Introduction
Intervertebral disc degeneration (IDD) is a common
clinical diagnosis and is the leading cause of debilitating spinal
disorders and resulting lower back pain (LBP) and physical
disability (1). It is difficult
to economically manage health problems such as LBP due to the
escalating costs of healthcare services. Furthermore, it has a
pronounced impact on society, given that it leads to decreased work
efficiency among employees and disrupts the quality of life of
patients (2–4). Aging is the greatest risk factor for
developing IDD, as the incidence rate increases gradually with age
(5). Due to the complexities of
this disorder, the mechanisms of IDD are poorly understood. In this
study, we hypothesized that the changes occurring in IDD are
primarily manifested within cells and the extracellular matrix
within the disc. The altered extracellular matrix composition of
the disc directly defines the characteristics of IDD mechanics
(1,6). There are several possible causes of
disc degeneration, including the decline of cell viability,
modifications of extracellular matrix synthesis and distribution,
as well as excessive cell loss due to apoptosis. Indeed, the
extreme progression of apoptosis of nucleus pulposus cells can
directly affect intervertebral disc (IVD) function (7).
Tumour necrosis factor (TNF)-α is a pro-inflammatory
cytokine that plays a crucial role in disc degeneration. A number
of studies have indicated that TNF is expressed in the normal IVD
and that its expression increases with progressing age and disc
degeneration. Therefore, TNF-α may be a key initiating factor in
matrix degeneration. It may also act to upregulate interleukin
(IL)-1β expression, which is known to play a major role in
promoting pathological disc degradation (8–11).
Previous studies have demonstrated that insulin-like growth factor
(IGF)-1 stimulates the proliferation of human nucleus pulposus
cells (12). The stimulation of
bovine nucleus pulposus cells by IGF-1 and bone morphogenetic
