Introduction
Colorectal cancer (CRC) is a leading cause of
cancer-related mortality (1).
Metastasis is the most related cause of the majority of human
cancer-related deaths (2).
Metastasis is a complex process and many scientists have strived to
understand the mechanisms behind this phenomenon over the years
(3); however, the mechanisms
responsible for the occurrence of metastasis remain to be fully
elucidated. The treatment of colon cancer involves a comprehensive
therapeutic approach (4). Thus,
the more in depth understanding of the mechanisms responsible for
CRC metastasis may provide valuable direction and an experimental
basis for its treatment in future.
Epithelial-mesenchymal transition (EMT) had been
highlighted as a process through which epithelial cells lose their
characteristics and gain mesenchymal properties to be motile,
playing a critical role in the metastasis of cancer cells (5,6).
Among the EMT-related transcription factors, the Snail family of
zing finger transcription factors is prominent, particularly Slug
(Snail2) (7,8). Stimulating Slug expression has been
shown to suppress E-cadherin and to concomitantly upregulate
N-cadherin expression (9,10). Moreover, Slug induces vimentin
expression (11). Therefore, Slug
regulates E-cadherin and N-cadherin, and vimentin expression.
Sphingosine kinase 1 (SphK1) is an oncogenic enzyme
which promotes the transformation, proliferation and angiogenesis
of a number of human tumors (12,13). Recent studies have indicated that
SphK1 is involved in regulating the NF-κB pathways (14,15), AKT (16) and focal adhesion kinase (FAK)
(17) in cancer. We wished to
determine whether SphK1 potentially promotes the EMT process in CRC
cells, as it has been previously shown to promote EMT in lung
cancer cells (18).
To date, some researchers have discovered that FAK
plays an important role in EMT and influences the expression of
EMT-related makers (19–21). Thus, we hypothesized that SphK1
may affect metastasis, and the expression of Slug, E-cadherin,
N-cadherin and vimentin via the FAK pathway in CRC cells. In order
to confirm our hypothesis, in this study, we used various CRC
cells, and we found indeed, that SphK1 modulates the expression of
EMT-related markers by regulating the expression of p-FAK in CRC
cells.
Materials and methods
Cell lines and cell culture
The human colorectal carcinoma cell lines, Caco2
(Boster, Wuhan, China), HT29, RKO and HCT116 (R&S Biotechnology
Co., Ltd., Shanghai, China) were routinely cultured in DMEM
containing 100 ml/l fetal bovine serum (FBS; ExCell Bio, Shanghai,
China) and incubated at 37°C with 5% CO2.
Each of the cell lines was divided into 3 groups as
follows: the control group (N group), the SKI-II (an inhibitor of
SphK1) intervention group (SK group) and PF-562271 (an inhibitor of
FAK) intervention group (PF group). The cells in the SK group were
cultured for 24 h and then incubated with SKI-II (Selleck, Houston,
TX, USA) at 20 µM (22)
for 48 h. The cells in the PF group were also cultured for 24 h and
then incubated with PF-562271 (Selleck) 5 µM (23) for 48 h.
Transwell chamber assay
The cells were cultured in serum-free medium to
produce suspension, and the cell density was adjusted to
5×105/ml. Subsequently, 200 µl cell suspension
were added to the upper chamber of the Transwell (Corning, Inc.,
Corning, NY, USA), while 600 µl medium including 10% FBS
were added to the bottom chamber, followed by incubation for a
period of 24 h at 37°C. The assay was terminated as a result of the
removal of the medium from the upper well and the filter was fixed
with methanol for 10 min. The cells in the upper chamber were wiped
off and the cells migrating to the lower side of the upper chamber
were stained with 0.1% crystal violet (Beyotime, Shanghai, China)
for 30 min. Random fields were scanned (6 fields/filter) under a
fluorescent inverted phase contrast microscope (magnification,
×200; TS100-F; Nikon, Tokyo, Japan) for the presence of the cells
at the lower membrane side only.
RNA isolation, cDNA synthesis and
quantitative PCR (qPCR)
RNA isolation was performed using a RNA extraction
kit (Tiangen, Beijing, China) according to the manufacturer's
instruction. cDNA synthesis was performed using a reverse
transcription kit (Takara, Dalian, Japan). Fluorescence-based qPCR
was performed using 2 µl of cDNA and SYBR-Green (Takara) in
a total volume of 20 µl and using the ABI 7500 Real-time PCR
system (Applied Biosystems, Rockford, IL, USA). The cycling
parameters were: 95°C for 30 sec followed by 40 cycles at 95°C for
5 sec and 60°C for 34 sec, and a dissociation program that included
95°C for 15 sec, 60°C for 1 min,and 95°C for 15 sec ramping up at
0.2°C/sec. Gene expression levels were determined with the
2−ΔΔCq method using glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) as a reference and the non-stimulated
condition was set to 1. The forward and reverse primers (Takara) of
the genes are listed in Table
I.
 | Table IThe primers used in this study. |
Table I
The primers used in this study.
| Gene name (protein
name) | Sequence primers
(5′→3′) |
|---|
| GAPDH (GAPDH) | F:
GCACCGTCAAGGCTGAGAAC |
| R:
TGGTGAAGACGCCAGTGGA |
| SPHK1 (SphK1) | F:
GGCTTCATTGCTGATGTGGA |
| R:
AGGAAGGTGCCCAGAGTGAA |
| FAK (FAK) | F:
CAACCACCTGGGCCAGTATTATC |
| R:
CCATAGCAGGCCACATGCTTTA |
| SLUG (Slug) | F:
TTTGCAAGATCTGCGGCAAG |
| R:
CTGCAAATGCTCTGTTGCAGTG |
| VIM (vimentin) | F:
TGACATTGAGATTGCCACCTACAG |
| R:
TCAACCGTCTTAATCAGAAGTGTCC |
| CDH2
(N-cadherin) | F:
AGCACAGTGGCCACCTACAAAG |
| R:
CAGCTCCTGGCCCAGTTACA |
| CDH1
(E-cadherin) | F:
GAGTGCCAACTGGACCATTCAGTA |
| R:
AGTCACCCACCTCTAAGGCCATC |
Western blot analysis
The cells were collected using cell scrapers and
lysed with RIPA lysis buffer supplemented with 1% protease and 1%
phosphatase inhibitor for 30 min following 2 washes with cold
phosphate-buffered saline (PBS). The cells were subjected to
centrifugation at 12,000 × g/min for 15 min at 4°C to remove the
cell debris. Protein concentrations were measured by bicinchoninic
acid (BCA) assay (Solarbio, Beijing, China) according to the
standard protocol.
