Introduction
Tau, a microtubule-associated protein (MAP) has the
ability to interact with tubulin, thus promoting the assembly of
microtubules and structure stabilization (1). Human tau is coded for by a single
gene on chromosome 17, yielding several molecular isoforms due to
alternative mRNA splicing (2). In
the human brain, there are 6 tau isoforms based on different
combinations of the presence or absence of inserts encoded by
exon-2 (29-aa) and/or by exon-3 (29-aa) in the amino-terminal half,
and a 31-aa repeat (R2) encoded by exon-10 in the carboxy-terminal
half of the protein. Tau contains 3 repeat segments in the domain
for microtubule-binding. Depending on the number of
inserts/repeats, tau isoforms are also nominated as 0N4R/tau383,
1N4R/tau412 and 2N4R/tau441 with 4 repeats, and as 0N3R/tau352,
1N3R/tau381 and 2N3R/tau410 with 3 repeats. It has been suggested
that tau isoforms containing 4 repeats are better at promoting
microtubule assembly than those with 3 repeats (3). The smallest size tau isoform
(0N3R/tau352) is the only form that is expressed in the human fetal
brain (1).
The aggregation of tau in brain tissue has been
described in a large number of neurodegenerative diseases, such as
Alzheimer’s disease (AD), progressive supranuclear palsy (PSP),
corticobasal degeneration (CBD), Pick’s disease (PiD), prion
disease (PrD), and frontotemporal dementia and parkinsonism linked
to chromosome 17 (FTDP-17); thus, these diseases have been
classified as ‘tauopathies’ (4–7).
However, the tau pathology among these disorders may vary greatly.
For instance, all brain tau isoforms with a hyperphosphorylated
state are the main component of the paired helical and straight
filaments in AD (8–10), and tau isoforms with 3 repeats
predominate in the neuronal deposits of PiD (11), whereas the filaments of PSP and
CBD are composed of 4-repeat tau isoforms (12,13). In addition, filaments from human
brains affected by tau pathology also exhibit a range of
morphologies and specific conformers of aggregated tau may give
rise to distinct tauopathies (6).
In a previous study, we prepared 3 tau exon-specific
polyclonal antibodies which react against exon-2, -3 and -10,
respectively (14). Different
disease-related tau profiles in cerebrospinal fluid (CSF) samples
from patients suffering from sporadic Creutzfeldt-Jakob disease
(sCJD) were observed in the antibody reactions against exon-2 or
-10, suggesting the potential usage of these 2 antibodies in the
further study of prion diseases, as well as in the study of other
neurodegenerative disorders (14). In the present study, to obtain
monoclonal antibodies (mAbs) against tau exon-2 and -10, purified
recombinant GST-fusion proteins were separately immunized into
Balb/c mice. Following cell fusion and selection, 2 strains of
hybridomas that produced the antibody against exon-2 or -10 of tau,
respectively, were obtained. The specificities and sensitivities of
the prepared mAbs were systematically analyzed. The tau patterns
and characteristics of the brain tissues of various mammals were
assessed in parallel with the prepared mAbs.
Materials and methods
Ethics statement
Human brain tissue was obtained from a healthy donor
who was killed in a car accident at the age of 56, as previously
described (15). The approval for
its use was obtained from his next of kin following written
informed consent and the approval of the Ethics Committee of the
National Institute for Viral Disease Control and Prevention at the
Chinese Center for Disease Control and Prevention (China CDC),
Beijing, China. The animal tissues used in this study were approved
by the Ethics Committee of the National Institute for Viral Disease
Prevention and Control at the China CDC. Housing and experimental
protocols were in accordance with the Chinese Regulations for the
Administration of Affairs Concerning Experimental Animals.
Preparation of mouse mAbs against tE2 and
tE10
The recombinant GST-fusion prokaryotic proteins,
GST-tE2 and GST-tE10, which were used as antigens in the
preparations of the mAbs, were expressed and purified according to
the protocol described in our previous study (16). One hundred micrograms of GST-tE2
or GST-tE10 were emulsified with an equal volume of Freund’s
complete adjuvant, and were immunized into 6- to 8-week-old Balb/c
female mice by intraperitoneal injection (3 mice per antigen). Two
weeks later, the animals were injected with a half-dose of each
antigen (50 μg) emulsified with an equal volume of Freund’s
incomplete adjuvant. A week later, blood samples were collected by
cutting the mouse tails for measuring the presence of specific
antibodies in sera. After the third immunization, blood was taken
to monitor the induction of the specific antibody. The mice were
administered a final booster of GST-tE2 or GST-tE10 (100 μg)
3 days before the fusing program. When the serum titers reached
5×103 in a tau-specific ELISA, the mice were sacrificed
under anesthesia with ether and their spleens were removed.
Splenocytes (1×108) were fused with
1×107 SP2/0 myeloma cells in 45% polyethylene glycol
(PEG). Hybridoma clones were screened in HAT medium (RPMI-1640 with
10% FBS, 10 mM sodium hypoxanthanine, 40 mM aminopterin and 1.6 mM
thymidine). An indirect tau-exon specific ELISA was used to screen
the secretion of the antibodies into the wells. The wells that were
positive for GST-tE2 but negative for GST-tE10 were selected as
positive wells for anti-tE2 and vice versa. The positive cells were
subsequently subcloned twice with a limiting dilution
procedure.
One week following treatment, the cloned hybridomas
producing anti-tE2 and anti-tE10 were separately intraperitoneally
injected into Balb/c mice with 0.5 ml pristane (Sigma, St. Louis,
MO, USA) per mouse. The animals were euthanized by ether 2–3 weeks
post-injection when the transplant tumors formed and ascites were
collected for antibody detection. The isotypes of the prepared
antibodies were determined using the Mouse Monoclonal Antibody
Isotyping kit (Roche, Basel, Switzerland; Cat. no. 11493027001).
The sensitivities and specificities of the antibodies were examined
by ELISA and western blot analysis.
Preparation of brain homogenates
Goat, bovine, rabbit, mouse and hamster brain
samples were obtained from animals euthanized with ether. The brain
tissue originated from a 56-year-old car accident victim. All brain
samples were homogenized in 10% lysis buffer (100 mM NaCl, 10 mM
EDTA, 0.5% Nonidet P-40, 0.5%
C24H40O4·Na, 10 mM Tris, pH 7.5)
according to the protocol used in the study by Zhang et al
(17). Tissue debris was removed
by low-speed centrifugation at 2,000 × g for 10 min and the
supernatants were collected for further analysis.
Biotin labeling
The labeling of the prepared mAbs with biotin was
conducted using a commercially supplied biotin label kit (EBLK0002;
Elabscience Biotechnology Co. Ltd., Wuhan, China) according to the
manufacturer’s instructions. The final concentration of the
biotin-labeled mAb was 1.5 mg/ml.
