Introduction
The incidence of retinal neovascular diseases,
including diabetic retinopathy, retinopathy of prematurity (ROP),
and age-related macular degeneration (1), has significantly increased in recent
years and is one of the major causes of blindness in China. ROP is
one of the leading causes of premature blindness in infants. In the
oxygen-induced retinopathy (OIR) model frequently used in retinal
neovascular research, retinal vessel constriction is induced by
high oxygen, resulting in retinal ischemia and hypoxia. Due to
immaturity, the contraction of the retinal vascular smooth muscle
in infants is sensitively regulated by oxygen concentration
(2). Inhalation of high
concentrations of oxygen may induce retinal vessel occlusion. Upon
removal of high oxygen conditions, hypoxia stimulates the formation
of new blood vessels, abnormal vascular proliferation and
expansion. The endothelial cells in these new blood vessels lack
normal vascular barrier properties. The absence of vascular
integrity results in retinal detachment and neonatal blindness due
to the proliferation of new blood vessels (2).
Neovascularization (i.e., angiogenesis) is the
physiological process that involves the growth of new blood vessels
from existing vessels (3). The
non-perfused (NP) region, i.e., the hypoxic-ischemic region, is the
site of neovascularization and release of vascular endothelial
growth factor (VEGF). Hypoxia-inducible factor-1α (HIF-1α)
(4) regulates VEGF transcription
via signal transduction proteins that in turn enhance
neovascularization in the retina. The B-cell lymphoma/leukemia-2
(Bcl-2) gene is an oncogene that inhibits apoptosis (5,6).
Caspase-3 degrades poly(ADP-ribose) polymerase (PARP), resulting in
inhibition of DNA repair, DNA degradation, and apoptosis.
Regulatory mechanisms of retinal apoptosis are involved in normal
development and reconstruction of the retina under hypoxic-ischemic
conditions (7). The
neovascularization of endothelial cells, disintegration of the
capillary basement membrane and intercellular adhesion have been
extensively investigated over the past decade (8,9).
Intercellular adhesion adhesion molecule-1 (ICAM-1), also known as
CD54, is a member of the immunoglobulin superfamily that includes
antibodies and T-cell receptors. ICAM-1 can be induced by
interleukin-1 and tumor necrosis factor-α and is expressed in the
vascular endothelium, macrophages and lymphocytes. ICAM-1 is a
ligand for integrin lymphocyte function-associated antigen-1
(LFA-1) (10). When activated,
leukocytes bind to endothelial cells via ICAM-1/LFA-1 and
transmigrate into the tissues (11,12). A potential role for ICAM-1 in
signal transduction has been suggested based on research involving
ICAM-1 in cell-cell adhesion, extravasation and infection.
Characterizing the role of ICAM-1 has been a major focus of
research over the past number of years.
Rho-associated protein kinases (ROCKs) belong to the
AGC (PKA/PKG/PKC) family of serine-threonine kinases (13). ROCKs were identified as the first
effectors of Rho in rats. They induce the formation of stress
fibers and focal adhesion by phosphorylating myosin light chains.
In response to ROCK phosphorylation, actin binds to myosin II and
increases the contractility of capillary endothelial cells. As
such, ROCKs regulate cell-cell adhesion. The loss of ROCK activity
disrupts the integrity of tight junctions in endothelial cells.
Active ROCK in endothelial cells interferes with
E-cadherin-mediated cell-cell contact by activating actomyosin
contractility (14). ROCKs are
key regulators in the formation of the epithelial apical junctional
complex (AJC) (15–17), which is involved in the collapse
of the vascular endothelial barrier and the induction of
intercellular adhesion. The AJC determines the polarity of retinal
capillary endothelial cells, maintains endothelial tight junctions
and ensures the integrity of the blood-ocular barrier. Although
ROCKs regulate cytoskeletal structure and form AJC structures,
cytoskeletal contraction regulated by the increased expression of
ROCK can destroy tight junctions and AJC epithelial cell integrity,
promoting the expression of inflammatory factors and intercellular
adhesion adhesion.
Fasudil (FSD) is a potent ROCK inhibitor and
vasodilator (18). Since its
development, FSD has been used to treat cerebral vasospasms, which
may result in subarachnoid hemorrhage and lead to cognitive decline
in stroke victims. It also is effective for the treatment of
pulmonary hypertension (19). In
general, ROCK inhibitors are valid treatment options for ischemic
cardiovascular and cerebrovascular diseases (20). Over the years, FSD has emerged as
a potential therapeutic agent for the treatment of neovascular
retinopathy due to its post-ischemic vasorelaxant and
neuroprotective effects (21).
There is some controversy as to the application of
ROCK inhibitors in the treatment of retinal neovascularization
(22). Amano et al
reported that ROCK inhibitors regulate the endothelial actin
cytoskeleton and the underlying microsystem, thereby determining
endothelial cell polarity (15).
They suggested that the increased expression of Rho-ROCKs improves
the vascular endothelial cell number in diabetic retinal diseases,
potentially promoting the development of neovascularization.
