Introduction
Nasal polyps (NPs) cause considerable morbidity,
including nasal congestion, rhinorrhea, anosmia, decreased taste,
sinusitis, olfactory dysfunction and headaches, which significantly
deteriorate the quality of life of patients (1). NPs are swellings of the lining of
the nasal passages and sinuses. They are characterized by tissue
remodeling consisting of basement membrane thickening, goblet cell
hyperplasia, epithelial proliferation, pseudocyst formation, focal
fibrosis, inflammatory cell infiltration and edema (1,2).
NPs are a common chronic inflammatory disease of the mucous
membranes with a high recurrence rate in the nose and paranasal
sinuses, and are generally related with chronic rhinosinusitis
(CRS) (3). While many studies on
NPs have been carried out, the pathogenesis of NPs has not been
elucidated thus far.
Many types of cells, such as epithelial cells, T
cells, mast cells and fibroblasts, are related with the
pathogenesis of NPs (3). Among
these, fibroblasts are the critical component of the polyp
organization. Fibroblasts are relevant regulators of local
inflammation due to a source of biological mediators in initiating
and amplifying inflammation (4).
Stimulated fibroblasts contribute towards the inflammatory response
by releasing inflammatory mediators. Pseudomonas aeruginosa (P.
aeruginosa) can be frequently found in nasal smears of patients
with persistent sinus symptoms after sinus surgery (5). Previous studies have shown that
lipopolysaccharide (LPS) derived from P. aeruginosa induces
interleukin-1 (IL-1), IL-6, monocyte chemoattractant protein-1
(MCP-1), and tumor necrosis factor-α (TNF-α) in airway inflammation
and goblet cell hyperplasia (6).
Among these inflammatory mediators, IL-6 and IL-8 were found to be
produced in LPS-stimulated NP-derived fibroblasts (NPDFs) (4). NPDF activation by LPS appears to
play a crucial role in the initiation and progression of NPs.
Therefore, regulation of NPDF activation and inhibition of these
inflammatory mediators may be therapeutic targets to reduce the
development of NPs.
Marine algae have been used as dietary and medicinal
component in Asia and are rich in dietary fibers, minerals,
vitamins, polysaccharides, proteins and various functional
poly-phenols (7). Previous
studies have supported the pharmaceutical and the medicinal
importance of seaweed in treating NFs (7,8).
The authors of the present study have found that the ethanolic
extract of Distromium decumbens (DDE) has the highest
quantity of total polyphenol content (21.27±0.09%). In our previous
study, DDE was found to have more than 89.57±0.43%
1,1-diphenyl-2-picrylhydrazyl radical scavenging activity (9). In addition, DDE inhibited nitric
oxide, reactive oxygen species, and prostaglandin E2
production in RAW cells when LPS was used as an inducer
(unpublished data). These inflammatory mediators serve important
roles in inflammation-related disorders. Taken together, these
results address the hypothesis that DDE may exert anti-inflammatory
effects in NPs.
Therefore, as a part of our ongoing study to screen
and evaluate the anti-inflammatory efficacy of natural bioactive
materials, we focused on the anti-inflammatory effects of DDE and
the mechanism related in NPDFs, which can be stimulated using LPS
to mimic the conditions of NPs in vitro.
Materials and methods
Reagents
LPS from P. aeruginosa (PA-LPS) was
purchased from Merck Millipore (Darmstadt, Germany). An antibody
against nuclear factor-κB (NF-κB) (cat. no. 14-6731) was purchased
from eBioscience (San Diego, CA, USA). Antibodies against phospho
(p)-extracellular signal-related kinase (ERK) (cat. no. 9106),
c-Jun N-terminal kinase (JNK) (cat. no. 9252), p-JNK (cat. no.
9251), p-p38 mitogen-activated protein kinase (MAPK) (cat. no.
9211), Akt (cat. no. 9272), and p-Akt (cat. no. 4058) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against ERK (cat. no. sc-94), and p38 MAPK (cat. no.
sc-535) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Inhibitors of ERK kinase (U0126) and Akt (LY294002)
were purchased from Calbiochem (Billerica, MA, USA) and were
dissolved in dimethyl sulfoxide (DMSO). BAY 11-7082 and
parthenolide were purchased from Santa Cruz Biotechnology,
Inc..
Preparation of ethanol extract from D.
decumbens
Marine brown alga, D. decumbens, was
collected from the Jeju Island, Korea. After collection, D.
decumbens was washed with tap water to remove slats, epiphytes,
and sand attached to the surface of the samples, and they were then
maintained at −20°C. The samples were lyophilized and homogenized
using a grinder. The dried powder was extracted with 70% EtOH (1:10
w/v) for 1 h (5 times) by sonication, and then the extract was
evaporated in vacuo. The extract was dissolved in DMSO prior
to use in the experiment.
NP-derived fibroblast culture
Fifteen subjects with NPs and 15 subjects with
deviated nasal septum were recruited from the Department of
Otorhinolaryngology, Inje University Busan Paik Hospital, Busan,
Republic of Korea. Based on the minimum criteria for chronic
sinusitis with NPs, individuals were diagnosed as having NPs
(10). All patients who
participated in this study provided informed consent and the
procedure was approved by our local ethics committee. A NP was
defined as the presence of an endoscopically visible bilateral
polyp growing from the middle nasal meatus into the nasal cavity,
and affecting the ethmoid and maxillary sinuses as observed using
computed tomography of the paranasal sinus. NPs were obtained from
the region of the middle meatus at the beginning of the surgical
procedure. The subjects had no history of nasal allergy, aspirin
sensitivity or asthma. No patient had prescribed steroids (topical
or systemic), nonsteroidal anti-inflammatory drugs, antihistamines,
or macrolide antibiotics during the 4 weeks before the biopsy.
