Introduction
Recent studies have suggested that angiogenesis in
the ischemic penumbra area may play a crucial role in neural
protection and tissue recovery. Angiogenesis is an important
structural adaptation to increase vascular perfusion, sprouting,
cell proliferation, migration and maturation (1,2).
Numerous regulatory angiogenic factors have been identified, and
their molecular modulations are associated with several angiogenic
disorders (3,4). Vascular endothelial growth factor
(VEGF) and nitric oxide (NO) from activated endothelial nitric
oxide synthase (eNOS) are key regulators of normal and pathological
angiogenesis (5,6). The survey and development of new
agents, which promote angiogenesis via growth factors, have become
a focus of therapeutic strategies for ischemic diseases (7).
Caveolin-1, a coat protein of caveolae, is involved
in many signaling pathways and functions of endothelial cells
(8,9). The caveolin-1 signaling pathway is
widely known for its role in regulating cellular functions,
including signal transduction, endocytosis, transcytosis, molecular
transport, embryonic vessel development, normal tissue growth, and
wound healing, as well as pathological processes such as ischemia
and tumor growth (10,11). The importance of caveolin-1 has
been confirmed in endothelial cells during angiogenesis. Gao et
al (12) found that
caveolin-1 and VEGF expression induced by treadmill exercise after
middle cerebral artery occlusion was consistent with the good
neurological outcomes of decreased infarct volumes and increased
microvessel densities. In addition, the expression of VEGF could be
significantly inhibited by a caveolin-1 inhibitor. Caveolin-1
overexpression has been reported to enhance endothelial capillary
tubule formation (13). Despite a
number of studies on the involvement of caveolin-1 in
VEGF-stimulated angiogenesis, whether caveolin-1 is a stimulator or
inhibitor of angiogenesis is still controversial. Thus, further
studies are needed to identify the mechanism by which caveolin-1
regulates VEGF. eNOS localized to the inner leaflet of caveolar
membranes and in the Golgi of endothelial cells is believed to be
maintained in an inactive state owing to a direct interaction with
caveolin-1 (14,15). It has been documented that
decreasing the association of caveolin-1 with eNOS results in
activation of eNOS (16,17). However, the mechanism of
caveolin-1-mediated inhibition of eNOS activation is unclear.
Regulation of eNOS by caveolin-1 is an important physiological
mechanism to control vascular reactivity.
Apigenin is a naturally occurring flavonoid that has
a variety of pharmacological activities, mainly including
antitumor, anti-oxidative stress, and anti-DNA damage activities
(18). Furthermore, apigenin has
vasorelaxing and anti-platelet properties that may reduce the risk
of coronary heart disease and improve endothelial functions
(19–21). Even though apigenin has potential
effects on both endothelial cells and neurons, it is unclear
whether there is an effect on the promotion of angiogenesis and the
underlying mechanisms. Nevertheless, recent data have indicated
that apigenin inhibits VEGF expression and tumor angiogenesis
(22,23). However, normal cells and cancer
cells have different characteristics, and the potential effects of
apigenin on the expression of VEGF have not been clarified in a
normal biological system. We previously demonstrated that treatment
of rats with apigenin after stroke enhanced the expression of both
VEGF and caveolin-1 at the brain-infarct border zone, and that
caveolin-1 expression correlates with improved functional recovery
(24,25). However, we have not further
explored the angiogenic effect of apigenin.
These findings suggest that caveolin-1 may also be
involved in apigenin-induced angiogenesis after
hypoxia-reoxygenation (HR) of human umbilical vein endothelial
cells (HUVECs). To test this hypothesis and explore the mechanisms
involved in apigenin-induced angiogenesis, we determined the
effects of apigenin on the caveolin-1-dependent signaling pathway
upon caveolin-1 silencing, as well as the expression of VEGF and
eNOS in vitro. This study may provide the basis for the
novel therapeutic management of stroke by angiogenesis.
Materials and methods
Chemicals, reagents and culture
medium
Apigenin (≥99% pure) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). It was diluted in dimethyl
sulfoxide purchased from Sigma-Aldrich at a final concentration of
40 mM. HUVECs were purchased from the Cell Bank of the Chinese
Academy of Sciences. Dulbecco's modified Eagle's medium (DMEM) was
purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
Fetal bovine serum (FBS) was purchased from Biosun Biotech
(Shanghai, China). Phosphate-buffered saline was purchased from
Yuanpei (Shanghai, China). Trypsin was purchased from Boguang
Biotech (Shanghai, China). Trizol was purchased from Takara Bio
(Dalian, China). Glutamine was purchased from Amresco (Solon, OH,
USA). Reverse transcriptase was purchased from Fermentas China
(Shanghai, China). Anti-caveolin-1 (ab2910) and -eNOS (ab199956)
antibodies were obtained from Abcam (Cambridge, MA, USA). An
anti-VEGF antibody (sc-1876) was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). SYBR-Green Mix was
purchased from Dongsheng Biotech (Guangzhou, China). Transwells
were purchased from Corning, Inc. (Corning, NY, USA). Crystal
violet was purchased from Genmed Scientifics Inc. (Shanghai,
China).
Cell culture and HR injury
HUVECs were cultured in DMEM containing 10% FBS, 1%
penicillin/streptomycin and 1% glutamine. For exposure to hypoxia,
the HUVECs were incubated in 1% O2 plus 5%
CO2 balanced with N2 for 0, 2 and 6 h.
Following exposur to hypoxia, the cells were returned to a standard
incubator with the normoxic condition of humidified air with 5%
CO2 for 24 and 48 h. Zero hours was defined as the onset
after cells were exposed to hypoxia.
Cell viability assay
Cell viability was measured by the Cell Counting
Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) as
described previously (26).
Briefly, HUVECs were subcultured in a 96-well plate at
5×103 cells/well in DMEM. Then, the cells were treated
with increasing concentrations of apigenin (10, 20, 50 and 80
µM). After hypoxia for 6 h and oxygenation for 24 h, 10
µl CCK-8 solution was added to each well. After incubation
for 2 h at 37°C in the dark, the absorbance was measured at 450 nm
using a Multiskan MK3 microplate reader (Thermo Labsystems, Paris,
France). Cells cultured in serum-free DMEM were used as a
background control. The absorbance for each apigenin concentration
alone in serum-free DMEM and CCK-8 solution was also measured at
450 nm. The appropriate concentration was chosen for the following
experiment. The cell viabilities of four groups (NC, NC + apigenin,
caveolin-1-KD and caveolin-1-KD + apigenin) were tested at 1, 2, 3,
4, 5 and 6 days after treatment with apigenin, caveolin-1 short
interfering RNA (siRNA), or negative control siRNA. The NC group
was the negative control group. The NC + apigenin group was the
negative control treated with apigenin. The caveolin-1-KD group was
subjected to caveolin-1 silencing. The caveolin-1-KD + apigenin
group was subjected to caveolin-1 silencing and treatment with
apigenin. Cell viability was calculated using the following
formula: cell viability (%) = (ODsample −
ODbackground)/(ODcontrol −
ODbackground) ×100%, where ODcontrol and
ODsample are the optical density (OD) of the negative
control and apigenin-treated groups, respectively. The absorbance
was directly correlated with the number of metabolically active
HUVECs. All conditions were measured in at least three independent
experiments. Data analysis was performed in a blinded manner.
Caveolin-1 siRNA generation and
transduction
Caveolin-1 was silenced in the HUVECs by lentiviral
siRNA transfection. The lentiviral vectors for caveolin-1 and
control siRNAs were obtained from R&S Biotechnology Co., Ltd.
