Introduction
Hypertension is a frequently occurring disease
causing cardio-vascular complications (1), which currently represents one of the
most serious public health concerns and a major socioeconomic
burden. Vascular remodeling is a critical pathological
characteristic of hypertension, leading to increased vascular
resistance and hemodynamic changes (1). Approximately 95% of the cases are
classed as essential hypertension (EH) (2-4);
however, to date, the exact etiology and pathogenesis of EH have
not been fully elucidated. Recent studies have found that the
pathogenic factors of EH do not only involve the interaction
between genetic and environmental factors (5,6),
but are also correlated with human cytomegalovirus (HCMV)
infection, microRNA (miRNA) regulation and inflammation activation,
as well as other mechanisms (7-9).
HCMV is a ubiquitous beta-herpes virus with the
largest genome among human herpesviruses, the gene products of
which play immunomodulatory roles in the host (10,11). The findings of epidemiological
studies indicate that the prevalence of HCMV in the population is
40-100%, depending on socio-economic and geographical factors
(12). CMV is reportedly
associated with EH (13,14). When HCMV infects humans, it may
enter a latent infection phase and cause disease by changing host
and viral miRNA expression (15).
miRNAs are single-stranded, non-coding RNAs composed of 20-25
nucleotides, which can inhibit protein synthesis by inhibiting the
expression of specific target genes or directly terminating the
translation of target mRNAs (16,17). Furthermore, accumulating evidence
suggests that miR-21 plays an important role in vascular remodeling
in hypertension (18).
Additionally, the deletion of miR-431-5p can prevent hypertension
and vascular injury caused by angiotensin II (Ang II) (19). However, decreased expression of
miR-181-5p participates in the phenotypic transformation of the
vascular smooth muscle cells (VSMCs) in Ang II-induced hypertension
by causing an increase in high mobility group box 1 (20). It was previously confirmed that
HCMV-encoded miR-UL112 promoted HCMV-mediated vascular disease by
inducing vascular endothelial cell dysfunction (21). It was also reported that miR-138
promoted the migration of human umbilical vein endothelial cells
(HUVECs) and tube formation in HCMV-infected HUVECs via the
SIRT1/P-STAT3 pathway (22).
However, the virus encodes at least 26 mature miRNAs (23-25). Given the regulatory network of
miRNAs and their important role in the pathogenesis of
hypertension, the recently discovered HCMV-encoded miRNAs have not
sufficiently elucidated the role and underlying mechanism of action
of CMV and associated miRNAs in hypertension. It remains unclear
whether miRNA changes in the host following CMV infection are
involved in the occurrence and development of hypertension and
vascular remodeling.
The nucleotide-binding domain, leucine-rich repeat
protein 3 (NLRP3) inflammasome, is a multiprotein complex
consisting of an NLRP3 scaffold, an adaptor apoptosis speck-like
protein containing a CARD (ASC) and the effector procaspase-1, and
participates in the activation of the inflammatory response.
Activation and assembly of inflammasomes promote proteolytic
cleavage, maturation and secretion of the pro-inflammatory
cytokines interleukin (IL)-1β and IL-18 (26). Accumulating evidence suggests that
EH is also associated with NLRP3 inflammasome activation (27,28) and, in turn, the inflammatory
response is involved in end-organ injury and vascular remodeling in
EH (29). Moreover, NLRP3 is
closely associated with certain miRNAs, and miR-181a has been
demonstrated to activate the NLRP3 inflammatory pathway by
targeting mitogen-activated protein kinase kinase 1 in
athero-sclerosis (30). Moreover,
miR-125a-5p negatively regulates the NLRP3 inflammasome by
targeting C-C motif chemokine ligand 4 in human VSMCs treated with
oxidized low-density lipoprotein (31). NLRP3 inflammasome activation also
plays an important role in the phenotypic transformation and
proliferation of human VSMCs and vascular remodeling (32,33).
We previously observed that blood pressure was
increased and miR-1929-3p was significantly downregulated in mouse
cytomegalovirus (MCMV)-infected-C57BL/6J mice (34). Moreover, using bioinformatics
analysis and the dual-luciferase reported assay, the endothelin A
receptor (Ednra) was validated as one of the
hypertension-associated target genes regulated by miR-1929-3p. It
has been demonstrated that endo-thelin-1 (ET-1) induces vascular
remodeling-related persistent inflammation through Ednra (35). Based on our previous experimental
results and the aforementioned associations among MCMV, miRNAs and
the NLRP3 inflammasome, it was hypothesized that MCMV infection may
decrease the expression of miR-1929-3p in mice, thereby relieving
the inhibition of Ednra and activating the NLRP3 inflammasome in
order to promote the occurrence and development of adverse vascular
remodeling and hypertension.
Materials and methods
Animals
All experimental procedures involving laboratory
animals were approved by the Institutional Animal Research
Committee at Shihezi University Medical College. A total of 200
C57BL/6J male mice, aged 7 months and weighing 25-30 g, were
purchased from Beijing Viton Lihua Laboratory Animal Co., Ltd.
(license no. SCXK 2016-0006). During the whole study period, the
animals were housed at room temperature with 12-h light/dark cycles
and allowed access to normal rat chow and water ad libitum.
The mice were randomly divided into groups (n=17/group) as follows:
Control, MCMV, MCMV + miR-NC and MCMV + miR-1929-3p. In the MCMV
group, mice were intraperitoneally injected with 5×104
PFU/animal, while the control group was inoculated with the same
amount of normal saline. In the miR-1929-3p and miR-1929-3p
negative control (miR-NC) groups, mice were injected via the tail
vein with rAAV-miR-1929-3p or rAAV-miR-1929-3p-NC
(1×1011 virion particles in 100 ml saline solution). The
mice were anesthetized using sodium pentobarbital (P3761,
Sigma-Aldrich; Merck KGaA) through intraperitoneal injection (30
mg/kg), and blood was collected from the inner canthus artery. The
mice were sacrificed by cervical dislocation and the aorta was
resected, fixed in 4% paraformaldehyde at 4°C for 24 h and embedded
in paraffin.
MCMV immediate early (IE) gene
detection
Samples of mouse aorta (30 mg) were homogenized into
a tissue suspension, and the tissue DNA was extracted and amplified
using a DNA extraction kit (Tiangen Biotech Co., Ltd.) using the
following primers: MCMV IE forward, 5′-ATG GTG AAG CTA TCA AAG ATG
TGC ATC TCA-3′ and reverse, 5′-ATC AAT CAG CCA TCA ACT CTG CTA CCA
CAC-3′. After agarose gel electrophoresis, the DNA was visualized
on the Image-Pro Plus software, version 6.0 (Media Cybernetics,
Inc.).
Blood pressure measurement
The C57BL/6 mice were anesthetized using sodium
pentobarbital (P3761; Sigma-Aldrich; Merck KGaA) through
intraperitoneal injection (30 mg/kg), after which time they were
fixed on the operating table. An incision was made along the
midline of the neck, and the neck tissues were carefully separated
to expose the common carotid artery without injuring the vagus
nerve. Next, a cannula was inserted in the artery and secured in
place with 6/0 silk threads, and the common carotid artery was
repositioned. Systolic blood pressure (SBP), diastolic blood
pressure (DBP) and mean arterial pressure (MAP) in the artery were
monitored. For each measurement, the data represented the mean of
at least 5 stable recordings.
Hematoxylin and eosin (H&E) and
Masson's trichrome staining
The blood vessels were placed in 4% paraformaldehyde
buffer (pH 7.0) and fixed at room temperature for 24 h. The tissues
were embedded in paraffin, cut into 4-µm sections, stained
with H&E (Solarbio) and Masson's trichrome stain (Beijing
Solarbio Science & Technology Co., Ltd.), and observed under a
light microscopy (IX73, Olympus Corporation). Five microscopic
fields (magnification, ×400) were randomly selected from each blood
vessel, and the collagen area was calculated as a percentage of the
field of view.
Colorimetry
After homogenizing the mouse blood vessel tissue,
the supernatant was collected and analyzed for nitric oxide (NO)
and endothelial NO synthase (eNOS) levels in mouse aortic
homogenates using a commercial kit (Nanjing Jiancheng Biological
Engineering Institute), according to the manufacturer's
instructions.
Western blotting
After treatment, all experimental samples were
collected and homogenized on ice in RIPA buffer containing 1%
proteinase inhibitor solution. Then, the homogenates were
centrifuged at 12,000 × g for 10 min at 4°C. The resulting
supernatant contained the extracted proteins. The concentration of
total extracted protein was determined using NanoDrop2000 (Thermo
Fisher Scientific, Inc.). A total of 10 µg protein per lane
were separated by electrophoresis on 10% SDS-PAGE gels before
transferring onto a PVDF membrane by SEMI-DRY TRANSFER CELL
(Bio-Rad Laboratories, Inc.). After blocking with 5% non-fat dry
milk for 3 h at room temperature, the membranes were incubated
overnight at 4°C with primary antibodies diluted in a 5% bovine
serum albumin (NeoFROXX GmbH) solution in 1X Tris-buffered saline
solution containing 0.1% Tween-20 (TBS-T). The antibodies used for
western blot analysis were as follows: β-actin (1:1,000, cat. no.
