Introduction
Parkinson’s disease (PD) is a recognized progressive
neurodegenerative disorder which is caused by a decrease in the
number of dopaminergic neurons located in the substantia nigra
(1,2). Although the etiology of the disease
remains unknown, postmortem studies have indicated that dying cells
not only bear signs of necrosis, but also of apoptosis, in
particular chromatin condensation, DNA fragmentation, oxidative
damage, mitochondrial dysfunction and caspase activation (3,4).
1-Methyl-4-phenylpyridinium (MPP+) is commonly used
in vitro and in vivo to produce models of PD
(5,6). Similar to other dopaminergic toxins,
MPP+ causes oxidative stress and the selective death of
dopaminerigic neuronal cells, such as PC12 (7) and SH-SY5Y cells (8).
A number of studies have reported that herbal
preparations and their natural compounds display broad protective
effects against neurotoxicity in various neurodegenerative diseases
(9,10). Glycyrrhizic acid (GA), a major
active ingredient separated from Radix Glycyrrhizae, possesses
anti-inflammatory and anti-viral effects (11,12). It has been well documented that GA
exerts marked neuroprotective effects against 6-hydroxydopamine- or
glutamate-induced damage to neuronal cells (13,14). However, the contribution of GA
toward MPP+-induced cell damage and the underlying
mechanisms have not yet been fully elucidated.
It is well known that extracellular signal-regulated
kinase (ERK) plays a key role in cell proliferation,
differentiation, survival and apoptosis (15). The phosphorylation of ERK has been
shown to be critical for mediating the neuroprotectives effects of
leptin (16). Combined with the
activation of ERK, mitochondrial depolarization is associated with
apoptotic cell death (17).
Another pathway involved in this process is the PI3K/AKT signaling
pathway, which is essential for rescuing neuronal cells from
oxidative stress (18).
We therefore hypothesized that GA exerts
neuroprotective effects against MPP+-induced cell
damage. To examine this hypothesis, in this study, we investigated
the inhibitory effects of GA on MPP+ cytotoxicity and
the underlying mechanisms. Our data revealed that GA attenuated
MPP+-induced cell death, the high apoptotic rate, the
intracellular Ca2+ overload, the overproduction of
lactate dehydrogenase (LDH), as well as mitochondrial dysfunction.
Further experiments indicated that the activation of ERK
contributes to the GA-mediated neuroprotection of dopaminergic
neuronal cells.
Materials and methods
Cell culture
PC12 cells (rat adrenal gland pheochromocytoma
cells; obtained from ATCC, Manassas, VA, USA; CRL-1721 passages
<10) were maintained as monolayer cultures in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% horse serum
(HS), 5% fetal bovine serum (FBS) and penicillin (100 IU/ml), and
streptomycin (100 μg/ml) (all from Invitrogen, Carlsbad, CA, USA),
under a humidified atmosphere containing 5/95% CO2/air
at 37 °C. The culture medium was changed every 3 days. The PC12
cells were treated with 20 ng/ml nerve growth factor (NGF;
Sigma-Aldrich, St. Louis, MO, USA) in DMEM supplemented with 1% FBS
and 1% HS and incubated for 72 h to induce differentiation.
Primary cultures of neurons were prepared from fetal
cortices of pregnant Sprague-Dawley rats [embryonic days (E) 17–18]
as previously described (19).
Briefly, the neurons were dissociated from the cerebral cortex of
embryonic rats and were plated in 96-well culture plates which had
been previously coated with poly-D-lysine (Invitrogen). The cells
were maintained in neurobasal medium supplemented with 2% B27 and
1% GlutaMAX (both from Invitrogen). The purity of the primary
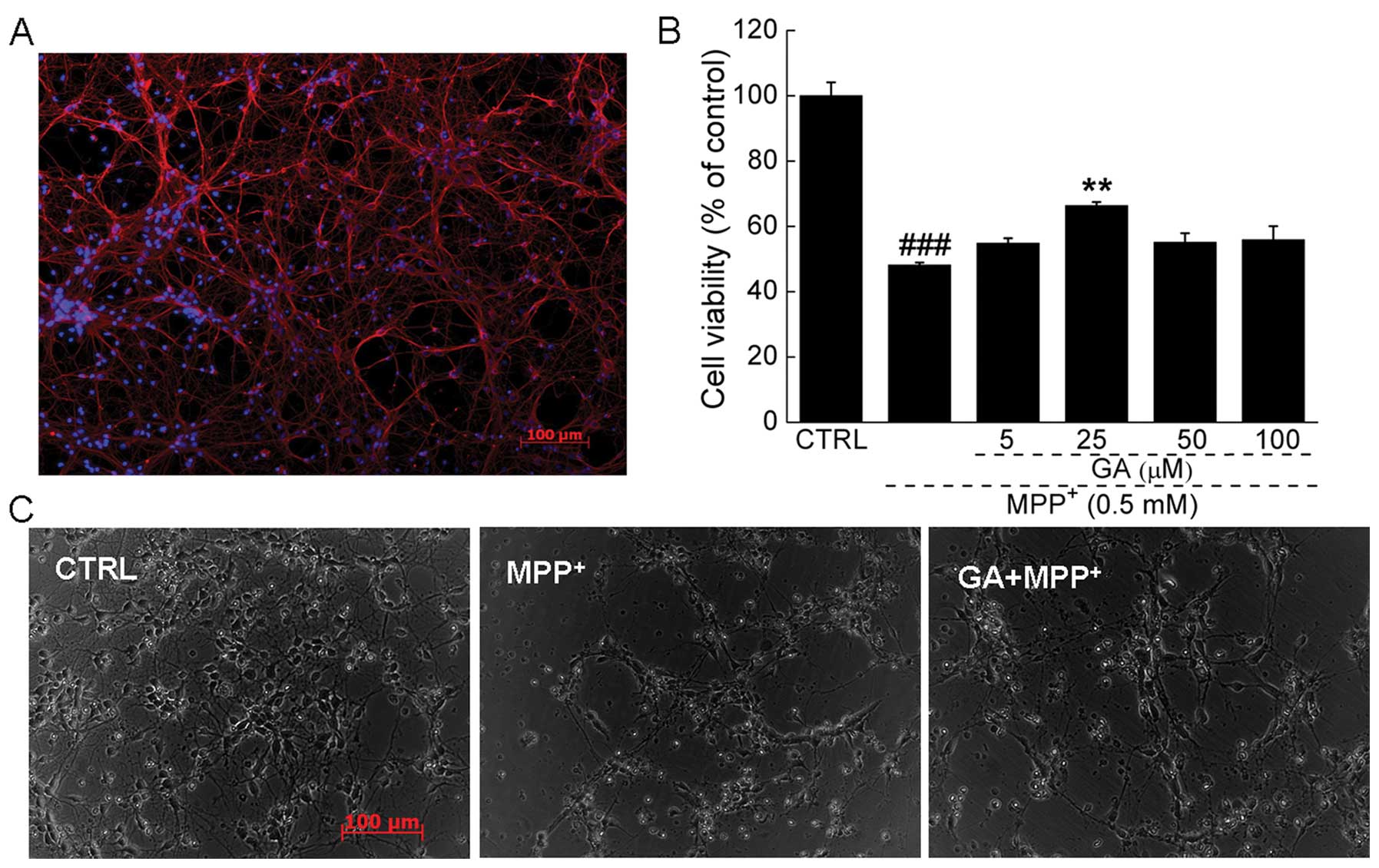
cortical neuronal cells was 88.1±5.6% (Fig. 2A) which was determined by
β3-tubulin (red fluorescence) staining using ImageJ software.
