Introduction
Cardiovascular diseases have reached epidemic
proportions in India, particularly among the younger population in
comparison to other ethnic groups (1). Patients who have survived a
first-time coronary event are at a greater risk of developing
recurrent episodes, as compared to asymptomatic subjects (2). Several biomarkers belonging to
different pathways have been identified in coronary artery disease
(CAD) and are being deployed to identify the subset of subjects who
are at a greater risk of developing acute coronary events. These
biomarkers are associated with inflammation (C-reactive protein),
hemodynamic stress (brain natriuretic peptide, N-terminal
prohormone brain natriuretic peptide) and kidney dysfunction
(albuminuria andcystatin-C) (3,4).
Pathogen burden caused due to the simultaneous infection by
multiple pathogens, such as Chlamydia pneumoniae,
cytomegalovirus (CMV), Helicobacter pylori, Herpes simplex
virus (5) and in particular,
neutralizing antibody titers against CMV along with inflammatory
markers have been shown to be important for risk stratification in
the Asian Indian population (6).
On the other hand, the use of global proteome
analysis can not only provide a better understanding of the
underlying disease process, but can also help to unearth novel
disease markers that can enhance the power to predict the risk of
acute coronary events when combined with other well-studied markers
as discussed above (7). This
includes the comparative proteomics approaches based on mass
spectrometric analysis of protein profiles in serum samples
obtained from normal and diseased subjects. Therefore, the aim of
this study was to perform global proteome analysis using the
surface-enhanced laser desorption ionization time-of-flight mass
spectrometry (SELDI-TOF MS) platform to possibly identify novel
biomarkers associated with recurrent coronary events in patients
with established CAD. We identified 2 infection-related proteins,
namely β-defensin-128 and histatin-3 as potential biomarkers for
recurrent CAD.
Materials and methods
Study population
The Indian Atherosclerosis Research Study (IARS) is
a prospective cohort study designed to investigate and understand
the molecular basis of CAD in the Indian population. The design and
details of data collection and the procedures used are described
elsewhere (8). The study was
designed in accordance with the principles and the guidelines of
World Medical Association Declaration of Helsinki and the Indian
Council of Medical Research and was approved by the Thrombosis
Research Institute Ethics Committee. The participants provided
their signed informed consent to participate in the study. In
phase-I of this study between 2004–2006, 2,332 subjects were
enrolled of which 772 (33%) were CAD-affected. A total of 152
subjects from this CAD-affected group experienced a recurrence of
cardiac events during the course of a 5-year follow-up period. All
the subjects in this study had a family history of CAD, and we
matched the age and gender of 85 subjects in the recurrent events
group to those of 85 subjects with first-time events, and the serum
samples of these subjects was used for global proteome analysis.
The baseline characteristics of the study participants are
presented in Table I. The
subjects were recruited based on the following criteria: i) age at
onset of CAD was ≤60 years for males and ≤65 years for females; ii)
the diagnosis of CAD was based on an electrocardiogram
(ECG)/echocardiography (echo)/biochemical or angiogram findings;
iii) patients who were posted for percutaneous transluminal
coronary angiography (PTCA)/coronary artery bypass graft (CABG).
The subjects who did not meet the above-mentioned criteria or had
any other major illness, as defined by the World Health
Organization, such as cancer, liver failure, etc. were excluded
from the study. All blood samples were collected after overnight
fasting for 12–14 h, and the plasma and serum aliquots were stored
at −80°C. Detailed information on medical history, demographics and
relevant information, including 3-generation pedigree was recorded
in a questionnaire after a personal interview. The diagnosis of
diabetes and hypertension was based on medical reports, while the
body mass index was calculated.
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Variables | First-time cases,
n=85 | Recurrent events,
n=85 | p-value |
|---|
| Age (years) | 55.13±1.00 | 54.45±1.12 | 0.649 |
| BMI
(kg/m2) | 25.83±0.416 | 26.95±0.486 | 0.080 |
| Sex, n (%) | | | |
| Male | 58 (68.2) | 59 (69.4) | 0.50 |
| Female | 27 (31.8) | 26 (30.6) | |
| Hypertension, n
(%) | 36 (42.4) | 51 (60.0) | 0.031 |
| Diabetes, n (%) | 39 (45.9) | 46 (54.1) | 0.357 |
| Systolic blood
pressure (mmHg) | 127.73±1.70 | 134.67±2.63 | 0.028 |
| Diastolic blood
pressure (mmHg) | 81.47±1.003 | 83.11±1.067 | 0.266 |
| Total cholesterol
(mg/dl) | 154.18±5.72 | 162.31±4.63 | 0.270 |
| TG (mg/dl) | 156.02±9.06 | 150.24±8.07 | 0.630 |
| HDL (mg/dl) | 36.76±1 | 35.54±1 | 0.391 |
| LDL (mg/dl) | 82.77±3.68 | 96.94±4.07 | 0.011 |
Telephonic assessment of cardiac health
status
The periodic telephonic follow-up of all study
participants was undertaken to obtain an update on the cardiac
health status and 5 such rounds of follow-up were completed.
Follow-up was performed once every 18 months on an average from the
time of recruitment with a successful follow-up rate of 60%.
Endpoints included non-fatal events (re-do PTCA/re-do CABG/heart
attack, stroke) and fatal events (fatal heart attack/fatal
stroke/sudden death/cardiac arrest). This information was verified
through either paper trail or hospital records whenever
available.
Global proteome analysis
Global proteome analysis of serum samples was
performed using SELDI-TOF MS. Sinapinic Acid (SPA) and CM10 chip
were purchased from Bio-Rad (Hercules, CA, USA) and all the other
reagents were from Sigma-Aldrich (St. Louis, MO, USA). Serum
samples were assayed on CM10 chip and data were analyzed using
Ciphergen Express Client software. Serum samples were thawed on ice
and centrifuged at 14,000 rpm for 5 min at 4°C. A total of 20
μl of supernatant and 30 μl of U9-buffer (9 M urea,
2% CAHPS, 1% DTT) were added to a tube and agitated for 30 min at
4°C on a platform shaker. Subsequently, 450 μl of Tris-HCl
(50 mM pH 7.5) were added to the U9/serum mixture and agitated at
4°C for 2 min. This was followed by the addition of 150 μl
of 50 mM Tris-HCl (pH 7.5) and agitation for 5 min twice to
activate the CM10 chips. Diluted samples (150 μl) were
spotted onto a bio-processor containing the ProteinChip arrays and
then agitated on a platform shaker for 60 min at room temperature.
