Introduction
Metastasis is the principal cause of morbidity and
mortality in cancer patients. It occurs during the late stages of
tumor progression and is an exceedingly complex event (1). Of note, certain types of cancer show
a predilection to metastasis to specific organs (2). In 1889, Stephen Paget set forth the
‘seed and soil’ hypothesis of metastasis, to explain the non-random
pattern of metastasis (3). The
theory states that metastasis is dependent on the cross-talk
between selected cancer cells (the ‘seeds’) and specific organ
microenvironments (the ‘soil’), and this theory still holds forth
today. However, the precise molecular mechanisms, regulatory
circuits and master metastasis-associated genes that govern these
fatal changes remain elusive (4).
Small cell lung cancer (SCLC) accounts for 15–20% of lung cancer
cases and presents an aggressive clinical behavior characterized by
rapid growth and spread to distant organs (5). Previously, Miki et al
(6) examined the ability of
various lung cancer cell lines to generate multiple organ
metastasis, particularly bone metastasis by intravenously injecting
the cancer cells into natural killer (NK) cell-depleted SCID mice;
they found that the human the SCLC cell line, SBC-5 was the only
one to generate bone metastasis; bone metastasis was not generated
by the SBC-3 cells (the SBC-3 cell line was originally established
from the bone marrow aspirate of a 24-year-old male patient with
SCLC). Although the SBC-5 and SBC-3 cells have a similar genetic
background, they differ in their potential to generate bone
metastasis. In this study, we compared the surface expression of
C-X-C chemokine receptor type 4 (CXCR4) proteins in SBC-5 and SBC-3
cells by flow cytometric analysis, and the results demonstrated
that CXCR4 protein expression was markedly higher in the SBC-5
cells compared with the SBC-3 cells. It has previously been
demonstrated that CXCR4 and stromal-derived-factor-1 (SDF-1)
regulate migration and metastasis in certain types of cancer
(7); however, the roles of
SDF-1-CXCR4 in the organ-selective metastasis of SCLC remain to be
elucidated.
The term intrakine (intracellular chemokine) refers
to the strategy used to genetically silence a certain (cytokine)
receptor. This is achieved by the fusion of the chemokine gene with
an endoplasmic reticulum (ER) retention signal (KDEL), termed
‘intrakine’, which can bind to the newly synthesized chemokine
receptor molecules within the cell and block their surface
expression. This method was originally proposed as gene therapy for
AIDS (8–10). Thereafter, it has been used as a
tool for the functional analysis of chemokines and their receptors
in vitro and in vivo (11).
In the present study, we knocked down CXCR4
expression in SBC-5 cells using the intrakine strategy and
evaluated the biological behavior of these cells, including
proliferation, apoptosis, cell cycle progression, invasion and
migration in vitro. In addition, we used the multiple organ
metastasis model of human SCLC cells to study organ metastasis
in vivo. Our results revealed that CXCR4 was a candidate
gene involved in metastasis to specific organs, such as the lungs,
liver and bone. However, the silencing of CXCR4 did not affect the
proliferation and apoptosis of the SBC-5 cells in vitro.
CXCR4 may prove to be a promising target for the prevention and
effective treatment of metastastic lesions due to SCLC.
Materials and methods
Cell culture
The human SCLC cell lines, SBC-5 and SBC-3, were
gifts from Professor Saburo Sone and Professor Seiji Yano
(Tokushima University, Tokushima, Japan). They were maintained at
37°C with 5% CO2 in RPMI-1640 supplemented with 10%
(v/v) heated-inactivated fetal bovine serum (FBS; both from Gibco,
Gaithersburg, MD, USA), 100 U/ml streptomycin and 100 U/ml
penicillin.
Flow cytometric analysis of CXCR4
expression in SBC-5 and SBC-3 cells
SBC-5 and SBC-3 cells (or stably transfected
SBC-5/S-K and SBC-5/neo cells) were collected and washed with
phosphate-buffered saline (PBS) supplemented with 0.5% BSA. The
cells were then resuspended to a final concentration of
4×106 cells/ml, 25 μl of which were extracted for
staining. In brief, this was followed by the addition of 10
μl carboxyfluorescein-conjugated mouse anti-human CXCR4
(clone 12G5) monoclonal antibodies (FAB170F; R&D Systems,
Minneapolis, MN, USA) and incubation for 30 min at 4°C.
Subsequently, the cells were washed with PBS to remove the
unreacted antibodies and were then resuspended in 200–400 μl
of PBS. The cell surface expression of CXCR4 was measured using a
flow cytometer (BD Biosciences, San Jose, CA, USA) using a 488 nm
wavelength laser excitation. The cells expressing CXCR4 were
fluorescently stained, with the intensity of staining directly
representing the density of CXCR4. The negative controls cells were
stained with PBS.
Construction and use of recombinant
plasmid, PCMV-S-K
The recombinant plasmid, PCMV-S-K, was gift from
Professor Ping Zhong Wang (Center of Diagnosis and Treatment for
Infectious Diseases, Tangdu Hospital, the Fourth Military Medical
University, Xi’an, China). It was transfected into competent
Escherichia coli DH5α cells, and then cultured in LB agar
plates to select colonies with inserted SDF-1-KDEL sequences using
colony polymerase chain reaction (PCR) with primers (sense,
5′-CACCATGAACGCCAAGGTC-3′, antisense,
5′-CAGCTCGTCCTTTTACTTGTTT-3′). Colonies with an inserted sequence
were identified by agarose gel electrophoresis. The sequence of the
plasmid correctly inserted with SDF-1-KDEL was verified by DNA
sequencing.
Stable transfection
The PCMV-S-K and the PCMV mock vector were
transfected into the SBC-5 cells using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) following the manufacturer’s
instructions. Plasmid GFP was transfected at the same time as
positive control to show its transfection efficiency. The
transfected cells were selected with medium containing 100
μg/ml of G418 sulfate (Geneticin; Invitrogen) from the
second day (100 μg/ml is the minimum concentration of
geneticin which can kill all SBC-5 cells within 2 weeks). After 1
month of transfection, G418-resistant colonies were isolated by
limiting dilution and then expanded. The stably transfected cells
were designated as the SBC-5/S-K and SBC-5/neo cells, respectively
and maintained in growth mediumcontaining 50 μg/ml of
Geneticin.
Detection of SDF-1 expression by
immunofluorescence
The SBC-5/S-K and SBC-5/neo cells in the logarithmic
growth phase were collected, washed, fixed and incubated for 5 min
at room temperature. They were then washed for 15 min with PBS
containing 0.5% Triton X-100, and incubated for 1 h at 37°C in
blocking solution. The rabbit-anti-human SDF-1 polyclonal antibody
(FL-93, sc-28876; 1:50 dilution; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) was added followed by incubation for 2 h at 37°C;
the cells were then washed and incubated in fluorescein
isothiocyanate (FITC)-conjugated goat-anti-rabbit IgG antibody
(H+L, bsF-0295G; Bioss, Woburn, MA, USA; 1:100 dilution with PBS
containing 0.01% Evans blue) at 37°C for 1 h. Finally, the cells
were washed and coverslips were mounted in 50% buffered glycerol
mounting solution. Microscopic images to detect the expression of
SDF-1 were obtained using an Olympus BX51 inverted fluorescence
microscope (Olympus, Tokyo, Japan). For the negative controls, the
process was carried out by substituting the primary antibody with
PBS.
