Introduction
Recently, steroid-related osteonecrosis of the
femoral head (ONFH) has been reported to occur in patients with
collagen vascular diseases following high-dose steroid treatment
(1). Several treatment strategies
for ONFH have been implented, including drug therapy and surgery.
However, these methods have barely been effective in treating the
disease. As steroid-related ONFH usually occurs in young and
middle-aged patients and is associated with a poor prognosis
(2), it is important to examine
the pathophysiology of steroid-related ONFH and develop a
preventive strategy.
Bone marrow-derived mesenchymal stem cells (BMMSCs)
are multipotent cells that can differentiate into adipocytes or
osteocytes (3). Changes in the
functional characteristics of the differentiation pathway of BMMSCs
may contribute to the pathogenesis of ONFH (4). Furthermore, interactions involving
the differentiation pathway of BMMSCs that promote adipogenesis and
decrease osteogenesis, are considered to be the major factors
leading to steroid-related ONFH (5). Adipogenesis and osteogenesis share
an inverse relationship in the bone marrow during the
differentiation of BMMSCs (6). In
ONFH, BMMSCs may differentiate preferentially into adipocytes
rather than osteoblasts (7).
Thus, the enhancement of osteogenesis coupled with a reduction in
adipogenesis may provide a potential therapeutic strategy for the
treatment of ONFH.
Wnt/β-catenin signaling functions as a molecular
switch that determines the balance between osteogenesis and
adipogenesis (8). Moreover,
Wnt/β-catenin signaling is reportedly activated in
osteoblast-committed MSCs, further indicating that it controls the
balance between the osteogenesis and adipogenesis of MSCs (9). β-catenin is phosphorylated by a
complex composed of adenomatous polyposis coli protein, glycogen
synthase kinase 3β (GSK-3β) and axin. Following phosphory-lation,
β-catenin is subsequently decomposed (10,11). Lithium, which has been used in the
treatment of bipolar disorder for years (12), may be used to inactivate GSK-3β,
thus resulting in the accumulation of cytoplasmic β-catenin
(13). Galli et al
reported that lithium chloride (LiCl) enhanced osteogenesis in
osteoblast-like MC3T3-E1 mouse cells (14). In addition, Itoigawa et al
and Sen et al demonstrated that LiCl reduces the efficacy of
adipogenic induction (15,16).
However, LiCl has rarely been used in the treatment of ONFH, and
the detailed mechanisms remain to be elucidated.
The benefits of LiCl are manifold; based on this
fact, we thus hypothesized that LiCl may attenuate the abnormal
osteogenic and adipogenic differentiation of BMMSCs obtained from
rats with steroid-related ONFH through the activation of the
β-catenin pathway.
Materials and methods
Animals
All the experimental procedures adhered to the
recommendations of the Experimental Animal Center of Xi'an Jiaotong
University, Xi'an, China, and were approved by the Ethics Committee
of Xi'an Jiaotong University. A total of 45 male Sprague Dawley
rats weighing 190-210 g were obtained from the Experimental Animal
Center of Xi'an Jiaotong University. All the animals were housed
under specific pathogen-free conditions in a clean, temperature and
humidity-controlled environment with unlimited access to food and
water and with a 12-h light/dark cycle.
Rat model of ONFH
A total of 30 rats underwent sequential drug
administration to establish the ONFH model of ONFH as previously
described (17). The remaining 15
rats were used as the normal controls. Briefly, the 30 rats were
administered 2 mg/kg lipopolysaccharide (Sigma, St. Louis, MO, USA)
intravenously for 2 days. One day later, 20 mg/kg of
methylprednisolone (Pfizer, Inc., New York, NY, USA) was injected
intramuscularly 3 times for 24 h. All the animals were sacrificed
(via an intravenous injection of excess phenobarbital sodium) for
the isolation of the BMMSCs 4 weeks after the final day of drug
administration.
Preparation of rat BMMSCs
The BMMSCs were prepared as previously described
(18). Briefly, BMMSCs were
obtained from the femoral shafts of the rats (from the rats with
ONFH and the normal rats) after the muscles and extraosteal tissues
were trimmed. Bone marrow was flushed and centrifuged on a 1.073
g/ml Percoll density gradient (Pharmacia, St. Louis, MO, USA). The
BMMSCs obtained from the rats with steroid-related ONFH are hereon
referred to as ONFH-BMMSCs. The cells were washed with PBS (Wuhan
Boster Biological Technology, Ltd., Wuhan, China), seeded into
25-cm2 cell culture flasks, and cultivated in L-DMEM
(Sigma) supplemented with 10% FBS (Sigma) and 20 mg
penicillin-streptomycin/ml (Sigma) in a humidified 5%
CO2 atmosphere at 37°C. The medium was changed every 3
days. When the cells became subconfluent, the cells were released
from the culture substratum using trypsin/EDTA (0.25% trypsin and
0.02% EDTA) (Sigma). The cell surface molecules, CD44 and CD34,
were analyzed on 3 cultures by flow cytometry (FACSCalibur; Becton,
Dickinson and Company, Franklin Lakes, NJ, USA).
ONFH-BMMSC viability assay
The ONFH-BMMSCs were plated into 96-well plates at a
density of 2,000 cells/well. Following 4 h of incubation, some of
the plates containing the ONFH-BMMSCs were treated with various
concentrations of LiCl (1, 5, 10 and 20 mM; Sigma). The cells were
then incubated at 37°C for 5 days. Following incubation, the cells
were treated with methyl thiazolyl tetrazolium (MTT; 0.5 mg/ml) for
4 h at 37°C. Following solubilization with dimethyl sulfoxide, the
absorbance was recorded at 570 nm using a microplate
spectrophotometer (SpectraMax; Molecular Devices LLC, Sunnyvale,
CA, USA).
Analysis of osteogenic
differentiation
The BMMSCs were trypsinized and replated in a 6-well
plate at a concentration of 2×105 cells/well. The medium
was replaced with osteogenic medium (low-glucose DMEM containing 5%
FBS, 10 nM dexamethasone, 50 µM L-ascorbic acid-2-phosphate
and 10 mM glycerophosphate; all from Sigma). The medium was added
every other day, and the cells were maintained at 37°C in a
humidified 5% CO2 atmosphere. The differentiating cells
were treated for 7, 14 and 21 days with osteogenic medium alone or
medium containing 5 mM LiCl. The rat BMMSCs were divided into the
following 3 groups: i) the normal group; ii) the ONFH-BMMSC group;
and iii) the LiCl + ONFH-BMMSC group. The expression levels of
osteocalcin (OCN) and Runx2 were measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
After 21 days, mineralization was measured using
Alizarin Red S (Sigma) staining. Briefly, the BMMSC cultures were
fixed in a 4% paraformaldehyde solution and stained with a 0.5%
Alizarin Red (Sigma) solution. We added 1 ml of 10% cetylpyridinium
chloride (Sigma) for quantitative analysis. The light absorbance of
the extracted dye was measured using a spectrophotometer (UV-1800:
Shimadzu, Kyoto, Japan) at 570 nm.
Analysis of adipogenic
differentiation
The BMMSCs were seeded in 6-well plates at a
concentration of 2×105 cells/well and cultured in
high-glucose DMEM supplemented with 10% FBS, 1%
penicillin-streptomycin, 10 µg/ml insulin, 1 µM
dexamethasone and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; all
from Sigma) at 37°C in a humidified 5% CO2 atmosphere.