ELISA
An indirect ELISA was established in order to
examine the specificity and sensitivity of the selected mAbs. The
fusion proteins, GST-tE2 or GST-tE10, were diluted to a final
concentration of 1 μg/ml in PBS and coated onto 96-well
microtiter plates overnight at 4°C. After washing 3 times with PBST
(phosphate-buffered saline, pH 7.6, containing 0.05% Tween-20), the
plates were blocked with 2% BSA in PBST at 37°C for 2 h and then
incubated with various dilutions of the tested sera, supernatants
of the cloned hybridoma or ascites. After rinsing thoroughly with
PBST 3 times, the possible captured mouse antibodies were further
identified by horseradish peroxidase (HRP)-conjugated anti-mouse
secondary antibody (Cat. no. 31430; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at a 1:10,000 dilution at 37°C for 1 h. Color was
developed with 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma) as a
substrate at 37°C for 30 min. Absorbance was measured at 450 nm
(A450 nm) after quenching the wells by the addition of 2
M H2SO4. The titration of the tested samples
was evaluated as positive when the P(n) value was ≥2.1.
Immunoprecipitation (IP)
In total, 200 μl of 10% brain homogenates of
the various mammals were incubated with Tau polyclonal anbitody
(pAb; H-150; Cat. no. sc-5587; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and 50 μl of Dynabeads® Protein G
(Invitrogen, Waltham, MA, USA) at 4°C overnight. Additionally, 15
μl of each 10% brain homogenate were used as input controls.
The immunocomplexes were collected and washed 6 times with
phosphate - buffered saline (PBS) containing 0.02% Tween-20,
supplemented with 50 μl 1X loading buffer and heated in
boiling water for 10 min. Protein G beads were removed with a
magnetic shelf (Invitrogen). The eluted products and the inputs
were separated with 12% SDS-polyacrylamide gelelectrophoresis
(SDS-PAGE), and subsequently detected by the biotin-labeled mAb
3E12- or 4A8-specific western blot analysis.
Western blot analysis
The analysis of the expression and purification of
the GST-tau-exon-fusion proteins, including GST-tE2, GST-tE3 and
GST-tE10, as well as 6 tau isoforms including tau441, tau412,
tau383, tau410, tau381 and tau352, were conducted as previously
described (14).
GST-tau-exon-fusion proteins were separated by 15% SDS-PAGE, while