In vitro cell proliferation assay
To measure the cell proliferation of the SBC-5/S-K
and SBC-5/neo cells, cells at 80% confluence were harvested and
placed into 96-well plates (1,000 cells/well). Each day 1 plate was
used for MTT assay. A total of 20 μl MTT solution (5 mg/ml)
was added to each well followed by incubation at 37°C for 4 h.
Subsequently, the MTT solution was removed and 150 μl of
dimethyl sulfoxide (DMSO) were added to dissolve the formazan
crystals. Absorbance was detected at reference wavelengths of 490
and 630 nm using an ELISA plate reader (Multiskan MK3; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). This assay was
performed 3 times.
Soft agar colony formation assay
Twenty-four-well plates were covered with 0.6% agar
in RPMI-1640 medium containing 10% FBS to prevent the attachment of
the cells to the plastic substratum. Cell suspensions
(1×103 cells/well) of the SBC-5/S-K and SBC-5/neo cells
with 0.3% agar were prepared and seeded on the foundation agar.
After 2 weeks of incubation at 37°C, the colonies containing at
least 50 cells were counted under an inverted microscope (IX53;
Olympus). All assays were performed 3 times.
Cell apoptosis and cell cycle
analysis
Cell apoptosis was determined by flow cytometry
using the Annexin V-FITC apoptosis detection kit (Calbiochem, La
Jolla, CA, USA). Cells (1×106) were collected and washed
twice with cool PBS. The cells were then resuspended in 1X binding
buffer. Annexin V-FITC and PI were then added and the cells were
incubated at room temperature for 15 min in the dark. The cells
were again washed with cool PBS twice. Finally, 500 μl PBS
were added to the mixture which was analyzed using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA).
Cell cycle distribution was determined by the
following steps: the cells were resuspended at 1×106
cells/ml, fixed with 75% ethanol at 4°C overnight, washed and
resuspended with cool PBS. Subsequently, 5 μl RNase (10
mg/ml) were added, and the cells were fixed for 1 h at 37°C; 100
μg/ml propidium iodide in a 0.1% sodium citrate/0.1% Triton
X-100 solution was then added, followed by incubation for 30 min at
room temperature in the dark. After the cells were washed, the
analysis of cellular DNA content was carried out using a flow
cytometer (BD Biosciences, San Jose, CA, USA) at an excitation
wavelength of 488 nm. The distribution of these cells in 3 major
phases of the cell cycle (G1, S and G2 phase) was analyzed using
CellQuest and ModFit software (BD Biosciences, San Jose, CA,
USA).
In vitro migration and invision
assay
To detect cell migration in vitro, 24-well
Transwell plates containing filters of 8.0 μm pore size
(Costar, Cambridge, MA, USA; Matrigel uncoated) were used. The
SBC-5/S-K and SBC-5/neo cells (5×104) in RPMI-1640
medium containing 0.1% BSA (Sigma-Aldrich Co., St. Louis, MO, USA)
were placed into the upper chamber of the wells and RPMI-1640
containing 10% FBS was added to the lower chamber. The Transwell
plates were incubated for 36 h. The filters were fixed with 10%
formalin, and stained with crystal violet. The cells on the upper
surface of the filters were removed by swabbing with a cotton swab
and the cells migrated to the lower surface were counted under a
microscope (IX53; Olympus) at x200 magnification. Ten fields for
each sample were counted and analyzed. All assays were performed 3
times.
Cell invasion assays were performed using
Matrigel-coated (BD Biosciences, Le Pont-de-Claix, France) 24-well
Transwell plates. The other steps were the same as those used for
the migration assay.
Determination of metastasis in vivo
All animal experiments were performed according to
the Guidelines on Animal Experimentation formulated by the Forth
Military Medical University, Xi’an, China. The SBC-5/S-K and
SBC-5/neo cells were harvested and only a single cell suspension
with >90% cell viability were used. NOD-SCID mice at 3–4 weeks
of age (from the Institute of Biochemistry and Cell Biology of
Shanghai Institute for Biological Sciences, Chinese Academy of
Sciences, China) were divided into 2 groups and each group
consisted of 2 male and 3 female mice. The SBC-5/S-K and SBC-5/neo
cells (1×106/300 μl) were injected into the tail
vein of the mice, which were maintained under specific
pathogen-free conditions. After 5 weeks, the mice were anesthetized
and bone metastases were visualized by X-ray images. The number of
osteolytic bone metastases on the X-ray images was evaluated
independently by 2 investigators (Dr Haichuan Su and Professor
Minzhang Tang, Department of Oncology, Tangdu Hospital, The Fourth
Military Medical University, Xi’an, China).
Subsequently, the mice were sacrificed by anesthesia
and all the major organs were removed. The number of metastatic
lesions >0.5 mm in diameter on the surface of the major organs
was counted macroscopically. The lungs were fixed in Bouin’s
solution for 24 h. The major organs with metastastic lesions were
fixed in 10% formalin. The bone specimens were decalcified in 10%
EDTA solution for 1 week and then embedded in paraffin.
Statistical analysis
The Wilcoxon rank sum test was used to determine the
significance of the differences in the number and incidence of
metastatic lesions in multiple organs/tissues (bone, lungs, liver
and kidneys) between 2 groups, and the other data were analyzed by
variance analyis or the t-test. Statistical tests were performed
using SPSS software version 13.0.0 (SPSS Inc., Chicago, IL, USA). A
value of P<0.05 was considered to indicate a statistically
significant difference and all statistical tests performed were
two-sided.
