Introduction
The abnormal proliferation of mesangial cells is a
common pathological alteration in proliferative glomerular
diseases, such as lupus nephritis (LN). The inflammatory reaction
or cell injury triggers the abnormal proliferation of mesangial
cells, and the activation and operation of the cell cycle, which
ultimately leads to glomerulosclerosis and end-stage renal failure.
The interference of the cell cycle at any stage can prevent cell
proliferation or promote cell apoptosis (1). Hormones, immunosuppressants,
immunomodulatory drugs and their combination therapies are commonly
used to address mesangial cell proliferation induced by
glomerulonephritis. However, these drugs have a narrow therapeutic
window and severe side-effects (2,3).
Clinically, the chemotherapeutic treatment of leukemia involves a
combination of various chemotherapeutic drugs, which are
administered sequentially in a planned manner according to the cell
cycle of leukemia cells. The leukemia cells are blocked at a
certain stage of cell cycle proliferation, and subsequently
non-specific drugs of the cell cycle are used to kill the viable
leukemia cells, so that remission and intensive treatment can be
achieved by repeating consolidation (4-6).
The combination of multiple immunosuppressive agents and sequential
therapy for renal transplant recipients and patients with myeloid
leukemia has been successful (7).
Liu et al (8), as well as
others (9,10) proposed the concept of multi-target
therapy, and carried out large-scale, randomized, open,
multi-center research, which achieved a good therapeutic effect.
Therefore, it was speculated that a complementary or sequential
immunosuppressive therapeutic strategy may be more effective in
inhibiting the proliferation of human mesangial cells (HMCs). In a
previous study by the authors, it was revealed that tacrolimus
(TAC) and mycophenolate mofetil (MMF) exerted their inhibitory
effect on mesangial cell proliferation by acting on different
phases of the cell cycle of mesangial cells (11). Thus, TAC combined with MMF at half
the concentration was selected in the present study for the
treatment of the proliferative stage of mesangial cells in
vitro and to explore the therapeutic effect in vivo. The
present study provides a theoretical basis for the treatment of
mesangial proliferative nephropathy by using multiple
immunosuppressants according to the cell cycle.
Materials and methods
Animals
A total of 6 female C57BL/6 mice (8 weeks old;
weighing ~20±5 g) were purchased from the Experimental Animal
Center of Shanxi Medical University, while 24 female MRL/lpr mice
(8 weeks old; weighing 27-31 g) were purchased from the Nanjing
Model Animal Research Institute (originally from Jackson
Laboratory). The animals were allowed to adapt to their
surroundings and fed until the age of 3 months in an environment
with constant temperature (22±1°C), relative humidity (65-70%) and
a 12 h:12 h light/dark cycle. The animals were provided with free
access to water and were fed a standard laboratory diet. The
experiments were performed according to protocols approved by the
Institutional Animal Care and Use Committee of Shanxi Medical
University.
Treatment protocols
A total of 6 C57BL/6 mice served as the control
group. The MRL/lpr mice were randomly divided into 4 groups
(n=6/group) as follows: The lupus group, the lupus + T group, the
lupus + M group and the lupus + 1/2T + 1/2M group. The mice in the
lupus + T group received an intraperitoneal injection of 1
mg/kg/day TAC (Sigma-Aldrich; Merck KGaA). The mice in the lupus +
M group received an intra-gastric administration of 60 mg/kg/day
MMF (Sigma-Aldrich; Merck KGaA). The mice in the lupus + 1/2T +
1/2M group received an intraperitoneal injection of 0.5 mg/kg/day
TAC + 30 mg/kg/day MMF. The mice in the lupus + T, lupus + M and
lupus + 1/2T + 1/2M groups received the drugs from the age of 3
months. At the age of 6 months, the experiment was completed. The
mice were anesthetized by an intraperitoneal injection of 2%
pentobarbital sodium at a dose of 45 mg/kg body weight. Following
saline lavage, both kidneys were immediately removed, and the renal
cortex was extracted. Some samples were fixed in formaldehyde
solution, and some were stored in a refrigerator at −70°C for
further analyses.
Cell culture and treatments
The HMC line, T-SV40 (12), used in the present study was
kindly donated by Professor Xuewang Li of Peking Union Medical
College Hospital. The HMCs were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal calf serum (Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd.) and placed in
a 37°C, 5% CO2 incubator. HMCs in logarithmic growth
phase were fused up to 60%. Following 24 h of culture in serum-free
DMEM, the cells were randomly divided into the CTL group, the PDGF
group, the PDGF + T group, the PDGF + M group, and the PDGF + 1/2T
+ 1/2M group. In the CTL group, the HMCs were cultured routinely;
in the PDGF group, the HMCs were stimulated with 20 ng/ml
platelet-derived growth factor (PDGF) for 48 h; in the PDGF + T
group, the HMCs were stimulated with 20 ng/ml PDGF and cultured for
24 h, and the cells were then treated with 5 µmol/l TAC and
further cultured for 24 h. In the PDGF + M group, the HMCs were
stimulated with 20 ng/ml PDGF and cultured for 24 h, and were then
treated with 10 µmol/l MMF and further cultured for an
additional 24 h. In the PDGF + 1/2T + 1/2M group, the HMCs were
stimulated with 20 ng/ml PDGF and cultured for 24 h. The cells were
then treated with 2.5 µmol/l TAC and 5 µmol/l MMF,
and further cultured for 24 h. For these treatments, the culture
conditions of the cells were the same as those above in a 37°C, 5%
CO2 incubator.
There were 2 grouping methods used in the present
study. One grouping method comprised: Normal HMCs, HMCs treated
with 5 ng/ml TGF-β1, HMCs treated with 5 µmol/l TAC and HMCs
treated with 5 ng/ml TGF-β1 + 5 µmol/l TAC for 48 h. Another
grouping method comprised: Normal HMCs, HMCs treated with 20 ng/ml
PDGF, HMCs treated with 10 µmol/l MMF and HMCs treated with
20 ng/ml PDGF + 10 µmol/l MMF for 48 h in a 37°C, 5%
CO2 incubator.
Patients and treatment
A total of 6 patients with LN were enrolled from the
Shanxi Provincial People's Hospital between January and February,
2019. The inclusion criteria were as follows: i) Patients were of
either sex, and between 15 and 65 years of age; ii) patients
fulfilled the 1997 revised criteria for the classification of SLE
(13); iii) patients had a
diagnosis of class IV LN; and iv) patients had proteinuria levels
≥1.5 g/24 h and serum creatinine levels <265 µmol/l,
SLEDAI ≥12. The key exclusion criteria were the following: i) The
use of any immunosuppressant drug prior to entering the study; ii)
severe infection; iii) central nervous system symptoms; iv)
abnormal index of liver function; v) type 1 or 2 diabetes; vi)
allergies to TAC or MMF; and vii) family planning, pregnancy and
lactation during the previous 6 months. The Ethics Committee of
Shanxi Provincial People's Hospital (Taiyuan, China) approved the
study. Written informed consent was obtained from each patient or
their legal representative, according to the Declaration of
Helsinki.
Patients who entered the study received combined
treatment with TAC (2 mg, twice daily), MMF (500 mg, twice daily)
and methyl prednisone for 6 months and were followed-up. At the end
of the 6-month follow-up period, the patients received a renal
biopsy. The expression of cyclin D1, Ki67 and PDGF in the renal
tissues was evaluated by immunohistochemistry. Changes in the
SLEDAI score, 24-h proteinuria levels, the levels of estimated
glomerular filtration rate (eGFR), serum albumin levels, anti-dsDNA
antibodies, complement 3 (C3) and other clinical indicators were
compared before and after treatment.
Immunofluorescence
The HMCs were washed with PBS for 10 min and fixed
with 4% paraformaldehyde for 20 min after drying, washed with PBS
containing 0.1% Triton for 10 min, sealed with Block solution
(11921673001, Sigma-Aldrich; Merck KGaA) for 30 min, incubated with
a rabbit anti-Ki67 primary antibody (1:200 diluted with block
solution; NCL-Ki67p, Novocastra Laboratories, British) at 4°C
over-night, washed with PBS without Triton 3 times for 5 min each,
incubated with Alexa Fluor conjugated goat anti-rabbit secondary
antibody (A32731, Invitrogen; Thermo Fisher Scientific, Inc.) in
the dark for 1 h, washed with PBS 3 times (5 min each), and
immediately observed under a fluorescence microscope (DM2500, Leica
Microsystems GmbH).