protein (BMP)-7 has a significant impact on anabolism through
complementary and synergistic mechanisms on matrix formation.
Compared to treatment with growth factor alone, the combination
therapy of BMP-7 and IGF-1 may hold considerable promise in the
treatment of IDD (13). If cells
can overexpress IGF-1 alone, this may have considerable, positive
impact on cell survival; however, to our knowledge, this has not
been demonstrated to date.
Gene therapy is considered a potential therarpy for
degenerative disc disease. In this study, our main objective was to
determine the effects of the IGF-1 gene on the TNF-α-induced
apoptosis of rabbit nucleus pulposus cells and to investigate the
potential anti-apoptotic mechanisms involved. To achieve these
objectives, we constructed an adenoviral vector expressing the
human IGF-1 (hIGF-1) gene and transfected nucleus pulposus cells
with this vector. We used western blot analysis to assess transgene
expression and terminal deoxynucleotidyl transferase (TdT)-mediated
dUTP-biotin nick end-labeling (TUNEL) and flow cytometry (FCM) to
quantify the apoptotic rate of nucleus pulposus cells.
Materials and methods
Cell culture
IVDs were obtained from lumbar spines of mature New
Zealand white rabbits immediately post-mortem. From the IVDs, the
nucleus pulposus was harvested, washed with Hanks’ balanced salt
solution (HBSS) and transported to our laboratory within 30 min of
harvesting. The nucleus pulposus tissue was rinsed 3 times in HBSS
and dissected into small fragments of ~1 mm3 in size.
The cells were isolated from the nucleus pulposus using sequential
enzyme digestion. Subsequently, 0.25% trypsin was used for carrying
out the initial 30-min digestion followed by a wash with HBSS and
incubation of the material in 0.1% collagenase type II at 37°C for
2–3 h. The cells were collected by filtering the digested tissue
through a 200-μm mesh nylon cell strainer and centrifugation of the
filtrate at 1,000 × g for 5 min. The cells were washed twice with
