Introduction
Triggering receptor expressed on myeloid cells-1
(TREM-1) is a novel receptor which participates in the
amplification of inflammatory responses, and it is mainly expressed
in neutrophils and monocytes/macrophages. Although the ligands of
TREM-1 have not yet been identified, an agonistic monoclonal
antibody elicits the interaction of TREM-1 with DNAX-activating
protein 12 (DAP12), which is a transmembrane adaptor molecule, in
order to activate the mitogen-activated protein kinase (MAPK) and
nuclear factor-κB (NF-κB) pathways, thereby inducing the production
of pro-inflammatory cytokines, such as interleukin (IL)-8, monocyte
chemotactic protein (MCP)-1, tumor necrosis factor (TNF)-α and
IL-1β (1-3). By contrast, silencing TREM-1
expression has been shown to suppress the production of
inflammatory cytokines, such as IL-1β, MCP-1, IL-10 and IL-2 by
lipopolysaccharide (LPS)-stimulated macrophages (4). Furthermore, silencing TREM-1
expression and blocking TREM-1 responses have also been shown to
suppress the production of cytokines (IL-1β, TNF-α and IL-6) and
prolong the survival of mice or rats with bacterial sepsis
(5–8). These observations suggest that
TREM-1 modulates the production of cytokines, thereby amplifying
the inflammatory response.
Based on the discovery that the mRNA expression of
TREM-1 is higher in human mature CD14+ monocytes
compared with that in progenitor CD34+ stem cells
(9), it has been demonstrated
that TREM-1 mRNA expression is increased by the maturation of
monocyte progenitor cells into monocytes (9). Consistently, the mRNA expression of
TREM-1 has been shown to increase following the treatment of U937
cells (a human monocytic cell line) with
1,25-dihydroxy-cholecalciferol (vitamin D3, hereon referred to as
VitD3), which promotes monocyte/macrophage maturation (9,10).
Of note, the expression of TREM-1 has also been shown to be
upregulated in monocytes/macrophages following stimulation with
Gram-negative and Gram-positive bacterial components, such as LPS
and lipoteichoic acid (LTA), respectively (5). Previous studies have noted that the
transcription factor NF-κB (p65) and hypoxia-inducible factor
(HIF)-1α are involved in the VitD3-induced upregulation of TREM-1
mRNA expression in phorbol myristate acetate (PMA)-matured U937
cells, based on the discovery that p65 and HIF-1α were increased by
treatment with VitD3 and the upregulation of TREM-1 mRNA expression
was inhibited by their inhibitors (10,11). By contrast, in a previous study of
ours, we demonstrated, using a mouse TREM-1 promoter and a murine
macrophage-cell line (RAW264.7), that the constitutive
transcription of the TREM-1 gene is regulated via the
interaction of CCAAT-enhancer-binding protein (C/EBP)α and p50/p50
homodimers with the cAMP response element (CRE) and the NF-κB site,
respectively, whereas the LPS-induced upregulation of the
TREM-1 gene is regulated via the interaction of c-Fos/c-Jun
with the activator protein-1 (AP-1) site in the promoter (12). However, the cis-regulatory
elements and transcription factors participating in the expression
of the human TREM-1 gene during maturation or stimulation of
monocytes/macrophages have not yet been elucidated.
Thus, in the present study, in order to elucidate
the regulatory mechanisms responsible for the basal, and VitD3- and
LPS-induced TREM-1 expression in human monocytes/macrophages, we
evaluated TREM-1 promoter activity (using a luciferase reporter
assay), the effects of transcription factor oligodeoxynucleotide
(ODN) decoys on TREM-1 mRNA expression, as well as the expression
of putative transcription factors (by western blot analysis) in
resting, as well as in VitD3- and LPS-treated THP-1 cells, a human
monocytic cell line.
Materials and methods
Reagents and antibodies
LPS [from Escherichia coli (E. coli)
serotype O111:B4] was purchased from Sigma Chemical Co. (St. Louis,
MO, USA); 1,25-dihydroxycholecalciferol (VitD3), rabbit anti-C/EBPα
polyclonal antibody (sc-9314), rabbit anti-C/EBPβ polyclonal
antibody (sc-150), rabbit anti-C/EBPζ polyclonal antibody
(sc-130709), rabbit anti-serum response factor (SRF) polyclonal
antibody (sc-335), rabbit anti-c-Jun polyclonal antibody (sc-1694)
and rabbit anti-c-Fos polyclonal antibody (sc-253) were obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-TATA
binding protein (TBP) monoclonal antibody (MA5-14739) was from
Thermo Fisher Scientific (Rockford, IL, USA); horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-conjugated
goat anti-mouse IgG/IgM were purchased from Chemicon International
(Temecala, CA, USA); PE-labeled anti-human CD14 monoclonal antibody
and PE-labeled anti-mouse IgG2b isotype control were from Beckman
Coulter (Brea, CA, USA); and PE-labeled anti-human toll-like
receptor (TLR)4 monoclonal antibody and PE-labeled anti-mouse IgG2a
K isotype control were obtained from eBioscience (San Diego, CA,
USA).
Cell culture
THP-1, a human acute monocytic leukemia cell line
was obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were grown and maintained in
Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Co.)
containing 10% fetal calf serum (FCS; endotoxin level <10 EU/ml;
Cell Culture Technologies, Herndon, VA, USA), penicillin (100 U/ml)
and streptomycin (0.1 mg/ml) at 37°C in an incubator with 5%
CO2.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
The THP-1 (1×106) cells were cultured in
RPMI-1640 supplemented with 10% FBS overnight in 35-mm dishes.
Thereafter, the cells were incubated for 24 h in the absence or
presence of VitD3 (100 nM), which acted as a
differentiation-inducing agent, and then further stimulated with
LPS (E. coli O111:B4, 100 ng/ml) for 24 h. Total RNA was
purified using an RNeasy Plus Mini kit and QIAshredder (Qiagen,
Valencia, CA, USA), and RT-PCR was performed using a ReverTra plus
RT-PCR kit (Toyobo Co., Ltd., Osaka, Japan) in a thermal cycler
(mastercycler gradient; Eppendorf, Hamburg, Germany) with the
following set of oligonucleotide primers: TREM-1 forward,
5′-ATGAGGAAGACCAGGCTC-3′ and reverse, 5′-CTAGGGTACAAATGACC-3′; and
GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. In brief, cDNA was synthesized by
reverse transcription of the total RNA (500 ng) using ReverTra Ace
reverse transcriptase and oligo(dT)20. PCR amplification was
performed with KOD -Plus- ver.2 polymerase in a thermal cycler. The
PCR profile consisted of pre-denaturation [94°C for 3 min, 24
cycles (for TREM-1) or 18 cycles (for GAPDH) at 96°C for 10 sec,
58°C for 30 sec and 68°C for 45 sec] and a final extension of 7
min. PCR products were resolved by 1% agarose gel electrophoresis
and stained with ethidium bromide. In our preliminary experiments,
we attempted to semi-quantitatively detect mRNA expression by using
different numbers of PCR cycles. The results indicated that the
amounts of RT-PCR products increased, depending on the number of
cycles. Thus, we decided to measure the mRNA levels by RT-PCR with
the number of cycles indicated above. The detected bands were
quantified using Multi Gauge (version 3.0; Fujifilm, Tokyo, Japan).
The mRNA expression of TREM-1 was normalized to that of GAPDH mRNA
expression, and expressed as a ratio relative to resting cells
incubated without VitD3 and LPS.
Flow cytometry
The THP-1 (1×106) cells were incubated
for 48 h in the presence or absence of VitD3 (100 nM), and washed
twice with cold phosphate-buffered saline (PBS). The cells
(1×106/200 µl) were incubated with PE-labeled
anti-human CD14 monoclonal antibody (1 µg), PE-labeled
anti-mouse IgG2b isotype control (1 µg), PE-labeled
anti-human TLR4 monoclonal antibody (2 µg) or PE-labeled
anti-mouse IgG2a K isotype control (2 µg) at 4°C for 15 min.
The cells were then washed with PBS containing 1% bovine serum
albumin (BSA) and 0.1% NaN3, and analyzed by flow
cytometry (FACSCalibur™; BD Biosciences, San Jose, CA, USA). The
expression of CD14 and TLR4 was determined by the mean fluorescence
intensity (MFI), which was corrected for non-specific binding by
subtracting the MFI values corresponding to the isotypematched
controls, and was expressed as a ratio relative to resting cells
incubated without VitD3.