Equal amounts of protein were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
then blotted onto 0.45 nitrocellulose membranes. The membranes were
subsequently blocked in non-fat milk [dissolved in Tris-buffered
saline with 5% Tween-20 (TBST)] buffer at room temperature for 30
min to block non-specific binding and incubated with antibodies
diluted by western blot antibody diluents overnight at 4°C, and
subsequently incubated with secondary fluorescent antibodies
(IRDye® 680RD Goat anti-Rabbit 925-68071; C60329-11;
dilution, 1:10,000; LI-COR Biosciences, Lincoln, NE, USA) for 1 h
at room temperature following 3 washes with TBST. The membranes
were scanned using an Odyssey infrared imaging system (LI-COR
Biosciences). Relevant signal intensity was determined using LI-COR
imaging software (LI-COR Biosciences). The strips were analyzed
using Quantity One software (Bio-Rad, Shanghai, China). Antibodies
against E-cadherin (3195S; 1:1,000) and p-FAK (Tyr397; 8556P;
1:1,000) were purchased from Cell Signaling Technology (CST,
Beverly, MA, USA). Antibodies against SphK1 (10670-1-AP; 1:500),
FAK (12636-1-AP; 1:1,000), Slug (12129-1-AP; 1:500), N-cadherin
(66219-1-1g; 1:500), vimentin (10366-1-AP; 1:500) and GAPDH
(10494-1-AP; 1:1,000) were purchased from Proteintech Group (PTG,
Rosemont, IL, USA).
Statistical analysis
Each experiment was repeated at least 3 times. Data
were presented as the means ± SD. The results of Transwell chamber
assay and western blot analysis were analyzed using the t-test. The
Mann-Whitney U test was used to analyze the results of PCR. Results
were considered statistically significant at P<0.05.
Results
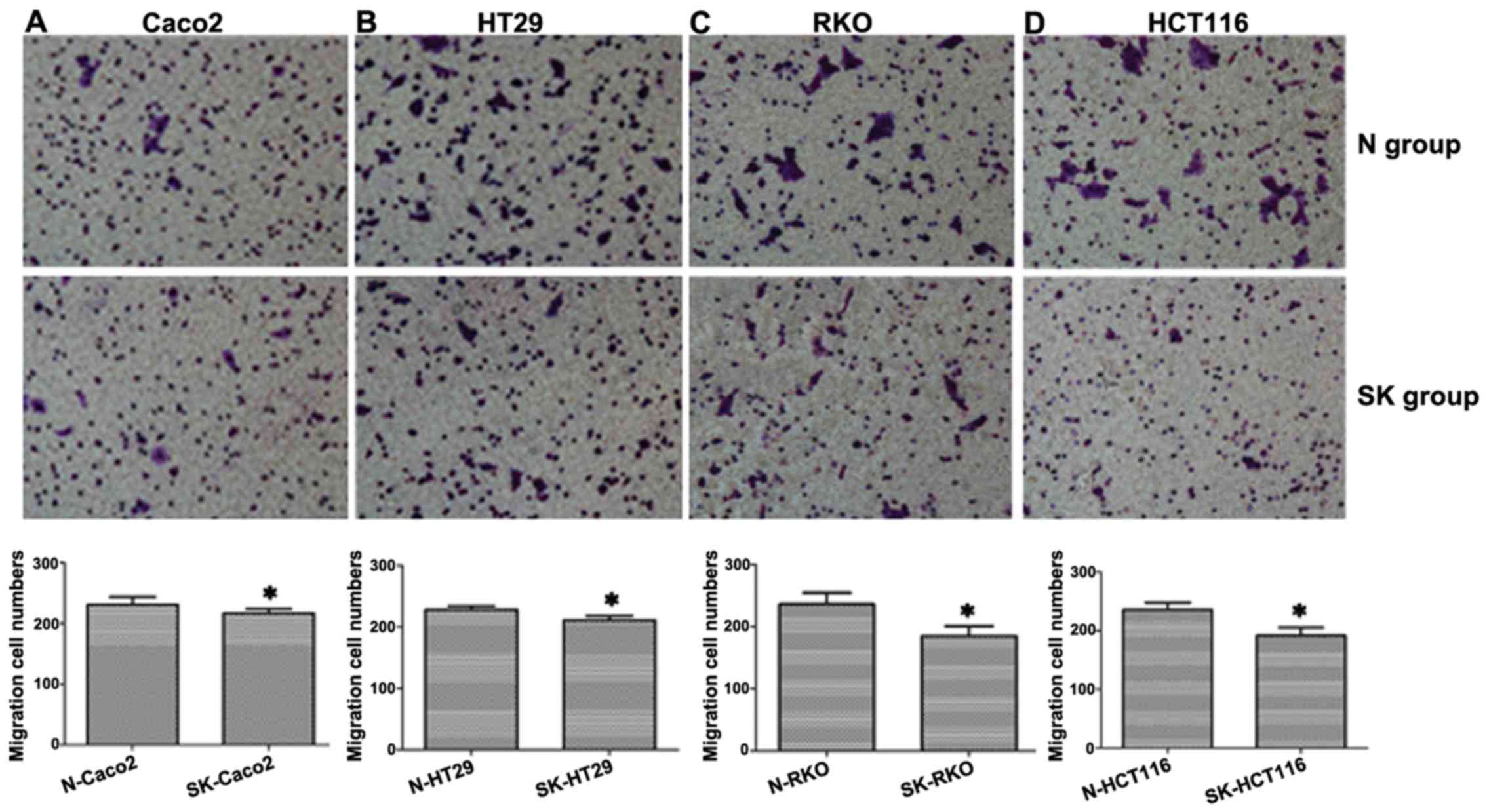
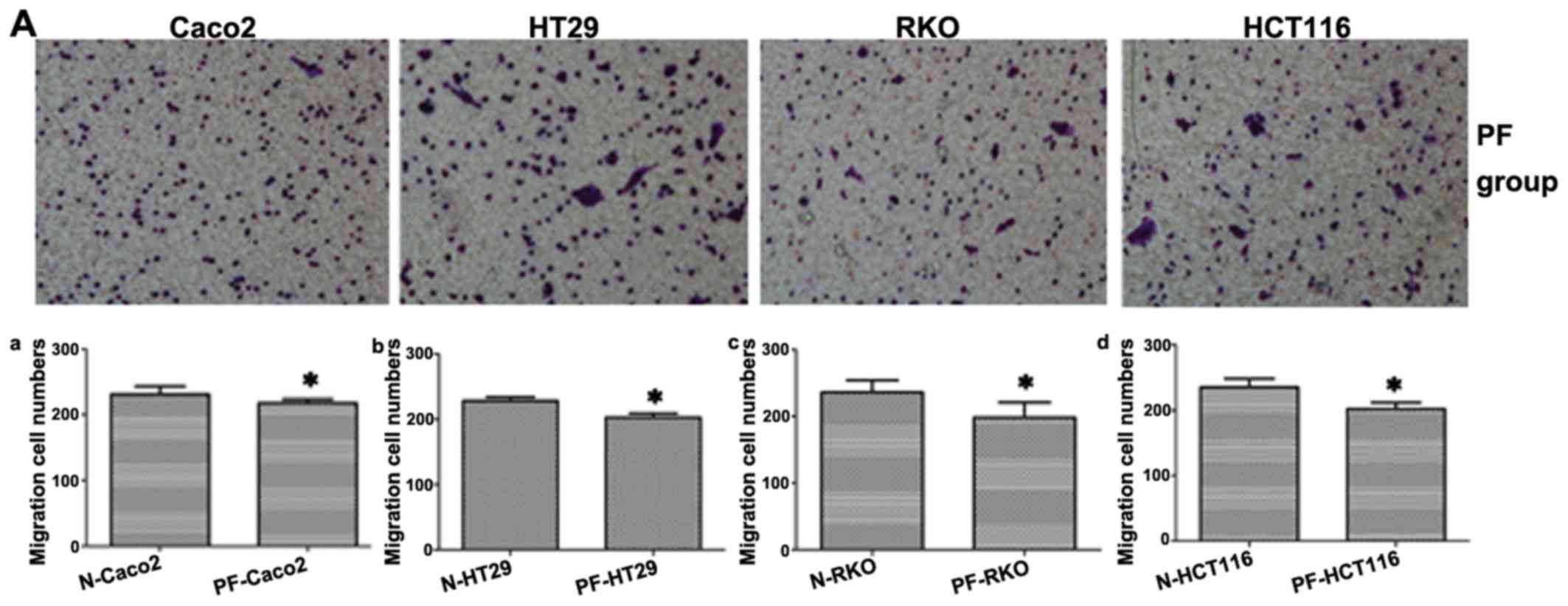
Suppression of SphK1 reduces the
migratory ability of CRC cells
To examine the effect of SphK1 on the migration of
human CRC cells, the cells were exposed to 20 µM SKI-II for
48 h, in order to inhibit SphK1 expression. The migrating cells
were observed under an inverted microscope. The results revealed
that the number of migrating cells in the SK group was
significantly lower than that of the cells in the N group in all 4
CRC cell lines (P<0.05; Fig.
1), indicating that SphK1 plays an important role in CRC cell
migration.
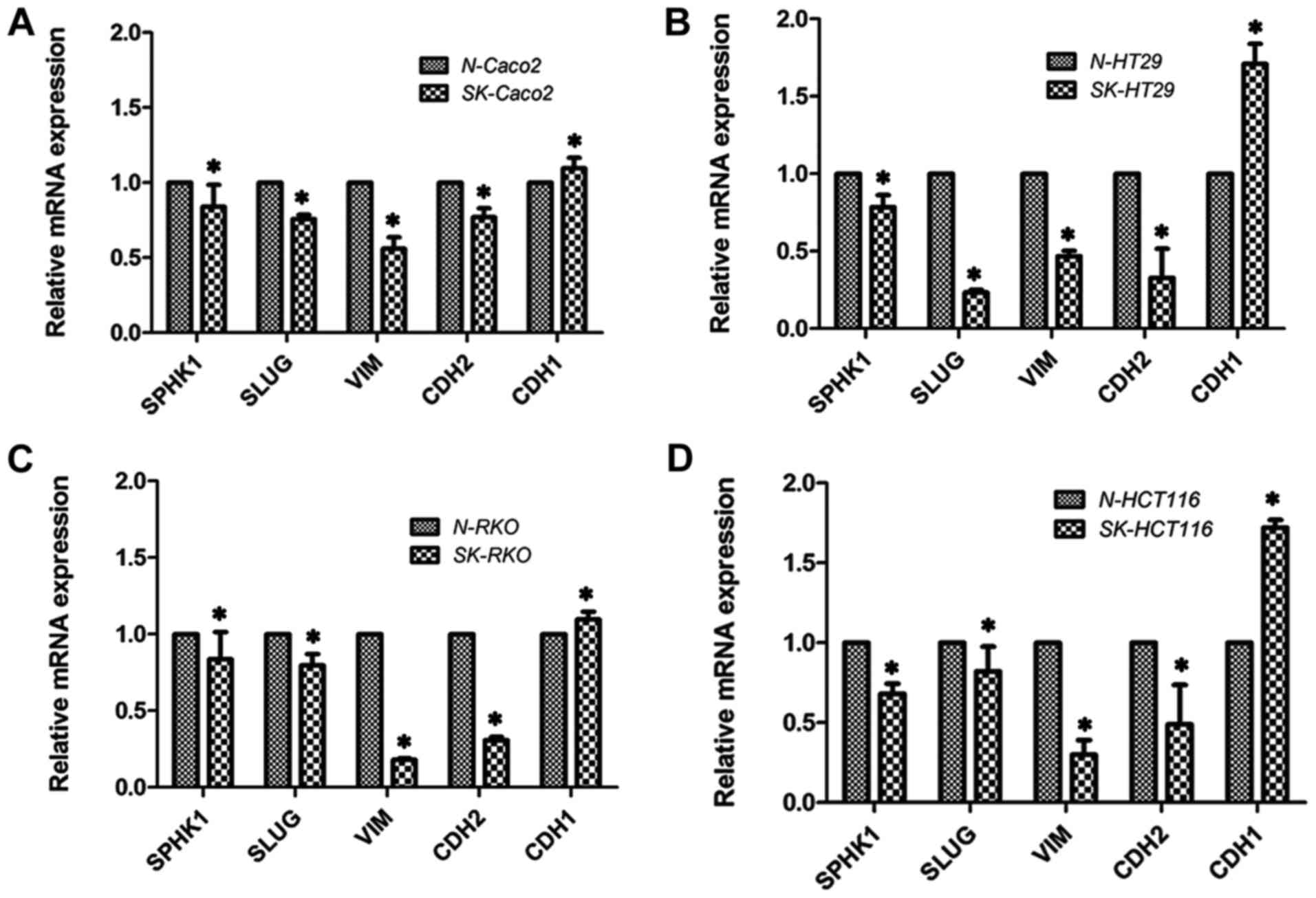
mRNA expression of SphK1, Slug, vimentin,
N-cadherin and E-cadherin detected by fluorescence-based qPCR
