Introduction
Patients with high altitude-associated polycythemia
(HAPC) often have excessive erythrocytosis [generally, females have
hemoglobin (Hb) levels >19 g/dl and males have Hb levels >21
g/dl]. The disease affects the majority of individuals residing at
a high altitude (1). More red
blood cells (RBCs) are produced to carry oxygen to the lungs
(2,3). The number of RBCs reaches a high
level in the majority of individuals following long-term exposure
to high-altitude situations. However, the number of RBCs continues
to increase and this results in serious complications.
Normally, gastric mucosal lesion (GML) is a
gastrointestinal disorder which is often associated with HAPC is
hard to overcome. GMLs usually lead to serious clinical
complications in the gastric system and extend from the mucosa into
the submucosa. There is evidence to indicate that blood flowing
through capillaries (4,5), arterioles (6) and collecting venues (7) is important for maintaining the
normal structure and functions of most organs. Generally, the
gastric micro-circulates are bypassed by arteriovenous shunting,
which results in severe injuries to the gastric mucosa (8). It can be hypothesized that
HAPC-related GML is linked with gastric mucosal ischemia, which is
caused by microvascular thrombosis due to polycythemia.
Additionally, under normal conditions, the physiological balance is
often kept between the secretion of peptic acid and the defense
mechanisms of the gastroduodenal mucosa. Mucosal injuries and
subsequent peptic ulcers can occur when the balance between the
factors of aggression and the defense mechanisms is disrupted. The
decrease in the defense system is often caused by a number of
factors, such as hypoxia (9).
Hypoxia is a main reason for the pathophysiological change at
high-altitude situations. Hypoxia usually decreases the blood
flowing to the gastric tissues, resulting in ischemia and the
subsequent destruction of the mucosal linings. Rat models of
hypoxia-ischemia have been widely used to explore the molecular
mechanisms through which hypoxia-ischemia often causes brain or
neonatal injuries (10–14). However, there are limited data
available on the effects of hypoxia-ischemia on HAPC-related GML
(15).
In this study, to better understand the molecular
mechanisms responsible for the development of HAPC-related GML, we
compared the gene expression profiles from patients with HAPC and
healthy residents at high altitudes in an aim to identify more
candidate molecules which are involve in the development of
HAPC.
Materials and methods
Study participants
All experimental protocols were conducted based on
the ethical guidelines of the Helsinki Declaration and approved by
Human Ethics Committee at the People's Hospital of Tibet Autonomous
Region (Lasha, China). Written informed consent was obtained from
all participants. In June 2014, 3 patients living at altitudes of
3,600 to 4,800 m (Tibetan Plateau), diagnosed with HAPC were
recruited as the HAPC group. At the same time, 3 healthy residents,
also living at altitudes of 3,600 to 4,800 m (Tibetan Plateau) and
receiving gastrointestinal endoscopy for the re-examination of
gastric submucosal injuries at the same time, were enrolled as the
control group. All participants were life-long residents at Lhasa
or Nagqu, and/or Rigaze of Tibet. Each patient with HAPC was
matched to a healthy resident in regards to factors, such as
gender, birthplace, age, lifestyle, diet, body mass index (BMI),
altitude and occupation. All subjects were native male Tibetans and
residents and were 40 to 45 years of age.
Prior to endoscopic examinations, peripheral venous
blood was obtained using vacuum tubes. The oxygen saturation of
arterial blood was measured using pulse oximetry. the inclusion
criteria were as follows: i) HAPC was diagnosed as defined at the
2004 Qinghai International High Altitude Medicine Conference,
namely concentrations of Hb >21 g/dl for males and >19 g/dl
for females (1); ii) the clinical
complications included the metabolic disturbance-related headaches
(16), cachexia, polycythemia and
hypercalcemia (17), digestive
disorders (18) and difficulty
sleeping (19). Finally, 30 HPAC
patients and 30 matched healthy subjects (with a similar lifestyle,
living altitude, age and gender distribution as the HPAC patients)
received endoscopy detection.
The exclusion criteria were as follows: i) chronic
pulmonary disorders, such as emphysema (20), bronchitis (21), alveolar fibrosis and lung cancer
(which may be caused by smoking) (22); ii) chronic respiratory
polycythemia; iii) severe other disorders, such as heart, brain,
liver and kidney disease; iv) alcohol and drug abuse, mental injury
or disease, which may make it difficult to perform gastroscopy; v)
pregnant or lactating women; vi) an obstructed gastrointestinal
tract; and vii) medical history, such as recent gastrointestinal
bleeding. Chronic gastritis was diagnosed based on the Chinese
Consensus on Chronic Gastritis, which was established in Shanghai
in 2006.
Endoscopic detection
A rigorous endoscopic surveillance was conducted in
all participants, as previously described (23). Ten milliliters viscous lidocaine
hydrochloride mucilage (Jiangsu Jichuan Pharmaceutical Co., Ltd.,
Jiangsu, China) was orally administered to all subjects. A
gastroscope (Olympus GIF-260; Olympus, Tokyo, Japan) was used to
observe the gastric tissues. All subjects were examined by the same
endoscope and one endoscopist. The light source strength and type
of endoscopic lamp used was also same in the study. The color
change of the gastrointestinal mucosa (the esophagus, cardia,
gastric fundus, gastric antrum, duodenal bulb and the descending
portion) was also overserved in all persons.
Bile influx measurement
One-channel MI catheters with unique sensor arrays
were used to traverse the working channels of the upper endoscopes.
Bile influx was measured in all subjects. Motility index was tested
at the sites of 2, 5 and 10 cm above the squamocolumnar junction.
The motility index values were compared at different levels along
the esophageal axis between the patients with HAPC and the healthy
subjects.
Histological analysis
The mucosa was collected at the Endoscopy Surgery
Department, the People's Hospital of the Tibet Autonomous Region
(Tibet, China). We analyzed 12 gastric antrum biopsies, including 6
biopsies from patients with HAPC and another 6 from the control
subjects. The samples were frozen in liquid nitrogen as soon as the
surgical excision was completed, and they were stored at −80°C.
Prior to histological analyses, the gastric mucosa tissues were
treated formalin and embedded in paraffin. The treated tissues were
cut into 5-µm-thick sections, and stained with hematoxylin
and eosin (Sigma, St. Louis, MO, USA). The results were analyzed by
two experts in a double-blinded manner. The mucosa was considered
injured if one or more of the following were observed:
non-continuous surface, expanded gland, hemorrhage, or cells with
destructed morphology (24).
RNA extraction
Gastric samples from the 3 patients with HAPC and 3
control subjects were collected. All samples were treated with 3 ml
TRIzol (Invitrogen, Carlsbad, CA, USA), and homogenized with a
homogenizer. Following extraction with chloroform, RNA was
precipitated with isopropanol. The resultant pellets were
re-suspended in TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA).
Following DNase digestion, the quantification and purity RNA was
measured at 260/280 nm using an Agilent-Bioanalyzer (Agilent
Technologies, Palo Alto, CA, USA). RNA integrity and genome DNA
contamination was identified using agarose gel electrophoresis.
Microarray data analysis
Single-color gene expression profiles were created
to compare data between the patients with HAPC patients and the
healthy controls. These profiles was constructed using 4×44 K
oligonucleotide microarrays (Agilent Technologies). The human
genome microarray has 41,000 genes and transcripts. The
representative sequences can be found at public databases. The RNA
samples were amplified and then labeled using a labeling kit
(Agilent Technologies) and hybridized with a human genome
microarray in the chambers of Agilent's SureHyb. Following
hybridization and washing, the slides were scanned using a DNA
microarray scanner (G2505B; Agilent Technologies) and analyzed
using Feature Extraction Analytics software (version 9.5.3; Agilent
Technologies). All the procedures were performed at KangChen
Bio-Tech (Shanghai, China). The Agilent GeneSpring GX software
(version 7.3; Agilent Technologies) was used to analyze the signal
intensities, which represent the gene levels. For data analysis,
fold changes were used to explore the differentially expressed
genes (DEGs) at the 2-fold change cut-off. Genes were regarded as
upregulated genes with changes in expression of ≥2-fold compared to
the controls. By contrast, genes were regarded as downregulated
genes with changes in expression of <2-fold. Changes in
experssion of 0.50–1.99-fold were not regarded as significant.
Gene Ontology (GO) analysis
The GO database was referred to in order to analyze
the DEGs involving biological functions and signaling pathways. The
GO project has a controlled vocabulary to show gene functions in
any organism (http://www.geneontology.org). The ontology has three
different domains: biological processes, cellular components and
molecular functions. Fisher's exact test was used to determine
whether there were overlaps between the DEGs list and the GO
annotation. A value of P<0.05 indicate that there were
statistically significant differences in GO enrichment terms in the
DEGs.