Plasmid construction
The transcription initiation site of human TREM-1
was estimated using the human chromosome 6 sequences and the mRNA
database (NCBI reference sequence nos. AL391903 and AF287008). A
1.2-kbp fragment of the human TREM-1 promoter (−1200 to +64) was
amplified from THP-1 genomic DNA using KOD -Plus- polymerase and a
set of oligonucleotide primers as follows: −1200 sense, 5′-GGG
ACGCGTCCTATACTTGAGTAGCAATC-3′ and +64 antisense,
5′-GGGAGATCTCCTTCCTGTGCACCAGC-3′ (underlined letters indicate the
restriction sites, MluI and BglII, respectively). The
PCR products were digested with MluI and BglII, and
subcloned into a promoterless firefly luciferase- expression
plasmid (pGL3-Basic; Promega, Madison, WI, USA) to generate a −1200
plasmid. The 0.6-, 0.4-, 0.2-, 0.1- and 0.05-kbp fragments of the
human TREM-1 promoter (−600, −400, −200, −100 and −50 to +64) were
amplified by PCR using a −1200 plasmid (as a template), KOD -Plus-
polymerase and a set of oligonucleotide primers containing
MluI and BglII restriction sites (indicated by
underlined letters): −600 sense, 5′-GGGACGCGTCTGTTCTTGTTGGGTGGTG-3′;
−400 sense, 5′-GGGACGCGTATGTTCTCACAAAAACCCTGAAG-3′;
−200 sense, 5′-GGGACGCGTGTTGAAAGGTAATTGT
CATTATTACC-3′; −100 sense, 5′-GGGACGCGTTCAGG AGTCAGAGCAACTGG-3′;
−50 sense, 5′-GGGACGCGT CCAGGAATGGCCTCATATCC-3′;
and +64 antisense, 5′-GGGAGATCTCCTTCCTGTGCACCAGC-3′. The
PCR products were digested and subcloned into pGL3-Basic. The
inserts were confirmed by sequencing with a BigDye®
Terminator v3.1 Cycle sequencing kit and a 3730xl DNA Analyzer
(both from Applied Biosystems, Foster City, CA, USA). The
cis-regulatory elements were investigated using the TFSEARCH
database (http://diyhpl.us/~bryan/irc/protocol-online/protocol-cache/TFSEARCH.html)
in the 5′ upstream region (−1200 to +64) of the human TREM-1
promoter.
The cis-acting motifs of AP-1-2 (−341 to
−331), AP-1-3 (−97 to −90), AP-1-4 (−58 to −48), SRE (−353 to
−342), CRE (−105 to −98), C/EBP-2 (−544 to −537), C/EBP-3 (−212 to
−199), C/EBP-4 (−195 to −180), C/EBP-5 (−31 to −18), GATA-3 (−479
to −470) and GATA-4 (−376 to −368), which were deduced using the
TFSEARCH database, were substituted by adenine nucleotides via
PCR-based site-directed mutagenesis using the −1200 or −600 plasmid
comprising of pGL3-Basic (as a template), KOD -Plus- polymerase and
appropriate oligonucleotide sense and antisense primers listed in
(Table I). Synthesized
blunt-ended PCR products were purified with a MinElute Gel
extraction kit (Qiagen), phosphorylated with polynucleotide kinase,
and then self-ligated with T4 DNA ligase. Mutated
cis-regulatory elements were confirmed by sequencing.
 | Table IOligonucleotide primers for PCR-based
site directed mutagenesis. |
Table I
Oligonucleotide primers for PCR-based
site directed mutagenesis.
| Putative
motifs | Primer
sequences |
|---|
| AP-1-2 | | −330 | −307 |
| Sense:
5′-AAAAAATCACTACACTAAACTGGATGTG-3′ |
| Antisense:
3′-GTCGGAGAGGGATACACCCTTTTT-5′ |
| −360 | −342 |
| −89 | −67 |
| AP-1-3 | Sense:
5′-AAAAAGCAACTGGTGATGAAACAGAAC-3′ |
| Antisense:
3′-CAGAGACATAATAACACAACTACAGTTTTT-5′ |
| −119 | −98 |
| −47 | −29 |
| AP-1-4 | Sense:
5′-AAAAAAGGAATGGCCTCATATCCTG-3′ |
| Antisense:
3′-CACTACTTTGTCTTGGGTTTGAGTTTTT-5′ |
| −81 | −59 |
| −341 | −322 |
| SRE | Sense:
5′-AAAAAACCTGACTCTCTTCACTACAC-3′ |
| Antisense:
3′-CTATGCTAACAGCGTCGGAGTTTTTT-5′ |
| −373 | −354 |
| −97 | −79 |
| CRE | Sense:
5′-AAAAGGAGTCAGAGCAACTGGTG-3′ |
| Antisense:
3′-ACACAATAATACAGAGACCAAGACTCTTTT-5′ |
| −131 | −106 |
| −536 | −519 |
| C/EBP-2 | Sense:
5′-AAAAGCTCCCGAGGCCATGTCTG-3′ |
| Antisense:
3′-GTTTAACAAAGACCCCAGGGATGTGTTTT-5′ |
| −569 | −545 |
| −198 | −173 |
| C/EBP-3 | Sense:
5′-AAAAAAAAAGGTAATTGTCATTATTACCAC-3′ |
| Antisense:
3′-GTAAGTTCATAGTAATTTTTTT-5′ |
| −227 | −213 |
| −179 | −158 |
| C/EBP-4 | Sense:
5′-AAAAAAAATTACCACAGAAAGGAAAACTGG-3′ |
| Antisense:
3′-GAGGGAATCTAAAACATTCCAACTTTTTTTT-5′ |
| −218 | −196 |
| −17 | +1 |
| C/EBP-5 | Sense:
5′-AAAAAAATCCGAAGCCTCTAGGTCA-3′ |
| Antisense:
3′-GGTCCTTACCGGAGTATGTTTTTTT-5′ |
| −49 | −32 |
| −469 | −451 |
| GATA-3 | Sense:
5′-AAAAAAGGAGGTGCACCCCAGGTC-3′ |
| Antisense:
3′-GTTTACGTCCCACACCGGGAGGATTTTT-5′ |
| −503 | −480 |
| −367 | −351 |
| GATA-4 | Sense:
5′-AAAAAATTGTCGCAGCCTCTCC-3′ |
| Antisense:
3′-CAAGAGTGTTTTTGGGACTTCTTTTT-5′ |
| −397 | −377 |
Transfection and luciferase assay
The THP-1 cells (5×106) were transfected
with the −1200 plasmid, −600 plasmid or adenine mutant reporter
plasmids (10 µg) with the Renilla luciferase control
reporter vector phRL-TK (500 ng, as an internal control; Promega)
by electroporation (220 mV, 960 mFD) using Gene Pulser™ (Bio-Rad,
Hercules, CA, USA). The cells were then incubated without or with
VitD3 (100 nM) for 48 h, or incubated with VitD3 for 24 h followed
by further incubation with LPS for 24 h. Thereafter, the cells were
washed twice with PBS and lysed in passive lysis buffer (Promega).
Firefly and Renilla luciferase activity was then measured
using a Dual-Luciferase® reporter assay system (Promega)
and a microplate luminometer (SpectraMax® L; Molecular
Devices, Sunnyvale, CA, USA). Promoter activity was normalized to
Renilla luciferase activity and expressed as a ratio
relative to the firefly luciferase activity of the −1200
plasmid-transfected cells incubated without VitD3 and LPS.
Western blot analysis
Nuclear extracts were prepared from resting THP-1
cells, THP-1 cells treated with VitD3 or LPS, or THP-1 cells
treated with VitD3 and LPS, as previously described (12,13). Aliquots of nuclear extracts (10
µg) were subjected to 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and resolved
proteins were then electrotransferred to polyvinylidene difluoride
membranes (Immobilon™-P; Millipore, Billerica, MA, USA) using a
Trans-Blot® SD semi-dry transfer cell (Bio-Rad). The
membranes were blocked in 5% BlockAce (Dainippon Pharmaceutical,
Osaka, Japan) in TBS-T (10 mM Tris-HCl pH 7.5, 100 mM NaCl 0.05%
Tween-20). The blotted membranes were probed with anti-C/EBPα
antibody (1:200), anti-C/EBPβ antibody (1:200), anti-C/EBPζ
antibody (1:200), anti-SRF antibody (1:200), anti-c-Fos antibody
(1:1,000), anti-c-Jun antibody (1:1,000) or anti-TBP antibody
(1:1,000). The membranes were washed with TBS-T 3 times and further
probed with HRP-conjugated goat anti-rabbit IgG (1:2,000) or goat
anti-mouse IgG/IgM (1:2,000). Proteins were visualized using
SuperSignal® West Pico Chemiluminescent Substrate
(Pierce, Rockford, IL, USA) and detected with an LAS-3000 Image
Analyzer (Fujifilm). TBP was used as a loading control to indicate
that equal amounts of proteins were analyzed in each sample.