Excess serum was discarded and the chips were washed thrice with
150 μl of Tris-HCl (50 mM, pH 7.5) and twice with 200
μl of dH2O, removed from the bioprocessor and
air-dried. Prior to SELDI analysis, 1 μl of a saturated
solution of SPA was applied onto each chip twice and air-dried.
Analysis of protein chip array and
protein identification
A set of different protocols was used on each spot
which varied in laser intensity. Pre-processing was done on
ProteinChip© software 3.1. Peaks which had less
background noise were considered for further analysis after
baseline subtraction. The peaks were normalized and those having ±2
standard deviation were removed. Finally, clusters were made by
expression differential matrix (EDM) within a range of 1,500–30,000
Da. Peaks having m/z <1,500 were considered as matrix noise. A
threshold of 20% and signal to noise ratio and valley depth of 5
was set as first pass and 0.3% mass window and second pass of 2 was
assigned. Mass accuracy was calibrated to <0.1% by all-in-one
peptide molecular mass standard (Ciphergen Biosystems, Fremont, CA,
USA).
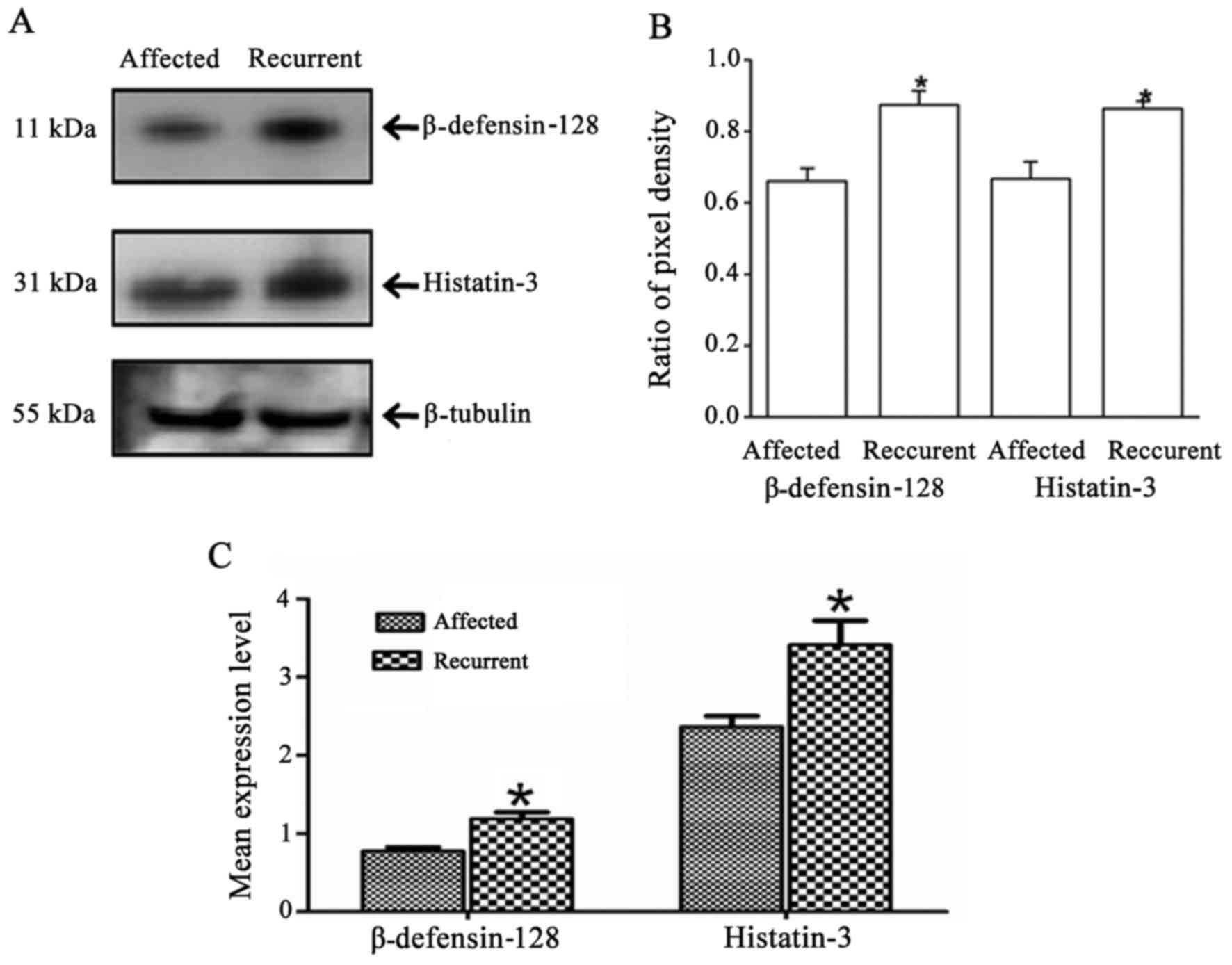
Biomarker validation assays
Three independent western blot analyses were
performed to validate the presence and levels of significant
proteins identified through global proteome analysis. Age- and
gender-matched serum samples from 10 CAD-affected and 10 CAD
patients with recurrent events were used for western blot analysis.
An equal amount of 50 μg protein was loaded after protein
estimation using the Bradford method. The blot was first probed for
β-defensin-128 (sc-51670, secondary antibody SC-2033; Santa Cruz
Biotechnology, Inc., Santa Cruz, Ca, USA) and then re-probed with
histatin-3 antibody (SAB1402234; Sigma-Aldrich; secondary antibody
sc-2005; Santa Cruz Biotechnology, Inc.). Finally, the same blot
was probed for loading control β-tubulin (sc-85539; Santa Cruz
Biotechnology, Inc.). Following 3 independent western blot
analyses, the images were scanned in Bio-Rad molecular imaging
ChemiDoc XRS plus (Bio-Rad), and bands were quantified using ImageJ
software (http://rsbweb.nih.gov/ij/index.html) and ratio of
β-defensin-128/β-tubulin and histatin-3/β-tubulin were calculated
(Fig. 2B). ELISAs were performed
for β-defensin-128 (CSB-EL006698HU) and histatin-3 (CSB-EL006698HU)
using kits from Cusabio (Hubei, China) with covariance percentage
of 1.21 and 7.04%, respectively.