Immunohistochemistry
Paraffin-embedded sections of the kidney tissues of
the mice and biopsy samples of the patients were deparaffinized
with xylene and rehydrated with ethanol. Endogenous peroxidase was
removed by 3% hydrogen peroxide, followed by washing and placing
the tissues in antigen repair solution. The tissues were repaired
in a microwave oven for 2 min, cooled down naturally to room
temperature and cleaned 3 times. After blocking in Block solution
(11921673001, Sigma-Aldrich; Merck KGaA) for 30 min, the mice
samples were incubated with primary antibodies against Ki67
(NCL-Ki67p, Novocastra Laboratories) and PDGF (ab23914; Abcam). The
patient samples were incubated with the human primary antibodies
against Ki67 (NCL-Ki67p, Novocastra Laboratories), PDGF (AF220;
R&D Systems, Inc.) and cyclin D1 (MAB4314; R&D Systems,
Inc.) (1:200 diluted with block solution) at 4°C overnight.
Following 3 rinses with PBS, secondary antibodies (PV-9000 or
PV-9003; ZSGB-BIO Technology, Co., Ltd.) were added, and incubated
at room temperature for 30 min. Following incubation with AB enzyme
at room temperature for 30 min, the samples were colored with DAB,
re-stained in hematoxylin, dehydrated, made transparent and
observed after sealing. Immunohistochemical analysis was performed
in a blinded manner by two independent investigators. Mice
glomeruli were examined in a blinded manner using a high-power
light microscope (DM2500, Leica Microsystems GmbH) (magnification,
×400). The Image-Pro Plus image analysis system was used to count
the number of positive cells and total glomerular cells. Relative
density was calculated as the ratio of positive cells to the total
glomerular cells. The results were scored as follows: of +, 1-25%
positive cells; ++, 26-50% positive cells; +++, 51-75% positive
cells; and ++++, 76-100% positive cells.
PAS and Masson's trichrome staining
Paraffin blocks of resected tissues from these mice
were cut into 5-µM-thick tissue sections and stained with
the hematoxylin and eosin staining kit (cat. no. C0105; Beyotime
Institute of Biotechnology, Inc.) and Masson's trichrome staining
(Masson's Trichrome Stain kit, cat. no. TR-1303; ZSGB-Bio Co.,
Ltd.), respectively, according to the manufacturer's instructions.
The stained tissue sections were then observed under a microscope
(DM2500, Leica Microsystems GmbH).
Western blot analysis
The HMCs were washed twice with PBS. After drying,
250 µl cell lysate (Sigma-Aldrich; Merck KGaA) was added to
each pore. Cells in the pore were gently scraped up and lysed on
ice for 30 min. The lysed cells were placed into an EP tube and
centrifuged for 20 min at 4°C and 12,000 × g. The supernatant was
separated, and the protein concentration was measured by the BCA
method. Proteins were denatured by mixing the sample with an equal
volume of 2X loading buffer and boiling at 100°C for 5 min, and
were then cooled down to room temperature. A 15% prefabricated glue
(Bio-Rad Laboratories, Inc.) with a higher concentration was
selected, and the channel of the prefabricated glue was rinsed with
Running Buffer (Beyotime Institute of Biotechnology) to ensure that
the sample size of each lane protein was 50 µg.
Electrophoresis was conducted at a constant voltage of 120 V, 100 V
trans-membrane for 1 h and 30 min. After blocking with Block
solution (11921673001, Sigma-Aldrich; Merck KGaA) for 30 min, the
following primary antibodies were added and incubated overnight at
4°C: Anti-β-actin (ZRB1312, Sigma-Aldrich; Merck KGaA), anti-cyclin
D1 (ab16663, Abcam), anti-Smad2 (700048, Invitrogen; Thermo Fisher
Scientific, Inc.), anti-phosphorylated (p)-p38 (sc-7973, Santa Cruz
Biotechnology, Inc.) and anti-p38 (sc-7972, Santa Cruz
Biotechnology, Inc.) (antibodies diluted 1:200 with block
solution). After washing with TBST solution, horseradish
peroxidase-conjugated secondary antibody (1:2,000) goat anti-rabbit
IgG (ZB-5301, ZSGB-BIO Technology, Co., Ltd.) or goat anti-mouse
IgG (ZB-5305, ZSGB-BIO Technology, Co., Ltd.) was added for
hybridization, and incubated at room temperature for 1 h. The
membrane was then washed with TBST solution for 10 min (3 times in
total). The absorbance of the results was analyzed using the
Quantity One analysis system, version 4.62 version (Bio-Rad
Laboratories, Inc.).
The mouse kidneys were cut and incubated with 3-fold
volume of cold lysis buffer (Beyotime Institute of Biotechnology).
Subsequently, 10 µl phosphatase inhibitors, 1 µl
protease inhibitors and 10 µl PMSF (1 mmol/l) were added per
1 ml lysis buffer. Upon incubation on ice for 15 min, the tissues
were fully grinded with a glass homogenizer, and centrifuged for 30
min at 4°C and 12,000 × g. The supernatant was then placed in
another EP tube. The subsequent experimental steps were the same as
described above. The following primary antibodies were applied:
Anti-β-actin (ZRB1312, Sigma-Aldrich; Merck KGaA), anti-cyclin D1
(ab16663, Abcam), anti-PDGF (ab23914; Abcam). The secondary
antibody used was horseradish peroxidase-conjugated goat
anti-rabbit antibody (ZB-5301).
MTT assay
Cell proliferation was assessed using a MTT Cell
Proliferation and Cytotoxicity Assay kit (Beijing Solarbio Science
& Technology Co., Ltd.) in accordance with the manufacturer's
protocols. HMCs were grown in 96-well plates at a density of
5,000-10,000 cells/well and placed in a 37°C, 5% CO2
incubator for 12 h. Following 24 h of culture in serum-free DMEM
and the following treatment, the supernatant was removed, and 90
µl fresh culture medium were added. This was followed by the
addition of 10 µl MTT solution and further culture for 4 h
in 37°C. The cell supernatant was then discarded, and 110 µl
formazan were administered with gentle shaking for 10 min. The
absorbance was recorded at 490 nm with a Microplate
Spectrophotometer (C-5000, Institute of Biophysics, Chinese Academy
of Sciences).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp). All experiments were repeated 3 times. The
results were expressed as the means ± standard deviation. One-way
ANOVA followed by Tukey's test was used to detect differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Combination of TAC and MMF at half the
concentration exerts a more prominent inhibitory effect on HMC
proliferation
Ki67 is present in all the active phases of the cell
cycle, but is absent in resting cells (G0) (14). Its expression indicates the entry
of the cell into the cell cycle. Therefore, Ki67 is a widely used
marker of cell proliferation. Cyclin D1 represents the
proliferation of mesangial cells, since it is a key protein that
regulated the cell cycle and is significantly upregulated in the
proliferative stage of mesangial cells (15). Thus, in the present study, these 2
markers were used to evaluate mesangial cell proliferation.