Tranesfection of transcription factor ODN
decoys
Single-stranded sense and antisense
phosphorothioate-bonded ODN decoys were synthesized by Operon
(Alameda, CA, USA). The single-stranded sense sequences of
consensus and mutant ODNs were as follows (the underlined letters
indicate phosphorothioate-bonded bases): C/EBP consensus ODN,
5′-TGCAGATTGCGCAATCTGCA-3′; C/EBP mutant ODN,
5′-TGCAGAGACTAGTCTCTGCA-3′; AP-1 consensus ODN,
5′-CGCTTGATGACTCAGCCGGAA-3′; AP-1 mutant ODN,
5′-CGCTTGATGACTTGGCCGGAA-3′; SRF consensus ODN,
5′-GGATGTCCATAT
TAGGACATCT-3′; and
SRF mutant ODN, 5′-GGATGTCCATATTATTACATCT-3′. The double-stranded
ODN was prepared by annealing the sense ODN to its antisense ODN.
This was achieved by heating equal amounts of each single-stranded
ODN at 95°C for 5 min in TE buffer [10 mM Tris-HCl, pH 7.4, 1 mM
ethylenediaminetetraacetic acid (EDTA)], and then slowly cooling
the mixture to room temperature. THP-1 cells (5×105) in
12-well plates were transfected with 250 pmol of consensus or
mutant ODN using X-tremeGENE HP (Roche Applied Science, Mannheim,
Germany) according to the manufacturer's instructions. After 24 h,
the transfected cells were incubated without or with VitD3 (100 nM)
for 48 h or incubated with VitD3 for 24 h followed by further
incubation with LPS for 24 h. The cells were then washed twice with
cold PBS, and total RNA was purified for use in RT-PCR.
Statistical analysis
The data are expressed as the means ± SD.
Statistical analysis was performed by one-way analysis of variance
(ANOVA), followed by Bonferroni's multiple comparison test or the
unpaired Student's t-test (GraphPad Prism; GraphPad Software, Inc.,
San Diego, CA, USA). A P-value <0.05 was considered to indicate
a statistically significant difference.
Results
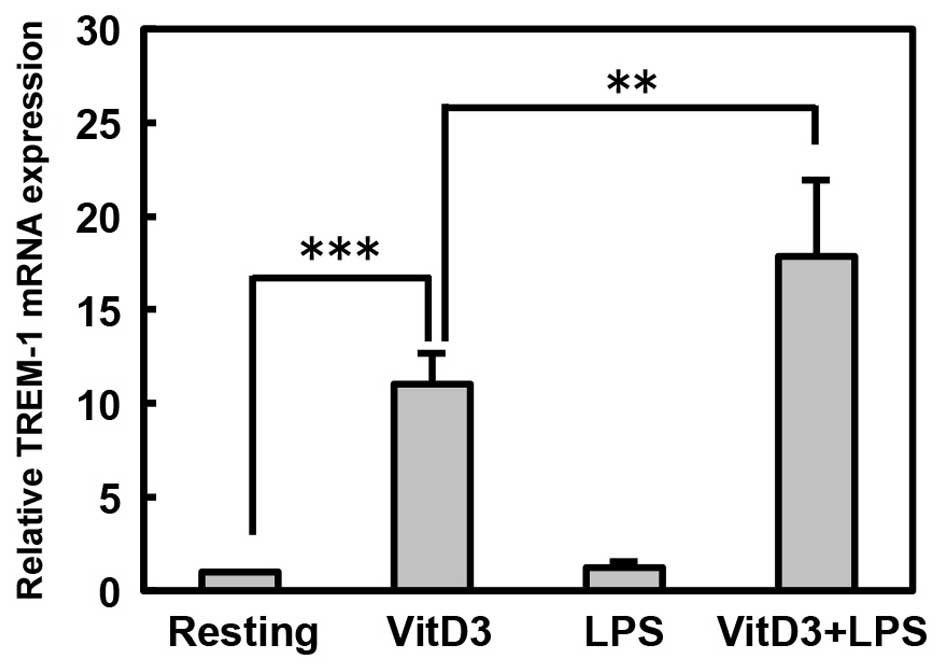
VitD3 and LPS induce human TREM-1 mRNA
expression in THP-1 cells
First, we examined the effects of treatment with
VitD3 or LPS on the mRNA expression of human TREM-1 in
monocytes/macrophages using THP-1 monocytic cells by RT-PCR. We
found that TREM-1 mRNA was constitutively expressed at a low level
in resting THP-1 cells that had not been treated with VitD3 and
LPS, and was upregulated by treatment with VitD3, but not by LPS
(P<0.001; Resting vs. VitD3; Fig.
1). Importantly, TREM-1 mRNA expression was further upregulated
by the stimulation of the VitD3-treated THP-1 cells with LPS
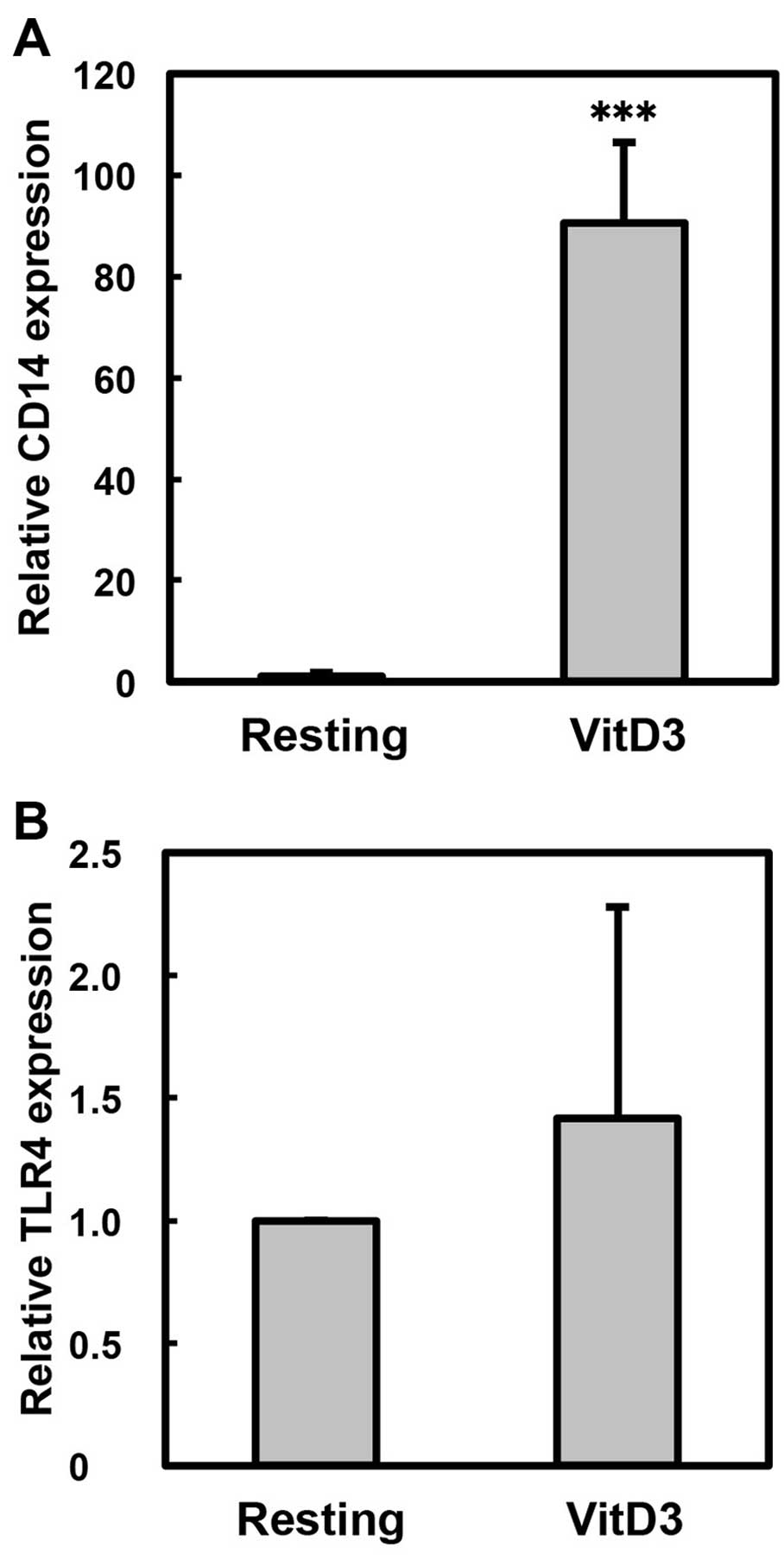
(P<0.01; VitD3 vs. VitD3 + LPS). In addition, we examined the
effect of VitD3 treatment on the expression of CD14 as a
differentiation marker of macrophages (Fig. 2A). VitD3, as a macrophage
differentiation agent, significantly increased the expression of
CD14 (approximately 90-fold, P<0.001; Resting vs. VitD3);
however, the expression of TLR4 was not markedly altered by
treatment with VitD3 (Fig. 2B).