Bioinformatics and statistical
analysis
We compared the differential pattern of protein
levels between the first-time CAD-affected group and the recurrent
event group. Prior to the analyses, all spectra were normalized,
and subjected to baseline correction and de-noising. A
non-parametric t-test (Mann-Whitney) was performed to identify the
most significant peaks. TagIdent software (www.expasy.org) was used to identify the proteins from
their corresponding m/z values (Table IIA) (9–12).
Backward logistic regression, logistic regression, Cox regression
analysis for hazards ratio (HR) and C-statistics were performed for
the ELISA data to estimate the improvement in risk assessment of
the predictive models using the two biomarkers. Using the
probabilities scores through logistic regression followed by ROC
analysis, we generated the area under the receiver operating
characteristic curve (AUC) for 3 different models: conventional
risk factors (CRFs) [age, gender, hypertension (HTN), diabetes
mellitus (DM), body mass index (BMI), waist-hip ratio, smoking,
total cholesterol (TC), triglycerides (TG), high-density
lipoprotein (HDL) and low-density lipoprotein (LDL)] alone, the
biomarkers alone, and for a combination of CRFs and biomarkers. The
significance of improvement or change in the models was calculated
using the DeLong method. The combined risk score was calculated for
the final model of biomarkers values adjusted to CRFs. We used the
following equation to calculate the combined risk score for these
two biomarkers (13): combined
score = (β1×β − biomarker1) + (β2×biomarker2) where, β1 and β2
denote the estimates of biomarker 1β coefficient (β-defensin-128)
and biomarker 2 (histatin-3), respectively. Participants were
categorized into quintiles of combined risk score with the lowest
two quintiles categorized as low risk, third and fourth quintiles
as intermediate, and the top quintile as high risk categories. The
Kaplan-Meier curve analysis was performed for the best models for
estimating the cumulative cardiovascular events in each group and
plots were generated for the low and high risk groups. The analysis
was performed using SPSS software (version 17.0) and R package
2.13.0.
 | Table IISignificant protein peaks classifying
between CAD cases and subjects with recurrent CAD. |
Table II
Significant protein peaks classifying
between CAD cases and subjects with recurrent CAD.
A, List of m/z
protein peaks showing significant differential levels in the
subjects with first-time CAD and those with recurrent events.
|
|---|
| Protein peaks
(m/z) | Identified
protein | Protein ID | m/z peak intensity
in CAD affected subjects | m/z peak intensity
in recurrent event subjects | p-value |
|---|
| 1864 | Histatin-3,
HIS3_HUMAN | P15516 | 60.53±10.61 | 118.21±14.48 | <0.001 |
| 22259 | Vascular
endothelial growth factor A, VEGFA_HUMAN | P15692 | 4.25±0.19 | 3.69±0.19 | 0.004 |
| 2022 | Complement C3,
CO3_HUMAN | P01024 | 143.75±14.44 | 204.44±18.34 | 0.006 |
| 18072 | Interleukin-24,
IL-24_HUMAN | Q13007 | 2.26±0.18 | 1.76±0.15 | 0.016 |
| 14680 | Choriogonadotropin
subunit β variant 1, CGB1_HUMAN | A6NKQ9 | 14.27±0.84 | 11.34±0.68 | 0.02 |
| 4146 | Peptide YY,
PYY | P10082 | 49.22±8.22 | 100.63±18.03 | 0.02 |
| 7604 | Growth-regulated α
protein, GROA_HUMAN | P09341 | 50.84±2.85 | 43.23±2.91 | 0.023 |
| 7040 | Defensin-5,
DEF5_HUMAN | Q01523 | 24.82±3.26 | 28.33±4.62 | 0.024 |
| 22466 | Metalloproteinase
inhibitor 4, TIMP4 | Q99727 | 4.45±0.24 | 3.87±0.21 | 0.025 |
| 4293 | Pro-neuropeptide Y,
NPY | P01303 | 78.46±14.48 | 92.52±12.96 | 0.03 |
| 7742 | Platelet factor 4,
PLF4_HUMAN | P02776 | 67.07±5.81 | 88.58±10.14 | 0.037 |
| 2498 | Natriuretic
peptides B, ANFB_HUMAN | P16860 | 51.32±2.79 | 46.55±2.69 | 0.039 |
| 6100 | Cardiac
phospholamban, PPLA_HUMAN | P26678 | 22.25±2.30 | 25.01±2.00 | 0.039 |
| 3185 | Lactotransferrin,
TRFL_HUMAN | P02788 | 100.13±10.87 | 124.79±12.32 | 0.042 |
| 8588 | β-defensin-128,
DB128_HUMAN | Q7Z7B8 | 369.09±25.49 | 424±28.20 | 0.042 |
| 17845 | Transcription
factor MafG, MAFG_HUMAN | O15525 | 2.33±0.21 | 1.86±0.16 | 0.049 |
B, Significant
peaks obtained with backward logistic regression from all the 16
m/z peaks
|
|---|
| m/z peak | Odds ratio | 95% CI | p-value |
|---|
| 8588
(β-defensin-128) | 7.49 | 2.57–21.90 | <0.001 |
| 1864 (HTN3) | 1.4 | 1.02–2.13 | 0.042 |
Results
Patient characteristics
Table I shows the
clinical characteristics of the study participants. The mean age,
gender distribution, BMI and lipid profile were comparable between
the patients with first-time CAD and those with recurrent events.
Hypertension and a higher systolic blood pressure were more
frequent, and LDL-c levels were higher in the latter group than in
the former group of patients. The subgroup with recurrent events
included 21 deaths due to cardiac arrest out of 85 subjects, while
none of the patients in the first-time CAD-affected group had any
event or death.