Analysis of cell viability and cellular
morphology
Cell viability was measured by a quantitative
colorimetric assay with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) as described in a previous study (20). Briefly, the differentiated PC12
(DPC12) cells and primary neurons were seeded into 96-well plates
at 5×104 cells per well. The cells were pre-treated with
5–100 μM GA for 3 h, co-treated with 0.5 or 4 mM MPP+
for 24 h, and then incubated with MTT solution (5 mg/ml) for a
further 4 h. A total of 100 μl dimethyl sulfoxide (DMSO) was added
to each well and then the absorbance was measured using a
microplate reader (Bio-Rad, Berkeley, CA, USA) at 540 nm. Cell
viability was expressed as a percentage of the value in the control
group (untreated cells). Prior to MTT assay, the morphology of the
primary neurons was detected by normal photography (10′, Axio
Observer Z1; Carl Zeiss, Inc., Oberkochen, Germany).
Measurement of LDH release
The release of LDH into the culture medium was
measured using an In Vitro Toxicology Assay kit
(Sigma-Aldrich). Briefly, the PC12 cells were seeded into 96-well
plates at 2×104 cells per well. Following
differentiation, the cells were pre-treated with 25 μM GA for 3 h
and co-treated with 4 mM MPP+ for 24 h. A total of 60 μl
mixed assay solution was added into 30 μl culture medium collected
from each group. Following 30 min of incubation at room temperature
in the dark, 10 μl 1 N HCl were added to terminate the reaction.
The absorbance was measured at a wavelength of 490 nm using a
microplate reader (Bio-Rad). The values of the treated cells were
expressed as a percentage of the corresponding untreated cells.
Mitochondrial membrane potential (MMP)
analysis
5,5′,6,6′-Tetrachloro-1,1′,3,3′
tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Sigma-Aldrich)
was used to measure changes in MMP. The PC12 cells
(1×105) were seeded in 12-well plates and allowed to
differentiate. The cells were pre-treated with 25 μM GA for 3 h and
co-treated with 4 mM MPP+ for 24 h. The treated DPC12
cells were incubated with 2 μM JC-1 at 37°C for 10 min. After 3
washes with phosphate-buffered saline (PBS), the changes in MMP of
the DPC12 cells were analyzed using a fluorescence microscope (×20
magnification; Axio Observer Z1, CCD camera; Carl Zeiss, Inc.).
Measurement of intracellular
Ca2+ concentration
Fluo-4 AM (Invitrogen) staining was used to measure
the intracellular Ca2+ concentration. The DPC12 cells
were pre-treated with 25 μM GA for 3 h and then co-treated with 4
mM MPP+ for a further 3 h. The cells were incubated with
Fluo-4 AM (final concentration, 5 μM) at 37°C for 30 min, and
washed 3 times to remove the excess probe. The fluorescence
intensity was determined using a fluorescence microscope (×40
magnification; Axio Observer Z1, CCD camera; Carl Zeiss). The
experiment was repeated 3 times and the average fluorescence
intensity for each cell was calculated using ImageJ software.
Flow cytometric analysis of
apoptosis
Flow cytometric analysis was used to assess the
membrane and nuclear events during apoptosis, as previously
described (7). The assay was
performed with a two-color analysis of fluorescein isothiocyanate
(FITC)-labeled Annexin V binding propidium iodide (PI)
(Becton-Dickinson Co., Miami, FL, USA). The DPC12 cells were
pre-treated with 25 μM GA for 3 h and then co-treated with 4 mM
MPP+ for a further 12 h. The cells were suspended
(1×106/ml) in binding buffer and incubated for 10 min
with 5 μl Anexin V-FITC (20 μg/ml) and 10 μl PI (50 μg/ml) at room
temperature in the dark. The level of fluorescence was analyzed
using a flow cytometer (Cytomics™ FC 500; Beckman Coulter, Inc.,
Brea, CA, USA). The experiment was repeated 3 times.
Western blot analysis
The treated cells were lysed with RIPA buffer
containing 1% protease inhibitor cocktails and 2%
phenylmethanesulfonyl fluoride (PMSF) (all from Sigma-Aldrich). For
the detection of ERK translocation, the preparation of cytoplasmic
and nuclear extracts was carried out as described in a previous
study by Yang et al (21).
After the supernatant collection, the protein concentration was
determined using the Bradford method. Proteins were separated on a
10% SDS-PAGE gel and transferred electrophoretically onto
nitrocellulose membranes (Bio Basic, Inc., Markham, Ontario,
Canada). The transferred membranes were then blotted with primary
antibodies (dilution of 1:1,000) to: phosphorylated ERK (p-ERKs),
total ERK (t-ERKs), phosphorylated AKT (p-AKT), total AKT (t-AKT),
cleaved poly(ADP-ribose) polymerase (PARP), glyceraldehyde
3-phosphate dehydrogenase (GAPDH) and lamin B (Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight, followed by
treatment with horseradish peroxidase-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Chemiluminescence was detected using ECL detection kits (GE
Healthcare, Buckinghamshire, UK). The intensity of the bands was
quantified by scanning densitometry using Quantity One 4.5.0
software (Bio Basic, Inc.).
