Introduction
Oxygen supplementation is widely used in the
treatment of acute and severe diseases suffered by newborns.
However, high concentrations of oxygen can produce a large number
of highly active superoxides, hydrogen peroxide and other toxic
products (1) resulting in not
only irreversible lung damage and pulmonary hypertension in
adulthood, but also damage to other organs (2). Simultaneously, oxygen exposure may
alter the developmental dynamics of the immune response and inhibit
the expression of key genes involved in adaptive immunity (3).
The intestine is relatively immature in the first
two weeks after birth, increasing the susceptibility of newborn
animals to hyperoxia (4). A
previous study found that neonatal hyperoxic exposure destroyed the
intestinal barrier of neonatal rats, and in this case, oxidative
stress stimulated the intestine to produce a variety of cytokines,
indicating that oxidative stress is the main cause of intestinal
injury in newborns (5).
The mammalian interleukin 17 (IL-17) family is
considered one of the most ancient cytokine families, and is
comprised of six cytokines, namely IL-17A, IL-17B, IL-17C, IL-17D,
IL-17E, and IL-17F. Members of the family can directly or
indirectly induce a variety of cytokines, chemokines, and
inflammatory factors, as well as participate in both innate and
adaptive immune responses. However, information regarding IL-17D is
currently limited. Several studies have found that IL-17D has an
important impact in inflammatory diseases, and host defense against
bacterial infection of vertebrates such as poultry and teleosts
(6-10). The expression of IL-6, IL-8,
granulocyte-macrophage colony-stimulating factor (GM-CSF) and other
cytokines can be induced by IL-17D, similar to those of other
family members (11). The
difference, though, is that IL-17D is not only expressed on immune
cells but also is preferentially expressed in skeletal muscle,
brain, adipose, heart, lung, and pancreas (12). It was reported that IL-17D can
regulate protective immunity during intracellular bacterial and
viral infections (6).
Furthermore, in tumors and in viral infections, IL-17D recruited
natural killer (NK) cells via the chemokine (C-C motif) ligand 2
(CCL2). In this specific process, nuclear factor erythroid
2-related factor 2 (Nrf2) is required for positively regulating
IL-17D (13-16). Nrf2 is the primary responder to
oxidative stress and is a sensitive factor of reactive oxygen
species (ROS). This study aimed to investigate the changes in
intestinal IL-17D during hyperoxia and to determine whether the
Nrf2/kelch-like ECH-associated protein 1 (keap1) pathway is
involved in regulating IL-17D.
Materials and methods
Animals
All Sprague-Dawley rats in this study were provided
by the Animal Center of Shengjing Hospital of China Medical
University. All surgical procedures were performed under anesthesia
and all efforts were made to minimize animal suffering. All studies
were performed in accordance with the protocol approved by the
Institutional Animal Care and Use Committee of the China Medical
University (no. 2018PS178K). All applicable institutional and/or
national guidelines for the care and use of animals were
followed.
Time-dated pregnant female Sprague-Dawley rats (42
female rats; 8 male mates; weight, 200-240 g; age,45-65 days) were
housed in individual cages with free access to laboratory food and
water, maintained on a 12:12-h light-dark cycle, and were allowed
to deliver vaginally at term. Newborn rats were randomly divided
into a model [exposure to hyperoxia (80-85% O2) from day
of birth], n≥10, and a control group [exposure to normoxia (21%
O2), n≥10, within 12 h of birth]. To avoid O2
toxicity, maternal rats within the model and control groups were
switched every 24 h.
Tissue harvest
At days 3, 7, 10 and 14 after the start of exposure
to either hyperoxia (n≥10) or normoxia (n≥10) newborn rats from the
model or control group were anesthetized with pentobarbital sodium
(50 mg/kg; intraperitoneal) and sacrificed by cervical dislocation.
The abdomen was opened through a midline incision, and the
intestinal tract was carefully removed. The section of small
intestine of each rat was flushed with saline to remove residual
fecal contents. The middle 2 cm of the small intestine was excised
and fixed with formalin, embedded in paraffin and sectioned using a
microtome (Thermo Fisher Scientific, Inc.) at 3 µm for
subsequent immunostaining. The remaining tissue was immediately
stored at −80°C to separate the protein at a later date.
Immunofluorescence Single
immunofluorescence
The tissue sections were deparaffinized in xylene
and rehydrated in a gradient ethanol series. For antigen retrieval,
the sections were microwaved in 0.01 M citrate buffer (pH 6.0) for
37 min, then incubated for 40 min at room temperature in normal
goat serum (OriGene Technologies) and 3% H2O2
to block nonspecific antibody binding and endogenous peroxidase
activity. Rabbit poly-clonal anti-IL-17D (dilution 1:100, cat. no.
bs-2612R, Bioss) was used for incubating the sections overnight at
4°C, while phosphate-buffered saline (PBS) was used in place of the
anti-body for negative controls. The sections were then treated for
4 h at room temperature with fluorochrome-conjugated goat
anti-rabbit IgG (dilution 1:100, cat. no. RS3611, Immunoway),
followed by incubation with 4′,6-diamidino-2-phenylindole (DAPI)
incubation for nuclear staining. Immunofluorescence images were
acquired using a confocal laser-scanning micro-scope
(magnification, ×400) (C1; Nikon, Tokyo, Japan).
Double immunofluorescence
After routine deparaffinization, the sections were
microwaved in 0.01 M citrate buffer. The sections were incubated
for 40 min at room temperature in normal goat serum (OriGene
Technologies) and 3% H2O2. A combination of
two primary antibodies; rabbit polyclonal anti-IL-17D (dilution
1:100, cat. no. bs-2612R, Bioss) and mouse monoclonal anti-CD4
(dilution 1:100, cat. no. sc-2007, Santa Cruz Biotechnology, Inc.),
or rabbit polyclonal anti-IL-17D (dilution 1:100, cat. no.
bs-2612R, Bioss) and mouse mono-clonal anti-CD19 (dilution 1:50,
cat. no. sc-373897, Santa Cruz Biotechnology, Inc.) were used for
incubating the sections overnight at 4°C. The sections were then
incubated with a mixture of two secondary antibodies, goat
anti-rabbit IgG (dilution 1:100, cat. no. RS3611, Immunoway) and
goat anti-mouse IgG (dilution 1:100, cat. no. RS3208, Immunoway)
for 4 h at room temperature, followed by DAPI incubation for
nuclear staining. Double immunofluorescence images were acquired
using a confocal laser-scanning microscope (magnification, ×400)
(C1; Nikon).
Immunohistochemistry
The experiment was performed using the streptavidin
peroxidase method (SPlink detection kit; OriGene Technologies)
according to the manufacturer's instructions. Paraffin-embedded
intestinal tissue sections were deparaffinized, rehydrated, blocked
with 10% goat serum and treated with 3% H2O2.
Then the sections were incubated overnight at 4°C with IL-17D
antibody (dilution 1:400, cat. no. bs-2612R, Bioss) or Nrf2
antibody (dilution 1:1,600, cat. no. ab 31163, Abcam), or keap1
antibody (dilution 1:1,600, cat. no. ab139729, Abcam). The sections
were then incubated with biotin-labeled goat anti-rabbit IgG
secondary antibody and streptavidin-horseradish peroxidase for 30
min at 37°C. Finally, the sections were developed with
3,3′-diaminobenzidine and counterstained with hematoxylin. All
immunostained sections were viewed using a digital camera Nikon
Eclipse E800 (Olympus Corp.). Semiquantitative analysis of the
optical density (OD) values of the positive staining in the villi
of each section at ×200 magnification was performed using ImageJ
6.0 (National Institutes of Health). The absorbance values of Nrf2,
keap1 and IL-17D were compared via Prism Graph version 5.0 software
(GraphPad Software, Inc.) after scanning.