Reverse transcription-quantitative PCR
(RT-qPCR)
The microarray results were repeatedly confirmed by
RT-qPCR for top DEGs, including KLK1, KLK3, KLK7, KLK8, KLK12 and
the actin gene was used as a loading control. In order to
compensate the shortage of the sample size of transcriptome
analysis, total RNA was isolated from gastric mucosal samples from
another 20 patients with HAPC patients and another 20 healthy
subjects living at the same altitude. RNA (5 µg) was
reverse-transcribed using a reverse transcriptase reaction kit
(Takara, Dalian, China). Using the primers shown in Table I, PCR was conducted in triplicate
using SYBR-Green PCR Master Mix and the 7500 Fast real-time PCR
detection system (ABI Biosystems, Foster City, CA, USA) with the
amplified conditions: 95°C for 10 min, 45 cycles of 95°C for 10
sec, 60°C for 34 sec and 60°C for 60 sec. The relative expression
values were calculated using the 2−ΔΔT method.
 | Table IPrimers and product sizes of genes
that were examined by RT-qPCR. |
Table I
Primers and product sizes of genes
that were examined by RT-qPCR.
| Gene | Length | Forward primer | Reverse primer |
|---|
| KLK1 | 140 bp |
GCTCTGTACCATTTCAGCAC |
GCTGTGTTTTCGTCGTCAAA |
| KLK3 | 160 bp |
ATCCTGTCTCGGATTGTGGG |
AGATCACGCTTTTGTTCCTG |
| KLK7 | 150 bp |
GCAGGAGAAGAAGCCCAGGG |
GTGGGCGGCAGTGAGCACCC |
| KLK8 | 140 bp |
GCCTGGGCAGGACACTCCAG |
CAGTTGCCACCTACAAGGAC |
| KLK12 | 151 bp |
GAGGGCACCAGCCTGCGCTG |
AGCCGCTGTGCCGGATCTGC |
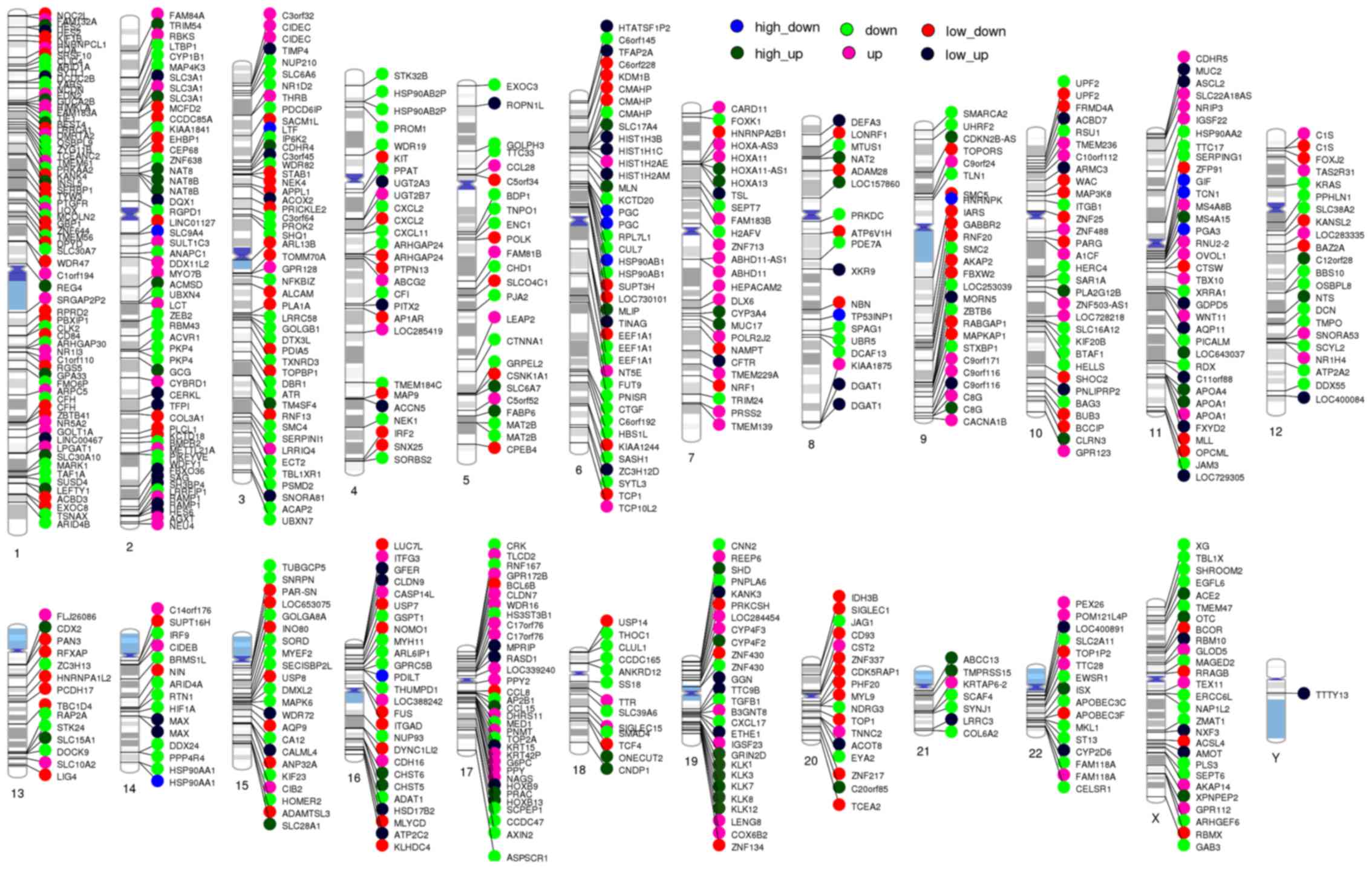
DEGs on chromosome locations
Chromosomes may be related to HAPC-induced GML
(25,26), which can be reflected by DEGs on
different chromosomes. Thus, in this study, all the DEGs were
marked on 24 chromosomes to visualize their distributions on all
chromosomes.
Remodeling the interaction between KLKs
and cholesterol
Cholosterol has been widely reported to be
associated with hypertension (27,28), while kallikrein hypertension is
also well known (29,30). Thus, in this study, we explored
the interaction between KLKs and cholesterol using the software
PyMOL v1.8 (https://www.pymol.org) and AutoDock
4.2.6 (http://autodock.scripps.edu).
Statistical analysis
Data are presented as the means ± SD. Hierarchical
cluster analysis was performed between HPAC and healthy groups
using transcriptional data and SPSS software. The Chi-square and
Student's t-test were used to calculate the statistical
significance of paired data where appropriate. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
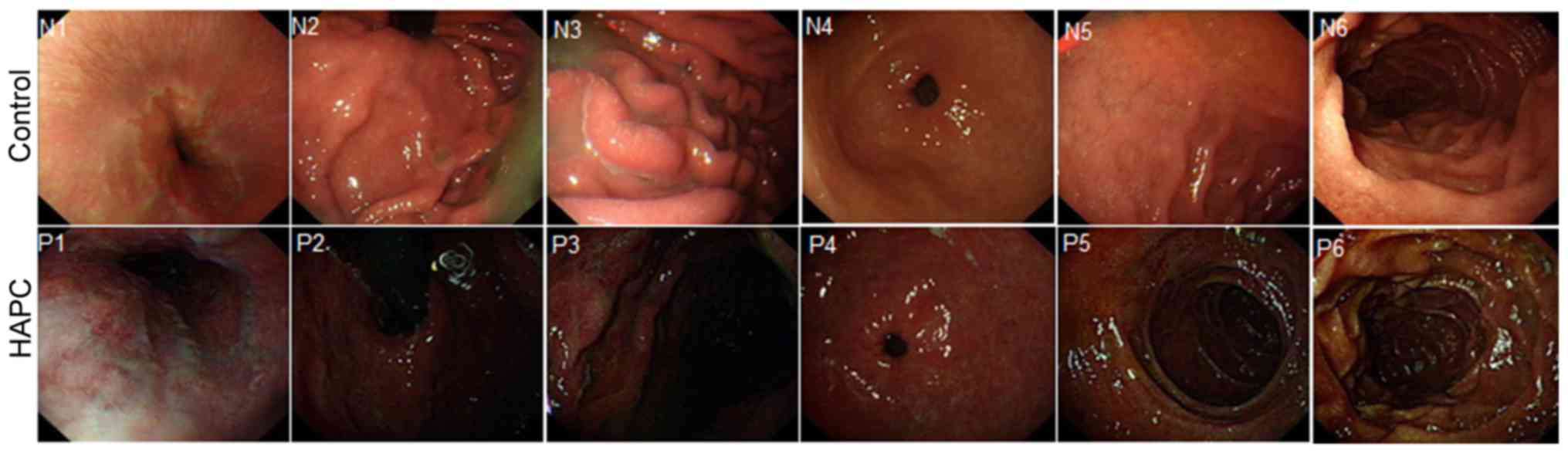
Endoscopic findings of the upper
gastrointestinal tract
The patients had a similar gender distribution, age,
living altitude and lifestyle with the healthy subjects (Table II). In the HAPC group, the
endoscopic findings reveaked darker colors in the upper
gastrointestinal tract when compared with the controls, including
the esophagus (N1, brown; P1, dark red), cardia (N2, thin brown;
P2, dark red brown), gastric fundus (N3, thin brown; P3, dark red
brown), gastric antrum and body (N4, brown; P4, red), the duodenal
bulb (N5, brown; P5, dark brown) and descending portion (N6, brown;
P6, dark brown) (Fig. 1). All the
N-marked numbers indicate the gastrointestinal tract from healthy
subjects, whereas all the P-marked numbers indicate the
gastrointestinal tract from patients with HAPC. Furthermore, in the
HAPC group, the mucosa was thin and red, with a fine meshwork of
vessels (Fig. 1, P1 and P4).