ZM-0001; Sugisuke Bridge), α-smooth muscle actin (α-SMA; 1:400,
cat. no. BM0002; Boster Biological Technology), osteopontin (OPN;
1:200, cat. no. ab8448; Abcam), proliferating cell nuclear antigen
(PCNA; 1:250, cat. no. BM0104; Boster Biological Technology), NLRP3
(1:500, cat. no. ab214185; Abcam) ASC (1:250, cat. no. ab47092;
Abcam), caspase-1 (1:1,000, cat. no. ab1872; Abcam), pro-caspase-1
(1:1,000, cat. no. ab179515; Abcam), IL-18 (1:1,000, cat. no.
ab71495; Abcam), IL-1β (1:1,000, cat. no. ab9722; Abcam) and
pro-IL-1β (1:1,000, cat. no. ab216995; Abcam). Subsequently, the
membranes were washed three times with TBS-T and treated with goat
anti-rabbit lgG (1:10,000; cat. no. ZB2301; Sugisuke Bridge,
Beijing, China) or goat anti-rat lgG (1:10,000; cat. no. ZB2305;
Sugisuke Bridge, Beijing, China) for 2 h at room temperature. The
signals were visualized with Supersensitive ECL Chemiluminescent
Kit, according to the manufacturer's instructions. The films w ere
scanned and analyzed using the Image-Pro Plus software, version 6.0
(Media Cybernetics, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the collected aortic
samples using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), treated with DNase I (Tiangen Biotech Co.,
Ltd.), reverse transcribed with SuperScript™ First Strand cDNA
System (Invitrogen; Thermo Fisher Scientific, Inc.), and mature
miRNA levels were assessed using SYBR Green Real-time PCR (Tiangen
Biotech Co., Ltd.) according to the manufacturer's instructions
with U6 and GAPDH as the reference genes. The thermocycling
conditions were as follows: 3 min at 95°C followed by 40 cycles of
amplification at 94°C for 20 sec and at 60°C for 34 sec. The
results were analyzed using the 2−ΔΔCq method (36). The sequences of the PCR primers
used were as follows: miR-1929-3p forward, 5′-ACA CTC CAG CTG GGC
AGC TCA TGG AGA CCT-3′ and reverse, 5′-TGG TGT CGT GGA GTC G-3′;
Ednra forward, 5′-TCA CCG TCT TGA ACC TCT GTG C-3′ and reverse,
5′-GAT GGA GAC GAT TTC AAT GGC GG-3′; U6 forward, 5′-GCT TCG GCA
GCA CAT ATA CTA AAA T-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT
GTC AT-3′; and GAPDH forward, 5′-TGG CCT TCC GTG TTC CTA C-3′ and
reverse, 5′-GAG TTG CTG TTG AAG TCG CA-3′.
Immunohistochemistry
To determine the expression of target proteins in
the aortic tissues, the samples were stained by
immunohistochemistry. Briefly, the aortic tissues were fixed in 4%
paraformaldehyde for 24 h at 4°C and embedded in paraffin. Then,
samples were cut into 3-µm sections, rehydrated with 3%
H2O2 after being deparaffinized, and washed
with 0.1 M PBS. The sections were then incubated with primary
antibodies at 4°C overnight. The antibodies used were as follows:
OPN (1:100, cat. no. ab8448; Abcam), PCNA (1:50, cat. no. ab92552;
Abcam), α-SMA (1:200, cat. no. ab5694; Abcam), NLRP3 (1:100, cat.
no. ab214185; Abcam), ASC (1:100, cat. no. ab47092; Abcam) and
caspase-1 (1:25, cat. no. ab1872; Abcam). After washing with PBS,
the sections were incubated with secondary antibody (Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 30 min.
Then, color in the aorta was developed using 3,3-diaminobenzidine.
The sections were then counterstained with hematoxylin at room
temperature for 4 min, incubated with ammonia for 20 min,
dehydrated with an ethanol gradient (80% ethanol for 5 min; 95%
ethanol for 5 min; and 100% ethanol for 5 min) and transparentized
twice with xylene (5 min each time), mounted on glass coverslips,
and sealed with neutral resin. Cells containing brown-strained
particles in the cytoplasm were considered as positive based on
imaging under a fluorescence microscope (Carl Zeiss AG).
Quantitative image analyses were performed with the Image-Pro Plus
software, version 6.0 (Media Cybernetics, Inc.). The mean of the
number of positive cells from at least 5 random fields was
calculated for each section and then averaged within the group. The
staining scores were based on percentage of cells stained and
intensity of staining: The level of protein accumulation was scored
as 0 (no detectable immunostaining), 1 (few scattered stained
nuclei), 2 (up to 10% stained nuclei), 3 (10-50% stained nuclei),
and 4 (>50% stained nuclei) (37).
ELISA
Commercially available enzyme immunoassay kits
(Jingmei Biotechnology) were used to detect MCMV IgG (cat. no.
JM-02340M1), MCMV IgM (cat. no. JM-02341M1), IL-18 (cat. no.
JM-02452M1), IL-1β (cat. no. JM-02323M1) and ET-1 (cat. no.
JM-02844M1) levels in the plasma or aortic tissues. A microplate
reader (Model 3550-UV; Bio-Rad Laboratories, Inc.) was used to
measure the absorbance at a wavelength of 450 nm.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was
used for statistical analyses. All the data are presented as mean ±
SD from at least three independent experiments, and all in
vitro experiments were conducted in triplicate. The SPSS 20.0
statistical software (IBM Corp.) was utilized for statistical
analysis. The Student's t-test was used for comparisons between two
groups. Multiple comparisons were performed using one-way or
two-way ANOVA. Tukey's multiple comparisons test was used for the
pairwise comparison following ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
Development of MCMV-infected C57BL/6J
mouse model
In order to establish an experimental animal model
for elevated blood pressure induced by MCMV infection, 7-month-old
C57BL/6J mice were intraperitoneally injected with the Smith strain
of MCMV, whereas control group mice were injected with the same
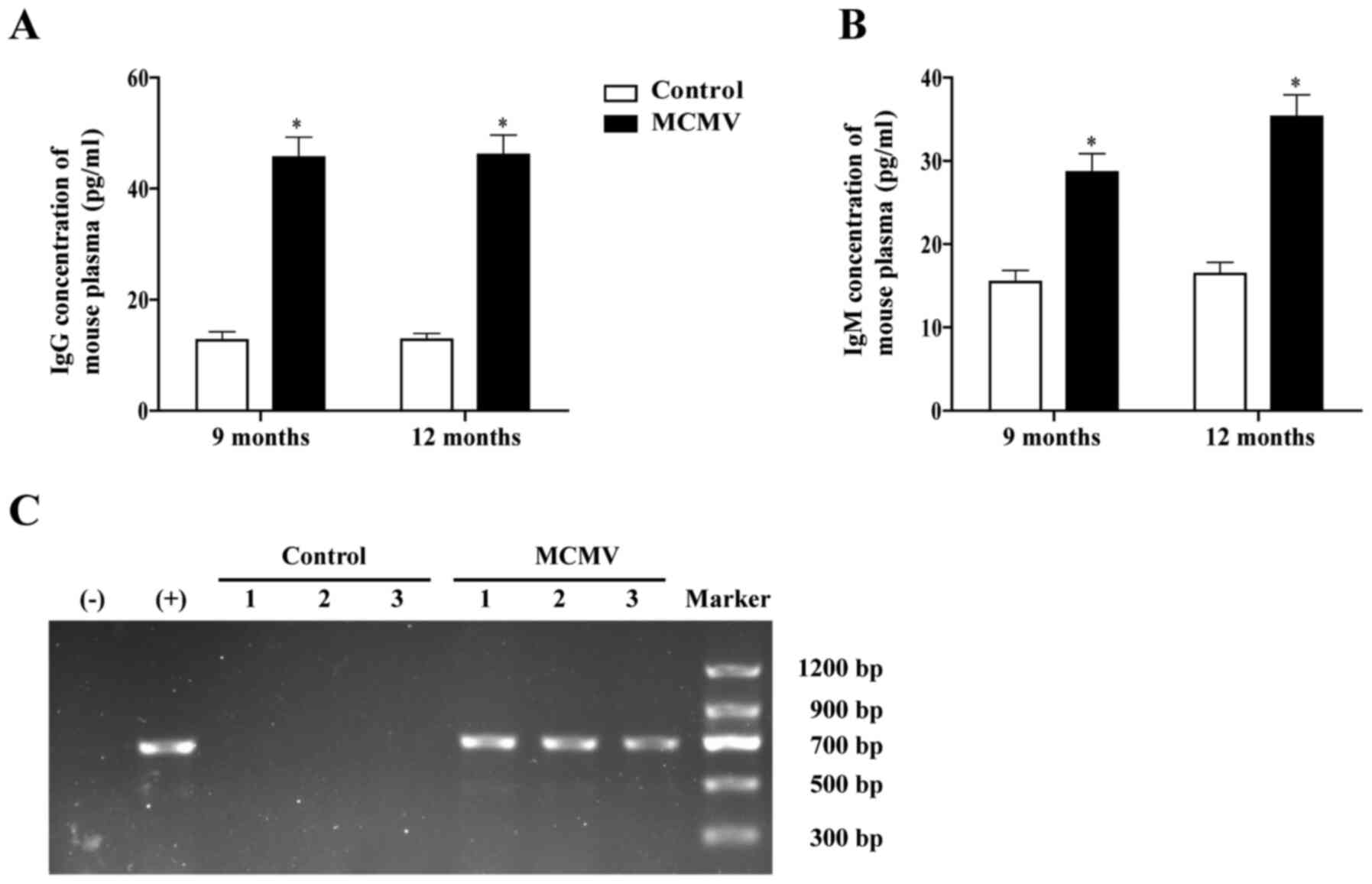
amount of normal saline. At 9 months of age, the serum
concentrations of MCMV IgG and IgM in the MCMV group were
significantly higher compared with those in control group mice of
the same age (Fig. 1A and B). PCR
analysis revealed that the expression of MCMV IE in the MCMV group
was positive, indicating that the mice had been infected with MCMV
(Fig. 1C).
