | 0.27 | -3.77 | 0.01 |
| Social | Model | 0.56 | 0.31 | 11.14 | 0.01 | | | | 0.01 |
| | PG-SGA | | | | | -1.73 | 0.42 | -4.13 | 0.01 |
| Fatigue | Model | 0.75 | 0.56 | 31.35 | 0.01 | | | | 0.01 |
| | PG-SGA | | | | | 2.11 | 0.27 | 7.75 | 0.01 |
| Nausea and
vomiting | Model | 0.54 | 0.29 | 10.16 | 0.01 | | | | 0.01 |
| | PG-SGA | | | | | 1.83 | 0.30 | 6.15 | 0.01 |
| Pain | Model | 0.63 | 0.40 | 43.24 | 0.01 | | | | |
| | PG-SGA | | | | | 1.98 | 0.22 | 9.13 | 0.01 |
| | Age | | | | | -0.41 | 0.16 | -2.57 | 0.01 |
| Appetite loss | Model | 0.73 | 0.53 | 27.46 | 0.01 | | | | |
| | PG-SGA | | | | | 2.45 | 0.29 | 8.54 | 0.01 |
| Constipation | Model | 0.56 | 0.31 | 18.84 | 0.01 | | | | |
| | PG-SGA | | | | | 1.27 | 0.17 | 7.35 | 0.01 |
| | Age | | | | | -0.27 | 0.12 | -2.30 | 0.02 |
| | Feeding route | | | | | -14.90 | 4.70 | -3.17 | 0.01 |
Discussion
Malnutrition is common in patients with cancer, with
a prevalence ranging from 20 to 70% depending on the cancer type,
stage and clinical setting. This is often detected through
screening tools and results primarily from inadequate food intake
due to nutrition impact symptoms caused by the tumor itself, cancer
treatments, or disease-related complications (18-20).
The present cross-sectional study identified a high prevalence of
malnutrition among NPC patients at Can Tho Oncology Hospital. The
majority of the participants in the present study were experiencing
moderate to severe malnutrition and were in need of nutritional
assistance. Only 1.6% of the patients were well-nourished, while
74% of the patients had severe malnutrition (PG-SGA ≥9). These
results align with those of previous longitudinal studies by Wei
et al (11) (80.7% with
PG-SGA ≥9) and Miao et al (22) (82% requiring nutritional
intervention when weight loss ≥5%), although the present study
observed a lower intervention rate in the patients with NPC. This
discrepancy likely stems from the cross-sectional design pf the
present study, which captured patients at varying treatment stages,
unlike the studies by Wei et al (11) and Miao et al (22), which followed patients from the
beginning to the end of radiotherapy or chemo-radiotherapy.
Consequently, the present study sample likely included newly
treated patients with an improved nutritional status compared to
those nearing the end of treatment. Consistent with this, the study
by Wei et al (11)
demonstrated a significant increase in moderate to severe
malnutrition post-treatment (PG-SGA ≥9: 0.7% pre-treatment vs.
80.7% post-treatment). Likewise, herein, the mean cross-sectional
PG-SGA score (15.98±9.3) was higher than that of untreated patients
(7.50±5.97), but lower than that of patients receiving prolonged
radiotherapy for 4 weeks (17.75±5.56) and 6 weeks (20.50±6.76), as
reported in the study by Ding et al (23).
Malnutrition and weight loss are widespread in
patients with cancer, with reported weight loss in 20-80% of cases
and up to 60% in patients with NPC (23,24).
Excessive weight loss, often related to tumor characteristics and
treatment-related side-effects, exacerbates chemotherapy toxicity,
causes treatment interruption and is associated with poorer
treatment outcomes (25,26). Previous research has demonstrated
that severe weight loss affects the progression and recurrence of
NPC, and excessive weight loss can be considered a factor related
to survival (28). In the present
study, to minimize recall bias, patient weight data were
re-recorded from medical records documented 1 month prior. For
weight 6 months prior, recall was aided by using suggestive
questioning, cross-checking information via repetition and family
input and linking times to events. If the patient still could not
recall, their usual pre-illness weight was recorded. This approach
improves the accuracy of reported weight changes. The result was
that >30% of patients experienced ≥5% weight loss in 1 month,
with an average weight loss of 2.9±5.3 kg. In northern China, 56%
of patients experience similar weight loss at diagnosis, and the
average weight loss post-treatment is 6.9 kg (26). Benkhaled et al (29) found that 86% of patients with NPC
lost >10% of body weight by week 7 of treatment with
intensity-modulated radiation therapy with or without chemotherapy.