Statistical analysis
Data are expressed as the means ± SD. One-way ANOVA
was applied to determine the statistical significance, followed by
post-hoc multiple comparisons (Dunn’s test). A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of GA on cell viability, LDH
release and apoptosis of DPC12 cells or primary cortical
neurons
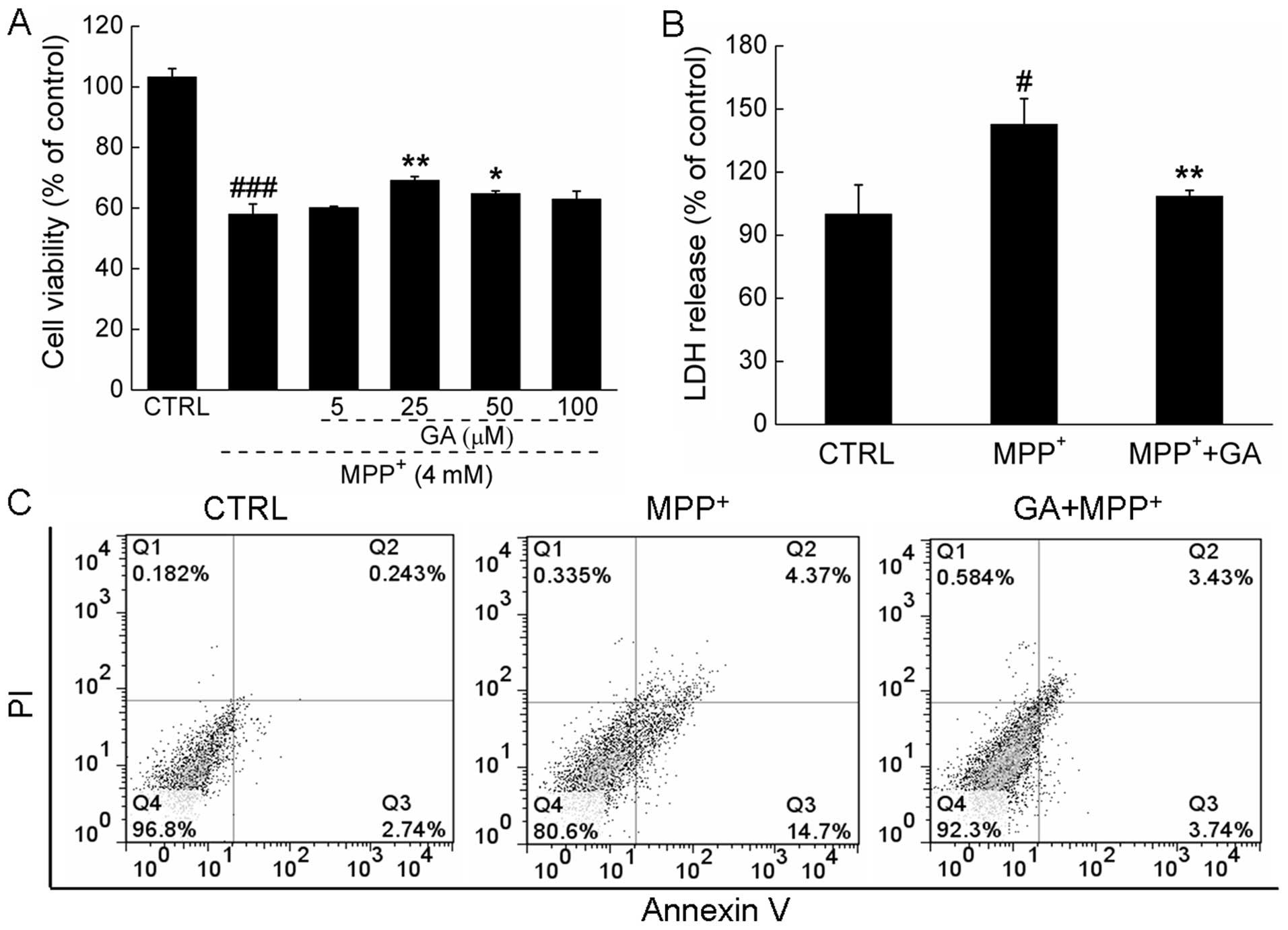
Exposure to 4 mM MPP+ for 24 h resulted
in 41.9±3.3% cell death compared with the control cells; however,
pre-treatment with 25 μM GA significantly attenuated the
MPP+-induced cytotoxicity and increased cell viability
(58.1±3.3 vs. 69.2±1.3%; P<0.01; Fig. 1A). The protective effects of GA
(25 μM) on cell viability were further confirmed in primary neurons
(48.2±0.8 vs. 65.3±1.1%; P<0.01; Fig. 2B). Pre-treatment with 25 μM GA for
3 h following exposure to 4 mM MPP+ for 24 h markedly
suppressed the overproduction of LDH induced by MPP+ in
the DPC12 cells (108.4±2.9 vs. 142.7±12.2%; P<0.01; Fig. 1B). Moreover, double-staining with
Annexin V-FITC and PI was used to detect the apoptotic rate of
DPC12 cells following treatment. The increase in the apoptotic rate
of the MPP+-exposed cells was reversed by pre-treatment
with 25 μM GA (7.2 vs. 19.1%; Fig.
1C). The morphological changes in the primary cortical neurons
were visualized by phase-contrast imaging. Compared with the
untreated cells, shrinkage and detachment were observed following
exposure to MPP+. Pre-treatment with 25 μM GA markedly
reversed the morphological damage caused by MPP+
(Fig. 2C).
