Introduction
Osteoclasts, mainly responsible for bone resorption,
are multinucleated giant cells formed by the fusion of precursor
cells of myeloid-originated hematopoietic monocyte/macrophage
lineage (1–3). The receptor activator of nuclear
factor-κB (NF-κB) ligand (RANKL)-RANK system is a key regulator of
osteoclastogenesis under physiological conditions. However, several
pro-inflammatory cytokines, including tumor necrosis factor-α
(TNF-α) and interleukin (IL)-1, in combination with the RANK-RANKL
complex, play a crucial role in enhancing osteoclast
differentiation and activation in chronic inflammatory conditions,
such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and
chronic gouty arthritis (4–7).
In in vitro studies, IL-1 has been found to be a pluripotent
cytokine that exhibits bone resorption activity through interaction
with the RANKL-RANK system or binding with the IL-1 receptor
(IL-1R) (8–10). Under the aegis of the p38
mitogen-activated protein kinase (MAPK), IL-1 mediates
TNF-α-induced RANKL expression by stromal cells and promotes the
differentiation of osteoclast precursor cells (10). This suggests that IL-1 is a
crucial cytokine for osteoclast differentiation and activation, and
can thus be considered a potent therapeutic target in the treatment
of bone disorders (11).
Bee venom (Apis mellifera) has been shown to
have anti-inflammatory and anti-nociceptive properties in human
(12) and animal models (13,14). Melittin is a major component of
bee venom (15) and is commonly
studied for its therapeutic potential in chronic inflammatory joint
diseases (14,16). These investigations have
demonstrated a distinct anti-arthritic effect through the
inactivation of the NF-κB signaling pathway and the induction of
the apoptosis of RA synoviocytes (14,16). With regard to the effect of
melittin on bone metabolism, Tashjian et al reported that
melittin was a potent stimulator of prostaglandin synthesis in bone
and bone resorption, which was inhibited by indomethacin (17). By contrast, the water-soluble
fraction of bee venom has been shown to significantly inhibit
pathological bone changes in a rat model of arthritis (13).
To the best of our knowledge, there are limited
studies available on the effects of melittin on osteoclast
formation or bone resorption (13,17). Thus, in the present study, we
investigated whether melittin influences RANKL-induced osteoclast
formation in murine RAW 264.7 cells and bone marrow-derived
macrophages (BMMs). In addition, we examined the effects of
melittin on IL-1β-mediated osteoclast formation in
vitro.
Materials and methods
Cell culture and osteoclast
differentiation
BMMs were isolated from 6-week-old male C57BL/6 mice
(Samtako Inc., Osan, Korea) as previously described (18), following the approval of the
Institutional Review Board Animal Trial Board Committee of Daegu
Catholic University Medical Center, Daegu, Korea. BMMs were
cultured in α-MEM containing 10% fetal bovine serum (FBS) and 10
ng/ml macrophage colony-stimulating factor (M-CSF) for 24 h.
Non-adherent cells were removed the following day and cultured in
the presence of 30 ng/ml M-CSF for 3 days. The attached cells were
then used to generate osteoclasts from BMMs. The cells
(5×104 cells/well) were cultured with various treatment
combinations of M-CSF (30 ng/ml), RANKL (50 ng/ml) and IL-1β (100
ng/ml) in 24-well tissue culture plates.
The RAW 264.7 cells (Korean Cell Line Bank, Seoul,
Korea) were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% FBS and 1% antibiotics. The cells were grown
at 37°C, 5% CO2 in fully humidified air. For the
establishment of osteoclast differentiation, the RAW 264.7 cells
were incubated at 8×104 cells/well in 6-well plates for
7 days with DMEM containing RANKL (50 ng/ml). The medium was
refreshed every 2 days. Cell viability was assessed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra zolium bromide (MTT)
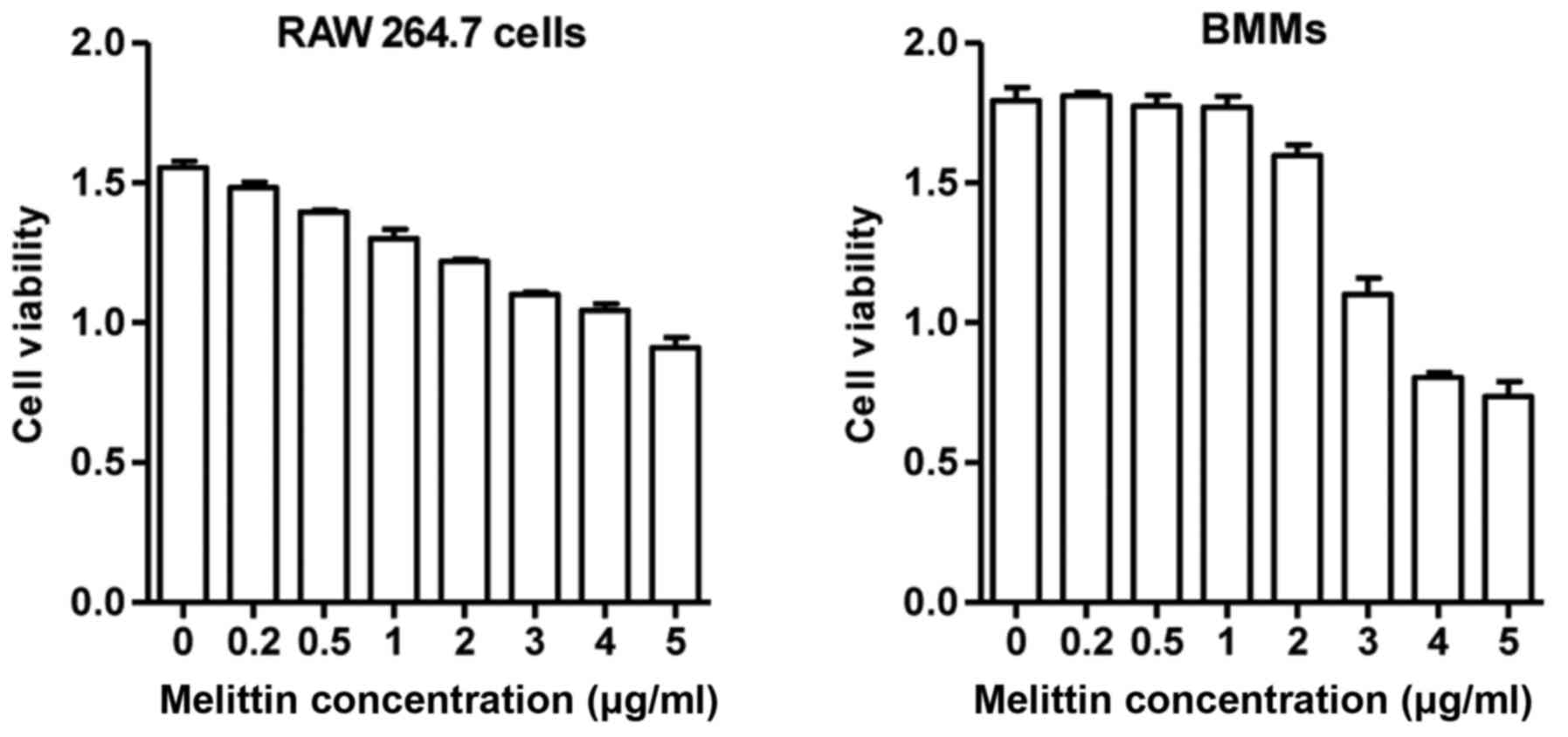
assay (Sigma, St. Louis, MO, USA). The RAW 264.7 cells and BMMs
treated with melittin up to a concentration of 1 µg/ml
exhibited >80% viability after 24 h (Fig. 1). However, the viability of the
RAW 264.7 cells and BMMs treated with melittin at 5 µg/ml
was significantly decreased. Based on these results, the
non-cytotoxic concentration of melittin (1 µg/ml) was used
in our experiments.