Such weight loss is linked to increased treatment toxicity,
interruptions and worse outcomes. Despite its clinical importance,
weight loss often goes undetected. Routine monitoring of weight and
nutritional status should be integrated into NPC management
pathways.
Timely nutritional support is crucial for patients
with NPC undergoing radiation therapy who are at risk of developing
malnutrition (6,18). Early nutritional intervention can
maintain nutritional status and improve treatment tolerance
(30). Diet and nutritional
education are continuous, emphasizing healthy cooking and small,
frequent meals. Oral nutritional supplements are used when diet and
education are insufficient (31).
In the case that oral nutritional supplements are inadequate,
enteral nutrition is initiated, and parenteral nutrition is
considered if enteral nutrition fails to meet nutritional needs
(32). The study Wang et al
(33) on the association of
nutritional counseling with the severity of radiation-induced oral
mucositis in patients with NPC demonstrated that nutritional
counseling was beneficial in reducing severe radiation-induced oral
mucositis and PG-SGA ≥4. On the other hand, radiotherapy or
chemo-radiotherapy lead to severe swallowing impairment in 50-70%
of patients, necessitating enteral nutrition during or immediately
post-treatment (34). In addition,
the present study also found an association between nutritional
status and feeding route (100% of patients with NPC need
nutritional intervention when feeding through a tube; P<0.05;
Table III). Enteral nutrition is
indicated in patients who have nutritional issues (unable to
tolerate at least 60% of their energy and protein needs orally for
7-14 days, even with education, medication and supplements)
(20,35) combined with dietary habits,
finances and inadequate nutritional knowledge that can further
complicate nutritional management. The truth is that at the
authors' research site, a common option for nasogastric tube
feeding is diluted white porridge, favored for its easy pump,
particularly among patients with NPC and other types of cancer.
However, its low nutritional content and the limited inclusion of
energy-dense foods often result in an inadequate daily energy
intake, heightening the risk of malnutrition. This highlights the
critical need for improved nutritional strategies, such as
integrating energy-rich foods into tube feeds and enhancing
nutritional education for both patients and caregivers, and
choosing locally available food sources to reduce costs. These
measures are essential for preventing malnutrition and promoting
improved health outcomes in patients with NPC.
The present study found that malnutrition
significantly reduced global and functional QOL scores, while
increasing symptom burden, particularly fatigue, nausea and
vomiting, pain, appetite loss and constipation (P<0.05), which
is similar to findings from previous studies (36,37).
Social and role function were found to be most affected by
radiotherapy, likely due to physical changes (e.g., ulcerated skin
and fatigue), emotional distress and body image concerns (38), resulting in social withdrawal
during the treatment. Symptoms of pain (mainly caused by
mucositis), fatigue, dry mouth and abnormal taste changes increased
during radiotherapy, which markedly affects the appetite and eating
habits of patients (30,31). In addition, almost all patients
experienced abnormal taste, decreased salivation and dry mouth,
which may be the main reasons for loss of appetite in patients with
NPC undergoing radiotherapy (37).
Fatigue is a common and distressing symptom experienced by patients
with cancer. The combination of anorexia and early satiety in
patients with cancer is associated with poorer overall health
perception, role function and increased fatigue. These prevalent
appetite disorders significantly impair the nutritional status and
QOL of patients, particularly when occurring in conjunction. This
can have profound effects on QOL and physical functioning (41,42).
Fatigue is further exacerbated by concurrent chemotherapy and
radiotherapy (43). In addition to
sociodemographic and tumor-related factors and disease-specific
symptoms, during treatment, tube feeding also has an impact on
weight, nutritional status and QOL. It has been demonstrated that
dietary counseling helps avoid weight loss and improves QOL.
Previous studies have demonstrated that patients with head and neck
cancer who received nutritional counseling during radiation therapy
had a lower likelihood of weight loss, as well as a lower
likelihood of deterioration in symptoms, functional scores and
overall QOL (44,45). Early nutritional monitoring may
prevent malnutrition and improve the QOL of patients with cancer,
as suggested by a study on 312 patients that found an association
between early monitoring and improved outcomes (46). Therefore, nutritional monitoring
could be implemented during the early stages for NPC to improve its
outcomes.
Convenience sampling and study samples being
collected solely from one research center may bias results,
limiting generalizability to all NPC. In the present study, the
inclusion of all patients undergoing treatment limited the
evaluation of how individual treatment-related side-effects affect
nutrition and QOL. Future research is thus require to focus on
single chemotherapy or radiotherapy regimens for more specific
results. The present cross-sectional study cannot definitively
prove causality between nutritional status and quality of life.