Furthermore, congestion was detected in areas where veins were
slightly wider. Additionally, due to the significantly red
appearance of the esophageal mucosa, there were no evident
boundaries that were distinguished between the esophageal mucosa
and gastric mucosa. The color was orange below the line. Diffuse
hyperemia, edema and changes of congestion, were observed in HAPC
group, whereas no such symptoms were observed in the control group.
In addition, the duodenal bulb was slightly enlarged with marked
hyperemia and swelling in the HAPC group when compared with the
control group (Fig. 1).
 | Table IIBaseline characters between HPAC
patients and healthy subjects. |
Table II
Baseline characters between HPAC
patients and healthy subjects.
| HPAC patients (n=30
cases) | Healthy controls
(n=30 cases) | P-values |
|---|
| Male, n (%) | 13 (43.3) | 13 (43.3) | 1 |
| Age, years | 42.5±2.5 | 42.5±2.5 | 1 |
| Smokers, n (%) | 15 (50.0) | 15 (50.0) | 1 |
| Drinkers, n
(%) | 12 (40.0) | 12 (40.0) | 1 |
| Spouse, n (%) | 21 (70.0) | 21 (70.0) | 1 |
| Living altitude
(m) | 4,200±600 | 4,200±600 | 1 |
| Bile reflux, n
(%) | 26 (86.7) | 5 (16.7) | 0.00 |
| Score of gastric
mucosal damage | 3.04±0.31 | 0.26±0.05 | 0.00 |
| Hyperemia and
bleeding, n (%) | 54 (90.0) | 5 (8.3) | 0.00 |
Abnormal contraction and relaxation only occurred in
approximately 12.5% of the subjects, and most pyloric antra
contracted and relaxed normally in the control group (P<0.01).
Additionally, in the HAPC group, bile was observed in both the
fundus and body of the stomach, as well as in the pyloric antrum
and varying degrees of bile reflux were detected in approximately
86.7% of the patients. By contrast, in the control group, gastric
bile was observed in only 16.7% of the subjects (P<0.001)
(Table II).
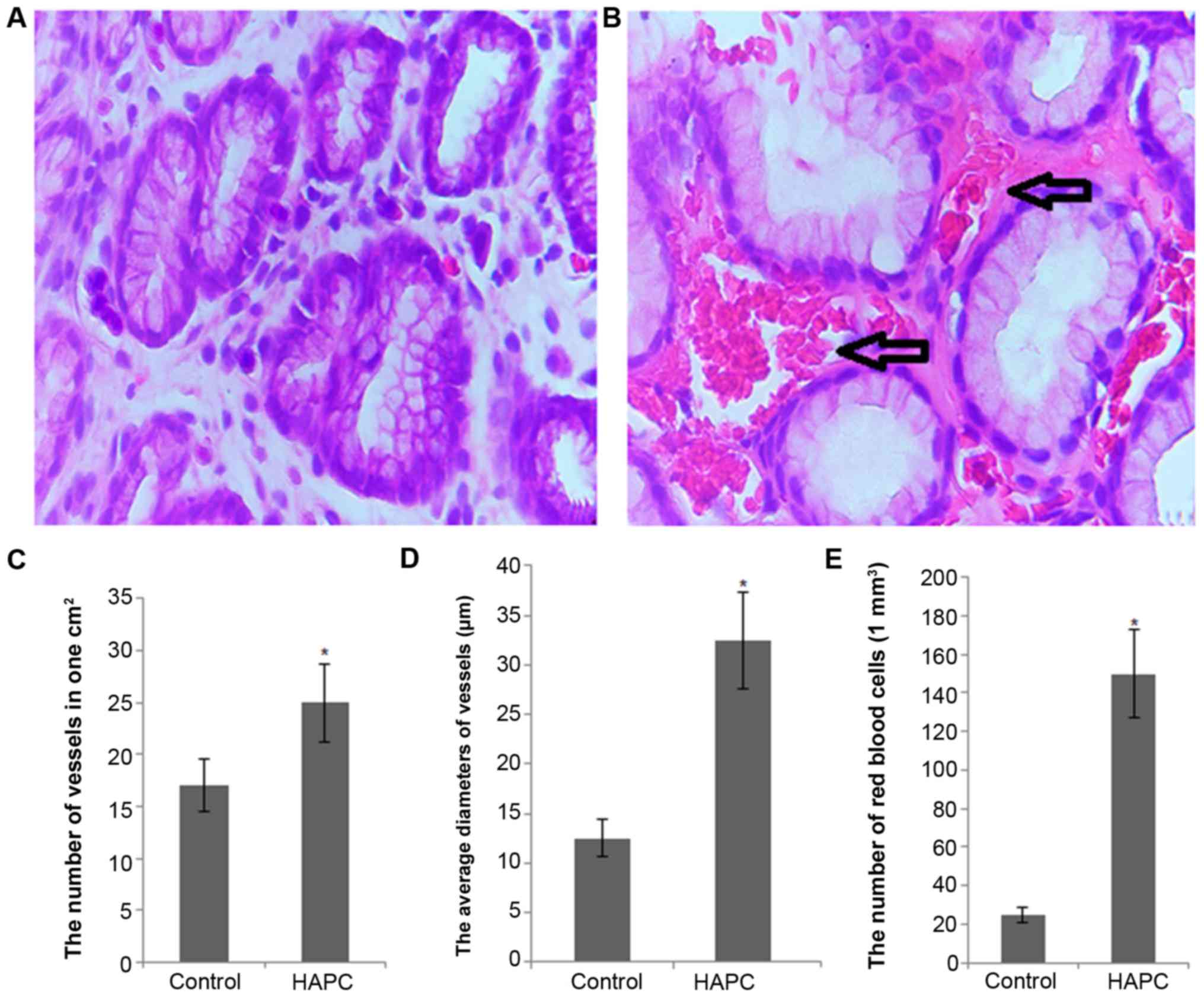
Histopathological changes
As shown in Fig.
2A, histopathological analysis revealed that HAPC induced
severe congestion, edema and multiple hemorrhagic erosions in the
gastric antrum mucosa from patients with HAPC. By contrast, no
significant damage was observed in the gastric mucosa from the
control group subjects. On the other hand, the mean score of
gastric mucosal damage was 3.04±0.31 in the patients with HAPC,
which was higher than the score of 0.26±0.05 in the control
subjects (P<0.01) (Table
II).
Under a microscope, a variety of changes in vessels
within the gastric mucosa was observed in the HAPC group, such as
dilation and distortion accompanied by hyperemia and bleeding;
these changes were greater than those of the control group, with
statistically significant differences (90.0 vs. 8.3%, P<0.001)
(Table II). Under high
magnification, the number of vessels/cm2 was
significantly higher in the HAPC group than in the control group
(24.68±4.38 vs. 11.79±2.43, P<0.05) (Fig. 2C). Similarly, the average vessel
diameter was significantly higher in the HAPC group than in the
control group (39.2±11.5 vs. 15.9±4.5, P<0.05) (Fig. 2D). Furthermore, the RBC counts
were also higher in the HAPC group than in the control group
(160.91±62.53 vs. 30.33±15.98, P<0.05) (Fig. 2E).
