Introduction
Oxygen therapy is a very common administration for
neonates with critical respiratory diseases. However, the high
concentration and long-term exposure to oxygen are known to cause
oxygen toxicity, acute lung injury (ALI), lung developmental
disorders and even death. It is also one of the main etiological
factors of bronchopulmonary dysplasia (BPD). Due to the lack of
effective treatments, ALI and BPD represent a major cause of
mortality and morbidity among premature infants (1–4).
Hyperoxia can result in severe epithelial and endothelial damage
(5–7). Epithelial cell death plays a
critical role in hyperoxia-induced lung injury. In order to
maintain normal pulmonary function, rapid and efficient self-repair
is very important to the injured alveolar epithelium. Although the
repair of the lung alveolar epithelium may include respiratory stem
or progenitor cells (8), it has
also been demonstrated that the proliferation and differentiation
of alveolar epithelial type II (AECII) cells plays an important
role in repairing the injured alveolus (9). Unfortunately, current treatments for
lung alveolar epithelial injury at best provide symptomatic relief,
but offer no prospect for the repair of the damaged epithelium.
As a 37-amino acid neuropeptide, calcitonin
gene-related peptide (CGRP) is secreted by a dense network of
sensory C-fibers, and has broad regulatory effects throughout the
body, particularly in the cardiovascular and respiratory system
(10). In the lungs, CGRP has
been reported to play a role in immunomodulation, vasodilatation,
bronchial protection, the proliferation of epithelial and
endothelial cells and the regulation of airway responsiveness, and
is associated with a number of respiratory diseases (11). CGRP has also had been researched
in alveolar epithelial cells (12). In vitro, a previous study
carried out in our laboratory primarily demonstrated that CGRP
promoted the proliferation of AECII cells and partially relieved
the effects of 60% oxygen on AECII cells (13). It seemed to play a protective role
against lung injury through its antioxidant properties when the
AECII cells were exposed to 60% oxygen (13). However, the effects of CGRP under
conditions with higher oxygen concentrations (>90%), have not
yet been elucidated.
Lung alveolar interstitial fibroblasts and their
communications with adjacent epithelial cells play a critical role
in lung development and injury/repair (14). A number of signaling pathways,
such as the JAK/STAT (15), PI3
kinase/Akt (16) and
mitogen-activated protein kinase pathways (17), have been demonstrated to play
important roles under several conditions. The Sonic hedgehog (SHH)
signaling pathway consists chiefly of the Shh, Ptc1, Smo, Gli1,
Gli2 and Gli3 molecules. It has long been known that this pathway
is essential for embryonic development, and it has been shown to
regulate cell migration, proliferation, differentiation and
apoptosis (18). This signaling
cascade is crucial for the patterning of early lung morphogenesis
(19). However, its role in AECII
cells, particularly those exposed to hyperoxia, remains to be
determined. In particular, it is unclear whether the SHH signaling
pathway is associated with the protective effects of CGRP against
hyperoxia-induced injury to AECII cells.
Thus, in the present study, we examined the changes
of the two important members of this signaling pathway, Shh and its
receptor Ptc1, under conditions of normal air, hyeroxia and
following treatment with CGRP.
Materials and methods
Experimental animals
All animal experiments were carried out with home
office and local ethical committee approval (approval was obtained
from the Ethics Committee of Chongqing Medical University,
Chongqing, China). All animals received care according to the
'Guide for the Care and Use of Laboratory Animals'. This study also
followed the institutional and National Institutes of Health
guidelines for laboratory animal care. A total of 32 healthy
pregnant specific-pathogen-free Sprague-Dawley rats (weighing
200–220 g; gestational age, 19 days) used in this study were
obtained from the Experimental Animal Center of the Third
Affiliated Hospital of the Third Military Medical University
(Chongqing, China).
Isolation of AECII cells from premature
rats
According the modified method previously described
(20), following anaesthesia by
an intraperitoneal injection of pentobarbital (200 mg/kg), delivery
was induced in the pregnant rats by uterine incision and the
premature rats were thus removed from the rat womb on day 19 (full
term, 22 days). The fetal lungs were obtained, minced and digested
with 0.125% trypsin and 10 mg/ml DNAse for 20 min at 37°C. The
trypsin reaction was terminated with DMEM/F12 with 10% fetal calf
serum (FCS) (Trypsin, DNAse, DMEM/F12 and FCS were obtained from
Gibco, Grand Island, NY, USA) and then centrifuged at 800 × g for 5
min. The supernatants were removed and the cell pellets were
resuspended in collagenase (Sigma, St. Louis, MO, USA), followed by
incubation for 15 min at 37°C. The collagenase reaction was
terminated by the addition of FCS, followed by centrifugation. The
cell pellets were then resuspended and transferred into culture
flasks for differential adherence to remove the fibroblasts. Thus,
the AECII cells were isolated quickly. Subsequently, the isolated
cells were stained by modified Papanicolaou stain and trypan blue;
the purity and survival rates were >90%. Finally, the purified
AECII cells were seeded into 6-well plates at a density of
1×106 in DMEM/F12 supplemented with 10% fetal bovine
serum (FBS). Morphological changes of AECII cells were observed
under a Nikon TS100 inverted microscope (Nikon Instruments Inc.,
Melville, NY, USA).
Cell treatment and experimental
groups
The AECII cells were inoculated into 6-well plates
and allowed to grow to approximately 80% confluence. The medium was
changed, and 10−7 M/l α-CGRP (AnaSpec, Inc., Fremont,
CA, USA), according to the experimental conditions, were added
before placing the plates in special chambers and exposure to air
or hyperoxia. The air condition was accomplished by filling the
chamber with air (21% oxygen) containing 5% CO2. The
hyperoxia condition was achieved by flushing the chamber with 95%
medical oxygen containing 5% CO2 until equilibrium. Then
both the air and hyperoxia chambers were sealed and placed into a
37°C incubator for 24 h with continuous monitoring of the oxygen
fraction by an input high-accuracy smart oxygen meter. At 24 h
following exposure, the cells and culture supernatant were
collected for further experiments. For the experiments, the cells
were divided into 4 groups as follows: i) air group, cells were
cultured under air conditions; ii) the air + CGRP group, cell
medium was added with CGRP prior to culture under air conditions;
iii) the hyperoxia group (HG), the cells were cultured under
hyperoxic conditions; iv) the hyperoxia + CGRP group, CGRP was
added to the medium prior to exposure to hyeroxia.
Intracellular reactive oxygen species
(ROS) assay
The dichlorofluorescin diacetate (DCFH-DA) molecular
probe (KeyGen Biotech Co., Ltd., Nanjing, China) was used to detect
the levels of intracellular ROS. The AECII cells were washed with
DMEM and cultured with 10 µmol/l DCFH-DA at 37°C for 25 min,
and then digested with 0.25% trypsin after being washed with
phosphate-buffered saline (PBS) 3 times. Thereafter, the cells were
collected by centrifugation (800 × g, 25°C) and the mean
fluorescence intensity was detected using a flow cytometer
(Becton-Dickinson, San Jose, CA, USA) with an excitation wavelength
of 488 nm and an emission wavelength of 525 nm.
Measurement of malondialdehyde (MDA) and
superoxide dismutase (SOD) activities
To evaluate the damage caused by hyperoxia and the
effect of CGRP, we measured the activities of malondialdehyde (MDA;
indicator of oxidative damage) and superoxide dismutase (SOD;
antioxidant indicator) with spectrophotometry in the culture
supernatant of AECII cells using commercially available kits
(KeyGen Biotech Co., Ltd.) according to directions provided by the
the manufacturer. MDA was measured using the thiobarbituric acid
method. This technique measures the degradation product of lipid
peroxidation, which condenses with penthiobarbital and leads to a
red product with measurable absorbance using a UV
spectrophotometer. SOD was measured using the xanthine oxidase
method; according to this method, superoxide anion radicals lead to
the oxidation of hydroxylamine, resulting in a purple nitrite
compound measurable by pectrophotometric analysis, with a maximum
absorptive length of 568 nm. SOD activity leads to the reduction of
the nitrite compound, thus, allowing for a measurably lower
absorbance that correlates with SOD presence.