the tau isoforms and brain homogenates of various mammals were
separated in 12% SDS-PAGE. Following electrotransfer onto PVDF
membranes (Millipore, Billerica, MA, USA) using a semi-dry blotting
system (Bio-Rad Laboratories, Hercules, CA, USA), the membranes
were blocked with 5% dried non-fat milk in PBS and incubated with
various primary antibodies at 4°C overnight, including 1:1,000
diluted mAb tau-13 and pAb Tau (H-150) (Cat. no. sc-5587; Santa
Cruz Biotechnology) and 1:1,000 diluted prepared mAb anti-tE2 (4A8)
or anti-tE10 (3E12). Subsequently, after washing with TBS
containing Tween-20 (TBST, 10 mM Tris-HCl, 133 mM NaCl, pH 7.4),
the blots were incubated with 1:10,000 diluted HRP-conjugated
secondary antibody (Thermo Fisher Scientific). After washing with
TBST, the reactive signals were visualized by an enhanced
chemiluminescence (ECL) kit (PE Applied Biosystems, Foster City,
CA, USA). Images were captured using a ChemiDoc™ XRS+ Imager
(Bio-Rad Laboratories) and quantified using NIH ImageJ software.
The signals were normalized to the loading controls.
Results
Preparations of murine mAbs against tE2
and tE10
To prepare the specific mAbs against the fragments
encoded by exon-2 and exon-10 of human tau, the Balb/c mice were
separately immunized with the purified recombinant GST-fusion
proteins, GST-tE2 or GST-tE10. Following two immunizations, blood
samples were collected from the mouse tails and the presence of the
specific antibodies was evaluated by an indirect ELISA coated with
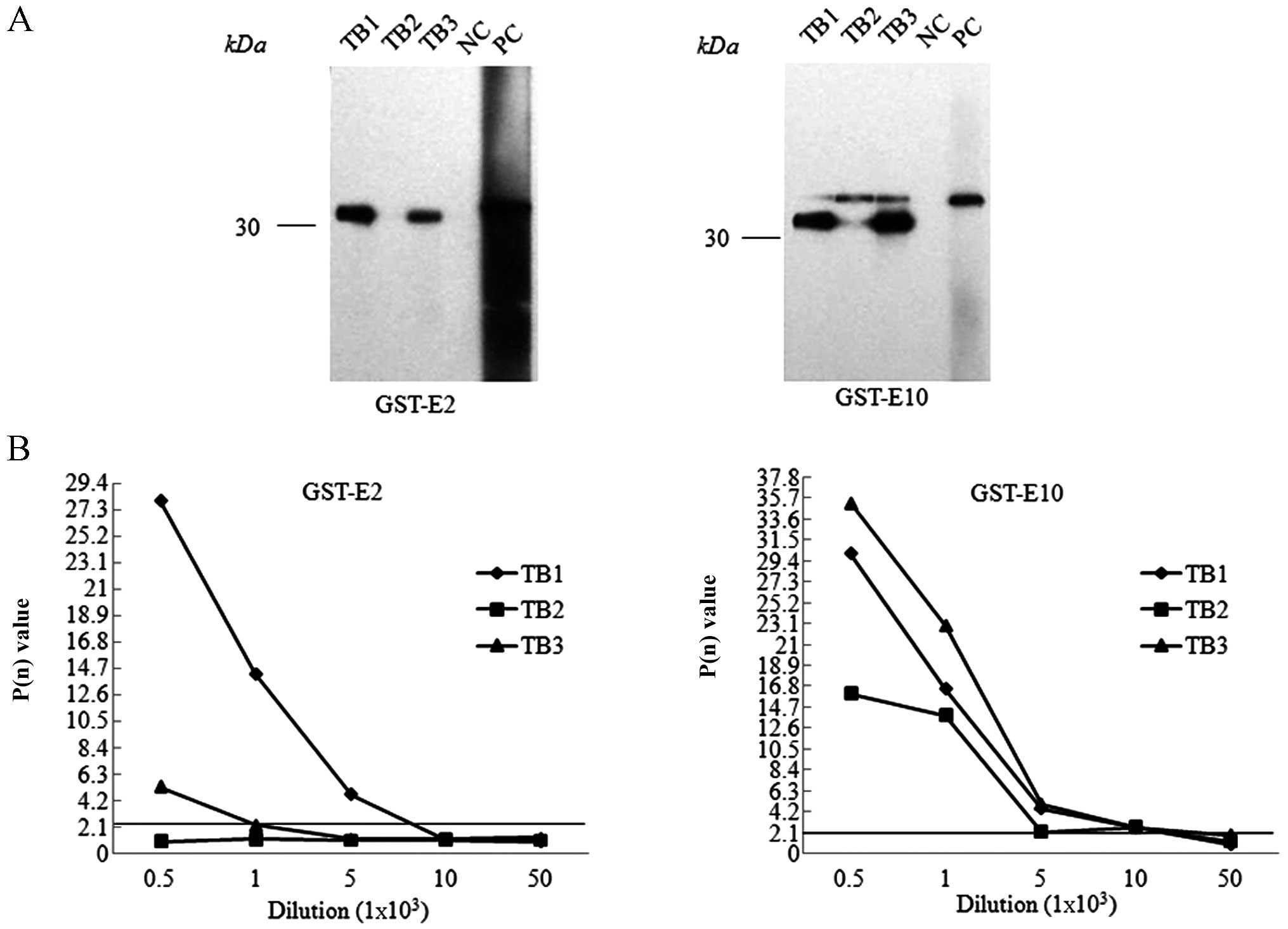
tau exon proteins. In total, 1 out of 3 GST-tE2-immunized mice and
2 out 3 GST-tE10-immunized mice showed positive immunoreactivities
(P(n) >2.1 at the dilution of 1:1,000) (Fig. 1). Through the procedures of cell
fusion and multiple clone selections, dozens of hybridoma cell
clones producing antibodies against either tE2 or tE10 were
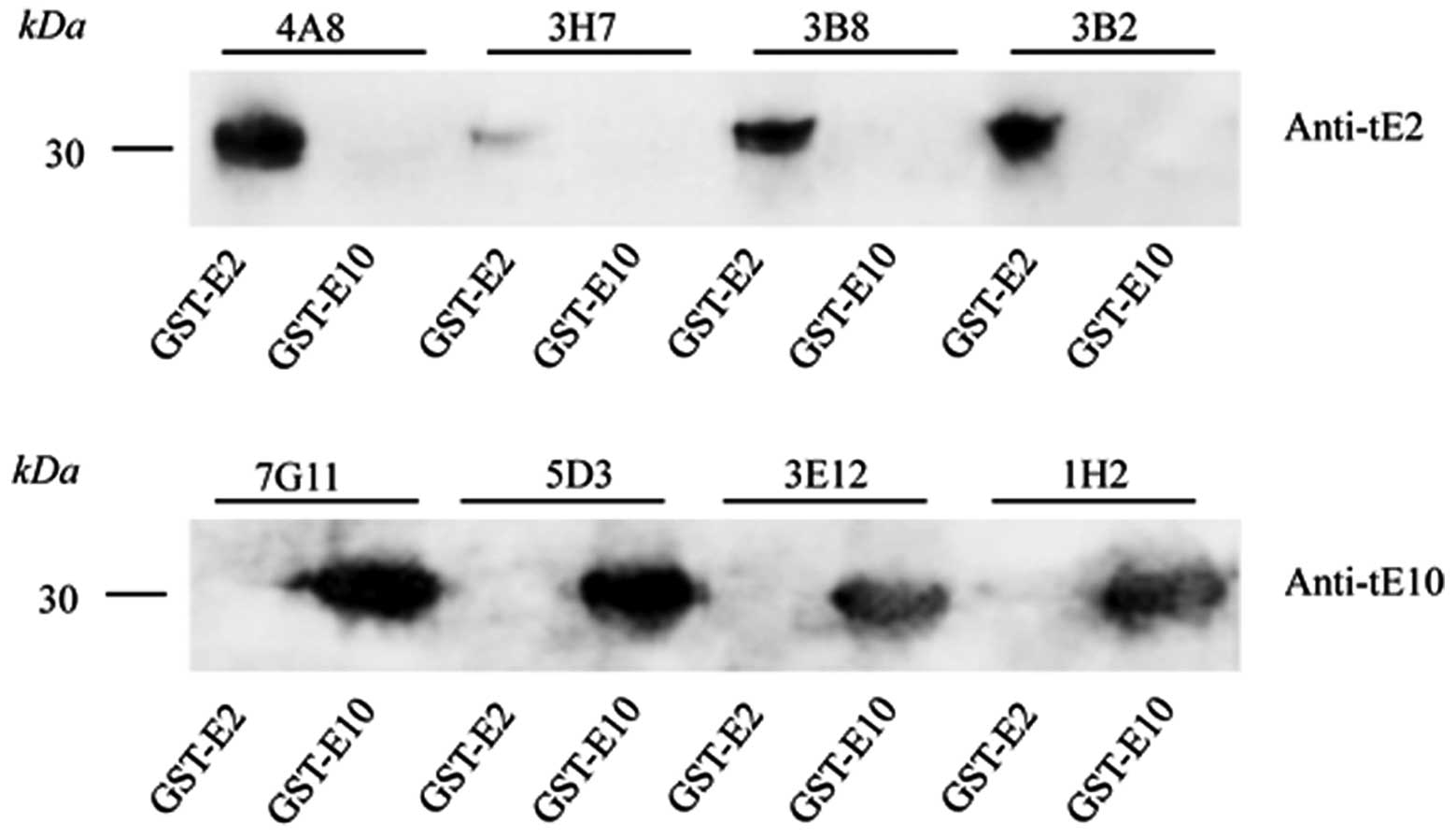
selected. Western blot analysis revealed that the supernatants of
the selected cell clones reacted specifically with the individual
GST-tE2 or GST-tE10 fusion proteins (Fig. 2). Further indirect ELISA
highlighted that the hybridoma clone 4A8 showed the highest
anti-tE2 titer and that clone 3E12 showed the highest anti-tE10
titer (data not shown). Mouse ascites were prepared by
intraperitoneal injections of these 2 hybridoma cells.
