Introduction
Doxorubicin (Dox) is an anthracycline, which are
effective anticancer drugs widely used in clinical practice
(1). Despite the effectiveness of
Dox against cancer, it is associated with severe dose-limiting
cardiotoxicity, which typically manifests as subclinical cardiac
dysfunction or clinical heart failure at the end of chemotherapy or
after several years (2). This
late-onset form of anthracycline cardiotoxicity is referred to as
chronic and has a complex pathogenesis (3,4).
Cardiomyocyte senescence has been previously suggested as a
mechanism underlying Dox-induced cardiotoxicity (5), which can, at least partly, result in
cardiac remodeling and dysfunction (6). Therefore, senescence is pivotal to
the progression of delayed anthracy-cline cardiomyopathy in mice
(7), as well as in humans
(8). Accordingly, regulation of
cardiomyocyte senescence may promote the development of
cardioprotective strategies and reduce the incidence and mortality
of cardiovascular disease in the early and late stages of cancer
treatment (9). However, the
clinical effectiveness of anti-senescence therapies remains poor.
Therefore, investigating novel therapeutic strategies to alleviate
Dox-induced cardiomyocyte senescence remains a major challenge.
Honokiol (Hnk) is an effective ingredient extracted
from the bark of Magnolia officinalis and is widely used in
Chinese medicine. In previous studies, Hnk was found to have a wide
spectrum of pharmacological properties, such as antitumor (10), antibacterial (11) and antihypertensive (12). In addition, recent studies
demonstrated that Hnk can protect against pressure
overload-mediated cardiac hypertrophy (13), myocardial ischemia/reperfusion
injury (14) and Dox-induced
cardiomyopathy (15). Several
mechanisms underlying the role of Hnk in cardioprotection have been
reported, including the inhibition of oxidative stress and
apoptosis (16), as well as
improvement of autophagy (14)
and mitochondrial function (13).
In addition, a recent study revealed the association between Hnk
and senescence in skin cells (17). However, to the best of our
knowledge, it remains unknown whether inhibition of cardiomyocyte
senescence is involved in the protective effect of Hnk on the
heart. Investigating whether Hnk protects heart cells from
senescence may further support the biological effects of Hnk on the
heart and uncover its potential clinical applications.
Inflammasomes are multimeric complexes of innate
immune receptors and their formation promotes caspase-1 activation,
which subsequently results in the processing and secretion of
inflammatory cytokines, including interleukin (IL)-1β and IL-18
(18). A number of studies have
demonstrated that the activation of the NOD-like receptor family
pyrin domain-containing 3 (NLRP3) inflammasome directly
participates in the regulation of inflammation, oxidative stress
and apoptosis in cultured cardiomyocytes (19). Recently, additional studies have
demonstrated a pivotal role of the NLRP3 inflammasome in
accelerating endothelial cell senescence (20,21). However, the role of the NLRP3
inflammasome in cardiomyocyte senescence remains to be fully
elucidated.
Thioredoxin-interacting protein (TXNIP), also termed
thioredoxin-binding protein-2/vitaminD3 upregulated protein 1,
belongs to the arrestin superfamily and inhibits the disulfide
reductase activity of thioredoxin (22). Studies in mammals have
demonstrated that TXNIP plays a key role in regulating cell growth
(23), apoptosis (24), metabolism (25) and immune responses (26). Previous studies have reported that
TXNIP acts as a link between redox regulation and the pathogenesis
of senescence (27,28). Additionally, TXNIP may be
upregulated during the process of senescence, and upregulation of
TXNIP in young cells resulted in typical signs of senescence
(29). Furthermore, a study
demonstrated that the TXNIP/NLRP3 inflammasome pathway contributes
to the senescence of vascular endothelial cells (27), thus providing a novel mechanism
for TXNIP/NLRP3 inflammasome-mediated cellular senescence. Despite
increasing evidence supporting pivotal roles of TXNIP in the
regulation of cardiomyocyte fatty acid oxidation (30) and apoptosis (31), further research is required to
determine whether TXNIP and the NLRP3 inflammasome are involved in
cardiomyocyte senescence.
The aim of the present study was to investigate the
effects of Hnk on Dox-induced cardiomyocyte senescence, and to
examine the roles of TXNIP and the NLRP3 inflammasome in
Hnk-mediated inhibition of cardiomyocyte senescence.
Materials and methods
Reagents
The following materials were purchased from Thermo
Fisher Scientific, Inc.: Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS), penicillin, streptomycin, dimethyl
sulfoxide (DMSO), TRIzol reagent and chemiluminescence reagents.
The following materials were purchased from Beyotime Institute of
Biotechnology: Senescence Cells Histochemical Staining kit (cat.
no. C0602), Cell Counting Kit-8 (CCK-8; cat. no. C0038), RIPA lysis
buffer, BCA protein assay kit and polyvinylidene difluoride (PVDF)
membrane. PrimeScript RT Master mix kit and SYBR Green Master
Mixture were from Takara Biotechnology Co., Ltd. Hnk (cat. no.
HY-N0003) was obtained from MedChemExpress. Doxorubicin
hydrochloride (cat. no. D1515) was from Sigma-Aldrich; Merck KGaA.
Anti-p16INK4A (cat. no. ARG57377) was from Arigo
Biolaboratories. Anti-p21 (cat. no. ab109199) was from Abcam.
Anti-TXNIP (cat. no. sc-166234) was from Santa Cruz Biotechnology,
Inc. The β-tubulin antibody (cat. no. 66240-1-Ig) was from
ProteinTech Group, Inc. Secondary antibodies conjugated to
horseradish peroxidase and enhanced chemiluminescence reagents were
from AntGene Biotechnology Co., Ltd.
Cell culture and treatments
Experiments were performed on the rat embryonic
cardiac cell line H9c2, which was purchased from the American Type
Culture Collection (cat. no. CRL-1446). H9c2 cardiomyocytes were
cultured in DMEM with 10% FBS, 1% penicillin and 1% streptomycin at
37°C under an atmosphere of 5% CO2, as reported
previously (32), and treated
after reaching a confluence of ~80%.
Generation of recombinant adenovirus
Replication-defective recombinant adenovirus
containing the entire coding sequence of TXNIP (Ad-TXNIP) was
constructed with the Adenovirus Expression Vector kit (Takara Bio,
Inc.). An adenovirus-only-carrying green fluorescence protein was
used as a negative control (Ad-NC). To generate adenovirus
expressing shRNA against TXNIP (Ad-shTXNIP), three siRNAs for rat
TXNIP were designed. The one with the optimal knockdown efficiency
evaluated by western blotting was selected to create shRNA and then
recombined into adenoviral vectors. The target sequence was as
follows: 5′-GAA GAA AGT TTC CTG CAT GTT C-3′. The negative control
adenovirus was designed to express non-targeting 'universal
control' shRNA (Ad-shNC). Amplification and purification of
recombinant adenovirus was performed according to the
manufacturer's instructions (Takara Bio, Inc.).
Experimental design
To investigate the protective effects of Hnk on
Dox-induced senescence, H9c2 cardiomyocytes were pretreated with
Hnk at a dose of 2.5 or 5.0 µM for 24 h. The cells were then
incubated with or without 0.1 µM Dox for 48 h (33,34) and analyzed for each experiment. To
further investigate whether the anti-senescence effects of Hnk were
associated with the inhibition of TXNIP, H9c2 cells were infected
with either Ad-TXNIP or Ad-shTXNIP 48 h prior to Hnk treatment
under the control of Ad-NC or Ad-shNC, and subsequently incubated
with or without 0.1 µM Dox for 48 h prior to analysis. Since
both Hnk and Dox were dissolved in 0.1% DMSO, an equivalent amount
of vehicle was added to both the control and the virus-infected
cells when the experiments were conducted with drug treatments.
