Introduction
It is well-known that the small intestine is the
first organ to be affected when oxidative damage occurs, and acute
small intestinal damage has an adverse effect on the overall health
of animals. Oxidative damage is caused by an imbalance between the
antioxidant system and the generation of free radicals, resulting
in an increase in the level of reactive oxygen species (ROS), which
mediate tissue damage and cause the release of inflammatory
cytokines (1,2). Mitochondria generate adenosine
triphosphate (ATP) by respiration, which is accompanied by the
synthesis of ROS, thus making them sensitive to oxidative damage.
The excessive generation of ROS results in an imbalanced redox cell
state, leading to a decrease in the mitochondrial membrane
potential and disruption of mitochondrial function (3). It has been reported that
subcutaneous injection of indomethacin in mice results in serious
mucosal damage to the gastrointestinal tract, and this was used as
the basis for a study on the clinical risks of small intestine
failure due to damage (4).
Dimethylglycine (DMG) improves the body's immune
system and relieves oxidative damage by scavenging excess free
radicals (5). A previous study
showed that DMG is a small water-soluble lipophilic molecule that
effectively enters the body via the cell membrane and is absorbed
by the intestinal cells, which improves the utilization of oral DMG
(6). In addition, DMG may
increase digestive enzyme capacity and enhance the ability of the
intestinal brush border to come into contact with nutrients,
subsequently promoting the digestion and absorption of nutrients in
the small intestine (7). The
biological consumption of choline is reduced in the metabolic
pathway, suggesting DMG, as a methyl donor, is rapidly digested and
absorbed, resulting in the body needing more ATP and inevitably
increasing the generation of ROS (7). It has been reported that DMG-Na is
similar to choline and betaine, and improves antioxidant capacity
by acting as an important raw material for the glutathione
synthesis pathway (8). A previous
study indicated that DMG relieves oxidative damage and improves an
animal's health and growth performance (9). Our previous results also suggested
that DMG-Na protects against LPS-induced oxidative stress by
increasing free radical scavenging capacity and antioxidant system
activity (10). The present study
was designed to study the effects of DMG-Na on oxidative damage and
mitochondrial dysfunction in the small intestines of mice.
Materials and methods
Reagents and animals
Indomethacin (IN) was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). DMG-Na was obtained from Qilu
Shenghua Pharmaceutical Co. Ltd. (Shandong, China). All other
chemicals were commercially available and of reagent grade. Male
Kunming mice (n=100; 40 days old) with a body weight (BW) of 20-25
g were obtained from the Animal Multiplication Centre of Qinglong
Mountain (Nanjing, China) (11-13) and raised for 4 weeks. Mice were
raised under controlled conditions at 25±3°C, 60±10% humidity, and
a 12/12 h light-dark cycle. All mice were fed common basal diets
for 14 days. They had access to water and food ad libitum.
On day 29, mice were randomly assigned to five groups (n=20 per
group): i) Mice gastric intubation with 0.3 ml sterile saline
solution (once), then subcutaneously injected with sterile saline
solution (0.5 ml) after 1 h (CON); ii) mice gastric intubation with
12 mg DMG-Na/0.3 ml of sterile saline solution once (10), then subcutaneously injected with
sterile saline solution (0.5 ml) 1 h later (D); iii) mice gastric
intubation with 0.3 ml sterile saline solution once, then
subcutaneously injected with indomethacin (10 mg/kg BW) 1 h later
(IN) (4); iv) mice gastric
intubation with 12 mg DMG-Na/0.3 ml sterile saline solution once,
then subcutaneously injected with indomethacin (10 mg/kg BW) 1 h
later (DIN); and v) mice subcutaneously injected with indomethacin
(10 mg/kg BW), then gastrically intubated with 12 mg DMG-Na/0.3 ml
sterile saline solution once after 1 h (IND). Previous studies
found that gastric intubation in mice with 12 mg DMG-Na/0.3 ml
sterile saline solution is beneficial to mice performance,
including growth and antioxidant capacity (10). This study was approved and
conducted under the supervision of Animal Care and Use Committee of
Nanjing Agriculture University (Nanjing, China; approval no. SYXK
2017-0007). The health of each mouse was monitored every 6 h and
humane endpoints were strictly adhered to, to determine when mice
should be euthanized. All the mice were euthanized by
intraperitoneal injection of 100 mg/kg BW pentobarbital
(Sigma-Aldrich; Merck KGaA) and death was confirmed by limb
paralysis or if mice were unable to right themselves in 15 sec when
placed on their side. Following sacrifice, blood (0.3 ml) and the
small intestines were collected.
Histological study
Small intestine (jejunum and ileum) samples were
fixed in 4% buffered formaldehyde and dehydrated using a graded
series of xylene and ethanol, following which they were embedded in
paraffin for histological analysis. The small intestine samples (5
mm) were subsequently deparaffinized using xylene and rehydrated
with graded dilutions of ethanol. In total, ten slides for each
sample were obtained and images were acquired using an optical
binocular microscope (×400). The villus length (L), crypt depth,
villus width (W), muscle thickness, and mucosa thickness were
measured. The villus area was calculated using the following
formula (14):
Antioxidant enzymes assays
Mouse small intestine (1 g; jejunum and ileum) was
homogenized at 6,800 × g for 10 sec in 9 ml 0.9% sodium chloride
solution on ice. The homogenate was centrifuged at 3,500 × g for 15
min at 4°C. The supernatant and serum were each used to measure the
activity of superoxide dismutase (SOD; cat. no. A001), glutathione
peroxidase (GSH-Px; cat. no. A005), glutathione (GSH1; cat. no.
A006), and glutathione reductase (GR; cat. no. A062) according to
the methods described by Panchenko et al (15) and Lawrence and Burk (16) using commercial diagnostic kits, as
per the respective protocols provided by the manufacturer (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Isolation of mice small intestine
mitochondria and its antioxidant enzyme assays
Mitochondria from the small intestines of mice were
isolated according to the method described by Roediger and Truelove
(17) with a mitochondria
isolation kit (cat. no. MITOISO2-1KT; Sigma-Aldrich; Merck KGaA).
The mitochondria were lysed and the protein content was determined
using the Micro Bicinchoninic Acid (BCA) protein assay kit (Nanjing
Jiancheng Institute of Bioengineering). The levels of manganese
superoxide dismutase (MnSOD; cat. no. A001-2), glutathione
peroxidase (GPx; cat. no. A005), glutathione (GSH2; cat. no. A006),
GR (cat. no. A062) and γ-glutamylcysteine ligase (γ-GCL; cat. no.
A120) in the small intestinal mitochondria were determined
according to the methods described by Langston et al
(18), Van et al (19) and Lawrence and Burk (16). All assays were conducted in
triplicate according to the protocols provided by the manufacturer
(Nanjing Jiancheng Bioengineering Institute).
Lipid peroxidation, protein oxidation and
8-hydroxy-2′- deoxyguanosine assays
Lipid peroxidation, expressed as malondialdehyde
concentration, was determined according to the methods of Botsoglou
et al (20). With a
malondialdehyde (MDA; cat. no. A003) assay kit (Nanjing Jiancheng
Bioengineering Institute). Protein oxidation in mouse small
intestinal mitochondria was calculated as the concentration of the
protein carbonyls using a spectrophotometer, which was measured as
previously described (21) and
was expressed as nmol/mg protein. The content of
8-hydroxy-2′-deoxyguano-sine (8-OHdG) in mitochondria from the
small intestines of mice was measured using an ELISA kit (cat. no.
H165; Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions, and was expressed as ng/mg
protein.
Mitochondrial ROS and mitochondrial
membrane potential (MMP) assays
The concentration of ROS in mouse small intestinal
mitochondria was determined using a previously described method
(22) and a ROS assay kit (cat.
no. E004; Nanjing Jiancheng Bioengineering Institute). Alterations
in the MMP in were detected according to a previously described
method (23) using a MMP assay
kit (cat. no. G009; Nanjing Jiancheng Bioengineering
Institute).
