Introduction
Neuropathic pain is a chronic type of pain caused by
a primary lesion or dysfunction in both the central and peripheral
nervous system (1). The causes of
neuropathic pain are diverse, including alcoholism, amputation or
spinal surgery (2). Nowadays, a
variety of clinical treatment methods are tested, such as Aleve or
Motrin, but serious side effects limit the scope and effect of
treatment (3). Therefore, it is
necessary to discover a novel and effective medical treatment for
this condition.
MicroRNAs (miRs/miRNAs) are small non-coding RNAs
that are 21-25 nucleotides in length (4). miRNAs play a post-transcriptional
regulatory role by binding to the 3′-untranslated region (UTR)
region or accidental 5′-UTR region of the target gene (5). Jiangpan et aldemonstrated
that the dysfunction of miRNAs is associated with various types of
pathological conditions, including neuropathic pain (4). miR-142-3p is a highly conserved
miRNA across vertebrates, which plays an essential role in the
development and pathophysiology of the nervous system (5). Wang et al revealed that
miR-142-3p expression was increased following sciatic nerve
conditioning injury and is a target of adenylate cyclase 9 (AC9),
and intracellular AC9 induced the upregulation of cAMP (6). However, Ouyang et al found
that miR-142-3p was downregulated, instead of upregulated, in the
spinal cord following chronic compression injury (CCI) (7). Therefore, the role of miR-142-3p in
rats with sciatic nerve injury needs to be further clarified.
A previous study demonstrated that miR-142-3p is a
novel regulator of inflammation that regulates proinflammatory
mediators, including NF-κB, TNF-ɑ and IL-1β (8). Numerous studies have revealed that
neuroinflammatory cytokines are involved in the maintenance and
occurrence of neuropathic pain (9-11).
Meanwhile, these inflammatory cytokines also accelerate the system
of neuropathic pain, eventually leading to central and peripheral
sensitization (10). The sciatic
nerve tissue plays important roles in pain modulation and
perception (11). Pain
hypersensitivity in the sciatic nerve may result from neuronal
sensitization and glial activation (11). It is therefore important to study
the relationship between miR-142-3p and inflammatory cytokines in
rats with sciatic nerve injury.
AMP-activated protein kinase (AMPK) is an essential
sensor of cellular energy status and plays an important role in
cellular energy balance (12).
AMPK has been implicated in various diseases associated with energy
metabolism, including neuropathic pain (13). In addition to the regulation of
cellular energy metabolism, AMPK may mediate the pain response in
preclinical pain models, and targeting AMPK is considered to be a
novel strategy for the prevention and treatment of pain (14).
Based on previous studies, the present study
hypothesized that miR-142-3p targets AC9 to regulate sciatic nerve
injury-induced neuropathic pain via regulating the cAMP/AMPK
signalling pathway.
Materials and methods
Animals
A total of 77 healthy male Sprague-Dawley rats,
weighing 200-220 g [permission no. SCXK (Beijing) 20160006], aged 8
weeks, were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. The rats were fed freely in a clean and quiet
specific pathogen-free laboratory at 23±2°C, 55±5% humidity and a
12-h light/dark cycle. The animal study protocol was in line with
the National Institutes of Health (pub. no. 85-23, revised 1996).
The study was approved by the Institutional Animal Care and Use
Committee of Yantaishan Hospital (approval no. 20180705-0012).
Cell culture
293T cells (cat. no. BNCC290119; BeNa Culture
Collection) were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA), 100
U/ml penicillin and 100 mg/ml streptomycin. All cells were
incubated at 37°C with 5% CO2. Cells in the logarithmic
growth phase were collected and used for experiments.
Sciatic nerve injury model
A rat model was established using the CCI model
method (15). Briefly, following
the administration of 3% sodium pentobarbital (50 mg/kg) via
intraperitoneal injection, the sciatic nerve of the right hind limb
was exposed. Next, the sciatic nerve was ligated with a 4-0 catgut
to tie four consecutive channels. The distance between each two
channels was 1.0 mm. The ligation intensity was appropriate when
the nerve was slightly depressed, but the blood supply of the
sciatic nerve was not blocked when observed under a microscope.
Following surgery, the muscles and skin were sutured layer by layer
and disinfected with iodophor, and the rats were fed in single
cages.
Grouping and administration
In order to analyse the expression of miR-142-3p,
AC9 and cAMP in the sciatic nerve of CCI rats, the rats were
divided into two groups, with six rats per group: i) The sham group
(Sham), in which the sciatic nerve of the right hind limb of the
rats was exposed without ligation, the incisions were sutured layer
by layer and ii) the CCI model group (CCI), in which the CCI model
was established. In each group, three rats were sacrificed.
The intrathecal implantation was performed by
inserting a PE-10 polyethylene catheter into the cisterna magna.
For each intrathecal administration, miR-142-3p mimic (5′-UGU AGU
GUU UCC UAC UUU AUG GA-3′), mimic negative control (NC; 5′-CAG UAC
UUU UGU GUA GUA CAA-3′), miR-142-3p small interfering RNA (siRNA;
5′-UCC AUA AAG UAG GAA ACA CUA CA-3′), siRNA control (5′-CAG UAC
UUU UGU GUA GUA CAA-3′), expression plasmid AC9 (5′-UUC UUU UCU UAA
AAA UGU GAU GG-3′), plasmid control (5′-CAG UAC UUU UGU GUA GUA
CAA-3′), AC9 siRNA (5′-AAG GAG ATG GTG AAC ATG AGA-3′) and AC9
siRNA control (5′-CAG UAC UUU UGU G UA GUA CAA-3′) (all from
Shanghai GenePharma Co., Ltd.) was injected into the intrathecal
catheter using a microinjection syringe.
To determine the role of miR-142-3p in sciatic nerve
injury of CCI rats, the rats were divided into six groups, with
five rats per group; i) The sham group (Sham), in which an
intrathecal injection of 10 µl normal saline was
administered daily for 3 consecutive days; ii) the CCI model group
(CCI), in which an intrathecal injection of 10 µl normal
saline was administered daily for 3 consecutive days; iii) the
miR-142-3p mimic treatment group (miR-142-3p mimic), in which the
CCI model was established and then the rats were intrathecally
injected with 10 µl 10 µM miR-142-3p mimic (16) every day for 3 consecutive days;
iv) the miR-142-3p mimic NC group (mimic-NC), in which the CCI
model was established and then the rats were intrathecally injected
with 10 µl 10 µM mimic NC daily for 3 consecutive
days; v) the miR-142-3p siRNA treatment group (miR-142-3p siRNA),
in which the CCI model was established and then the rats were
intrathecally injected with 10 µl 10 µM miR-142-3p
siRNA every day for 3 consecutive days and vi) the miR-142-3p siRNA
NC group (siRNA-NC), in which the CCI model was established and
then the rats were intrathecally injected with 10 µl 10
µM siRNA NC daily for 3 consecutive days.
To investigate the role of AC9 in sciatic nerve
injury in CCI rats, the rats were divided into four groups, with
five rats per group: i) The expression plasmid AC9 treatment group
(pcDNA-AC9), in which the CCI model was established and then the
rats were intrathecally injected with 3 µl 0.005
mg/µl pcDNA-AC9 (16)
every day for 3 consecutive days; ii) the plasmid control group
(pc-NC), in which the CCI model was established and then the rats
were intrathecally injected with 3 µl 0.005 mg/µl
plasmid control vector daily for 3 consecutive days; iii) the AC9
siRNA treatment group (AC9 siRNA), in which the CCI model was
established and then the rats were intrathecally injected with 3
µl 0.005 mg/µl AC9 siRNA every day for 3 consecutive
days and iv) the AC9 siRNA control group (si-NC), in which the CCI
model was established and then the rats were intrathecally injected
with 3 µl 0.005 mg/µl of siRNA NC daily, for 3
consecutive days.
To reveal the effects of the interaction between AC9
and miR-142-3p on sciatic nerve injury-induced neuropathic pain,
the rats were further divided into three groups, with five rats per
group: i) The AC9 siRNA and miR-142-3p siRNA treatment group
(miR-142-3p siRNA + AC9 siRNA), in which the CCI model was
established and then the rats were intrathecally injected with 3
µl 0.005 mg/µl AC9 siRNA and 10 µl 10
µM miR-142-3p siRNA every day for 3 consecutive days; ii)
the cAMP activator group (Forskolin), in which the CCI model was
established and then the rats were intrathecally injected with 3
µM forskolin (17) (cat.
no. S1612-5 mg; Beyotime Institute of Biotechnology) daily for 3
consecutive days; iii) the miR-142-3p siRNA and cAMP inhibitor
treatment group (miR-142-3p siRNA + SQ22536), in which the CCI
model was established and then the rats were intrathecally injected
with 10 µl 10 µM miR-142-3p siRNA and 500 µM
SQ22536 (17) (MB3735; Dalian
Meilun Biology Technology Co., Ltd.) every day for 3 consecutive
days.