Effects of GA on mitochondrial
dysfunction, intracellular Ca2+ overload and the
expression of cleaved PARP
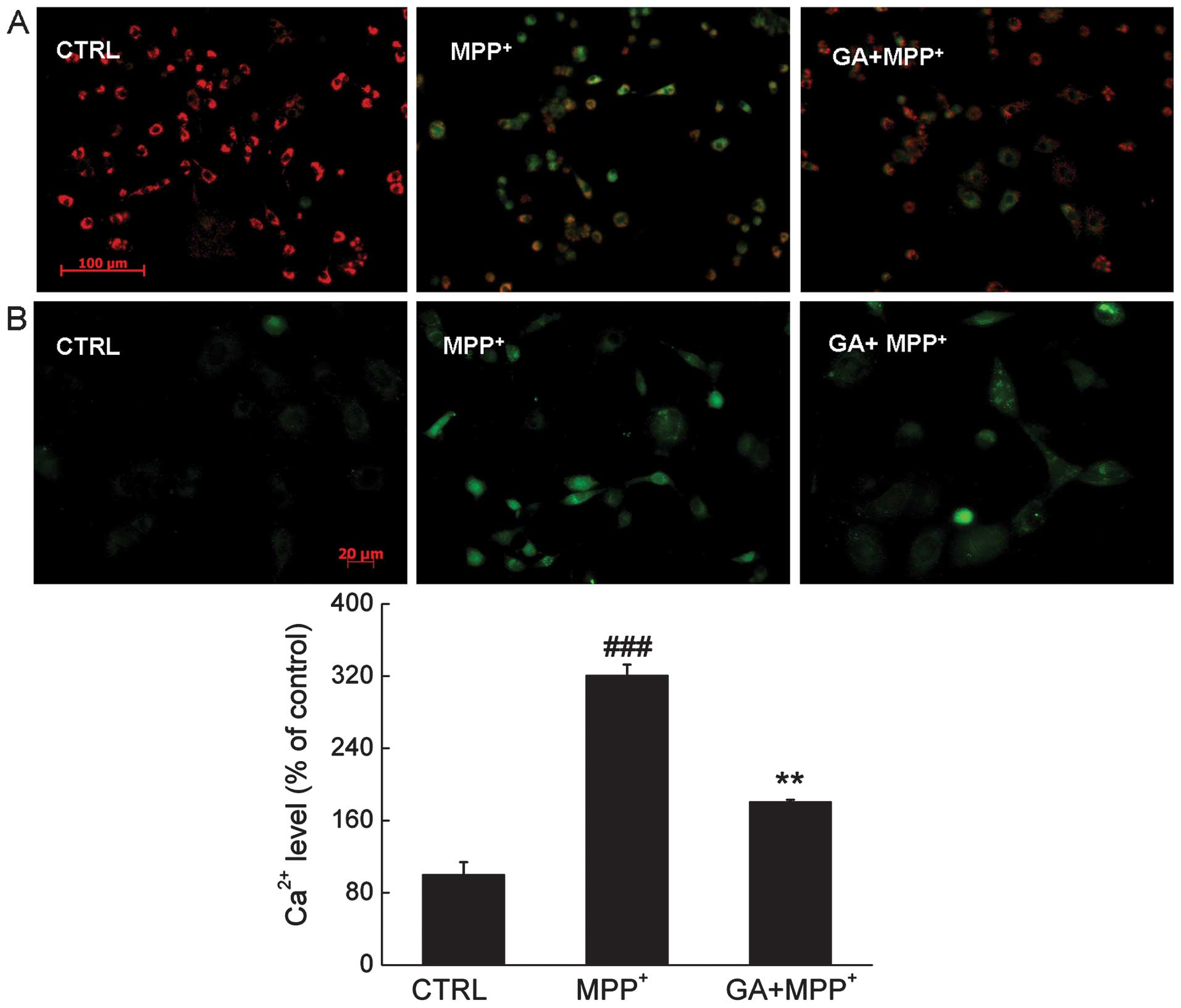
MMP related to mitochondrial permeability plays an
important role in the cell apoptotic pathway. Compared with the
MPP+-exposed cells, after 12 h of co-treatment, 25 μM GA
restored the dissipation of MMP indicated by an increase in the
emission of red fluorescence (Fig.
3A).
The results from Fluo-4 AM staining showed that
after 3 h of incubation of the PC12 cells, GA mitigated the calcium
overload caused by MPP+, indicated by a reduction in the
emission of green fluorescence (Fig.
3B).
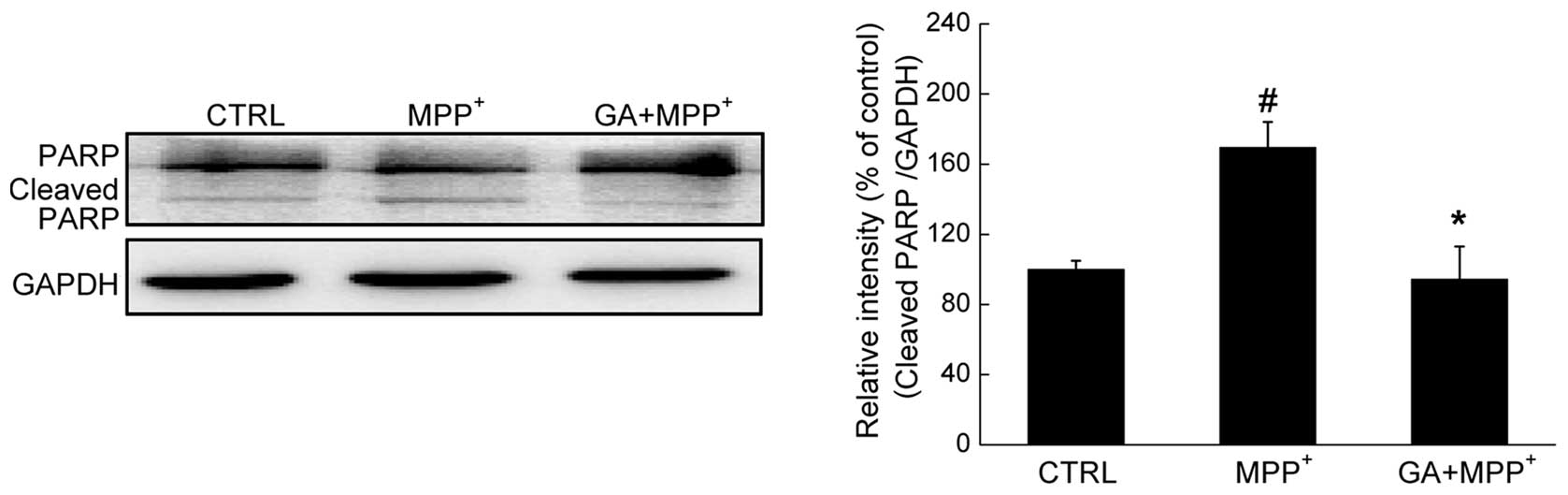
Furthermore, the level of cleaved PARP, a hallmark
of apoptosis, was determined by western blot analysis. A
significant enhancement in the expression of cleaved PARP was
observed in the DPC12 cells exposed to 4 mM MPP+ for 24
h. Conversely, pre-treatment with 25 μM GA reduced the
overexpression of cleaved PARP (P<0.05; Fig. 4).
The ERK, but not the AKT pathway
contributes to the GA-mediated neuroprotective effects
Exposure to MPP+ significantly suppressed
the expression of p-ERK, but not that of t-ERK (Fig. 5A). Treatment with GA (25 μM) alone
increased the phosphorylation of ERK after 30 min of incubation
(P<0.01; Fig.
