Introduction
POP is a global health concern that is associated
with increasing morbidity and economic burden, and it is estimated
to affect almost 50% of women above 60 years of age (1). Approximately 11% of females require
at least one surgery for prolapse in their lifetime (2), and the direct cost incurred is
equivalent to billions of dollars annually in the United States
(3). POP contributes to a low
quality of life and has become one of the most common indications
for gynecological surgery among post-menopausal women (4). The pathophysiological mechanisms of
POP, however, have not yet been fully elucidated. The pelvic organs
are mainly connected and supported by the levator ani muscle
complex, the cardinal and uterosacral ligament (USL) and endopelvic
fascia. The parauterine ligaments along with the endopelvic fascia
compose a connective tissue network, supporting the maintenance of
the normal position of the vagina and uterus. The disruption and
dysfunction in this connective tissue network may lead to the
weakening of support, and eventually, urogenital prolapse (5,6).
The present study focused on the metabolic
alterations in connective tissue in USL as it has been considered
to be one of the most important apparatuses in the pelvic
supporting network (7). USL is
composed of vessels, nerves, smooth muscle and connective tissue,
and collagen fibers constitute 70–80% of the connective tissue
(8). Collagen and elastin are the
main extracellular matrix (ECM) components in USL, which provide
strength and flexibility. Collagen type I (COL1) strengthens the
connective tissues, whereas collagen type III (COL3) and elastin
contribute to flexibility (9).
Precursor collagens (COL1A1 and COL3A1) and elastin are synthesized
by fibroblasts, and are then secreted into the ECM as the raw
material of fibril assembly (6,22–24). The degradation of collagens
depends on the activity of the interstitial matrix
metalloproteinases (MMPs). MMPs are divided into subgroups
according to substrate. MMP-1, -8 and -13 (collagenase) cleave
pro-collagen fibers into fragments. The cleaved collagen fragments
and elastin are then digested into amino acids by MMP-2/9
(gelatinases) (10). Tissue
inhibitor of matrix metalloproteinases (TIMP) is the specific
antagonist of MMPs 11. TGF-β1,
as an important regulator of fibrotic metabolism, and has been
widely reported in the process of fibrosis and degenerative
fibrotic disease (6,12); however, the role of TGF-β1 in USL
with POP has not been reported to date, at least to the best of our
knowledge. Based on the available data, we hypothesized that TGF-β1
may play a regulatory role in POP.
The importance of ECM metabolism in USL has been
well documented. However, the majority of the published data was
established on the investigation of the vaginal wall (13,14) and endopelvic fascia (15,16); research data on USL are lacking.
Additionally, there are many controversies regarding the trends in
the alterations of the levels of collagen, elastin, MMPs and TIMP,
which has led to uncertainty and is not beneficial for further
study. In the present study, we aimed to identify the metabolic
status the ECM proteins mentioned above in USL from women with POP,
and to explore the exact role of TGF-β1 in the pathogenesis of POP
and the related mechanisms.
Materials and methods
Participants and specimen collection
Human USL biopsies were collected from patients
after obtaining informed consent with the approval of the Ethics
Committee of Renmin Hospital of Wuhan University, Wuhan, China. A
total of 60 subjects were selected among female patients who
underwent hysterectomy for benign indications during the period of
March 2011 to June 2014 at the Department of Gynecology of Renmin
Hospital of Wuhan University. Based on POP quantitative examination
(17), 30 patients with POP
stages II-IV who underwent hysterectomy as part of pelvic
reconstruction surgery were included in the POP group. The other 30
subjects with non-POP or asymptomatic POP (POP grade I) who
underwent hysterectomy for cervical intraepithelial neoplasia or
dysfunctional uterine bleeding comprised the control group. All the
included subjects were post-menopausal for at least 1 year. Women
with malignant tumors, pelvic endometriosis and acute pelvic
inflammation, as well as those undergoing hormone replacement
therapy (HRT) were excluded from the study. The 2 groups were
matched for age, menopause status, parity and body mass index
(BMI). Biopsies were obtained from the left USL during
hysterectomy. Specimens were prepared and preserved according to
various protocols.
Masson's trichrome staining
The specimens were fixed with 4% paraformaldehyde,
embedded in paraffin, cut into 4-μm-thick sections, and
mounted onto coated slides. Masson's trichrome staining was
performed to quantify collagen fibers using the HT15 kit (Sigma,
St. Louis, MO, USA) according to the standard protocol.
Immunochemistry (IHC)
The UltraSensitive SP kit-9710 (Maixin Biotech Co.,
Ltd., Fuzhou, China) was used for IHC. The slides were heated for
30 min at 60°C, de-paraffinized and rehydrated in a graded alcohol
series. Antigen retrieval was performed through boiling in citrate
(pH <6) or EDTA buffer (pH >9), according to the product
datasheets. This was followed by incubation with 3%
H2O2 for 10 min at room temperature to
inactivate endogenous peroxidase, and blocking with 5% goat serum
for 15 min, and incubationg with primary antibodies overnight at
4°C. Following incubation with streptavidin peroxidase for 10 min
at room temperature, secondary antibodies were added. Finally,
immune reaction was visualized using the DAB-0031 kit (Maixin
Biotech Co., Ltd.). The specimens were washed with
phosphate-buffered saline (PBS) after each step in the protocol.
The primary antibody was replaced with PBS for the negative
controls.
The antibodies used were as follows: COL1A1 antibody
(diluted at 1/100; BA0325; Boster Inc., Wuhan, China), COL3A1
antibody (diluted at 1/150; BA0326; Boster Inc.), elastin antibody
(diluted at 1/100; ab23747; Abcam, Cambridge, UK), MMP-2 antibody
(diluted at 1/75; BA3716; Boster Inc.), MMP-9 antibody (diluted at
1/100; BA0573; Boster Inc.), TIMP-2 antibody (diluted at 1/100;
sc-5539; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
TGF-β1 antibody (diluted at 1/100; ab92486; Abcam).
Immunoactivity was quantified with the IOD,
integrated optical density (IOD) value and the mean density was
captured using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA); mean density =
IOD/area.
Western blot analysis
Tissue samples were homogenized in RIPA buffer and
quantified using the BCA Protein Assay kit (P0012; Beyotime
Biotech, Jiangsu, China), prior to mixing with loading buffer.
Following degeneration, the protein samples were separated in 10%
SDS-PAGE and transferred onto PVDF membranes at 200 mA for 1–3 h at
4°C. The blots were blocked with 5% non-fat milk in TBS for 1 h at
room temperature and incubated with primary antibody overnight at
4°C. The PVDF membranes were washed with TBST thrice. Subsequently,
the blots were incubated with IRDye 800CW goat anti-rabbit/mouse
secondary antibodies (diluted at 1/10,000; LI-COR Inc., Lincoln,
NE, USA) at room temperature for 1 h. The Odyssey imaging system
(LI-COR Inc.) was used for western blot analysis.