Western blotting
Western blotting was performed using standard
protocols. In brief, total proteins were extracted from small
intestine tissue and mixed with loading buffer. Equal amounts of
protein were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to polyacrylamide
difluoride (PVDF) membranes. After blocking with 5% skim milk for 2
h at room temperature, the membranes were incubated with
anti-IL-17D (dilution 1:1,000, cat. no. bs-2612R, Bioss), anti-Nrf2
(dilution 1:1,000, cat. no. ab31163, Abcam), and anti-keap1
(dilution 1:1,000, cat. no. ab139729, Abcam), or anti-β-actin
(dilution 1:2,000, cat. no. bs-0061R, Bioss) and left shaking
gently overnight at 4°C. The next day, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
anti-body (dilution 1:5,000; cat. no. SA00001-2, Proteintech) for 2
h at room temperature, washed in 10 mM Tris/HCl, 150 mM NaCl, and
0.05% Tween-20, pH 7.5 TBST buffer three times, and developed using
enhanced chemiluminescence reagents (NCM Biotech). Densitometry
values were computed using ImageJ 6.0 (National Institutes of
Health) and standardized to β-actin.
Statistical analysis
For each experiment, we tested at least 10
generations of each group. The data from all groups are reported as
the means ± standard deviations. Statistical anal-ysis was
performed using SPSS 25.0 software (IBM Corp.). A P-value (P) of
<0.05 was considered as indicative of a statistically
significant difference. The statistical significance of the data
was determined using two-way ANOVA with Bonferroni's post hoc test,
and correlation analysis was performed via Pearson's test.
Results
IL-17D expression in rat intestinal
epithelial cells under hyperoxia
The expression of IL-17D in small intestine tissues
from both the control and model groups was analyzed by
immunofluorescence staining. Red fluorescence represents IL-17D. As
indicated in Fig. 1, IL-17D was
expressed in intestinal epithelial cells of both the model group
