Introduction
The abuse of drugs in modern society has increased
steeply during the last decade and has become a severe psychiatric
problem worldwide. Cocaine is one such widely abused and addictive
drug that acts on the central nervous system (CNS). Currently,
approximately 3.6 million Americans use cocaine on a regular basis
(1).
The toxic effect of cocaine is observed in nearly
all vital organs of the body. However, cocaine is most notably
known for its psychostimulant effects on the CNS by binding to
various neurotransmitter transporters with high affinity. Cocaine
binding prevents the reuptake of dopamine by pre-synaptic neurons
in the nucleus accumbens of the brain (2), and the excess extraneuronal dopamine
at the synaptic cleft causes a euphoric feeling due to its repeated
binding to post-synaptic neurons. Unfortunately, the injurious
aspect of cocaine is that it causes severe toxicity to various
brain cells.
To understand the mechanism of cocaine toxicity,
many in vitro studies have been conducted. Most have been
carried out for long exposure times such as 1 day (3–5) or
6 days (6) or 7 days (7). These extended endpoints may not
provide an accurate picture of cocaine cytotoxicity since cocaine
is removed rapidly from the body as demonstrated by its 1-h
half-life (8–10). Thus, the various deleterious
effects on CNS cells of cocaine users are experienced within this
short period. With regards to the short half-life of cocaine, in
vitro toxicity studies with shorter incubations will have more
in vivo relevance in terms of understanding the cytotoxicity
profile. Thus far no studies have been attempted to identify the
short term impact of cocaine in different types of CNS cells.
One of the CNS cell types that is first affected by
cocaine owing to their abundance is astrocytes. Since neurons
depend on astrocytes for tropic support, cocaine-induced death of
astrocytes may lead to neuronal dysfunction in cocaine addicts.
Drugs which can prevent cocaine-induced death in astrocytes could
avert neuronal dysfunction in cocaine addicts. Yet, currently there
is no specific pharmacological medication available for this
purpose.
In the present study, we investigated the potential
role of N-acetyl-L-cysteine (NAC) against cocaine-induced toxicity
in astrocytes. NAC is commonly used as a nutritional supplement for
various health benefits in the US. Albeit NAC is a known
antioxidant compound, its mechanism of protection in the context of
a 1-h cocaine exposure has not been studied. We employed rat C6
astroglial cells in this study. These cells are astrocytes in
origin and have several merits as previously outlined (11,12), making it a suitable model cell
line for pharmacological studies. One-hour treatment was selected
based on the 1-h half-life of cocaine (9).
Materials and methods
Materials
RPMI-1640, fetal bovine serum (FBS),
penicillin/streptomycin sulfate, amphotericin B, phosphate-buffered
saline (PBS) and L-glutamine were purchased from Mediatech
(Herndon, VA, USA). Cocaine hydrochloride, crystal violet,
2′,7′-dichlorodihydrofluorescein diacetate dye
(H2DCFDA), 2,2-diphenyl-1-picrylhydrazyl,
ethylenediaminetetraacetic acid (EDTA), L-glutaraldehyde, NAC and
trypan blue were supplied by Sigma Chemical Co. (St. Louis, MO,
USA). All other routine chemicals were of analytical grade.
Preparation of drug solutions
A known amount of NAC was dissolved in PBS as a 0.5
M stock. Various working stocks of NAC (40–200 mM) were prepared in
the media and added to the cells. Cocaine stock and working stocks
were prepared in PBS as previously described (13) just prior to the studies and added
to the cells to achieve 2 to 4 mM.
Cell culture studies
The CNS-derived rat C6 astroglial cell line
(CCL-107) was purchased from the American Type Culture Collection
(Rockville, MD, USA) and maintained as an adherent monolayer
culture in complete RPMI-1640 (modified) medium, 2 mM L-glutamine,
10% (v/v) FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate
and 0.25 μg/ml amphotericin B. Cells were grown in a humidified
atmosphere of 95% air, 5% CO2 at 37°C in an incubator,
and subcultured twice a week. For the cytotoxic studies, the
culture was harvested by treatment with 0.05% EDTA in PBS for 2 min
or less, resulting in a single-cell suspension. The cell count was
assessed by 0.4% trypan blue dye exclusion assay using a
hemocytometer under a light microscope.