NPDFs were separated from tissues by enzymatic treatment with
collagenase (500 U/ml), hyaluronidase (30 U/ml), and DNase (10
U/ml) (all from Sigma-Aldrich, St. Louis, MO, USA). Dulbecco's
modified Eagle's medium was applied to culture the cells containing
10% (v/v) heat-inactivated fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 1,000 µg/ml
streptomycin (Invitrogen, Carlsbad, CA, USA), and 1,000 U/ml
penicillin. The purity of the NPDFs was confirmed by flow cytometry
and characteristic spindle-shaped cell morphology. Experimental
cells were used in the fourth cell passage.
Cell line culture
RPMI-1640 medium supplemented with fetal bovine
serum (10%) was used to maintain HL-60 cells. To achieve the
expression of the neutrophilic phenotype, the HL-60 cells were
induced to differentiate (dHL-60) with 1.75% (vol/vol) DMSO for 3–4
days.
Determination of cell viability
The Cell Counting Kit-8 (CCK-8; Dojindo
Laboratories, Kumamoto, Japan) method was applied to determine the
cellular viability. Briefly, wells (1×105 cells/ml) were
incubated with DDE for 24 h and then the cells were washed with PBS
twice. CCK-8 was added to each well and incubated at 37°C for 1 h,
followed by analysis at 450 nm using a plate reader (model EL800;
Bio-Tek Instruments, Inc., Winooski, VT, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The cytokine levels in the culture media were
detected using ELISA. ELISA kits, which were used to measure IL-6
and IL-8 expression levels, were purchased from BioLegend (San
Diego, CA, USA). The absorbance at 450 nm was measured using a
plate reader (model EL800; Bio-Tek Instruments, Inc.).
Cell migration assay
The dHL-60 cell migration was measured using a
24-well plate system at densities of 1×106 cells/ml. The
dHL-60 cells were added to the upper chambers of Transwell cluster
plates (24-well companion plate; BD Biosciences, Franklin Lakes,
NJ, USA) with 3-µm pore filters. PA-LPS-induced NPDFs
were added to the lower wells of the plates. The cells were allowed
to migrate for 24 h at 37°C in 5% CO2. The transferred
cells from the lower chamber were collected and then centrifuged at
400 × g for 10 min. The number of cells that had migrated to the
lower well was counted with a hemocytometer. Each experiment was
performed in triplicate and repeated at least 3 times.
Western blot analysis
The cells were lysed with lysis buffer (Mammalian
Cell-PE LB; G-Biosciences, St. Louis, MO, USA). Equal amounts of
protein were separated on 10% SDS-polyacrylamide mini-gels and
transferred onto nitrocellulose membranes (GE Healthcare Life
Sciences, Chalfont, UK). Following incubation with the appropriate
primary antibodies (ERK, p-ERK, p38, p-p38, JNK, p-JNK, Akt, p-Akt
and NF-κB) at a dilution of 1:1,000 overnight at 4°C, the membranes
were incubated with horseradish peroxidase conjugated anti-rabbit
(cat. no. 31460; Pierce Biotechnology, Inc., Rockford, IL, USA) or
anti-mouse (cat. no. sc-2031; Santa Cruz Biotechnology, Inc.)
secondary antibodies. Following three washing with Tris-buffered
saline Tween-20 (TBST), immunoreactive bands were visualized using
an enhanced chemiluminescence (ECL) detection system (Pierce
Biotechnology, Inc.).
Electrophoretic mobility shift assay
(EMSA)
Nuclear extract was prepared using the NE-PER
nuclear extraction reagent (Pierce Biotechnology, Inc.). An
oligonucleotide containing the immunoglobulin κ-chain binding site
(κB, 5′-GATCTCAGA GGGGACTTTCCGAGAGA-3′) was synthesized as a probe
for the gel retardation assay. A non-radioactive method, in which
the 3′ end of the probe was labeled with biotin, was used (Pierce
Biotechnology, Inc.). The binding reactions contained 5 µg
nuclear extract protein, buffer (10 mM Tris, pH 7.5, 50 mM KCl, 5
mM MgCl2, 1 mM dithiothreitol, 0.05% Nonidet P-40, and
2.5% glycerol), 50 ng poly(dI-dC) and 20 fM biotin-labeled DNA. The
reactions were incubated at room temperature for 20 min to a final
volume of 20 µl. The competition reactions were performed by
adding a 100-fold excess of unlabeled κB to the reaction mixture.
The mixture was then separated by electrophoresis on a 5%
polyacrylamide gel in 0.5X Tris-borate buffer and transferred to
nylon membranes. The biotin-labeled DNA was assessed using a
LightShift Chemiluminescent EMSA kit (Pierce Biotechnology,
Inc.).
Statistical analysis
Data values represent the means ± standard
deviation. To analyze the data produced from the experiments with
two independent variables, one-way analysis of variance was
performed using GraphPad Prism software (GraphPad Software, Inc.,
La Jolla, CA, USA). Values of P<0.05 and P<0.01 were
considered significant.
