Introduction
As a pathological state with multiple possible
etiologies, osteonecrosis of the femoral head (ONFH), also known as
avascular necrosis, results in decreased vascular supply to the
subchondral bone of the femoral head, ultimately resulting in
osteocyte death and the collapse of the articular surface (1). Several studies have examined the
pathogenesis of ONFH, which has been demonstrated to involve the
apoptosis of osteoblasts and osteocytes (2,3),
adipogenesis (4), venous
congestion (5,6) and mutations in the COL2A1 gene
(7). However, the specific
mechanisms underlying the pathology of ONFH remain poorly
understood.
The potential of muscle, cartilage, bone and adipose
tissue differentiation has resulted in the use of human bone
marrow-derived mesenchymal stem cells (hBMSCs) (8) therapeutically in a clinical setting
(9-13). As a key function of hBMSCs,
osteogenic differentiation plays a crucial role in the formation
and remodeling of bone. Long non-coding RNAs (lncRNAs) are
transcripts with a length of >200 nucleotides that do not code
for any proteins. The critical roles of lncRNAs in various
physiological and pathological processes have been proven (14-18). It has also been demonstrated that
lncRNAs may also participate in hBMSC osteogenic differentiation
(19). Moreover, the abnormal
expression of lncRNAs may lead to the development of diseases due
to variations in the osteogenic differentiation capacity of hBMSCs.
However, the differential expression profiles of lncRNAs expressed
in hBMSCs from patients with ONFH have not yet been fully
elucidated. Therefore, the present study aimed to examine the role
of lncRNAs expressed during the osteogenic differentiation of
abnormal hBMSCs obtained from patients with ONFH.
Materials and methods
Cells and cell culture
Patients who had undergone total hip arthroplasty
(THA) due to femoral neck fracture or ONFH were included in the
present study, whereas patients who had undergone THA for
rheumatoid arthritis, ankylosing spondylitis and other diseases
were excluded. In total, 3 patients who had undergone THA for the
treatment of femoral neck fracture provided normal bone marrow
tissue: All 3 were Chinese; 2 were females, aged 60 and 67 years,
while the other patient was a 66-year-old male. ONFH bone marrow
tissue was also obtained from another 3 patients, of which 2 were
females, aged 55 and 61 years and the other patient was a
58-year-old male, on whom THA had been performed for ONFH. Bone
marrow tissue was collected at the Affiliated Hospital of Qingdao
University from January, 2018 to May, 2018. The femoral bone marrow
tissue samples were used to extract the hBMSCs with density
gradient separation, as previously described (20). Bone marrow diluted with an equal
volume of PBS was layered over lymphocyte separate medium. The
mononuclear cell layer was collected following centrifugation. The
hBMSCs extracted were cultured in a stem cell medium in a
humidified atmosphere with 5% CO2 at 37°C. The Ethics
Committee of the Affiliated Hospital of Qingdao University approved
the study, while informed consent was obtained from all
participants.
Flow cytometric analysis
Surface antigen markers on the hBMSCs were detected
using an Apogee A50-MICRO flow cytometer (Apogee Corporation). The
hBMSCs were suspended in PBS at a concentration of approximately
106 cells/ml and washed twice with PBS. Approximately
5x105 cells per 500 µl were incubated and stained with 5
ml of mouse anti-human CD34-fluorescein isothiocyanate (FITC)
(560942), CD45-FITC (560976), CD73-FITC (561254) and CD90-FITC
(561969) antibodies for 20 min at room temperature. All antibodies
used were purchased from BD Biosciences.
Osteogenic differentiation of hBMSCs
The hBMSCs (passage 3) were plated in growth medium
in 6-well plates. When 80% confluency was reached, mesenchymal stem
cell osteogenic differentiation medium was used as the growth
medium. The medium was changed every 3 days. The RT-qPCR analysis
of osteogenic differentiation markers [alkaline phosphatase (ALP),
Runt-related transcription factor 2 (RUNX2), osteopontin (OPN) and
bone sialoprotein (BSP)] and staining (ALP staining and Alizarin
Red staining) were adopted to detect the osteogenic ability of the
hBMSCs. All experiments were performed in triplicate.
RAN extraction and RT-qPCR
Total RNA extraction was performed form the hBMSCs
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
following instructions provided by the manufacturer. The expression
of osteogenesis- and adipogenesis-related genes, including OPN,
BSP, Runx2, ALP and peroxisome proliferator-activated receptor γ
(PPARγ) were determined by RT-qPCR. Total RNA was reverse
transcribed using oligo-dT primers. The cDNA was utilized as a
template to amplify target genes with the SYBR Premix Ex Tag kit
(Takara Bio, Inc.). The primers of these genes are listed in
Table I. Each RNA sample was
evaluated in triplicate and PCR cycles were as follows: 94°C for 5
min, 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec (35
cycles), 94°C for 5 min. Relative expression of mRNA was evaluated
using the 2-ΔΔCq method and normalized to the expression
of GAPDH (21).
 | Table IPrimers of lncRNAs and the related
osteogenic genes. |
Table I
Primers of lncRNAs and the related
osteogenic genes.
| Gene | Primer sequence
5'-3' |
|---|
| GAPDH-F |
GGTCACCAGGGCTGCTTTTA |
| GAPDH-R |
GGATCTCGCTCCTGGAAGATG |
| ALP-F CC |
ACGTCTTCACATTTGGTG |
| ALP-R |
AGACTGCGCCTGGTAGTTGT |
| OPN-F |
ACTCGAACGACTCTGATGATGT |
| OPN-R |
GTCAGGTCTGCGAAACTTCTTA |
| RUNX2-F |
TGTCATGGCGGGTAACGAT |
| RUNX2-R |
AAGACGGTTATGGTCAAGGTGAA |
| BSP-F |
TGGATGAAAACGAACAAGGCA |
| BSP-R |
AAACCCACCATTTGGAGAGGT |
| PPARγ-F CC |
TATTGACCCAGAAAGCGATT |
| PPARγ-R C |
ATTACGGAGAGATCCACGGA |
| CEBP-α-F |
AGGAACACGAAGCACGATCAG |
| CEBP-α-R C |
GCACATTCACATTGCACAA |
|
hsa-lncRNA-AC107070.1-F C |
ACATTCCAGCCAAGGTAG |
|
hsa-lncRNA-AC107070.1-R C |
AGCCTCTCAGACCACATTC |
|
hsa-lncRNA-linc-ANKRD20A1-4-F |
TGGAGTTGGACATTTGTGG |
|
hsa-lncRNA-linc-ANKRD20A1-4-R |
TGGAGTTGGACATTTGTGG |
|
hsa-lncRNA-LINC00473-F |
GAGGTCTGAGTCCGAAGTTG |
|
hsa-lncRNA-LINC00473-R |
AGCAGGCAGATTCCAAAG |
|
hsa-lncRNA-MAPT-AS1-F |
TCCGCTGGAAAGAGAACTC |
|
hsa-lncRNA-MAPT-AS1-R CC |
TGTGAGGGCATACACC |
|
hsa-lncRNA-RP11-794G24.1-F |
GGCGTGGATCTTGGAGAGTC |
|
hsa-lncRNA-RP11-794G24.1-R |
GATGCTGGACGAATCCCAGT |
|
hsa-lncRNA-AP005273.1-F |
TTCTTGACCCTCTCCAATGTGA |
|
hsa-lncRNA-AP005273.1-R |
ACTGTCCAATAGCTTCCATCAGG |
Staining
Alizarin Red and ALP staining were adopted to
evaluate the osteogenic differentiation capacity of the hBMSCs. An
ALP staining kit (Tianjin Blood Research Institute) was used on day
3, as instructed by the manufacturer, to conduct ALP staining. The
cells are processed according to the following procedures: No. 1
solution was added at room temperature for 1 min, followed by
rinsing for 2 min. The staining solution was then added followed by
incubation at 37°C for 2 h and rinsing for 2 min. No. 5 solution
was then added for re-staining for 5 min, followed by rinsing for 2
min, and drying. For Alizarin Red staining, after washing the cells
twice with PBS, fixing was performed using 95% ethanol for 20 min,
and the cells were washed 3 times using distilled water, and were
stained using Alizarin Red solution (Sigma-Aldrich; Merck KGaA) for
30 min at 37°C. Oil Red O staining was performed to evaluate the
adipogenic differentiation capacity of the hBMSCs. Cells were
washed twice with PBS and fixed with 10% formalin for 10 min at
room temperature. After fixation, cells were stained with filtered
Oil Red O solution (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature.
Microarray analysis
Three normal cell samples of osteogenic
differentiation were used as the controls, while 3 osteogenic
differentiation samples obtained from patients with ONFH were used
as the experimental group. TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from the
hBMSCs, while a mirVana miRNA Isolation kit (Ambeon; Thermo Fisher
Scientific, Inc.) was used as instructed by the manufacturer for
purification. Following RNA extraction, labeling, hybridization and
amplification, the CapitalBiotech human array used 4 identical
arrays for each slide to design the lncRNA Array V4.0, while each
array contained probes that could interrogate approximately 41,000
human lncRNAs. The probes were used to detect each RNA and the
process was repeated for confirmation. A total of 4,974 control
probes (Agilent Technologies, Inc.) constituted the array. The
analysis of the lncRNA array data was conducted using GeneSpring
software V13.0 (Agilent Technologies, Inc.) for data summarization,
normalization and quality control. The differentially expressed
genes were obtained using a t-test P-value of 0.05 and a fold
change of ≥2 and ≤-2 as the threshold values. The adjust data
function of CLUSTER 3.0 software was applied for log2
transformation of the data and median centering by the genes, while
further analysis was conducted using hierarchical clustering with
an average linkage.