Treatment of cells
Treatments were performed in 96-well microtiter
plates. The cells were seeded at a starting density of
2×104 cells/well in a total volume of 195 μl growth
medium supplemented with 10% FBS. The cells were then allowed to
adhere to the wells in the incubator prior to drug exposure. Cells
which were typically ~80–90% confluent were treated with 2, 3 and 4
mM cocaine in a final volume of 5 μl. In some experiments, 5 μl NAC
from different working stocks was added per well to achieve the
final concentrations of 1 to 5 mM. Controls and the treated samples
were always present in different wells of the same 96-well
microtiter culture plate. These plates were incubated for 1 h in an
incubator in 5% CO2 at 37°C.
Evaluation of cytotoxicity
At the end of the 1-h treatment, 100 μl of 0.25%
glutaraldehyde was added per well and incubation was carried out
for 30 min to fix the cells to the polystyrene surface of the
culture plates. The plates were gently washed three times and air
dried. The cells were then stained with crystal violet as described
previously (14,15). The dye in each well was extracted
with 100 μl of 50 mM sodium phosphate monobasic solution,
containing 50% ethyl alcohol. The plates were gently vortexed, and
the optical density (OD) measurements of the incorporated dye in
the viable cells were carried out at 540 nm using a microplate
reader (BioTek Instruments, Inc., Wincoski, VT, USA). From the
treated and control absorbance values, the percentage of cells
killed was determined using the following equation: [1 − (T/C)] ×
100, where T is the average absorbance values of the treated cells,
and C is the average absorbance values of the control cells.
Antioxidation activity of NAC
The experiment was carried out at different final
concentrations of NAC (0.02–0.1 mM) in 1-ml Eppendorf tubes without
cells with ethanol as a solvent. Free radical
2,2-diphenyl-1-picrylhydrazyl was added to the samples at a final
concentration of 0.1 mM. Milk thistle, a known antioxidant, was
used as a positive control (0.1 mg/ml). After a 30-min incubation
at room temperature, the solution in each tube was transferred to a
96-well plate. Absorbance at 517 nm was read using a microplate
reader. The percentage of scavenging activity was calculated
(16) by the following equation:
[(OD of control - OD of NAC)/OD of control] × 100.
Cocaine pro-oxidant study
The pro-oxidant activity of cocaine was assessed in
phenol red-free RPMI-1640 (modified) media in 96-well microtiter
plates without employing the cells. The assay utilized
H2DCFDA dye. Different concentrations of cocaine (final
2–4 mM) were incubated with 0.1 mM (final) H2DCFDA for 1
h in an incubator with the plates capped in a normal fashion. The
plates were read with the excitation filter set at 485 nm and the
emission filter at 530 nm in a Microplate Fluorometer Model 7620,
version 5.02 (Cambridge Technology, Inc., Watertown, MA, USA).
Hydrogen peroxide (0.1 mM) was used as a positive control.
Nitric oxide (NO) assay
NO production with cocaine treatment (2–4 mM) was
assessed as reported previously (17) in the media lacking phenol red. A
5-point standard curve was generated by using various
concentrations of sodium nitrite (25–400 μM) in culture media. The
absorbance readings at 546 nm were measured using a microplate
reader.
Estimation of cellular glutathione (GSH)
levels
Cellular GSH was determined according to Smith et
al(18). In brief, at the end
of the incubation, the cells were deproteinized with 2%
5-sulfosalicylic acid (10 μl/well) for 30 min at 37°C, followed by
addition of 90 μl of the reaction mixture containing 0.416 mM
sodium EDTA, 0.416 mM nicotinamide adenosine dinucleotide phosphate
(NADPH), 0.835 mM 5,5-dithiobis-2-nitrobenzoic acid (DTNB), 0.083
mM sodium phosphate buffer, pH 7.5. The mixture was incubated at
37°C for 30 min, and the absorbance was measured at 412 nm using a
plate reader.