Determinations of the tau exon mAbs, 4A8
and 3E12
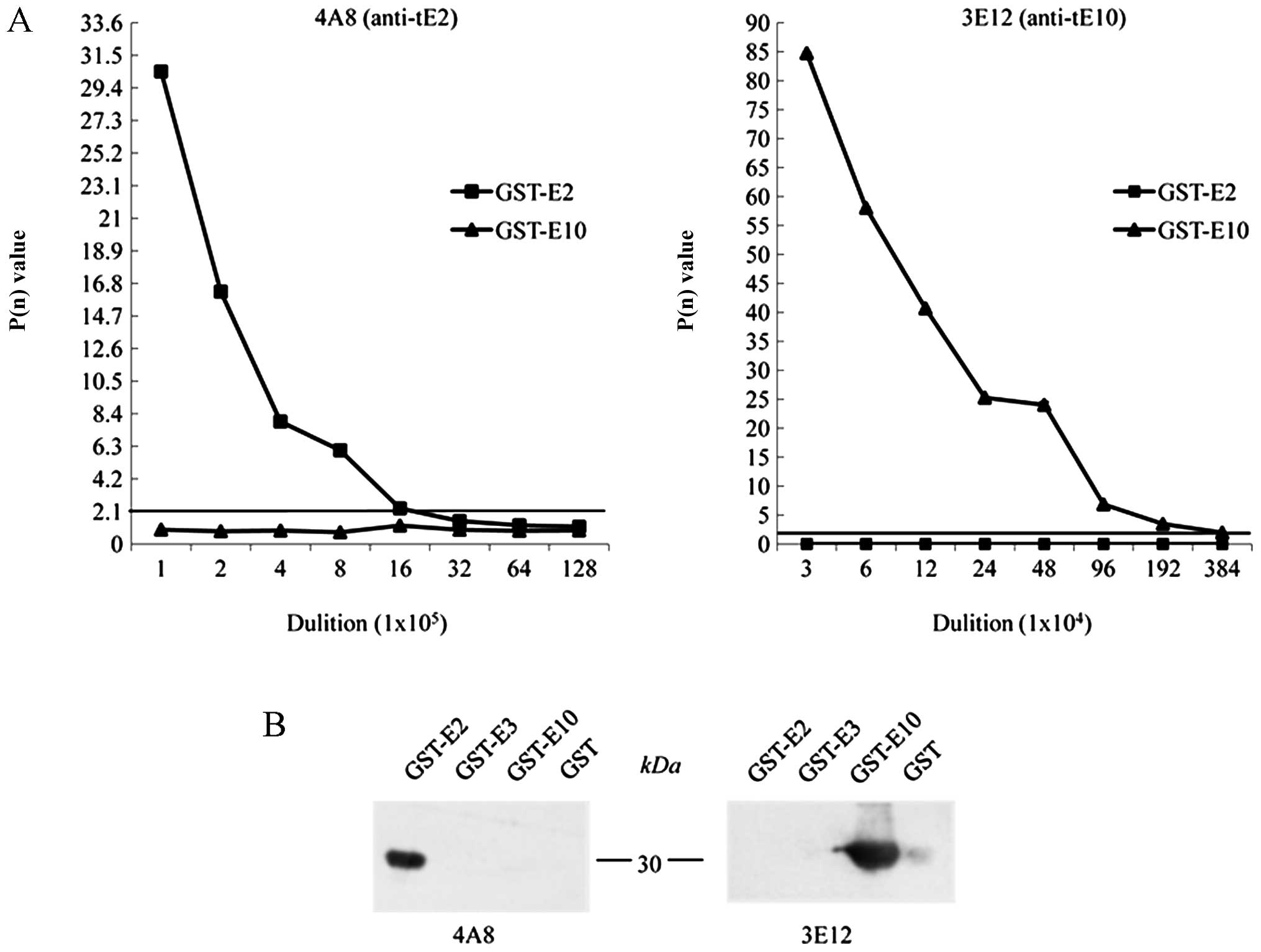
To examine the sensitivities and specificities of
the anti-tE2 mAb, 4A8, and the anti-tE10, mAb 3E12, the prepared
ascites were subjected to an indirect ELISA coated with equal
amounts of the purified GST-tE2 and GST-tE10. The results revealed
that the mAb 4A8 reacted only with GST-tE2, while 3E12 reacted only
with GST-tE10, again revealing their reliable immune specificities.
Based on the rule of a P(n) value ≥2.1, the ELISA titers of the
mAbs, 4A8 and 3E12, with the individual recombinant proteins
reached 1.6×106 and 1.9×106, respectively
(Fig. 3A). Furthermore, the
specificities of the mAbs, 4A8 and 3E12, were examined by western
blot analysis containing the purified recombinant proteins,
GST-tE2, GST-tE3 and GST-tE10. At the dilution of 1:500 ascites,
only GST-tE2 in the reaction of mAb 4A8 and GST-tE10 in that of mAb
3E10 showed positive signals at the expected positions (Fig. 3B). No cross-reaction was
identified with the other tau exon fusion proteins or GST protein
under this experimental condition.
Using the commercial mouse mAb isotyping kit, the
tE2 mAb, 4A8, was identified as IgG2b, κ chain and the
tE10 mAb, 3E12, as IgG1, κ chain.
Specific recognition of the tau exon
mAbs, 4A8 and 3E12, with the corresponding recombinant tau
isoforms
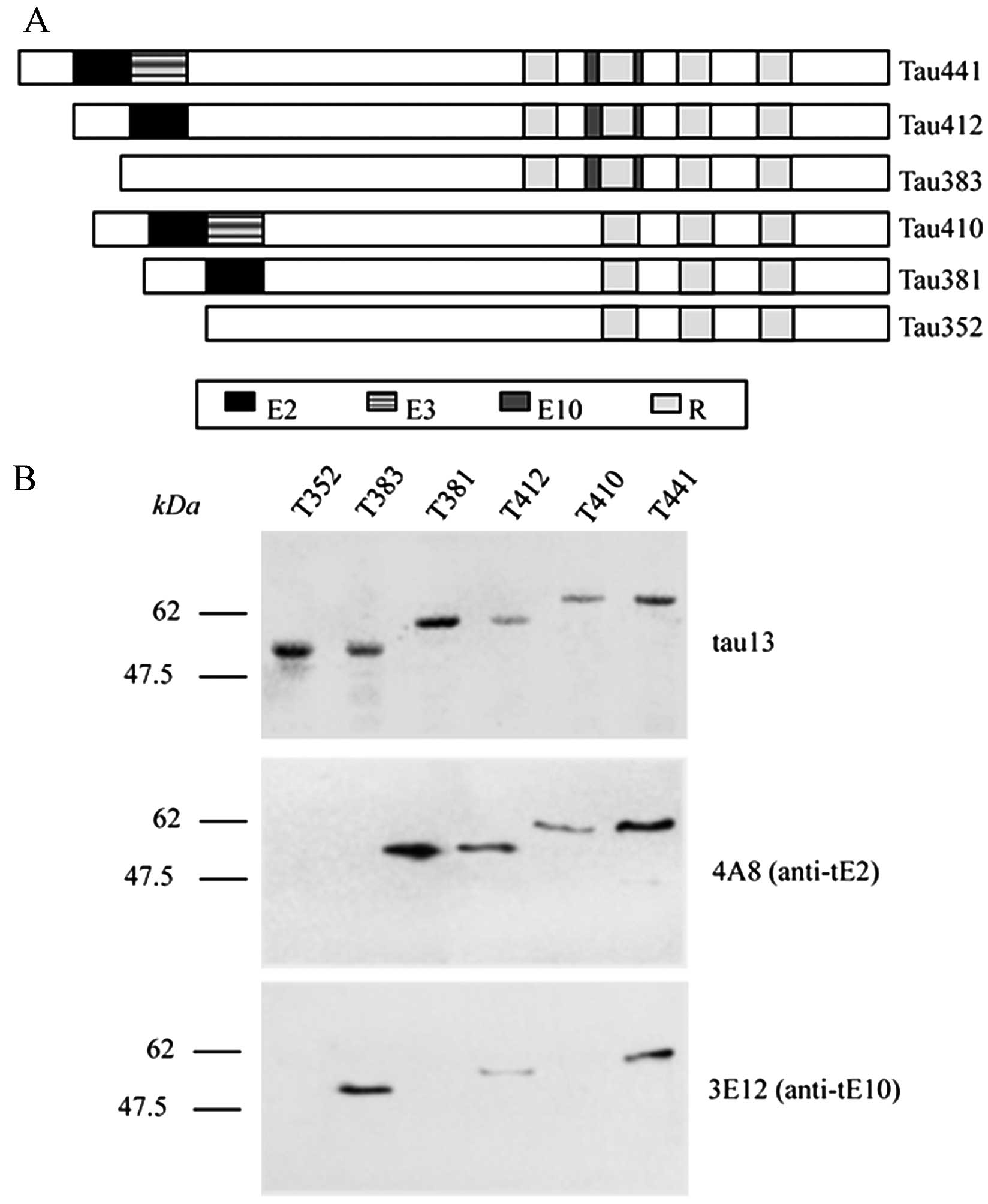
Six human tau isoforms have been addressed, varying
on the basis of different tau exon segment insertions and the
number of repeated regions (1).