phosphate-buffered saline (PBS) and then resuspended and grown in
Dulbecco’s modified Eagle’s medium with Ham’s F-12 nutrient mixture
(DMEM-F12) supplemented with 10% (vol/vol) fetal bovine serum (FBS)
plus 1% penicillin and streptomycin. The culture medium was changed
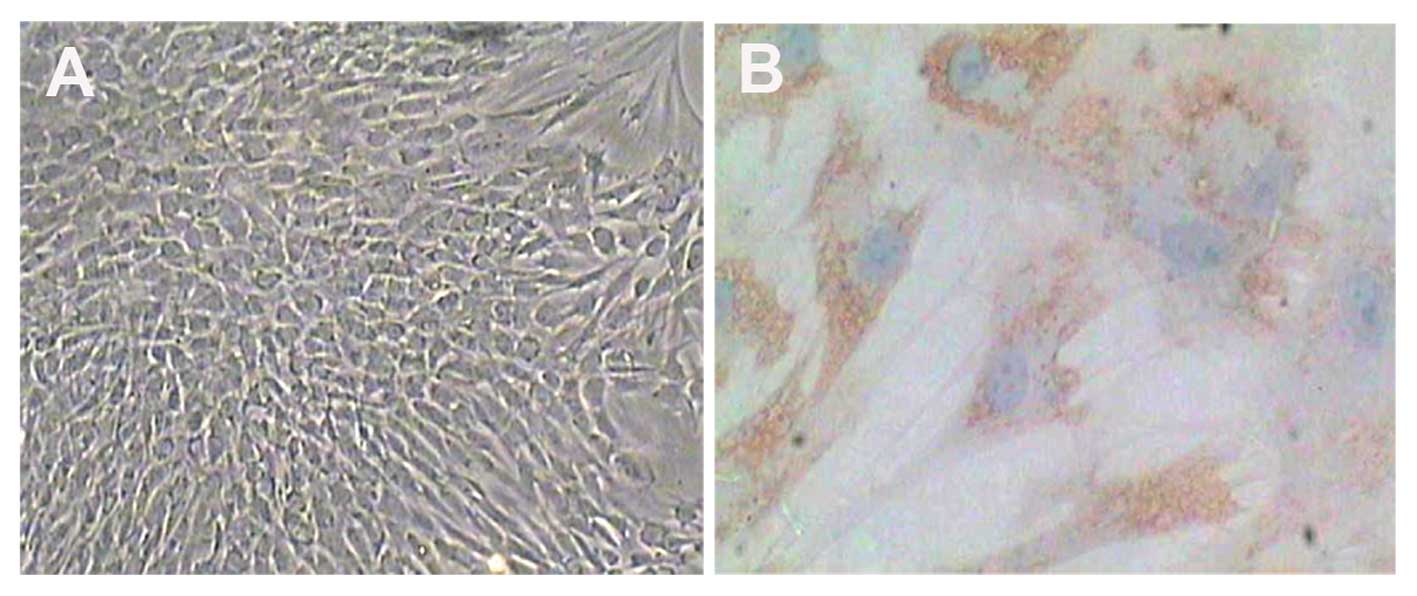
every 2–3 days. The phenotype of the nucleus pulposus cells was
confirmed using positive immunostaining for type II collagen. Once
the nucleus pulposus cells had formed a monolayer, they were
allowed to expand exponentially until ~80% confluence.
Second-passage chondrocytes were used in all the experiments
(Fig. 1A).
Construction and generation of
recombinant adenoviral vector expressing hIGF-1 (Ad-hIGF-1)
The adenoviral vector containing the hIGF-1 gene was
constructed by Biowit Technologies Co., Ltd. (Shenzhen, China).
Second-passage chondrocytes were digested using trypsin,
transferred to 6-well plates and grown to a density of
1×105/ml in DMEM-F12 supplemented with 10% (vol/vol)
FBS. After 24 h of adherence, the medium was changed to DMEM-F12
with 10% FBS containing TNF-α (100 ng/ml) with or without Ad-hIGF-1
for 48 h [the adenoviral titer was 4.0×109 plaque
forming units (PFU)/ml]. A subset of cells grown in DMEM-F12 with
10% FBS for 48 h served as the controls.
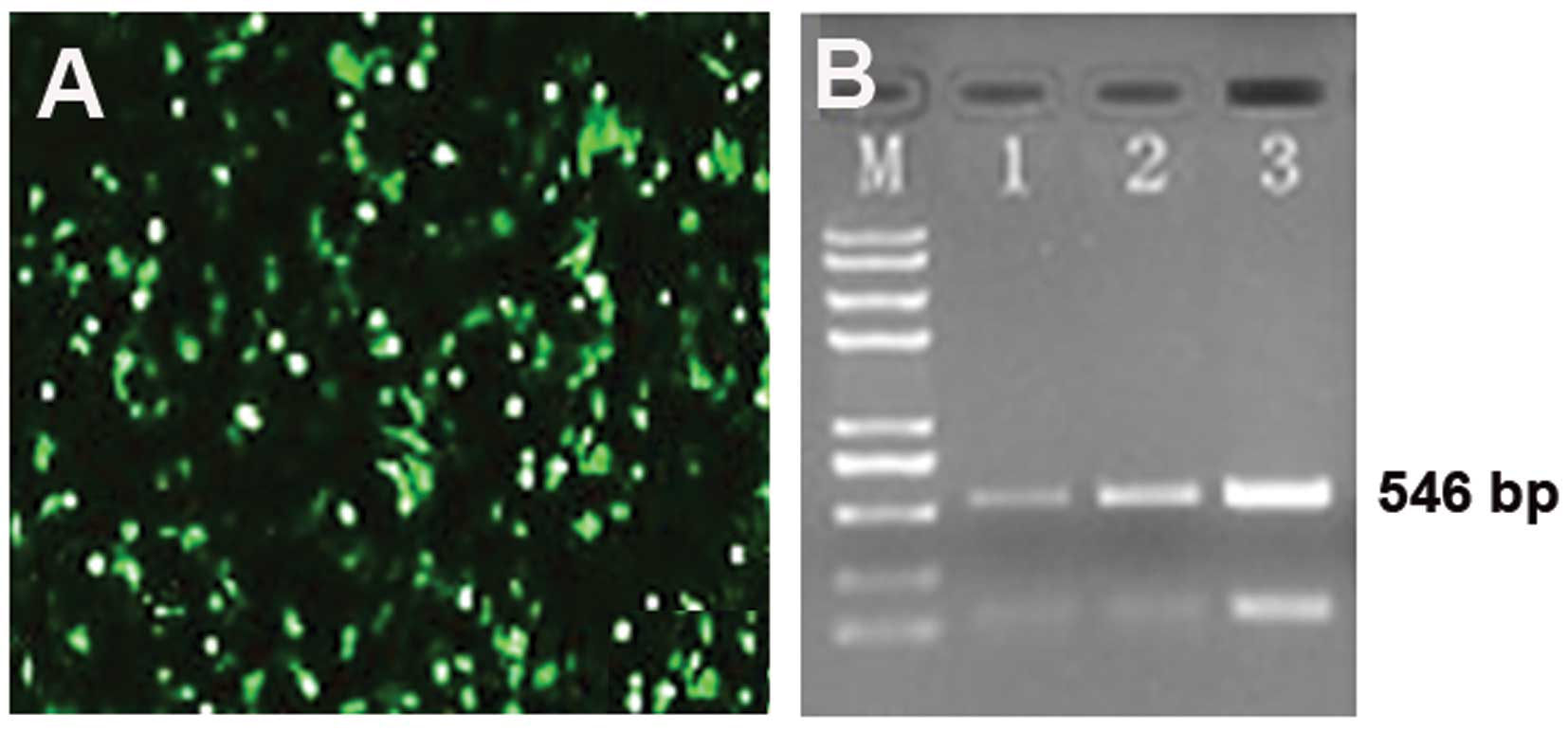
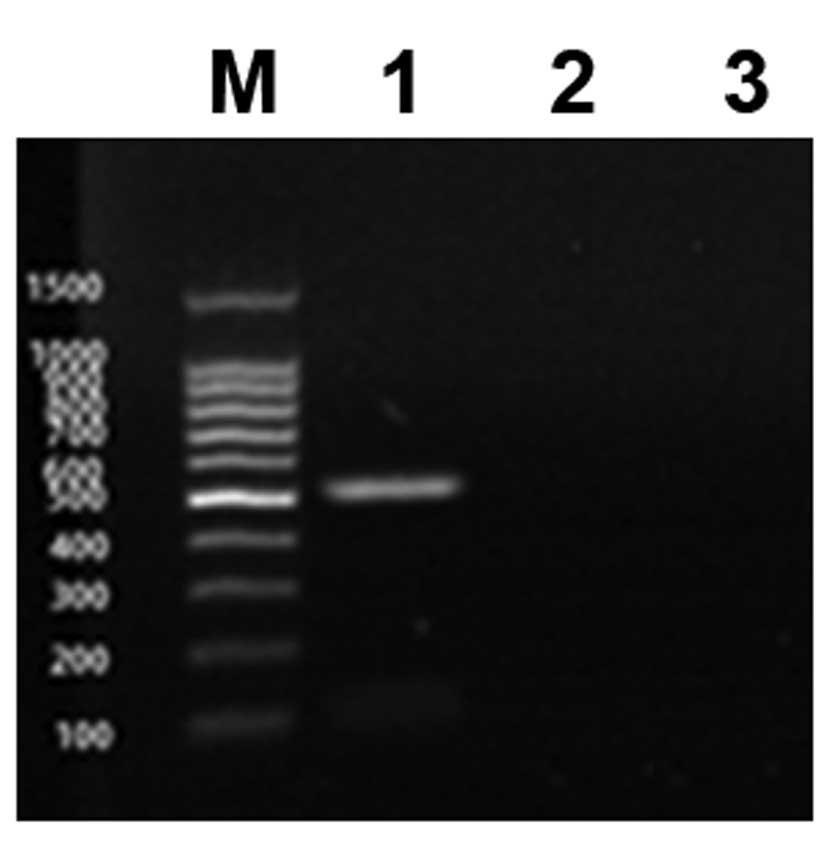
RT-PCR
Total RNA was extracted from the nucleus pulposus
cells using TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing,
China) according to the manufacturer’s instructions. The primers
for hIGF-1 (546 bp) were 5′-TGGCTTATCGAAATT AATACGACTC-3′ (forward)
and 5′-GGCTGATCAGCGGG TTTAAACTTAA-3′ (reverse). The PCR procedure
was as follows: denaturation at 94°C for 2 min; 30 cycles of 94°C
for 30 sec, 55°C for 30 sec, and 72°C for 30 sec followed by a 60°C
elongation step for 10 min. Electrophoresis of the RT-PCR products
was performed using an agarose gel, and the results were analyzed
by a gel imaging system.
