Introduction
Ischemia/reperfusion (I/R) injury causes the
apoptosis of cardiomyocytes and cardiac fibrosis, which is
characterized as the transdifferentiation of fibroblasts to
myofibroblasts and collagen deposition (1–3).
Attenuating the apoptosis of cardiomyocytes and cardiac fibrosis
effectively reduces the extent of myocardial I/R injury and the
degree of heart failure (4,5).
Therefore, revealing the underlying molecular mechanism responsible
for these effects is important for the development of therapeutic
strategies for myocardial I/R injury.
MicroRNAs (miRNAs or miRs), a class of non-coding
RNAs 18–25 nucleotides in length, directly bind to the
3′-untranslational region (3′UTR) of their target miRs, which may
cause further degradation of miR or inhibit protein translation
(6). Through inhibition of the
expression of their target genes at the post-transcriptional level,
miRs have been demonstrated to play a key role in the regulation of
a variety of cellular processes, such as cell proliferation,
differentiation and apoptosis, as well as fibrosis (7,8).
miR-142-3p has previously been reported to be associated with
cardiovascular diseases (9–11).
For example, miR-142-3p was upregulated in the peripheral blood
mononuclear cells derived from chronic heart failure patients
affected by non-ischemic dilated cardiomyopathy (12). Kee et al reported that
miR-142-5p inhibited the proliferation of vascular smooth muscle
cells by directly targeting B cell translocation gene 3 (13). However, whether miR-142-3p plays a
role in hypoxia/reoxygenation (H/R)-induced apoptosis of
cardiomyocytes and cardiac fibrosis has never previously been
studied, to the best of our knowledge.
High-mobility group box 1 (HMGB1) is a non-histone,
nuclear DNA binding protein that belongs to the HMGB superfamily.
HMGB1 has been found to participate in the organization of DNA and
regulate gene transcription, playing a role in several cellular
processes, including inflammation, cell differentiation and tumor
cell migration (14,15). Moreover, HMGB1 has been implicated
in I/R-induced myocardial injury (16,17). The protein expression of HMGB1 is
significantly increased in cardiac tissues following I/R injury,
and the inhibition of HMGB1 effectively attenuates the extent of
the tissue injury and improves cardiac performance (16,17). Therefore, HMGB1 is a promising
therapeutic target for the treatment of myocardial I/R injury. In
addition, transforming growth factor-β1 (TGF-β1)/Smad3 signaling
has been found to be involved in HMGB1-mediated pulmonary fibrosis
(18). However, the regulatory
mechanism of HMGB1 expression during myocardial I/R injury remains
largely unknown.
In the present study, we aimed to reveal the
regulatory mechanism of miR-142-3p in H/R-induced apoptosis of
cardiomyocytes and cardiac fibrosis. Moreover, we also studied the
association between miR-142-3p and HMGB1 in H/R-treated
cardiomyocytes, as well as the downstream signaling pathway.
Materials and methods
Cell culture and H/R treatment
The mouse cardiomyocyte line M6200 was purchased
from ScienCell Research Laboratories (San Diego, CA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS; both from Life Technologies,
Carlsbad, CA, USA) at 37°C in a humidified incubator containing 5%
CO2. For H/R treatment, the M6200 cells were cultured
under hypoxic conditions for 24 h, followed by reoxygenation for 1
h. Subsequently, M6200 cells were harvested for analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Life
Technologies) according to the manufacturer's instructions. TaqMan
MicroRNA Reverse Transcription kit (Life Technologies) was then
used to synthesize cDNA, according to the manufacturer's
instructions. SYBR-Green Universal qPCR Master Mix (Bio-Rad,
Hercules, CA, USA) was then used to perform qPCR on an ABI 7500
thermocycler (Life Technologies) in a total volume of 20 µl
reaction system, including 10 µl 2X SYBR-Green qPCR Mix, 1
µl primer (10 µmol/l), 1 µl cDNA and 7
µl H2O. The thermal cycling condition was set as
95°C for 3 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 30 sec. The following primer sequences were used: HMGB1 sense,
GCTGACAAGGCTCGTTATGAA and antisense, CCTTTGATTTTGGGGCGGTA;
fibronectin sense, 5′-TCTGTGCCTCCTATCTATGTGC-3′ and antisense,
5′-GAGGGACCACGACAACTCTTC-3′; COL1A1 sense, GCTCCTCTTAGGGGCCACT and
antisense, ATTGGGGACCCTTAGGCCAT; COL1A2 sense, TCGTGCCTAGCAACATGCC
and antisense, CCATAGCTGAACTGAAAACCACC; COL2A1 sense,
GGGTCACAGAGGTTACCCAG and antisense, ACCAGGGGAACCACTCT CAC; COL3A1
sense, CTGTAACATGGAAACTGGGGAAA and antisense,
CCATAGCTGAACTGAAAACCACC; COL4A1 sense, TCCGGGAGAGATTGGTTTCC and
antisense, CTGGCCTATAAGCCCTGGT; and GAPDH sense,
AGGTCGGTGTGAACGGATTTG and antisense, GGGGTCGTTGATGGCAACA. GAPDH was
used as the internal control. Gene expression was determined by
2−ΔΔCt, where ΔCt = (Ctgene −
CtGAPDH) and Ct is the threshold cycle.
Western blot analysis
The cells were solubilized in cold RIPA Lysis and
Extraction buffer (Life Technologies), and 50 µg protein was
separated with 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and transferred onto a polyvinylidene
difluoride membrane (Pierce Chemical Co., Rockford, IL, USA), which
was incubated with PBS containing 5% milk overnight at 4°C; and
then with rabbit anti-cleaved caspase-3 (1:100; ab32042), mouse
Bcl-2 (1:100; ab692), mouse anti-fibronectin (1:200; ab2413), mouse
anti-collagen I (1:100; ab90395), mouse anti-collagen II (1:100;
ab3092), mouse anti-collagen III (1:100; ab7778), mouse
anti-collagen IV (1:50; ab6311), mouse anti-HMGB1 (1:200; ab11354),
mouse anti-TGF-β1 (1:50; ab64715), mouse anti-Smad3 (1:100;
ab75512), mouse anti-GAPDH (1:200; ab8245) monoclonal antibodies
and rabbit anti-phosphorylated (p-)Smad3 (1:200; ab63403)
polyclonal antibody (all from Abcam, Cambridge, MA, USA) at room
temperature for 3 h; and then with rabbit anti-mouse monoclonal IgG
(1:5,000; ab190475; unconjugated) or goat anti-rabbit monoclonal
IgG (1:10,000; ab190492; unconjugated) secondary antibodies at room
temperature for 1 h, followed by chemiluminescence for
visualization with an ECL kit (Pierce Chemical Co.). The relative
protein expression was analyzed by Image-Pro Plus 6.0 software,
represented as the density ratio versus GAPDH.
Cell proliferation assay
M6200 cells were plated into a 96-well plate, and
cultured at 37°C with 5% CO2 for 12, 24, 48 or 72 h.