The medium was changed every 2 days. The differentiating cells were
treated for 3, 7 and 14 days with adipogenic medium alone or medium
containing 5 mM LiCl. The expression levels of
proliferator-activated receptor γ (PPARγ) and fatty acid binding
protein 4 (Fabp4) were measured by RT-qPCR.
After 14 days, the BMMSCs were stained using Oil Red
O. Briefly, the cells were fixed with 10% formalin. The cells were
then washed with 60% isopropyl alcohol and stained with 2% Oil Red
O reagent (Sigma). The staining was quantified by extracting the
Oil Red O stain with 100% isopropyl alcohol, after which the
absorbance was measured using a spectrophotometer (UV-1800;
Shimadzu) at 510 nm.
RT-qPCR
Briefly, total cellular RNA was extracted from the
cultured cells using TRIzol reagent. Total RNA was then reverse
transcribed into cDNA using an RT-PCR mixture (Takara Bio, Otsu,
Japan). qPCR was performed in a reaction mixture containing
SYBR-Green PCR Master Mix (Roche Diagnostics, Basel, Switzerland).
β-actin was used as an internal control. The following primers were
used in the present study: OCN sense, 5′-GAC AAG TCC CAC ACA GCA
AC-3′ and antisense, 5′-CCG GAG TCT ATT CAC CAC CT-3′; Runx2 sense,
5′-TCA CAA ATC CTC CCC AAG TGG-3′ and anti-sense, 5′-GAA TGC GCC
CTA AAT CAC TGA-3′; PPARγ sense, 5′-TCC TCC TGT TGA CCC AGA GCA
T-3′ and anti-sense, 5′-AGC TGA TTC CGA AGT TGG TGG-3′; Fabp4
sense, 5′-GGA ATT CGA TGA AAT CAC CCC-3′ and anti-sense, 5′-TGG TCG
ACT TTC CAT CCC ACT-3′; and β-actin sense, 5′-AGT ACC CCA TTG AAC
ACG GC-3′ and antisense, 5′-TTT TCA CGG TTA GCC TTA GG-3′. The
results were analyzed using SDS 2.0 software (Life Technologies,
Grand Island, NY, USA).
Western blot analysis
To measure the protein expression of phosphorylaed
GSK-3β at Tyr-216 (p-Tyr216 GSK-3β) and β-catenin, we conducted
western blot analysis. Total proteins were separated on 10%
SDS-PAGE gels and then transferred onto polyvinylidene fluoride
blotting membranes. After blocking non-specific binding with 5% BSA
in Tween-Tris-buffered saline, the membranes were incubated with
the following primary antibodies: anti-p-Tyr216 GSK-3β (ab75745;
1:500 dilution; Abcam, Cambridge, CA, USA), anti-β-catenin
(ab32572; 1:1,000 dilution; Abcam) and anti-β-actin (BM0627; 1:200
dilution; Wuhan Boster Biological Technology, Ltd.) antibodies
overnight. The membranes were then incubated for 2 h with secondary
antibodies (ZDR-5306, 1:2,000 dilution; Zhongshan Golden Bridge
Biotechnology, Co., Ltd., Beijing, China) labeled with horseradish
peroxidase. The immunoreactive proteins on the blots were
visualized using ECL™ western blot detection reagents, and the
signals were detected using Image Station 4000R (Kodak, Rochester,
NY, USA).
Inhibition of β-catenin pathway
activation
Quercetin (Que; Calbiochem, San Diego, CA, USA), an
inhibitor of the β-catenin pathway (19,20), was used to further investigate the
role of LiCl in the β-catenin pathway. The ONFH-BMMSCs were treated
with 5 mM LiCl in the presence of Que (50 µM) and then
underwent osteogenic and adipogenic differentiation. The LiCl +
ONFH-BMMSC and ONFH-BMMSC groups were used as the controls in this
experiment. The mRNA expression levels of Runx2 and PPARγ were
measured by RT-qPCR at the end of induction. The expression levels
of OCN and Fabp4 were measured by immunofluorescence. Briefly, the
BMMSCs were fixed and treated with 50 µg/ml 4′,
6-diamidino-2-phenylindole (DAPI) for nuclear staining. The primary
antibodies were diluted 1:100 [anti-OCN (ab13418) and anti-Fabp4
(ab92501), from Abcam]. The cells were then stained with anti-OCN
and anti-Fabp4 antibodies and visualized with a secondary antibody
conjugated with tetraethyl rhodamine isothiocyanate (TRITC). The
controls were also stained, but without the primary antibodies.
Fluorescence images were acquired using a fluorescence microscope
(FluoView 500; Olympus, Tokyo, Japan). Data were analyzed using
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). Data analysis was performed using SPSS 13.0
software (SPSS Inc., Chicago, Il, USA). One-way analysis of
variance followed by an LSD test was used for comparisons among the
experimental groups. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Characterization of rat BMMSCs
The BMMSCs were successfully and rapidly expanded
into colonies of confluent spindle-shaped cells. The results of
flow cytometry for CD34 and CD44 revealed that the cells were
negative for CD34 (0.57%) and positive for CD44 (99.43%) (data not
shown). The cultured cells were thus considered BMMSCs.
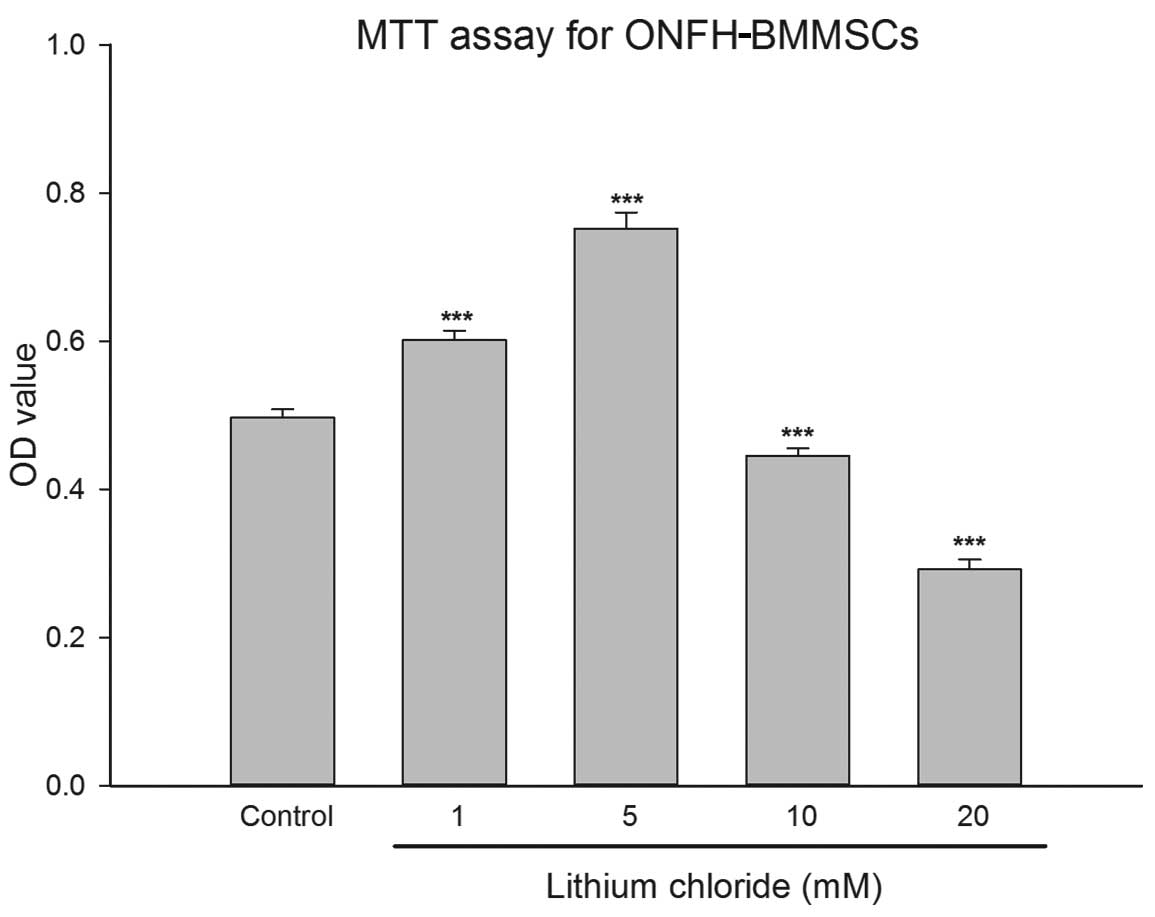
Effect of LiCl on the viability of
ONFH-BMMSCs
We tested a range of LiCl concentrations to
determine the optimal concentration to treat the ONFH-BMMSCs with.