Cell viability assay
The cell viability was determined by the CCK-8
assay. Briefly, H9c2 cells were seeded in 96-well plates at a
density of 1×105/ml, and 100 µl DMEM with 10% FBS
was added to each well followed by incubation overnight. Following
drug treatment, CCK-8 (10 µl/well) was added, and the cells
were cultured for a further 2 h at 37°C. The absorbance at 450 nm
was measured using a Multiscan microplate reader (Thermo Fisher
Scientific, Inc.). The cell viability for the control group was set
at 100%, while the viability for the other groups was expressed as
a percentage of the control group.
Assessment of senescence
To detect senescence, two different features of
senescence were investigated: i) Enhanced β-galactosidase activity
(SA-β-gal) associated with increased lysosomal content; and ii)
expression of the cell cycle inhibitor p16INK4A and p21
(35). Staining for SA-β-gal was
performed with a Senescence Cells Histochemical Staining kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. Briefly, cultured H9c2 cardiomyocytes
were fixed in 4% formaldehyde for 15 min at room temperature and
then washed three times in phosphate-buffered saline (PBS) at room
temperature. The slides were immersed in freshly prepared SA-β-gal
staining solution and incubated at 37°C without CO2
overnight. Stained sections were washed twice with PBS. Senescence
was quantitated by visual inspection of blue/green stained cells
with an inverted microscope (magnification ×200). In total, >6
observations were included in each group.
Western blot analysis
Total protein was prepared with RIPA lysis buffer at
4°C for 30 min and quantified using a BCA kit. Equal amounts of
protein (60 µg) were separated by 10% SDS-PAGE,
electrotransferred to a PVDF membrane and blocked in 5% milk
diluted with TBS/0.1% Tween-20 (TBST) buffer for 2 h at room
temperature. The membranes were incubated with the appropriate
primary antibody at 4°C overnight. The primary antibodies used were
as follows: Anti-β tubulin (1:1,000), anti-TXNIP (1:100);
anti-p16INK4A (1:1,000) and anti-p21 (1:1,000). The PVDF
membranes were washed three times with TBST buffer and then
incubated with secondary antibody at room temperature for 2 h.
After washing three times, the bands were evaluated with a
chemiluminescence detection system.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total mRNA was extracted using TRIzol reagent. Equal
amounts of mRNA (1 µg) were reversed-transcribed into
complementary DNA (cDNA) using the PrimeScript RT Master Mix kit
(Takara Bio, Inc.). The reverse transcription conditions were as
follows: 37°C for 15 min and 85°C for 5 sec. Subsequently, cDNA was
used for qPCR with SYBR Green Master Mixture (Takara Bio, Inc.) on
the ABI StepOnePlus RT-PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The cycling conditions were as follows:
95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for 15 sec.
qPCR was performed with three replicates of each sample. The
β-actin housekeeping gene was used as a control. The relative
expression quantity was calculated using the 2−ΔΔCq
method (36). The primers were as
follows: β-actin forward, 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′ and
reverse, 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′; TXNIP forward,
5′-TAG TGT CCC TGG CTC CAA GAA A-3′ and reverse, 5′-GGA TGT TTA GGT
CTA TCC AGC TCA T-3′; NLRP3 forward, 5′-CCA GGA GTT CTT TGC GGC
TA-3′ and reverse, 5′-GCC TTT TTC GAA CTT GCC GT-3′; caspase 1
forward, 5′-AAA CTG AAC AAA GAA GGT GGC G-3′ and reverse, 5′-GCA
AGA CGT GTA CGA GTG GGT G-3′; and IL-1β forward, 5′-GAC AGA ACA TAA
GCC AAC AAG-3′ and reverse, 5′-GTC AAC TAT GTC CCG ACC ATT-3′.
Statistical analysis
The results are representative of at least three
independent experiments. Data are presented as the mean ± standard
error of the mean. Differences in the data were analyzed by an
unpaired, two-tailed Student's t-test for two groups or one-way
analysis of variance followed by Newman-Keuls test for multiple
groups. All statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Hnk protects H9c2 cardiomyocytes against
Dox-induced senescence
According to previous research (37), the present study selected 0.1
µM as the most suitable concentration to induce senescence
of H9C2 cardiomyocytes. Dose-response experiments by CCK-8 assay
were then performed to identify the optimal concentration of Hnk
for the following experiments. It was observed that Hnk suppressed
Dox (0.1 µM)-induced reductions in cell viability, with
maximum inhibition observed when Hnk was used at 2.5 and 5
µM (Fig. 1A). Therefore,
2.5 and 5 µM were selected to study the effect of Hnk on
aging. The 48-h treatment of H9c2 cells with Dox significantly
increased the percentage of senescent cells, as assessed by
SA-β-gal staining, while this effect was significantly reversed by
Hnk treatment at both 2.5 and 5.0 µM (Fig. 1B and C). In addition,
p16INK4A and p21 protein expression levels were
increased in Dox-treated H9c2 cardiomyocytes compared with the
vehicle group, whereas Hnk pretreatment antagonized this effect
elicited by Dox (Fig. 1D-F).
These results demonstrated for the first time that Hnk can protect
H9c2 cardiomyocytes against Dox-induced senescence.
