Introduction
Vascular calcification is prevalent in patients with
atherosclerosis, aging, end-stage renal failure, uremia and type 2
diabetes and is a strong predictor of cardiovascular morbidity and
mortality (1–3). In previous years, vascular
calcification was considered to be a passive process. However,
vascular calcification is now recognized as an organized, highly
regulated process which is similar to mineralization in bone tissue
(4,5). A number of factors, such as
bioactive peptides, cytokines, leptin and elevated oxidative
stress, are involved in the regulation of vascular calcification
(6–9).
Several vascular cell types, such as pericytes,
vascular smooth muscle cells and fibroblasts, undergo phenotypic
change during vascular calcification (10). Vascular smooth muscle cells
(VSMCs) have been shown to promote the formation of vascular
calcifications (11). Several
bone markers have been detected in atherosclerotic plaque,
including collagen I, osteocalcin, osteopontin, matrix GLA protein
and osteoglycin, indicating that VSMCs can be transdifferentiated
into osteoblast-like cells (12).
Connective tissue growth factor (CTGF) is a member
of the CCN (cef10/cyr61/ccn1, ctgf/ccn2 and nov/ccn3) family, and
has been shown to be upregulated in atherosclerotic plaque
(13). CTGF is involved in a
variety of autocrine or paracrine functions in several cell types,
including fibroblasts, vascular endothelial, neuronal, epithelial
and vascular smooth muscle cells, as well as cells of supportive
skeletal tissue (14). CTGF is
considered to contribute to the development of atherosclerotic
lesions by increasing VSMC migration, proliferation, adhesion,
apoptosis and the secretion of matrix components (15–17). However, the effect of CTGF on
vascular calcification and the transdifferentiation of VSMCs has
not yet been fully elucidated.
There is increasing evidence suggesting that
extracellular signal-regulated kinase (ERK) plays a crucial role in
VSMC calcification (18–21). Furthermore, evidence has
accumulated indicating that CTGF activates intracellular signaling
pathways, including the ERK, c-Jun N-terminal kinase (JNK) and p38
MAPK pathways (21–25).
The present study aimed to investigate the role of
CTGF in VSMC calcification and to determine whether the ERK
signaling pathway is involved in this process.
Materials and methods
Methods
All experiments were carried out in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the United States National Institutes of Health. Study approval was
granted by the Ethics Committee of the Second Xiangya Hospital of
Central South University, Changsha, China.
Reagents
Recombinant human CTGF was purchased from PeproTech
(Rocky Hill, NJ, USA). Antibodies for phosphorylated ERK1/2
(p-ERK1/2), phosphorylated p38 (p-p38), phosphorylated JNK (p-JNK),
ERK1/2, p38, or JNK and anti-mouse and anti-rabbit IgG horseradish
peroxidase (HRP)- conjugated antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Waltham, MA, USA). The ERK-specific
inhibitor, PD98059, was purchased from Calbiochem Corp. (San Diego,
CA, USA). β-glycerophosphate was purchased from Sigma (St. Louis,
MO, USA). Kunming mice were purchased from the Animal Center, the
Second Xiangya Hospital of Central South University. To isolate
primary VSMCs, the mice received a 150 mg/kg intraperitoneal dose
of pentobarbital sodium prior to euthanasia which was confirmed by
the absence of breathing and a heartbeat.
VSMCs culture
Primary VSMCs used in the present study were
obtained from mouse thoracic aortas by enzymatic digestion as
previously described and identified by immunostaining for smooth
muscle specific α-actin antibody (α-SMA) (26). Briefly, a fragment of mouse
thoracic aorta was stripped of adventitia and intima. VSMCs were
obtained from the remaining medial layer. The cells were grown in
Dulbecco's modified Eagle's medium (DMEM; high glucose, 4.5 g/l;
Gibco-BRL, Carlsbad, CA, USA) containing 5–10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA). The culture medium were changed
once every 2 or 3 days.
In vitro calcification
VSMC calcification was induced by the addition of
CTGF to the osteogenic mediaum containing 5–10% FBS in the presence
of 0.25 mM ascorbic acid and 10 mM β-glycerophosphate for 14 days.