and the control group. In the control group, there was no change in
IL-17D expression in a day-dependent manner. Compared with the
control group, there was no difference in IL-17D expression in the
model group on postnatal day 3 (Fig.
1A, B and I). On postnatal day 7, IL-17D expression in the
intestinal epithelial cells in the model group was significantly
increased compared to the control group (P<0.01) (Fig. 1C, D and I). On postnatal days 10
and 14, IL-17D expression of intestinal epithelial cells in the
model and control groups was reduced compared to that of postnatal
day 7, but IL-17D expression in the model group was significantly
decreased when compared with the control group (P<0.05)
(Fig. 1E-I). At the same time,
IL-17D was expressed on the lamina propria cells in small
intestines in both groups.
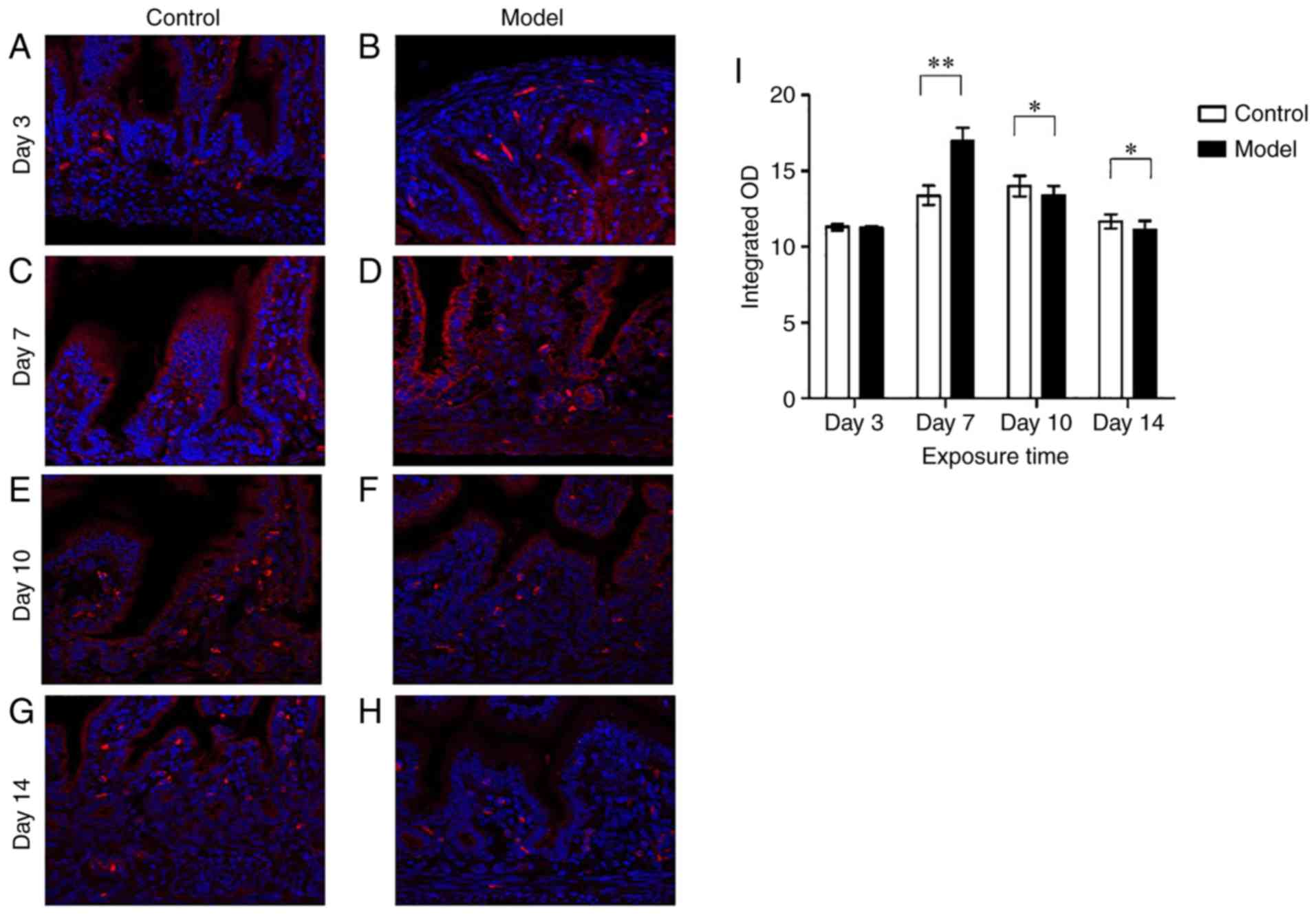 | Figure 1Intestinal IL-17D expression. Red
fluorescence represents IL-17D. Intestinal IL-17D staining in the
model group (A, C, E and G) on postnatal days 3, 7, 10 and 14,
respectively. Intestinal IL-17D staining in the control group (B,
D, F and H) on postnatal day 3, 7, 10 and 14, respectively. (I)
Integrated optical density (OD) of IL-17D. Scale bars, 50 µm
(n≥10 for each time point). *P<0.05 and
**P<0.01. IL-17D, interleukin 17D. |
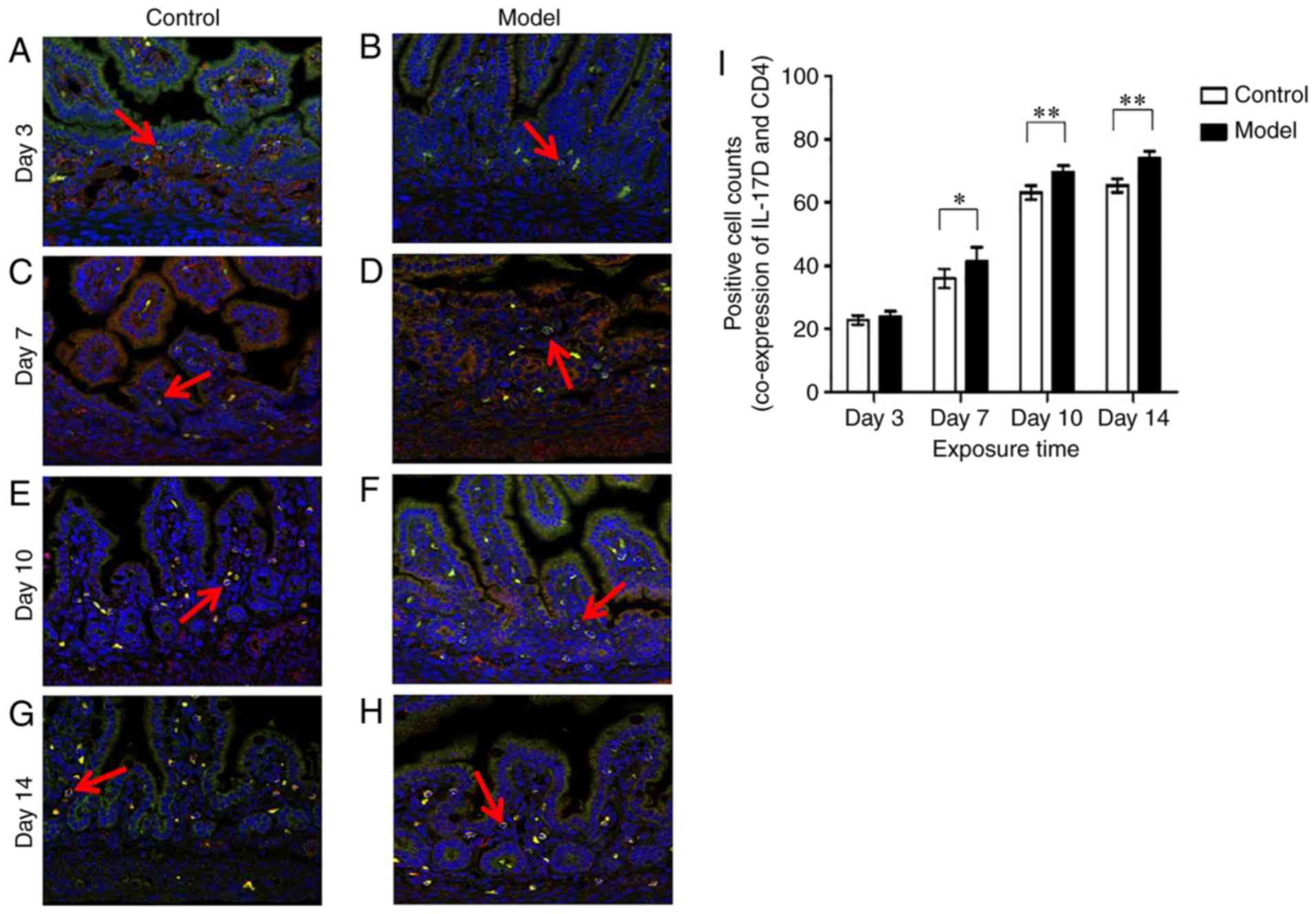
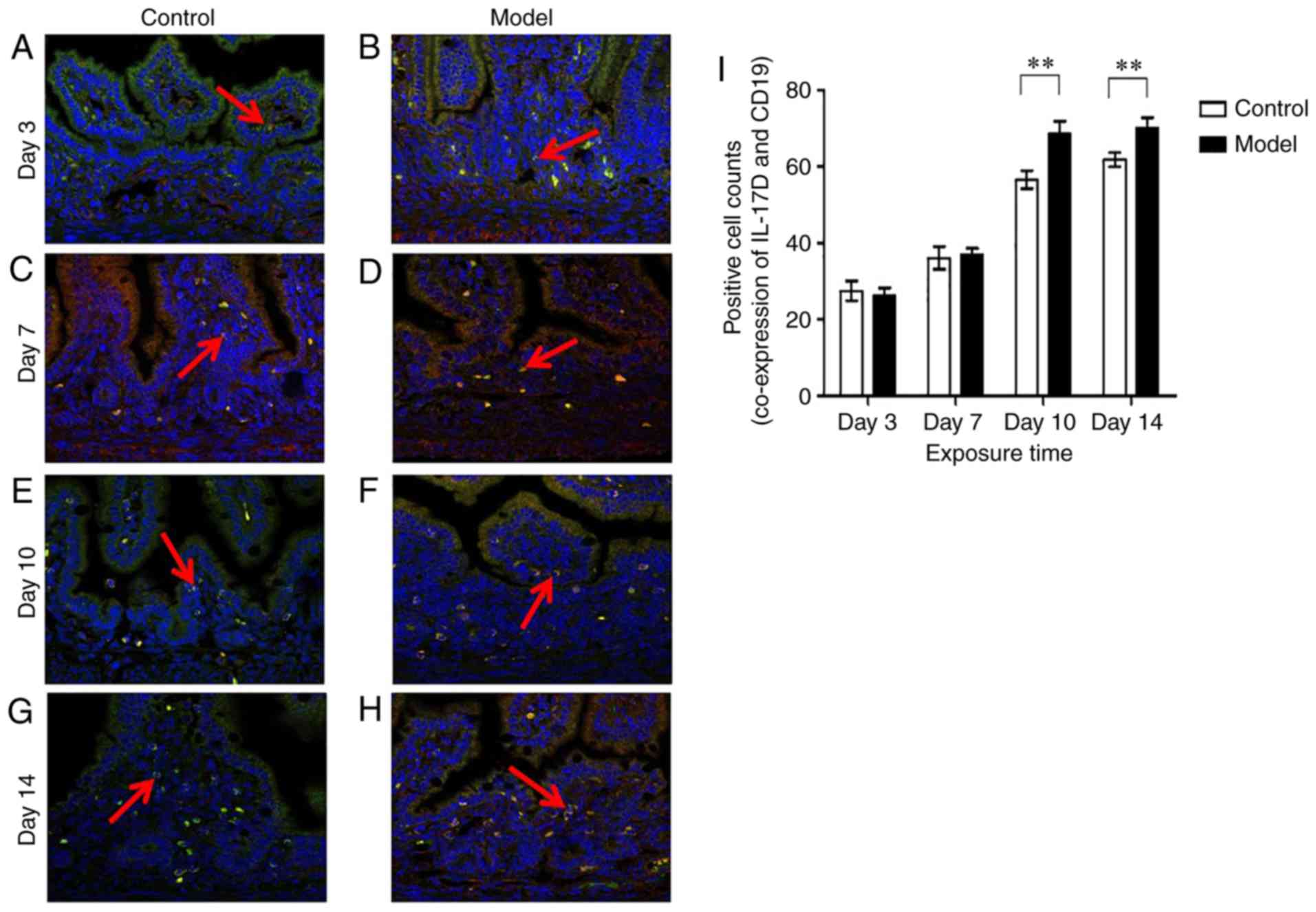
IL-17D expression in neonatal intestinal
lamina propria T and B lymphocytes under hyperoxia
Glycoproteins CD4 and CD19 are selectively expressed
on the surface of T and B lymphocytes, respectively. Green
fluorescence represents CD4 or CD19, red fluorescence represents
IL-17D, and co-expression of CD4 and IL-17D or CD19 and IL-17D
appears as yellow or orange fluorescence. As shown in Figs. 2 and 3, IL-17D was expressed only on a small
number of CD4+ T cells and CD19+ B cells in
lamina propria on postnatal day 3.