(Shanghai China). Briefly, caveolin-1 was amplified and subcloned
into the pcDNA6.2 vector with the enhanced green fluorescent
protein (EGFP) gene, and then EGFP-caveolin-1 was cloned into the
pLenti6.3-MCS/V5 DEST vector. The resulting plasmid was analyzed
and verified by gel electrophoresis and sequencing. To obtain
lentiviruses, 293T cells were co-transfected with caveolin-1 or
control siRNA lentivirus expression plasmids and a packaging
plasmid mix (Invitrogen; Thermo Fisher Scientific Inc.) using
POLOdeliverer™ 3000 transfection reagent (Ruisai Inc., Shanghai,
China) according to the instructions provided by the manufacturer.
Following lentivirus infection for 48 h, HUVECs transduced with
fluorescently tagged proteins were selected and verified by
fluorescence microscopy. The cells were applied to the following
experiments.
Transwell migration assay
The bottom chambers of Transwells were filled with
DMEM and the top chambers were seeded with HUVECs transfected with
siRNA for 72 h and then treated with 10 µM apigenin, hypoxia
for 6 h, and reoxygenation 24 h. Cells on the top surface of the
membrane (non-migrated cells) were removed with a cotton swab, and
cells that had migrated onto the bottom sides of the membrane were
fixed in 4% paraformaldehyde and stained with 2% crystal violet.
The absorbance was measured at 570 nm using a Multiskan MK3
microplate reader. The OD value indicated the number of migrated
cells. Each experiment was performed in triplicate. All data
analysis was performed in a blinded manner.
Tube formation assay
An endothelial cell tube formation assay is a useful
indicator of angiogenesis potential. In the present study, the tube
formation assay was performed using the branch point method
(27). In brief, HUVECs
(5×104/well) were seeded onto a 96-well plate pre-coated
with Matrigel (50 µl) and cultured at 37°C in a 5%
CO2 incubator. The formed network of tubes was
visualized at ×100 magnification by light microscopy. The number of
branch points was counted to calculate the branch point density.
Each experiment was performed in triplicate. All data analysis was
performed in a blinded manner.
Quantitative polymerase chain reaction
(qPCR) analysis of caveolin-1
Gene expression was measured by qPCR in endothelial
cells harvested after treatments. qPCR was performed to investigate
the expression of caveolin-1. Total RNA was isolated and purified
using TRIzol® Plus RNA purification kit (Takara Bio),
according to the manufacturer's instructions. RNA was reverse
transcribed into cDNA that was utilized as a template in the
subsequent qPCR amplifications using a SYBR-Green Mix kit
(Dongsheng Biotech). The qPCR primers included: caveolin-1
(forward, CGCAGGGACATCTCTACACC and reverse, CTTCCAAATGCCGTCAAAAC,
233 bp). Each experiment was repeated at least three times.
Western blot analysis of caveolin-1, VEGF
and eNOS levels
Cells subjected to various treatments were lysed in
lysis buffer. Equal amounts of total proteins were separated on
sodium dodecyl sulfate polyacrylamide gels (caveolin-1, 12%; VEGF,
10%; eNOS, 8%) and then electrophoretically transferred to a
polyvinylidene difluoride membrane. The membrane was blocked with
5% dry skim milk for 1 h, incubated with anti-caveolin-1 (1:800),
anti-VEGF (1:200), and anti-eNOS (1:500) antibodies at 4°C
overnight, and then incubated with HRP-labeled goat anti-rabbit
antibody (1:3,000; A0208, Beyotime Biotech, Jiangsu, China) for 1
h. Labeled proteins were detected using an enhanced
chemiluminescence detection kit. The relative quantity of proteins