Pretreatments with NAC
Experiments were performed in 96-well culture plates
under the conditions described above in complete media. In brief,
the C6 astrocytes were pretreated with 5 μl of 0.2 M NAC to obtain
a final concentration of 5 mM for 30 min. The media was discarded,
and the cells were washed gently to remove NAC. Then 195 μl fresh
media were added per well, followed by treatments with 5 μl of
80–160 mM cocaine working stocks to achieve final concentrations of
2–4 mM for 1 h. In the case of NAC-cocaine mixture studies, 0.2 M
NAC was incubated with 80–160 mM cocaine in Eppendorf tubes for 30
min in an incubator at 37°C and added to the cells to achieve final
concentrations of 5 mM NAC and 2, 3 and 4 mM cocaine. At the end of
the incubation, cell viability and GSH levels were determined as
described above.
Statistical analysis
The experimental results are presented as means ±
standard error of the mean (SEM). The data were analyzed for
significance by one-way ANOVA and then compared by Dunnett’s
multiple comparison tests using GraphPad Prism Software, version
3.00 (GraphPad Software, Inc., San Diego, CA, USA). The test value
of P<0.05 was considered significant. The LC50 or
ED50 values representing the millimolar concentration of
cocaine needed to show a 50% response were determined from the
graphs (19).
Results
Dose-response study with NAC
Prior to evaluating any pro-survival action of NAC
on C6 astroglial cells against acute cocaine treatment, we first
assessed NAC effects on cell viability at concentrations of 1, 2,
3, 4 and 5 mM. The treatment was continued for 1 h, and the data
are shown in Fig. 1. It was
observed that NAC did not cause cell death (P>0.05, n=6) at any
concentration compared to the control (Fig. 1). The viability results were
corroborated by the microscopic observations of crystal
violet-stained cells. The photomicrographs revealed that the
morphology of the NAC-treated cells was similar in resemblance to
the untreated control cells (Fig.
2B). Lack of NAC toxicity in astroglial cells corroborated a
similar viability result with ascorbic acid as previously reported
(20).
Scavenging activity
NAC is a well-known antioxidant. In this study, we
aimed to determine its activity under our experimental conditions.
Initial studies with concentrations ranging between 0.1 and 1 mM
NAC showed high antioxidation activity. In the subsequent
experiments, the concentrations were adjusted between 0.01 and 0.1
mM. The data are presented in Fig.