MCMV infection increases blood pressure
and induces vascular remodeling in C57BL/6J mice
To determine the role of MCMV infection in mice with
hypertension, blood pressure in mice was determined by carotid
arterial pressure measurement. The results revealed that SBP, DBP
and MAP in the MCMV group of 9- and 12-month mice were
significantly higher compared with those in control group mice of
the same age (Fig. 2A and C).
This indicated that MCMV infection promoted an increase in blood
pressure in mice. Concurrently, the miR-1929-3p expression level in
the MCMV group decreased significantly with the increase in blood
pres-sure (Fig. 2D). H&E and
Masson's trichrome staining revealed increased vascular media
thickness and collagen accumulation in aortic tissue sections of
12-month-old mice in the MCMV group (Fig. 2E-H). Taken together, these results
indicated that MCMV infection caused increased blood pressure and
vascular remodeling in C57BL/6J mice.
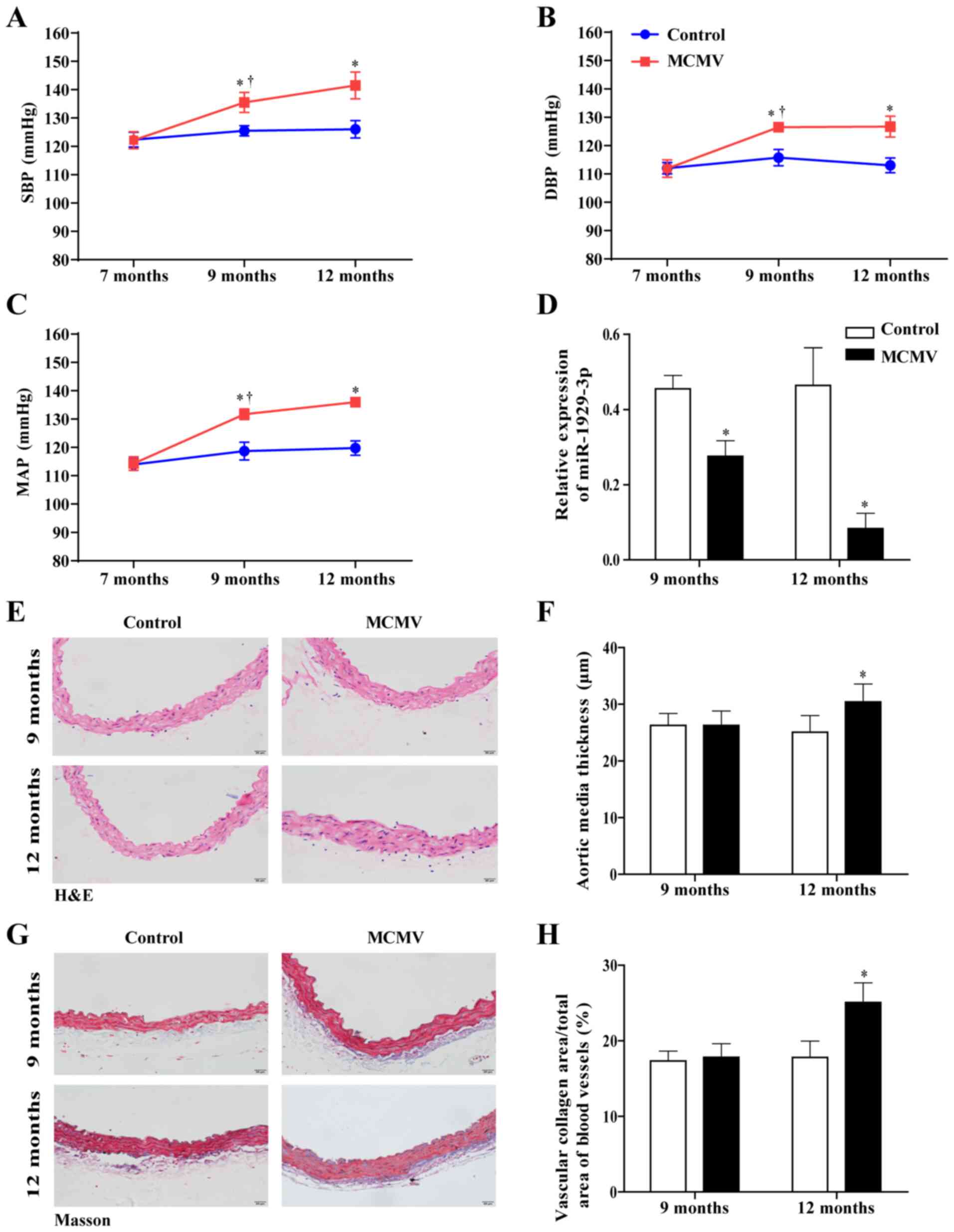 | Figure 2Increased blood pressure and vascular
remodeling were caused by MCMV infection in C57BL/6J mice. (A) SBP,
(B) DBP and (C) MAP were determined by carotid artery pressure
measurement. (D) The expression of miR-1929-3p was detected by
reverse transcription-quantitative PCR anal-ysis. (E and F) Effect
of MCMV infection on blood vessel wall thickness in mice assessed
using hematoxylin and eosin staining (magnification, ×100). (G and
H) The area of vascular fibrosis was evaluated by Masson's
trichrome staining (magnification, ×100). *P<0.05 vs.