Western blot analysis
Arter resolution by electrophoresis, proteins were
separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis (PAGE). The proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% non-fat dried milk in TBST
(10 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween-20, pH 7.5) for 1 h
at room temperature and then incubated with antibodies against
hIGF-1 (1:200 dilution; Boster Biological Technology Ltd., Wuhan,
Hubei, China) for 1 h at 37°C. After washing twice with TBST
buffer, the membranes were incubated with horseradish
peroxidase-conjugated anti-mouse IgG secondary antibodies for 1 h
at room temperature and detected using a Bio-Rad digital imaging
system (Bio-Rad, Hercules, CA, USA).
TUNEL assay
Nucleus pulposus cells were collected and cultured
in 6-well plates. Each well was covered with a 1×1-cm cover glass.
The cover glasses were removed after 12 h, and the cells adherent
to the glass were fixed in 4% paraformaldehyde for 60 min. Methanol
containing 3% H2O2 was applied for 5 min to
inactivate endogenous peroxidase. The cells were then exposed to
0.1% Triton X-100 at 4°C for 2 min. These cells were subsequently
incubated with 50 μl of the TUNEL reaction mixture (5 μl TdT-enzyme
solution and 45 μl of nucleotide mixture solution) in the dark at
37°C for 60 min. The cells were then exposed to
3,3′-diaminobenzidine (DAB) and then counterstained with
hematoxylin. Finally, the cells were dehydrated using graded
ethanol and covered with a xylene-based mounting medium. The
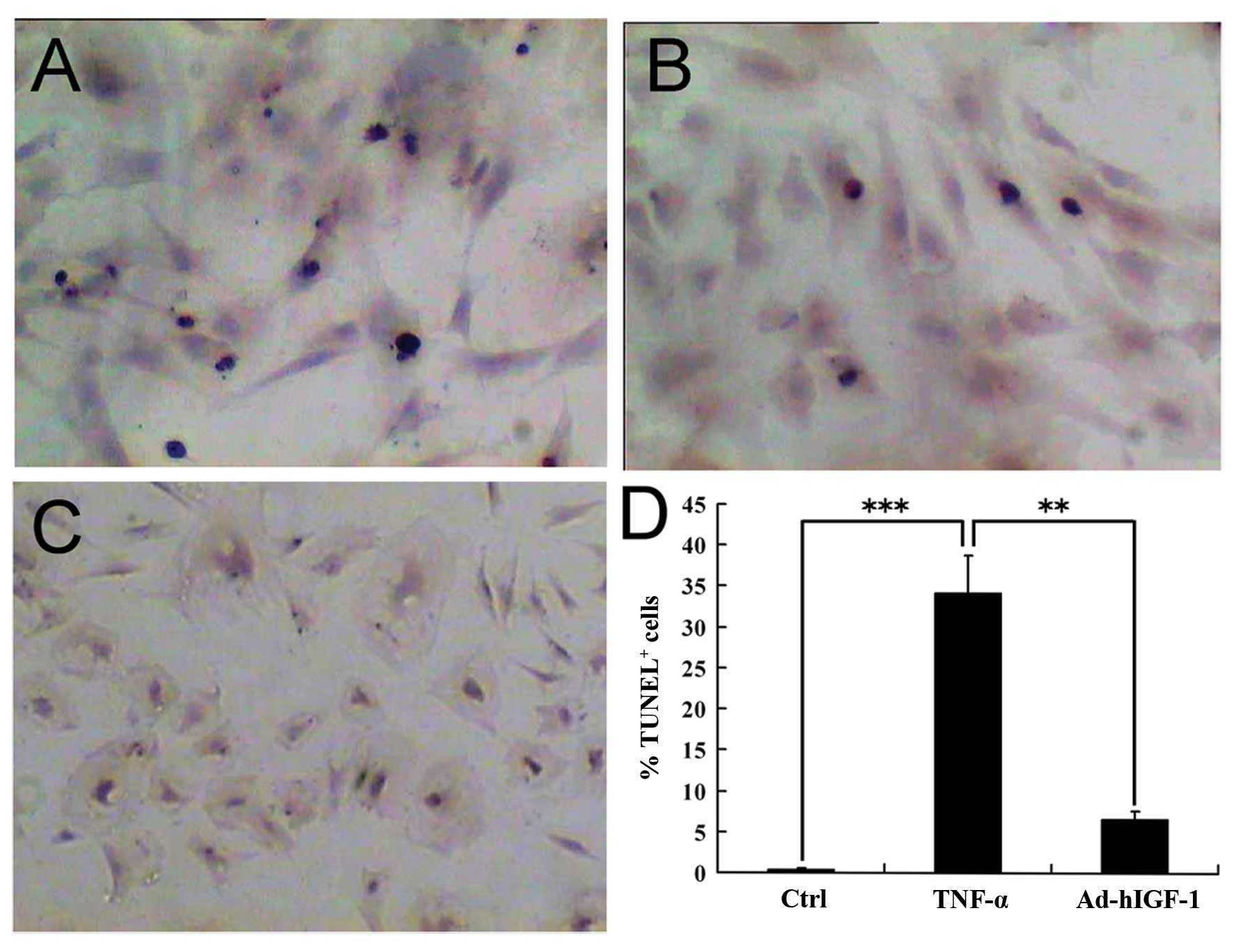
percentage of TUNEL-positive cells from the control, TNF-α and
TNF-α + Ad-hIGF-1 groups was quantified within 10 non-continuous
low-power fields (magnification, ×100).