The antibodies used were as follows: elastin
antibody (1:200 dilution; ab23747; Abcam), MMP-2 antibody (1:200
dilution, sc-53630), MMP-9 antibody (1:100 dilution, sc-13520),
TIMP-2 antibody (1:200 dilution; sc-5539), COL1A1 antibody (1:400
dilution; sc-8784), COL3A1 antibody (1:400 dilution; sc-28888) (all
from Santa Cruz Biotechnology, Inc.), TGF-β1 antibody (1:250
dilution; ab92486; Abcam) and GAPDH antibody (1:1,000 dilution;
sc-20357; Santa Cruz Biotechnology, Inc.), p-Smad3 (1:1,000
dilution, #9520), Smad3 (1:1,000 dilution, #9523), p-Smad2 (1:1,000
dilution, #3108), Smad2 (1:1,000 dilution, #5339), Smad4 (1:1,000
dilution, #38454; all from Cell Signaling Technology, Inc.,
Danvers, MA, USA).
Primary cell culture
Human uterosacral ligamental fibroblasts (hUSLFs)
were developed from fresh tissue of USL biopsies, according to
Gibco/Thermo Fisher Scientific, Inc. (Waltham, MA, USA) protocols
for primary culture, as previously described (18,19). The cells at passages 4–6 were used
for the subsequent experiments. Recombinant human TGF-β1 protein
was obtained from PeproTech China (Suzhou, China).
Mechanical strain loading
hUSLFs were seeded on flexible plates which were
pre-coated with rat tail collagen type I (25 μg/ml in 0.02 N
acetic acid; Sigma). Following reaching confluence, cells were
pre-treated with recombinant human TGF-β1 at the indicated
concentrations for 24 h, then incubated in serum-free DMEM for 12 h
and prepared for mechanical strain. To exert cyclic mechanical
stretching (CMS) on the cells, a 4-point bending device (SXG4201;
Chengdu Miracle Chemicals Co., Ltd., Chengdu, China) was used
(19,20). The mechanical strain parameter was
set at 0.3 Hz of 5,333 με (loading displacement is 4 mm) for
4 h.
Cell counting kit-8 (CCK-8) assay
Cytotoxicity was measured using a CCK-8 assay
(Beyotime Biotech). According to the protocol, following treatment
with recombinant human TGF-β1 and/or CMS loading, cell samples
suspended with DMEM (100 μl; Gibco/Thermo Fisher Scientific,
Inc.) were inoculated into a 96-well plate (5,000 cells/well) and
incubated at 37°C for 24 h, each sample was loaded in triplicate.
Subsequently, 10 μl CCK-8 solution were added to each well
and incubated for 4 h at 37°C. The absorbance was measured at 450
nm using a microplate spectrophotometer (Victor3-1420 Multilable
Counter; PerkinElmer, Inc., Waltham, MA, USA). The viability of the
treatment group was expressed as a percentage of the control group,
which was designated as 100%.
Gelatin zymography
A gelatin zymography assay kit (P1700; Applygen
Technologies, Inc., Beijing, China) was used to examine the
gelatinolytic activity of the conditioned medium. In brief, the
samples (30 μl of conditioned medium) were electrophoresed
on a 10% SDS polyacrylamide gel containing 1 mg/ml gelatin under
non-reducing conditions. The volume of conditioned medium loaded
per lane was standardized on the basis of the cell count at
harvest. To activate the proteinases, the gels were incubated at
37°C overnight in an incubation buffer containing 50 mM Tris-HCl
(pH 7.5), 10 mM CaCl2, and 0.02 mM NaN3. The
gels were subsequently fixed and stained with 0.25% Coomassie
brilliant blue R-250 (Maixin Biotech Co., Ltd.) for 1 h and washed
in 25% methanol and 7% acetic acid to visualize the bands of
proteolytic activity. White lysis zones indicated the degrading
activity.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed with
2 μg of total RNA in a reaction volume of 20 μl
according to the protocol of RevertAid First Strand cDNA Synthesis
kit (K1622; Fermentas, Glen Burnie, MD, USA). Quantitative PCR
(qPCR) assays for relative quantification were performed using the
SYBR Premix Ex Taq system (RR820A; Takara Bio, Inc., Otsu, Japan)
and an ABI 7500 Fast Real-Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The total reaction volume was
20 μl. SYBR-Green was used to amplify detection. The cycling
conditions were as follows: stage 1, 30 sec at 95°C; stage 2, 40
cycles of 5 and 34 sec at 95°C and 60°C, respectively; stage 3, 15
sec, 1 min, 15 sec, and 15 sec at 95°C, 60°C, 95°C, and 60°C,
respectively. Primers were synthesized by Sangon Biotech (Shanghai,
China). Sequence data are presented in Table I.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Primer | Accession no. | Sequence
(5′→3′) | Product length
(bp) |
|---|
| COL1A1 | NM_000088.3 | Forward:
CAAGACGAAGACATCCCACCAATC
Reverse: ACAGATCACGTCATCGCACAACA | 135 |
| COL3A1 | NM_000090.3 | Forward:
TCGCTCTGCTTCATCCCACTAT
Reverse: CTTCCAGACATCTCTATCCGCAT | 101 |
| Elastin | NM_006329.3 | Forward:
CGCTCTAGCATCCCTCCTCT
Reverse: GCAAGGTGGCTATTCCCAGT | 122 |
| MMP-2 | NM_001127891.2 | Forward:
AGTTTCCATTCCGCTTCCAG
Reverse: CGGTCGTAGTCCTCAGTGGT | 100 |
| MMP-9 | NM_004994.2 | Forward:
GTCCACCCTTGTGCTCTTCC
Reverse: GACTCTCCACGCATCTCTGC | 117 |
| TIMP-2 | NM_003255.4 | Forward:
TCTGGAAACGACATTTATGG
Reverse: GTTGGAGGCCTGCTTATGGG | 508 |
| TGF-β1 | NM_000660.5 | Forward:
TATTGAGCACCTTGGGCACT
Reverse: ACCTCTCTGGGCTTGTTTCC | 130 |
| GAPDH | NM_001289746.1 | Forward:
GAAGGTGAAGGTCGGAGTC
Reverse: GAAGATGGTGATGGGATTTC | 226 |
A preprogrammed dissociation protocol was used
following amplification to ensure that all samples exhibited a
single amplification. Gene expression was normalized to the
housekeeping genes, GAPDH. mRNA levels were determined using the
ΔΔCq method (Applied Biosystems Life Technologies) and expressed
relative to an external calibrator on each plate.
In order to examine the clinical relevance of TGF-β1
mRNA expression with the severity of POP, all data were re-grouped
into 5 subgroups according to the grade of POP, including the
non-POP (n=20), POP I (n=10), POP II (n=10), POP III (n=10) and POP
IV group (n=10).