Cell apoptosis, ATP concentration and
mitochondrial (mt)DNA copy number assays
The number of apoptotic and necrotic cells was
measured according to a previously described method (24). An Alexa Fluor® 488
Annexin V/Dead Cell Apoptosis kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used. The concentration of cellular adenosine
triphosphate (ATP) in mouse small intestines was determined
according to the method described by Liu et al (25). The mtDNA copy number in the small
intestine of mice was determined using a real-time fluorescence
quantitative polymerase chain reaction (PCR) kit (Tli RNaseH Plus;
Takara Bio, Inc., Otsu, Japan) (26). In brief, 20 μl PCR mixture
consisting of 10 μl SYBR Premix Ex Taq (2X), 0.4 μl
upstream primer, 0.4 μl downstream primer, 0.4 μl ROX
dye (50X), 6.8 μl ultra-pure water, and 2 μl cDNA
template was used. The sequence of the MtD-loop gene
upstream primer was 5′-AGG ACT ACG GCT TGA AAA GC-3′, and the
sequence of the downstream primer was 5′-CAT CTT GGC ATC TTC AGT
GCC-3′; the length of the target fragment was 198 bp. The sequence
of the β-actin upstream primer was 5′-TTC TTG GGT ATG GAG
TCC TG-3′ and that of the downstream primer was 5′-TAG AAG CAT TTG
CGG TGG-3′; the length of the target fragment was 150 bp. The
amplification for each mouse small intestine sample was performed
in triplicate. The fold-expression of each gene was calculated
according to the 2−ΔΔCq method (27), in which β-actin was used as
an internal standard.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extracted from mouse small intestines
using TRIzol® reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and was reverse-transcribed using a Perfect Real
Time, SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.) according to
manufacturer's instructions. The mRNA expression levels of specific
genes were quantified by PCR, using a SYBR Premix Ex Taq II (Tli
RNaseH Plus; Takara Bio, Inc.) in an ABI 7300 Fast Real-Time PCR
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to manufacturer's instructions. The SYBR-Green PCR
mixture consisted of 10 μl SYBR Premix Ex Taq (2X), 0.4
μl each of the forward and reverse primers, 0.4 μl
ROX reference dye (50X), 6.8 μl ddH2O and 2
μl cDNA template. The amplification for each mouse small
intestine was performed in triplicate, and the fold-expression of
each gene was calculated according to the 2−ΔΔCq method
(27), in which β-actin
was used as an internal standard. The primer sequences used are
provided in Table I.
 | Table IPrimer sequences used for the reverse
transcription-quantitative polymerase chain reaction assay. |
Table I
Primer sequences used for the reverse
transcription-quantitative polymerase chain reaction assay.
| Gene name | Direction | Sequence
(5′-3′) | GenBank accession
number |
|---|
| β-actin | F |
CTGTCCCTGTATGCCTCTG | NM_007393.3 |
| R |
ATGTCACGCACGATTTCC | |
| Nrf2 | F |
GATGTCACCCTGCCCTTAG | NM_205117.1 |
| R |
CTGCCACCATGTTATTCC | |
| HO1 | F |
GGTCCCGAATGAATGCCCTTG | HM237181.1 |
| R |
ACCGTTCTCCTGGCTCTTGG | |
|
Cu/ZnSOD | F |
CCGGCTTGTCTGATGGAGAT | NM_205064.1 |
| R |
TGCATCTTTTGGTCCACCGT | |
| GSH-Px | F |
GACCAACCCGCAGTACATCA | NM_001277853.1 |
| R |
GAGGTGCGGGCTTTCCTTTA | |
| MnSOD | F |
AGGAGGGGAGCCTAAAGGAGA | NM_204211.1 |
| R |
CCAGCAATGGAATGAGACCTG | |
| Sirt1 | F |
TGCAGACGTGGTAATGTCCAAAC | NM_019812.2 |
| R |
ACATCTTGGCAGTATTTGTGGTGAA | |
| γ-GCLc | F |
TGCGGTTCTGCACAAAATGG | XM_419910.3 |
| R |
TGCTGTGCGATGAATTCCCT | |
| γ-GCLm | F |
CCAGAACGTCAAAGCACACG | NM_001007953.1 |
| R |
TCCTCCCATCCCCCAGAAAT | |
| Trx2 | F |
AGTACGAGGTGTCAGCAGTG | NM_001031410.1 |
| R |
CACACGTTGTGAGCAGGAAG | |
| Trx-R2 | F |
CCGGGTCCCTGACATCAAA | NM_001122691.1 |
| R |
TAGCTTCGCTGGCATCAACA | |
| Prx3 | F |
ACCTCGTGCTCTTCTTCTACC | XM_004942320.1 |