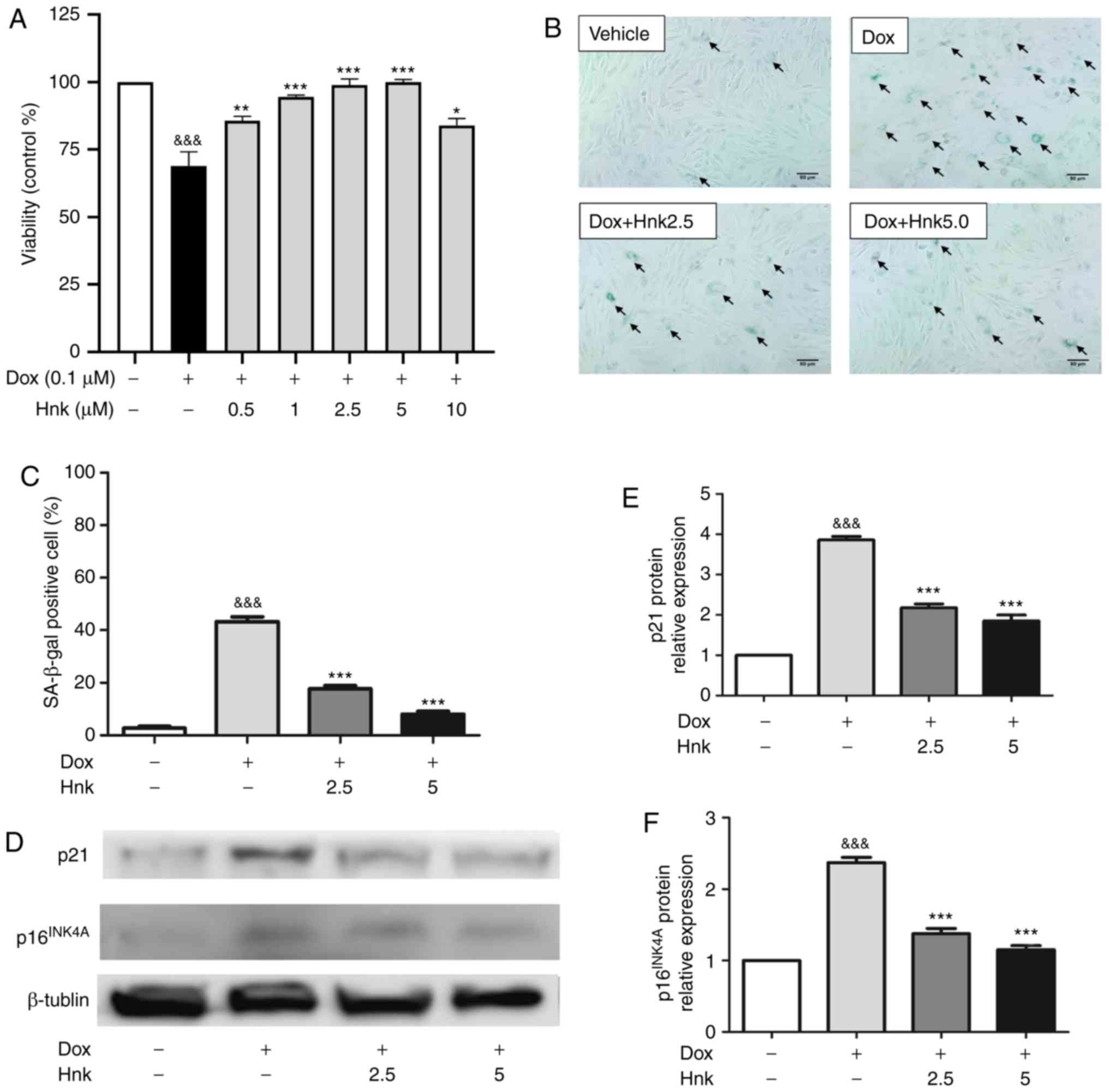 | Figure 1Honokiol (Hnk) protects H9c2
cardiomyocytes against Dox-induced senescence. (A) H9c2
cardiomyocytes were pretreated with different concentrations of Hnk
(0.5-10 µM) for 24 h prior to Dox (0.1 µM) exposure
for 48 h, which was followed by a CCK-8 assay to evaluate cell
viability. (B) Cultured H9c2 cardiomyocytes were pretreated with
Hnk (0, 2.5 or 5 µM), followed by treatment with Dox (0.1
µM) for 48 h. Representative images of the co-staining for
SA-β-gal-positive cells (blue; arrows). Magnification ×200. Scale
bar, 50 µm. (C) Percentage of SA-β-gal-positive H9c2 cells.
(D) Detection of p16INK4A and p21 protein levels. (E and
F) Quantification of p16INK4A and p21 protein levels.
The results are representative of at least three independent
experiments. &&&P<0.001 vs. vehicle
group; *P<0.05, **P<0.01,
***P<0.001 vs. Dox group. SA-β-gal,
senescence-associated β-galactosidase; Dox, doxorubicin; Hnk2.5,
honokiol 2.5 µM; Hnk5.0, honokiol 5 µM. |
Hnk inhibits Dox-induced TXNIP expression
and NLRP3 inflammasome activation in H9c2 cardiomyocytes
TXNIP is a recognized activator of the NLRP3
inflammasome (38). In the
present study, the protein and mRNA levels of TXNIP were found to
be increased significantly in Dox-treated H9c2 cardiomyocytes
compared with the vehicle group (Fig.
2A-C). Furthermore, Hnk-pretreatment at both 2.5 and 5.0
µM in cultured H9c2 cells significantly downregulated the
expression of TXNIP compared with the Dox group (Fig. 2A-C). As a key modulator of
senescence, the NLRP3 inflammasome was analyzed at the mRNA level
in H9c2 cardiomyocytes. Dox-stimulated H9c2 cardiomyocytes
exhibited higher mRNA levels of NLRP3, caspase-1 and IL-1β compared
with the vehicle group (Fig.
2D-F). Consistently, cells pretreated with 2.5 and 5.0
µM exhibited markedly downregulated mRNA levels of NLRP3,
caspase-1 and IL-1β compared with the vehicle group (Fig. 2D-F). These results indicated that
Hnk was able to inhibit TXNIP expression and NLRP3 inflammasome
activation in Dox-treated H9c2 cardiomyocytes.
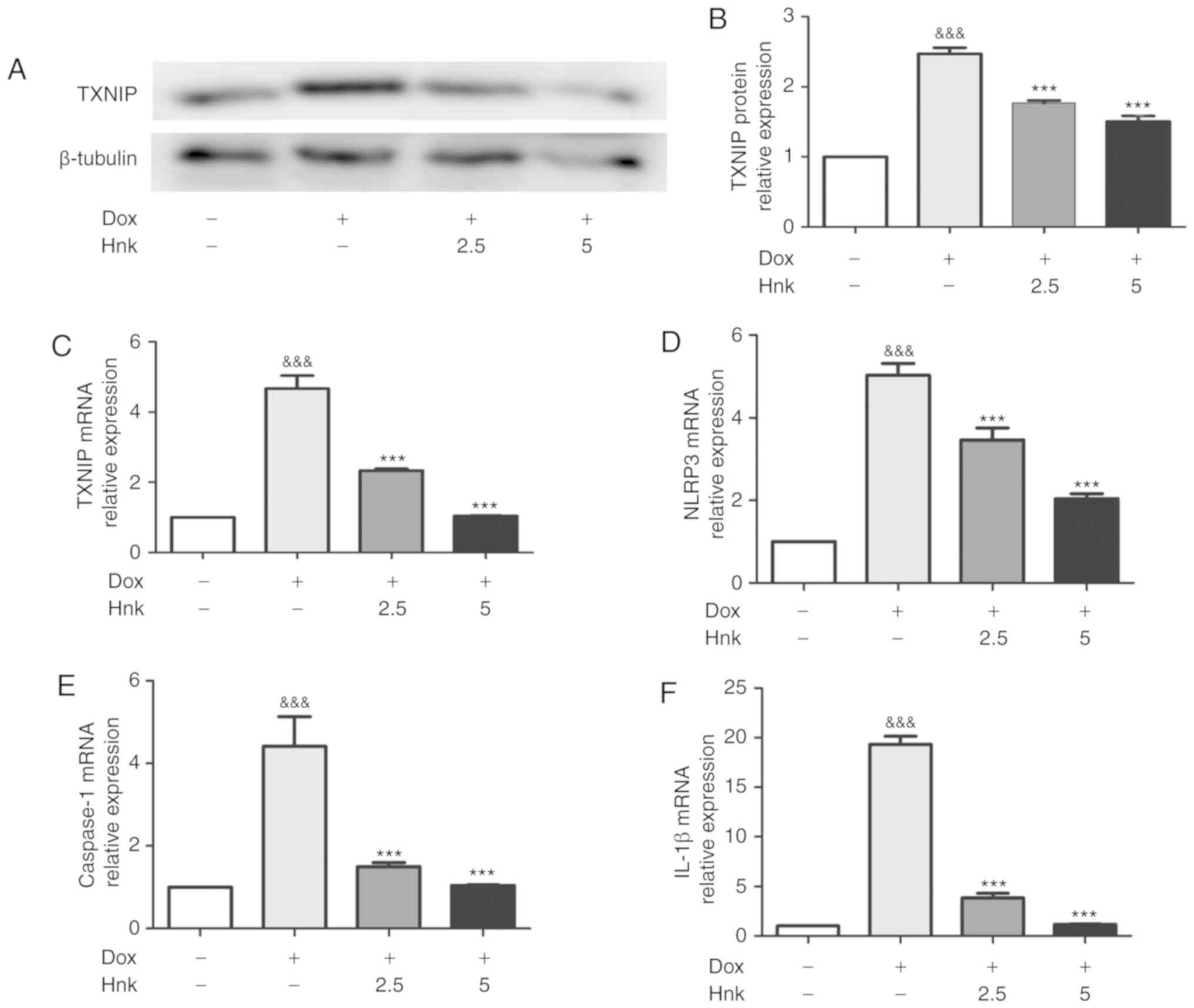 | Figure 2Honokiol (Hnk) inhibits Dox-induced
TXNIP expression and NLRP3 inflammasome activation in H9c2
cardiomyocytes. (A and B) Detection of TXNIP protein levels after
no treatment or exposure to Dox with or without prior incubation
with Hnk, and quantitative analysis. (C-F) Detection of relative
TXNIP, NLRP3, caspase-1 and IL-1β mRNA levels after no treatment or
exposure to Dox with or without prior incubation with Hnk. The
results are representative of at least three independent
experiments. &&&P<0.001 vs. vehicle
group; ***P<0.001 vs. Dox group. SA-β-gal,
senescence-associated β-galactosidase; Dox, doxorubicin; Hnk2.5,
honokiol 2.5 µM; Hnk5.0, honokiol 5 µM; TXNIP,
thioredoxin-interacting protein; NLRP3, NOD-like receptor family
pyrin domain-containing 3; IL, interleukin. |
TXNIP gene silencing enhances the effect
of Hnk treatment on Dox-induced senescence in H9c2
cardiomyocytes
To determine whether TXNIP is involved in the
protective effect of Hnk on H9c2 cardiomyocytes against Dox-induced
senescence, TXNIP expression was knocked down by
adenovirus-mediated gene silencing (Fig. 3A). The Hnk + Dox + Ad-shTXNIP
group displayed further reduced expression of TXNIP compared with
the Hnk + Dox group (Fig. 3B-D).