control of the same age; †P<0.05 vs. MCMV group at 7
months of age. In panels A-C, data were analyzed using two-way
ANOVA and Tukey's multiple comparisons post hoc test; in panels D,
F and H, data were analyzed using the Student's t-test. n=5 per
group. MCMV, murine cytomegalovirus; SBP, systolic blood pressure;
DBP, diastolic blood pressure; MAP, mean arterial pressure. |
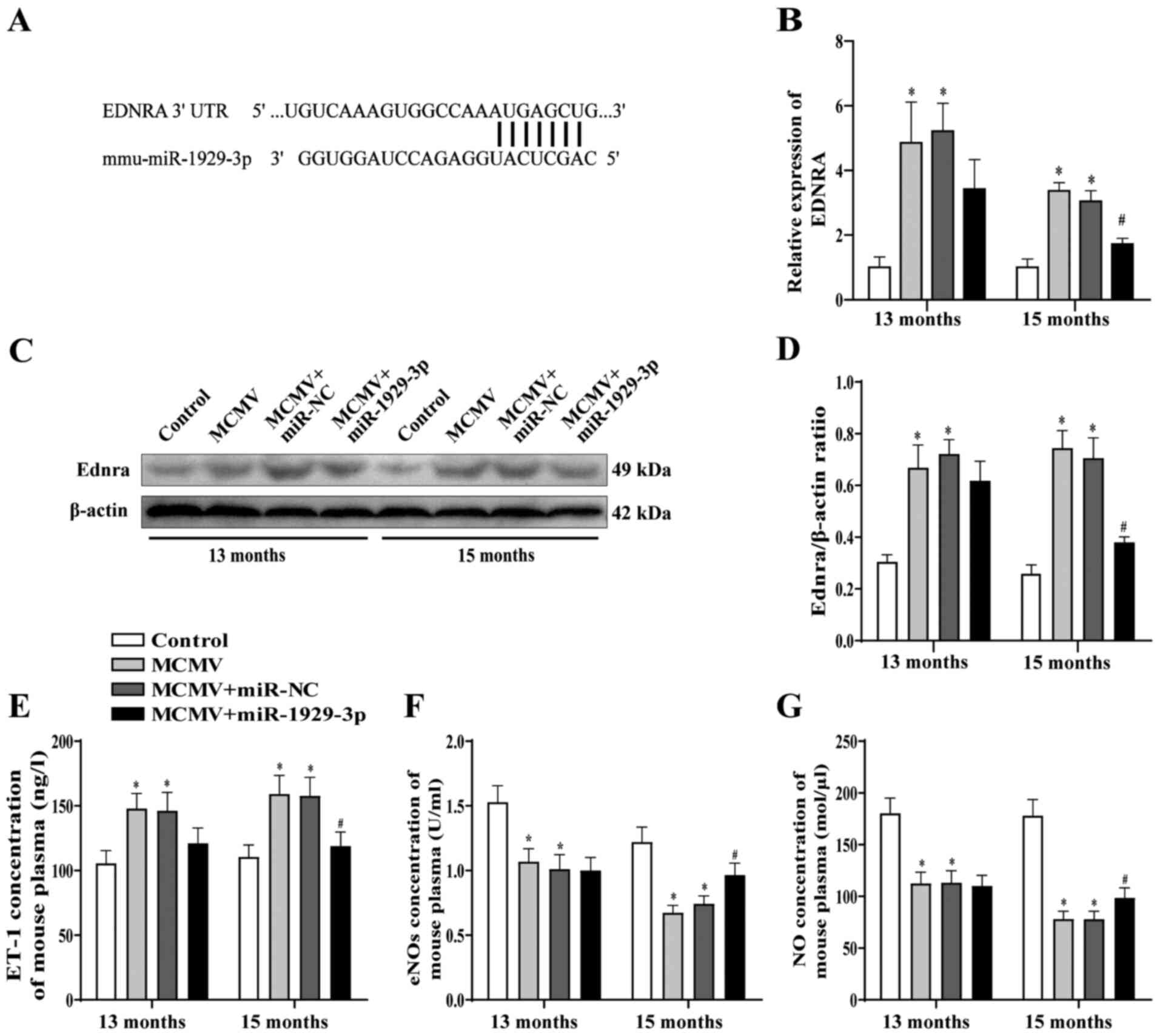
Overexpression of miR-1929-3p improves
vascular remodeling in MCMV-induced hypertensive mice
To investigate the role of miR-1929-3p in vascular
remodeling, the type 9 recombinant adeno-associated virus (rAAV9)
system was used in MCMV-induced hypertensive C57BL/6J mice. At 13
and 15 months of age, the expression of miR-1929-3p in the MCMV
group was significantly downregulated, while in the MCMV +
miR-1929-3p group, miR-1929-3p expression was significantly
increased (Fig. 3A). At the age
of 14 months, blood pressure in MCMV + miR-1929-3p group mice was
restored. This indicated that the overexpression of miR-1929-3p
attenuated the MCMV-induced elevation of DBP, MAP and SBP (Fig. 3B-D).
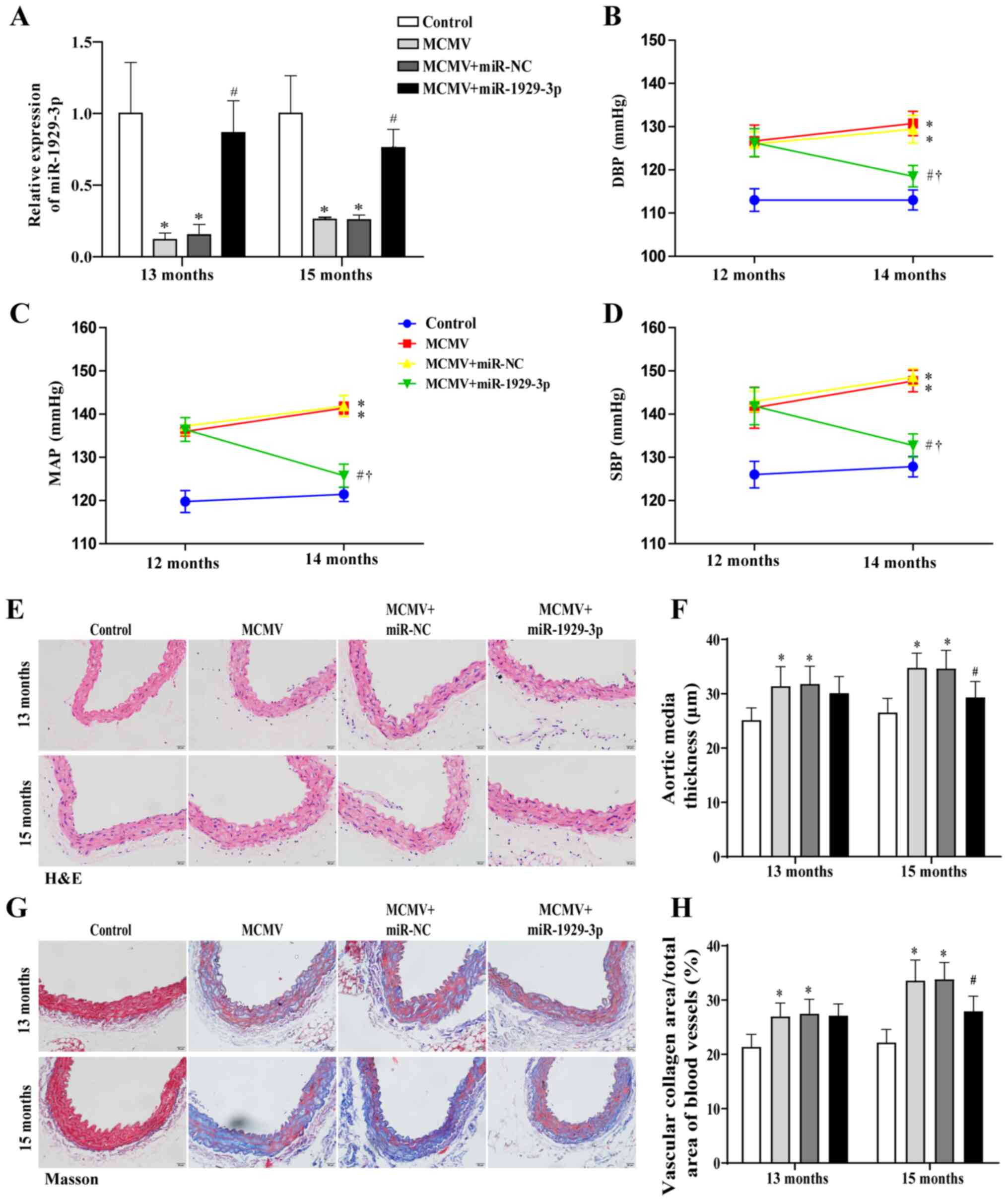 | Figure 3miR-1929-3p overexpression alleviated
MCMV-induced vascular remodeling in mice. (A) The expression of
miR-1929-3p following transfection with AAV-miR-1929-3p was
detected by reverse transcription-quantitative PCR analysis. (B)
DBP, (C) MAP and (D) SBP were determined by carotid artery pressure
measurement. (E and F) Staining images of aortic tissues following
hematoxylin and eosin staining; the pathological changes in aortic
tissues were semi-quantitatively evaluated based on histological
examination (magnification, ×100). (G and H) The area of vascular
fibrosis was evaluated by Masson's trichrome staining
(magnification, ×100). *P<0.05 vs. control of the
same age; #P<0.05 vs. MCMV + miR-NC group of the same
age; †P<0.05 vs. MCMV group at 7 months of age. In
panels A, F and H, data were analyzed using one-way ANOVA, while in
panels B-D, data were analyzed using two-way ANOVA, followed by
Tukey's multiple comparisons post hoc test; n=5 per group. MCMV,
murine cytomegalovirus; SBP, systolic blood pressure; DBP,
diastolic blood pressure; MAP, mean arterial pressure. |
A characteristic change caused by the increase in
blood pressure is vascular remodeling due to the proliferation of
VSMCs (4), with the morphological
changes mainly comprising thickening of the media, increased
rigidity of the vessel wall, and decreased vascular compliance
(13). In the present study, the
MCMV + miR-1929-3p group mice exhibited no significant changes at
13 months of age compared with the MCMV group. However, H&E and
Masson's trichrome staining of aortic tissue in mice aged 15 months
revealed that miR-1929-3p significantly reduced the thickness of
the aortic media and the fibrotic area of aortic tissue, which were
caused by MCMV infection (Fig.
3E-H).
To further determine the role of miR-1929-3p in
vascular remodeling in MCMV-infected aortic tissues, the protein
expression of α-SMA, OPN and PCNA was analyzed using
immunohistochemistry and western blotting. The results revealed
that, at 15 months of age, the miR-1929-3p over-expression group
exhibited an increase in the contractile phenotype of α-SMA
expression and a decrease in the synthetic phenotype of OPN and
PCNA expression compared with the MCMV group (Fig. 4A-J). These data indicated that
miR-1929-3p overexpression inhibited vascular smooth muscle
thickening and exerted a protective effect against vascular
remodeling. These findings indicated that MCMV infection promoted
aortic remodeling by inhibiting the expression of miR-1929-3p,
thereby leading to hypertension.