5B).Pre-treatment with GA (25 μM) markedly reversed the
decrease in the expression of p-ERK caused by MPP+ at 10
and 30 min of treatment (Fig.
5C). Furthermore, the translocation of activated ERK from the
cytoplasm to the nucleus was determined. Following exposure to 4 mM
MPP+ for 60 min, the expression of p-ERK in the nucleus
was reduced; by contrast, pre-treatment with GA for 3 h
significantly reversed the MPP+-induced suppression of
the translocation of ERK to the nucleus (Fig. 5E).
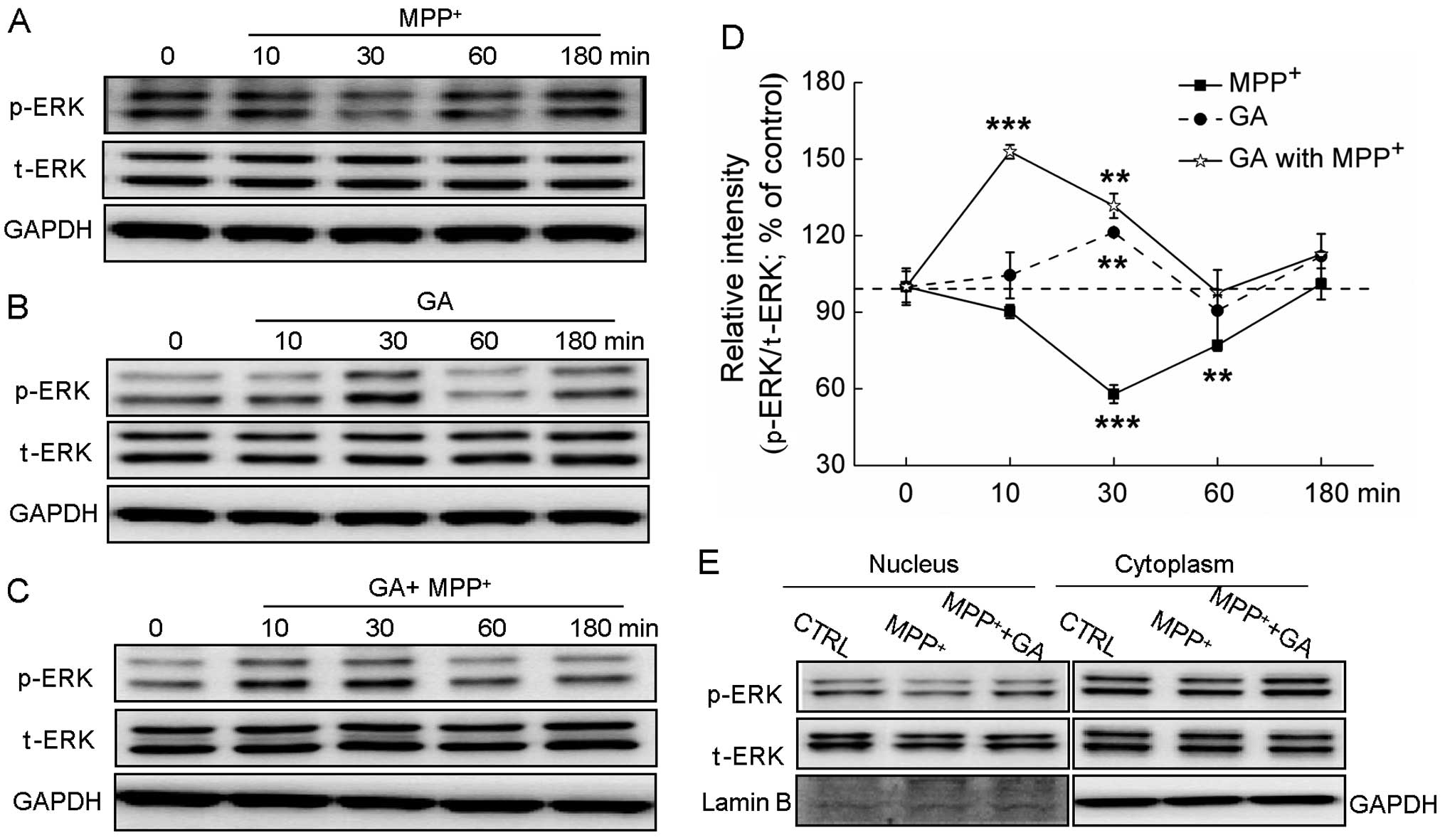 | Figure 5The extracellular signal-regulated
kinase (ERK) signaling pathway contributes to the glycyrrhizic acid
(GA)-mediated neuroprotective effects against
1-methyl-4-phenylpyridinium (MPP+) neurotoxicity. (A)
Differentiated PC12 (DPC12) cells were treated with 4 mM
MPP+ and collected at 0, 10, 30, 60 and 180 min. (B)
DPC12 cells were pre-treated with 25μM GA for 3 h, and then
collected at 0, 10, 30, 60 and 180 min. (C) Following pre-treatment
with 25 μM GA for 3 h, cells were collected at 0, 10, 30, 60 and
180 min after exposure to 4 mM MPP+. (D) Quantification
data of the expression of phosphorylated ERK (p-ERK) were
normalized to the corresponding values of total ERK (t-ERK) and
expressed as a percentage of corresponding cells collected at 0
min. (E) GA enhanced the migration of p-ERK from the cytoplasm to
the nucleus. DPC12 cells were pre-treated with or without 25 μM GA
for 3 h and then co-treated with MPP+ for 1 h. Data are
expressed as the means ± SD (n=3) and analyzed by one-way ANOVA.
**P<0.01 and ***P<0.001 vs. non-treated
cells. CTRL, control. |
Additionally, treatment with MPP+ alone,
GA alone and MPP+ plus GA had no effects on the
expression of p-AKT and t-AKT (Fig.
6A–D).