Reagents and antibodies
Recombinant mouse RANKL, M-CSF and IL-1β were
purchased from PeproTech (Rocky Hill, NJ, USA). Melittin was
purchased from Sigma. Components of cell culture media were
obtained as follows: DMEM (GeneDEPOT, Barker, TX, USA), α-MEM
(Gibco-BRL, Grand Island, NY, USA) and 10% FBS (HyClone, Logan, UT,
USA). The antibodies used were as follows: phosphorylated (p-)c-Jun
(sc-16312), nuclear factor of activated T cells, cytoplasmic 1
(NFATc1; sc-7294), TNF receptor-associated factor-6 (TRAF6;
sc-7221), p-extracellular signal-regulated kinase (ERK; sc-7383),
ERK (sc-94), β-actin (sc-47778) and goat anti-rabbit IgG-HRP (all
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
osteoprotegerin (OPG; ab73400-100), IL-1β (ab9722), p-p38 (ab47363)
and mouse IgG-HRP (all from Abcam, Cambridge, MA, USA), RANK (AM56)
and RANKL (AM57) (from Calbiochem, San Diego, CA, USA), p-p65
subunit of NF-κB (#3033), p-JNK (#9252), p-c-Fos (#5348), p38
(#9212) and JNK (#9252) (all from Cell Signaling Technology,
Beverly, MA, USA).
Tartrate-resistant acid phosphatase
(TRAP) staining
The RAW 264.7 cells were plated in a 6-well culture
dish and stimulated with RANKL (50 ng/ml). After 7 days of culture,
the cells were stained for TRAP using a leukocyte acid phosphatase
kit (Takara Bio, Inc., Shiga, Japan) according to the
manufacturer's instructions. After 5 days of culture, the BMMs were
also stained for TRAP. The cultured cells were fixed in a fixation
solution for 5 min at room temperature and washed with distilled
water. TRAP-positive MNCs with at least 3 or more nuclei were
defined as osteoclasts. Three random selected areas at each well
were used to count the number of osteoclast-like MNCs. This
procedure was repeated 3 times. The number of osteoclast-like MNCs
was expressed as an average.
Pit formation assay and F-actin ring
staining
The RAW 264.7 cells were plated in a 24-well culture
dish and stimulated with RANKL (50 ng/ml) for 10 days. To examine
bone resorption, we used a pit formation assay plate kit (Corning
Inc., Corning, NY, USA). After 10 days of osteoclast culture, the
conditioned medium was removed from each well and the cells were
treated with 5% sodium hypochlorite for 5 min. After washing the
plate with water and allowing it to dry, regions of each well were
photographed using a microscope. For F-actin ring staining,
osteoclasts differentiated from RAW 264.7 cells and BMMs were
cultured for 7 days. Staining was carried out using Phalloidin
CruzFluor™ 488 Conjugate (Santa Cruz Biotechnology, Inc.). The
cells were washed with phosphate-buffered saline (PBS), fixed in
3.5% neutral buffered formalin for 10 min at room temperature, and
permeabilized for 10 min with 0.1% Triton X-100 in PBS. The cells
were blocked with 1% BSA for 1 h at room temperature and incubated
with Phalloidin CruzFluor™ 488 Conjugate (Santa Cruz, Heidelberg,
Germany) (1:5,000 dilution) for 15 min. The cells were then washed
3 times with PBS and photographed using a fluorescence microscope
(Olympus, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for analysis of gene
expression
To evaluate the mRNA levels of TRAP, cathepsin K,
matrix metalloproteinase-9 (MMP-9) and carbonic anhydrase II, the
RAW 264.7 cells were stimulated with RANKL, treated with melittin
and cultured for the indicated periods of time (0, 3, 5, 7, 9 and
11 days). Total RNA was extracted using TRIzol reagent (Gibco-BRL).
cDNA synthesis was performed using the GeNet Bio kit (GeNet Bio,
Cheonan, Korea). Quantitative PCR amplification was performed using
a RT-PCR machine (Bio-Rad, Hercules, CA, USA) and SYBR-Green
Supermix (Toyobo, Tokyo, Japan). We used the following PCR
protocol: 95°C for 3 min; 40 cycles (15 sec, 95°C/1 min, 58–62°C);
and 72°C/45 sec; and 60°C to 95°C per cycle for melting curve
analysis. The oligonucleotide primers used in this analysis are
listed in Table I.
 | Table ISpecific primer sequences used in
quantitative RT-PCR. |
Table I
Specific primer sequences used in
quantitative RT-PCR.
| Gene | Sequence
(5′→3′) |
|---|
| TRAP | F: AAG GCG AGA GAT
TCT TTC CCT G |
| R: ACT GGG GAC AAT
TCA CTA GAG C |
| Cathepsin K | F: CAG CAG AAC GGA
GGC ATT GA |
| R: CCT TTG CCG TGG
CGT TAT AC |
| MMP-9 | F: GCC CTG GAA CTC
ACA CGA CA |
| R: TTG GAA ACT CAC
ACG CCA GAA G |
| Carbonic | F: CAT TAC TGT CAG
CAG CGA GCA |
| anhydrase II | R: GAC GCC AGT TGT
CCA CCA TC |
| IL-1β | F: GCC TCG TGC TGT
CGG ACC |
| R: TGT CGT TGC TTG
GTT CTC CTT G |
| GAPDH | F: AAG GCT GTG GGC
AAG GTC ATC |
| R: CAG GCG GCT CAG
ATC C |
Western blot analysis
The RAW 264.7 cells were pre-treated with melittin
(1 µg/ml) for 2 h and stimulated with RANKL for the
indicated periods of time. The cells were harvested after washing
with PBS buffer. Total protein was extracted in an IPH buffer [1 M
Tris (pH 8.0), 5 M sodium chloride, 10% Nonidet P-40 and protease
inhibitor cocktail (Roche, Indianapolis, IN, USA)]. The protein
concentration was determined using the Bio-Rad protein assay kit
(Bio-Rad, Hercules, CA, USA). Cell lysates of equal protein
concentrations were prepared in Laemmli protein sample (LDS) buffer
(Bio-Rad). Cell lysates were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel
electrophoresis and transferred onto nitrocellulose membranes
(Bio-Rad). The membranes were then rinsed and blocked with 5%
non-fat dry milk in TBS/0.1% Tween-20 for 1 h, incubated with
primary antibodies overnight at 4°C, and then with a horseradish
peroxidase-conjugated secondary antibody for 1 h. Immunoreactive
proteins were detected using the ELC western blot detection system
kit (Amersham, Braunschweig, Germany).