Results
Expression levels of CXCR4 in SBC-5 cells
are higher than those in SBC-3 cells
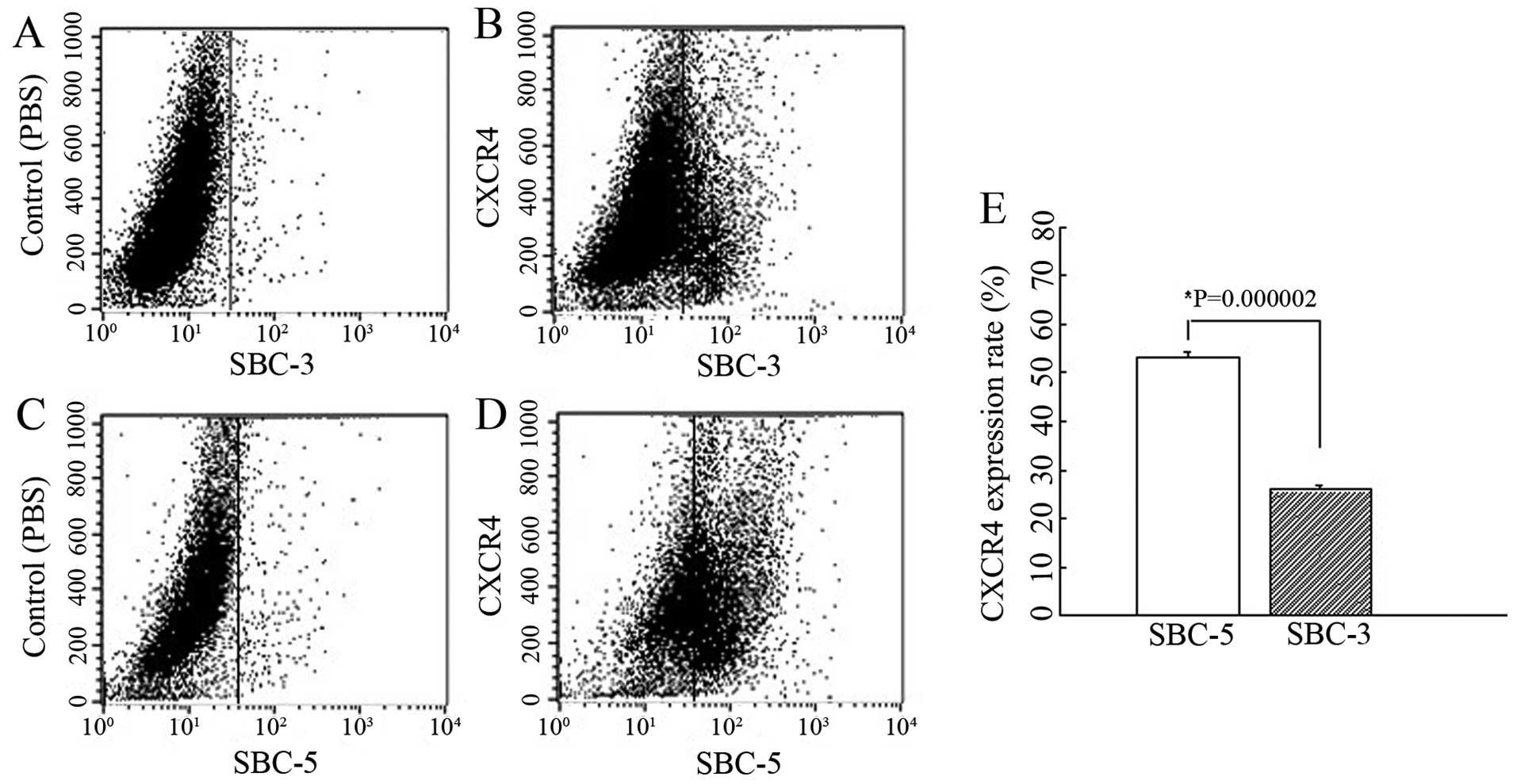
We determined the expression levels of CXCR4 in the
SBC-5 and SBC-3 cells by flow cytometric analysis and found that
the CXCR4 expression rate was 53.04±1.35% in the SBC-5 cells and
25.91±0.78% in the SBC-3 cells (P=0.000002) (Fig. 1). This assay indicated that the
surface expression of CXCR4 in the SBC-5 cells was markedly higher
than that in the SBC-3 cells.
Expression of the recombinant fusion
protein SDF-KDEL in SB-5/S-K cells
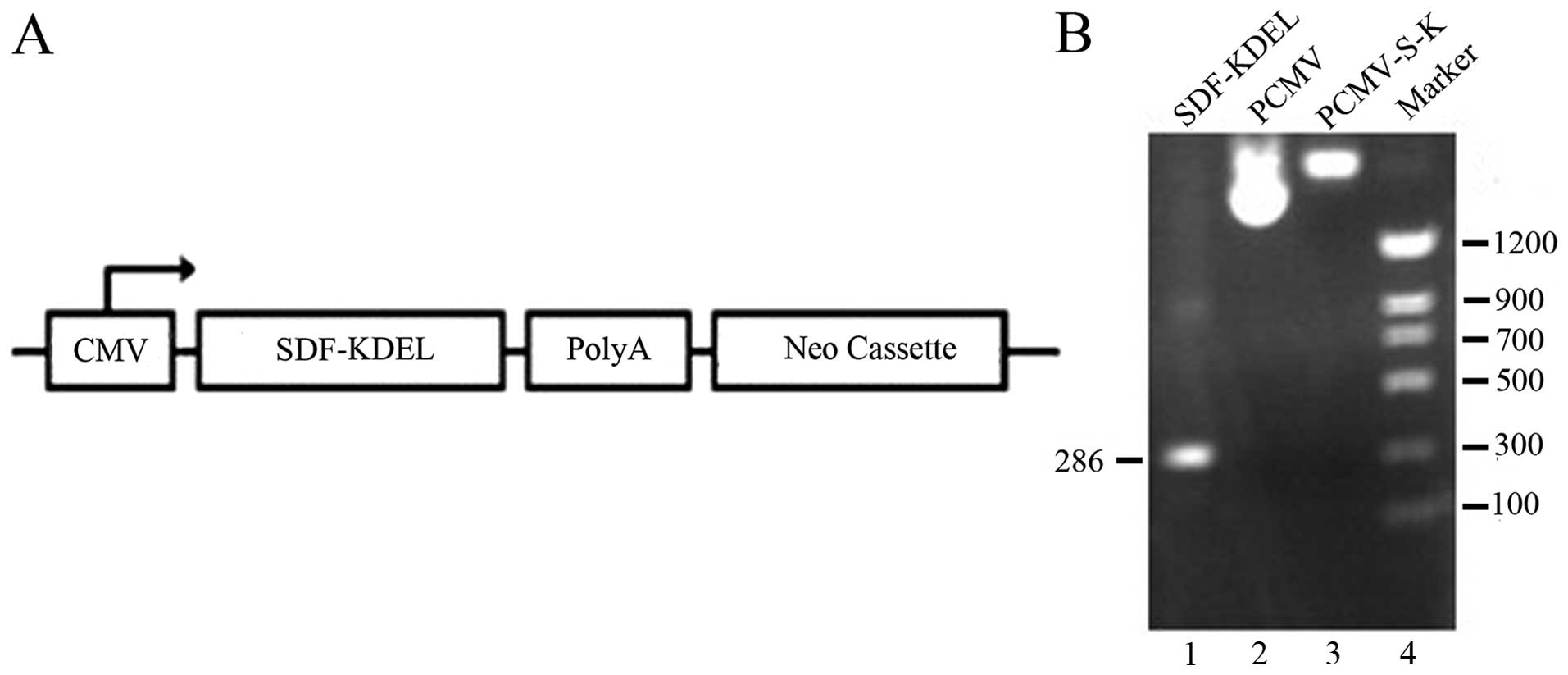
After we confirmed the recombinant plasmid PCMV-S-K
by colony PCR and DNA sequencing (Fig. 2), the PCMV-S-K vector and the PCMV
mock vector were transfected into the SBC-5 cells and the stably
transfected cells were designated as SBC-5/S-K and SBC-5/neo cells,
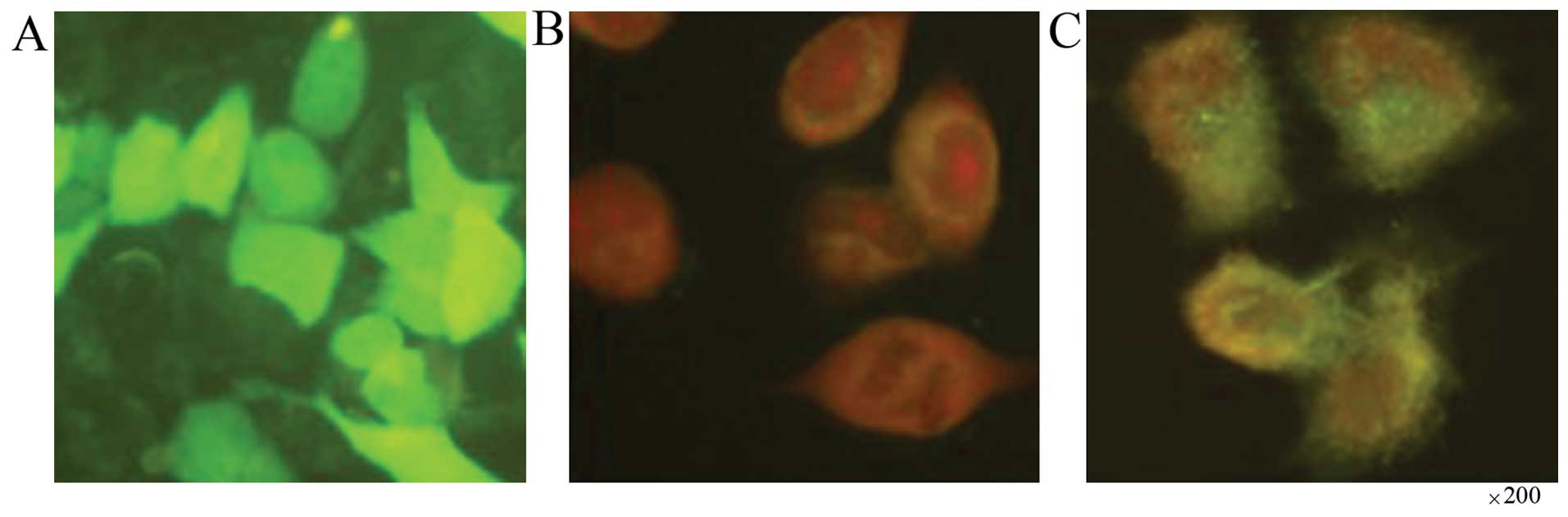
respectively. Of these cells, 80% were GFP-positive within 24 h