| R |
ACCACCTCGCAGTTCACATC | |
| OCLN | F CC |
GTAACCCCGAGTTGGAT | NM_205128.1 |
| R |
ATTGAGGCGGTCGTTGATG | |
| CLDN2 | F CC |
TGCTCACCCTCATTGGAG | NM_001277622.1 |
| R |
GCTGAACTCACTCTTGGGCT | |
| CLDN3 | F CCC |
GTCCCGTTGTTGTTTTG | NM_204202.1 |
| R CCCC |
TTCAACCTTCCCGAAA | |
| ZO1 | F |
TGTAGCCACAGCAAGAGGTG | XM_413773.4 |
| R C |
TGGAATGGCTCCTTGTGGT | |
Western blotting assay
Antibodies against α-tubulin, nuclear factor
erythroid 2-related factor 2 (Nrf2), heme oxygenase 1 (HO1), and
sirtuin 1 (Sirt1) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Samples were lysed using Laemmli sample
buffer [2% SDS, 62 mM Tris, 10% glycerol, 5% β-mercaptoethanol,
0.005% bromphenol blue (pH 6.8)]. The protein content of the sample
was assayed using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China). For western blot analysis, 50 mg
protein from each sample was subjected to SDS-PAGE (10% gel) and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked in 5% non-fat dry milk for 12 h at 4°C, followed by
incubation with antibodies against Nrf2 (cat. no. 12721; 1:1,000),
HO1 (cat. no. 82206; 1:1,000), Sirt1 (cat. no. 9475; 1:1,000) and
α-tubulin (cat. no. 2125; 1:1,000) for 10 h at 4°C. Next, membranes
were incubated with goat-anti-rabbit secondary antibody (1:1,000;
Cell Signaling Technology, Inc.) for 2 h at 4°C. Bands were
visualized using enhanced chemiluminescence reagents (ECL-Kit;
Beyotime Institute of Biotechnology,) followed by autoradiography.
Images of the membranes were taken using a Luminescent Image
Analyzer-4000 system (Fujifilm, Tokyo, Japan) and quantified using
ImageJ 1.42q software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of three experimental repeats, and were analyzed by
one-way analysis in Statistical Analysis System software (version
9.1; SAS Institute, Inc., Cary, NC, USA). This was followed by the
Tukey's test, when significant differences were found. P<0.05
was considered to indicate a statistically significant
difference.
Results
Histological study
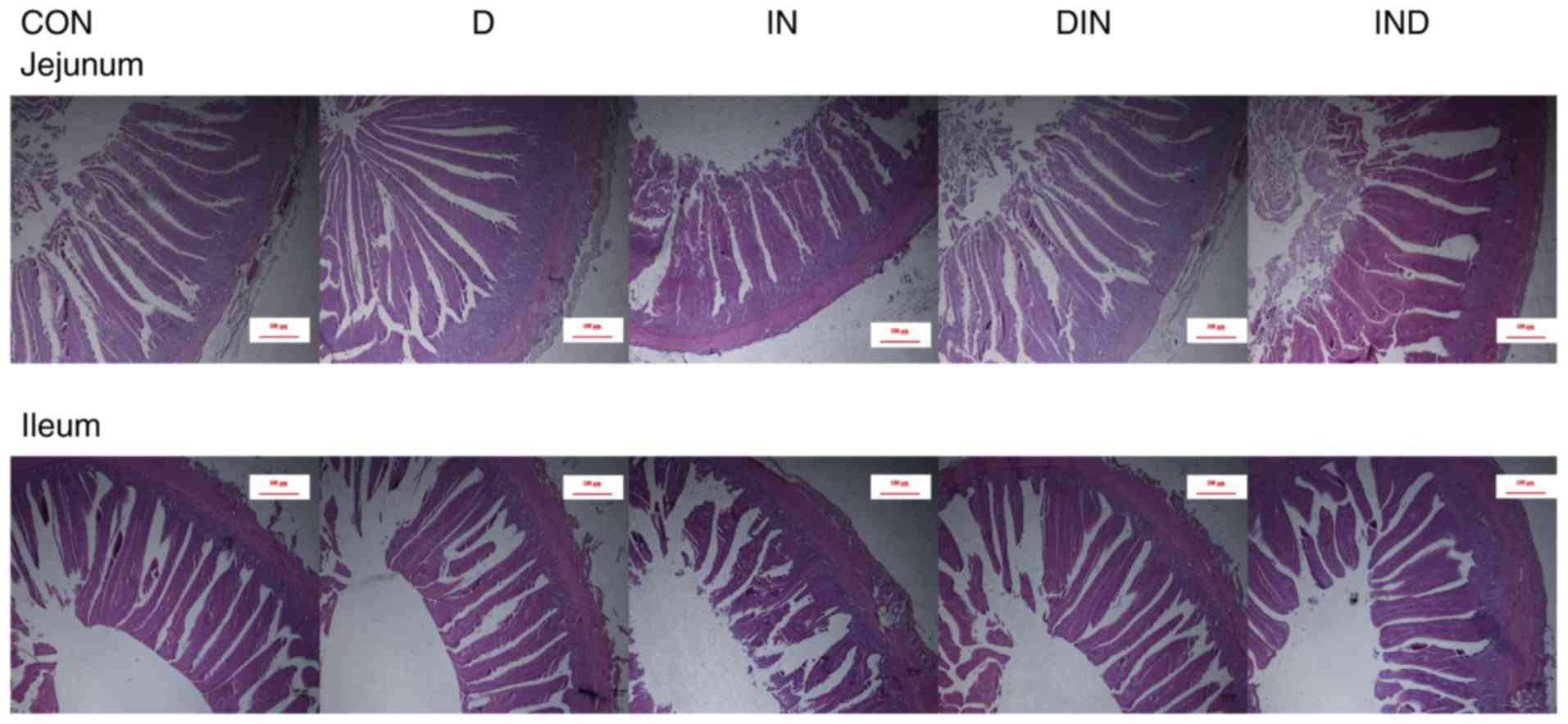
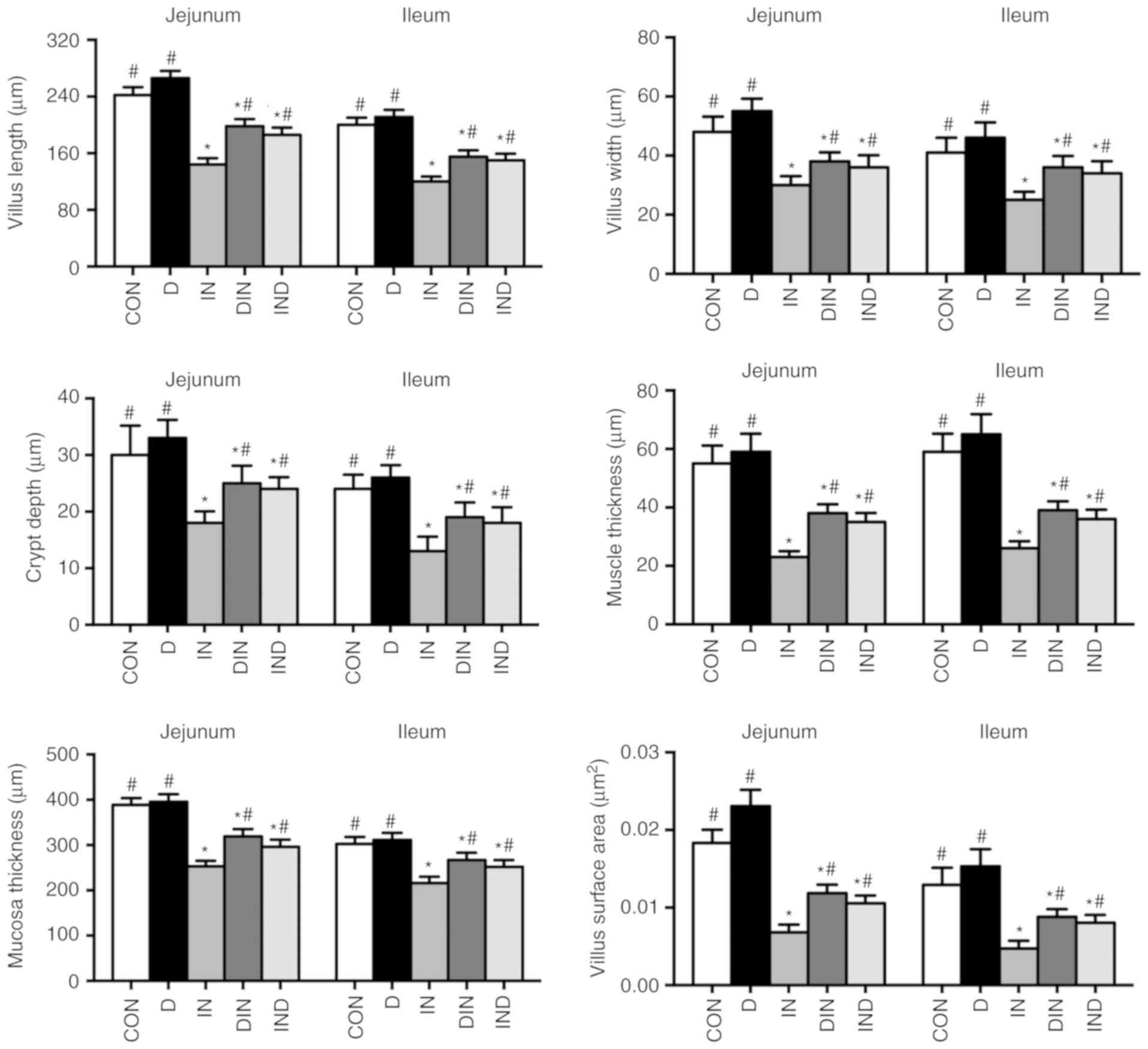
Histological analysis revealed that the villus
length, crypt depth, villus width, muscle thickness, mucosa
thickness, and villus area values of the small intestine (jejunum
and ileum) were lower (P<0.05) in the IN group, compared to the
CON group (Figs. 1 and 2). However, compared with mice in the IN
group, histological analysis showed that the L, crypt depth, W,
muscle thickness, mucosa thickness, and villus area values of the
small intestine were higher (P<0.05) in the DIN and IND groups
(Figs. 1 and 2). There was no significant difference
between the DIN and IND group in the histological analysis. The
results indicated that DMG-Na could prevent damage to the small
intestine.
Antioxidant enzymes assays
The effects of DMG-Na on serum and small intestinal
antioxidant capacity, including SOD, GSH-Px, GSH1, GR and MDA are
shown in Figs. 3 and 4. The IN group had lower activity levels
(P<0.05) of antioxidant enzymes and a higher (P<0.05)
concentration of MDA in both serum and the small intestine,
compared with the CON group. However, the DIN and IND groups had
higher activity levels (P<0.05) of antioxidant enzymes and a
lower (P<0.05) MDA concentration in serum and the small
intestine, compared with the IN group. There was no significant
difference between DIN and IND groups on the antioxidant enzymes
activity of mice small intestine. The results suggested that DMG-Na
could improve antioxidant capacity, thus to relieve the oxidative
damage in small intestine.
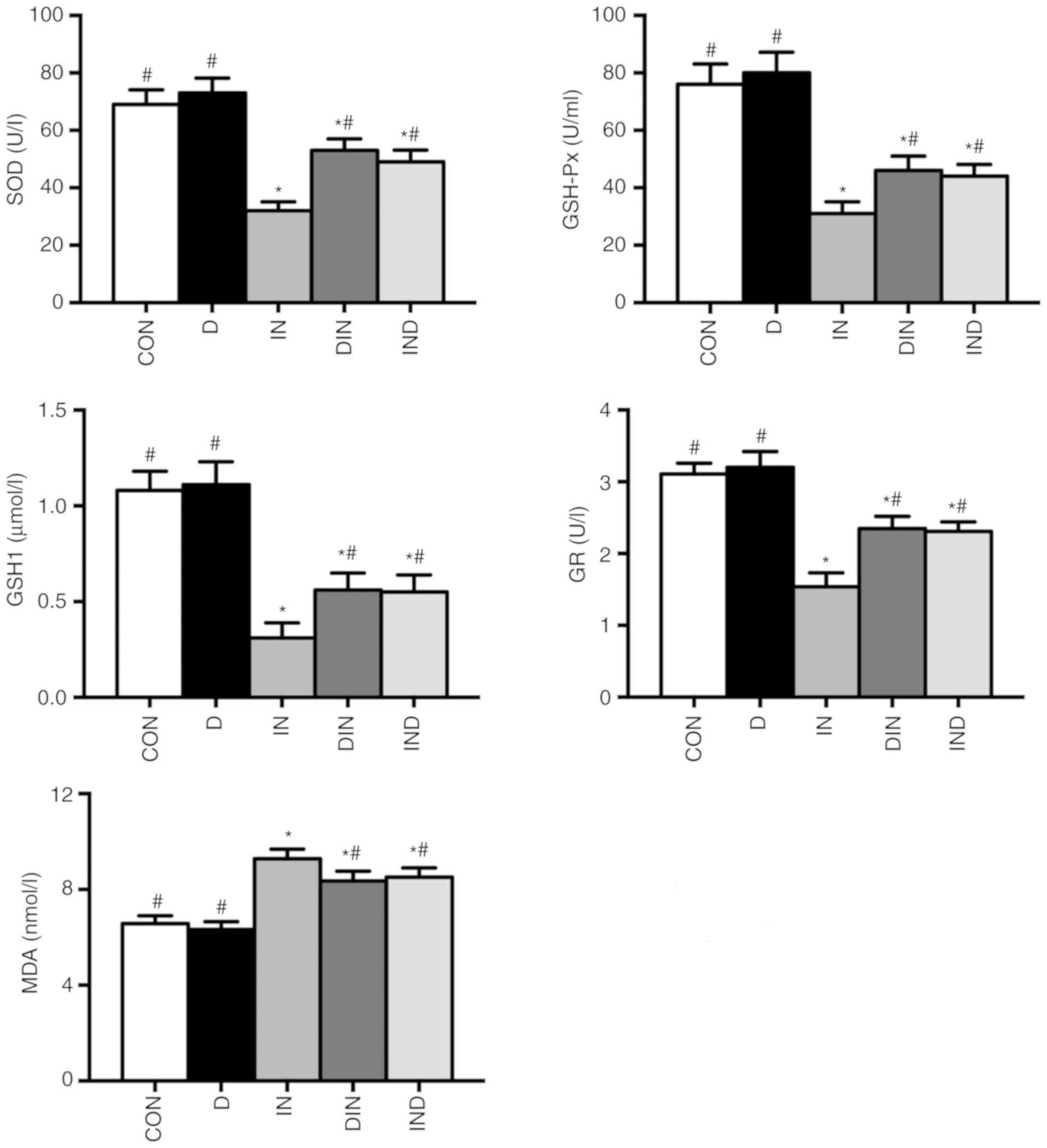 | Figure 3Effects of DMG-Na on serum
antioxidant capacity in indomethacin-injected mice. Data are
expressed as the mean ± standard error of the mean (n=20).