Using RT-qPCR, the identity of 6 lncRNAs
[AC107070.1, linc-ANKRD20A1-4, RP11-794G24.1, long intergenic
non-protein coding RNA 473 (LINC00473), MAPT antisense RNA 1
(MAPT-AS1) and AP005273.1] was further confirmed. The primers used
for the lncRNAs are listed in Table
I.
Furthermore, hBMSCs were islated from another 30
samples, including 15 normal and 15 patients with ONFH who
underwent THA in the Department of Joint surgery at The Affiliated
Hospital of Qingdao University from October, 2018 to September,
2019. Informed consent form was obtained from all participants. The
expression levels of 2 upregulated lncRNAs (AC107070.1 and
linc-ANKRD20A1-4) and 2 downregulated lncRNAs (LINC00473 and
MAPT-AS1) were examined.
Target gene prediction
In the present study, the functions of cis
and transtarget mRNAs were used to predict the target genes
of the lncRNAs. Protein-coding genes within a 100 kb genomic
distance from the lncRNA were defined as potentially
cis-regulated target genes, and protein-coding genes
co-expressed with the lncRNA with a Pearson's correlation
coefficient (|r|>0.95) and a >100 kb genomic distance from
the lncRNA or in different chromosomes were defined as potentially
trans-regulated target genes.
Lentivirus vector construction and
infection of hBMSCs for MAPT-AS1
The pRLenti-EF1a-EGFP-CMV-MAP T-AS1-overexpression
lentivirus (H12785) was obtained from OBiO Technology. The hBMSCs
were infected with MAPT-AS1-overexpression lentivirus at a final
multiplicity of infection (MOI) of 100 containing 5 µg/ml
polybrene, and observed for the expression of green fluorescent
protein (GFP) using an inverted fluorescence microscope (EVOS FL,
Invitrogen; Thermo Fisher Scientific, Inc.) after 24 h. The
transfection efficiency of MAPT-AS1 was determined by RT-qPCR after
3 days.
Bioinformatics analysis
Gene Ontology (GO) was utilized to identify the
molecular functions of the differentially expressed genes. The GO
category was also calculated. Furthermore, the differentially
expressed lncRNAs were used in a pathway analysis that was
performed using the latest Kyoto Encyclopedia of Genes and Genomes
(KEGG) database to analyze the potential functions of target
genes.
Statistical analysis
All statistical analyses were conducted using SPSS
statistical software v.16.0 (SPSS, Inc.). All data are expressed as
the means ± standard deviation. Comparisons between 2 variables of
microarray data was performed using the Student's t-test.
Comparisons between multiple groups were performed using the
Kruskal-Wallis test along with Dunn's post hoc test. The
χ2 test and Fisher's exact test were adopted for the GO
and KEGG analyses. Statistical significance was considered to be
indicated by P-values of <0.05.
Results
hBMSCs and osteogenic
differentiation
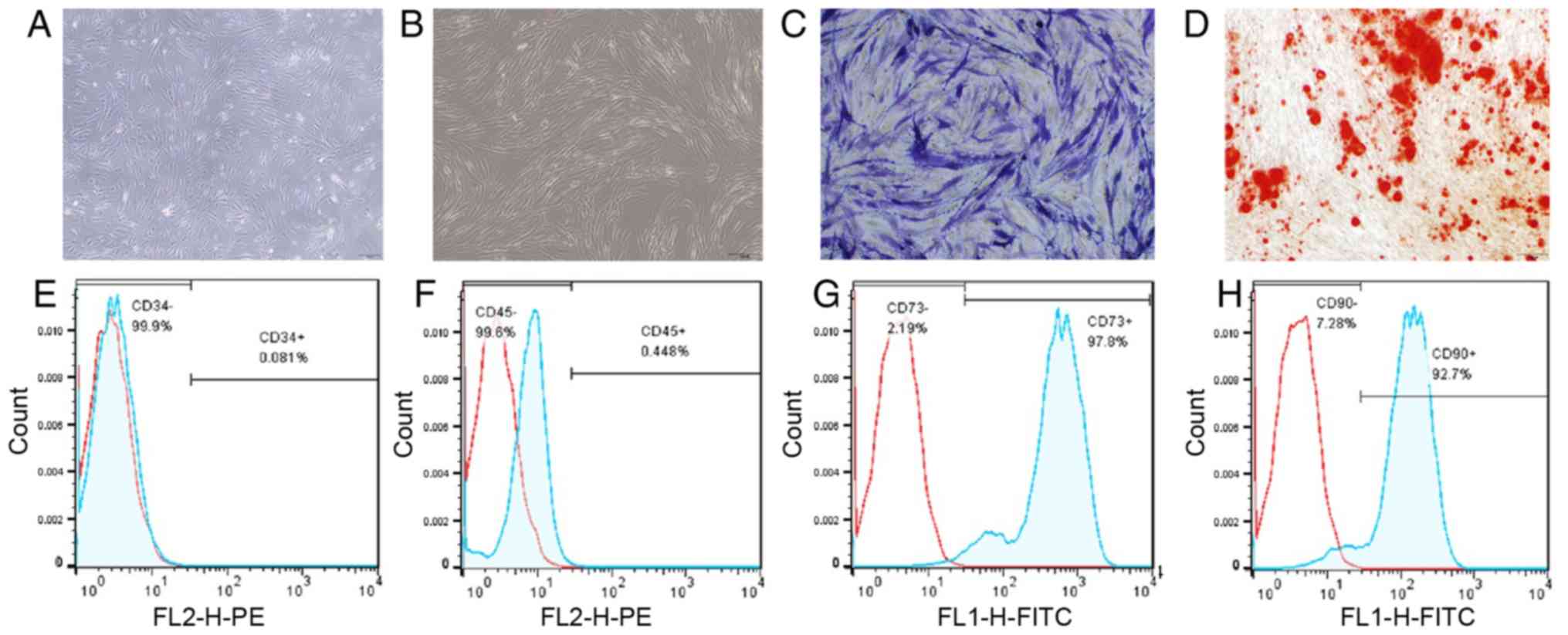
The hBMSCs isolated from the bone marrow samples
were spindle-shaped cells (Fig.
1) and no morphological differences were found between the 2
groups. ALP staining, Alizarin Red staining and osteogenic markers,
including ALP, RUNX2, OPN and BSP, were used to detect the
osteogenic ability of the hBMSCs. Positive ALP staining and
Alizarin Red staining results revealed mineral deposits and bone
formation (Fig. 1). The results
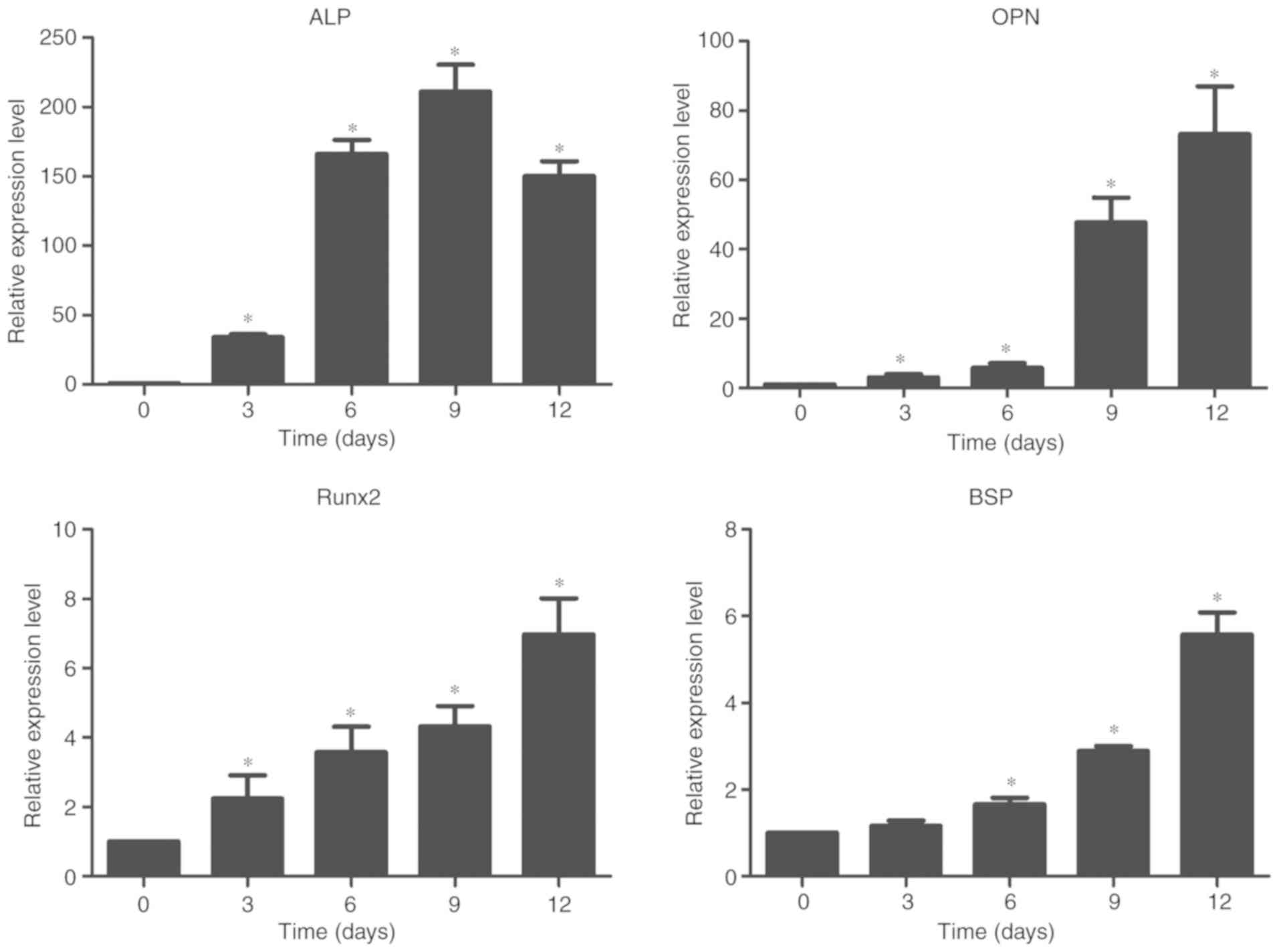
of flow cytometric analysis are also shown in Fig. 1. The results of RT-qPCR revealed
the elevated expression levels of OPN, RUNX2, ALP and BSP (Fig. 2).