Hierarchical cluster analysis of the DEGs
in the gastric mucosa of patients with HAPC
We analyzed the expression spectrum of the DEGs in
the gastric mucosa of patients with HAPC and the control group
subjects. Transcriptome analysis revealed mRNA subtype-specific and
clinical subtype-specific patterns of DEGs. DEGs with >2-fold
changes in expression at the transcriptional level were
considerably more diverse in the HAPC group than in the control
group. However, the number of overall upregulated DEGs was higher
in the control group than in the HAPC group.
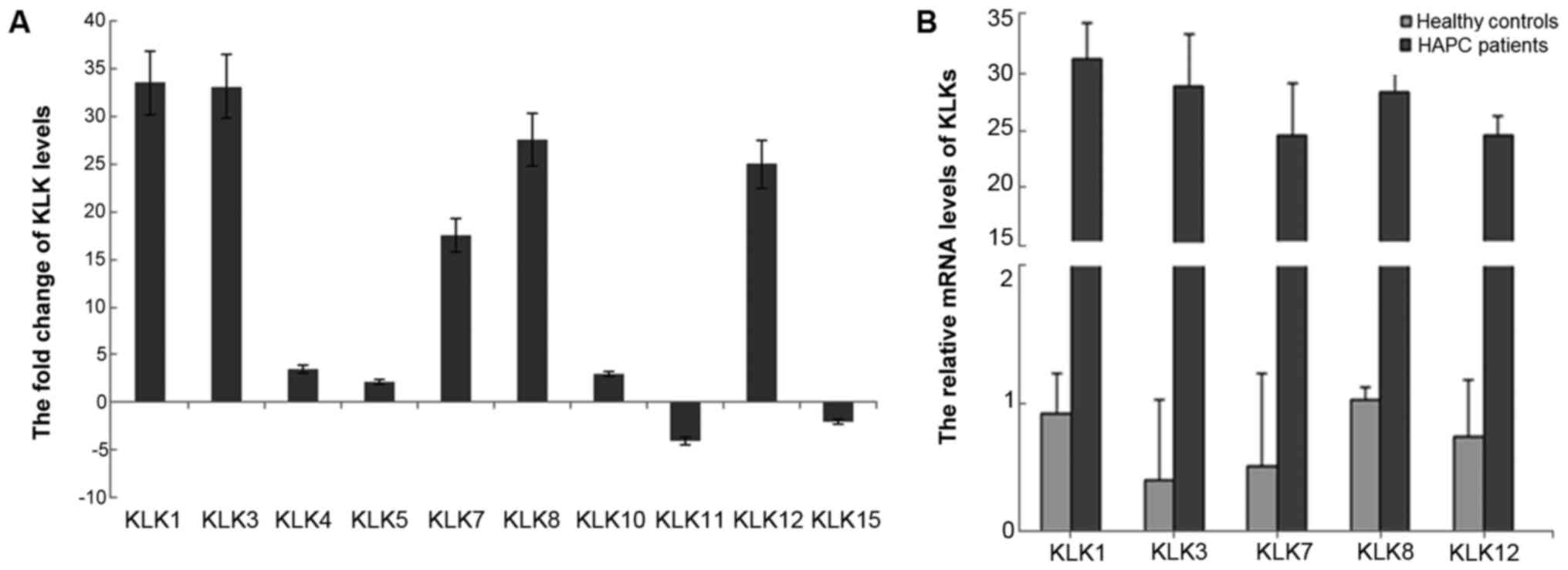
Hierarchical cluster analysis revealed 4,341
upregulated DEGs and 5,363 downregulated DEGs in the gastric mucosa
of patients with HAPC. In particular, the kallikrein gene cluster
(KLK1/3/7/8/12) was upregulated >17-fold in the patietns with
HAPC compared to the controls (Fig.
3).
Results of RT-qPCR
Transcriptome analysis revealed the changes in all
KLK members and 10 members were DEGs (Fig. 3A). Among these members,
KLK1/3/7/8/12 were significantly upregulated, KLK11 and KLK15 were
downregulated, and KLK4/5/10 were slightly upregulated. No
significant differences were observed in the other members
(KLK2/6/9/14) as shown by RT-qPCR analysis of these genes in 3
pairs of tissues from patients wtih HAPC-induced GML; KLK1 and KLK3
were upregulated >30-fold, while KLK5 and KLK11 were
downregulated 3-fold in the tissues from patients with HAPC-induced
GML compared with the healthy tissues. To confirm the microarray
results, RT-qPCR was performed to measure 5 top upregulated genes
from 20 HAPC patients and 20 healthy subjects, including the 3
pairs of patients and healthy subjects analyzed by transcriptome
analysis. The results revealed that the KLK1, KLK3, KLK7, KLK8 and
KLK12 expression patterns (Fig.
3B) were similar to those observed in the microarray
experiments (Fig. 3A).
Biological function analysis of DEG
The identified differential genes were annotated in
the GO format for biological function classification and in the
pathway analysis format for the elucidation of whole chains of
events in the gastric mucosa tissues from patients with HAPC
compared with the controls. In the GO analysis, the upregulated
DEGs were involved in responses to tissue injuries, inducing smooth
muscles, increasing vascular permeability and complex formation,
and others. The downregulated DEGs were involved in intrinsic
GTPase activity, cellular processes, recruiting PH domain proteins,
cell growth and proliferation, as well as others (data not
shown).
The map of DEGs on 24 chromosomes
All DEGs were marked on 24 chromosomes (Fig. 4). KLK1, KLK3, KLK7, KLK8 and KLK12
were all located on chromosome 19q13.3–13.4 and were highly
upregulated in the HAPC group compared with the controls.
Interaction between KLK members and
cholesterol
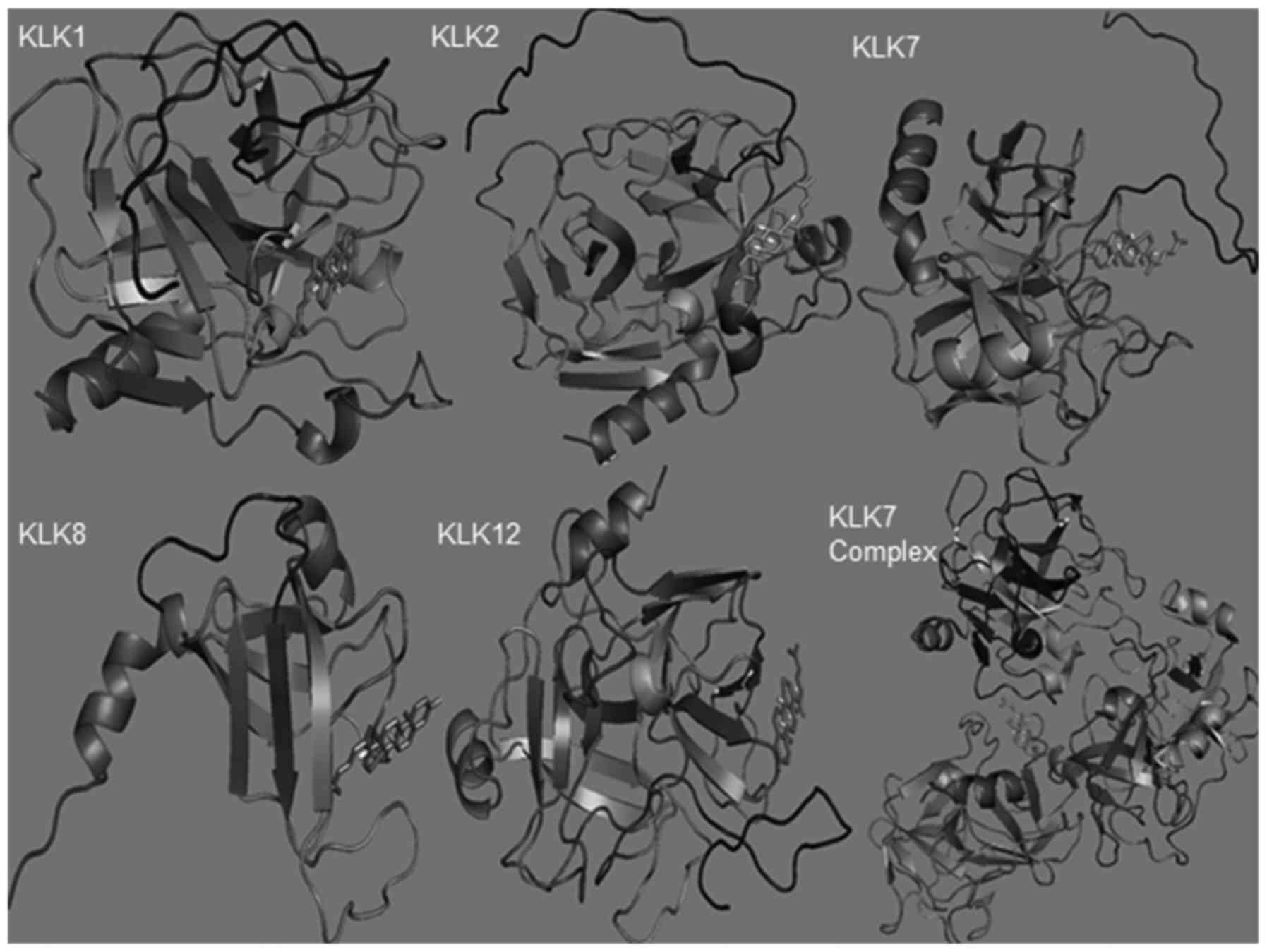
AutoDock analysis revaled that 5 members
(KLK1/2/7/8/12) had high-score binding ability with cholesterol
(Table III). All the members
had different binding sites with cholesterol. Cholesterol tended to
bind the sites near the C-terminal KLK1and KLK2 sequences, the
N-terminal KLK7 and KLK12 sequences and the middle KLK8 sequence
(Fig. 5). Using the polymer of
KLK7 as the model, the results revealed that the trimers tended to
have high score for binding cholesterol (Fig. 5 and Table III).