Measurement of paw withdrawal mechanical
threshold (PWMT) and paw withdrawal thermal latency (PWTL) in
rats
PWMT and PWTL values were measured once a day before
surgery, which constituted the preoperative values. The two
indicators were tested again on days 4, 7, 10, 14 and 21 following
surgery, according to Hargreaves et al (18) and Shao et al (19). Briefly, rats were individually
placed in a 1-mm thick plastic chamber (7×9×11 cm3) with
a smooth glass surface on the base for PWTL measurement. The rats
underwent a familiarization period 45 min before the behavioural
tests. To avoid tissue damage, an automatic 20 sec cut-off value
was set. The hind paw of each right posterior limb was repeated
three times with a 5-min interval. Mechanical allodynia was
determined by foot withdrawal with Von Frey filaments (North Coast
Medical Inc.). Briefly, rats were laid individually on a wire mesh
floor in a 20×25×15-cm3 plastic box with a 45-min
adoption before the text. The filaments were perpendicularly placed
to the plantar surface of the hind paws with increasing forces
until the maximum stimulus (4.0 g) or 10 positive responses. The
mechanical allodynia was determined as the force of a minimum
detectable withdrawal on 50% of the tests at the same force
level.
Sample collection
On the 10th day following surgery, the rats were
injected intraperitoneally with 3% pentobarbital sodium (50 mg/kg)
and then perfused with 200 ml old saline. The L4-6 sciatic nerve on
the right side of the rats was removed. Some parts were fixed with
4% paraformaldehyde at room temperature for 24 h for
immunohistochemistry, while others were stored in liquid nitrogen
for western blotting and reverse transcription-quantitative PCR
(RT-qPCR) analysis.
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was then synthesized using TaqMan reverse transcription
kits (Thermo Fisher Scientific, Inc.). RT-PCR was performed using
Mastercycler® Nexus X2 (Eppendorf) with the SYBR Premix
Ex Taq II kit (cat. no. RR820A; Takara Bio, Inc.). The conditions
were 95°C for 15 sec, 60°C for 60 sec, and 72°C for 40 sec (35
cycles). Data were processed by the 2−ΔΔCq method
(20).
The primer sequences were designed as follows:
miR-142-3p forward, 5′-CTC CTG TAG TGT TTC CTA C-3′ and reverse,
5′-GAC TGT TCC TCT CTT CCT C-3′; U6 forward, 5′-TCG CTT CGG CAG CAC
ATA-3′ and reverse, 5′-TTT GCG TGT CAT CCT TGC-3′; AC9 forward,
5′-AAC AGC ACC AAG GCT TCT GGA GGA C-3′ and reverse, 5′-TCT TGA ACC
TCA GCG GAA GGA GAG C-3′ and GAPDH forward, 5′-CAT CAC TGC CAC CCA
GAA GAC TG-3′ and reverse, 5′-ATG CCA GTG AGC TTC CCG TTC
AG-3′.
ELISA
Sciatic nerve samples were prepared using 0.1N HCl
and centrifuged at 10,000 × g at 4°C for 20 min to eliminate
impurities. The acquired supernatant was neutralized with 1 N NaOH.
cAMP ELISA kit (cat. no. BP-E30574-2; R&D Systems, Inc.) was
used to detect the levels of cAMP in the supernatant according to
the manufacturer's instructions.
The sciatic nerve samples were treated with lysis
buffer (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) and benzylsulfonyl fluoride, incubated at 4°C for 10
min, then centrifuged at 10,000 × g at 4°C for 15 min to collect
the supernatant. The levels of pro-inflammatory cytokines TNF-α
(cat. no. RAT00) and IL-1β (cat. no. RLB00) were measured by ELISA
kits (R&D Systems, Inc.) according to the manufacturer's
instructions.
Immunohistochemistry
Following routine sectioning, specimens embedded in
paraffin were baked at 65°C for 100 min, dewaxed with xylene and
hydrated with a serial ethanol solution. The solution was
inactivated by adding 3% H2O2 methanol
solution for 20 min at room temperature, heat-fixed with high
temperature antigen in citrate buffer (pH 6.0) for 10 min and
blocked with 5% BSA (cat. no. SW3015; Beijing Solarbio Science
& Technology Co., Ltd.) for 20 min at room temperature. Rabbit
anti-rat IL-1β (1:1,000; cat. no. ab9722; Abcam) polyclonal
antibody and TNF-ɑ (1:1,000; cat. no. ab66579; Abcam) polyclonal
antibody were added and incubated over-night at 4°C. Washing with
phosphate buffer saline (PBS) at room temperature, 5 min, 3 times,
the samples were incubated with goat anti-rabbit HRP-conjugated
secondary antibody (1:1,000; cat. no. ABIN101988; antibodies-online
GmbH). Slides were stained with diaminobenzidine (Beyotime
Institute of Biotechnology) and re-dyed with haematoxylin. After
dehydrating with gradient ethanol solution, the slices were sealed
with neutral gum. Results were observed at ×400 magnification under
an upright microscope (CX43; Olympus Corporation) and analysed by
ImageJ 5.0 software (National Institutes of Health). Cells positive
for IL-1β and TNF-ɑ expression were stained brown. Positive cells
(%) = numbers of positive cells/numbers of total cells ×100%.
Western blotting
Total protein from sciatic nerve tissues were
extracted using RIPA buffer (Beyotime Institute of Biotechnology)
and then quantified using a Pierce™ BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). A total of 40 µg protein/lane was
separated via 10% SDS-PAGE (Mini-Protean-3; Bio-Rad Laboratories,
Inc.) and transferred to a polyvinylidene difluoride membrane (EMD
Millipore). The membrane was blocked with 5% skimmed milk powder
solution for 1 h at room temperature and then incubated with the
following primary antibodies at 4°C overnight: Anti-rabbit
phosphorylated (p)-AMPKɑ (1:1,000; cat. no. 4184; Cell Signaling
Technology, Inc.), AMPKɑ (1:1,000; cat. no. 2532; Cell Signaling
Technology, Inc.), p-NF-κB p65 (1:1,000; cat. no. 3033, Cell
Signaling Technology, Inc.), NF-κB p65 (1:1,000, cat. no. 4746;
Cell Signaling Technology, Inc.), p-IκBɑ (1:1,000; cat. no.
vk10582; Guangzhou Weiboxin Biotechnology Co., Ltd.), IκBɑ
(1:1,000; cat. no. A2457; ABclonal Biotech Co., Ltd.), AC9
(1:1,000; cat. no. ab191423; Abcam) and GAPDH (1:1,000; cat. no.
AC033; ABclonal Biotech Co., Ltd.). The membranes were then
incubated with goat anti-rabbit immunoglobulin G secondary antibody
(1:1,000; cat. no. ABIN101988; antibodies-online GmbH) at room
temperature for 2 h and treated with enhanced chemiluminescence
solution (Thermo Fisher Scientific, Inc.). GAPDH was used as the
internal reference and the integrated density values of protein
bands were quantitatively analysed by ImageJ 5.0 software (National
Institutes of Health).
Target prediction for miR-142-3p
Potential target genes of miR-142-3p were predicted
by TargetScan (targetscan.org/), which is a
web-based resource used for the prediction of biological targets of
miRNAs by searching for the presence of 8-, 7- and 6-mer sites that
match the seed region of each miRNA.
Dual-luciferase reporter assay
The 3′-UTR regions of AC9 containing the predicted
miR-142-3p specific binding sites were amplified by RT-PCR and
cloned into the pmirGLO firefly luciferase reporter vector (Promega
Corporation) to obtain the wild-type luciferase reporter plasmids
(wt-AC9). In order to generate mutant reporter plasmids (mut-AC9),
certain nucleotides in AC9 3′-UTR lacking miR-142-3p binding sites
were mutated using RT-PCR. The constructed luciferase reporter
plasmids were separately co-transfected with 50 nM miR-142-3p mimic
or mimic NC into 293T cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were lysed
using lysis buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.) for 15 min at room temperature and luciferase
activities of gene plasmid and phRL-TK were assayed at 48 h
post-transfection using a Dual-Luciferase Reporter Assay system
(Promega Corporation) according to the manufacturer's
instructions.
