Introduction
Heavy menstrual bleeding (HMB; >80 ml/menstrual
episode), is common in women of reproductive age, accounting for
>20% of gynecological outpatient visits. Although HMB is often
associated with fibroids and carcinoma, approximately 50% of HMB
cases occur in the absence of recognized uterine pathology. Such
idiopathic HMB of endometrial origin (HMB-E) has been estimated to
account for 9–14% of gynecological outpatient visits (1). HMB can lead to iron deficiency and
anemia and may require hysterectomy, and its psychological, social
and employment consequences for many women have led to its
designation as a public health concern (2).
The disturbance of endometrial angiogenesis is one
of several mechanisms that have been suggested to play a role in
HMB (3). In previous studies, we
demonstrated that the agonist-receptor pathway of the vascular
endothelial growth factor (VEGF) is upregulated in patients with
HMB-E, as they displayed significantly higher numbers of
microvessels that expressed VEGF-A, or VEGF receptors-1, -2 and -3
than did the healthy controls (4). Likewise, angiopoietin-1 was
upregulated in patients with HMB-E in the secretory phase of the
menstrual cycle (5). Moreover, we
found that endometrial microvessels possessed a distinct morphology
characterized by endothelial cell gaps, and that these gaps (as
well as the microvessel perimeters) were larger in patients with
HMB-E. Endothelial cell gaps highly correlated with the
overexpression of VEGF-A and VEGF receptor-1. These findings
suggested that microvessel wall cells, such as endothelial cells
and possibly pericytes, expressed an aberrant phenotype that may
contribute to HMB-E, for instance, by rendering microvessels
fragile (6).
An important step in the formation of functional and
stable microvessels is the recruitment of pericytes, cells of
mesenchymal origin that cover the abluminal surface of endothelial
tubes, to form microvessels. Pericytes tightly interact and adhere
to endothelial cells through pores in their common basement
membrane (7). Pericytes are
mostly recruited to endothelial tubes through the expression of
platelet-derived growth factor (PDGF), which interacts with its
receptor (PDGFR-β) on the pericyte surface. PDGF is secreted mainly
by endothelial cells on the tip of the sprouting microvessel, and
the amount of PDGF secreted usually determines the extent of
pericyte recruitment (8). If the
pericytes are insensitive to PDGF due to mutations in the PDGF gene
or its receptor, the microvessels become immature and leaky, which
leads to widespread hemorrhaging (9). Furthermore, it has been shown that
in some cases, pericytes can initiate angiogenesis through the
production of VEGF-A, which stimulates endothelial cell
proliferation and tube formation (7).
Pericyte coverage can be dense, e.g., in the blood
brain barrier, where little and highly regulated transfer of
substances occurs, or sparse, e.g., in organs with a high molecule
exchange rate across the microvessel wall. Pericytes may also
regulate microvessel blood flow, through the contraction of the
microvessels (10). One important
function of pericytes is to regulate the remodeling of microvessels
through pruning of the endothelial network (11).
In the present study, we hypothesized that
endometrial microvessels in patients with HMB-E are immature and
fragile due to the loss or rearrangement of pericytes. We used
previously published data for reported endothelial cell gaps and
the expression of VEGF-A, VEGFR1-2 and angiopoietin-1 (6,12).
Subjects and methods
Study subjects
The data and biological samples used in the present
study have been described previously (6). Subjects in this study were recruited
from an outpatient clinic for women with HMB. In order to avoid
selection bias, patients were enrolled consecutively. Women with a
pictorial blood loss assessment chart (PBAC) score >100 were
considered to have HMB (13).
Healthy, ovulating women with a PBAC score <80 were considered
as the control subjects. All women underwent examination and
transvaginal ultrasound, which revealed no pathology in the uterine
cavity.
Endometrial biopsies and blood samples for hormones
were obtained from 17 control subjects (mean age, 41 years) and
from 10 patients (mean age, 42 years) with a normal menstrual cycle
and a history of HMB-E of <5 years. None of the study subjects
smoked, had used drugs (with the exception of tranexamic acid) or
hormonal or intrauterine contraception for at least 3 months prior
to the biopsy, or had abnormal pre-operative values for blood
platelets, activated prothrombin, thromboplastin time,
international normalized ratio bleeding time, or were positive for
the von Willebrand factor. The uterine cavity of the patients with
HMB-E appeared normal as evaluated by hysteroscopy. The date of the
last menstruation, the analysis of estradiol and progesterone
levels and the histological assessment of endometrial biopsies
(which showed no abnormal findings) indicated that 8 patients with
HMB-E and 5 control subjects were in the proliferative phase (cycle
day 8) and 9 patients and 5 control subjects were in the secretory
phase of the menstrual cycle (ovulation peak + 6 days) at the time
of biopsy. The subjects identified the day of luteinizing hormone
surge by testing their morning urine.
Ethics statement
All women provided informed consent to participate
in the study, which was approved by the Ethics Committee of
Karolinska Institutet/Karolinska University Hospital, Sweden.
