Introduction
Osteoporosis is an increasingly serious
life-threatening medical issue (1-4).
It often results in fractures, with associated complications such
as hypostatic pneumonia, deep-vein thrombosis and bedsores, which
can be fatal (5). Osteoporosis
occurs due to a decrease in the number/activity of osteoblasts
and/or increase in the number/activity of osteoclasts (6). The pathogenesis of osteoporosis
involves complex signaling pathway regulation and protein
modification, and a lot about this process remains unknown
(7-9). In the past years, research has
identified key signalling molecules that modulate bone formation
and/or bone resorption, including Wnt (10,11), Akt (12,13), MAPK (14,15), AMP-activated protein kinase (AMPK)
(16,17), receptor activator of nuclear
factor-κB ligand (RANKL) (18,19), osteoprotegerin (OPG) (20,21) and tumor necrosis factor
superfamily member 14 (22).
Currently, there is no efficacious anti-osteoporosis treatment with
minimal side effects; thus, there is an urgent unmet medical need
for novel treatment strategies.
Bone homeostasis is maintained through osteogenesis
and osteoclastogenesis (23).
Osteoclasts originate via a process of differentiation from
hematopoietic stem cells or monocytes (24). Osteoclast differentiation (or
osteoclastogenesis) involves multiple steps, which include
transformation into tartrate-resistant acid phosphatase
(TRAP)-positive cells, merging into multinucleated cells,
activating bone resorption and finally undergoing spontaneous
apoptosis (25). RANK, its
cellular ligand RANKL, macrophage colony-stimulating factor (M-CSF)
and OPG (a decoy soluble receptor for RANKL) are four critical
factors for osteoclastogenesis (26,27). RANKL is highly conserved and is a
member of the TNF family (28).
RANK (encoded by Tnfrsf11a) is a receptor activator of
nuclear factor-κB, which is expressed via M-CSF stimulation on the
surface of Raw264.7 cells (29).
OPG competitively inhibits the binding of RANKL to RANK, and
polymorphisms in the OPGgene are associated with
osteoporosis (30,31). Furthermore, the interaction
between M-CSF and CSF1 receptor is crucial for proliferation and
differentiation in osteoporosis (32).
The existing drugs for osteoporosis are inefficient
and produce unsatisfactory results (33); therefore, it is critical to
develop safe and effective treatment options. Natural substances
provide a new avenue for the treatment of osteoporosis. Melatonin
is a methoxyindole that is synthesized in, and secreted
predominantly from, the pineal gland (34). Although it is also synthesized in
mitochondria, virtually every cell can produce melatonin, including
cells of the bone marrow (35).
This secretion is performed at night as part of the circadian
rhythm; the schedule of the circadian rhythm is orchestrated by the
suprachiasmatic nuclei and synchronized with the light/dark cycle
(34). Furthermore, light can
inhibit melatonin production (36). The biological rhythm of melatonin
can be estimated by the melatonin content in the plasma/saliva or
by measuring urinary 6-sulfatoxymelatonin, the primary hepatic
metabolite (34,37,38). Some studies have determined that
melatonin may reinforce the coupling of rhythms, such as the
sleep/wake cycle and core temperature, although these findings are
based on clinical trial information (34,39,40). Sánchez-Barceló et al
(41) summarized the
physiological effect of melatonin on the bone. Specifically, low
concentrations (in the µM range) of melatonin were found to
promote the proliferation and differentiation of osteoblasts and
the expression levels of bone differentiation markers (such as type
I collagen, osteopontin, bone sialoprotein and osteocalcin)
(41). Additionally, melatonin
can simultaneously inhibit osteoclast differentiation by promoting
OPG secretion and eliminating the free radicals produced by
osteoclasts (41). However, the
underlying mechanism by which melatonin exerts its effects on
individuals afflicted by osteoporosis remains to be identified.
Thus, the current study aimed to investigate the effects of
melatonin on RANKL-induced osteoclastogenesis in Raw264.7
cells.
Materials and methods
Cell culture
Raw264.7 cells (The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) were cultured in
DMEM containing 10% FBS and 1% streptomycin and penicillin (all
HyClone; Cytiva) in a 37°C incubator with 5% CO2 and
maximum humidity. The culture medium was replenished daily. Cells
were incubated in serum-free medium 24 h before treatment. Raw264.7
cells were cultured for 7 days with 100 ng/ml RANKL (R&D
Systems, Inc.) and 30 ng/ml M-CSF (R&D Systems, Inc.) in the
presence of varying concentrations (0.1 or 1 µmol) of
melatonin (Sigma-Aldrich; Merck KGaA; Fig. 1A) for 48 h at 37°C. Raw264.7 cells
were cultured in the presence of varying concentrations of SR9009
(5, 10 and 15 µmol; MedChemExpress; Fig. 1B) or SR8278 (5, 10 and 15
µmol; MedChemExpress; Fig.
1C) for 48 h at 37°C.
Synthetic RNA oligonucleotides and
transfection
MicroRNA (miR)-882 mimics, miR-882 mimic negative
control (NC), miR-882 inhibitors and miR-882 inhibitor NC were
obtained from Shanghai GenePharma Co., Ltd. Raw264.7 cells were
transfected with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 6-8 h at 37°C, according to the
manufacturer's instructions. The type of NCs used was a non-sense
sequence. The concentration of miR-882 mimics/inhibitors used for
transfection was 20 µM. RNA was extracted 24 h after
transfection, and protein was extracted 48 h after transfection.
The sequences of all miR-882 mimics and inhibitors were as follows:
miR-882 mimics sense, 5′-AGG AGA GAG UUA GCG CAU UAG U-3′ and
antisense, 5′-UAA UGC GCU AAC UCU CUC CUU U-3′; miR-882 mimics NC
sense, 5′-UUC UCC GAA CGU GUC ACG UTT -3′ and antisense, 5′-ACG UGA
CAC GUU CGG AGA ATT -3′; miR-882 inhibitors, 5′-ACU AAU GCG CUA ACU
CUC UCC U-3′; miR-882 inhibitors NC, 5′-CAG UAC UUU UGU GUA GUA CAA
-3.
RNA extraction and reverse
transcription-quantitative PCR
The TRIzol® Reagent kit (Qiagen Sciences,
Inc.) was used to extract total RNA from cells according to the
manufacturer's protocol. miRNA and mRNA reverse-transcription PCR
was performed using the Mir-X miRNA qRT-PCR TB Green®
kit (cat. no. 638314; Clontech Laboratories, Inc.) for miRNA and
the PrimeScript™ RT reagent kit with gDNA Eraser (cat. no. RR047A;
Takara Biotechnology Co., Ltd.) for mRNA according to the
manufacturer's protocol. The QuantiTect SYBR-Green PCR kit (cat.
no. RR820A; Takara Biotechnology Co., Ltd.) was used to perform
real-time quantitative PCR. The thermocycling conditions are as
follows: Initial denaturation at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec, one cycle at 95°C for
5 sec, 60°C for 1 min and 95°C, and finally annealing at 50°C for
30 sec. The results were analyzed using a Roche Light
Cycler® 480 II system (Roche Diagnostics). The relative
expression of miR-882 was standardized to U6 RNA expression, while
mRNA relative expression was standardized to GAPDH mRNA expression
using the well-accepted 2−ΔΔCq method (42). For detailed information see
section Performing a Basic Relative Quantification Experiment. The
following primer sequences were used: miR-882 forward, 5′-CGC AGG
AGA GAG TTA GCG CAT TAG T-3′ and reverse primer was taken by
Universal sequence; U6 forward, 5′-CGC TTC GGC AGC ACA TAT AC-3′
and reverse, 5′-TTC ACG AAT TTG CGT GTC AT-3′; cathepsin K forward,
5′-GAA GAA GAC TCA CCA GAA GCA G-3′ and reverse, 5′-TCC AGG TTA TGG
GCA GAG ATT -3′; NR1D1 forward, 5′-TAC ATT GGC TCT AGT GGC TCC -3′
and reverse, 5′-CAG TAG GTG ATG GTG GGA AGT A-3′; and GAPDH
forward, 5′-AGG TCG GTG TGA ACG GAT TTG -3′ and reverse, 5′-TGT AGA
CCA TGT AGT TGA GGT CA-3′.