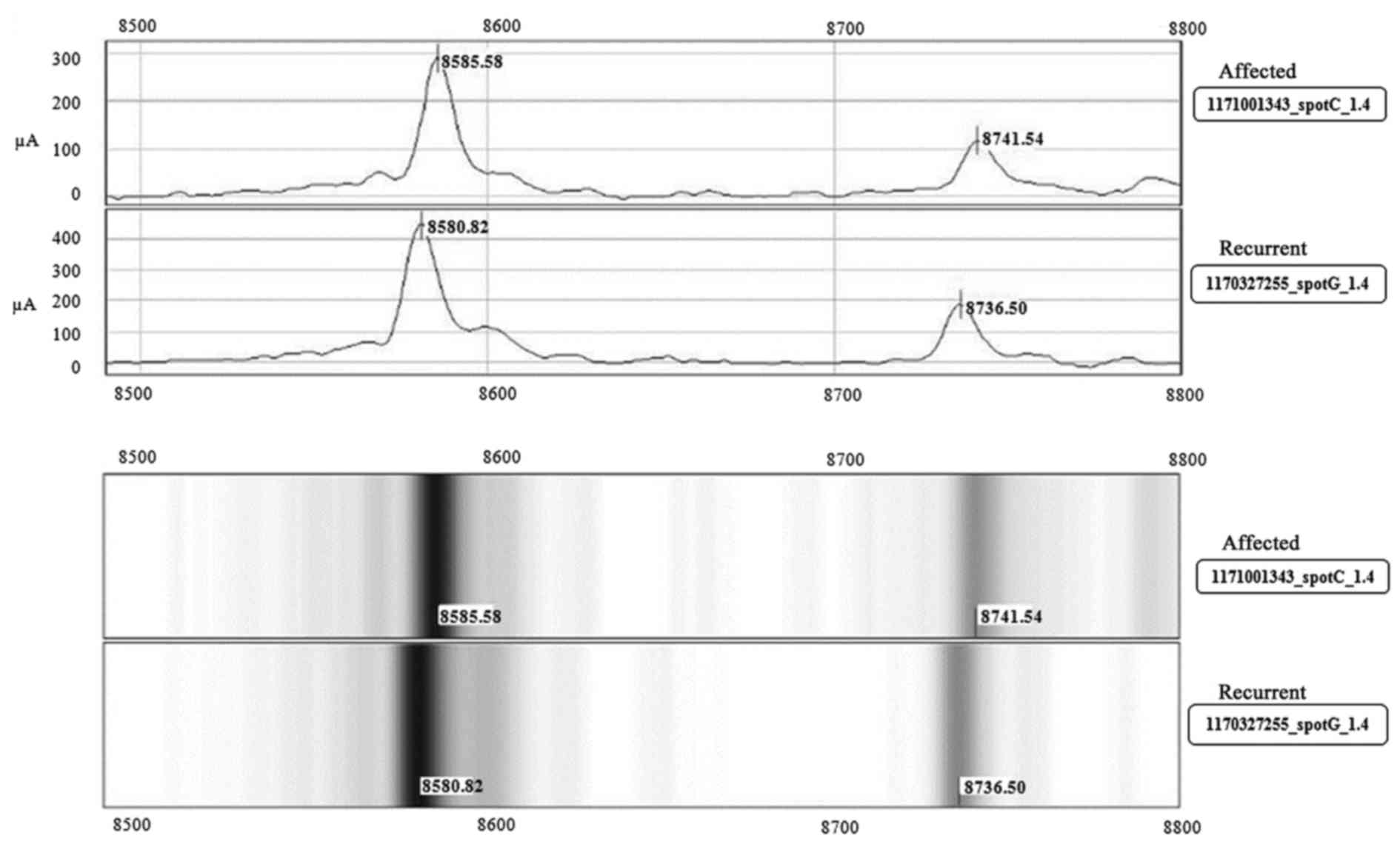
Protein profiling
The proteomic profile of the 85 first-time
CAD-affected and 85 subjects with recurrent events using SELDI-TOF
MS yielded 39 differentially expressed protein peaks
[representative peak and pseudo gel (Fig. 1)], of which 16 had p-values
<0.05 (Table IIA), belonging
to different pathways and functions, as suggested in the SWISSPROT
database (www.expasy.org). Of the 16 proteins, 4
of them were related to infection [histatin-3 (m/z 1864),
defensin-5 (m/z 7040), lactotransferrin (m/z 3185) and
β-defensin-128 (m/z 8588)]. The defensin proteins and complement C3
(m/z 2022) play an important role in immune regulatory mechanisms.
A total of 5 of the other proteins, vascular endothelial growth
factor A (m/z 22259), choriogonadotrophin subunit β-variant-1 (m/z
14680), peptide-YY (m/z 4146), growth-regulated α protein (m/z
7604) and pro-neuropeptide Y (m/z 4293) were all related to growth
and hormone pathways. The other members included
inflammation-related proteins, interleukin-24 (m/z 18072),
metalloproteinase inhibitor 4 (m/z 22466), platelet factor 4 (m/z
7742) and natriuretic peptide-B (m/z 2498), a well known CAD risk
marker, cardiac phospholamban (m/z 6100) and transcription factor
MAFG (m/z 17845).
Backward logistic regression analysis resulted in
the identification of peaks with m/z 8588 and m/z 1864 identified
as β-defensin-128 and histatin-3 (HTN-3), respectively, to be
significantly associated with odds ratios of 7.49 (95% CI,
2.71–21.90, p-value <0.001) and 1.4 (95% CI, 1.02–2.13,
p-value=0.042), respectively (Table
IIB). These markers were further used for validation.
Biomarker validation
The two protein peaks, m/z 8588 and m/z 1864,
corresponding to β-defensin-128 and histatin-3 were validated using
western blot analysis (Fig. 2A).
Densitometric analysis (Fig. 2B)
of the protein bands suggested that the levels of both proteins
were higher in subjects with recurrent events than in the subjects
with first-time CAD. Furthermore, ELISAs (Fig. 2C) performed for these two proteins
in all the 170 subjects confirmed that both proteins were
significantly differentially expressed. The mean levels of
β-defensin-128 in the first-time CAD-affected subjects was
0.779±0.045 ng/ml and in the recurrent subjects was 1.19±0.082
ng/ml (p-value=2.2×10−15). Similarly, the histatin-3
level was 2.38±0.130 μg/ml in the first-time CAD affected
subjects and 3.42±0.306 μg/ml in the recurrent subjects
(p-value of 0.002).