Statistical analysis
Data are summarized as the means ± standard
deviation (SD). The Chi-square test and Student's t-test were was
used to determine statistical difference between 2 groups, for
numeration data and measurement data, respectively. The statistical
comparison among multiple groups was processed with one-way ANOVA,
and Bonferroni's multiple comparisons test was used for inter-group
comparison. The detail statistical treatments and graph plotting
were performed with the software of SPSS version 19.0 (IBM SPSS,
Armonk, NY, USA) and GraphPad Prism version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Well-matched demographic and clinical
characteristics
A total of 60 women were enrolled in the present
study: 30 with symptomatic POP (POP II-IV) constituted the POP
group, another 30 with non-POP or asymptomatic POP (POP I) served
as the control group. The participants from each group were well
matched in terms of age, parity, BMI and post-menopausal duration;
accordingly, there was no significant difference between the POP
group and the control group (P>0.05; Table II).
 | Table IIDemographic and clinical
characteristics of all participants. |
Table II
Demographic and clinical
characteristics of all participants.
| Parameters | POP group
(n=30) | Control group
(n=30) | t-value or
χ2-value | P-value |
|---|
| Age (years, mean ±
SD) | 58.6±4.1 | 57.1±3.3 | 1.877a | NS |
| Parity (mean ±
SD) | 2.3±1.6 | 2.4±1.1 | 0.337b | NS |
| BMI (mean ±
SD) | 24.6±2.7 | 25.2±2.4 | 1.100a | NS |
| Post-menopausal
duration (months, means ± SD) | 43.8±8.6 | 40.7±7.9 | 1.761a | NS |
Decreased expression of collagen and
elastin fibers in the POP group
In order to examine the histomorphological changes
of collagen and elastin fibers in the slices of USL tissue with
symptomatic POP, the methods of Masson's trichrome and IHC staining
were used. As shown in Fig. 1,
collagen fibers in the slices of USL tissue from the POP group were
much more loose, and decreased significantly compared with the
control group (P<0.05). Elastin fibers became shortened,
condensed and cluttered in rearrangements, and the immunochemical
activity of elastin in the POP group was significantly lower than
that of the control group (P<0.05; Fig. 1A). The RT-qPCR data revealed the
mRNA expression levels of COL1A1 (P<0.01), COL3A1 (P<0.01)
and elastin (P<0.05) were significantly decreased in the POP
group (Fig. 1B). These results
suggest that the hypo-anabolism and morphological changes of
collagen and elastin fibers may be related to the pathogenesis of
POP.
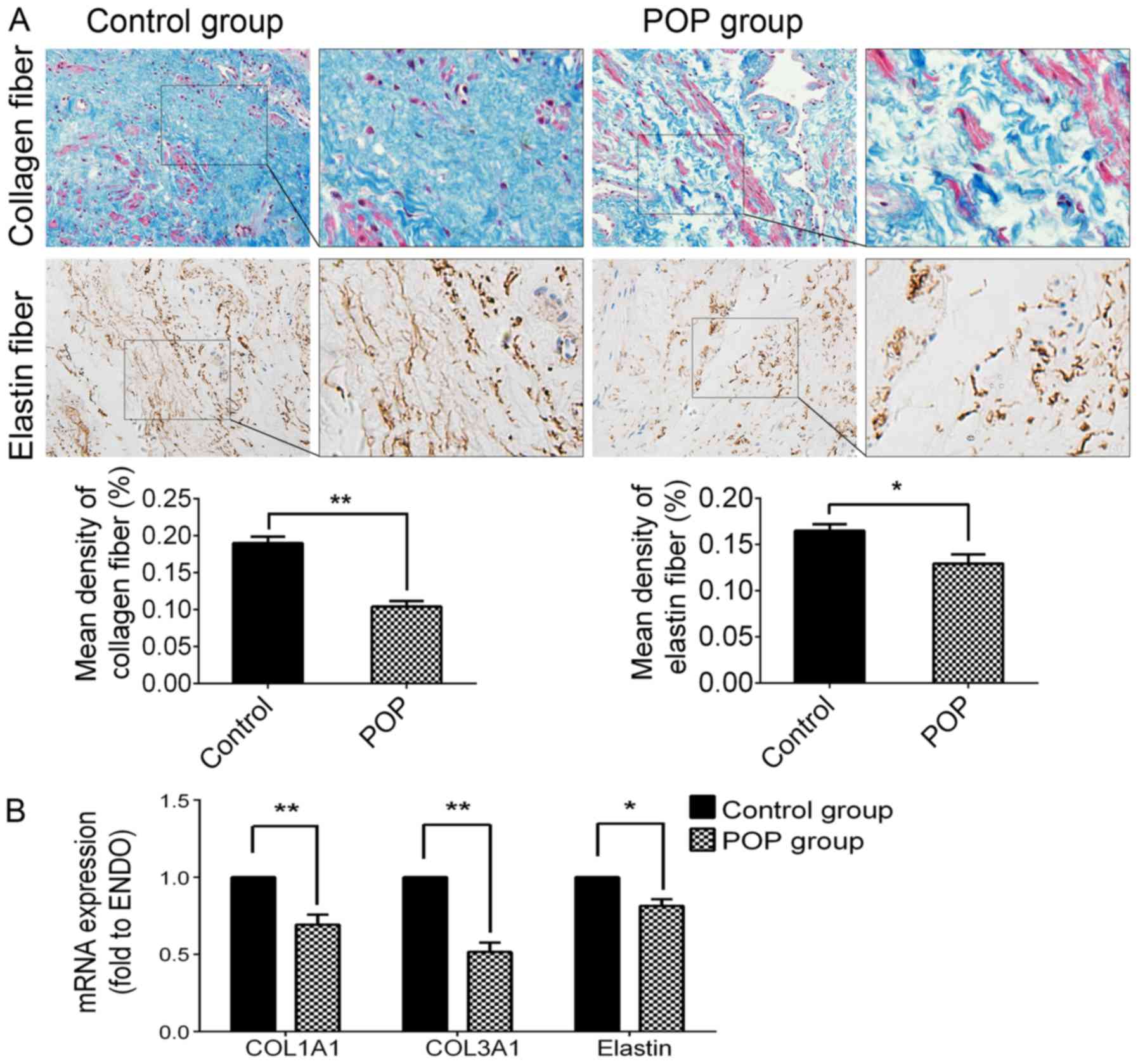 | Figure 1Expression levels of collagen fibers
and elastin in USL tissue from the control group and the POP group.
(A) Upper panel, by Masson's trichrome staining, collagen fibers
are stained blue, smooth muscle and cytoplasm are stained red, cell
nuclei are stained black; second panel, immunohistochemical
staining of elastin fiber, strong positive in the control group,
moderately positive in the POP group; third panel, statistical
analysis based on the IOD value and mean density captured by
Image-Pro plus 6.0 software. (B) The mRNA expression levels of
COL1A1, COL3A1 and elastin detected by RT-qPCR. Data are presented
as the means ±standard deviation for at least 3 independent
experiments. n=30; *P<0.05 vs. the control group,
**P<0.01 vs. the control group. USL, uterosacral
ligament; POP, pelvic organ prolapse; COL, collagen; IOD,
integrated optical density. |
Upregulated expression of MMP-2/9 and
downregulated expression of TIMP-2 in the POP group
The methods of IHC and RT-qPCR were used to
investigate the expression of MMP-2/9 and TIMP-2 (Fig. 2). In the POP group, the
immunochemical activity of either MMP-2 (P<0.01) or MMP-9
(P<0.01) was significantly stronger than that of the control
group. Moreover, there was a significantly lower expression of
TIMP-2 in the POP group than in the control group (P<0.01;
Fig. 2A). The trends in the
alterations in mRNA expression were consistent with the results of
IHC (Fig. 2B). The
above-mentioned data suggest that the increased degradation of ECM
proteins may be involved in the pathogenesis of POP.