Alternatively, the use of ROCK inhibitors may decrease
neovascularization (15). Another
study indicated that ROCK inhibitors promote VEGF-mediated
proliferation and the neovascularization of endothelial cells in
vitro (24). In the present
study, we established a rat model of OIR as previously described
(25–28) to determine whether ROCK affects
retinal neovascularization and to determine the effects of the ROCK
inhibitor, FSD, on the expression of ICAM-1, HIF-1α, Bcl-2 and
caspase-3. Using this model, we observed the NP region and retinal
blood vessel networks around the optic nerve, examined the mRNA
expression of ICAM-1, Bcl-2, caspase-3 and HIF-1α in samples from 3
treatment groups at different time points, and assessed the effects
of FSD on the NP region and vascularization in the retina of rats
with OIR.
Materials and methods
Animal model of ROP (OIR in vivo)
Newborn Sprague-Dawley rats and their mothers were
obtained from the Animal Experimental Center at China Medical
University, Shenyang, China. All animal experiments adhered to the
Association for Research in Vision and Ophthalmology Statement for
the Use of Animals in Ophthalmic and Vision Research. Newborn rats
were divided into 3 groups: group 1 [normoxia control (N) n=6] that
was not exposed to hyperoxia; group 2 [hyperoxia (H) only, n=6]
that was subjected to hyperoxia without treatment; and group 3 [H +
FSD (HF) n=6] that was subjected to hyperoxia and treated with FSD
injection.
We implemented a cyclic oxygen exposure protocol
that was modified from previous studies on oxygen-induced
retinopathy using rats (25–27). Hyperoxic experiments were
conducted in an airtight glass container constructed in our
laboratory. The chamber was 40×40×50 cm3 (4.5 l) in
volume and equipped with inlet and outlet ports. The inlet port
received 100% medical-grade oxygen. Throughout the entire exposure
period, airflow from the outlet was monitored for oxygen content
using an oxygen monitor. The interior of the chamber was maintained
at room temperature. Cyclic hyperoxic conditions of 80% oxygen for
1 day followed by 21% oxygen for 1 day were applied from postnatal
day (P)1 to P14. FSD was injected intraperitoneally daily (15
mg/kg) from P7 to P21, and intraocularly on P12, and the rats were
sacrificed on P12, P13, P14, P15, P17, P18, P19 and P21.
Experimental reagents
FSD was purchased from Asahi Kasei Pharma Corp.
(Tokyo, Japan). Rat ICAM-1, Bcl-2, caspase-3, HIF-1α and GAPDH
primers for reverse transcription (RT)-PCR were synthesized by
Takara Bio, Inc. (Shiga, Japan). RT kits, the Finnzymes SYBR Premix
Ex Taq qPCR kit and TRIzol reagent were purchased from Takara Bio,
Inc. Rabbit anti-rat ICAM-1, caspase-3, HIF-1α polyclonal
antibodies, a streptavidin-biotin complex (SABC) antibody and
diaminobenzidine (DAB) were obtained from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China).
Retinal flat mounts
The rats were sacrificed on P14 or P18. Following
intracardial perfusion with saline, both eyes were excised,
injected intraocularly with approximately 5 μl of 4%
paraformaldehyde and immersed in 4% paraformaldehyde at 4°C. After
24 h, 1 eye was prepared as a retinal flat mount with adenosine
diphosphate (ADP) histochemical staining and the other eye was
embedded in paraffin. For retinal flat mounts, the anterior segment
was removed and 4 or 5 radial cuttings were made. The cuttings were
rinsed, incubated in ADP and lead nitrate, stained with ammonium
sulfide and covered with coverslips. Micrographs (×40 and ×100)
were obtained using a MetaMorph/Evolution MP5.0/BX51 medical
imaging system (Olympus, Tokyo, Japan). The NP area, the whole
retina area (WRA) and the NP/WRA ratio (%) were measured (mean ±
standard deviation).
RNA isolation and real-time PCR
Total RNA was isolated from the tissues using TRIzol
reagent. cDNA was synthesized using RNA reverse transcription
reagents. The 10 μl reaction volume for cDNA synthesis included
total RNA (1.0 μl), 5X PrimerScript buffer (2.0 μl), PrimerScript
RT Enzyme Mix I (0.5 μl), 50 μM Oligo(dT) primers (0.5 μl), 100 μM
random 6-mers (0.5 μl) and RNase-free dH2O (5.5 μl). For
cDNA synthesis, the samples were incubated at 37°C for 15 min. The
reaction was stopped at 85°C for 5 sec, and the samples were
preserved at 4°C.