Immunohistochemical staining for the
presence of pericytes
All analyses were performed on the functionalis
layer of the endometrium. The processing of endometrial biopsies
has been previously described (14). Briefly, 5-μm-thick sections
of formaldehyde-fixed, paraffin-embedded endothelial tissue were
stained with antibodies to the CD34 glycoprotein (Cat. no.
ABIN343723; QBEnd/10; BioGenex, San Ramon, CA, USA) alone, or
together with smooth muscle actin-α (SMAα; M0874; with a FITC
conjugated IgG2a; Dako, Glostrup, Denmark), as previously described
(4,5). We have previously reported that
pericytes in human tissue rarely express PDGFR-β and human desmin,
but SMAα has been found to reliably identify pericytes in most
tissues (6). Thus, the presence
of pericytes was determined by SMAα positivity in this study, as
previously reported (6).
For secondary antibodies, we used Alexa 488 (I36933)
or Alexa 568 (Z-25006; Molecular Probes, Eugene, OR, USA),
respectively, as previously described (15). The specificity of these antibodies
and their negative controls has been described previously (14).
Quantification of microvessels with
pericyte coverage
We used the Leica Q550IW image analysis system, a
Leica DM RXA color video camera, and software based on Leica QWin
Image Analysis to measure microvessel dimensions following SMAα
staining. Ten randomly selected and cross-sectioned CD34-stained
microvessels, with a clear lumen area (the area of the open space
in the microvessel), were captured per slide with a x63 oil
immersion objective. Firstly, microvascular density (MVD) was
determined, i.e., the number of microvessels per high-power field
(HPF). Thereafter, we counted the number of microvessels covered
with pericytes. Microvessels with >50% of their perimeter
covered with pericytes were defined as pericyte-positive
microvessels.
Transmission electron microscopy
Small sections of endometrial biopsies were fixed
for 30 min at room temperature, and then for 24 h at 4°C with 2%
glutaraldehyde + 0.5% paraformaldehyde in 0.1 M sodium cacodylate
buffer containing 0.1 M sucrose and 3 mM CaCl2, pH 7.4
and further processed as previously described (16). To evaluate the thickness of the
endothelial cell layer, we performed a volume density measurement,
as previously described (17).
The total microvessel area was measured on digital images using the
software provided with the electron microscope. The total
microvessel area (reference area) was calculated using the basement
membrane as the outer border. The lumen area was then measured.
Dividing the lumen area by the reference area yielded a ratio that
represented the volume of endothelial cells; this endothelial area
is given as percentage of the reference area.
All endometrial biopsies in the present study were
coded and analyzed by two independent observers. Each observer
examined the slides on least on two different occasions.
Differences in opinion between the observers were resolved by
simple discussion and microscopic evaluation. We used previously
published data for MVD, endothelial cell gap sizes and the number
of microvessels expressing VEGF-A, VEGF receptors 1–3 and
angiopoietin-1 (4,5).
Statistical analysis
Data are presented as median values and 95%
confidence intervals (CIs). The Kruskal-Wallis, Mann-Whitney, and
Spearman tests were performed with the Statistica software package.
A P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
MVD, endothelial cell gaps,
VEGF-A-positivity and microvessel perimeters
In this study, previously published results
(4–6,14)
are given, as they were used for correlation analyses. Briefly, MVD
did not differ between the patients with HMB-E and the control
subjects during the menstrual cycle [median value of 19.5
microvessels/HPF; standard deviation (SD) 6, 0; 95% CI in patients
with HMB-E compared to the median value of 16.0 microvessels/HPF,
SD 2, 5; 95% CI for the control subjects; P>0.05]. Endothelial
cell gaps (i.e., discontinuities in CD34, CD31, as well as in von
Willebrand factor immunostainings of the endothelial cell layer)
have been described in detail in our previous study (6), and were larger in endometrial
biopsies from patients with HMB-E than in those from the control
subjects. The relative size of endothelial cell gaps (i.e., the
percentage of the circumference of the microvessels occupied by
gaps) in the patients with HMB-E was 1.7-fold that in the control
subjects (P=0.000002), regardless of the phase of the menstrual
cycle. The number of microvessels expressing VEGF-A was
significantly (P=0.001) greater in the patients with HMB-E (median
17; 95% CI, 16–22) than in the control subjects (median 10; 95% CI,
9–15). The perimeter of endometrial microvessels (measured at the
inner, luminal profile of CD34 staining) in the patients with HMB-E
in the secretory phase of the menstrual cycle (median, 9.3
μm; 95% CI, 6.1–14.9 μm) was significantly (P=0.0007)
larger than that for the control subjects in the same phase (3.6
μm; 95% CI, 1.3–7.2 μm).