*P<0.05 vs. CON; #P<0.05 vs. IN.
DMG-Na, dimethylglycine sodium salt; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; GSH1, glutathione; GR, glutathione
reductase; MDA, malondialdehyde; CON, intubation and injection with
saline; D, intubation with DMG-Na and injection with saline; IN,
intubation with saline and injection with indomethacin; DIN,
intubation with DMG-Na and injection with indomethacin; IND,
injection with indomethacin and intubation with DMG-Na. |
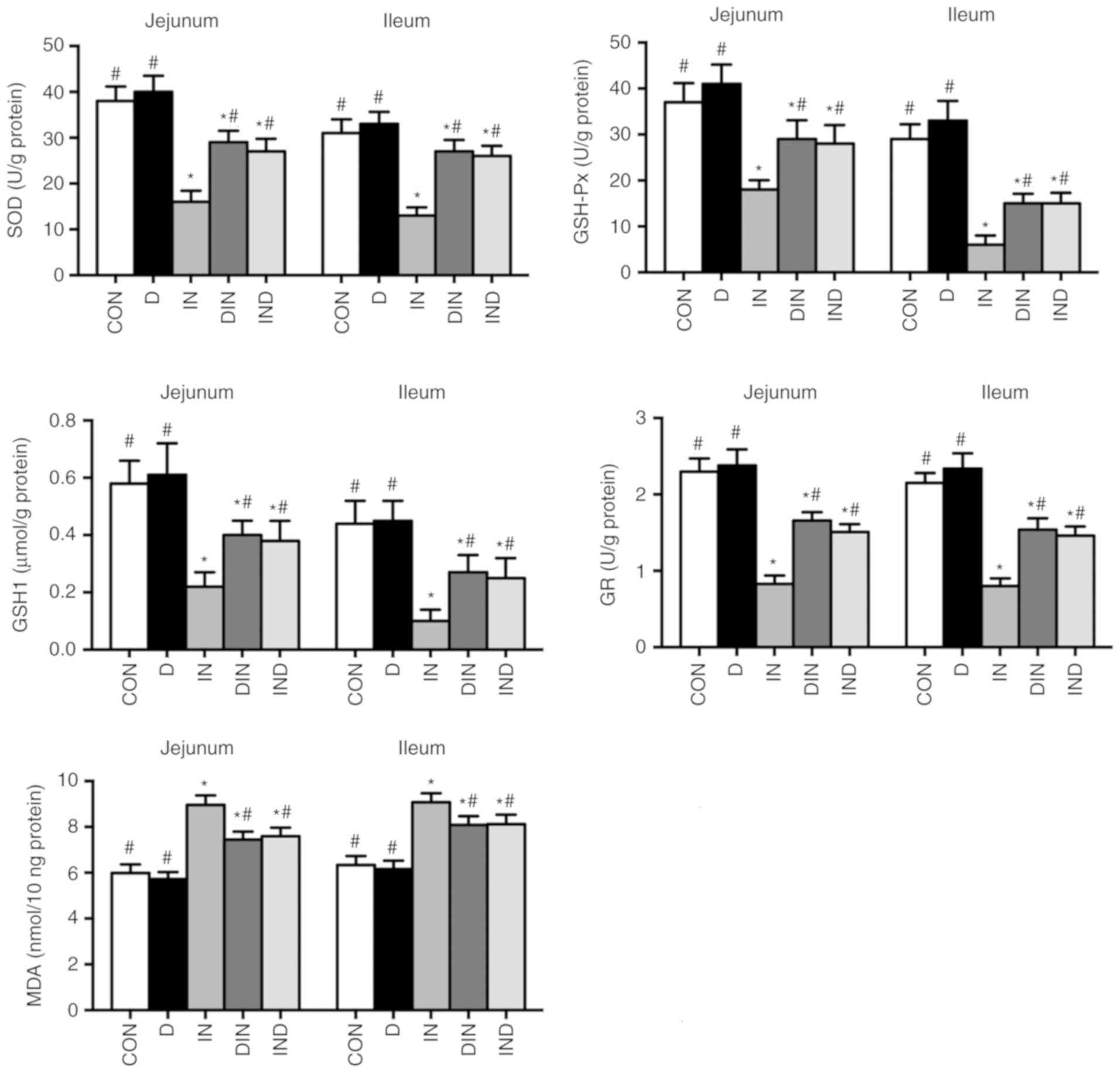 | Figure 4Effects of DMG-Na on small intestinal
antioxidant capacity in indomethacin-injected mice. Data are
expressed as the mean ± standard error of the mean (n=20).
*P<0.05 vs. CON; #P<0.05 vs. IN.
DMG-Na, dimethylglycine sodium salt; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; GSH1, glutathione; GR, glutathione
reductase; MDA, malondialdehyde; CON, intubation and injection with
saline; D, intubation with DMG-Na and injection with saline; IN,
intubation with saline and injection with indomethacin; DIN,
intubation with DMG-Na and injection with indomethacin; IND,
injection with indomethacin and intubation with DMG-Na. |
Small intestine mitochondrial antioxidant
enzyme assays
The IN group had lower (P<0.05) levels of MnSOD,
GPx, GSH2, GR and γ-GCL in mitochondria from the small intestine,
compared with the CON group (Fig.
5). The DIN and IND groups, however, had higher (P<0.05)
levels of MnSOD, GPx, GSH2, GR, and γ-GCL in small intestinal
mitochondria, compared with the IN group. There was no significant
difference between DIN and IND groups. The results showed that
DMG-Na could suppress the oxidative damage via enhancing
mitochondrial antioxidant capacity in small intestine.
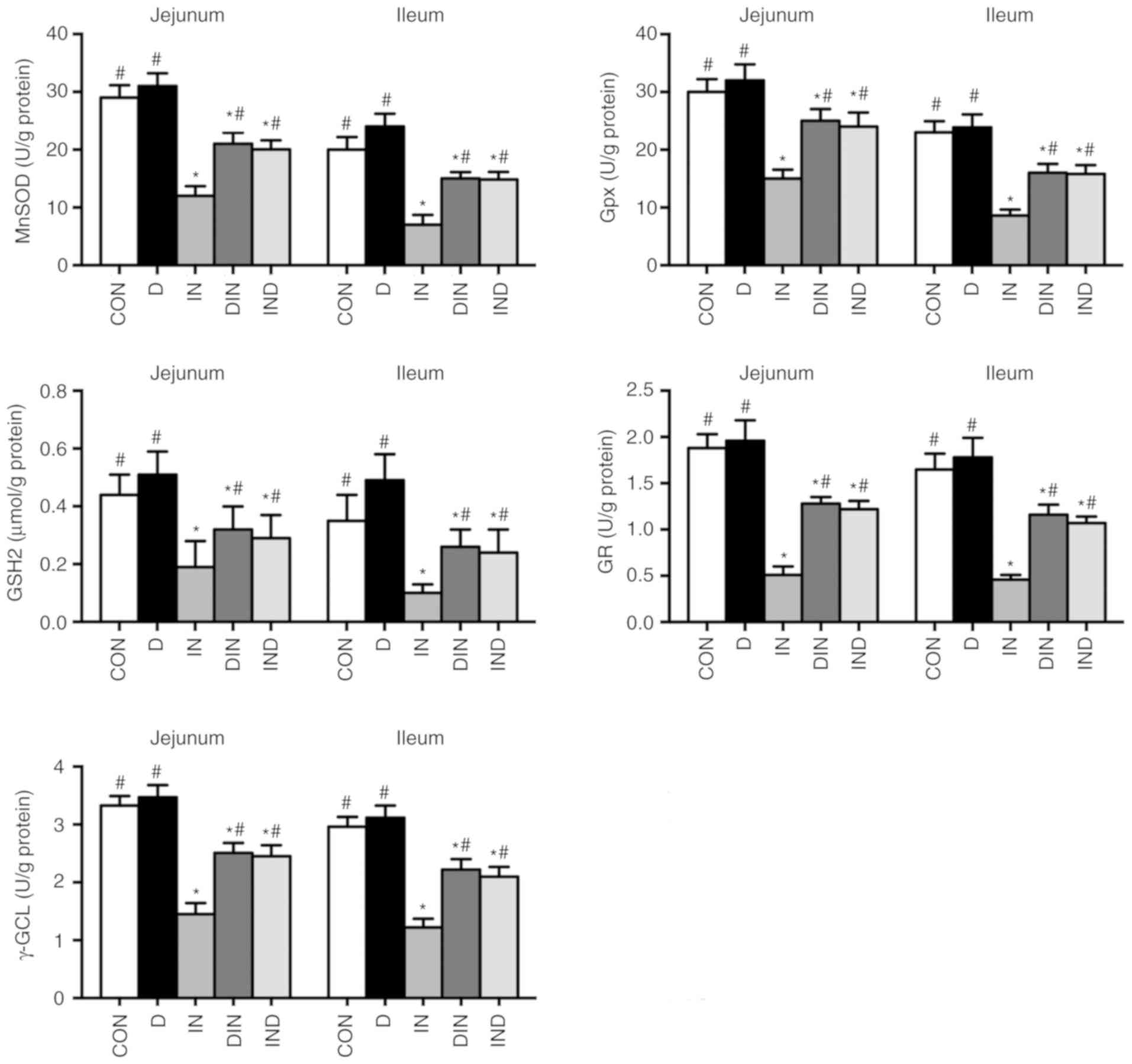 | Figure 5Effects of DMG-Na on small intestine
mitochondrial antioxidant capacity in indomethacin-injected mice.