Quantitative PCR amplification was performed in
32-well PCR plates using the primers listed in Table I. The 20-μl reaction volume
included 2X SYBR Premix Ex Taq (10 μl), 10 μM forward primers (0.4
μl), 10 μM reverse primers (0.4 μl), a cDNA template (2 μl) and
water (7.2 μl). The reaction conditions were as follows:
pre-denaturation at 95°C for 10 sec and PCR amplification for 40
cycles at 95°C for 10 sec and 65°C for 20 sec. The data were
analyzed using LightCycler software (Roche Diagnostics,
Indianapolis, IN, USA).
 | Table ISequences of rat GAPDH, caspase-3,
Bcl-2, ICAM-1 and HIF-1α primers used for RT-PCR and mRNA
quantification. |
Table I
Sequences of rat GAPDH, caspase-3,
Bcl-2, ICAM-1 and HIF-1α primers used for RT-PCR and mRNA
quantification.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| GAPDH |
GCAAGTTCAACGGCACA |
CATTTGATGTTAGCGGGAT |
| Caspase-3 |
CAGACAGTGGAACTGACGAT |
TTTCAGCATGGCGCAAAGTG |
| Bcl-2 |
TGAACCGGCATCTGCACA |
CGTCTTCAGAGACAGCCAGGAG |
| ICAM-1 |
ACCAGACCCTGGAGATGGAGA |
ACCGTGGGCTTCACACTTCA |
| HIF-1α |
CCAGATTCAAGATCAGCCAGCA |
CTGTCCACATCAAAGCAGTACTCA |
Electrophoresis and western blot
analysis
Equal amounts of protein (30 μg) were loaded onto 6
or 12% gels for sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) following denaturation in 5X SDS gel
loading buffer [60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 14.4
mM 2-mercaptoethanol and 0.1% bromophenol blue]in boiling water for
10 min. Electrophoresed proteins were electrotransferred onto a
polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA,