after transfection (Fig. 3A). We
examined the location of the recombinant fusion protein, SDF-KDEL,
in the SBC-5/S-K and SBC-5/neo cells by immunofluorescence
staining. SDF-1 was mainly expressed in the cytoplasm of the
SBC-5/S-K cells, particularly in the perinuclear region (Fig. 3C). However, the expression of
SDF-1 was not detected in the SBC-5/neo cells (Fig. 3B).
Downregulation of CXCR4 in SBC-5/S-K
cells compared with SBC-5/neo cells
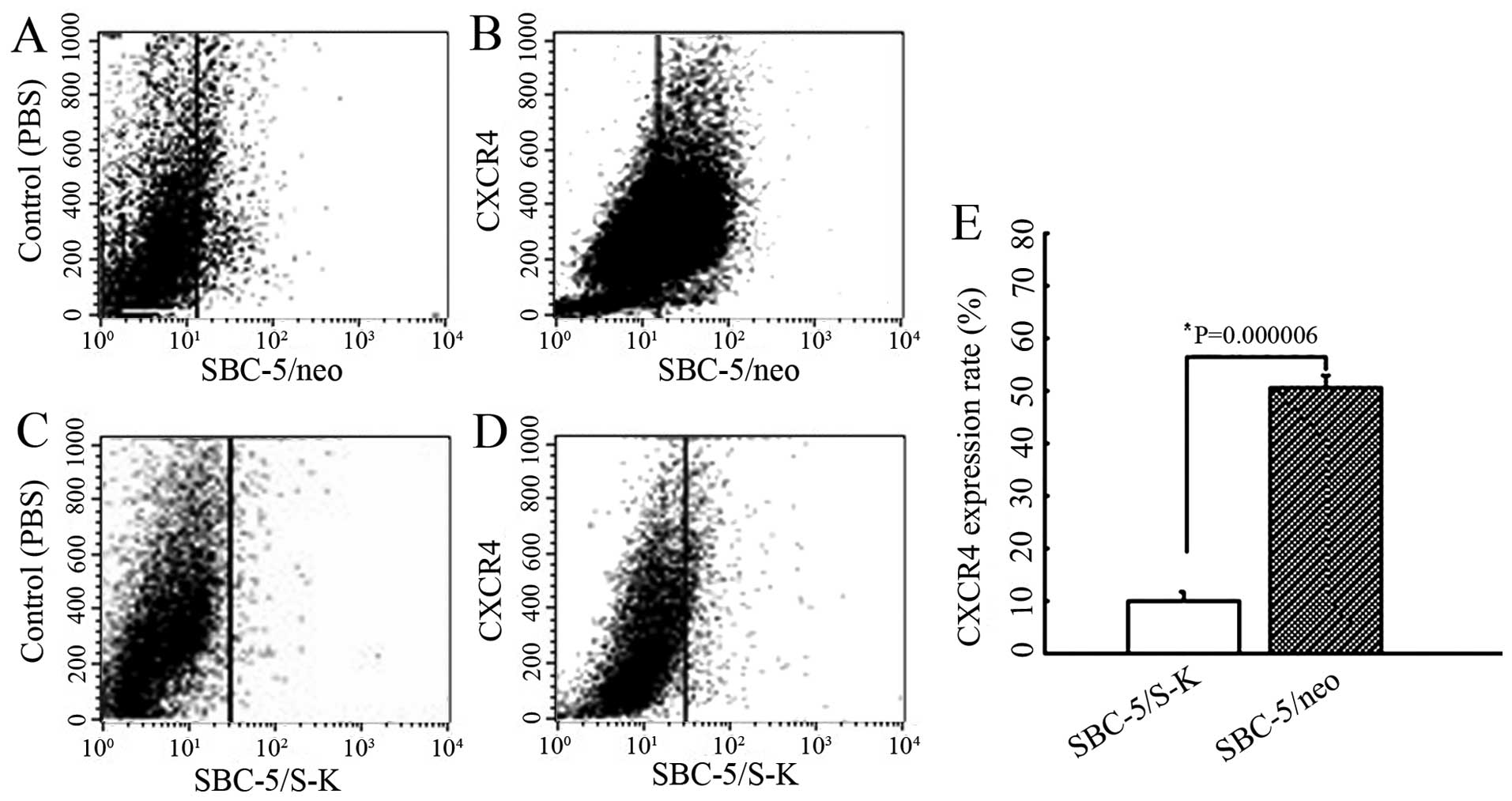
To examine the biological activity of the produced
SDF-KDEL fusion protein, the expression of CXCR4 in the SBC-5/S-K
cells was monitored by flow cytometry. The CXCR4 expression rates
in the SBC-5/S-K and SBC-5/neo cells were 10.08±1.49 and
50.50±2.31%, respectively (P=0.000006) (Fig. 4). These results indicated that the
cells transfected with the recombinant plasmid, pCMV-S-K, presented
with a significantly reduced expression of CXCR4 on the cell
surface.