The OD value provided by the MTT assay in the low-dose LiCl-treated
groups (1 and 5 mM) was significantly higher than that of the
control group (P<0.001; Fig.
1). Treatment with LiCl at up to 5 mM increased cell viability
in a dose-dependent manner. On the contrary, in the high-dose
LiCl-treated groups (10 and 20 mM), LiCl exerted an inhibitory
effect on cell viability (P<0.001; Fig. 1).
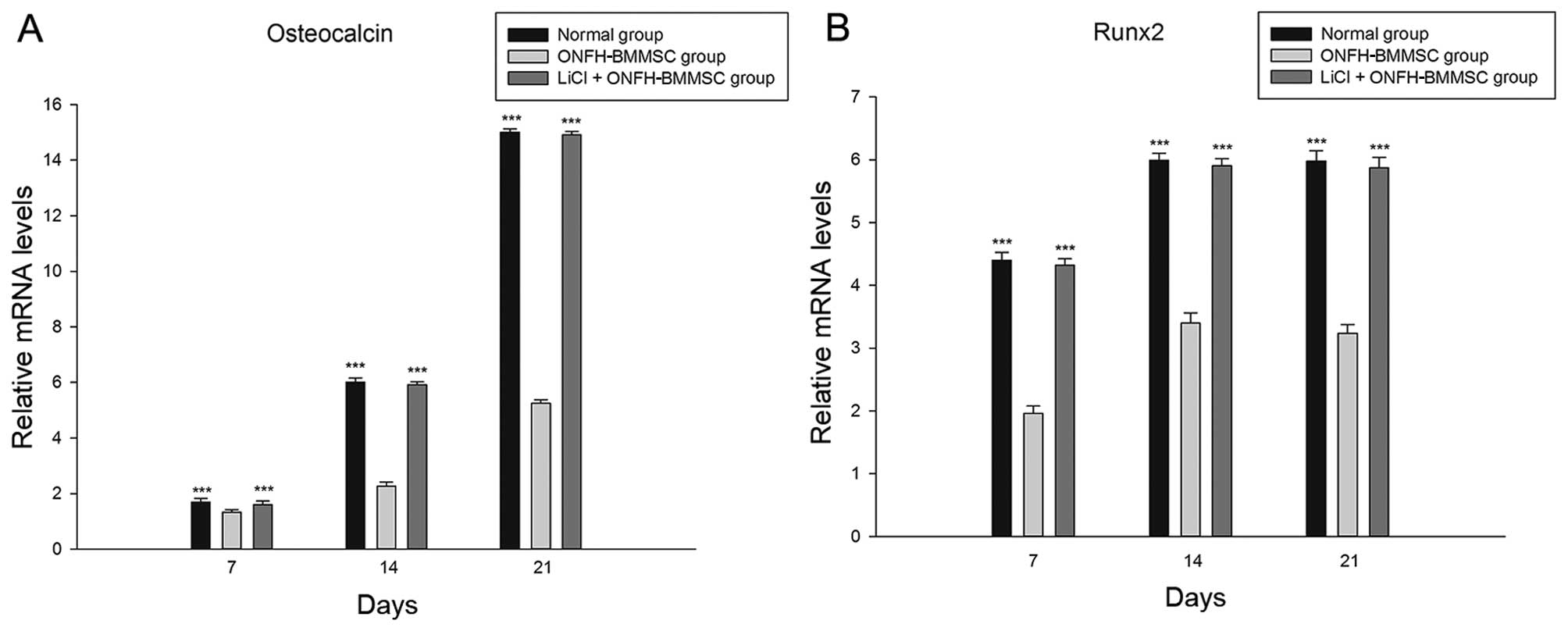
LiCl enhances the osteogenesis of
ONFH-BMMSCs
The results revealed that the 5 mM LiCl treatment
group and the normal group exhibited a significant increase in the
mRNA expression of OCN and Runx2 in a time-dependent manner
compared with the ONFH-BMMSC group (P<0.001; Fig. 2). Furthermore, we found that the
OCN and Runx2 mRNA levels did not significantly differ between the
LiCl + ONFH-BMMSC group and the normal BMMSC group (P>0.05;
Fig. 2).
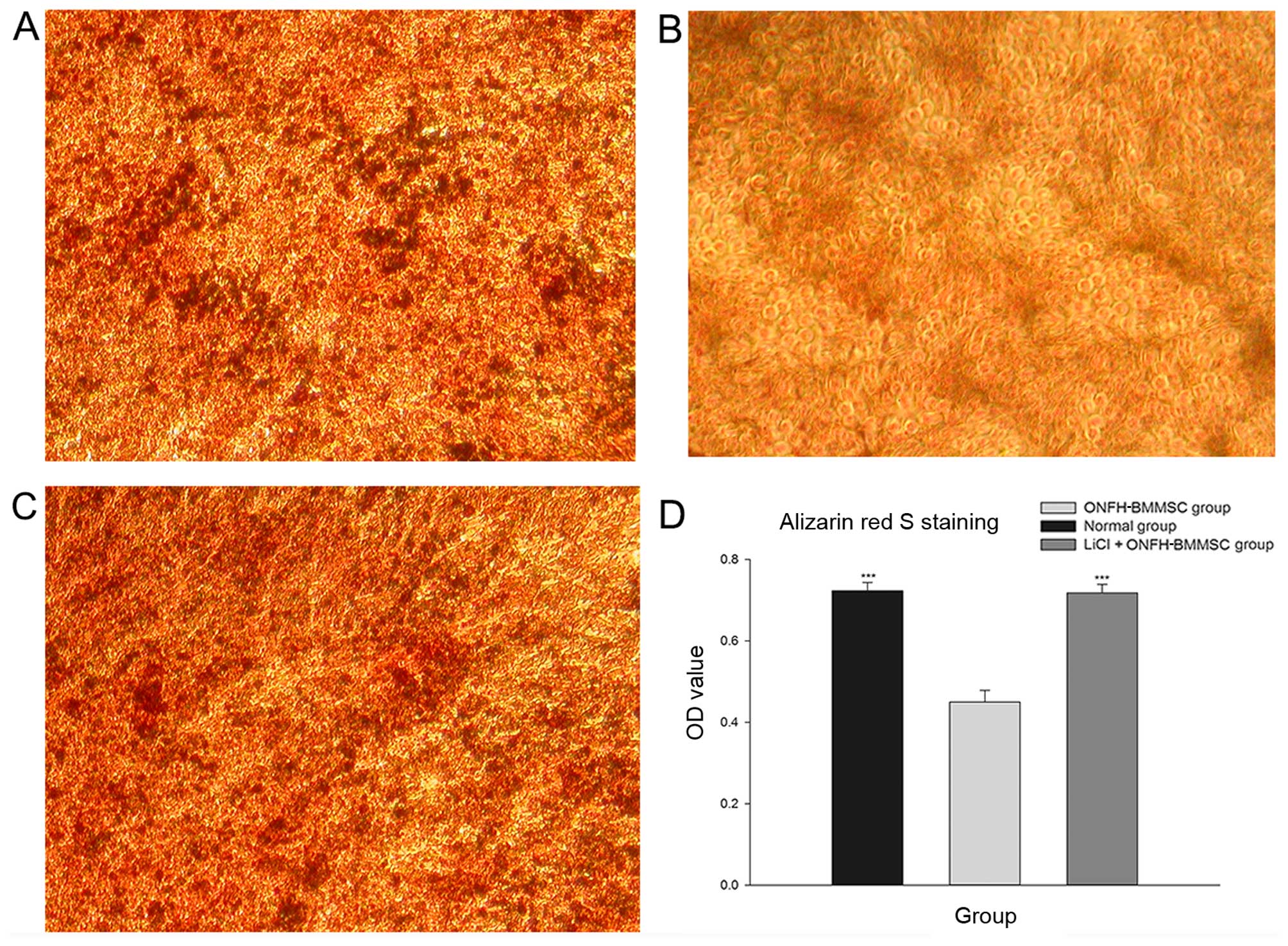
The results revealed that the normal and the LiCl +
ONFH-BMMSC groups presented with a greater number of mineralized
nodules than the ONFH-BMMSC group (Fig. 3A-C). Furthermore, the light
absorbance analysis revealed that the normal and LiCl + ONFH-BMMSC
groups had significantly higher OD values than the ONFH-BMMSC group
(P<0.001); by contrast, the OD values did not differ between the
normal and the LiCl + ONFH-BMMSC groups (P>0.05; Fig. 3D).