3. A significant (P<0.01, n=9) dose-dependent increase in
the scavenging activity of NAC was noted. The ED50 value
was found to be 0.047 mM.
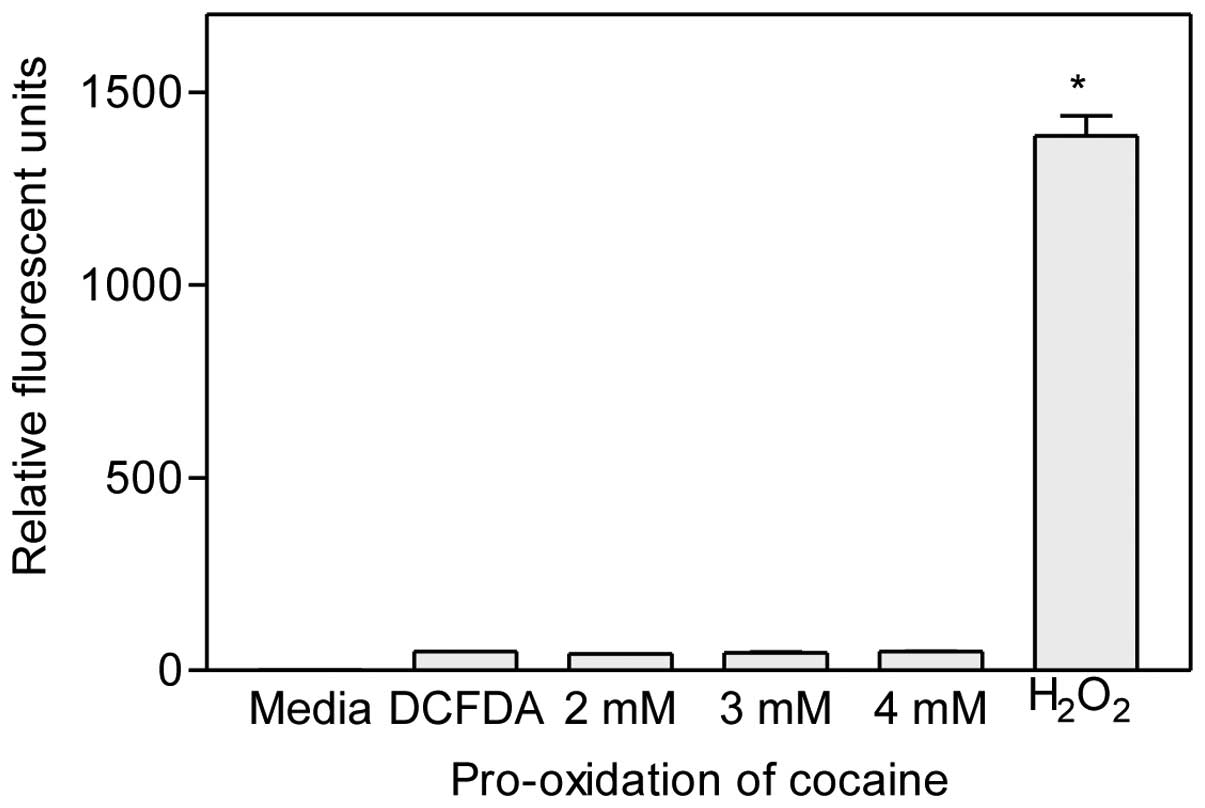
Pro-oxidant activity of cocaine
Various concentrations of cocaine (final 2–4 mM)
were mixed with H2DCFDA dye (final 0.1 mM) and incubated
without cells for 1 h at 37°C. Since this dye is converted to
fluorescent dichlorodihydrofluorescein (DCF) molecules upon
oxidation by reactive oxygen species (ROS), it represents a
convenient method to determine whether cocaine exhibits pro-oxidant
activity during treatment periods in cells. Results indicated that
there was no progressive increase (P>0.05, n=6) in the amount of
fluorescence from the dye following cocaine treatment at any
concentration (Fig. 4). These
results clearly indicate that cocaine did not exhibit pro-oxidant
activity. In contrast, 0.1 mM H2O2, the
positive control, released very high levels of fluorescence from
the H2DCFDA dye, demonstrating the reliability of the
method.
Lack of NO release
Overproduction of NO contributes to various
pathological conditions in the CNS. In order to study the role of
cocaine in NO production, the C6 astrocytes were treated with
cocaine (2–4 mM) for 1 h. The cells were not challenged with
lipopolysaccharide or interferon-γ as these cells contain NO
synthase (21) and our objective
was to determine whether cocaine induces this enzyme. It was
observed that acute cocaine did not induce (P>0.05, n=12)
cellular NO synthase for NO production when compared to the
controls (Fig. 5A). On the other
hand, sodium nitrite standard exhibited a linear response (Fig. 5B).
Attenuation of cocaine toxicity by
NAC
Based on the non-toxicity of NAC to the C6
astrocytes (Fig. 1), we selected
5 mM NAC to determine its efficacy for cell protection against
cocaine-induced toxicity. For this purpose, the cells were
initially pretreated with NAC at a final concentration of 5 mM for
30 min. Then the media were discarded completely, and cells were
re-fed with fresh media, followed by treatment with acute cocaine
at 2, 3 and 4 mM for 1 h. The cell viability results indicated that
NAC rendered 90–100% protection (P<0.01, n=16) against cocaine
cytotoxicity (Fig. 6A). The
LC50 value was found to be >4 mM cocaine. In the
absence of NAC pretreatment, a significant (P<0.01, n=16)
dose-dependant cell death by cocaine was noted with an
LC50 value of 2.857 mM. The cell morphology of
pretreated cells following cocaine exposure remained the same as
that of the control cells (Fig.