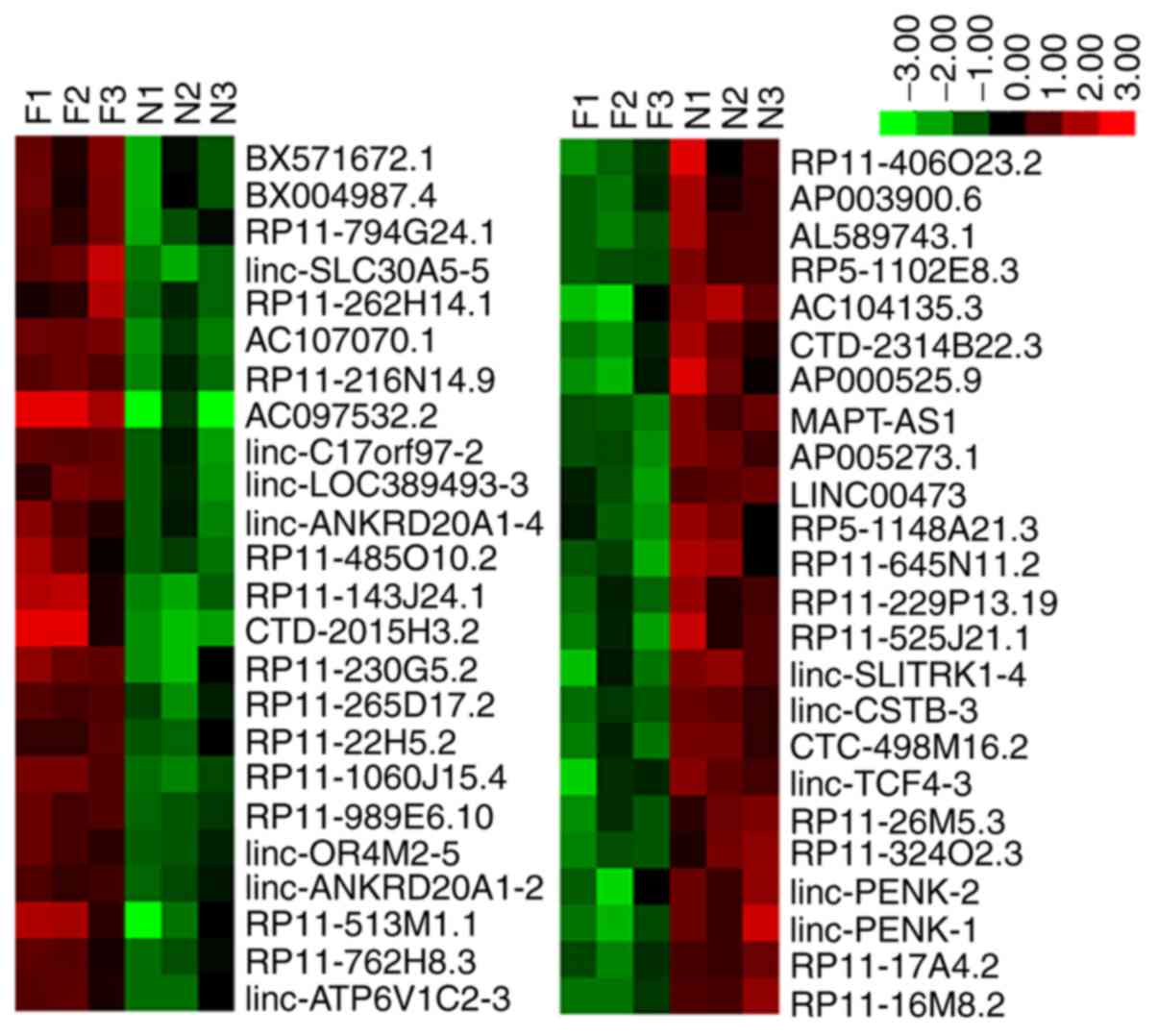
Expression profiles of lncRNAs in hBMSCs
from patients with ONFH
lncRNA expression levels during hBMSC osteogenic
differentiation were detected using lncRNA microarray chips (The
CapitalBiotech human lncRNA Array V4.0). A total of 48
differentially expressed lncRNAs were identified, including 24
lncRNAs, which were upregulated (RP11-216N14.9, RP11-989E6.10,
BX571672.1, RP11-230G5.2, linc-SLC30A5-5, BX004987.4, AC107070.1,
RP11-265D17.2, RP11-1060J15.4, RP11-794G24.1, linc-ANKRD20A1-4,
RP11-485O10.2, AC097532.2, RP11-143J24.1, RP11-762H8.3,
CTD-2015H3.2, linc-C17orf97-2, RP11-513M1.1, RP11-22H5.2,
linc-ANKRD20A1-2, linc-OR4M2-5, RP11-262H14.1, linc-LOC389493-3 and
linc-ATP6V1C2-3) and 24 lncRNAs, which were downregulated
(AC104135.3, RP11-26M5.3, MAPT-AS1, AL589743.1, linc-SLITRK1-4,
RP11-229P13.19, AP005273.1, RP11-406O23.2, AP003900.6,
RP11-525J21.1, linc-PENK-1, linc-PENK-2, RP5-1102E8.3, linc-CSTB-3,
CTD-2314B22.3, RP5-1148A21.3, LINC00473, CTC-498M16.2, RP11-16M8.2,
AP000525.9, linc-TCF4-3, RP11-645N11.2, RP11-17A4.2 and
RP11-324O2.3). The differential expression of the 48 lncRNAs is
presented in brief in Table II.
Hierarchical clustering analysis revealed the expression profiles
of the lncRNAs during the osteogenic differentiation of hBMSCs from
patients with ONFH and healthy subjects (Fig. 3).
 | Table IIDifferential expression of the
lncRNAs during the osteogenic differentiation of hBMSCs from
patients with ONFH. |
Table II
Differential expression of the
lncRNAs during the osteogenic differentiation of hBMSCs from
patients with ONFH.