Western blot analysis
Raw264.7 cells were lysed in RIPA buffer containing
phenylmethanesulfonyl fluoride (both Beyotime Institute of
Biotechnology), followed by centrifugation at 4°C at 12,000 × g for
30 min. Quantitative analysis of protein concentration was
performed using a BCA assay kit (Beyotime Institute of
Biotechnology), and each sample was loaded at a concentration of 3
µg/µl in RIPA and loading buffer. Samples were
separated via 10% SDS-PAGE at 80 V and the proteins were
transferred to polyvinylidene difluoride membranes at 200 mA for 60
min. The membranes were blocked with 5% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 2 h at room temperature and
incubated with primary antibodies diluted in TBS-Tween (TBST;
Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
T1081) at concentrations according to the manufacturer's
instructions overnight at 4°C. The following primary antibodies
were used: Anti-cathepsin K (Abcam; cat. no. ab19027; 1:1,000),
anti-nuclear receptor subfamily 1 group D member 1 (NR1D1/Rev-erbα;
Abcam; cat. no. ab174309; 1:5,000) and anti-GAPDH (ProteinTech
Group, Inc.; cat. no. 10494-1-AP; 1:10,000). After primary antibody
incubation, the membranes were washed thrice and incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (ProteinTech
Group, Inc.; cat. no. SA00001-2; 1:10,000) diluted in TBST for 2 h
at room temperature. An Ultrasensitive Enhanced Chemiluminescence
Detection kit (ProteinTech Group, Inc.; cat. no. PK10002) was used
as the visualization reagent. ImageJ software (v1.52; National
Institutes of Health) was used for densitometry.
TRAP staining
Raw264.7 cells were plated in 6-well plates (3,000
cells/cm2) and cultured in medium containing 100 ng/ml
RANKL and 30 ng/ml M-CSF for 7 days at 37°C prior to TRAP staining,
with or without 0.1 or 1 µM melatonin. Plates were washed
with PBS and fixed with 4% paraformaldehyde for 15 min at 37°C.
TRAP staining working solution was added to the plates and
incubated for 60 min at 37°C in the dark. TRAP staining was
performed using an acid phosphatase leukocyte kit (Sigma-Aldrich;
Merck KGaA; cat. no. 387) in accordance with the manufacturer's
protocol. Representative images were acquired using an Eclipse Ti
fluorescence microscope (Nikon Corporation; magnification, ×200 and
400). TRAP-positive cells containing >3 nuclei were recorded as
osteoclasts.
Cell Counting Kit-8 (CCK-8) assay for
cell viability
Raw264.7 cells were plated in 96-well plates at a
concentration of 5×103 cells/well. After 24 h, melatonin
(0.1, 1 or 10 µmol), the Rev-erbα agonist SR9009 (Fig. 1B) or antagonist SR8278 (Fig. 1C) was added. After 48 h at 37°C,
CCK-8 reagent (Dojindo Molecular Technologies, Inc.) was added for
30-60 min to detect cell activity according to the manufacturer's
instructions. The results were analyzed with an automated
enzyme-linked immunosorbent assay reader ELx808 (BioTek
Instruments, Inc.; Agilent Technologies, Inc.) at 450 nm. Cell
activity was expressed as OD value.
Target gene prediction
Potential miRNAs that could target Rev-erbα were
first predicted by collecting information from databases including
TargetScan (http://www.targetscan.org/), miRDB (http://mirdb.org/) and DIANA (http://diana.imis.athena-innovation.gr/), followed by
organizing and consolidating these data.
Dual-luciferase reporter assay
Plasmid transfections for luciferase assays in 293T
cells (purchased from The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences, cultured with DMEM with 10% FBS in
a 37°C incubator with 5% CO2) were performed with 0.1
µg reporter constructs (pMIR-REPORT-wild-type-Rev-erbα or
pMIR-REPORT-mutant-Rev-erbα plasmids; Shanghai GeneChem Co., Ltd.)
and 0.4 µg miR-882 expression plasmid (Shanghai GeneChem
Co., Ltd.), in a 24-well plate using Roche X-tremeGENE HP (Roche
Diagnostics; cat. no. 06366236001) according to the manufacturer's
instructions. Luciferase activity was measured 48 h
post-transfection using the Dual Luciferase Reporter Assay System
according to the manufacturer's instructions (Promega Corporation).
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
All data were analyzed using SPSS 22.0 software (IBM
Corp.) and GraphPad Prism 6.0 (GraphPad Software, Inc.). Each group
of experiments was repeated thrice independently, and the values
are expressed as the mean ± SD. In the present study, one-way ANOVA
followed by Dunnett's post-hoc test was used for determining
whether ≥3 groups were statistically different from each other,
while an unpaired t-test was used to determine whether 2 groups
were statistically different from each other. P<0.05 was used to
indicate a statistically significant difference.
Results
Melatonin inhibits RANKL-induced
osteoclastogenesis in Raw264.7 cells
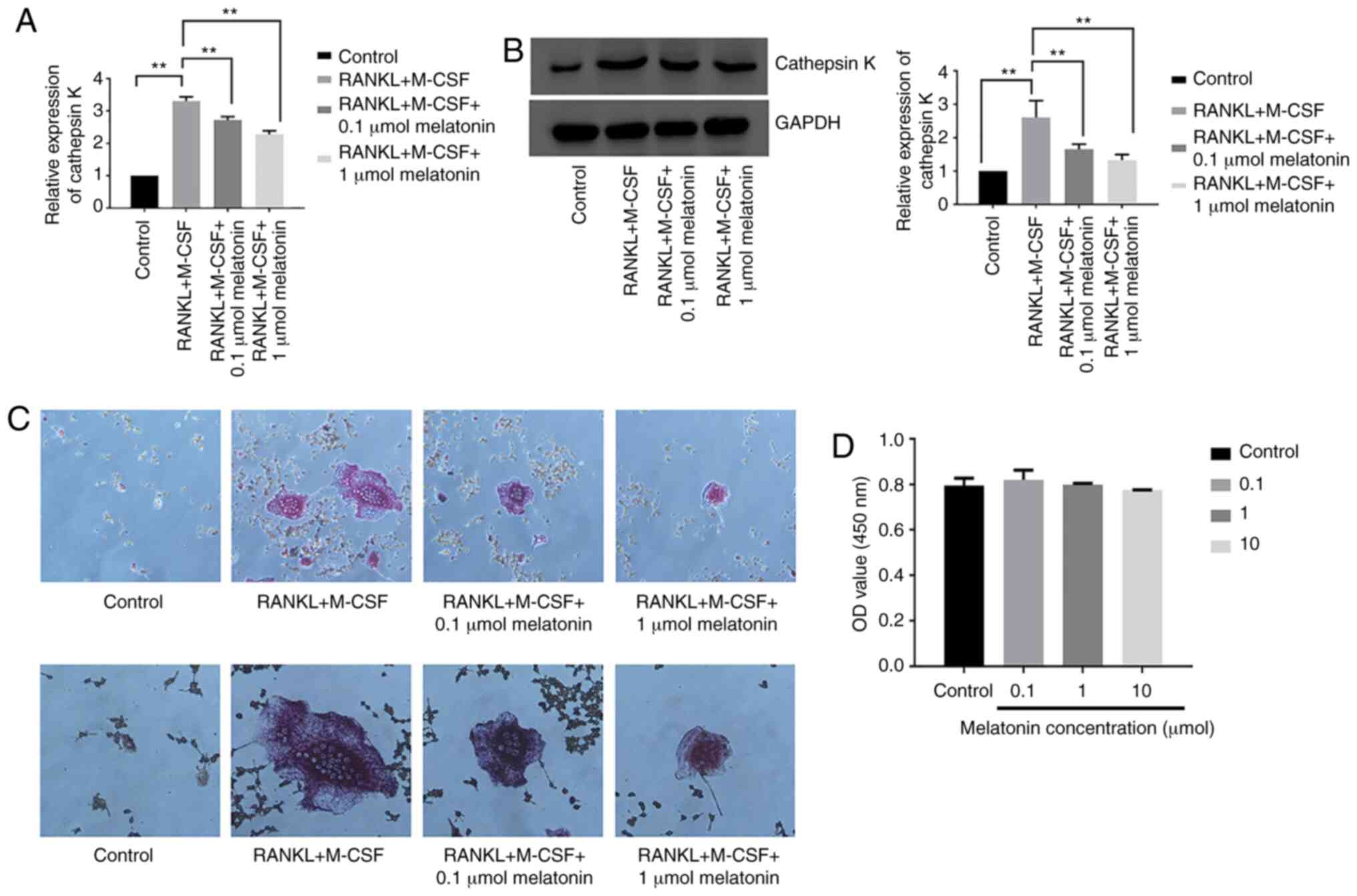
To study the effect of melatonin on
osteoclastogenesis, the mRNA and protein expression levels of
cathepsin K were examined in Raw264.7 cells cultured for 7 days
with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of
varying concentrations (0.1 or 1 µmol) of melatonin for 48
h. Cathepsin K was analyzed since its expression represents the
level of osteoclastogenesis (43). Melatonin decreased both the mRNA
and protein expression levels of cathepsin K following RANKL and
M-CSF treatment (Fig. 2A and B).