Subsequently, 20 µl MTT (5 mg/ml; Life Technologies) was
added. Following incubation at 37°C for 4 h, 150 µl
dimethylsulphoxide (DMSO) was added. After incubation at room
temperature for 10 min, the production of formazan was detected by
determining the optical density (OD) at 570 nm, using a Multiskan
FC enzyme immunoassay analyzer (Thermo Fisher Scientific, Waltham,
MA, USA).
Cell apoptosis assay
An Annexin V apoptosis detection kit (Life
Technologies) was used for apoptosis detection. Following H/R
treatment, the M6200 cells were washed with cold phosphate buffer
saline (PBS; Life Technologies) and resuspended with 500 µl
binding buffer. Subsequently, 5 µl propidium iodide (PI) and
5 µl Annexin V-FITC were added and mixed. The cells were
then incubated at room temperature in darkness for 30 min.
Thereafter, the apoptosis of M6200 cells was analyzed using a BD C6
flow cytometer (BD Biosciences, San Jose, CA, USA).
Transfection
Lipofectamine 2000 (Life Technologies) was used to
perform cell transfection according to the manufacturer's
instructions. Briefly, miR-142-3p mimic, miR-142-3p inhibitor,
HMGB1 siRNA and Lipofectamine 2000 were diluted with serum-free
medium, respectively. The diluted Lipofectamine 2000 was added into
the diluted miR-142-3p mimic, miR-142-3p inhibitor, or HMGB1 siRNA
plasmid, respectively, and incubated for 20 min at room
temperature; and then added into the M6200 cell suspension. The
M6200 cells were then incubated at 37°C with 5% CO2 for
6 h. Subsequently, the medium in each well was replaced by DMEM
with 10% FBS, and cultured for 48 h prior to the following
analyses.
Bioinformatics prediction and
Dual-Luciferase reporter assay
The TargetScan online software (http://www.targetscan.org/vert_60/) was used to
analyze the putative target genes of miR-142-3p. For the luciferase
reporter experiments, the wild-type (WT) of the 3′UTR segment of
the HMGB1 gene containing the miR-142-3p binding sequences was
amplified by PCR from human genomic DNA. A Site-Directed
Mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to
construct the mutant type (MT) of HMGB1 3′UTR lacking
complementarity with miR-142-3p seed sequence, in accordance with
the manufacturer's instructions. Subsequently, the WT or MT of
HMGB1 3′UTR was cloned into the psiCHECK-2 vector (Promega,
Madison, WI, USA), downstream of the Renilla luciferase
gene, respectively (Amspring, Changsha, China). The M6200 cells
were then transfected with the WT or MT HMGB1-3′UTR-psiCHECK-2
combined with miR-142-3p mimic or miR negative control (NC) mimic,
respectively, using Lipofectamine 2000 according to the
manufacturer's instructions. Following transfection for 48 h, the
M6200 cells were lysed with a 1X passive lysis buffer, and
lucif-erase activity was measured using the Dual-Luciferase
reporter assay system (Promega) on an LMax multiwell Luminometer
(Molecular Devices, Sunnyvale, CA, USA), in accordance with the
manufacturer's instructions.
Statistical analysis
All data in this study are presented as the means ±
SD. GraphPad Prism 5 software (GraphPad Software, La Jolla, CA,
USA) was used to perform statistical analysis. Comparisons between
groups were performed by one-way analysis of variance (ANOVA). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with H/R induces the apoptosis
and fibrosis of cardiomyocytes as well as the inhibition of
miR-142-3p expression
The M6200 cells were exposed to hypoxic conditions
for 24 h, followed by reoxygenation for 1 h. An MTT assay was
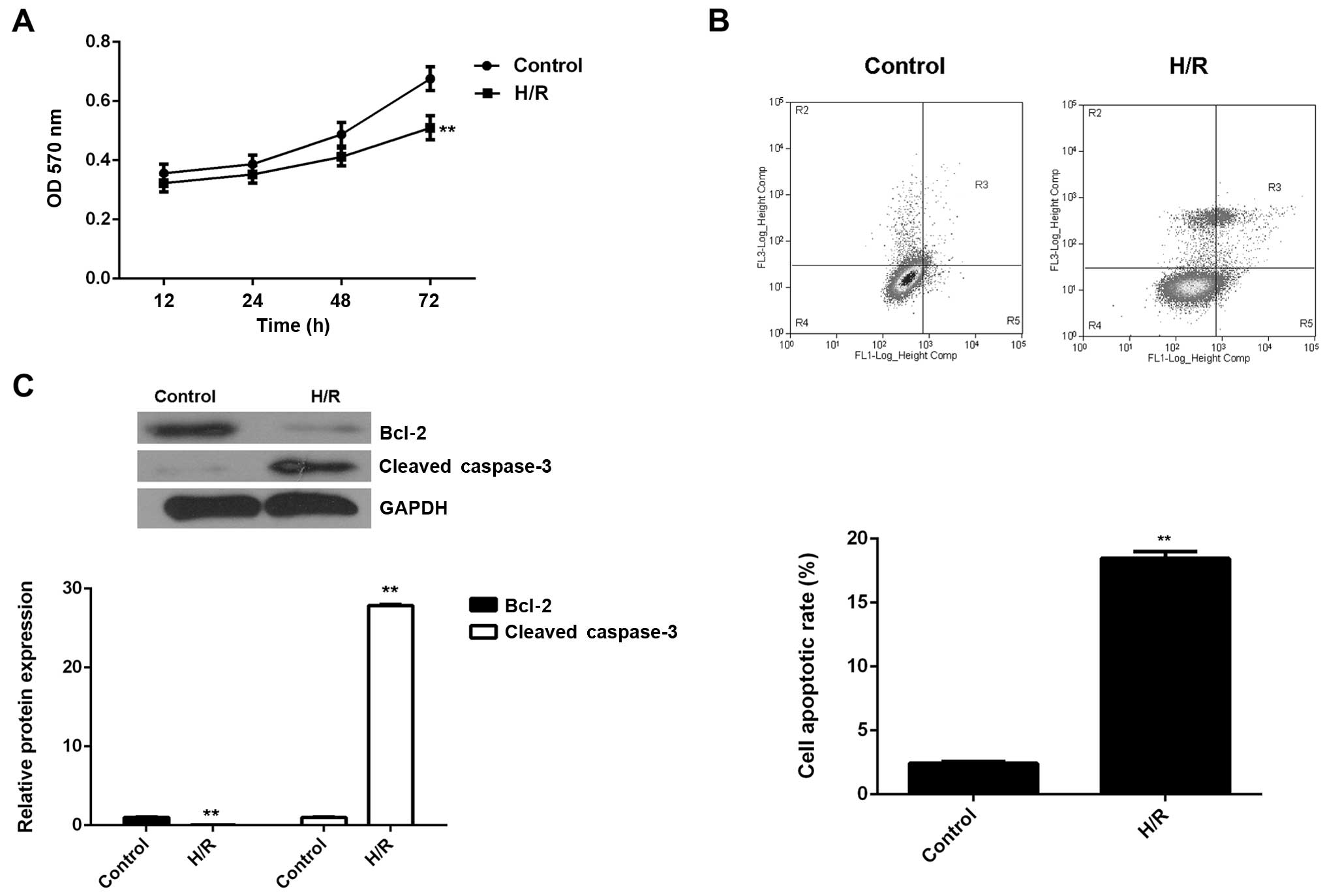
conducted to examine cell proliferation. As shown in Fig. 1A, H/R treatment led to a
significant decrease in the proliferation of M6200 cells. We
speculated that the downregulation of cell proliferation may be
caused by the induction of cell apoptosis. Therefore, we
subsequently performed flow cytometry in order to determine the
level of apoptosis in M6200 cells treated with H/R. As shown in
Fig. 1B, the apoptosis rate was
significantly increased after H/R treatment. To further confirm
these findings, western blot analysis was performed to evaluate the
expression of apoptosis-related proteins. Our data showed that
Bcl-2, a key inhibitor of cell apoptosis, was markedly
downregulated, whereas cleaved caspase-3, a marker of cell
apoptosis, was significantly upregulated in M6200 cells following
H/R (Fig. 1C). Accordingly, H/R
treatment inhibited the proliferation of M6200 cells probably
through the induction of cell apoptosis.