| lncRNA name | Regulation | Chromosome | Strand | start | End | Gene | Class | Database |
|---|
| RP11-216N14.9 | Up | 1 | - | 153723458 | 153724652 |
ENSG00000233222.2 | Antisense | ENSEMBL |
| RP11-989E6.10 | Up | 16 | - | 33344308 | 33348279 |
ENSG00000261200.1 | Intergenic | ENSEMBL |
| BX571672.1 | Up | 1 | + | 143119060 | 143158077 |
ENSG00000230850.2 | Intergenic | ENSEMBL |
| RP11-230G5.2 | Up | 12 | - | 65917042 | 65932479 |
ENSG00000250748.2 | Intergenic | ENSEMBL |
| linc-SLC30A5-5 | Up | 5 | + | 67802061 | 67807619 | XLOC_004413 | Intergenic |
HumanLincRNACatalog |
| BX004987.4 | Up | 1 | - | 143429871 | 143467644 |
ENSG00000185044.8 | Intergenic | ENSEMBL |
| AC107070.1 | Up | 2 | - | 4007683 | 4021626 |
ENSG00000237401.2 | Intergenic | ENSEMBL |
| RP11-265D17.2 | Up | 11 | - | 12282972 | 12284720 |
ENSG00000254680.1 | Antisense | ENSEMBL |
| RP11-1060J15.4 | Up | 12 | - | 27849488 | 27857344 |
ENSG00000256377.1 | Antisense | ENSEMBL |
| RP11-794G24.1 | Up | 11 | - | 61306987 | 61309731 |
ENSG00000256443.1 | Intronic | ENSEMBL |
|
linc-ANKRD20A1-4 | Up | 9 | + | 67051144 | 67269646 | XLOC_007374 | Intergenic |
HumanLincRNACatalog |
| RP11-485O10.2 | Up | 15 | + | 49075386 | 49076318 |
ENSG00000259670.1 | Antisense | ENSEMBL |
| AC097532.2 | Up | 2 | - | 133043367 | 133051950 |
ENSG00000230803.1 | Intergenic | ENSEMBL |
| RP11-143J24.1 | Up | 15 | - | 30297645 | 30338051 |
ENSG00000259647.1 | Intergenic | ENSEMBL |
| RP11-762H8.3 | Up | 15 | - | 78549886 | 78556498 |
ENSG00000259708.1 | Antisense | ENSEMBL |
| CTD-2015H3.2 | Up | 18 | + | 1655177 | 1779956 |
ENSG00000266450.1 | Intergenic | ENSEMBL |
|
linc-C17orf97-2 | Up | 17 | + | 56208 | 56601 | XLOC_012065 | Intergenic |
HumanLincRNACatalog |
| RP11-513M1.1 | Up | 18 | + | 10893614 | 10894803 |
ENSG00000263952.1 | Intronic | ENSEMBL |
| RP11-22H5.2 | Up | 16 | + | 82806923 | 82863243 |
ENSG00000260862.1 | Intronic | ENSEMBL |
|
linc-ANKRD20A1-2 | Up | 9 | + | 67340516 | 67343501 | XLOC_007377 | Intergenic |
HumanLincRNACatalog |
| linc-OR4M2-5 | Up | 15 | + | 20505642 | 20531084 | XLOC_011157 | Intergenic |
HumanLincRNACatalog |
| RP11-262H14.1 | Up | 9 | + | 66457284 | 66466010 |
ENSG00000238113.2 | Intergenic | ENSEMBL |
|
linc-LOC389493-3 | Up | 7 | - | 56683915 | 56685484 | XLOC_006459 | Intergenic |
HumanLincRNACatalog |
|
linc-ATP6V1C2-3 | Up | 2 | + | 10702420 | 10706471 | XLOC_001343 | Intergenic |
HumanLincRNACatalog |
| AC104135.3 | Down | 2 | + | 75155365 | 75160151 |
ENSG00000204792.2 | Intergenic | ENSEMBL |
| RP11-26M5.3 | Down | 8 | + | 53063379 | 53067452 |
ENSG00000254314.1 | Intronic | ENSEMBL |
| MAPT-AS1 | Down | 17 | - | 43921016 | 43972966 |
ENSG00000264589.1 | Antisense | ENSEMBL |
| AL589743.1 | Down | 14 | + | 19650041 | 19718563 |
ENSG00000225210.5 | Intergenic | ENSEMBL |
| linc-SLITRK1-4 | Down | 13 | - | 85685978 | 85722284 | XLOC_010679 | Intergenic |
HumanLincRNACatalog |
| RP11-229P13.19 | Down | 9 | + | 139869545 | 139871433 |
ENSG00000238268.2 | Divergent | ENSEMBL |
| AP005273.1 | Down | 11 | + | 64268324 | 64272858 |
ENSG00000232500.1 | Intergenic | ENSEMBL |
| RP11-406O23.2 | Down | 9 | - | 112522639 | 112534323 |
ENSG00000232939.1 | Intronic | ENSEMBL |
| AP003900.6 | Down | 21 | + | 11169787 | 11184046 |
ENSG00000271308.1 | | ENSEMBL |
| RP11-525J21.1 | Down | 4 | - | 60633533 | 60657832 |
ENSG00000249892.1 | Intergenic | ENSEMBL |
| linc-PENK-1 | Down | 8 | - | 57432371 | 57472069 | XLOC_007087 | Intergenic |
HumanLincRNACatalog |
| linc-PENK-2 | Down | 8 | - | 57447959 | 57449765 | XLOC_007088 | Intergenic |
HumanLincRNACatalog |
| RP5-1102E8.3 | Down | 1 | + | 77102561 | 77103024 |
ENSG00000272855.1 | | ENSEMBL |
| linc-CSTB-3 | Down | 21 | - | 45225402 | 45230480 | XLOC_014107 | Intergenic |
HumanLincRNACatalog |
| CTD-2314B22.3 | Down | 14 | - | 19883800 | 19925345 |
ENSG00000244306.5 | Intergenic | ENSEMBL |
| RP5-1148A21.3 | Down | 6 | - | 64280909 | 64282313 |
ENSG00000266680.1 | Antisense | ENSEMBL |
| LINC00473 | Down | 6 | - | 166361710 | 166401536 |
ENSG00000223414.2 | Intergenic | ENSEMBL |
| CTC-498M16.2 | Down | 5 | - | 87705663 | 87734907 |
ENSG00000250156.2 | Intergenic | ENSEMBL |
| RP11-16M8.2 | Down | 8 | - | 57432873 | 57472243 |
ENSG00000246430.2 | Intergenic | ENSEMBL |
| AP000525.9 | Down | 22 | - | 16158828 | 16159470 |
ENSG00000206195.6 | | ENSEMBL |
| linc-TCF4-3 | Down | 18 | - | 53727806 | 53735555 | XLOC_012851 | Intergenic |
HumanLincRNACatalog |
| RP11-645N11.2 | Down | 7 | + | 102613968 | 102629303 |
ENSG00000230257.1 | Intronic | ENSEMBL |
| RP11-17A4.2 | Down | 8 | + | 57401656 | 57439695 |
ENSG00000254254.1 | Intergenic | ENSEMBL |
| RP11-324O2.3 | Down | 10 | - | 114166031 | 114169248 |
ENSG00000232934.2 | Antisense | ENSEMBL |
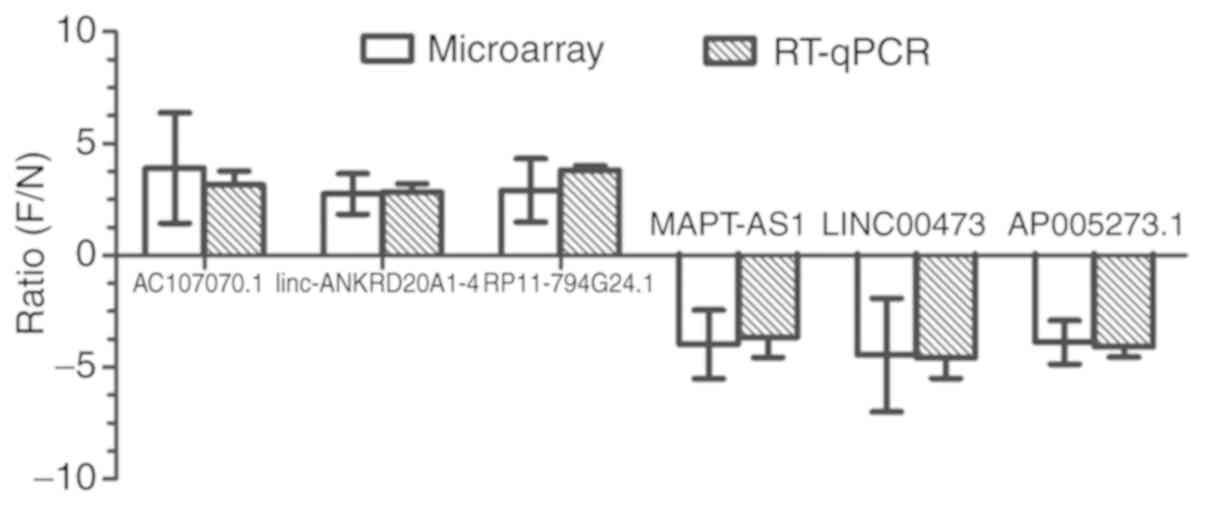
Comparison between the RT-qPCR and
microarray analyses
The microarray data analysis revealed 3 upregulated
lncRNAs (AC107070.1, linc-ANKRD20A1-4 and RP11-794G24.1) and 3
downregulated lncRNAs (LINC00473, MAPT-AS1 and AP005273.1) which
were selected for RT-qPCR analysis in the hBMSCs. The results of
RT-qPCR revealed that the expression trends of these 4 lncRNAs were
consistent with those of the microarray results, which are shown in
Fig. 4.