Apoptosis assay
Following culture for 24 h, the cells were collected
in a tube and washed twice with PBS and suspended in a binding
buffer containing 5 µl Annexin V-FITC and 5 µl
propidium iodide (PI) (KeyGen Biotech Co., Ltd.), then incubated at
room temperature for 10 min in a dark room. Apoptosis and necrosis
was assayed by flow cytometry (Becton-Dickinson) according to the
manufacturer's instructions.
Detection of Shh and Ptc1 mRNA levels by
RT-qPCR
The Shh and Ptc1 mRNA levels were detected by
RT-qPCR. The cells were collected and the total RNA was extracted
using an RNA TRIzol kit (Invitrogen Life Technologies, Paisley, UK)
according to the manufacturer's instructions. Template cDNAs were
obtained by the reverse transcription of total RNA using oligo(dT)
primer and superscript II reverse transcriptase (Takara, Otsu,
Japan). Amplification was carried out using SYBR-Green qPCR Master
Mix (Takara). The expression level of β-actin was used as an
internal control. The PCR primers for Shh, Ptc1 and β-actin were
designed and synthesized by Shinegene Molecular Biotech, Inc.
(Shanghai, China). The sequences of the primers used (rat) were as
follows: Shh forward, 5′-TCGTGCTACGCAGTCATCG-3′ and reverse,
5′-CGCTTCCGCTACAGATTGC-3′; Ptc1 forward, 5′-TGTGGCAACAGGACGGAAC-3′
and reverse, 5′-CCAGAGTGTCAGCAGAAGAAAAG-3′; and β-actin forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. All qPCR reactions were performed with
a FTC2000 machine (Funglyn Biotech Inc., Scarborough, ON, Canada)
using the following thermocycling conditions: 94°C for 4 min, 1
cycle; 94°C for 20 sec, 60°C for 30 sec; and 72°C for 30 sec, 35
cycles. β-actin was used for each test sample along with target
genes. Gene expression was quantitatively analyzed using the
comparative CT (ΔCT) method, in which CT is the threshold cycle
number. As the target genes, the Shh and Ptc1 mRNA levels were
calculated using the following formula (21): ΔΔCt, experimental group
(Cttarget gene − CTβ-actin) - control group
(Cttarget gene − CTβ-actin),
2−ΔΔCT, amount of target. Finally, the formula
2−ΔΔCT was used to calculate the target RNA amount in
comparison with the control.
Detection of protein levels of Shh and
Ptc1 by western blot analysis
The protein expression levels of Shh and Ptc1 were
examined by with western blot analysis. Total cellular proteins
were extracted using ice-cold lysis buffer (RIPA buffer) containing
50 mM Tris·HCl (pH 7.5), 1 mM EGTA, 1 mM EDTA, supplemented with 1
mM PMSF, phosphatase inhibitor and complete proteinase inhibitor
cocktail (Sigma Chemical Co.). The samples were sonicated and then
centrifuged at 500 × g for 20 min at 4°C to remove cellular debris.
Fifty micrograms of total protein for each sample were denatured by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) sample buffer and electrophoresed in a 10% SDS
polyacrylamide gel. The resolved samples were then transferred onto
PVDF membranes (ImmobilonP; Millipore, Bedford, MA, USA), which,
after blocking with TBS-Tween-20 (TBST) + 5% milk, the membranes
were incubated with the following primary antibodies: anti-rat Shh
(AV44235; 1:100) and Ptc1 (P0088; 1:100), or anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH; G5262; 1:1,000) rabbit antibody
(Sigma Chemical Co.) overnight at 4°C, followed by horseradish
peroxidase-conjugated goat anti-rabbit IgG (sc-2091; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Photographic film was
used to capture the protein bands, and densitometric analysis was
performed to measure the intensity of these bands using Quantity
One 4.6 software (Bio-Rad Laboratories, Hercules, CA, USA). Protein
band intensities were normalized for loading using the
corresponding GAPDH signals and expressed as arbitrary units
(AU).
Measurement of Shh expression by
immunofluorescence
The AECII cells were grown on slides and cultured
for 15–18 h, and then treated with CGRP prior to exposure to air or
95% oxygen as mentioned above. Twenty-four hours later, the slides
were taken out and rinsed 3 times with ice-cold PBS. The cells were
then fixed with methanol for 15 min at −20°C, rehydrated twice with
PBS, and blocked with 1% BSA for 10 min at room temperature.
Following overnight incubation with a specific Shh antibody
(AV44235), the slides were rinsed extensively with PBS, and further
incubated with a FITC secondary antibody (F0382; Sigma Chemical
Co.) for 1 h at 25°C in the dark room. Cellular morphology was
observed by DAPI staining. Visualization was performed using a
fluorescence microscope (Nikon TS100; Nikon Instruments Inc.).
Statistical analysis
Biochemical experiments were carried out at least 3
independent times. All the data are expressed as the means ± SEM
and analyzed using SPSS statistical software (16.0 for Windows;
SPSS Inc., Chicago, IL, USA). The statistical significance of the
differences between the means of the groups was determined by
one-way ANOVA or two-tailed Student's t-tests. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
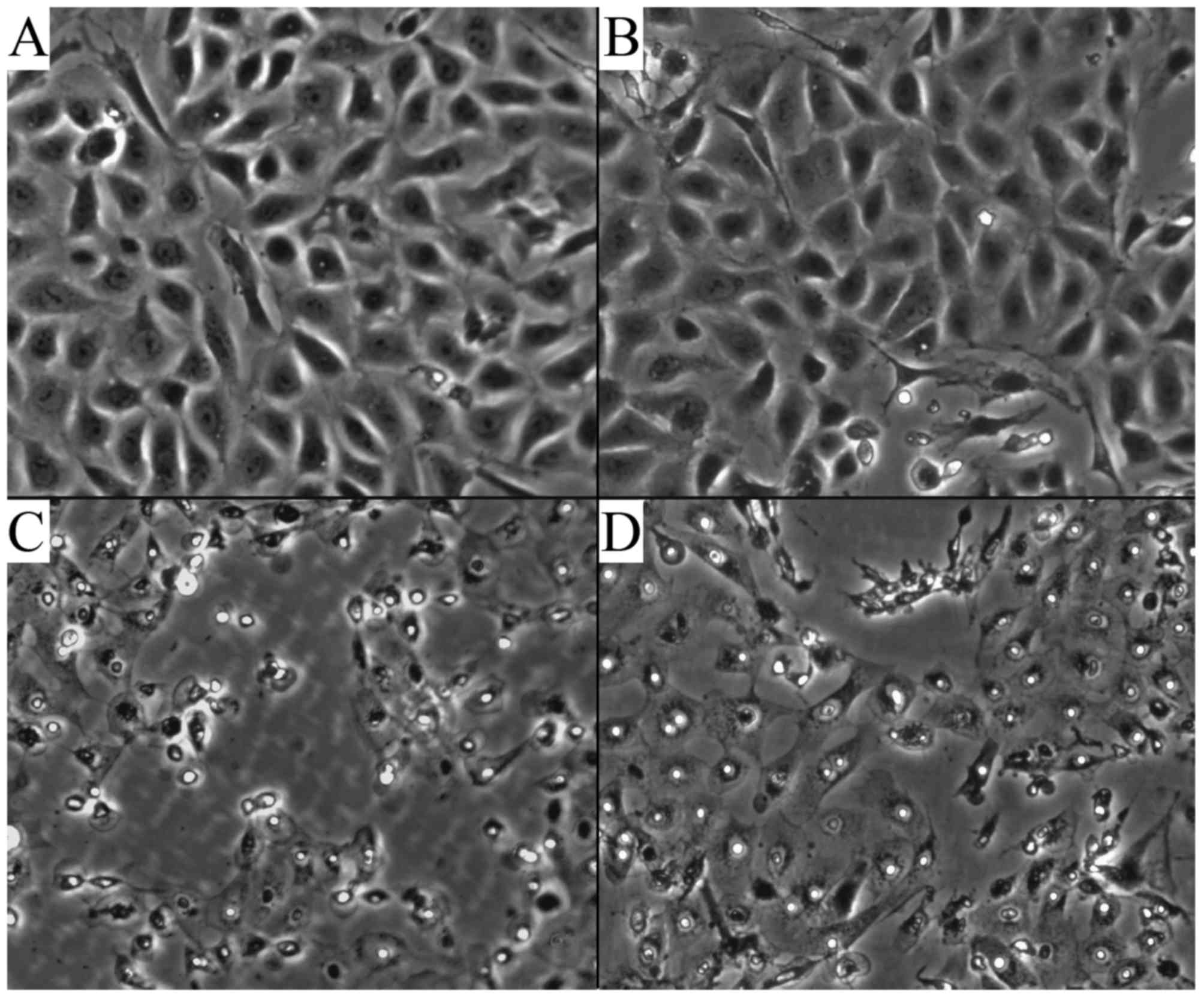
Morphological alteration of the AECII
cells
The cells had spread and contained many lamellar
bodies, and clung to the bottom of the wells. The cells were
connected closely and grew in a good condition in the air and air +
CGRP group (Fig. 1A and B). In
the hyperoxia group, the cell number decreased, and the cells
became deformed, stopped growing, underwent apoptosis, the number
of lamellar bodies was decreased, and the intercellular space
expanded and intracellular vacuoles could be observed (Fig. 1C). Following treatment with CGRP,
however, these changes in cell morphology were attenuated to a
certain extent (Fig. 1D).