These observations indicate that VitD3 induces the mRNA expression
of TREM-1, which is accompanied by the differentiation of THP-1
cells into macrophages, and LPS further upregulates the
VitD3-induced expression of TREM-1.
Sequence analysis of the 5′ upstream
flanking region of the human TREM-1 gene
In order to elucidate the mechanisms controlling the
basal, VitD3- and LPS-induced human TREM-1 gene transcription, we
analyzed the cis-regulatory elements in the TREM-1 promoter
(from -1200 to +64) using the TFSEARCH database (version 1.3); the
transcription initiation site (+1) was estimated using the sequence
of human chromosome 6 and the mRNA database (NCBI reference
sequencenos. AL391903 and AF287008). As shown in Fig. 3, the human TREM-1 promoter
contained multiple potential binding motifs for the AP-1 family
(AP-1-1, -2, -3 and -4), SRF, GATA (GATA-1, -2, -3 and -4), C/EBP
(C/EBP-1, -2, -3, -4 and -5) and CRE, although the TATA-box
sequence could not be detected in the promoter.
 | Figure 3Sequence of the 5′ upstream region of
the human TREM-1 gene. Transcription initiation site was
estimated using the sequences of human chromosome 6 and mRNA
database (NCBI reference sequence nos. AL391903 and AF287008).
Analysis with a TFSEARCH program (version 1.3) revealed that the 5′
upstream region of the human TREM-1 gene contained putative
cis-regulatory motifs, such as AP-1 sites, SRE (−353 to
−342), GATA-1 sites, CCAAT-enhancer-binding proteins (C/EBP) sites
and CRE (−105 to −98); however, a TATA-box sequence cannot be
detected in the promoter. AP-1 sites are termed AP-1-1 (−1114 to
−1106), AP-1-2 (−341 to −331), AP-1-3 (−97 to −90) and AP-1-4 (−58
to −48); GATA-1 sites are termed GATA-1 (−1090 to −1081), GATA-2
(−889 to −879), GATA-3 (−479 to −470) and GATA-4 (−376 to −368);
C/EBP sites are termed C/EBP-1 (−753 to −740), C/EBP-2 (−544 to
−537), C/EBP-3 (−212 to −199), C/EBP-4 (−195 to −180) and C/EBP-5
(−31 to −18). |
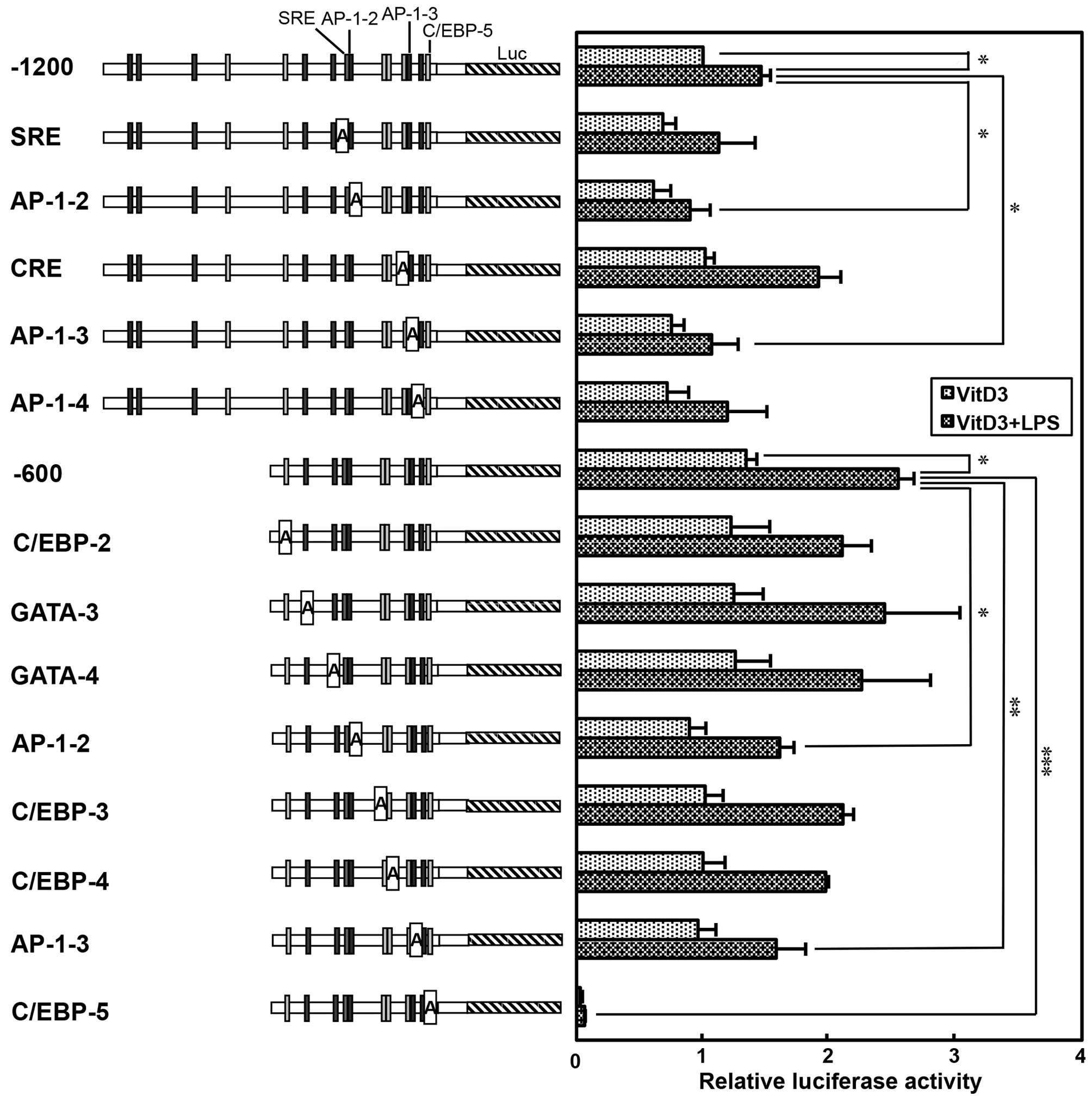
Luciferase assay of the human TREM-1
promoter containing adenine substitution mutants
In order to elucidate the potential
cis-regulatory elements involved in the basal, VitD3- and
LPS-induced transcription of human TREM-1 gene, the motifs
of AP-1-2 (−341 to −331), AP-1-3 (−97 to −90), AP-1-4 (−58 to −48),
C/EBP-2 (−544 to −537), C/EBP-3 (−212 to −199), C/EBP-4 (−195 to
−180), C/EBP-5 (−31 to −18), CRE (−105 to −98), GATA-3 (−479 to
−470), GATA-4 (−376 to −368) and SRE (−353 to −342) in the promoter
were substituted by adenine nucleotides (Fig. 4). The luciferase vectors
containing these adenine-substituted promoter sequences were
transfected into THP-1 cells and incubated without (Resting) or
with 100 nM VitD3. As shown in Fig.
4, the plasmid containing the −1200 or −600 upstream region
substantially enhanced the luciferase activity, which is consistent
with the finding that the TREM-1 gene is constitutively
transcribed in resting THP-1 cells (Fig. 1). Importantly, the promoter
activity of the −1200 plasmid was significantly enhanced by
treatment with VitD3 (approximately 3.7-fold; Resting vs. VitD3,
P<0.001). By contrast, VitD3-induced promoter activity was
significantly decreased by the mutation of SRE (−1200 vs. SRE,
P<0.05). In addition, promoter activity was significantly
reduced by the mutation of the AP-1-2 or AP-1-3 site (−1200 vs.
AP1-2, −1200 vs. AP1-3, P<0.05). Moreover, other
cis-regulatory elements in the TREM-1 promoter were analyzed
using a −600 plasmid containing adenine-substituted promoter
sequences. Similar to the results obtained with the −1200 plasmid,
the promoter activity of the −600 plasmid was significantly
enhanced by treatment with VitD3 (approximately 4.8-fold; Resting
vs. VitD3, P<0.001; Fig. 4).
Moreover, promoter activity was significantly decreased by the
mutation of the AP-1-2 or AP-1-3 site (−600 vs. AP1-2, −600 vs.
AP1-3, P<0.01). By contrast, promoter activity was not
significantly affected by the mutations of C/EBP-2, -3 and -4 and
GATA-3 and -4 sites. Notably, the mutation of the C/EBP-5 site
markedly decreased not only the basal, but also the VitD3-induced
promoter activity (−600 vs. C/EBP-5, P<0.001; Fig. 4). These findings suggest that the
C/EBP-5 site is essential for the basal and VitD3-induced promoter
activity, whereas the SRE, AP-1-2 and AP-1-3 sites are involved in
the VitD3-induced promoter activity of the human TREM-1
gene.