 | Table IIIInteraction between the KLK1/2/7/8/12
gene cluster members and cholesterol. |
Table III
Interaction between the KLK1/2/7/8/12
gene cluster members and cholesterol.
| KLK members | Score | Area | Atomic contact
energy | Transformation |
|---|
| KLK1 | 4284 | 487.40 | −339.50 | 2.36, −0.29, −2.37,
121.70, 37.95, −11.19 |
| KLK2 | 4944 | 553.90 | −204.99 | 2.48, 0.98, 0.14,
−13.00, 16.86, 13.76 |
| KLK7 | 4386 | 481.70 | −205.67 | −3.04, −0.67, 2.14,
−0.62, 10.67, 10.93 |
| KLK8 | 4342 | 456.00 | −305.37 | 0.29, −0.67, −0.59,
−11.48, 21.89, 4.73 |
| KLK12 | 4734 | 564.50 | −252.11 | −1.68, −0.14,
−0.24, −24.08, −16.63, −7.34 |
| KLK7 complex | 5534 | 613.60 |
−62.14 | 2.90, −0.66, 2.05,
−27.73, −5.61, 25.19 |
Discussion
The Qinghai-Tibetan Plateau is the largest and
highest plateau, which contains the largest high-altitude
population worldwide. Due to the increase in the concentrations of
RBC, there is a significant increase in blood viscosity and both
microcirculation disturbances and systemic disorders can occur.
However, no effective prevention and control measures have yet been
utilized. Generally, the incidence of HAPC increases with the
elevation of altitude. However, due the varieties of living
environments such as different altitudes, and diverse ethnic
groups, the incidence of HAPC is differs around the world (31).
The pathogenesis of HAPC is complex and it is
difficult to overcome. It has been widely reported that the
increased synthesis and release of erythropoietin, induced by
long-term exposure to high-altitude hypoxic conditions, is a key
factor in the development of HAPC (32). In this study, Hb concentrations,
RBC counts, the number of microvessels and the diameter of
microvessels in the HAPC group were significantly higher than those
in control group. This was also associated with the notion that the
initiating factor of HAPC was the high-altitude hypoxia-induced
enhancement of bone marrow erythropoiesis, which induced RBC
hyperplasia and related clinical manifestations. In addition, the
oxygen saturation of arterial blood in the HAPC group was
significantly lower than that in control group, indicating that
this may be associated with excessive RBC hyperplasia, as well as
increased blood viscosity and flow resistance in patients with
HAPC.
Studies have shown that in the presence of high
altitude-induced hypoxia, the dynamic equilibrium between in
vivo nitric oxide and endothelin is disrupted, causing enhanced
vasoconstriction and increased systemic peripheral resistance. This
leads to the dilation of mucosal vessels. Moreover, HAPC caused by
high altitude-induced hypoxia leads to increased blood viscosity,
decreased blood flow and severe local mucosal congestion, resulting
in severe vascular hyperemia and even rupture. As a consequence,
microcirculation disturbances occur in the gastric mucosa (15). Considering the environment
contributing to long-term high altitude-induced hypoxia, the
increase in the numbers of RBC can cause damage to the
gastrointestinal mucosa and compromise normal physiological
functions, such as digestion and absorption.
The Whole Human Genome Oligo Microarray is a broad
view that represented all known genes and transcripts in the human
genome. Sequences were compiled from a broad source survey, and
then verified and optimized by alignment to the assembled human
genome. In this study, gastric mucosa tissues samples from patients
with HAPC and healthy controls were analyzed using genome
microarrays, in which 4,941 genes (fold change ≥2) were upregulated
and 5,363 genes (fold change ≤0.5) were downregulated in the
patients with GML. Using GO and pathway analyses, we then examined
the function of DEGs. Our results indicated that HAPC-induced GML
was a process involving multiple genes and pathways.
Certainly, there are some limitations to the present
study. Firstly, the use of only 6 subjects seems to be a small
population with which to explore the molecular mechanisms of GML.
Furthermore, the more detailed molecular mechanisms remain to be
explored. The pathogenesis of HAPC-induced GML was not explored in
this study this was one of initial aims. The disease can be
elucidated in vitro using gastric cells from GML-related
induced pluripotent stem (iPS) cells (33).
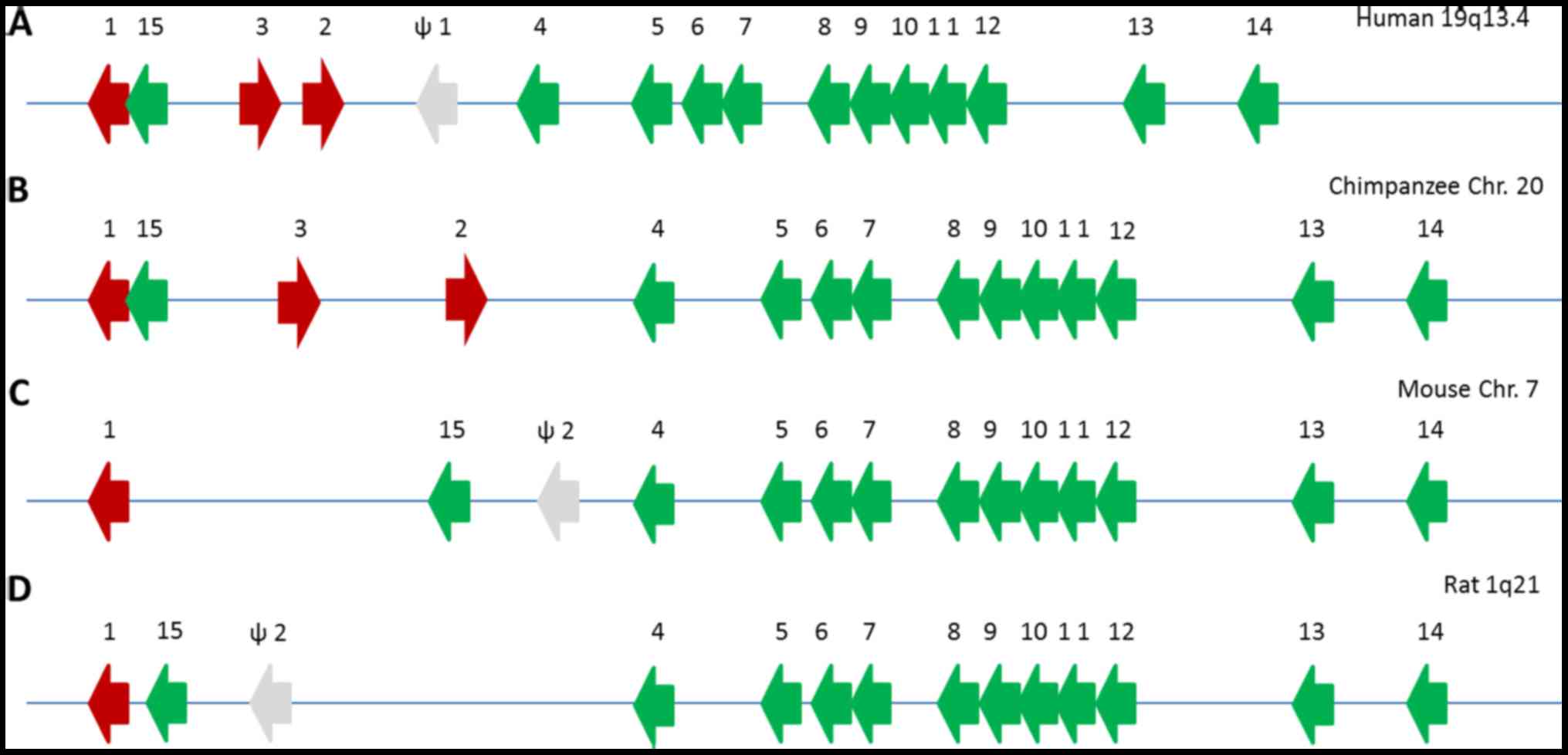
Kallikreins are a group of 15 serine proteases,
which are encoded by the largest gene cluster of proteases. KLK
loci have been described in the human (34), chimpanzee (35), mouse (36) and rat (37) genomes (Fig. 6). Generally, KLK loci consist of a
single copy of KLK4-KLK15 genes with different numbers of classical
KLK genes and pseudogenes, which are caused by gene duplication. In
humans, the KLK loci span approximately 300 kb on chromosome 19 in
the cytogenic region 13.3–13.4. The KLK genes are clustered
together and are not intervened by other genes. The three KLK genes
(KLK1, KLK2 and KLK3) are clustered within KLK15, while KLK4-KLK14
and the ΨKLK1 pseudogene are located telomeric to KLK2. The
transcriptional direction of KLK genes is from telomere to
centromere exception of KLK2 and KLK3.