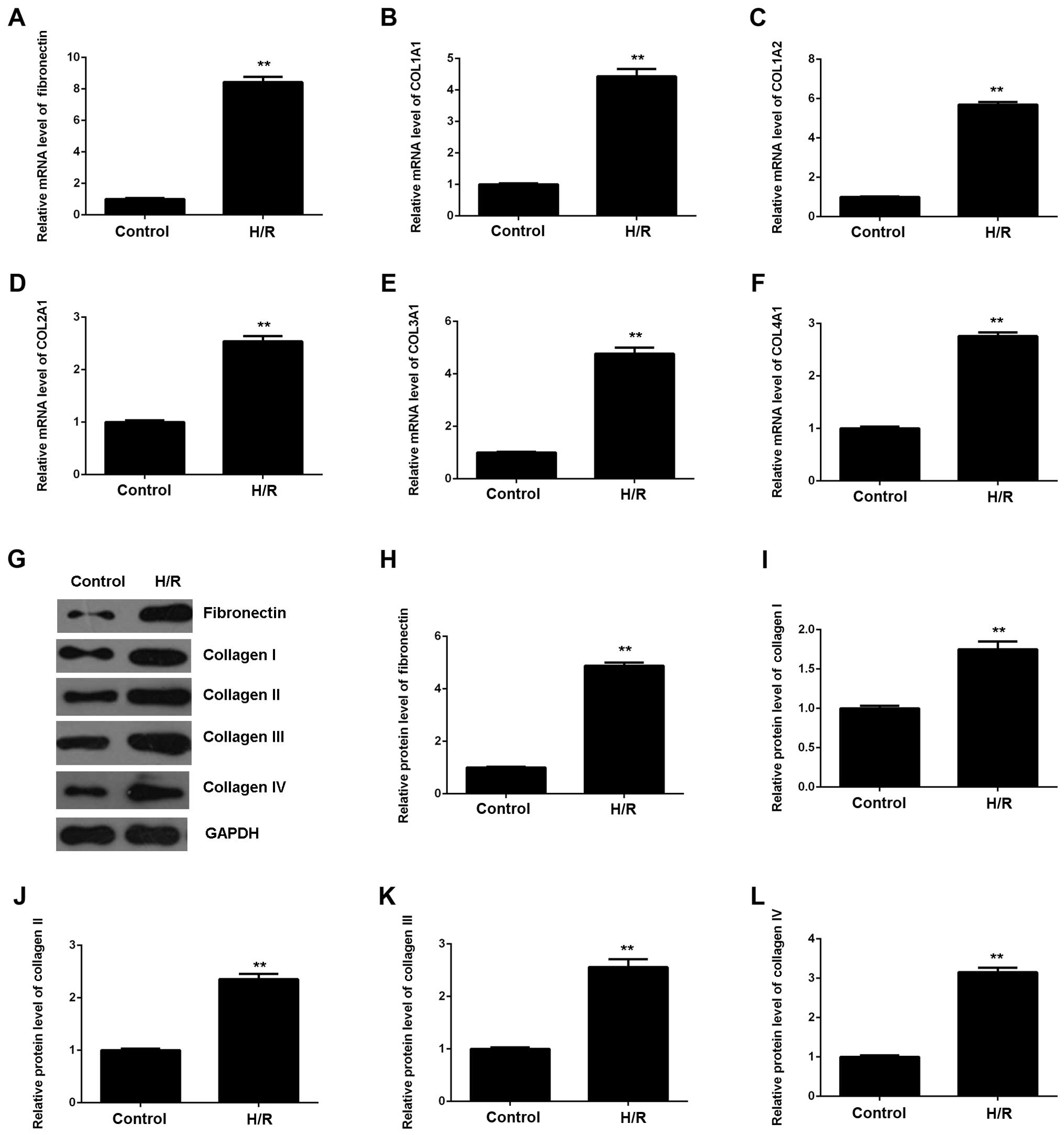
As myocardial I/R injury may also cause cardiac
fibrosis, we then evaluated the expression of fibrosis-related
proteins using RT-qPCR and western blot analysis. As shown in
Fig. 2, the mRNA and protein
expression of fibronectin and collagen I, II, III and IV were all
significantly upregulated in M6200 cells following H/R treatment,
when compared with the control group, respectively. Therefore, H/R
treatment also induced the fibrosis of M6200 cardiomyocytes.