In the model group the numbers of CD4+ T
cells which expressed IL-17D were higher than those in the control
group (P<0.05) (Fig. 2A-D and
I) on postnatal day 7, while the numbers of CD19+ B
cells which expressed IL-17D had no significant difference between
the model and control group on postnatal day 7 (Fig. 3A-D and I). On days 10 and 14, the
CD4+ T cells and CD19+ B cells which
expressed IL-17D were significantly increased. In the model group,
the numbers of CD4+ T cells and CD19+ B cells
which expressed IL-17D were significantly higher than those in the
control group (P<0.01) (Figs.
2E-I and 3E-I). This showed
that the IL-17D expression on intestinal epithelial cells was
exactly opposed to those of the intestinal lamina propria T and B
lymphocytes. This demonstrated that the IL-17D of intestinal
epithelial cells may play a unique immune role during
hyperoxia.
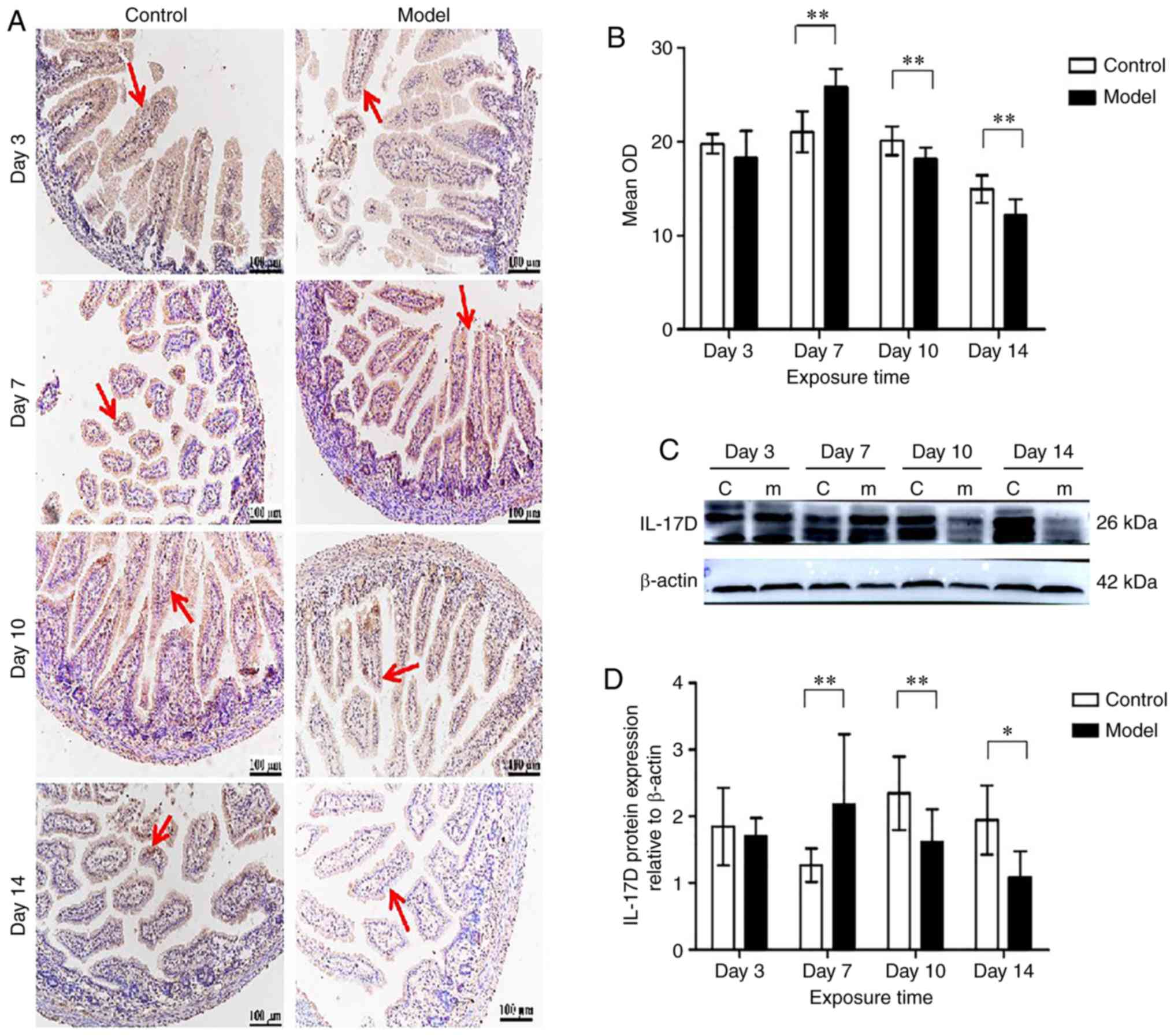
IL-17D expression in neonatal intestines
under hyperoxia
The immunohistochemistry results showed that at
postnatal days 3, 7, 10 and 14, IL-17D protein was expressed in the
cytoplasm of intestinal epithelial cells and intestinal lamina
propria T and B cells (Fig. 4A).
On postnatal day 3, there was no difference in the expression of
IL-17D between the model group and the control group. IL-17D
expression increased and peaked on day 7 in the model group
compared with the control group (P<0.01), and then decreased
gradually on days 10 and 14, remaining significantly lower than
that of the control group (P<0.01) (Fig. 4B). Western blotting results showed
clear and specific bands of IL-17D (26 kDa) (Fig. 4C). On days 3, 10 and 14 of
hyperoxia exposure, IL-17D expression was lower than that of the
control group, but increased and was significantly higher than that
of the control group on day 7 (Fig.
4D). During hyperoxia exposure, IL-17D expression increased,
and reached a peak on day 7 (P<0.01), and decreased gradually on
days 10 and 14 (P<0.05).