Association of β-defensin-128 and
histatin-3 in CAD-affected subjects with recurrent events
We evaluated individual and combined association of
these biomarkers with recurrent events. As shown in Table III, the HR for β-defensin-128
was 1.715 (95% CI, 1.32–2.24; p-value=8.14×10−5) and
that for histatin-3 was 1.133 (95% CI, 1.04–1.24; p-value=0.004).
However, when combined with the CRFs, the HRs improved to 1.729
(95% CI, 1.28–2.34; p-value=3.51×10−4) for
β-defensin-128 and 1.147 (95% CI, 1.04–1.26; p-value=0.004) for
histatin-3. The combined HR of β-defensin-128 and histatin-3
together was 1.699 (95% CI, 1.29–2.24; p-value=1.6×10−4)
before adjustment and 1.833 (95% CI, 1.34–2.50;
p-value=1.3×10−4) after adjustment for the conventional
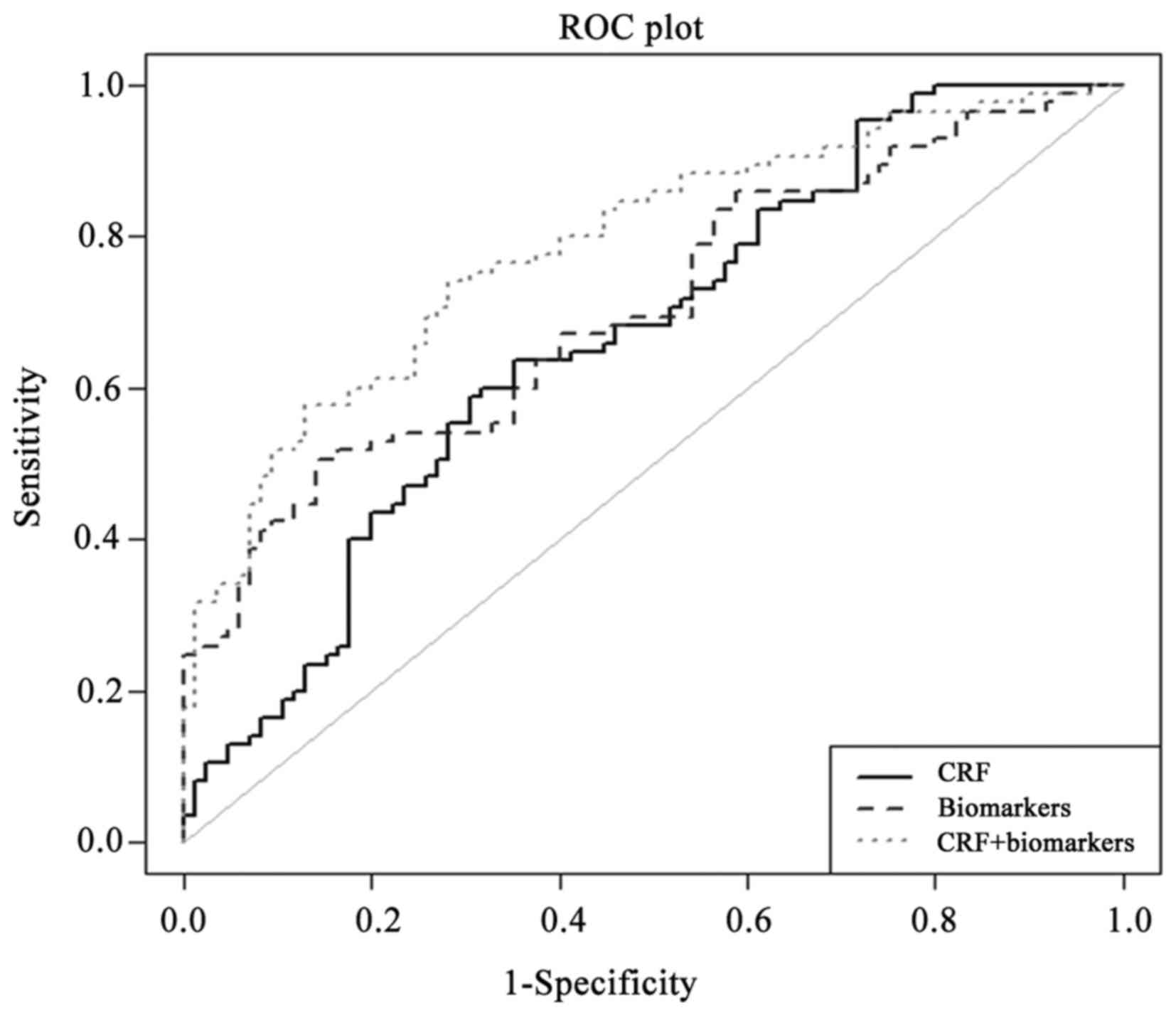
risk factors. The analysis of C-statistics (Fig. 3) suggested that CRFs alone yielded
an AUC of 0.677 (95% CI, 0.601–0.714; p-value=5.1×10−5).
Upon the addition of biomarkers, the AUC increased to 0.800 (95%
CI, 0.702–0.844; p-value=1.37×10−9) and the difference
was statistically significant (p-value=0.001) (Table IV) as evaluated using DeLong
analysis.
 | Table IIIHazards ratio for histatin-3 and
β-defensin-128 adjusted for conventional risk factors. |
Table III
Hazards ratio for histatin-3 and
β-defensin-128 adjusted for conventional risk factors.
| Model | Hazards ratio | 95% CI | p-value |
|---|
| DB128 | 1.715 | 1.32–2.24 |
8.14×10−5 |
| DB128 adjusted for
CRF | 1.729 | 1.28–2.34 |
3.51×10−4 |
| HTN-3 | 1.133 | 1.04–1.24 | 0.004 |
| HTN-3 adjusted for
CRF | 1.147 | 1.04–1.26 | 0.004 |
| DB128 + HTN3 | 1.699 | 1.29–2.24 |
1.60×10−4 |
| DB128 + HTN3
adjusted for CRF | 1.833 | 1.34–2.50 |
1.30×10−4 |
 | Table IVC-statistic analysis showing
improvement of the Area Under the Curve (AUC) for the combined
model of biomarkers and CRF over the CRF alone. |
Table IV
C-statistic analysis showing
improvement of the Area Under the Curve (AUC) for the combined
model of biomarkers and CRF over the CRF alone.