Overexpression of miR-142-3p suppresses
H/R-induced apoptosis and fibrosis of M6200 cells
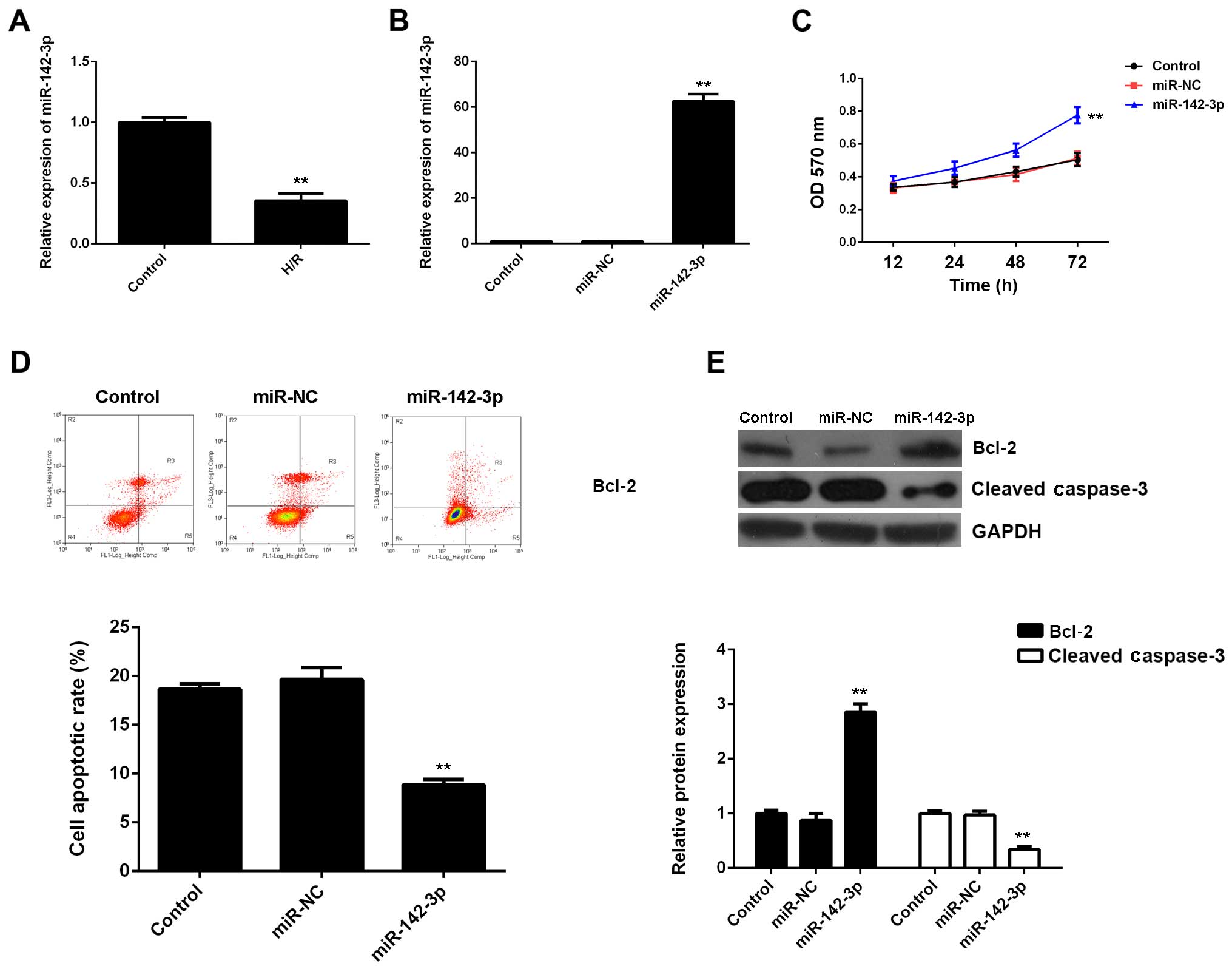
Subsequently, we evaluated the expression of
miR-142-3p in M6200 cells treated with H/R. Our data showed that
miR-142-3p was markedly downregulated following H/R treatment, when
compared with the control group (Fig.
3A), suggesting that it may be involved in myocardial I/R
injury. To further reveal the role of miR-142-3p in H/R-treated
cardiomyocytes, the gain-of-function model of miR-142-3p was then
conducted. Following transfection with miR-142-3p mimic, miR-142-3p
expression was significantly increased compared with the control
group (Fig. 3B). Thereafter, we
found that cell proliferation was higher in
miR-142-3p-overexpressing M6200 cells after H/R treatment when
compared with that in the control group (Fig. 3C). Moreover, the apoptosis level
was lower following the overexpression of miR-142-3p in M6200 cells
treated with H/R (Fig. 3D), and
this was accompanied by the increased expression of Bcl-2 and the
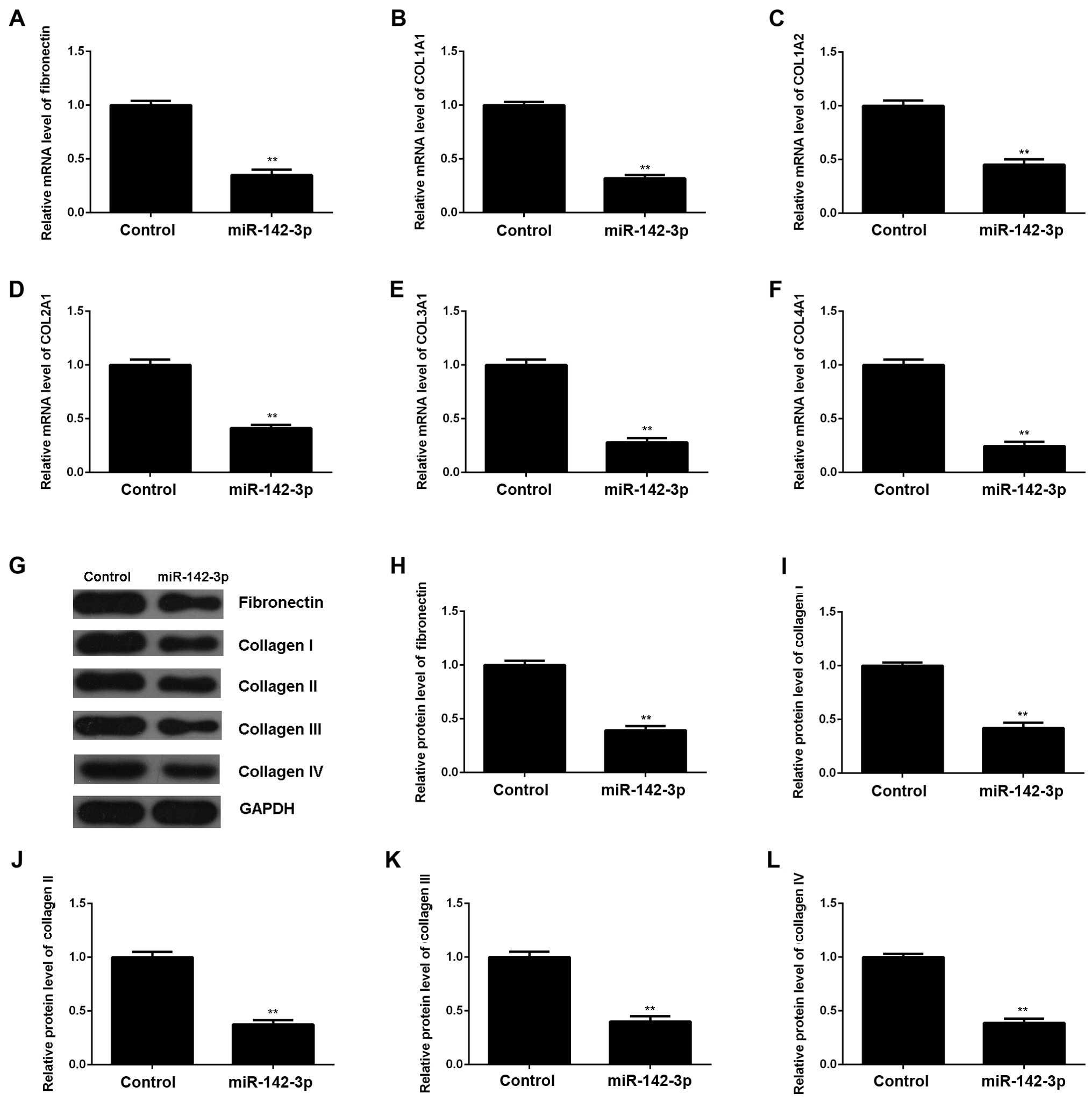
decreased expression of cleaved caspase-3 (Fig. 3E). In addition, the upregulation
of miR-142-3p significantly suppressed the mRNA and protein levels
of fibrosis-related proteins (Fig.