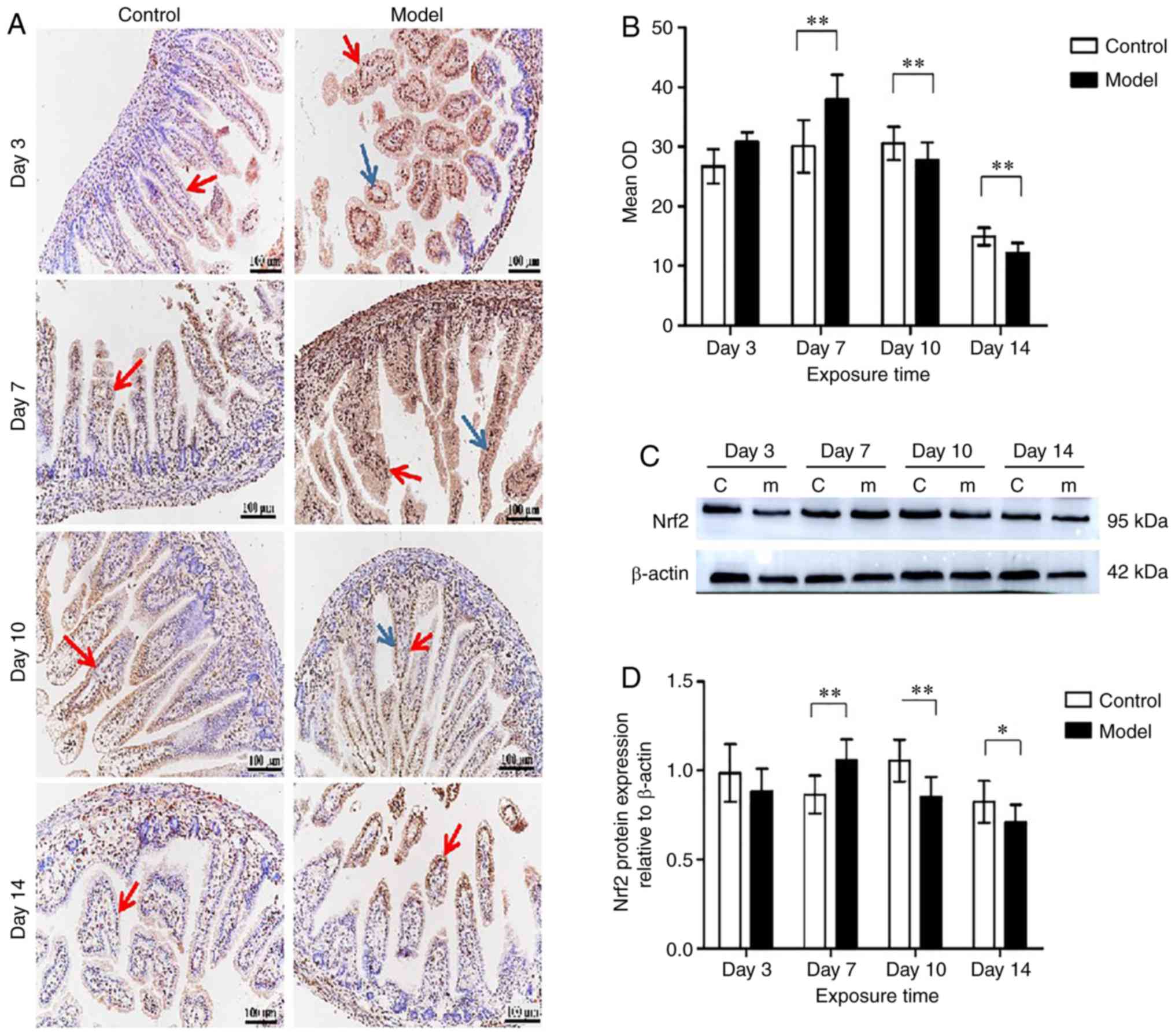
Nrf2 expression in neonatal intestines in
hyperoxia
As indicated in Fig.
5A, Nrf2 was expressed in the nucleus and the cytoplasm of
intestinal epithelial cells and lamina propria cells. Compared with
the control group, Nrf2 expression in the model group increased
significantly, reaching a peak when exposed to hyperoxia for 7 days
(P<0.01); however, Nrf2 expression was downregulated at days 10
and 14 (P<0.05) (Fig. 5B).
Nrf2 protein levels of the small intestinal tissues were examined
by western blotting (Fig. 5C).
Compared with the control group, Nrf2 expression level in the model
group was no different on day 3, but was significantly increased at
day 7 (P<0.01). Subsequently, on days 10 and 14, Nrf2 protein
levels in the model group were significantly downregulated compared
with the control group (P<0.05) (Fig. 5D).
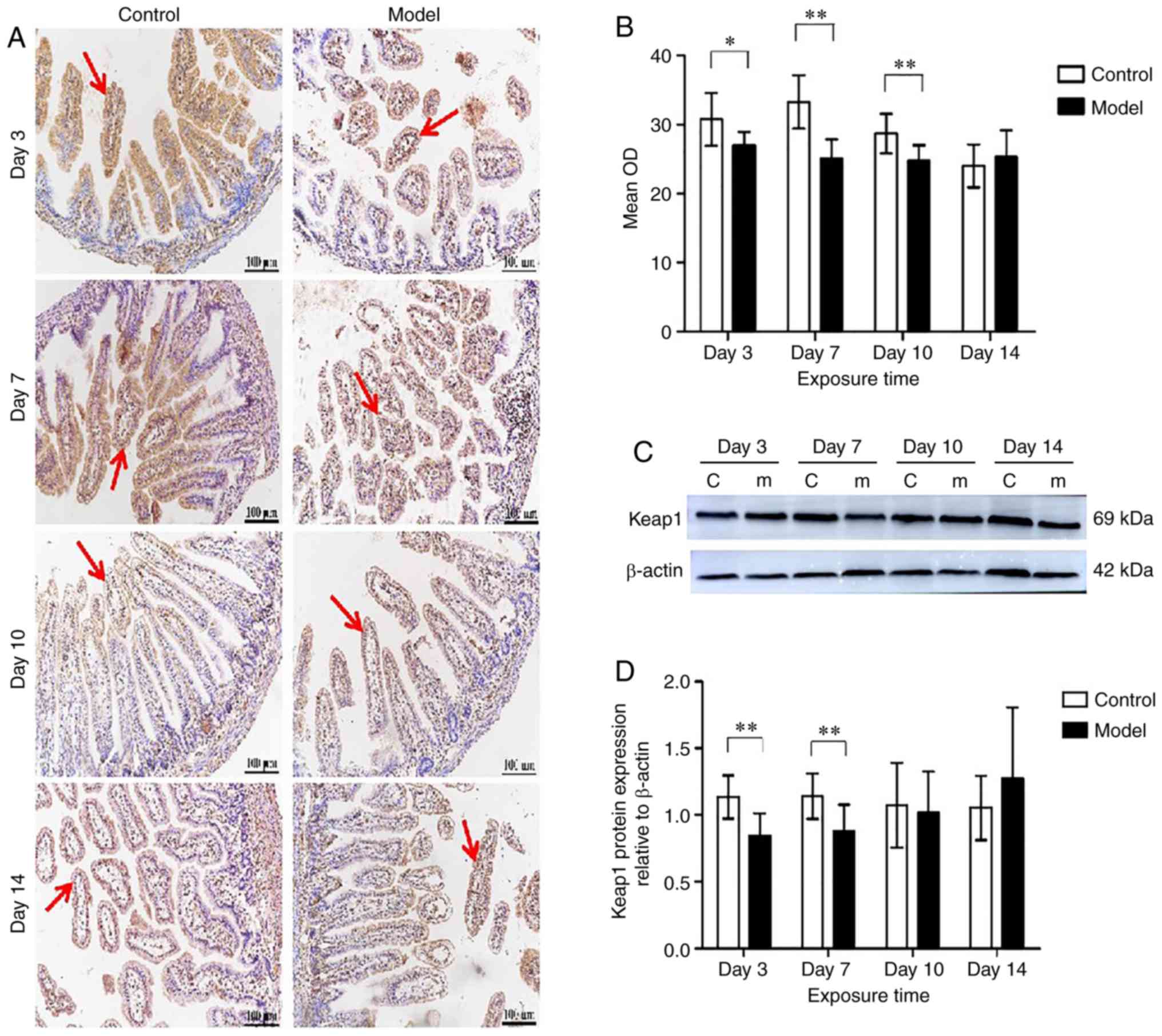
Keap1 expression in neonatal intestines
under hyperoxia
Keap1 was expressed in the cytoplasm of intestinal
epithelial cells and lamina propria cells (Fig. 6A). Keap1 protein expression was
downregulated after hyperoxia exposure at day 3 (P<0.05) and was
decreased significantly at day 7 (P<0.01) under hyperoxia. When
hyperoxia exposure was prolonged to 14 days, there was no
difference between the model and the control group in keap1
expression (Fig. 6B). In the
model group, keap1 expression was no different in a day-dependent
manner. Keap1 protein levels in total small intestine tissues were
significantly downregulated in the model group at days 3 and 7
compared with the control group, as demonstrated by western blot
analysis (P<0.01) (Fig. 6C and
D).