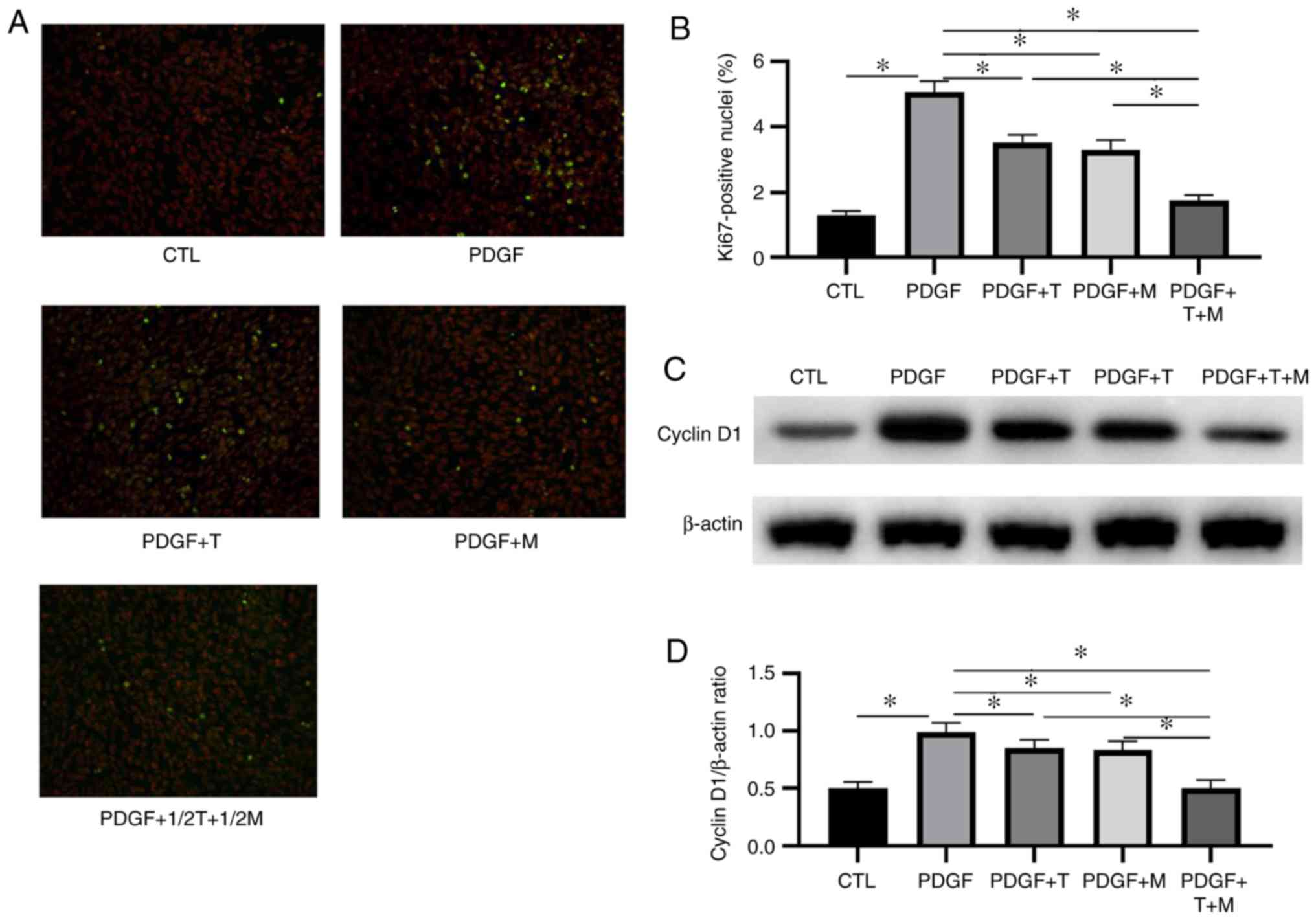
To investigate whether the combination of TAC and
MMF at half the concentration exerted a more prominent inhibitory
effect on HMC proliferation in vitro than the use of a
single drug, the HMCs were divided into 5 groups as follows: CTL,
PDGF, PDGF + 5 µmol/l T, PDGF + 10 µmol/l M and PDGF
+ 2.5 µmol/l plus TAC + 5 µmol/l MMF. The ratio of
Ki67-positive nuclei and the expression of cyclin D1 in each group
were measured by fluorescence staining and western blot analysis,
respectively. The results revealed that the ratio of Ki67-positive
nuclei and the expression of cyclin D1 in the PDGF group were
significantly higher than those in the CTL group. Following
treatment with TAC, MMF or 1/2TAC + 1/2MMF, the ratio of
Ki67-positive nuclei and the expression of cyclin D1 were
significantly decreased. These changes indicated that all 3
treatment regimens inhibited mesangial cell proliferation.
Furthermore, the ratio of Ki67-positive nuclei and the expression
of cyclin D1 were lower following treatment with 1/2TAC + 1/2MMF
compared with following treatment with either TAC or MMF alone, and
the difference was statistically significant, indicating that the
combination of TAC and MMF was more effective than mono-therapy
in vitro (Fig. 1).
Combination of TAC and MMF at half the
concentration exerts a more prominent inhibitory effect on
mesangial cell proliferation in mice with LN than using a single
drug
Spontaneous lupus MRL/lpr mice are one of the
recognized pathological models for the study of mesangial
proliferative nephropathy (16).
At the age of 3-6 months, the disease in these mice manifests as a
simple hyperplasia of mesangial cells. The present study commenced
when the mice were 3 months old and was completed when the animals
had an age of 6 months. The proliferation of mesangial cells in the
kidneys of the mice with lupus treated with the various regimens
(TAC, MMF and 1/2TAC + 1/2MMF) was observed.
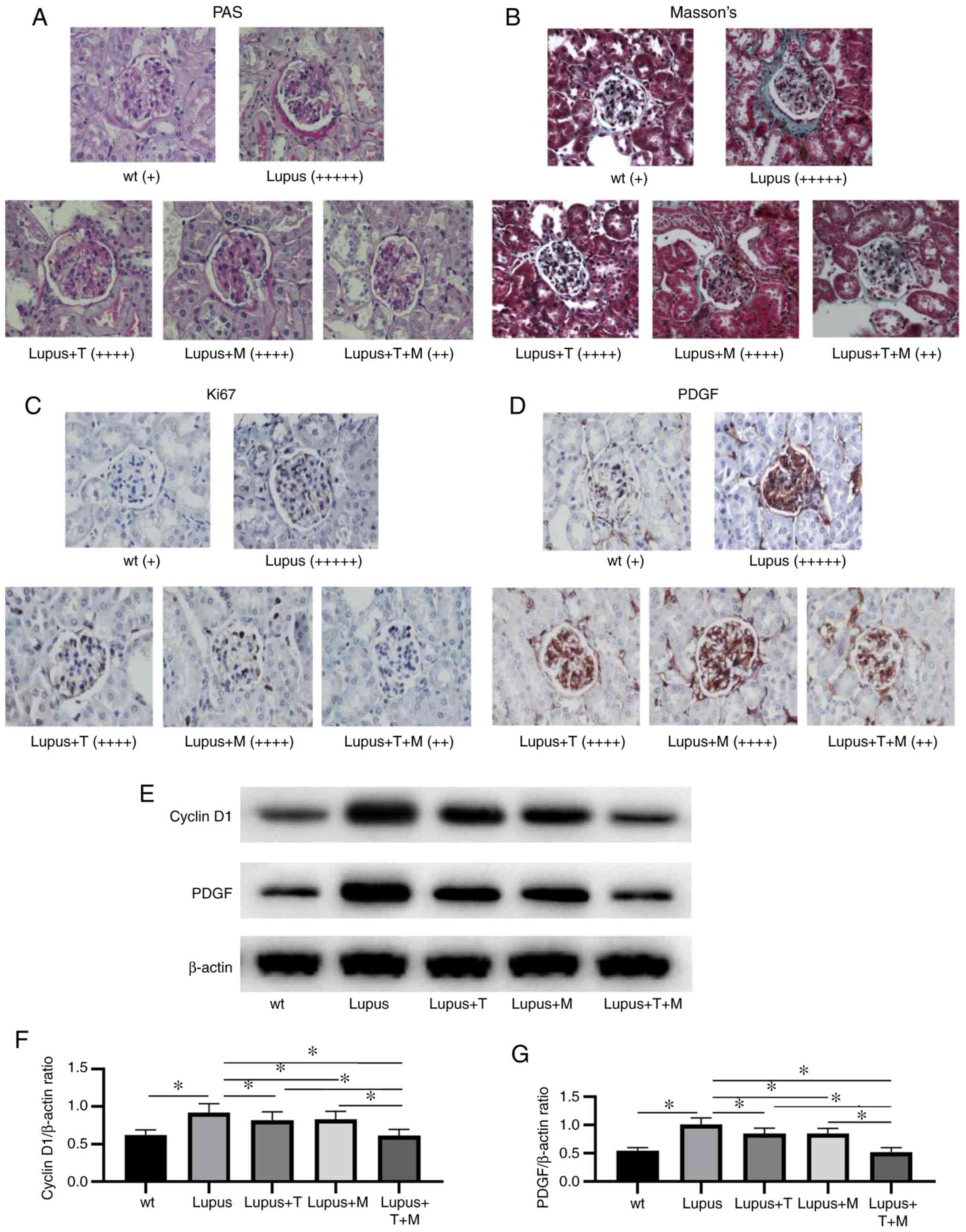
PAS staining and Masson's trichrome staining
revealed that, at the end of the experiment, the renal tissue
structure of the wild-type mice was normal under a light
microscope, while the lupus group exhibited evident pathological
changes, namely the proliferation of mesangial cells, the
destruction of glomerular structure and a large number of
infiltrated inflammatory cells. The pathological changes in the
lupus + T and lupus + M groups were less than those in the lupus
group: The mesangial cells proliferated less, glomerular structure
destruction was not as evident, and inflammatory cell infiltration
was lower. Compared with those in the lupus + T and the lupus + M
groups, the pathological changes in the lupus + 1/2T + 1/2M group
were markedly attenuated, the mesangial cells proliferated less,
the glomerular structure was not markedly damaged, and the number
of infiltrated inflammatory cells was lower (Fig. 2A and B).