| Model | AUC | p-value | 95% CI | DeLong analysis for
significance (p-value) in the improvement of AUC from CRF model to
CRF + biomarkers |
|---|
| CRF | 0.677 |
5.100×10−5 | 0.601–0.714 | 0.001 |
| CRF + HTN3 +
DB128 | 0.800 |
1.37×10−9 | 0.702–0.844 | |
The combined risk score (Table V) of β-defensin-128 and histatin-3
adjusted to the CRFs reclassified the subjects into 3 groups,
namely low (combined risk score of <0.6256, reference tertile),
intermediate (combined risk score of 0.625 to <0.956; HR, 2.22;
95% CI, 1.008–4.891; p-value=0.048) and high risk (combined risk
score of ≥0.956; HR, 2.962; 95% CI, 1.675–5.23;
p-value=1.89×10−4). The analysis of the complete study
group (n=170) revealed that 81 subjects were at high risk of which
31 belonged to the CAD-affected group and 51 were subjects with
recurrent events (13 deaths and 3 subjects with a third reported
event). Similarly, 32 subjects were reclassified into the
intermediate risk group, of which 17 were from the affected group,
while 15 belonged to the recurrent events group (3 deaths and 2
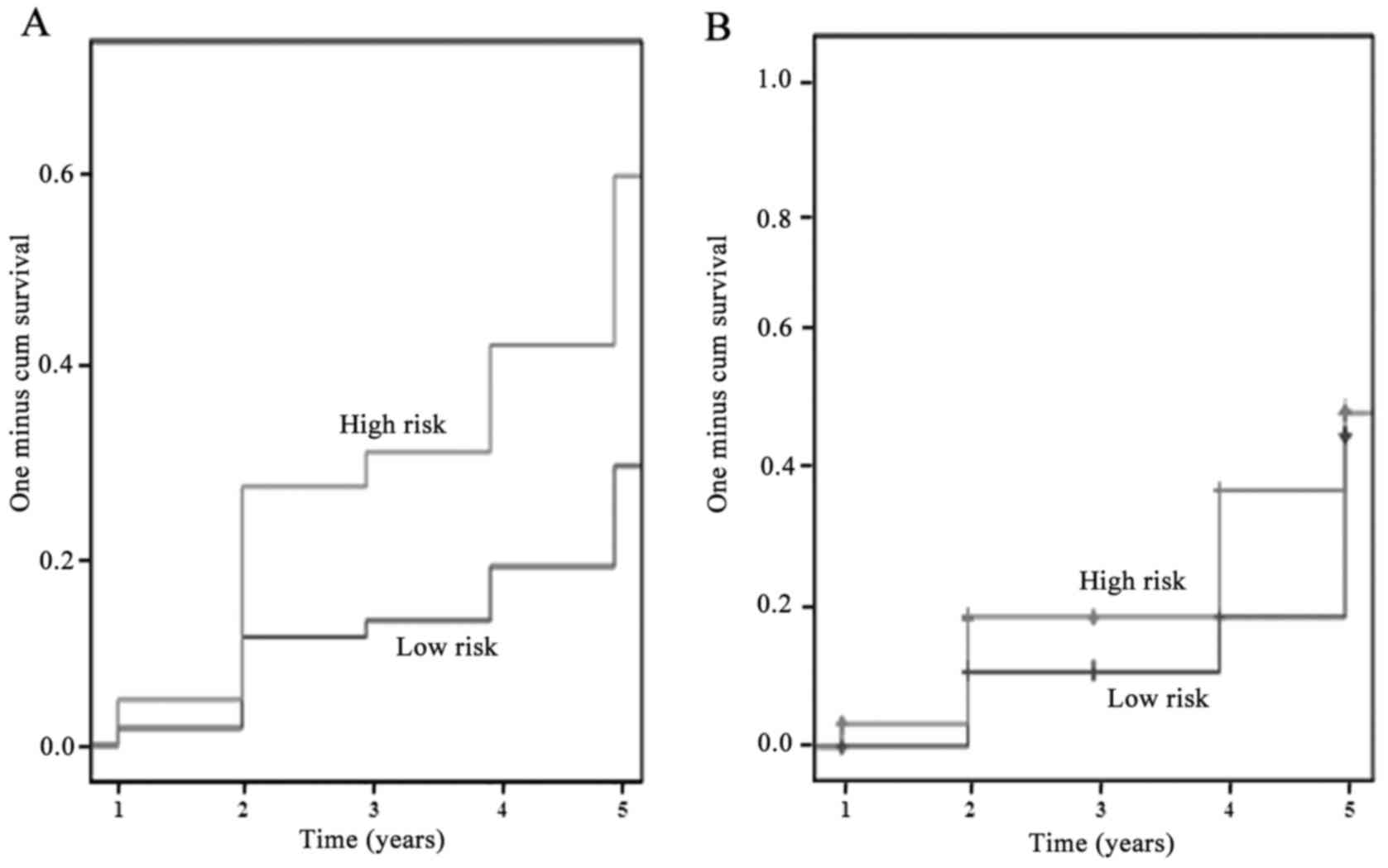
subjects with 3 events reported). Kaplan-Meier survival analysis
for the cumulative probability of recurrent event for the model
containing β-defensin-128 and histatin-3 adjusted to the CRFs,
suggested that subjects in the high-risk group had a low survival
rate as compared to those in the low-risk group (Fig. 4A). Similar analysis on the
cumulative probabilities of death due to cardiovascular events or
occurrence of triple events for recurrent event subjects alone also
suggested that the high-risk group had a low survival rate as
compared to the low-risk subjects (Fig. 4B).
 | Table VCombined score of histatin-3 and
β-defensin128 adjusted for CRFs stratifying low, intermediate and
high risk groups in the study population. |
Table V
Combined score of histatin-3 and
β-defensin128 adjusted for CRFs stratifying low, intermediate and
high risk groups in the study population.