SMAα positivity in endometrial
microvessels
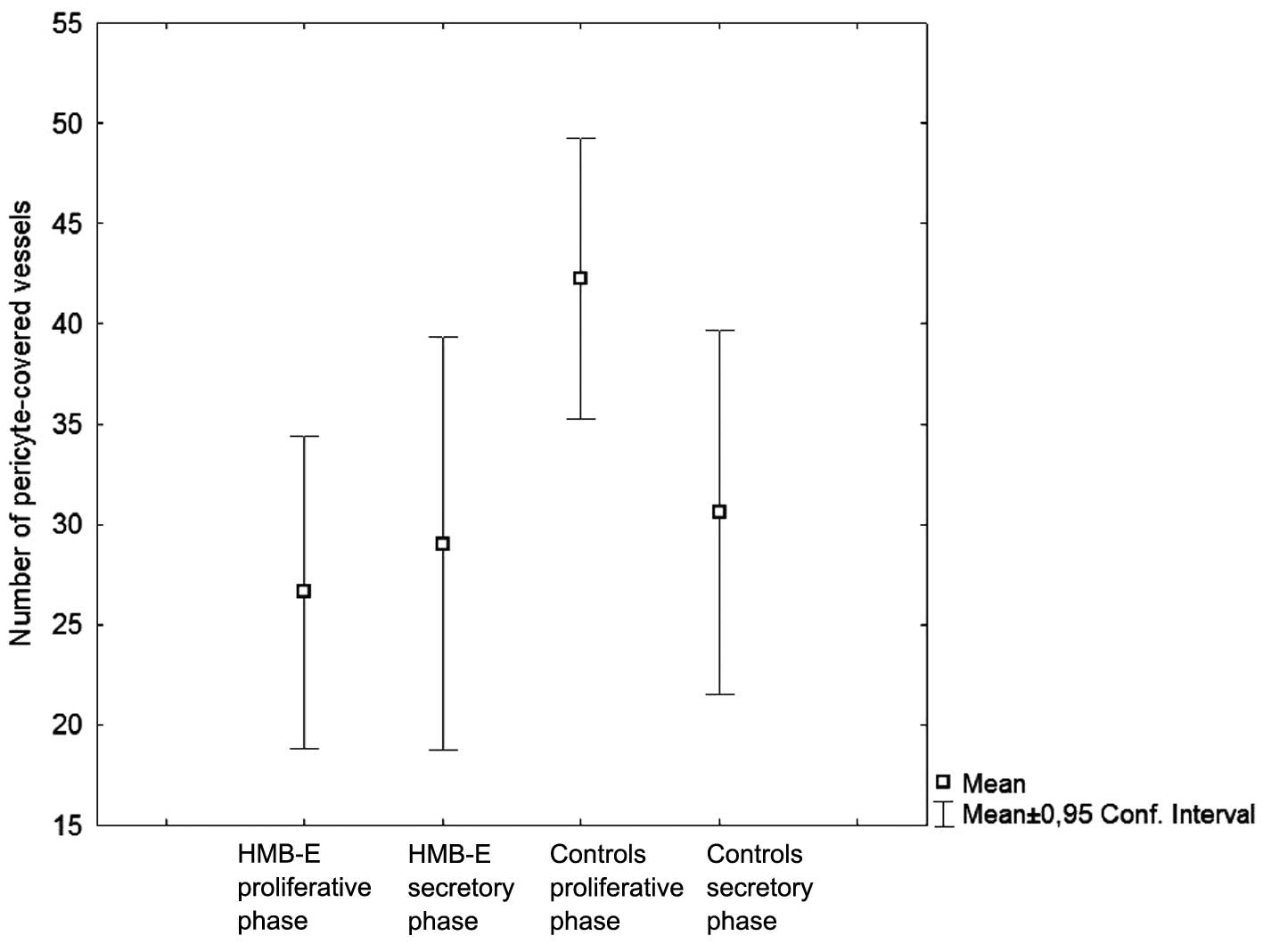
The number of SMAα-positive microvessels in
endometrial biopsies from the patients with HMB-E than in the
control subjects (Fig. 1).
However, there were no significant differences observed in
endometrial biopsies obtained during the secretory phase
(P=0.87).
When we compared the number of SMAα-positive
microvessels in the control subjects only, we found significantly
lower numbers of SMAα-positive microvessels in the secretory phase
(P=0.004) compared with the proliferative phase. By contrast, the
number of SMAα-positive microvessels did not differ among the
patients with HMB-E between the two phases. Thus, the control
subjects displayed a menstrual cycle-related variation in pericyte
coverage, but the patients with HMB-E did not.