Results
Effects of DDE on the viability of
NPDFs
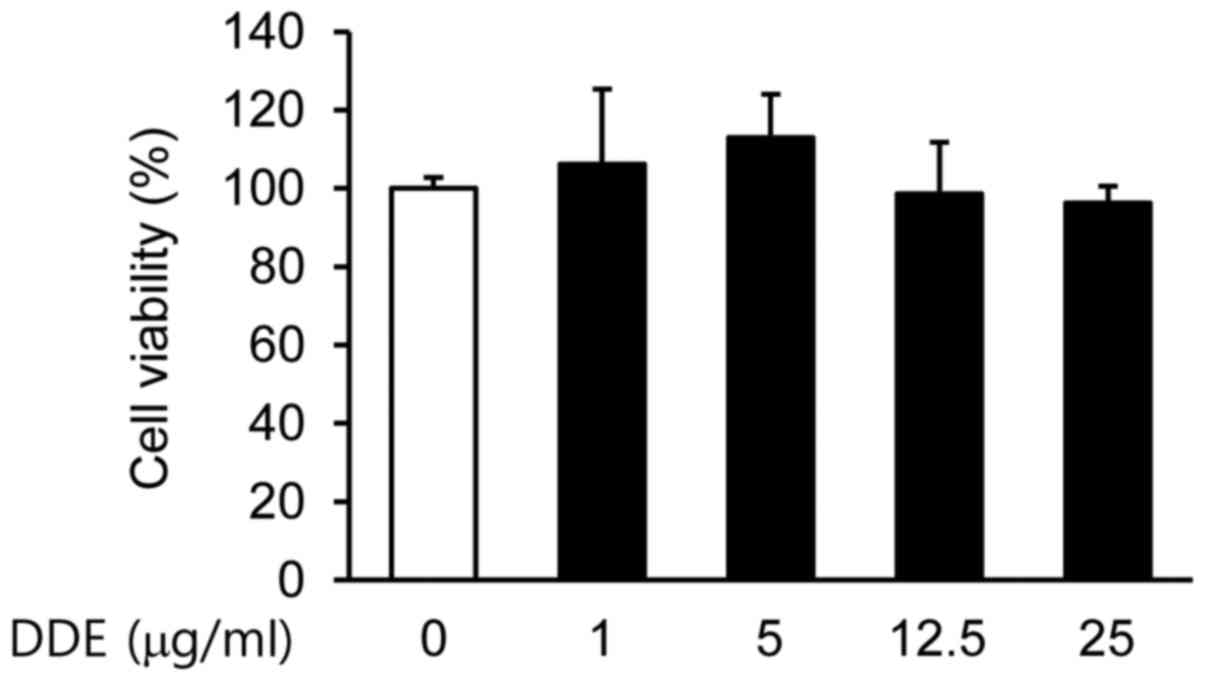
Initially, we examined the viability of NPDFs
treated with DDE by using a CCK-8 assay. No significant
cytotoxicity to NPDFs was observed at doses up to 25 µg/ml
(Fig. 1). Based on these results,
a concentration range of 1 to 25 µg/ml was selected for the
subsequent experiments.
Effects of DDE on the expression of IL-6
and IL-8 in PA-LPS-induced NPDFs
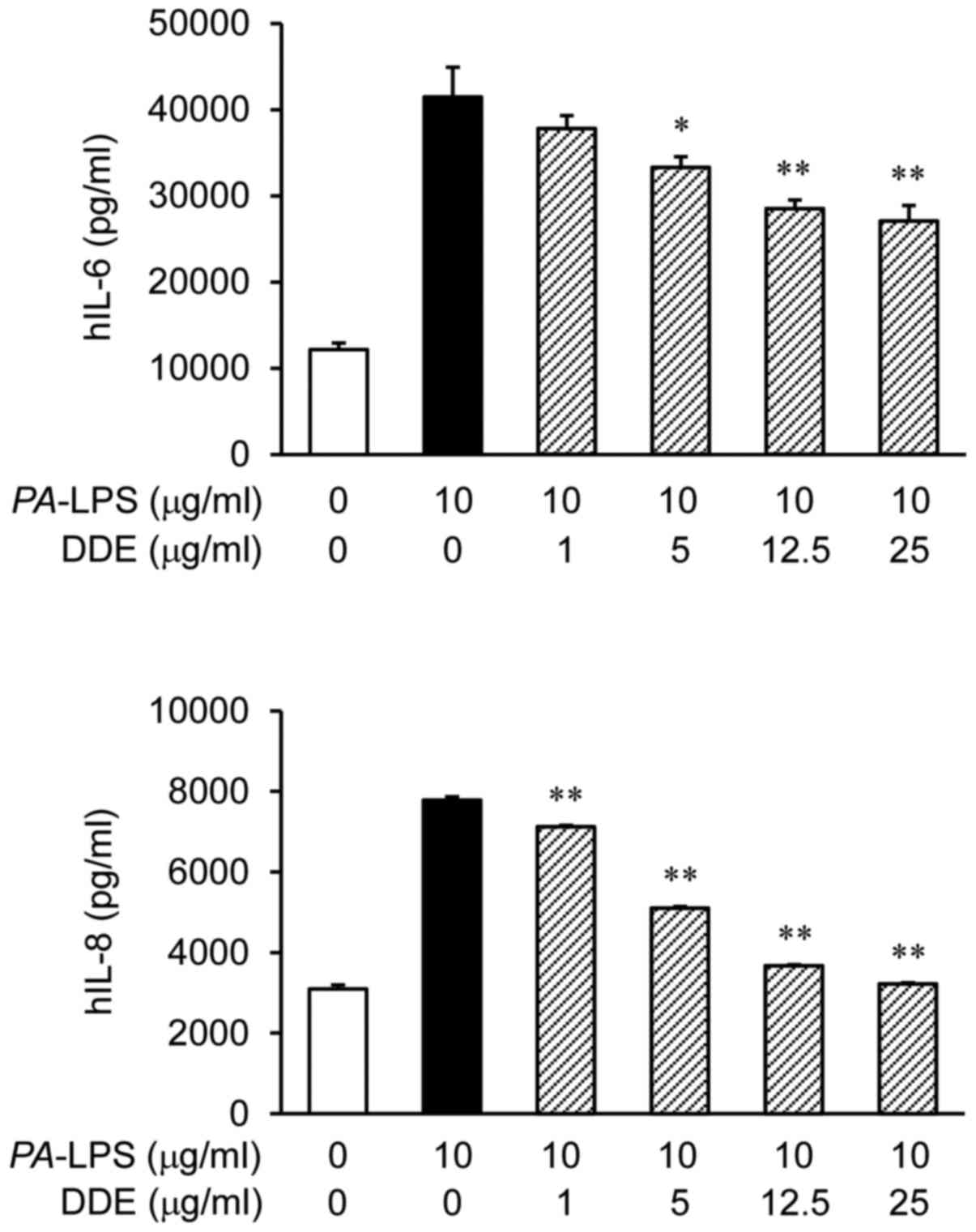
The protein expression levels of IL-6 and IL-8 were
markedly increased after the stimulation of NPDFs with
PA-LPS (Fig. 2). To assess
the effect of DDE on the protein production of IL-6 and IL-8 in
NPDFs, we pretreated the cells with DDE (1, 5, 12.5 and 25
µg/ml) before stimulation with PA-LPS (10
µg/ml). The treatment with DDE attenuated the LPS-induced
expression of IL-6 and IL-8 proteins.