Data are expressed as the mean ± standard error of the mean (n=20).
*P<0.05 vs. CON; #P<0.05 vs. IN. MnSOD,
manganese superoxide dismutase; GPx, glutathione peroxidase; GSH2,
glutathione; GR, glutathione reductase; γ-GCL, γ-glutamylcysteine
ligase; DMG-Na, dimethylglycine sodium salt; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; GSH1, glutathione; GR,
glutathione reductase; MDA, malondialdehyde; CON, intubation and
injection with saline; D, intubation with DMG-Na and injection with
saline; IN, intubation with saline and injection with indomethacin;
DIN, intubation with DMG-Na and injection with indomethacin; IND,
injection with indomethacin and intubation with DMG-Na. |
Oxidative damage assays
Higher (P<0.05) levels of ROS, PC, 8-OHdG,
apoptosis, and necrosis, as well as a higher ATP/mitochondrial DNA
(mtDNA) ratio and lower (P<0.05) levels of MMP, ATP and mtDNA
were observed in the IN group, compared with the CON group
(Fig. 6). The opposite effects
were observed in the DIN and IND groups, compared with the IN group
(P<0.05; Fig. 6). There was no
significant difference between DIN and IND groups. In the present
study, DMG-Na could relieve the oxidative damage possibly due to
the increasing of antioxidant capacity in small intestine.
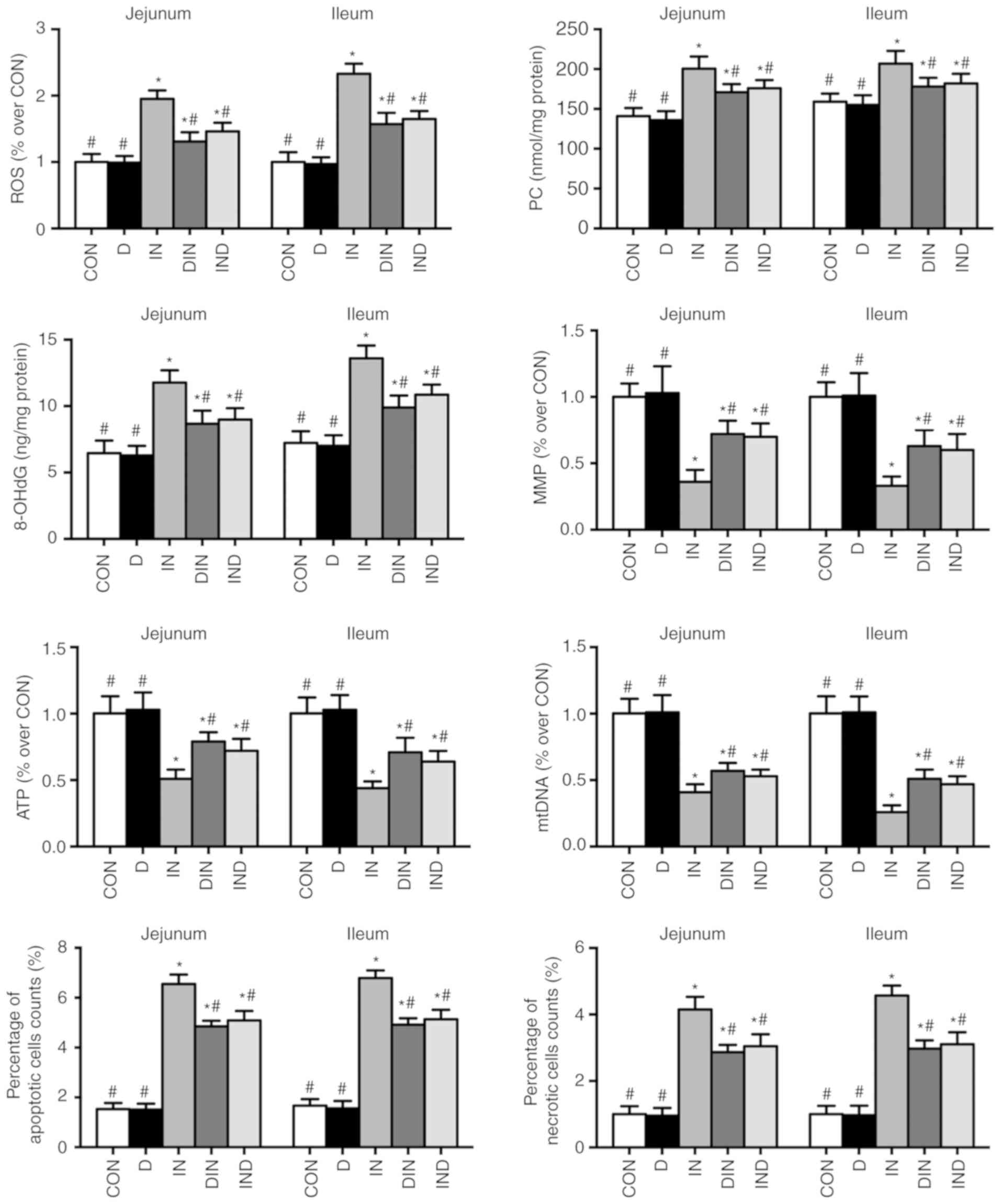 | Figure 6Effects of DMG-Na on small intestinal
oxidative damage in indomethacin-injected mice. Data are expressed
as the mean ± standard error of the mean (n=20).
*P<0.05 vs. CON; #P<0.05 vs. IN. ROS,
reactive oxygen species; PC, protein carbonyls; 8-OHdG,
8-hydroxy-2-deoxyguanosine; MMP, mitochondrial membrane potential;
ATP, adenosine triphosphate; mtDNA, mitochondria DNA; DMG-Na,
dimethylglycine sodium salt; CON, intubation and injection with
saline; D, intubation with DMG-Na and injection with saline; IN,
intubation with saline and injection with indomethacin; DIN,
intubation with DMG-Na and injection with indomethacin; IND,
injection with indomethacin and intubation with DMG-Na. |
Gene expression
The expression of antioxidant-associated genes in
the small intestine (Nrf2, HO-1, CLDN2,
CLDN3, Cu/ZnSOD, GSH-Px, Sirt1,
γ-GCLc, γ-GCLm, OCLN and ZO1) and in
the small intestinal mitochondria (Trx2, Trx-R2,
Prx3 and MnSOD) was lower (P<0.05) in the IN
group, compared with the CON group (Fig. 7). Expression of the same
antioxidant-associated genes in the small intestine and small
intestinal mitochondria was higher (P<0.05) in the DIN and IND
groups, compared with the IN group (Fig. 7). There was no significant
difference between DIN group and IND groups.
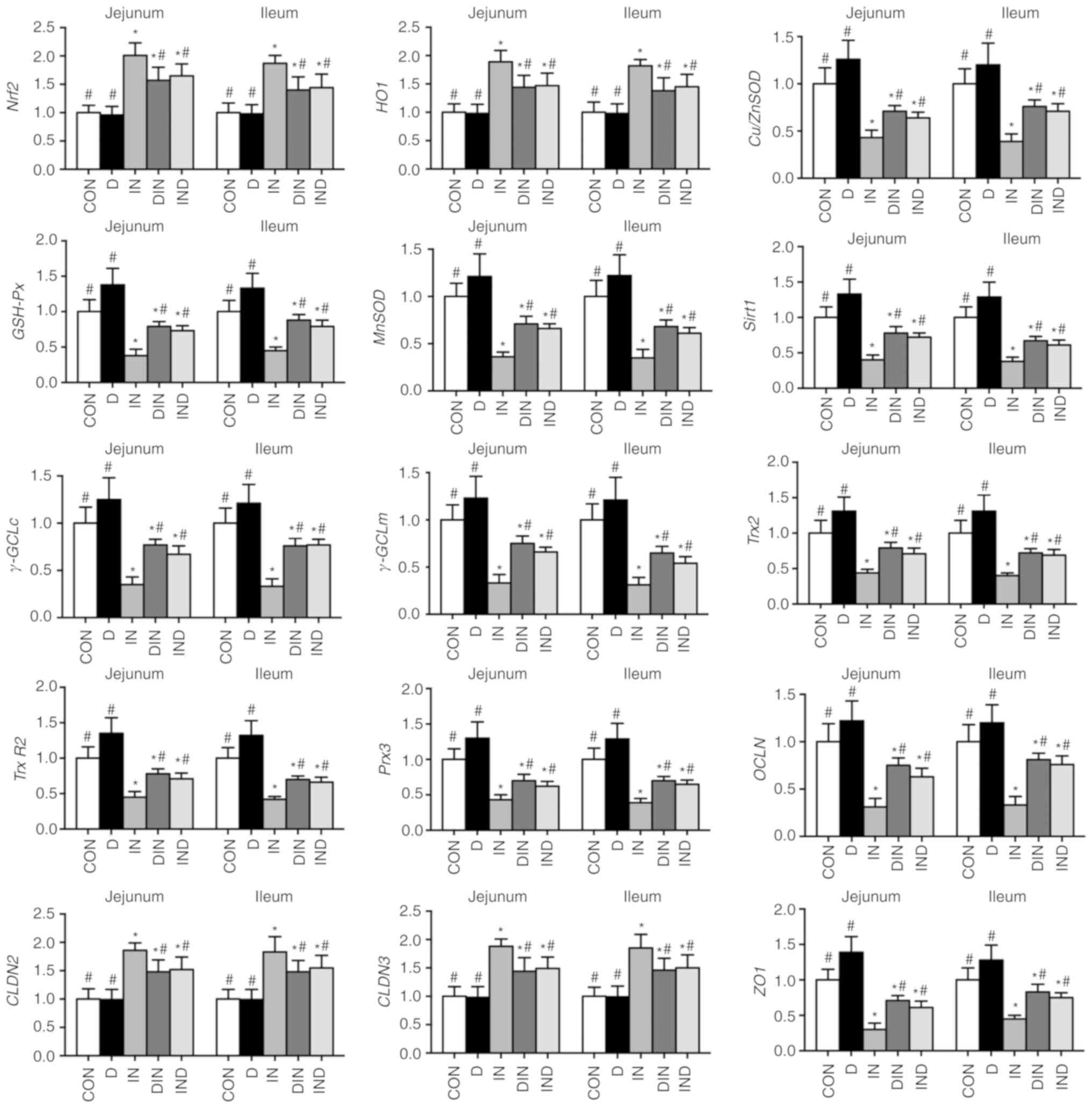 | Figure 7Effects of DMG-Na on small intestine
gene expression in indomethacin-injected mice. Data are expressed
as the mean ± standard error of the mean (n=20).