In some experiments, the cells were incubated with CTGF (0, 10, 20,
50, 100 and 200 ng/ml) for 14 days with medium changes every 2–3
days.
Quantification of calcium deposition
Measurement of total calcium in the extracellular
matrix was performed as previously described (27). The cells were decalcified with
0.60 M HCl for 24 h. The calcium content was determined by
measuring the concentrations of calcium in the HCl supernatant by
atomic absorption spectroscopy. Following decalcification, the
cells were washed 3 times with phosphate-buffered solution and
solubilized with 0.1 M NaOH/0.1% sodium dodecyl sulfate. The total
protein content was determined using a Bradford protein assay. The
calcium content of the cell layer was normalized to the protein
content.
Alizarin Red S Staining
The calcifying cells that were characterized by the
appearance of multilayer nodules were determined by Alizarin Red S
staining as previously described (28). Briefly, the cells were washed with
PBS 3 times, and fixed in 95% ethanol for 10 min at room
temperature. The cells were then washed with distilled water 3
times and exposed to 1.0% Alizarin Red S/Tris-HCl solution (pH
>8.3) for 30 min at 37°C. After staining, the cells were washed
with distilled water to eliminate non-specific staining.
Quantitative real-time RT-PCR
The effect of CTGF on the expression of bone-related
gene markers in the VSMCs was determined by quantitative real-time
RT-PCR using a Roche Molecular LightCycler (Roche Applied Science,
Indianapolis, IN, USA). Total RNA was isolated from the cultured
VSMCs using TRIzol reagent (Invitrogen, Carslbad, CA, USA) and
reverse-transcribed into cDNA according to the recommended protocol
provided with the reverse transcription kit (Fermentas, Hanover,
MD, USA). PCR were performed using specific primers as follows:
osteoprotegerin (OPG) sense, 5′-AGTCCGTGAA GCAGGAGT-3′ and
antisense, 5′-CCATCTGGACATTTTTT GCAAA-3′; osteocalcin (OC) sense,
5′-CTGACAAAGCCTT CATGTCCAA-3′ and antisense, 5′-GCGCCGGAGTCTGTT
CACTA-3′; core-binding factor subunit α1 (Cbfα1)/runt-related
transcription factor 2 (Runx2) sense, 5′-CCGGTCTCCTTC CAGGAT-3′ and
antisense, 5′-GGGAACTGCTGTGGC TTC-3′; alkaline phosphatase (ALP)
sense, 5′-GACCTCCTC GGAAGACACTC-3′ and antisense, 5′-AGGCCCATTGCC
ATACAG-3′; and GAPDH sense, 5′-GGCTGCCCAGAACA TCAT-3′ and
antisense, 5′-CGGACACATTGGGGGTAG-3′; SYBR-Green-based real-time PCR
was performed according to the recommendations of the SYBR-Green
kit using SYBR Premix Ex Taq [Takara Biotechnology (Dalian) Co.,
Ltd., Dalian, China].
Western blot analysis
Western blot analysis was performed to investigate
the expression of Runx2 protein and the activity of the downstream
intracellular signaling pathways. VSMC extracts were prepared, and
the protein concentration was quantified using a Bradford protein
assay. A total of 18 μg of protein from each cell layer or 30 μl
conditioned medium from VSMC cultures were loaded onto a 8.0%
polyacrylamide gel. Proteins were separated by electrophoresis on
an SDS-PAGE polyacrylamide gel and then were transferred to a
0.45-mm nitrocellulose membrane. The membrane was incubated at 4°C
overnight with antibodies for Runx2, p-ERK1/2, ERK1/2, JNK, p-JNK,
p38 or p-p38 and then with the appropriate HRP-conjugated secondary
antibodies. Immunoreactive proteins were detected using an ECL kit
and then exposed to X-ray film.
Statistical analysis
SPSS 13.0 software was used for statistical
analyses. The results are expressed as the means ± SD. One-way
analysis of variance was used to compare the experimental with the
control groups. All experiments were repeated at least 3 times. A
P-value <0.05 was considred to indicate a statistically
significant difference.