Effects of DDE on the migration of dHL-60
cells towards PA-LPS-stimulated NPDFs
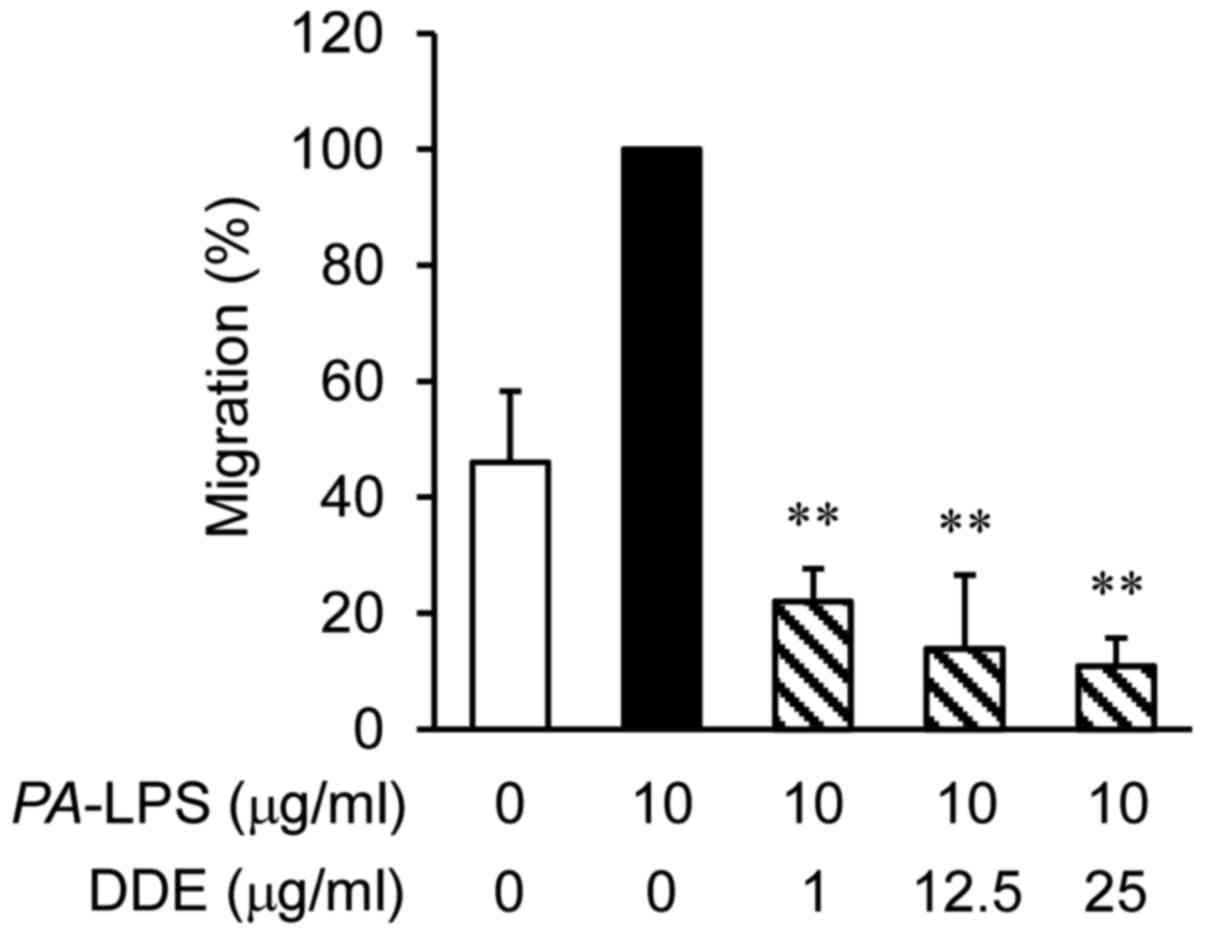
To examine whether DDE affects the migration of
dHL-60 cells, migration assays were performed using Transwell
cluster plates (Fig. 3). The
number of migrated dHL-60 cells co-cultured with
PA-LPS-treated NPDFs was 2 times greater than that of the
vehicle-treated dHL-60 cells. However, the levels of infiltration
of the dHL-60 cells were significantly attenuated by DDE treatment
in a concentration-dependent manner when compared with the
infiltration of the PA-LPS-induced group.