The inhibitory effect of Hnk on Dox-induced NLRP3 inflammasome
activation was enhanced when H9c2 cardiomyocytes were pre-infected
with Ad-shTXNIP under the control of Ad-shNC (Fig. 3E-G). Furthermore, SA-β-gal
staining was performed to detect the senescence of H9c2
cardiomyocytes (Fig. 3H).
Pre-infecting H9c2 cells with Ad-shTXNIP led to a further reduction
in Dox-induced H9c2 cardiomyocyte senescence compared with Hnk
treatment alone (Fig. 3I). In
addition, western blotting was performed to detect the protein
expression levels of p16INK4A and p21. Accordingly, Hnk
decreased the expression levels of p16INK4A and p21,
which were augmented by pre-infecting H9c2 cells with Ad-shTXNIP
(Fig. 3J-L). In summary, these
results demonstrated that TXNIP silencing significantly enhanced
the effect of Hnk treatment on Dox-induced senescence of H9c2
cardiomyocytes.
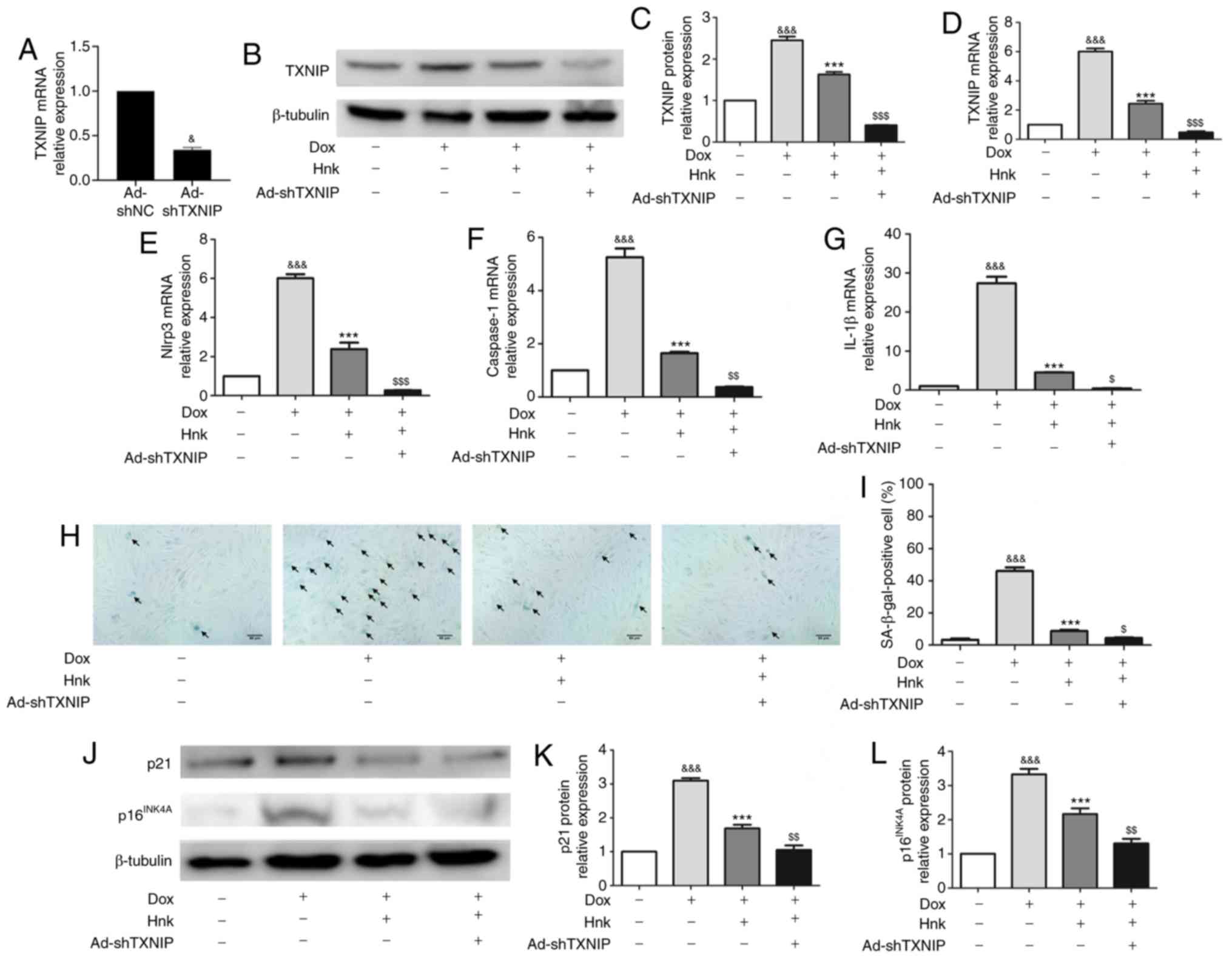 | Figure 3TXNIP gene silencing enhances the
effect of honokiol (Hnk) treatment on Dox-induced senescence in
H9c2 cardiomyocytes. Cultured H9c2 cardio-myocytes were infected
with Ad-shNC or Ad-shTXNIP for 48 h, followed by treatment with Hnk
(5 µM) for 24 h, and then exposure to Dox (0.1 µM)
for 48 h. (A) mRNA levels of TXNIP in H9c2 cells infected with
TXNIP knockdown adenovirus. (B and C) Detection of TXNIP protein
levels and quantitative analysis. (D-G) Detection of relative
TXNIP, NLRP3, caspase-1 and IL-1β mRNA levels. (H) Representative
images of the co-staining for SA-β-gal-positive cells (blue; black
arrows). Magnification ×200. Scale bar, 50 µm. (I)
Percentage of SA-β-gal-positive H9c2 cells. (J) Detection of
p16INK4A and p21 protein levels. (K and L)
Quantification of p21 and p16INK4A protein levels. The
results are representative of at least three independent
experiments. &P<0.05,
&&&P<0.001 vs. vehicle group.