was analyzed using Quantity One software and normalized to the
loading control. Each experiment was repeated at least three
times.
Statistical analyses
Data are presented as the mean ± standard error of
the mean (SEM). The results were analyzed by SPSS 20.0 software
(SPSS Inc. Chicago, IL, USA). Mean values were derived from at
least three independent experiments. Statistical comparisons
between experimental groups were performed by one-way analysis of
variance. The level of statistical significance was set at
P<0.05.
Results
Determination of optimal durations for
HR
To determine appropriate durations for HR, HUVECs
were subjected to HR for various times, and then expression of
caveolin-1, VEGF and eNOS was assessed by qPCR and western
blotting. The results showed that the mRNA expression levels of
caveolin-1, VEGF, and eNOS at 6 h of hypoxia were lower than those
at 0 h. After 48 h of reoxygenation, the mRNA expression of
caveolin-1, VEGF, and eNOS was recovered for any duration of
hypoxia (Fig. 1A). The protein
expression of caveolin-1 declined slightly, but the expression of
VEGF and eNOS increased during hypoxia for 6 h and reoxygenation
for 24 h, indicating that the protein expression was not consistent
with the mRNA expression. After 48 h of reoxygenation for any
duration of hypoxia, expression was restored for all three proteins
(Fig. 1B). Cell viability was
markedly decreased and cell necrosis was increased by hypoxia for 6
h and reoxygenation for 24 h (Fig.
1C). Considering these results, we induced hypoxia for 6 h and
reoxygenation for 24 h in further experiments.
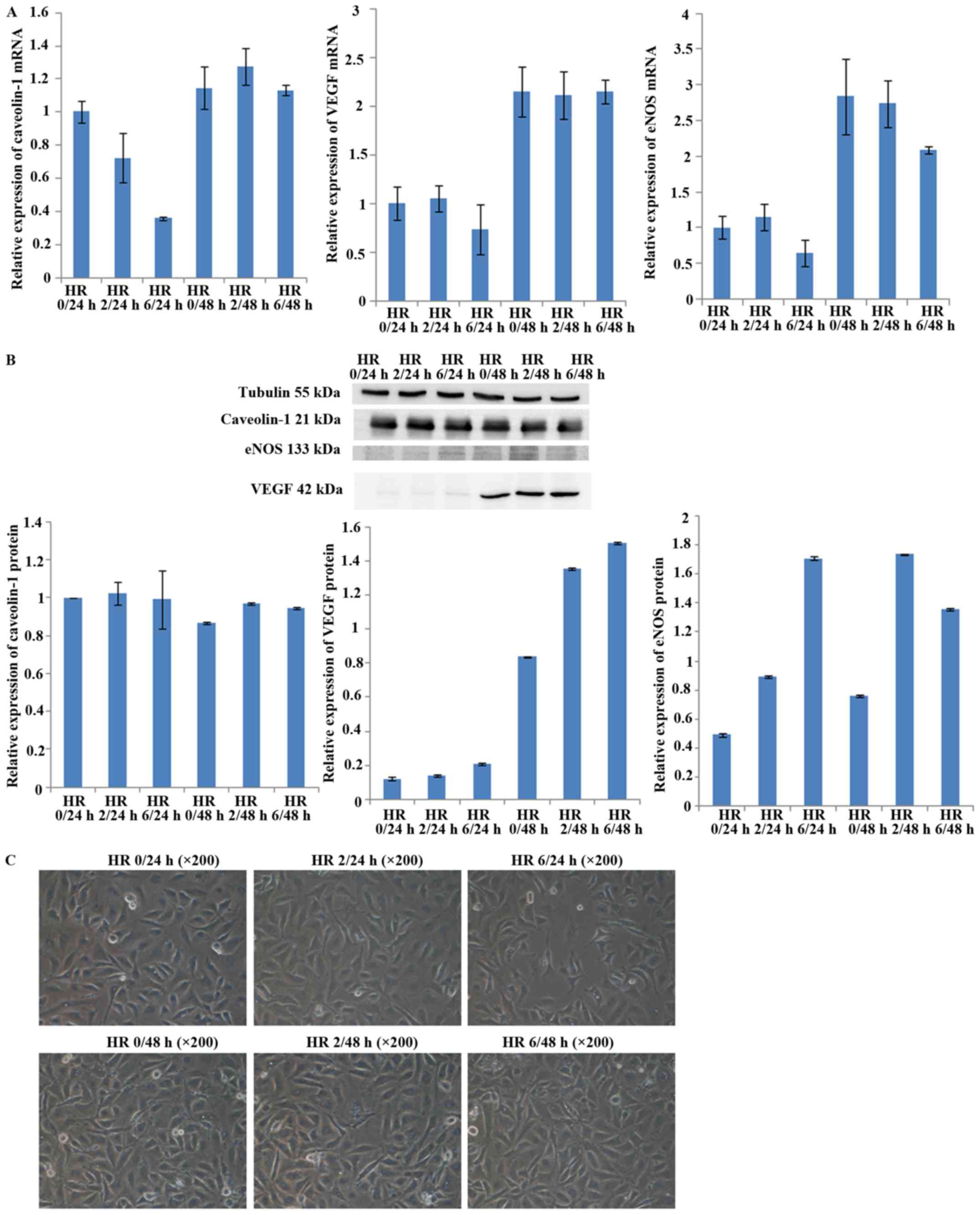 | Figure 1Expression of caveolin-1, vascular
endothelial growth factor (VEGF), and endothelial nitric oxide
synthase (eNOS) in human umbilical vein endothelial cells (HUVECs)
after various durations of hypoxia-reoxygenation (HR). Cells were
incubated under hypoxic conditions (1% O2) for 0, 2 or 6
h and then reoxygenated for 24 or 48 h. (A) RT-PCR analysis of
caveolin-1, VEGF and eNOS in HUVECs. mRNA expression of caveolin-1,
VEGF and eNOS was decreased after hypoxia for 6 h and reoxygenation
for 24 h. The mRNA expression of caveolin-1, VEGF and eNOS was
restored after reoxygenation for 48 h compared with reoxygenation
for 24 h. (B) Western blot analysis of caveolin-1, VEGF and eNOS in
HUVECs. The protein expression of caveolin-1 was decreased
slightly, while VEGF and eNOS protein expression was increased
after hypoxia for 6 h and reoxygenation for 24 h. However, compared
with reoxygenation for 24 h, VEGF and eNOS protein expression was
recovered after reoxygenation for 48 h. (C) Cell viability at
various durations of HR. The example shown is representative of six
independent experiments. The cell viability was markedly decreased
and cell necrosis was increased by hypoxia for 6 h and
reoxygenation for 24 h. |
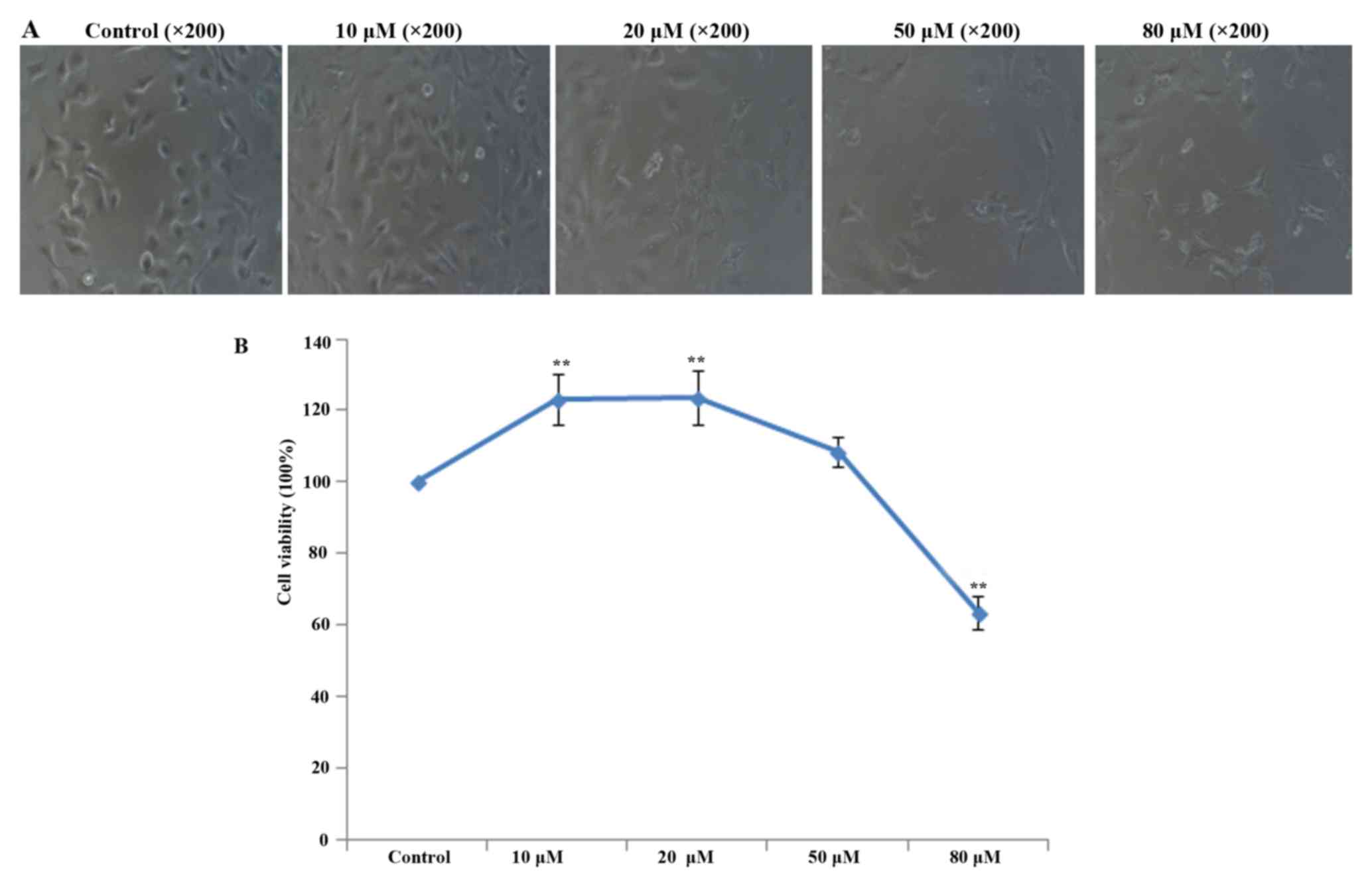
Determination of the appropriate
concentration of apigenin
To ascertain the appropriate non-toxic concentration
of apigenin for the treatment of HUVECs, the effect of apigenin on
HUVEC viability was examined by CCK-8 assays. The results showed
that 10 and 20 µM apigenin had protective effects on HUVECs
after HR, while apigenin reduced cell viability at concentrations
≥50 (Fig. 2). The viability of
HUVECs treated with 10 µM apigenin was higher than that of
HUVECs treated with 20 µM apigenin. Therefore, we chose 10
µM as the appropriate concentration of apigenin for the
following experiments.