The schematic structures of the 6 tau isoforms are presented in
Fig. 4A. To prove the
recognitions of the prepared tau exon mAbs, 4A8 and 3E12, on
various tau isoforms in the context of a full-length protein, the 6
purified human tau isoforms were reacted with the prepared tau exon
mAbs, as well as a commercial mAb, tau-13, able to recognize all
isoforms in a western blot analysis. As observed previously, all 6
tau isoforms were detectable in the reaction with mAb tau-13,
mobilizing from 48 to 67 kDa (Fig.
4B, upper panel). Four tau isoforms containing exon-2 domain
(tau-381,-412, -410 and -441) showed positive bands in the reaction
with mAb 4A8 (Fig. 4B, middle
panel), while 3 containing the exon-10 domain (tau-383, -412 and
-441) positively reacted with mAb 3E12 (Fig. 4B, lower panel). No false- or
cross-reaction was observed between the 2 prepared mAbs, suggesting
reliable specificities of the mAbs, 4A8 and 3E12, in recognizing
the relevant tau isoforms in the context of full-length
proteins.
Recognition of the tau exon mAbs, 4A8 and
3E12, with endogenous brain tau proteins from a series of
mammals
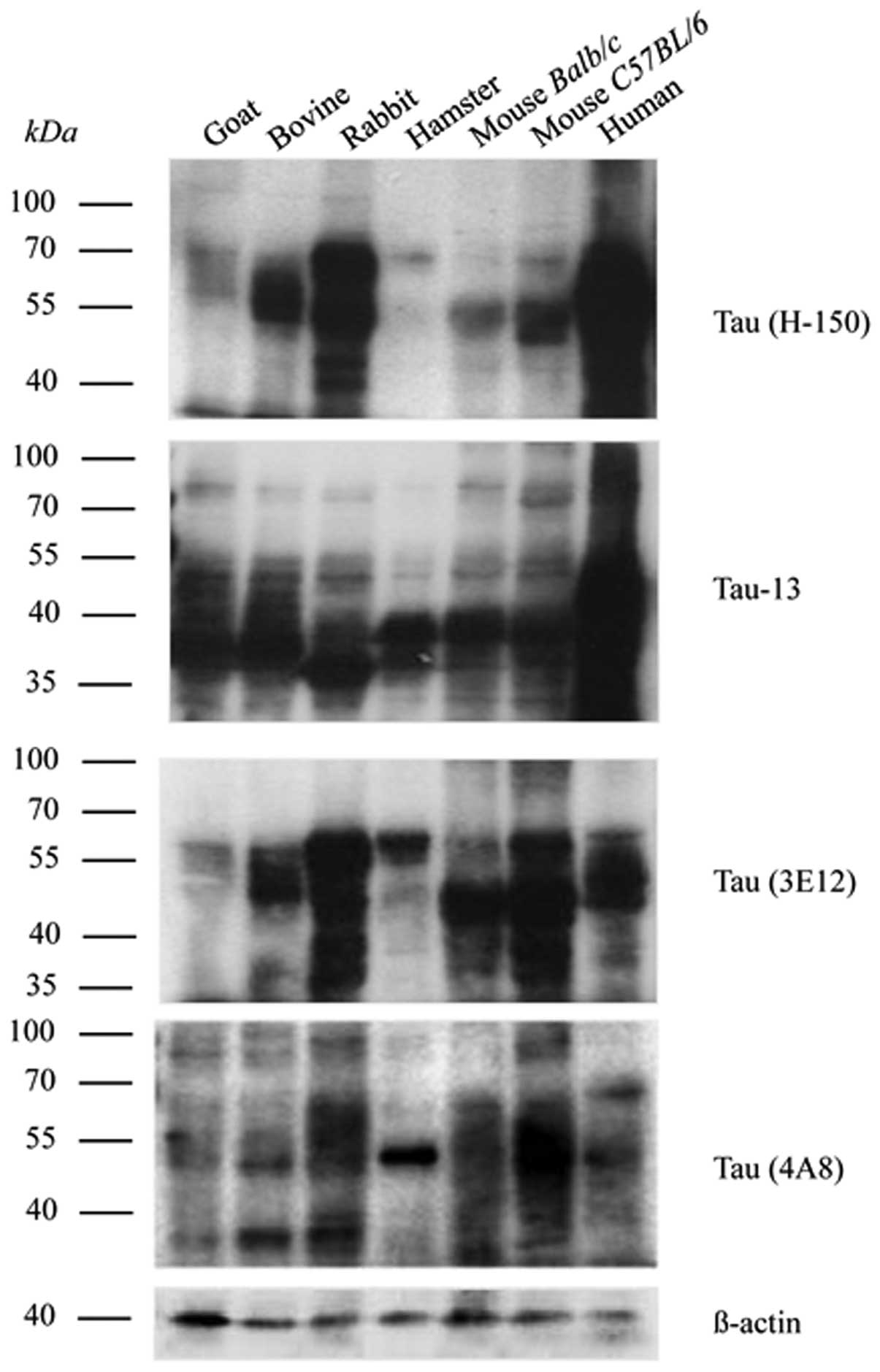
To examine the immunoreactivities of the prepared
tau exon mAbs with endogenous tau proteins, brain homogenates of
human origin and various mammals comprising goat, bovine, rabbit,
mouse (strains Balb/c and C57BL/6) and hamster tissues were
prepared and run on a western blot analysis with the mAbs, 4A8 and
3E12, as well as with the commercial mouse-derived mAb, tau-13, and
the rabbit-derived pAb, tau (H-150), respectively. As shown in
Fig. 5, numerous bands were
detected in the reactions with the mAb tau-13 and pAb tau (H-150),
ranging from 45 to 70 kDa. Generally, the profiles of the
immunoreactivities of mAb tau-13 and pAb tau (H-150) in the brain
tissues of the tested mammals were similar, indicating a conserved
feature of tau. However, the tau signal intensities among the
tested samples varied significantly.
The prepared tau exon mAbs, 4A8 and 3E12, resulted
in clear reactive bands with human brain homogenates, ranging from
45–70 kDa (Fig. 5). Positive
signals were also detected at positions <40 kDa. Similar
reactive patterns were also observed in the preparations of other
tested samples, with the difference in the signal intensities among
species. These data highlight that our prepared tau exon specific
mAbs may recognize endogenous tau proteins not only in humans, but
also in brain tissue from various mammalian sources.