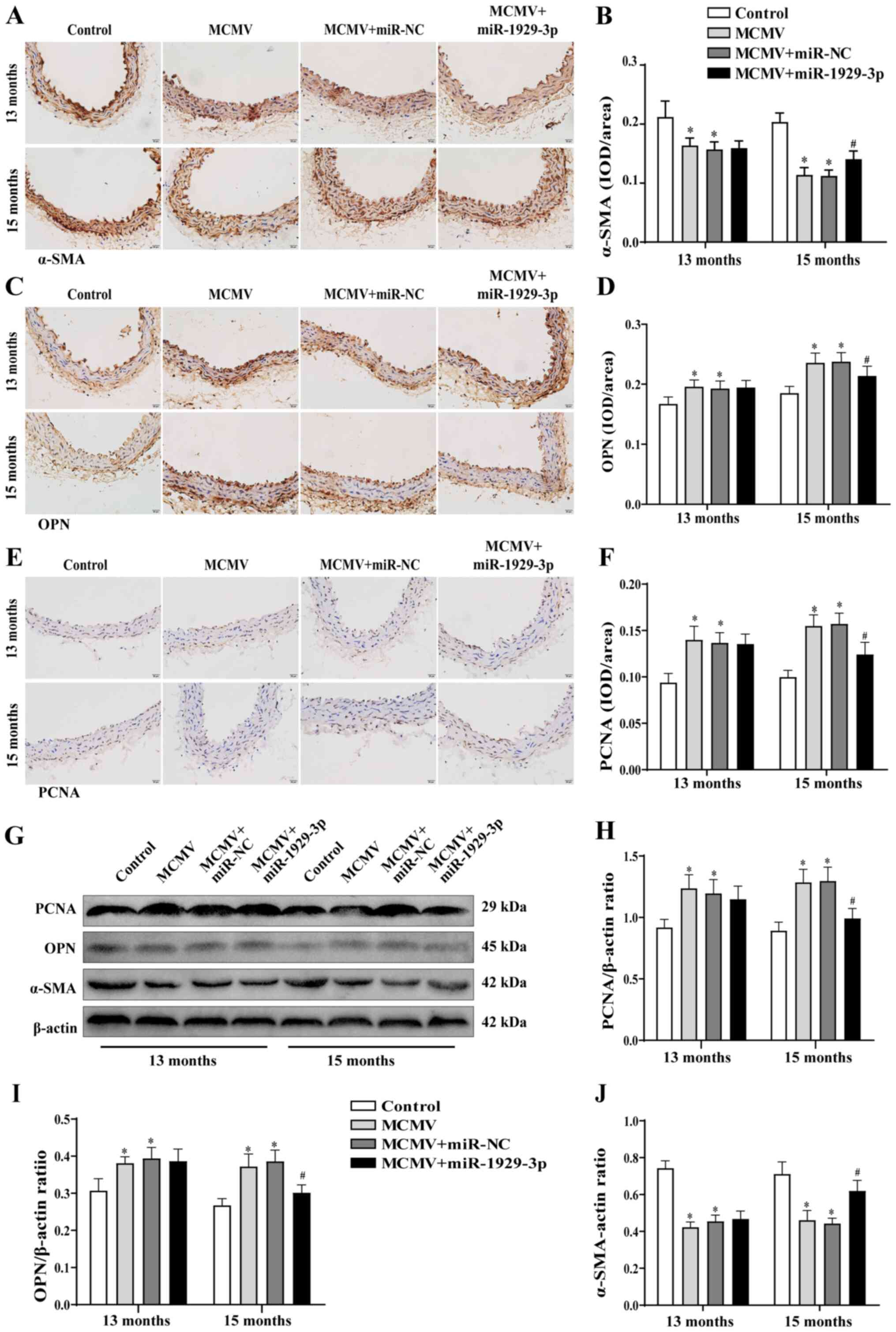 | Figure 4Improved MCMV-induced vascular
phenotypic transformation by miR-1929-3p. (A-F) Expression of (A)
α-SMA, (C) OPN and (E) PCNA in mouse aorta as detected by
immunohistochemical staining (magnification, ×100), and statistical
analysis of (B) α-SMA, (D) OPN and (F) PCNA. (G) Western blotting
and (H-J) statistical analysis of the expression of (H) PCNA, (I)
OPN and (J) α-SMA in aortic tissues. *P<0.05 vs.
control of the same age; #P<0.05 vs. MCMV + miR-NC
group of the same age. In panels A, D, F, H, I and J, data were
analyzed using one-way ANOVA followed by Tukey's multiple
comparisons post hoc. n=5 per group. MCMV, murine cytomegalovirus;
SMA, smooth muscle actin; OPN, osteopontin; PCNA, proliferating
cell nuclear antigen. |
Targeted regulation of endothelin A
receptor by miR-1929-3p alleviates endothelial dysfunction induced
by MCMV infection
Ednra was previously identified as the target of
miR-1929-3p (34). To confirm
this finding, the expression level of Ednra was detected after
overexpression of miR-1929-3p and, consistently with the results of
the previous study, Ednra mRNA and protein expressions were
upregulated in the MCMV group compared with those in the control
group. However, Ednra mRNA and protein expression levels were
significantly downregulated in the MCMV + miR-1929-3p group
(Fig. 5B-D). These results
indicated that MCMV infection downregulated miR-1929-3p and
increased the expression of its target gene, Ednra, while
miR-1929-3p overexpression alleviated the MCMV infection-induced
increase of Ednra expression. It has been reported that the
overexpression of ET-1 causes persistently elevated blood pressure
and vascular injury through Ednra (38). Moreover, it has been demonstrated
that endothelial cell dysfunction is an important component of
inflammation caused by CMV infection (39). To assess the role of miR-1929-3p
in MCMV-induced endothelial dysfunction, eNOS activity and NO
content were measured as indicators of endothelial cell function in
aortic tissue, and the content of ET-1 in plasma was also measured.
Compared with the control group, plasma ET-1 concentration in mice
in the MCMV group was significantly increased, while eNOS activity
in aortic tissues was downregulated. However, plasma ET-1
concentration and aortic eNOS activity were significantly decreased
in the MCMV group relative to the MCMV + miR-NC group after
miR-1929-3p overexpression, up to 15 months of age. Additionally,
the concentration of NO in the aortic tissues displayed the same
trend as that of the activity of eNOS (P<0.05; Fig. 5E-G).
These data suggested that MCMV infection may lead to
endothelial cell dysfunction by inhibiting miR-1929-3p, increasing
Ednra expression and promoting the production of endothelial injury
factors, whereas overexpression of miR-1929-3p protects against
MCMV-induced endothelial function disruption.
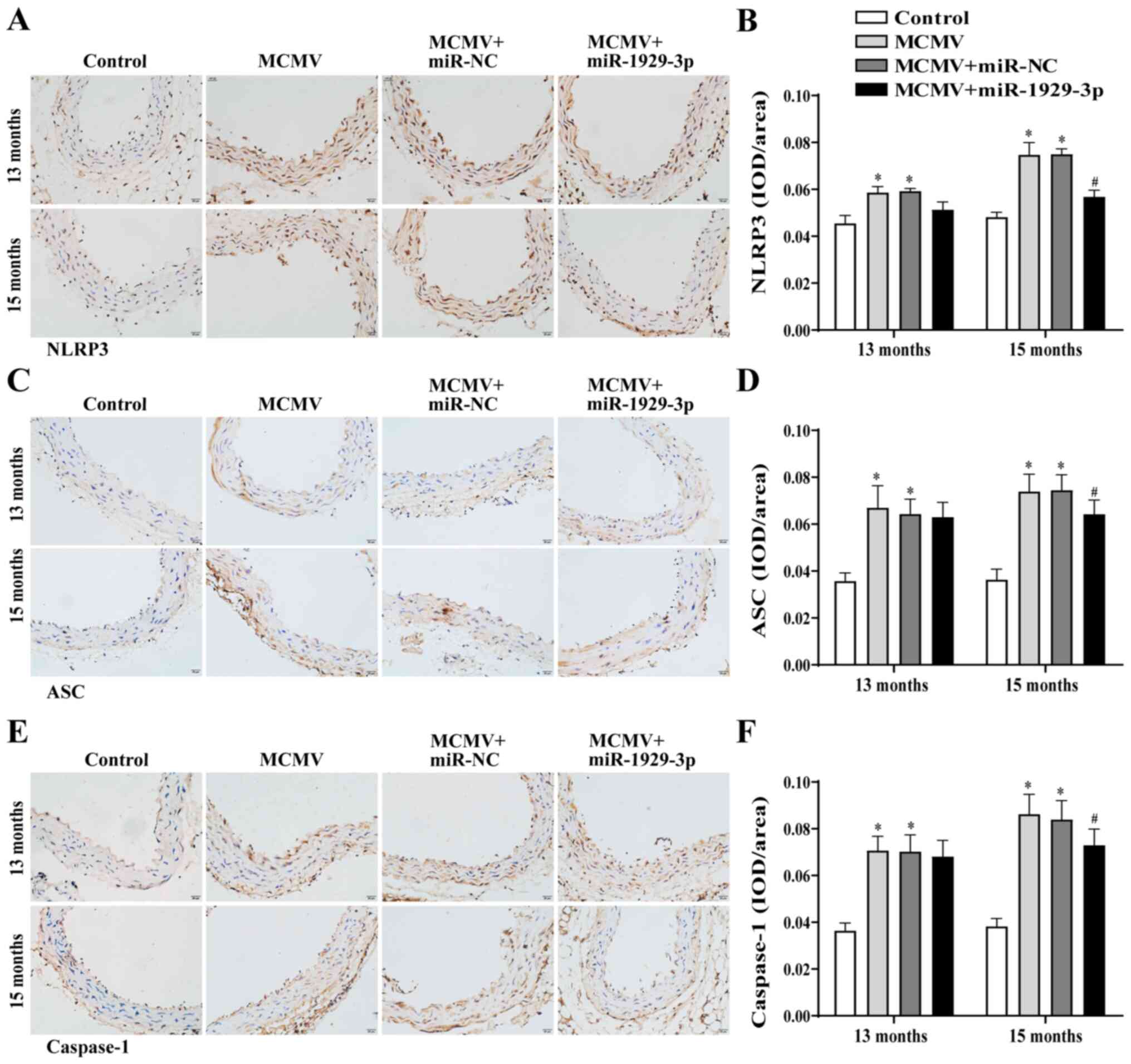
miR-1929-3p is involved in vascular
remodeling associated with MCMV infection by activating the NLRP3
inflammasome
NLRP3 inflammasome activation reportedly triggers an
inflammatory response and promotes vascular remodeling (8). Furthermore, it has been reported
that ET-1-activated Ednra promotes the activation of the NLRP3
inflammasome (40). To explore
the role of the NLRP3 inflammasome during the overexpression of
miR-1929-3p in improving MCMV-induced hypertension vascular
remodeling, the expression of the NLRP3 inflammasome in mouse blood
vessels was evaluated.