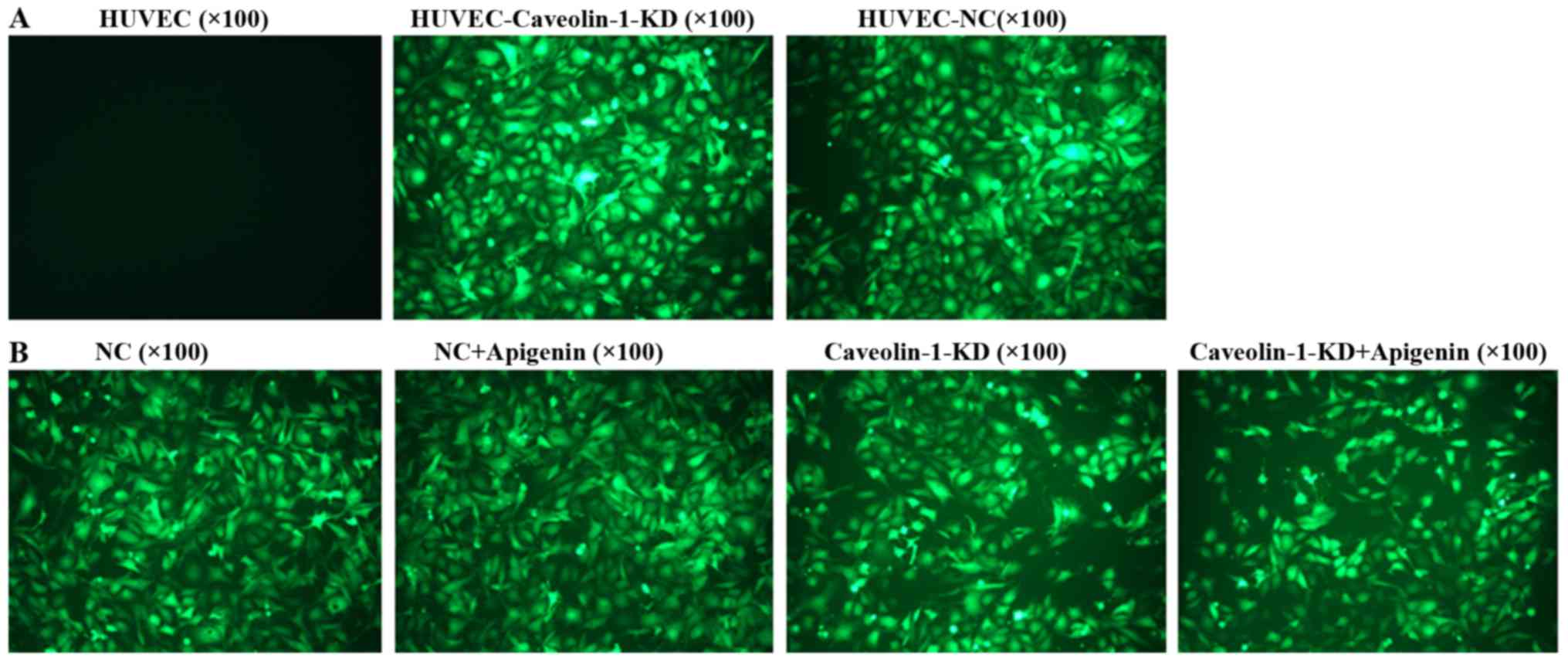
Transfection of caveolin-1 siRNA into
HUVECs
Cells were transfected with caveolin-1 or control
siRNAs for 72 h. EGFP-positive cells were observed by fluorescence
microscopy. The positive cells (Fig.
3) showed that the HUVECs were successfully transfected with
the siRNA vectors.
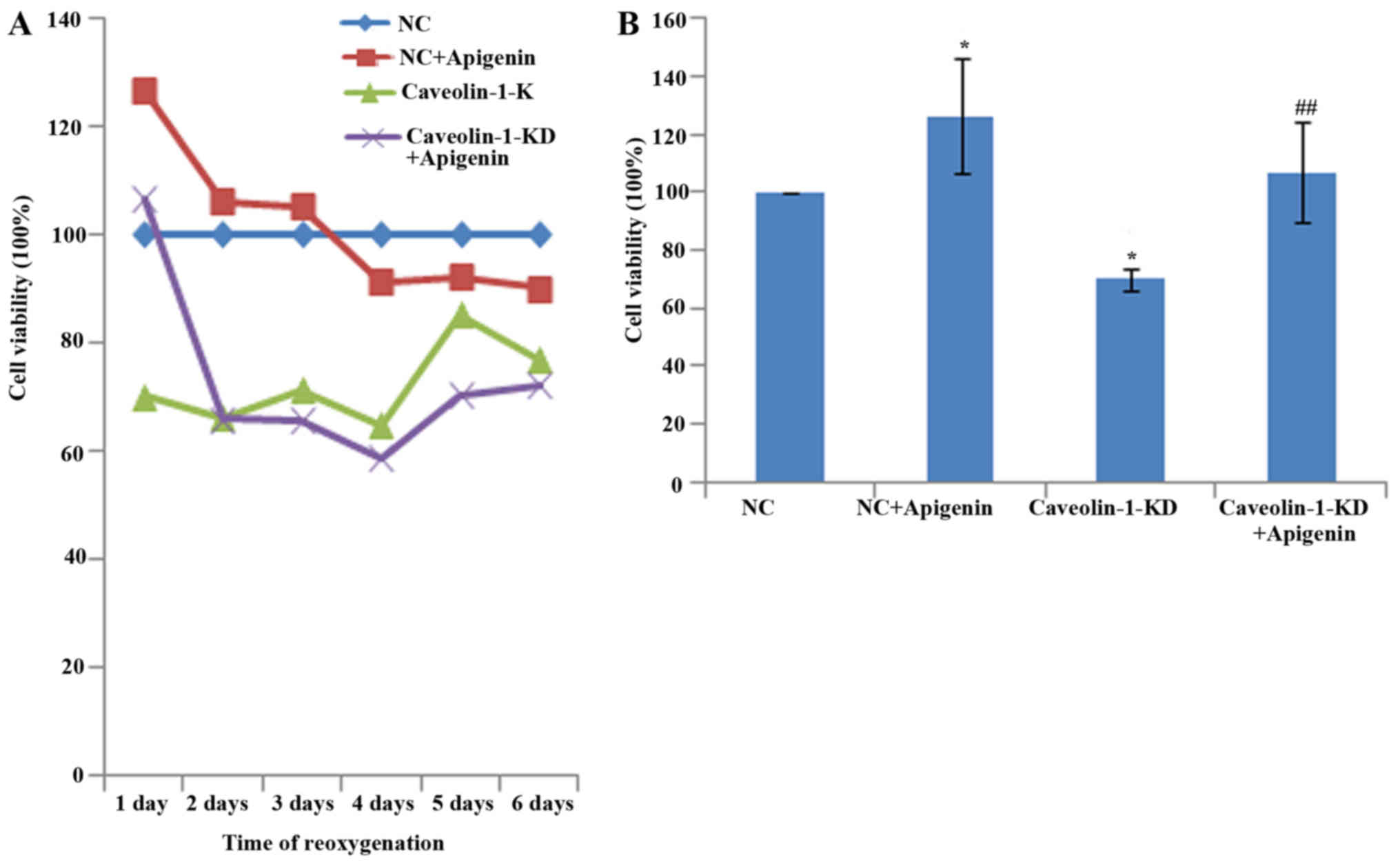
Effects of apigenin on caveolin-1-induced
angiogenesis of HUVECs after HR
Cell viability, migration and tube formation were
analyzed as indicators of angiogenesis. To investigate the effects
of apigenin on HUVEC viability, we performed CCK-8 assays. We found
that cell viability was markedly decreased after HR. However,
apigenin did not promote the viability of HUVECs reoxygenated for
more than 3 days (Fig. 4A). HUVEC
viability was increased by treatment with apigenin (Fig. 4B). Caveolin-1 silencing inhibited
the viability of HUVECs, and apigenin recovered their viability
(Fig. 4B).