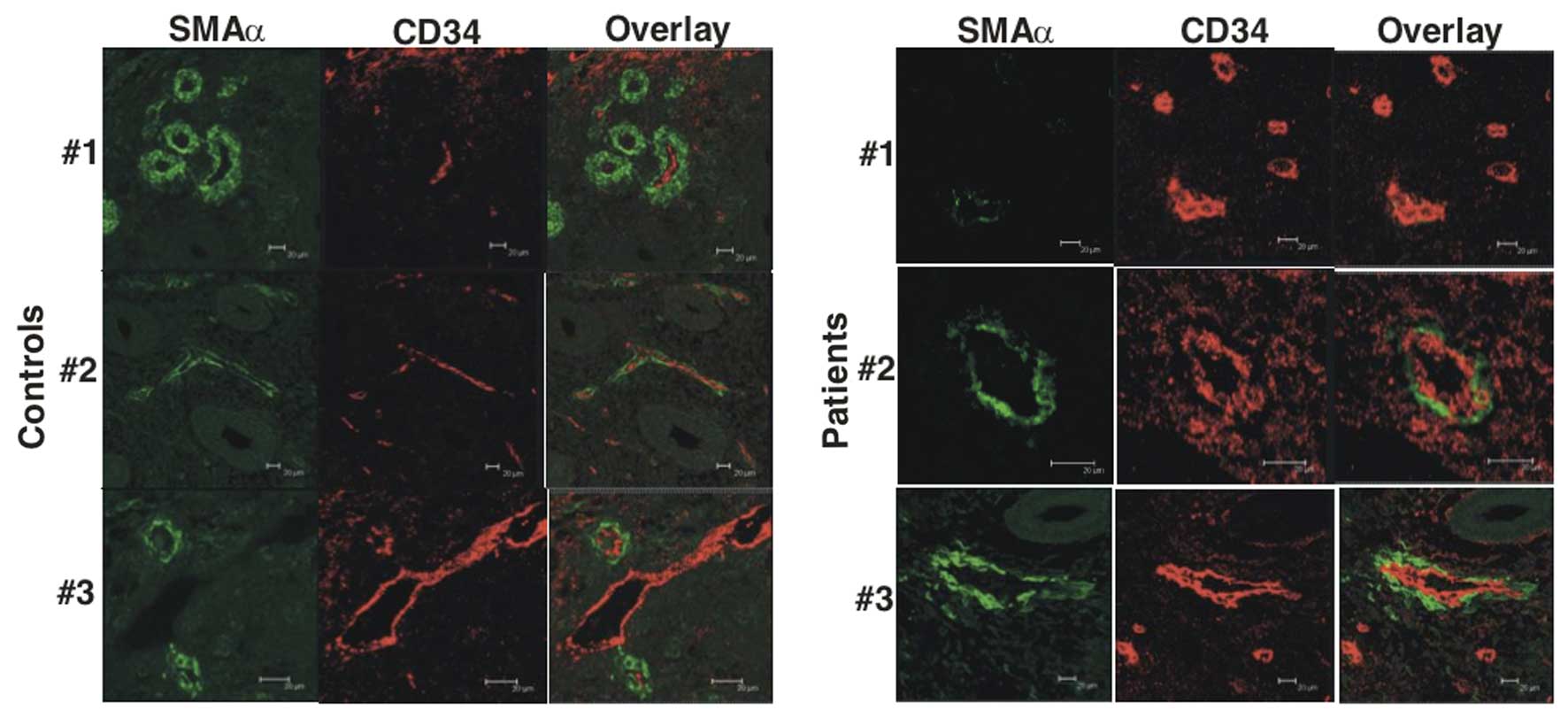
Using confocal imaging, we found that the control
subjects regularly had CD34-positive microvessels (Fig. 2, control subjects #1–3). The
classic pericyte structure, as disclosed by electron microscopy, is
further detailed in Fig. 3 for
the control subjects #1 and #2. Occasionally, no CD34-positive
microvessels were detected, but instead typical pericytes tubes
were present (Fig. 2, control
subject #1); in other settings such structures have been
interpreted as remodeling or regressing microvessels, where
pericytes remain after endothelial cells have disappeared (18). Only rarely did the control
subjects display endothelial tubes without pericytes (Fig. 2, control subject #3). By contrast,
the patients with HMB-E exhibited many endothelial structures
without pericytes (Fig. 2, HMB-E
patient #1). Nonetheless, some microvessels showed a combination of
endothelial and pericyte structures (Fig. 2, HMB-E patients #2 and #3). There
was also an impression that microvessels in patients with HMB-E
were larger and coarser than those of the control subjects, in that
both the CD34 and SMAα stained endothelial cell layers were thicker
in the patients with HMB-E than in the control subjects (Fig. 2, HMB-E patient #2 and #3). A
formal analysis of this finding was performed by means of electron
microscopy (see below).