2D). In contrast, the cell morphology of the non-pretreated
cells with cocaine was altered significantly (Fig. 2C). In addition, several
cytoplasmic vacuoles were observed in these cells. In the case of
the NAC-cocaine mixture studies, the cell viability remained 100%
(P<0.01, n=16) in the presence of NAC, while in its absence, the
viability was significantly decreased (P<0.01, n=16) (Fig. 6B). Thus, either pretreatment with
NAC followed by cocaine or co-treatment of NAC and cocaine produced
very similar results (Fig.
6).
NAC pretreatment enhances GSH
synthesis
Based on the significant results of cell protection
by NAC pretreatment against cocaine exposure (Fig. 6), we subsequently evaluated the
GSH levels in these cells. In the non-pretreated cells, cocaine
treatment at 2, 3 and 4 mM decreased the GSH levels (Fig. 7). However, with a 30-min
pretreatment with 5 mM NAC, followed by its discard, the GSH levels
were increased significantly (P<0.05, n=8) in all treated
cells.
Discussion
Cocaine is hydrolyzed rapidly by various esterases
in the body (22). In addition,
it is also degraded non-enzymatically by auto-oxidation at basic
physiological pH (23). In spite
of these losses, entry of a small fraction of intact cocaine into
various domains of the brain can cause cell death or deformation of
cell morphology to a high extent. In the present study, cocaine
treatment of astroglial cells was evidenced by the presence of
cytoplasmic vacuoles (Fig. 2C).
Previous studies showed that cocaine interaction decreased both
membrane potential (13) and the
general respiratory status (17)
of mitochondria. This type of association often results in high ROS
release in cells (24). It is
possible that a portion of the released ROS accounts for cocaine’s
pro-oxidation action. We employed a fluorometric method to detect
this activity. The data clearly indicated that cocaine did not have
pro-oxidation activity (Fig. 4)
during the study period. Thus, the generated ROS was solely due to
cocaine interaction with mitochondria.
Cocaine interaction with mitochondria also creates a
condition similar to hypoxia. This situation in astroglial cells
favors inflammation and causes excess release of NO by inducible NO
synthase (25). Dysfunctional
mitochondria (26) and excess
release of NO (27) have been
implicated in several late-onset neurodegenerative diseases such as
Parkinson’s disease (28),
schizophrenia and Alzheimer’s disease (29). There are some speculations that
drug addicts are more likely to be affected by these diseases at an
early age. Unfortunately, few reports are available to link the
release of NO from astroglial cells in drug addicts to
neurodegenerative diseases. Our data clearly rule out that
astroglial cells are the source of NO release (Fig. 5). However, it may still be
possible that NO is released from different CNS cells such as
neurons or activated microglial cells following cocaine exposure.
Lack of NO production in our study suggests that cocaine toxicity
was not through the NO pathway in astroglial cells.
It is known that cocaine induces damage to different
types of CNS cells. Of the three main types of cells found in the
brain, namely neurons, astroglial cells and oligodendrocytes,
astrocytes are the most abundant cells (30). These cells not only function in
neuronal survival but also maintain the fundamental patterns of
circuitry. Furthermore, due to close contacts with endothelial
cells of the blood-brain barrier (BBB) (31), it is possible that astrocytes are
the first cells to face toxic insults by cocaine. Since neuronal
survival depends on astrocytes, death of astrocytes by cocaine
could immediately trigger neuronal dysfunction. This may lead to
cognitive defects, an observation commonly found in drug addicts.
Counter measures, which inhibit cocaine-induced death in
astrocytes, may prevent eventual neuronal dysfunction. In the
absence of timely remedial measures, it is possible that a strong
stage may be set for long-term effects such as neuronal death or
drug tolerance in cocaine addicts. Therefore, the main question is
how to decrease or prevent the toxic effects of cocaine in
cells.
To this end, we explored the effects of the
pretreatment of cells with antioxidants in cultures. We evaluated
the role of NAC, a sulfhydryl compound, against acute
cocaine-induced toxicity in C6 astroglial cells. The antioxidant
activity of NAC is well known (32). Prior to testing its attenuating
effect, a dose-response study was conducted to determine the
optimum dose for further studies. The non-toxicity of NAC following
a 1-h exposure to astroglial cells was evident in the viability
study (Fig. 1). Even at a dose of
5 mM, all cells were alive and maintained the same morphological
features as the untreated controls (Fig. 2A and B). Thus, we pretreated the
astroglial cells with 5 mM NAC.