Transfection with IL-1β siRNA
For the determination of IL-1β-mediated osteoclast
differentiation, the RAW 264.7 cells were seeded at
2×105 cells/well in 6-well plates in DMEM. The cells
were transfected with mouse IL-1β siRNA (Invitrogen, Carlsbad, CA,
USA) to a final concentration of 50 nM using Lipofectamine RNAiMax
(Invitrogen). Negative control siRNA used was non-targeting
negative control siRNA (Med GC; Invitrogen). Negative control siRNA
was also used to refer to 'mock-transfected cells' in the
experiments. After 72 h, the medium was replaced with
differentiation medium and the cells were treated.
Statistical analysis
Statistical analysis for significant differences was
performed using a non-parametric Wilcoxon signed rank sum test
between two groups. Statistical significance was determined at
p-values <0.05. All statistical analyses were carried out using
IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA).
Results
Melittin inhibits the formation of
osteoclast-like MNCs
The gene expression of TRAP, cathepsin K, MMP-9 and
carbonic anhydrase II gradually increased in the RAW 264.7 cells
during stimulation with RANKL (50 ng/ml) (Fig. 2A). Maximal expression was observed
on day 7 for TRAP and cathepsin K and on day 9 for MMP-9 and
carbonic anhydrase II. The expression of these genes decreased
thereafter. The increased expression of these osteoclastogenic
genes in the RAW 264.7 cells cultured with RANKL for 7 days was
significantly inhibited by pre-treatment with melittin for 3 and 5
days (Fig. 2B). In addition,
melittin (ranging from 0.2 to 1.0 µg/ml) dose-dependently
suppressed TRAP, cathepsin K, MMP-9 and carbonic anhydrase II
expression in RAW 264.7 cells treated with RANKL (50 ng/ml)
(Fig. 2C).
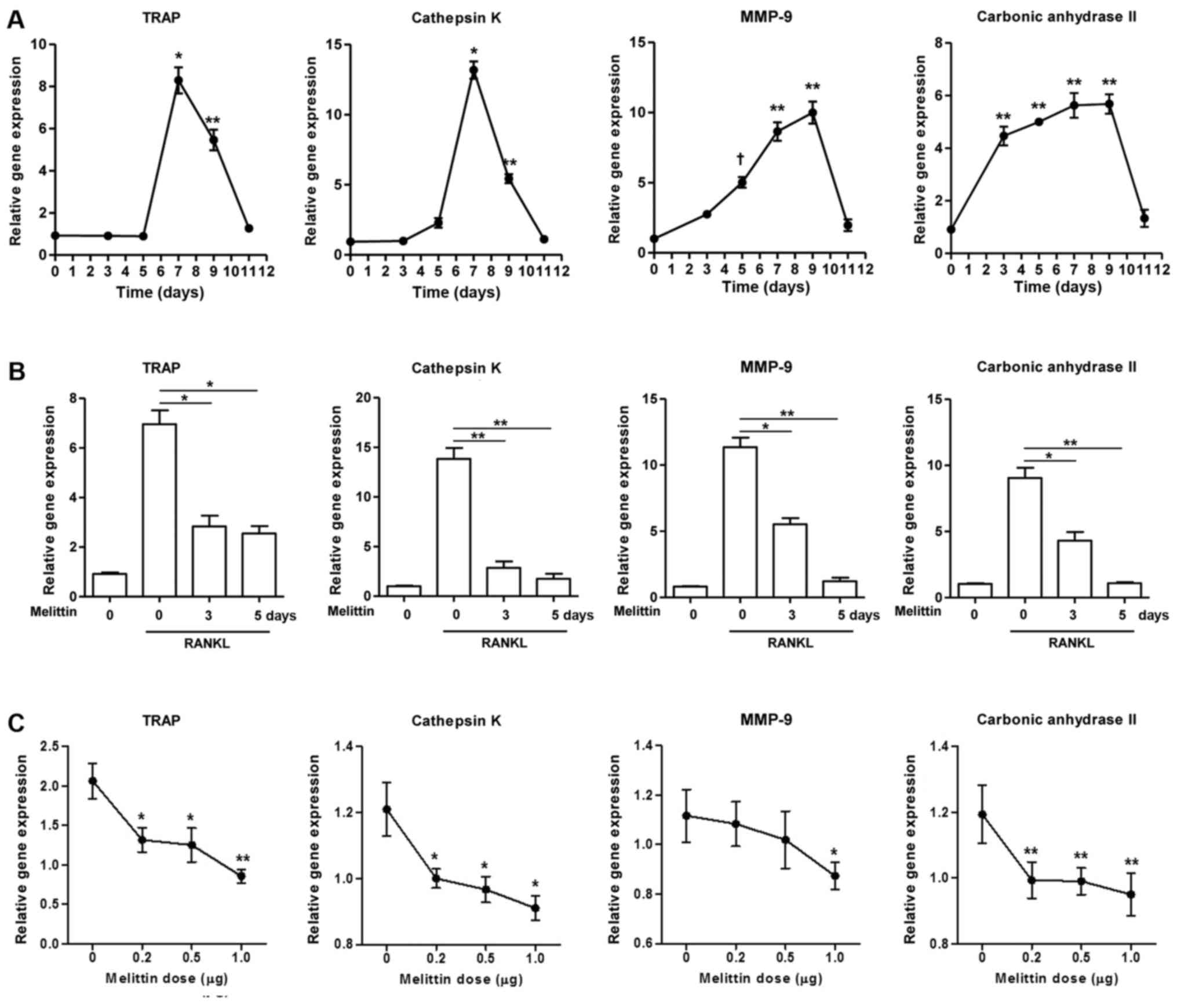 | Figure 2Melittin influencea osteoclast
formation in time- and dose-dependent manner. (A) Osteoclastogenic
genes, such as TRAP, cathepsin K, matrix metalloproteinase-9
(MMP-9) and carbonic anhydrase II, in the course of
osteoclastogenesis in RAW 264.7 cells incubated with receptor
activator of nuclear factor-κB ligand (RANKL) (50 ng/ml) for 0, 3,
5, 7, 9 and 11 days were measured (*p<0.001,
**p<0.01 and †p<0.05 compared to
non-treated cells). (B) Melittin (1 µg/ml) was added to the
RAW 264.7 cells on days 3 and 5 following stimulation with RANKL.