According to the results of immunohistochemistry,
the expression of Ki67 and PDGF in the lupus group was
significantly higher than that in the wild-type group. The
expression levels of Ki67 and PDGF in the lupus + T, lupus + M and
lupus + 1/2T + 1/2M groups were markedly decreased compared with
those in the lupus group. Compared with that in the lupus + T and
lupus + M groups, the expression of Ki67 and PDGF decreased
markedly in the lupus + 1/2T + 1/2M group (Fig. 2C and D).
The results of western blot analysis indicated that
the expression of cyclin D1 and PDGF decreased following treatment
with TAC, MMF and 1/2T + 1/2M. Compared with that following
treatment with TAC and MMF alone, the expression of cyclin D1 and
PDGF significantly decreased following treatment with 1/2T + 1/2M
(Fig. 2E-G).
These results indicated that TAC and MMF monotherapy
or TAC combined with MMF at half the concentration exerted an
inhibitory effect on mesangial cell proliferation in mice with LN,
and the therapy regime of the combination of TAC and MMF at half
the concentration was more effective than monotherapy in mice with
LN.
Combination of TAC and MMF inhibits the
proliferation of mesangial cells in patients with LN
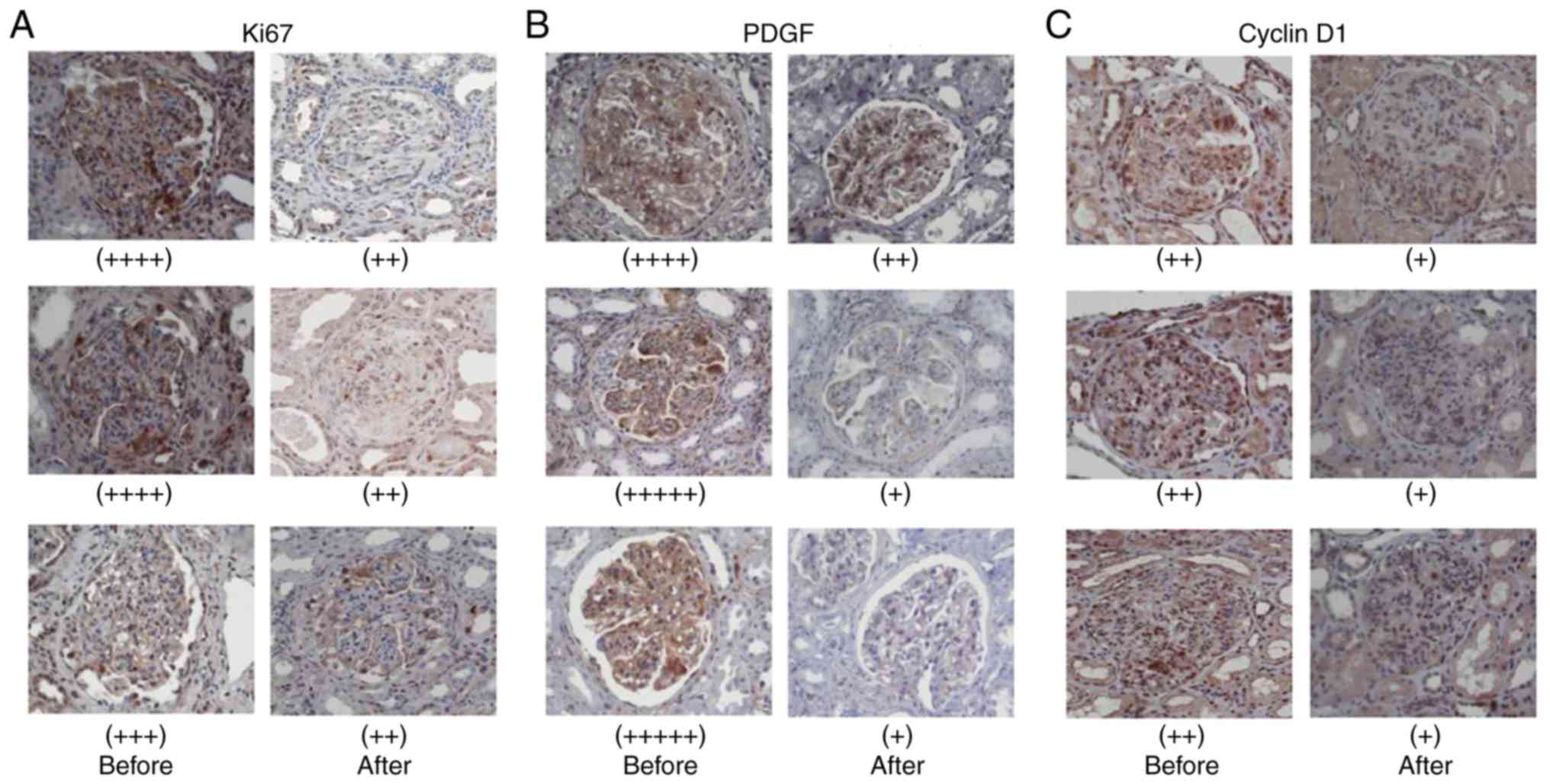
In total, 6 patients with biopsy-confirmed LN
(classes IV) received combined treatment TAC and MMF and methyl
prednisone for 6 months. The levels of the cell proliferation
markers, Ki67 and PDGF, and the cell cycle-related protein, cyclin
D1, were measured in the kidney biopsy samples of the patients by
immunohistochemistry before and after 6 months of treatment.
Following combination therapy for 6 months, the Ki67, PDGF and
cyclin D1 levels were markedly decreased in the mesangial cells
(Fig. 3).