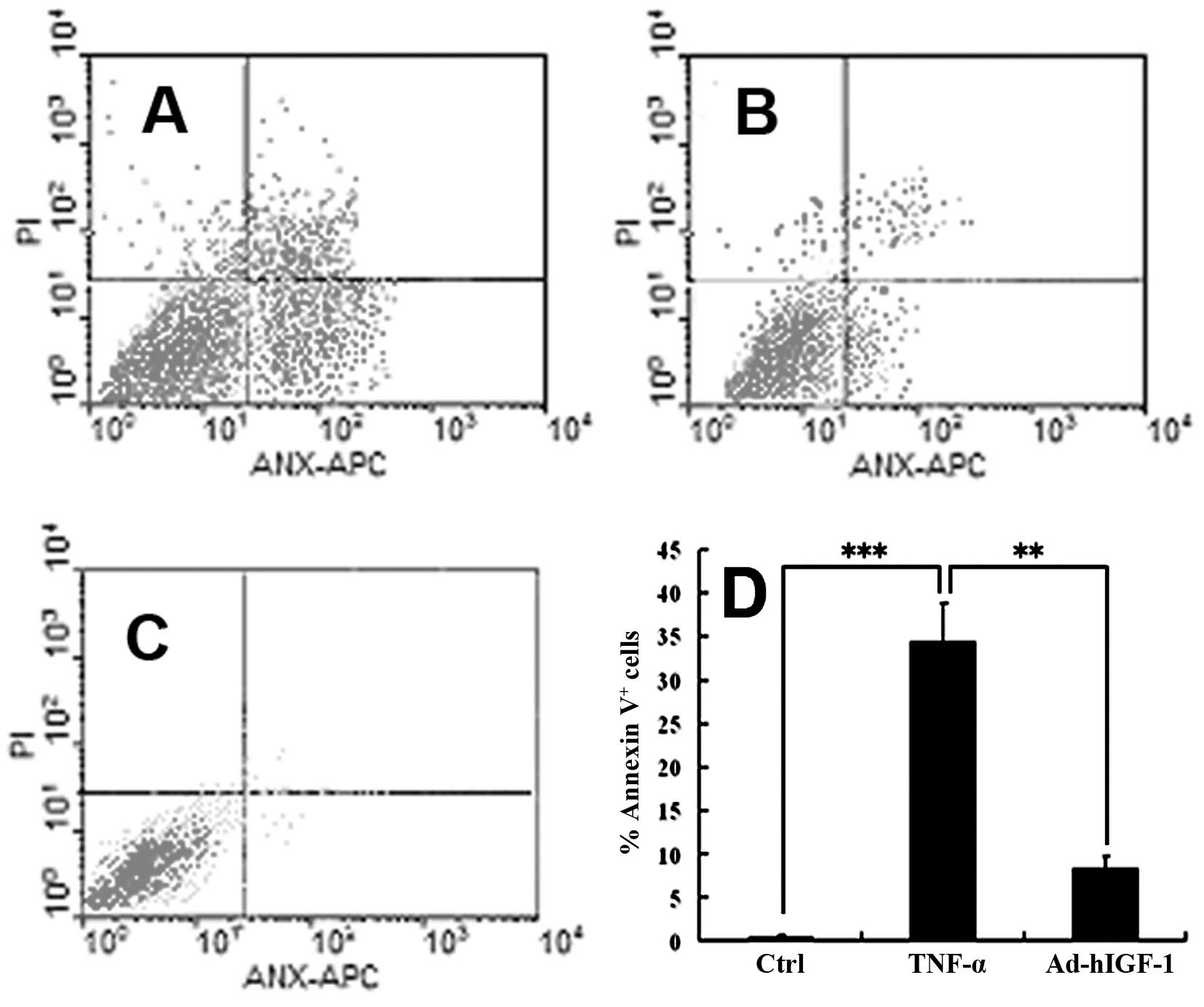
FCM
The cells were cultured in DMEM-F12 containing TNF-α
(100 ng/ml) with or without Ad-hIGF-1 for 48 h. Cells cultured in
DMEM-F12 with 1% FBS for 48 h served as the controls. The cells
were digested using trypsin, then collected and washed in FCM
buffer. The collected cells were re-suspended in FCM wash buffer.