Immunohistochemical staining revealed that the
levels of NLRP3, ASC and caspase-1 were markedly upregulated in the
aortic tissues in the MCMV group at 13 and 15 months of age. NLRP3,
ASC and caspase-1 expression levels did not change significantly in
the MCMV + miR-1929-3p group at the age of 13 months, but were
significantly downregulated at 15 months (Fig. 6A-F). The western blot analysis
revealed that the expression of the NLRP3 inflammasome-related
proteins, NLRP3, ASC, pro-caspase-1, caspase-1, pro-IL-1 and IL-1β
were significantly increased in the MCMV-infected group. MCMV
activated caspase-1 and IL-1β; however, no significant difference
in IL-18 expression was observed. These upregulated indicators were
alleviated by miR-1929-3p overexpression in 15-month-old mice
(Fig. 7A-J). Similarly, ELISA
detected IL-18 and IL-1β in the plasma, and the results of this
assay corresponded to those of the western blot analysis (Fig. 7K and L). These data demonstrated
that miR-1929-3p overexpression can improve MCMV-induced vascular
remodeling, possibly by inhibiting the activation of the NLRP3
inflammasome via ET-1/Ednra.
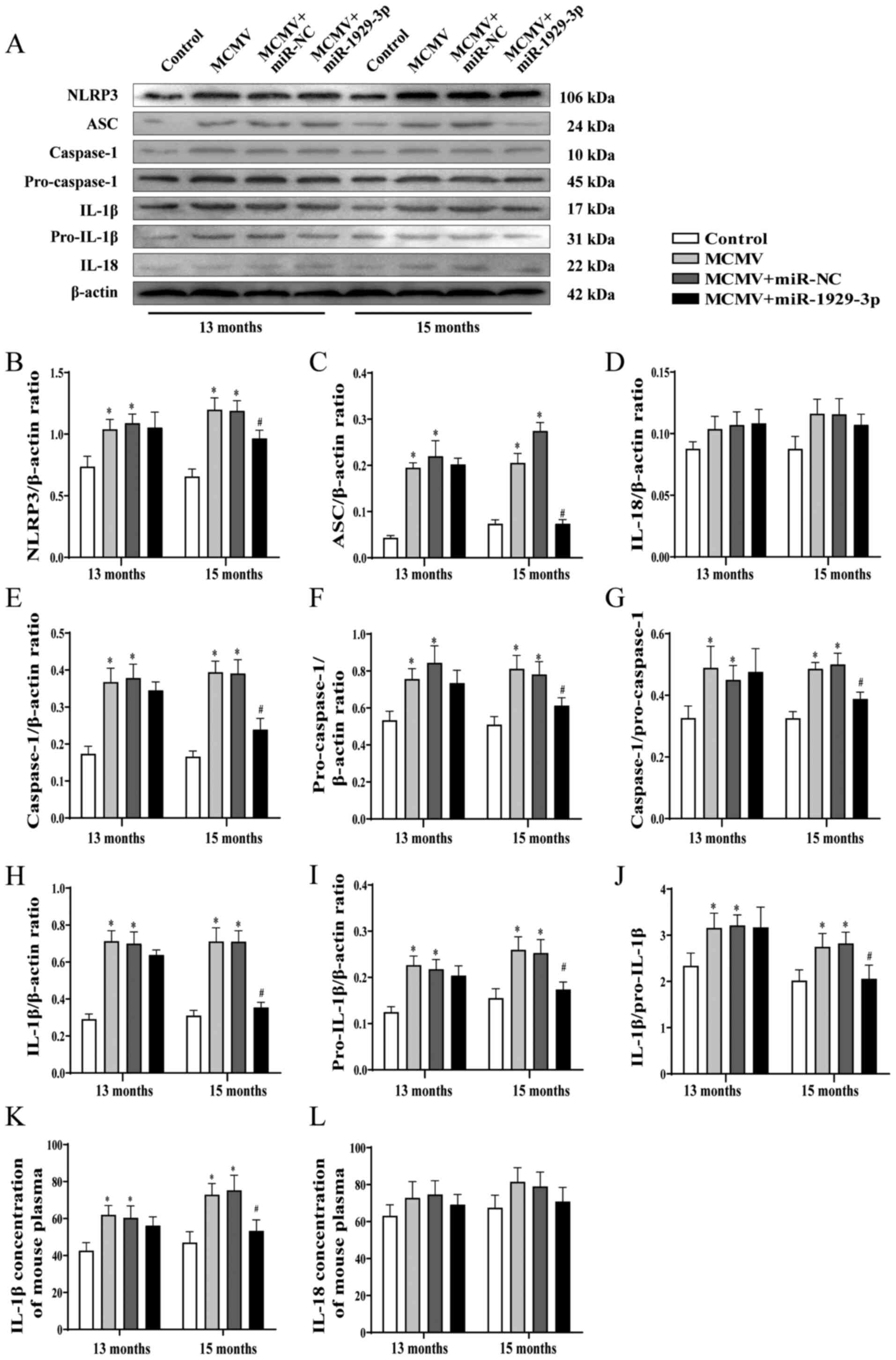 | Figure 7Overexpression of miR-1929-3p
alleviates MCMV-induced NLRP3 inflammasome activation. (A) Western
blotting and (B-L) statistical analysis of the expression of
inflammatory factors in aortic tissues of mice after MCMV infection
and miR-1929-3p overexpression. (B) NLRP3, (C) ASC, (D) IL-18, (E)
caspase-1, (F) pro-caspase-1, (G) caspase-1/pro-caspase-1 ratio;
(H) IL-1β, (I) pro-IL-1β, (J) IL-1β/pro-IL-1β ratio and (K) IL-1β
in mouse plasma. *P<0.05 vs. control of the same age;
#P<0.05 vs. MCMV + miR-NC group of the same age. In
panels B-L, data were analyzed using one-way ANOVA followed by
Tukey's multiple comparisons post hoc test. n=5 per group. MCMV,
murine cytomegalovirus; NLRP3, nucleotide-binding oligomerization
domain-like receptor pyrin domain-containing 3; ASC,
apoptosis-associated speck-like protein containing a CARD; IL-1β,
interleukin 1β; IL-18, interleukin 18. |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that miR-1929-3p plays an important role
in mediating hypertensive vascular remodeling in MCMV infection. It
was observed that MCMV-infected mice had elevated blood pressure,
thickened blood vessels, and decreased miR-1929-3p expression. When
miR-1929-3p was overexpressed, the expression of its target gene,
Ednra, decreased, while the activation of the NLRP3 inflammasome
was inhibited, which improved MCMV-induced vascular remodeling. In
other words, miR-1929-3p overexpression may suppress MCMV-induced
hypertensive vascular remodeling, indicating its potential value as
a therapeutic target for hypertension. To the best of our
knowledge, this is the first attempt at targeting miR-1929-3p for
MCMV-induced hypertension therapeutics.