***P<0.001 vs. Dox group. $P<0.05,
$$P<0.01, $$$P<0.001 vs. Dox + Hnk
group. Ad, adenovirus; SA-β-gal, senescence-associated
β-galactosidase; Dox, doxorubicin; Hnk, honokiol 5 µM;
TXNIP, thioredoxin-interacting protein; NLRP3, NOD-like receptor
family pyrin domain-containing 3; IL, interleukin. |
TXNIP overexpression abrogates the effect
of Hnk treatment on Dox-induced senescence of H9c2
cardiomyocytes
To determine whether TXNIP mediates the protective
effect of Hnk against Dox-induced H9c2 cardiomyocyte senescence,
TXNIP expression was upregulated by adenovirus-mediated gene
overexpression (Fig. 4A).
Ad-TXNIP partially reversed the inhibitory effect of Hnk on TXNIP
expression (Fig. 4B-D). Indeed,
the inhibitory effect of Hnk on Dox-induced NLRP3 inflammasome
activation was lost when H9c2 cardiomyocytes were pre-infected with
Ad-TXNIP (Fig. 4E-G).
Furthermore, SA-β-gal staining was performed to detect the
cardiomyocyte senescence (Fig.
4H). Pre-infecting H9c2 cells with Ad-TXNIP abrogated the
reduction in Dox-induced H9c2 cardiomyocyte senescence attained by
Hnk (Fig. 4I). In addition, Hnk
almost normalized the expression levels of p16INK4A and
p21, and this effect was abolished by pre-infecting H9c2 cells with
Ad-TXNIP (Fig. 4J-L).
Collectively, these data suggest that Hnk reverses Dox-induced H9c2
cardiomyocyte senescence in a TXNIP/NLRP3 inflammasome
dependent-manner.
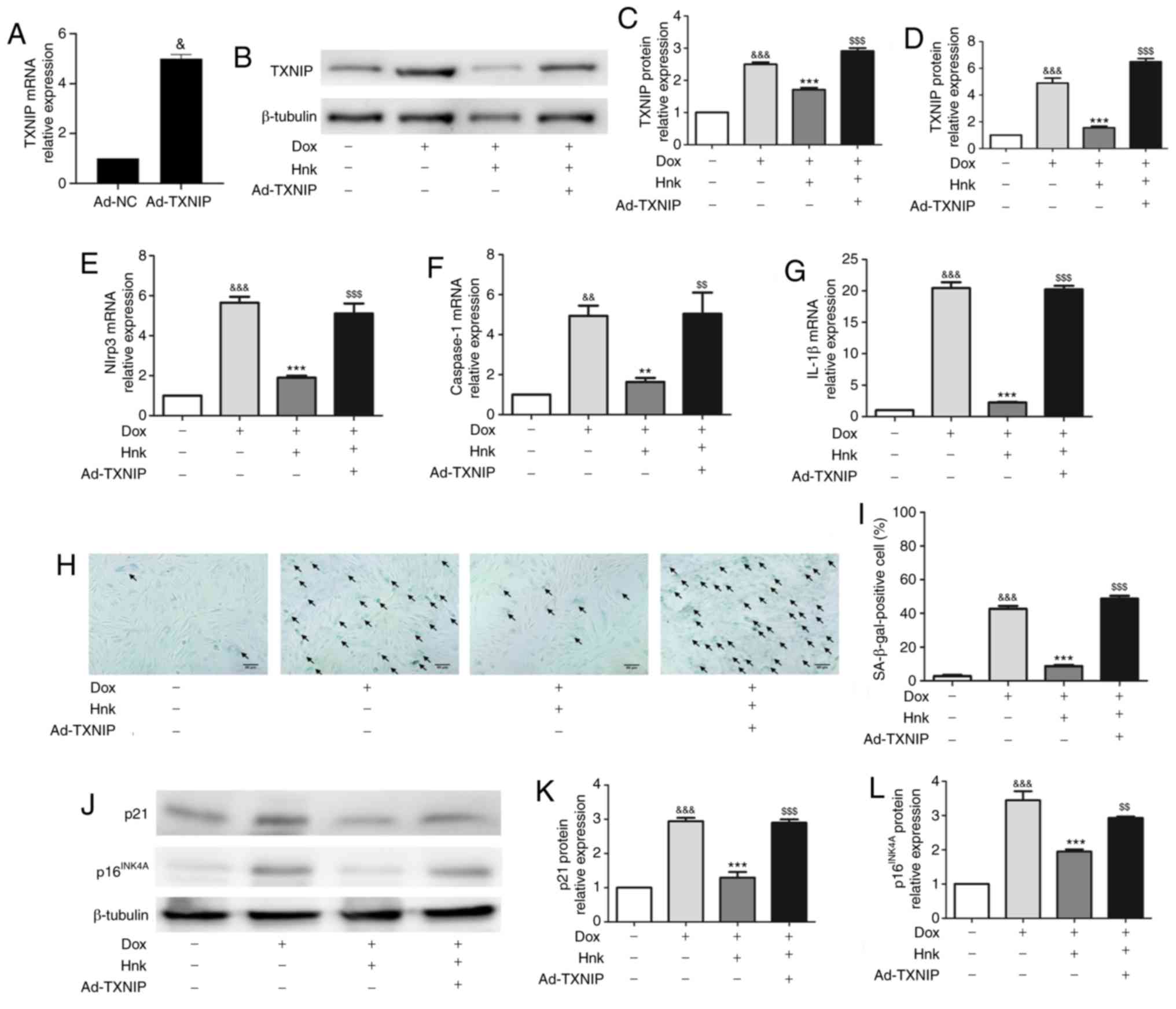 | Figure 4TXNIP overexpression abrogates the
effect of honokiol (Hnk) treatment on Dox-induced senescence in
H9c2 cardiomyocytes. Cultured H9c2 cardiomyocytes were transfected
with Ad-NC or Ad-TXNIP for 48 h, followed by treatment with Hnk (5
µM) for 24 h and subsequent exposure to Dox (0.1 µM)
for 48 h. (A) mRNA levels of TXNIP in H9c2 cells infected with
TXNIP overexpression adenovirus. (B and C) Detection of TXNIP
protein levels and quantitative analysis. (D-G) Detection of
relative TXNIP, NLRP3, caspase-1 and IL-1β mRNA levels. (H)
Representative images of co-staining for SA-β-gal-positive cells
(blue; arrows). Magnification ×200. Scale bars, 50 µm. (I)
Percentage of SA-β-gal-positive H9c2 cells. (J) Detection of
p16INK4A and p21 protein levels. (K and L)
Quantification of p21 and p16INK4A protein levels. The
results are representative of at least three independent
experiments. &P<0.05,
&&P<0.01,
&&&P<0.001 vs. vehicle group;
**P<0.01, ***P<0.001 vs. Dox group;
$$P<0.01, $$$P<0.001 vs. Dox + Hnk
group. Ad, adenovirus; SA-β-gal, senescence-associated
β-galactosidase; Dox, doxorubicin; Hnk, honokiol 5 µM;
TXNIP, thioredoxin-interacting protein; NLRP3, NOD-like receptor
family pyrin domain-containing 3; IL, interleukin. |
Discussion
Dox is an anthracycline that is effective against a
wide range of tumors (1). Despite
its beneficial effects against cancer, the clinical application of
Dox is associated with severe cardiotoxicity (2). Dox induces a senescent-like
phenotype in cardiomyocytes, with an abnormal pattern of troponin
phosphorylation that may result in inefficient cardiac contraction
(5). Senescence of cardiac cells
has been identified to be crucial for the development of
anthracycline-related cardiomyopathy (39). Consistent with these previous
findings, the data in the present study suggest that Dox-induced
cytotoxicity is mediated by the development of cellular senescence,
which is accompanied by increased expression of senescence-related
genes and SA-β-gal activity. Therefore, it is of great interest to
identify effective therapies that inhibit cardiomyocyte senescence
in order to prevent Dox-induced cardiotoxicity.