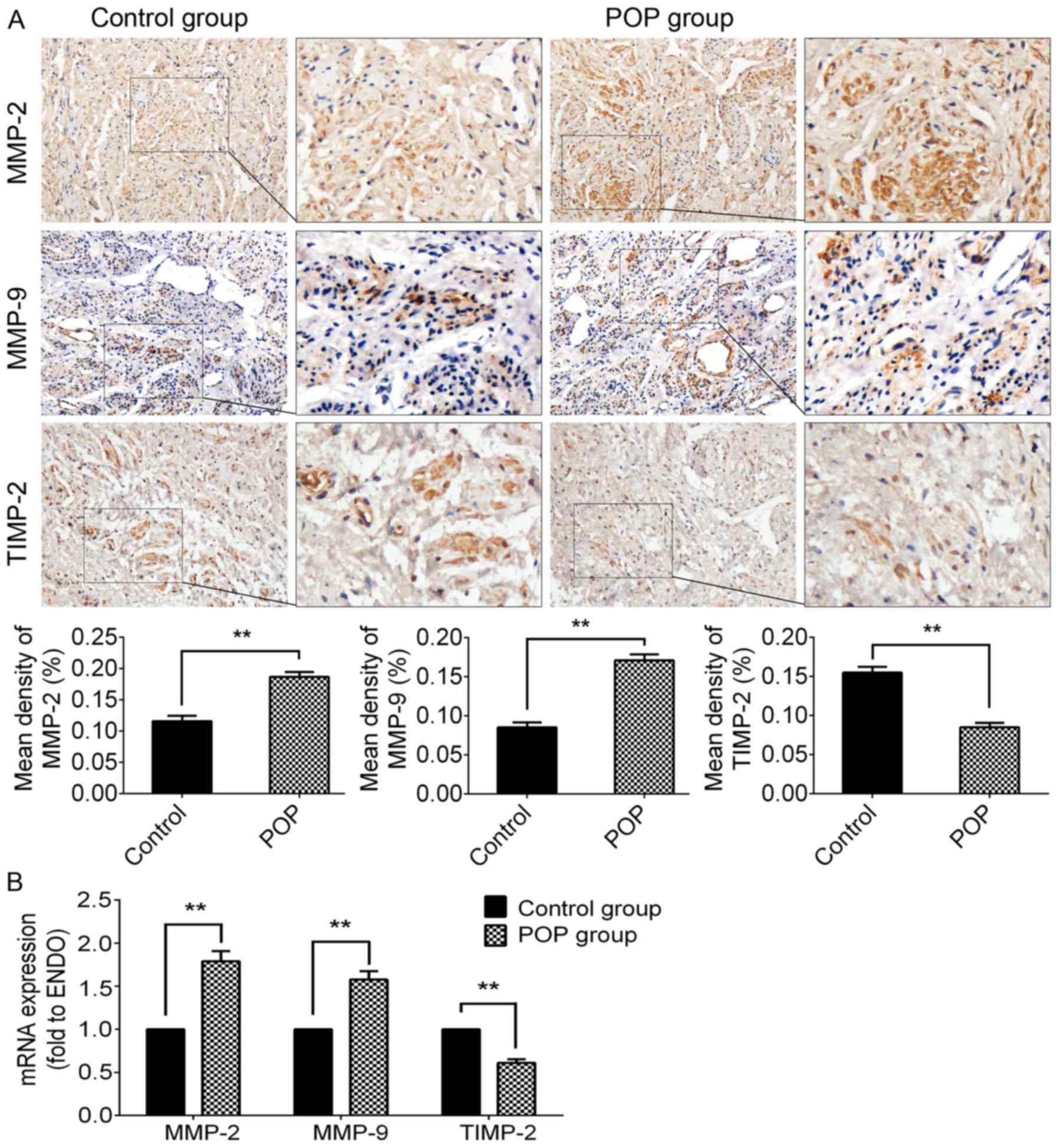 | Figure 2Expression levels of MMP-2, MMP-9 and
TIMP-2 in USL tissue from the control group and the POP group. (A)
Immunohistochemical staining of USL sections; top panel, for MMP-2,
moderate positive staining was observed in the control group, and
strong positive staining in the POP group; second panel, for MMP-9,
mildly positive staining was observed in the control group, while
moderately positive staining was observed in the POP group; third
panel, for TIMP-2, moderately positive staining was observed in the
control group, and mildly positive staining in the POP group;
fourth panel, statistical comparison between the control and POP
group. (B) The mRNA expression levels of MMP-2, MMP-9, and TIMP-2,
examined by RT-qPCR. Data are presented as the means ± standard
deviation for at least 3 independent experiments. n=30;
**P<0.01 vs. the control group. MMP, matrix
metalloproteinase; TIMP, tissue inhibitor of MMP; USL, uterosacral
ligament; POP, pelvic organ prolapse. |
Expression level of TGF-β1 has clinical
relevance with the severity of POP
IHC and RT-qPCR were performed using USL tissue to
examine the expression of TGF-β1 (Fig. 3). In the POP group, the expression
of TGF-β1 was significantly lower than that of the control group,
as shown by both IHC and RT-qPCR (n=30, P<0.05).
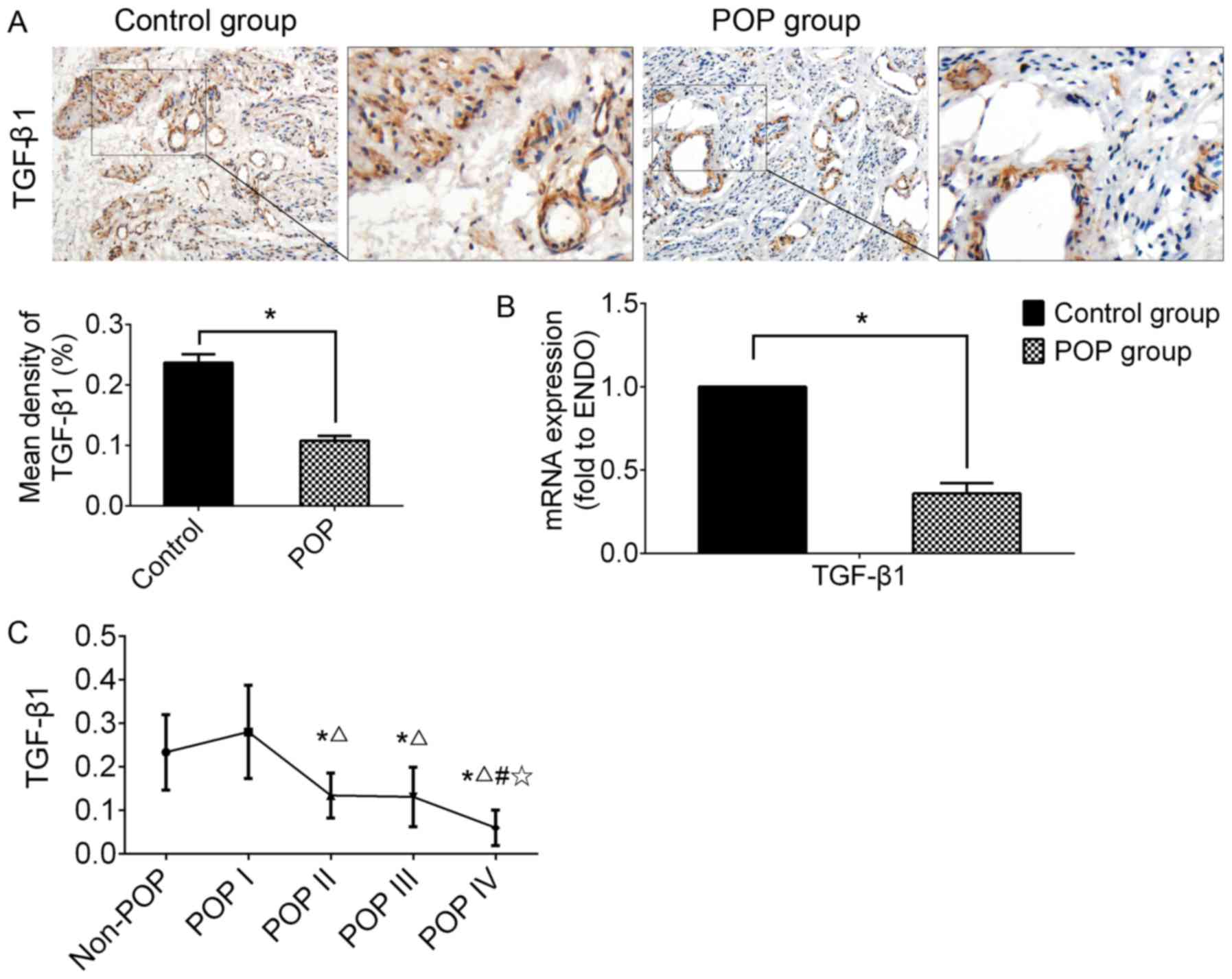 | Figure 3The expression of TGF-β1 in USL
tissue from the control group (including non-POP and asymptomatic
POP of grade I) and the POP group (POP II-IV). (A)
Immunohistochemical staining of USL sections; strongly positive
staining was observed in the control group, and mildly positive in
the POP group; n=30; (B) the mRNA expression levels of TGF-β1
examined by RT-qPCR. *P<0.05 vs. the control group.