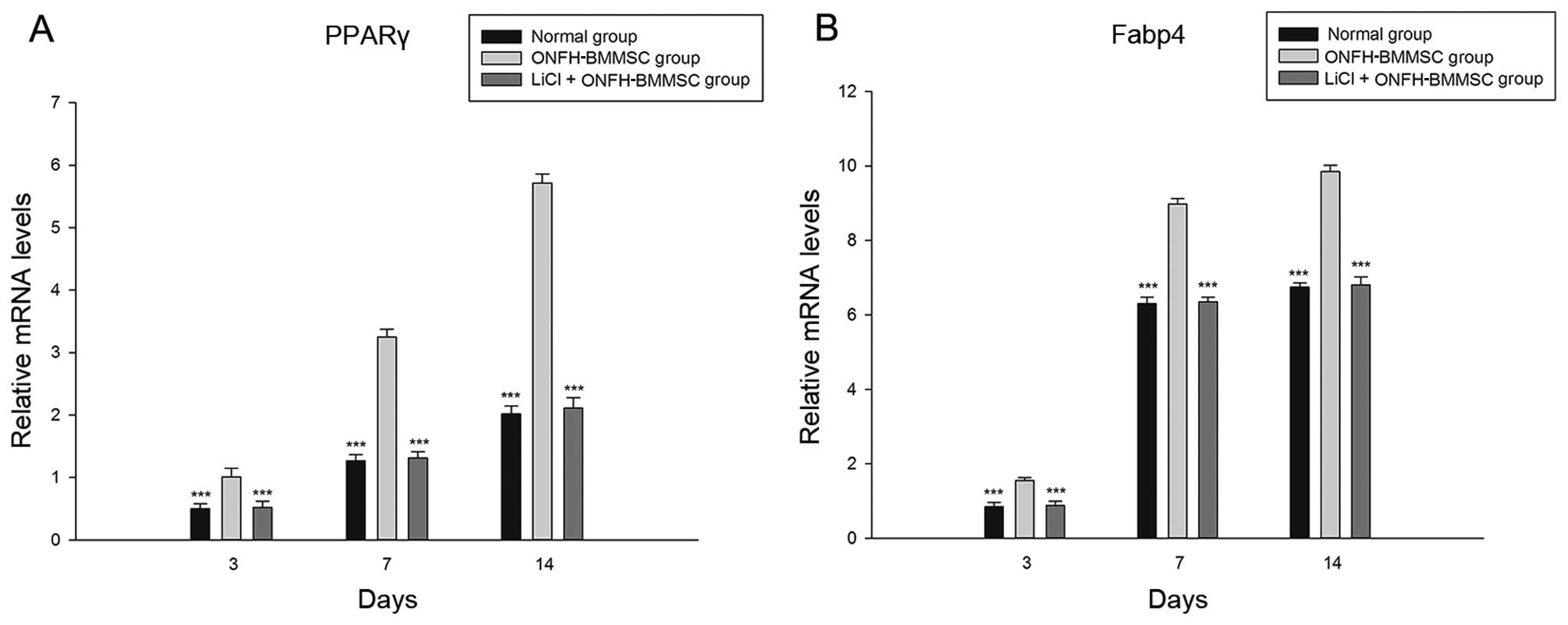
LiCl reduces the adipogenesis of
ONFH-BMMSCs
The LiCl treatment group and normal group exhibited
a significant decrease in the mRNA expression of PPARγ and Fabp4
compared with the ONFH-BMMSC group (P<0.001, Fig. 4). Furthermore, we found that the
LiCl treatment group showed no statistically significant
differences in the PPARγ and Fabp4 mRNA expression levels compared
with the normal BMMSC group (P>0.05; Fig. 4).