Over the past decades, a number of studies have
demonstrated important pathophysiological roles for these
kallikreins in various tissues (38). Therefore, kallikreins are
considered as attractive targets for the development of novel
therapies for airway disorders (39), cardiovascular disease (40), brain injury (41), skin inflammation and allergy
(42) and neoplastic disorders
(43). Increasing evidence
indicates that many kallikreins are implicated in carcinogenesis
(44) and are utilized as
potential biomarkers for head and neck squamous cell carcinoma
(45) and colorectal
adenocarcinoma (46). There are
15 kallikrein members located on chromosome 19. These proteins have
conserved functions in their capacities to release the vasoactive
peptide (47) and Lys-bradykinin
from low-molecular-weight kininogen (48).
KLK1 belongs to a member of the peptidase S1 family
and is a serine protease, which involves kinin production for the
normal functions of cardiac and arterial tissues. A previous study
demonstrated that KLK1 is involved in the normal development of
uterine endometrial tissues (49). Bradykinin released from the
endothelium plays a critical role in the regulation of the human
cardiovascular system. Endothelial cells significantly express
de novo kallikrein, and it plays an important role in the
generation of vasoactive kinins (50). Kallikrein-kinin system has been
shown to be involved in some functions of kidneys, such as salt
homeostasis. Therefore, reduced levels of KLK1 contribute to
salt-sensitive hypertension in Dahl salt-sensitive animal models
(51). In this study, we found
increased levels of KLK1 in patients with HPAC, which may play a
protective role in preventing the development of hypertension.
Polycythemia innovles an increase in the amount of RBC which
circulate in the blood stream. Thus, these changes increase the
viscosity of the blood and result in high blood pressure.
KLK3 is also known as prostate-specific antigen or
γ-seminoprotein, which is a glycoprotein enzyme encoded by the KLK3
gene. PSA is secreted by the epithelial cells from the prostate
gland and is a widely used biomarker for prostate cancer (52). Elevated levels of serum PSA
concentrations are often found in patients with prostate cancer
compared with healthy individuals. According to associations found
in healthy adults between the seminal plasma and serum
concentrations of PSA, the mutants of KLK2 and KLK3 can be
benefical to adjust the cut-off value in the PSA model for prostate
cancer (53). Obese individuals
have been observed to have low levels of serum PSA. Delayed early
detection in obese men may result in the risk of prostate cancer
(54). Of note, patients with
polycythemia tend to have a higher risk for cancer (55). Moreover, the association between
polycythemia and obesity has been widely reported (56).
KLK7 is a serine protease encoded by the KLK7 gene
located on chromosome 19q13. KLK7 is initially purified from the
epidermis and is a stratum corneum chymotryptic enzyme (57). KLK7 has been shown to be
aberrantly expressed in colon cancer and to be involved in cell
proliferation in vitro and in vivo. Thus, KLK7 is a
potential therapeutic target for human colon cancer (58). KLK7 specifically participates in
pathophysiological events associated with skin disorder,
gastrointestinal tract injury and central nervous system diseases
(59). In this study, KLK7 was
found to be highly expressed in gastric tissue and it was found
that i may cause gastric injury, as indicated by gastrointestinal
endoscopy.
KLK8 is a tryptic serine protease with a few types
of substrates. KLK is expressed in many developing organs, while
its expression is restricted to limited regions such as the
hippocampus. In the hippocampus, KLK8 is involved in
activity-dependent synaptic changes, including long-term
potentiation, which can be suppressed in KLK8-knockout mice. KLK8
is expressed in oligodendrocytes following injury to the central
nervous system. KLK8 is also highly expressed in the epidermis of
the skin and is involves in desquamation by the degradation of
adhesive molecules which connect layers of the epidermis.
Therefore, KLK8 may play a role in tissue development and
rearrangement (60). On the other
hand, KLK8 has been shown to be associated with the progression of
inflammation (61). In our study,
we demonstrated that high levels of KLK8 may contribute to the
development of gastric inflammation.
The kallikrein family consists of 15 genes, most of
which have been found to be differentially expressed in various
types of cancer and may be used as biomarkers for cancer prognosis.
The levels of KLK5, KLK6, KLK12 and KLK13 are consistently related
the risk of prostate cancer and tumor aggressiveness (62). Furthermore, KLK12 plays a critical
role in angiogenesis by modulating the bioavailability of
proangiogenic factor and activating the kinin-receptor-B2 signaling
pathway. However, the proangiogenic activity of KLK12 is different
from KLK1 and is not related to kinin release in lung endothelial
cells (63). Although little is
known about angiogenesis and polycythemia, our results suggest that
elevated levels of KLK12 cause angiogenesis which may be associated
with polycythemia.
Most importantly, KLKs are associated with
hypertension (29,30), while cholesterol is also
associated with hypertension (27,28) which is an important clinical
symptoms of HAPC. Thus, in this study, we explored the interaction
between KLKs and cholesterol using AutoDock remodeling. The results
suggested that all five members (KLK1/2/7/8/12) had high-score
binding ability with cholesterol by binding it from different
sites. Moreover, the trimer of KLK7 was used to find that polymers
could bind cholesterol well. All the findings implied that KLKs are
important proteins for binding cholesterol through different
mechanisms, causing signs of KLK hypertension, which may be closely
associated with HPAC-induced GML.
As already stated above, there are some limitations
to the present study. Firstly, only three subjects were selected in
each group for transcriptome analysis and some bias may exist. To
avoid this bias, RT-qPCR was performed using tissues from 20
healthy subjects and 20 patients with HAPC residing at the same
high altitudes, and the results revealed the same trend: KLK1,
KLK3, KLK7, KLK8 and KLK12 were all upregulated in patients with
HAPC compared with the controls. Thus, the trend was not caused by
group bias. Secondly, the exact functions of KLK1, KLK3, KLK7, KLK8
and KLK12 were not determined in the present study. Animal models
should be used to confirm our findings. Thirdly, the interaction of
these molecules was not identified. Finally, only a few crystal
structures of KLKs are available, thus the interaction of polymers
of KLK1/2/8/12 and cholesterol cannot be explored by molecular
remodeling. Thus, further research is required in order to fully
understand the molecular mechanisms responsible for the development
of HAPC and for HAPC-related diseases.
In conclusion, as demonstrated by the
above-mentioned data, the elevated levels of KLK1, KLK3, KLK7, KLK8
and KLK12 may be closely associated with hypertension,
inflammation, obesity and other gastric injuries associated with
polycythemia. From the interacting partners of KLK members
(Table III), the five members,
KLK1, KLK3, KLK7, KLK8 and KLK12, may have multiple functions. The
interaction of KLKs and cholesterol may play an important role in
hypertension in patients with HAPC, which results in gastric
injuries in these patients. The results of this study originated
from the whole genome microarray data com paring patients with HAPC
with healthy controls, providing evidence of the molecular
mechanisms responsible for HAPC-induced GML. Therefore, the present
findings indicated that HAPC-induced GML leads to the activation of
protective responses through the upregulation of the levels of the
KLK1/3/7/8/12 gene cluster. These results may also have
implications in the treatment of gastric lesions.