| Category | Combined-marker
score for event | HR | 95% CI for
Exp(B) | p-value | Total subjects
reclassification n=170 | Affected subjects
reclassification n=85 | Recurrent subjects
reclassification n=85 |
|---|
| Low | <0.6256 | 1.00 (Ref) | – | – | 57 | 37 | 20 (6 deaths) |
| Intermediate | 0.625 to
<0.956 | 2.220 | 1.008–4.891 | 0.048 | 32 | 17 | 15 (3 deaths and 2
subjects with 3rd event) |
| High | ≥0.956 | 2.962 | 1.675–5.23 |
1.89×10−4 | 81 | 31 | 50 (13 deaths and 3
subjects with 3rd event) |
Discussion
Using comparative global proteome analysis, we
identified β-defensin-128 and histatin-3 as novel biomarkers
associated with recurrent coronary events. Recently, we also
demonstrated that pathogen burden (5) and in specific CMV-NA titer is
strongly associated with recurrent CAD events (6). Several other studies have focused on
using putative candidate biomarkers for improving risk assessment
in cases with recurrent CAD events, and these have provided a
modest improvement in the risk discrimination (4). These findings suggest that there is
a need to improve these discriminative models, as it is well known
that subjects with established CAD have a high risk of further
vascular events compared to disease-free subjects (14). In such a scenario, the use of
global proteomic analysis help to unearth newer biomarkers that can
substantially improve risk assessment. Global proteome analysis
using SELDI-TOF MS commonly used for biomarker discovery in complex
diseases has also been described with regard to network biomarkers
associated with the incidence of CAD (15), major adverse cardiac events (MACE)
(16) and the identification of
soluble tumor necrosis factor-like weak inducer of apoptosis
(sTWEAK) as a possible biomarker of subclinical atherosclerosis
(17).
Of the 16 significant peaks (Table IIA), logistic regression analysis
identified 2 significantly associated proteins, β-defensin-128 and
histatin-3 (Table IIB).
Furthermore, western blot analysis (Fig. 2A and B) and ELISAs (Fig. 2C) in individual serum samples also
revealed differential levels of these proteins in the 2 groups.
β-defensins play an important role in immunoregulation (18), and have been shown to be
significantly associated progressive cardiovascular disease
(19). Furthermore, genetic
polymorphisms and copy number variation of β-defensin-4 gene have
been shown to be associated with ischemic stroke (20). Other studies have shown that in
oral infections by spirochetes, which constitute 40% of the
microflora in disease sites, are also linked to cardiovascular
diseases (21,22). In our analysis, β-defensin-128 was
shown to be associated with recurrent events, suggesting that this
may be a novel biomarker belonging to the defensin family, linking
infection and immunity to CAD. Similarly, gene polymorphisms in
histatin-3 (23) and gene
expression (24) have been shown
to play a major role in oral and other infections.
Of the other proteins identified (Table IIA), we found peptides, such as
natriuretic peptide-B, pro-neuropeptide-Y and peptide-YY which are
associated with cardiovascular diseases (25). However, in the present study,
infection-related biomarkers, β-defensin-128 and histatin-3 in
particular seem to play a major role in the discrimination between
first-time CAD-affected subjects and those with recurrent events
(Tables III–V and Figs.
3 and 4). This may be due to
the importance of active infection of CMV in these subjects, as our
previous study using the same subset of patients demonstrated that
higher titer values of CMV-NA (6)
were associated with first-time CAD and to recurrent CAD (26).
Several studies have shown that chronic infection
can lead to pro-atherogenic effects (27), and can lead to vascular damage
(27–29). The Asian population has been
earlier reported to have a higher pathogen burden, which includes
CMV infection (5,6,26)
and the identification of these two biomarkers suggests that they
may add better value proposition in conjunction with other
biomarkers. Earlier studies have reported that the combination of
biomarkers, such as Nt-proBNP, albuminuria and CRP (4) or CMV-NA with IL-6 (6) has a marginal improvement in
C-statistics. Therefore, the development of a multimarker approach
by including β-defensin-128 and histatin-3, where there was a
significant improvement in the AUC (Table IV), may help to better
discriminate subjects with recurrent CAD. Furthermore, the combined
risk scores of β-defensin-128 and histatin-3 (Table V) and Kaplan-Meier curve
cumulative probability analysis of cardiovascular events (Fig. 4A) or deaths/3rd event (Fig. 4B) suggested that 68 out of the 85
subjects with recurrent events were correctly classified into the
intermediate- and high-risk groups.
Understanding the limitations of the present study,
further validation of these biomarkers in larger cohort of subject
is needed. In order to understand the role of other proteins,
protein-protein interactions network (15), pathways, regulome analysis
(30) and greater dissection of
all the 16 significant proteins in our data set (Table IIA) may provide a comprehensive
understanding of the crosstalk that occurs among the different
pathways and their collective contribution to recurrent coronary
events.
In conclusion, this study identified two members of
the danger-recognizing protein family, β-defensin-128 and
histatin-3 which are associated with recurrent CAD events, and also
demonstrated their potential use in discrimination of subjects.
Acknowledgments
We are grateful for the support of the funding
agencies Department of Biotechnology, Ministry of Science and
Technology, Government of India (BT/01/CDE/08/07), the Tata Social
Welfare Trust, India (TSWT/IG/SNB/JP/Sdm), Bharati Foundation,
India, Garfield Weston Foundation, UK and Foundation Bay, UK. The
sponsors did not participate in the design, conduct, sample
collection, analysis and interpretation of the data or in the
preparation, review or approval of the manuscript. The authors
would like to thank all the investigators, staff and administrative
teams and participants of IARS from Narayana Hrudayalaya, Bangalore
and Asian Heart Centre, Mumbai for their contribution. The authors
are grateful to the patients and their family members for
participating in the study.
References
|
1
|
Sharma M and Ganguly NK: Premature
coronary artery disease in Indians and its associated risk factors.
Vasc Health Risk Manag. 1:217–225. 2005.
|
|
2
|
Briffa TG, Hobbs MS, Tonkin A, Sanfilippo
FM, Hickling S, Ridout SC and Knuiman M: Population trends of
recurrent coronary heart disease event rates remain high. Circ
Cardiovasc Qual Outcomes. 4:107–113. 2011. View Article : Google Scholar
|
|
3
|
Sabatine MS, Morrow DA, de Lemos JA,
Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP and
Braunwald E: Multimarker approach to risk stratification in non-ST
elevation acute coronary syndromes: Simultaneous assessment of
troponin I, C-reactive protein, and B-type natriuretic peptide.