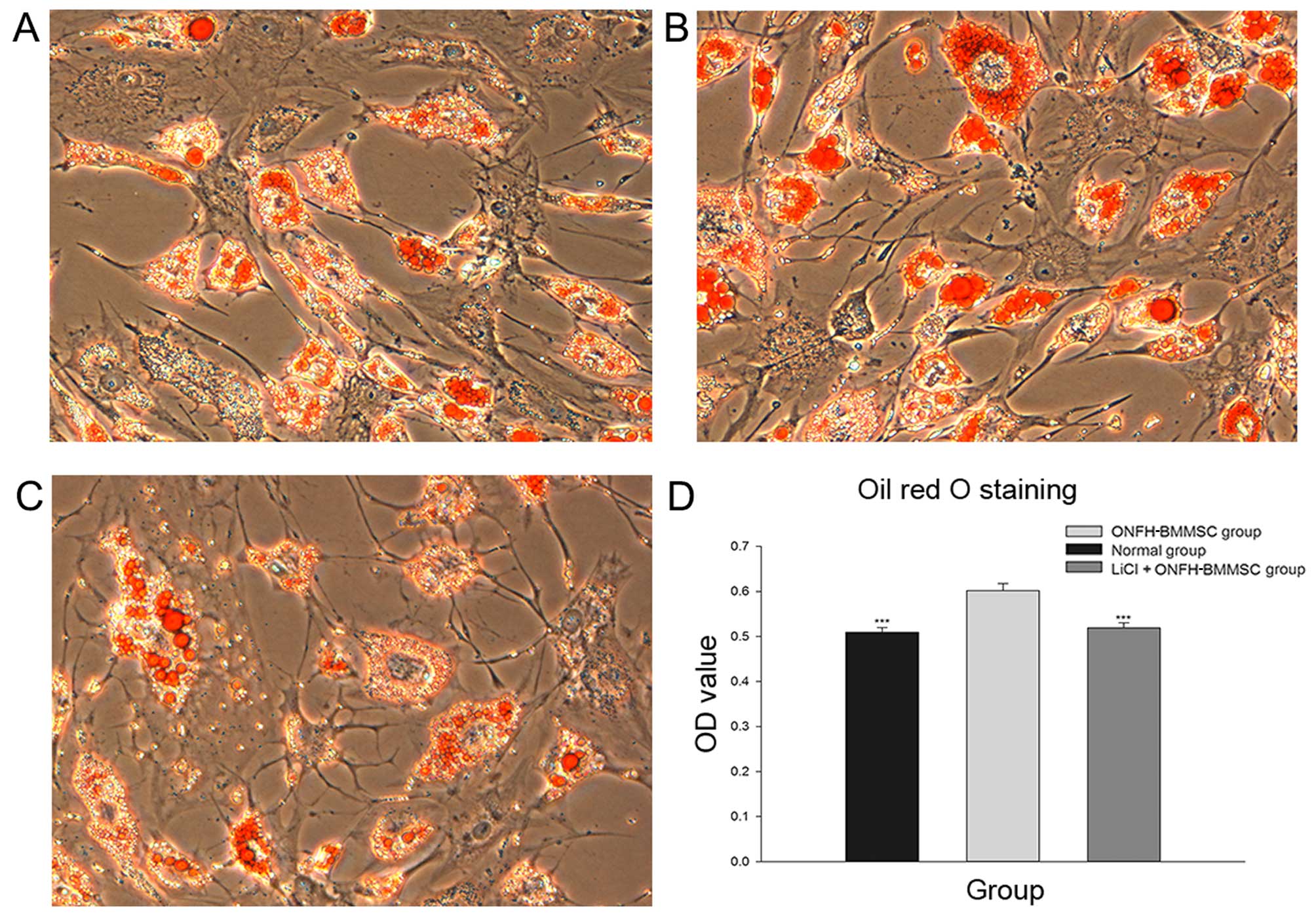
The results obtained from Oil Red O staining
revealed that the normal and LiCl + ONFH-BMMSC groups presented
with fewer lipid droplets than the ONFH-BMMSC group (Fig. 5A-C). Moreover, the light
absorbance analysis revealed that the normal and LiCl + ONFH-BMMSC
groups had significantly lower OD values than the ONFH-BMMSC group
(P<0.001), while the OD values between the normal and the LiCl +
ONFH-BMMSC groups did not differ (P>0.05; Fig. 5D).
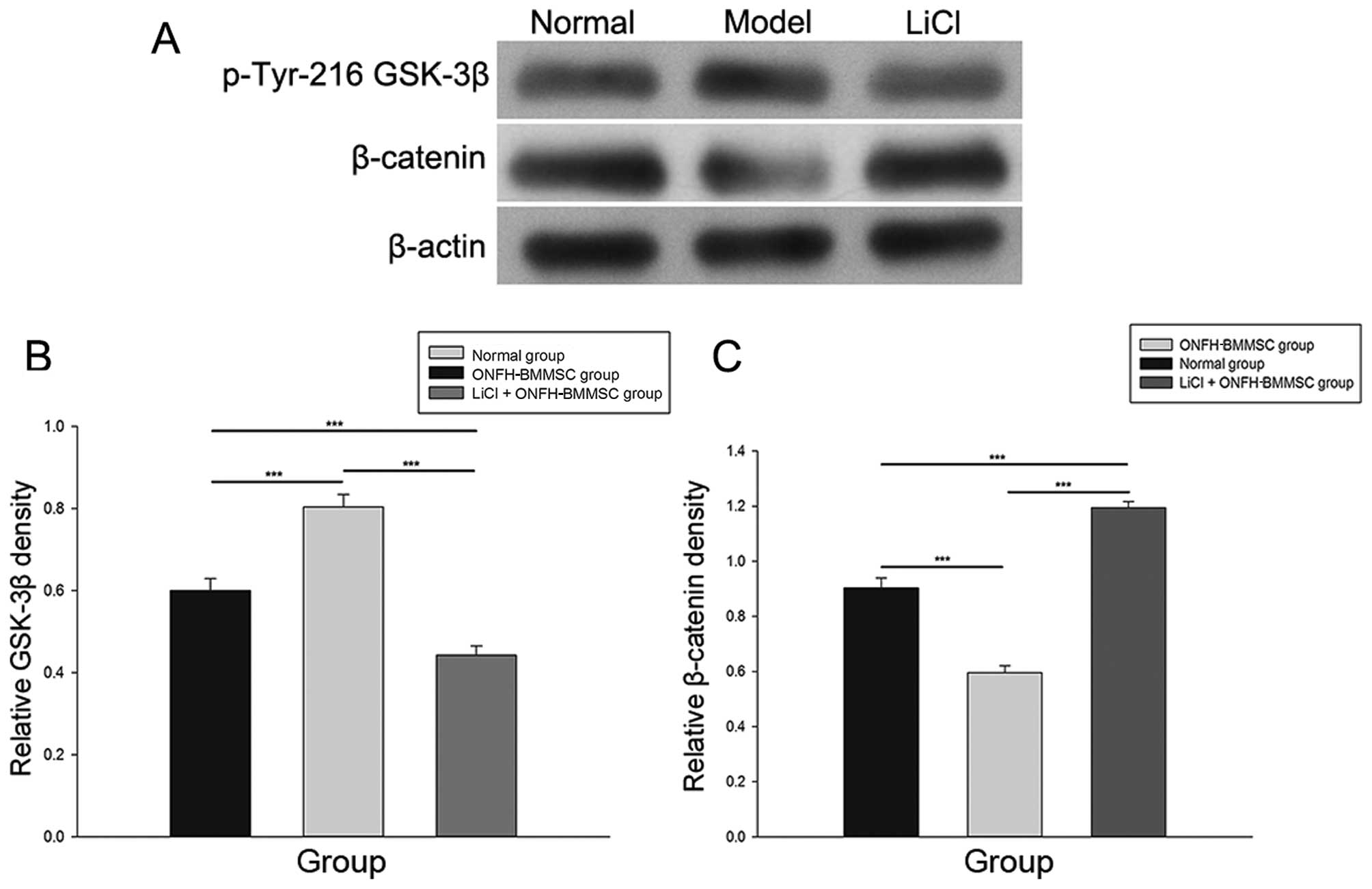
LiCl activates the β-catenin pathway
As shown in Fig.
6, treatment with LiCl markedly increased the expression of
β-catenin and decreased the p-Tyr216 GSK-3β protein levels compared
with the ONFH-BMMSC group (P<0.001). The normal group exhibited
a higher expression of β-catenin and a lower expression of p-Tyr216
GSK-3β compared with the ONFH-BMMSC group (P<0.001).