Inhibitory effect of melittin (1 µg/ml) treatment for 0, 3
and 5 days on osteoclastogenic genes was assessed
(*p<0.01 and **p<0.001 compared to
RANKL-stimulated cells without melittin). (C) RAW 264.7 cells
treated with RANKL (50 ng/ml) were cultured at different dosages of
melittin (0.2 to 1.0 µg/ml) (*p<0.05 and
**p<0.01 compared to non-treated cells). (D) Melittin
was added to the RAW 264.7 cells on days 3 and/or 5 following
stimulation with RANKL. The in vitro morphological and
functional analyses for osteoclastogenesis were performed using
TRAP staining, pit formation, and F-actin ring staining assays in
RAW 264.7 cells treated with RANKL with or without melittin
(*p<0.01 compared to non-treated cells) (E) The
assessment of osteoclast-like cells differentiated from bone
marrow-derived macrophages (BMMs) was done using TRAP staining and
F-actin ring staining assays. Data are representative of 3
independent experiments. |
TRAP staining and F-actin ring staining demonstrated
that, for the RAW 264.7 cells, incubation with RANKL (50 ng/ml) for
7 days markedly induced the formation of TRAP(+) and F-actin(+)
MNCs. This was significantly attenuated by pretreatment with
melittin (1 µg/ml) for 3 and 5 days (Fig. 2D). The pit formation assay for
bone resorption revealed that stimulating the RAW 264.7 cells with
RANKL for 10 days promoted the generation of numerous and
variable-sized pits. The addition of melittin for 3 and 5 days
prominently reduced the number and size of the pits (Fig. 2D).
Treatment of the BMMs with M-CSF (30 ng/ml) alone to
failed to induce the formation of osteoclast-like cells (Fig. 2E). However, TRAP staining assay
illustrated that the BMMs stimualted with RANKL (50 ng/ml) and
M-CSF (30 ng/ml) for 5 days differentiated into osteoclast-like
MNCs, as shown by the increased number of TRAP(+) cells; this
effect was significantly suppressed by treatment with melittin (1
µg/ml). The F-actin ring formation assay also revealed
larger and numerous F-actin ring(+) MNCs following stimulation with
RANKL, and this effect was also significantly suppressed by
treatment with melittin (1 µg/ml) (Fig. 2E).
Melittin regulates the activation of
MAPKs, c-Fos and NFATc1 in RANKL-induced osteoclastogenesis
After stimulating the RAW 264.7 cells with RANKL (50
ng/ml), OPG protein expression was markedly decreased compared to
the levels in the non-stimulated RAW 264.7 cells (Fig. 3A). By contrast, there was an
increased protein expression of RANKL and RANK in the RAW 264.7
cells stimulated with RANKL. However, melittin (1 µg/ml)
enhanced OPG protein expression in a time-dependent manner, whereas
it decreased the protein expression of RANKL and RANK.
The protein expression of TRAF6, p-ERK, p-JNK and
p-p65 in the RAW 264.7 cells stimulated with RANKL (50 ng/ml) which
was increased by RANKL at 15 and 30 min was markedly attenuated by
pre-treatment with melittin (1 µg/ml) for 2 h, in comparison
to the levels in culture medium-treated cells; however, the protein
level of p-p38 was not altered by melittin treatment (Fig. 3B). In the assessment of the
downstream of RANK-RANKL system in osteoclastogenesis, the results
of western blot analysis revealed that the increased p-c-Fos and
NFATc1 expression induced by RANKL in the RAW 264.7 cells was
prominently suppressed by treatment with melittin (1 µg/ml);
however, the phosphorylation of c-Jun was not significantly
affected (Fig. 3C).
IL-1β has a crucial effect on the
formation of osteoclast-like MNCs
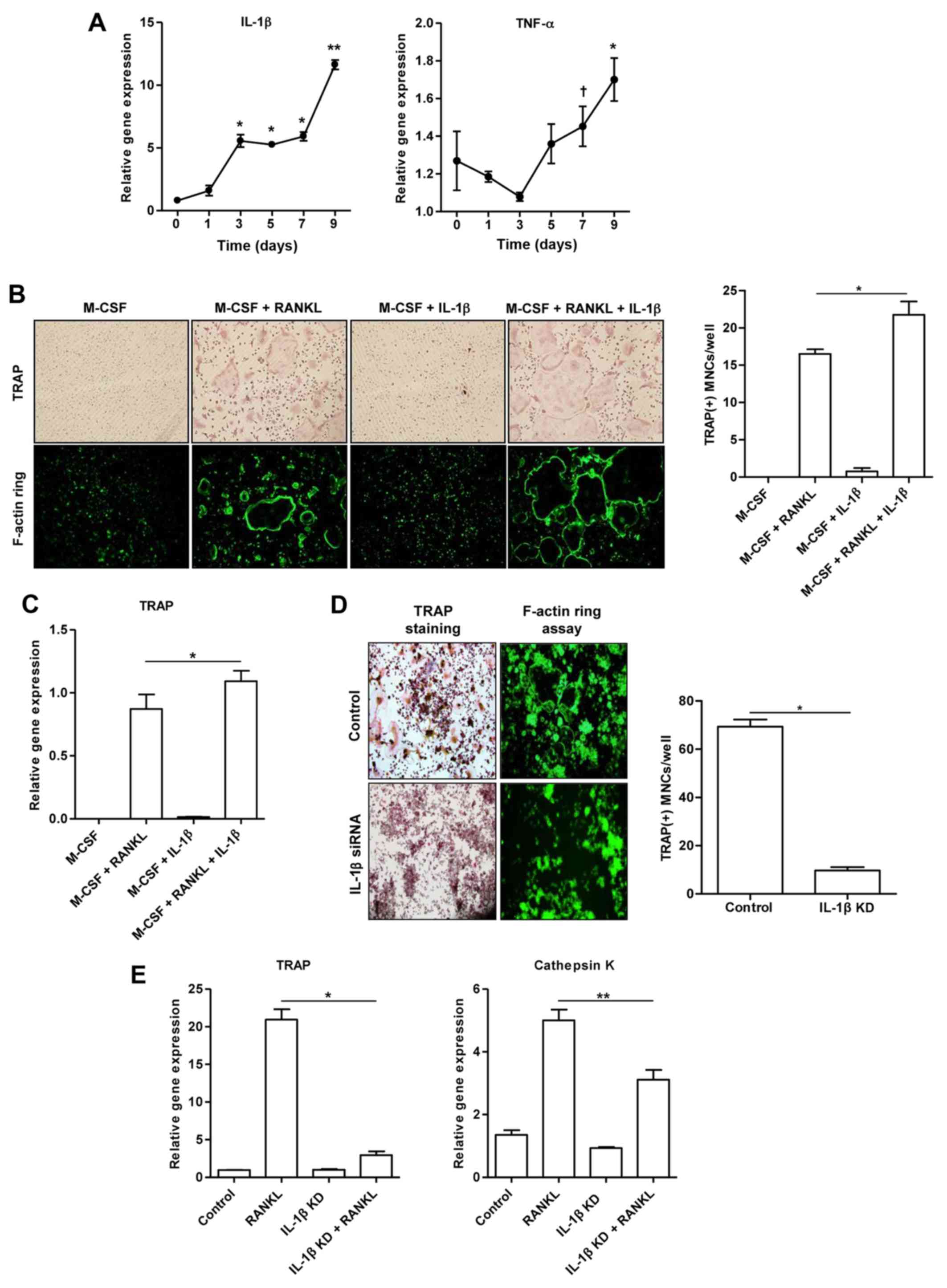
This experiment was designed to assess whether IL-1β
contributes to the development of mature osteoclasts. Fig. 4A illustrates that the mRNA
expression of IL-1β in the RAW 264.7 cells stimulated with RANKL
increased in a time-dependent manner. In addition, the
RANKL-stimulated RAW 264.7 cells exhibited a trend towards an
increased TNF-α mRNA expression. However, treatment of the BMMs
with M-CSF alone or M-CSF combined with IL-1β (100 ng/ml) did not
induce the formation of osteoclast-like MNCs, as shown by TRAP and
F-actin ring assays (Fig. 4B).