*P<0.05 vs. CON; #P<0.05 vs. IN.
Nrf2, nuclear factor erythroid 2-related factor 2;
HO1, heme oxygenase 1; Cu/ZnSOD, copper and zinc
superoxide dismutase; GSH-Px, glutathione peroxidase;
MnSOD, manganese superoxide dismutase; Sirt1, sirtuin
1; γ-GCLc; γ-glutamylcysteine ligase c; γ-GCLm,
γ-glutamylcysteine ligase m; Trx2, thioredoxin 2;
Trx-R2, thioredoxin reductase 2; Prx3, peroxiredoxin
3; OCLN, occluding; CLDN, claudin; ZO1, zonula
occludens-1; DMG-Na, dimethylglycine sodium salt; CON, intubation
and injection with saline; D, intubation with DMG-Na and injection
with saline; IN, intubation with saline and injection with
indomethacin; DIN, intubation with DMG-Na and injection with
indomethacin; IND, injection with indomethacin and intubation with
DMG-Na. |
Protein expression
The IN group had lower (P<0.05) Sirt1 and higher
(P<0.05) Nrf2 and HO-1 expression (Fig. 8), compared with the CON group. The
DIN and IND groups had higher (P<0.05) Sirt1 expression
accompanied by lower (P<0.05) Nrf2 and HO-1 expression, compared
with the IN group (Fig. 8). There
was no significant difference between the DIN and IND groups. In
the current study, DMG-Na could improve the antioxidant-related
gene and protein level, which was consistent with the above results
of antioxidant system, thus confirmed its used as an antioxidant to
improve the oxidative damage in small intestine.
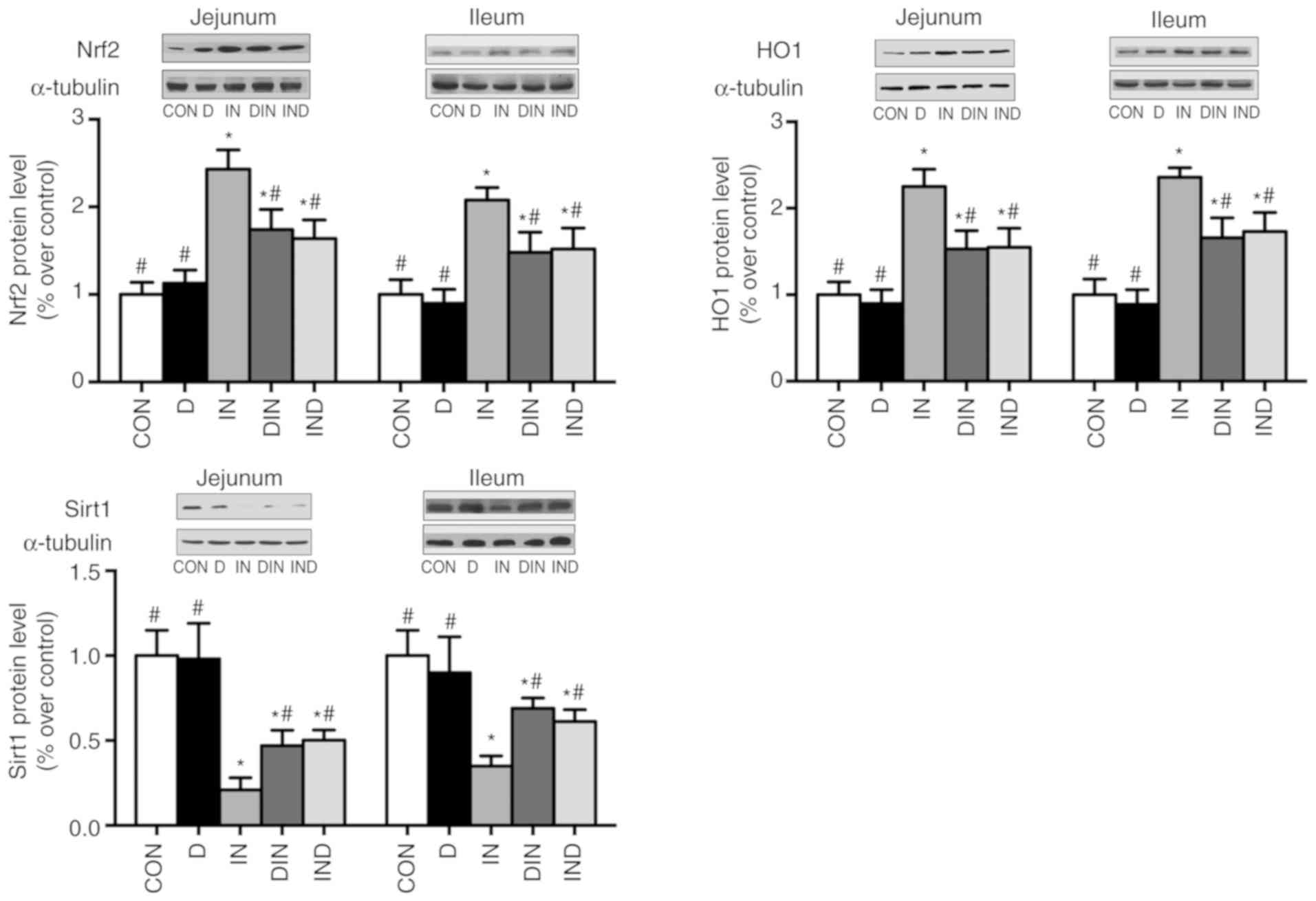 | Figure 8Effects of DMG-Na on Nrf2, HO1, and
Sirt1 protein level of small intestine in indomethacin-injected
mice. Data are expressed as the mean ± standard error of the mean
(n=20). *P<0.05 vs. CON; #P<0.05 vs.
IN. Nrf2, nuclear factor erythroid 2-related factor 2; HO1, heme
oxygenase 1; Sirt1, sirtuin 1; DMG-Na, dimethylglycine sodium salt;
CON, intubation and injection with saline; D, intubation with
DMG-Na and injection with saline; IN, intubation with saline and
injection with indomethacin; DIN, intubation with DMG-Na and
injection with indomethacin; IND, injection with indomethacin and
intubation with DMG-Na. |
Discussion
The small intestine, which is the main organ
involved in digestion and the absorption of nutrients, is the first
line of defense to protect the body from oxidative damage induced
by external pathogenic microorganisms and toxins (28). Substances depend on diffusion or
active transport to cross cell membranes in the small intestine,
and this movement is regulated by the structure of the cell-to-cell
contacts (29). Thus, good
intestinal morphology is crucial for maintaining health. It has
previously been shown that DMG, which is a raw material for the
synthesis of glutathione, is an important antioxidant required to
simultaneously maintain the normal histological morphology of the
small intestine and to protect the body from oxidative damage
(8). The protective effect of DMG
against oxidative damage to cells has also been shown in another
study (30), and this may be one
potential explanation for the better histological morphology of the
small intestine in mice from the DMG-Na groups, compared with that
in the IN groups. In the present study, DMG-Na administered to mice
as a preventative measure or as a treatment for IN exposure,
improved the histological morphology of the small intestine
compared to that of mice given IN alone, suggesting that DMG-Na
could prevent or reverse damage to the small intestine induced by
the injected IN.