Inhibition of β-catenin pathway
diminishes the effects of LiCl
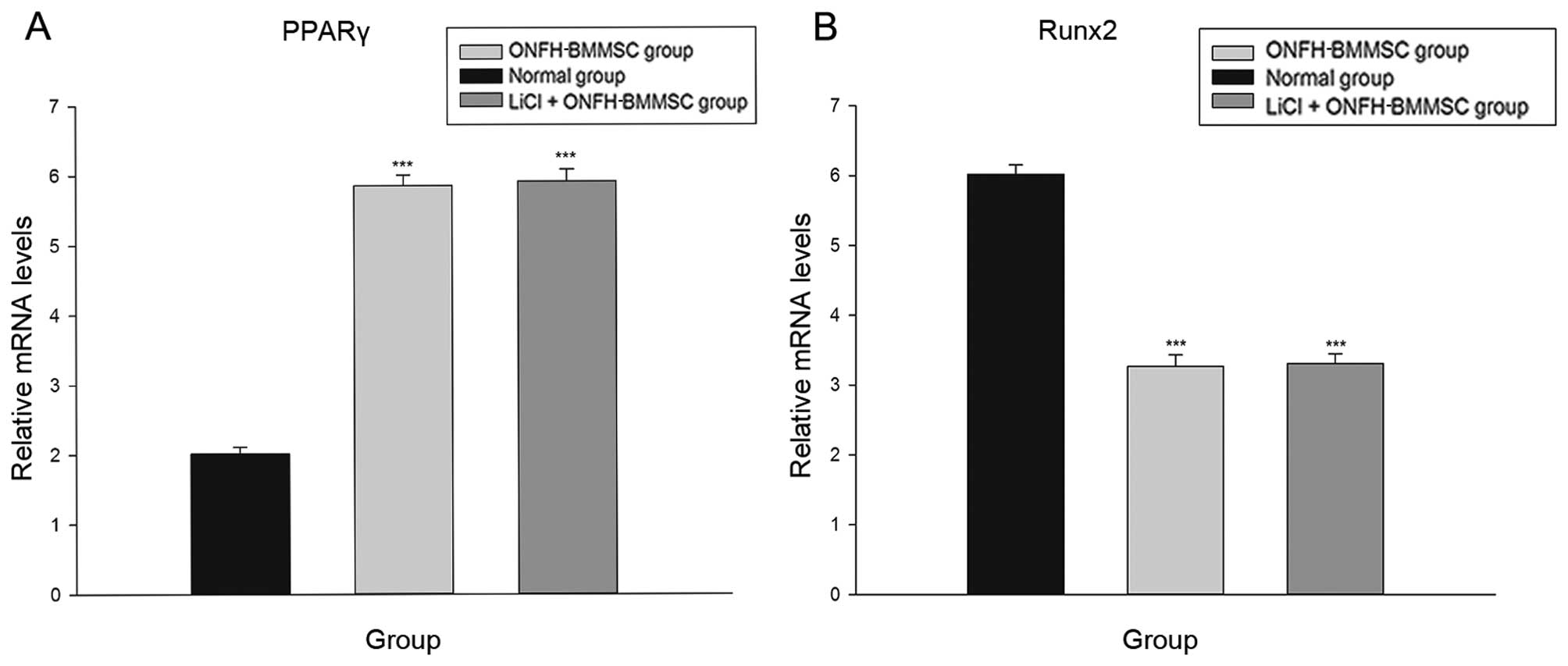
The LiCl + Que + ONFH-BMMSC and ONFH-BMMSC groups
exhibited a significant increase in the mRNA expression of PPARγ
compared with the LiCl + ONFH group (P<0.001; Fig. 7A). By contrast, the LiCl + Que +
ONFH-BMMSC and ONFH-BMMSC groups exhibited a statistically
significant decrease in the mRNA expression of Runx2 (P<0.001;
Fig. 7B). Moreover, the LiCl +
Que + ONFH-BMMSC group exhibited no statistically significant
difference in PPARγ and Runx2 mRNA levels compared with the
ONFH-BMMSC group (P>0.05; Fig.
7).
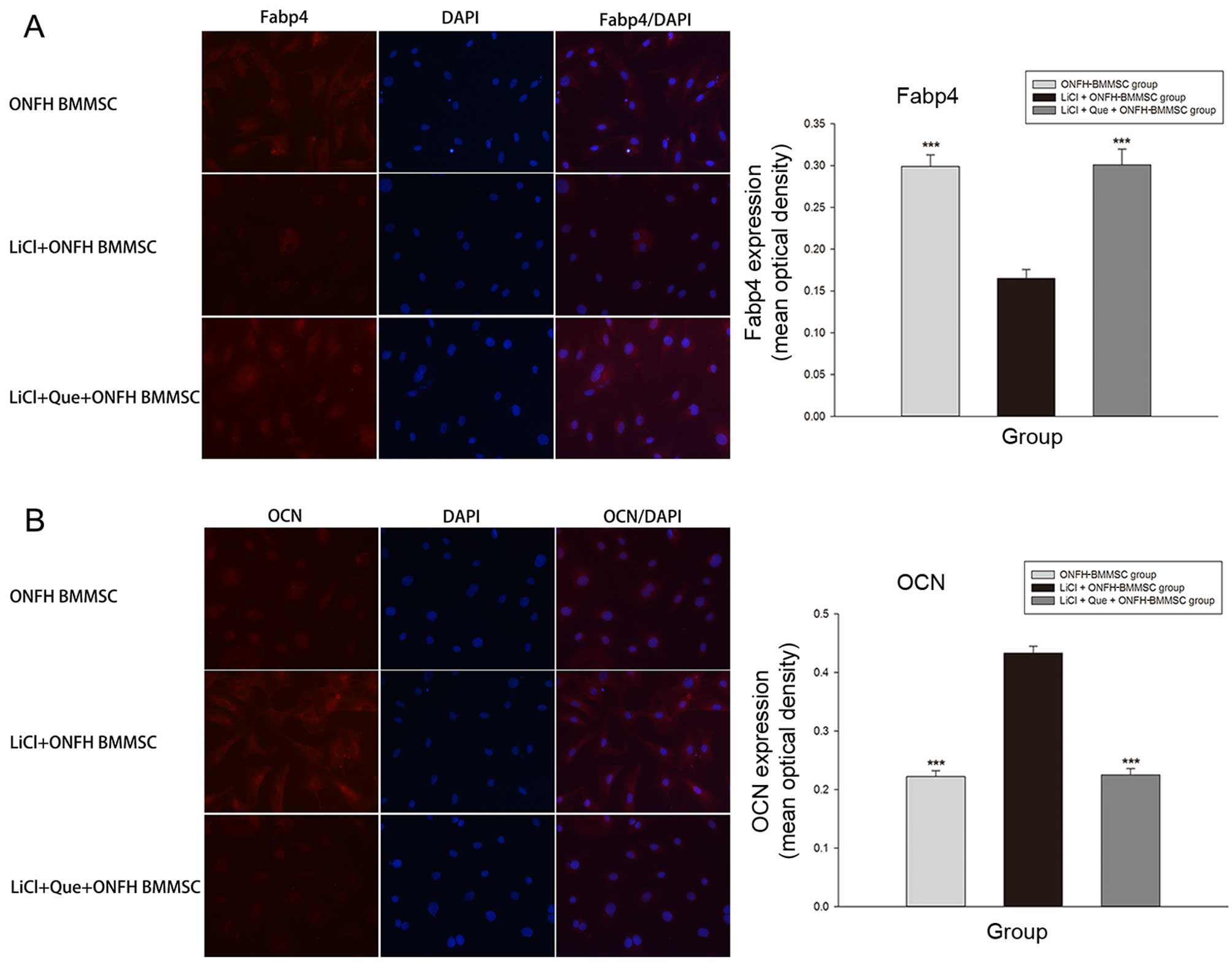
Immunofluorescence staining revealed that the LiCl +
Que + ONFH-BMMSC and ONFH-BMMSC groups exhibited a significant
increase in Fabp4 expression compared with the LiCl + ONFH group
(P<0.001; Fig. 8A). By
contrast, the LiCl + Que + ONFH-BMMSC and ONFH-BMMSC groups
exhibited a significantly lower expression of OCN (P<0.001;
Fig. 8B). Moreover, we also found
that the LiCl + Que + ONFH-BMMSC group exhibited no statistically
significant difference in Fabp4 and OCN expression compared with
the ONFH-BMMSC group (P>0.05; Fig.