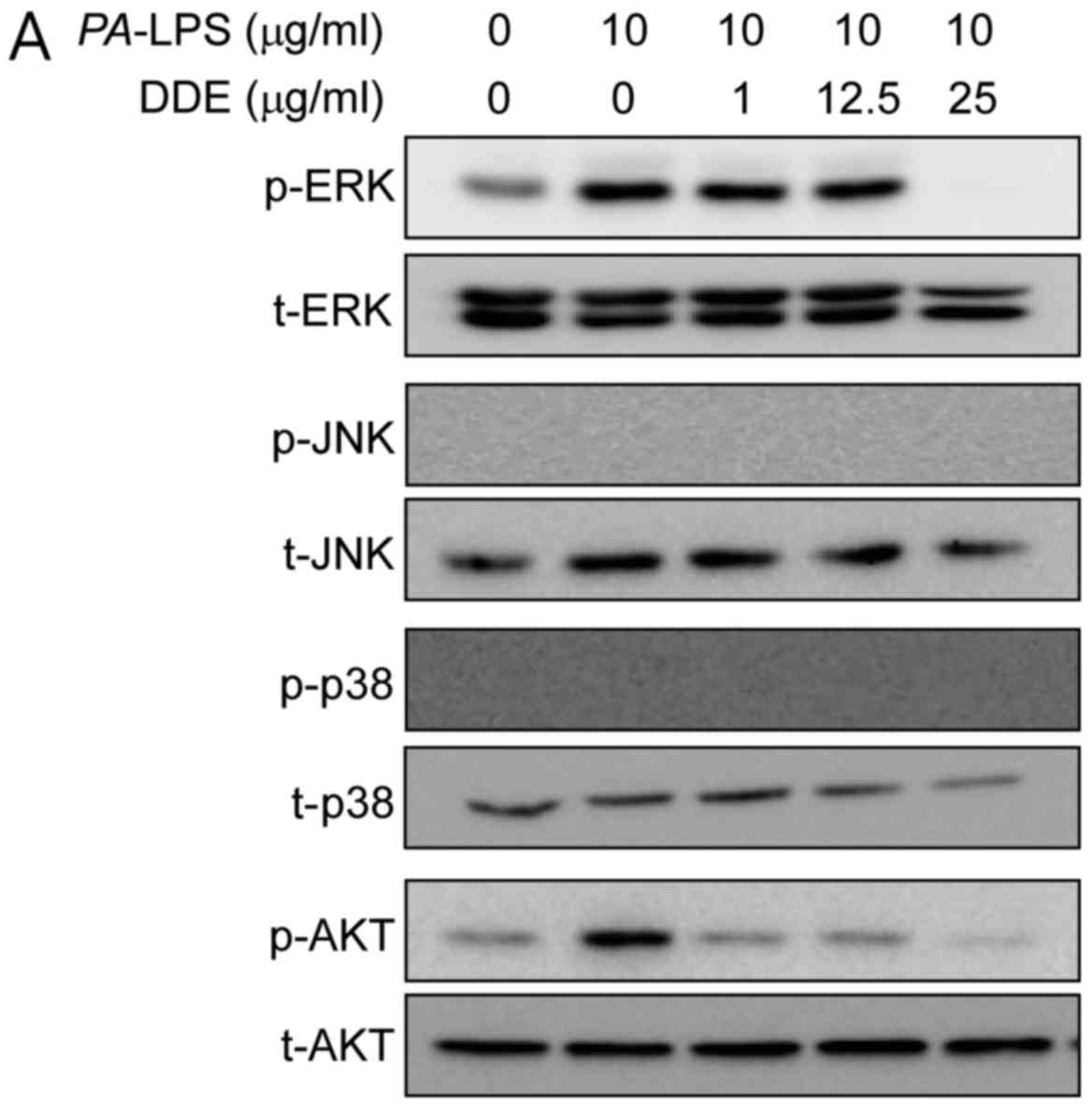
Effects of DDE on the activation of the
Akt and MAPK signaling pathways in PA-LPS-induced NPDFs
To clarify the mechanisms underlying the effects of
DDE on the expression of inflammatory mediators, we inspected the
activation of Akt and MAPKs using western blot analysis. The
stimulation of NPDFs with PA-LPS resulted in an increase in
the phosphorylation of Akt and ERK, but not of JNK and p38.
Pretreatment for 1 h with DDE (1, 12.5 and 25 µg/ml)
attenuated the phosphorylation of Akt and ERK induced by a 2-h
incubation with 10 µg/ml PA-LPS (Fig. 4A). To verify whether the Akt and
ERK signaling pathways are involved in the expression of IL-6 and
IL-8, NPDFs were treated with PA-LPS with or without DDE,
Akt or ERK inhibitors. The upregulated expression of IL-6 and IL-8
was significantly inhibited by U0126 (an inhibitor of ERK) and
LY294002 (an inhibitor of Akt) (Fig.
4B).
Effects of DDE on the activation of NF-κB
in PA-LPS-stimulated NPDFs
The production of pro-inflammatory cytokines is
regulated by the transcription factor NF-κB in PA-LPS
stimulation (11). Therefore, to
inspect the mechanism by which DDE affects the expression of IL-6
and IL-8, we assessed the effects of DDE on NF-κB activation. We
found that DDE inhibited the PA-LPS-induced translocation of
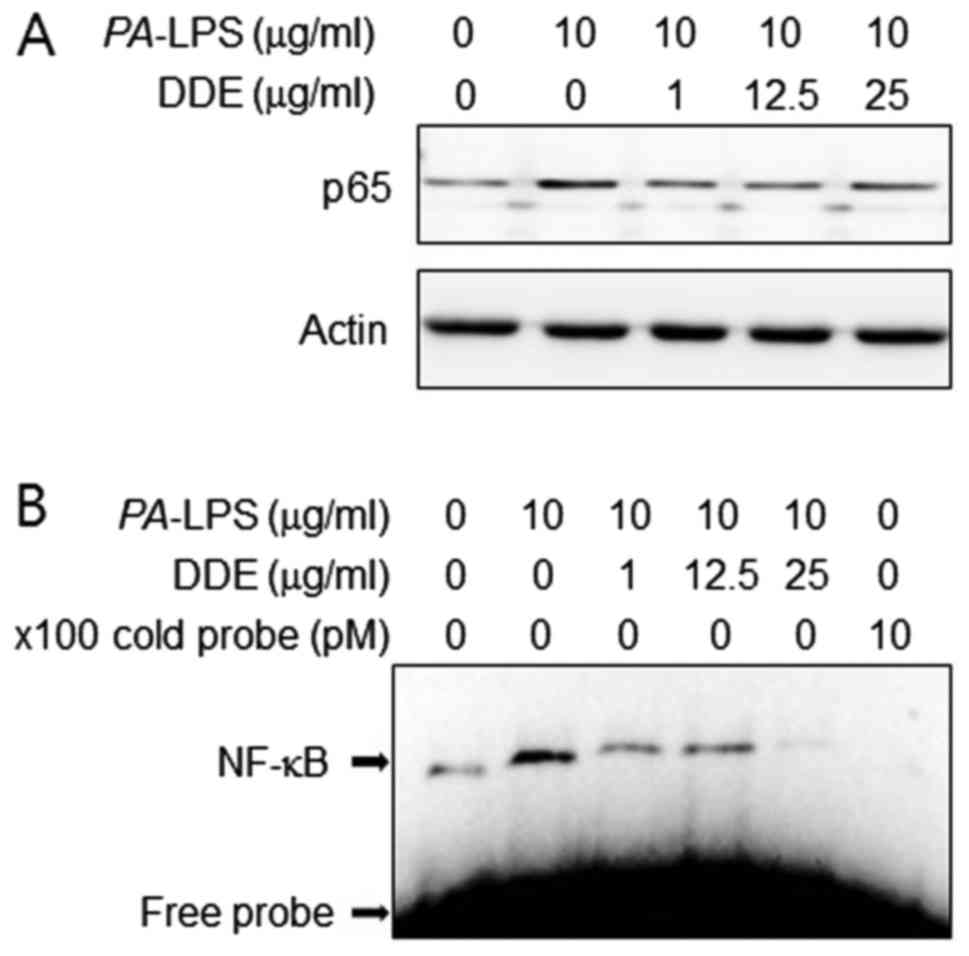
NF-κB p65 into the nuclear compartment (Fig. 5A). We next examined the effect of
DDE on the DNA-binding activity of NF-κB using EMSA (Fig. 5B). PA-LPS treatment caused
a significant increase in the DNA-binding activity of NF-κB,
whereas treatment with DDE markedly reduced the
PA-LPS-induced DNA-binding activity of NF-κB.