4). Accordingly, the restoration of miR-142-3p expression
inhibited the H/R-induced apoptosis and fibrosis of M6200
cells.
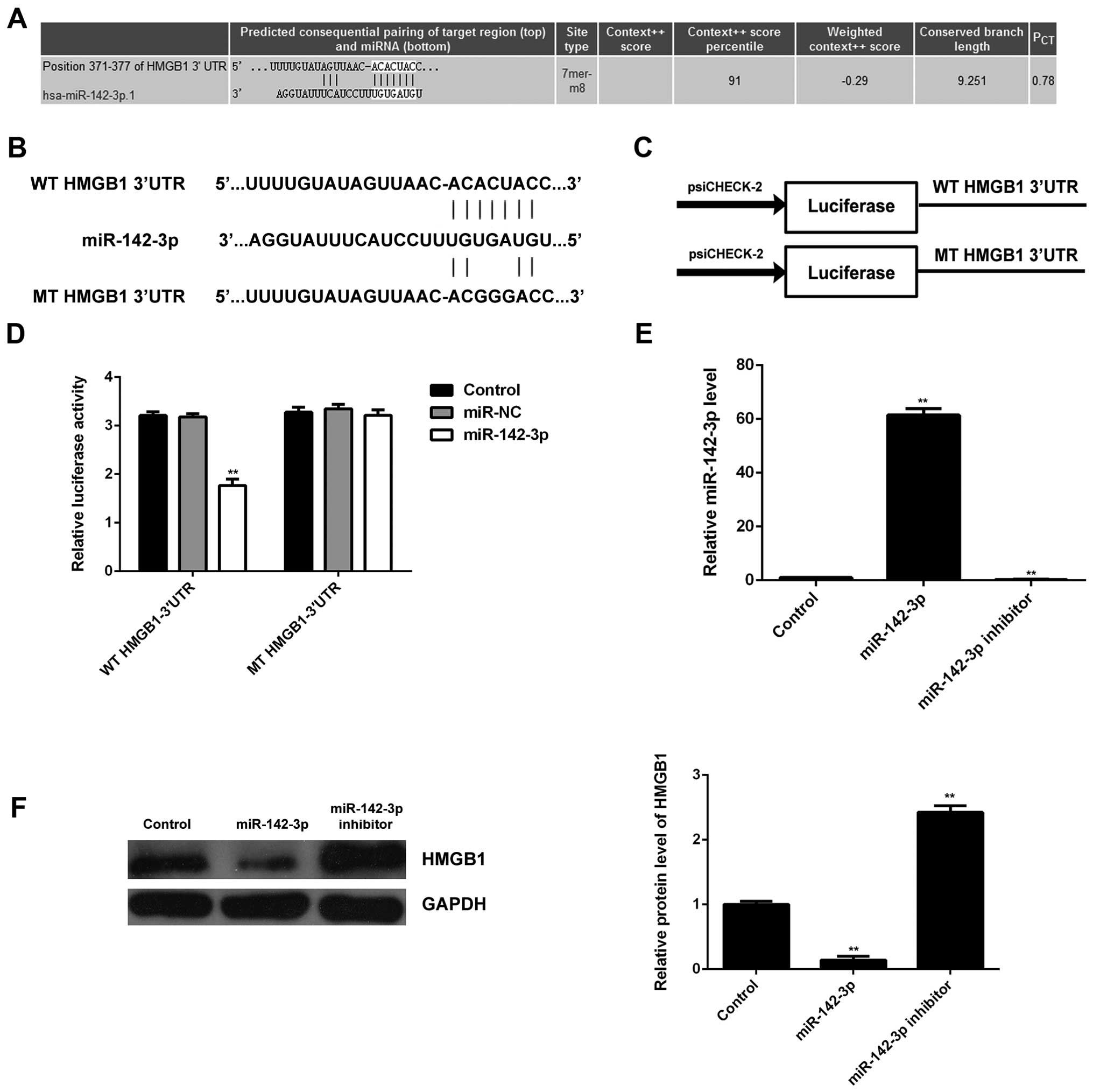
Identification of HMGB1 as a target gene
of miR-142-3p and the negatively mediated expression of HMGB1 by
miR-142-3p in M6200 cells
Bioinformatics analysis was performed in order to
predict the target genes for miR- 142-3p. HMGB1 was predicted to be
a candidate target of miR-142-3p (Fig. 5A). In order to confirm whether
HMGB1 was indeed functionally targeted by miR-142-3p, the WT or MT
of HMGB1 3′UTR was cloned into the psiCHECK-2 vector downstream of
the Renilla luciferase gene, respectively (Fig. 5B and C). M6200 cells were then
transfected with the WT or MT HMGB1-3′UTR-psiCHECK-2, combined with
miR-142-3p mimic or miR-NC mimic, respectively, and the
Dual-Luciferase reporter assay was then performed. Our data showed
that the luciferase activity was significantly reduced in M6200
cells co-transfected with the WT HMGB1-3′UTR-psiCHECK-2 vector and
miR-142-3p mimics, whereas luciferase activity was unchanged in the
cells co-transfected with MT HMGB1-3′UTR-psiCHECK-2 vector and
miR-142-3p mimics, when compared with the control group (Fig. 5D). Accordingly, HMGB1 was
identified as a target gene of miR-142-3p.
Subsequently, we examined the expression of HMGB1 in
M6200 cells transfected with miR-142-3p mimic or inhibitor,
respectively. As shown in Fig.
5E, transfection with miR-142-3p mimic enhanced the miR-142-3p
level, whereas transfection with miR-142-3p inhibitor decreased the
miR-142-3p level in M6200 cells when compared with that in the
control group, respectively. Western blot analysis was performed to
examine the protein level of HMGB1. Our data indicated that
overexpression of miR-142-3p led to a significant decrease in the
protein level of HMGB1, whereas knockdown of miR-142-3p upregulated
HMGB1 protein expression in M6200 cells when compared with that in
the control group, respectively (Fig.
5F). These data indicated that HMGB1 was negatively mediated by
miR-142-3p at the post-transcriptional level in M6200 cells.