We observed that a 30-min NAC pretreatment decreased
the cocaine toxicity by 90% in the cells (Fig. 6A). Furthermore, the cells treated
with NAC-cocaine mixture were rendered 100% protection (Fig. 6B). Our study demonstrated that
cocaine exposure caused a decrease in the GSH levels in cells.
However, NAC pretreatment boosted these levels similar to the
controls even in the presence of cocaine (Fig. 7). It is known that hydrolysis of
NAC provides an L-cystein precursor for GSH biosynthesis in cells.
Comparison of the controls indicated that there was a clear
insignificant increase in GSH level in the NAC-pretreated cells
than that in the non-pretreated cells (Fig. 7). This observation suggests that
the L-cystein precursor from hydrolyzed NAC was utilized in
intracellular GSH synthesis during the pretreatment period. These
results clearly suggest that the observed cell protection against
cocaine-induced toxicity was through increased GSH levels. The
results of cocaine toxicity and NAC protection appear to imply that
both compounds share similar target sites in these cells. Another
important finding in our study was that we observed no cell death
following NAC-cocaine co-treatment (Fig. 6B). We plan to use our treatment
in vivo to determine whether these same protective effects
are observed in animals.
Acknowledgements
This study was supported by the NIH grant NCRR/RCMI
G12 RR03020 and the NIH grant NIMHD 8G12 MD 007582–28 of the
USA.
Abbreviations:
|
BBB
|
blood-brain barrier
|
|
CNS
|
central nervous system
|
|
DCF
|
dichlorodihydrofluorescein
|
|
DTNB
|
5,5-dithiobis-2-nitrobenzoic acid
|
|
EDTA
|
ethylene diamine tetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
GSH
|
glutathione
|
|
H2DCFDA
|
2′,7′-dichlorodihydrofluorescein
diacetate dye
|
|
NAC
|
N-acetyl-L-cysteine
|
|
NADPH
|
nicotinamide adenosine dinucleotide
phosphate
|
|
NO
|
nitric oxide
|
|
OD
|
optical density
|
|
PBS
|
phosphate-buffered saline
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
National Institute on Drug Abuse. NIDA
Research Report Series. National Institutes of Health Publications;
Bethesda, MD: Revised May, 2009.
|
|
2
|
Ritz MC, Lamb RJ, Goldberg SR and Kuhar
MJ: Cocaine receptors on dopamine transporters are related to
self-administration of cocaine. Science. 237:1219–1223. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee C, Chen J, Hayashi T, et al: A
mechanism for the inhibition of neural progenitor cell
proliferation by cocaine. PLoS Med. 5:e1172008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunha-Oliveira T, Rego AC, Cardoso SM, et
al: Mitochondrial dysfunction and caspase activation in rat
cortical neurons treated with cocaine or amphetamine. Brain Res.
1089:44–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garg UC, Turndorf H and Bansinath M:
Effect of cocaine on macromolecular syntheses and cell
proliferation in cultured glial cells. Neuroscience. 57:467–472.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu J, Yassini P, Goldberg G, et al:
Cocaine cytotoxicity in serum-free environment: C6 glioma cell
culture. Neurotoxicology. 14:19–22. 1993.PubMed/NCBI
|
|
7
|
Hu S, Cheeran MC, Sheng WS, et al: Cocaine
alters proliferation, migration, and differentiation of human fetal
brain-derived neural precursor cells. J Pharmacol Exp Ther.
318:1280–1286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilkinson P, Van Dyke C, Jatlow P, et al:
Intranasal and oral cocaine kinetics. Clin Pharmacol Ther.
27:386–394. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barnett G, Hawks R and Resnick R: Cocaine
pharmacokinetics in humans. J Ethnopharmacol. 3:353–366. 1981.
View Article : Google Scholar
|
|
10
|
Chou MJ, Ambre JJ, Ruo TI, et al: Kinetics
of cocaine distribution, elimination, and chronotropic effects.