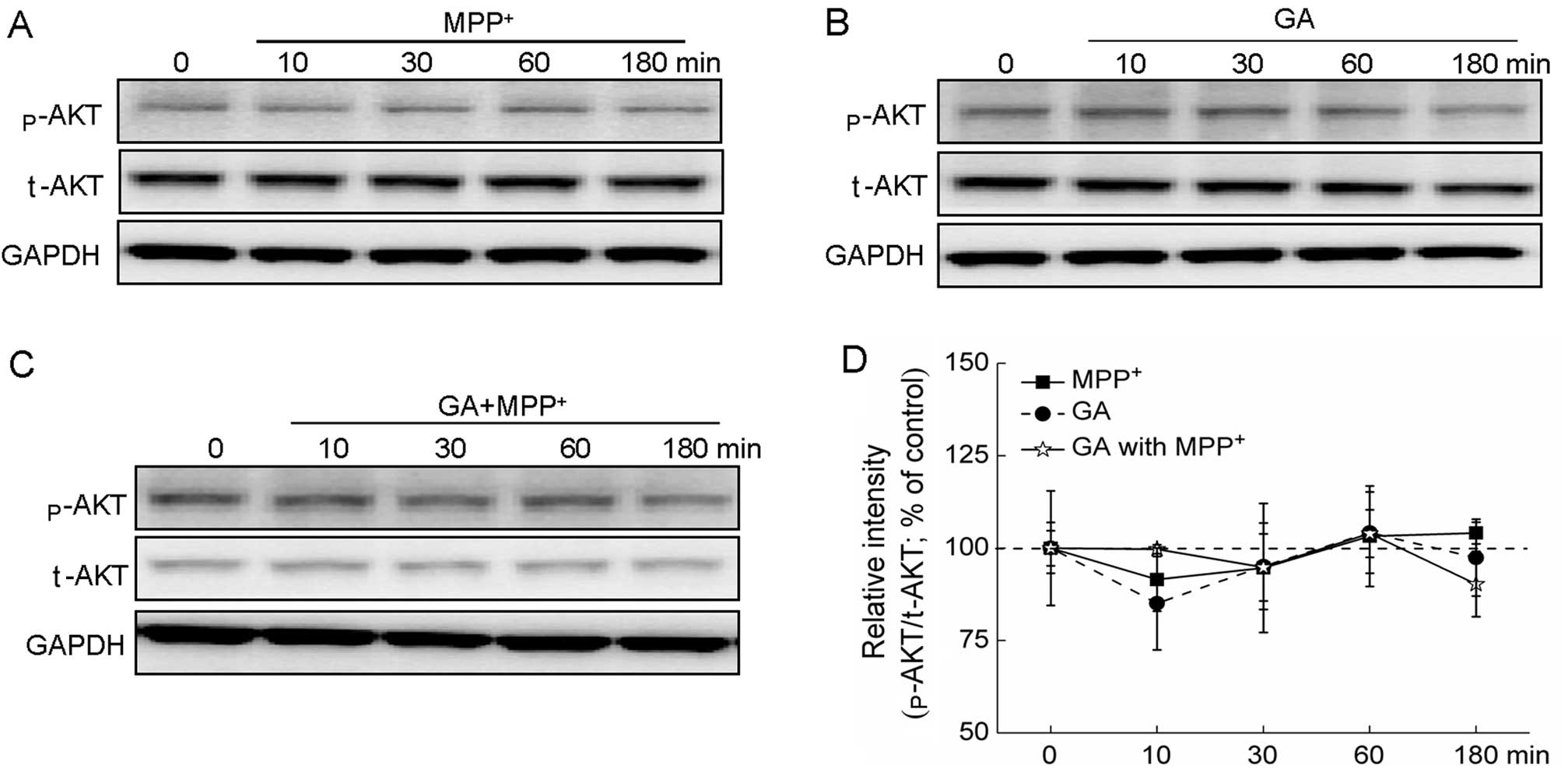 | Figure 6AKT signaling pathway is not involved
in the glycyrrhizic acid (GA)-mediated neuroprotective effects
against 1-methyl-4-phenylpyridinium (MPP+). (A)
Differentiated PC12 (DPC12) cells were collected at 0, 10, 30, 60
and 180 min after exposure to 4 mM MPP+. (B) DPC12 cells
were pre-treated 25 μM GA for 3 h, and then collected at 0, 10, 30,
60 and 180 min. (C) Following pre-treatment with 25 μM GA for 3 h,
cells were collected at 0, 10, 30, 60 and 180 min after exposure to
4 mM MPP+. (D) Quantification data of the expression of
phosphorylated AKT (p-AKT) were normalized to the corresponding
values of total AKT (t-AKT) and expressed as a percentage of
corresponding cells collected at 0 min. Data are the means ± SD of
3 replicate values in 3 separate experiments and analyzed by
one-way ANOVA. |
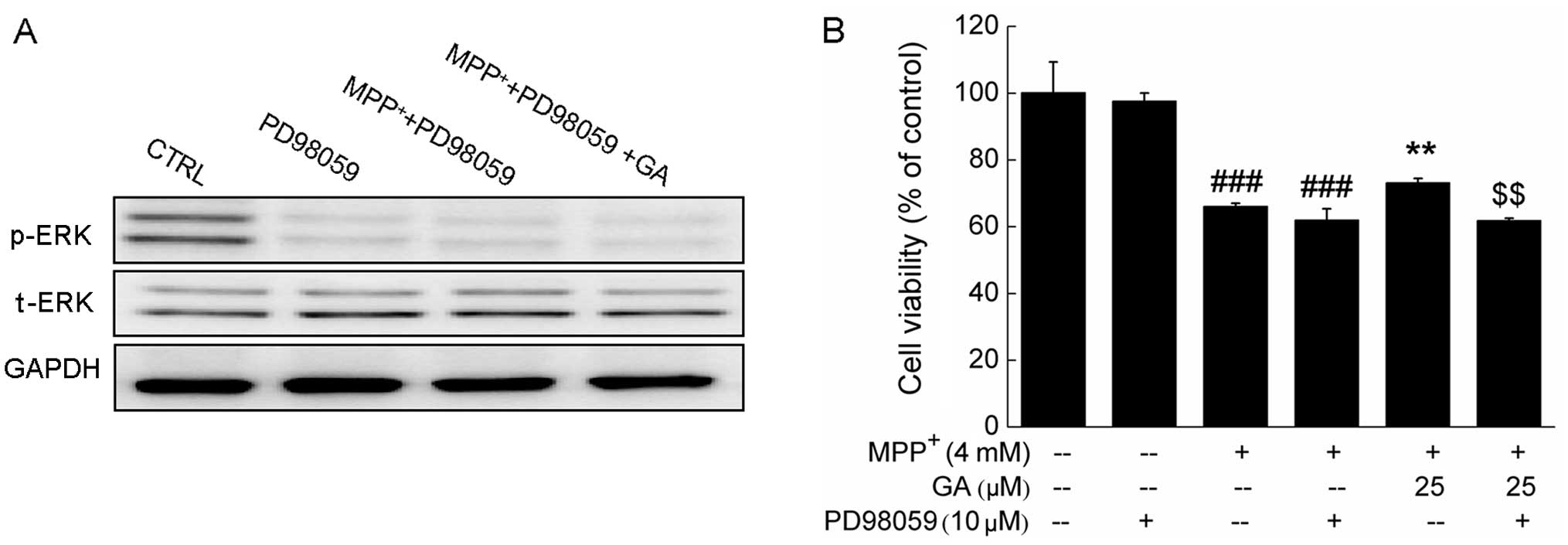
Further experiments revealed that the GA-induced
increase in the phosphorylation of ERK and the improvement in cell
viability were markedly abrogated by pre-treatment with 10 μM
PD98059, an ERK inhibitor (Fig. 7A
and B). Collectively, our data suggest that the ERK, but not
the AKT signaling pathway contributes to the GA-mediated
neuroprotective effects against MPP+-induced DPC12 cell
damage.