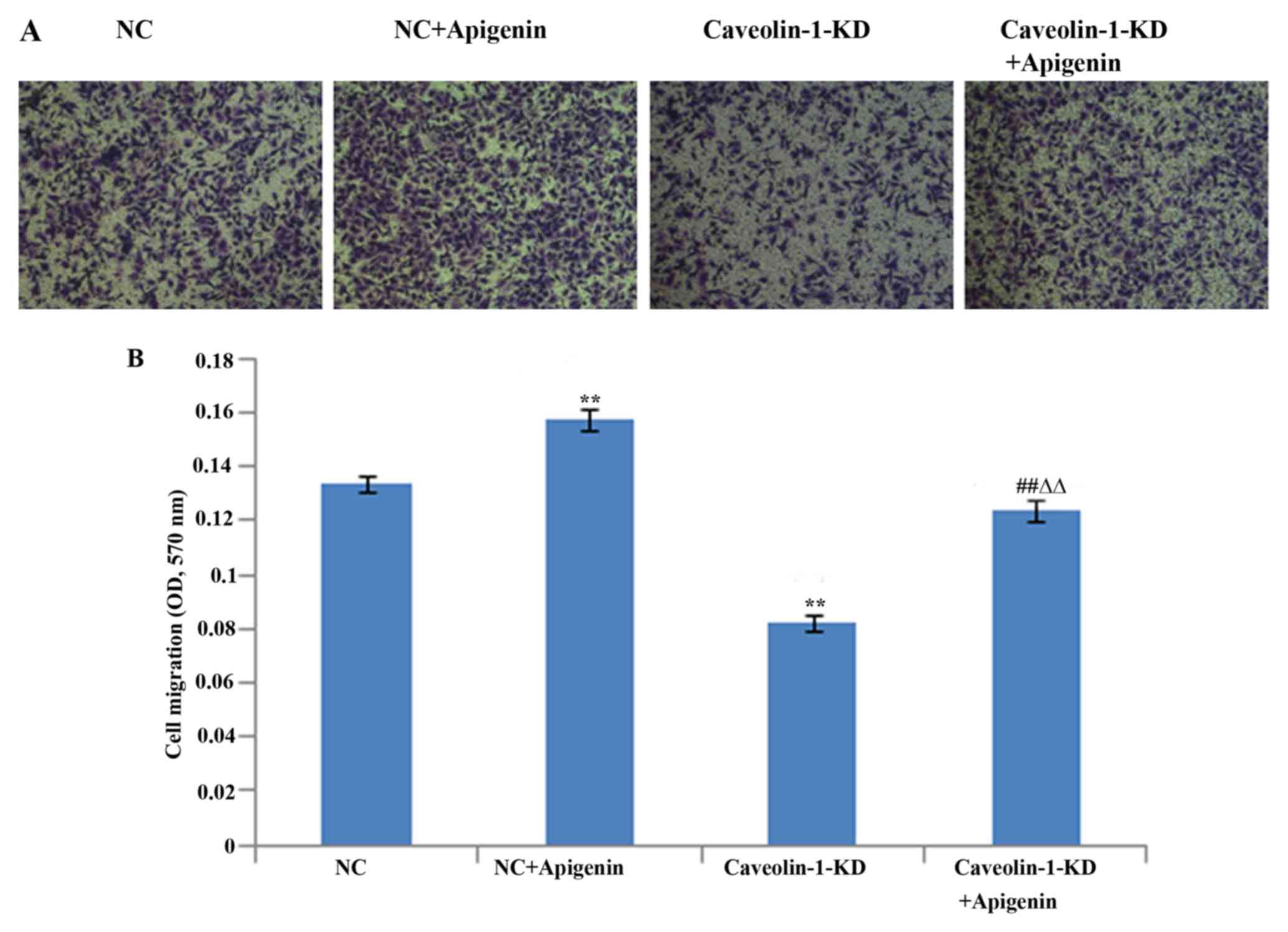
Migration of endothelial cells plays a key role in
angiogenesis. Therefore, we determined the effects of apigenin on
HUVEC migration after HR (Fig.
5). We found that apigenin promoted the migration of HUVECs.
Caveolin-1 siRNA suppressed the HUVEC migration. However, the
inhibition of HUVEC migration was reversed following treatment with
apigenin.
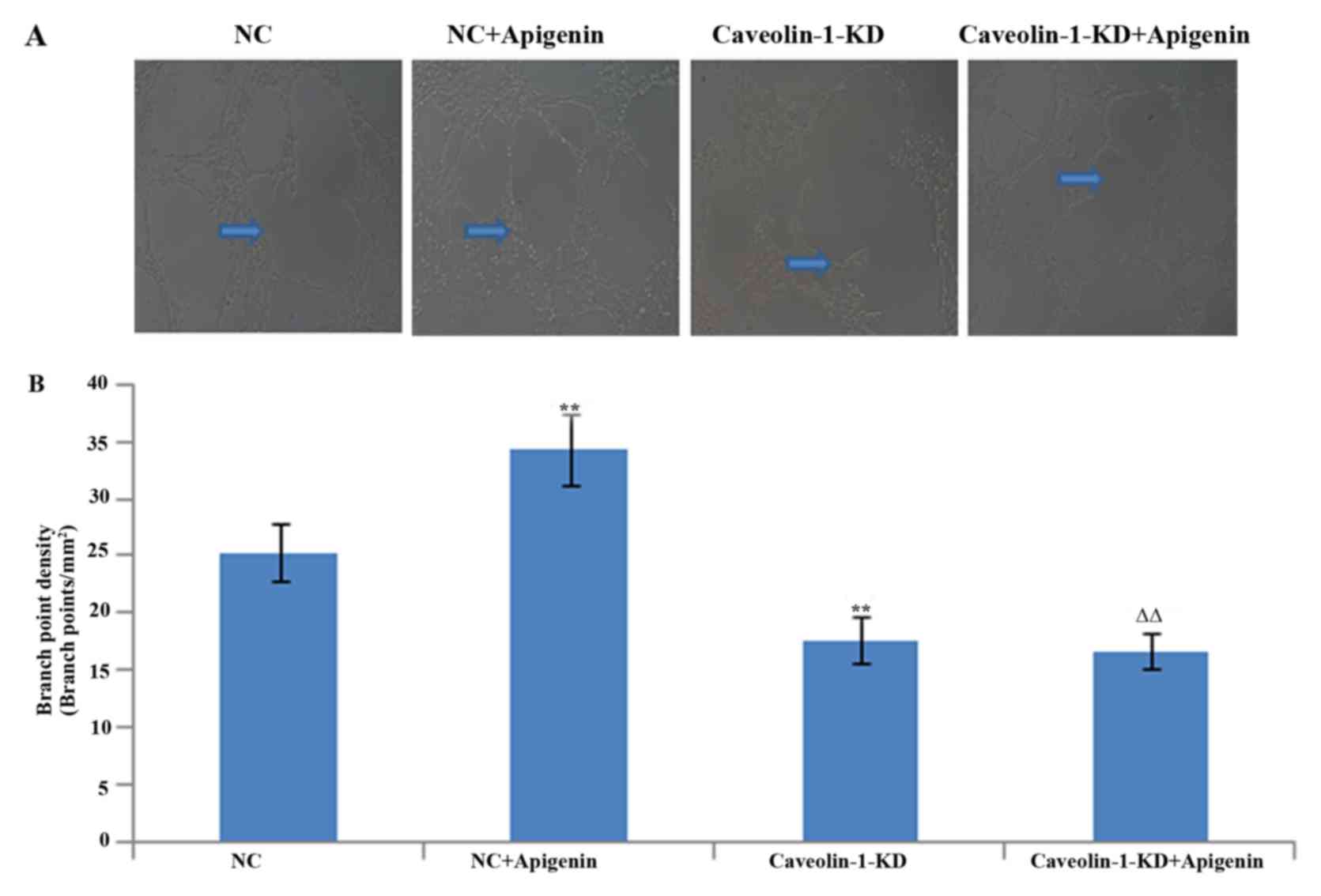
To evaluate the role of apigenin in regulating
angiogenesis, we next assessed the effects of apigenin on tube
formation of HUVECs. As expected, apigenin promoted tube formation
after HR. To verify the involvement of caveolin-1 in the process of
tube formation, we used RNA interference to selectively silence
caveolin-1. The tube formation was decreased after caveolin-1
silencing. Apigenin had no effect on tube formation inhibited by
caveolin-1 silencing (Fig.