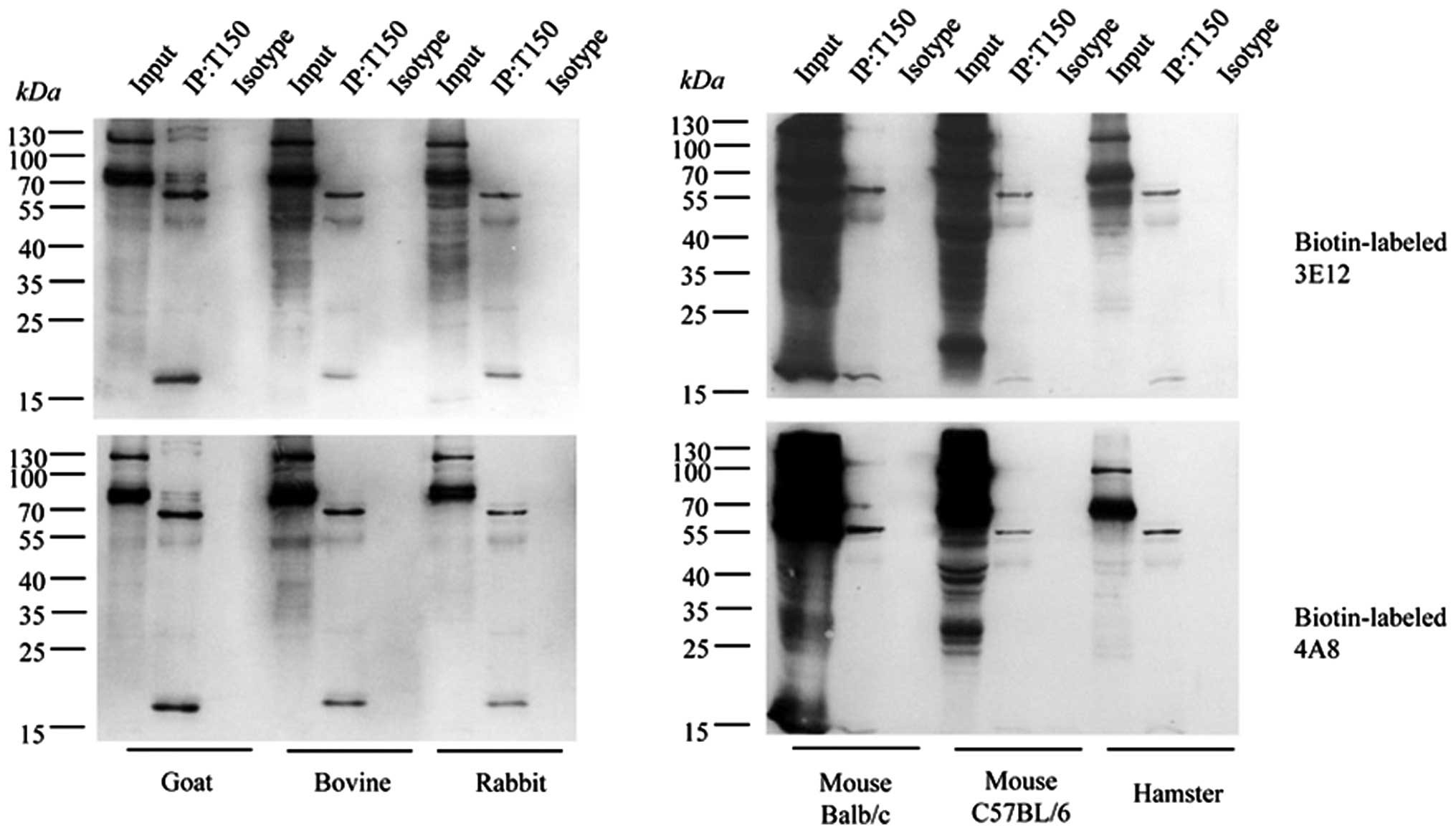
Reliable specificity of the tau exon
mAbs, 4A8 and 3E12, with endogenous brain tau proteins
To further assess the specificities of the prepared
tau exon mAbs in the recognition of endogenous brain tau proteins,
immunoprecipitation assays were conducted with brain homogenates
from goat, bovine, rabbit, hamster and mouse. To exclude the
possibility of cross-reaction of the IgG heavy-chain due to the
HRP-labeled secondary antibody during immunoprecipitation, the tau
exon mAbs, 4A8 and 3E12, were separately labeled by biotin with the
help of a commercial labeling kit. The brain homogenates were first
precipitated with pAb tau (H-150) and blotted with the
biotin-labeled mAbs, 4A8 and 3E12. As shown in Fig. 6, 2 predominately reactive bands that were
roughly 65 and 55 kDa were observed in all the precipitated
products from all tested samples, while a detectable signal was
observed in the preparations precipitated with rabbit IgG as the
isotype antibody. These 2 positive bands coincided well with the
tau-reactive bands in the brain extracts used as controls. Besides
these 2 bands, a smaller band (approximately 17 kDa) was also
identified in all reactions, while several larger specific bands
were also seen in some reactions (Fig. 6). The reactive patterns in the
precipitated products were quite comparable among the tested
animals. These data indicate that the tau exon mAbs, 4A8 and 3E12,
possess reliable specificity in the recognition of endogenous brain
tau proteins.