Cell differentiation was further studied using TRAP staining in
Raw264.7 cells cultured for 7 days with RANKL and M-CSF in the
presence of melatonin for 48 h. Melatonin treatment decreased the
number of TRAP-positive cells following RANKL treatment (Fig. 2C). Subsequently, the viability of
Raw264.7 cells was analyzed in the presence of varying
concentrations (0.1, 1 or 10 µmol) of melatonin for 48 h
using the CCK-8 assay. Cell viability was expressed as OD value.
The results revealed that melatonin was not cytotoxic to Raw264.7
cells (Fig. 2D). The present
findings indicated that melatonin significantly inhibited
RANKL-induced osteoclastogenesis in Raw264.7 cells without any
observed cytotoxicity.
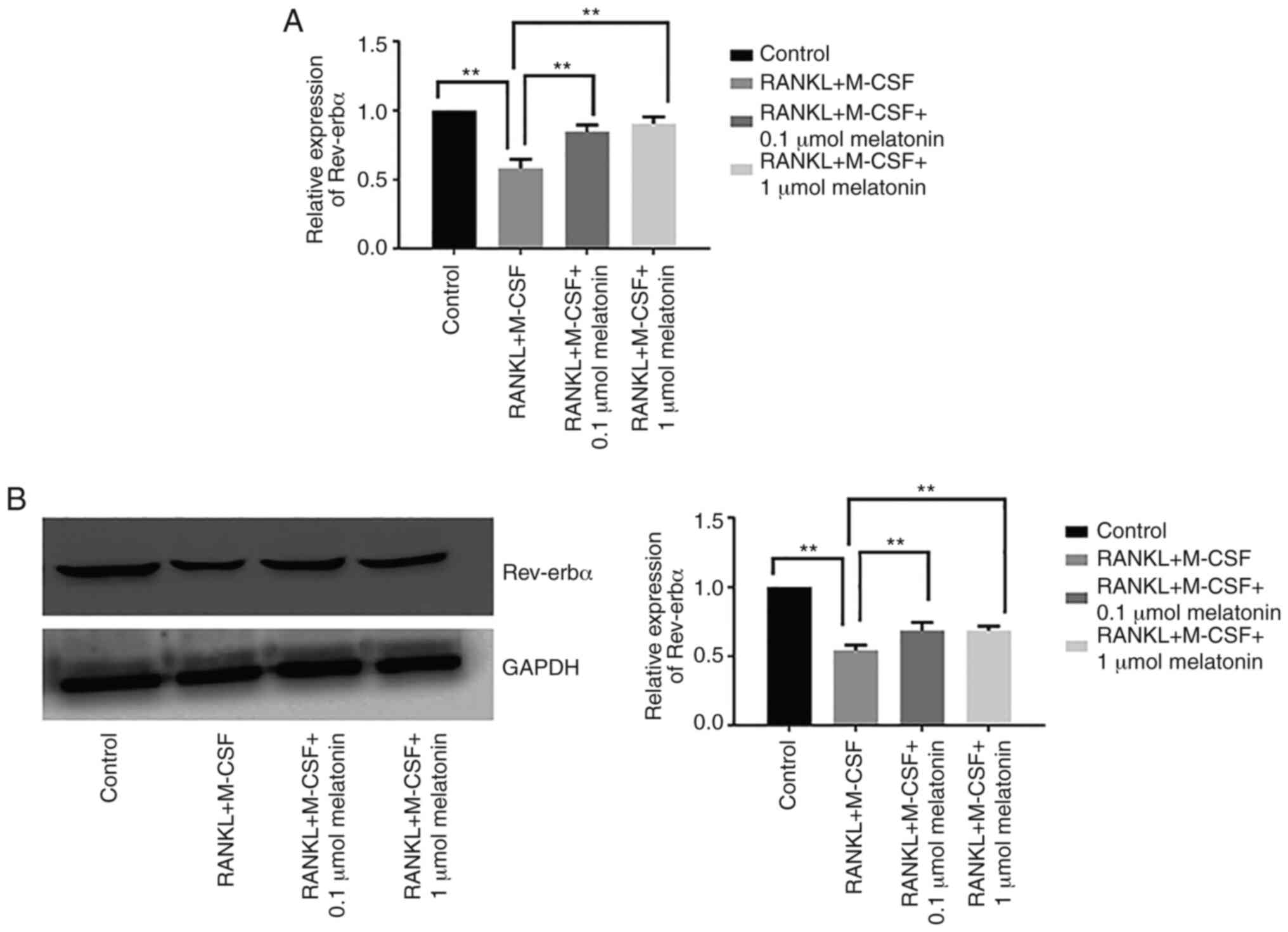
Melatonin enhances Rev-erbα expression in
Raw264.7 cells
During RANKL-induced osteoclast differentiation,
both mRNA and protein expression levels of Rev-erbα were suppressed
compared with the control group, which indicated that the
inhibitory effect of melatonin on osteoclastogenesis may be
associated with Rev-erbα (Fig. 3A and
B). By contrast, the addition of melatonin (0.1 or 1
µmol) significantly upregulated Rev-erbα mRNA and protein
expression in Raw264.7 cells cultured with RANKL and M-CSF
(Fig. 3A and B).