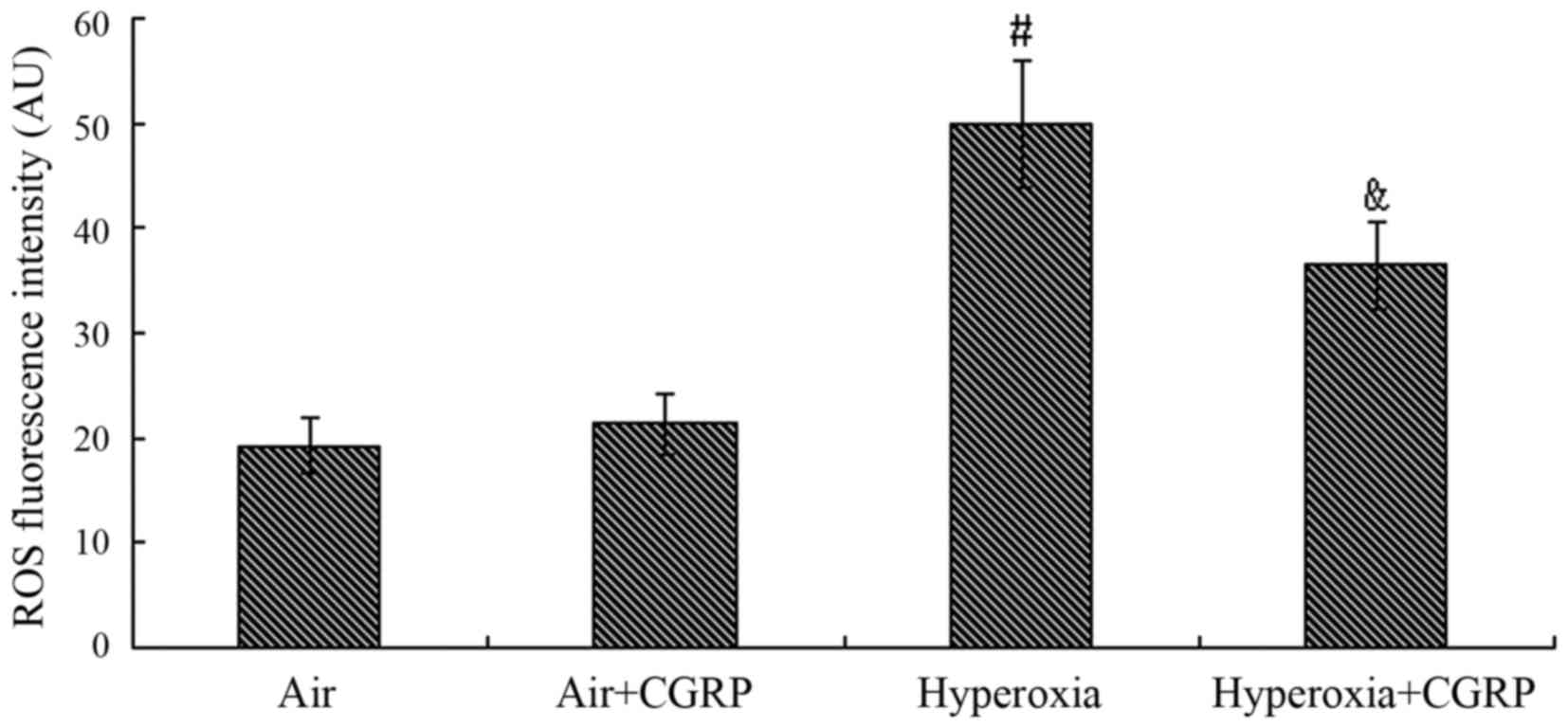
Effect of CGRP on ROS
The production of intracellular ROS did not exhibit
a significant difference between the air control and the air + CGRP
group (P>0.05). Compared with the normal air group, the level of
ROS following exposure to hyperoxia was markedly increased
(P<0.05); however, this increase was partly inhibited by
treatment with CGRP 10−7 M (P<0.05) (Fig. 2).
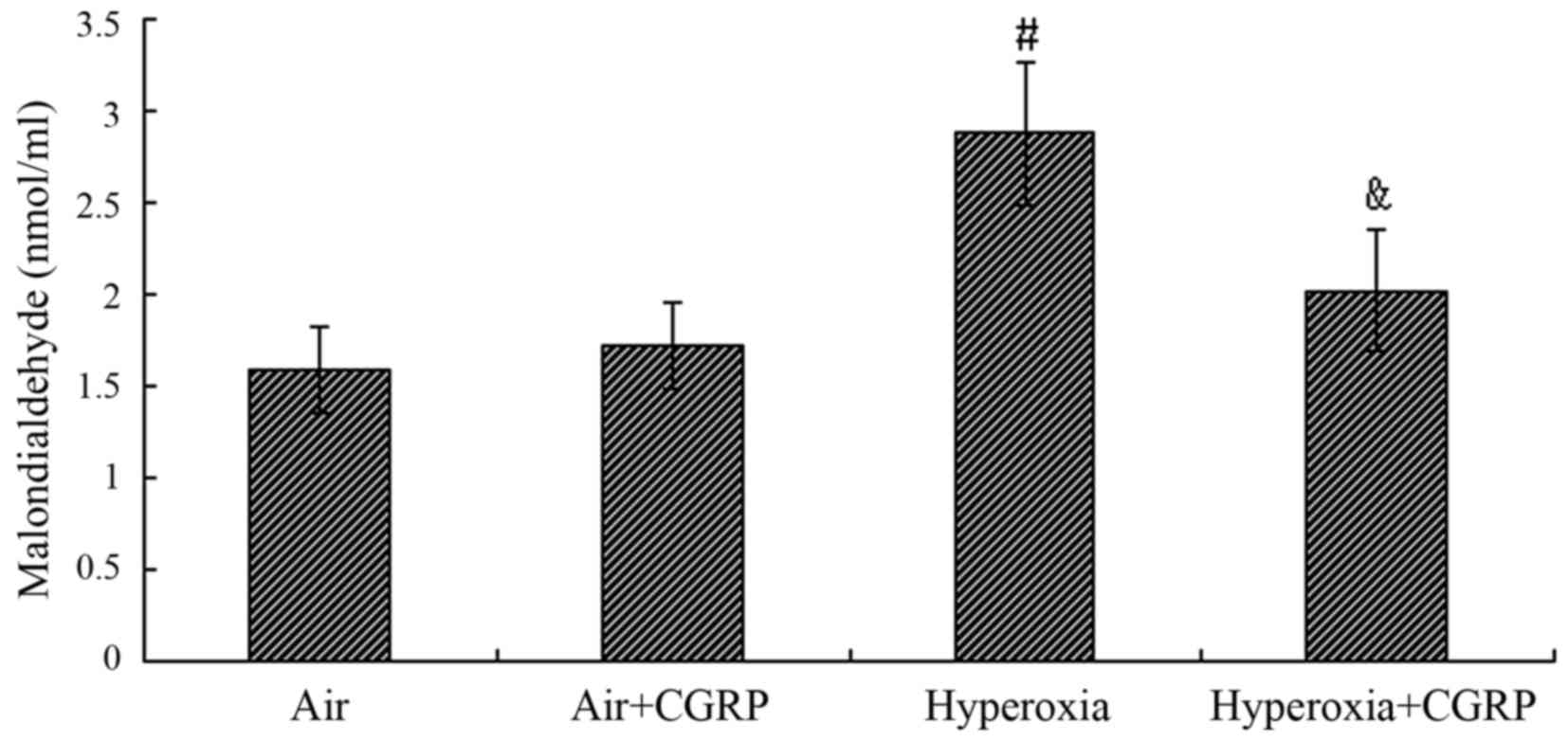
Malondialdehyde activity in the culture
supernatant of AECII cells
The level of MDA in the culture supernatant of AECII
cells did not differ significantly between the normal air control
group and the air + HG group (P>0.05); however, the level was
markedly increased approximately 2-fold under the condition of
hyperoxia compared with the air control group (P<0.05). In the
hyperoxia + CGRP group, CGRP significantly reduced the MDA level
compared with the hyperoxia group (P<0.05) (Fig. 3).