n=30; (C) analyzing the clinical relevance of TGF-β1 mRNA
expression level with the grade of POP. Data are presented as the
means ± standard deviation for at least 3 independent experiments;
n=10; *, △, # and ✩
represent P<0.05 when compared with the non-POP, POP I, POP II,
and POP III group, respectively. TGF, transforming growth factor;
USL, uterosacral ligament; POP, pelvic organ prolapse. |
In order to examine the clinical relevance of TGF-β1
mRNA expression with the severity of POP, all data were re-grouped
into 5 subgroups according to the grade of POP, including the
non-POP (n=20), POP I (n=10), POP II (n=10), POP III (n=10) and POP
IV group (n=10). The results revealed that the mRNA expression
level of TGF-β1 in the POP group (POP II-IV) was significantly
lower than that of the control group (non-POP or POP I).
Additionally, Bonferroni's multiple comparisons test among the
subgroups from POP I to POP IV was carried out to determine the
correlation between TGF-β1 and the severity of disease. The results
revealed a tendency for a negative correlation between the mRNA
expression of TGF-β1 and the grade of POP in a severity-dependent
manner; however, there was no significant difference between the
non-POP and POP I subgroups (Fig.
3C).
TGF-β1 attenuates the CMS-induced
degradation of ECM proteins in a cell model established using
hUSLFs in vitro
To determine whether TGF-β1 plays a role in ECM
remodeling generated by hUSLFs in the pathogenesis of POP, we
replicated the cell model induced by CMS with certain parameters as
described in our previous study (19,20). In cell experiments in the present
study, hUSLFs at passages 4–6 were pre-treated with recombinant
human TGF-β1 at concentrations of 0, 5, or 10 ng/ml for 24 h, and
then exposed to CMS with a strain of 5,333 με for 4 h. As
shown in Fig. 4, pre-treatment
with TGF-β1 significantly attenauted the decrease in cell viability
induced by CMS. In the absence of TGF-β1, CMS not only reduced the
synthesis of COL1A1 (P<0.05), COL3A1 (P<0.05) and elastin
(P<0.05), but also promoted their degradation through the
inhibition of the expression of TIMP-2 (P<0.05) and the
promotion of the expression of MMP-2/9 (P<0.05). In addition,
pre-treatment with TGF-β1 significantly attenuated the CMS-induced
degradation of COL1A1 and elastin by inhibiting the proteolytic
activities of MMP-2/9 (P<0.05). The above-mentioned data
indicate that TGF-β1 treatment plays a therapeutic role in the
CMS-induced excessive degradation of the ECM.
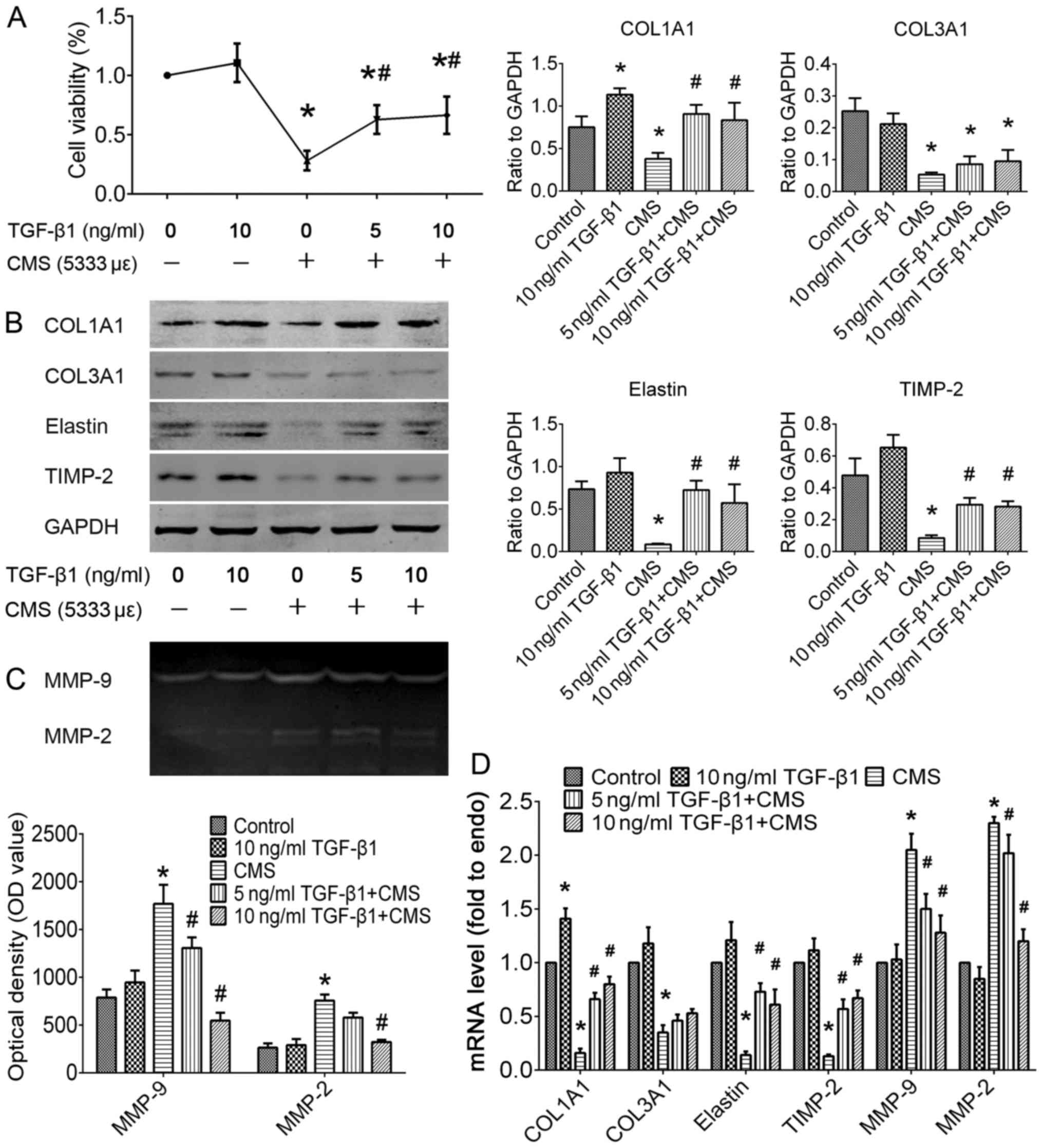 | Figure 4Effect of TGF-β1 on cell viability
and ECM metabolism in the control or hUSLFs subjected to CMS. In
the study groups, hUSLFs were incubated with recombinant human TGF-