The number of TRAP(+) osteoclast-like MNCs derived from the BMMs
treated together with M-CSF, RANKL and IL-1β increased in
comparison to the BMMs treated with M-CSF and RANKL (p<0.05;
Fig. 4B). Consistently, we
observed an increased TRAP gene expression in BMMs treated together
with M-CSF, RANKL and IL-1β, compared to that in the cells treated
with M-CSF and RANKL (p<0.05; Fig.
4C).
To confirm the synergistic effect of IL-1β and RANKL
on osteoclast formation, the presence of TRAP(+) and F-actin
ring(+) osteoclast-like MNCs was detected in the control cells and
IL-1β siRNA-transfected cells stimulated with RANKL alone (Fig. 4D). Few osteoclast-like MNCs were
detected in the RAW 264.7 cells transfected with IL-1β siRNA
(p<0.001; Fig. 4D). The
increased expression of TRAP (p<0.001) and cathepsin K
(p<0.05) genes induced by RANKL was significantly suppressed in
the RAW 264.7 cells transfected with IL-1β siRNA (Fig. 4E).
Melittin synergistically attenuates the
effects of RANKL on osteoclastogenesis in the cells transfected
with IL-1β siRNA
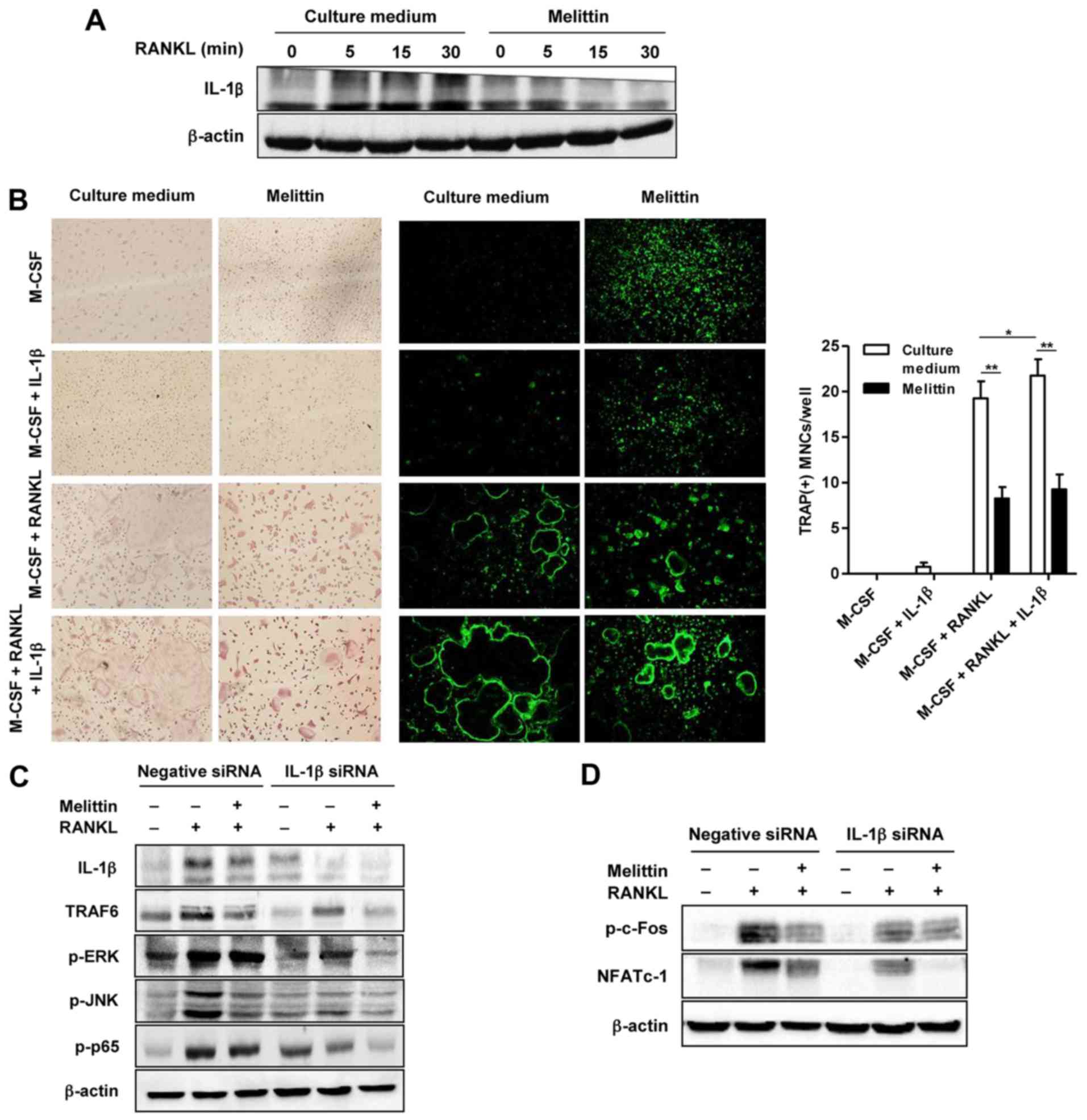
The increased protein expression of IL-1β in the
cells not treated with melittin and stimulated with RANKL was
noted, as shown by western blot analysis (Fig. 5A). By contrast, melittin markedly
suppressed IL-1β protein expression in the RANKL-stimulated RAW
264.7 cells (Fig. 5A). Analysis
using TRAP staining and F-actin staining revealed numerous TRAP(+)
and F-actin ring(+) osteoclast-like MNCs in the BMMs co-stimulated
with RANKL (50 ng/ml) and M-CSF (30 ng/ml) (Fig. 5B). The addition of IL-1β (100
ng/ml) was shown to have a synergistic effect on
osteoclastogenesis, illustrated by the more frequent detection of
larger TRAP(+) and F-actin ring(+)osteoclast-like MNCs compared to
those in the BMMs stimulated with M-CSF and RANKL, but without
IL-1β. Consistently, the increased number of TRAP(+) MNCs in the
BMMs co-stimulated with RANKL and M-CSF, with or without IL-1β, was
attenuated by melittin treatment (Fig. 5B).