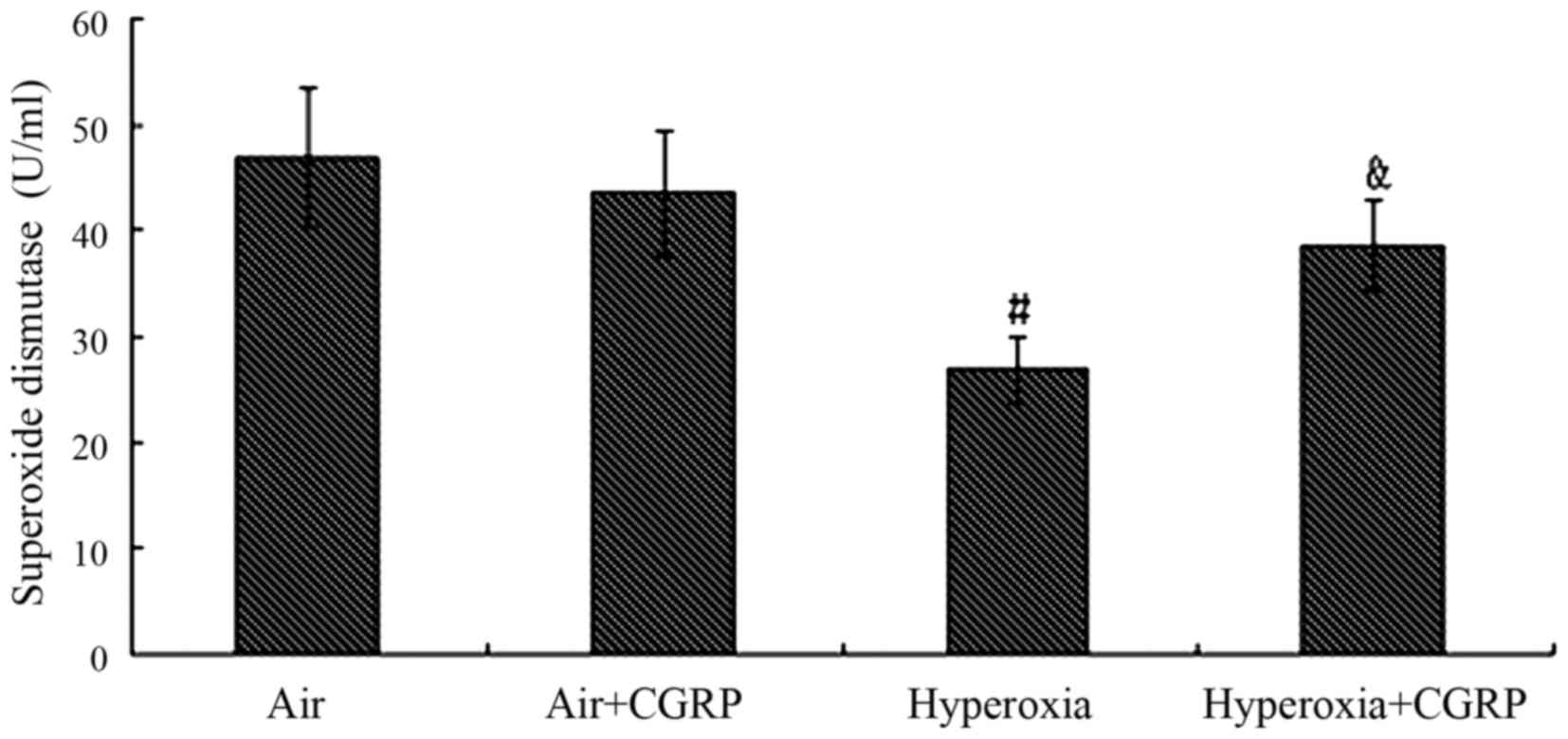
Superoxide dismutase activity in the
culture supernatant of AECII cells
Under the normal air condition, the activity of SOD
was not markedly decreased in the air + CGRP group (P>0.05), but
was significantly decreased in the hyperoxia group compared with
the air group (P<0.05). In the hyperoxia + CGRP group, the
activity of SOD significantly increased as compared with the
hyperoxia group (P<0.05) (Fig.
4).