Results
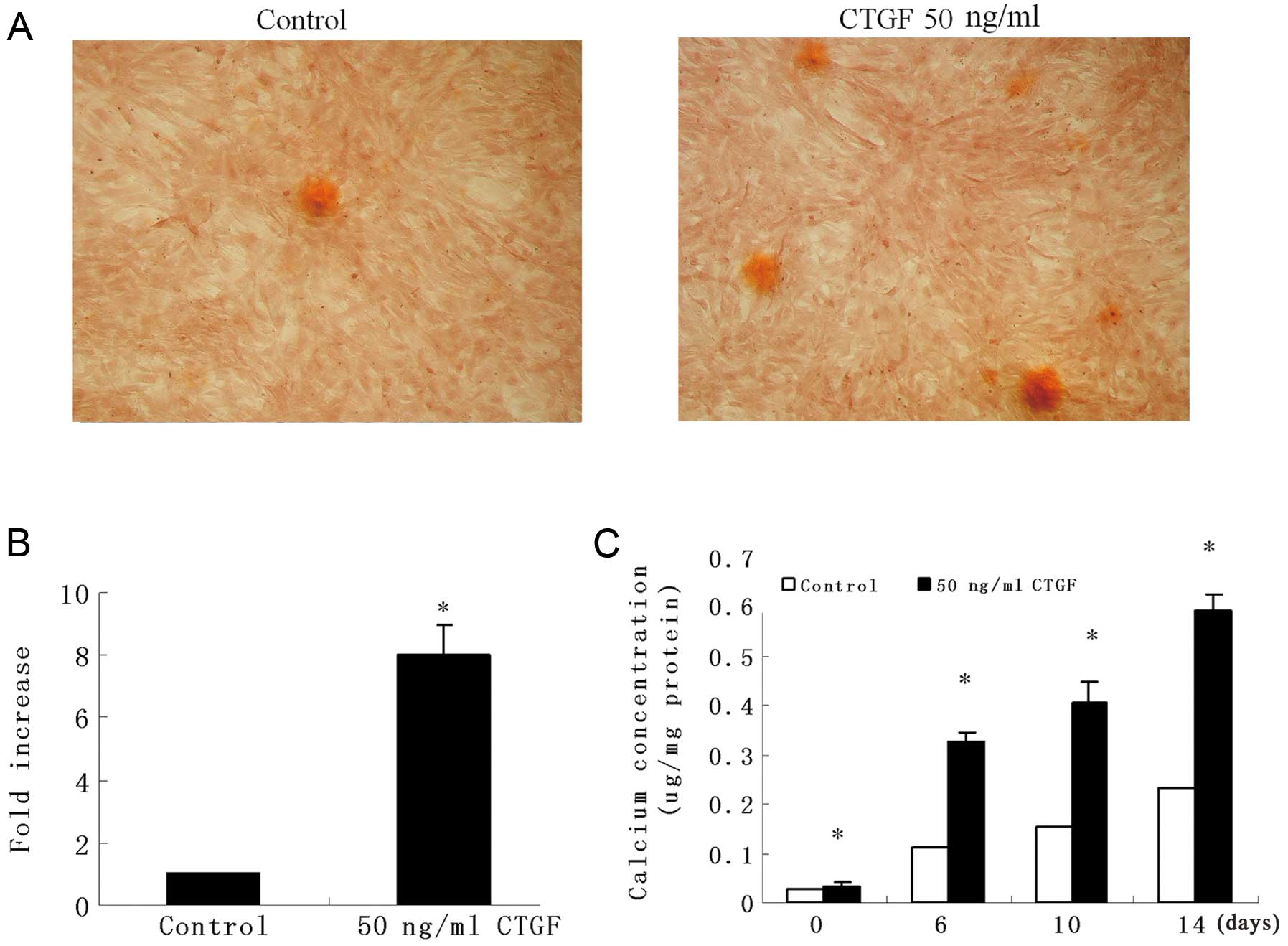
CTGF induces VSMC calcification
To determine the role of CTGF in vascular
calcification, we incubated VSMCs from mouse thoracic aortas in
DMEM supplemented with 0.25 mM ascorbic acid and 10 mM
β-glycerophosphate in the presence or absence of CTGF for 14 days,
and then the amount of calcification in the cells was determined by
Alizarin Red S staining. After 14 days, calcifying nodules appeared
in CTGF-treated (50 ng/ml) VSMCs, but fewer nodules appeared in the
controls (Fig. 1A and B). We then
measured calcification using 50 ng/ml CTGF. Likewise, the results
showed that CTGF promoted the formation of calcium deposits in a
time-dependent manner (Fig. 1C).