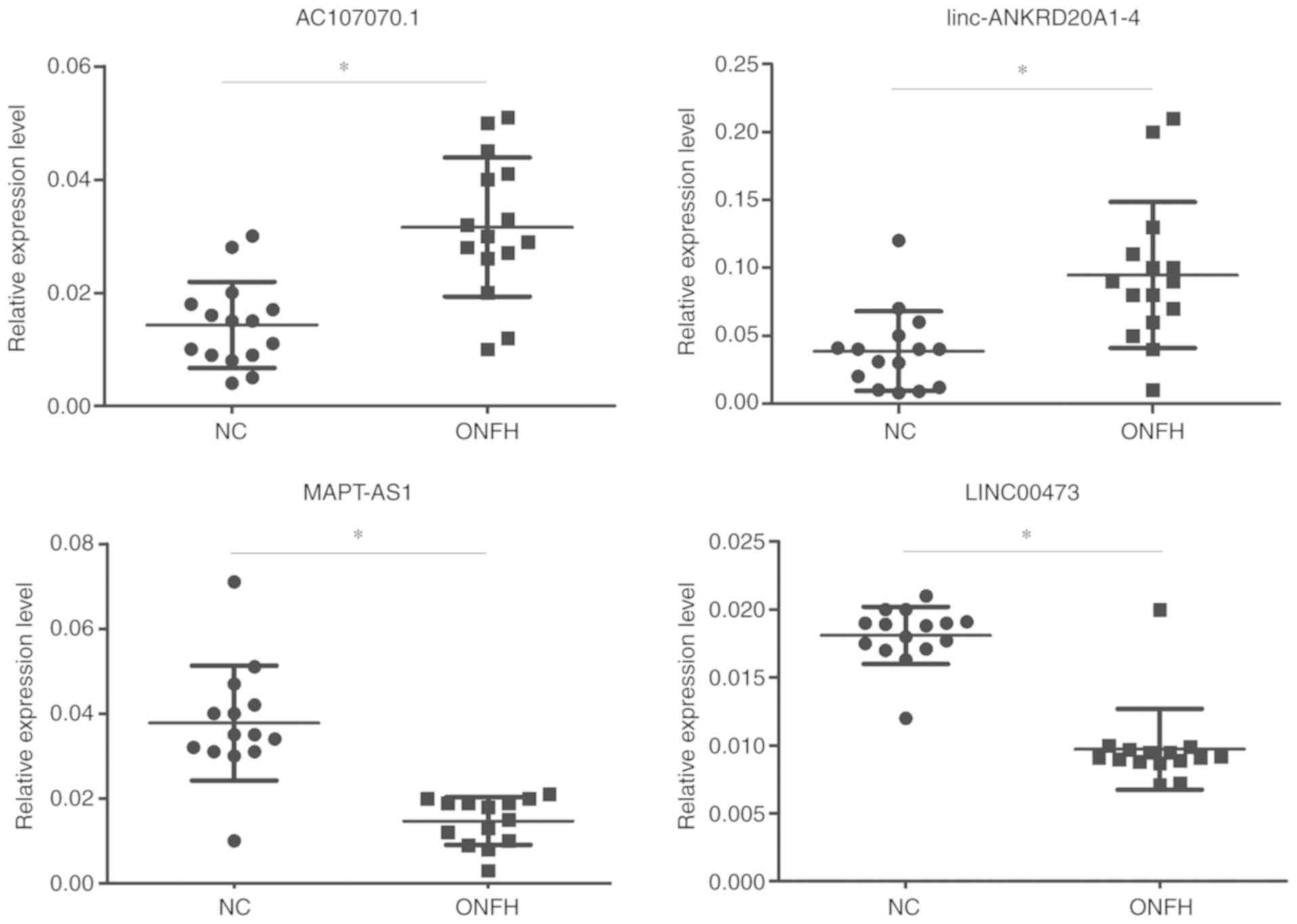
The expression levels of AC107070.1,
linc-ANKRD20A1-4, LINC00473 and MAPT-AS1 (Fig. 5) in the samples were also
consistent with those of the results of the microarray
analysis.
Target gene prediction and association
study
In the present study, the genes involved were
predicted based on the functional annotations of their related
cisand transtarget mRNAs. Further results are
presented in detail in Table
III.
 | Table IIIPredicted target genes of
differentially expressed lncRNAs. |
Table III
Predicted target genes of
differentially expressed lncRNAs.
| lncRNA | Target gene |
|---|
| A, Partial lncRNAs
overexpressed in hBMSCs during osteogenic differentiation in
ONFH |
| RP11-216N14.9 | GATAD2B, ILF2,
SLC27A3, CHTOP |
| RP11-989E6.10 | TP53TG3E |
| RP11-230G5.2 | HMGA2 |
| RP11-265D17.2 | MICAL2, PARVA, |
| RP11-1060J15.4 | MANSC4, MRPS35,
KLHL42, |
| RP11-794G24.1 | TMEM138, DDB1,
CYB561A3, PGA3, TKFC, |
| RP11-485O10.2 | GALK2, COPS2,
SECISBP2L |
| AC097532.2 | NCKAP5 |
| RP11-143J24.1 | CHRFAM7A,
GOLGA8R |
| RP11-762H8.3 | HYKK, CHRNA3,
PSMA4, PSMA4, IREB2 |
|
linc-C17orf97-2 | DOC2B, SCGB1C2 |
| RP11-513M1.1 | PIEZO2 |
| RP11-22H5.2 CD | H13 |
|
linc-ANKRD20A1-2 | SPATA31A3 |
| linc-OR4M2-5 | GOLGA6L6 |
|
linc-ATP6V1C2-3 | ATP6V1C2, NOL10,
PDIA6, |
| B, Partial lncRNAs
underexpressed in hBMSCs during osteogenic differentiation in
ONFH |
| AC104135.3 | TACR1 |
| MAPT-AS1 | TMEM101, MPP2,
CD300LG, LSM12, PYY |
| AL589743.1 | OR11H2, OR4Q3,
OR4N2, OR4M1 |
| linc-SLITRK1-4 | SLITRK6 |
| AP005273.1 | NUDT22, GPR137,
TRPT1, FKBP2, TRMT112, VEGFB |
| RP11-406O23.2 | KIAA1958,
HSDL2 |
| RP5-1102E8.3 | PIGK,
ST6GALNAC5 |
| linc-CSTB-3 | ADARB1,
POFUT2, |
| CTD-2314B22.3 | OR4K1, OR4K14,
OR4K2, OR4N2, OR4K15 |
| RP5-1148A21.3 | EYS |
| LINC00473 | ILF2, MMP2, USP9X,
CHUK, STK11, RPS6KA2 |
| RP11-645N11.2 | RASA4, POLR2J3,
SPDYE2, |
| RP11-324O2.3 | CCDC186, TDRD1,
VWA2, |
Bioinformatics analysis of the DNA
sequence
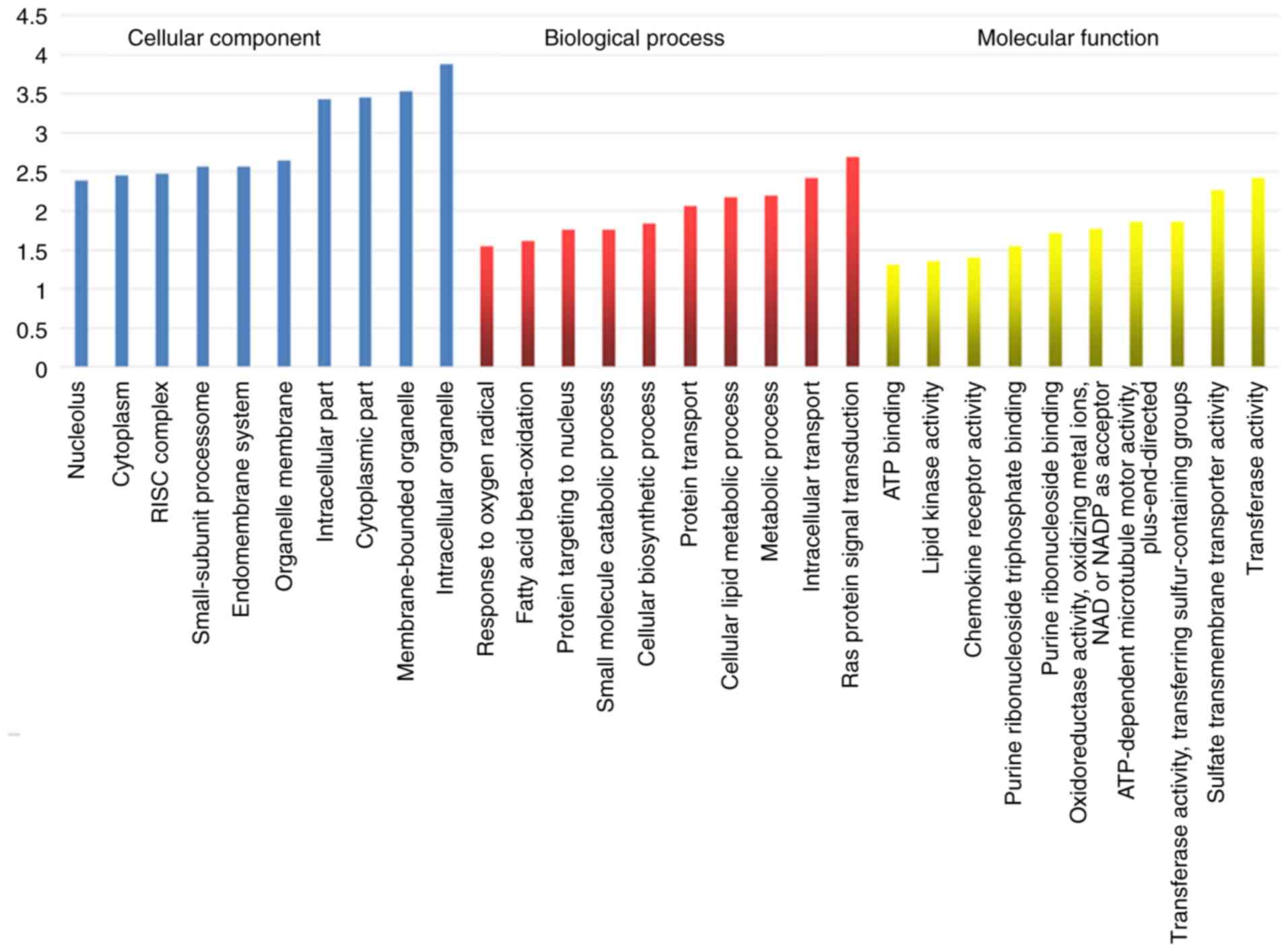
GO analysis mainly analyzes cellular components,
biological processes and molecular functions. Cellular components
involved were found to include the nucleolus, cytoplasm, RISC
complex, small-subunit proteasome, endomembrane system, organelle
membrane, intracellular part, cytoplasmic part, membrane-bounded
organelle and intracellular organelle. Biological processes
involved were found to include response to oxygen radical, fatty
acid beta-oxidation, protein targeting to nucleus, small molecule
catabolic process, cellular biosynthetic process, protein
transport, cellular lipid metabolic process, metabolic process,
intracellular transport and Ras protein signal transduction.