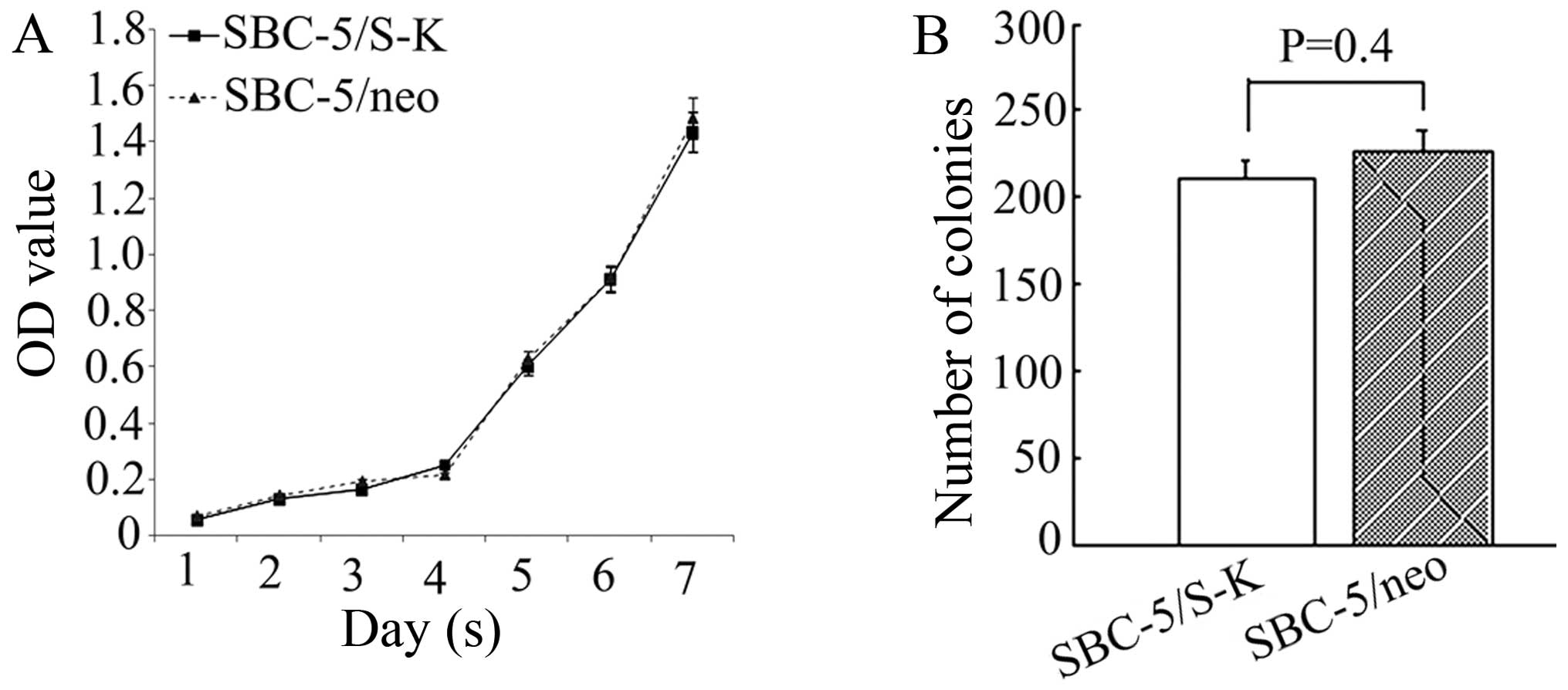
Downregulation of CXCR4 has no effect on
cell proliferation
Subsequently, the effects on cell proliferation of
the downregulation of CXCR4 in the SBC-5 cells were analyzed by MTT
assay and soft agar colony formation assay. From the cell
proliferation curve, we concluded that the proliferation of the
SBC-5/S-K cells did not differ significantly from that of the
SBC-5/neo cells (Fig. 5A).
Furthermore, the colonies formed by these 2 types of cells also
showed no differences (210.75±10.89 and 226.25±13.31, P=0.4;
Fig. 5B). These results suggested
that the downregulation of CXCR4 had no effect on the proliferation
of the SBC-5 cells.
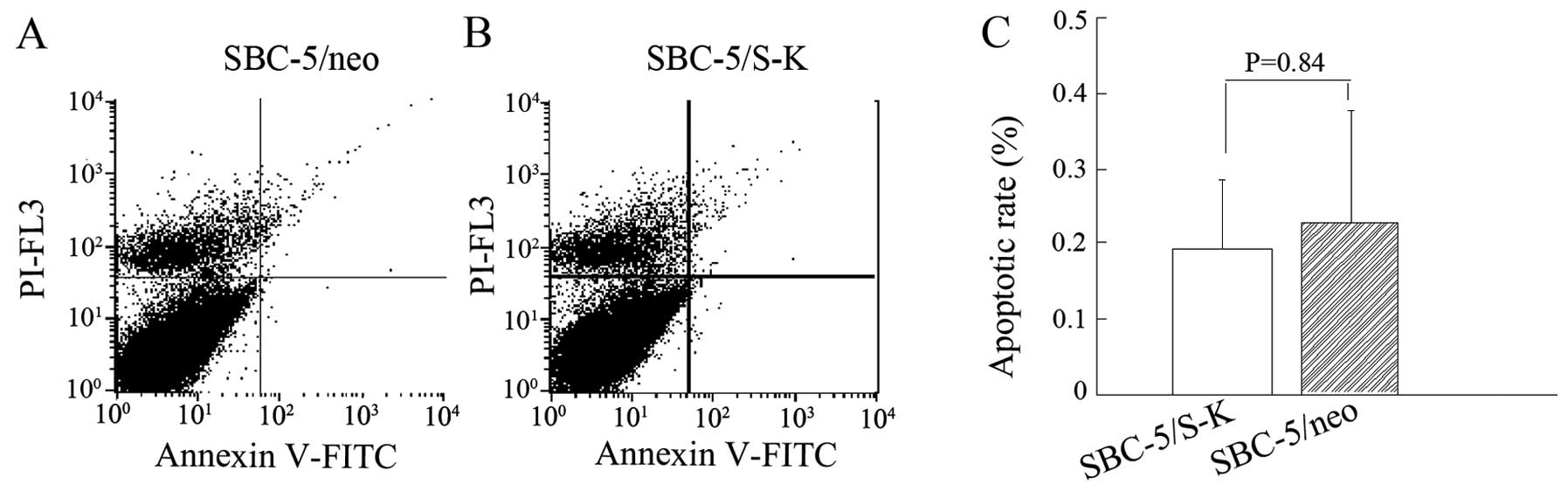
Downregulation of CXCR4 does not affect
the apoptotic rate of SBC-5 cells
The analysis of cell apoptosis revealed that the
apoptotic rates of the SBC-5/S-K and SBC-5/neo cells were
0.19±0.0967 and 0.2275±0.15%, respectively (P=0.84; Fig. 6). This assay indicated that the
downregulation of CXCR4 did not affect the apoptotic potential of
the SBC-5 cells.