Rev-erbα activation boosts the effect of
melatonin on the inhibition of osteoclastogenesis, whereas Rev-Erbα
inhibition promotes osteoclastogenesis
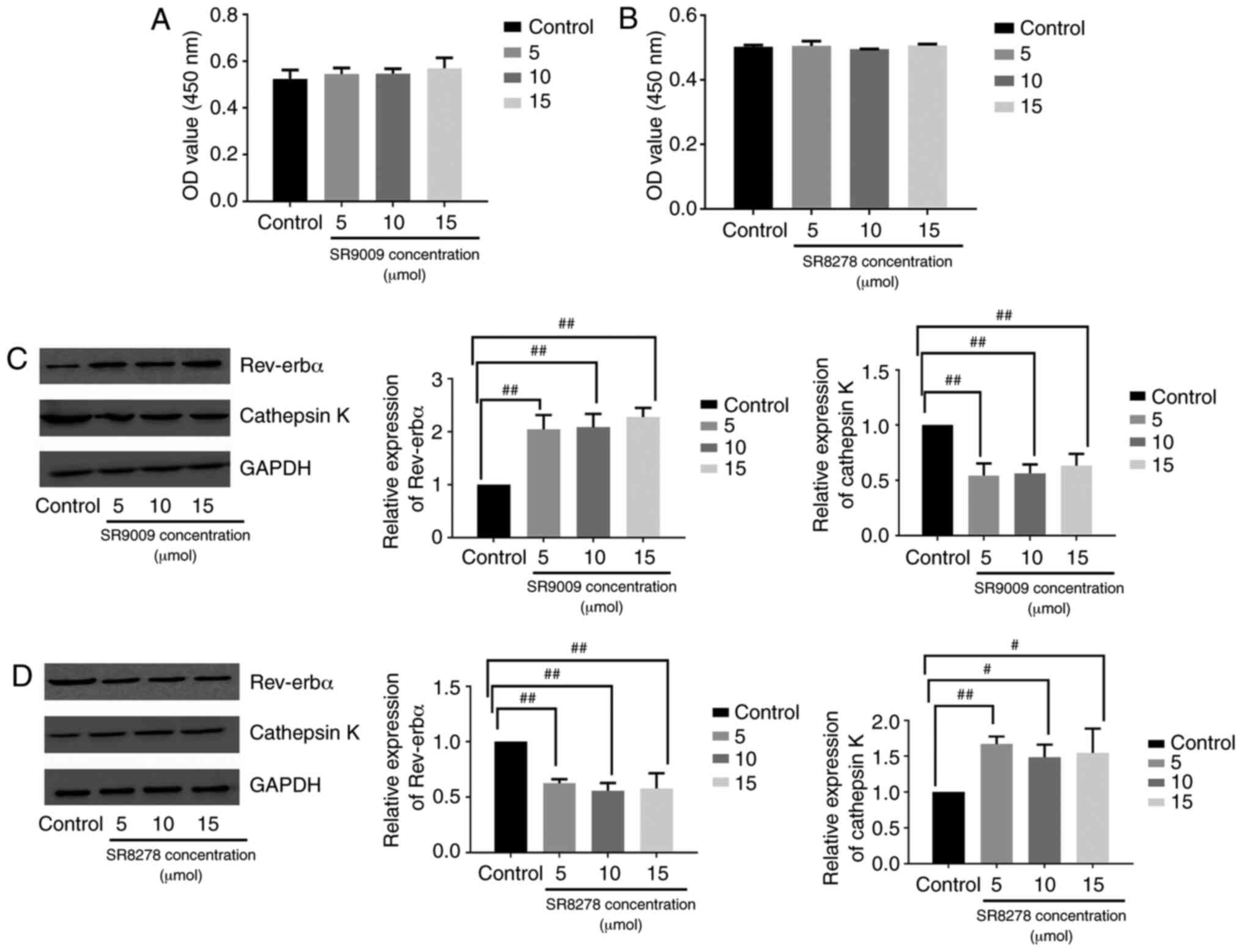
Raw264.7 cells were treated with the Rev-erbα
agonist SR9009 or antagonist SR8278 to assess the function of
Rev-erbα in osteoclastogenesis. The viability of Raw264.7 cells was
analyzed in the presence of varying concentrations of SR9009 (5, 10
and 15 µmol) or SR8278 (5, 10 and 15 µmol) for 48 h
via CCK-8 assay. SR9009 and SR8278 were not toxic to Raw264.7 cells
at the concentrations tested (Fig. 4A
and B). Western blotting revealed that in Raw264.7 cells
cultured with RANKL, M-CSF and melatonin (1 µmol),
osteoclastogenesis was inhibited by melatonin more significantly in
cells treated with the Rev-erbα agonist SR9009 compared with the
osteoclastogenesis process in cells cultured without SR9009
(Fig. 4C). The Rev-erbα
antagonist SR8278 hampered the ability of melatonin to influence
osteoclastogenesis in Raw264.7 cells cultured with RANKL, M-CSF and
1 µmol melatonin (Fig.
4D). Overall, these results indicated that the inhibitory
effect of melatonin on osteoclastogenesis may be mediated by
Rev-erbα.
miR-882 regulates Rev-erbα protein
expression
Subsequently, whether Rev-erbα expression was
regulated by miRNAs was investigated. Potential miRNAs that could
target Rev-erbα were first predicted by collecting information from
databases including TargetScan, miRDB and DIANA, followed by
organizing and consolidating these data. The overlapping miRNAs
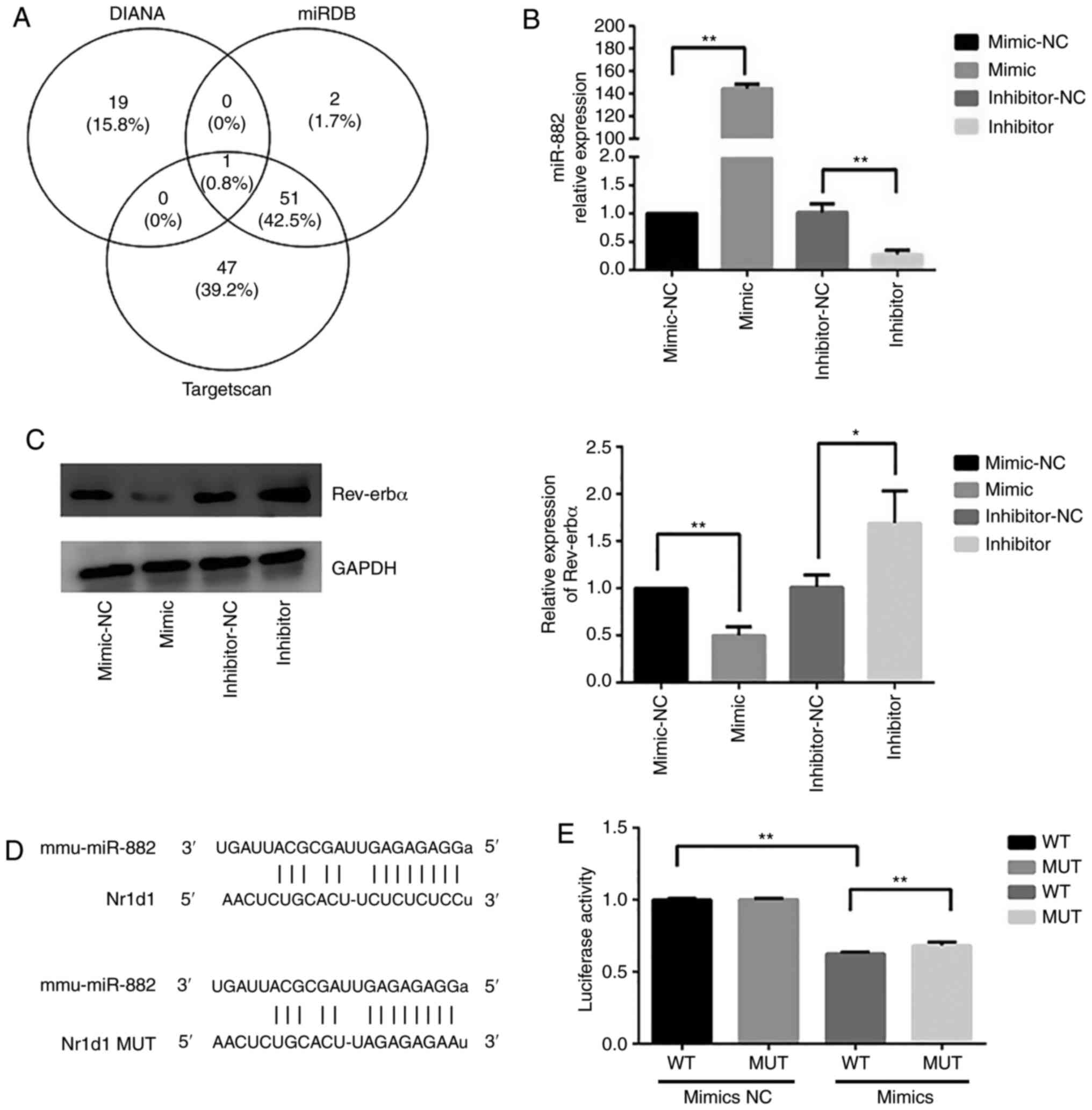
across different databases are shown in the Venn diagram in
Fig. 5A. Since miR-882 was
identified by the intersection of the three databases, it was
hypothesized that miR-882 may regulate Rev-erbα mRNA expression.