Molecular functions involved were found to include ATP binding,
lipid kinase activity, chemokine receptor activity, purine
ribonucleoside triphosphate binding, purine ribonucleoside binding,
oxidoreductase activity, oxidizing metal ions, NAD or NADP as
acceptor, ATP-dependent microtubule motor activity,
plus-end-directed, transferase activity, transferring
sulfur-containing groups, sulfate transmembrane transporter
activity and transferase activity. The results of GO analysis are
presented in Fig. 6.
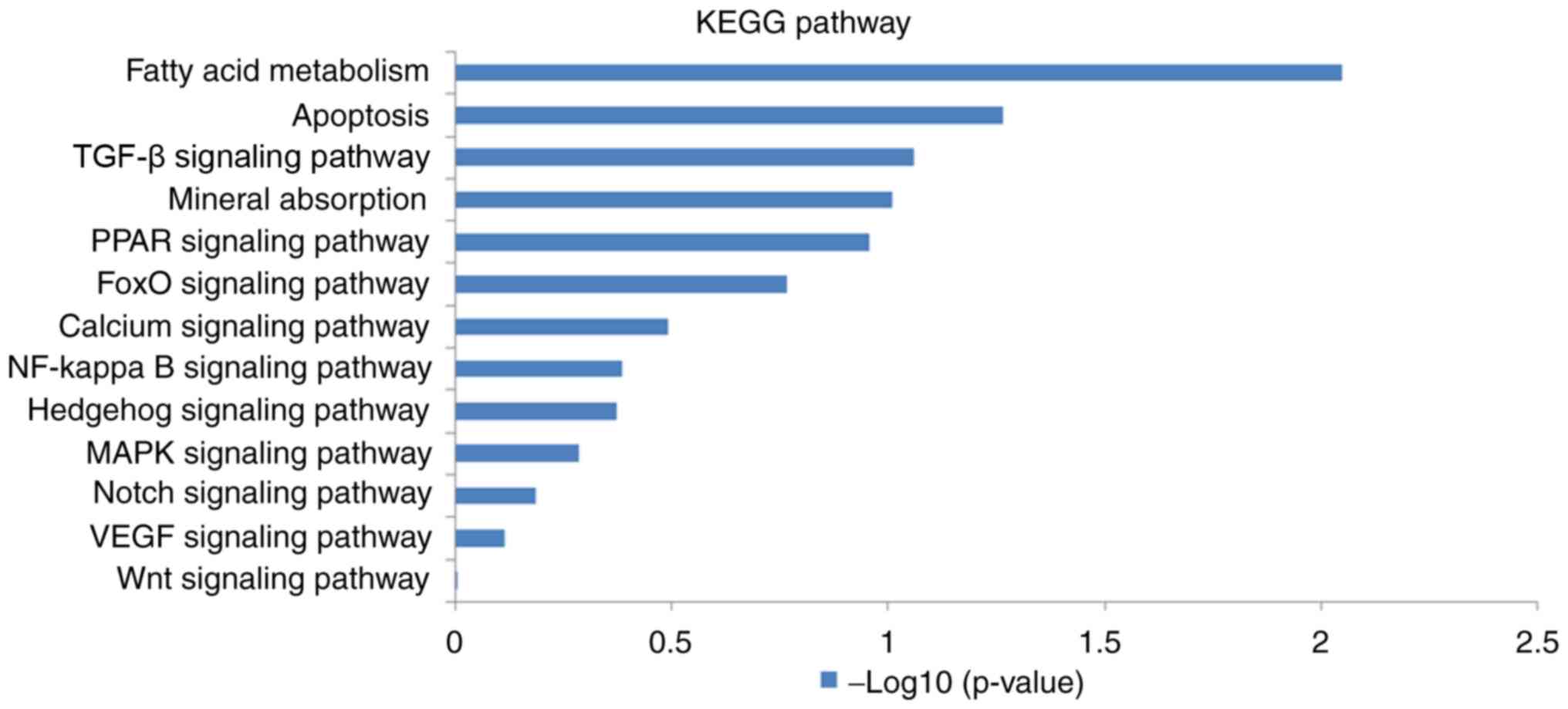
Pathway analysis was conducted using the KEGG
database. It was found that several pathways (Wnt, VEGF, Notch,
MAPK, hedgehog, NF-κB, calcium, FoxO, PPAR and TGF-β signaling
pathways, as well as mineral absorption, apoptosis and fatty acid
metabolism) were involved in osteogenic differentiation in the 2
groups, as illustrated in Fig.
7.
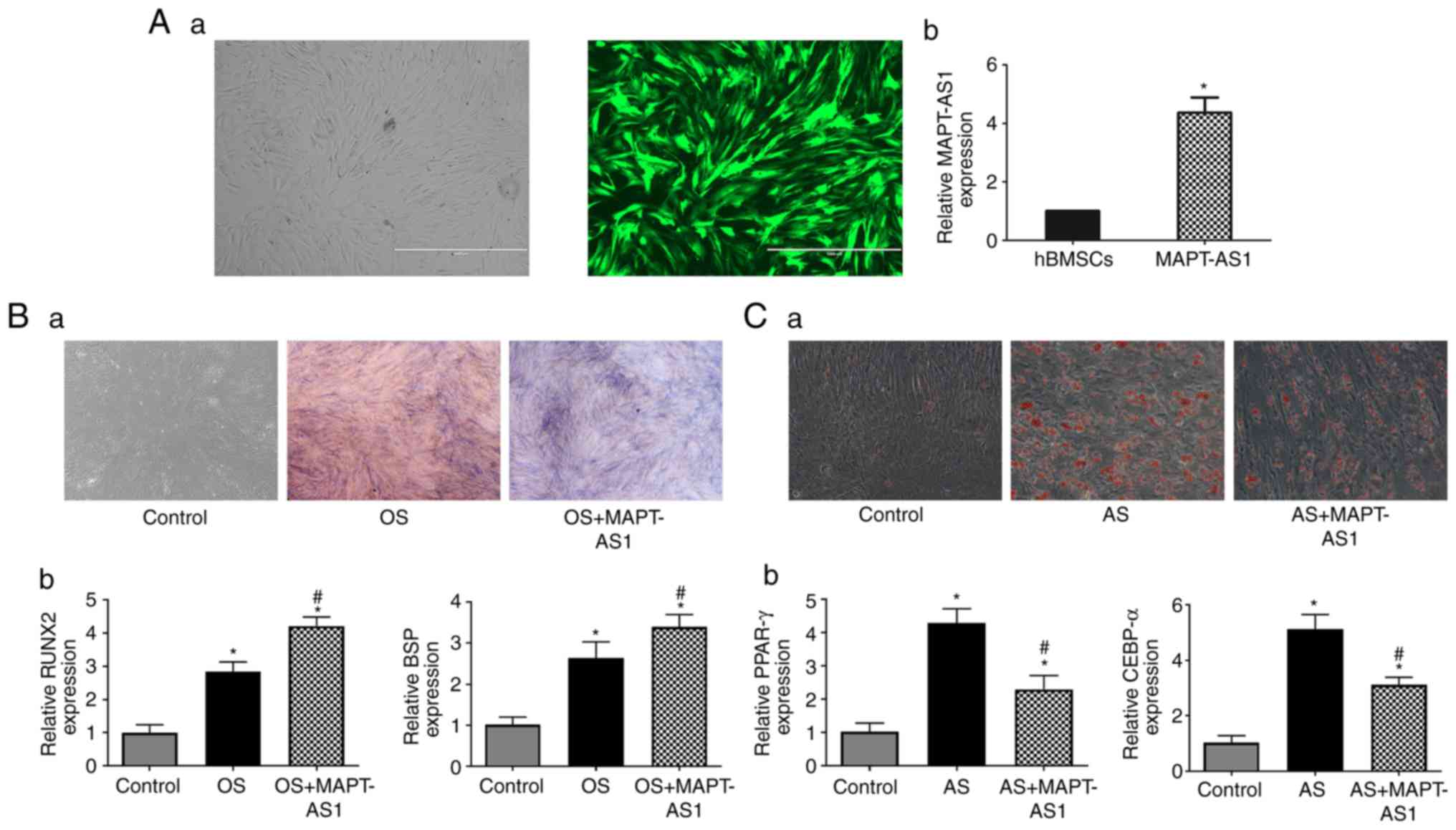
MAPT-AS1 promotes osteogenesis and
inhibits the adipo- genesis of hBMSCs
MAPT-AS1, which was downregulated in hBMSCs from
patients with ONFH during osteogenic differentiation, was selected
for functional analysis and for the further verification of the
findings. The results revealed that the overexpression of MAPT-AS1
significantly promoted osteogenic differentiation, as indicated by
ALP staining for the mineralization and expression of the
osteogenic transcription factors, Runx2 and BSP (Fig. 8). On the contrary, the
upregulation of MAPT-AS1 inhibited adipogenic differentiation, as
indicated by Oil Red O staining and the expression of the
adipogenic transcription factors, CEBP-α and PPARγ (Fig. 8).