In order to investigate the signaling pathways
involved in the regulatory effects of SphK1 on the migratory
abitlity of CRC cells, some EMT-related markers were examined in
the present study. EMT is known to play a critical role in the
metastasis of cancer cells (24,25). The mRNA expression of EMT-related
makers was detected, including the expression of the transcription
factor, Slug (26), the
mesenchymal cell markers, vimentin and N-cadherin, and the
epithelial cell marker, E-cadherin (27–29). As shown in Fig. 2, the mRNA levels of Slug, vimentin
and N-cadherin decreased, whereas that of E-cadherin increased
(P<0.05). This indicated that the suppression of SphK1
suppressed Slug, vimentin and N-cadherin gene expression, while it
promoted E-cadherin gene expression.
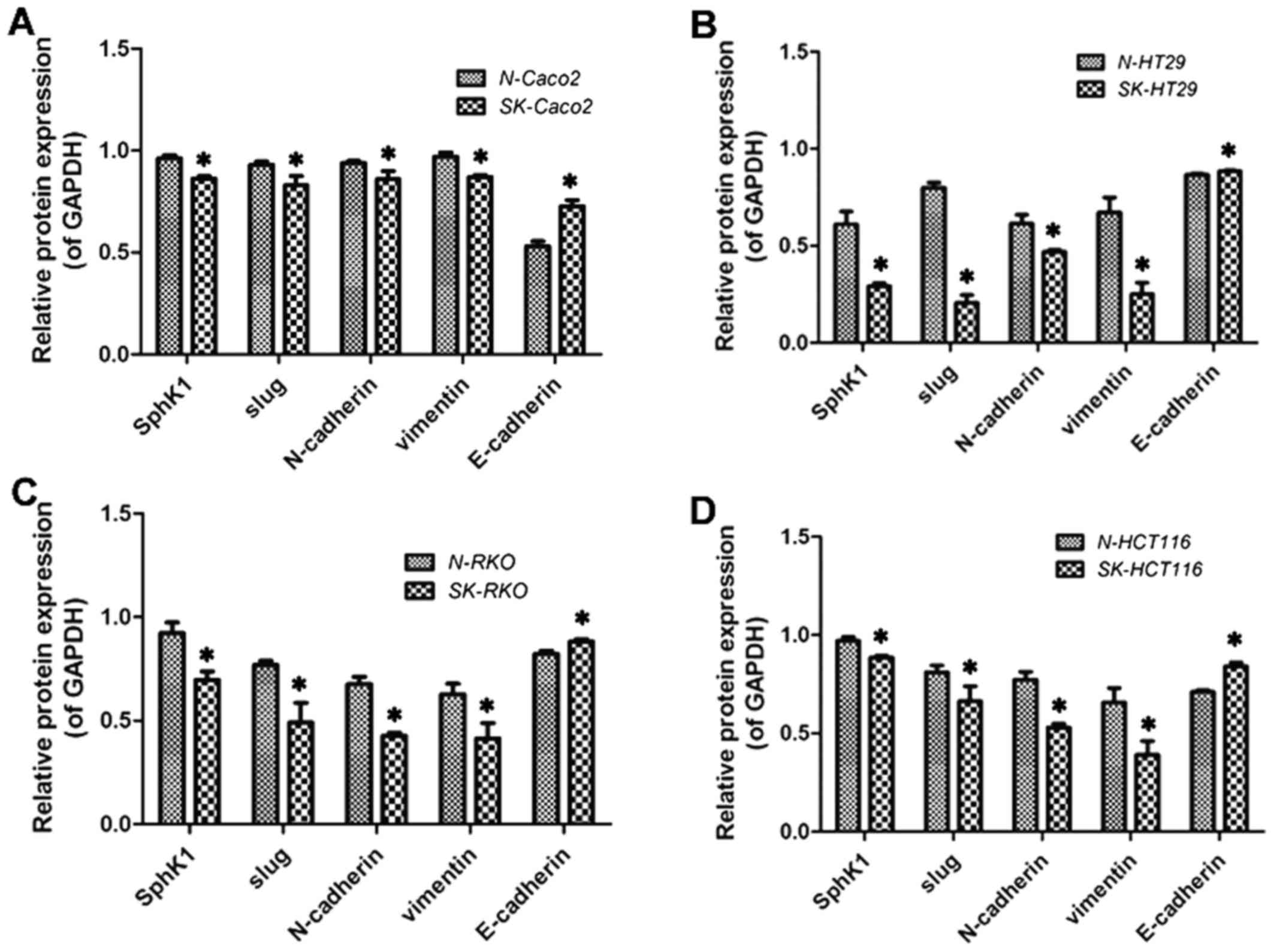
Protein expression of SphK1, Slug,
vimentin, N-cadherin and E-cadherin detected by western blot
analysis
The results revealed that the expression of Slug,
vimentin and N-cadherin decreased, whereas that of E-cadherin
increased following the suppression of SphK1 (P<0.05; Figs. 3 and 4). These results were consistent with
those obtained by PCR.