Effects of NF-κB inhibitors on the
expression of IL-6 and IL-8 in PA-LPS-induced NPDFs
To confirm whether the NF-κB p65 signaling pathway
is involved in the expression of IL-6 and IL-8, NPDFs were treated
with PA-LPS with or without DDE or NF-κB inhibitors (BAY
11-7082 and parthenolide, respectively). As shown in Fig. 5C, BAY 11-7082 and parthenolide
inhibited the expression of IL-6 and IL-8 in the
PA-LPS-stimulated NPDFs.
Discussion
Seaweed has long been traditionally consumed for
medicinal and dietary purposes. Marine algae are known to produce
natural bioactive materials, such as dietary fibers, protein,
polysaccharides, vitamins, minerals and various functional
polyphenols (12). They are rich
sources of anti-inflammatory therapeutic agents. A pro-inflammatory
response is initiated as a defense reaction following stimulation
by environmental stimuli such as pathogens and damage, resulting in
the expression of pro-inflammatory mediators. These
pro-inflammatory factors are regarded as responsible for the
pathological states associated with NPs (4). Therefore, the present study was
attempted to examine the pharmacological effects of DDE on the
expression of inflammatory cytokines and the migration of
neutrophils. In addition, the mechanism of action of
PA-LPS-induced stimulation of NPDFs was investigated.
Inflammatory factors are expressed in response to
gram-negative bacterial infections, mediated by LPS, a cell wall
component of gram-negative bacteria. LPS is a pathogen-associated
molecular pattern that provokes pathogenicity. Fibroblasts are the
major structural components of tissues and play an augmenting
effector role in the inflammatory response (13). Nasal fibroblasts are the principal
structural components of the nose and are involved in NPs, which
are common findings in chronic rhinosinusitis (14). Nasal fibroblasts produce
pro-inflammatory cytokines, including IL-6, and chemokines,
including IL-8, in nasal fibroblasts upon LPS stimulation (3).
We first investigated PA-LPS-induced
production of IL-6 and IL-8 in HNDFs. IL-6, a pleiotropic cytokine,
has a wide range of biological activity, which is associated with
the production of acute phase proteins. IL-6 is associated with the
pathogenesis of diverse inflammatory diseases, including multiple
myeloma, rheumatoid arthritis, Crohn's disease, asthma and lupus
(15-18). IL-6-induced signaling can be
mostly seen in a relatively stingy number of IL-6-reactive cells,
whereas in a chronic inflammatory phase it is able to activate
nearly all cells of the body (19). A recent study confirmed elevated
IL-6 protein expression in polyp tissue compared to its expression
in middle turbinate in the same patients with CRS with NPs (CRSwNP)
(20). These results suggest that
IL-6 plays a pathogenic role in CRSwNP. Although IL-8 synthesis
aims at combating and eliminating the pathogen, persistent
production of IL-8 plays a crucial role in the accumulation of
neutrophils in sites of inflammation (21). The concentrations of IL-6 and IL-8
are increased in nasal lavage and NPs in CRS, and they activate NP
growth (22). As shown in
Fig. 2 DDE inhibited the
PA-LPS-induced production of IL-6 and IL-8 at concentrations
of DDE that were not cytotoxic to the NPDFs. Next, we performed
functional validation by assessing the attenuation effect of DDE on
the migration of neutrophils in vitro. Neutrophils release
matrix metalloproteinases and induce tissue destruction (23,24). Because fibroblasts can produce a
variety of chemokines, they are considered responsible for local
recruitment of inflammatory cells (25,26). A human promyelocytic HL-60 cell
line that can be induced to differentiate using agents such as DMSO
is a useful model system for studying neutrophil migration
(27). We therefore used dHL-60
cells to evaluate the anti-migratory effects of DDE. Fig. 3 shows that treatment of dHL-60
cells with DDE (1, 12.5 or 25 µg/ml) decreased migration
dose-dependently.