Clin Pharmacol Ther. 38:318–324. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Monte DA, Wu EY, Delanney LE, et al:
Toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in primary
cultures of mouse astrocytes. J Pharmacol Exp Ther. 261:44–49.
1992.
|
|
12
|
Sibenaller ZA, Etame AB, Ali MM, et al:
Genetic characterization of commonly used glioma cell lines in the
rat animal model system. Neurosurg Focus. 19:E12005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Badisa RB, Darling-Reed SF and Goodman CB:
Cocaine induces alterations in mitochondrial membrane potential and
dual cell cycle arrest in rat c6 astroglioma cells. Neurochem Res.
35:288–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Badisa RB, Tzakou O, Couladis M and
Pilarinou E: Cytotoxic activities of some Greek Labiatae herbs.
Phytother Res. 17:472–476. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano M, Lin AW, McCurrach ME, et al:
Oncogenic ras provokes premature cell senescence associated
with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997.
|
|
16
|
Patel RM and Patel NJ: In vitro
antioxidant activity of coumarin compounds by DPPH, super oxide and
nitric oxide free radical scavenging methods. JAPER. 1:52–68.
2011.
|
|
17
|
Badisa RB and Goodman CB: Effect of
chronic cocaine in rat C6 astroglial cells. Int J Mol Med.
30:687–692. 2012.PubMed/NCBI
|
|
18
|
Smith IK, Vierheller TL and Thorne CA:
Assay of glutathione reductase in crude tissue homogenates using
5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem. 175:408–413.
1988.PubMed/NCBI
|
|
19
|
Ipsen J and Feigl P: Bancroft’s
Introduction to Biostatistics. Harper and Row; New York: pp.
4551970
|
|
20
|
Hardaway CM, Badisa RB and Soliman KF:
Effect of ascorbic acid and hydrogen peroxide on mouse
neuroblastoma cells. Mol Med Rep. 5:1449–1452. 2012.PubMed/NCBI
|
|
21
|
Green SJ and Nacy CA: Antimicrobial and
immunopathologic effects of cytokine-induced nitric oxide
synthesis. Curr Opin Infect Dis. 6:384–396. 1993.
|
|
22
|
Stewart DJ, Inaba T, Tang BK and Kalow W:
Hydrolysis of cocaine in human plasma by cholinesterase. Life Sci.
20:1557–1563. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das Gupta V: Stability of cocaine
hydrochloride solutions at various pH values as determined by
high-pressure liquid chromatography. Int J Pharm. 10:249–257.
1982.
|
|
24
|
Yang Y, Yao H, Lu Y, et al: Cocaine
potentiates astrocyte toxicity mediated by human immunodeficiency
virus (HIV-1) protein gp120. PLoS One. 5:e134272010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawase M, Kinouchi H, Kato I, et al:
Inducible nitric oxide synthase following hypoxia in rat cultured
glial cells. Brain Res. 738:319–322. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blass JP: Glucose/mitochondria in
neurological conditions. Int Rev Neurobiol. 51:325–376. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulz JB, Lindenau J, Seyfried J and
Dichgans J: Glutathione, oxidative stress and neurodegeneration.
Eur J Biochem. 267:4904–4911. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hunot S, Boissière F, Faucheux B, et al:
Nitric oxide synthase and neuronal vulnerability in Parkinson’s
disease. Neuroscience. 72:355–363. 1996.
|
|
29
|
Dorheim MA, Tracey WR, Pollock JS and
Grammas P: Nitric oxide synthase activity is elevated in brain
microvessels in Alzheimer’s disease. Biochem Biophys Res Commun.
205:659–665. 1994.
|
|
30
|
Kandel ER: Nerve cells and behavior.
Principles of Neural Science. Kandel ER, Schwartz JH and Jessell
TM: 3rd edition. Appleton and Lange; Norwalk, CT: pp. 18–32.
1991
|
|
31
|
Attwell D: Glia and neurons in dialogue.
Nature. 369:707–708. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Estany S, Palacio JR, Barnadas R, et al:
Antioxidant activity of N-acetylcysteine, flavonoids and
alpha-tocopherol on endometrial cells in culture. J Reprod Immunol.
75:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|