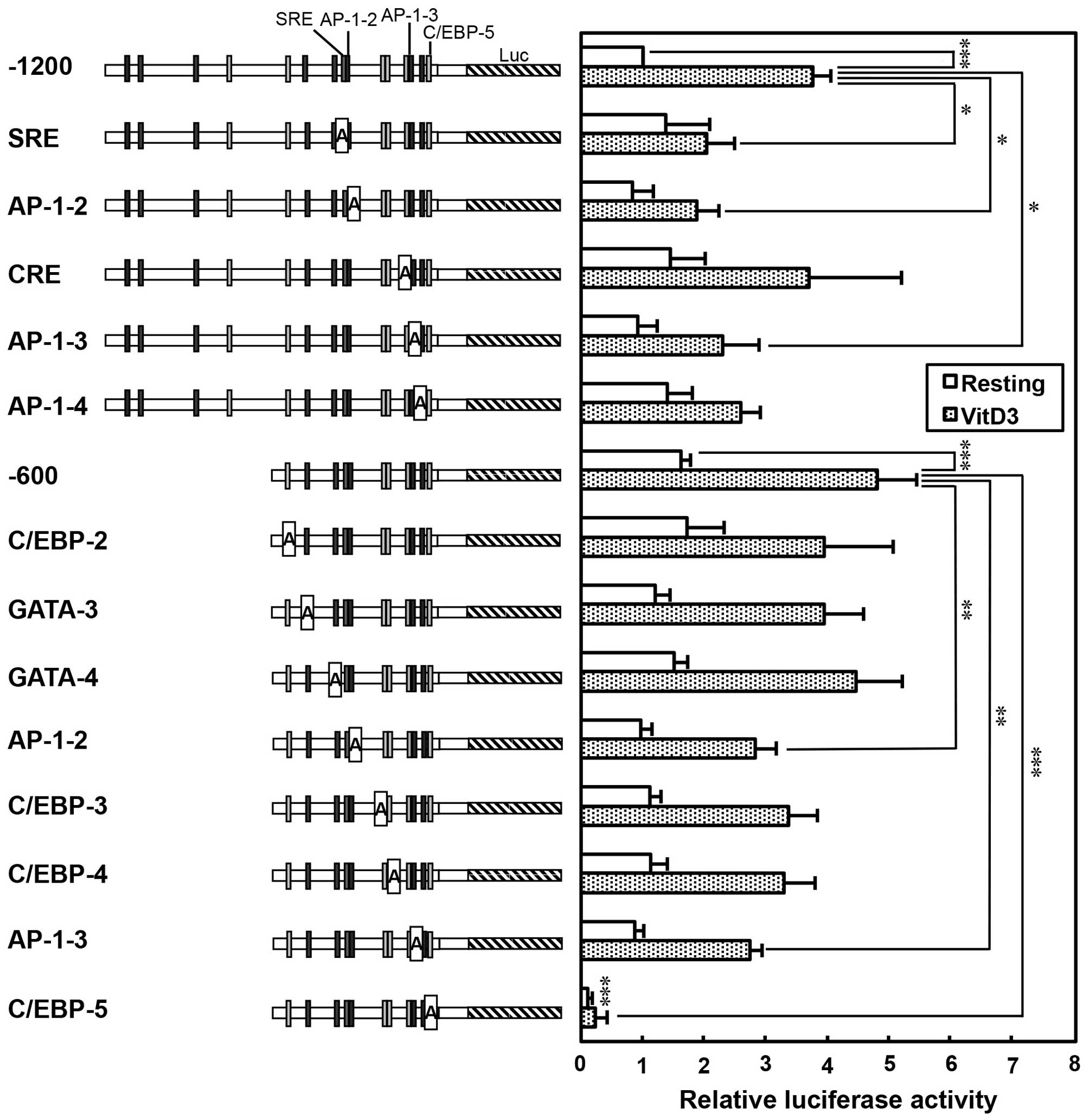 | Figure 4Basal and vitamin D3 (VitD3)-induced
luciferase activity of the human TREM-1 promoter containing
adenine-substituted constructs. Positions of putative
cis-regulatory motifs (AP-1, SRE, CCAAT-enhancer-binding
proteins (C/EBP), GATA and CRE) are indicated on the TREM-1
promoter flanking luciferase gene (Luc). THP-1 cells
(5×106) were transfected with −1200 plasmid, −600
plasmid or adenine mutant-reporter plasmids (10 µg) with
phRL-TK (500 ng, an internal control) by electroporation. Then,
tThe cells were then incubated without VitD3 (Resting) or with
VitD3 (100 nM) for 48 h (VitD3), and then firefly and
Renilla luciferase activity was measured. Promoter activity
was normalized to Renilla luciferase activity, and expressed
as a ratio relative to the firefly luciferase activity of −1200
plasmid-transfected cells incubated without VitD3. Values are the
means ± SD of at least 4 independent experiments. Values are
compared between −1200 or −600 plasmids and adenine-substituted
constructs in resting and VitD3-treated cells.
*P<0.05, **P<0.01,
***P<0.001. |
Next, we analyzed the cis-regulatory elements
involved in the LPS-induced transcription of the TREM-1 gene
by transfecting the THP-1 cells with luciferase vectors containing
adenine-substituted promoter sequences; the VitD3-treated cells
were then stimulated with LPS. In accordance with the results
obtained from the anlaysis of TREM-1 mRNA expression (Fig. 1), the promoter activity of the
−1200 and −600 TREM-1 promoter sequences was enhanced by 1.5- to
1.9-fold, respectively, following the stimulation of the
VitD3-treated cells with LPS (VitD3 vs. VitD3 + LPS, P<0.05)
(Fig. 5). Of note, the
LPS-induced promoter activity of the -1200 and -600 promoter
sequences was significantly decreased by the mutation of the AP-1-2
or AP-1-3 site (−1200 vs. AP-1-2 or AP-1-3, P<0.05; −600 vs.
AP-1-2, P<0.05; −600 vs. AP-1-3, P<0.01) (Fig. 5). Moreover, the mutation of the
C/EBP-5 site markedly decreased the LPS-induced promoter activity
(−600 vs. C/EBP-5, P<0.001), as well as the VitD3-induced
promoter activity (Fig. 5).
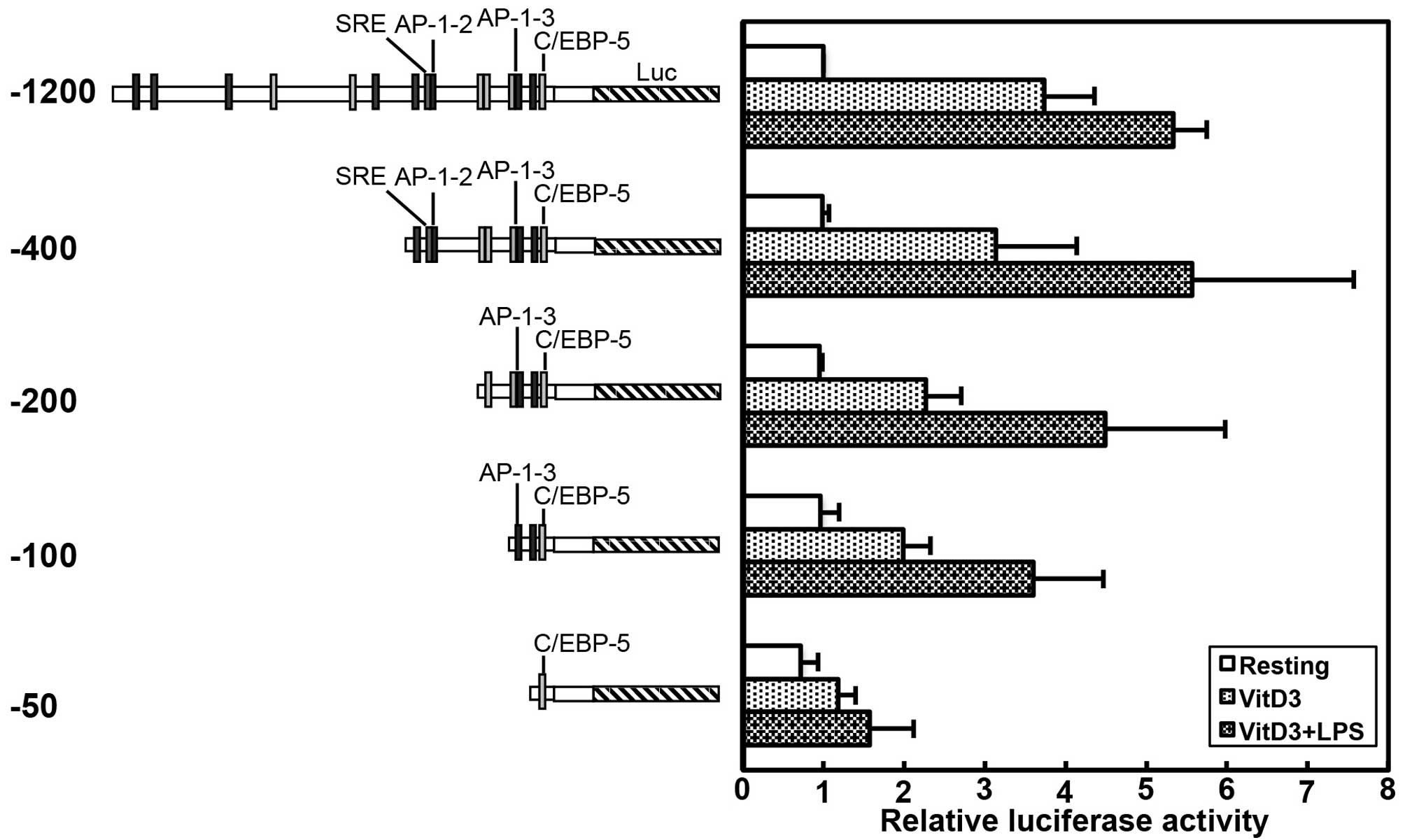
Taken together, these findings indicate that the
AP1-2 and AP1-3 sites participate in both the VitD3- and
LPS-induced promoter activity of the human TREM-1 gene,
whereas the C/EBP-5 site is involved not only in the basal, but
also in the VitD3- and LPS-induced promoter activity of the human
TREM-1 gene. These conclusions were supported by the
experiments using the luciferase vectors containing 5′ truncated
promoter sequences, which indicated that the basal promoter
activity of the −50 plasmid containing only C/EBP-5 was almost the
same as that of the −1200 plasmid, and the VitD3- and LPS-induced
promoter activity of the −400 plasmid containing AP-1-2, AP-1-3 and
C/EBP-5 was equal to that of the −1200 plasmid (Fig. 6). Moreover, the VitD3- and
LPS-induced promoter activity was slightly decreased by the
deletion of the AP-1-2 site in the −200 plasmid, compared with that
of the −400 plasmid; however, the VitD3- and LPS-induced promoter
activity of the −100 plasmid containing the AP-1-3 site was almost
the same as that of the −200 plasmid containing AP-1-3 (Fig. 6).