RANKL induced an increase in the expression of
IL-1β, TRAF6, p-ERK, p-JNK and NF-κB p65 in the RAW 264.7 cells
transfected with negative siRNA, and this effect was significantly
attenuated in the cells transfected with IL-1β siRNA (Fig. 5C). Treatment with melittin exerted
a suppressive effect on the protein expression of TRAF6, p-JNK and
p-p65 in the RAW 264.7 cells transfected with negative siRNA. In
the RAW 264.7 cells transfected with IL-1β siRNA, melittin
treatment also additionally inhibited the expression of these
proteins, including TRAF6, p-ERK and p-p65.
In addition, stimulation with RANKL increased
p-c-Fos and NFATc1 expression in the RAW 264.7 cells transfected
with negative siRNA; this effect was markedly suppressed in the
cells transfected with IL-1β siRNA (Fig. 5D). Melittin synergistically
inhibited NFATc1 expression in the RAW 264.7 cells transfected with
IL-1β siRNA (Fig. 5D).
Discussion
Osteoclast differentiation and activation are mainly
regulated by the RANK-RANKL system (1,2). A
pathogenic role for osteoclast differentiation and activation has
been noted in conditions of chronic inflammatory arthritis,
including RA, PsA and gouty arthritis, as diverse pro-inflammatory
cytokines trigger bone erosion upon the activation of the
RANK-RANKL system (4–7). In particular, melittin has shown to
have potent anti-arthritic properties in in vitro and in
vivo studies, as indicated by the attenuation of inflammatory
responses through the inhibition of NF-κB activation and the
induction of the apoptosis of fibroblast-like synoviocytes and
murine or human macrophages (14,16). Based on these data, we
hypothesized that melittin has the potential to protect against
bone erosion by blocking osteoclast formation in inflammatory
conditions. To the best of our knowledge, to date, there is no
evidence of the effect of melittin on osteoclast formation at the
molecular level. In the present study, we revealed that melittin
inhibited osteoclast formation in RAW 264.7 cells and BMMs through
the inhibition of the RANKL-RANK system and of the osteoclastogenic
effect of IL-1β.
Melittin, a major component of whole bee venom, is a
small protein with 26 amino acid residues and a water-soluble
tetramer that acts as a lytic agent (15). Whole bee venom is composed of 90%
water-soluble, 5% ethyl acetate-soluble and 5% hexane-soluble
components (13). Bee venom has
been found to exert an anti-arthritic effect through the
attenuation of inflammatory responses in rat models of
adjuvant-induced (13,14) and type II collagen-induced
arthritis (19). Kwon et
al demonstrated that treatment with the water-soluble fraction
of bee venom significantly suppressed pathological bone changes in
affected joints, whereas the ethyl acetate fraction of bee venom
did not interfere with bone damage to arthritic joints (13). They proposed that melittin may be
a potent candidate for anti-inflammatory therapeutics in arthritis
as it is a main component of the water-soluble fraction of bee
venom. By contrast, Tashjian et al reported that melittin
was a stimulator of prostaglandin E2 (PGE2)
synthesis and bone resorption in neonatal mouse calvaria (17). In the present study, the increased
gene expression of osteoclast markers induced by RANKL, such as
TRAP, cathepsin K, MMP-9 and carbonic anhydrase II, was
significantly attenuated by melittin treatment in RAW 264.7 cells.
In addition, melittin was found to inhibit the formation of
osteoclast-like MNCs, as shown by TRAP staining and F-actin ring
assays in RAW 264.7 cells and BMMs. This evidence indicates that
melittin inhibits the formation of osteoclast-like MNCs
differentiated from macrophages, which is in accordance with the
findings of a previous study (13).
RANK is a member of the TNF receptor (TNFR)
superfamily, which sequentially recruits TRAFs (1,2).
TRAF proteins are adaptor proteins which play an important role in
signal transduction after the binding of RANK-RANKL. Among the TRAF
proteins, TRAF6 seems to be important in RANKL-induced
osteoclastogenesis, as shown by the defective activation of
osteoclasts differentiated from osteoclast precursor cells in
TRAF6−/− mice (20).
TRAF6 mediates the activation of RANKL-induced NF-κB and MAPK
signaling, which have been well recognized as downstream targets in
osteoclastogenesis (1,2). RANKL, through the degradation of
IκBα, is involved in the activation of the NF-κB dimer, p50/p65, in
both BMMs and RAW 264.7 cells (21). ERK activity in osteoclast-like
cells has been found to be closely related to osteoclast survival,
but not bone resorption by the regulation of apoptosis (22). BMMs derived from mice which are
JNK1-/-, but not JNK2-/-, exhibit reduced
differentiation into RANKL-induced osteoclasts (23). It has been well established that
the NF-κB and MAPK signaling pathways are targets for inflammatory
responses regulated by melittin (24). In the present study, we
investigated whether melittin regulates the activation of the NF-κB
and MAPK cascades, and participates in the process of the formation
of osteoclast-like MNCs. Our results demonstrated that melittin
treatment inhibited the increased protein expression of TRAF6,
p-ERK, p-JNK and NF-κB (p65) induced by RANKL in RAW 264.7 cells,
but not that of p-p38 MAPK.
The dimeric AP-1 transcription factor in
osteoclastogenesis is a downstream target of the ERK and JNK
pathway (1,2). In addition, NFATc1 is also
considered a transcriptional regulator of osteoclastogenesis, which
can be activated by ERK. ERK activates AP-1 transcriptional
activity by the phosphorylation of c-Fos, while c-Jun activation is
induced by JNK. In this study, we found that RANKL-induced c-Fos,
and NFATc1 were activated in RAW 264.7 cells, but not c-Jun.
Sequentially, melittin treatment was shown to inhibit the
phosphorylation of c-Fos and NFATc1. This suggests that downstream
ERK in signal transduction may be major determinant during
osteoclastogenesis.