USA) at a constant voltage of 10 V for 30 min. The membranes were
washed twice with 1X Tris-buffered saline (TBS) with 0.1% Tween-20
(TBST, pH 7.4) and pre-incubated with blocking buffer (5% non-fat
dry milk in TBST) at room temperature for 1 h. The blots were
incubated with rabbit anti-rat polyclonal ICAM-1 and β-actin
primary antibodies in TBST (1:1,000) at 4°C overnight. Following
incubation with the primary antibodies, the blots were incubated
with horseradish peroxidase-conjugated anti-rabbit secondary
antibody (1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at room temperature for 1 h. The PVDF membranes were washed
and developed using ECL Plus Western Blotting Detection Reagent (GE
Healthcare, Amersham, UK). The band densities were quantified by
densitometry (Multi Gauge software; Fuji Photo film, Tokyo,
Japan).
Statistical analysis
SPSS 15.0 software was used to conduct the
statistical analyses. Data are presented as the means ± standard
deviation. One-way ANOVA and the least significant difference (LSD)
were used for group comparisons. The correlation between multiple
variables was analyzed with Pearson's correlation test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
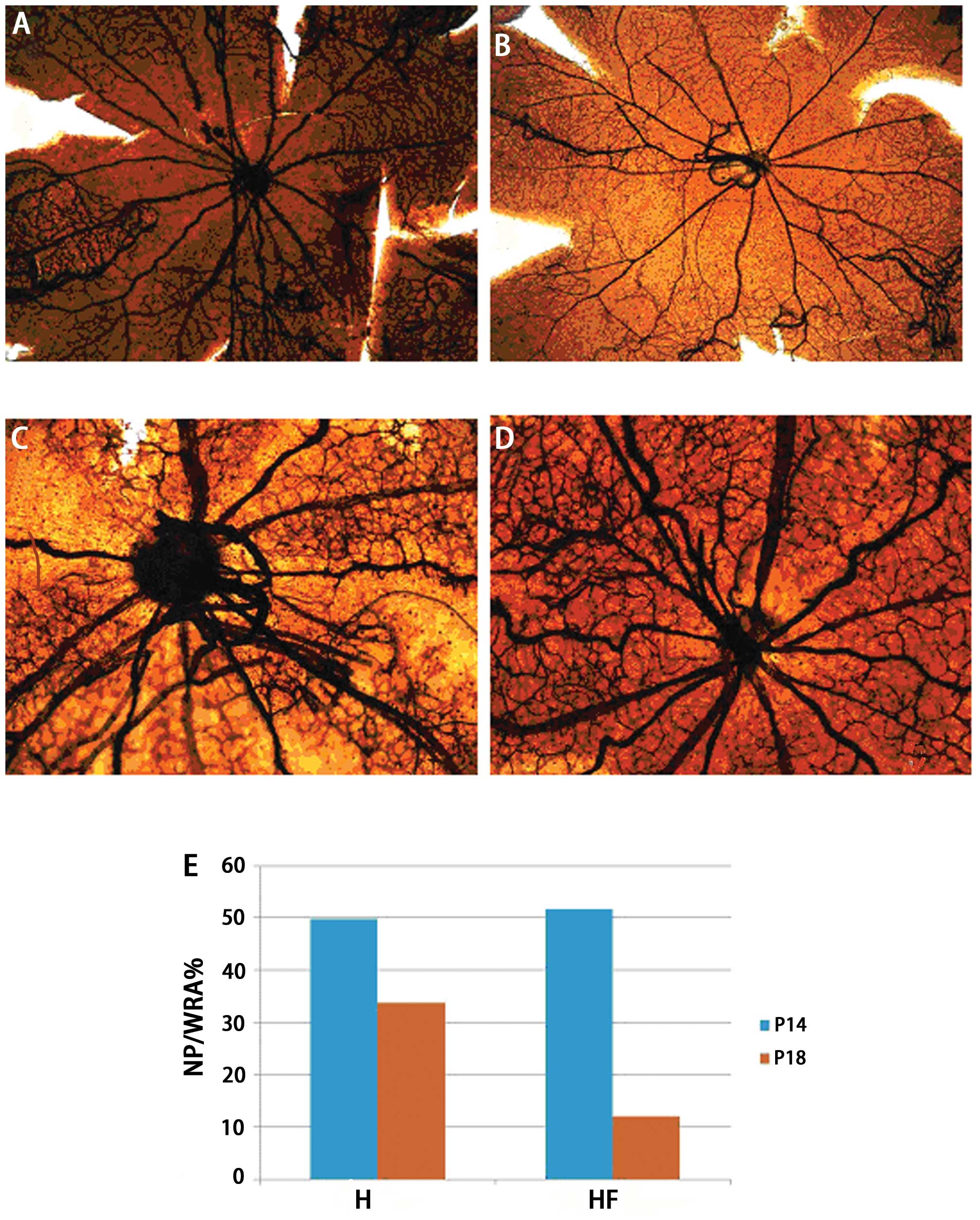
The retinal flat mounts indicated that the posterior
poles in the H (Fig. 1A) and HF
groups (Fig. 1B) appeared
avascular on P14 (t=2.311, P=0.237, n=6). The central retinal veins
were more circuitous and the vascular walls were less smooth in the
H group (Fig. 1A) compared with