Correlation between pericyte coverage and
VEGF-A expression or endothelial cell gaps
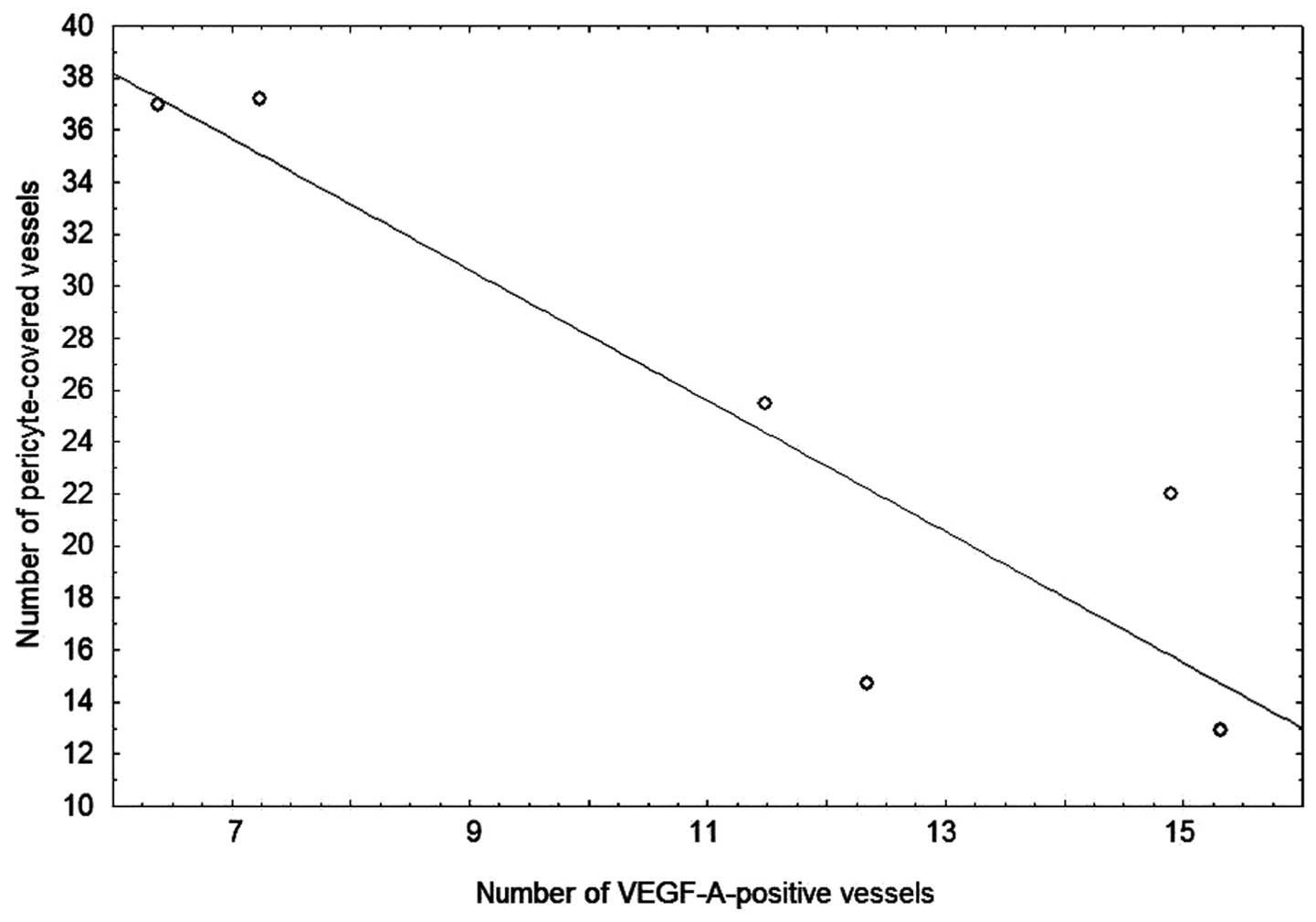
Analysis of the data from all patients with HMB-E
and the control subjects in the proliferative phase revealed a
significant negative correlation between the number of
VEGF-A-positive microvessels and the number of microvessels covered
with pericytes (r = 0.8; P= 0.04) (Fig. 4). Thus, the higher the VEGF-A
expression, the lower the pericyte coverage was.
Analysis of the data from all patients with HMB-E
and the control subjects in the proliferative phase revealed no
significant correlation between pericyte coverage and the relative
size of the endothelial cell gaps, or VEGF receptor-1, -2 or -3 or
angiopoietin-1 microvessel expression (data not shown).