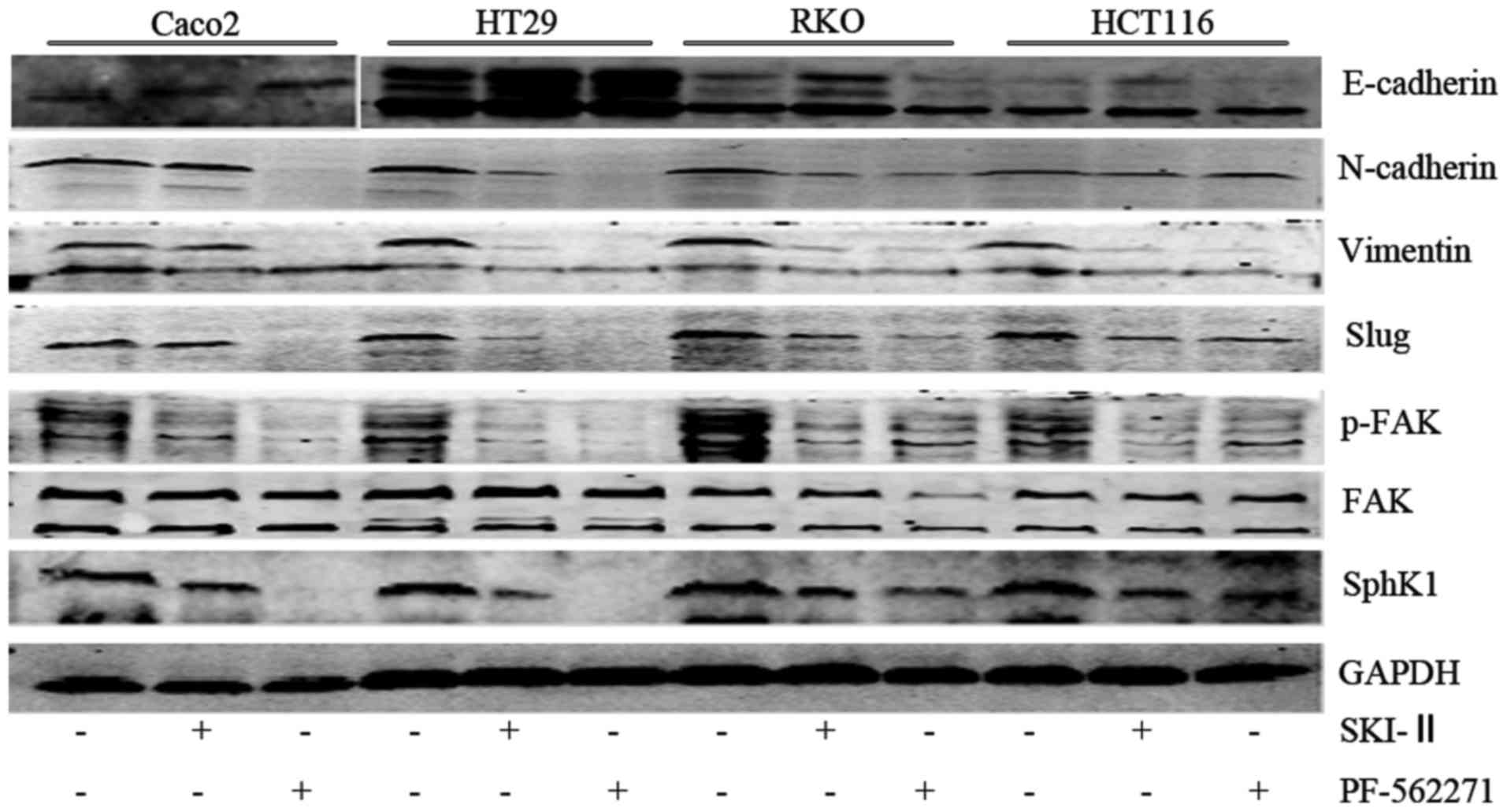 | Figure 3Protein expression of sphingosine
kinase 1 (SphK1), FAK, p-FAK, Slug, vimentin, N-cadherin,
E-cadherin in Caco2, HT29, RKO, HCT116 cells. '−' indicates culture
without SKI-II or PF-562271, while '+' indicates treatment with
SKI-II 20 µM for 48h or PF-562271 5 µM for 48 h. |
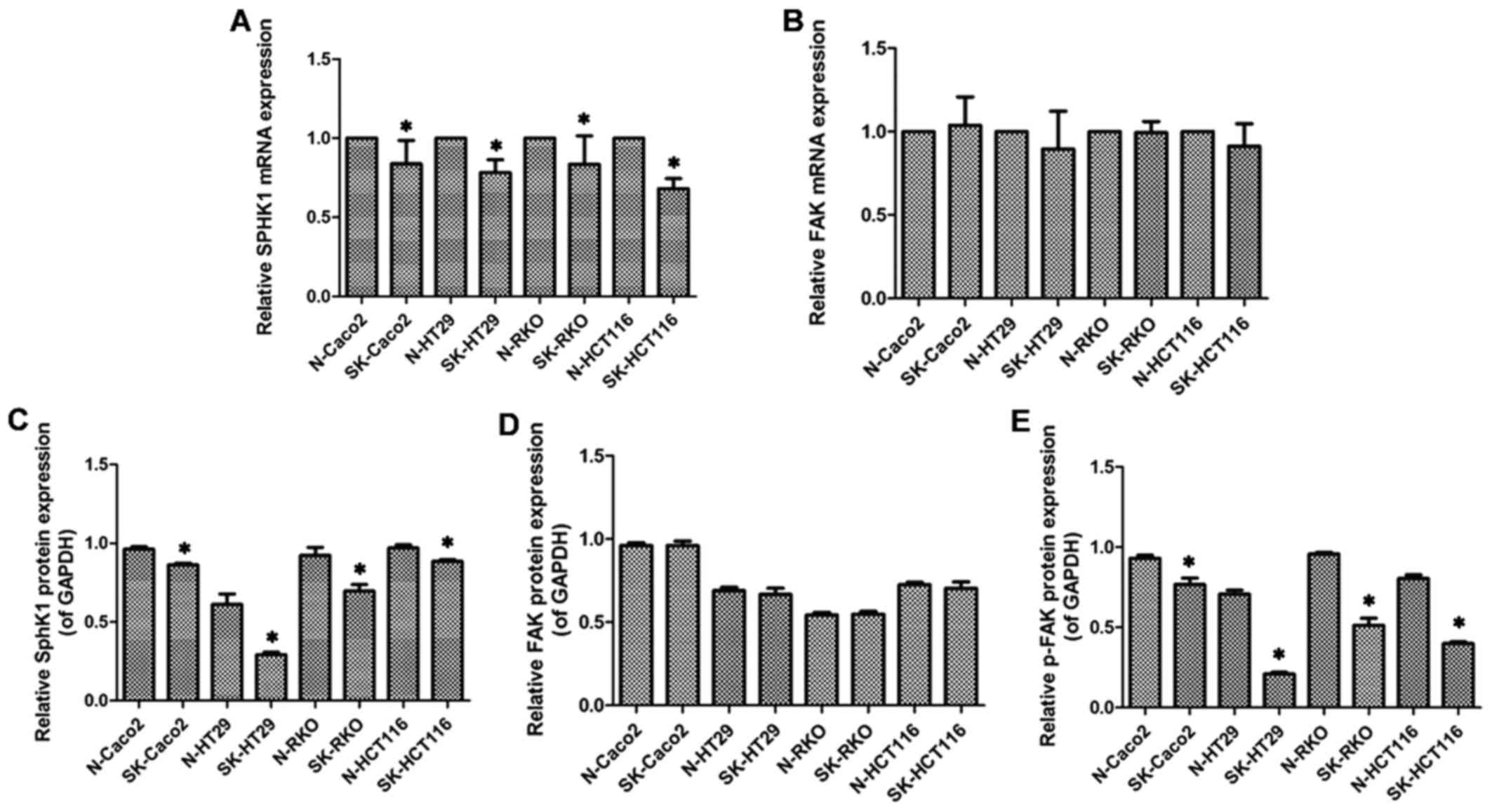
SphK1 is involved in modulating the
expression of p-FAK
Compared to the N group, although in the SK group
FAK mRNA and total FAK protein expression exhibited no significant
difference (P>0.05; Fig. 5B and
D), p-FAK protein expression was markedly decreased (P<0.05;
Figs. 3 and 5E). This indicated that SphK1 regulated
the expression of p-FAK, suggesting that SphK1 is involved in
modulating the FAK pathway.
Inhibition of FAK affects the migratory
ability, and the mRNA and protein expression of Slug, vimentin,
N-cadherin and E-cadherin in CRC cells
To date, some researchers have demonstrated that FAK
is involved in regulating the EMT process in cancer cells (30). Moreover, FAK affects the
expression of Slug (31). In this
study, our results revealed that the numbers of migrating cells in
the PF group were lower than those in the N group in the Caco2,
HT29, RKO and HCT116 cells (P<0.05; Fig. 1 and 6A). In addition, compared with the N
group, the mRNA expression levels of FAK, Slug, vimentin and
N-cadherin in the PF group were decreased, whereas the mRNA
expression level of E-cadherin was increased (P<0.05; Fig. 6B). Moreover, the protein
expression levels of FAK, p-FAK, Slug, vimentin and N-cadherin in
the PF group were lower than the levels in the N group (P<0.05;
Figs. 3 and 6C). Similar with the mRNA expression,
the protein expression of E-cadherin in the PF group was higher
than that in the N group (P<0.05; Figs. 3 and 6C). Thus, the results revealed that FAK