Raw264.7 cells were transfected with miR-882 mimics, inhibitors or
corresponding NCs. miR-882 expression in Raw264.7 cells transfected
with miR-882 mimics was significantly higher than that with the
mimic-NC; additionally, Raw264.7 cells transfected with miR-882
inhibitors exhibited the opposite effect (Fig. 5B). Subsequently, whether Rev-erbα
expression may be modulated by miR-882 was examined. Transfection
with miR-882 mimics significantly decreased Rev-erbα protein
expression in Raw264.7 cells, whereas the inhibition of miR-882
produced the opposite effect (Fig.
5C). Next, the wild-type 3′-untrans-lated region (UTR) of
Rev-erbα mRNA was cloned with the presumed miR-882-binding sites,
along with the mutant 3′-UTR located upstream of the
luciferase-coding sequence (Fig.
5D). Luciferase activity was decreased in cells co-transfected
with miR-882 mimics and Rev-erbα mRNA wild-type 3′-UTR fragments
compared with in cells co-transfected with miR-882 mimics NC and
Rev-erbα mRNA wild-type 3′-UTR fragments, and compared with in
cells co-transfected with miR-882 mimics and Rev-erbα mRNA mutant
3′-UTR fragments (Fig. 5E). These
results indicated that Rev-erbα may be a direct target of miR-882
and implied that miR-882 may exert its influence on
osteoclastogenesis by targeting Rev-erbα.
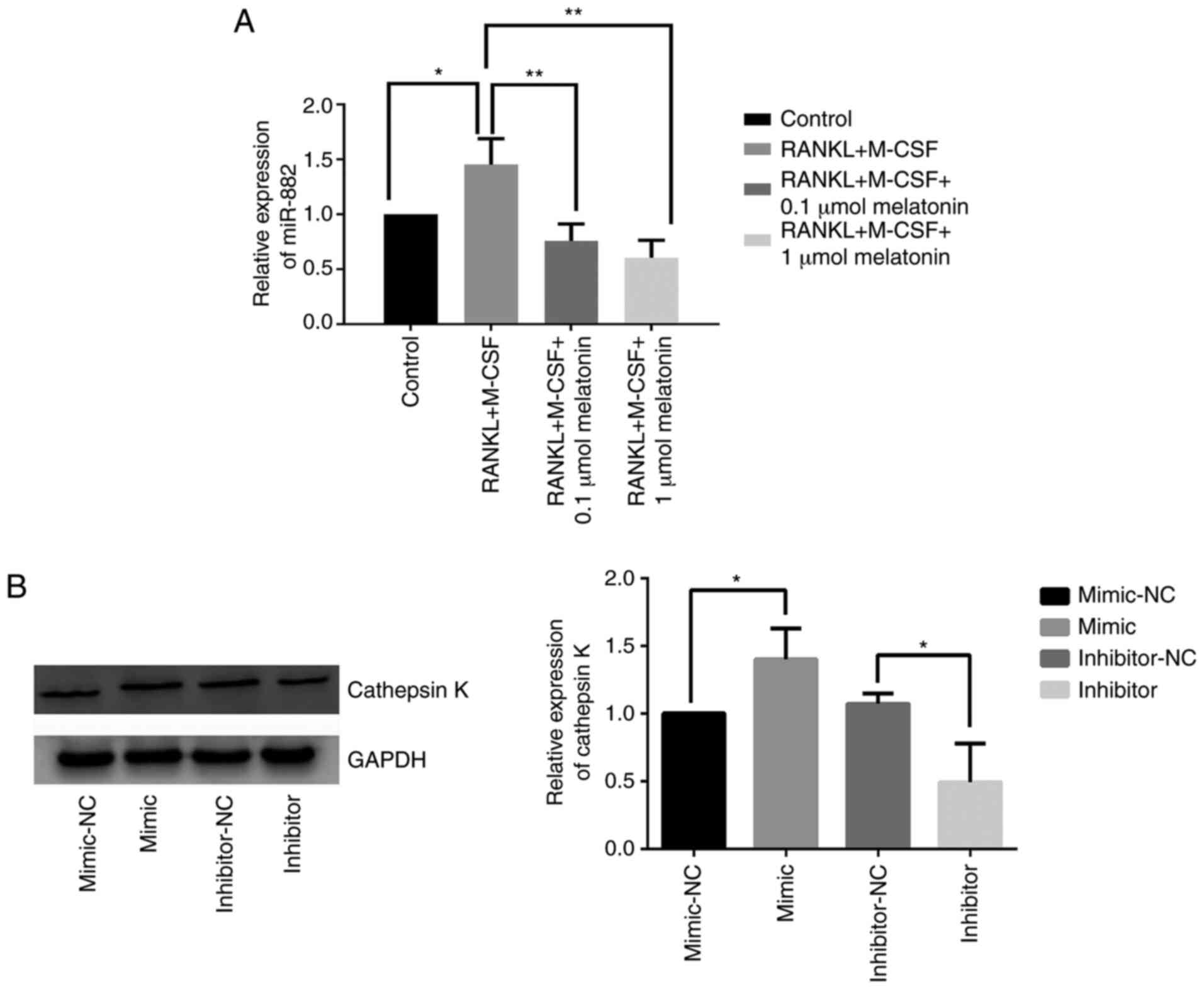
Melatonin downregulates miR-882
expression and the inhibition of miR-882 hinders RANKL-induced
osteoclastogenesis
After demonstrating that Rev-erbα is a target gene
of miR-882, whether miR-882 could regulate osteoclastogenesis was
further explored. To determine the role of miR-882 in the
inhibition of RANKL-induced osteoclastogenesis by melatonin,
miR-882 expression was examined in Raw264.7 cells cultured with
RANKL and M-CSF in the presence of varying concentrations of
melatonin via reverse transcription-quantitative PCR. The results
indicated that melatonin augmented Rev-erbα expression by
decreasing miR-882 expression, resulting in decreased miR-882
expression compared with RANKL and M-CSF treatment (Fig. 6A). To further explore the function
of miR-882 upon melatonin treatment, miR-882 mimics, inhibitors or
corresponding NCs were transfected into Raw264.7 cells, and
cathepsin K expression was examined. The overexpression of miR-882
upregulated cathepsin K expression compared with the mimic-NC;
additionally, transfection with inhibitors decreased cathepsin K
expression compared with the inhibitor-NC (Fig. 6B). The current results indicated
that miR-882 inhibition may inhibit osteoclastogenesis to prevent
osteoporosis, whereas the overexpression of miR-882 may promote
osteoclastogenesis.