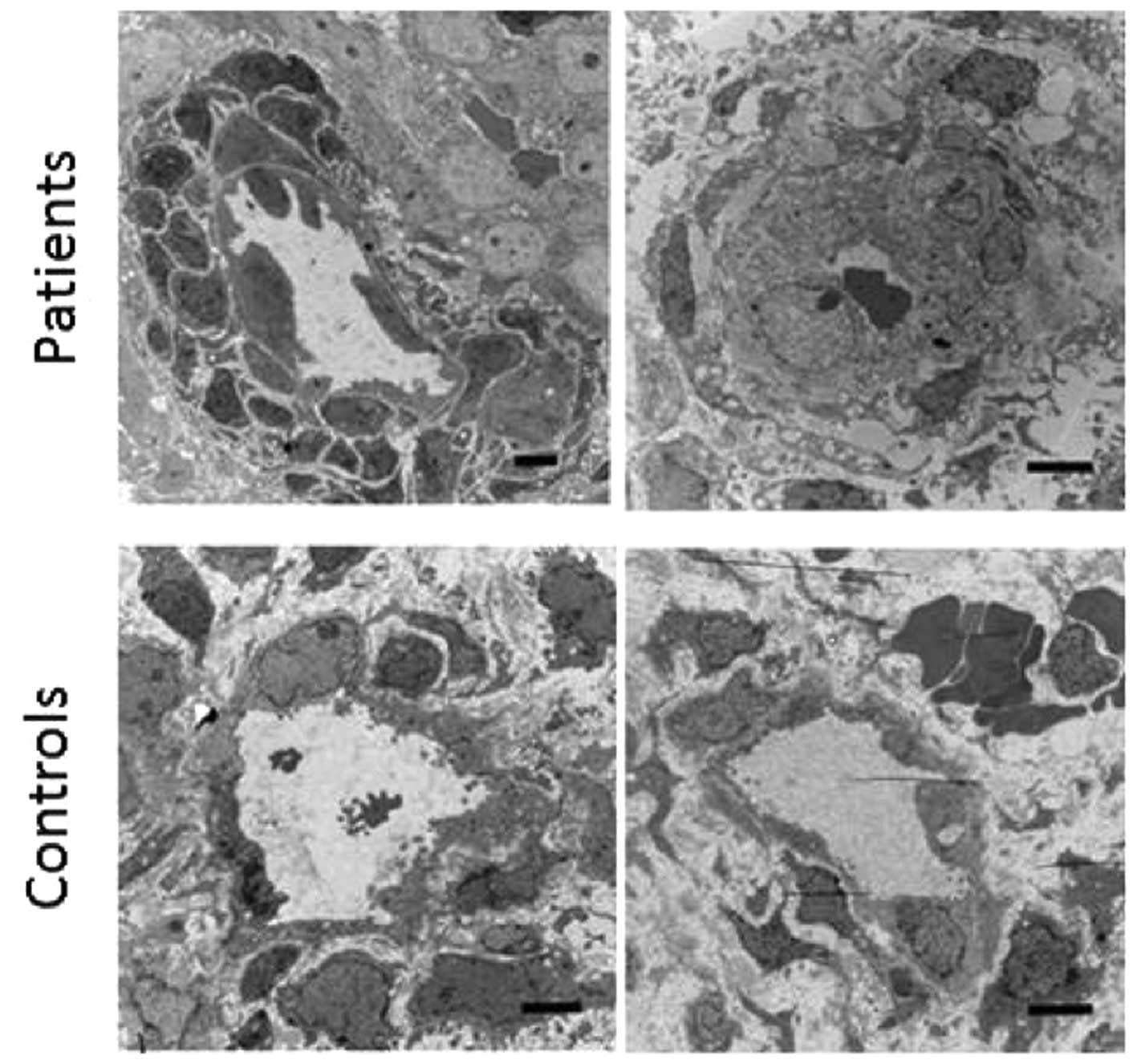
Electron microscopy findings
In 2 HMB-E patients and 2 control subjects, 1 HMB-E
patient exhibited highly unusual multilayer accumulations of
pericytes around the endothelial layer (Fig. 3, left top panel). This was
observed in >90% of the 7 microvessels examined in this patient.
By contrast, the 2 control subjects and the 2nd HMB-E patient
displayed traditional pericyte monolayers in all microvessels (32
microvessels examined for the 3 subjects) (Fig. 3, right top and bottom panels).
With this technique it was not possible to enumerate the pericyte
coverage further.
We then analyzed the thickness of the endothelial
layer in all microvessels identified in the electron microscopy
sections. The patients with HMB-E displayed a significantly higher
endothelial cell percentage area compared to the control subjects
(median value of 77.4%, 95% CI: 70.4–81.8, n=17 microvessels and
53.3%, 95% CI: 49.6–58.4, n=24 microvessels, respectively;
P<0.001). There was no evidence of defect contact (e.g., focal
adhesion points, caveole) between the pericytes and endothelial
cells, mitochondrial morphology abnormalities in the pericytes, or
signs of apoptosis or autophagy.
Discussion
In this study, to the best of our knowledge, we
demonstrate for the first time that that the microvessels in the
endometrium of women with HMB-E show a dysregulated pericyte
coverage: the number of microvessels that were covered with
pericytes was significantly lower in the patients with HMB-E during
the proliferative phase of the menstrual cycle compared to the
control subjects, and the accumulation of pericytes into
multilayers occurred in a few women. Moreover, the number of
microvessels covered with pericytes negatively correlated with VEGF
expression during the proliferative phase. Finally, endothelial
linings were thicker in the patients with HMB-E than in the control
subjects. Our findings point to a dysregulation of the interaction
of endothelial cells and pericytes and suggest that deviant VEGF
signaling may be involved in this process.