may affect the mRNA and protein expression levels of Slug,
vimentin, N-cadherin and E-cadherin, as well as the cell migratory
ability.
On the whole, according to our data, it can be
suggested that SphK1 modulates the expression of Slug, E-cadherin,
N-cadherin and vimentin, as well as CRC cell metastasis by
regulating the expression of p-FAK in Caco2, HT29, RKO, HCT116 CRC
cell lines.
Discussion
SphK1 is a lipid kinase which catalyzes the
phosphorylation of sphingosine to S1P. There is evidence to
indicate that SphK1 is an oncogenic enzyme, and its activation is
closely associated with the transformation, proliferation and
survival of tumor cells (32). In
a previous study, SphK1 was shown to be overexpressed in colon
cancer tissue compared with normal colonic tissue. In addition, the
expression of SphK1 was found to correlate with the Dukes' stage,
histological grading, lymphnode metastasis and distant metastasis.
These data indicate that SphK1 may contribute to the metastasis and
the malignant phenotype of colon cancer (17).
EMT has been confirmed to be an important step in
metastasis and in the change to the malignant phenotype. After EMT
has occurred in the cell, cell migratory ability increases, along
with an increase in the expression of mesenchymal cell surface
markers, and a decrease in the expression of epithelial cell
surface markers and vice versa (33–35). Slug is one of the important
transcription factors of EMT (26,36). E-cadherin is the molecular marker
of epithelial cells (27–29). N-cadherin and vimentin are
molecular markers of mesenchymal cells. In a word, they are all
indispensable biological markers in the process of EMT. However, it
is not yet clear whether SphK1 modulates the expression of
EMT-related markers, though SphK1 may contribute to metastasis and
to the change to the malignant phenotype.