the HF group (Fig. 1B). On P18,
the avascular areas in the posterior poles were larger in the H
group (Fig. 1C) than the HF
group. In the H group, retinal neovascular sprouting was evident
and disorganized. By contrast, the avascular areas of the posterior
poles in the HF group (Fig. 1D)
were almost repaired, and retinal double-layer vascular networks
were indistinct. In the HF group, the NP/WRA ratio (Fig. 1E) was significantly lower than
that in the H group on P18 (t=6.793, P=0.001, n=6).
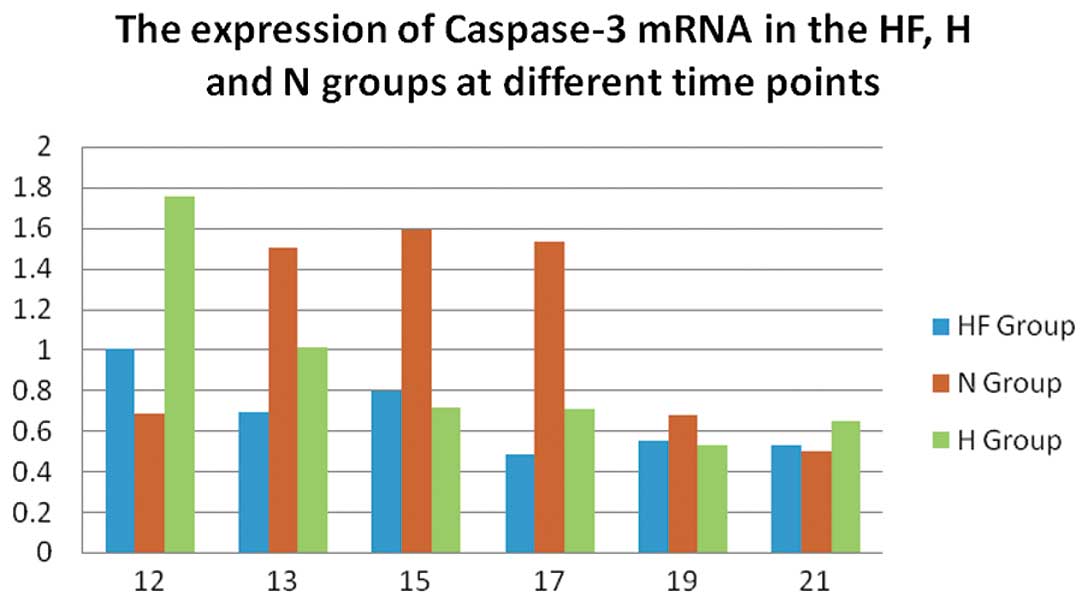
In the N group, the mRNA expression of caspase-3
(Fig. 2) was significantly
increased compared with the H and HF group on P13, P15 and P17
(P<0.01). In the H group, the mRNA expression of caspase-3
(Fig. 2) was significantly
increased to a maximum level on P12 compared with the HF group
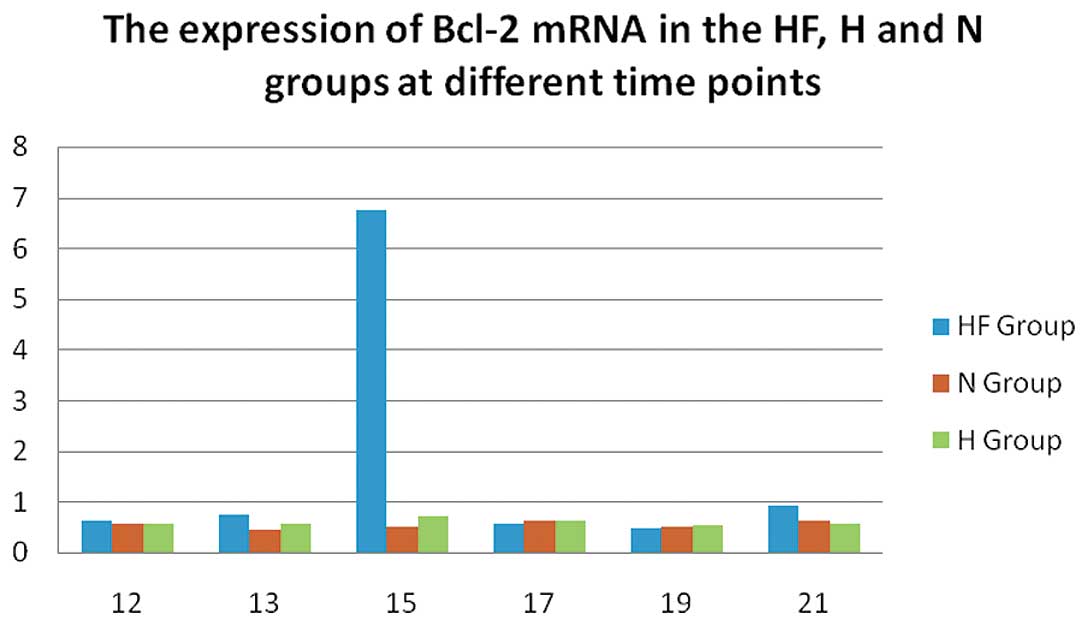
(P<0.01). In the HF group, the mRNA expression of Bcl-2
(Fig. 4) was significantly
increased on P15 compared with the N and H group (P<0.01). On
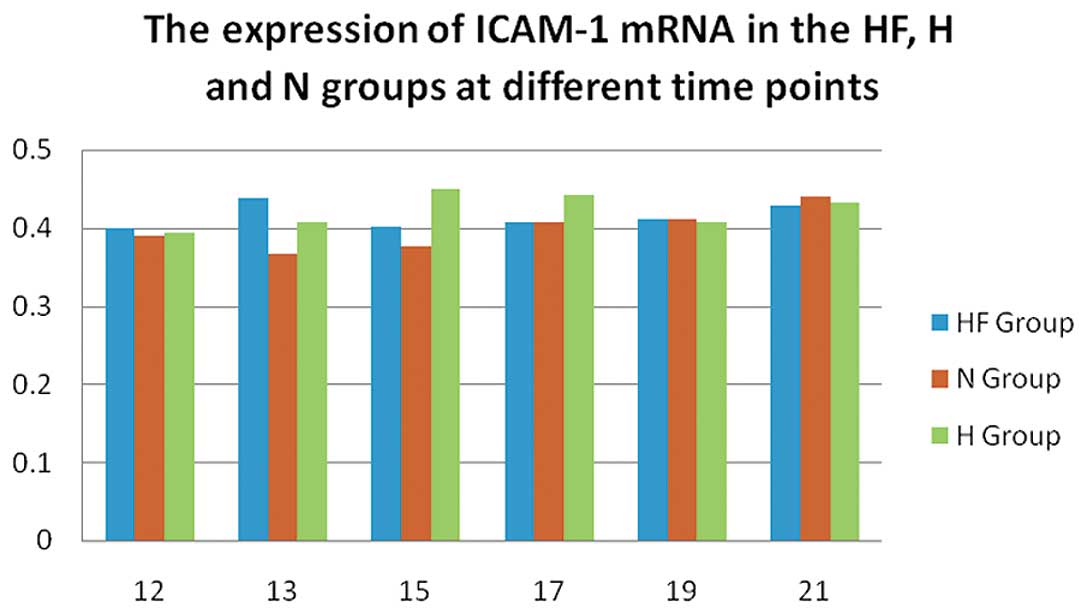
P15 and P17 in the H group and on P13 in the HF group, the mRNA
expression of ICAM-1 (Fig. 3) was
significantly increased compared with the other groups (P<0.05).
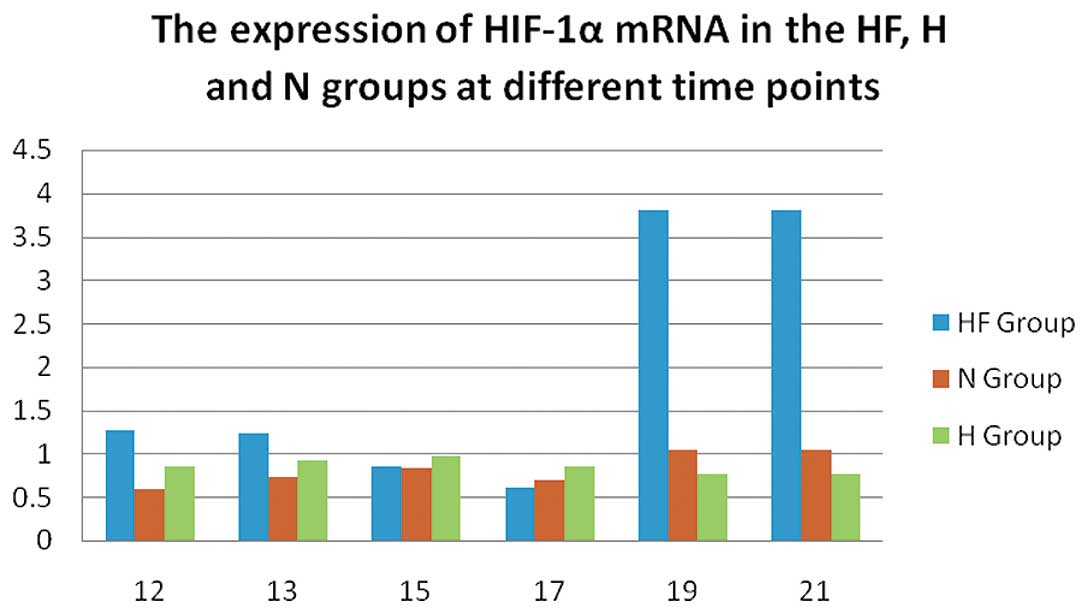
In the H and HF group, the expression of HIF-1α (Fig. 5) was significantly increased on
P12 compared with the N group (P<0.05). Of note, HIF-1α
expression was significantly increased to a maximum level on P19
and P21 in the HF group compared with the H and N group
(P<0.01).