Hnk is a bioactive natural compound obtained from
the genus Magnolia. Hnk has been reported to possess diverse
biological properties, including antiarrhythmic, antihy-pertensive
and anticancer properties, without appreciable toxicity (12,40,41). It has been demonstrated that Hnk
acts as an anti-inflammatory agent by blocking the classical
nuclear factor-κB pathway (42).
Due to its prominent anti-inflammatory properties, there has been
increasing attention on its favorable effects on cardiac
performance. Tan et al (14) demonstrated that Hnk post-treatment
alleviated myocardial ischemia/reperfusion injury by enhancing
autophagic flux and reducing intracellular reactive oxygen species
(ROS) production. Furthermore, Pillai et al (13) suggested that Hnk blocked and
reversed cardiac hypertrophy in mice by activating mitochondrial
sirtuin 3. Although Huang et al (15) demonstrated that Hnk pretreatment
could reduce cardiac damage by improving mitochondrial function in
Dox-treated mouse hearts, the effects and the underlying mechanisms
of Hnk in Dox cardiomyopathy remain to be fully elucidated. The
present study confirmed that Hnk was able to attenuate Dox-induced
cardiomyocyte senescence, as indicated by the significantly
decreased number of SA-β-gal-positive cells, as well as decreased
expression levels of p16INK4A and p21. This new evidence
supports the previous research demonstrating that Hnk protected
Dox-treated mice against cardiotoxicity. Based on these results,
Hnk may be of value for suppressing the pathogenesis of Dox-induced
cardiomyopathy via modulating cardiac senescence. However, detailed
information regarding the precise mechanism underlying the
protective effect of Hnk against Dox-induced cardiomyocyte
senescence remains to be clearly determined.
Subsequently, the present study focused on
elucidating how Hnk exerts its protective effects on cardiomyocytes
subjected to Dox stimulation. A recent study reported that Hnk was
able to inhibit the activation of the NLRP3 inflammasome in nucleus
pulposus cells (43). However, to
the best of our knowledge, it is not clear whether Hnk affects the
NLRP3 inflammasome signaling pathway in cardiomyocyte senescence.
The presence of low-grade inflammation is considered a definitive
characteristic of senescence progression (44). In addition, this chronic
pro-inflammatory state can aggravate cell senescence (45). During the process of endothelial
cell senescence, the NLRP3 inflammasome is activated, which leads
to the production of cytokines, such as IL-1β, thereby activating
the senescent signaling pathway and leading to cellular senescence
(20,46). Consistent with these findings, the
present study observed activated NLRP3 inflammasome and increased
expression of IL-1β. Therefore, activation of the NLRP3
inflammasome may be involved in promoting Dox-induced cardiomyocyte
senescence. Notably, Hnk successfully inhibited NLRP3 inflammasome
activation and decreased the expression of IL-1β in Dox-treated
cardiomyocytes. Taken together, these results indicate that Hnk is
a novel antagonist of the NLRP3 inflammasome in Dox-induced
cardiomyocyte senescence.
TXNIP, an endogenous inhibitor of thioredoxin,
suppresses the ROS scavenging function of thioredoxin, which leads
to cellular oxidative stress (22). A previous study demonstrated that
TXNIP overexpression switches the function of TXNIP from a
thioredoxin repressor to a NLRP3 inflammasome activator under
hyperglycemic conditions (47).
Furthermore, TXNIP was confirmed to directly activate the NLRP3
inflammasome and promote the release of IL-1β upon oxidative stress
(48). However, the effects of
TXINP on Hnk-mediated inhibition of the NLRP3 inflammasome in
Dox-induced cardio-myocyte senescence are not well understood. In
the present study, the expression of TXNIP was significantly
upregulated by Dox stimulation, whereas Hnk pretreatment
successfully inhibited the upregulation of TXNIP induced by Dox in
H9c2 cardiomyocytes. Furthermore, silencing of TXNIP resulted in
enhanced inhibitory effects of Hnk on Dox-induced cardio-myocyte
senescence, while TXNIP overexpression abrogated the inhibitory
effect of Hnk on Dox-induced senescence of cardiomyocytes,
indicating that TXNIP functions as a checkpoint in the protective
effects of Hnk against Dox-induced cardiomyocyte senescence. Based
on these results, it may be concluded that Hnk prevents
cardiomyocyte senescence depending on reduced TXNIP expression and
the subsequent attenuation of NLRP3 inflammasome activation. The
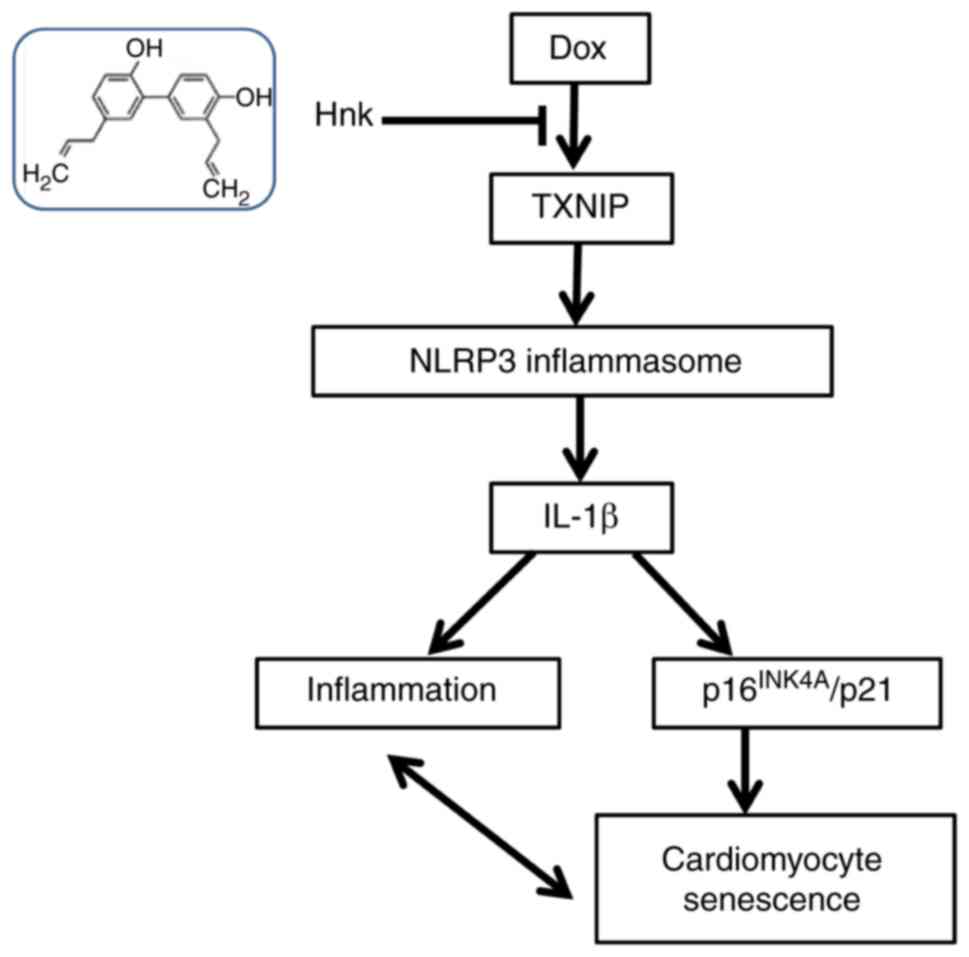
proposed regulatory pathway of Hnk is presented in Fig. 5.