Statistical analysis
All data were analysed by SPSS 19.0 software (IBM
Corp.) and presented as the mean ± SD. Comparisons between groups
were conducted by t-test. In addition, multiple comparisons were
performed using one-way ANOVA and Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of miR-142-3p, AC9 and cAMP
following sciatic nerve injury
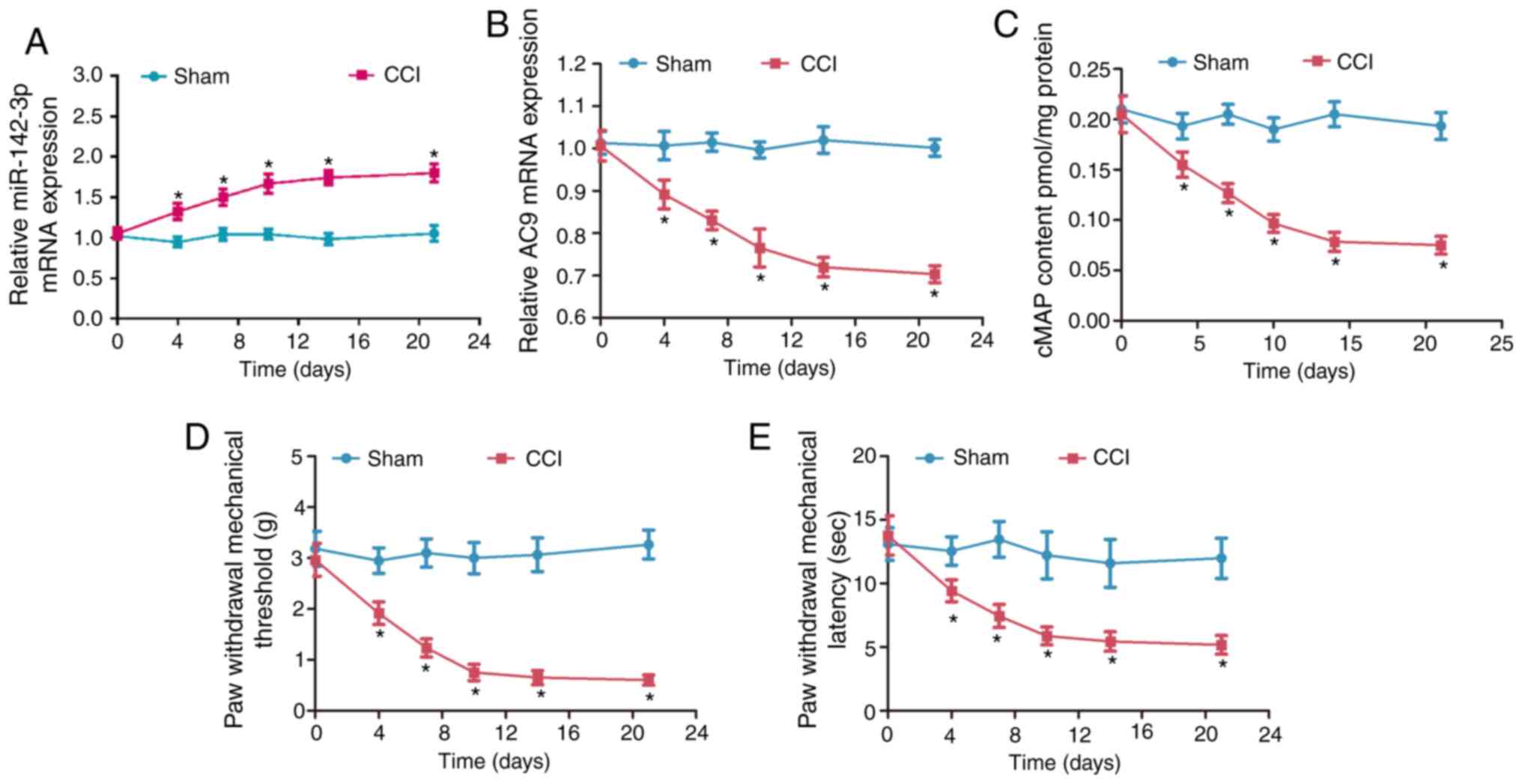
To characterize the potential role of mir-142-3p in
neuropathic pain, the expression of miR-142-3p (Fig. 1A) and AC9 (Fig. 1B) were detected by RT-qPCR.
Compared with the sham group, miR-142-3p was significantly
upregulated and AC9 mRNA was significantly downregulated in the CCI
group (P<0.05). cAMP levels significantly decreased in the CCI
group compared with the sham group (P<0.05; Fig. 1C). PWMT and PWTL values were
significantly decreased in the CCI group compared with the sham
group (P<0.05; Fig. 1D and E).
These data showed that the miR-142-3p expression was significantly
increased, but the AC9 and cAMP expression was clearly decreased in
the sciatic nerve of CCI rats.
Effects of miR-142-3p on sciatic pain and
the AMPK pathway
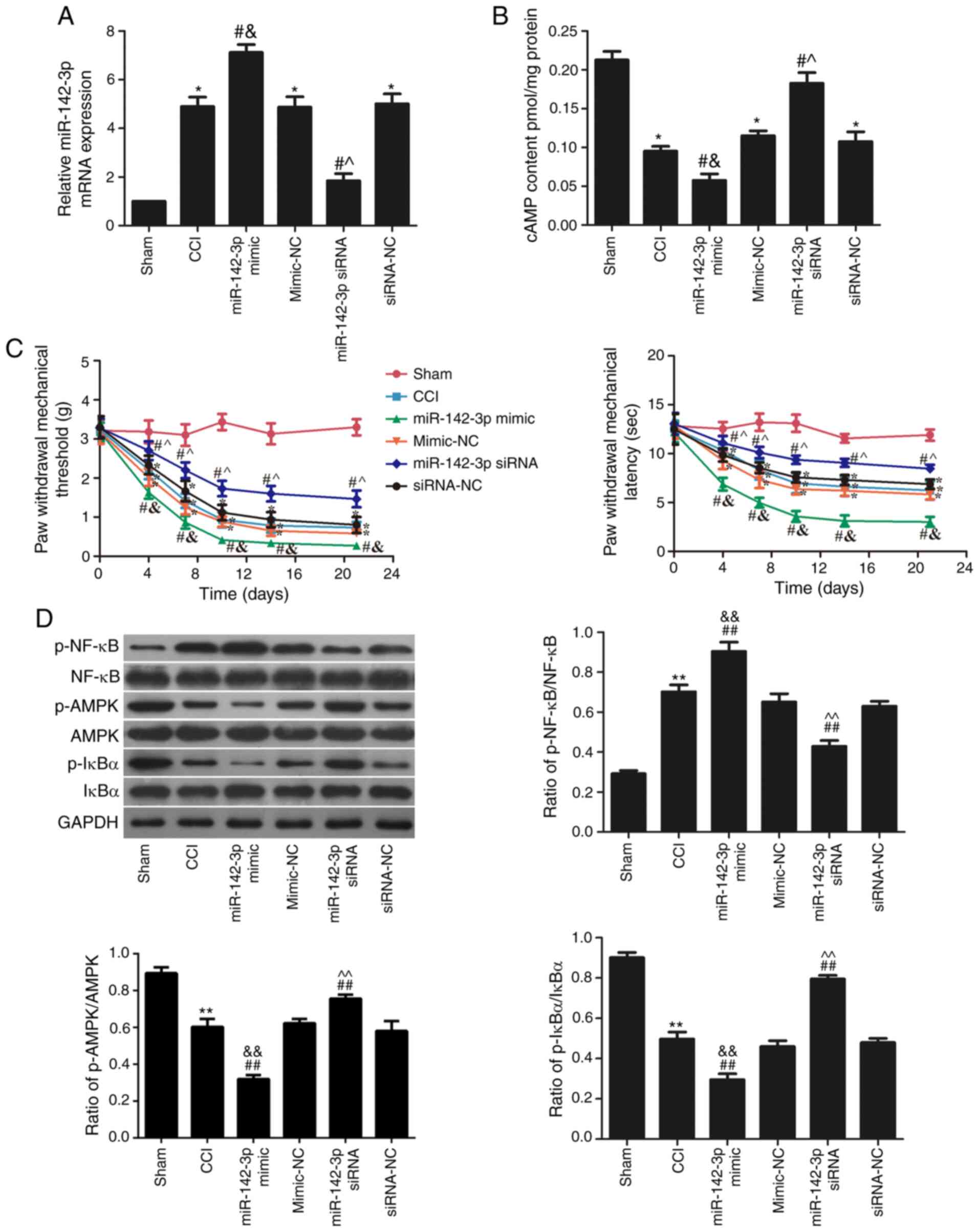
To investigate the association between miR-142-3p
and neuropathic pain, miR-142-3p mimic and siRNA were injected
intrathecally, and the expression of miR-142-3p in each group is
shown in Fig. 2A. miR-142-3p
expression was significantly increased in the miR-142-3p mimic
group and significantly decreased in the miR-142-3p siRNA group
when compared with their respective NC groups (P<0.05). In
addition, the cAMP content was significantly reduced in the
miR-142-3p mimic group and increased in the miR-142-3p siRNA group
compared with their respective NC groups (P<0.05; Fig. 2B).