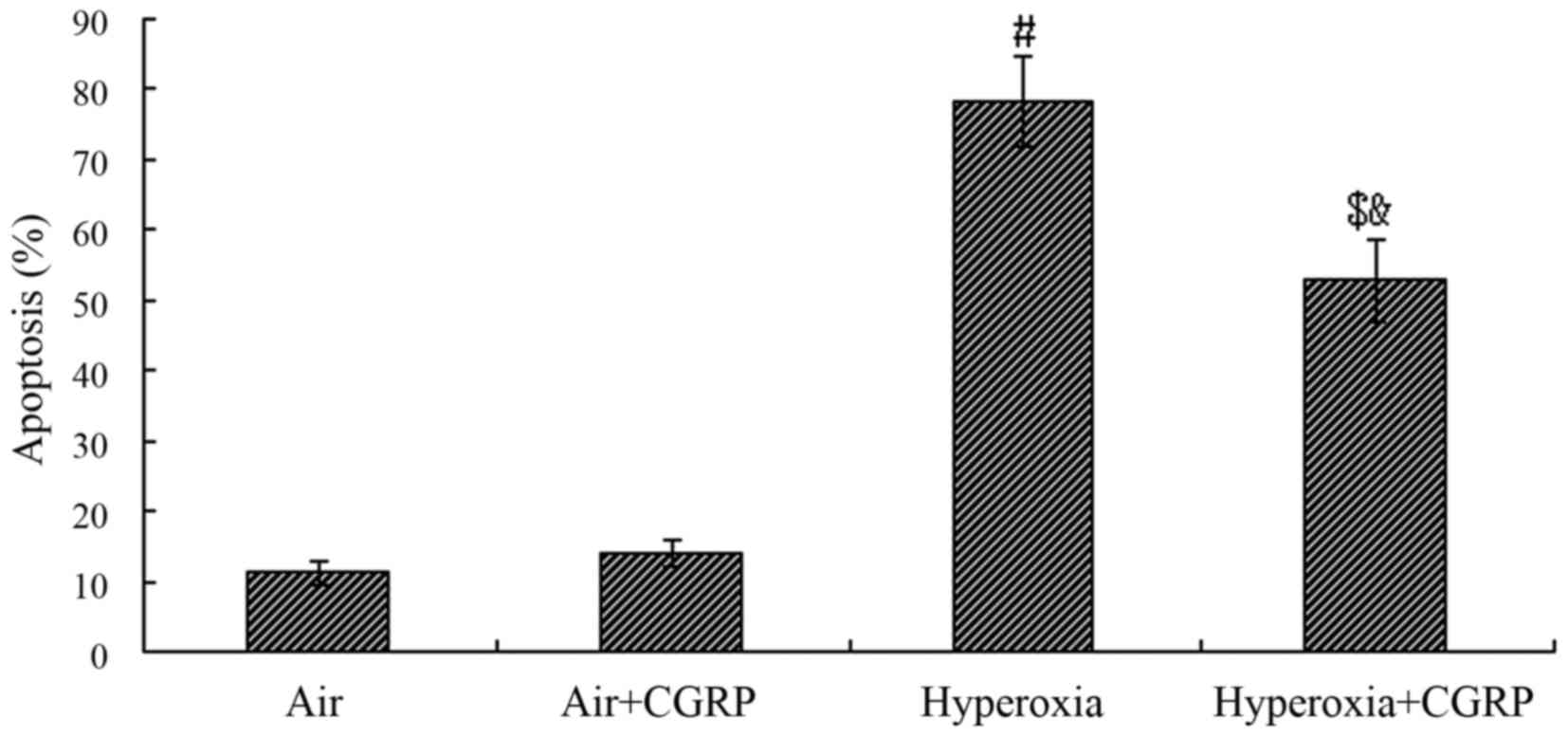
Effect of CGRP on apoptosis following
exposure to hyperoxia
Compared with the normal air group, the percentage
of AECII cell apoptosis did not differ significantly between the
normal air and tte air + CGRP group (P>0.05); however, in the
hyperoxia group, the percentage apoptosis significantly increased
and reached a peak level of approximately 78% (P<0.05). The
percentage apoptosis was significantly decreased following
treatment with CGRP (P<0.05). Nevertheless, it was still much
higher than the normal air group (P<0.05) (Fig. 5).
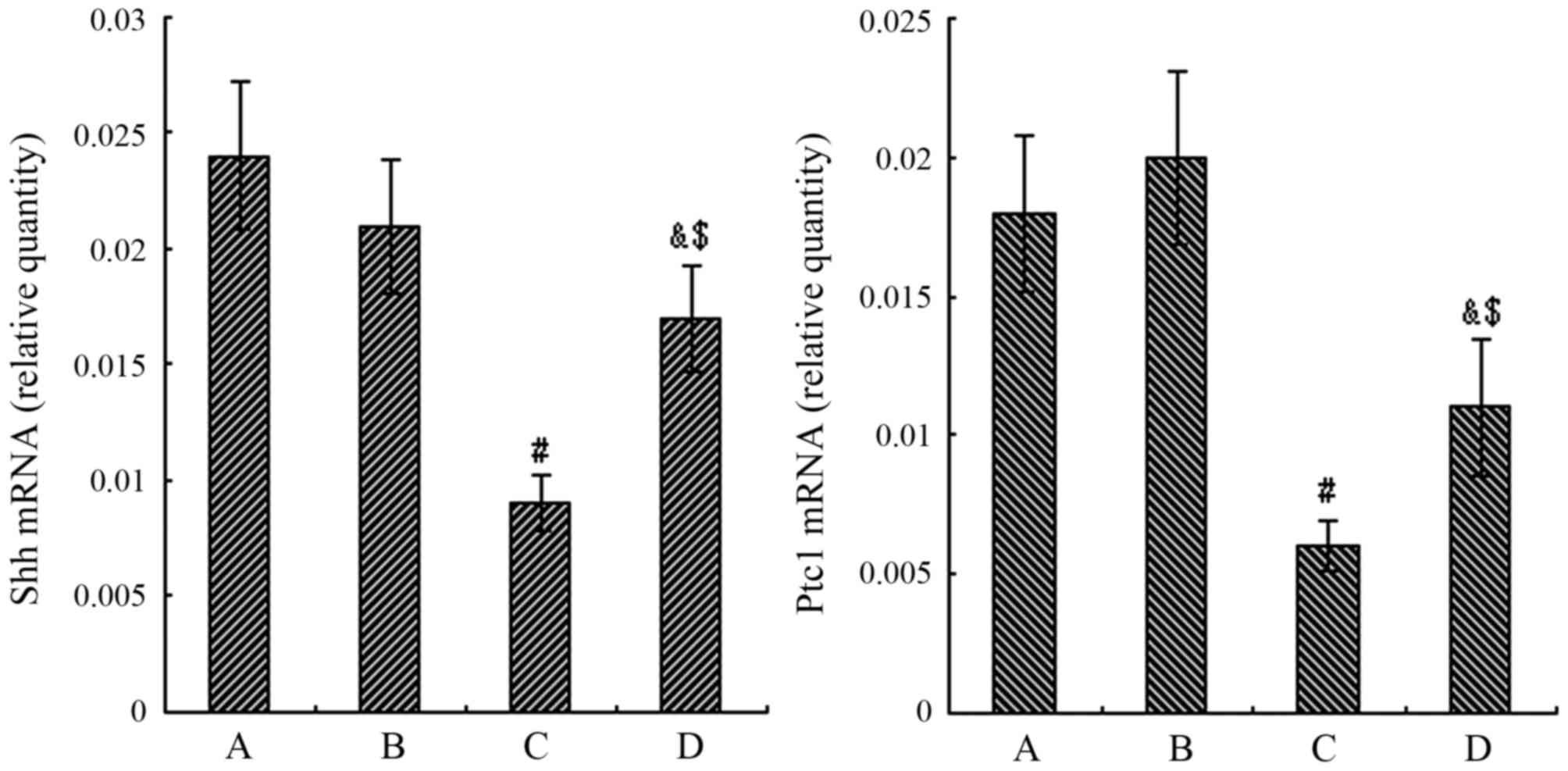
mRNA expression of Shh and Ptc1
As shown by RT-qPCR, no significant differences were
observed in the mRNA levels of Shh and Ptc1 between the normal air
and air + CGRP group (P>0.05). However, a significant decrease
in the mRNA levels of Shh and Ptc1 was observed in the hyperoxia
group compared to the air group at 24 h (P<0.05). In the
hyperoxia + CGRP group, the mRNA expression levels of Shh and Ptc1
markedly increased compared with the hyperoxia group (P<0.05),
but were still significantly higher than the levels in the normal
air group (P<0.05) (Fig.
6).