These results suggest that CTGF increases vascular
calcification.
CTGF induces the expression of bone
markers and smooth muscle cell markers
Recent studies have suggested that the process of
vascular calcification is similar to the procedure of osteogenesis
(29,30). Therefore, we examined the effect
of CTGF on osteogenic transdifferentiation by assessing the
expression of bone and VSMC markers. The data from the real-time
PCR experiments revealed that the mRNA expression of bone markers,
such as ALP, OPG and OC was significantly upregulated after the
VSMCs were exposed to 50 ng/ml CTGF for 14 days (Fig. 2A). Western blot analysis was
performed to determine the expression of the VSMC marker, α-SMA
(Fig. 2B). The results revealed
that the level of α-SMA was also increased during the osteogenic
differentiation of VSMCs. These data suggest that CTGF induces the
transdifferentiation of VSMCs into osteoblasts. CTGF may promote
the proliferation of VSMCs as previously described by Fan et
al (15).
CTGF increases Runx2 expression in
VSMCs
Runx2, one of the phenotypic markers of osteoblasts,
is thought to be a key regulator of osteoblast differentiation and
is thought to play an important role in the expression of several
bone markers (31). Thus, we
examined whether CTGF affects the expression and transactivity of
Runx2 during the process of VSMC calcification. Real-time
quantitative RT-PCR analysis and western blot analysis were
performed to examine the effects of CTGF on the expression of
Runx2. The data from western blot analysis showed that the protein
expression of Runx2 was increased in the VSMCs exposed to CTGF (0,
10, 20, 50, 100 and 200 ng/ml) for 14 days (Fig. 3A) in a concentration-dependent
manner. In addition, real-time PCR demonstrated a 4.1-fold increase
in Runx2 mRNA expression after the VSMCs were treated with 50 ng/ml
CTGF for 14 days (Fig. 3B). Thus,
these results indicate that CTGF induces the expression of
Runx2.
ERK signaling mediates CTGF-induced Runx2
activation and VSMC calcification
ERK plays an essential role in controlling vascular
calcification, and CTGF is involved in the activation of
extracellular signaling pathways, including the ERK, JNK and p38
MAPK pathways. Therefore, we determined whether the activation of
these signaling pathways by CTGF is required for VSMC
calcification. Western blot analysis revealed that CTGF activated
ERK and the peak activation of ERK occurred at the 30-min time
point (Fig. 4A). However, CTGF
had no effect on the phosphorylation of JNK and p38 (Fig. 4A). The effect of the ERK inhibitor
(PD98059) on ERK activation was determined by western blot
analysis, demonstrating the inhibition of p-ERK in the VSMCs
(Fig. 4B). To futher determine
whether CTGF stimulates vascular calcification through the ERK
pathway, we examined the effect of the ERK signaling pathway
inhibitor, PD98059, on VSMC calcification induced by CTGF.
Treatment of the VSMCs with the inhibitor of ERK signaling
(PD98059) abolished CTGF-induced VSMC calcification (Fig. 4C), suggesting that CTGF
accelerates VSMC calcification through the ERK signaling pathway.
Furthermore, we found that the induction of Runx2 expression by
CTGF was prevented by the ERK inhibitor (Fig. 4D and E). In conclusion, our data
indicate that the ERK signaling pathway plays an important role in
CTGF-induced VSMC calcification.
Discussion
CTGF is a 38-kDa cysteine-rich protein that belongs
to the CCN gene family and is known to play an important role in
the development of atherosclerosis. CTGF is abundantly expressed in
the zone of provisional calcification and in VSMCs around
calcification lesions (13,32). CTGF exerts favorable effects on
the osteogenic differentiation of mesenchymal stem cells (33). Previous studies have indicated
that CTGF promotes atherosclerosis by stimulating VSMC growth,
migration, adhesion, apoptosis and the production of extracellular
matrix components (15,17,34). However, to our knowledge, there
are no data available on the role of CTGF in the osteogenic
differentiation of VSMCs. In the present study, we demonstrate that
CTGF, at concentrations of 10 to 200 ng/ml, induces the osteogenic
differentiation and calcification of VSMCs in a
concentration-dependent manner, and that the effect of CTGF on the
calcification of VSMCs appears to occur through the activation of
the ERK signaling pathway.