In summary, the present study revealed that Hnk, a
major active molecule in the traditional Chinese medicine Hou Po,
effectively prevented Dox-induced cardiomyocyte senescence. This
cytoprotective effect was demonstrated to occur via the inhibition
of TXNIP and the subsequent suppression of the NLRP3 inflammasome.
The understanding of the mechanism underlying this effect may
support the further development of Hnk as a potential
cardioprotective drug through inhibition of cardiomyocyte
senescence.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 8157051059), the
National Nature Science Foundation of China (grant no. 81601217)
and the Natural Science Foundation of Hubei Province (grant no.
2017CFB627).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PPH designed and performed the study, analyzed the
data and wrote the manuscript. JF was involved in conducting the
experiments, analyzing the data and writing the manuscript. LHL
contributed to the writing of the manuscript and data analysis. KFW
and HXL were involved in conducting the study. BMQ contributed to
data analysis and interpretation. BLQ and YL conceived the study,
participated in its design and helped draft the manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
committee of Tongji Medical College, Huazhong University of Science
and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Octavia Y, Tocchetti CG, Gabrielson KL,
Janssens S, Crijns HJ and Moens AL: Doxorubicin-induced
cardiomyopathy: From molecular mechanisms to therapeutic
strategies. J Mol Cell Cardiol. 52:1213–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh ET and Bickford CL: Cardiovascular
complications of cancer therapy: Incidence, pathogenesis,
diagnosis, and management. J Am Coll Cardiol. 53:2231–2247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hahn VS, Lenihan DJ and Ky B: Cancer
therapy-induced cardiotoxicity: Basic mechanisms and potential
cardioprotective therapies. J Am Heart Assoc. 3:e0006652014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tocchetti CG, Carpi A, Coppola C,
Quintavalle C, Rea D, Campesan M, Arcari A, Piscopo G, Cipresso C,
Monti MG, et al: Ranolazine protects from doxorubicin-induced
oxidative stress and cardiac dysfunction. Eur J Heart Fail.
16:358–366. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maejima Y, Adachi S, Ito H, Hirao K and
Isobe M: Induction of premature senescence in cardiomyocytes by
doxorubicin as a novel mechanism of myocardial damage. Aging Cell.
7:125–136. 2008. View Article : Google Scholar
|
|
6
|
Sun R, Zhu B, Xiong K, Sun Y, Shi D, Chen
L, Zhang Y, Li Z and Xue L: Senescence as a novel mechanism
involved in β-adrenergic receptor mediated cardiac hypertrophy.
PLoS One. 12:e01826682017. View Article : Google Scholar
|
|
7
|
Huang C, Zhang X, Ramil JM, Rikka S, Kim
L, Lee Y, Gude NA, Thistlethwaite PA, Sussman MA, Gottlieb RA and
Gustafsson AB: Juvenile exposure to anthracyclines impairs cardiac
progenitor cell function and vascularization resulting in greater
susceptibility to stress-induced myocardial injury in adult mice.
Circulation. 121:675–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piegari E, De Angelis A, Cappetta D, Russo
R, Esposito G, Costantino S, Graiani G, Frati C, Prezioso L,
Berrino L, et al: Doxorubicin induces senescence and impairs
function of human cardiac progenitor cells. Basic Res Cardiol.
108:3342013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baar MP, Brandt RMC, Putavet DA, Klein
JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van
Willigenburg H, Feijtel DA, et al: Targeted apoptosis of senescent
cells restores tissue homeostasis in response to chemotoxicity and
aging. Cell. 169:132–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan J, Lee Y, Zhang Q, Xiong D, Wan TC,
Wang Y and You M: Honokiol decreases lung cancer metastasis through
inhibition of the STAT3 signaling pathway. Cancer Prev Res (Phila).
10:133–141. 2017. View Article : Google Scholar
|
|
11
|
Greenberg M, Urnezis P and Tian M:
Compressed mints and chewing gum containing magnolia bark extract
are effective against bacteria responsible for oral malodor. J
Agric Food Chem. 55:9465–9469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang GS, Wang RJ, Zhang HN, Zhang GP, Luo
MS and Luo JD: Effects of chronic treatment with honokiol in
spontaneously hypertensive rats. Biol Pharm Bull. 33:427–431. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pillai VB, Samant S, Sundaresan NR,
Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP,
Gius D and Gupta MP: Honokiol blocks and reverses cardiac
hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun.
6:66562015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Z, Liu H, Song X, Ling Y, He S, Yan Y,
Yan J, Wang S, Wang X and Chen A: Honokiol post-treatment
ameliorates myocardial ischemia/reperfusion injury by enhancing
autoph-agic flux and reducing intracellular ROS production. Chem
Biol Interact. 1:82–90. 2019. View Article : Google Scholar
|
|
15
|
Huang L, Zhang K, Guo Y, Huang F, Yang K,
Chen L, Huang K, Zhang F, Long Q and Yang Q: Honokiol protects
against doxorubicin cardiotoxicity via improving mitochondrial
function in mouse hearts. Sci Rep. 7:119892017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Zhai M, Li B, Liu Z, Li K, Jiang
L, Zhang M, Yi W, Yang J, Yi D, et al: Honokiol ameliorates
myocardial isch-emia/reperfusion injury in type 1 diabetic rats by
reducing oxidative stress and apoptosis through activating the
SIRT1-Nrf2 signaling pathway. Oxid Med Cell Longev.
2018:31598012018.
|
|
17
|
Costa A, Facchini G, Pinheiro A, da Silva
MS, Bonner MY, Arbiser J and Eberlin S: Honokiol protects skin
cells against inflammation, collagenolysis, apoptosis, and
senescence caused by cigarette smoke damage. Int J Dermatol.
56:754–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mangali S, Bhat A, Udumula MP, Dhar I,
Sriram D and Dhar A: Inhibition of protein kinase R protects
against palmitic acid-induced inflammation, oxidative stress, and
apoptosis through the JNK/NF-κB/NLRP3 pathway in cultured H9C2
cardiomyocytes. J Cell Biochem. 120:3651–3663. 2019. View Article : Google Scholar
|
|
20
|
Sun C, Diao Q, Lu J, Zhang Z, Wu D, Wang
X, Xie J, Zheng G, Shan Q, Fan S, et al: Purple sweet potato color
attenuated NLRP3 inflammasome by inducing autophagy to delay
endothelial senescence. J Cell Physiol. 234:5926–5939. 2019.