8).
Discussion
ONFH is a common degenerative disease that mostly
occurs in young and middle age. The disease critically affects the
quality of life of patients and is associated with a high
morbidity. Hernigou et al demonstrated that the decreased
activity of BMMSCs may contibute to the pathogenesis of ONFH
(4). In a prevoius study, in
patients who developed osteonecrosis following treatment with
corticosteroids, abnormal adipogenesis was discovered in the bone
marrow, with a decreased number of osteogenic cells (21). These findings indicate that
steroids may disrupt the osteogenic/adipogenic activity of stem
cells in ONFH.
To explore the potential impairment in the
osteogenic/adipogenic activity of BMMSCs in ONFH, we established a
rat model of steroid-related ONFH previously reported by Chen et
al (17). According to their
study, the necrosis generated in this model is similar to that
observed in steroid-treated patients. In the present study,
Alizarin Red S staining determined the osteogenic function of
BMMSCs in the model group, which was impaired compared with the
normal BMMSCs. By contrast, Oil Red O staining indicated that the
adipogenic function of BMMSCs isolated from rats with
steroid-related ONFH was enhanced compared with normal BMMSCs.
These results indicated that the differentiation of ONFH-BMMSCs was
impaired, which may be the cause of steroid-related ONFH.
Lithium has been used in the treatment of bipolar
disorder for decades, and its safety has been well confirmed
(22). Studies have reported that
lithium inhibits the adipogenic effect (15,16). Mai et al also confirmed the
anti-adipogenic effects of LiCl (23). LiCl has also been reported to
stimulate osteoblast differentiation (14). However, it remains unknown as to
whether lithium can reverse the abnormal osteogenic/adipogenic
activity of BMMSCs from rats with steroid-related ONFH. In the
present study, we examined the possibility of using LiCl to reverse
the abnormal differentiation of ONFH-BMMSCs. The effects of lithium
have been reported to be highly dependent on its concentration
(24,25); thus, we tested a series of LiCl
concentrations to find the optimal concentration. We found that 5
mM LiCl exerted the greatest stimulatory effect on the viability of
ONFH-BMMSCs. Thus, we selected 5 mM LiCl as the optimal
concentration.
Runx2 is essential for osteoblast differentiation
and skeletal morphogenesis. Moreover, OCN, also known as bone
gamma-carboxyglutamic acid-containing protein (BGLAP), is a
non-collagenous protein found in bone (26). It has been implicated in bone
mineralization and calcium ion homeostasis (27). In the present study, we found the
decreased expression of Runx2 and OCN in the ONFH-BMMSCs,
suggesting that steroid-related ONFH reduces the osteogenic
activity of BMMSCs. Following treatment with LiCl, the expression
of Runx2 and OCN significantly increased, and this was consistent
with the results of Alizarin Red S staining. These results indicate
that LiCl can reverse the abnormal osteogenic differentiation of
ONFH-BMMSCs.
By contrast, PPARγ, as a member of the nuclear
receptor gene superfamily of ligand-activated transcription
factors, functions as a stimulator of bone marrow adipogenesis and
an inhibitor of osteogenesis. Fabp4 is a carrier protein for fatty
acids that is considered to be a terminal differentiation marker
for adipocytes (28). In the
present study, we found the increased expression of PPARγ and Fabp4
in the BMMSCs from rats with steroid-related ONFH, indicating that
steroid-related ONFH may cause BMMSCs to change into an adipogenic
phenotype. Following treatment with LiCl, the expression of PPARγ
and Fabp4 significantly decreased. These results were consistent
with those of Oil Red O staining, suggesting that lithium
attenuates the impairment of excessive adipogenic differentiation
of ONFH-BMMSCs.
Lithium, as used in the present study, is known to
be a GSK-3β inhibitor that can raise the β-catenin concentration
(14). The Wnt/β-catenin pathway
has been reported to inhibit adipocyte differentiation (29), as well as enhance osteoblast
differentiation (30). As a key
component of this pathway, the activation of β-catenin leads to the
direct suppression of PPARγ and the prevention of 3T3-L1 cell
adipogenic differentiation (31,32). By contrast, the disruption of the
β-catenin pathway increases adipogenesis (33) and reduces bone formation (29). In addition, GSK-3β activity is
regulated by phosphorylation and Tyr216 phosphorylation increases
its activity (34) and Garza
et al (35) reported that
steroids inhibit GSK-3β/β-catenin signaling by activating GSK-3β.
This activation inhibited the activity of β-catenin (35) and was consistent with our present
findings that the ONFH-BMMSCs had decreased levels of β-catenin and
increased levels of p-Tyr216 GSK-3β (active form of GSK-3β)
compared with the normal BMMSCs. The results of western blot
analysis in the present study revealed that LiCl, as a GSK-3β
inhibitor, activated β-catenin by reducing p-Tyr216 GSK-3β.
However, whether lithium attenuated the abnormal
osteogenic/adipogenic differentiation of ONFH-BMMSCs through the
β-catenin pathway remains uncertain. To further elucidate the
possible mechanisms of action of lithium in ONFH-BMMSCs, we used
Que, an inhibitor of the β-catenin pathway. We found that the
treatment effects of LiCl (the attenuation of the abnormal
osteogenic and adipogenic differentiation) on ONFH-BMMSCs were
abolished by treatment with Que. In this respect, we speculated
that lithium may act as a switch to reduce both the abnormal
adipogenic activity and increase the osteogenic differentiation of
ONFH-BMMSCs by activating the β-catenin pathway.
In conclusion, our data indicate that the normal
osteogenic/adipogenic activity of BMMSCs is impaired during
steroid-related ONFH. The present study also provides evidence to
suggest that LiCl attenuates the abnormal osteogenic/adipogenic
differentiation of BMMSCs obtained from rats with steroid-related
ONFH by activating the β-catenin signaling pathway. These findings
highlight the treatment effects of LiCl on steroid-related ONFH and
provide an effective therapeutic strategy.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81101363, 81371944
and 81572145) and the Fundamental Research Funds for the Central
Universities.
Abbreviations:
|
ONFH
|
osteonecrosis of the femoral head
|
|
BMMSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
GSK-3β
|
glycogen synthase kinase 3β
|
|
MSC
|
mesenchymal stem cell
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
OCN
|
osteocalcin
|
|
IBMX
|
3-isobutyl-1-methylxanthine
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
SDS-PAGE
|
sodium dodecyl sulphate-polyacrylamide
gel electrophoresis
|
|
QUE
|
quercetin
|
|
BGLAP
|
bone gamma-carboxyglutamic
acid-containing protein
|
References
|
1
|
Sun Z, Yang S, Ye S, Zhang Y, Xu W, Zhang
B, Liu X, Mo F and Hua W: Aberrant CpG islands' hypermethylation of
ABCB1 in mesenchymal stem cells of patients with steroid-associated
osteonecrosis. J Rheumatol. 40:1913–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lieberman JR, Berry DJ, Mont MA, Aaron RK,
Callaghan JJ, Rajadhyaksha AD and Urbaniak JR: Osteonecrosis of the
hip: management in the 21st century. Instr Course Lect. 52:337–355.