Cross-sectional data limits our ability to determine if nutrition
affects quality of life, or vice versa, or if other factors
influence both. Future large cohort studies are warranted to
confirm associations and the direction of influence.
In conclusion, malnutrition is highly prevalent
among patients with NPC and is associated with significant weight
loss, symptom burden and a reduced QOL. The present study
highlights the need for early nutritional screening (e.g., PG-SGA),
individualized dietary counseling and optimized enteral feeding
using energy-dense, locally available foods. Enhancing caregiver
education and initiating timely interventions can prevent
nutritional decline, improve treatment tolerance, and ultimately
enhance the clinical outcomes and QOL of patients.
Acknowledgements
Not applicable.
Funding
Funding: The author(s) received no financial support for the
research, authorship, and/or publication of this article.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors (KVV, HTTP, HTL, KND and ALTN)
contributed to the conception and design of the study. HTTP, HTL
and KND carried out the statistical analysis of the data. The
study's investigators included HTTP, HTL, KND, ALTN and KVV. HTTP
and HLT worked together to interpret the data. HTTP, ALTN and KND
contributed to the initial draft of the text. HTTP, HTL, ALTN and
KVV helped write, evaluate and revise the manuscript. HTTP and HTL
confirm the authenticity of all the raw. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hanoi Medical University (Hanoi, Vietnam) under
Decision no. NCS2024/GCN-HMUIRB, dated May 15, 2024. It is
understood that the study team provided the potential participants
with study-related information on the contents and objectives of
the study and sought their consent to participate by signing the
consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bossi P, Chan AT, Licitra L, Trama A,
Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J,
et al: Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
32:452–465. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
International Agency for Research on
Cancer (IARC): Global Cancer Observatory. IARC, Lyon, 2024.
https://gco.iarc.fr/. Accessed April 17,
2024.
|
|
4
|
Miao J, Xiao W, Wang L, Han F, Wu H, Deng
X, Guo X and Zhao C: The value of the prognostic nutritional index
(PNI) in predicting outcomes and guiding the treatment strategy of
nasopharyngeal carcinoma (NPC) patients receiving
intensity-modulated radiotherapy (IMRT) with or without
chemotherapy. J Cancer Res Clin Oncol. 143:1263–1273.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng J, He Y, Sun XS, Li JM, Xin MZ, Li
WQ, Li ZX, Nie S, Wang C, Li YZ, et al: Construction of a
comprehensive nutritional index and its correlation with quality of
life and survival in patients with nasopharyngeal carcinoma
undergoing IMRT: A prospective study. Oral Oncol. 98:62–68.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bischoff SC, Austin P, Boeykens K,
Chourdakis M, Cuerda C, Jonkers-Schuitema C, Lichota M, Nyulasi I,
Schneider SM, Stanga Z and Pironi L: ESPEN guideline on home
enteral nutrition. Clin Nutr. 39:5–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ji J, Jiang DD, Xu Z, Yang YQ, Qian KY and
Zhang MX: Continuous quality improvement of nutrition management
during radiotherapy in patients with nasopharyngeal carcinoma. Nurs
Open. 8:3261–3270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shu Z, Zeng Z, Yu B, Huang S, Hua Y, Jin
T, Tao C, Wang L, Cao C, Xu Z, et al: Nutritional status and its
association with radiation-induced oral mucositis in patients with
nasopharyngeal carcinoma during radiotherapy: A prospective study.
Front Oncol. 10(594687)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hong JS, Wu LH, Su L, Zhang HR, Lv WL,
Zhang WJ and Tian J: Effect of chemoradiotherapy on nutrition
status of patients with nasopharyngeal cancer. Nutr Cancer.
68:63–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wan M, Zhang L, Chen C, Zhao D, Zheng B,
Xiao S, Liu W, Xu X, Wang Y, Zhuang B, et al: GLIM criteria-defined
malnutrition informs on survival of nasopharyngeal carcinoma
patients undergoing radiotherapy. Nutr Cancer. 74:2920–2929.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xueyan W, Ying L and Desheng H:
Nutritional status and its influencing factors of nasopharyngeal
carcinoma patients during chemoradiotherapy. Zhongliu Fangzhi
Yanjiu. 47:524–530. 2020.(In Chinese).
|
|
12
|
Zhuang B, Zhang L, Wang Y, Zhang T, Jin
SL, Gong L, Fang Y, Xiao S, Zheng B, Lu Q and Sun Y: Malnutrition
and its relationship with nutrition impact symptoms and quality of
life at the end of radiotherapy in patients with head and neck
cancer. Chinese Journal of Clinical Nutrition. 28:207–213.