MCMV is widely invasive and can infect a variety of
cells, including endothelial and smooth muscle cells (41). The fact that CMV infection may
lead to an increase in blood pressure has also been observed in
human studies (42). Hypertension
is a common chronic disease, which is associated with age (43). Therefore, 7-month-old C57BL/6J
mice infected MCMV were used to construct an animal model of
hypertension (44). Blood
pressure and vascular morphology detection were performed monthly.
It was observed that SBP, DBP and MAP were significantly increased
following MCMV infection at 9 months of age, while vascular
morphology did not change significantly, suggesting that the blood
pressure increase caused by MCMV infection occurred before the
morphological changes of the blood vessels. Vascular remodeling is
a characteristic phenotype in hypertension (45). This morphological change is mainly
manifested by the thickening of the media, increased wall rigidity
and decreased vascular compliance (46). It was observed that typical
vascular remodeling, such as increased aortic thickness and
collagen deposition in the aorta, occurred at 12 months of age in
the MCMV group. The markers are shown primarily at 9 months of age,
when blood pressure began to change, and at 12 months of age, when
blood vessel function began to change. In addition, it was observed
that blood pressure increases following MCMV infection precede
changes in vascular function. This may be partly due to the fact
that CMV re-infection usually presents with high levels of antibody
titers and is involved in the development of cardiovascular disease
(39,47). However, the degree of MCMV
infection at 9 months of age was significantly lower compared with
that at 12 months of age. This finding was consistent with our
previous population survey, which found that an increase in HCMV
IgG titer was an independent risk factor for EH and target organ
damage during HCMV infection (48). Another important reason may be
that elevated blood pressure is a hemodynamic factor that induces
vascular remodeling (49).
A study by Li et al (50) reported that miRNAs may serve as a
key link between HCMV and EH. We previously found that CMV
infection was associated with EH in Kazakh and Han males in
Xinjiang (51). Subsequently,
RNA-SEQ screening of miRNAs that were differentially expressed in
peripheral blood mononuclear cells from mice with MCMV-induced
hypertension revealed that 118 miRNAs were detected between the two
groups. Among these, 91 were upregulated and 27 were downregulated,
including virus-encoded miRNAs and mouse-encoded miRNAs. This
finding indicated that MCMV infection induced differential
expression of MCMV and host miRNAs, suggesting that the occurrence
and development of MCMV-induced hypertension may involve the
interaction of the two miRNAs. Bioinformatics analyses and RT-qPCR
validation of peripheral blood monocytes, blood vessels and
myocardial tissues revealed that the MMU-miR-1929-3p and
MCMV-miR-m01-4-5p results were consistent with those of miRNAs-SEQ
sequencing and bioinformatics analysis. However, comparative
analysis of the sequence targets of MCMV-miR-m01-4 did not identify
any genes associated with hypertension. Therefore, miR-1929-3p was
finally selected as the differentially expressed miRNA in
MCMV-induced EH. Using bioinformatics analysis and the
dual-luciferase reporter assay, Ednra was confirmed as the
EH-related target of miR-1929-3p (34). In the present study, RT-qPCR
analysis demonstrated that the relative expression level of
miR-1929-3p in the MCMV group was significantly decreased at the
age of 9 months. However, there was no significant change in the
expression of miR-1929-3p at the age of 9-15 months, indicating
that the expression of miR-1929-3p did not decrease progressively
with the duration of MCMV infection. miRNAs, a class of non-coding
single-stranded RNAs with a length of 21-25 nucleotides, are
widespread in eukaryotes and play important roles in various
biological processes, including cell proliferation,
differentiation, migration and apoptosis (52,53). Indeed, a large body of evidence
indicates that miRNAs are involved in the occurrence and
development of EH (54), as well
as vascular remodeling (55-57). For example, miR-150 can prevent
hypoxia-induced pulmonary vascular remodeling, fibrosis and
abnormal proliferation of pulmonary artery smooth muscle cells and
endothelial cells (58). These
findings indicate that miRNAs are key regulators of the phenotypic
transformation of VSMCs and the adverse remodeling of vessels in
hypertension (55). Therefore, it
was hypothesized that miR-1929-3p played an important role in MCMV
infection of C57BL/6J mice by increasing blood pressure and
inducing adverse vascular remodeling.
It was also investigated whether miR-1929-3p played
a role in EH and MCMV-induced vascular remodeling using a
recombinant adeno-associated virus-coated miR-1929-3p
overexpression plasmid. The data demonstrated that the relative
miR-1929-3p expression level in the aortic tissues of C57BL/6J mice
was significantly higher compared with that in the MCMV group after
1 month of rAAV-miR-1929-3p intervention, and was even
significantly higher after a further 3 months. These results
indicated that the targeted vascular delivery of miR-1929-3p
mediated by type 9 rAAV signifi-cantly increased the relative
miR-1929-3p expression level in the aortic tissues of C57BL/6J
mice. This finding was consistent with a previous study reporting
that type 9 rAAV can be stably transfected into adult mice
(59). During the detection of
blood pressure in mice, it was observed that the blood pressure in
MCMV + miR-1929-3p group mice increased at the age of 14 months,
but the vascular function did not improve significantly. However,
vascular remodeling was significantly reversed in the MCMV +
miR-1929-3p group at 15 months of age. This is consistent with our
previous findings, indicating that changes in blood pressure
precede vascular remodeling. Furthermore, it was proven that
miR-1929-3p overexpression inhibited MCMV-induced VSMC
proliferation and differentiation, and exerted a protective effect
on blood vessels.
MicroRNAs are post-transcriptional regulators of
gene expression that participate in various developmental and
cellular processes by inhibiting the translation of target genes
(60). Our group identified Ednra
as a direct target of miR-1929-3p, which was also verified in the
present study. Ednra was significantly upregulated following
MCMV-induced miR-1929-3p down-regulation. Mainly located in VSMCs,
Ednra is primarily involved in the occurrence and development of
vasoconstriction, cardiac hypertrophy, inflammation and
cardiovascular diseases (61-63). Ednra is activated by ET-1 and
induces the increase of O2−, promotes oxidative stress
and causes endothelial cell injury (64,65). Ednra inhibitors can downregulate
iNOS to alleviate endotoxin-induced liver injury in cirrhotic
patients (61). The present study
demonstrated that the down-regulation of miR-1929-3p by MCMV
increased Ednra levels, thereby leading to increased expression of
the endothelial injury factor ET-1 and reduced eNOS activity and NO
levels. Other studies have also demonstrated that the activation of
Ednra enhances NF-κB activation, oxidative stress and production of
pro-inflammatory cytokines (35,66). Additionally, TLR-induced NF-κB
activation has been found to lead to the activation of NLRP3,
pro-IL-1 and pro-IL-18 (28).
However, the association between Ednra and NLRP3 in hypertension
has not been widely studied. Therefore, the expression of NLRP3
inflammasome components and downstream IL-1β and IL-18 was
examined, and it was observed that IL-1β was significantly
inhibited when miR-1929-3p was overexpressed. However, there were
no significant effects of miR-1929-3p on IL-18 expression and
concentration in the plasma, possibly because the activation of
NLRP3 by different cofactors induced different levels of IL-18 and
IL-1β processing and secretion (67). Furthermore, Pirhonen et al
reported in their studies on the influenza A and Sendai viruses
that monocytes and macrophages have different capacities to produce
IL-1β and IL-18 due to their different sensitivities to viral
infection. In that study, the authors found that the expression of
IL-18 was induced only by the Sendai virus, while both the
influenza A and Sendai viruses induced IL-1β expression (68), an observation which may partly
explain our results. Therefore, it may be inferred that miR-1929-3p
inhibits the expression of Ednra, downregulates the activation of
the NLRP3 inflammasome and decreases the production of IL-1β in
MCMV-infected hypertensive mice. A limitation of the present study
was that the mechanism underlying the involvement of Ednra/NLRP3 in
the development of hypertension and vascular remodeling following
MCMV-induced downregulation of miR-1929-3p in mice was not fully
elucidated. Future work will aim to study these mechanisms in
further detail at the cellular and molecular level. Furthermore, it
is well known that miRNAs have a complex regulatory network, which
can either regulate the expression of multiple genes through a
single miRNA, or regulate the expression of a single gene via
multiple miRNAs (69). Although
miR-1929-3p was found to improve MCMV-induced hypertensive vascular
remodeling by inhibiting Ednra, whether other targets of
miR-1929-3p may be partially involved in promoting the
miR-1929-3p-mediated proliferation of blood vessels cannot be ruled
out. Moreover, the possibility that MCMV-induced differential
expression of other miRNAs and proteins may affect blood pressure
and vascular remodeling in mice must be considered. Furthermore,
since miR-1929-3p is a murine miRNA, it cannot be confirmed whether
it was specifically differentially expressed only in response to
MCMV infection.
In summary, the present study demonstrated that MCMV
induced an increase in blood pressure and promoted vascular
remodeling in mice, which was likely caused by the downregulation
of miR-1929-3p and the activation of ET-1/Ednra/NLRP3 inflammasome.