A previous study demonstrated that the silencing of
SphK1 decreased the expression of vimentin and enhanced the
expression of E-cadherin in non-small cell lung cancer.
Regretfully, Slug and N-cadherin expression was not detected
(18). In the present study, the
expression of Slug, N-cadherin and vimentin was decreased, whereas
that of E-cadherin was increased following the inhibition of SphK1.
These results indicate that SphK1 plays a role in regulating the
EMT process. Moreover, the migratory ability of CRC cells was found
to weaken following the inhibition of the expression of SphK1,
which is consistent with the findings of other researchers
(37). Thus, SphK1 may regulate
cell migration and the EMT process in CRC cells.
However, it is also important to determine the
potential mechanism behind the modulatory effects of SphK1 on the
expression of EMT-related markers. It was previously found that the
FAK pathway is involved in the SphK1-mediated acquisition of the
malignant phenotype in colon cancer cells (17). FAK is a 125 kDa non-receptor
protein tyrosine kinase that has been shown to be important in the
tumorigenesis and development of human tumors (38). Studies have suggested that the FAK
pathway positively participates in the EMT process, which is
accompanied by a decrease in the expression of E-cadherin, and an
increase in the expression of Slug, N-cadherin and vimentin
(32,39,40). In our study, the expression of
E-cadherin increased, while the expression of Slug, N-cadherin and
vimentin decreased following the suppression of FAK. This study
also confirmed that the inhibition of SphK1 suppressed the
expression of p-FAK in the CRC cell lines, Caco2, HT29, RKO and
HCT116, suggesting that SphK1 may be involved in the modulation of
the FAK pathway in CRC cells. On the other hand, the results
revealed that the inhibition of SphK1 and FAK expression using
specific inhibitors had similar effects on the cell migratory
ability and on the expression of EMT-related markers. This suggests
that SphK1 modulates the EMT process and cell migration by
regulating the expression of p-FAK in CRC cells.
In conclusion, in this study, we demonstrated that
SphK1 modulated the expression of EMT-related markers and cell
migration by regulating the expression of p-FAK in the CRC cell
lines, Caco2, HT29, RKO and HCT116. Our results provide evidence of
the functional role of SphK1 in mediating the EMT process in colon
cancer. Thus, SphK1 may be a promising therapeutic target in
CRC.
Acknowledgments
This study was supported in part by grants from the
National Nature Science Foundation of China (nos. 81260365 and
81460380), and the Nature Science Foundation of Guangxi (no.
2013GXNSFAA019159).
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2015. Atlanta: American Cancer Society; 2015,
https://www.cancer.org/research/cancer-facts-statistics.html.
|
|
2
|
Xie J, Dong H, Chen H, Zhao R, Sinko PJ,
Shen W, Wang J, Lu Y, Yang X, Xie F, et al: Exploring cancer
metastasis prevention strategy: Interrupting adhesion of cancer
cells to vascular endothelia of potential metastatic tissues by
antibody-coated nanomaterial. J Nanobiotechnology. 13:92015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arvelo F, Sojo F and Cotte C: Cancer and
the metastatic substrate. Ecancermedicalscience. 10:7012016.
View Article : Google Scholar
|
|
4
|
Xu J, Qin X, Wang J, Zhang S, Zhong Y, Ren
L, Wei Y, Zeng S, Wan D and Zheng S; Society of Surgery; Chinese
Medical Association; Committee of Colorectal Cancer, Chinese
Anticancer Association: Chinese guidelines for the diagnosis and
comprehensive treatment of hepatic metastasis of colorectal cancer.
J Cancer Res Clin Oncol. 137:1379–1396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Wang G, Yang Y, Mei Z, Liang Z, Cui
A, Wu T, Liu CY and Cui L: Increased TEAD4 expression and nuclear
localization in colorectal cancer promote epithelial-mesenchymal
transition and metastasis in a YAP-independent manner. Oncogene.
35:2789–2800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atmaca A, Wirtz RW, Werner D, Steinmetz K,
Claas S, Brueckl WM, Jäger E and Al-Batran SE: SNAI2/SLUG and
estrogen receptor mRNA expression are inversely correlated and
prognostic of patient outcome in metastatic non-small cell lung
cancer. BMC Cancer. 15:3002015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan H, Kajiyama H, Ito S, Chen D, Shibata
K, Hamaguchi M, Kikkawa F and Senga T: HOXB13 and ALX4 induce SLUG
expression for the promotion of EMT and cell invasion in ovarian
cancer cells. Oncotarget. 6:13359–13370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palma-Nicolás JP and López-Colomé AM:
Thrombin induces slug-mediated E-cadherin transcriptional
repression and the parallel upregulation of N-cadherin by a
transcription-independent mechanism in RPE cells. J Cell Physiol.
228:581–589. 2013. View Article : Google Scholar
|
|
10
|
Xie Y, Liu S, Lu W, Yang Q, Williams KD,
Binhazim AA, Carver BS, Matusik RJ and Chen Z: Slug regulates
E-cadherin repression via p19Arf in prostate tumorigenesis. Mol
Oncol. 8:1355–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar
|
|
12
|
Xia J, Wu Z, Yu C, He W, Zheng H, He Y,
Jian W, Chen L, Zhang L and Li W: miR-124 inhibits cell
proliferation in gastric cancer through downregulation of SPHK1. J
Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan J, Tao YF, Zhou Z, Cao BR, Wu SY,
Zhang YL, Hu SY, Zhao WL, Wang J, Lou GL, et al: An novel role of
sphingosine kinase-1 (SPHK1) in the invasion and metastasis of
esophageal carcinoma. J Transl Med. 9:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Yan Z, Yuan Z, Sun Y, He H and
Mai C: SPHK1 inhibitor suppresses cell proliferation and invasion
associated with the inhibition of NF-κB pathway in hepatocellular
carcinoma. Tumour Biol. 36:1503–1509. 2015. View Article : Google Scholar
|
|
15
|
Chen K, Pan Q, Gao Y, Yang X, Wang S,
Peppelenbosch MP and Kong X: DMS triggers apoptosis associated with
the inhibition of SPHK1/NF-κB activation and increase in
intracellular Ca2+ concentration in human cancer cells.