Discussion
Previous research has demonstrated that melatonin
impacts osteoclastogenesis (44-47). Melatonin results in the
concentration-dependent inhibition of osteoclastogenesis at
pharmacological concentrations (44), which is consistent with the
present findings. Notably, the inhibitory effect of melatonin may
not be associated with the melatonin receptor, as demonstrated in a
previous study (45). In an in
vitro experiment with Transwell or layered mesenchymal stem
cells and peripheral blood monocytes, melatonin inhibited
osteoclastogenesis in the layered culture, but not the Transwell
culture (46). Moreover, in
vivo, melatonin can inhibit titanium particle-induced
osteolysis (47). Thus, the
present study examined the effect of melatonin on
osteoclastogenesis, and the current data demonstrated that
melatonin inhibited RANKL-induced osteoclastogenesis by promoting
Rev-erbα expression via miR-882. The present results highlight the
potential for melatonin in the treatment of osteoporosis.
Osteoporosis is associated with clock genes, such as Rev-erbα
(48), Bmal1 (49) and cryptochrome circadian clocks 2
(50). Osteoporosis intervention
such as oral salmon calcitonin, administration of teriparatide and
pulsed electromagnetic field therapy at different time points in
one day can provide different levels of bone protection,
demonstrating the role of the circadian rhythm in the mechanisms of
osteoporosis (51). The
parathyroid hormone-responsive circadian clock serves a crucial
role in the process of mouse femur fracture healing (52).
A previous study has demonstrated that certain
proteins encoded by clock genes, such as Rev-erbα, a member of the
NR1D1, are expressed rhythmically in Raw264.7 cells (53). Rev-erbα, which is present in
abundant levels in adipose cells, macrophages and muscle cells, was
reported to govern the circadian rhythm, as well as lipid and
glucose metabolism (54).
Additionally, it serves a crucial role in inflammatory reactions
and diseases, including diabetes and atherosclerosis, by
suppressing the transcription and translation of down-stream genes
(55,56). Rev-erbα is a critical component of
the biological clock and one of the important participants in
regulating biological rhythms (57). Furthermore, other studies have
demonstrated that the abnormal expression levels of Rev-erbα are
closely associated with diseases, such as osteoporosis (48), acute myocardial infarction
(58), Alzheimer's disease
(59) and skeletal muscle
myopathies (60). SR9009, the
biological effect of which is caused by an interaction with
Rev-erbα, is a Rev-erbα agonist (61). SR9009 can enhance basal metabolism
by raising oxygen consumption, enriching mitochondrial content and
accelerating glucose and fatty acid metabolism in skeletal muscle
(62). In addition, SR9009
decreases the synthesis of lipids, cholesterol and bile acid in the
liver, and downregulates fat reserves in white adipose tissue based
on in vitro and in vivo experiments (63). SR8278 is structurally similar to
SR9009, but functionally different (64). SR8278 can promote microglia
polarization toward a phagocytic M2-like phenotype during which
purinergic receptor P2Y12R expression is upregulated
(65). Previous studies
corroborate the present finding that Rev-erbα impacts
osteoclastogenesis (48,66). For example, the Rev-erbα agonist
SR9009 inhibits osteoclastogenesis in postmenopausal mice by
upregulating fatty acid binding protein 4 (66). This demonstrates that Rev-erbα is
a crucial component in the inhibition of osteoclast
differentiation. Furthermore, it may be closely associated with the
occurrence and development of osteoporosis.
miRNAs are non-coding RNAs that contain 21-23
nucleotides and exert their influence by binding to the 3′-UTR of
target mRNAs to inhibit their translation (67). The present study identified a new
interaction between miRNAs and Rev-erbα during osteoclastogenesis
using online databases. The current results indicated that Rev-erbα
could be regulated by miRNAs binding to the 3′-UTR of Rev-erbα
mRNA. miR-882 is localized to chromosome 12 on GRCm38.p6, and, to
the best of our knowledge, an association between miR-882 and
osteoporosis has not yet been reported. The present results
indicated that miR-882 promoted osteoporosis by binding to the
Rev-erbα 3′-UTR, inhibiting Rev-erbα translation, and thereby
negatively regulating Rev-erbα expression. In the present study,
miR-882 expression was decreased upon melatonin treatment.
Additionally, the inhibition of miR-882 hampered
osteoclastogenesis, whereas the miR-882 overexpression promoted
osteoclastogenesis. Thus, the down-regulation of miR-882 may
represent a potential strategy to treat osteoporosis by stalling
osteoclastogenesis. However, further studies on the role of miR-882
in protein signalling pathways to regulate diverse biological
behaviours are required.
Calcium and vitamin D can be used to treat
osteoporosis, but they result in severe side effects (68). A prior meta-analysis reported that
the small risk of significant adverse effects, such as kidney
stones, myocardial infarction, hypercalcemia and hospitalization
with acute gastrointestinal symptoms, together with the moderate
risk of minor side effects, including constipation, probably
outweighs any benefits of calcium supplements used for the
treatment of fractures (68).
In conclusion, the present study demonstrated that
the miR-882/Rev-erbα axis may serve a vital role in osteoporosis,
suggesting that melatonin may first decrease miR-882 expression,
followed by elevating Rev-erbα expression and then lowering
cathepsin K expression, and finally inhibiting osteoclastogenesis
(Fig. 7). Thus, miR-882 and
Rev-erbα comprise a potential novel therapeutic dual-target
mechanism through which melatonin may impact osteoporosis. The role
of non-coding RNAs and circadian rhythms in the progression of
osteoporosis was explored to provide a basis for the application of
melatonin to sensitize osteoporotic cells, and potentially
patients, to drug treatment in the future.
Abbreviations:
|
AMPK
|
AMP-activated protein kinase
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
OPG
|
osteoprotegerin
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
UTR
|
untranslated region
|
|
Rev-erbα/NR1D1
|
nuclear receptor subfamily 1 group D
member 1
|
Funding
The present study was supported by the Natural
Science Foundation of Liao Ning (grant no. 2019-BS-294) and the
Construction of Clinical Medical Research Center of Orthopaedics
and Sports Rehabilitation Diseases in Liaoning Province (grant no.
2019416030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the authors on reasonable request.
Authors' contributions
YT and YZ contributed to the conception and design.
YT, ZG and RZ performed cell cultures. YT, ZG, RZ and YZ
contributed to acquisition and analysis of data, revising the
manuscript critically for important intellectual content, and
approved the manuscript for publication. YT and ZG performed the
statistical analysis and manuscript preparation. YZ obtained
funding for the study and approved the final version of the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Acknowledgments
Not applicable.
References
|
1
|
Gosch M, Kammerlander C and Nicholas JA:
Treatment of osteoporosis in older adults. Panminerva Med.
56:133–143. 2014.PubMed/NCBI
|
|
2
|
Kurra S, Fink DA and Siris ES:
Osteoporosis-associated fracture and diabetes. Endocrinol Metab
Clin North Am. 43:233–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeda SS and Lazaretti-Castro M: An
overview on the treatment of postmenopausal osteoporosis. Arq Bras
Endocrinol Metabol. 58:162–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yun H, Delzell E, Saag KG, Kilgore ML,
Morrisey MA, Muntner P, Matthews R, Guo L, Wright N, Smith W, et
al: Fractures and mortality in relation to different osteoporosis
treatments. Clin Exp Rheumatol. 33:302–309. 2015.
|
|
5
|
Hegyi L: The risk of immobility in
geriatrics. Eurorehab. 3:151–154. 2001.
|
|
6
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malluche HH, Koszewski N, Monier-Faugere
MC, Williams JP and Mawad H: Influence of the parathyroid glands on
bone metabolism. Eur J Clin Invest. 36(Suppl 2): S23–S33. 2006.