1 at concentrations of 0, 5, or 10 ng/ml for 24 h, then exposed to
CMS at a strain of 5,333 με for 4 h. The normal control
group was not treated with TGF-β1 or CMS. (A) Cell viability was
detected using the Cell Counting kit-8 (CCK-8) method. Expression
levels of COL1A1, COL3A1, elastin, TIMP-2, MMP-9 and MMP-2 were
analyzed by (B) western blot analysis, (C) gelatin zymography, or
(D) RT-qPCR. Data represent the means ± standard deviation for at
least 3 independent experiments; n=3; *P<0.05 vs. the
control group; #P<0.05 vs. the CMS group. TGF,
transforming growth factor ECM, extracellular matrix; CMS, cyclic
mechanical stretching; hUSLF, human USL fibroblast; COL, collagen;
TIMP, tissue inhibitor of MMP; MMP, matrix metallloproteinase. |
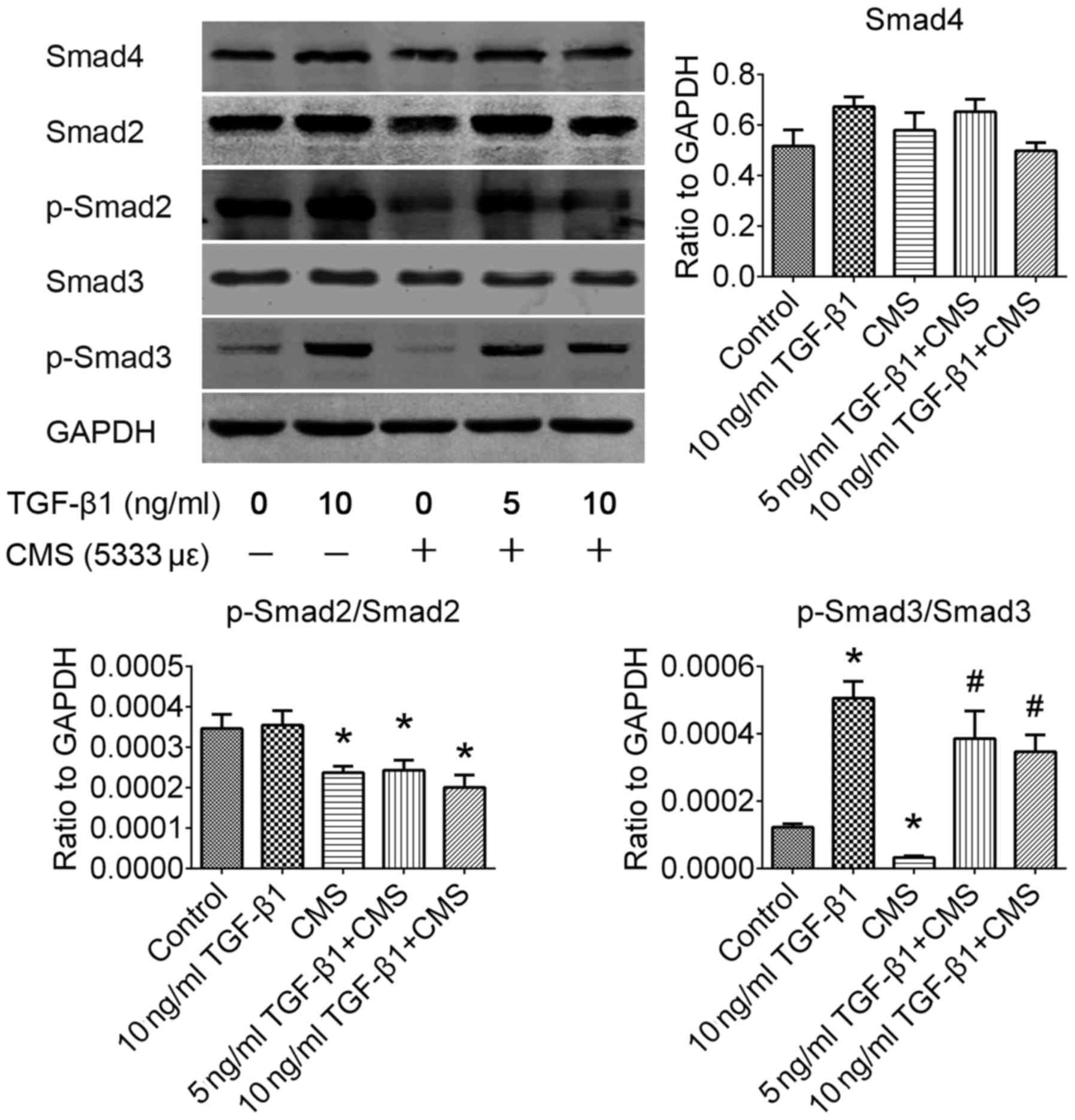
Phosphorylation of the Smad3 signaling
pathway is significantly improved by pre-treatment with TGF-β1
following CMS
The TGF-β1/Smad signaling pathway plays an important
role in fibrosis and degenerative fibrotic disease. In order to
identify the exact regulatory mechanisms through which TGF-β1
attenuates the ECM remodeling induced by CMS, the alterations in
downstream Smad signaling pathways were investigated by western
blot analysis (Fig. 5). In the
present study, it was observed that the phosphorylation rates of
both Smad2 and 3 were significantly inactivated by CMS in the
hUSLFs (P<0.05). Pre-treatment with TGF-β1 significantly
improved the phosphorylation rate of Smad3 (P<0.05), but had no
significant effect on the phosphorylation of Smad2 (P>0.05). In
addition, western blot analysis revealed that the Smad4 signaling
pathway exhibited no significant alteration induced following CMS
(P>0.05). It was thus suggested that the Smad3 signaling pathway
is the downstream regulatory mechanism activated by TGF-β1 in
hUSLFs subjected to CMS.
Discussion
POP is currently considered to be a disorder of
pelvic floor dysfunction, which is caused by the degenerative
weakening of pelvic supportive structures (21). Based on previous studies, collagen
and elastin, as the essential components of connective tissue,
contribute the most to the maintenance of resistance ability
(6,22). Although the histopathological
alterations in connective tissue in POP women have been mentioned
previously (22,23), there are disputes as to the trends
in the alterations of the levels of collagen I, III and elastin.
Vulic et al (24) and Chen
et al (25) reported that
the amounts of collagen I and III were significantly decreased in
USL with POP. However, there are different opinions, as Yucel et
al (26) clarified that the
expression of collagen III was upregulated in women with POP.