An important step in the formation of functional and
stable microvessels is the recruitment of pericytes. The presence
of pericytes is regarded as a sign of vascular maturity (15), while the absence of pericytes is
associated with microvessel malformations and microvessel fragility
(19,20). Fenestrated microvessels, i.e.,
microvessels with few pericytes, are more common in the vascular
beds of certain organs, whereas other organs have a very tight
pericyte surface layer (e.g., the blood brain barrier). Parts of
the microvessel where the direct exchange of gas and nutrients
takes place are mostly free of pericytes (7), probably as a way to increase the
diffusion capacity, whereas the microvessel branchings have high
pericyte coverage, believed to be of significance for regulation of
blood flow and stress (21).
In the present study, we demonstrated that the
number of microvessels covered with pericytes was significantly
lower in the patients with HMB-E during the proliferative menstrual
phase compared to the control subjects. Moreover, using electron
microscopy, we also observed signs of remodeling or regressing
microvessels, where pericytes remained after the endothelial cells
had disappeared. The lower pericyte coverage in the proliferative
phase may lead to the impaired regulation of blood flow,
microvessel growth, endothelial cell proliferation and microvessel
integrity (10). In all, this
would result in poorer microvessel tubes, ultimately leading to a
higher probability of microvessel fragility.
We also observed significantly higher pericyte
coverage during the proliferative phase than in the secretory phase
in the control subjects, while the amount of pericytes during these
phases did not differ in the patients with HMB-E. Estrogen
receptor-β has been found on endothelial cells, which stimulates
angiogenesis directly and makes them more sensitive to VEGF-A
stimulation (22); therefore the
observed differences may depend on the upregulation of VEGF-A by
estradiol stimulation. However, the mechanisms through which VEGF-A
production and activity is controlled by estrogen and progesterone
remains unclear. Estrogen can be either pro- or anti-angiogenic in
the endometrium. Studies have indicated that angiogenesis also
increases in response to progesterone, and this effect is not
inhibited by estrogen (23,24). These same reports demonstrated a
significant increase in SMAα positivity in the microvessels of mice
treated with progesterone; however, the mechanisms through which
these hormones affect the recruitment of pericytes remain unclear.
On the other hand, another study illustrated how progestin-only
contraceptives reduced the amount of SMAα in the endometrial
microvasculature (25), which
could indicate an inhibitory role for progesterone on pericytes.
The fact that the amount of pericytes did not differ in the
patients with HMB-E in our study between the phases of the
menstrual cycle may indicate a pathological insensitivity to sex
hormone stimulation either by the endothelial cells or the
pericytes themselves.
Furthermore, we demonstrated that the high local
expression of VEGF-A was negatively associated with pericyte
coverage. These results are in agreement with the previous
demonstration that VEGF-A confers pericyte insensitivity to the
recruiting activity of PDGF-β, ablating pericyte coverage of
nascent vascular sprouts, and leading to microvessel
destabilization (26).
We did not find a significant correlation between
the amount of endothelial cell gaps and the number of microvessels
with pericyte coverage. As described in our previous study,
endothelial cell gaps were found to be covered with mural cells
containing SMAα, and these cells were assumed to be pericytes
(6). What should be noted is that
no leakage of blood was observed near endothelial cell gaps, which
may indicate a protective role for pericytes.
In conclusion, our data agree well with, and extend,
our previous finding that HMB-E is associated with aberrant
angiogenesis. In this study, we demonstrate that an upregulation of
the agonist-receptor pathway of VEGF in idiopathic HMB-E affects
the recruitment of pericytes, which increases microvessel fragility
and possibly leads to excessive blood loss. The evidence of
increased fragility of microvessels in the endometrium in patients
with HMB-E may provide novel opportunities for investigations and
therapeutic interventions.
Acknowledgments
We thank Annette Hofmann from the Center for
Infectious Medicine, Karolinska Institutet, for providing
assistance with confocal microscopy. This study was supported by
grants from the Swedish Medical Research Council (19X-05991 and
71XS-13135), Karolinska Institutet, the regional agreement on
medical training and clinical research (ALF) between Stockholm
County Council and the Karolinska Institutet, Huddinge University
Hospital, and the Swedish Labour Market Insurance (AFA).
Abbreviations:
|
CI
|
confidence interval
|
|
HMB-E
|
heavy menstrual bleeding of
endometrial origin
|
|
HPF
|
high-power field
|
|
MVD
|
microvascular density
|
|
PBAC
|
pictorial blood loss assessment
chart
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Hallberg L, Högdahl AM, Nilsson L and Rybo
G: Menstrual blood loss - a population study. Variation at
different ages and attempts to define normality. Acta Obstet
Gynecol Scand. 45:320–351. 1966. View Article : Google Scholar
|
|
2
|
Gath D, Osborn M, Bungay G, Iles S, Day A,
Bond A and Passingham C: Psychiatric disorder and gynaecological
symptoms in middle aged women: a community survey. Br Med J (Clin
Res Ed). 294:213–218. 1987. View Article : Google Scholar
|
|
3
|
Salamonsen LA, Kovacs GT and Findlay JK:
Current concepts of the mechanisms of menstruation. Baillieres Best
Pract Res Clin Obstet Gynaecol. 13:161–179. 1999. View Article : Google Scholar
|
|
4
|
Mints M, Blomgren B and Palmblad J:
Expression of vascular endothelial growth factor receptor-3 in the
endometrium in menorrhagia. Int J Mol Med. 19:909–913.