Discussion
Using a classic hybridoma technique with the
recombinant human tau fusion proteins, GST-tE2 and GST-tE10, as
immunogens, we created 2 hybridoma cell lines that secrete tau
exon-specific mAbs against tau exon-2 (mAb 4A8) and exon-10 (mAb
3E12). We confirmed that our prepared mAbs correctly recognize
recombinant human tau proteins that contain corresponding exons by
western blot analysis. Both two tau exons contain only 29 and 31
amino acids. These two small segments seem to have strong
immunogenicity in the form of GST-fusion protein, both exon-2 and
-10 efficiently elicit high antibody titer when immunized into
mice. On the other hand, our data verified that there was no
detectable cross-reaction between these 2 antibodies. Analysis of
the sequences of these exons indicated that, although the molecular
weights of the peptides encoded by these 2 exons were quite
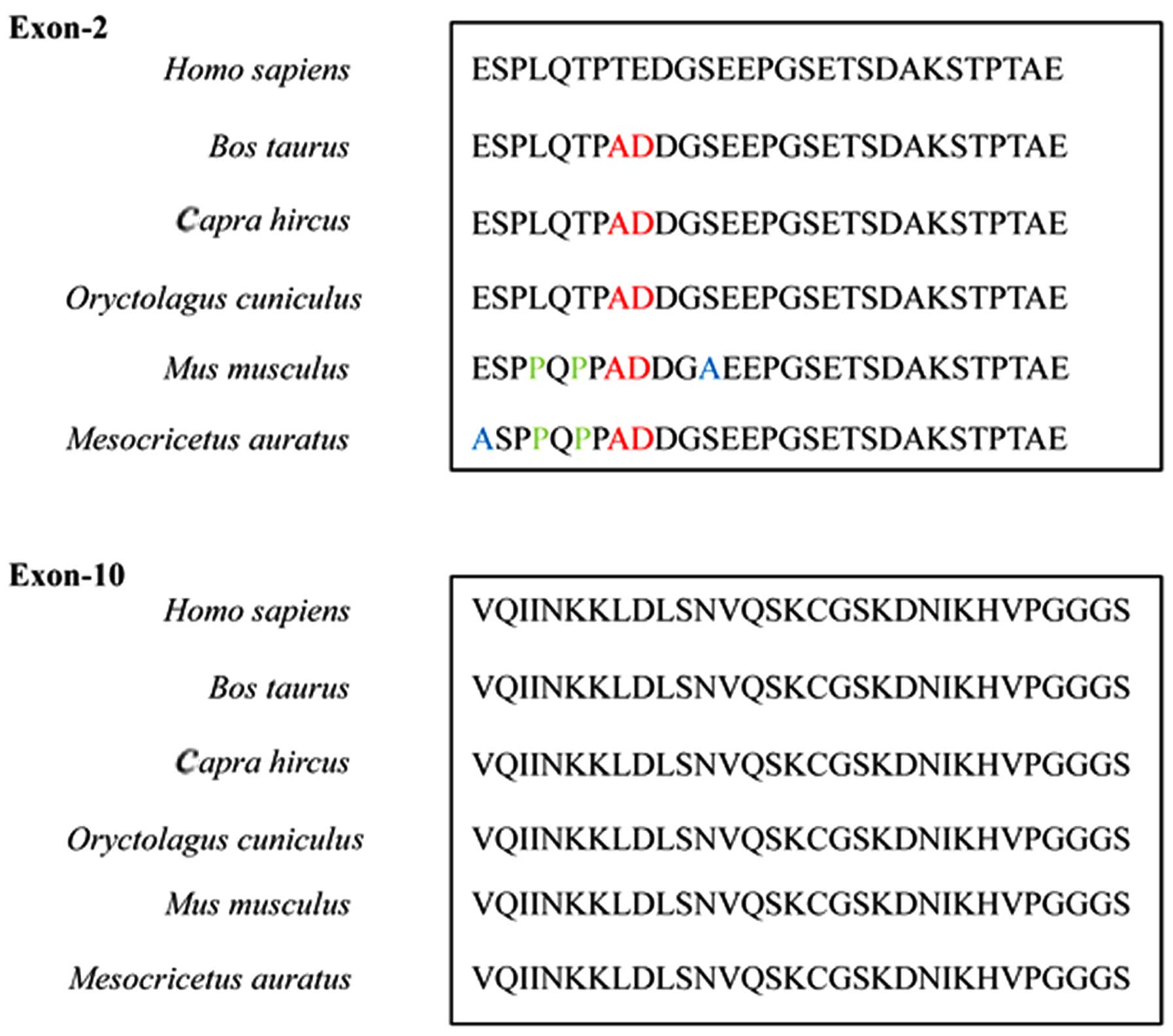
similar, the sequences of each exon varied greatly (Fig. 7). This trait may supply the
molecular basis for preparing individual specific antibodies.
Our tau exon mAbs showed a wide range of
immunore-activity among different mammalian species. As a conserved
protein (2), the peptide
sequences of tau exon-2 and -10 also share homology among human and
various mammals. The screening of tau sequences of relevant mammals
confirmed that the N-terminus of exon-2 in humans and various
mammals only differed by 2–5 amino acids, while the exon-10 did not
differ at all (Fig. 7). The high
homology in the peptide sequences of tau exon-2 and -10 supplies
the molecular basis for the wide range of recognition of the mAbs,
4A8 and 3E12, to the endogenous tau proteins among the tested
mammals.
Tau proteins may present different isoforms in
mammalian tissues, based on the different components of the repeat
segments (2). In humans, 6 tau
isoforms have been addressed, ranging from 352 to 441 amino acids
in length (1). Studies have
suggested the presence of 5 tau isoforms in mice, 4 in bovines and
2 in goats (18,19), ranging from 341 to 448 amino acids
in length (Table I). Relevant
data for rabbits and hamsters is relatively limited (Table I). However, the majority of tau
isoforms contain segments of exon-2 and/or -10, which may help to
explain the presence of multiple reactive tau bands in western
blots with the prepared tau exon mAbs. Cellular tau protein
undergoes post-translational modification under physiological
conditions, i.e., phosphorylation (1,2).
The common phosphorylating sites within human tau are believed to
be located at the tubulin binding region, including
Ser-202/Thr-205, Ser-214/Ser-212, Thr-231/Ser-235 and
Ser-396/Ser-404 (20). Thereby,
the apparent molecular weights of tau in tissue usually vary
greatly (14,21), which leads to more than expected
tau-specific bands in western blot analysis with tau-specific
antibodies. Therefore, it is difficult to distinguish the
endogenous tau isoforms in tissues based on their diversity in
molecular weights, such as by western blot analysis, without
complete dephosphorylation. In the reactions with commercial tau
mAb tau-13 and pAb tau (H-150) in western blot analysis, some
smaller (<40 kDa) positive reactive bands were also detected in
the brain tissues with tau-exon mAbs 4A8 and 3E12, suggesting that
apart from mature tau proteins, there are numerous truncated tau
proteins containing exon-2 and/or-10 in brain tissues.
 | Table IHomology comparison of exon-2 and -10
of different tau isoforms among humans and various mammals
according to published studies. |
Table I
Homology comparison of exon-2 and -10
of different tau isoforms among humans and various mammals
according to published studies.
| Species | Accession no. | No. of amino
acids | Contain/similar to
the sequence of human-derived
| Comments |
|---|
| Exon-2 | Exon-10 |
|---|
| Goat | | | | | |
| (Capra
hircus) | O02828 | 403 | ✓ | ✓ | Isoform A: contain
exon of 1, 4, 5, 7, 9, 11, 12, 13
Isoform B: contain exon of 1, 2, 4, 5, 7, 9, 10, 11, 12,
13
Nelson et al (19) |
| Bovine | | | | | |
| (Bos
taurus) | NP_776531 | 448 | ✓ | ✓ | Isoform A: 1, 2, 4,
5, 7, 9, 11, 12, 13
Isoform B: 1, 2, 4, 5, 7, 9, 10, 11, 12, 13
Isoform C: 1, 2, 3, 4, 5, 7, 9, 11, 12, 13
Isoform D: 1, 2, 3, 4, 5, 7, 9, 10, 11, 12, 13
Himmler (18) |
| Rabbit | | | | | |
| (Oryctolagus
cuniculus) | XP_008269868 | 840 | ✓ | ✓ | Predicted sequence
from NCBI |
| Mouse | | | | | |
| (Mus
musculus) | NP_001033698 | 430 | x | ✓ | Isoform a |
| NP_034968 | 372 | ✓ | ✓ | Isoform b |
| NP_001272383 | 364 | x | x | Isoform c |
| NP_001272384 | 350 | x | ✓ | Isoform d |
| NP_001272385 | 341 | x | x | Isoform e |
| Hamster | | | | | |
| (Mesocricetus
auratus) | NP_001268808 | 432 | ✓ | ✓ | |
The presence of tau isoforms in human brains may
also vary distinctly depending on different physiological and
pathological situations. Physiologically, the amounts of 3R-tau and
4R-tau are comparable in the adult cerebral cortex, while 0N-tau is
predominant in developing brains (1). In many neurodegenera-tive diseases,
tau may conform to different patterns. In AD, the neuronal
fibrillary tangles (NFTs) are made up of paired helical filaments
(PHFs) comprised of hyperphosphorylated tau, which are composed of
six central nervous system tau isoforms (2,8).