Gabriel et al (8)
concluded that there was no significant difference in the
expression of collagen I between women with POP and or women with
non-POP, but an increased ratio of collagen I/III may be a salient
feature of POP. Methodological issues may account for these
discrepancies. A common feature in the above-mentioned results is
that most of these experimental data were obtained from a single
IHC investigation; such evidence would inevitably have limitations
and one-sidedness, as IHC data are always limited by the biopsy
site and sample size. Different biopsy sites, particularly those in
prolapsed tissue, also increase variability as the differences in
stress loads can upregulate different proteins. Additionally,
pathological diagnosis is susceptible to the subjective judgement
of the pathologist. To a certain extent, RT-qPCR data is more
objective. In order to obtain more objective experimental data, in
addition to IHC, we also carried out RT-qPCR as a further
complement. In the present study, our experimental data validated
that the expression levels of COL1A1, COL3A1 and elastin were
significantly decreased in USL tissue from the POP group compared
with the control group (Fig. 1).
It can be inferred that the loss of collagen and elastin is
associated with the development of POP.
MMPs and TIMPs are considered key regulators in the
degradation of ECM, which are commonly involved in physiological
and pathological proteolytic processes. A previous study found a
lower amount of collagen, and higher expression levels of MMP-2/9
in vaginal biopsies from women with POP (14). A significantly higher
immunoreactivity in MMP-1, but no difference in MMP-2, was observed
in women with POP compared with the controls (24,27). Gabriel et al (28) reported that an increased MMP-2
expression in USL correlated with the incidence of POP. Dviri et
al (29) found higher
expression levels of MMP-1/9 in vaginal wall biopsies from women
with POP compared with the controls. To analyze the reasons for
discrepancies in the above-mentioned findings, firstly, the effects
of confounding factors, such as age, parity, obesity, menopausal
status and a history of HRT should be considered. Secondly,
differences in research methods may lead to divergent conclusions.
Compared with western blot analysis, gelatin zymography,
immunochemistry and RT-qPCR are more suitable for the study of MMP
expression, as gelatin zymography directly exhibits the activity of
proteolytic enzymes, immunochemistry additionally shows the
distribution of MMPs, and RT-qPCR reflects changes at the
transcriptional level, while western blot analysis can only reflect
the protein content of MMPs. In the present study, the method of
either immunochemistry or gelatin zymography combined with RT-qPCR
was selected to study MMPs. A significantly higher expression of
MMP-2/9, as well as a lower expression of TIMP-2, was observed in
USL from women with POP compared with the asymptomatic controls
after being matched in age, parity, BMI and menopausal duration
(Fig. 2). The present results are
highly in accordance with those of previous reports (30,31). According to the experimental
results, we suggest not only a reduced synthesis, but also an
increased degradation of ECM, which are the metabolic abnormalities
which contribute to the incidence of POP.
TGF-β is currently considered as an important
regulator widely involved in the pathogenesis of fibrosis and
degenerative fibrotic diseases, that may induce fibroblast
differentiation, promote collagen synthesis and reduce degradation
by inhibiting MMPs and upregulating TIMPs (12). Previous researchers have clarified
that the activity of gelatinase/type IV collagenase (MMP-2) was
regulated by TGF-β1 in human gingival fibroblasts (32). TGF-β1 reduced the level of
collagenase mRNA and increased the level of TIMP mRNA. A sparse,
but growing evidence of TGF-β1 modulation in pelvic connective
tissue has been found. The TGF-β/Smad signaling pathway and MMPs
are involved in stress urinary incontinence induced by vaginal
delivery in animal studies (15).
However, to the best of our knowledge, TGF-β1 has rarely been
investigated in women with POP. In the present study, TGF-β1 was
found to be expressed at significantly lower levels in USL tissue
from women with POP than in the controls, and further statistical
analysis after re-grouping according to the grade of POP indicated
the mRNA expression level of TGF-β1 partially negatively correlated
with the severity of POP (Fig.
3). Perhaps more statistically significant results may have
been obtained if a greater number of participants were enrolled in
this study. Taken together with the published theory in the
relative field, at least we could predict the possible involvement
of TGF-β1 in the pathogenesis and development of POP by regulating
the metabolism of ECM.
To identify the exact role of TGF-β1 in the
development of POP, in the follow-up experiment in vitro, we
introduced a cellular disease model that mimicked ECM remodeling in
POP, which was established on hUSLFs subjected to CMS loading with
a strain of 5,333 με for 4 h as described in our previous
study (19,20). Since it has been conducted in a
previous study (19,20), we did not describe the dose-effect
investigation in detail in this study. To verify the rationality of
the cell model, it was observed that CMS loading not only
significantly stimulated the degradation of ECM proteins, but also
reduced its synthesis (Fig. 4);
these effects are metabolic features in POP. In vitro cell
experimental results indicated that pre-treatment with TGF-β1
significantly attenuated the CMS-induced loss of ECM proteins by
inhibiting the proteolytic activities of MMP-2/9, as well as by
stimulating synthesis of COL1A1 and elastin. Further experiments
revealed that the phosphorylation of the Smad3 signaling pathway is
the downstream regulatory mechanism activated by TGF-β1 in hUSLFs
subjected to CMS loading (Fig.
5).
There are still some shortcomings to the present
study. Firstly, we used a relatively small sample size. With a
larger patient number, perhaps more statistically significant
results may have been obtained following re-grouping according to
the grade of POP. Secondly, IHC is not the most ideal method for
the study of the expression of MMPs at the tissue level, since the
secretion and activity of MMPs may always change quantitatively,
and in theory, gelatin zymography is the more suitable method.
However, due to the limited source of clinical specimens and the
experimental budget, we selected IHC as the study method, as IHC
has a certain advantage in detecting the localization of MMP
expression in tissue to a certain extent. Thirdly, due to time and
experimental technology constraints, the present study was limited,
resulting in the lack of adequate evidence to confirm whether the
Smad3 signaling pathway is the only downstream regulatory
mechanism. In addition, the present conclusion may be more
convincing if further validation was available at the animal level
experiment in vivo.
In conclusion, in the present study, we provide
evidence to indicate that the reduced anabolism and increased
catabolism of ECM proteins in USL are the pathological
characteristics of POP. TGF-β1 not only can be used as a biomarker
to predict the severity of POP, but also has a therapeutic effect
in cell model of POP, and it is suggested that the TGF-β1/Smad3
signaling pathway may be a novel therapeutic target for POP.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (NSFC) (grant no. 81270684) and the
Special Fund for Basic Scientific Research in National central
Colleges and Universities (grant no. 2042017kf0114). We would like
to thank Professor Xu Xue-Xian, Ms. Li Yan-Bo, Ms. Cheng Yan-Xiang,
Mr. Luo Ruo-Yu, at the Department of Gynecology and Obstetrics,
Renmin Hospital of Wuhan University for assisting with the specimen
biopsies.