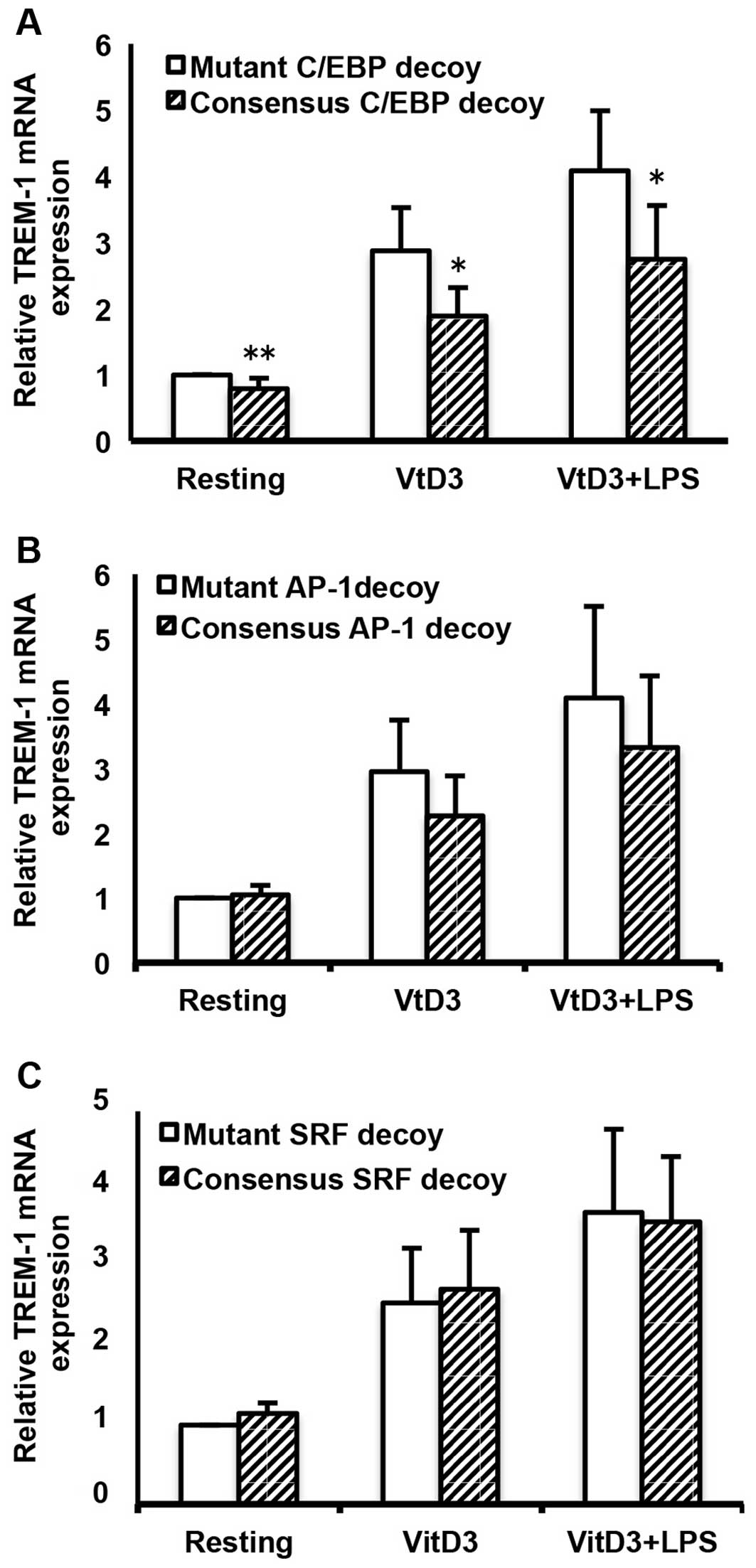
Effects of C/EBP and AP-1 ODN decoys on
TREM-1 mRNA expression
To further determine the involvement of C/EBP, AP-1
and SRF in the basal, as well as in the VitD3- and LPS-induced
transcription of the human TREM-1 gene, we examined the
effects of C/EBP, AP-1 and SRF ODN decoys on the mRNA expression of
TREM-1 by transfecting ODN decoys into the cells, followed by
incubation with or without VitD3 and LPS. As shown in Fig. 7, the C/EBP consensus ODNs
significantly suppressed not only the basal, but also the VitD3-
and VitD3/LPS-induced TREM-1 mRNA expression, as compared with the
mutant ODNs (resting cells, P<0.01; VitD3- and VitD3 +
LPS-treated cells, P<0.05; Fig.
7A). In addition, AP-1 consensus ODNs substantially suppressed
the VitD3- and VitD3/LPS-induced TREM-1 mRNA expression, although
the effects were not statistically significant (Fig. 7B). By contrast, the SRF consensus
ODNs did not affect the VitD3-and VitD3/LPS-induced TREM-1 mRNA
expression (Fig. 7C).
Furthermore, we examined the expression levels of
C/EBP, AP-1 and SRF in resting, VitD3-, LPS- and VitD3/LPS-treated
THP-1 cells by western blot analysis. As shown in Fig. 8, of the members of the C/EBP
family, C/EBPα was constitutively expressed in resting cells; its
expression was enhanced by treatment with VitD3 and further
increased by treatment with LPS (VitD3 +LPS). Moreover, c-Fos and
c-Jun (members of the AP-1 family) were constitutively expressed in
resting cells; their expression was enhanced by treatment with
VitD3/LPS, but not by treatment with VitD3 alone. SRF was
constitutively expressed in resting cells; however, its expression
was not altered by treatment with VitD3, but was increased by
treatment with VitD3 and LPS.