View Article : Google Scholar
|
|
21
|
Sun C, Fan S, Wang X, Lu J, Zhang Z, Wu D,
Shan Q and Zheng Y: Purple sweet potato color inhibits endothelial
premature senescence by blocking the NLRP3 inflammasome. J Nutr
Biochem. 26:1029–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshihara E, Masaki S, Matsuo Y, Chen Z,
Tian H and Yodoi J: Thioredoxin/Txnip: Redoxisome, as a redox
switch for the pathogenesis of diseases. Front Immunol. 4:5142014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zark JW, Lee SH, Woo GH, Kwon HJ and Kim
DY: Downregulation of TXNIP leads to high proliferative activity
and estrogen-dependent cell growth in breast cancer. Biochem
Biophys Res Commun. 498:566–572. 2018. View Article : Google Scholar
|
|
24
|
Ji L, Wang Q, Huang F, An T, Guo F, Zhao
Y, Liu Y, He Y, Song Y and Qin G: FOXO1 overexpression attenuates
tubulointerstitial fibrosis and apoptosis in diabetic kidneys by
ameliorating oxidative injury via TXNIP-TRX. Oxid Med Cell Longev.
2019:32869282019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alhawiti NM, Al Mahri S, Aziz MA, Malik SS
and Mohammad S: TXNIP in metabolic regulation: Physiological role
and therapeutic outlook. Curr Drug Targets. 18:1095–1103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du SQ, Wang XR, Zhu W, Ye Y, Yang JW, Ma
SM, Ji CS and Liu CZ: Acupuncture inhibits TXNIP-associated
oxidative stress and inflammation to attenuate cognitive impairment
in vascular dementia rats. CNS Neurosci Ther. 24:39–46. 2018.
View Article : Google Scholar
|
|
27
|
Yin Y, Zhou Z, Liu W, Chang Q, Sun G and
Dai Y: Vascular endothelial cells senescence is associated with
NOD-like receptor family pyrin domain-containing 3 (NLRP3)
inflammasome activation via reactive oxygen species
(ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J
Biochem Cell Biol. 84:22–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huy H, Song HY, Kim MJ, Kim WS, Kim DO,
Byun JE, Lee J, Park YJ, Kim TD, Yoon SR, et al: TXNIP regulates
AKT-mediated cellular senescence by direct interaction under
glucose-mediated metabolic stress. Aging Cell. 17:e128362018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuo de X, Niu XH, Chen YC, Xin DQ, Guo YL
and Mao ZB: Vitamin D3 up-regulated protein 1(VDUP1) is regulated
by FOXO3A and miR-17-5p at the transcriptional and
post-transcriptional levels, respectively, in senescent
fibroblasts. J Biol Chem. 285:31491–31501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Young ME, Chatham JC, Crossman DK,
Dell'Italia LJ and Shalev A: TXNIP regulates myocardial fatty acid
oxidation via miR-33a signaling. Am J Physiol Heart Circ Physiol.
311:H64–H75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Otaki Y, Takahashi H, Watanabe T, Funayama
A, Netsu S, Honda Y, Narumi T, Kadowaki S, Hasegawa H, Honda S, et
al: HECT-Type ubiquitin E3 ligase ITCH interacts with
thioredoxin-interacting protein and ameliorates reactive oxygen
species-induced cardio-toxicity. J Am Heart Assoc. 5:e0024852016.
View Article : Google Scholar
|
|
32
|
Spallarossa P, Altieri P, Garibaldi S,
Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A,
Brunelli C and Barsotti A: Matrix metalloproteinase-2 and -9 are
induced differently by doxorubicin in H9c2 cells: The role of MAP
kinases and NAD(P H oxidase. Cardiovasc Res. 69:736–745. 2006.
View Article : Google Scholar
|
|
33
|
Altieri P, Barisione C, Lazzarini E,
Garuti A, Bezante GP, Canepa M, Spallarossa P, Tocchetti CG,
Bollini S, Brunelli C and Ameri P: Testosterone antagonizes
doxorubicin-induced senescence of cardiomyocytes. J Am Heart Assoc.
5:e0023832016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spallarossa P, Altieri P, Aloi C,
Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A and
Brunelli C: Doxorubicin induces senescence or apoptosis in rat
neonatal cardiomyocytes by regulating the expression levels of the
telomere binding factors 1-2. Am J Physiol Heart Circ Physiol.
297:H2169–H2181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bernardes de Jesus B and Blasco MA:
Assessing cell and organ senescence biomarkers. Circ Res.
111:97–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Zhang XL, Ji XT, Sun B, Qian LL, Hu XL,
Lou HX and Yuan HQ: Anti-cancer effect of marchantin C via inducing
lung cancer cellular senescence associated with less secretory
phenotype. Biochim Biophys Acta Gen Subj. 1863:1443–1457. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Guo Q, Zhu Q, Tan R, Bai D, Bu X,
Lin B, Zhao K, Pan C, Chen H and Lu N: Flavonoid VI-16 protects
against DSS-induced colitis by inhibiting Txnip-dependent NLRP3
inflammasome activation in macrophages via reducing oxidative
stress. Mucosal Immunol. 12:1150–1163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Angelis De A, Piegari E, Cappetta D,
Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H,
Hosoda T, Rota M, et al: Anthracycline cardiomyopathy is mediated
by depletion of the cardiac stem cell pool and is rescued by
restoration of progenitor cell function. Circulation. 121:276–292.
2010. View Article : Google Scholar
|
|
40
|
Sengupta S, Nagalingam A, Muniraj N,
Bonner MY, Mistriotis P, Afthinos A, Kuppusamy P, Lanoue D, Cho S,
Korangath P, et al: Activation of tumor suppressor LKB1 by honokiol
abrogates cancer stem-like phenotype in breast cancer via
inhibition of oncogenic Stat3. Oncogene. 36:5709–5721. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sungnoon R and Chattipakorn N:
Anti-arrhythmic effects of herbal medicine. Indian Heart J.
57:109–113. 2005.PubMed/NCBI
|
|
42
|
Wu H, Yin Z, Wang L, Li F and Qiu Y:
Honokiol improved chondrogenesis and suppressed inflammation in
human umbilical cord derived mesenchymal stem cells via blocking
nuclear factor-κB pathway. BMC Cell Biol. 18:292017. View Article : Google Scholar
|
|
43
|
Tang P, Gu JM, Xie ZA, Gu Y, Jie ZW, Huang
KM, Wang JY, Fan SW, Jiang XS and Hu ZJ: Honokiol alleviates the
degeneration of intervertebral disc via suppressing the activation
of TXNIP-NLRP3 inflammasome signal pathway. Free Radic Biol Med.
120:368–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Minguzzi M, Cetrullo S, D'Adamo S,
Silvestri Y, Flamigni F and Borzi RM: Emerging players at the
intersection of chon-drocyte loss of maturational arrest, oxidative
stress, senescence and low-grade inflammation in osteoarthritis.
Oxid Med Cell Longev. 2018:30752932018. View Article : Google Scholar
|
|
45
|
Del Pinto R and Ferri C:
Inflammation-Accelerated senescence and the cardiovascular system:
Mechanisms and perspectives. Int J Mol Sci. 19:E37012018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cordero MD, Williams MR and Ryffel B:
AMP-activated protein kinase regulation of the NLRP3 inflammasome
during aging. Trends Endocrinol Metab. 29:8–17. 2018. View Article : Google Scholar
|
|
47
|
Schroder K, Zhou R and Tschopp J: The
NLRP3 inflammasome: A sensor for metabolic danger? Science.
327:296–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Davis BK and Ting JP: NLRP3 has a sweet
tooth. Nat Immunol. 11:105–106. 2010. View Article : Google Scholar : PubMed/NCBI
|