Previous studies have demonstrated that vascular
calcification is an active and cell-regulated process resembling
skeletal mineralization. It is well known that VSMCs can
transdifferentiate from contractile into distinct osteoblast-like
cells during the process of vascular calcification (35). VSMCs can undergo reversible
differentiation into various cells types, including osteoblasts,
adipocytes and chondrocytes in atherosclerosis (36,37). Accumulating evidence suggests that
the imbalance of phosphate and calcium metabolism, high glucose,
chronic inflammation and oxidative stress induce the expression of
osteogenic differentiation markers in VSMCs and may contribute to
the development of vascular calcification (38,39). We found that CTGF induces the
expression of osteocyte phenotype markers and contributes to the
phenotypic transition of VSMCs and VSMC calcification.
Bone differentiation markers have been detected in
calcified areas, and these proteins are commonly used as markers to
indicate the osteogenic/chondrogenic differentiation of VSMCs into
osteoblast-like cells (39).
These bone-related proteins and genes include transcription
factors, such as Gas6, Cbfα1 or Msx2 (40), factors that may contribute to the
mineralization process, including ALP, bone sialoprotein and OC, as
well as inhibitors of osteochondrogenic mineralization, such as
osteopontin, matrix γ-carboxyglutamic acid and OPG (41). The CTGF-induced VSMC calcification
is associated with the induction of bone markers, including ALP, OC
and OPG, demonstrating the transdifferentiation of VSMCs into
osteoblast-like cells. It has been demonstrated in previous studies
that ALP genes are early markers of the transdifferentiation of
VSMCs into phenotypically osteoblast-like cells, and that OC
proteins are indicators of the late stage of transdifferentiation
(42,43). OPG is a key regulator of
osteoclastogenesis and may protect cells from arterial
calcification (44). Runx2, a key
transcription factor for osteoblast and chondrocyte
differentiation, regulates the phenotypic conversion of VSMCs.
Previous studies have indicated that Runx2 plays an essential role
in oxidative stress-induced VSMC calcification in vitro, and
Runx2 alone is sufficient to induce VSMC calcification (38). In this study, we found that CTGF
promoted the phenotypic switch of VSMCs, with the CTGF-treated
VSMCs showing significant increases in ALP, OC, OPG and Runx2
expression. CTGF induced VSMC calcification with the induction of
bone markers; however, it is interesting that CTGF is unlike other
factors, such as oxidative stress and fibronectin that can
downregulate the expression of the VSMC-specific marker, α-SMA
(18,38). Perhaps CTGF can not only induce
the osteogenic differentiation of VSMCs but can also promote the
growth of VSMCs, both of which contribute to vascular
calcification.
We further determined the molecular signals involved
in CTGF-induced VSMC calcification. A number of studies have
demonstrated that the ERK pathway is involved in vascular
calcification with the ERK pathway activated by oxidized
low-density lipoprotein (LDL), PI3-kinase, homocysteine,
fibronectin and fibroblast growth factor-2 (18,19,45–47). p38 MAPK and JNK also contribute to
the induction of vascular calcification. Advanced glycation end
products induce the calcification of VSMCs via the p38 MAPK
signaling pathway (48). In this
study, we found that the CTGF-activated ERK signaling, but not p38
MAPK or JNK signaling, was required for the process of vascular
calcification. Furthermore, PD98059, an ERK-specific inhibitor,
significantly suppressed the effect of CTGF on VSMC calcification
and osteoblastic expression. These findings suggest that CTGF
enhances vascular calcification and the osteogenic differentiation
of VSMCs through the ERK signaling pathway.