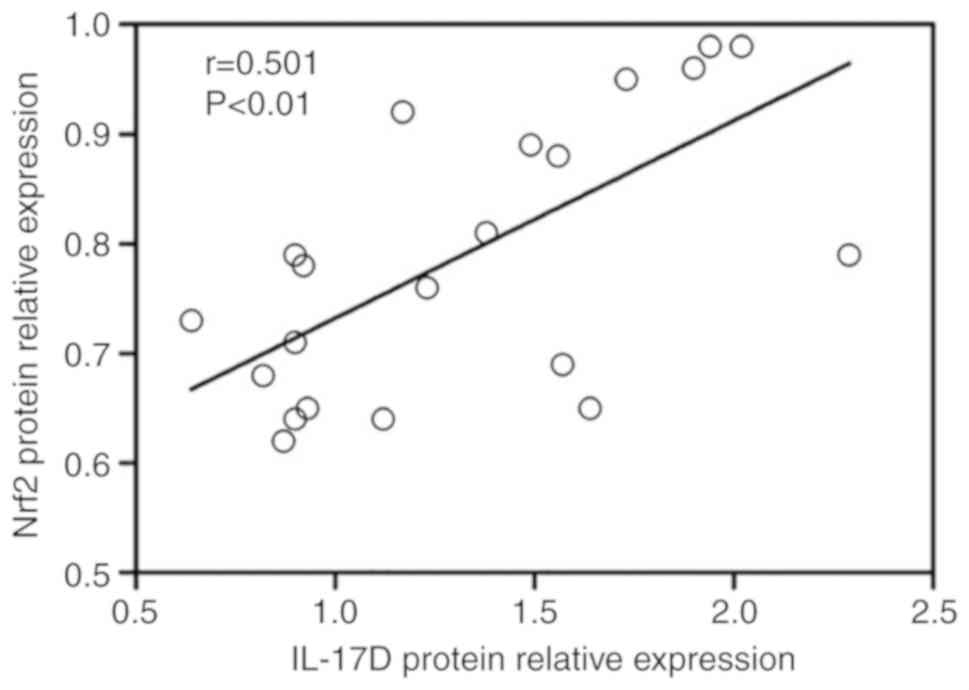
Correlation between IL-17D expression and
Nrf2 or keap1 expression in neonatal intestines
To confirm the correlation between IL-17D protein
expression and Nrf2, a correlation analysis was performed. Protein
expression of IL-17D was significantly positively correlated with
Nrf2 (r=0.501, P<0.01) (Fig.
7), while IL-17D showed a poor correlation with keap1 protein
(data not shown).
Discussion
In the interleukin (IL)-17 family, IL-17A plays an
important role in host defense against bacterial and fungal
infections, and IL-17F is mainly involved in mucosal host defense
(17). Another family member,
IL-17B, is widely expressed in various tissues and has the
potential to affect tumor progression, whereas IL-17E enhances T
helper cell 2 (Th2) immune responses by inducing Th2 cytokines,
contributing to the host defense against nematodes and allergic
disorders (18,19). IL-17C promotes Th17 cell responses
via the IL-17 receptor E, which is critical in preventing
intestinal pathogen infection and the pathogenesis of a variety of
autoimmune diseases, such as psoriasis, inflammatory bowel disease,
and multiple sclerosis (20-23). However, little research has been
conducted into investigating the expression and potential role of
IL-17D. Currently, it has only been reported that IL-17D can
regulate the production of cytokines and inhibit hematopoiesis
in vitro (24,25). In addition to this, it has been
reported that expression of IL-17D changes during the development
of certain diseases: For example, IL-17D expression is decreased in
psoriatic skin (26), but
increased in rheumatoid nodules (27). It appears that the main role of
tumor-expressed IL-17D is to initiate antitumor immunity and
stimulate the infiltration of natural killer (NK) cells into the
tumor microenvironment (28).
Recent studies have found that IL-17D inhibits bacterial
phagocytosis in macrophages by mediating downregulation of NF-κB
activation in macrophages, which increases mortality in patients
with sepsis (29). IL-17D is
expressed in a variety of tissues and is expressed in only resting
CD4+ T and CD19+ B cells in immune cells
(18). It is well known that the
intestinal epithelial cells include differentiated cells of various
lineages such as absorptive intestinal cells, goblet cells,
enteroendocrine cells, Paneth cells, tufted cells and microfold
cells (M cells), which fold to form crypts and villous structures
(30). In this study, we observed
that IL-17D was expressed on intestinal epithelial cells of small
intestinal villi. Compared with the control group, IL-17D
expression in intestinal epithelial cells during hyperoxia
increased and reached a peak during early postnatal stages.
However, along with neonatal growth and development, in the later
stages of hyperoxia, IL-17D was lower in intestinal epithelial
cells. Prior research has shown that in hyperoxia the apoptotic
rate of intestinal epithelial cells is significantly increased, and
that increased expression of tumor necrosis factor α (TNFα) induces
the production of intracellular reactive oxygen species (ROS)
(31), which may be the cause of
the above results. At the same time, IL-17D was also expressed in
intestinal lamina propria lymphocytes (such as CD4+ T
cells and CD19+ B cells), and IL-17D expression in
intestinal lamina propria T and B lymphocyte cells was exactly
opposed to those of intestinal epithelial cells. In the later
stages of hyperoxia, the numbers of CD4+ T cells and
CD19+ B cells expressing IL-17D were significantly
increased. These results seemed to indicate that IL-17D acts
through different mechanisms in different cells. At present, the
research on IL-17D mainly focuses on its role in antitumor and
viral infections, but there is not enough clinical data to confirm
its affect in intestinal epithelial cells in the hyperoxia
response. Therefore, it is important to further explore the
potential mechanism of IL-17D in hyperoxia treatment in the
future.
The gastrointestinal tract represents the largest
mucosal membrane surface in the body and is one of the most complex
human organs. Intestinal epithelial cells not only constitute the
first line of defense for the intestine, but also constantly pass
signal information between the gut lumen and immune cells (32). Although hyperoxia is an
indispensable treatment in clinical critical care for various
neonatal diseases, the neonatal small intestine is highly sensitive
to oxygen (30). The structure of
intestinal villi in neonatal rats was destroyed in the hyperoxic
environment, which may have affected the barrier function of their
intestines, making them susceptible to bacterial insult (33). Culturing of intestinal epithelial
cells in vitro showed that hyperoxia could inhibit cell
growth, and destroy intestinal epithelial cells, which would
promote the invasion of intestines by bacteria (33). Hyperoxemia caused intestinal
damage and predisposed premature neonates to necrotizing
enterocolitis (5). Lee et
al found that IL-17D expression in hepatocytes decreased during
listeria monocytogenes expressing ovalbumin (LM-OVA) infection,
which indicated that IL-17D could be inhibited during LM-OVA
infection (34). In the present
study, after exposure to hyperoxia, the expression level of
intestinal total IL-17D in the model group reached a peak during
the early postnatal stages, and subsequently decreased, indicating
that IL-17D expression is repressed in the later postnatal stages.
This indicates that inflammation occurred during hyperoxia. This is
also likely as the hyperoxic exposure destroyed the intestinal
epithelial cells, the apoptotic rate of the intestinal epithelial
cells increased, and thus decreased the level of IL-17D expressed
by intestinal epithelial cells. Although the number of
CD4+ T cells and CD19+ B cells which
expressed IL-17D increased on days 10 and 14 during hyperoxia
exposure, low proportion of CD4+ T cells and
CD19+ B cells compared to the intestinal epithelial
cells was observed, and the intestinal total IL-17D was lower. Thus
it is necessary to explore the different mechanisms of IL-17D in
different cells under hyperoxia in the future.