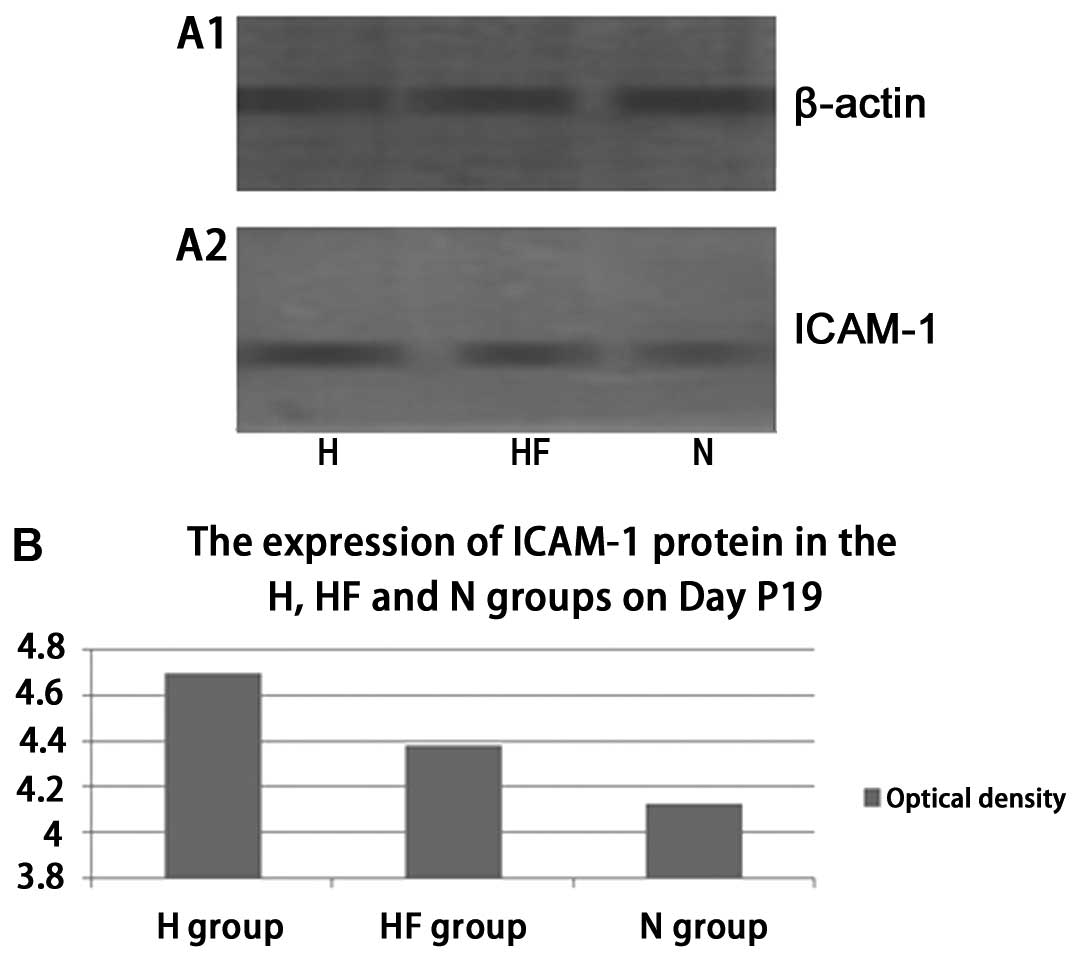
On P19, western blot analysis indicated that the
protein expression of ICAM-1 (Fig.
6) was significantly increased in the H group compared with the
HF group (t=3.768, P=0.013). ICAM-1 expression (Fig. 6) in the N group was lower compared
with the HF group (t=−7.357, P=0.001). There was a statistically
significant difference in the expression of ICAM-1 between the H
and N groups (Fig. 6) (t=9.022,
P=0.001). Western blot analysis on P19 indicated that the protein
expression of caspase-3 (Fig. 7)
was significantly increased in the HF group compared with the H
group (t=4.524, P=0.011). Caspase-3 expression in the HF and H
group was significantly lower compared with the N group (Fig. 7) (t=−7.357, −5.688; P=0.002,
0.006). On P19, western blot analysis indicated that the protein
expression of HIF-1α (Fig. 8) was
significantly increased in the HF group compared with the H group
(t=4.524, 3.765; P=0.003, 0.007).