View Article : Google Scholar
|
|
8
|
Li B, Wang Y, Liu Y, Ma J and Li Y:
Altered gene expression involved in insulin signaling pathway in
type II diabetic osteoporosis rat model. Endocrine. 43:136–146.
2013. View Article : Google Scholar
|
|
9
|
Kameda Y, Takahata M, Mikuni S, Shimizu T,
Hamano H, Angata T, Hatakeyama S, Kinjo M and Iwasaki N: Siglec-15
is a potential therapeutic target for postmenopausal osteoporosis.
Bone. 71:217–226. 2015. View Article : Google Scholar
|
|
10
|
Canalis E: Wnt signalling in osteoporosis:
Mechanisms and novel therapeutic approaches. Nat Rev Endocrinol.
9:575–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manolagas SC: Wnt signaling and
osteoporosis. Maturitas. 78:233–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu J, Mao Z, He S, Zhan Y, Ning R, Liu W,
Yan B and Yang J: Icariin protects against glucocorticoid induced
osteoporosis, increases the expression of the bone enhancer DEC1
and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling
pathway. Biochem Pharmacol. 136:109–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Wang X, Chang H, Gao X, Dong C, Li
Z, Hao J, Wang J and Fan Q: Mongolian Medicine echinops prevented
postmenopausal osteoporosis and induced ER/AKT/ERK pathway in
BMSCs. Biosci Trends. 12:275–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y,
Zhang ZL, Ao J, Li B and Liu H: p38alpha MAPK regulates
proliferation and differentiation of osteoclast progenitors and
bone remodeling in an aging-dependent manner. Sci Rep. 7:459642017.
View Article : Google Scholar
|
|
15
|
Pan BL, Tong ZW, Li SD, Wu L, Liao JL,
Yang YX, Li HH, Dai YJ, Li JE and Pan L: Decreased microRNA-182-5p
helps alendronate promote osteoblast proliferation and
differentiation in osteoporosis via the Rap1/MAPK pathway. Biosci
Rep. 38:BSR201806962018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong W, Qi M, Wang Y, Feng X and Liu H:
Zoledronate and high glucose levels influence osteoclast
differentiation and bone absorption via the AMPK pathway. Biochem
Biophys Res Commun. 505:1195–1202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Pablos RM, Espinosa-Oliva AM,
Hornedo-Ortega R, Cano M and Arguelles S: Hydroxytyrosol protects
from aging process via AMPK and autophagy, a review of its effects
on cancer, metabolic syndrome, osteoporosis, immune-mediated and
neurodegenerative diseases. Pharmacol Res. 143:58–72. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchwald ZS, Yang C, Nellore S, Shashkova
EV, Davis JL, Cline A, Ko J, Novack DV, DiPaolo R and Aurora R: A
bone anabolic effect of RANKL in a murine model of osteoporosis
mediated through FoxP3+ CD8 T cells. J Bone Miner Res.
30:1508–1522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi J, Hu KS and Yang HL: Roles of TNF-α,
GSK-3β and RANKL in the occurrence and development of diabetic
osteoporosis. Int J Clin Exp Pathol. 8:11995–2004. 2015.
|
|
20
|
Guo L, Tang K, Quan Z, Zhao Z and Jiang D:
Association between seven common OPG genetic polymorphisms and
osteoporosis risk: A meta-analysis. DNA Cell Biol. 33:29–39. 2014.
View Article : Google Scholar
|
|
21
|
Lin H, Zhang G, Chen X, Wu X, Wu C, Ca H
and Hu Z: The relationship between the g.27450A>T genetic
variant of OPG gene and osteoporosis in Chinese postmenopausal
women. Int Immunopharmacol. 21:464–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brunetti G, Storlino G, Oranger A,
Colaianni G, Faienza MF, Ingravallo G, Di Comite M, Reseland JE,
Celi M, Tarantino U, et al: LIGHT/TNFSF14 regulates estrogen
deficiency-induced bone loss. J Pathol. 250:440–451. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Liang T, Zhu Y, Qiu J, Qiu X, Lian
C, Gao B, Peng Y, Liang A, Zhou H, et al: Melatonin prevents bone
destruction in mice with retinoic acid-induced osteoporosis. Mol
Med. 25:432019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An J, Hao D, Zhang Q, Chen B, Zhang R,
Wang Y and Yang H: Natural products for treatment of bone erosive
diseases: The effects and mechanisms on inhibiting
osteoclastogenesis and bone resorption. Int Immunopharmacol.
36:118–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ledesma-Colunga MG, Adán N, Ortiz G,
Solís-Gutiérrez M, López-Barrera F, Martínez de la Escalera G and
Clapp C: Prolactin blocks the expression of receptor activator of
nuclear factor κB ligand and reduces osteoclastogenesis and bone
loss in murine inflammatory arthritis. Arthritis Res Ther.
19:932017. View Article : Google Scholar
|
|
27
|
Chai L, Zhou K, Wang S, Zhang H, Fan N, Li
J, Tan X, Hu L and Fan X: Psoralen and bakuchiol ameliorate M-CSF
plus RANKL-induced osteoclast differentiation and bone resorption
via inhibition of AKT and AP-1 pathways in vitro. Cell Physiol
Biochem. 48:2123–2133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W and Zhang X: Receptor activator of
nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in
bone and other tissues (review). Mol Med Rep. 11:3212–3218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI
|
|
30
|
Naidu VG, Dinesh Babu KR, Thwin MM, Satish
RL, Kumar V and Gopalakrishnakone P: RANKL targeted peptides
inhibit osteoclastogenesis and attenuate adjuvant induced arthritis
by inhibiting NF-κB activation and down regulating inflammatory
cytokines. Chem Biol Interact. 203:467–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin S, Zhang Q and Zhang L: Effect of OPG
gene mutation on protein expression and biological activity in
osteoporosis. Exp Ther Med. 14:1475–1480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai XM, Ryan GR, Hapel AJ, Dominguez MG,
Russell RG, Kapp S, Sylvestre V and Stanley ER: Targeted disruption
of the mouse CSF-1 receptor gene results in osteopetrosis,
mono-nuclear phagocyte deficiency, increased primitive progenitor
cell frequencies and reproductive defects. Blood. 99:111–120. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng C, Wentworth K and Shoback DM: New
frontiers in osteoporosis therapy. Annu Rev Med. 71:277–288. 2020.
View Article : Google Scholar
|
|
34
|
Claustrat B and Leston J: Melatonin:
Physiological effects in humans. Neurochirurgie. 61:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan DX and Reiter RJ: Mitochondria: The
birth place, battle ground and the site of melatonin metabolism in
cells. Melat Res. 2:44–66. 2019. View Article : Google Scholar
|
|
36
|
Claustrat B, Geoffriau M, Brun J and
Chazot G: Melatonin in humans: A biochemical marker of the
circadian clock and an endogenous synchronizer. Neurophysiol Clin.
25:351–359. 1995.In French. View Article : Google Scholar
|
|
37
|
Vural EM, van Munster BC and de Rooij SE:
Optimal dosages for melatonin supplementation therapy in older
adults: A systematic review of current literature. Drugs Aging.
31:441–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Faassen M, Bischoff R and Kema IP:
Relationship between plasma and salivary melatonin and cortisol
investigated by LC-MS/MS. Clin Chem Lab Med. 55:1340–1348. 2017.