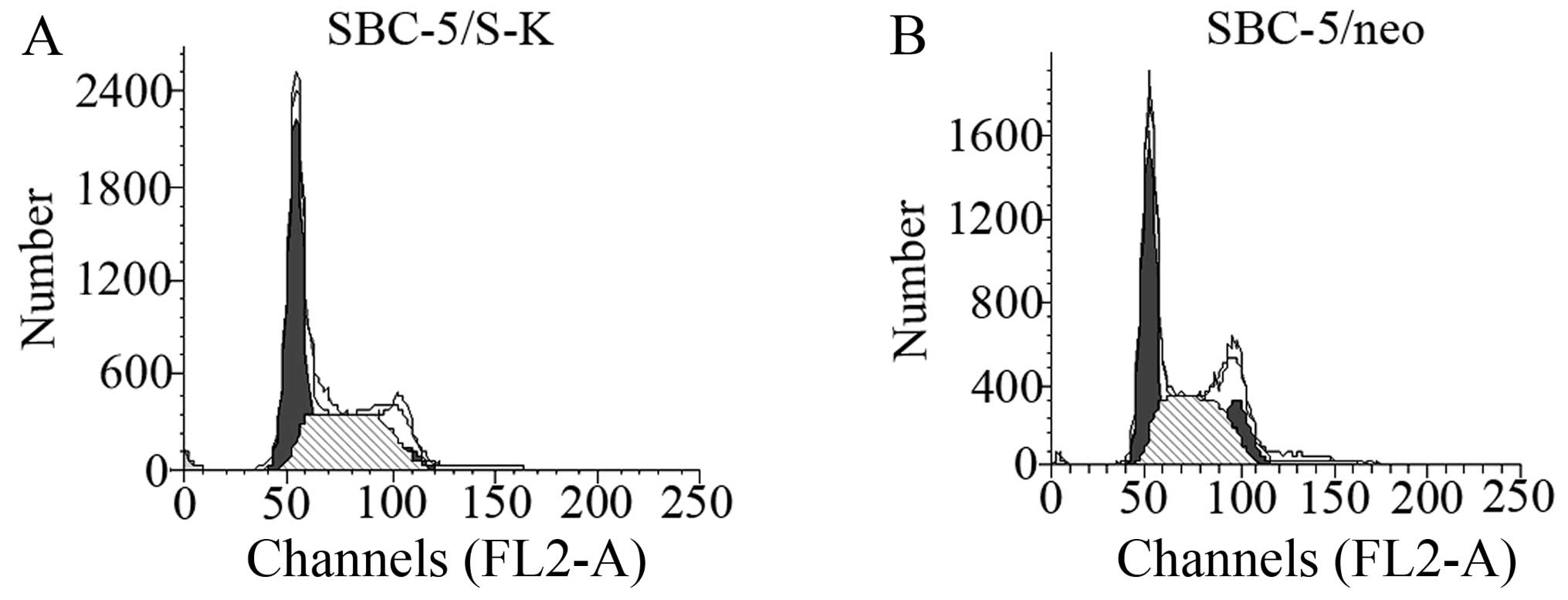
Downregulation of CXCR4 does not affect
the cell cycle distribution of SBC-5 cells
The percentage of SBC-5/S-K and SBC-5/neo cells in
the G1 phase of the cell cycle of was 49.64±5.65 and 44.775±2.21%,
respectively (P=0.44), and that in the S phase was 46.7±6.50 and
50.49±1.32%, respectively (P=0.6) (Fig. 7 and Table I). These result indicated that the
downregulation of CXCR4 did not affect the cell cycle distribution
of SBC-5 cells.
 | Table ICell cycle distribution of SBC-5/S-K
and SBC-5/neo cells. |
Table I
Cell cycle distribution of SBC-5/S-K
and SBC-5/neo cells.
| Cell group | Cell cycle phase
|
|---|
| G1 (%) | S (%) | G2 (%) |
|---|
| SBC-5/S-K | 49.64±5.65 | 46.7±6.50 | 5.32±2.13 |
| SBC-5/neo | 44.775±2.21 | 50.49±1.32 | 4.73±1.58 |
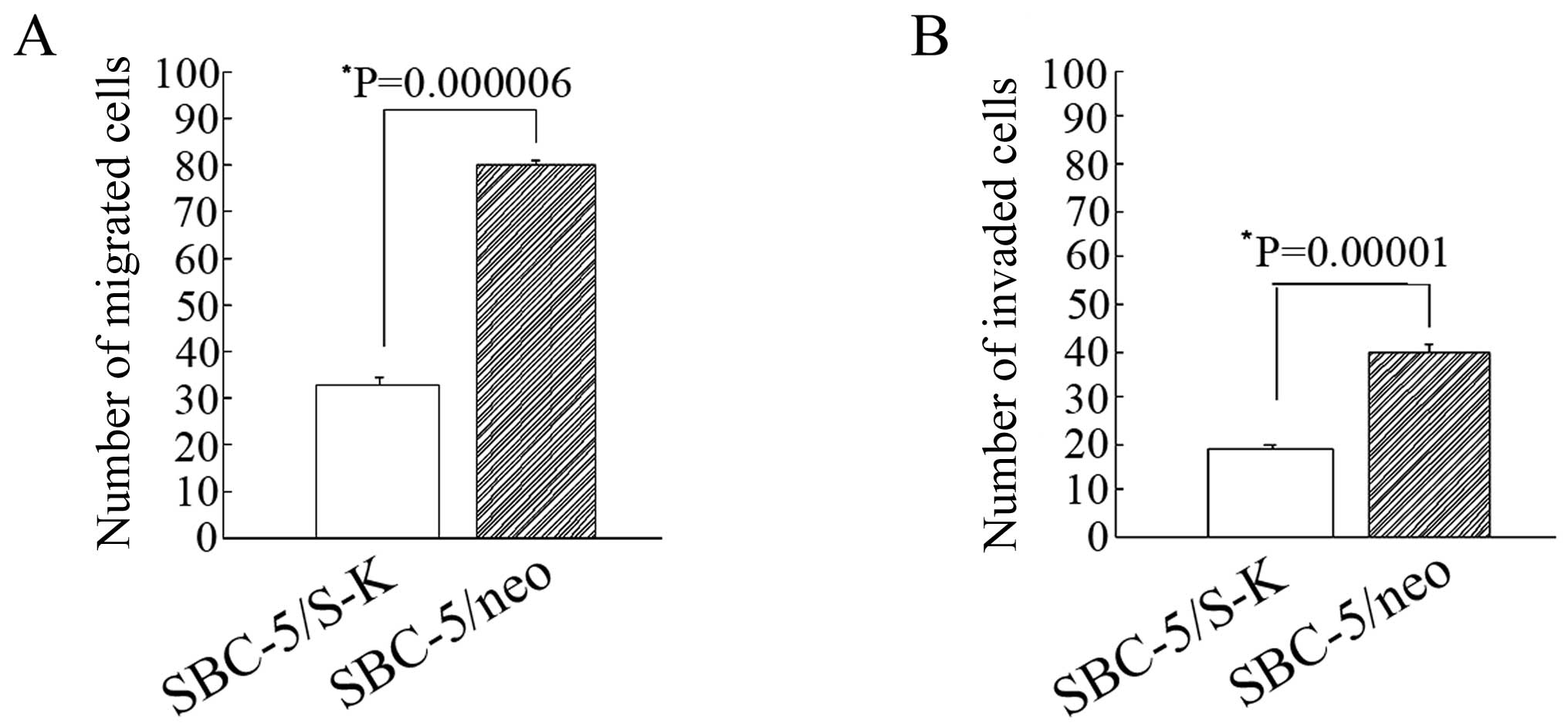
Downregulation of CXCR4 inhibits the
migration and invasion of SBC-5 cells
Through preliminary experiments, we found that there
was a marked difference in the number of SBC-5/S-K and SBC-5/neo
cells that had migrated through the insert (33±1.73 and 80.2±4.2,
P=0.000006; Fig. 8A). The number
of invaded SBC-5/S-K and SBC-5/neo cells was 19.2±0.86 and
39.6±1.96, respectively (P=0.00001; Fig. 8B). These results indicated that
the downregulation of CXCR4 significantly decreased the invasion
and migration capability of the SBC-5 cells.