The transcription factor, nuclear factor erythroid
2-related factor 2 (Nrf2), is a highly conserved basic leucine
zipper (bZip) belonging to the cap-n-Collar (CNC) regulatory
protein family, that plays a central role in oxidative stress. The
Nrf2 gene is expressed in most cell types and activates a
wide range of cellular defense processes, thereby enhancing the
overall capacity of cells to detoxify and eliminate harmful
substances (35). Suzuki et
al found that Nrf2 contains hundreds of target genes (36). It has been reported that Nrf2
gene-knockout mice develop inflammation-related diseases such as
autoimmune diseases, indicating that Nrf2 may be an important
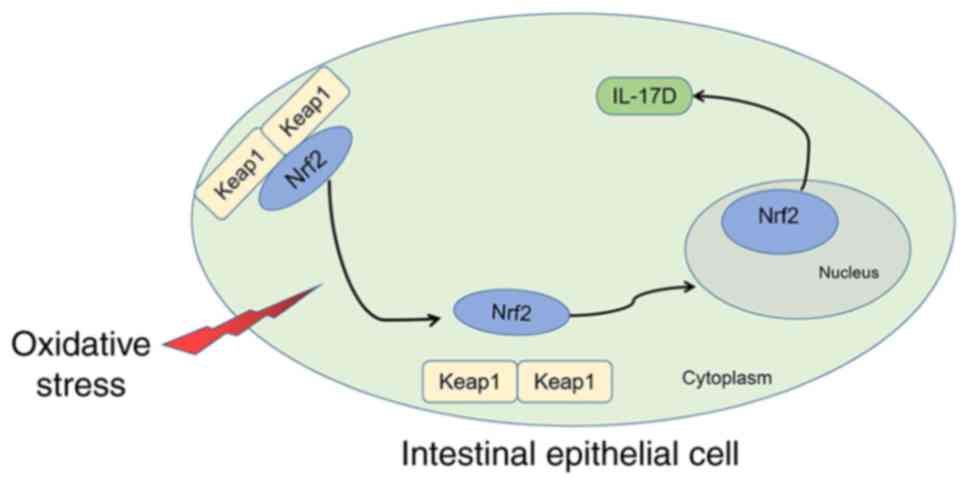
endogenous protective factor for autoimmune supervision (37). The adaptor subunit of Cullin
3-based E3 ubiquitin ligase, keap1, is the negative regulator of
Nrf2 (38). Under basal
conditions, keap1 binds to Nrf2 in the cytoplasm to form a dimer,
which keeps the cells in a stable state (Fig. 8). When keap1 and Nrf2 dissociate
under oxidative stress conditions, Nrf2 then translocates into the
nucleus and binds to the small Maf protein to form a heterodimer,
which binds to the antioxidant response element (ARE) in the gene
promoter, exerting antioxidant capacity accordingly (39,40). At present, the application of this
antioxidant system protection mechanism in many treatments has
entered the preclinical research stage, including multiple
sclerosis, psoriasis and retinal vascular disease (41). The present study demonstrated that
keap1 levels were lower at the early stage, but the change in keap1
was not obvious in the later stage. In contrast, expression of Nrf2
in the model group reached a peak in the early stages and decreased
in the later stages compared with the control group. On days 10 and
14, the expression of Nrf2 in total small intestines in the model
group was also reduced, which was consistent with IL-17D
expression. In tumor and viral infection, Nrf2 involvement in the
expression of IL-17D has been extensively reported (14-16). This study showed that Nrf2 was
consistent with IL-17D expression in the small intestine under
hyperoxia and was positively correlated with IL-17D. Based on these
findings, we can deduce that intestinal IL-17D expression may be
modulated by Nrf2 to exert biological effects on the intestines
(Fig. 8). As IL-17D can induce
the expression of inflammatory cytokines (24,25), and in hyperoxia TNFα is increased
(31), it can be concluded that,
in the early stage of hyperoxia, IL-17D expressed by intestinal
epithelial cells aggravates intestinal inflammation by inducing the
production of several inflammatory cytokines. However, IL-17D
expressed by intestinal immune cells can also exert protective
effects on the intestinal epithelium by controlling inflammation
and mediating immune responses. These effects and underlying
mechanisms need further investigation.
In conclusion, the expression of intestinal IL-17D
and Nrf2 were simultaneously altered following neonatal development
under hyperoxia, indicating that Nrf2 may be involved in regulating
the expression of IL-17D in intestinal epithelial cells. Moreover,
IL-17D in intestinal epithelial cells may play a unique
immunological role during hyperoxia. Although the use of Nrf2
agonists can reduce the inflammatory response caused by hyperoxia
(42), the specific process of
IL-17D regulated by Nrf2 under hyperoxia and the mechanism of
action of IL-17D remain unclear. Therefore, further research is
required to clarify the induction of inflammatory factors by IL-17D
in intestinal epithelial cells under hyperoxia and whether Nrf2 is
necessary to regulate IL-17D in this process.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (81170604, 30871158), the Key Research
and Development Joint Project of Liaoning Province (2020JH
2/10300136), the Education Department Foundation of Liaoning
Province (LK201620), and the Outstanding Scientific Fund of
Shengjing Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and QC acquired, analyzed, and interpreted the
experimental data. DZ acquired the data. SS drafted the work and
revised it critically for important intellectual content. DL
conceptualized and designed the study, and gave the final approval
of the submitted manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal studies were ethically approved by the
Institutional Animal Care and Use Committee of the China Medical
University (no. 2018PS178K) for animal experimentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Zhang A, Li Y, Xu J, Huang F, Zhao
M, Wu B and He S: Effect of intermittent hypoxia or hyperoxia on
lung development in preterm rat neonates during constant oxygen
therapy. J Cell Biochem. 120:17545–17554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar VHS, Wang H, Kishkurno S, Paturi BS,
Nielsen L and Ryan RM: Long-term effects of neonatal hyperoxia in
adult mice. Anat Rec (Hoboken). 301:717–726. 2018. View Article : Google Scholar
|
|
3
|
Kumar VHS, Wang H and Nielsen L: Adaptive
immune responses are altered in adult mice following neonatal
hyperoxia. Physiol Rep. 6:e135772018. View Article : Google Scholar :
|
|
4
|
Chou HC and Chen CM: Neonatal hyperoxia
disrupts the intestinal barrier and impairs intestinal function in
rats. Exp Mol Pathol. 102:415–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CM and Chou HC: Hyperoxia disrupts
the intestinal barrier in newborn rats. Exp Mol Pathol. 101:44–49.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong YH, Lillehoj HS, Park DW, Lee SH, Han
JY, Shin JH, Park MS and Kim JK: Cloning and functional
characterization of chicken interleukin-17D. Vet Immunol
Immunopathol. 126:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz JA, Kim WH, Fernandez CP, Jeong J,
Afrin F, Lillehoj HS, Kim S, Kim S, Dalloul RA and Min W:
Identification and expression analysis of duck interleukin-17D in
Riemerella anatipestifer infection. Dev Comp Immunol. 61:190–197.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumari J, Larsen AN, Bogwald J and Dalmo
RA: Interleukin-17D in Atlantic salmon (Salmo salar): Molecular
characterization, 3D modelling and promoter analysis. Fish
Shellfish Immunol. 27:647–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du L, Qin L, Wang X, Zhang A, Wei H and
Zhou H: Characterization of grass carp (Ctenopharyngodon idella)
IL-17D: Molecular cloning, functional implication and signal
transduction. Dev Comp Immunol. 42:220–228. 2014. View Article : Google Scholar
|
|
10
|
Ding Y, Ao J and Chen X: Comparative study
of interleukin-17C (IL-17C) and IL-17D in large yellow croaker
Larimichthys crocea reveals their similar but differential
functional activity. Dev Comp Immunol. 76:34–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal S and Gurney AL: IL-17: Prototype
member of an emerging cytokine family. J Leukoc Biol. 71:1–8.