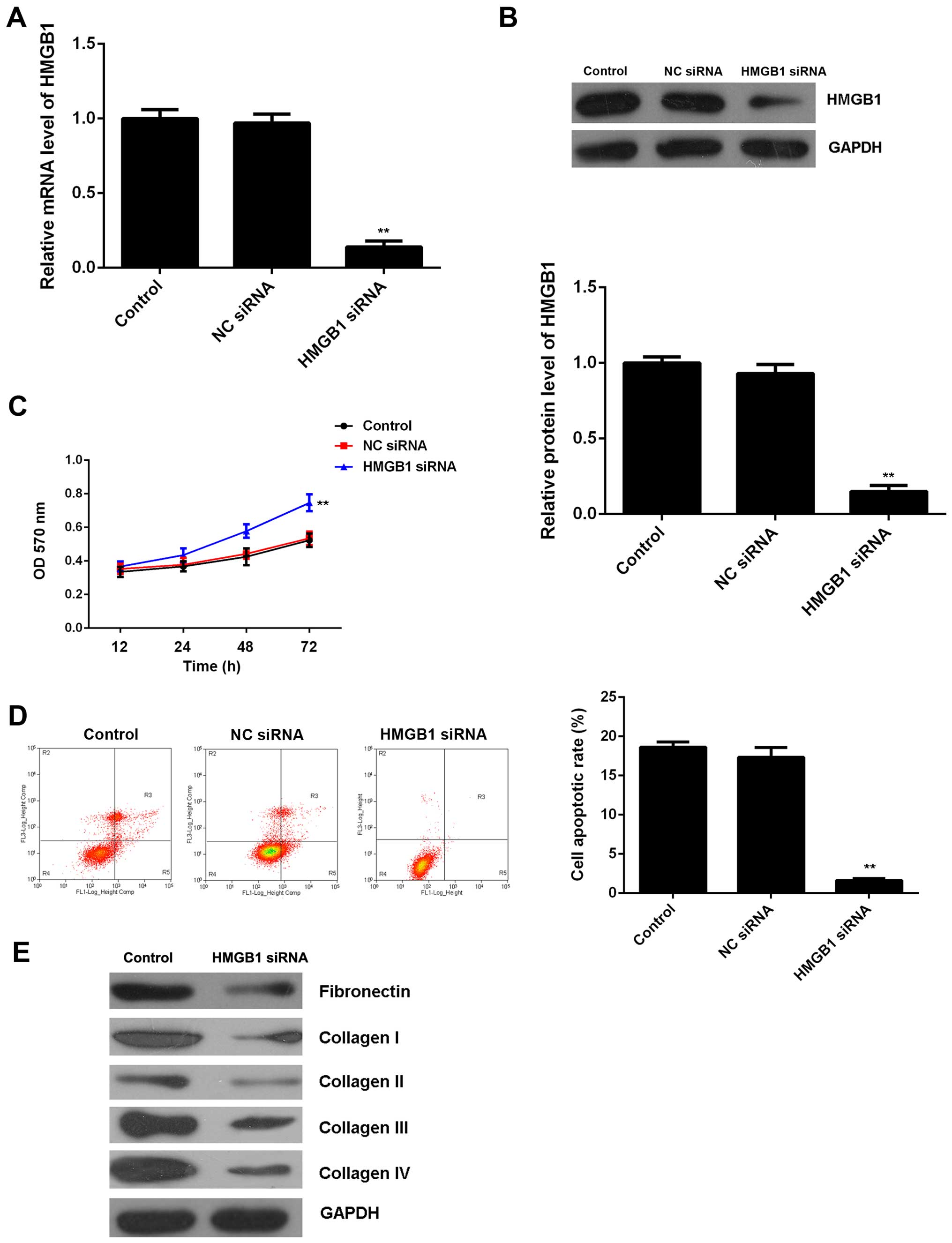
Knockdown of HMGB1 suppresses H/R-induced
apoptosis and fibrosis of M6200 cells
To further clarify whether HGMB1 was involved in the
miR-142-3p-mediated proliferation, apoptosis and fibrosis of M6200
cells treated with H/R, HMGB1 siRNA was transfected into M6200
cells. Following transfection, both the mRNA and protein expression
of HMGB1 were significantly reduced when compared with the control
group, (Fig. 6A and B). Moreover,
the cell proliferation was higher after knockdown of HMGB1 in the
M6200 cells following H/R treatment, and this was accompanied by a
lower level of cell apoptosis (Fig.
6C and D). Furthermore, we found that knockdown of HMGB1 also
suppressed the protein levels of fibrosis-related proteins
(Fig. 6E). Accordingly, these
findings demonstrate that knockdown of HMGB1 suppresses H/R-induced
apoptosis and fibrosis of M6200 cells, which is similar to the
effects of miR-142-3p upregulation.
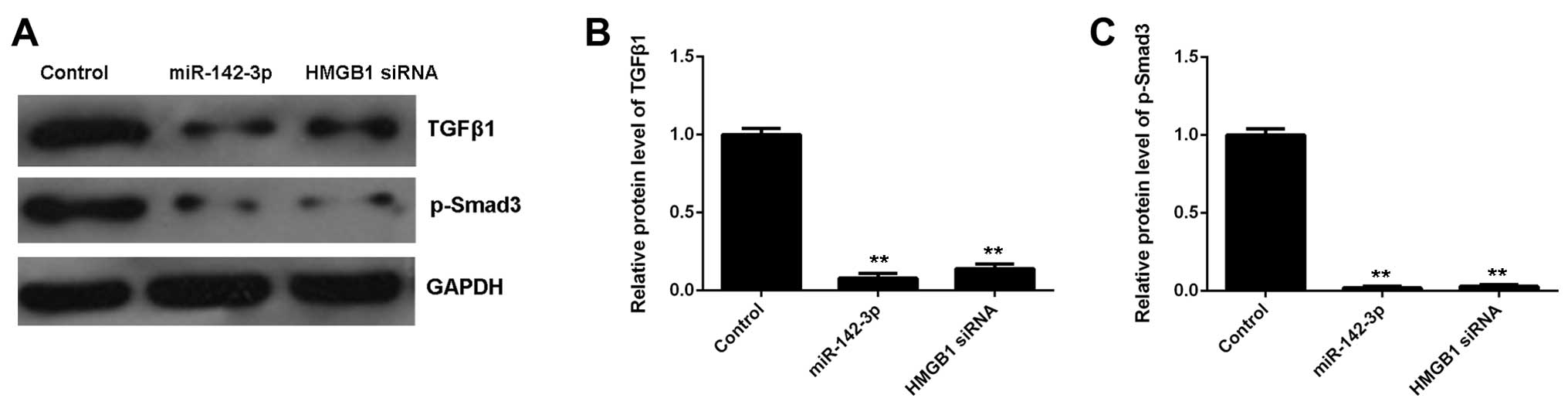
Involvement of TGF-β1/Smad3 signaling in
the miR-142-3p/HMGB1-mediated apoptosis and fibrosis of M6200 cells
treated with H/R
As the TGF-β1/Smad3 signaling pathway has been
reported to be mediated by HMGB1 and involved in HMGB1-mediated
apoptosis and fibrosis, we performed western blot analysis in order
to measure the activity of this signaling pathway in M6200 cells in
each group. Our data showed that the protein levels of TGF-β1 and
p-Smad3 were significantly decreased in miR-142-3p-overexpressing
M6200 cells treated with H/R, when compared with the control group
(Fig. 7). As overexpression of
miR-142-3p may inhibit the protein expression of HMGB1, we suggest
that the TGF-β1/Smad3 signaling pathway is involved in the
miR-142-3p/HMGB1-mediated apoptosis and fibrosis of M6200 cells
treated with H/R.