Acknowledgments
This study was supported by a grant from the
National 'Twelfth Five-Year' Plan for Science and Technology
Support of China (2013BAI05B04) and the Key project for Natural
Science Foundation in Tibet Autonomous Region, China (2012).
References
|
1
|
León-Velarde F, Maggiorini M, Reeves JT,
Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T,
Moore LG, et al: Consensus statement on chronic and subacute high
altitude diseases. High Alt Med Biol. 6:147–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang R, Xiang Y, Ran Q, Deng X, Xiao Y,
Xiang L and Li Z: Involvement of calcium, reactive oxygen species,
and ATP in hexavalent chromium-induced damage in red blood cells.
Cell Physiol Biochem. 34:1780–1791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lücker A, Weber B and Jenny P: A dynamic
model of oxygen transport from capillaries to tissue with moving
red blood cells. Am J Physiol Heart Circ Physiol. 308:H206–H216.
2015. View Article : Google Scholar
|
|
4
|
Watts T, Barigou M and Nash GB: Effects of
vessel size, cell sedimentation and haematocrit on the adhesion of
leukocytes and platelets from flowing blood. Biorheology.
52:391–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Freund JB and Vermot J: The wall-stress
footprint of blood cells flowing in microvessels. Biophys J.
106:752–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nair PK, Huang NS, Hellums JD and Olson
JS: A simple model for prediction of oxygen transport rates by
flowing blood in large capillaries. Microvasc Res. 39:203–211.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu C, Holroyd E, Cheng Y and Lau JT:
Institutional incentives for altruism: Gifting blood in China. BMC
Public Health. 13:5242013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida M, Wakabayashi G, Ishikawa H,
Kawachi S, Tanabe M, Otani Y, Shimazu M, Kubota T and Kitajima M:
Arteriovenous shunting blood flow is intravitally observed in the
stomach after thermal injury in rats. Keio J Med. 51:193–200. 2002.
View Article : Google Scholar
|
|
9
|
Kurata JH: Ulcer epidemiology: An overview
and proposed research framework. Gastroenterology. 96(Suppl 2):
569–580. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Przyslupski AM, Armstrong E, Shen K and
Yager JY: Sulforaphane is not additive in combination with
hypothermia in a neonatal rat model of hypoxia-ischemia. Int J Dev
Neurosci. 47:552015. View Article : Google Scholar
|
|
11
|
Ferraz MM, Sab IM, Silva MA, Santos DA and
Ferraz MR: Prenatal Hypoxia Ischemia Increases Male Rat Sexual
behavior. J Sex Med. 12:2013–2021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blanco-Alvarez VM, Soto-Rodriguez G,
Gonzalez-Barrios JA, Martinez-Fong D, Brambila E, Torres-Soto M,
Aguilar-Peralta AK, Gonzalez-Vazquez A, Tomás-Sanchez C, Limó ID,
et al: Prophylactic subacute administration of zinc increases CCL2,
CCR2, FGF2, and IGF-1 expression and prevents the long-term memory
loss in a rat model of cerebral hypoxia-ischemia. Neural Plast.
2015:3753912015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang HZ, Wen XH and Liu H: Sex
differences in brain MRI abnormalities and neurodevelopmental
outcomes in a rat model of neonatal hypoxia-ischemia. Int J
Neurosci. 126:647–657. 2016. View Article : Google Scholar
|
|
14
|
Min JW, Hu JJ, He M, Sanchez RM, Huang WX,
Liu YQ, Bsoul NB, Han S, Yin J, Liu WH, et al: Vitexin reduces
hypoxiaischemia neonatal brain injury by the inhibition of
HIF-1alpha in a rat pup model. Neuropharmacology. 99:38–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li K, Gesang L, Dan Z, Gusang L, Dawa C
and Nie Y: Transcriptome reveals 1400-fold upregulation of
APOA4-APOC3 and 1100-fold downregulation of GIF in the patients
with polycythemia-induced gastric injury. PLoS One.
10:e01405342015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ickenstein GW, Klotz JM and Langohr HD:
Headache caused by polycythemia vera. Classification of a headache
under the heading of metabolic disturbances. Schmerz. 13:279–282.
1999.In German. View Article : Google Scholar
|
|
17
|
Ding GX, Feng CC, Song NH, Fang ZJ, Xia
GW, Jiang HW, Hua LX and Ding Q: Paraneoplastic symptoms: Cachexia,
polycythemia, and hypercalcemia are, respectively, related to
vascular endothelial growth factor (VEGF) expression in renal clear
cell carcinoma. Urol Oncol. 31:1820–1825. 2013. View Article : Google Scholar
|
|
18
|
Jia C, Chen Y, Hu Z and Lu X: Right
hepatic artery thrombosis in an essential polycythemia vera patient
following pancreatobiliary surgery for severe pancreatitis. J
Thromb Thrombolysis. 34:135–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pathak R, Giri S, Karmacharya P and Aryal
MR: Obstructive sleep apnea syndrome and secondary polycythemia:
Analysis of the nationwide inpatient sample. Sleep Med. 16:205–206.
2015. View Article : Google Scholar
|
|
20
|
Jepson JH: Polycythemia: Diagnosis,
pathophysiology and therapy. I. Can Med Assoc J. 100:271–277.
1969.PubMed/NCBI
|
|
21
|
Thiele J, Kvasnicka HM, Muehlhausen K,
Walter S, Zankovich R and Diehl V: Polycythemia rubra vera versus
secondary polycythemias. A clinicopathological evaluation of
distinctive features in 199 patients. Pathol Res Pract. 197:77–84.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugiura Y, Nemoto E, Shinoda H, Nakamura N
and Kaseda S: Surgery for lung adenocarcinoma with smokers'
polycythemia: A case report. BMC Res Notes. 6:382013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manninen P, Karvonen AL, Laukkarinen J,
Aitola P, Huhtala H and Collin P: Colorectal cancer and
cholangiocarcinoma in patients with primary sclerosing cholangitis
and inflammatory bowel disease. Scand J Gastroenterol. 50:423–428.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Argenio G, Mazzone G, Tuccillo C,
Grandone I, Gravina AG, Graziani G, Fogliano V and Romano M: Apple
polyphenol extracts prevent aspirin-induced damage to the rat
gastric mucosa. Br J Nutr. 100:1228–1236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tombak A, Ay OI, Erdal ME, Sungur MA, Ucar
MA, Akdeniz A and Tiftik EN: MicroRNA expression analysis in
patients with primary myelofibrosis, polycythemia vera and
essential thrombocythemia. Indian J Hematol Blood Transfus.
31:416–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cornea MI, Levrat E, Pugin P and Betticher
DC: BCR-ABL1-positive chronic myeloid leukemia with erythrocytosis
presenting as polycythemia vera: A case report. J Med Case Reports.
9:302015. View Article : Google Scholar
|
|
27
|
Acuna L, Sanchez P, Soler L and Alvis LF:
Total cholesterol (Tc), Low-density lipoprotein cholesterol (Ldl-C)
and high-density lipoprotein cholesterol (Hdl-C) levels in patients
with hypertension (Ht), diabetes (Dm), both (Ht And Dm) and chronic
kidney disease (Ckd). Value Health. 18:A405–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Zhang N, Zhou Y, Li J, Gu Y, Wang J
and Liu C: A 50-year-old woman with haemoptysis, cough and
tachypnea: Cholesterol pneumonia accompanying with pulmonary artery
hypertension. Clin Respir J. Jun 15–2015.Epub ahead of print.
|
|
29
|
Ceravolo GS, Montezano AC, Jordão MT,
Akamine EH, Costa TJ, Takano AP, Fernandes DC, Barreto-Chaves ML,
Laurindo FR, Tostes RC, et al: An interaction of renin-angiotensin
and kallikrein-kinin systems contributes to vascular hypertrophy in
angiotensin II-induced hypertension: In vivo and in vitro studies.
PLoS One. 9:e1111172014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katori M and Majima M: Renal (tissue)
kallikrein-kinin system in the kidney and novel potential drugs for
salt-sensitive hypertension. Prog Drug Res. 69:59–109.
2014.PubMed/NCBI
|
|
31
|
Jiang C, Chen J, Liu F, Luo Y, Xu G, Shen
HY, Gao Y and Gao W: Chronic mountain sickness in Chinese Han males
who migrated to the Qinghai-Tibetan plateau: Application and
evaluation of diagnostic criteria for chronic mountain sickness.