6).
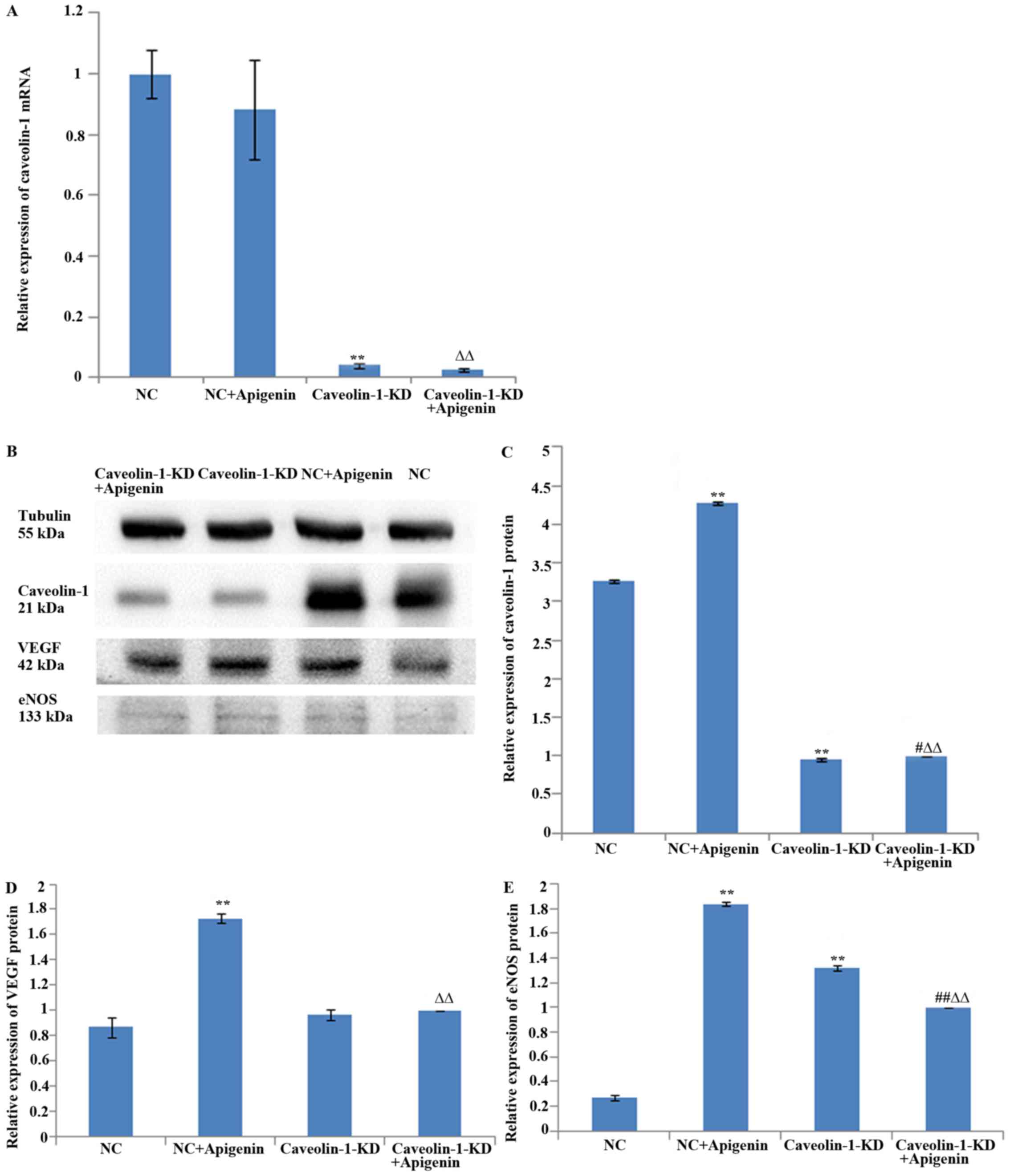
Effects of apigenin on caveolin-1 levels
in HUVECs
Caveolin-1 expression levels were evaluated by
western blotting and qPCR. Apigenin did not affect caveolin-1 mRNA
expression (Fig. 7A), but it
increased caveolin-1 protein expression in HUVECs after HR
(Fig. 7B and C). To verify the
effects of apigenin on caveolin-1 expression, we transfected the
HUVECs with caveolin-1 siRNA. The results showed that caveolin-1
silencing inhibited the mRNA (Fig.
7A) and protein expression of caveolin-1 (Fig. 7B and C). Apigenin increased
caveolin-1 protein expression after the transfection of HUVECs with
caveolin-1 siRNA (Fig. 7B and C),
but it did not upregulate the expression of caveolin-1 mRNA
(Fig. 7A). These results
indicated that apigenin may regulate translation and/or
post-translational processing of caveolin-1 protein but not the
transcription of caveolin-1 mRNA.
Effects of apigenin on VEGF levels in
HUVECs
To examine the effect of apigenin on angiogenesis,
we assessed the well-established pro-angiogenic mediator VEGF by
western blot analysis. We found that apigenin promoted VEGF
expression after HR. The results revealed that transfection with
caveolin-1 siRNA did not significantly affect VEGF protein
expression; apigenin increased VEGF expression, but it did not
upregulate VEGF expression after caveolin-1 silencing (Fig. 7B and D).
Effects of apigenin on eNOS levels in
HUVECs
Since eNOS also plays a critical role in
angiogenesis and vascular permeability, we next examined the role
of eNOS in apigenin-induced angiogenesis. Incubation of HUVECs with
apigenin rapidly increased the protein expression of eNOS. Compared
with the NC group, blocking the caveolin-1 pathway with caveolin-1
siRNA significantly increased the protein level of eNOS, whereas
compared with the caveolin-1-KD group, apigenin downregulated the
level of eNOS (Fig. 7B and
E).
Discussion
Angiogenesis is involved in physiological processes,
such as development, and pathological states such as stroke,
cancer, and inflammatory diseases. Recent studies suggest that
angiogenesis in the ischemic penumbra area may play a crucial role
in neural protection and tissue recovery. Apigenin is orally
bioavailable and non-toxic (28)
and has potential effects on both endothelial and nerve cells. We
previously showed that apigenin increases the expression of both
VEGF and caveolin-1 at the brain-infarct border zone after stroke
in rats, and that caveolin-1 expression correlates with improved
functional recovery. However, we did not investigate the angiogenic
effects of apigenin after stroke. Therefore, in the present study,
we first investigated the angiogenic effects of apigenin on HUVECs
after HR injury and the underlying mechanism. Caveolin-1 has
attracted attention due to its contribution to angiogenesis.
Caveolin-1 plays an important role in the mechanisms of cellular
repair in many pathological conditions including stroke (10,29). Previous studies, including our
own, have demonstrated that ischemia/reperfusion increases
caveolin-1 protein expression in association with increased
microvessel density and better outcomes (24,25,30). In the present study, we explored
the involvement of caveolin-1 in the angiogenic effects of
apigenin.
For this purpose, we examined the effects of
apigenin on the viability, migration and tube formation of HUVECs
after HR. In vitro angiogenesis can be evaluated by
migration and tube formation assays. As expected, our results
showed that apigenin promoted cell viability, migration, and tube
formation. However, this stimulatory effect was significantly
inhibited by caveolin-1 siRNA, suggesting that caveolin-1 induction
by apigenin is important to control angiogenesis. Apigenin could
not recover tube formation, but increased the migration and
proliferation of HUVECs, which were inhibited by caveolin-1
siRNA.
The underlying mechanisms of the angiogenic effect
of apigenin remain unclear. In the present study, we demonstrated
for the first time that apigenin promotes angiogenesis via
caveolin-1 in HUVECs after HR. As shown previously, caveolin-1 is
downregulated in the core and penumbra in ischemic brains (31,32). Our results showed that HR
decreased caveolin-1 expression in HUVECs. We also found that
apigenin treatment restored high caveolin-1 protein levels.