In order to detect cell apoptosis, the cells were incubated in the
dark with 5 μl of Annexin V-FITC incubation reagent for 15 min at
4°C. This was followed by incubation with 10 μl of propidium iodide
(PI)-phycoerythrin (PE) for 5 min at 4°C. The samples were analyzed
within 30 min by FCM.
Statistical analysis
Data are presented as the means ± SD (n=3). Groups
of data were compared statistically using the Mann-Whitney U test.
Values of P<0.05 were considered to indicate statistically
significant differences.
Results
Isolation and culture of nucleus pulposus
cells
Primary nucleus pulposus cells were obtained by
sequential enzyme digestion and cultured in DMEM-F12 10% FBS. To
identify the phenotype of nucleus pulposus cells, immunostaining
was used to detect type II collagen (Fig. 1B). The results revealed that these
cells expressed type II collagen.
Generation of recombinant adenoviral
vector Ad-hIGF-1 expressing hIGF-1 exogenously
The adenoviral vector containing the hIGF-1 gene was
successfully constructed by Biowit Technologies Co., Ltd. A
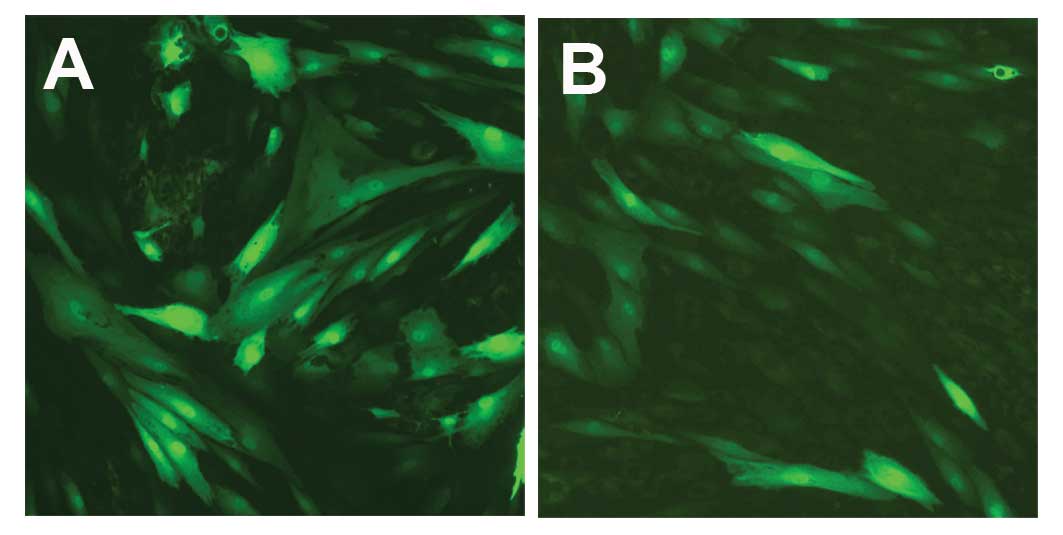
built-in green fluorescent protein (GFP) contained in the Ad-hIGF-1
adenoviral vector was a unique feature of the Ad-hIGF-1 vector. As
a result of GFP expression, we were able to effectively track the
gene expression in the transfected cells. For the controls, we also
constructed a recombinant adenoviral vector that only expressed
GFP. HEK293 cells were transfected with the viral vector, Ad-GFP
(Fig. 2). The viral transfection
efficiency positively correlated with the viral titer (Fig. 2B). When the cells were ~60 to 70%
confluent, transfection with Ad-hIGF-1 or the control vector,
Ad-GFP, was performed. At 24 h post-transfection, fluorescence was
the brightest, as observed under a fluorescence microscope;
however, this fluorescence weakened after 48 h and disappeared
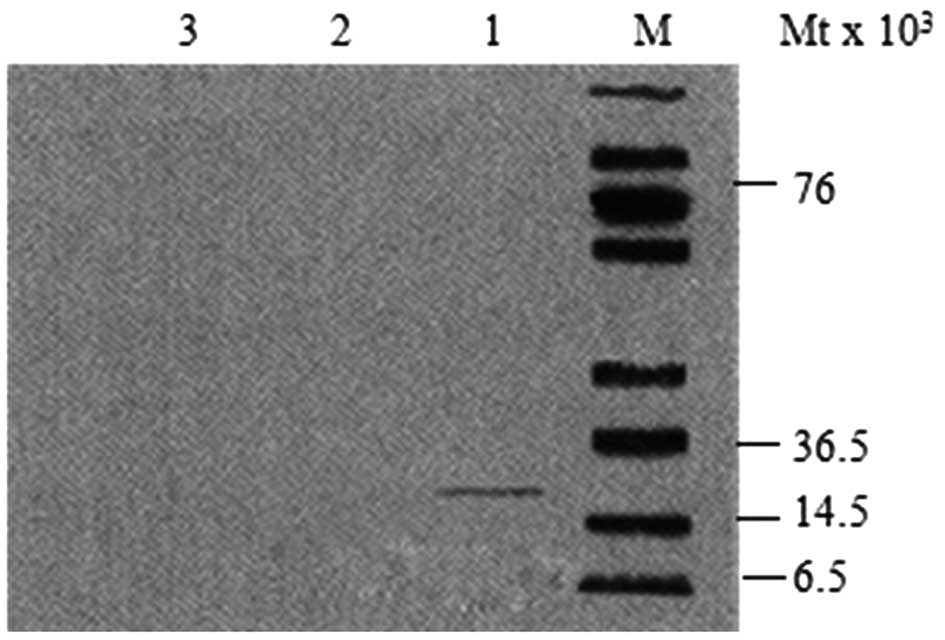
after 72 h (Fig. 3). Western blot
analysis revealed that IGF-1 in the nucleus pulposus was
significantly expressed in the Ad-hIGF-1-transfected group, whereas
no expression was reported in the TNF-α-treated group or the
control group (Fig. 4). The
results of western blot analysis were confirmed by RT-PCR (Fig. 5).