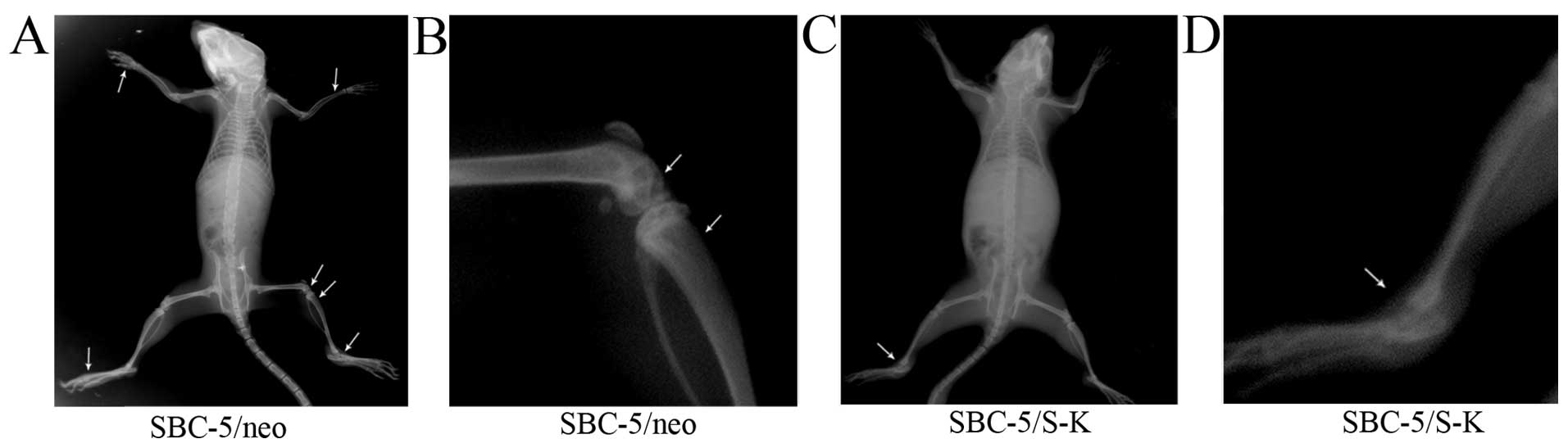
Downregulation of CXCR4 inhibits
metastasis in vivo
Finally, we examined the role played by CXCR4 in
vivo. At the 5th week after inoculation, paralysis (possibly
caused by spinal cord compression and bone metastases in the hind
limbs) occurred in 1/5 mice in the SBC-5/S-K group and in 5/5 mice
in the SBC-5/neo group. In addition, bone metastasis occurred in
3/5 mice in the SBC-5/S-K group, but in 100% of the mice in the
SBC-5/neo group (Table II). The
number of metastatic lesions observed in the bone, liver and lungs
in the SBC-5/S-K group was significantly decreased compared with
that in the SBC-5/neo group (P=0.01; Table II); however, the number of
metastatic lesions observed in the kidneys did not differ
significantly the 2 groups (P>0.05). These results revealed that
the downregulation of CXCR4 significantly inhibited specific
metastasis to the bone, liver and lungs. It can thus be
hypothesized that SDF-1-CXCR4 is involved in the organ-selective
metastasis to the lungs, liver and bone. The images of bone
metastatic lesions are shown in Fig.
9.
 | Table IIMetastatic lesions formed in NOD-SCID
mice following inoculation with SBC-5/S-K and SBC-5/neo cells for 5
weeks. |
Table II
Metastatic lesions formed in NOD-SCID
mice following inoculation with SBC-5/S-K and SBC-5/neo cells for 5
weeks.
| Cell type | Bone
| Lung
| Liver
| Kidney
|
|---|
| Incidence | N | Incidence | N | Incidence | N | Incidence | N |
|---|
| SBC-5/S-K | 3/5 | 1 (0–3)a | 5/5 | 3 (2–5)a | 5/5 | 3 (2–4)a | 5/5 | 5 (4–6) |
| SBC-5/neo | 5/5 | 5 (4–6) | 5/5 | 8 (7–9) | 5/5 | 8 (7–11) | 5/5 | 6 (3–7) |
Discusion
Chemokines are a large family of small, structurally
related heparin-binding proteins classified as XCL, CXCL, CCL and
CX3CL1 subfamilies depending on the number and spacing of conserved
cysteine residues near the N-terminus. Chemokines interact with
seven-transmembrane G protein-coupled chemokine receptors. More
than 40 chemokines and 18 chemokine receptors have been discovered,
and some chemokines bind to multiple chemokine receptors or vice
versa (12). Chemokines were
noted initially for their ability to stimulate the directional
migration of nearly all classes of leukocytes (13). Recent evidence indicates that
members of the chemokines and their receptors may play critical
roles in tumorigenesis and/or metastasis (14).
CXCR4 is by far the most common chemokine receptor
that has been demonstrated to be overexpressed in a broad array of
human cancer tissues, but its expression is low or absent in many
normal tissues (15). Its sole
ligand, CXCL12, was found mainly in 2 isoforms α and β, and isoform
α is constitutively produced in multiple tissues, including lung,
liver and bone tissue (16).
There is growing evidence that CXCR4 and SDF-1 regulate migration
and metastasis in various types of cancer (17). It has been reported that high
levels of CXCR4 expression positively correlate with bone
metastasis in breast cancer patients (18). GST-NT21MP, an antagonist of CXCR4
was identified to inhibit the progression of breast cancer
(19). Therefore, targeting CXCR4
may be a promising strategy for the treatment of human cancer. The
overexpression of CXCR4 in pancreatic cancer, melanoma and
neuroblastoma plays an important role in the progression and
organ-specific metastasis (20–23). Thus, CXCR4 is considered one of
several genes which contribute to bone metastasis in cancer
(24). Endothelial cell-derived
CXCL12 may trigger the integrin activation to promote the adhesion
of cancer cells to the extracellular matrix or accessory cells
within the tumor microenvironment. Of note, cancer cells can be
retained in the metastatic microenvironment which may confer
protection against chemotherapy, and which may be responsible for
residual disease and relapses (25,26).
In this study, the experssion of CXCR4 was knocked
down using the intrakine strategy with the recombinant plasmid,
PCMV-S-K. It was confirmed that the downregulation of CXCR4
significantly inhibited invasion and migration in vitro and
metastasis in vivo. Although the number of metastatic
lesions observed in the lungs, bone and liver was decreased in the
mice injected with PCMV-S-K cells, metastasis to the kidneys was
not suppressed. These results indicated that SDF-1-CXCR4 mediated
organ-specific metastasis in SCLC. The mechanisms of action of the
SDF-1-CXCR4 axis remain unclear, and thus future studies are
required on this issue.