View Article : Google Scholar
|
|
39
|
Claustrat B, Brun J and Chazot G: The
basic physiology and pathophysiology of melatonin. Sleep Med Rev.
9:11–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amaral FGD and Cipolla-Neto J: A brief
review about melatonin, a pineal hormone. Arch Endocrinol Metab.
62:472–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sánchez-Barceló EJ, Mediavilla MD, Tan DX
and Reiter RJ: Scientific basis for the potential use of melatonin
in bone diseases: Osteoporosis and adolescent idiopathic scoliosis.
J Osteoporos. 2010:8302312010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Jacome-Galarza CE, Percin GI, Muller JT,
Mass E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M,
et al: Developmental origin, functional maintenance and genetic
rescue of osteoclasts. Nature. 568:541–545. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou L, Chen X, Yan J, Li M, Liu T, Zhu C,
Pan G, Guo Q, Yang H, Pei M and He F: Melatonin at pharmacological
concentrations suppresses osteoclastogenesis via the attenuation of
intracellular ROS. Osteoporos Int. 28:3325–3337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim HJ, Kim HJ, Bae MK and Kim YD:
Suppression of osteoclastogenesis by melatonin: A melatonin
receptor-independent action. Int J Mol Sci. 18:11422017. View Article : Google Scholar :
|
|
46
|
Sifat M, Samsonraj RM, Munmun F, Glas J,
Silvestros M, Kotlarczyk MP, Rylands R, Dudakovic A, van Wijnen AJ,
Enderby LT, et al: Biological effects of melatonin on
osteoblast/osteoclast cocultures, bone, and quality of life:
Implications of a role for MT2 melatonin receptors, MEK1/2, and
MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res.
64:2018.
|
|
47
|
Ping Z, Wang Z, Shi J, Wang L, Guo X, Zhou
W, Hu X, Wu X, Liu Y, Zhang W, et al: Inhibitory effects of
melatonin on titanium particle-induced inflammatory bone resorption
and osteoclastogenesis via suppression of NF-κB signaling. Acta
Biomater. 62:362–371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song C, Tan P, Zhang Z, Wu W, Dong Y, Zhao
L, Liu H, Guan H and Li F: REV-ERB agonism suppresses
osteoclastogenesis and prevents ovariectomy-induced bone loss
partially via FABP4 upregulation. FASEB J. 32:3215–3228. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou X, Yu R, Long Y, Zhao J, Yu S, Tang Q
and Chen L: BMAL1 deficiency promotes skeletal mandibular
hypoplasia via OPG downregulation. Cell Prolif. 51:e124702018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang Z, Xu T, Li Y, Fei W, Yang G and Hong
Y: Inhibition of CRY2 by STAT3/miRNA-7-5p promotes osteoblast
differentiation through upregulation of CLOCK/BMAL1/P300
expression. Mol Ther Nucleic Acids. 19:865–876. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song C, Wang J, Kim B, Lu C, Zhang Z, Liu
H, Kang H, Sun Y, Guan H, Fang Z and Li F: Insights into the role
of circadian rhythms in bone metabolism: A promising intervention
target? BioMed Res Int. 2018:91564782018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kunimoto T, Okubo N, Minami Y, Fujiwara H,
Hosokawa T, Asada M, Oda R, Kubo T and Yagita K: A PTH-responsive
circadian clock operates in ex vivo mouse femur fracture healing
site. Sci Rep. 6:224092016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gibbs JE, Blaikley J, Beesley S, Matthews
L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW and
Loudon AS: The nuclear receptor REV-ERBα mediates circadian
regulation of innate immunity through selective regulation of
inflammatory cytokines. Proc Natl Acad Sci USA. 109:582–587. 2012.
View Article : Google Scholar
|
|
54
|
Vieira E, Merino B and Quesada I: Role of
the clock gene Rev-erbalpha in metabolism and in the endocrine
pancreas. Diabetes Obes Metab. 17(Suppl 1): S106–S114. 2015.
View Article : Google Scholar
|
|
55
|
Lam MT, Cho H, Lesch HP, Gosselin D, Heinz
S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, et al:
Rev-Erbs repress macrophage gene expression by inhibiting
enhancer-directed transcription. Nature. 498:511–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sato S, Sakurai T, Ogasawara J, Takahashi
M, Izawa T, Imaizumi K, Taniguchi N, Ohno H and Kizaki T: A
circadian clock gene, Rev-erbα, modulates the inflammatory function
of macrophages through the negative regulation of Ccl2 expression.
J Immunol. 192:407–417. 2014. View Article : Google Scholar
|
|
57
|
Mazzoccoli G, Cai Y, Liu S, Francavilla M,
Giuliani F, Piepoli A, Pazienza V, Vinciguerra M, Tamamoto T and
Takumi T: REV-ERBalpha and the clock gene machinery in mouse
peripheral tissues: A possible role as a synchronizing hinge. J
Biol Regul Homeost Agents. 26:265–276. 2012.PubMed/NCBI
|
|
58
|
Wang S, Gu X, Zhang Q, Zhang X, Li Y, Yao
Y, Yu B and Zhang Y: Angiotensin II suppresses Rev-erbα expression
in THP-1 macrophages via the Ang II type 1 receptor/liver X
receptor α pathway. Cell Physiol Biochem. 46:303–313. 2018.
View Article : Google Scholar
|
|
59
|
Roby DA, Ruiz F, Kermath BA, Voorhees JR,
Niehoff M, Zhang J, Morley JE, Musiek ES, Farr SA and Burris TP:
Pharmacological activation of the nuclear receptor REV-ERB reverses
cognitive deficits and reduces amyloid-β burden in a mouse model of
Alzheimer's disease. PloS One. 14:e02150042019. View Article : Google Scholar
|
|
60
|
Welch RD and Flaveny CA: REV-ERB and ROR:
Therapeutic targets for treating myopathies. Phys Biol.
14:0450022017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Solt LA, Wang Y, Banerjee S, Hughes T,
Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, et al:
Regulation of circadian behavior and metabolism by synthetic
REV-ERB agonists. Nature. 485:62–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Thevis M and Schänzer W: Emerging drugs
affecting skeletal muscle function and mitochondrial biogenesis -
Potential implications for sports drug testing programs. Rapid
Commun Mass Spectrom. 30:635–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mazzarino M, Rizzato N, Stacchini C, de la
Torre X and Botrè F: A further insight into the metabolic profile
of the nuclear receptor Rev-erb agonist, SR9009. Drug Test Anal.
10:1670–1681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kojetin D, Wang Y, Kamenecka TM and Burris
TP: Identification of SR8278, a synthetic antagonist of the nuclear
heme receptor REV-ERB. ACS Chem Biol. 6:131–134. 2011. View Article : Google Scholar :
|
|
65
|
Lee J, Kim DE, Griffin P, Sheehan PW, Kim
DH, Musiek ES and Yoon SY: Inhibition of REV-ERBs stimulates
microglial amyloid-beta clearance and reduces amyloid plaque
deposition in the 5XFAD mouse model of Alzheimer's disease. Aging
Cell. 19:e130782020. View Article : Google Scholar
|
|
66
|
Kim K, Kim JH, Kim I, Seong S and Kim N:
Rev-erbα Negatively Regulates Osteoclast and Osteoblast
Differentiation through p38 MAPK Signaling Pathway. Mol Cells.
43:34–47. 2020.PubMed/NCBI
|
|
67
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bolland MJ, Grey A and Reid IR: Should we
prescribe calcium or vitamin D supplements to treat or prevent
osteoporosis. Climacteric. 18(Suppl 2): S22–S31. 2015. View Article : Google Scholar
|