Discussion
PC12 cells, which possess a dopamine synthesis,
metabolism and transporting system (22), can shift their phenotype, changing
from proliferating, undifferentiated cells into post-mitotic,
differentiated, neurite-bearing neurons following incubation with
NGF (23). Studies have
demonstrated that DPC12 cells are more sensitive to neurotoxins
(24). The present study clearly
confirms that GA exerts marked neuroprotective effects, as
evidenced by our results: GA greatly ameliorated the
MPP+-induced reduction in cell viability, the increased
apoptotic rate, the intracellular Ca2+ overload and the
dissipation in MMP in the DPC12 cells. GA also exerted suppressive
effects on the overproduction of intracellular LDH caused by
MPP+. Consistent with the results of previous studies
(25,26), this suppressive effect on the LDH
level is a consequence of the protective effects of GA against
neurotoxicity. PARP is a family of proteins involved in a number of
cellular processes, including DNA repair and programmed cell death
(27). GA markedly suppressed the
enhanced expression of cleaved PARP caused by MPP+.
The pro-survival activation of ERK is well known in
dopaminergic neurons (16,28).
The phosphorylation of ERK in SH-SY5Y cells has been shown to be
suppressed after 4 h of exposure to MPP+ (29). Our study confirmed that
MPP+ reduced the phosporylation of ERK; by contrast, GA
reversed the MPP+-mediated inhibition of ERK activation.
Additionally, the presence of PD98059 eradicated the effects of GA
on ERK activation and protection of cell viability. Compared with
the MPP+-treated cells, treatment with GA resulted in an
increase in p-ERK nuclear migration. It has previously been
demonstrated that activated ERK migrates to the nucleus where it
regulates transcription factors, leading to changes in gene
expression and cell proliferation (30). Collectively, our data indicate
that the ERK signaling pathway contributes to the GA-mediated
protective effects against MPP+-induced DPC12 cell
damage.
Our data further indicated that pre-treatment with
GA markedly blocked the calcium influx caused by MPP+.
Excessive cytosolic calcium causes a wide range of subcellular
pathological responses, in particular the dysfunction of
mitochondrial membrane permeability (31). Growing experimental evidence
suggests that the mitochondrial-dependent pathway plays a central
role in cell apoptosis (17). A
key feature of mitochondrial apoptosis is the disruption of the
membrane potential, mainly caused by increased membrane
permeability (32). The present
study demonstrated that GA restored the dissipation of MMP and
promoted the activation of ERK. As reported previously, the
activation of ERK regulates mitochondrial function (33,34). Previous studies have demonstrated
that ERK inhibitors downregulate the expression of B-cell
lymphoma-2 (Bcl-2) and Bcl-extra large (Bcl-xL) (33), which are located in the outer
membrane of the mitochondria and regulate mitochondrial function
(35). However, the association
between the activation of ERK and mitochondrial function and their
involvement in the GA-mediated neuroprotective effects require
further investigation.
It has been demonstrated that GA exerts
neuroprotective effects against 6-hydroxydopamine-induced
cytotoxicity in PC12 cells via PI3K/AKT pathway (13); however, in our study, we did not
observe any significant effects on the activation of AKT following
treatment with GA or MPP+. In another separate
experiment, GA did not exert any effects on the glutamate-induced
decrease in the level of p-AKT. The different results noted in our
study may due to the different microenvironmental system.
Furthermore, the accumulation of cells in the G1 phase is
considered one of the factors responsible for
MPP+-induced cell damage (36). In our study,
MPP+-induced G1 phase arrest was observed in the DPC12
cells; however, pre-treatment with GA failed to reverse this effect
(data not shown).
In conclusion, our data demonstrate that GA exerts
significant protective effects on neuronal cells against
MPP+ neurotoxicity, as indicated by the suppression of
the intracellular Ca2+ overload, the restoration of
mitochondrial dysfunction, and the increase in the expression and
migration of p-ERK. The present findings provide pharmacological
evidence to support the therapeutic application of GA in the
treatment of neurodegenerative diseases.
Acknowledgements
This study was supported by the National Science and
Technology support program of P.R. China (grant no. 2012BAL29B05)
and the Major Scientific and Technological Double Ten Project in
Jilin Province (20130201006ZY).
References
|
1
|
Forno LS: Neuropathology of Parkinson’s
disease. J Neuropathol Exp Neurol. 55:259–272. 1996.
|
|
2
|
Surendran S and Rajasankar S: Parkinson’s
disease: oxidative stress and therapeutic approaches. Neurol Sci.
31:531–540. 2010.
|
|
3
|
Hisahara S and Shimohama S: Toxin-induced
and genetic animal models of Parkinson’s disease. Parkinsons Dis.
2011:9517092010.
|
|
4
|
Schulz JB and Falkenburger BH: Neuronal
pathology in Parkinson’s disease. Cell Tissue Res. 318:135–147.
2004.
|
|
5
|
Sonsalla PK, Zeevalk GD and German DC:
Chronic intraventricular administration of
1-methyl-4-phenylpyridinium as a progressive model of Parkinson’s
disease. Parkinsonism Relat Disord. 14(Suppl 2): S116–S118.
2008.
|
|
6
|
Li X, Ye X, Sun X, et al: Salidroside
protects against MPP(+)-induced apoptosis in PC12 cells by
inhibiting the NO pathway. Brain Res. 1382:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin R, Li X, Li G, et al: Protection by
tetrahydroxystilbene glucoside against neurotoxicity induced by
MPP+: the involvement of PI3K/Akt pathway activation.