Whether CXCR4 is involved in the survival and
proliferation of tumor cells remains controversial, perhaps as this
is tumor dependent. The activation of extracellular
signal-regulated kinase and Akt can both potentially contribute to
the survival and growth of tumor cells (27,28). SDF-1-CXCR4 has been implicated in
the organ-specific metastases of many types of cancer, including
bone-specific metastasis. The bone marrow is a hypoxic
microenvironment and is rich in hypoxia-inducible factor-1 (HIF-1).
HIF-1α and CXCR4 co-operate to regulate the adaptation and survival
of cancer- and metastasis-initiating cells (29). In addition, CXCR4 expression can
be upregulated by the hypoxia response element, HIF-1α (30,31). In a relay multistep navigation
process, the hypoxia-HIF-1α-CXCR4 pathway may regulate trafficking
in and out of hypoxic tissue microenvironments, which is in favor
of cell proliferation, migration, invasion and the formation of
tumor/metastases.
Metastases arise following the spread and subsequent
growth of cancer cells from a primary site to distant tissues.
There are likely to be some special requirements for the formation
of metastasis, as evidenced by the existence of a class of genes
termed ‘metastasis-related genes’, which regulate the development
of metastatic tumors, but have relatively little effect on the
growth of primary lesions. In view of these biological functions
mediated by CXCR4, it can be concluded that CXCR4 is a gene
involved in metastasis in SCLC, and the blockade of the interaction
between SDF-1 and CXCR4 may lead to the development of a novel
therapeutic strategy for the prevention and treatment of
organ-specific metastases in SCLC.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81272345 and
81172011).
References
|
1
|
Robert J: Biology of cancer metastasis.
Bull Cancer. 100:333–342. 2013.In French. PubMed/NCBI
|
|
2
|
Guise T: Examining the metastatic niche:
targeting the microenvironment. Semin Oncol. 37(Suppl 2): S2–S14.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
4
|
Patel LR, Camacho DF, Shiozawa Y, Pienta
KJ and Taichman RS: Mechanisms of cancer cell metastasis to the
bone: a multistep process. Future Oncol. 7:1285–1297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miki T, Yano S, Hanibuchi M and Sone S:
Bone metastasis model with multiorgan dissemination of human
small-cell lung cancer (SBC-5) cells in natural killer
cell-depleted SCID mice. Oncol Res. 12:209–217. 2000.
|
|
7
|
Ben-Baruch A: Organ selectivity in
metastasis: regulation by chemokines and their receptors. Clin Exp
Metastasis. 25:345–356. 2008. View Article : Google Scholar
|
|
8
|
Chen JD, Bai X, Yang AG, Cong Y and Chen
SY: Inactivation of HIV-1 chemokine co-receptor CXCR-4 by a novel
intrakine strategy. Nat Med. 3:1110–1116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai X, Chen JD, Yang AG, Torti F and Chen
SY: Genetic co-inactivation of macrophage- and T-tropic HIV-1
chemokine coreceptors CCR-5 and CXCR-4 by intrakines. Gene Ther.
5:984–994. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JC, Sun L, Nie QH, et al:
Down-regulation of CXCR4 expression by SDF-KDEL in CD34(+)
hematopoietic stem cells: An anti-human immunodeficiency virus
strategy. J Virol Methods. 161:30–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma WF, Du J, Fu LP, Fang R, Chen HY and
Cai SH: Phenotypic knockout of CXCR4 by a novel recombinant protein
TAT/54R/KDEL inhibits tumors metastasis. Mol Cancer Res.
7:1613–1621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bachelerie F, Ben-Baruch A, Burkhardt AM,
et al: International Union of Basic and Clinical Pharmacology.
#x0005B;corrected]. LXXXIX. Update on the extended family of
chemokine receptors and introducing a new nomenclature for atypical
chemokine receptor. Pharmacol Rev. 66:1–79. 2013. View Article : Google Scholar
|
|
13
|
Hayashi H: A review on the natural
mediators of inflammatory leucotaxis. Acta Pathol Jpn. 32(Suppl 2):
271–284. 1982.PubMed/NCBI
|
|
14
|
Roy I, Evans DB and Dwinell MB: Chemokines
and chemokine receptors: update on utility and challenges for the
clinician. Surgery. 155:961–973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cojoc M, Peitzsch C, Trautmann F,
Polishchuk L, Telegeev GD and Dubrovska A: Emerging targets in
cancer management: role of the CXCL12/CXCR4 axis. Onco Targets
Ther. 6:1347–1361. 2013.PubMed/NCBI
|
|
16
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gebura K and Bogunia-Kubik K: Clinical
relevance of chemokine receptor CXCR4. Postepy Hig Med Dosw
(Online). 66:252–266. 2012.In Polish. View Article : Google Scholar
|
|
18
|
Hung CS, Su HY, Liang HH, et al:
High-level expression of CXCR4 in breast cancer is associated with
early distant and bone metastases. Tumour Biol. 35:1581–1588. 2014.
View Article : Google Scholar
|
|
19
|
Yang Q, Zhang F, Ding Y, et al: Antitumour
activity of the recombination polypeptide GST-NT21MP is mediated by
inhibition of CXCR4 pathway in breast cancer. Br J Cancer.
110:1288–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong W, Chen W, Zhang D, et al:
CXCL12/CXCR4 axis plays pivotal roles in the organ-specific
metastasis of pancreatic adenocarcinoma: A clinical study. Exp Ther
Med. 4:363–369. 2012.PubMed/NCBI
|
|
21
|
Toyozawa S, Kaminaka C, Furukawa F,
Nakamura Y, Matsunaka H and Yamamoto Y: Chemokine receptor CXCR4 is
a novel marker for the progression of cutaneous malignant
melanomas. Acta Histochem Cytochem. 45:293–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Feng C, Liao W, Zhang H and Tang S:
Expression of CXC chemokine receptor-4 and forkhead box 3 in
neuroblastoma cells and response to chemotherapy. Oncol Lett.
7:2083–2088. 2014.PubMed/NCBI
|
|
23
|
Ma M, Ye JY, Deng R, Dee CM and Chan GC:
Mesenchymal stromal cells may enhance metastasis of neuroblastoma
via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Lett. 312:1–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Loberg R and Taichman RS: The
pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis.
Cancer Metastasis Rev. 25:573–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang B, Wang W, Niu W, et al: SDF-1/CXCR4
axis promotes directional migration of colorectal cancer cells
through upregulation of integrin αvβ6. Carcinogenesis. 35:282–291.
2014. View Article : Google Scholar
|
|
27
|
Barbieri F, Bajetto A, Porcile C, et al:
CXC receptor and chemokine expression in human meningioma:
SDF1/CXCR4 signaling activates ERK1/2 and stimulates meningioma
cell proliferation. Ann NY Acad Sci. 1090:332–343. 2006. View Article : Google Scholar
|
|
28
|
Wong D and Korz W: Translating an
antagonist of chemokine receptor CXCR4: from bench to bedside. Clin
Cancer Res. 14:7975–7980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Wei L, Chen Q and Terek RM:
CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion
through ERK signaling and increased MMP1 expression. Mol Cancer.
9:172010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo M, Cai C, Zhao G, et al: Hypoxia
promotes migration and induces CXCR4 expression via HIF-1α a
activation in human osteosarcoma. PLoS One. 9:e905182014.
View Article : Google Scholar
|