As DDE inhibited the expression of IL-6 and IL-8 in
PA-LPS-stimulated NPDFs, we further investigated the
mechanism underlying the inhibitory actions of DDE. LPS promotes
the transcription of a variety of inflammatory genes related to
inflammatory responses, including MAPKs (ERK, JNK and p38 MAPK) and
the Akt pathway, in various types of cells (28). MAPKs play an important role in the
control of cell responses. MAPKs have been implicated as important
mediators of the signaling pathway that appears to play a critical
role in the inflammatory response (29). In addition, Akt is essential in
LPS-stimulated inflammation progression (30), since LPS induces inflammatory
responses via Akt signaling in fibroblasts (31). Therefore, modulation of the Akt
axis is considered to be an effective approach towards the
treatment of many inflammatory disorders. A previous study
demonstrated that LPS stimulates IL-6 and IL-8 expression through
the MAPK and Akt signaling pathways in NPDFs (4). As expected, when NPDFs were treated
with PA-LPS, ERK and Akt were phosphorylated, whereas
pretreatment with DDE significantly reduced the LPS-induced
phosphorylation of ERK and Akt (Fig.
4A). In addition, treatment with these kinase inhibitors
effectively inhibited PA-LPS-stimulated expression of IL-6
and IL-8 (Fig. 4B). Therefore,
the present study demonstrated a significant inhibition of
PA-LPS-stimulated phosphorylation of ERK and Akt by DDE in
NPDFs.
Transcription factor NF-κB is known to be a
multifunctional regulator of various genes involved in the
expression of many inflammatory mediators involved in inflammatory
process (32). Activation of
NF-κB causes phosphorylation, ubiquitination and
proteasome-mediated degradation of the inhibitory IκB proteins and
then nuclear transfer (33).
Therefore, we examined the effects of DDE on the
PA-LPS-stimulated activation of NF-κB by NPDFs. Since
activated NF-κB enters the nucleus and induces IL-6 and IL-8
expression, we studied the nuclear translocation of the NF-κB
subunit. Western blot analysis showed that stimulation with
PA-LPS induced translocation of NF-κB to the nuclear
compartment. However, nuclear translocation of
PA-LPS-induced NF-κB was attenuated in the presence of DDE.
According to EMSA data, PA-LPS stimulation increased the
DNA-binding activity of NF-κB. As shoen in Fig. 5B, pretreatment with DDE
effectively inhibited the PA-LPS-stimulated DNA-binding
activity of NF-κB. To confirm the above results, we investigated
the effects of the NF-κB inhibitors, BAY 11-7082 and parthenolide,
on the PA-LPS-induced production of IL-6 and IL-8 by NPDFs
(Fig. 5C). These data
demonstrated that DDE inhibits NF-κB activity in
PA-LPS-stimulated NPDFs by suppressing the nuclear
translocation of p65, resulting in attenuation of IL-6 and IL-8
production.
In conclusion, the results of the present study
demonstrated that PA-LPS increased the release of IL-6 and
IL-8 from cultured NPDFs. Pretreatment with DDE significantly
attenuated the expression of IL-6 and IL-8 in NPDFs under
PA-LPS stimulated inflammatory conditions by suppressing the
activation of the ERK, Akt, and NF-κB signaling pathways. These
results suggest that the anti-inflammatory activity of bioactive
compounds contained in D. decumbens ethanolic bioactive
extract have the potential to be developed as therapeutic agents
for the management of NPs.
Acknowledgments
This study was supported by the National Marine
Biodiversity Institute of Korea Research program (no.
2017M01400).
References
|
1
|
Pawankar R: Nasal polyposis: an update:
editorial review. Curr Opin Allergy Clin Immunol. 3:1–6. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaaban MR, Walsh EM and Woodworth BA:
Epidemiology and differential diagnosis of nasal polyps. Am J
Rhinol Allergy. 27:473–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho JS, Han IH, Lee HR and Lee HM:
Prostaglandin E2 induces IL-6 and IL-8 production by the
EP receptors/Akt/NF-κB pathways in nasal polyp-derived fibroblasts.
Allergy Asthma Immunol Res. 6:449–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho JS, Kang JH, Um JY, Han IH, Park IH
and Lee HM: Lipopolysaccharide induces pro-inflammatory cytokines
and MMP production via TLR4 in nasal polyp-derived fibroblast and
organ culture. PLoS One. 9:e906832014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer JE, Harder J, Görögh T, Schröder JM
and Maune S: hBD-2 gene expression in nasal mucosa.
Laryngorhinootologie. 79:400–403. 2000.In German. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris JF, Aden J, Lyons CR and Tesfaigzi
Y: Resolution of LPS-induced airway inflammation and goblet cell
hyperplasia is independent of IL-18. Respir Res. 8:242007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DS, Park WS, Heo SJ, Cha SH, Kim D,
Jeon YJ, Park SG, Seo SK, Choi JS, Park SJ, et al: Polyopes affinis
alleviates airway inflammation in a murine model of allergic
asthma. J Biosci. 36:869–877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Athukorala Y, Jung WK, Park PJ, Lee YJ,
Kim SK, Vasanthan T, No HK and Jeon YJ: Evaluation of biomolecular
interactions of sulfated polysaccharide isolated from Grateloupia
filicina on blood coagulation factors. J Microbiol Biotechnol.
18:503–511. 2008.PubMed/NCBI
|
|
9
|
Kim TH, Ko SC, Oh GW, Park HH, Lee DS, Yim
MJ, Lee JM, Yoo JS, Kim CS, Choi IW, et al: Studies on bioactive
substances and antioxidant activities of marine algae from Jeju
Island. J Mar Biosci Biotechnol. 8:30–38. 2016. View Article : Google Scholar
|
|
10
|
Meltzer EO, Hamilos DL, Hadley JA, Lanza
DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L and
Chinchilli VM: Rhinosinusitis initiative: Rhinosinusitis:
Developing guidance for clinical trials. Otolaryngol Head Neck
Surg. 135(Suppl 5): S31–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paeng SH, Park WS, Jung WK, Lee DS, Kim
GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, et al: YCG063
inhibits Pseudomonas aeruginosa LPS-induced inflammation in human
retinal pigment epithelial cells through the TLR2-mediated
AKT/NF-κB pathway and ROS-independent pathways. Int J Mol Med.