Toxicol Lett. 202:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halvorsen EM, Dennis J, Keeney P, Sturgill
TW, Tuttle JB and Bennett JB Jr: Methylpyridinium (MPP(+))-and
nerve growth factor-induced changes in pro- and anti-apoptotic
signaling pathways in SH-SY5Y neuroblastoma cells. Brain Res.
952:98–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim EH, Jang MH, Shin MC, Shin MS and Kim
CJ: Protective effect of aqueous extract of Ginseng radix against
1-methyl-4-phenylpyridinium-induced apoptosis in PC12 cells. Biol
Pharm Bull. 26:1668–1673. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi JG, Kim HG, Kim MC, et al: Polygalae
radix inhibits toxin-induced neuronal death in the Parkinson’s
disease models. J Ethnopharmacol. 134:414–421. 2011.PubMed/NCBI
|
|
11
|
Matsui S, Matsumoto H, Sonoda Y, et al:
Glycyrrhizin and related compounds down-regulate production of
inflammatory chemokines IL-8 and eotaxin 1 in a human lung
fibroblast cell line. Int Immunopharmacol. 4:1633–1644. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cinatl J, Morgenstern B, Bauer G, Chandra
P, Rabenau H and Doerr HW: Glycyrrhizin, an active component of
liquorice roots, and replication of SARS-associated coronavirus.
Lancet. 361:2045–2046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kao TC, Shyu MH and Yen GC:
Neuroprotective effects of glycyrrhizic acid and
18beta-glycyrrhetinic acid in PC12 cells via modulation of the
PI3K/Akt pathway. J Agric Food Chem. 57:754–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cherng JM, Lin HJ, Hung MS, Lin YR, Chan
MH and Lin JC: Inhibition of nuclear factor kappaB is associated
with neuroprotective effects of glycyrrhizic acid on
glutamate-induced excitotoxicity in primary neurons. Eur J
Pharmacol. 547:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YL, Wang GJ, Huang CL, et al:
Ligusticum chuanxiong as a potential neuroprotectant for preventing
serum deprivation-induced apoptosis in rat pheochromocytoma cells:
functional roles of mitogen-activated protein kinases. J
Ethnopharmacol. 122:417–423. 2009. View Article : Google Scholar
|
|
16
|
Weng Z, Signore AP, Gao Y, et al: Leptin
protects against 6-hydroxydopamine-induced dopaminergic cell death
via mitogen-activated protein kinase signaling. J Biol Chem.
282:34479–34491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CS, Kim YJ, Lee MS, Han ES and Lee SJ:
18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa
cells and exhibits a synergistic effect against antibiotic
anti-cancer drug toxicity. Life Sci. 83:481–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu S, Lu C, Han Q, et al: Adipose-derived
mesenchymal stem cells protect PC12 cells from glutamate
excitotoxicity-induced apoptosis by upregulation of XIAP through
PI3-K/Akt activation. Toxicology. 279:189–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JY, Chi SI and Hwang CP: Effects of
various nitric oxide synthase inhibitors on NMDA-induced neuronal
injury in rat cortical neurons. Chin J Physiol. 39:227–233.
1996.PubMed/NCBI
|
|
20
|
Ocazionez RE, Meneses R, Torres FA and
Stashenko E: Virucidal activity of Colombian Lippia
essential oils on dengue virus replication in vitro. Mem Inst
Oswaldo Cruz. 105:304–309. 2010.PubMed/NCBI
|
|
21
|
Yang CL, Chik SC, Li JC, Cheung BK and Lau
AS: Identification of the bioactive constituent and its mechanisms
of action in mediating the anti-inflammatory effects of black
cohosh and related Cimicifuga species on human primary blood
macrophages. J Med Chem. 52:6707–6715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tuler SM, Hazen AA and Bowen JM: Release
and metabolism of dopamine in a clonal line of pheochromocytoma
(PC12) cells exposed to fenthion. Fundam Appl Toxicol. 13:484–492.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar
|
|
24
|
Tahir SK, Trogadis JE, Stevens JK and
Zimmerman AM: Cytoskeletal organization following cannabinoid
treatment in undifferentiated and differentiated PC12 cells.
Biochem Cell Biol. 70:1159–73. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao BY, Yang YP, Luo WF, et al:
Paeoniflorin, a potent natural compound, protects PC12 cells from
MPP+and acidic damage via autophagic pathway. J
Ethnopharmacol. 131:122–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zandbergen EG, de Haan RJ and Hijdra A:
Systematic review of prediction of poor outcome in anoxic-ischaemic
coma with biochemical markers of brain damage. Intensive Care Med.
27:1661–1667. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piskunova TS, Yurova MN, Ovsyannikov AI,
et al: Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1)
accelerates aging and spontaneous carcinogenesis in mice. Curr
Gerontol Geriatr Res. 2008:7541902008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hetman M and Gozdz A: Role of
extracellular signal regulated kinases 1 and 2 in neuronal
survival. Eur J Biochem. 271:2050–2055. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu JH, Horbinski C, Guo F, Watkins S,
Uchiyama Y and Chu CT: Regulation of autophagy by extracellular
signal-regulated protein kinases during
1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol.
170:75–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuber J, Tchernitsa OI, Hinzmann B, et al:
A genome-wide survey of RAS transformation targets. Nat Genet.
24:144–152. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giorgi C, Baldassari F, Bononi A, et al:
Mitochondrial Ca(2+) and apoptosis. Cell Calcium. 52:36–43. 2012.
View Article : Google Scholar
|
|
32
|
Mayer B and Oberbauer R: Mitochondrial
regulation of apoptosis. News Physiol Sci. 18:89–94. 2003.
|
|
33
|
Boucher MJ, Morisset J, Vachon PH, Reed
JC, Laine J and Rivard N: MEK/ERK signaling pathway regulates the
expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of
human pancreatic cancer cells. J Cell Biochem. 79:355–369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galante JM, Mortenson MM, Bowles TL,
Virudachalam S and Bold RJ: ERK/BCL-2 pathway in the resistance of
pancreatic cancer to anoikis. J Surg Res. 152:18–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: from mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai J, Nakamura H, Ueda S, et al:
Proteasome-dependent degradation of cyclin D1 in
1-methyl-4-phenylpyridinium ion (MPP+)-induced cell
cycle arrest. J Biol Chem. 279:38710–38714. 2004. View Article : Google Scholar : PubMed/NCBI
|