IL-1β is a multifunctional cytokine that targets
various cells and tissues. In bone metabolism, IL-1β is known to be
responsible for each step of osteoclast-induced bone resorption,
such as differentiation and activation (9,10,25). In addition, this cytokine plays an
important role in increasing osteoclast survival time, which may be
due to IL-1β working together with RANKL to induce NF-κB activation
(25). Tanabe et al showed
that IL-1 alone could not drive osteoclast formation (26). By contrast, Kim et al
demonstrated that IL-1 alone directly induced osteoclast
differentiation if the IL-1 receptor was expressed on osteoclast
precursor cells, such as BMMs, in the RANKL-dependent or
RANKL-independent pathway (9).
Anakinra, a recombinant human IL-1 receptor antagonist, was shown
to significantly reduce radiographic joint erosion in RA (11). In this study, we used BMMs to
identify a synergistic role for IL-1β in the formation of
osteoclast-like MNCs. This was supported by the decreased
expression of osteosclast-specific genes, such as TRAP and
cathepsin K, and decreased osteoclast-like MNCs formation in RAW
264.7 cells lacking IL-1β. Melittin has the potential to suppress
IL-1 expression, as illustrated through the blocked activation of
the NF-κB and MAPK pathway by exposure to Propionibacterium
acnes in HaCaT cells (24),
although Stuhlmeier demonstrated that neither BV nor melittin
blocked IL-1β expression in experimental cells (27). This study also revealed that
melittin inhibited endogenous IL-1β production in RANKL-stimulated
cells.
Our results suggest that the effect of melittin on
RANKL-induced osteoclast formation may be dependent on the
activation of c-Fos and NFATc1 downstream of MAPKs, particularly
ERK, as these signal transduction molecules were assessed to
delineate the mechanism of IL-1β on osteoclast-like MNC formation.
This study demonstrated that the activation of NFATc1 and the
phosphorylation of NF-κB, ERK, JNK and c-Fos were markedly
attenuated in IL-1β siRNA transfected RAW 264.7 cells. In
particular, c-Fos may be a crucial component in osteoclastogenesis,
either by a RANKL-dependent or RANKL-independent mechanism
(9). The induction of the IL-1
receptor by overexpression of c-Fos could contribute to the
formation of osteoclast-like cells stimulated by IL-1 alone in a
RANKL-independent mechanism.
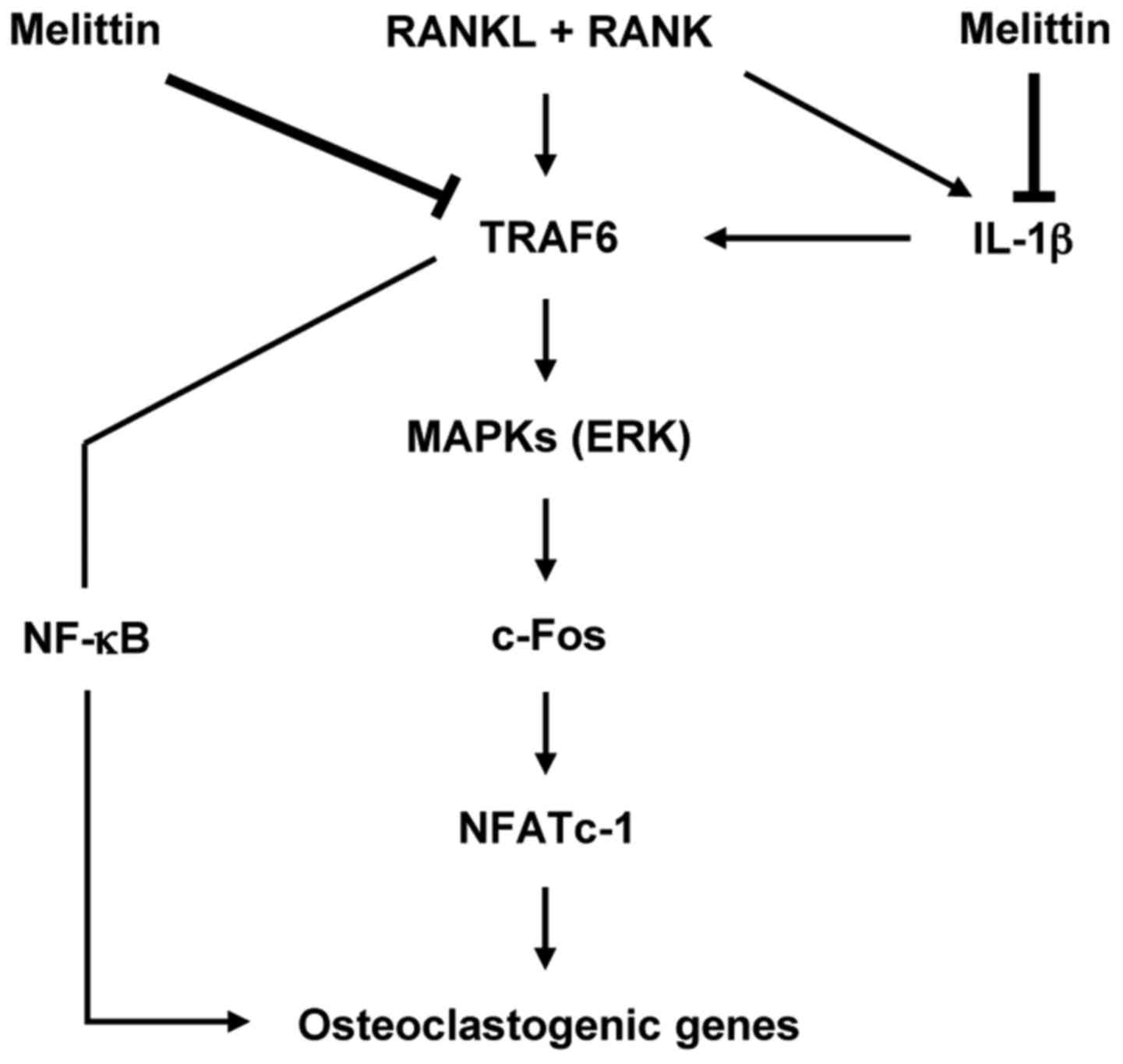
In conclusion, the importance of this study is that
melittin has the ability to potently suppress osteoclast-like MNC
formation through the inhibition of c-Fos and NFATc1 downstream of
the MAPK signaling pathway in osteoclastogenesis (Fig. 6). It is also worth noting that
IL-1β is an important cytokine for regulating osteoclast-like MNC
formation, and may be considered as a promising therapeutic target
for bone loss or erosion in chronic inflammatory arthritis.
Acknowledgments
The present study was supported by the Daegu
Catholic University Medical Center Regional Center for Rheumatic
Diseases and Degenerative Arthritis research grant. We would like
to thank Dr Ki-Yeun Park for technical assistance of TRAP and
F-actin ring assays.