TNF-α induces the apoptosis of nucleus
pulposus cells
TUNEL staining revealed that the percentage of
TUNEL-positive cells was considerably greater in the TNF-α-treated
group compared with the controls (Fig. 6A–C). The results from TUNEL assay
were confirmed by FCM (Fig.
7A–C). IGF-1 suppressed the TNF-α-induced apoptosis of nucleus
pulposus cells. TUNEL analysis indicated that the TNF-α-induced
apoptosis was significantly suppressed (P<0.01) in the Ad-hIGF-1
group (Fig. 6A and B). This
suggests that hIGF-1 inhibited the TNF-α-induced apoptosis of
nucleus pulposus cells. The results of FCM revealed that the
percentage of nucleus pulposus cells exhibiting signs of early
stages of apoptosis was significantly higher in the TNF-α group
compared with the controls (P<0.01) (Fig. 7A–C), but was significantly lower
in the Ad-hIGF-1-treated group compared with the TNF-α-treated
group (P<0.01) (Fig. 7A and
B).
Discussion
Over the years, a number of studies have focused on
elucidating the role of cytokines in the pathogenesis of IDD. IL-1β
and TNF-α are considered principal pro-inflammatory cytokines.
TNF-α is closely associated with IDD, as it suppresses matrix gene
expression, but also regulates cell-cell and cell-matrix
communication and cell growth in the invertebral disc (14). It has been demonstrated that TNF-α
inhibits the expression of prostaglandins and type II collagen by
increasing the expression of matrix metalloproteinases (MMPs) and a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)-4 which are among the major factors in IVD degeneration
(14). Late-stage disc
degeneration is characterized by a reduction in the number of
nucleus pulposis cells and both IL-1β and TNF-α can induce nucleus
pulposus cell apoptosis (9,15).
Therefore, we hypothesized that IL-1β and TNF-α can significantly
contribute to this loss. In addition, TNF-α can upregulate IL-1β
expression and may be an important initiating factor in matrix
degeneration (10).
IGF-1 effectively stimulates the proliferation of
human nucleus pulposus cells (16). As recently demonstrated, IGF-1
stimulates the proliferation of human IVD cells through the MEK/ERK
and PI-3K/Akt pathways (12,18). Studies have proven the importance
of gene therapy in the treatment of disc degeneration, and
adenoviral vectors are considered an effective and reliable method
for delivering exogenous genes to the IVD (19–21).
In this study, nucleus pulposus cells were
successfully transfected with Ad-hIGF-1, and based on the results
of RT-PCR and western blot analysis, we deduced that the
transfected cells effectively expressed hIGF-1. The results from
TUNEL assay and FCM indicated that the rate of apoptosis was
particularly high in the nucleus pulposus cells treated with TNF-α
compared with the controls. Ad-hIGF-1 significantly reduced
apoptosis induced by TNF-α, suggesting that hIGF-1 reversed the
TNF-α-mediated apoptosis of nucleus pulposus cells in vitro.
The results from FCM also suggested that TNF-α induced both the
early- and late-stage apoptosis of nucleus pulposus cells.
Moreover, apoptosis was suppressed by hIGF-1. Taken together, these
results indicate that Ad-hIGF-1 may contribute to the development
of clinical interventions for disc degeneration. In the IVD, the
relatively encapsulated and avascular environment of the nucleus
pulposus may prohibit elevated immune response and support the
prolonged and enhanced expression of the IGF-1 gene.