Discussion
The results of the present study demonstrate the
significant effects of FSD on NP areas and retinal
neovascularization in rats with OIR. Retinal flat mounts were
successfully prepared before performing immunohistochemistry. The
development of retinal NP areas in the OIR model associated with
neovascularization damaged the blood-retinal barrier. We observed
different microcirculatory alterations in the H and HF group on P14
(Fig. 1). Compared with the H
group, retinal vascularization in the HF group was characterized by
dilation. On P14, the NP area and retinal vascularization in the HF
group exhibited greater shrinkage and more orderly
neovascularization compared with the H group (Fig. 1). The internal limiting membranes
(ILMs) of the animals in the H group were not integral and a
greater number of neovascular endothelial cell nuclei were observed
to break through the ILM into the vitreous. In the HF group, the
ILM was integral, and more neovascular endothelial cell nuclei were
observed under the ILM in the retinal nerve fiber layer. This
suggests that the intraocular concentrations of FSD were
effective.
On P14, the retinal flat mounts demonstrated
significantly less expansion in retinal vascular tortuosity in the
H group compared with the HF group, indicating the expanding effect
of FSD on vascular spasms. However, a large NP area in both the H
and HF group was observed in the posterior pole. On P18, the HF
group displayed smooth walls in the retinal arteries and veins, and
the NP area was significantly reduced compared with the H
group.
These results suggest that FSD directly inhibits
cytoskeletal contraction (15),
protects the integrity of tight junctions in vascular endothelial
cells, inhibits ICAM-1 transcription and translation, and regulates
the distribution and aggregation of ICAM-1. In turn, these
activities inhibit leukocyte adhesion in endothelial cells and
control retinal inflammation and edema at the posterior pole. The
results from the present study confirmed that ICAM-1 mRNA (Fig. 3) and protein expression (Fig. 6) in the HF group were
significantly reduced compared with the H group on P17 in
vivo. The RT-PCR and western blot analysis results were
concordant with our retinal flat mount findings. In the OIR model,
FSD protected endothelial cell tight junctions in the retinal
vasculature and promoted the progressive formation of new vascular
networks in the surface layers of the retinal nerve fibers.
In mRNA quantification, the expression of caspase-3
(Fig. 2) in the N group peaked
from P13 to P17, indicating possible apoptosis during normal
retinal development (6). The
expression of caspase-3 in the H group increased until P12 and then
decreased, which suggests that apoptosis occurred due to retinal
vascular contraction under hypoxic conditions. The expression of
caspase-3 in the HF group on P12 was significantly decreased
compared with the H group, indicating the inhibitory effect of FSD
on caspase-3 expression. The mRNA expression of Bcl-2 (Fig. 4) in the HF group peaked on P15 and
its expression levels were higher or the same compared with the
other 2 groups on the other days. The possible effects of apoptosis
due to hypoxia and apoptosis during normal development require
further investigation. This study demonstrates that FSD has an
inhibitory effect on caspase-3 expression and a stimulatory effect
on Bcl-2 expression, thereby inhibiting retinal apoptosis in the
rat model of OIR.
The mRNA expression of HIF-1α (Fig. 5) in the HF group was slightly
higher than that in the N and H group on P12, followed by a stable
decline on P15 and P17, which is consistent with the western blot
analysis results (Fig. 8). The
expression of HIF-1α in the HF group peaked on P19 and P21; on
these days its expression was markedly higher compared with in the
H and N group. The increase in HIF-1α expression may be associated
with the repair of tissue damage induced by hypoxia (28). FSD stimulated the repair process
and had a positive effect on nerve protection. However, the
increase in HIF-1α expression led to an increase in VEGF
expression, which further induced retinal neovascularization and
the possible development of proliferative retinopathy. In the
stretched preparation of the retina, reconstruction of the
doubled-layered retinal blood vessels in the HF group was observed
on P18, suggesting that reconstruction of the compensatory retinal
circulatory system was possible following exposure to FSD during
development, but that long-term efficacy requires further
confirmation.
In conclusion, in our OIR model, FSD protected the
nerves in the particularly thin layer of the retinal nervous
system. Specifically, FSD inhibited caspase-3 and enhanced Bcl-2
expression, which prevented retinal apoptosis, suppressed ICAM-1
expression, reduced inflammation and improved retinal blood
circulation, while maintaining the normal structure of the retina.
However, the increase in HIF-1α expression upregulated VEGF
expression. In this model, FSD interfered with the repair of
retinal vessels, escalated the contraction of the NP region of the
retina, increased the inhibition of apoptosis and reduced
inflammation. The reconstruction of neovascularization that was
improved by retinal circulation and the accelerated formation of
neovascularization requires further evaluation. It is anticipated
that the damaging effects of accelerated neovascularization will be
dominant over the protective effects in the long-term.