Discussion
Myocardial I/R injury may cause apoptosis of
cardiomyocytes as well as cardiac fibrosis, which may further lead
to heart failure. Therefore, understanding the underlying molecular
mechanism is important for the development of effective therapeutic
strategies for myocardial I/R injury. Herein, we examined the role
of miR-142-3p in H/R-induced apoptosis and fibrosis of M6200
cardiomyocytes. Our data showed that treatment with H/R induced
cell apoptosis and the upregulation of fibrosis-related proteins,
namely fibronectin and collagen I, II, III and IV, which was
accompanied by the downregulation of miR-142-3p in M6200 cells.
Further investigation indicated that miR-142-3p suppressed
H/R-induced apoptosis and fibrosis of M6200 cells partly at least,
through the direct inhibition of HMGB1 expression in M6200 cells.
Moreover, we found that the TGF-β1/Smad3 signaling pathway was
involved in the miR-142-3p/HMGB1-mediated apoptosis and fibrosis of
M6200 cells treated with H/R.
Previous studies have focused on the role of miR-142
in human cancers (19,20). For example, the serum level of
miR-142-3p was found to be significantly decreased in patients with
colorectal carcinoma, suggesting that miR-142-3p may serve as a
novel, non-invasive biomarker in the diagnosis of colorectal cancer
(21). On the contrary,
miR-142-3p was markedly upregulated in the plasma of patients with
head and neck squamous cell cancer, and its upregulation correlated
with a poorer prognosis (22).
Isobe et al reported that miR-142 enhanced the
tumorigenicity of human breast cancer stem cells partly at least,
by directly targeting the canonical Wnt signaling pathway and
regulating the expression of miR-150 (23). In addition, the downregulation of
miR-142-3p was found to enhance thyroid follicular tumorigenesis by
enhancing the expression of ASH1L and MLL1 (24). Recently, miR-142-3p has been
implicated in cardiovascular diseases (9). In the present study, we found that
H/R treatment led to a significant decrease in the miR-142-3p level
as well as inducing the apoptosis and fibrosis of M6200 cells. We
speculated that the downregulation of miR-142-3p may play a role in
H/R-induced apoptosis and fibrosis of M6200 cells. Therefore, M6200
cells were transfected with miR-142-3p mimic to upregulate its
expression. Our data indicated that the restoration of miR-142-3p
markedly suppressed the H/R-induced apoptosis and fibrosis of M6200
cells, which confirmed our speculation.
As miRs function through negative mediation of their
target genes (25), we further
focused on the investigation of the putative targets of miR-142-3p
using bioinformatics analysis and a Dual-Luciferase reporter assay.
We identified HMGB1 as a direct target gene of miR-142-3p and
indicated that the expression of HMGB1 was negatively regulated by
miR-142-3p in M6200 cells. HMGB1 can be produced by various types
of cell such as immune cells, hepatocytes or endothelial cells, as
well as cardiomyocytes, and has been found to play a critical role
in cell death and survival (26–29). Moreover, HMGB1 is an important
pro-inflammatory cytokine in cardiovascular diseases (30). The cardiac expression of HMGB1 is
significantly increased in I/R injury, which makes it a potential
therapeutic target (16). The
inhibition of HMGB1 may protect against I/R-induced myocardial
injury and improve cardiac performance (17). In our study, knockdown of HMGB1
markedly suppressed H/R-induced apoptosis and fibrosis in M6200
cells, similar to the effects of miR-142-3p overexpression. Based
on these findings, we suggest that the suppressive role of
miR-142-3p on the H/R-induced apoptosis and fibrosis of M6200 cells
occurs partly at least through the direct inhibition of HMGB1
expression.
The TGF-β1/Smad3 signaling pathway plays an
important role in tissue fibrosis through regulating the production
of collagens as well as other extracellular matrix (ECM) components
(33). Previous research has found that HMGB1 participates in the
epithelial-to-mesenchymal transition (EMT) in pulmonary fibrosis
through regulating the activity of TGF-β1/Smad3 signaling (18). Li et al found that HMGB1
enhanced TGF-β1 expression and triggered Smad2/3 phosphorylation,
whereas TGF-β1 deficiency ameliorated HMGB1-mediated EMT with
reduced p-Smad2/3 in A549 cells (18). In our study, we found that both
miR-142-3p overexpression and HMGB1 knockdown inhibited the
activity of the TGF-β1/Smad3 signaling pathway in M6200 cells
treated with H/R. Based on these findings, we suggest that
overexpression of miR-142-3p inhibits HMGB1 expression, which
suppresses the activation of TGF-β1/Smad3 signaling, and
subsequently suppresses ECM production and fibrosis in H/R-treated
M6200 cells.
In conclusion, the present study demonstrates that
miR-142-3p inhibits H/R-induced apoptosis and fibrosis of
cardiomyocytes partly at least, by the direct inhibition of HMGB1
expression. Therefore, these findings have expanded our
understanding of the pathogenesis of H/R-induced myocardial
injury.
References
|
1
|
Yong KW, Li Y, Huang G, Lu TJ, Safwani WK,
Pingguan-Murphy B and Xu F: Mechanoregulation of cardiac
myofibroblast differentiation: implications for cardiac fibrosis
and therapy. Am J Physiol Heart Circ Physiol. 309:H532–H542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korkmaz-Icöz S, Lehner A, Li S, Vater A,
Radovits T, Hegedűs P, Ruppert M, Brlecic P, Zorn M, Karck M and
Szabó G: Mild Type 2 diabetes mellitus reduces the susceptibility
of the heart to ischemia/reperfusion injury: identification of
underlying gene expression changes. J Diabetes Res.
2015:3964142015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan
MK, Nylander S and Birnbaum Y: Ticagrelor protects the heart
against reperfusion injury and improves remodeling after myocardial
infarction. Arterioscler Thromb Vasc Biol. 35:1805–1814. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Ji SY, Liu SZ, Jing R and Lou WJ:
Cardioprotective effect of breviscapine: inhibition of apoptosis in
H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following
simulated ischemia/reperfusion injury. Pharmazie. 70:593–597.