Efficacy and safety of combination of TAC
and MMF and methyl prednisone for the treatment of patients with
LN
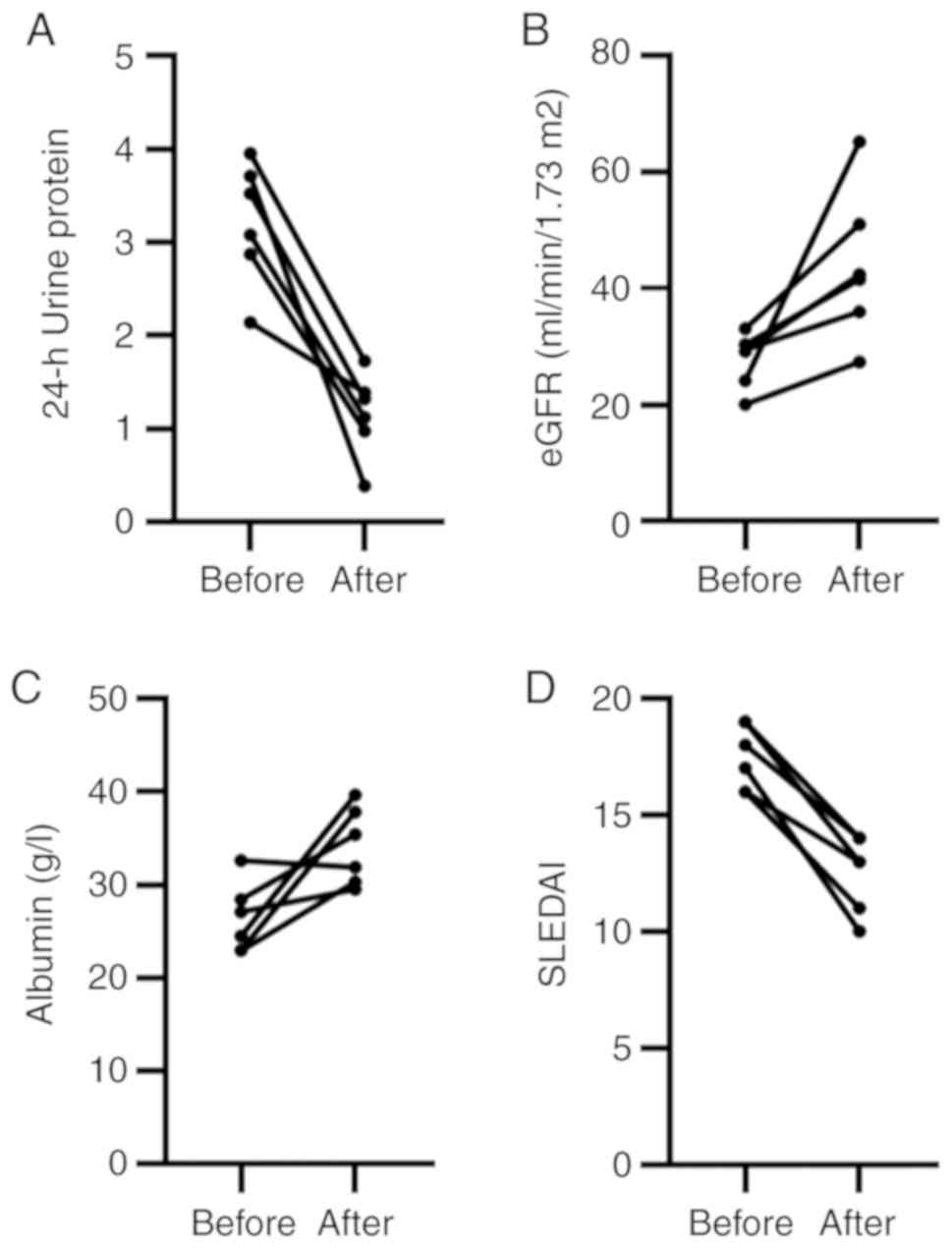
Changes in 24-h proteinuria, eGFR, albumin, SLEDAI,
anti-dsDNA antibodies and C3 before and after treatment were
compared to evaluate the efficacy and safety of the combination of
TAC, MMF and methyl prednisone in the treatment of LN. Following
combination therapy for 6 months, the levels of 24-h proteinuria,
eGFR, albumin and SLEDAI were significantly improved (Fig. 4). In 3 out of 5 patients,
anti-dsDNA antibody turned negative, and in 4 out of 6 patients, C3
levels were elevated (Table I).
Some adverse effects occurred during the treatment period; however,
none of these were considered serious (Table II).
 | Table IChanges in anti-dsDNA antibody and C3
levels in 6 patients before and after 6 months of treatment. |
Table I
Changes in anti-dsDNA antibody and C3
levels in 6 patients before and after 6 months of treatment.
| Patient no. | Age (years) | Disease history
(months) | Before treatment
| After treatment
|
|---|
| anti-dsDNA Ab | C3 (<0.79
g/l) | anti-dsDNA Ab | C3 (<0.79
g/l) |
|---|
| 1 | 21 | 12 | + | + | - | - |
| 2 | 26 | 3 | - | + | - | - |
| 3 | 31 | 8 | + | + | + | + |
| 4 | 24 | 4 | + | + | + | - |
| 5 | 19 | 7 | + | + | - | + |
| 6 | 36 | 15 | + | + | - | - |
 | Table IIAdverse effects noted during the
treatment period. |
Table II
Adverse effects noted during the
treatment period.
| Patient no. | Dyspepsia | Transient abnormal
liver function | Transient elevation
of serum creatinine | Leucopenia | Severe
infection | Arrhythmia | Menstrual
disorder |
|---|
| 1 | + | - | - | - | - | - | + |
| 2 | - | - | - | - | - | - | + |
| 3 | - | + | - | - | - | - | - |
| 4 | + | - | + | - | - | - | + |
| 5 | - | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - | - |
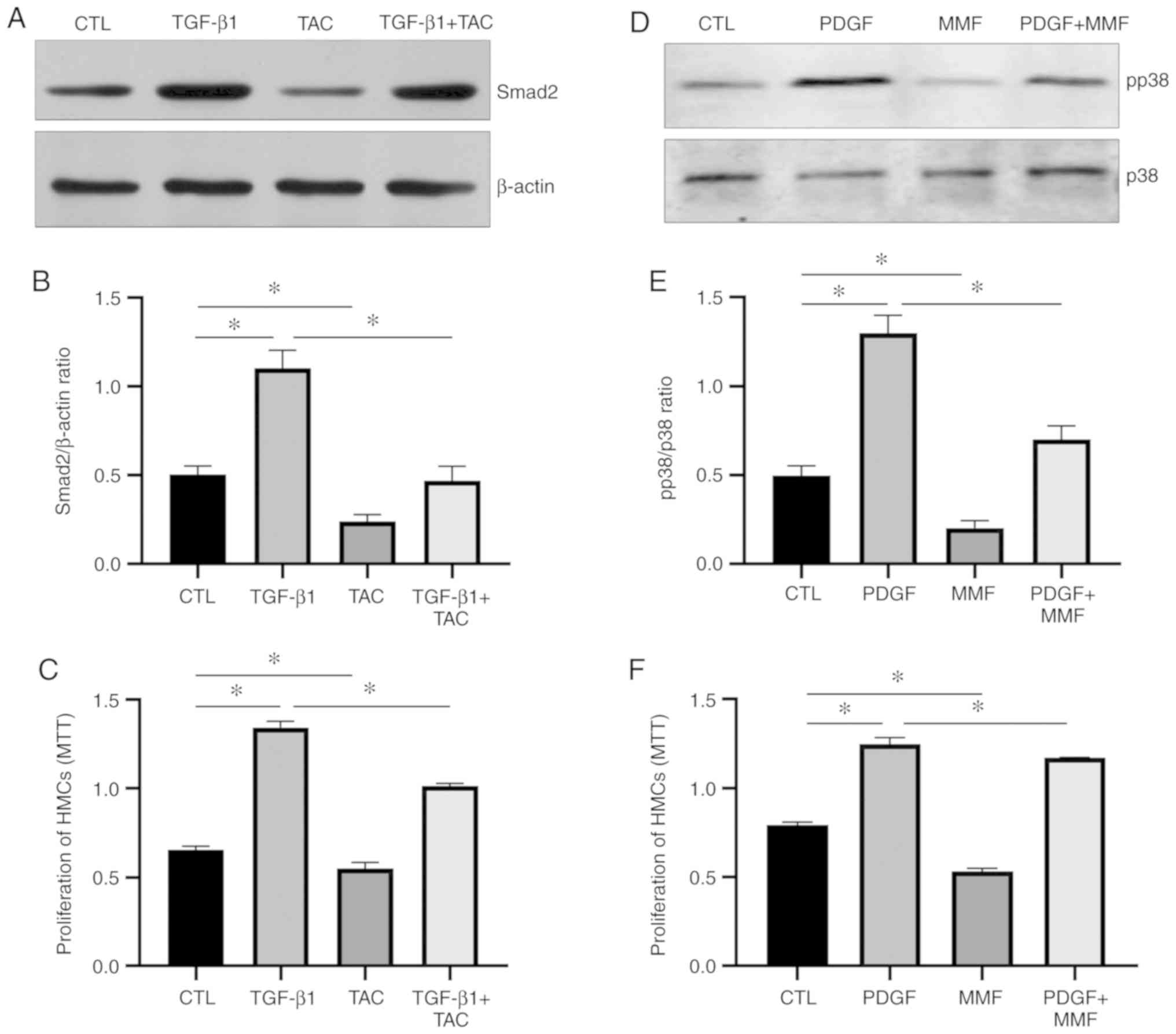
TAC and MMF inhibit HMC proliferation
through different mechanisms
To further investigate the mechanisms responsible
for the inhibition of the proliferation of HMCs by TAC and MMF,
TGF-β1 and PDGF were used to stimulate the HMCs. A number of HMCs
were cultured normally, while other HMCs were treated with 5 ng/ml
TGF-β1, 5 µmol/l TAC or 5 ng/ml TGF-β1 + 5 µmol/l TAC
for 48 h. Western blot analysis was used to detect the expression
of Smad2, and MTT assay was used to measure the cell proliferation
rate. Detectable levels of Smad2 were found in the control HMCs.