2002.PubMed/NCBI
|
|
12
|
Starnes T, Broxmeyer HE, Robertson MJ and
Hromas R: Cutting edge: IL-17D, a novel member of the IL-17 family,
stimulates cytokine production and inhibits hemopoiesis. J Immunol.
169:642–646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Sullivan T, Saddawi-Konefka R, Gross E,
Tran M, Mayfield SP, Ikeda H and Bui JD: Interleukin-17D mediates
tumor rejection through recruitment of natural killer cells. Cell
Rep. 7:989–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saddawi-Konefka R, Seelige R, Gross ET,
Levy E, Searles SC, Washington A Jr, Santosa EK, Liu B, O'Sullivan
TE, Harismendy O and Bui JD: Nrf2 induces IL-17D to mediate tumor
and virus surveillance. Cell Rep. 16:2348–2358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seelige R, Saddawi-Konefka R, Adams NM,
Picarda G, Sun JC, Benedict CA and Bui JD: Interleukin-17D and Nrf2
mediate initial innate immune cell recruitment and restrict MCMV
infection. Sci Rep. 8:136702018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seelige R, Washington A Jr and Bui JD: The
ancient cytokine IL-17D is regulated by Nrf2 and mediates tumor and
virus surveillance. Cytokine. 91:10–12. 2017. View Article : Google Scholar :
|
|
17
|
Iwakura Y, Ishigame H, Saijo S and Nakae
S: Functional specialization of interleukin-17 family members.
Immunity. 34:149–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fort MM, Cheung J, Yen D, Li J, Zurawski
SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al: IL-25
induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in
vivo. Immunity. 15:985–995. 2001. View Article : Google Scholar
|
|
19
|
Wang YH, Angkasekwinai P, Lu N, Voo KS,
Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, et al:
IL-25 augments type 2 immune responses by enhancing the expansion
and functions of TSLP-DC-activated Th2 memory cells. J Exp Med.
204:1837–1847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song X and Qian Y: The activation and
regulation of IL-17 receptor mediated signaling. Cytokine.
62:175–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin
SD and Qian Y: IL-17RE is the functional receptor for IL-17C and
mediates mucosal immunity to infection with intestinal pathogens.
Nat Immunol. 12:1151–1158. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramirez-Carrozzi V, Sambandam A, Luis E,
Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, et al:
IL-17C regulates the innate immune function of epithelial cells in
an autocrine manner. Nat Immunol. 12:1159–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang SH, Reynolds JM, Pappu BP, Chen G,
Martinez GJ and Dong C: Interleukin-17C promotes Th17 cell
responses and autoimmune disease via interleukin-17 receptor E.
Immunity. 35:611–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yagi Y, Andoh A, Inatomi O, Tsujikawa T
and Fujiyama Y: Inflammatory responses induced by interleukin-17
family members in human colonic subepithelial myofibroblasts. J
Gastroenterol. 42:746–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurasawa K, Hirose K, Sano H, Endo H,
Shinkai H, Nawata Y, Takabayashi K and Iwamoto I: Increased
interleukin-17 production in patients with systemic sclerosis.
Arthritis Rheum. 43:2455–2463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johansen C, Usher PA, Kjellerup RB,
Lundsgaard D, Iversen L and Kragballe K: Characterization of the
interleukin-17 isoforms and receptors in lesional psoriatic skin.
Br J Dermatol. 160:319–324. 2009. View Article : Google Scholar
|
|
27
|
Stamp LK, Easson A, Lehnigk U, Highton J
and Hessian PA: Different T cell subsets in the nodule and synovial
membrane: Absence of interleukin-17A in rheumatoid nodules.
Arthritis Rheum. 58:1601–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saddawi-Konefka R, O'Sullivan T, Gross ET,
Washington A Jr and Bui JD: Tumor-expressed IL-17D recruits NK
cells to reject tumors. Onco Immunology. 3:e9548532015.
|
|
29
|
Yan X, Tu H, Liu Y, Chen T and Cao J:
Interleukin-17D aggravates sepsis by inhibiting macrophage
phagocytosis. Crit Care Med. 48:e58–e65. 2020. View Article : Google Scholar
|
|
30
|
Nakamura T: Recent progress in organoid
culture to model intestinal epithelial barrier functions. Int
Immunol. 31:13–21. 2019. View Article : Google Scholar
|
|
31
|
Zhao M, Tang S, Xin J, Wei Y and Liu D:
Reactive oxygen species induce injury of the intestinal epithelium
during hyperoxia. Int J Mol Med. 41:322–330. 2018.
|
|
32
|
Adachi T, Kakuta S, Aihara Y, Kamiya T,
Watanabe Y, Osakabe N, Hazato N, Miyawaki A, Yoshikawa S, Usami T,
et al: Visualization of probiotic-mediated Ca2+
signaling in intestinal epithelial cells in vivo. Front Immunol.
7:6012016. View Article : Google Scholar
|
|
33
|
Liu DY and Li JJ: Effect of hyperoxia on
the intestinal IgA secretory component in neonatal rats and on
intestinal epithelial cells in vitro. Braz J Med Biol Res.
43:1034–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee Y, Clinton J, Yao C and Chang SH:
Interleukin-17D promotes pathogenicity during infection by
suppressing CD8 T cell activity. Front Immunol. 10:11722019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki T and Yamamoto M: Molecular basis
of the Keap1-Nrf2 system. Free Radic Biol Med. 88(Pt B): 93–100.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki T, Motohashi H and Yamamoto M:
Toward clinical application of the Keap1-Nrf2 pathway. Trends
Pharmacol Sci. 34:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson DA, Amirahmadi S, Ward C, Fabry Z
and Johnson JA: The absence of the pro-antioxidant transcription
factor Nrf2 exacerbates experimental autoimmune encephalomyelitis.
Toxicol Sci. 114:237–246. 2010. View Article : Google Scholar :
|
|
38
|
Furukawa M and Xiong Y: BTB protein Keap1
targets anti-oxidant transcription factor Nrf2 for ubiquitination
by the Cullin 3-Roc1 ligase. Mol Cell Biol. 25:162–171. 2005.
View Article : Google Scholar :
|
|
39
|
Wakabayashi N, Itoh K, Wakabayashi J,
Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F,
Roop DR, et al: Keap1-null mutation leads to postnatal lethality
due to constitutive Nrf2 activation. Nat Genet. 35:238–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taguchi K, Maher JM, Suzuki T, Kawatani Y,
Motohashi H and Yamamoto M: Genetic analysis of cytoprotective
functions supported by graded expression of Keap1. Mol Cell Biol.
30:3016–3026. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cuadrado A, Rojo AI, Wells G, Hayes JD,
Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler
TW and Dinkova-Kostova AT: Therapeutic targeting of the NRF2 and
KEAP1 partnership in chronic diseases. Nat Rev Drug Discov.
18:295–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dunigan K, Li Q, Li R, Locy ML, Wall S and
Tipple TE: The thioredoxin reductase inhibitor auranofin induces
heme oxygenase-1 in lung epithelial cells via Nrf2-dependent
mechanisms. Am J Physiol Lung Cell Mol Physiol. 315:L545–L552.
2018. View Article : Google Scholar : PubMed/NCBI
|