Meanwhile, the PWMT and PWTL values were used to
study the role of the miR-142-3p expression in sciatica pain. The
results indicated that miR-142-3p inhibition effectively increased
the PWMT and PWTL values following surgery compared with the
siRNA-NC group (P<0.05; Fig.
2C). However, miR-142-3p overexpression reduced the PWMT and
PWTL values following surgery compared with the mimic-NC group
(P<0.05; Fig. 2C). These
results suggested that miR-142-3p downregulation suppressed sciatic
pain in CCI rats.
The levels of p-AMPK/AMPK, p-NF-κB/NF-κB and
p-IκBɑ/IκBɑ in the sciatic nerve were detected using western
blotting (Fig. 2D). Compared with
the sham group, the phosphorylation levels of AMPK and IκBɑ were
significantly reduced (P<0.05), while the phosphorylation levels
of NF-κB was significantly increased (P<0.05) in the CCI group.
Following miR-142-3p mimic transfection, compared with the CCI
group, the phosphorylation levels of AMPK and IκBɑ were
significantly reduced (P<0.05), while the phosphorylation levels
of NF-κB was further increased (P<0.05). However, transfection
of miR-142-3p siRNA significantly elevated the phosphorylation
levels of AMPK and IκBɑ and inhibited the phosphorylation levels of
NF-κB compared with the CCI group (P<0.05).
Effects of miR-142-3p on inflammatory
cytokine content following sciatic nerve injury
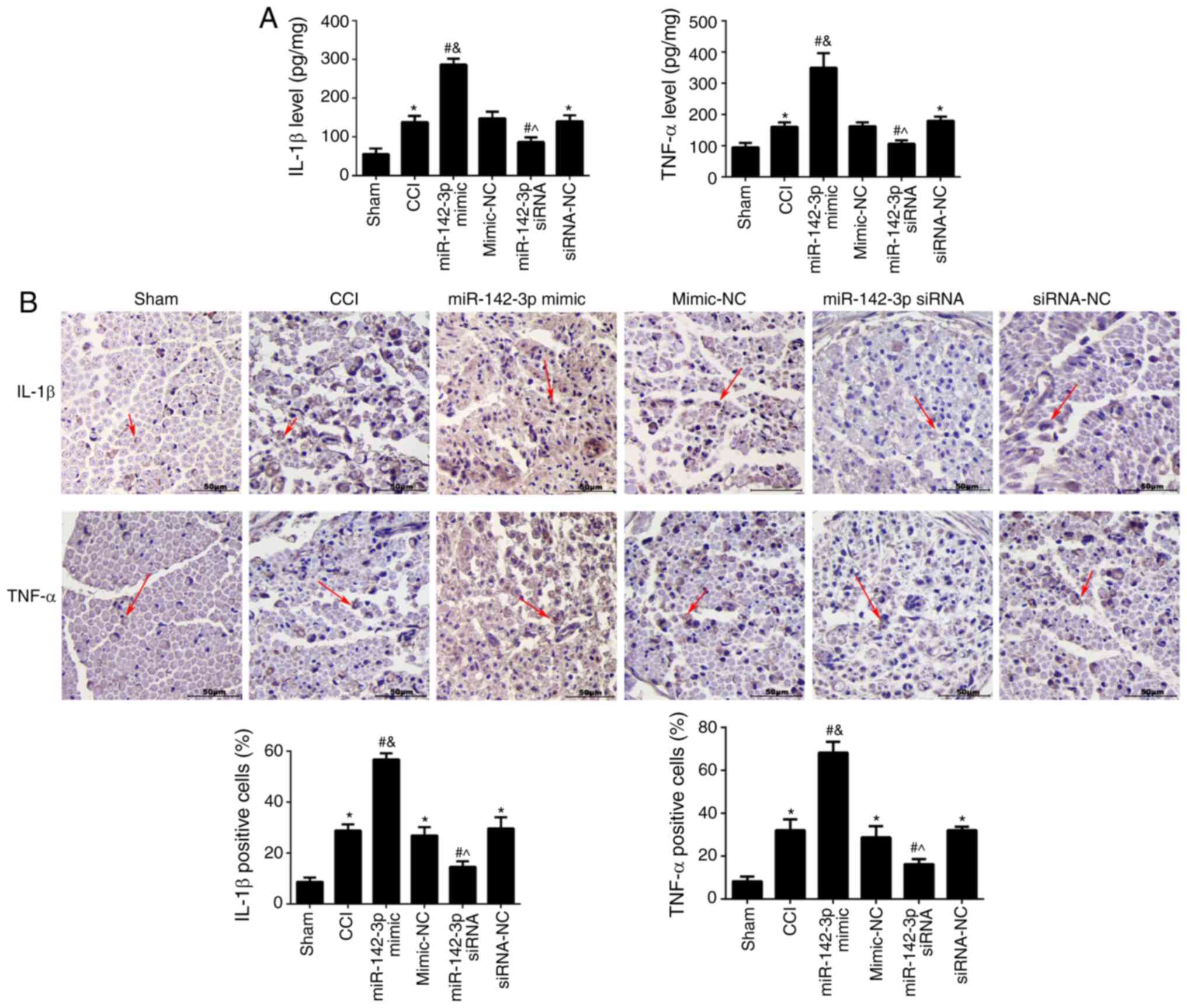
The effects of miR-142-3p on IL-1β and TNF-α in the
sciatic nerve was analysed using ELISA (Fig. 3A) and immunohistochemistry
(Fig. 3B). The results showed
that the contents of IL-1β and TNF-α in the CCI group were
significantly higher compared with the sham group (P<0.05).
Compared with the CCI group, miR-142-3p overexpression
significantly increased the levels of IL-1β and TNF-ɑ (P<0.05),
while miR-142-3p-silencing showed opposite effects (P<0.05).
These results revealed that miR-142-3p-over-expression increased
the secretion of inflammatory cytokines in rats with sciatic nerve
injury.