Although the protein level was increased in cells treated with
apigenin and exposed to HR, transcription of caveolin-1 was
unaltered, suggesting that apigenin may affect post-translational
processes of caveolin-1 expression.
We also investigated the involvement of apigenin in
promotion of angiogenesis through VEGF and eNOS pathways. VEGF and
eNOS-derived NO are important regulators of angio-genesis. However,
the underlying mechanism regarding the modulation of caveolin-1 in
the secretion of VEGF and eNOS remain ambiguous. VEGF is an
angiogenesis accelerator, and caveolin-1 may play an important role
in angiogenesis induced by VEGF (33). It is well established that VEGF is
induced when tissue is subjected to hypoxic and ischemic attack.
VEGF stimulates the proliferation and migration of vascular
endothelial cells and increases the permeability of vessels as well
as the differentiation of endothelial cells into capillary tubes
(34,35). In this study, VEGF expression was
increased in HUVECs after HR, and apigenin upregulated VEGF
expression, indicating that apigenin may directly activate
angiogenic signaling pathways. Intriguingly, after treatment of
endothelial cells with caveolin-1 siRNA, the expression of VEGF
protein did not significantly change. This result may be attributed
to the association of VEGF with other molecules in the cells. It
has been demonstrated that VEGF enhances the expression of eNOS in
native and cultured endothelial cells, an effect that may be
important in the process of VEGF-induced angiogenesis. VEGF-induced
vascular permeability and angiogenesis were found to be markedly
reduced in eNOS-deficient mice (36). In this study, the increased
expression of eNOS may have upregulated VEGF expression. Therefore,
further studies are needed to confirm the involvement of the
eNOS-VEGF pathway in the angiogenic effect of apigenin. However,
apigenin-increased VEGF protein expression was inhibited by
caveolin-1 silencing. Therefore, the increase in angiogenesis by
apigenin may be partly mediated via caveolin-1 regulating VEGF
expression.
Several lines of evidence have shown upregulation of
eNOS activation by hypoxia (37).
This study produced the same results. The only direct
protein-protein interaction demonstrated in vivo between
caveolin-1 and a non-homologous protein is with eNOS. Studies have
demonstrated that caveolin-1 functions as an endogenous negative
regulator of eNOS activity (38–41). Binding of eNOS to caveolin-1 often
leads to its inactivation (42).
Our results demonstrated that apigenin promoted eNOS protein
expression in HUVECs after HR, suggesting the involvement of eNOS
in angiogenesis after HR injury. Furthermore, our data showed an
increase in the protein expression of eNOS after caveolin-1
silencing, which is supported by a previous study (43). They showed that caveolin-1
deficiency induced the activation of eNOS and the generation of NO.
It is important that eNOS is downregulated when cells are treated
with apigenin. We believe that upon treatment with apigenin after
caveolin-1 silencing, caveolin-1 was upregulated by apigenin, which
bound to eNOS and inhibited its activity. Activation of eNOS
promoted caveolin-1 phosphorylation and eNOS/caveolin-1 binding,
creating an inhibitive feedback loop for eNOS (44). Nevertheless, our data suggest that
caveolin-1 modulated angiogenesis of HUVECs after HR in part
through its negative regulation of eNOS activity. Therefore,
apigenin may promote angiogenesis through the caveolin-1/eNOS
pathway.
Although apigenin treatment enhanced angiogenesis of
HUVECs via caveolin-1 after HR, other studies showed that apigenin
is a natural chemopreventive agent with clear anti-angiogenic
effects in tumors (45,46). Tong et al (47) showed that 50 µM apigenin
affected ultraviolet B-induced cutaneous proliferation and
angiogenesis. In the present study, we examined the viability of
HUVECs treated with various concentrations of apigenin after HR.
The results showed that ≥50 µM apigenin had a cytotoxic
effect, but had protective effects on cells at lower
concentrations. Therefore, we speculate that the effects were
dose-dependent in these assays. Furthermore, the effects of
apigenin may be different in various tissues and cell types.
Taken together, our findings suggest that apigenin
may be a novel agent to promote angiogenesis after HR injury.
However, there are several important caveats to consider. Although
our data provide cellular and pharmacological proof of principle
for apigenin in endothelial cell angiogenesis, in vitro
endothelial cell cultures may not recapitulate similar changes
occurring in primary cultures or stroke models in vivo.
Therefore, the pro-angiogenic utility of apigenin as a potential
stroke recovery therapy should be explored in future studies.
Acknowledgments
This study was supported by the Medical Research
Project of Zhejiang Province (2016KYB201), and the Wenzhou
Municipal Science and Technology Bureau Project Funding
(Y20150017), and the Key Construction Disciplines (Children's
Rehabilitation) at the Second Affiliated Hospital of Wenzhou
Medical University.
Glossary
Abbreviations
Abbreviations:
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
HR
|
hypoxia-reoxygenation
|
|
VEGF
|
vascular endothelial growth factor
|
|
NO
|
nitric oxide
|
|
eNOS
|
endothelial nitric oxide synthase
|
References
|
1
|
Semenza GL: Vasculogenesis, angiogenesis,
and arteriogenesis: Mechanisms of blood vessel formation and
remodeling. J Cell Biochem. 102:840–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kofler S, Nickel T and Weis M: Role of
cytokines in cardiovascular diseases: A focus on endothelial
responses to inflammation. Clin Sci (Lond). 108:205–213. 2005.
View Article : Google Scholar
|
|
4
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–C970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shergill U, Das A, Langer D, Adluri R,
Maulik N and Shah VH: Inhibition of VEGF- and NO-dependent
angiogenesis does not impair liver regeneration. Am J Physiol Regul
Integr Comp Physiol. 298:R1279–R1287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida D, Akahoshi T, Kawanaka H,
Yamaguchi S, Kinjo N, Taketomi A, Tomikawa M, Shirabe K, Maehara Y
and Hashizume M: Roles of vascular endothelial growth factor and
endothelial nitric oxide synthase during revascularization and
regeneration after partial hepatectomy in a rat model. Surg Today.
41:1622–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan TP, Yeh JC, Leung KW, Yue PY and Wong
RN: Angiogenesis: From plants to blood vessels. Trends Pharmacol
Sci. 27:297–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahman A and Swärd K: The role of
caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf).
195:231–245. 2009. View Article : Google Scholar
|
|
9
|
Sowa G: Caveolae, caveolins, cavins, and
endothelial cell function: New insights. Front Physiol. 2:1202012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mundy DI, Machleidt T, Ying YS, Anderson
RG and Bloom GS: Dual control of caveolar membrane traffic by
microtubules and the actin cytoskeleton. J Cell Sci. 115:4327–4339.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Navarro A, Anand-Apte B and Parat MO: A
role for caveolae in cell migration. FASEB J. 18:1801–1811. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Zhao Y, Pan J, Yang L, Huang T,
Feng X, Li C, Liang S, Zhou D, Liu C, et al: Treadmill exercise
promotes angiogenesis in the ischemic penumbra of rat brains
through caveolin-1/VEGF signaling pathways. Brain Res. 1585:83–90.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Wang XB, Park DS and Lisanti MP:
Caveolin-1 expression enhances endothelial capillary tubule
formation. J Biol Chem. 277:10661–10668. 2002. View Article : Google Scholar
|
|
14
|
Frank PG, Woodman SE, Park DS and Lisanti
MP: Caveolin, caveolae, and endothelial cell function. Arterioscler
Thromb Vasc Biol. 23:1161–1168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gratton JP, Bernatchez P and Sessa WC:
Caveolae and caveolins in the cardiovascular system. Circ Res.