Vascular calcification correlates with osteoporosis,
and the vascular RANKL system is a common mechanism of osteoporosis
and vascular calcification. A recent study found that estrogen
inhibits vascular calcification via the vascular RANKL system
(49). Our previous study showed
that estrogen inhibits the expression of CTGF in a dose-dependent
manner (50). It is not known
whether the effect of estrogen on vascular calcification is
associated with the effect of CTGF on VSMC calcification and
osteoblast differentiation, and whether estrogen inhibits vascular
calcification by inhibiting the expression of CTGF. Therefore,
further studies are warranted to explore the combined effect of
CTGF and estrogen on VSMC calcification and osteoblast
differentiation.
Taken together, our results suggest that CTGF
enhances in vitro calcification by inducing the osteogenic
differentiation and calcification of VSMCs, and that this effect
may be occur through the activation of the ERK pathway. The
ERK-specific inhibitor, PD98059, suppressed nodule formation,
calcium deposition and the expression of bone markers. Our findings
reveal the function of CTGF in vascular calcification and may aid
in the development of novel therapies for the prevention and
treatment of diseases associated with vascular calcification.
Acknowledgements
This study was supported by the China National
Natural Scientific Foundation (grant nos. 30600661, 30572078 and
81070246). We thank Zhongjian Xie, Rongrong Cui and Qiuhua Liang
for their assistance and advice.
References
|
1
|
London GM, Marchais SJ, Guerin AP and
Metivier F: Arteriosclerosis, vascular calcifications and
cardiovascular disease in uremia. Curr Opin Nephrol Hypertens.
14:525–531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mackenzie NC, Zhu D, Longley L, Patterson
CS, Kommareddy S and MacRae VE: MOVAS-1 cell line: A new in
vitro model of vascular calcification. Int J Mol Med.
27:663–668. 2011.PubMed/NCBI
|
|
3
|
Mikhaylova L, Malmquist J and Nurminskaya
M: Regulation of in vitro vascular calcification by BMP4, VEGF and
Wnt3a. Calcif Tissue Int. 81:372–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Otto CM, Kuusisto J, Reichenbach DD, Gown
AM and O'Brien KD: Characterization of the early lesion of
‘degenerative’ valvular aortic stenosis. Histological and
immunohistochemical studies. Circulation. 90:844–853. 1994.
|
|
5
|
Watson KE, Bostrom K, Ravindranath R, Lam
T, Norton B and Demer LL: TGF-beta 1 and 25-hydroxycholesterol
stimulate osteoblast-like vascular cells to calcify. J Clin Invest.
93:2106–2113. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurabayashi M: Differentiation of smooth
muscle cells and vascular calcification. Clin Calcium.
20:1637–1644. 2010.(In Japanese).
|
|
7
|
Nakagami H, Osako MK and Morishita R: New
concept of vascular calcification and metabolism. Curr Vasc
Pharmacol. 9:124–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parhami F, Morrow AD, Balucan J, et al:
Lipid oxidation products have opposite effects on calcifying
vascular cell and bone cell differentiation. A possible explanation
for the paradox of arterial calcification in osteoporotic patients.
Arterioscler Thromb Vasc Biol. 17:680–687. 1997. View Article : Google Scholar
|
|
9
|
Shioi A, Katagi M, Okuno Y, et al:
Induction of bone-type alkaline phosphatase in human vascular
smooth muscle cells: roles of tumor necrosis factor-alpha and
oncostatin M derived from macrophages. Circ Res. 91:9–16. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan XY, Xie PL, Ma YL and Tang SY:
Omentin inhibits osteoblastic differentiation of calcifying
vascular smooth muscle cells through the PI3K/Akt pathway. Amino
Acids. 41:1223–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iyemere VP, Proudfoot D, Weissberg PL and
Shanahan CM: Vascular smooth muscle cell phenotypic plasticity and
the regulation of vascular calcification. J Intern Med.
260:192–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi A: Vascular calcification and
bone-related factors. Clin Calcium. 12:1078–1083. 2002.(In
Japanese).
|
|
13
|
Oemar BS, Werner A, Garnier JM, et al:
Human connective tissue growth factor is expressed in advanced
atherosclerotic lesions. Circulation. 95:831–839. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moussad EE and Brigstock DR: Connective
tissue growth factor: what's in a name. Mol Genet Metab.