References
|
1
|
Pastor JC, de la Rúa ER and Martín F:
Proliferative vitreoretinopathy: risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartnett ME: The effects of oxygen
stresses on the development of features of severe retinopathy of
prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol.
120:25–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Penn JS: Retinal and Choroidal
Angiogenesis. Springer; Dordrecht: pp. 1192008
|
|
4
|
Näpänkangas U, Lindqvist N, Lindholm D and
Hallböök F: Rat retinal ganglion cells upregulate the pro-apoptotic
BH3-only protein Bim after optic nerve transection. Brain Res Mol
Brain Res. 120:30–37. 2003.PubMed/NCBI
|
|
5
|
Zhang Z, Qin X, Zhao X, Tong N, Gong Y,
Zhang W and Wu X: Valproic acid regulates antioxidant enzymes and
prevents ischemia/reperfusion injury in the rat retina. Curr Eye
Res. 37:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cellerino A, Bähr M and Isenmann S:
Apoptosis in the developing visual system. Cell Tissue Res.
301:53–69. 2000. View Article : Google Scholar
|
|
7
|
Ozaki H, Yu AY, Della N, Ozaki K, Luna JD,
Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL and
Campochiaro PA: Hypoxia inducible factor-1alpha is increased in
ischemic retina: temporal and spatial correlation with VEGF
expression. Invest Ophthalmol Vis Sci. 40:182–189. 1999.PubMed/NCBI
|
|
8
|
Joussen AM, Murata T, Tsujikawa A,
Kirchhof B, Bursell SE and Adamis AP: Leukocyte-mediated
endothelial cell injury and death in the diabetic retina. Am J
Pathol. 158:147–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barouch FC, Miyamoto K, Allport JR, Fujita
K, Bursell SE, Aiello LP, Luscinskas FW and Adamis AP:
Integrin-mediated neutrophil adhesion and retinal leukostasis in
diabetes. Invest Ophthalmol Vis Sci. 41:1153–1158. 2000.PubMed/NCBI
|
|
10
|
Rothlein R, Dustin ML, Marlin SD and
Springer TA: A human intercellular adhesion molecule (ICAM-1)
distinct from LFA-1. J Immunol. 137:1270–1274. 1986.
|
|
11
|
Samarin S and Nusrat A: Regulation of
epithelial apical junctional complex by Rho family GTPases. Front
Biosci. 14:1129–1142. 2007.PubMed/NCBI
|
|
12
|
Smith A, Bracke M, Leitinger B, Porter JC
and Hogg N: LFA-1-induced T cell migration on ICAM-1 involves
regulation of MLCK-mediated attachment and ROCK-dependent
detachment. J Cell Sci. 116:3123–3133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cytoskeleton. Mol Cell
Biol. 16:5313–5327. 1996.PubMed/NCBI
|
|
14
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warner SJ and Longmore GD: Distinct
functions for Rho1 in maintaining adherens junctions and apical
tension in remodeling epithelia. J Cell Biol. 185:1111–1125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brady DC, Alan JK, Madigan JP, Fanning AS
and Cox AD: The transforming Rho family GTPase Wrch-1 disrupts
epithelial cell tight junctions and epithelial morphogenesis. Mol
Cell Biol. 29:1035–1049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abman SH: Recent advances in the
pathogenesis and treatment of persistent pulmonary hypertension of
the newborn. Neonatology. 91:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Budzyn K, Marley PD and Sobey CG:
Targeting Rho and Rho-kinase in the treatment of cardiovascular
disease. Trends Pharmacol Sci. 27:97–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimokawa H: Rho-kinase as a novel
therapeutic target in treatment of cardiovascular diseases. J
Cardiovasc Pharmacol. 39:319–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hata Y, Miura M, Nakao S, et al:
Antiangiogenic properties of fasudil, a potent Rho-Kinase
inhibitor. Jpn J Ophthalmol. 52:16–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bryan BA, Dennstedt E, Mitchell DC, et al:
RhoA/ROCK signaling is essential for multiple aspects of
VEGF-mediated angiogenesis. FASEB J. 24:3186–3195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arita R, Hata Y, Nakao S, et al: Rho
kinase inhibition by fasudil ameliorates diabetes-induced
microvascular damage. Diabetes. 58:215–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin L, Morishige K, Takahashi T, et al:
Fasudil inhibits vascular endothelial growth factor-induced
angiogenesis in vitro and in vivo. Mol Cancer Ther. 6:1517–1125.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Penn JS, Tolman BL and Lowery LA: Variable
oxygen exposure causes preretinal neovascularization in the newborn
rat. Invest Ophthalmol Vis Sci. 34:576–585. 1993.PubMed/NCBI
|
|
27
|
Kim WT and Suh ES: Retinal protective
effects of resveratrol via modulation of nitric oxide synthase on
oxygen-induced retinopathy. Korean J Ophthalmol. 24:108–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharp FR, Ran R, Lu A, et al: Hypoxic
preconditioning protects against ischemic brain injury. NeuroRx.
1:26–35. 2004. View Article : Google Scholar : PubMed/NCBI
|