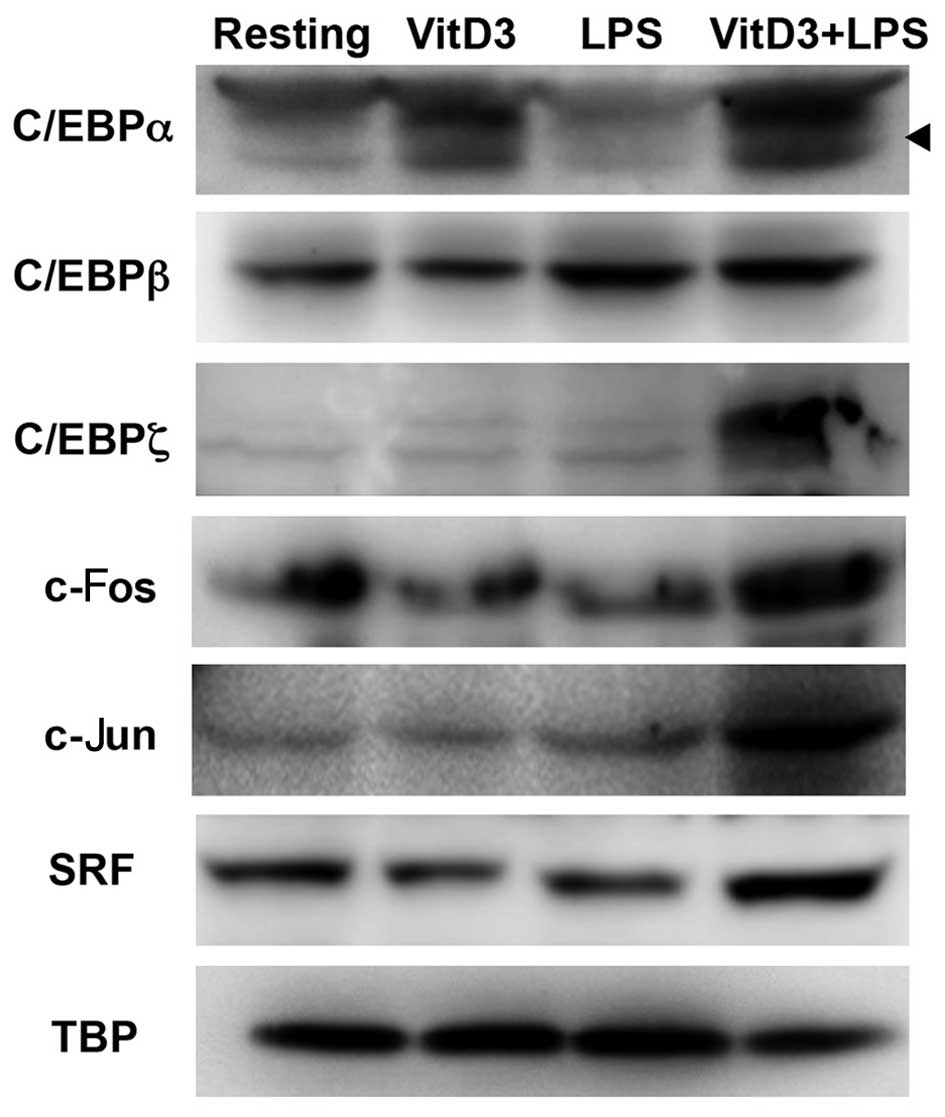 | Figure 8Western blot analysis of
transcription factors in resting, vitamin D3 (VitD3)- and
lipopolysaccharide (LPS)-treated THP-1 cells. THP-1 cells
(1×106) were incubated without (Resting) or with VitD3
for 48 h (VitD3), incubated with VitD3 for 24 h, followed by
further incubatedion with LPS for 24 h (VitD3 + LPS), or incubated
with LPS alone for 24 h (LPS). Nuclear extracts (10 µg) were
subjected to 10% SDS-PAGE, and then resolved proteins were
electrotransferred onto PVDF membranes. The membranes were blocked
and probed with anti-CCAAT-enhancer-binding proteins (C/EBP)α,
anti-C/EBPβ, anti-C/EBPζ, anti-c-fos, anti-c-jun, anti-SRF and
anti-TBP antibody. The membrane was further probed with
HRP-conjugated goat anti-rabbit IgG and anti-mouse IgG/IgM.
Proteins were visualized with a chemiluminescent substrate. The
arrowhead indicates C/EBPα. Images are representative of at least 3
separate experiments. |
Discussion
In the present study, the regulatory mechanisms
responsible for the transcription of the human TREM-1 gene
were examined using a human monocytic cell line (THP-1 cells).
RT-PCR revealed that TREM-1 mRNA was constitutively expressed at a
low level in resting cells, and was upregulated by treatment with
VitD3, but not by LPS (Fig. 1).
Importantly, TREM-1 mRNA expression was further upregulated by the
stimulation of the VitD3-treated THP-1 cells with LPS (Fig. 1). In addition, a luciferase
reporter assay revealed that the SRE site was involved in the
VitD3-induced promoter activity (Fig.
4), whereas the AP-1 sites participated in the VitD3- and
LPS-induced promoter activity (Fig.
5). Of note, the C/EBP site contributed not only to the basal,
but also to the VitD3- and LPS-induced promoter activity (Figs. 4 and 5). Transfection with transcription
factor ODN decoys indicated that the transcription factors of the
C/EBP and AP-1 families were likely involved in the basal, VitD3-
and LPS-induced TREM-1 transcription (Fig. 7); however, the role of SRF in
TREM-1 transcription could not be clarified. Notably, western blot
analysis indicated that, of the members of the C/EBP family, C/EBPα
was constitutively expressed in resting cells; its expression was
enhanced by VitD3 and was further increased by LPS. Moreover, the
expression of c-Fos and c-Jun (members of the AP-1 family) was
augmented by treatment with both VitD3 and LPS (Fig. 8). Taken together, these findings
indicate that members of the C/EBP family participate not only in
the basal, but also in the VitD3- and LPS-induced promoter activity
of the human TREM-1 gene, whereas members of the AP-1 family
are involved in the VitD3- and LPS-induced promoter activity.
CD14 and TLR4 function as receptors for LPS
(14–17). The expression of CD14 was markedly
upregulated by treatment of the THP-1 cells with VitD3. By
contrast, TLR4 was constitutively expressed in the THP-1 cells, and
its expression was not markedly altered by treatment with VitD3. Of
note, the expression of TREM-1 was increased by treatment of the
THP-1 cells with VitD3, and its expression was further upregulated
by LPS. By contrast, TREM-1 expression was not increased by the
stimulation of resting THP-1 cells with LPS, which expressed a low
level of CD14. These observations suggest that the LPS-induced
upregulation of TREM-1 in VitD3-treated cells is largely dependent
on the increased expression of CD14 following the maturation of
THP-1 cells with VitD3.
The transcription factors NF-κB (p65) and HIF-1α
have been shown to be involved in the VitD3-induced upregulation of
TREM-1 mRNA expression in PMA-matured U937 cells, based on the
findings that p65 and HIF-1α were increased by treatment with
VitD3, and the upregulation of TREM-1 mRNA expression was inhibited
by their inhibitors (Bay 11-7082, YC-1) (10,11). However, in the present study, the
putative motifs of NF-κB and HIFs could be identified in the 5′
upstream region (−1200 to +64) of the human TREM-1 promoter
(Fig. 3). Thus, it remains to be
elucidated whether NF-κB and HIF-1α are involved in the regulation
of TREM-1 gene expression in VitD3-treated THP-1 cells.
Furthermore, it has been reported that the vitamin D receptor (VDR)
participates in the upregulation of TREM-1 mRNA expression in
VitD3-treated human airway epithelial cells, based on the findings
that a vitamin D receptor response element (VDRE) was identified in
the TREM-1 promoter using computer-based analysis (MatInspector;
www.genomatix.de) and the protein level of VDR
was increased by VitD3 (18). In
this study, we localized a putative VDRE (−209 to −186) in the
human TREM-1 promoter using the MatInspector algorism. However, the
luciferase assay revealed that the mutations of the C/EBP-3 and
C/EBP-4 sites overlapping VDRE (−209 to −186) did not significantly
affect the VitD3-induced promoter activity of the TREM-1
gene (Fig. 4). Thus, it is
unlikely that putative VDRE was involved in the VitD3-induced
TREM-1 promoter activity in monocytes/macrophage using U937 cells
(10,11).
The luciferase assay indicated that the C/EBP-5 site
plays a role in the basal, and in the VitD3- and LPS-induced TREM-1
promoter activity. In line with this, the transfection of ODN
decoys revealed that transcription factors of the C/EBP family were
involved in the basal, and in the VitD3- and LPS-induced TREM-1
mRNA expression. Furthermore, western blot analysis revealed that,
of the members of the C/EBP family, C/EBPα was constitutively
expressed in resting cells; its expression was enhanced by
treatment with VitD3 and was further increased by treatment with
VitD3 and LPS. These observations suggest that members of the C/EBP
family, possibly C/EBPα, participate not only in the basal, but
also in the VitD3- and LPS-induced promoter activity of the human
TREM-1 gene.
In addition, the luciferase assay indicated that the
AP1-2 and AP1-3 sites are involved in both the VitD3- and the
LPS-induced promoter activity of the human TREM-1 gene.
Consistently, the transfection of ODN decoys revealed that
transcription factors of the AP-1 family contribute to the VitD3-
and LPS-induced TREM-1 mRNA expression. Of note, the expression of
c-Fos and c-Jun (members of the AP-1 family) was markedly increased
by treatment with both VitD3 and LPS, suggesting that c-Fos and
c-Jun may participate in the VitD3/LPS-induced TREM-1 gene
transcription. However, the levels of c-Fos and c-Jun were not
affected by treatment with VitD3 alone, although VitD3 enhanced
TREM-1 promoter activity. Importantly, it has been reported, using
U937 and HL-60 cells, that treatment with VitD3 induces the
activation of the c-Jun-N-terminal (JNK) kinase, and that
phosphorylated c-Jun can bind with AP-1 sites to enhance the
transcription of target genes (19). Thus, it is interesting to
hypothesize that c-Jun, which is phosphorylated by JNK, may
interact with AP-1 sites and increase the TREM-1 promoter activity
without substantially altering the level of c-Jun level, although
we did not confirm the phosphorylation of c-Jun in the present
study.