Expression of AC9 in sciatic pain and the
AMPK pathway
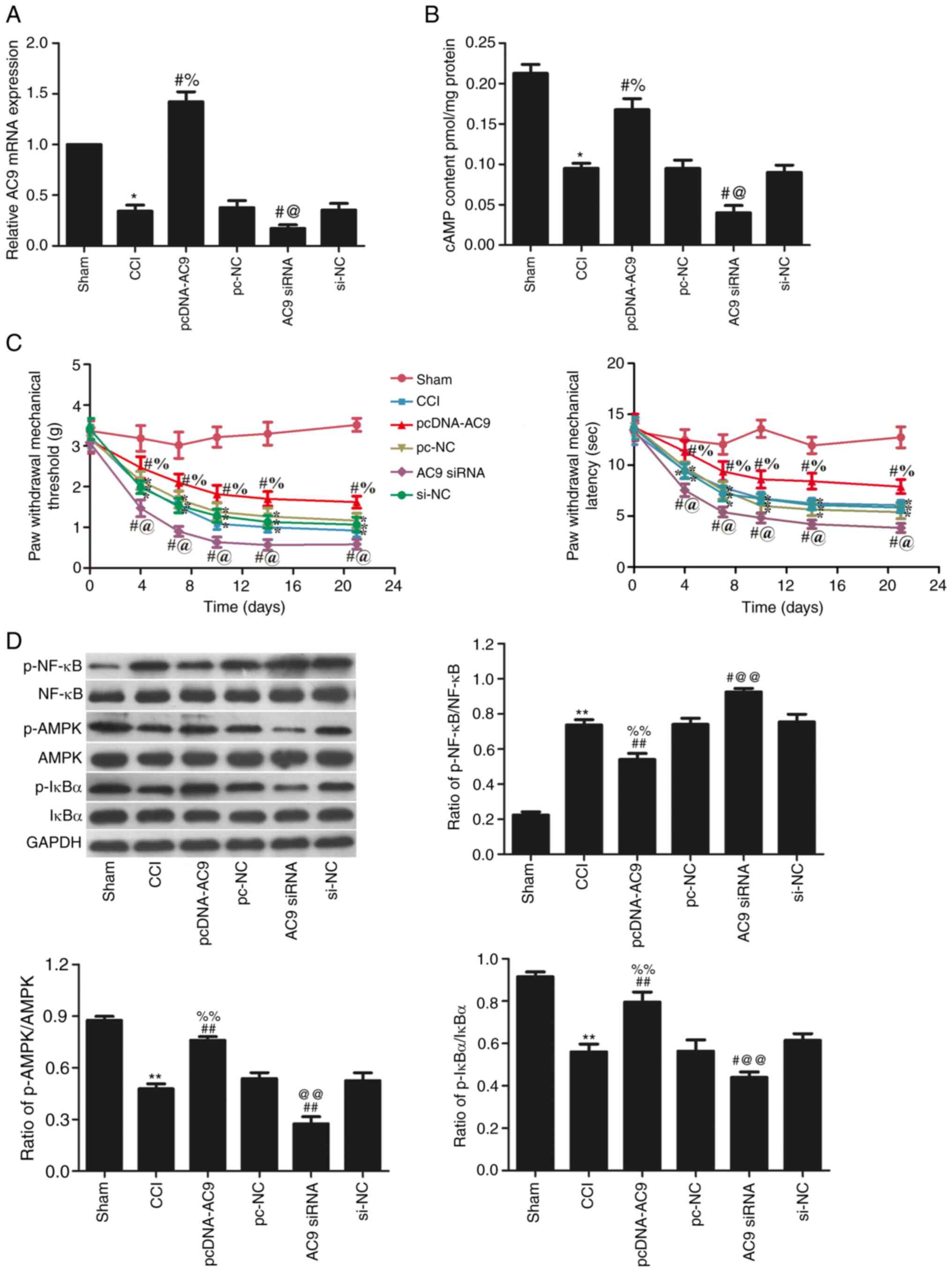
RT-qPCR was used to analyse AC9 expression in the
L4-6 sciatic nerve (Fig. 4A).
Following the injection of pcDNA-AC9, AC9 expression was
significantly increased (P<0.05), whereas injection of AC9 siRNA
significantly decreased AC9 expression (P<0.05) compared with
the CCI groups. The cAMP content was significantly decreased in the
AC9 siRNA group, but significantly increased in the pcDNA-AC9 group
compared with the CCI group (P<0.05; Fig. 4B).
The results from PWMT and PWTL analysis (Fig. 4C) indicated that AC9
overexpression effectively alleviated sciatic pain following
surgery, while AC9 silencing obviously exacerbated sciatic pain
following surgery compared with the CCI group (P<0.05). These
results suggested that AC9 down-regulation leads to neuropathic
pain in CCI rats.
The levels of p-AMPK/AMPK, p-NF-κB/NF-κB and
p-IκBɑ/IκBɑ in the sciatic nerve were detected using western
blotting (Fig. 4D). Following
pcDNA-AC9 transfection, compared with the CCI group, the
phosphorylation levels of AMPK and IκBɑ were significantly
increased (P<0.05), while the phosphorylation levels of NF-κB
was further decreased (P<0.05). However, transfection of AC9
siRNA significantly decreased the phosphorylation levels of AMPK
and IκBɑ, and increased those of NF-κB, as compared with the CCI
group (P<0.05).
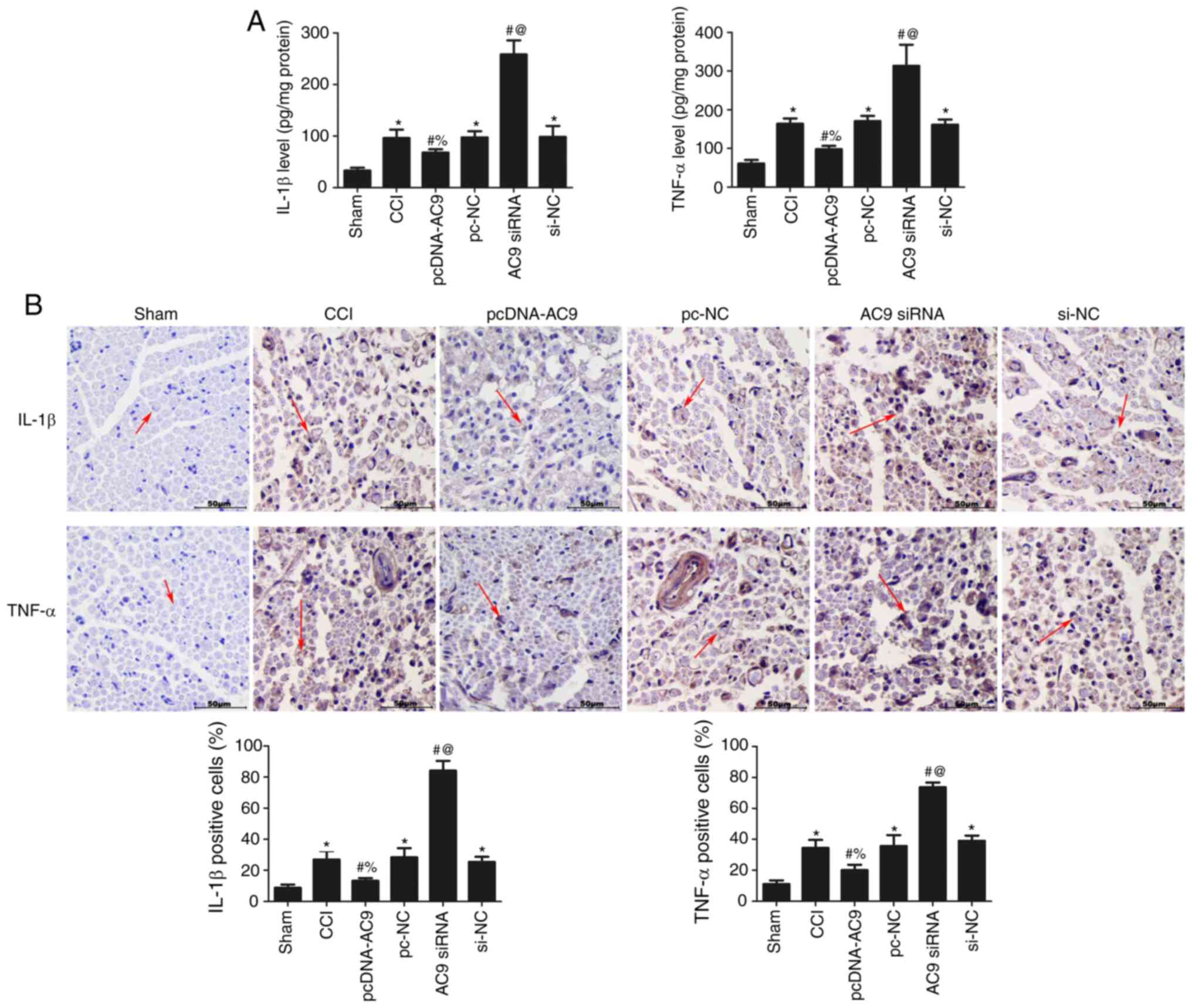
Effects of AC9 on inflammatory cytokine
content following sciatic nerve injury
The levels of IL-1β and TNF-α were measured using
ELISA (Fig. 5A) and
immunohistochemistry (Fig. 5B) to
analyse the effects of AC9 on IL-1β and TNF-α in the sciatic nerve
of each group. AC9 overexpression significantly reduced the
expression of IL-1β and TNF-ɑ (P<0.05), while AC9 silencing
significantly increased the contents of IL-1β and TNF-ɑ compared
with the CCI group (P<0.05). These results revealed that AC9
overexpression suppressed the secretion of inflammatory cytokines
in rats with sciatic nerve injury.