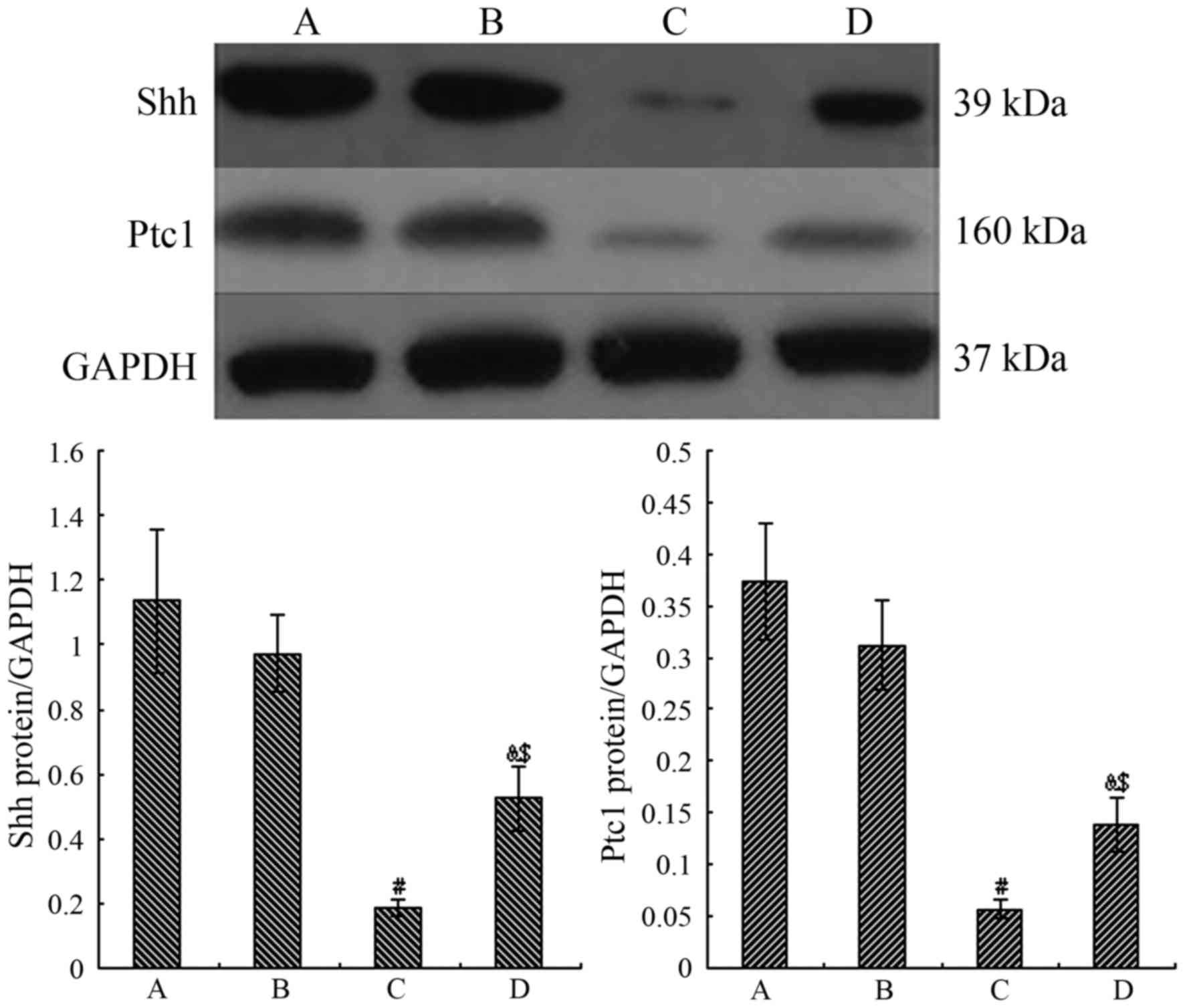
Proteins expression of Shh and Ptc1
We then examined the protein expression levels of
Shh and Ptc1 in the AECII cells. The Shh and Ptc1 protein levels
did not differ significantly between the air control and the air +
CGRP group (P>0.05). In the hyperoxia group, however, the mean
values of Shh and Ptc1 were all highly decreased compared to the
normal air control group (P<0.05). Following treatment with
CGRP, the mean values of Shh and Ptc1 significantly increased
(P<0.05), but were still lower than those in the normal air
group (P<0.05) (Fig. 7).
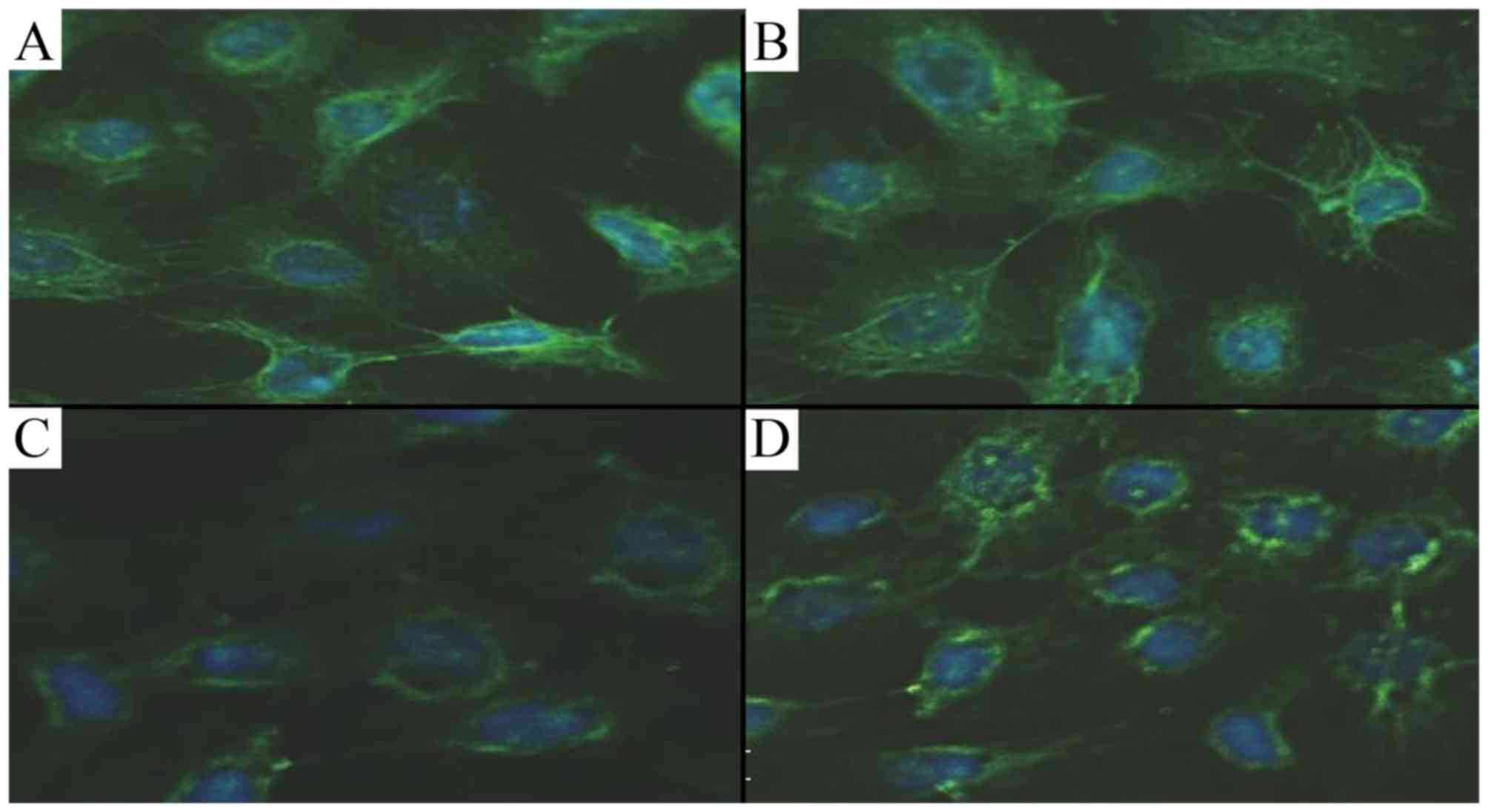
By immunofluorescence, Shh fluorescence in the AECII
cells was markedly decreased in the hyperoxia group compared with
the air group, and treatment with CGRP partially attenuated this
effect. However, the fluorescence did not differ between the groups
cultured in air and with CGRP (Fig.
8).