36:808–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung WK, Choi I, Oh S, Park SG, Seo SK,
Lee SW, Lee DS, Heo SJ, Jeon YJ, Je JY, et al: Anti-asthmatic
effect of marine red alga (Laurencia undulata) polyphenolic
extracts in a murine model of asthma. Food Chem Toxicol.
47:293–297. 2009. View Article : Google Scholar
|
|
13
|
Flavell SJ, Hou TZ, Lax S, Filer AD,
Salmon M and Buckley CD: Fibroblasts as novel therapeutic targets
in chronic inflammation. Br J Pharmacol. 153(Suppl 1): S241–S246.
2008. View Article : Google Scholar
|
|
14
|
Carroll WW, O'Connell BP, Schlosser RJ,
Gudis DA, Karnezis TT, Lawrence LA, Soler ZM and Mulligan JK:
Fibroblast levels are increased in chronic rhinosinusitis with
nasal polyps and are associated with worse subjective disease
severity. Int Forum Allergy Rhinol. 6:162–168. 2016. View Article : Google Scholar
|
|
15
|
Houssiau FA, Devogelaer JP, Van Damme J,
de Deuxchaisnes CN and Van Snick J: Interleukin-6 in synovial fluid
and serum of patients with rheumatoid arthritis and other
inflammatory arthritides. Arthritis Rheum. 31:784–788. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atreya R, Mudter J, Finotto S, Müllberg J,
Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et
al: Blockade of interleukin 6 trans signaling suppresses T-cell
resistance against apoptosis in chronic intestinal inflammation:
Evidence in crohn disease and experimental colitis in vivo. Nat
Med. 6:583–588. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doganci A, Eigenbrod T, Krug N, De Sanctis
GT, Hausding M, Erpenbeck VJ, Haddad B, Lehr HA, Schmitt E, Bopp T,
et al: The IL-6R α chain controls lung
CD4+CD25+ Treg development and function
during allergic airway inflammation in vivo. J Clin Invest.
115:313–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polgár A, Brózik M, Tóth S, Holub M, Hegyi
K, Kádár A, Hodinka L and Falus A: Soluble interleukin-6 receptor
in plasma and in lymphocyte culture supernatants of healthy
individuals and patients with systemic lupus erythematosus and
rheumatoid arthritis. Med Sci Monit. 6:13–18. 2000.
|
|
19
|
Allocca M, Jovani M, Fiorino G, Schreiber
S and Danese S: Anti-IL-6 treatment for inflammatory bowel
diseases: Next cytokine, next target. Curr Drug Targets.
14:1508–1521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peters AT, Kato A, Zhang N, Conley DB, Suh
L, Tancowny B, Carter D, Carr T, Radtke M, Hulse KE, et al:
Evidence for altered activity of the IL-6 pathway in chronic
rhinosinusitis with nasal polyps. J Allergy Clin Immunol.
125:397–403.e10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimmermann HW, Seidler S, Gassler N,
Nattermann J, Luedde T, Trautwein C and Tacke F: Interleukin-8 is
activated in patients with chronic liver diseases and associated
with hepatic macrophage accumulation in human liver fibrosis. PLoS
One. 6:e213812011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JA, Cho JH, Park IH, Shin JM, Lee SA
and Lee HM: Diesel exhaust particles upregulate interleukins IL-6
and IL-8 in nasal fibroblasts. PLoS One. 11:e01570582016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Romanic AM, White RF, Arleth AJ, Ohlstein
EH, Barone FC and Dawson VL: Matrix metalloproteinase expression
increases after cerebral focal ischemia in rats: Inhibition of
matrix metal-loproteinase-9 reduces infarct size. Stroke.
29:1020–1030. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salamonsen LA: Current concepts of the
mechanisms of menstruation: A normal process of tissue destruction.
Trends Endocrinol Metab. 9:305–309. 1998. View Article : Google Scholar
|
|
25
|
Kawka E, Witowski J, Fouqet N, Tayama H,
Bender TO, Catar R, Dragun D and Jörres A: Regulation of chemokine
CCL5 synthesis in human peritoneal fibroblasts: A key role of
IFN-γ. Mediators Inflamm. 2014:5906542014. View Article : Google Scholar
|
|
26
|
Xia Y, Entman ML and Wang Y: CCR2
regulates the uptake of bone marrow-derived fibroblasts in renal
fibrosis. PLoS One. 8:e774932013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Millius A and Weiner OD: Chemotaxis in
neutrophil-like HL-60 cells. Methods Mol Biol. 571:167–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Intayoung P, Limtrakul P and Yodkeeree S:
Antiinflammatory Activities of crebanine by inhibition of NF-κB and
AP-1 activation through suppressing MAPKs and Akt signaling in
LPS-induced RAW264.7 macrophages. Biol Pharm Bull. 39:54–61. 2016.
View Article : Google Scholar
|
|
29
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy–from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang QB, Sun LY, Gong ZD and Du Y:
Veratric acid inhibits LPS-induced IL-6 and IL-8 production in
human gingival fibroblasts. Inflammation. 39:237–242. 2016.
View Article : Google Scholar
|
|
32
|
Wang YB, Tan B, Mu R, Chang Y, Wu M, Tu
HQ, Zhang YC, Guo SS, Qin XH, Li T, et al: Ubiquitin-associated
domain-containing ubiquitin regulatory X (UBX) protein UBXN1 is a
negative regulator of nuclear factor κB (NF-κB) signaling. J Biol
Chem. 290:10395–10405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Bosscher K, Vanden Berghe W and
Haegeman G: The interplay between the glucocorticoid receptor and
nuclear factor-kappaB or activator protein-1: Molecular mechanisms
for gene repression. Endocr Rev. 24:488–522. 2003. View Article : Google Scholar : PubMed/NCBI
|