TAC significantly decreased the level of Smad2 and inhibited HMC
proliferation compared with the control group. Incubation with
TGF-β1 induced an increase in Smad2 expression and HMC
proliferation, which was significantly abrogated by TAC (Fig. 5A-C). These data demonstrated that
TAC inhibited HMC proliferation by affecting the Smad2 signaling
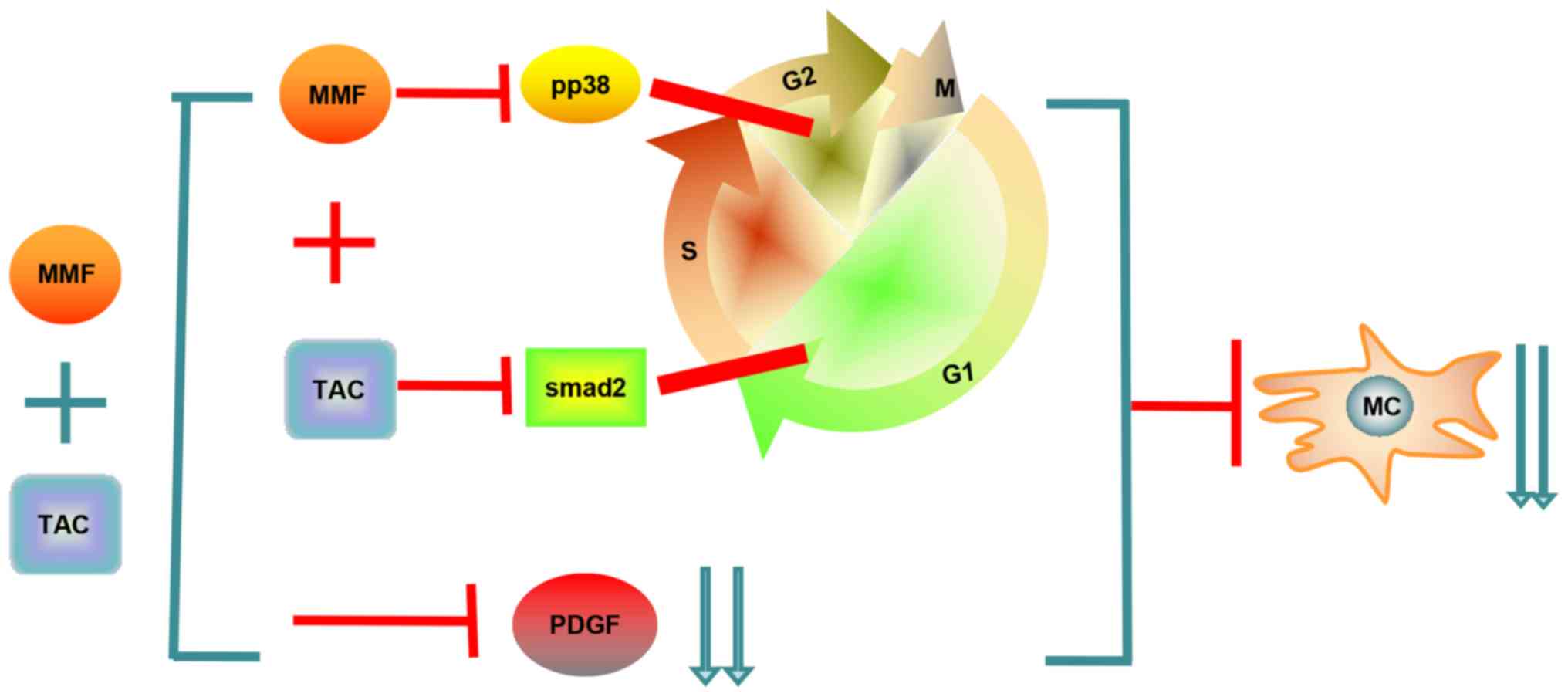
pathway (Fig. 6).
In addition, a group of HMCs was cultured normally,
while other groups of cells were treated with 20 ng/ml PDGF, 10
µmol/l MMF or 20 ng/ml PDGF + 10 µmol/l MMF for 48 h.
The protein expression levels of p-p38 and p38 were examined by
western blot analysis. Cell proliferation was measured by MTT
assay. The results revealed that MMF decreased the level of p38 and
inhibited HMC proliferation. PDGF induced the phosphorylation of
p38 and HMC proliferation. However, following treatment with MMF,
the opposite trend was observed (Fig.
5D-F). These data demonstrated that MMF inhibits HMC
proliferation by affecting the p38 signaling pathway (Fig. 6).
Discussion
Glomerular mesangial cells have multiple functions,
including the structural support of capillary clusters, regulation
of glomerular hemodynamics and the phagocytic clearance of
macromolecules and immune complexes (17,18). In addition, they have a complex
interaction with infiltrating inflammatory cells, and play an
important role in the development of inflammation, fibrosis and
glomerular sclerosis (19). The
abnormal proliferation of mesangial cells is a common pathological
change of proliferative glomerular disease (such as IgA
nephropathy, nephrotic syndrome and LN). Under pathological
conditions, mesangial cells proliferate in large quantities,
release various inflammatory mediators and secrete extracellular
matrix, which then causes glomerular sclerosis, fibrosis and
ultimately leads to end-stage renal failure (20). Since the 1950s, hormones and
immunosuppressants have found clinical applications. The
multi-targeted use of immunosuppressants based on the cell cycle is
a new direction in the treatment of kidney disease.
In a previous study, it was confirmed that TAC can
block mesangial cells in the G0/G1 phase, preventing them from
entering the S phase. MMF prevents mesangial cells in S phase from
entering the G2/M phase, thus inhibiting their proliferation
(1). However, the effect of
combining TAC and MMF based on cell cycle to inhibit the
proliferation of mesangial cells, and whether it is more promiment
than the effect of a single drug, remain unknown. In the present
study, the inhibitory effect of TAC combined with MMF on mesangial
cell proliferation was explored.
Three treatment regimens, TAC, MMF or 1/2TAC +
1/2MMF, were applied to assess the proliferative stage of HMCs and
mice with LN. The levels of antigen Ki67 and the cell cycle
regulatory protein, cyclin D1, which reflect the proliferation of
mesangial cells, were reduced following these treatments, and the
changes indicated that all three treatment regimens inhibited
mesangial cell proliferation. The expression of Ki67 and cyclin D1
in the 1/2T + 1/2M treatment group was lower than that in the other
2 treatment groups at the end of the experiment, and the
differences were statistically significant. These results are
consistent with the results of PAS and Masson's trichrome staining
of mice with LN, indicating that the combination of TAC and MMF was
more effective than monotherapy in vitro and in vivo.
Furthermore, following the treatment of patients, biopsy-confirmed
LN (classes IV) with combination TAC and MMF at half the dose, the
levels of Ki67, PDGF and cyclin D1 were decreased significantly in
the patient kidney samples. In addition, clinical indicators were
also improved, and there were no severe adverse effects. These
results confirmed that the combination of TAC and MMF at half the
dose is an effective and safe regiment in the therapy of glomerular
proliferative disease.
In fact, combination therapy with a steroid, TAC and
MMF has achieved a good therapeutic effect in clinical practice in
LN. Previous studies have found that the combination of TAC and MMF
is more effective and safer than conventional treatment
(intravenous cyclophosphamide and steroid) (9,10,21,22). Some studies have reported that TAC
combined with MMF treatment may be a beneficial option for patients
with LN who exhibit an inadequate response to either
cyclophosphamide or MMF treatment, or had lupus flares after
achieving a complete response (23-25). However, whether the effect of TAC
combined with MMF is better than single-drug treatment has not been
reported to date. The present study compared the effect of combined
TAC and MMF with single-drug therapy, and found that the
combination therapy was more effective than monotherapy in
vitro and in vivo. Additional clinical trials are
required to verify this result.