Oxidative damage increases the level of ROS, which
decreases antioxidant capacity and disrupts the mitochondrial
structure (31). The tissue
damage can be improved by activity of the SOD enzyme in cells,
which catalyzes the conversion of endogenous superoxide anions to
hydrogen peroxide through disproportionation, which is finally
neutralized by the intracellular GSH-Px enzyme (31). The mitochondrial antioxidant
system is composed of enzymes and non-enzymatic antioxidants that
protect the body from oxidative damage (32). It has been reported that the MnSOD
enzyme and GSH-related metabolic enzymes are crucial for
suppressing oxidative damage in mitochondria (33). The γ-GCL enzyme is an important
rate-limiting enzyme in the synthesis of γ-glutamylcysteine that is
crucial in relieving oxidative damage (18). Previous studies have demonstrated
that DMG could act as an important additive to improve the body's
antioxidant capacity (8,10). In support of this, the present
study reported that DMG-Na, given for prevention or as a treatment,
reduced oxidative damage in the small intestines of mice by
increasing their antioxidant capacity. It was speculated that
DMG-Na may have improved antioxidant capacity by scavenging excess
ROS, thereby maintaining the balance of the intracellular redox
state.
In the oxidative damage model of the mouse small
intestine, blockade of the oxidative phosphorylation reaction
increased ROS levels (34).
Excessive ROS generation damages biological macromolecules, such as
membrane lipids and proteins, and exacerbates oxidative damage
through lipid peroxidation and protein oxidation reactions
(35). In conditions of oxidative
damage, excessive ROS may destroy the structural integrity of the
mitochondrial bilayer by damaging unsaturated fatty acids and
structural proteins in the mitochondrial membrane (36). The present study showed that
oxidative damage in the mouse small intestine resulted in a higher
concentration of MDA, PC, 8-OHdG and ROS compared with control
group mice. It has previously been shown that DMG, a natural
antioxidant, effectively neutralizes free radicals to alleviate
oxidative damage (5). Therefore,
it was speculated that DMG-Na may inhibit lipid peroxidation and
protein oxidation reactions by scavenging excess ROS.
The level of MMP, which is negatively associated
with ROS concentration, is an indicator of mitochondria-dependent
apoptosis. As the level of MMP decreases, mitochondrial oxida-tive
phosphorylation becomes uncoupled, which results in an increase in
ROS levels and excessive ATP consumption (32). ATP is the product of the oxidative
phosphorylation reaction associated with mitochondrial function
(37), and it also acts as an
important substrate for RNA synthesis (38). Mitochondria have their own mtDNA
genome that is responsible for the synthesis and regulation of
proteins required for mitochondrial function. Alterations in mtDNA
copy number serve as indicators of mitochondrial dysfunction, which
contributes to many diseases (39). Previous studies have reported that
oxidative damage could decrease the mtDNA copy number and damage
the mitochondrial DNA by altering the reductive environment of
cells and mitochondria (39,40). The present study also indicated
that the lower level of apoptosis may be due to suppression by
DMG-Na of oxidative damage, which verifies the results of previous
studies that suggested natural antioxidants inhibit apoptosis by
protecting cells from oxidative damage (8,41).
Furthermore, the present study showed that DMG-Na prevention and
treatment reduced the effects of oxidative damage on MMP, ATP,
mtDNA and apoptosis in the small intestine of mice. Considering the
current results, it was hypothesized that there was a correlation
between the anti-apoptotic effect of DMG-Na and the protective
effects of DMG-Na on mitochondrial function in the damaged small
intestines of mice.
The present study demonstrated that DMG-Na increased
the expression of antioxidant-associated genes in the mouse model
of small intestinal damage. Activation of the Nrf2 (42), HO1 (43) and SIRT1 (44) genes is important in reducing
oxidative damage, and also for regulating the expression of genes
encoding antioxidant enzymes. The mitochondria are rich in
Trx2 (45), Trx-R2
(46) and Prx3 (46) that compose a unique antioxidant
system to relieve oxidative damage by scavenging free radicals and
regulating the mitochondria-dependent apoptotic pathways (47). It has been indicated that the gene
ZO1 is correlated with paracellular permeability.
Furthermore, together with the genes OCLN and CLDN
(48) from the same gene family,
it is a key regulator of intestinal permeability, which indirectly
shows the level of oxidative damage (49). The expression of
antioxidant-associated genes in the small intestine of mice
administered DMG-Na as a prevention or a treatment was increased,
compared with mice in the IN group. This is in line with previous
studies that suggested natural antioxidants are beneficial in the
regulation of antioxidant-associated gene expression (10,41). The current study indicated that
the regulation of antioxidant-associated genes following prevention
and treatment with DMG-Na may be one possible mechanism by which
oxidative damage was reduced and mitochondrial function maintained
in the small intestines of mice. Some positive results have been
reported in this manuscript, however, further study is required to
determine the specific mechanisms by which DMG-Na improves small
intestine damage.
In conclusion, the present study demonstrated that
DMG-Na was a potential antioxidant that effectively alleviated
indomethacin-induced damage in the small intestines of mice. Both
prevention and treatment with DMG-Na scavenged excess ROS,
prevented mitochondrial dysfunction and inhibited cell apoptosis
that was induced by oxidative damage. It was speculated that DMG-Na
directly neutralized the excessive free radicals generated, and
acted indirectly to improve the activity of antioxidant enzymes and
to inhibit the abnormal expression of stress-associated factors. Of
note, the results highlighted that the effects of DMG-Na when
administered as a preventative measure were stronger than when
DMG-Na was administered as a treatment. This therefore suggested
that DMG-Na served as a health-promoting preventative additive and
could be used in the field of disease prevention.
Abbreviations:
|
DMG-Na
|
dimethylglycine sodium salt
|
|
BW
|
body weight
|
|
ROS
|
reactive oxygen species
|
|
L
|
villus length
|
|
W
|
villus width
|
|
SOD
|
superoxide dismutase
|
|
GSH-Px
|
glutathione peroxidase
|
|
GSH
|
glutathione
|
|
GR
|
glutathione reductase
|
|
MnSOD
|
manganese superoxide dismutase
|
|
GPx
|
glutathione peroxidase
|
|
γ-GCL
|
γ-Glutamylcysteine ligase
|
|
MDA
|
malondialdehyde
|
|
PC
|
protein carbonyls
|
|
8-OHdG
|
8-hydroxy-2-deoxyguanosine
|
|
MMP
|
mitochondrial membrane potential
|
|
ATP
|
adenosine triphosphate
|
|
mtDNA
|
mitochondria DNA
|
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KB and LJ analyzed and interpreted the data. KB, LJ,
SZ, CF, YZ, LZ and TW performed the experiments, and LJ was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Animal Care and Use Committee of Nanjing Agricultural University
(Nanjing, Jiangsu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ma K, Zhang Y, Zhu D and Lou Y: Protective
effects of asiatic acid against
D-galactosamine/lipopolysaccharide-induced hepatotoxicity in
hepatocytes and kupffer cells co-cultured system via
redox-regulated leukotriene C4 synthase expression pathway. Eur J
Pharmacol. 603:98–107. 2009. View Article : Google Scholar
|
|
2
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue J, Miki I, Tanahashi T, Kawauchi S,
Azuma T and Mizuno S: Mo2016 Effect of ghrelin on
indomethacin-induced Ssmall intestinal injury in mice.
Gastroenterology. 144(Suppl 1): S7192013. View Article : Google Scholar
|
|
5
|
Hariganesh K and Prathiba J: Effect of
dimethylglycine on gastric ulcers in rats. J Pharm Pharmacol.
52:1519–1522. 2000. View Article : Google Scholar
|
|
6
|
Cupp MJ and Tracy TS: Dimethylglycine (N,
N-Dimethylglycine). Dietary Suppl. 149–160. 2003.
|
|
7
|
Pere C and Maria RI: Amino acid-based
surfactants: Enzymatic synthesis, properties and potential
applications. Cheminform. 20:215–233. 2009.
|
|
8
|
Friesen RW, Novak EM, Hasman D and Innis
SM: Relationship of dimethylglycine, choline, and betaine with
oxoproline in plasma of pregnant women and their newborn infants. J
Nutr. 137:2641–2646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clapes P and Infante MR: Amino acid-based
surfactants: Enzymatic synthesis, properties and potential
applications. Cheminform. 20:215–233. 2009.
|
|
10
|
Bai KW, Xu W, Zhang JF, Kou T, Niu Y, Wan
X, Zhang L, Wang C and Wang T: Assessment of free radical
scavenging activity of dimethylglycine sodium salt and its role in
providing protection against lipopolysaccharide-induced oxidative
stress in mice. PLoS One. 11:e01553932016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma P, Wu Y, Zeng Q, Gan Y, Chen J, Ye X
and Yang X: Oxidative damage induced by chlorpyrifos in the hepatic
and renal tissue of Kunming mice and the antioxidant role of
vitamin E. Food Chem Toxicol. 58:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long M, Yang SH, Han JX, Li P, Zhang Y,
Dong S, Chen X, Guo J, Wang J and He JB: The protective effect of
grape-seed proanthocyanidin extract on oxidative damage induced by
zearalenone in Kunming mice liver. Int J Mol Sci. 17:8082016.