94:1408–1417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fleming I and Busse R: Molecular
mechanisms involved in the regulation of the endothelial nitric
oxide synthase. Am J Physiol Regul Integr Comp Physiol. 284:R1–R12.
2003. View Article : Google Scholar
|
|
17
|
Ju H, Zou R, Venema VJ and Venema RC:
Direct interaction of endothelial nitric-oxide synthase and
caveolin-1 inhibits synthase activity. J Biol Chem.
272:18522–18525. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang QQ, Cheng N, Yi WB, Peng SM and Zou
XQ: Synthesis, nitric oxide release, and α-glucosidase inhibition
of nitric oxide donating apigenin and chrysin derivatives. Bioorg
Med Chem. 22:1515–1521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woodman OL and Chan EC: Vascular and
anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol
Physiol. 31:786–790. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olszanecki R, Gebska A, Kozlovski VI and
Gryglewski RJ: Flavonoids and nitric oxide synthase. J Physiol
Pharmacol. 53:571–584. 2002.
|
|
21
|
Guerrero JA, Lozano ML, Castillo J,
Benavente-García O, Vicente V and Rivera J: Flavonoids inhibit
platelet function through binding to the thromboxane A2 receptor. J
Thromb Haemost. 3:369–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu LZ, Fang J, Zhou Q, Hu X, Shi X and
Jiang BH: Apigenin inhibits expression of vascular endothelial
growth factor and angiogenesis in human lung cancer cells:
Implication of chemo-prevention of lung cancer. Mol Pharmacol.
68:635–643. 2005.PubMed/NCBI
|
|
23
|
Ansó E, Zuazo A, Irigoyen M, Urdaci MC,
Rouzaut A and Martínez-Irujo JJ: Flavonoids inhibit hypoxia-induced
vascular endothelial growth factor expression by a HIF-1
independent mechanism. Biochem Pharmacol. 79:1600–1609. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XM, Niu WZ and Chen X: Effect of
apigenin on expression of VEGF in cerebral ischemia and reperfusion
rats. Chin J Pathophysiol. 26:2473–2477. 2010.
|
|
25
|
Niu WZ, Li XM, Wang G, Han X and Chen X:
Effects of apigenin on caveolin -1 expression of focal cerebral
ischemia-reperfusion in rats. Chin Tradit Herbal Drugs.
41:1658–1662. 2010.
|
|
26
|
Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y,
Li D and Cai S: Overexpression of forkhead Box C2 promotes tumor
metastasis and indicates poor prognosis in colon cancer via
regulating epithelial-mesenchymal transition. Am J Cancer Res.
5:2022–2034. 2015.PubMed/NCBI
|
|
27
|
Taylor AC, Seltz LM, Yates PA and Peirce
SM: Chronic whole-body hypoxia induces intussusceptive angiogenesis
and microvascular remodeling in the mouse retina. Microvasc Res.
79:93–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopez-Jornet P, Camacho-Alonso F,
Gómez-Garcia F, Molina Miñano F, Cañas X, Serafín A, Castillo J and
Vicente-Ortega V: Effects of potassium apigenin and verbena extract
on the wound healing process of SKH-1 mouse skin. Int Wound J.
11:489–495. 2014. View Article : Google Scholar
|
|
29
|
Liu P, Rudick M and Anderson RG: Multiple
functions of caveolin-1. J Biol Chem. 277:41295–41298. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chidlow JH Jr and Sessa WC: Caveolae,
caveolins, and cavins: Complex control of cellular signalling and
inflammation. Cardiovasc Res. 86:219–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen J, Ma S, Chan P, Lee W, Fung PC,
Cheung RT, Tong Y and Liu KJ: Nitric oxide down-regulates
caveolin-1 expression in rat brains during focal cerebral ischemia
and reperfusion injury. J Neurochem. 96:1078–1089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Luo J, Lau WM, Zheng G, Fu S, Wang
TT, Zeng HP, So KF, Chung SK, Tong Y, et al: Caveolin-1 plays a
crucial role in inhibiting neuronal differentiation of neural
stem/progenitor cells via VEGF signaling-dependent pathway. PLoS
One. 6:e229012011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tahir SA, Park S and Thompson TC:
Caveolin-1 regulates VEGF-stimulated angiogenic activities in
prostate cancer and endothelial cells. Cancer Biol Ther.
8:2286–2296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roy S and Sen CK: miRNA in wound
inflammation and angiogenesis. Microcirculation. 19:224–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng W, Yu Y, Li T, Zhu Y and Chen H: The
effects of small interfering RNA-targeting tissue factor on an in
vitro model of neovascularization. Mol Vis. 19:1296–1303.
2013.PubMed/NCBI
|
|
36
|
Fukumura D, Gohongi T, Kadambi A, Izumi Y,
Ang J, Yun CO, Buerk DG, Huang PL and Jain RK: Predominant role of
endothelial nitric oxide synthase in vascular endothelial growth
factor-induced angiogenesis and vascular permeability. Proc Natl
Acad Sci USA. 98:2604–2609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qing M, Görlach A, Schumacher K, Wöltje M,
Vazquez-Jimenez JF, Hess J and Seghaye MC: The hypoxia-inducible
factor HIF-1 promotes intramyocardial expression of VEGF in infants
with congenital cardiac defects. Basic Res Cardiol. 102:224–232.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Drab M, Verkade P, Elger M, Kasper M, Lohn
M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al:
Loss of caveolae, vascular dysfunction, and pulmonary defects in
caveolin-1 gene-disrupted mice. Science. 293:2449–2452. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Razani B, Engelman JA, Wang XB, Schubert
W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, et
al: Caveolin-1 null mice are viable but show evidence of
hyper-proliferative and vascular abnormalities. J Biol Chem.
276:38121–38138. 2001.PubMed/NCBI
|
|
40
|
Zhao YY, Liu Y, Stan RV, Fan L, Gu Y,
Dalton N, Chu PH, Peterson K, Ross J Jr and Chien KR: Defects in
caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension
in knockout mice. Proc Natl Acad Sci USA. 99:11375–11380. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maniatis NA, Shinin V, Schraufnagel DE,
Okada S, Vogel SM, Malik AB and Minshall RD: Increased pulmonary
vascular resistance and defective pulmonary artery filling in
caveolin-1−/−mice. Am J Physiol Lung Cell Mol Physiol.
294:L865–L873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mehta D and Malik AB: Signaling mechanisms
regulating endothelial permeability. Physiol Rev. 86:279–367. 2006.
View Article : Google Scholar
|
|
43
|
Siddiqui MR, Komarova YA, Vogel SM, Gao X,
Bonini MG, Rajasingh J, Zhao YY, Brovkovych V and Malik AB:
Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and
endothelial permeability. J Cell Biol. 193:841–850. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Bakhshi FR, Shajahan AN, Sharma T,
Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG,
Skidgel RA, et al: Nitric oxide-dependent Src activation and
resultant caveolin-1 phosphorylation promote eNOS/caveolin-1
binding and eNOS inhibition. Mol Biol Cell. 23:1388–1398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tong X and Pelling JC: Targeting the
PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer
Agents Med Chem. 13:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X
and Jiang BH: Apigenin inhibits tumor angiogenesis through
decreasing HIF-1alpha and VEGF expression. Carcinogenesis.
28:858–864. 2007. View Article : Google Scholar
|
|
47
|
Tong X, Mirzoeva S, Veliceasa D, Bridgeman
BB, Fitchev P, Cornwell ML, Crawford SE, Pelling JC and Volpert OV:
Chemopreventive apigenin controls UVB-induced cutaneous
proliferation and angiogenesis through HuR and thrombospondin-1.
Oncotarget. 5:11413–11427. 2014. View Article : Google Scholar : PubMed/NCBI
|