BMC Public Health. 14:7012014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li K, Gesang L, Dan Z and Gusang L:
Genome-wide transcriptional analysis reveals the protection against
hypoxia-induced oxidative injury in the intestine of Tibetans via
the inhibition of GRB2/EGFR/TPN11 pathways. Oxid Med Cell Longev.
2016:69673962016. View Article : Google Scholar
|
|
33
|
Kamiya A and Chikada H: Human pluripotent
stem cell-derived cholangiocytes: Current status and future
applications. Curr Opin Gastroenterol. 31:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yousef GM, Chang A, Scorilas A and
Diamandis EP: Genomic organization of the human kallikrein gene
family on chromosome 19q13.3–q13.4. Biochem Biophys Res Commun.
276:125–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
López-Otín C and Overall CM: Protease
degradomics: A new challenge for proteomics. Nat Rev Mol Cell Biol.
3:509–519. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olsson AY and Lundwall A: Organization and
evolution of the glandular kallikrein locus in Mus musculus.
Biochem Biophys Res Commun. 299:305–311. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olsson AY, Lilja H and Lundwall A:
Taxon-specific evolution of glandular kallikrein genes and
identification of a progenitor of prostate-specific antigen.
Genomics. 84:147–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prassas I, Eissa A, Poda G and Diamandis
EP: Unleashing the therapeutic potential of human
kallikrein-related serine proteases. Nat Rev Drug Discov.
14:183–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sexton DJ, Chen T, Martik D, Kuzmic P,
Kuang G, Chen J, Nixon AE, Zuraw BL, Forteza RM, Abraham WM and
Wood CR: Specific inhibition of tissue kallikrein 1 with a human
monoclonal antibody reveals a potential role in airway diseases.
Biochem J. 422:383–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kolte D and Shariat-Madar Z: Plasma
kallikrein inhibitors in cardiovascular disease: An innovative
therapeutic approach. Cardiol Rev. 24:99–109. 2016. View Article : Google Scholar
|
|
41
|
Albert-Weissenberger C, Mencl S, Hopp S,
Kleinschnitz C and Sirén AL: Role of the kallikrein-kinin system in
traumatic brain injury. Front Cell Neurosci. 8:3452014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Furio L and Hovnanian A: Netherton
syndrome: Defective kallikrein inhibition in the skin leads to skin
inflammation and allergy. Biol Chem. 395:945–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Czokało M, Pałka M, Kralisz P and
Filipkowski T: Kallikreinkinin system in patients with neoplastic
diseases. Rocz Akad Med Bialymst. 41:417–428. 1996.
|
|
44
|
Diamandis EP, Yousef GM, Luo LY, Magklara
A and Obiezu CV: The new human kallikrein gene family: Implications
in carcinogenesis. Trends Endocrinol Metab. 11:54–60. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schrader CH, Kolb M, Zaoui K,
Flechtenmacher C, Grabe N, Weber KJ, Hielscher T, Plinkert PK and
Hess J: Kallikrein-related peptidase 6 regulates
epithelial-to-mesenchymal transition and serves as prognostic
biomarker for head and neck squamous cell carcinoma patients. Mol
Cancer. 14:1072015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Christodoulou S, Alexopoulou DK, Kontos
CK, Scorilas A and Papadopoulos IN: Kallikrein-related peptidase-6
(KLK6) mRNA expression is an independent prognostic tissue
biomarker of poor disease-free and overall survival in colorectal
adenocarcinoma. Tumour Biol. 35:4673–4685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nishikawa K, Shibayama Y, Kuna P,
Calcaterra E, Kaplan AP and Reddigari SR: Generation of vasoactive
peptide bradykinin from human umbilical vein endothelium-bound high
molecular weight kininogen by plasma kallikrein. Blood.
80:1980–1988. 1992.PubMed/NCBI
|
|
48
|
Makevnina LG, Levina GO and Yatzimirsky
AK: Kinetics of Lys-bradykinin release by porcine pancreatic
kallikrein from rabbit low molecular weight kininogen. Agents
Actions Suppl. 38:89–97. 1992.PubMed/NCBI
|
|
49
|
Rajapakse S, Yamano N, Ogiwara K, Hirata
K, Takahashi S and Takahashi T: Estrogen-dependent expression of
the tissue kallikrein gene (Klk1) in the mouse uterus and its
implications for endometrial tissue growth. Mol Reprod Dev.
74:1053–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dedio J, Wiemer G, Rütten H, Dendorfer A,
Schölkens BA, Müller-Esterl W and Wohlfart P: Tissue kallikrein
KLK1 is expressed de novo in endothelial cells and mediates
relaxation of human umbilical veins. Biol Chem. 382:1483–1490.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Iwai N, Yasui N, Naraba H, Tago N,
Yamawaki H and Sumiya H: Klk1 as one of the genes contributing to
hypertension in Dahl salt-sensitive rat. Hypertension. 45:947–953.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rodriguez S, Al-Ghamdi OA, Burrows K,
Guthrie PA, Lane JA, Davis M, Marsden G, Alharbi KK, Cox A, Hamdy
FC, et al: Very low PSA concentrations and deletions of the KLK3
gene. Clin Chem. 59:234–244. 2013. View Article : Google Scholar
|
|
53
|
Sävblom C, Halldén C, Cronin AM, Säll T,
Savage C, Vertosick EA, Klein RJ, Giwercman A and Lilja H: Genetic
variation in KLK2 and KLK3 is associated with concentrations of hK2
and PSA in serum and seminal plasma in young men. Clin Chem.
60:490–499. 2014. View Article : Google Scholar
|
|
54
|
Bañez LL, Hamilton RJ, Partin AW, Vollmer
RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC
Jr, et al: Obesity-related plasma hemodilution and PSA
concentration among men with prostate cancer. JAMA. 298:2275–2280.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hasselbalch HC: Perspectives on the
increased risk of second cancer in patients with essential
thrombocythemia, polycythemia vera and myelofibrosis. Eur J
Haematol. 94:96–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weil MH: Polycythemia associated with
obesity. J Am Med Assoc. 159:1592–1595. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Giusti B, Serratì S, Margheri F, Papucci
L, Rossi L, Poggi F, Magi A, Del Rosso A, Cinelli M, Guiducci S, et
al: The antiangiogenic tissue kallikrein pattern of endothelial
cells in systemic sclerosis. Arthritis Rheum. 52:3618–3628. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Walker F, Nicole P, Jallane A,
Soosaipillai A, Mosbach V, Oikonomopoulou K, Diamandis EP, Magdolen
V and Darmoul D: Kallikrein-related peptidase 7 (KLK7) is a
proliferative factor that is aberrantly expressed in human colon
cancer. Biol Chem. 395:1075–1086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oliveira JR, Bertolin TC, Andrade D,
Oliveira LC, Kondo MY, Santos JA, Blaber M, Juliano L, Severino B,
Caliendo G, et al: Specificity studies on Kallikrein-related
peptidase 7 (KLK7) and effects of osmolytes and glycosaminoglycans
on its peptidase activity. Biochim Biophys Acta. 1854:73–83. 2015.
View Article : Google Scholar
|
|
60
|
Yoshida S: Klk8, a multifunctional
protease in the brain and skin: Analysis of knockout mice. Biol
Chem. 391:375–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shingaki K, Taniguchi M, Kanazawa S,
Matsuzaki S, Maeda T, Miyata S, Kubo T, Torii K, Shiosaka S and
Tohyama M: NGF-p75 and neuropsin/KLK8 pathways stimulate each other
to cause hyperkeratosis and acanthosis in inflamed skin. J Dermatol
Sci. 67:71–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lose F, Batra J, O'Mara T, Fahey P,
Marquart L, Eeles RA, Easton DF, Al Olama AA, Kote-Jarai Z, Guy M,
et al Australian Prostate Cancer BioResource: Common variation in
Kallikrein genes KLK5, KLK6, KLK12, and KLK13 and risk of prostate
cancer and tumor aggressiveness. Urol Oncol. 31:635–643. 2013.
View Article : Google Scholar
|
|
63
|
Kryza T, Lalmanach G, Lavergne M, Lecaille
F, Reverdiau P, Courty Y and Heuzé-Vourc'h N: Pro-angiogenic effect
of human kallikrein-related peptidase 12 (KLK12) in lung
endothelial cells does not depend on kinin-mediated activation of
B2 receptor. Biol Chem. 394:385–391. 2013. View Article : Google Scholar
|