2015.PubMed/NCBI
|
|
5
|
Li R, Xiao J, Qing X, Xing J, Xia Y, Qi J,
Liu X, Zhang S, Sheng X, Zhang X and Ji X: Sp1 mediates a
therapeutic role of MiR-7a/b in angiotensin II-induced cardiac
fibrosis via mechanism involving the TGF-β and MAPKs pathways in
cardiac fibroblasts. PLoS One. 10:e01255132015. View Article : Google Scholar
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji X, Wu B, Fan J, Han R, Luo C, Wang T,
Yang J, Han L, Zhu B, Wei D, et al: The anti-fibrotic effects and
mechanisms of microRNA-486-5p in pulmonary fibrosis. Sci Rep.
5:141312015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Li W and Wang S, Wu Y, Li Z, Wang
W, Liu R, Ou J, Zhang C and Wang S: MiR-142-3p attenuates the
migration of CD4+ T cells through regulating actin
cytoskeleton via RAC1 and ROCK2 in arteriosclerosis obliterans.
PLoS One. 9:e955142014. View Article : Google Scholar
|
|
10
|
Nair N, Kumar S, Gongora E and Gupta S:
Circulating miRNA as novel markers for diastolic dysfunction. Mol
Cell Biochem. 376:33–40. 2013. View Article : Google Scholar
|
|
11
|
Ellis KL, Cameron VA, Troughton RW,
Frampton CM, Ellmers LJ and Richards AM: Circulating microRNAs as
candidate markers to distinguish heart failure in breathless
patients. Eur J Heart Fail. 15:1138–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voellenkle C, van Rooij J, Cappuzzello C,
Greco S, Arcelli D, Di Vito L, Melillo G, Rigolini R, Costa E, Crea
F, et al: MicroRNA signatures in peripheral blood mononuclear cells
of chronic heart failure patients. Physiol Genomics. 42:420–426.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kee HJ, Park S, Kwon JS, Choe N, Ahn Y,
Kook H and Jeong MH: B cell translocation gene, a direct target of
miR-142-5p, inhibits vascular smooth muscle cell proliferation by
down-regulating cell cycle progression. FEBS Lett. 587:2385–2392.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gruber HE, Hoelscher GL, Bethea S, Ingram
J, Cox M and Hanley EN Jr: High-mobility group box-1 gene, a potent
proinflammatory mediators, is upregulated in more degenerated human
discs in vivo and its receptor upregulated by TNF-α exposure in
vitro. Exp Mol Pathol. 98:427–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen M, Liu Y, Varley P, Chang Y, He XX,
Huang H, Tang D, Lotze MT, Lin J and Tsung A: High-mobility group
box 1 promotes hepatocellular carcinoma progression through
miR-21-mediated matrix metalloproteinase activity. Cancer Res.
75:1645–1656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diao H, Kang Z, Han F and Jiang W:
Astilbin protects diabetic rat heart against ischemia-reperfusion
injury via blockade of HMGB1-dependent NF-κB signaling pathway.
Food Chem Toxicol. 63:104–110. 2014. View Article : Google Scholar
|
|
17
|
Ding HS, Yang J, Chen P, Yang J, Bo SQ,
Ding JW and Yu QQ: The HMGB1-TLR4 axis contributes to myocardial
ischemia/reperfusion injury via regulation of cardiomyocyte
apoptosis. Gene. 527:389–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li LC, Li DL, Xu L, Mo XT, Cui WH, Zhao P,
Zhou WC, Gao J and Li J: High-mobility group box 1 mediates
epithelial-to-mesenchymal transition in pulmonary fibrosis
involving transforming growth factor-β1/Smad2/3 signaling. J
Pharmacol Exp Ther. 354:302–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai S, Tong M, Ng KY, Kwan PS, Chan YP,
Fung TM, Lee TK, Wong N, Xie D, Yuan YF, et al: Regulatory role of
miR-142-3p on the functional hepatic cancer stem cell marker CD133.
Oncotarget. 5:5725–5735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghanbari R, Mosakhani N, Asadi J, Nouraee
N, Mowla SJ, Yazdani Y, Mohamadkhani A, Poustchi H, Knuutila S and
Malekzadeh R: Downregulation of plasma miR-142-3p and miR-26a-5p in
patients with colorectal carcinoma. Iran J Cancer Prev.
8:e23292015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Summerer I, Unger K, Braselmann H,
Schuettrumpf L, Maihoefer C, Baumeister P, Kirchner T, Niyazi M,
Sage E, Specht HM, et al: Circulating microRNAs as prognostic
therapy biomarkers in head and neck cancer patients. Br J Cancer.
113:76–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isobe T, Hisamori S, Hogan DJ, Zabala M,
Hendrickson DG, Dalerba P, Cai S, Scheeren F, Kuo AH, Sikandar SS,
et al: miR-142 regulates the tumorigenicity of human breast cancer
stem cells through the canonical WNT signaling pathway. Elife.
3:e019772014. View Article : Google Scholar :
|
|
24
|
Colamaio M, Puca F, Ragozzino E, Gemei M,
Decaussin-Petrucci M, Aiello C, Bastos AU, Federico A, Chiappetta
G, Del Vecchio L, et al: miR-142-3p down-regulation contributes to
thyroid follicular tumorigenesis by targeting ASH1L and MLL1. J
Clin Endocrinol Metab. 100:E59–E69. 2015. View Article : Google Scholar
|
|
25
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Wang Z, Han G, Huang L, Jiang J and
Li S: Ketamine attenuates high mobility group box-1-induced
inflammatory responses in endothelial cells. J Surg Res.
200:593–603. 2015. View Article : Google Scholar
|
|
27
|
Lea JD, Clarke JI, McGuire N and Antoine
DJ: Redox-dependent HMGB1 isoforms as pivotal co-ordinators of
drug-induced liver injury: mechanistic biomarkers and therapeutic
targets. Antioxid Redox Signal. 24:652–665. 2016. View Article : Google Scholar
|
|
28
|
Zhang J, Yong Y, Li X, Hu Y, Wang J, Wang
YQ, Song W, Chen WT, Xie J, Chen XM, et al: Vagal modulation of
high mobility group box-1 protein mediates
electroacupuncture-induced cardioprotection in ischemia-reperfusion
injury. Sci Rep. 5:155032015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh V, Roth S, Veltkamp R and Liesz A:
HMGB1 as a key mediator of immune mechanisms in ischemic stroke.
Antioxid Redox Signal. 24:635–651. 2016. View Article : Google Scholar
|
|
30
|
Cai J, Wen J, Bauer E, Zhong H, Yuan H and
Chen AF: The role of HMGB1 in cardiovascular biology: danger
signals. Antioxid Redox Signal. 23:1351–1369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lytle KA, Depner CM, Wong CP and Jump DB:
Docosahexaenoic acid attenuates western diet-induced hepatic
fibrosis in Ldlr−/− mice by targeting the TGFβ-Smad3
pathway. J Lipid Res. 56:1936–1946. 2015. View Article : Google Scholar : PubMed/NCBI
|