The calcineurin inhibitor, TAC, is a macrolide
immuno-suppressant isolated from Streptomyces sinensis, TAC
can combine with a protein in the cytoplasm (FKBP12) to form a
complex, specifically bind to and inhibit calcineurin, inhibit the
transmission of calcium-dependent signals generated by T cells, and
prevent the activation of the T cell-specific transcription factor
NF-AT, thereby inhibiting lymphocyte proliferation and lymphokine
production (26). As an
immunosuppressive agent, MMF is often used in the treatment of
organ transplantation to prevent rejection and various immune
diseases. Its active metabolite is mycophenolic acid, which is an
inhibitor of IMPDH, a catalyst essential for the classical
synthetic pathway of guanine nucleotides, which is essential for
lymphocyte activation. Therefore, MMF inhibits the synthesis and
proliferation of lymphocytes in the G1-S phase (27). Previous studies have demonstrated
that the mechanisms through which combination therapy is more
beneficial than single therapy are as follows: The additive
suppression of T- and B-cell activation (28), anti-inflammatory and podocyte
protection (29,30). The present study uncovered novel
synergistic mechanisms in TAC and MMF combination therapy for
mesangial proliferative kidney disease.
PDGF is a platelet-derived mitogenic factor, and its
proliferative effect on cells is mediated mainly by PDGF-BB.
Previous in vivo and in vitro studies have indicated
that PDGF-BB is the most important mitogenic factor that causes
abnormal proliferation of mesangial cells, and plays a key role in
the pathogenesis of glomerulonephritis. In addition, PDGF can
stimulate the abnormal proliferation of HMCs (31). Thus, HMCs stimulated by PDGF were
selected in the present study to establish a model of HMC
hyperplasia. A previous study revealed that MMF decreased PDGF
protein expression (32). In the
present study, the expression of PDGF in mice with LN and in kidney
tissues of patients with LN was detected by immunohistochemistry
and western blot analysis. following TAC, MMF or 1/2TAC + 1/2MMF
treatment, the PDGF levels were decreased in mice with LN and in
kidney tissues of patients with LN. Compared with that following
treatment with TAC or MMF alone, the expression of PDGF following
treatment with TAC combined with MMF at half the concentration was
lower. These results demonstrated that both TAC and MMF reduced the
expression of PDGF, and the combination of TAC and MMF exerted a
synergistic effect in decreasing the expression of PDGF and
inhibiting mesangial cell proliferation. This may be partially
responsible for the inhibitory effect of TAC and MMF on mesangial
cell proliferation.
TGF-β1, as a multifunctional cytokine, plays an
important role in regulating the physiological processes of cell
growth, differentiation and biosynthesis (33). TGF-β1 plays an important role in
cell proliferation. After TGF-β1 binds to the serine/threonine
kinase receptor on the surface of mesangial cells, the signal is
transferred from the cell membrane to the nucleus mediated by Smad
proteins (34,35). In the present study, TGF-β1 was
used to induce HMC proliferation. When the HMCs were treated with
TGF-β1, the expression of Smad2 protein was enhanced, which
confirmed the existence of the Smad signaling pathway in mesangial
cells. Treatment of the HMCs with TAC decreased the expression of
Smad2 protein, which suggested that TAC inhibited the proliferation
of mesangial cells at least in part by affecting the Smad signaling
pathway.
Han et al reported the p38 signaling pathway
for the first time (36). The p38
signaling pathway is one of the most important signaling pathway in
cells. It plays an important role in multiple biological reactions,
including cell cycle regulation, cell proliferation, development,
differentiation, aging, apoptosis, immune response and
tumorigenesis. Numerous drugs promote mesangial cell proliferation
through the p38 signaling pathway (37,38). When exposed to various stimuli,
p38 is phosphorylated, p-p38 is transferred into the nucleus,
further regulating downstream signaling molecules and the activity
of transcription factors. In the present study, the phosphorylation
rate of p38 increased significantly in PDGF-stimulated HMCs, which
confirmed that the proliferation of HMCs involves the p38 signaling
pathway. The ratio of p38 protein phosphorylation in HMCs
stimulated by PDGF and treated with MMF was significantly lower
than that in HMCs not treated with MMF, which suggested that MMF
inhibited mesangial cell proliferation at least in part by
affecting the p38 signaling pathway.
In conclusion, TAC and MMF exert their inhibitory
effects on mesangial cell proliferation by acting on different
phases of the cell cycle of mesangial cells through different
pathways: TAC can block mesangial cells in the G0/G1 phase,
preventing them from entering the S phase via influencing the
TGF-β1/Smad signaling pathway; MMF prevents mesangial cells in the
S phase from entering G2/M phase via the p38 signaling pathway.
Furthermore, the results confirmed that the combination of TAC and
MMF therapy based on the cell cycle with half the concentration was
more effective than monotherapy in inhibiting mesangial cell
proliferation in vitro and in vivo. This synergistic
effect on the inhibition of mesangial cell proliferation is
mediated, at least in part, by a decrease in the expression of PDGF
(Fig. 6).
The combination of TAC and MMF at half the
concentration can inhibit the proliferation of mesangial cells more
effectively than TAC and MMF alone, suggesting that, according to
the cell cycle, treatment of proliferative glomerular disease with
multiple immunosuppressants agents can improve the therapeutic
effect and reduce the dosage of immunosuppressive agents used in
the treatment, thus reducing the occurrence of adverse reactions
and providing a novel strategy for the treatment of mesangial
proliferative disease. The findings suggest that, in the treatment
of mesangial proliferative nephropathy, if the disease requires the
combination of immunosuppressive agents, attention should be paid
to the use of immunosuppressive agents acting at different sites
and different phases of the cell cycle. These agents should be
sequentially combined based on the cell cycle to avoid the
simultaneous use of multiple immunosuppressive agents acting on the
same site and at the same phase of the cell cycle. This can fully
exert the therapeutic effect of immunosuppressants via different
mechanisms, and can effectively avoid excessive inhibition of
immunity, which may lead to adverse reactions. Additional clinical
studies are warranted to assess the efficacy and safety of a
multi-target therapy consisting of TAC, MMF and steroid compared
with other therapies for glomerular proliferative disease.
Acknowledgments
The authors would like to thank Dr Xuewang Li of
Peking Union Medical college Hospital for providing the human
mesangial cells.
Funding
The present study was supported by the Province
Natural Science Foundation of Shanxi (grant no. 201701D221263).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YG performed the experiments and was a major
contributor to the writing of the manuscript. HY performed the
experiments. YW performed the histological examination of the
kidneys. JT analyzed and interpreted the patient data. RL designed
the study and reviewed the manuscript. XZ conceived and designed
the study and performed the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Shanxi Provincial People's
Hospital (Taiyuan, China) approved the study. Written informed
consent was obtained from each patient or their legal
representative, according to the Declaration of Helsinki. The
animal experiments were performed according to protocols approved
by the Institutional Animal Care and Use Committee of Shanxi
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pastukhov O, Schwalm S, Römer I,
Zangemeister-Wittke U, Pfeilschifter J and Huwiler A: Ceramide
kinase contributes to proliferation but not to prostaglandin E2
formation in renal mesangial cells and fibroblasts. Cell Physiol
Biochem. 34:119–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chhabra S, Liu Y, Hemmer MT, Costa L,
Pidala JA, Couriel DR, Alousi AM, Majhail NS, Stuart RK, Kim D, et
al: Comparative analysis of calcineurin inhibitor-based
methotrexate and mycophenolate mofetil-containing regimens for
prevention of graft-versus-host disease after reduced-intensity
conditioning allogeneic transplantation. Biol Blood Marrow
Transplant. 25:73–85. 2019. View Article : Google Scholar
|
|
3
|
Akool ES, Doller A, Babelova A, Tsalastra
W, Moreth K, Schaefer L, Pfeilschifter J and Eberhardt W: Molecular
mechanisms of TGFbeta receptor-triggered signaling cascades rapidly
induced by the calcineurin inhibitors cyclosporin A and FK506. J
Immunol. 181:2831–2845. 2008. View Article : Google Scholar
|
|
4
|
Huls G, Chitu DA, Havelange V,
Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ, Sinnige H,
Hodossy B, Graux C, Kooy RVM, et al: Azacitidine maintenance after
intensive chemotherapy improves DFS in older AML patients. Blood.
133:1457–1464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang KH, Sanchez-Aguilera A, Shen S,
Sengupta A, Madhu MN, Ficker AM, Dunn SK, Kuenzi AM, Arnett JL,
Santho RA, et al: Vav3 collaborates with p190-BCR-ABL in lymphoid
progenitor leukemogenesis, proliferation, and survival. Blood.