References
|
1
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|
|
3
|
Teitelbaum SL: Osteoclasts; culprits in
inflammatory osteolysis. Arthritis Res Ther. 8:2012006. View Article : Google Scholar :
|
|
4
|
Udagawa N, Kotake S, Kamatani N, Takahashi
N and Suda T: The molecular mechanism of osteoclastogenesis in
rheumatoid arthritis. Arthritis Res. 4:281–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colucci S, Brunetti G, Cantatore FP,
Oranger A, Mori G, Quarta L, Cirulli N, Mancini L, Corrado A,
Grassi FR and Grano M: Lymphocytes and synovial fluid fibroblasts
support osteoclastogenesis through RANKL, TNFα, and IL-7 in an in
vitro model derived from human psoriatic arthritis. J Pathol.
212:47–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalbeth N, Smith T, Nicolson B, Clark B,
Callon K, Naot D, Haskard DO, McQueen FM, Reid IR and Cornish J:
Enhanced osteoclastogenesis in patients with tophaceous gout: Urate
crystals promote osteoclast development through interactions with
stromal cells. Arthritis Rheum (Munch). 58:1854–1865. 2008.
View Article : Google Scholar
|
|
7
|
Lee SJ, Nam KI, Jin HM, Cho YN, Lee SE,
Kim TJ, Lee SS, Kee SJ, Lee KB, Kim N and Park YW: Bone destruction
by receptor activator of nuclear factor κB ligand-expressing T
cells in chronic gouty arthritis. Arthritis Res Ther. 13:R1642011.
View Article : Google Scholar
|
|
8
|
Gowen M, Wood DD, Ihrie EJ, Meats JE and
Russell RG: Stimulation by human interleukin 1 of cartilage
breakdown and production of collagenase and proteoglycanase by
human chondrocytes but not by human osteoblasts in vitro. BBA.
797:186–193. 1984.PubMed/NCBI
|
|
9
|
Kim JH, Jin HM, Kim K, Song I, Youn BU,
Matsuo K and Kim N: The mechanism of osteoclast differentiation
induced by IL-1. J Immunol. 183:1862–1870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei S, Kitaura H, Zhou P, Ross FP and
Teitelbaum SL: IL-1 mediates TNF-induced osteoclastogenesis. J Clin
Invest. 115:282–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strand V and Kavanaugh AF: The role of
interleukin-1 in bone resorption in rheumatoid arthritis.
Rheumatology (Oxford). 43(Suppl 3): iii10–iii16. 2004. View Article : Google Scholar
|
|
12
|
Billingham ME, Morley J, Hanson JM,
Shipolini RA and Vernon CA: Letter: An anti-inflammatory peptide
from bee venom. Nat Lett. 245:163–164. 1973. View Article : Google Scholar
|
|
13
|
Kwon YB, Lee HJ, Han HJ, Mar WC, Kang SK,
Yoon OB, Beitz AJ and Lee JH: The water-soluble fraction of bee
venom produces antinociceptive and anti-inflammatory effects on
rheumatoid arthritis in rats. Life Sci. 71:191–204. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park HJ, Lee SH, Son DJ, Oh KW, Kim KH,
Song HS, Kim GJ, Oh GT, Yoon DY and Hong JT: Antiarthritic effect
of bee venom: Inhibition of inflammation mediator generation by
suppression of NF-κB through interaction with the p50 subunit.
Arthritis Rheum (Munch). 35:3504–3515. 2004. View Article : Google Scholar
|
|
15
|
Terwilliger JT and Eisenberg D: The
structure of melittin. II. Interpretation of the structure. J Biol
Chem. 257:6016–6022. 1982.PubMed/NCBI
|
|
16
|
Kim SK, Park KY, Yoon WC, Park SH, Park
KK, Yoo DH and Choe JY: Melittin enhances apoptosis through
suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3
activation and Bcl-2 expression for human fibroblast-like
synoviocytes in rheumatoid arthritis. Joint Bone Spine. 78:471–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tashjian AH Jr, Ivey JL, Delclos B and
Levine L: Stimulation of prostaglandin production in bone by
phorbol diesters and melittin. Prostaglandins. 16:221–232. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park H, Jung YK, Park OJ, Lee YJ, Choi JY
and Choi Y: Interaction of Fas ligand and Fas expressed on
osteoclast precursors increases osteoclastogenesis. J Immunol.
175:7193–7201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JD, Kim SY, Kim TW, Lee SH, Yang HI,
Lee DI and Lee YH: Anti-inflammatory effect of bee venom on type II
collagen-induced arthritis. Am J Chin Med. 32:361–367. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naito A, Azuma S, Tanaka S, Miyazaki T,
Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T and
Inoue J: Severe osteopetrosis, defective interleukin-1 signalling
and lymph node organogenesis in TRAF6-deficient mice. Genes Cells.
4:353–362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei S, Teitelbaum SL, Wang MW and Ross FP:
Receptor activator of nuclear factor-κB ligand activates nuclear
factor-κB in osteoclast precursors. Endocrinology. 142:1290–1295.
2001.PubMed/NCBI
|
|
22
|
Miyazaki T, Katagiri H, Kanegae Y,
Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM,
Oda H, et al: Reciprocal role of ERK and NF-κB pathways in survival
and activation of osteoclasts. J Cell Biol. 148:333–342. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
David JP, Sabapathy K, Hoffmann O,
Idarraga MH and Wagner EF: JNK1 modulates osteoclastogenesis
through both c-Jun phosphorylation-dependent and -independent
mechanisms. J Cell Sci. 115:4317–4325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee WR, Kim KH, An HJ, Kim JY, Chang YC,
Chung H, Park YY, Lee ML and Park KK: The protective effects of
melittin on Propionibacterium acnes-induced inflammatory responses
in vitro and in vivo. J Invest Dermatol. 134:1922–1930. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jimi E, Akiyama S, Tsurukai T, Okahashi N,
Kobayashi K, Udagawa N, Nishihara T, Takahashi N and Suda T:
Osteoclast differentiation factor acts as a multifunctional
regulator in murine osteoclast differentiation and function. J
Immunol. 163:434–442. 1999.PubMed/NCBI
|
|
26
|
Tanabe N, Maeno M, Suzuki N, Fujisaki K,
Tanaka H, Ogiso B and Ito K: IL-1a stimulates the formation of
osteoclast-like cells by increasing M-CSF and PGE2
production and decreasing OPG production by osteoblasts. Life Sci.
77:615–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stuhlmeier KM: Apis mellifera venom and
melittin block neither NF-κB-50-DNA interactions nor the activation
of NF-κB, instead they activate the transcription of
proinflammatory genes and the release of reactive oxygen
intermediates. J Immunol. 179:655–664. 2007. View Article : Google Scholar : PubMed/NCBI
|