View Article : Google Scholar :
|
|
13
|
Long M, Liu Y, Cao Y, Wang N, Dang M and
He J: Proanthocyanidins attenuation of chronic lead-induced liver
oxidative damage in kunming mice via the Nrf2/ARE pathway.
Nutrients. 8:E6562016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong L, Zhong X, He JT, Zhang L, Bai K, Xu
W, Wang T and Huang X: Supplementation of tributyrin improves the
growth and intestinal digestive and barrier functions in
intrauterine growth-restricted piglets. Clin Nutr. 35:399–407.
2016. View Article : Google Scholar
|
|
15
|
Panchenko LF, Brusov OS, Gerasimov AM and
Loktaeva TD: Intramitochondrial localization and release of rat
liver super-oxide dismutase. FEBS Lett. 55:84–87. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lawrence RA and Burk RF: Glutathione
peroxidase activity in selenium-deficient rat liver. Biochem
Biophys Res Commun. 71:952–958. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roediger WE and Truelove SC: Method of
preparing isolated colonic epithelial cells (colonocytes) for
metabolic studies. Gut. 20:484–488. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langston JW, Li W, Harrison L and Aw TY:
Activation of promoter activity of the catalytic subunit of
γ-glutamylcysteine ligase (GCL) in brain endothelial cells by
insulin requires antioxidant response element 4 and altered
glycemic status: Implication for GCL expression and GSH synthesis.
Free Radic Biol Med. 51:1749–1757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Remmen H, Ikeno Y, Hamilton M,
Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ,
Huang TT, et al: Life-long reduction in MnSOD activity results in
increased DNA damage and higher incidence of cancer but does not
accelerate aging. Physiol Genomics. 16:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Botsoglou NA, Fletouris DJ, Papageorgiou
GE, Vassilopoulos VN, Mantis AJ and Trakatellis AG: Rapid,
sensitive, and specific thiobarbituric acid method for measuring
lipid peroxidation in animal tissue, food, and feedstuff samples. J
Agric Food Chem. 42:1931–1937. 1994. View Article : Google Scholar
|
|
21
|
Wei QY, Chen WF, Zhou B, Yang L and Liu
ZL: Inhibition of lipid peroxidation and protein oxidation in rat
liver mitochondria by curcumin and its analogues. Biochim Biophys
Acta. 1760:70–77. 2006. View Article : Google Scholar
|
|
22
|
Sang H, Zhang L and Li J:
Anti-benzopyrene-7,8-diol-9,10-epoxide induces apoptosis via
mitochondrial pathway in human bronchiolar epithelium cells
independent of the mitochondria permeability transition pore. Food
Chem Toxicol. 50:2417–2423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Zou P, Zhan H, Zhang M, Zhang L,
Ge RS and Huang Y: Dihydrolipoamide dehydrogenase and cAMP are
associated with cadmium-mediated Leydig cell damage. Toxicol Lett.
205:183–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddhuraju P and Manian S: The antioxidant
activity and free radical-scavenging capacity of dietary phenolic
extracts from horse gram [Macrotyloma uniflorum (Lam.) Verdc.]
seeds. Food Chem. 105:950–958. 2007. View Article : Google Scholar
|
|
25
|
Liu H, Jiang Y, Luo Y and Jiang W: A
simple and rapid determination of ATP, ADP and AMP concentrations
in pericarp tissue of litchi fruit by high performance liquid
chromatography. Food Technol Biotechnol. 44:531–534. 2006.
|
|
26
|
Cavelier L, Johannisson A and Gyllensten
U: Analysis of mtDNA copy number and composition of single
mitochondrial particles using flow cytometry and PCR. Exp Cell Res.
259:79–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Chen D, Yao Y, Yu B, Mao X, He J,
Huang Z and Zheng P: Intrauterine growth retardation increases the
susceptibility of pigs to high-fat diet-induced mitochondrial
dysfunction in skeletal muscle. PLoS One. 7:e348352012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nusrat A, Parkos CA, Verkade P, Foley CS,
Liang TW, Innis-Whitehouse W, Eastburn KK and Madara JL: Tight
junctions are membrane microdomains. J Cell Sci. 113:1771–1781.
2000.PubMed/NCBI
|
|
30
|
Look MP, Riezler R, Berthold HK, Stabler
SP, Schliefer K, Allen RH, Sauerbruch T and Rockstroh JK: Decrease
of elevated N,N-dimethylglycine and N-methylglycine in human
immunodeficiency virus infection during short-term highly active
antiretroviral therapy. Metabolism. 50:1275–1281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca
AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E and Akcay A: Curcumin
protects against ischemia/reperfusion injury in rat kidneys. World
J Urol. 26:285–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kowaltowski AJ and Vercesi AE:
Mitochondrial damage induced by conditions of oxidative stress.
Free Radic Biol Med. 26:463–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang Y, Chao G, Xing M, Li Y, Zhu L, Wang
D, Yang X, Liu L and Yao P: Quercetin prevents ethanol-induced
dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol.
50:1194–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY,
Park YS, Kwon Y, Choi KH, Jo I, Park SI, et al: The role of
STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial
transmembrane potential during hepatic cell death induced by
LPS/d-GalN. J Mol Biol. 369:967–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilhelm EA, Jesse CR, Roman SS, Nogueira
CW and Savegnago L: Hepatoprotective effect of 3-alkynyl
selenophene on acute liver injury induced by D-galactosamine and
lipopoly-saccharide. Exp Mol Pathol. 87:20–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen JJ and Yu BP: Alterations in
mitochondrial membrane fluidity by lipid peroxidation products.
Free Radic Biol Med. 17:411–418. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang R, Kang KA, Piao MJ, Chang WY, Maeng
YH, Chae S, Lee IK, Kim BJ and Hyun JW: Butin reduces oxidative
stress-induced mitochondrial dysfunction via scavenging of reactive
oxygen species. Food Chem Toxicol. 48:922–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng T, Zhang CL, Zhu ZP, Yu LH, Zhao XL
and Xie KQ: Diallyl trisulfide (DATS) effectively attenuated
oxidative stress-mediated liver injury and hepatic mitochondrial
dysfunction in acute ethanol-exposed mice. Toxicology. 252:86–91.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK,
Ma YS and Wei YH: Oxidative stress-related alteration of the copy
number of mitochondrial DNA in human leukocytes. Free Radic Res.
37:1307–1317. 2003. View Article : Google Scholar
|
|
40
|
Morikawa A, Sugiyama T, Kato Y, Koide N,
Jiang GZ, Takahashi K, Tamada Y and Yokochi T: Apoptotic cell death
in the response of D-galactosamine-sensitized mice to
lipopoly-saccharide as an experimental endotoxic shock model.
Infect Immun. 64:734–738. 1996.PubMed/NCBI
|
|
41
|
Zhang J, Xu L, Zhang L, Ying Z, Su W and
Wang T: Curcumin attenuates
D-galactosamine/lipopolysaccharide-induced liver injury and
mitochondrial dysfunction in mice. J Nutr. 144:1211–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kode A, Rajendrasozhan S, Caito S, Yang
SR, Megson IL and Rahman I: Resveratrol induces glutathione
synthesis by activation of Nrf2 and protects against cigarette
smoke-mediated oxidative stress in human lung epithelial cells. Am
J Physiol Lung Cell Mol Physiol. 294:L478–L488. 2008. View Article : Google Scholar
|
|
43
|
Morse D, Lin L, Choi AM and Ryter SW: Heme
oxygenase-1, a critical arbitrator of cell death pathways in lung
injury and disease. Free Radic Biol Med. 47:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hwang J, Yao H, Caito S, Sundar IK and
Rahman I: Redox regulation of sirt1 in inflammation and cellular
senescence. Free Radic Biol Med. 61:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holmgren A, Johansson C, Berndt C, Lönn
ME, Hudemann C and Lillig CH: Thiol redox control via thioredoxin
and glutare-doxin systems. Biochem Soc Trans. 33:1375–1377. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pérez VI, Lew CM, Cortez LA, Webb CR,
Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, et al:
Thioredoxin 2 haploinsufficiency in mice results in impaired
mitochondrial function and increased oxidative stress. Free Radic
Biol Med. 44:882–892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Michelet L, Zaffagnini M, Massot V, Keryer
E, Vanacker H, Miginiac-Maslow M, Issakidis-Bourguet E and Lemaire
SD: Thioredoxins, glutaredoxins, and glutathionylation: New
cross-talks to explore. Photosynth Res. 89:225–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zeissig S, Bürgel N, Günzel D, Richter J,
Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M and
Schulzke JD: Changes in expression and distribution of claudin 2, 5
and 8 lead to discontinuous tight junctions and barrier dysfunction
in active Crohn's disease. Gut. 56:61–72. 2007. View Article : Google Scholar
|
|
49
|
Gu L, Li N, Gong J, Li Q, Zhu W and Li J:
Berberine ameliorates intestinal epithelial tight-junction damage
and down-regulates myosin light chain kinase pathways in a mouse
model of endotoxinemia. J Infect Dis. 203:1602–1612. 2011.
View Article : Google Scholar : PubMed/NCBI
|