The luciferase reporter assay indicated that SRE was
involved in the VitD3-induced TREM-1 promoter activity. However,
the VitD3-induced mRNA expression of TREM-1 was not affected by
transfection with consensus SRF ODN decoys that bind with SRF.
Thus, it may be speculated that transcription factors other than
SRF may bind to the SRE in the TREM-1 promoter to regulate the
VitD3-induced promoter activity, since the homology of the SRE
(CCCTATGTGG) is at most 80% of the consensus SRE sequence
CC(A/T)6GG, and the SRE is neighbored with the functional AP-1-2
site.
In a previous study, we revealed the transcriptional
regulation of the mouse TREM-1 gene using RAW264.7
macrophage-like cells (12). In
another study, pairwise sequence alignment using the EMBOSS
Stretcher Algorithm indicated only 47.4% homology between the
1.2-kbp human and mouse TREM-1 promoters (http://www.ebi.ac.uk/Tools/psa/emboss_
stretcher/nucletide.html) (20).
Our previous and present studies indicate that an NF-κB site (-743
to -730 in the mouse promoter) involved in the basal and
LPS-induced transcription of the mouse TREM-1 gene was not
identified in the human TREM-1 promoter. By contrast, several AP-1
sites were identified in both mouse and human promoters: an AP-1
site (−907 to −898) was involved in the LPS-induced transcription
of the mouse TREM-1 gene, whereas AP-1-2 (−341 to −331) and
AP-1-3 (−97 to −90) sites participated in the VitD3- and
LPS-induced transcription of the human TREM-1 gene.
Furthermore, CRE was identified in both mouse and human promoters;
however, it is not likely that CRE (−105 to −98) was involved in
the transcription of the human TREM-1 gene (Figs. 4 and 5), although CRE (−99 to −92)
participated in the basal and LPS-induced transcription of the
mouse TREM-1 gene. Notably, the C/EBP-5 site (−31 to −18)
was involved in the basal, and VitD3- and LPS-induced transcription
of the human TREM-1 gene; however, it is not likely that a
C/EBP-like sequence (−38 to −25), which was identified in the mouse
promoter, contributed to the transcription of the mouse
TREM-1 gene, since a −50 plasmid-retaining C/EBP sequence
completely lost the basal and LPS-induced transcription of the
mouse TREM-1 gene. These observations suggest that the
transcription of mouse and human TREM-1 genes is
differentially regulated by multiple cis-acting motifs and
transcription factors, although members of the AP-1 family may play
a role in the modulation of both genes.
In conclusion, the present study revealed the
mechanisms responsible for the basal, and VitD3- and LPS-induced
promoter activity of the human TREM-1 gene, which encodes a
novel inflammation-amplifying molecule. It is interesting to note
that silencing or blocking TREM-1 represses cytokine production,
thereby prolonging the survival of animal models with bacterial
sepsis (5–8). Thus, the findings of the present
study provide important insight into the modulation of TREM-1
expression, a therapeutic target in inflammation, which may assist
in controlling inflammatory disorders, based on the regulatory
mechanisms responsible for the transcription of the TREM-1
gene in humans.
Acknowledgments
The present study was supported in part by a
Grant-in-Aid for Young Scientists B (grant no. 24790424) for
Scientific Research from the Japan Society for the Promotion of
Science, and a Grant-in-Aid (Grant no. S1201013) from the Ministry
of Education, Culture, Sports, Science and Technology, Japan. The
authors thank the Laboratory of Molecular and Biochemical Research,
Research Support Center, Juntendo University, Graduate School of
Medicine.
References
|
1
|
Bouchon A, Dietrich J and Colonna M:
Cutting edge: Iinflammatory responses can be triggered by TREM-1, a
novel receptor expressed on neutrophils and monocytes. J Immunol.
164:4991–4995. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bleharski JR, Kiessler V, Buonsanti C,
Sieling PA, Stenger S, Colonna M and Molin RL: A role for
triggering receptor expressed on myeloid cells-1 in host defense
during the early-induced and adaptive phases of the immune
response. J Immunol. 170:3812–3818. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dower K, Ellis DK, Saraf K, Jelinsky SA
and Lin LL: Innate immune responses to TREM-1 activation: Ooverlap,
divergence, and positive and negative cross-talk with bacterial
lipopolysaccharide. J Immunol. 180:3520–3534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ornatowska M, Azim AC, Wang X, Christman
JW, Xiao L, Joo M and Sadikot RT: Functional genomics of silencing
TREM-1 on TLR4 signaling in macrophages. Am J Physiol Lung Cell Mol
Physiol. 293:L1377–L1384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouchon A, Facchetti F, Weigand MA and
Colonna M: TREM-1 amplifies inflammation and is a crucial mediator
of septic shock. Nature. 410:1103–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibot S, Alauzet C, Massin F, Sennoune N,
Faure GC, Béné MC, Lozniewski A, Bollaert PE and Lévy B: Modulation
of the triggering receptor expressed on myeloid cells-1 pathway
during pneumonia in rats. J Infect Dis. 194:975–983. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gibot S, Buonsanti C, Massin F, Romano M,
Kolopp-Sarda MN, Benigni F, Faure GC, Béné MC, Panina-Bordignon P,
Passini N and Lévy B: Modulation of the triggering receptor
expressed on the myeloid cell type 1 pathway in murine septic
shock. Infect Immun. 74:2823–2830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibot S, Massin F, Marcou M, Taylor V,
Stidwill R, Wilson P, Singer M and Bellingan G: TREM-1 promotes
survival during septic shock in mice. Eur J Immunol. 37:456–466.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gingras MC, Lapillonne H and Margolin JF:
TREM-1, MDL-1, and DAP12 expression is associated with a mature
stage of myeloid development. Mol Immunol. 38:817–824. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim TH, Lee B, Kwon E, Choi SJ, Lee YH,
Song GG, Sohn J and Ji JD: Regulation of TREM-1 expression by
1,25-dihydroxyvi-tamin D3 in human monocytes/macrophages. Immunol
Lett. 154:80–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee B, Kwon E, Kim Y, Kim JH, Son SW, Lee
JK, Kim DW, Sohn J, Kim TH and Ji JD: 1α,25-Dihydroxyvitamin D3
upregulates HIF-1 and TREM-1 via mTOR signaling. Immunol Lett.
163:14–21. 2015. View Article : Google Scholar
|
|
12
|
Hosoda H, Tamura H, Kida S and Nagaoka I:
Transcriptional regulation of mouse TREM-1 gene in RAW264.7
macrophage-like cells. Life Sci. 89:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsutsumi-Ishii Y and Nagaoka I: NF-kappa
B-mediated transcriptional regulation of human beta-defensin-2 gene
following lipopolysaccharide stimulation. J Leukoc Biol.
71:154–162. 2002.PubMed/NCBI
|
|
14
|
Fenton MJ and Golenbock DT: LPS-binding
proteins and receptors. J Leukoc Biol. 64:25–32. 1998.PubMed/NCBI
|
|
15
|
da Silva Correia J, Soldau K, Christen U,
Tobias PS and Ulevitch RJ: Lipopolysaccharide is in close proximity
to each of the proteins in its membrane receptor complex. transfer
from CD14 to TLR4 and MD-2. J Biol Chem. 276:21129–21135. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagaoka I, Hirota S, Niyonsaba F, Hirata
M, Adachi Y, Tamura H and Heumann D: Cathelicidin family of
antibacterial peptides CAP18 and CAP11 inhibit the expression of
TNF-alpha by blocking the binding of LPS to CD14(+) cells. J
Immunol. 167:3329–3338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Triantafilou M and Triantafilou K:
Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation
cluster. Trends Immunol. 23:301–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rigo I, McMahon L, Dhawan P, Christakos S,
Yim S, Ryan LK and Diamond G: Induction of triggering receptor
expressed on myeloid cells (TREM-1) in airway epithelial cells by
1,25(OH)2 vitamin D3. Innate Immun.
18:250–257. 2012. View Article : Google Scholar
|
|
19
|
Wang Q, Wang X and Studzinski GP: Jun
N-terminal kinase pathway enhances signaling of monocytic
differentiation of human leukemia cells induced by
1,25-dihydroxyvitamin D3. J Cell Biochem. 89:1087–1101. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rice P, Longden I and Bleasby A: EMBOSS:
The European Molecular Biology Open Software Suite. Trends Genet.
16:276–277. 2000. View Article : Google Scholar : PubMed/NCBI
|