2003.PubMed/NCBI
|
|
3
|
Webster RA, Blaber SP, Herbert BR, Wilkins
MR and Vesey G: The role of mesenchymal stem cells in veterinary
therapeutics - a review. N Z Vet J. 60:265–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernigou P and Beaujean F: Abnormalities
in the bone marrow of the iliac crest in patients who have
osteonecrosis secondary to corticosteroid therapy or alcohol abuse.
J Bone Joint Surg Am. 79:1047–1053. 1997.PubMed/NCBI
|
|
5
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis (Egypt).
2012:6017632012.
|
|
6
|
Abdallah BM and Kassem M: New factors
controlling the balance between osteoblastogenesis and
adipogenesis. Bone. 50:540–545. 2012. View Article : Google Scholar
|
|
7
|
Sheng H, Sheng CJ, Cheng XY, Zhang G, Lee
KM, Leung KS, Qu S and Qin L: Pathomorphological changes of bone
marrow adipocytes in process of steroid-associated osteonecrosis.
Int J Clin Exp Pathol. 6:1046–1050. 2013.PubMed/NCBI
|
|
8
|
Taipaleenmäki H, Abdallah BM, AlDahmash A,
Säämänen AM and Kassem M: Wnt signalling mediates the cross-talk
between bone marrow derived pre-adipocytic and pre-osteoblastic
cell populations. Exp Cell Res. 317:745–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franceschi RT and Xiao G: Regulation of
the osteoblast-specific transcription factor, Runx2: responsiveness
to multiple signal transduction pathways. J Cell Biochem.
88:446–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Young W: Review of lithium effects on
brain and blood. Cell Transplant. 18:951–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wexler EM, Geschwind DH and Palmer TD:
Lithium regulates adult hippocampal progenitor development through
canonical Wnt pathway activation. Mol Psychiatry. 13:285–292. 2008.
View Article : Google Scholar
|
|
14
|
Galli C, Piemontese M, Lumetti S, Manfredi
E, Macaluso GM and Passeri G: GSK3b-inhibitor lithium chloride
enhances activation of Wnt canonical signaling and osteoblast
differentiation on hydrophilic titanium surfaces. Clin Oral
Implants Res. 24:921–927. 2013. View Article : Google Scholar
|
|
15
|
Itoigawa Y, Kishimoto KN, Sano H, Kaneko K
and Itoi E: Molecular mechanism of fatty degeneration in rotator
cuff muscle with tendon rupture. J Orthop Res. 29:861–866. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sen B, Xie Z, Case N, Ma M, Rubin C and
Rubin J: Mechanical strain inhibits adipogenesis in mesenchymal
stem cells by stimulating a durable beta-catenin signal.
Endocrinology. 149:6065–6075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Li J, Peng H, Zhou J and Fang H:
Administration of erythropoietin exerts protective effects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Int J Mol Med. 33:840–848. 2014.PubMed/NCBI
|
|
18
|
Panepucci RA, Siufi JLC, Silva WA Jr,
Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT and Zago
MA: Comparison of gene expression of umbilical cord vein and bone
marrow-derived mesenchymal stem cells. Stem Cells. 22:1263–1278.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boku S, Nakagawa S, Masuda T, Nishikawa H,
Kato A, Kitaichi Y, Inoue T and Koyama T: Glucocorticoids and
lithium reciprocally regulate the proliferation of adult dentate
gyrus-derived neural precursor cells through GSK-3beta and
beta-catenin/TCF pathway. Neuropsychopharmacology. 34:805–815.
2009. View Article : Google Scholar
|
|
20
|
Park CH, Chang JY, Hahm ER, Park S, Kim HK
and Yang CH: Quercetin, a potent inhibitor against beta-catenin/Tcf
signaling in SW480 colon cancer cells. Biochem Biophys Res Commun.
328:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui Q, Wang GJ and Balian G:
Pluripotential marrow cells produce adipocytes when transplanted
into steroid-treated mice. Connect Tissue Res. 41:45–56. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jope RS: Lithium and GSK-3: One inhibitor,
two inhibitory actions, multiple outcomes. Trends Pharmacol Sci.
24:441–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mai Y, Zhang Z, Yang H, Dong P, Chu G,
Yang G and Sun S: BMP and activin membrane-bound inhibitor (BAMBI)
inhibits the adipogenesis of porcine preadipocytes through
Wnt/beta-catenin signaling pathway. Biochem Cell Biol. 92:172–182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Boer J, Wang HJ and Van Blitterswijk C:
Effects of Wnt signaling on proliferation and differentiation of
human mesenchymal stem cells. Tissue Eng. 10:393–401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregory CA, Perry AS, Reyes E, Conley A,
Gunn WG and Prockop DJ: Dkk-1-derived synthetic peptides and
lithium chloride for the control and recovery of adult stem cells
from bone marrow. J Biol Chem. 280:2309–2323. 2005. View Article : Google Scholar
|
|
26
|
Makita N, Suzuki M, Asami S, Takahata R,
Kohzaki D, Kobayashi S, Hakamazuka T and Hozumi N: Two of four
alternatively spliced isoforms of RUNX2 control osteocalcin gene
expression in human osteoblast cells. Gene. 413:8–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin GT, Tseng HF, Chang CK, Chuang LY, Liu
CS, Yang CH, Tu CJ, Wang EC, Tan HF, Chang CC, et al: SNP
combinations in chromosome-wide genes are associated with bone
mineral density in Taiwanese women. Chin J Physiol. 51:32–41.
2008.PubMed/NCBI
|
|
28
|
Mackay DL, Tesar PJ, Liang LN and
Haynesworth SE: Characterizing medullary and human mesenchymal stem
cell-derived adipocytes. J Cell Physiol. 207:722–728. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XL, Wang N, Zheng LZ, Xie XH, Yao D,
Liu MY, Yao ZH, Dai Y, Zhang G, Yao XS and Qin L: Phytoestrogenic
molecule desmethylicaritin suppressed adipogenesis via
Wnt/β-catenin 1signaling pathway. Eur J Pharmacol. 714:254–260.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corrado A, Neve A, Macchiarola A, Gaudio
A, Marucci A and Cantatore FP: RANKL/OPG ratio and DKK-1 expression
in primary osteoblastic cultures from osteoarthritic and
osteoporotic subjects. J Rheumatol. 40:684–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ross SE, Hemati N, Longo KA, Bennett CN,
Lucas PC, Erickson RL and MacDougald OA: Inhibition of adipogenesis
by Wnt signaling. Science. 289:950–953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J and Farmer SR: Regulating the
balance between peroxisome proliferator-activated receptor gamma
and beta-catenin signaling during adipogenesis. A glycogen synthase
kinase 3beta phosphorylation-defective mutant of beta-catenin
inhibits expression of a subset of adipogenic genes. J Biol Chem.
279:45020–45027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bennett CN, Ross SE, Longo KA, Bajnok L,
Hemati N, Johnson KW, Harrison SD and MacDougald OA: Regulation of
Wnt signaling during adipogenesis. J Biol Chem. 277:30998–31004.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hughes K, Nikolakaki E, Plyte SE, Totty NF
and Woodgett JR: Modulation of the glycogen synthase kinase-3
family by tyrosine phosphorylation. EMBO J. 12:803–808.
1993.PubMed/NCBI
|
|
35
|
Garza JC, Guo M, Zhang W and Lu XY: Leptin
restores adult hippocampal neurogenesis in a chronic unpredictable
stress model of depression and reverses glucocorticoid-induced
inhibition of GSK-3β/β-catenin signaling. Mol Psychiatry.
17:790–808. 2012. View Article : Google Scholar :
|