In conclusion, the findings of this study illustrate
that the adenoviral vector, Ad-hIGF-1, can successfully infect
nucleus pulposus cells in vitro and can effectively enhance
the expression of IGF-1. IGF-1 can reverse the TNF-α-induced
apoptosis of nucleus pulposus cells. Due to such beneficial
effects, transfection with Ad-hIGF-1 may be a prospective
therapeutic strategy and may aid the development of novel clinical
interventions for disc degeneration.
Acknowledgements
This study was supported by the Key Research
Foundation of Education Bureau of Anhui Province, China
(KJ2011A204).
References
|
1
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Becker A, Held H, Redaelli M, et al: Low
back pain in primary care: costs of care and prediction of future
health care utilization. Spine (Phila Pa 1976). 35:1714–1720. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dagenais S, Caro J and Haldeman S: A
systematic review of low back pain cost of illness studies in the
United States and internationally. Spine J. 8:8–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asche CV, Kirkness CS, McAdam-Marx C and
Fritz JM: The societal costs of low back pain: data published
between 2001 and 2007. J Pain Palliat Care Pharmacother. 21:25–33.
2007.PubMed/NCBI
|
|
5
|
Nasto LA, Wang D, Robinson AR, et al:
Genotoxic stress accelerates age-associated degenerative changes in
intervertebral discs. Mech Ageing Dev. 134:35–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith LJ, Nerurkar NL, Choi KS, et al:
Degeneration and regeneration of the intervertebral disc: lessons
from development. Dis Model Mech. 4:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KW, Kim YS, Ha KY, et al: An autocrine
or paracrine Fas-mediated counterattack: a potential mechanism for
apoptosis of notochordal cells in intact rat nucleus pulposus.
Spine (Phila Pa 1976). 30:1247–1251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bachmeier BE, Nerlich AG, Weiler C, et al:
Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors,
and the activating TNF-alpha-converting enzyme suggests activation
of the TNF-alpha system in the aging intervertebral disc. Ann NY
Acad Sci. 1096:44–54. 2007. View Article : Google Scholar
|
|
9
|
Zhang CC, Zhou JS, Hu JG, et al: Effects
of IGF-1 on IL-1β-induced apoptosis in rabbit nucleus pulposus
cells in vitro. Mol Med Rep. 7:441–444. 2013.
|
|
10
|
Millward-Sadler SJ, Costello PW and
Freemont AJ: Regulation of catabolic gene expression in normal and
degenerate human intervertebral disc cells: implications for the
pathogenesis of intervertebral disc degeneration. Arthritis Res
Ther. 11:R652009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiler C, Nerlich AG, Bachmeier BE and
Boos N: Expression and distribution of tumor necrosis factor alpha
in human lumbar intervertebral discs: a study in surgical specimen
and autopsy controls. Spine (Phila Pa 1976 ). 30:44–53.
2005.PubMed/NCBI
|
|
12
|
Pratsinis H, Constantinou V, Pavlakis K,
et al: Exogenous and autocrine growth factors stimulate human
intervertebral disc cell proliferation via the ERK and Akt
pathways. J Orthop Res. 30:958–964. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JS, Ellman MB, An HS, et al:
Insulin-like growth factor 1 synergizes with bone morphogenetic
protein 7-mediated anabolism in bovine intervertebral disc cells.
Arthritis Rheum. 62:3706–3715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Séguin CA, Pilliar RM, Roughley PJ and
Kandel RA: Tumor necrosis factor-alpha modulates matrix production
and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976 ).
30:1940–1948. 2005.
|
|
15
|
Wei A, Brisby H, Chung SA and Diwan AD:
Bone morphogenetic protein-7 protects human intervertebral disc
cells in vitro from apoptosis. Spine J. 8:466–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang R, Ruan D and Zhang C: Effects of
TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus
cells in medium with different serum concentrations. J Orthop Surg
Res. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mavrogonatou E and Kletsas D: Effect of
varying osmotic conditions on the response of bovine nucleus
pulposus cells to growth factors and the activation of the ERK and
Akt pathways. J Orthop Res. 28:1276–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Zhou Q, Liang QQ, et al: IGF-1
regulation of type II collagen and MMP-13 expression in rat
endplate chondrocytes via distinct signaling pathways.
Osteoarthritis Cartilage. 17:100–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paul R, Haydon RC, Cheng H, et al:
Potential use of Sox9 gene therapy for intervertebral degenerative
disc disease. Spine (Phila Pa 1976). 28:755–763. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon SH, Nishida K, Gilbertson LG, et al:
Biologic response of human intervertebral disc cells to gene
therapy cocktail. Spine (Phila Pa 1976). 33:1850–1855. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao D, Quan Z, Jiang D, et al: Effect of
adenovirus human bone morphogenetic protein 4 on human degenerative
lumbar intervertebral disc cells. Zhongguo Xiu Fu Chong Jian Wai Ke
Za Zhi. 6:1442–1447. 2012.(In Chinese).
|