Circulation. 105:1760–1763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shlipak MG, Ix JH, Bibbins-Domingo K, Lin
F and Whooley MA: Biomarkers to predict recurrent cardiovascular
disease: The Heart and Soul Study. Am J Med. 121:50–57. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mundkur LA, Rao VS, Hebbagudi S, Shanker
J, Shivanandan H, Nagaraj RK and Kakkar VV: Pathogen burden,
cytomegalovirus infection and inflammatory markers in the risk of
premature coronary artery disease in individuals of Indian origin.
Exp Clin Cardiol. 17:63–68. 2012.PubMed/NCBI
|
|
6
|
Mundkur LA, Shivanandan H, Hebbagudi S,
Endrész V, Varma M, Rao V, Gonczol E and Kakkar VV: Human
cytomegalovirus neutralising antibodies and increased risk of
coronary artery disease in Indian population. Heart. 98:982–987.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasan RS: Biomarkers of cardiovascular
disease: Molecular basis and practical considerations. Circulation.
113:2335–2362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shanker J, Maitra A, Rao VS, Mundkur L,
Dhanalakshmi B, Hebbagodi S and Kakkar VV: Rationale, design and
preliminary findings of the Indian Atherosclerosis Research Study.
Indian Heart J. 62:286–295. 2010.
|
|
9
|
Lomthaisong Khemika, Boonmaleerat
Kanchanit and Wongpia Aphinya: Proteomic study of recombinant
Escherichia coli expressing Beauveria bassiana vhitinase gene.
Chiang Mai J Sci. 35:324–330. 2008.
|
|
10
|
Wilkins MR, Gasteiger E, Bairoch A,
Sanchez JC, Williams KL, Appel RD and Hochstrasser DF: Protein
identification and analysis tools in the ExPASy server. Methods Mol
Biol. 112:531–552. 1999.PubMed/NCBI
|
|
11
|
Wilkins MR, Gasteiger E, Sanchez JC, Appel
RD and Hochstrasser DF: Protein identification with sequence tags.
Curr Biol. 6:1543–1544. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilkins MR, Gasteiger E, Tonella L, Ou K,
Tyler M, Sanchez JC, Gooley AA, Walsh BJ, Bairoch A, Appel RD, et
al: Protein identification with N and C-terminal sequence tags in
proteome projects. J Mol Biol. 278:599–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TJ, Gona P, Larson MG, Tofler GH,
Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, et
al: Multiple biomarkers for the prediction of first major
cardiovascular events and death. N Engl J Med. 355:2631–2639. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosamond W, Flegal K, Furie K, Go A,
Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2008
update: A report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
117:e25–e146. 2008. View Article : Google Scholar
|
|
15
|
Vangala RK, Ravindran V, Kamath K, Rao VS
and Sridhara H: Novel network biomarkers profile based coronary
artery disease risk stratification in Asian Indians. Adv Biomed
Res. 2:592013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin G, Zhou X, Wang H, Zhao H, Cui K,
Zhang XS, Chen L, Hazen SL, Li K and Wong ST: The
knowledge-integrated network biomarkers discovery for major adverse
cardiac events. J Proteome Res. 7:4013–4021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blanco-Colio LM, Martín-Ventura JL,
Muñóz-García B, Orbe J, Páramo JA, Michel JB, Ortiz A, Meilhac O
and Egido J: Identification of soluble tumor necrosis factor-like
weak inducer of apoptosis (sTWEAK) as a possible biomarker of
subclinical atherosclerosis. Arterioscler Thromb Vasc Biol.
27:916–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weinberg A, Jin G, Sieg S and McCormick
TS: The yin and yang of human beta-defensins in health and disease.
Front Immunol. 3:2942012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vordenbäumen S, Sander O, Bleck E,
Schneider M and Fischer-Betz R: Cardiovascular disease and serum
defensin levels in systemic lupus erythematosus. Clin Exp
Rheumatol. 30:364–370. 2012.PubMed/NCBI
|
|
20
|
Tiszlavicz Z, Somogyvári F, Szolnoki Z,
Sztriha LK, Németh B, Vécsei L and Mándi Y: Genetic polymorphisms
of human β-defensins in patients with ischemic stroke. Acta Neurol
Scand. 126:109–115. 2012. View Article : Google Scholar
|
|
21
|
Brissette CA and Lukehart SA: Treponema
denticola is resistant to human beta-defensins. Infect Immun.
70:3982–3984. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Koning J, Hoogkamp-Korstanje JA, van
der Linde MR and Crijns HJ: Demonstration of spirochetes in cardiac
biopsies of patients with Lyme disease. J Infect Dis. 160:150–153.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujigaki Y, Imamura Y, Oomori Y, Ouryouji
K, Miyazawa H and Wang PL: Polymorphism of salivary histatin gene
and periodontal disease in the Japanese population. J Int Acad
Periodontol. 11:220–225. 2009.PubMed/NCBI
|
|
24
|
Burgener A, Mogk K, Westmacott G, Plummer
F, Ball B, Broliden K and Hasselrot K: Salivary basic proline-rich
proteins are elevated in HIV-exposed seronegative men who have sex
with men. AIDS. 26:1857–1867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hung CC, Pirie F, Luan J, Lank E, Motala
A, Yeo GS, Keogh JM, Wareham NJ, O'Rahilly S and Farooqi IS:
Studies of the peptide YY and neuropeptide Y2 receptor genes in
relation to human obesity and obesity-related traits. Diabetes.
53:2461–2466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji YN, An L, Zhan P and Chen XH:
Cytomegalovirus infection and coronary heart disease risk: A
meta-analysis. Mol Biol Rep. 39:6537–6546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Epstein SE, Zhu J, Najafi AH and Burnett
MS: Insights into the role of infection in atherogenesis and in
plaque rupture. Circulation. 119:3133–3141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albert LJ and Inman RD: Molecular mimicry
and autoimmunity. N Engl J Med. 341:2068–2074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Epstein SE: The multiple mechanisms by
which infection may contribute to atherosclerosis development and
course. Circ Res. 90:2–4. 2002.PubMed/NCBI
|
|
30
|
Vangala RK, Ravindran V, Ghatge M, Shanker
J, Arvind P, Bindu H, Shekar M and Rao VS: Integrative
bioinformatics analysis of genomic and proteomic approaches to
understand the transcriptional regulatory program in coronary
artery disease pathways. PLoS One. 8:e571932013. View Article : Google Scholar : PubMed/NCBI
|