In Down syndrome, tau is hyperphosphorylated yielding a pattern
similar to that of AD (2). In
CBD, tau is also present in the hyperphosphorylated form, but only
approximately 68 and 64 kDa are detectable by electrophoresis
(1). In FTDP-17, tau inclusions
are observed both in neurons and in glial cells, with 2 types of
hyperphosphrylated tau forms (22). Due to the lack of tau isoform- or
exon-specific antibodies, the exact situations under which various
brain tau isoforms can be found and their exact contributions
during disease progression remain unknown. Therefore, the tau
exon-specific mAbs prepared in the present study may provide
potentially useful materials for tau profile assays in the brains
of humans and other mammals, physiologically and
pathologically.
Acknowledgments
The present study was supported by grants from the
Chinese National Natural Science Foundation Grants (81401670,
81100980), the China Mega-Project for Infectious Disease
(2011ZX10004-101, 2012ZX10004215) and the SKLID Development Grant
(2012SKLID102, 2011SKLID104).
References
|
1
|
Goedert M: Tau protein and
neurodegeneration. Semin Cell Dev Biol. 15:45–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Avila J, Lucas JJ, Perez M and Hernandez
F: Role of tau protein in both physiological and pathological
conditions. Physiol Rev. 84:361–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goedert M and Jakes R: Expression of
separate isoforms of human tau protein: correlation with the tau
pattern in brain and effects on tubulin polymerization. EMBO J.
9:4225–4230. 1990.PubMed/NCBI
|
|
4
|
Williams DR: Tauopathies: classification
and clinical update on neurodegenerative diseases associated with
microtubule-associated protein tau. Intern Med J. 36:652–660. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee VM, Goedert M and Trojanowski JQ:
Neurodegenerative tauopathies. Annu Rev Neurosci. 24:1121–1159.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández F and Avila J: Tauopathies. Cell
Mol Life Sci. 64:2219–2233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giaccone G, Mangieri M, Capobianco R,
Limido L, Hauw JJ, Haïk S, Fociani P, Bugiani O and Tagliavini F:
Tauopathy in human and experimental variant Creutzfeldt-Jakob
disease. Neurobiol Aging. 29:1864–1873. 2008. View Article : Google Scholar
|
|
8
|
Alonso AD, Zaidi T, Novak M, Barra HS,
Grundke-Iqbal I and Iqbal K: Interaction of tau isoforms with
Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro
phosphorylation into the disease-like protein. J Biol Chem.
276:37967–37973. 2001.PubMed/NCBI
|
|
9
|
Sjögren M, Davidsson P, Tullberg M,
Minthon L, Wallin A, Wikkelso C, Granérus AK, Vanderstichele H,
Vanmechelen E and Blennow K: Both total and phosphorylated tau are
increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry.
70:624–630. 2001. View Article : Google Scholar
|
|
10
|
Hampel H, Blennow K, Shaw LM, Hoessler YC,
Zetterberg H and Trojanowski JQ: Total and phosphorylated tau
protein as biological markers of Alzheimer’s disease. Exp Gerontol.
45:30–40. 2010. View Article : Google Scholar
|
|
11
|
Delacourte A, Robitaille Y, Sergeant N,
Buée L, Hof PR, Wattez A, Laroche-Cholette A, Mathieu J, Chagnon P
and Gauvreau D: Specific pathological Tau protein variants
char-acterize Pick’s disease. J Neuropathol Exp Neurol. 55:159–168.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flament S, Delacourte A, Verny M, Hauw JJ
and Javoy-Agid F: Abnormal Tau proteins in progressive supranuclear
palsy. Similarities and differences with the neurofibrillary
degeneration of the Alzheimer type. Acta Neuropathol. 81:591–596.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ksiezak-Reding H, Morgan K, Mattiace LA,
Davies P, Liu WK, Yen SH, Weidenheim K and Dickson DW:
Ultrastructure and biochemical composition of paired helical
filaments in cortico-basal degeneration. Am J Pathol.
145:1496–1508. 1994.PubMed/NCBI
|
|
14
|
Chen C, Shi Q, Zhang BY, Wang GR, Zhou W,
Gao C, Tian C, Mei GY, Han YL, Han J and Dong XP: The prepared tau
exon-specific antibodies revealed distinct profiles of tau in CSF
of the patients with Creutzfeldt-Jakob disease. PLoS One.
5:e118862010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Q, Xie WL, Zhang B, Chen LN, Xu Y,
Wang K, Ren K, Zhang XM, Chen C, Zhang J and Dong XP: Brain
microglia were activated in sporadic CJD but almost unchanged in
fatal familial insomnia and G114V genetic CJD. Virol J. 10:2162013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao C, Lei YJ, Han J, Shi Q, Chen L, Guo
Y, Gao YJ, Chen JM, Jiang HY, Zhou W and Dong XP: Recombinant
neural protein PrP can bind with both recombinant and native
apolipoprotein E in vitro. Acta Biochim Biophys Sin (Shanghai).
38:593–601. 2006. View Article : Google Scholar
|
|
17
|
Zhang J, Chen L, Zhang BY, Han J, Xiao XL,
Tian HY, Li BL, Gao C, Gao JM, Zhou XB, et al: Comparison study on
clinical and neuropathological characteristics of hamsters
inoculated with scrapie strain 263K in different challenging
pathways. Biomed Environ Sci. 17:65–78. 2004.PubMed/NCBI
|
|
18
|
Himmler A: Structure of the bovine tau
gene: Alternatively spliced transcripts generate a protein family.
Mol Cell Biol. 9:1389–1396. 1989.PubMed/NCBI
|
|
19
|
Nelson PT, Stefansson K, Gulcher J and
Saper CB: Molecular evolution of tau protein: implications for
Alzheimer’s disease. J Neurochem. 67:1622–1632. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buée L, Bussière T, Buée-Scherrer V,
Delacourte A and Hof PR: Tau protein isoforms, phosphorylation and
role in neurodegenerative disorders. Brain Res Brain Res Rev.
33:95–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goedert M, Spillantini MG, Potier MC,
Ulrich J and Crowther RA: Cloning and sequencing of the cDNA
encoding an isoform of microtubule-associated protein tau
containing four tandem repeats: Differential expression of tau
protein mRNAs in human brain. EMBO J. 8:393–399. 1989.PubMed/NCBI
|
|
22
|
Hong M, Zhukareva V, Vogelsberg-Ragaglia
V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D,
Goate A, et al: Mutation-specific functional impairments in
distinct tau isoforms of hereditary FTDP-17. Science.
282:1914–1917. 1998. View Article : Google Scholar : PubMed/NCBI
|