Glossary
Abbreviations
Abbreviations:
|
POP
|
pelvic organ prolapse
|
|
ECM
|
extracellular matrix
|
|
COL
|
collagen
|
|
PCR
|
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
TGF
|
transforming growth factor
|
|
IOD
|
integrated optical density
|
|
TIMP
|
tissue inhibitor of matrix
metalloproteinases
|
|
MMPs
|
matrix metalloproteinases
|
|
USL
|
uterosacral ligament
|
|
CMS
|
cyclic mechanical stretching
|
|
hUSLF
|
human uterosacral ligamental
fibroblast
|
|
Smad
|
small mothers against
decapentaplegic
|
References
|
1
|
Barber MD and Maher C: Epidemiology and
outcome assessment of pelvic organ prolapse. Int Urogynecol J
Pelvic Floor Dysfunct. 24:1783–1790. 2013. View Article : Google Scholar
|
|
2
|
Olsen AL, Smith VJ, Bergstrom JO, Colling
JC and Clark AL: Epidemiology of surgically managed pelvic organ
prolapse and urinary incontinence. Obstet Gynecol. 89:501–506.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subak LL, Waetjen LE, van den Eeden S,
Thom DH, Vittinghoff E and Brown JS: Cost of pelvic organ prolapse
surgery in the United States. Obstet Gynecol. 98:646–651. 2001.
|
|
4
|
Merrill RM: Hysterectomy surveillance in
the United States 1997 through 2005. Med Sci Monit. 14:CR24–CR31.
2008.
|
|
5
|
De Landsheere L, Munaut C, Nusgens B,
Maillard C, Rubod C, Nisolle M, Cosson M and Foidart JM: Histology
of the vaginal wall in women with pelvic organ prolapse: A
literature review. Int Urogynecol J Pelvic Floor Dysfunct.
24:2011–2020. 2013. View Article : Google Scholar
|
|
6
|
Chen B and Yeh J: Alterations in
connective tissue metabolism in stress incontinence and prolapse. J
Urol. 186:1768–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cole EE, Leu PB, Gomelsky A, Revelo P,
Shappell H, Scarpero HM and Dmochowski RR: Histopathological
evaluation of the uterosacral ligament: Is this a dependable
structure for pelvic reconstruction? BJU Int. 97:345–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabriel B, Denschlag D, Göbel H, Fittkow
C, Werner M, Gitsch G and Watermann D: Uterosacral ligament in
postmenopausal women with or without pelvic organ prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 16:475–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goh JT: Biomechanical and biochemical
assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol.
15:391–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ra HJ and Parks WC: Control of matrix
metalloproteinase catalytic activity. Matrix Biol. 26:587–596.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sampson N, Berger P and Zenzmaier C: Redox
signaling as a therapeutic target to inhibit myofibroblast
activation in degenerative fibrotic disease. BioMed Res Int.
2014:1317372014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosier E, Lin VK and Zimmern P:
Extracellular matrix expression of human prolapsed vaginal wall.
Neurourol Urodyn. 29:582–586. 2010. View Article : Google Scholar
|
|
14
|
Budatha M, Roshanravan S, Zheng Q,
Weislander C, Chapman SL, Davis EC, Starcher B, Word RA and
Yanagisawa H: Extracellular matrix proteases contribute to
progression of pelvic organ prolapse in mice and humans. J Clin
Invest. 121:2048–2059. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klutke J, Ji Q, Campeau J, Starcher B,
Felix JC, Stanczyk FZ and Klutke C: Decreased endopelvic fascia
elastin content in uterine prolapse. Acta Obstet Gynecol Scand.
87:111–115. 2008. View Article : Google Scholar
|
|
16
|
Li BS, Hong L, Min J, Wu DB, Hu M and Guo
WJ: The expression of glutathione peroxidase-1 and the anabolism of
collagen regulation pathway transforming growth
factor-beta1-connective tissue growth factor in women with uterine
prolapse and the clinic significance. Clin Exp Obstet Gynecol.
40:586–590. 2013.PubMed/NCBI
|
|
17
|
Bump RC, Mattiasson A, Bø K, Brubaker LP,
DeLancey JO, Klarskov P, Shull BL and Smith AR: The standardization
of terminology of female pelvic organ prolapse and pelvic floor
dysfunction. Am J Obstet Gynecol. 175:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Yang Q, Fang G, Li BS, Wu DB, Guo
WJ, Hong SS and Hong L: Collagen metabolic disorder induced by
oxidative stress in human uterosacral ligament derived fibroblasts:
A possible pathophysiological mechanism in pelvic organ prolapse.
Mol Med Rep. 13:2999–3008. 2016.PubMed/NCBI
|
|
19
|
Li BS, Guo WJ, Hong L, Liu YD, Liu C, Hong
SS, Wu DB and Min J: Role of mechanical strain-activated PI3K/Akt
signaling pathway in pelvic organ prolapse. Mol Med Rep.
14:243–253. 2016.PubMed/NCBI
|
|
20
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015.PubMed/NCBI
|
|
21
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon YJ, Choi JR, Jeon MJ, Kim SK and Bai
SW: Alteration of elastin metabolism in women with pelvic organ
prolapse. J Urol. 185:1786–1792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han L, Wang L, Wang Q, Li H and Zang H:
Association between pelvic organ prolapse and stress urinary
incontinence with collagen. Exp Ther Med. 7:1337–1341.
2014.PubMed/NCBI
|
|
24
|
Vulic M, Strinic T, Tomic S, Capkun V,
Jakus IA and Ivica S: Difference in expression of collagen type I
and matrix metallo-proteinase-1 in uterosacral ligaments of women
with and without pelvic organ prolapse. Eur J Obstet Gynecol Reprod
Biol. 155:225–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen HY, Lu Y, Qi Y, Bai WP and Liao QP:
Relationship between the expressions of mitofusin-2 and procollagen
in uterosacral ligament fibroblasts of postmenopausal patients with
pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol.
174:141–145. 2014. View Article : Google Scholar
|
|
26
|
Yucel N, Usta A, Guzin K, Kanter M, Bilgic
E, Ozel NO and Ozgul M: Immunohistochemical analysis of connective
tissue in patients with pelvic organ prolapse. J Mol Histol.
44:97–102. 2013. View Article : Google Scholar
|
|
27
|
Strinic T, Vulic M, Tomic S, Capkun V,
Stipic I and Alujevic I: Matrix metalloproteinases-1, -2 expression
in uterosacral ligaments from women with pelvic organ prolapse.
Maturitas. 64:132–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabriel B, Watermann D, Hancke K, Gitsch
G, Werner M, Tempfer C and zur Hausen A: Increased expression of
matrix metalloproteinase 2 in uterosacral ligaments is associated
with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct.
17:478–482. 2006. View Article : Google Scholar
|
|
29
|
Dviri M, Leron E, Dreiher J, Mazor M and
Shaco-Levy R: Increased matrix metalloproteinases-1,-9 in the
uterosacral ligaments and vaginal tissue from women with pelvic
organ prolapse. Eur J Obstet Gynecol Reprod Biol. 156:113–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alarab M, Kufaishi H, Lye S, Drutz H and
Shynlova O: Expression of extracellular matrix-remodeling proteins
is altered in vaginal tissue of premenopausal women with severe
pelvic organ prolapse. Reprod Sci. 21:704–715. 2014. View Article : Google Scholar :
|
|
31
|
Liang CC, Huang HY and Chang SD: Gene
expression and immunoreactivity of elastolytic enzymes in the
uterosacral ligaments from women with uterine prolapse. Reprod Sci.
19:354–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Overall CM, Wrana JL and Sodek J:
Transcriptional and post-transcriptional regulation of 72-kDa
gelatinase/type IV collagenase by transforming growth factor-beta 1
in human fibroblasts. Comparisons with collagenase and tissue
inhibitor of matrix metalloproteinase gene expression. J Biol Chem.
266:14064–14071. 1991.PubMed/NCBI
|