Discussion
ONFH occurs in young individuals aged 20-40 years
and 15,000-20,000 new cases of femoral head necrosis are reported
annually (22,23). The causes of femoral head necrosis
mainly include hormones, alcohol abuse and hip trauma. Among these,
steroid-induced osteonecrosis of the femoral head (SONFH) accounts
for 46.03% of total femoral head necrosis cases (23) and is the most common type of
femoral head necrosis. For patients with early-stage ONFH, although
early intervention can be performed through drug therapy, core
decompression, interventional therapy, etc., their outcomes are not
satisfactory. Approximately 65-85% of patients with femoral head
necrosis will continue to develop the disease, leading to the
collapse of the femoral head (24), resulting in the need for total hip
replacement surgery. The majority of patients with ONFH are young
adults, and the life of their prosthesis is limited; thus, they may
require multiple revision surgeries in the future, which leads to a
tremendous economic burden to the family and society. The aim of
the present study was to explore the possible mechanisms of ONFH
and provide a basis for further intervention treatment of
early-stage ONFH. To the best of our knowledge, the present study
is the first to describe the role of lncRNAs in hBMSCs during
osteogenic differentiation in osteonecrosis of the femoral head and
it has more practical clinical significance.
In recent years, scholars at home and abroad have
conducted extensive research on the pathogenesis of ONFH and have
proposed a multi-strand bone necrosis theory, including the
intraosseous hypertension theory (25), coagulation mechanism change theory
(26), lipid metabolism disorder
theory (27), osteoporosis theory
(28,29), bone cell apoptosis theory
(30), membrane particle theory
(31), gene polymorphism
(32) and immune factors
(33). Additionally, the disease
has been found to be associated with the proliferation, osteogenic
and adipogenic differentiation of hBMSCs. For example, the
proliferative capability of hBMSCs has been found to be inhibited
in patients with ONFH compared with healthy individuals (34). miRNA-22 has been shown to inhibit
the adipogenic differentiation of hBMSCs through the protein
expression of HDAC6 (35), while
miRNA-100 may target BMPR2, which leads to the inhibition of
osteogenic differentiation of hBMSCs (36). However, the association between
lncRNAs and the osteogenic differentiation of hBMSCs during the
pathogenesis of ONFH remains unclear. To date, at least to the best
of our knowledge, only one study conducted focused on lncRNAs
involved in femoral head necrosis (37), and studies have not been conducted
on the characteristics of the osteogenic differentiation of hBMSCs
from patients with ONFH.
In the present study, differentially expressed
lncRNAs during the osteogenic differentiation of hBMSCs in
steroid-induced femoral head necrosis were identified using the
CapitalBiotech human lncRNA Array V4.0. Bioinformatics analyses,
including GO and pathway analysis of differentially expressed
lncRNAs, were also conducted. CNC and ceRNA networks were also
analyzed. The lncRNAs identified were further verified by
RT-qPCR.
The expression levels of 24 downregulated and 24
upregulated lncRNAs were determined during the osteogenic
differentiation of hBMSCs in ONFH. Targets of these lncRNAs were
involved in processes, such as cell proliferation, differentiation
and tumor metastasis. In total, 6 lncRNAs (AC107070.1,
linc-ANKRD20A1-4, RP11-794G24.1, LINC00473, MAPT-AS1 and
AP005273.1) in the hBMSCs were identified and confirmed by RT-qPCR.
The results of RT-qPCR revealed that the expression trends of the 4
lncRNAs were consistent with those of the microarray analysis.
Moreover, hBMSCs were isolated from another 30 samples, including
15 normal and 15 patients with ONFH. The expression levels of 2
upregulated lncRNAs (AC107070.1 and linc-ANKRD20A1-4) and 2
downregulated lncRNAs (LINC00473 and MAPT-AS1) were also consistent
with those of the microarray and RT-qPCR analyses, which verified
the accuracy of the results.
Since the majority of the lncRNAs in current
databases have not yet been functionally annotated, their functions
were predicted based on the functional annotations of their related
cis and trans target mRNAs. From the information
presented in Table III, it was
found that one lncRNA can control multiple genes, such as
RP11-794G24.1, RP11-762H8.3 and AP005273.1, while one gene can be
regulated by several lncRNAs. For example, OR4N2 was found to be
targeted by CTD-2314B22.3 and AL589743.1. The functions of these
lncRNAs were also investigated using GO analysis to determine the
biological processes, cellular components and molecular functions
involved. Intracellular organelle, membrane-bounded organelle and
cytoplasmic part were the 3 cellular components identified. Ras
protein signal transduction, intracellular transport and metabolic
process were the 3 biological processes identified. Moreover, the 3
most obvious aspects of change in molecular functions were found in
transferase activity, sulfate transmembrane transporter activity
and transferase activity, as well as transferring sulfur-containing
groups. Fatty acid metabolism, apoptosis and TGF-β signaling
pathway were the 3 signaling pathways that exhibited the highest
level of correlation in the KEGG pathways analysis. These 3
signaling pathways are inextricably linked to femoral head
necrosis. For example, adipogenic overdifferentiation, osteoblast
apoptosis and the inhibition of osteogenic differentiation through
TGF-β play vital roles in the occurrence and development of femoral
head necrosis. However, carbon metabolism, the pentose phosphate
pathway and biosynthesis of amino acids were significantly
upregulated in the KEGG analysis (37). It was hypothesized that the
difference in the results was due to two aspects. First, the method
used differed. In the experiments in the present study, the hBMSCs
underwent osteogenic differentiation prior to microarray analysis,
while in the other study, the hBMSCs were screened using microarray
analysis before undergoing osteogenic differentiation. Second,
differences may also be due to individual differences in the cases
included.
In the present study, LINC00473, also known as
LNC473, C6orf176, bA142J11.1, and is located in the 6q27 region and
LINC00473, was downregulated in the hBMSCs following osteogenic
differentiation. It has been found that LINC00473 was involved in
the development of a number of diseases including preeclampsia
(38), colorectal cancer
(39), gastric cancer (40) and others. It has been demonstrated
that LINC00473 is involved in the pathogenesis and development of
preeclampsia and may be a candidate biomarker, as well as a
therapeutic target for preeclampsia (38). Wang et al found that
LINC00473 promoted Taxol resistance via miR-15a in colorectal
cancer (39). Zhang and Song
demonstrated that LINC00473 is an lncRNA that is associated with
prognosis and malignancy in gastric cancer, while it also regulates
gastric cancer cell invasion and migration (40). In the present study, the
expression of LINC00473 in ONFH was found to be lower than that in
normal hBMSCs, and it was hypotehsized that LINC00473 plays an
important role in the osteogenic and adipogenic differentiation of
stem cells via related signaling pathways.
The MAPT-AS1 gene is located in the 17q21.31 region
and in the present study, MAPT-AS1 was found to be down-regulated
during the osteogenic differentiation of hBMSCs. MAPT-AS1 is
involved in the occurrence and development of tumors and
Parkinson's disease. MAPT-AS1 overexpression has not been found in
breast cancer; however, in triple-negative type (TNBC), a high
MAPT-AS1 expression has been found to be associated with a longer
patient survival (41).
Additionally, MAPT-AS1 levels have been shown to be associated with
MAPT expression, which is associated with breast cancer survival.
The results of that study indicated that MAPT-AS1 may function as a
potential breast cancer survival prediction biomarker (41). Pan et al (42) found that patients with ER-negative
breast cancer who had larger tumors (≥2 cm), were of a younger age
(<60), were at stages (III-IV) and had metastatic lymph nodes,
exhibited higher levels of MAPT-AS1 expression. The regulation of
natural comparable sense MAPT transcripts in cells of ER-negative
breast cancer leads to the association between MAPT-AS1 and
paclitaxel resistance, invasiveness and cell growth. Research has
indicated that overexpression may partially protect the MAPT mRNA
from degradation by the overexpression of MAPT-AS1, while the
knockdown of MAPT-AS1 decreases MAPT mRNA stability. Moreover, the
knockdown of MAPT also decreases MAPT-AS1 mRNA expression. MAPT-AS1
expression is coordinated with that of MAPT in breast tumor tissues
(42). Moreover, MAPT-AS1 and
DNMT1 have been identified as potential epigenetic regulators of
MAPT expression in Parkinson's disease across 4 different brain
regions and an increased MAPT expression may be associated with the
disease state, but not with the neuropathology severity of
Parkinson's disease (43). In the
present study, the overexpres-sion of MAPT-AS1 significantly
promoted the osteogenic differentiation and inhibited the
adipogenic differentiation of hBMSCs at the cellular and mRNA
level, as indicated by relevant staining and RT-qPCR analysis.
There were several limitations to the present study.
First, the sample size of the present study was small, which may
affect the results of the microarray analysis. Second, the majority
of the lncRNAs require further validation by RT-qPCR. Third, the
specific functions, as well as mechanisms of lncRNAs warrant
further investigation.