Discussion
The type II alveolar epithelium is important as it
can secrete many cytokines and bioactive compounds. The AECII cell,
as the epithelial stem cell, plays an important role in the
maintenance of alveolar integrity. The fFunction of AECII cells
directly determines the pathological turnover following lung injury
(22). In the lungs, the
proliferation and differentiation of AECII cells are key steps in
the alveolarization process. The balance of AECII cells between
survival and death is very important, as if homeostasis is
undermined, this leads to pulmonary dysfunction and injury to the
lungs (23). In pre-term infants,
particularly those receiving mechanical ventilation and respiratory
support, hyperoxia is very useful. However, oxygen inhalation in
the long-term and at high concentrations maybe cause ALI, which can
result in respiratory failure and may then develop into BPD, and
can even lead to death. The lack of and the disregulation of
re-epithelialization of the damaged alveolus is regarded as a key
factor in the pathogenesis of ALI and BPD. AECII cell injury is the
early manifestation of hyperoxia induced ALI. Early research has
reported that hyperoxia can inhibit cell growth (24). Recently, some studies have
suggested that the survival and apoptosis of AECII cells may be
involved in the pathological changes of hyperoxia-induced ALI and
BPD, which determines the outcome of repair following lung injury
(25,26). Previous studies have also found
that the excessive apoptosis of AECII cells aggravated pulmonary
pathological alteration, which may induce an inflammatory reaction
and may aggravate the injury to residual lung tissue (23,27–29).
Although the precise mechanisms of hyperoxia-induced
ALI are unclear, the deregulation of oxidant and antioxidant
enzymes and the inflammatory response are believed to play a
pivotal role in the pathogenesis of hyperoxia-induced ALI and BPD
(30,31). In this study, we found that the
direct exposure of AECII cells to 95% oxygen for 24 h resulted in
serve damage, as the cells stopped growing, apoptosis occurred and
vacuolar degeneration was obsrved. These changes seemed to be in
conjunction with the abnormal changes in the activity of oxidant
and antioxidant enzymes, such as MDA and SOD, and increased levels
of ROS in the AECII cells. These findings possibly suggest that
hyperoxia leads to AECII cells damage and this is mainly mediated
by oxygen radicals.
Studies have shown that CGRP is one of the most
potent microvascular vasodilators (32,33). Thus, CGRP has long been considered
to be involved in the development of inflammation. In vivo,
however, an earlier study demonstrated that the ablation of the
sensory fibers results in a marked increase in the severity of
inflammation (34). Perhaps the
sensory neurons and its peptide CGRP may contribute to the
maintenance of tissue integrity by regulating inflammatory
responses. As a peptide structurally related to CGRP,
adrenomedullin (AM), can be acted via both CGRP and AM receptors,
and has also been demonstrated to play antioxidant roles in ROS
generation (35–37). CGRP has also been found to play an
important role in protecting myocytes, kidneys, gastric mucosa,
pancreas and the liver (38–43). Despite the endogenous repair
capacity of the alveolar epithelium, this is often not sufficient,
and can be inadequate, delayed, or impaired. In the present study,
CGRP treatment significantly improved the pathological changes of
the AECII cells exposed to hyperoxia. Our data indicated that CGRP
exerted antioxidant effects, such as the attenuation of the
hyperoxia-induced increase in MDA and the decrease in SOD activity,
markers of lipid peroxidation and antioxidation, and the reduction
of ROS generation. These results suggest that CGRP can decrease
oxidative damage in AECII cells and that treatment with CGRP can
protect against hyperoxia-induced injury to AECII cells via the
inhibition of the oxidative stress response.
We thus presumed that CGRP may be one of the
regulators of AECII cell regeneration and restoration in the
process of hyperoxia-induced injury, and may play an important role
in modulating the occurrence of ALI and BPD. Under the condition of
hyperoxia, the exact protective mechanisms of CGRP in AECII cells
have not yet been identified. It has been suggested that the CGRP
receptors are expressed on the surface of AECII cells, and when
CGRP binds to the receptor, the receptor-coupled G proteins are
activated, which leads to an induction in intracellular cyclic AMP
formation (44). The accumulated
cAMP inhibits the accumulation of nuclear factor-κB (NF-κB)
complexes in the nucleus by preventing phosphorylation and
degradation of the NF-κB inhibitor (45). The inhibition of NF-κB activity
would trigger the protective mechanisms of CGRP to confine the
inflammatory response (46).
The SHH pathway is a critically important
developmental signaling system, which is most active at sites of
immature organs. When there is no Shh, Ptc1 inhibits the activity
of Smo, and thus inhibits the expression of target genes. Once the
Shh protein binds to Ptc1, the inhibition of Smo is attenuated and
allows Gli protein to enter the cell nucleus and induce the
expression of target genes (47).
Shh, known to be expressed at low levels in normal lungs, is
enhanced during the repair of damaged airway epithelium in lung
fibrotic diseases and fibrosis-associated inflammatory processes
(48); however, little is known
about its function and importance in AECII cells, and has not been
studied under the normal and hyperoxic conditions, particularly its
expression in the intervention process of CGRP on hyperoxia-induced
AECII cell injury. Our results demonstrated that hyperoxia markedly
inhibited the expression of Shh and Ptc1 in AECII cells isolated
from premature rats following exposure to 95% oxygen for 24 h, but
this inhibition was partly attenuated following treatment with
CGRP. Under hyperoxic conditions, supplementary CGRP improved cell
survival, reduced apoptosis, decreased the level of ROS, and
downregulated MDA and upregulated SOD activity, accompanied by an
increase in the levels of Shh and Ptc1. CGRP may be responsible for
this protection and may be associated with the SHH signaling
pathway.
According to the findings of our study, CGRP
interference may be used to protect AECII cells from
hyperoxia-induced damage, and the promotion of the activation of
the SHH signaling pathway may be one of the underlying mechanisms
responsible for this protective effect. However, the more detailed
mechanisms and the concrete association between the the SHH pathway
and CGRP are far from being established. For further
investigations, it is necessary to develop other novel and specific
strategies.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (nos. 81071573, 30973218 and 81402126) and by
the Basic and Frontier Research Program of Chongqing Yuzhong
District [No. (2015)16–13].
References
|
1
|
Kinsella JP, Greenough A and Abman SH:
Bronchopulmonary dysplasia. Lancet. 367:1421–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al Hazzani F and Khadawardi E: Effects of
targeting higher vs lower arterial oxygen saturations on death or
disability in extremely preterm infants: The Canadian Oxygen Trial.
J Clin Neonatol. 2:70–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perrone S, Bracciali C, Di Virgilio N and
Buonocore G: Oxygen use in neonatal care: A two-edged sword. Front
Pediatr. 4:1432017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panwar R: The unknowns about oxygen
therapy in critically ill patients. J Thorac Dis. 8:E1543–E1546.
2016. View Article : Google Scholar
|
|
5
|
Altemeier WA and Sinclair SE: Hyperoxia in
the intensive care unit: Why more is not always better. Curr Opin
Crit Care. 13:73–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Fang F and Xu F: Effects of
different states of oxidative stress on fetal rat alveolar type II
epithelial cells in vitro and ROS induced changes in Wnt signaling
pathway expression. Mol Med Rep. 7:1528–1532. 2013.PubMed/NCBI
|
|
7
|
Hilgendorff A and O'Reilly MA:
Bronchopulmonary dysplasia early changes leading to long-term
consequences. Front Med (Lausanne). 2:22015.
|
|
8
|
Stripp BR: Hierarchical organization of
lung progenitor cells: Is there an adult lung tissue stem cell?