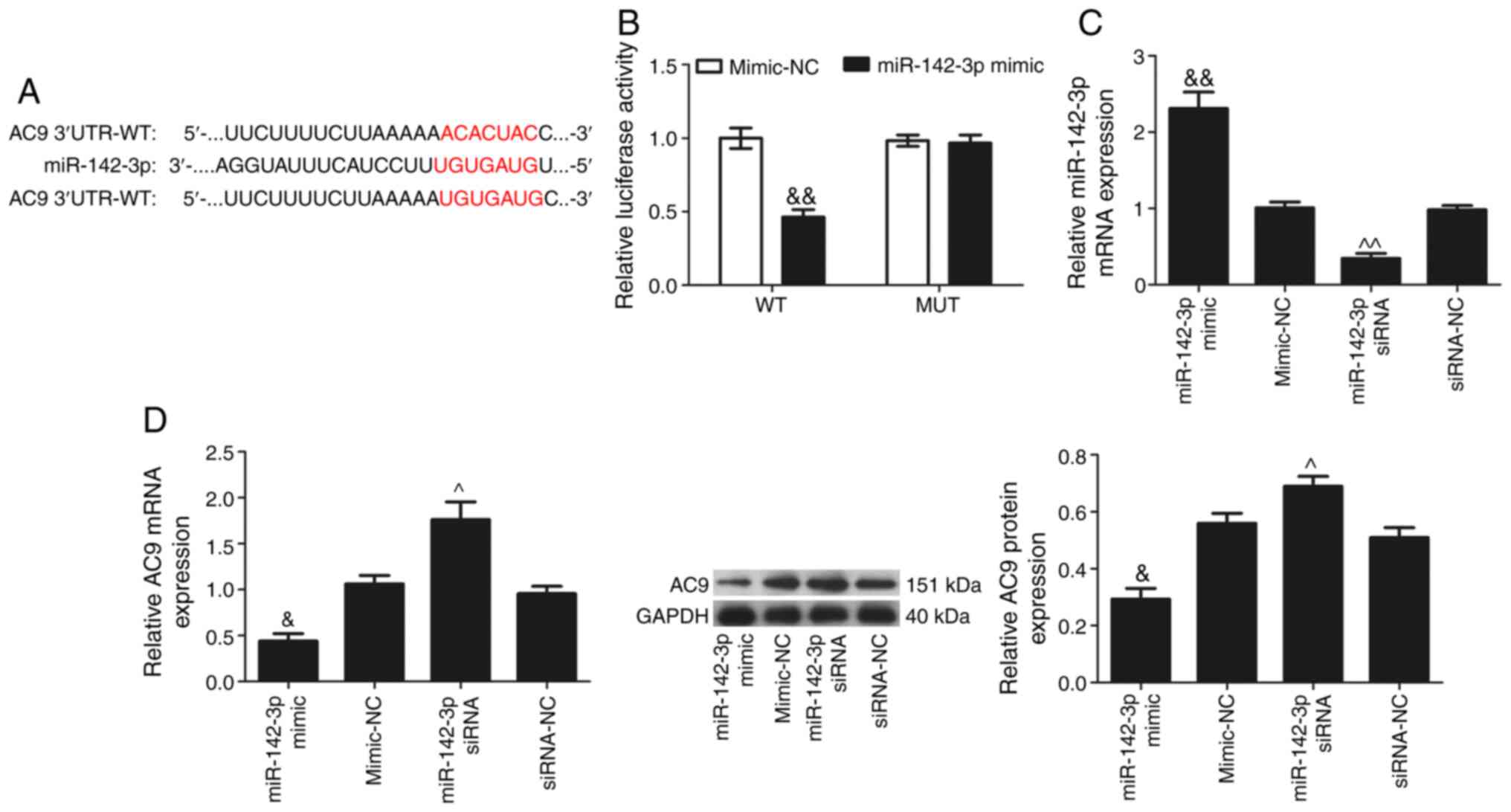
AC9 is the target gene of miR-142-3p
AC9 was predicted as a candidate target gene of
miR-142-3p using TargetScan (http://www.targetscan.org; Fig. 6A). Next, the present study
confirmed whether miR-142-3p is directly binds to the 3′-UTR of
AC9. The AC9 3′-UTR containing wild-type or mutated miR-142-3p
target site in a firefly luciferase reporter vector was cloned to
obtain the reporter plasmids wt-AC9 or mut-AC9, respectively. When
miR-142-3p mimic was co-transfected with wt-AC9, the luciferase
activity was significantly attenuated, whereas a reversal in
luciferase expression was observed when miR-142-3p mimic was
co-transfected with mut-AC9 (Fig.
6B). The transfection efficiency of miR-142-3p in each group
was analysed by RT-qPCR (Fig.
6C). miR-142-3p expression was significantly increased in the
miR-142-3p mimic group and significantly decreased in the
miR-142-3p siRNA group when compared with their respective NC
groups (P<0.05). RT-qPCR and western blotting (Fig. 6D) results showed that
miR-142-3p-overexpression significantly reduced AC9 expression, and
miR-142-3p silencing significantly increased AC9 expression
(P<0.05). These data provided evidence that miR-142-3p could
inhibit AC9 expression by directly binding to the AC9 3′-UTR.
miR-142-3p regulates sciatic pain via the
AC9/cAMP/AMPKA axis in rats with sciatic nerve injury
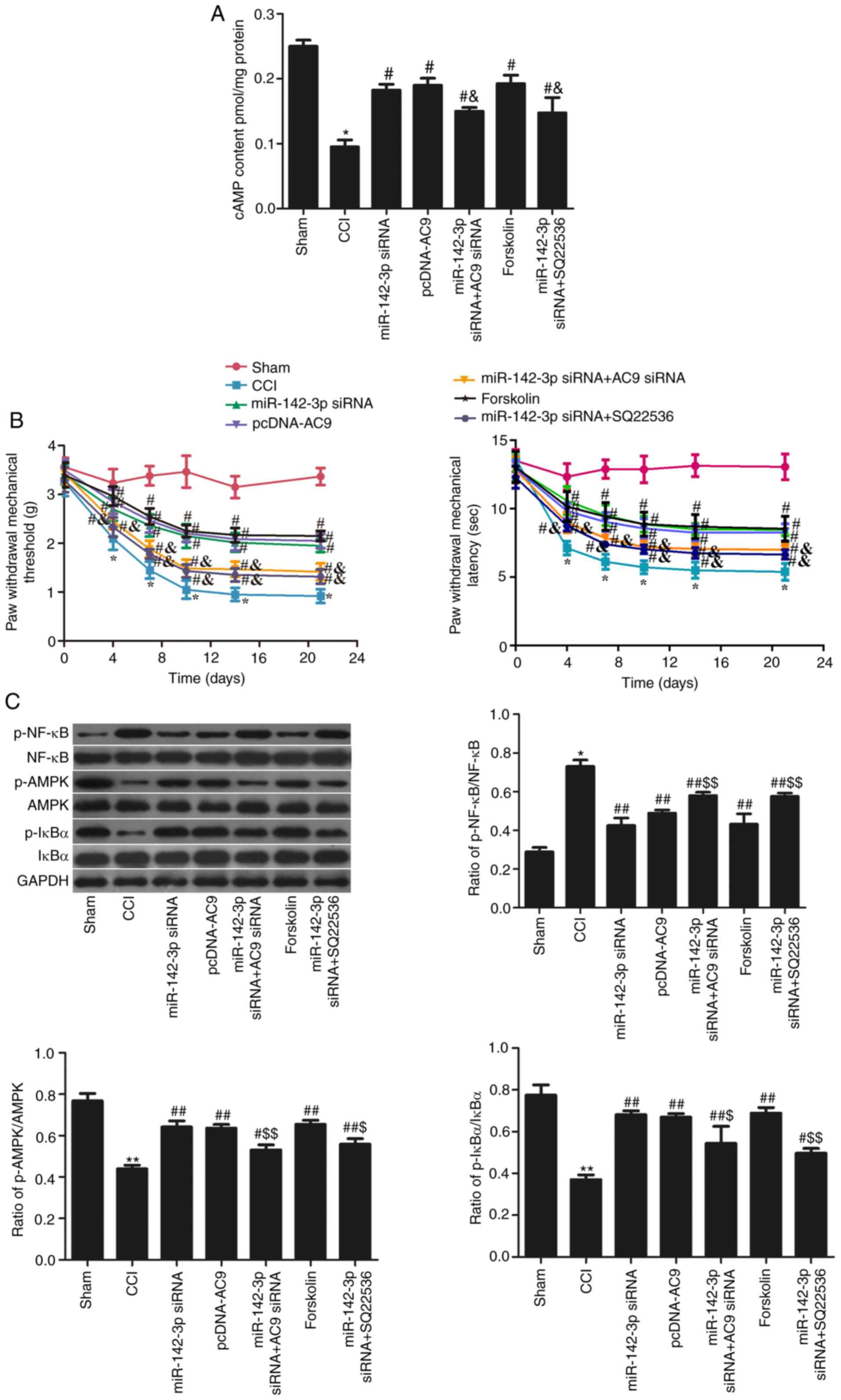
Following simultaneous intrathecal injection of
miR-142-3p siRNA and AC9 siRNA, cAMP levels were significantly
decreased compared with intrathecal injection of miR-142-3p siRNA
or AC9 pcDNA-AC9 alone (P<0.05; Fig. 7A). In addition, the administration
of cAMP activator forskolin significantly increased cAMP levels
compared with the sham group (P<0.05). Following simultaneous
intrathecal injection of miR-142-3p siRNA and cAMP inhibitor
(SQ22536), cAMP levels significantly decreased compared with the
miR-142-3p siRNA group (P<0.05). Fig. 7B showed that the simultaneous
intrathecal injection of miR-142-3p siRNA and AC9 siRNA
significantly decreased the PWMT and PWTL values (P<0.05)
compared with the miR-142-3p siRNA group (P<0.05). Following
forskolin administration, the PWMT and PWTL values were increased
compared with the CCI group (P<0.05). Following the simultaneous
intrathecal injection of miR-142-3p siRNA and SQ22536, the PWMT and
PWTL values were decreased compared with the miR-142-3p siRNA group
(P<0.05). As shown in Fig. 7C,
the levels of AMPK pathway-related proteins were measured. Compared
with the miR-142-3p siRNA group, the levels of p-AMPK and p-IκBɑ
decreased, and p-NF-κB p65 levels increased in the miR-142-3p siRNA
+ AC9 siRNA group (P<0.05). Compared with the CCI group,
forskolin significantly increased the levels of p-AMPK and p-IκBɑ
and reduced that of p-NF-κB p65 (P<0.05). The levels of p-AMPK
and p-IκBɑ were significantly reduced, and that of p-NF-κB p65 was
increased in the miR-142-3p siRNA and SQ22536 groups compared with
the miR-142-3p siRNA group (P<0.05). These data indicated that
miR-142-3p silencing reduced neuropathic pain in CCI rats by
upregulating AC9/cAMP/AMPK.