71:276–292. 2000. View Article : Google Scholar
|
|
15
|
Fan WH, Pech M and Karnovsky MJ:
Connective tissue growth factor (CTGF) stimulates vascular smooth
muscle cell growth and migration in vitro. Eur J Cell Biol.
79:915–923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hishikawa K, Oemar BS, Tanner FC, Nakaki
T, Fujii T and Luscher TF: Overexpression of connective tissue
growth factor gene induces apoptosis in human aortic smooth muscle
cells. Circulation. 100:2108–2112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanazawa S, Miyake T, Kakinuma T, Tanemoto
K, Tsunoda T and Kikuchi K: The expression of platelet-derived
growth factor and connective tissue growth factor in different
types of abdominal aortic aneurysms. J Cardiovasc Surg (Torino).
46:271–278. 2005.PubMed/NCBI
|
|
18
|
Ding HT, Wang CG, Zhang TL and Wang K:
Fibronectin enhances in vitro vascular calcification by promoting
osteoblastic differentiation of vascular smooth muscle cells via
ERK pathway. J Cell Biochem. 99:1343–1352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Chai S, Tang C and Du J:
Homocysteine potentiates calcification of cultured rat aortic
smooth muscle cells. Life Sci. 74:451–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao XB, Zhou XM, Li JM, et al: Taurine
inhibits osteoblastic differentiation of vascular smooth muscle
cells via the ERK pathway. Amino Acids. 34:525–530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang M, Huang H, Li J, Li D and Wang H:
Tyrosine phosphorylation of the LDL receptor-related protein (LRP)
and activation of the ERK pathway are required for connective
tissue growth factor to potentiate myofibroblast differentiation.
FASEB J. 18:1920–1921. 2004.
|
|
22
|
Huang HC, Yang M, Li JZ and Wang HY:
Connective tissue growth factor promotes the proliferation of
myofibroblast through Erk-1/2 signaling pathway. Zhonghua Yi Xue Za
Zhi. 85:1322–1326. 2005.(In Chinese).
|
|
23
|
Johnson PR, Burgess JK, Ge Q, et al:
Connective tissue growth factor induces extracellular matrix in
asthmatic airway smooth muscle. Am J Respir Crit Care Med.
173:32–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ponticos M, Holmes AM, Shi-wen X, et al:
Pivotal role of connective tissue growth factor in lung fibrosis:
MAPK-dependent transcriptional activation of type I collagen.
Arthritis Rheum. 60:2142–2155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yosimichi G, Nakanishi T, Nishida T,
Hattori T, Takano-Yamamoto T and Takigawa M: CTGF/Hcs24 induces
chondrocyte differentiation through a p38 mitogen-activated protein
kinase (p38MAPK), and proliferation through a
p44/42MAPK/extracellular-signal regulated kinase (ERK). Eur J
Biochem. 268:6058–6065. 2001. View Article : Google Scholar
|
|
26
|
Ross R, Glomset J, Kariya B and Harker L:
A platelet-dependent serum factor that stimulates the proliferation
of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA.
71:1207–1210. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wada T, McKee MD, Steitz S and Giachelli
CM: Calcification of vascular smooth muscle cell cultures:
inhibition by osteopontin. Circ Res. 84:166–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alexander MY, Wilkinson FL, Kirton JP, et
al: Identification and characterization of vascular
calcification-associated factor, a novel gene upregulated during
vascular calcification in vitro and in vivo. Arterioscler Thromb
Vasc Biol. 25:1851–1857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shioi A: Molecular mechanisms of vascular
calcification. Clin Calcium. 20:1611–1619. 2010.(In Japanese).
|
|
30
|
Sinha S, Eddington H and Kalra PA:
Vascular calcification: lessons from scientific models. J Ren Care.
35(Suppl 1): 51–56. 2009. View Article : Google Scholar
|
|
31
|
Nakano-Kurimoto R, Ikeda K, Uraoka M, et
al: Replicative senescence of vascular smooth muscle cells enhances
the calcification through initiating the osteoblastic transition.