In conclusion, to the best of our knowledge, this is
the first study to elucidate the hBMSC expression profiles during
osteogenic differentiation in ONFH. A total of 24 downregulated
lncRNAs and 24 upregulated lncRNAs were found to be expressed
during the osteogenic differentiation of hBMSCs from patients with
ONFH. A bioinformatics analysis of the functions and mechanisms of
the identified lncRNAs was conducted. The present study may provide
a new perspective of the pathogenesis of ONFH and a novel direction
for the early treatment of ONFH.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81802151), the
Shandong Province Natural Science Foundation (grant nos.
ZR2016HQ05, no. ZR2017BH089 and ZR2019MH012), the China
Postdoctoral Science Foundation (grant no. 2018M642616) and the
Qingdao Applied Foundational Research Youth Project (grant no.
19-6-2-55-cg).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL, YX, KX and YR performed the experiments and
analyzed the results; YX, KX and YR wrote and drafted the
manuscript; HZ and XW wrote, reviewed and edited the manuscript; HZ
and YW conceived the methodology; while YJ and XW designed the
research study and was a major contributor in recruiting the
donors. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University and
written informed consent was obtained from all donors included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Guerado E and Caso E: The physiopathology
of avascular necrosis of the femoral head: An update. Injury.
47(Suppl 6): S16–S26. 2016. View Article : Google Scholar
|
|
2
|
Youm YS, Lee SY and Lee SH: Apoptosis in
the osteonecrosis of the femoral head. Clin Orthop Surg. 2:250–225.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calder JD, Buttery L, Revell PA, Pearse M
and Polak JM: Apoptosis-a significant cause of bone cell death in
osteonecrosis of the femoral head. J Bone Joint Surg Br.
86:1209–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Q, Wang GJ, Su CC and Balian G: The
otto aufranc award. Lovastatin prevents steroid induced
adipogenesis and osteonecrosis. Clin Orthop Relat Res. 8–19.
1997.PubMed/NCBI
|
|
6
|
Wang GJ, Sweet DE, Reger SI and Thompson
RC: Fat-cell changes as a mechanism of avascular necrosis of the
femoral head in cortisone-treated rabbits. J Bone Joint Surg Am.
59:729–35. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kannu P, O'Rielly DD, Hyland JC and Kokko
LA: Avascular necrosis of the femoral head due to a novel C
propeptide mutation in COL2A1. Am J Med Genet A. 155A:1759–1762.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SL, Fang WW, Ye F, Liu YH, Qian J,
Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S and Sun JP: Effect on
left ventricular function of intracoronary transplantation of
autologous bone marrow mesenchymal stem cell in patients with acute
myocardial infarction. Am J Cardiol. 94:92–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Qu X and Zhao RC: Clinical
applications of mesenchymal stem cells. J Hematol Oncol. 5:192012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hare JM, Fishman JE, Gerstenblith G,
DiFede VD, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston
PV, Brinker JA, et al: Comparison of allogeneic vs autologous bone
marrow-derived mesenchymal stem cells delivered by transendocardial
injection in patients with ischemic cardiomyopathy: The POSEIDON
randomized trial. JAMA. 308:2369–2379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang J, Li X, Zhang H, Wang D, Feng X,
Wang H, Hua B, Liu B and Sun L: Allogeneic mesenchymal stem cells
transplantation in patients with refractory RA. Clin Rheumatol.
31:157–161. 2012. View Article : Google Scholar
|
|
13
|
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang
L, Liu B and Yu X: Treatment of early stage osteonecrosis of the
femoral head with autologous implantation of bone marrow-derived
and cultured mesenchymal stem cells. Bone. 50:325–330. 2012.
View Article : Google Scholar
|
|
14
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guttman M, Donaghey J, Carey BW, Garber M,
Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al:
lincRNAs act in the circuitry controlling pluripotency and
differentiation. Nature. 477:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Wu Z, Fu X and Han W: Long noncoding
RNAs: Insights from biological features and functions to diseases.
Med Res Rev. 33:517–553. 2013. View Article : Google Scholar
|
|
18
|
Lee JT and Bartolomei MS: X-inactivation,
imprinting, and long noncoding RNAs in health and disease. Cell.
152:1308–1323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Wang Y, Li Z, Li Z and Yu B:
Differential expression of long noncoding ribonucleic acids during
osteogenic differentiation of human bone marrow mesenchymal stem
cells. Int Orthop. 39:1013–1019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418. pp. 41–49. 2002, View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Moya-Angeler J, Gianakos AL, Villa JC, Ni
A and Lane JM: Current concepts on osteonecrosis of the femoral
head. World J Orthop. 6:590–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papakostidis C, Tosounidis TH, Jones E and
Giannoudis PV: The role of 'cell therapy' in osteonecrosis of the
femoral head. A systematic review of the literature and
meta-analysis of 7 studies. Acta Orthop. 87:72–78. 2016. View Article : Google Scholar
|
|
24
|
Pepke W, Kasten P, Beckmann NA, Janicki P
and Egermann M: Core decompression and autologous bone marrow
concentrate for treatment of femoral head osteonecrosis: A
randomized prospective study. Orthop Rev (Pavia). 8:61622016.
View Article : Google Scholar
|
|
25
|
Mukisi MM, Bashoun K and Burny F:
Sickle-cell hip necrosis and intraosseous pressure. Orthop
Traumatol Surg Res. 95:134–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar MN, Belehalli P and Ramachandra P:
PET/CT study of temporal variations in blood flow to the femoral
head following low-energy fracture of the femoral neck.
Orthopedics. 37:e563–e570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng X, Zhan K, Zhang L, Zeng D, Yu W,
Zhang X, Zhao M, Lai Z and Chen R: The impact of high total
cholesterol and high low-density lipoprotein on avascular necrosis
of the femoral head in low-energy femoral neck fractures. J Orthop
Surg Res. 12:302017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones LC and Hungerford DS: The
pathogenesis of osteonecrosis. Instr Course Lect. 56:179–196.
2007.PubMed/NCBI
|
|
29
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: A new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jilka RL, Noble B and Weinstein RS:
Osteocyte apoptosis. Bone. 54:264–271. 2013. View Article : Google Scholar :
|
|
31
|
Wu Z, Ji C, Li H, Qiu G, Gao C and Weng X:
Elevated level of membrane microparticles in the disease of
steroid-induced vascular osteonecrosis. J Craniofac Surg.
24:1252–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng L, Wang W, Ni J, Li Z and Xiao T:
The association of eNOS gene polymorphism with avascular necrosis
of femoral head. PLoS One. 9:e875832014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian L, Wen Q, Dang X, You W, Fan L and
Wang K: Immune response associated with Toll-like receptor 4
signaling pathway leads to steroid-induced femoral head
osteonecrosis. BMC Musculoskelet Disord. 15:182014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JS, Lee JS, Roh HL, Kim CH, Jung JS
and Suh KT: Alterations in the differentiation ability of
mesenchymal stem cells in patients with nontraumatic osteonecrosis
of the femoral head: Comparative analysis according to the risk
factor. J Orthop Res. 24:604–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang S, Wang S, Bian C, Yang Z, Zhou H,
Zeng Y, Li H, Han Q and Zhao RC: Upregulation of miR-22 promotes
osteogenic differentiation and inhibits adipogenic differentiation
of human adipose tissue-derived mesenchymal stem cells by
repressing HDAC6 protein expression. Stem Cells Dev. 21:2531–2540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng Y, Qu X, Li H, Huang S, Wang S, Xu Q,
Lin R, Han Q, Li J and Zhao RC: MicroRNA-100 regulates osteogenic
differentiation of human adipose-derived mesenchymal stem cells by
targeting BMPR2. FEBS Lett. 586:2375–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Yang Q, Chen G, Du Z, Ren M, Wang
A, Zhao H, Li Z, Zhang G and Song Y: LncRNA expression profiling of
BMSCs in osteonecrosis of the femoral head associated with
increased adipogenic and decreased osteogenic differentiation. Sci
Rep. 8:91272018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu D, Xu Y, Zou Y, Zuo Q, Huang S, Wang S,
Lu X, He X, Wang J, Wang T and Sun L: Long noncoding RNA 00473 is
involved in preeclampsia by LSD1 binding-regulated TFPI2
transcription in trophoblast cells. Mol Ther Nucleic Acids.
12:381–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Zhang X, Sheng L, Qiu C and Luo R:
LINC00473 promotes the Taxol resistance via miR-15a in colorectal
cancer. Biosci Rep. 38:BSR201807902018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W and Song Y: LINC00473 predicts
poor prognosis and regulates cell migration and invasion in gastric
cancer. Biomed Pharmacother. 107:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang D, Li J, Cai F, Xu Z, Li L, Zhu H,
Liu W, Xu Q, Cao J, Sun J and Tang J: Overexpression of MAPT-AS1 is
associated with better patient survival in breast cancer. Biochem
Cell Biol. 97:158–164. 2019. View Article : Google Scholar
|
|
42
|
Pan Y, Pan Y, Cheng Y, Yang F, Yao Z and
Wang O: Knockdown of LncRNA MAPT-AS1 inhibites proliferation and
migration and sensitizes cancer cells to paclitaxel by regulating
MAPT expression in ER-negative breast cancers. Cell Biosci.
8:72018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coupland KG, Kim WS, Halliday GM, Hallupp
M, Dobson-Stone C and Kwok JB: Role of the long non-coding RNA
MAPT-AS1 in regulation of microtubule associated protein Tau (MAPT)
expression in Parkinson's disease. PLoS One. 11:e01579242016.
View Article : Google Scholar : PubMed/NCBI
|