Proc Am Thorac Soc. 5:695–698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason RJ: Biology of alveolar type II
cells. Respirology. 11(Suppl): S12–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watson RE, Supowit SC, Zhao H, Katki KA
and Dipette DJ: Role of sensory nervous system vasoactive peptides
in hypertension. Braz J Med Biol Res. 35:1033–1045. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dakhama A, Larsen GL and Gelfand EW:
Calcitonin gene-related peptide: Role in airway homeostasis. Curr
Opin Pharmacol. 4:215–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawanami Y, Morimoto Y, Kim H, Nakamura T,
Machida K, Kido T, Asonuma E, Yatera K, Yoshii C and Kido M:
Calcitonin gene-related peptide stimulates proliferation of
alveolar epithelial cells. Respir Res. 10:82009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu HM, Xu F, Liu CJ, Kuang FW, Huang B,
Fang F and Fu YQ: Influence of 60% oxygen inhalation on type II
alveolar epithelial cells and protective role of calcitonin
gene-related peptide from their damage induced in premature rat.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 20:578–581. 2008.PubMed/NCBI
|
|
14
|
Demayo F, Minoo P, Plopper CG, Schuger L,
Shannon J and Torday JS: Mesenchymal-epithelial interactions in
lung development and repair: Are modeling and remodeling the same
process? Am J Physiol Lung Cell Mol Physiol. 283:L510–L517. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Severgnini M, Takahashi S, Rozo LM, Homer
RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL,
et al: Activation of the STAT pathway in acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 286:L1282–L1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yum HK, Arcaroli J, Kupfner J, Shenkar R,
Penninger JM, Sasaki T, Yang KY, Park JS and Abraham E: Involvement
of phosphoinositide 3-kinases in neutrophil activation and the
development of acute lung injury. J Immunol. 167:6601–6608. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schuh K and Pahl A: Inhibition of the MAP
kinase ERK protects from lipopolysaccharide-induced lung injury.
Biochem Pharmacol. 77:1827–1834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watson S, Serrate C and Vignot S: Sonic
Hedgehog signaling pathway: From embryology to molecular targeted
therapies. Bull Cancer. 97:1477–1483. 2010.
|
|
19
|
Jacob L and Lum L: Deconstructing the
hedgehog pathway in development and disease. Science. 318:66–68.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thome UH, Davis IC, Nguyen SV, Shelton BJ
and Matalon S: Modulation of sodium transport in fetal alveolar
epithelial cells by oxygen and corticosterone. Am J Physiol Lung
Cell Mol Physiol. 284:L376–L385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Miyake Y, Kaise H, Isono K, Koseki H,
Kohno K and Tanaka M: Protective role of macrophages in
noninflammatory lung injury caused by selective ablation of
alveolar epithelial type II cells. J Immunol. 178:5001–5009. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J, Xu F and Chen J: Repair, survival
and apoptosis of type II alveolar epithelial cells and the change
of bcl-2/53 in oxidative stress. Zhonghua Er Ke Za Zhi. 46:74–75.
2008.In Chinese. PubMed/NCBI
|
|
24
|
Yee M, Vitiello PF, Roper JM, Staversky
RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN and
O'Reilly MA: Type II epithelial cells are critical target for
hyperoxia-mediated impairment of postnatal lung development. Am J
Physiol Lung Cell Mol Physiol. 291:L1101–L1111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuwano K: Epithelial cell apoptosis and
lung remodeling. Cell Mol Immunol. 4:419–429. 2007.
|
|
26
|
Fattman CL: Apoptosis in pulmonary
fibrosis: Too much or not enough? Antioxid Redox Signal.
10:379–385. 2008. View Article : Google Scholar
|
|
27
|
Huang B, Fu H, Yang M, Fang F, Kuang F and
Xu F: Neuropeptide substance P attenuates hyperoxia-induced
oxidative stress injury in type II alveolar epithelial cells via
suppressing the activation of JNK pathway. Lung. 187:421–426. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ao X, Fang F and Xu F: Role of vasoactive
intestinal peptide in hyperoxia-induced injury of primary type II
alveolar epithelial cells. Indian J Pediatr. 78:535–539. 2011.
View Article : Google Scholar
|
|
29
|
Ao X, Fang F and Xu F: Vasoactive
intestinal peptide protects alveolar epithelial cells against
hyperoxia via promoting the activation of STAT3. Regul Pept.
168:1–4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhandari V: Molecular mechanisms of
hyperoxia-induced acute lung injury. Front Biosci. 13:6653–6661.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhandari V: Hyperoxia-derived lung damage
in preterm infants. Semin Fetal Neonatal Med. 15:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smillie SJ and Brain SD: Calcitonin
gene-related peptide (CGRP) and its role in hypertension.
Neuropeptides. 45:93–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brain SD and Grant AD: Vascular actions of
calcitonin gene-related peptide and adrenomedullin. Physiol Rev.
84:903–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szallasi A and Blumberg PM: Vanilloid
receptors: New insights enhance potential as a therapeutic target.
Pain. 68:195–208. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Itoh T, Obata H, Murakami S, Hamada K,
Kangawa K, Kimura H and Nagaya N: Adrenomedullin ameliorates
lipopolysac-charide-induced acute lung injury in rats. Am J Physiol
Lung Cell Mol Physiol. 293:L446–L452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rahman M, Nishiyama A, Guo P, Nagai Y,
Zhang GX, Fujisawa Y, Fan YY, Kimura S, Hosomi N, Omori K, et al:
Effects of adrenomedullin on cardiac oxidative stress and collagen
accumulation in aldosterone-dependent malignant hypertensive rats.
J Pharmacol Exp Ther. 318:1323–1329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SM, Kim JY, Lee S and Park JH:
Adrenomedullin protects against hypoxia/reoxygenation-induced cell
death by suppression of reactive oxygen species via thiol redox
systems. FEBS Lett. 584:213–218. 2010. View Article : Google Scholar
|
|
38
|
Song S, Liu N, Liu W, Shi R, Guo KJ and
Liu YF: The effect of pretreatment with calcitonin gene-related
peptide on attenuation of liver ischemia and reperfusion injury due
to oxygen free radicals and apoptosis. Hepatogastroenterology.
56:1724–1729. 2009.
|
|
39
|
Kroeger I, Erhardt A, Abt D, Fischer M,
Biburger M, Rau T, Neuhuber WL and Tiegs G: The neuropeptide
calcitonin gene-related peptide (CGRP) prevents inflammatory liver
injury in mice. J Hepatol. 51:342–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng G, Xu X, Wang Q, Liu Z, Li Z and Liu
G: The protective effects of calcitonin gene-related peptide on
gastric mucosa injury after cerebral ischemia reperfusion in rats.
Regul Pept. 160:121–128. 2010. View Article : Google Scholar
|
|
41
|
Machado J, Manfredi LH, Silveira WA,
Gonçalves DA, Lustrino D, Zanon NM, Kettelhut IC and Navegantes LC:
Calcitonin gene-related peptide inhibits autophagic-lysosomal
proteolysis through cAMP/PKA signaling in rat skeletal muscles. Int
J Biochem Cell Biol. 72:40–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Carnevale KA, Dipette DJ and Supowit
SC: Renal protective effects of alpha-calcitonin gene-related
peptide in deoxycorticosterone-salt hypertension. Am J Physiol
Renal Physiol. 304:F1000–F1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schneider L, Hartwig W, Flemming T,
Hackert T, Fortunato F, Heck M, Gebhard MM, Nawroth PP, Bierhaus A,
Buchler MW and Werner J: Protective effects and anti-inflammatory
pathways of exogenous calcitonin gene-related peptide in severe
necrotizing pancreatitis. Pancreatology. 9:662–669. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Drissi H, Lasmoles F, Le Mellay V, Marie
PJ and Lieberherr M: Activation of phospholipase C-beta1 via
Galphaq/11 during calcium mobilization by calcitonin gene-related
peptide. J Biol Chem. 273:20168–20174. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brigelius-Flohé R, Banning A, Kny M and
Böl GF: Redox events in interleukin-1 signaling. Arch Biochem
Biophys. 423:66–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Piette J, Piret B, Bonizzi G, Schoonbroodt
S, Merville MP, Legrand-Poels S and Bours V: Multiple redox
regulation in NF-kappaB transcription factor activation. Biol Chem.
378:1237–1245. 1997.
|
|
47
|
Cohen MM Jr: The hedgehog signaling
network. Am J Med Genet A. 123A:5–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|