2020.
|
|
13
|
Teixeira AC, Mariani MGC, Toniato TS,
Valente KP, Petarli GB, Pereira TSS and Guandalini VR: Scored
Patient-Generated Subjective Global Assessment: risk identification
and need for nutritional intervention in cancer patients at
hospital admission. Nutrición Clínica y Dietética Hospitalaria.
38:95–102. 2018.
|
|
14
|
Singh S, Raj E and Santhosh G:
Patient-generated subjective global assessment (PG-SGA) as a
nutrition assessment tool in patients with cancer. IP Journal of
Nutrition, Metabolism and Health Science. 7:60–67. 2024.
|
|
15
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB and de Haes JC: The European Organization for Research and
treatment of cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
EORTC-Quality of Life: EORTC Quality of
Life Group. Giving a voice to patients. https://qol.eortc.org/.
|
|
17
|
Bauer J, Capra S and Ferguson M: Use of
the scored patient-generated subjective global assessment (PG-SGA)
as a nutrition assessment tool in patients with cancer. Eur J Clin
Nutr. 56:779–785. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rogers SN, Semple C, Babb M and Humphris
G: Quality of life considerations in head and neck cancer: United
Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 130
(Suppl 2):S49–S52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arends J: Malnutrition in cancer patients:
Causes, consequences and treatment options. Eur J Surg Oncol.
50(107074)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arends J, Bachmann P, Baracos V,
Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring
E, Kaasa S, et al: ESPEN guidelines on nutrition in cancer
patients. Clin Nutr. 36:11–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marshall KM, Loeliger J, Nolte L, Kelaart
A and Kiss NK: Prevalence of malnutrition and impact on clinical
outcomes in cancer services: A comparison of two time points. Clin
Nutr. 38:644–651. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miao J, Wang L, Ong EHW, Hu C, Lin S, Chen
X, Chen Y, Zhong Y, Jin F, Lin Q, et al: Effects of induction
chemotherapy on nutrition status in locally advanced nasopharyngeal
carcinoma: A multicentre prospective study. J Cachexia Sarcopenia
Muscle. 14:815–825. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ding H, Dou S, Ling Y, Zhu G, Wang Q, Wu Y
and Qian Y: Longitudinal body composition changes and the
importance of fat-free mass index in locally advanced
nasopharyngeal carcinoma patients undergoing concurrent
chemoradiotherapy. Integr Cancer Ther. 17:1125–1131.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bozzetti F, Gavazzi C, Miceli R, Rossi N,
Mariani L, Cozzaglio L, Bonfanti G and Piacenza :
Perioperative total parenteral nutrition in malnourished,
gastrointestinal cancer patients: A randomized, clinical trial. J
Parenter Enteral Nutr. 24:7–14. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Langius JAE, van Dijk AM, Doornaert P,
Kruizenga HM, Langendijk JA, Leemans CR, Weijs PJ and Verdonck-de
Leeuw IM: More than 10% weight loss in head and neck cancer
patients during radiotherapy is independently associated with
deterioration in quality of life. Nutr Cancer. 65:76–83.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiu C, Yang N, Tian G and Liu H: Weight
loss during radiotherapy for nasopharyngeal carcinoma: A
prospective study from Northern China. Nutr Cancer. 63:873–879.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ng K, Leung SK, Johnson PJ and Woo J:
Nutritional consequences of radiotherapy in nasopharynx cancer
patients. Nutr Cancer. 49:156–161. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ou Q, Cui C, Zeng X, Dong A, Wei X, Chen
M, Liu L, Zhao Y, Li H and Lin W: Grading and prognosis of weight
loss before and after treatment with optimal cutoff values in
nasopharyngeal carcinoma. Nutrition. 78(110943)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Benkhaled S, Dragan T, Beauvois S, De
Caluwé A and Van Gestel D: Weight loss in nasopharyngeal cancer is
mainly associated with pre-treatment dental extraction, a European
Single-Center Experience. J Cancer Sci Ther. 11(3)2019.
|
|
30
|
Meng L, Wei J, Ji R, Wang B, Xu X, Xin Y
and Jiang X: Effect of early nutrition intervention on advanced
nasopharyngeal carcinoma patients receiving chemoradiotherapy. J
Cancer. 10:3650–3656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Langius JAE, Zandbergen MC, Eerenstein
SEJ, van Tulder MW, Leemans CR, Kramer MHH and Weijs PJ: Effect of
nutritional interventions on nutritional status, quality of life
and mortality in patients with head and neck cancer receiving
(chemo)radiotherapy: A systematic review. Clin Nutr. 32:671–678.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fan X, Cui H and Liu S: Summary of the
best evidence for nutritional support programs in nasopharyngeal
carcinoma patients undergoing radiotherapy. Front Nutr.