miR-142-3p regulates inflammatory
cytokines via the AC9/cAMP/AMPKA axis in rats with sciatic nerve
injury
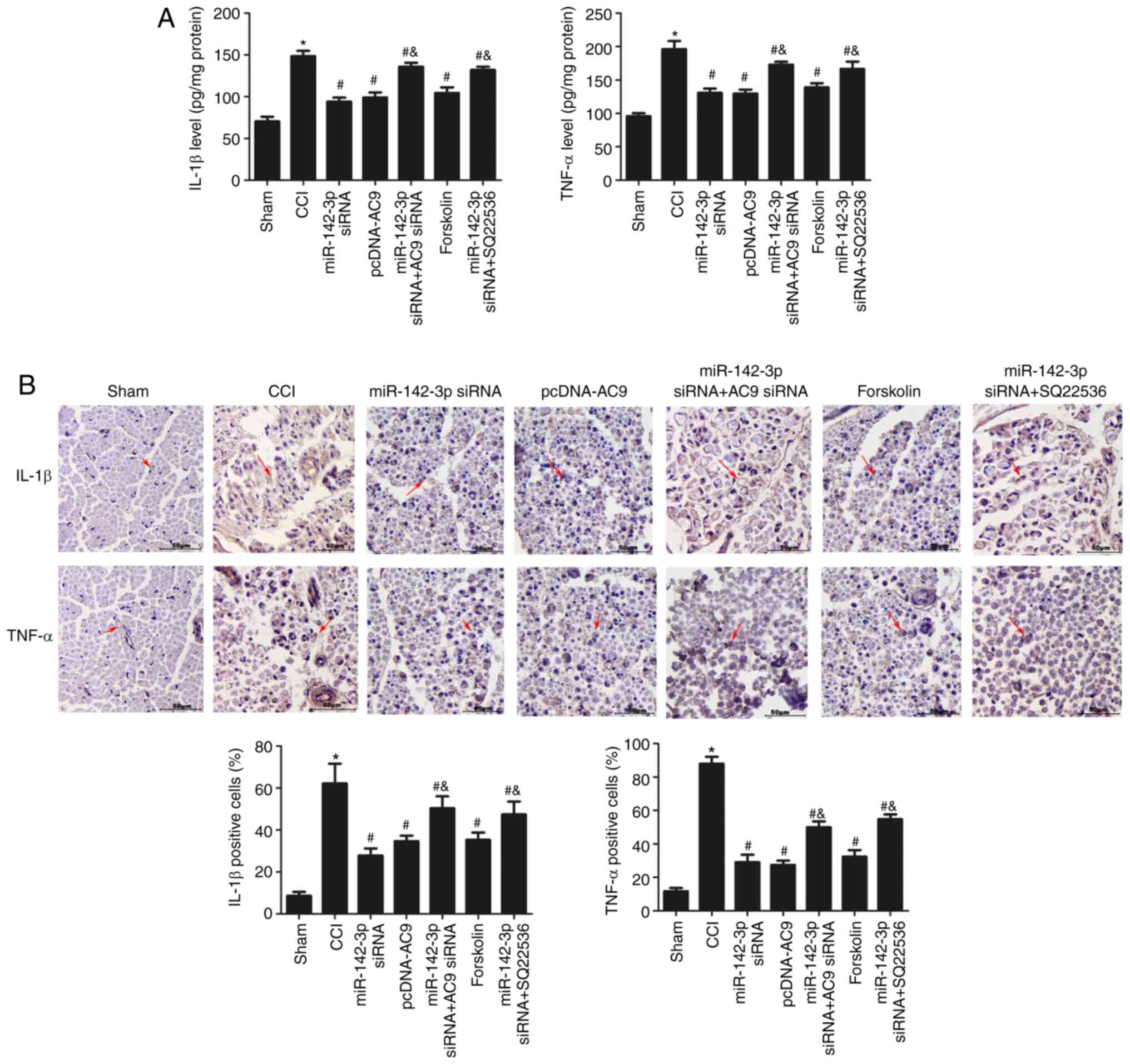
In addition, inflammatory factors were analysed in
each group using ELISA (Fig. 8A)
and immunohistochemistry (Fig.
8B). As shown in Fig. 8A,
compared with the miR-142-3p siRNA group, the expression of IL-1β
and TNF-ɑ significantly decreased in the miR-142-3p siRNA + AC9
siRNA group (P<0.05). Compared with the CCI group, forskolin
administration reduced the expression of these inflammatory
cytokines in the sciatic nerve of CCI rats (P<0.05), and no
differences were observed among the miR-142-3p siRNA, pcDNA-AC9 and
forskolin groups (P>0.05), while the levels of IL-1β and TNF-ɑ
were significantly higher in the miR-142-3p siRNA + SQ22536 group
compared with the miR-142-3p siRNA group. As shown in Fig. 8B, the immunohistochemical results
were consistent with the ELISA results, indicating that miR-142-3p
silencing restrained inflammatory cytokine expression via the
AC9/cAMP/AMPKA axis in rats with sciatic nerve injury.
Discussion
In the present study, it was found that miR-142-3p
was highly expressed, but AC9 was lowly expressed in the sciatic
nerve of CCI rats. miR-142-3p inhibition increased the expression
of AC9 and cAMP in the sciatic nerve of CCI rats. Furthermore, the
inhibition of miR-142-3p activated AMPK activity and inhibited the
release of IL-1β and TNF-ɑ in the sciatic nerve of CCI rats. AC9
exerted the opposite effects in rats with sciatic nerve injury
compared to miR-142-3p. miR-142-3p could inhibit AC9 expression by
directly binding to the AC9 3′-UTR. Consequently, these results
suggested that miR142-3p targets AC9 to reduce sciatic nerve
injury-induced neuropathic pain by regulating the cAMP/AMPK
signalling pathway.
Neuropathic pain often results from disease or
injury which influences the steady state of the nervous or central
nervous system (21). For the
past few years, miRNAs have gradually emerged as the functional
regulators of consistent neuropathic pain conception and
development (22). Increasing
evidence has shown that miRNA-based medical treatment may be an
effective means of blocking neuropathic pain (4,23).
A study revealed that miR-195 induced by spinal nerve
ligation-aggravated neuropathic pain by inhibiting Atg14 (24). At the same time, miR-221, miR-19a
and miR-155 alleviated neuropathic pain by targeting the suppressor
of cytokine signalling (25-27). Studies have demonstrated that
miR-146a-5p overexpression lessened neuropathic pain by suppressing
TNF receptor-associated factor-6-mediated neuro-inflammation
(28). miR-144, miR-132-3p,
miR-124a and miR-15b have been shown to be involved in the process
of neuropathic pain (25,28,29). Evidence has suggested that miRNAs
are emerging as novel and promising factors for neuropathic
treatment. A previous study demonstrated that miR-142-3p served as
a key miRNA for relieving neuropathic pain (5). Another study also revealed that
miR-142-3p targets high mobility group box 1 to positively regulate
neuropathic pain (30). However,
Wang et al (6) found that
miR-142-3p played a negative role in sciatic nerve conditioning
injury by regulating the AC9/cAMP axis. The present study also
demonstrated that miR-142-3p expression was higher in rats with
sciatic nerve injury.