2007.PubMed/NCBI
|
|
5
|
Mints M, Blomgren B and Palmblad J:
Expression of angiopoietins 1, 2 and their common receptor tie-2 in
relation to the size of endothelial lining gaps and expression of
VEGF and VEGF receptors in idiopathic menorrhagia. Fertil Steril.
94:701–707. 2010. View Article : Google Scholar
|
|
6
|
Mints M, Hultenby K, Zetterberg E,
Blomgren B, Falconer C, Rogers R and Palmblad J: Wall
discontinuities and increased expression of vascular endothelial
growth factor-A and vascular endothelial growth factor receptors 1
and 2 in endometrial blood vessels of women with menorrhagia.
Fertil Steril. 88:691–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerhardt H and Betsholtz C:
Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res.
314:15–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hellström M, Kalén M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.PubMed/NCBI
|
|
9
|
Levéen P, Pekny M, Gebre-Medhin S, Swolin
B, Larsson E and Betsholtz C: Mice deficient for PDGF B show renal,
cardiovascular, and hematological abnormalities. Genes Dev.
8:1875–1887. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirschi KK and D’Amore PA: Pericytes in
the microvasculature. Cardiovasc Res. 32:687–698. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellström M, Gerhardt H, Kalen M, Li X,
Eriksson U, Wolburg H and Betsholtz C: Lack of pericytes leads to
endothelial hyperplasia and abnormal vascular morphogenesis. J Cell
Biol. 153:543–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hewett P, Nijjar S, Shams M, Morgan S,
Gupta J and Ahmed A: Down-regulation of angiopoietin-1 expression
in menorrhagia. Am J Pathol. 160:773–780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higham JM, O’Brien PM and Shaw RW:
Assessment of menstrual blood loss using a pictorial chart. Br J
Obstet Gynaecol. 97:734–739. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mints M, Blomgren B, Falconer C,
Fianu-Jonasson A and Palmblad J: Microvascular density, vascular
endothelial growth factor A, and its receptors in endometrial blood
vessels in patients with menorrhagia. Fertil Steril. 84:692–700.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zetterberg E, Vannucchi AM, Migliaccio AR,
Vainchenker W, Tulliez M, Dickie R, Hasselbalch H, Rogers R and
Palmblad J: Pericyte coverage of abnormal blood vessels in
myelofibrotic bone marrows. Haematologica. 92:597–604. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Förster C1, Mäkela S, Wärri A, Kietz S,
Becker D, Hultenby K, Warner M and Gustafsson JA: Involvement of
estrogen receptor beta in terminal differentiation of mammary gland
epithelium. Proc Natl Acad Sci USA. 99:15578–15583. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weibel E: Stereological Methods: Practical
methods for biological morphometry. Academic Press; London:
1979
|
|
18
|
Mancuso MR, Davis R, Norberg SM, O’Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B,
Shalinsky DR, Hu-Lowe DD and McDonald DM: Rapid vascular regrowth
in tumors after reversal of VEGF inhibition. J Clin Invest.
116:2610–2621. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dulmovits BM and Herman IM: Microvascular
remodeling and wound healing: a role for pericytes. Int J Biochem
Cell Biol. 44:1800–1812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goddard LM and Iruela-Arispe ML: Cellular
and molecular regulation of vascular permeability. Thromb Haemost.
109:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sims DE and Westfall JA: Analysis of
relationships between pericytes and gas exchange capillaries in
neonatal and mature bovine lungs. Microvasc Res. 25:333–342. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Girling JE and Rogers PA: Recent advances
in endometrial angiogenesis research. Angiogenesis. 8:89–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Girling JE, Lederman FL, Walter LM and
Rogers PA: Progesterone, but not estrogen, stimulates vessel
maturation in the mouse endometrium. Endocrinology. 148:5433–5441.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Girling JE and Rogers PA: Regulation of
endometrial vascular remodelling: role of the vascular endothelial
growth factor family and the angiopoietin-TIE signalling system.
Reproduction. 138:883–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rogers PA, Plunkett D and Affandi B:
Perivascular smooth muscle alphaactin is reduced in the endometrium
of women with progestin-only contraceptive breakthrough bleeding.
Hum Reprod. 15(Suppl 3): S78–S84. 2000. View Article : Google Scholar
|
|
26
|
Greenberg JI, Shields DJ, Barillas SG,
Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS,
Angle N and Cheresh DA: A role for VEGF as a negative regulator of
pericyte function and vessel maturation. Nature. 456:809–813. 2008.
View Article : Google Scholar : PubMed/NCBI
|