120:800–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seo T, Fukushima T, Inoue H, Imamura S,
Urasaki Y, Yoshida A, Kawai Y, Yamauchi T, Iwasaki H, Tsutani H, et
al: Long-term follow-up of the clinical efficacy of chemotherapy
for acute myeloid leukemia at a single institute. J Infect
Chemother. 7:156–162. 2001. View Article : Google Scholar
|
|
7
|
Ekberg H, van Gelder T, Kaplan B and
Bernasconi C: Relationship of tacrolimus exposure and mycophenolate
mofetil dose with renal function after renal transplantation.
Transplantation. 92:82–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z,
Chen J, Lin H, Liu F, He Y, et al: Multitarget therapy for
induction treatment of lupus nephritis: A randomized trial. Ann
Intern Med. 162:18–26. 2015. View
Article : Google Scholar
|
|
9
|
Zhang H, Liu Z, Zhou M, Liu Z, Chen J,
Xing C, Lin H, Ni Z, Fu P, Liu F, et al: Multitarget therapy for
maintenance treatment of lupus nephritis. J Am Soc Nephrol.
28:3671–3678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT and
Li LS: Successful treatment of class V+IV lupus nephritis with
multitarget therapy. J Am Soc Nephrol. 19:2001–2010. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Workeneh B, Hu Z and Li R: Effect
of immunosuppression on the human mesangial cell cycle. Mol Med
Rep. 11:910–916. 2015. View Article : Google Scholar
|
|
12
|
Delarue F, Virone A, Hagege J, Lacave R,
Peraldi MN, Adida C, Rondeau E, Feunteun J and Sraer JD: Stable
cell line of T-SV40 immortalized human glomerular visceral
epithelial cells. Kidney Int. 40:906–912. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hochberg MC: Updating the American college
of rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Penault-Llorca F and Radosevic-Robin N:
Ki67 assessment in breast cancer: An update. Pathology. 49:166–171.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
incancer and the CDK inhibitorlandscape past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mai S, Zou L, Tian X, Liao X, Luan Y, Han
X, Wei Y, Wu Y, Kuang S, Yang Y, et al: Double-edged effect of
hydroxychloroquine on human umbilical cord-derived mesenchymal stem
cells treating lupus nephritis in MRL/lpr Mice. Mol Pharm.
15:1800–1813. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson RJ, Floege J, Yoshimura A, Iida H,
Couser WG and Alpers CE: The activated mesangial cell: A glomerular
'myofibroblast'? J Am Soc Nephrol. 2(10 Suppl): S190–S197.
1992.PubMed/NCBI
|
|
18
|
Alhassona F, Setha RK, Sarkara S, Kimonoa
DA, Albadrania MS, Dattaroya D, Chandrashekarana V, Scotta GI,
Raychoudhuryb S, Nagarkattic M, et al: High circulatory leptin
mediated NOX-2-peroxynitrite-miR21 axis activate mesangial cells
and promotes renal inflammatory pathology in nonalcoholic fatty
liver disease. Redox Biol. 17:1–15. 2018. View Article : Google Scholar
|
|
19
|
Pereira RL, Felizardo RJ, Cenedeze MA,
Hiyane MI, Bassi EJ, Amano MT, Origassa CS, Silva RC, Aguiar CF,
Carneiro SM, et al: Balance between the two kinin receptors in the
progression of experimental focal and segmental glomerulosclerosis
in mice. Dis Model Mech. 7:701–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scindia YM, Deshmukh US and Bagavant H:
Mesangial pathology in glomerular disease: Targets for therapeutic
inter-vention. Adv Drug Deliv Rev. 62:1337–1343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng J, Luo L, Zhu L and Xie H and Xie H:
Multitarget therapy versus intravenous cyclophosphamide in the
induction treatment oflupus nephritis: A metaanalysis of randomized
controlled trials. Turk J Med Sci. 48:901–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou T, Lin S, Yang S and Lin W: Efficacy
and safety of tacrolimus in induction therapy of patients with
lupus nephritis. Drug Des Devel Ther. 13:857–869. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park DJ, Kang JH, Lee KE, Bae SC, Chung
WT, Choe JY, Jung SY, Kim YS, Lee HS, Lee J, et al: Efficacy and
safety of mycophenolate mofetil and tacrolimus combination therapy
in patients with lupus nephritis a nationwide multicentre study.
Clin Exp Rheumatol. 37:89–96. 2019.
|
|
24
|
Mok CC, To CH, Yu KL and Ho LY: Combined
low-dose mycophenolate mofetil and tacrolimus for lupus nephritis
with suboptimal response to standard therapy: A 12-month
prospective study. Lupus. 22:1135–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi CB, Won S and Bae SC: Outcomes of
multitarget therapy using mycophenolate mofetiland tacrolimus for
refractory or relapsing lupus nephritis. Lupus. 27:1007–1011. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prytuła A and van Gelder T: Clinical
aspects of tacrolimus use in paediatric renal transplant
recipients. Pediatr Nephrol. 34:31–43. 2019. View Article : Google Scholar
|
|
27
|
Dubus I, Vendrely B, Christophe I,
Labouyrie JP, Delmas Y, Bonnet J and Combe C: Mycophenolic acid
antagonizes the activation of cultured human mesangial cells.
Kidney Int. 62:857–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiseman AC: Immunosuppressive medications.
Clin J Am Soc Nephrol. 11:332–343. 2016. View Article : Google Scholar :
|
|
29
|
Fu J, Wang Z, Lee K, Wei C, Liu Z, Zhang
M, Zhou1 M, Cai M, Zhang W, Chuang PY, et al: Transcriptomic
analysis uncovers novel synergistic mechanisms in combination
therapy for lupus nephritis. Kidney Int. 93:416–429. 2018.
View Article : Google Scholar
|
|
30
|
Manabe S, Nitta K and Nagata M: Direct
effects of immunomodulatory agents on podocytes in immune-mediated
glomerular diseases. Contrib Nephrol. 195:131–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shultz PJ, DiCorleto PE, Silver BJ and
Abboud HE: Mesangial cells express PDGF mRNAs and proliferate in
response to PDGF. Am J Physiol. 255(4 Pt 2): F674–F684.
1988.PubMed/NCBI
|
|
32
|
Sabuda-Widemann D, Grabensee B, Schwandt C
and Blume C: Mycophenolic acid inhibits the autocrine PDGF-B
synthesis and PDGF-BB-induced mRNA expression of Egr-1 in rat
mesangial cells. Nephrol Dial Transplant. 24:52–61. 2009.
View Article : Google Scholar
|
|
33
|
Liu HF, Liu H, Lv LL, Ma KL, Wen Y, Chen L
and Liu BC: CCN3 suppresses TGF-b1-induced extracellular matrix
accumulation in human mesangial cells in vitro. Acta Pharmacol Sin.
39:222–229. 2018. View Article : Google Scholar
|
|
34
|
Yoon J, Lee YJ, Namgung S, Han BH, Choi
ES, Kang DG and Lee HS: Samchuleum attenuates diabetic renal injury
through the regulation of TGF-β/Smad signaling in human renal
mesangial cells. Mol Med Rep. 17:3099–3108. 2018.
|
|
35
|
Zhang L, Han C, Ye F, He Y, Jin Y, Wang T,
Wu Y, Jiang Y, Zhang F and Jin X: Plasma gelsolin induced
glomerular fibrosis via the TGF-β1/Smads signal transduction
pathway in IgA nephropathy. Int J Mol Sci. 18:3902017. View Article : Google Scholar
|
|
36
|
Han J, Lee JD, Bibbs L and Ulevitch RJ: A
MAP kinase targeted by endotoxin and hyperosmolarity in mammalian
cells. Science. 265:808–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Wang L and Ma H: Betaine alleviates
high glucose-induced mesangial cell proliferation by inhibiting
cell proliferation and extracellular matrix deposition via the
AKT/ERK1/2/p38 MAPK pathway. Mol Med Rep. 20:1754–1760.
2019.PubMed/NCBI
|
|
38
|
Kim D, Li HY, Lee JH, Oh YS and Jun HS:
Lysophosphatidic acid increases mesangial cell proliferation in
models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp
Mol Med. 51:1–10. 2019.PubMed/NCBI
|