Int J Mol Med. 33:17–24. 2014.
|
|
16
|
Xiong H, Wang J, Guan H, Wu J, Xu R, Wang
M, Rong X, Huang K, Huang J, Liao Q, et al: SphK1 confers
resistance to apoptosis in gastric cancer cells by downregulating
Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 32:1369–1373.
2014.PubMed/NCBI
|
|
17
|
Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA
and Tang GD: Sphingosine kinase 1 promotes tumor progression and
confers malignancy phenotypes of colon cancer by regulating the
focal adhesion kinase pathway and adhesion molecules. Int J Oncol.
42:617–626. 2013.
|
|
18
|
Ni M, Shi XL, Qu ZG, Jiang H, Chen ZQ and
Hu J: Epithelial mesenchymal transition of non-small-cell lung
cancer cells A549 induced by SPHK1. Asian Pac J Trop Med.
8:142–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Golubovskaya VM: Targeting FAK in human
cancer: From finding to first clinical trials. Front Biosci
(Landmark Ed). 19:687–706. 2014. View
Article : Google Scholar
|
|
20
|
Wilson C, Nicholes K, Bustos D, Lin E,
Song Q, Stephan JP, Kirkpatrick DS and Settleman J: Overcoming
EMT-associated resistance to anti-cancer drugs via Src/FAK pathway
inhibition. Oncotarget. 5:7328–7341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ,
Chen MW, Jeng YM, Chiou J, Yu P, Chen PS, et al: Angiopoietin-like
protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin
Invest. 123:1082–1095. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gassowska M, Cieslik M, Wilkaniec A and
Strosznajder JB: Sphingosine kinases/sphingosine-1-phosphate and
death Signalling in APP-transfected cells. Neurochem Res.
39:645–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crompton BD, Carlton AL, Thorner AR,
Christie AL, Du J, Calicchio ML, Rivera MN, Fleming MD, Kohl NE,
Kung AL, et al: High-throughput tyrosine kinase activity profiling
identifies FAK as a candidate therapeutic target in Ewing sarcoma.
Cancer Res. 73:2873–2883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Gao D, Wang H, Li X, Yang J, Yan X,
Liu Z and Ma Z: Negative feedback loop between p66Shc and ZEB1
regulates fibrotic EMT response in lung cancer cells. Cell Death
Dis. 6:e17082015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhao Z, Xu C, Zhou Z, Zhu Z and You
T: HMGA2 induces transcription factor Slug expression to promote
epithelial-to-mesenchymal transition and contributes to colon
cancer progression. Cancer Lett. 355:130–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E-cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
da Silva SD, Morand GB, Alobaid FA, Hier
MP, Mlynarek AM, Alaoui-Jamali MA and Kowalski LP:
Epithelial-mesenchymal transition (EMT) markers have prognostic
impact in multiple primary oral squamous cell carcinoma. Clin Exp
Metastasis. 32:55–63. 2015. View Article : Google Scholar
|
|
30
|
Taliaferro-Smith L, Oberlick E, Liu T,
McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E,
Nagaraju GP, et al: FAK activation is required for IGF1R-mediated
regulation of EMT, migration, and invasion in mesenchymal triple
negative breast cancer cells. Oncotarget. 6:4757–4772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
John JK, Paraiso KH, Rebecca VW, Cantini
LP, Abel EV, Pagano N, Meggers E, Mathew R, Krepler C, Izumi V, et
al: GSK3β inhibition blocks melanoma cell/host interactions by
downregulating N-cadherin expression and decreasing FAK
phosphorylation. J Invest Dermatol. 132:2818–2827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Datta A, Loo SY, Huang B, Wong L, Tan SS,
Tan TZ, Lee SC, Thiery JP, Lim YC, Yong WP, et al: SPHK1 regulates
proliferation and survival responses in triple-negative breast
cancer. Oncotarget. 5:5920–5933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huo C, Kao YH and Chuu CP: Androgen
receptor inhibits epithelial-mesenchymal transition, migration, and
invasion of PC-3 prostate cancer cells. Cancer Lett. 369:103–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng G, Liu C, Sun X, Zhang L, Liu L,
Ouyang J and Li B: Visfatin promotes osteosarcoma cell migration
and invasion via induction of epithelial-mesenchymal transition.
Oncol Rep. 34:987–994. 2015.PubMed/NCBI
|
|
35
|
Räsänen K and Vaheri A: TGF-beta1 causes
epithelial-mesenchymal transition in HaCaT derivatives, but induces
expression of COX-2 and migration only in benign, not in malignant
keratinocytes. J Dermatol Sci. 58:97–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen MB, Yang L, Lu PH, Fu XL, Zhang Y,
Zhu YQ and Tian Y: MicroRNA-101 downregulates sphingosine kinase 1
in colorectal cancer cells. Biochem Biophys Res Commun.
463:954–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stewart JE, Ma X, Megison M, Nabers H,
Cance WG, Kurenova EV and Beierle EA: Inhibition of FAK and VEGFR-3
binding decreases tumorigenicity in neuroblastoma. Mol Carcinog.
54:9–23. 2015. View
Article : Google Scholar :
|
|
39
|
Hsieh YS, Chu SC, Hsu LS, Chen KS, Lai MT,
Yeh CH and Chen PN: Rubus idaeus L. reverses
epithelial-to-mesenchymal transition and suppresses cell invasion
and protease activities by targeting ERK1/2 and FAK pathways in
human lung cancer cells. Food Chem Toxicol. 62:908–918. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen KS, Shi MD, Chien CS and Shih YW:
Pinocembrin suppresses TGF-β1-induced epithelial-mesenchymal
transition and metastasis of human Y-79 retinoblastoma cells
through inactivating αvβ3 integrin/FAK/p38α signaling pathway. Cell
Biosci. 4:412014. View Article : Google Scholar
|