Am J Physiol Heart Circ Physiol. 297:H1673–H1684. 2009. View Article : Google Scholar
|
|
32
|
Yamaai T, Nakanishi T, Asano M, et al:
Gene expression of connective tissue growth factor (CTGF/CCN2) in
calcifying tissues of normal and cbfa1-null mutant mice in late
stage of embryonic development. J Bone Miner Metab. 23:280–288.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang JJ, Ye F, Cheng LJ, et al: Osteogenic
differentiation of mesenchymal stem cells promoted by
overexpression of connective tissue growth factor. J Zhejiang Univ
Sci B. 10:355–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Game BA, He L, Jarido V, et al:
Pioglitazone inhibits connective tissue growth factor expression in
advanced atherosclerotic plaques in low-density lipoprotein
receptor-deficient mice. Atherosclerosis. 192:85–91. 2007.
View Article : Google Scholar
|
|
35
|
Steitz SA, Speer MY, Curinga G, et al:
Smooth muscle cell phenotypic transition associated with
calcification: upregulation of Cbfa1 and downregulation of smooth
muscle lineage markers. Circ Res. 89:1147–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson RC, Leopold JA and Loscalzo J:
Vascular calcification: pathobiological mechanisms and clinical
implications. Circ Res. 99:1044–1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tyson KL, Reynolds JL, McNair R, Zhang Q,
Weissberg PL and Shanahan CM: Osteo/chondrocytic transcription
factors and their target genes exhibit distinct patterns of
expression in human arterial calcification. Arterioscler Thromb
Vasc Biol. 23:489–494. 2003. View Article : Google Scholar
|
|
38
|
Byon CH, Javed A, Dai Q, et al: Oxidative
stress induces vascular calcification through modulation of the
osteogenic transcription factor Runx2 by AKT signaling. J Biol
Chem. 283:15319–15327. 2008. View Article : Google Scholar
|
|
39
|
Liu Y and Shanahan CM: Signalling pathways
and vascular calcification. Front Biosci. 16:1302–1314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Son BK and Akishita M: Mechanism of
vascular calcification. Clin Calcium. 17:319–324. 2007.(In
Japanese).
|
|
41
|
Valdivielso JM: Vascular calcification:
types and mechanisms. Nefrologia. 31:142–147. 2011.(In
Spanish).
|
|
42
|
Gallop PM, Lian JB and Hauschka PV:
Carboxylated calcium-binding proteins and vitamin K. N Engl J Med.
302:1460–1466. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stinson RA and Hamilton BA: Human liver
plasma membranes contain an enzyme activity that removes membrane
anchor from alkaline phosphatase and converts it to a plasma-like
form. Clin Biochem. 27:49–55. 1994. View Article : Google Scholar
|
|
44
|
Zhang J, Fu M, Myles D, et al: PDGF
induces osteoprotegerin expression in vascular smooth muscle cells
by multiple signal pathways. FEBS Lett. 521:180–184. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bear M, Butcher M and Shaughnessy SG:
Oxidized low-density lipoprotein acts synergistically with
beta-glycerophosphate to induce osteoblast differentiation in
primary cultures of vascular smooth muscle cells. J Cell Biochem.
105:185–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakahara T, Sato H, Shimizu T, et al:
Fibroblast growth factor-2 induces osteogenic differentiation
through a Runx2 activation in vascular smooth muscle cells. Biochem
Biophys Res Commun. 394:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
You H, Yang H, Zhu Q, et al: Advanced
oxidation protein products induce vascular calcification by
promoting osteoblastic trans-differentiation of smooth muscle cells
via oxidative stress and ERK pathway. Ren Fail. 31:313–319. 2009.
View Article : Google Scholar
|
|
48
|
Tanikawa T, Okada Y, Tanikawa R and Tanaka
Y: Advanced glycation end products induce calcification of vascular
smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 46:572–580.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Osako MK, Nakagami H, Koibuchi N, et al:
Estrogen inhibits vascular calcification via vascular RANKL system:
common mechanism of osteoporosis and vascular calcification. Circ
Res. 107:466–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng Y, Wu M, Huang J, Xie H, Meng J and
Liao E: 17-β estradiol down-regulates the expression of CTGF in
vascular smooth muscle cells of mouse. In: The 10th National
Endocrinology Conference of Chinese Medical Association; Suzhou,
Jiangsu Province, P.R. China. 2011, (In Chinese).
|