11(1413117)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang SA, Zhu YH, Liu WJ, Haq IU, Gu JY, Qi
L, Yang M and Yang J: Association of nutritional counselling with
the severity of radiation-induced oral mucositis in patients with
nasopharyngeal carcinoma: A retrospective study. Nutrition Clinique
et Métabolisme. 38:244–250. 2024.
|
|
34
|
Karmakar-Mangaj S, Laskar SG and Talapatra
K: Choosing optimal feeding method in head-neck cancer patients
receiving radiation: Percutaneous endoscopic gastrostomy versus
nasogastric tube-is it pertinent? J Curr Oncol. 6:57–60. 2023.
|
|
35
|
Bechtold ML, Brown PM, Escuro A, Grenda B,
Johnston T, Kozeniecki M, Limketkai BN, Nelson KK, Powers J, Ronan
A, et al: When is enteral nutrition indicated? JPEN J Parenter
Enteral Nutr. 46:1470–1496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Löser A, Avanesov M, Thieme A, Gargioni E,
Baehr A, Hintelmann K, Tribius S, Krüll A and Petersen C:
Nutritional status impacts quality of life in head and neck cancer
patients undergoing (Chemo)Radiotherapy: Results from the
prospective HEADNUT trial. Nutr Cancer. 74:2887–2895.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kan Y, Yang S, Wu X, Wang S, Li X, Zhang
F, Wang P and Zhao J: The quality of life in nasopharyngeal
carcinoma radiotherapy: A longitudinal study. Asia Pac J Oncol
Nurs. 10(100251)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li JB, Guo SS, Tang LQ, Guo L, Mo HY, Chen
QY and Mai HQ: Longitudinal trend of health-related quality of life
during concurrent chemoradiotherapy and survival in patients with
stage II-IVb nasopharyngeal carcinoma. Front Oncol.
10(579292)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hua X, Chen LM, Zhu Q, Hu W, Lin C, Long
ZQ, Wen W, Sun XQ, Lu ZJ, Chen QY, et al: Efficacy of
controlled-release oxycodone for reducing pain due to oral
mucositis in nasopharyngeal carcinoma patients treated with
concurrent chemoradiotherapy: A prospective clinical trial. Support
Care Cancer. 27:3759–3767. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chan YW, Chow VLY and Wei WI: Quality of
life of patients after salvage nasopharyngectomy for recurrent
nasopharyngeal carcinoma. Cancer. 118:3710–3718. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stone P, Candelmi DE, Kandola K, Montero
L, Smetham D, Suleman S, Fernando A and Rojí R: Management of
fatigue in patients with advanced cancer. Curr Treat Options Oncol.
24:93–107. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Galindo DE, Vidal-Casariego A,
Calleja-Fernández A, Hernández-Moreno A, Pintor de la Maza B,
Pedraza-Lorenzo M, Rodríguez-García MA, Ávila-Turcios DM,
Alejo-Ramos M, Villar-Taibo R, et al: Appetite disorders in cancer
patients: Impact on nutritional status and quality of life.
Appetite. 114:23–27. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen LM, Yang QL, Duan YY, Huan XZ, He Y,
Wang C, Fan YY, Cai YC, Li JM, Chen LP and Qin HY: Multidimensional
fatigue in patients with nasopharyngeal carcinoma receiving
concurrent chemoradiotherapy: Incidence, severity, and risk
factors. Support Care Cancer. 29:5009–5019. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ravasco P, Monteiro-Grillo I, Vidal PM and
Camilo ME: Impact of nutrition on outcome: A prospective randomized
controlled trial in patients with head and neck cancer undergoing
radiotherapy. Head Neck. 27:659–668. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Isenring EA, Capra S and Bauer JD:
Nutrition intervention is beneficial in oncology outpatients
receiving radiotherapy to the gastrointestinal or head and neck
area. Br J Cancer. 91:447–452. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang YH, Xie FY, Chen YW, Wang HX, Tian
WX, Sun WG and Wu J: Evaluating the nutritional status of oncology
patients and its association with quality of life. Biomed Environ
Sci. 31:637–644. 2018.PubMed/NCBI View Article : Google Scholar
|