Previous studies have indicated that the
AMPK-related signalling pathway plays an essential role in the
biology of neuropathic pain (13,14). The underlying mechanism focuses on
AMPK-driven protein synthesis. The present study also demonstrated
that AMPK activity is activated by the increased miR-142-3p
expression. Other studies have revealed that AMPK activation
reduced mechanical hypersensitivity by restraining the expression
of inflammatory cytokines in a rat model of neuropathic pain, which
is linked to increased IL-1β, IL-6 and IL-8 expression (31,32). The present study demonstrated that
miR-142-3p overexpression increased the production of TNF-α and
IL-1β in sciatic nerve injury rats, supporting a pro-inflammatory
role of miR-142-3p.
However, the present study had several limitations.
For example, the cellular co-expression of miR-142-3p and AC9 in
the sciatic nerve has not been confirmed, and only the NF-κB p65
subunit was analyzed; other subunits need to be analyzed as well,
such as protein kinase A and mitogen-activated protein kinase. In
addition, whether there is any difference between the ipsilateral
and contralateral sciatic nerve also remains to be studied.
Neuropathic pain may be associated with multiple chronic diseases,
which exist in the balance of homeostasis.
In conclusion, miR-142-3p is highly expressed while
AC9 is lowly expressed in rats with sciatic nerve injury.
miR-142-3p inhibition could increase the expression of AC9 and
cAMP, and further activate the activity of AMPK. The
miR-142-3p/AC9/cAMP/AMPK axis can be used as an important signaling
pathway for sensory function recovery following sciatic nerve
injury.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XL and SW performed experimental work and the data
collection and interpretation. XL and XY participated in the design
and coordination of the experimental work and acquisition of the
data. SW and XY participated in the study design, data collection
and data analysis. XL and HC designed the study, analysed the data
and drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Yantaishan Hospital (approval no.
20180705-0012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Baron R: Peripheral neuropathic pain: From
mechanisms to symptoms. Clin J Pain. 16(Suppl 2): S12–S20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baron R: Mechanisms of disease:
Neuropathic pain-a clinical perspective. Nat Clin Pract Neurol.
2:95–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Connor AB and Dworkin RH: Treatment of
neuropathic pain: An overview of recent guidelines. Am J Med.
122(Suppl 10): S22–S32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiangpan P, Qingsheng M, Zhiwen Y and Tao
Z: Emerging role of microRNA in neuropathic pain. Curr Drug Metab.
17:336–344. 2016. View Article : Google Scholar
|
|
5
|
Lu X, Li X, He Q, Gao J, Gao Y, Liu B and
Liu F: miR-142-3p regulates the formation and differentiation of
hematopoietic stem cells in vertebrates. Cell Res. 23:1356–1368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T, Li B, Wang Z, Wang X, Xia Z, Ning
G, Wang X, Zhang Y, Cui L, Yu M, et al: Sorafenib promotes sensory
conduction function recovery via miR-142-3p/AC9/cAMP axis post
dorsal column injury. Neuropharmacology. 148:347–357. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouyang B, Tang Z, Hou X, Chen D, Guo Q and
Weng Y: Trichostatin A suppresses up-regulation of histone
deacetylase 4 and reverses differential expressions of miRNAs in
the spinal cord of rats with chronic constrictive injury. Nan Fang
Yi Ke Da Xue Xue Bao. 39:1421–1426. 2019.In Chinese.
|
|
8
|
Tahamtan A, Teymoori-Rad M, Nakstad B and
Salimi V: Anti-inflammatory MicroRNAs and their potential for
inflammatory diseases treatment. Front Immunol. 9:13772018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji RR, Nackley A, Huh Y, Terrando N and
Maixner W: Neuroinflammation and central sensitization in chronic
and widespread pain. Anesthesiology. 129:343–366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaue G, Shimaoka M, Fukuoka T, Hiroi T,
Inoue T, Hashimoto N, Sakaguchi T, Sawa Y, Morishita R, Kiyono H,
et al: NF-kappa B decoy suppresses cytokine expression and thermal
hyperalgesia in a rat neuropathic pain model. Neuroreport.
12:2079–2084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scholz J and Woolf CJ: The neuropathic
pain triad: Neurons, immune cells and glia. Nat Neurosci.
10:1361–1368. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Price TJ and Dussor G: AMPK: An emerging
target for modification of injury-induced pain plasticity. Neurosci
Lett. 557:9–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu L, Pan C, Chen L, Hu L, Wang C, Han Y,
Yang Y, Cheng Z and Liu WT: AMPK activation by peri-sciatic nerve
administration of ozone attenuates CCI-induced neuropathic pain in
rats. J Mol Cell Biol. 9:132–143. 2017. View Article : Google Scholar
|
|
14
|
Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK
and Song XJ: Chronic compression or acute dissociation of dorsal
root ganglion induces cAMP-dependent neuronal hyperexcitability
through activation of PAR2. Pain. 153:1426–1437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun
M, Luo X, Sun L and Cheung CW: miRNA-23a/CXCR4 regulates
neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome
axis. J Neuroinflammation. 15:292018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aglah C, Gordon T and Posse de Chaves EI:
cAMP promotes neurite outgrowth and extension through protein
kinase A but independently of Erk activation in cultured rat
motoneurons. Neuropharmacology. 55:8–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao H, Xue Q, Zhang F, Luo Y, Zhu H,
Zhang X, Zhang H, Ding W and Yu B: Spinal SIRT1 activation
attenuates neuropathic pain in mice. PLoS One. 9:e1009382014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Tsuda M: Microglia in the spinal cord and
neuropathic pain. J Diabetes Investig. 7:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andersen HH, Duroux M and Gazerani P:
MicroRNAs as modulators and biomarkers of inflammatory and
neuropathic pain conditions. Neurobiol Dis. 71:159–168. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai Z, Chu H, Ma J, Yan Y, Zhang X and
Liang Y: The regulatory mechanisms and therapeutic potential of
MicroRNAs: From chronic pain to morphine tolerance. Front Mol
Neurosci. 11:802018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z,
Ding J, Jia L and Yuan W: Increased miR-195 aggravates neuropathic
pain by inhibiting autophagy following peripheral nerve injury.
Glia. 61:504–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heyn J, Luchting B, Hinske LC, Hübner M,
Azad SC and Kreth S: miR-124a and miR-155 enhance differentiation
of regulatory T cells in patients with neuropathic pain. J
Neuroinflammation. 13:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia L, Zhang Y and Dong T: Inhibition of
MicroRNA-221 alleviates neuropathic pain through targeting
suppressor of cytokine signaling 1. J Mol Neurosci. 59:411–420.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Jiang Q, Wang M and Li D: miR-19a
targets suppressor of cytokine signaling 1 to modulate the
progression of neuropathic pain. Int J Clin Exp Pathol.
8:10901–10907. 2015.PubMed/NCBI
|
|
28
|
Lu Y, Cao DL, Jiang BC, Yang T and Gao YJ:
MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6
signaling in the spinal cord. Brain Behav Immun. 49:119–129. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leinders M, Üçeyler N, Pritchard RA,
Sommer C and Sorkin LS: Increased miR-132-3p expression is
associated with chronic neuropathic pain. Exp Neurol. 283:276–286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Mou J, Cao L, Zhen S, Huang H and
Bao H: MicroRNA-142-3p relieves neuropathic pain by targeting high
mobility group box 1. Int J Mol Med. 41:501–510. 2018.
|
|
31
|
Xiang HC, Lin LX, Hu XF, Zhu H, Li HP,
Zhang RY, Hu L, Liu WT, Zhao YL, Shu Y, et al: AMPK activation
attenuates inflammatory pain through inhibiting NF-kappaB
activation and IL-1beta expression. J Neuroinflammation. 16:342019.
View Article : Google Scholar
|
|
32
|
Hasanvand A, Amini-Khoei H, Hadian MR,
Abdollahi A, Tavangar SM, Dehpour AR, Semiei E and Mehr SE:
Anti-inflammatory effect of AMPK signaling pathway in rat model of
diabetic neuropathy. Inflammopharmacology. 24:207–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|