Overexpression of miR-1929-3p alleviated adverse MCMV-induced
vascular remodeling via a mechanism likely involving the inhibition
of ET-1/Ednra and subsequent deactivation of the NLRP3
inflammasome. Since miRNAs from different species are highly
homologous, miR-1929-3p may represent a novel CMV-mediated
hypertension biomarker and a potential target for regulating
vascular remodeling.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 31760291), the
National College Students innovation and Entrepreneurship Training
Program (no. 202010759027), and the Scientific Research Project of
Shihezi University (no. ZZZC201921A).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article or are available from
the corresponding author on reasonable request.
Authors' contributions
YS, FH and DX conceived and designed the
experiments. WZ, YS, DX, ZH, ZZ and YW performed the experiments.
NT, NL, LW, JP and YT analyzed the data. WZ, YL, HZ and FH wrote or
modified the manuscript. YS, ZH and FH confirm the authenticity of
all the raw data. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal studies were performed according to the
guide-lines of the Chinese Council on Animal Care, and the study
was approved by the Ethics Committee of Shihezi Medical University
(Shihezi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Renna NF, de Las Heras N and Miatello RM:
Pathophysiology of vascular remodeling in hypertension. Int J
Hypertens. 2013:8083532013.PubMed/NCBI
|
|
2
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiong JR: Controlling hypertension from a
public health perspective. Int J Cardiol. 127:151–156. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bloch MJ: Worldwide prevalence of
hypertension exceeds 1.3 billion. J Am Soc Hypertens. 10:753–754.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun F, Zhang K, Li X, He N, Zhao J and Qiu
C: Effects of angiotensin-converting enzyme gene and environment
interaction on essential hypertension in the Han nationality. Wei
Sheng Yan Jiu. 46:378–383. 2017.In Chinese.
|
|
6
|
Shah SFA, Iqbal T, Qamar R, Rafiq MA and
Hussain S: ARG1 gene polymorphisms and their association in
individuals with essential hypertension: A case-control study. DNA
Cell Biol. 37:609–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Samaranayake NR, Ong KL, Wong HK and
Cheung BM: Is human cytomegalovirus infection associated with
hypertension? The United States National Health and Nutrition
Examination Survey 1999-2002. PLoS One. 7:e397602012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao
MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, et al: NLRP3
inflammasome activation contributes to VSMC phenotypic
transformation and proliferation in hypertension. Cell Death Dis.
8:e30742017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hijmans JG, Diehl KJ, Bammert TD, Kavlich
PJ, Lincenberg GM, Greiner JJ, Stauffer BL and DeSouza CA:
Association between hypertension and circulating vascular-related
microRNAs. J Hum Hypertens. 32:440–447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy E, Yu D, Grimwood J, Schmutz J,
Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM and Shenk TE:
Coding potential of laboratory and clinical strains of human
cytomegalovirus. Proc Natl Acad Sci USA. 100:14976–14981. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatherer D, Seirafian S, Cunningham C,
Holton M, Dargan DJ, Baluchova K, Hector RD, Galbraith J, Herzyk P,
Wilkinson GW and Davison AJ: High-resolution human cytomegalovirus
transcriptome. Proc Natl Acad Sci USA. 108:19755–19760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cannon MJ, Schmid DS and Hyde TB: Review
of cytomegalo-virus seroprevalence and demographic characteristics
associated with infection. Rev Med Virol. 20:202–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Yang Y, Yang X and Cai J: Human
cytomegalovirus infection is a novel etiology for essential
hypertension. Med Hypotheses. 76:682–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emery VC: Recent progress in understanding
pathogenesis and control. QJM. 105:401–405. 2012. View Article : Google Scholar
|
|
15
|
Fu M, Gao Y, Zhou Q, Zhang Q, Peng Y, Tian
K, Wang J and Zheng X: Human cytomegalovirus latent infection
alters the expression of cellular and viral microRNA. Gene.
536:272–278. 2014. View Article : Google Scholar
|
|
16
|
Leite-Moreira AM, Lourenço AP,
Falcão-Pires I and Leite-Moreira AF: Pivotal role of microRNAs in
cardiac physiology and heart failure. Drug Discov Today.
18:1243–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar :
|
|
18
|
Li X, Wei Y and Wang Z: microRNA-21 and
hypertension. Hypertens Res. 41:649–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huo KG, Richer C, Berillo O, Mahjoub N,
Fraulob-Aquino JC, Barhoumi T, Ouerd S, Coelho SC, Sinnett D,
Paradis P and Schiffrin EL: miR-431-5p knockdown protects against
angiotensin II-induced hypertension and vascular injury.
Hypertension. 73:1007–1017. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li FJ, Zhang CL, Luo XJ, Peng J and Yang
TL: Involvement of the MiR-181b-5p/HMGB1 pathway in Ang II-induced
phenotypic transformation of smooth muscle cells in hypertension.
Aging Dis. 10:231–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen K, Xu L, Chen D, Tang W and Huang Y:
Human cytomeg-alovirus-encoded miR-UL112 contributes to
HCMV-mediated vascular diseases by inducing vascular endothelial
cell dysfunction. Virus Genes. 54:172–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi
L, Wang D and Wang J: miR-138 promotes migration and tube formation
of human cytomegalovirus-infected endothelial cells through the
SIRT1/p-STAT3 pathway. Arch Virol. 162:2695–2704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfeffer S, Sewer A, Lagos-Quintana M,
Sheridan R, Sander C, Grässer FA, van Dyk LF, Ho CK, Shuman S,
Chien M, et al: Identification of microRNAs of the herpesvirus
family. Nat Methods. 2:269–276. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meshesha MK, Veksler-Lublinsky I, Isakov
O, Reichenstein I, Shomron N, Kedem K, Ziv-Ukelson M, Bentwich Z
and Avni YS: The microRNA transcriptome of human cytomegalovirus
(HCMV). Open Virol J. 6:38–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Yu J and Liu Z: MicroRNAs
expressed by human cytomegalovirus. Virol J. 17:342020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elliott EI and Sutterwala FS: Initiation
and perpetuation of NLRP3 inflammasome activation and assembly.
Immunol Rev. 265:35–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishnan SM, Sobey CG, Latz E, Mansell A
and Drummond GR: IL-1β and IL-18: Inflammatory markers or mediators
of hyper-tension? Br J Pharmacol. 171:5589–5602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu D, Zeng X, Li X, Mehta JL and Wang X:
Role of NLRP3 inflammasome in the pathogenesis of cardiovascular
diseases. Basic Res Cardiol. 113:52018. View Article : Google Scholar
|
|
29
|
Intengan HD and Schiffrin EL: Vascular
remodeling in hypertension: Roles of apoptosis, inflammation, and
fibrosis. Hypertension. 38:581–587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Yang S, Yin R, Xiao Q, Ma A and
Pan X: MicroRNA-181a regulates the activation of the NLRP3
inflammatory pathway by targeting MEK1 in THP-1 macrophages
stimulated by ox-LDL. J Cell Biochem. 120:13640–13650. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wu Q, Yu J, Cao X and Xu Z:
miR-125a-5p inhibits the expression of NLRP3 by targeting CCL4 in
human vascular smooth muscle cells treated with ox-LDL. Exp Ther
Med. 18:1645–1652. 2019.PubMed/NCBI
|
|
32
|
Ren XS, Tong Y, Ling L, Chen D, Sun HJ,
Zhou H, Qi XH, Chen Q, Li YH, Kang YM and Zhu GQ: NLRP3 gene
deletion attenuates Angiotensin II-induced phenotypic
transformation of vascular smooth muscle cells and vascular
remodeling. Cell Physiol Biochem. 44:2269–2280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang R, Wu W, Li W, Huang S, Li Z, Liu R,
Shan Z, Zhang C, Li W and Wang S: Activation of NLRP3 inflammasome
promotes foam cell formation in vascular smooth muscle cells and
atherogenesis via HMGB1. J Am Heart Assoc. 7:e0085962018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yunzhong S, Xi D, Zhang X, Zhen H, Tang N,
YongMin L, Wang LM, Tang Y, Zhong H and He F: Screening and
validation of differentially expressed microRNAs and target genes
in hypertensive mice induced by cytomegalovirus infection. Biosci
Rep. BSR202023872020.
|
|
35
|
Yeager ME, Belchenko DD, Nguyen CM, Colvin
KL, Ivy DD and Stenmark KR: Endothelin-1, the unfolded protein
response, and persistent inflammation: Role of pulmonary artery
smooth muscle cells. Am J Respir Cell Mol Biol. 46:14–22. 2012.
View Article : Google Scholar :
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Shiao YH, Palli D, Caporaso NE, Alvord WG,
Amorosi A, Nesi G, Saieva C, Masala G, Fraumeni JF Jr and Rice JM:
Genetic and immunohistochemical analyses of p53 independently
predict regional metastasis of gastric cancers. Cancer Epidemiol
Biomarkers Prev. 9:631–633. 2000.PubMed/NCBI
|
|
38
|
Touyz RM and Schiffrin EL: Role of
endothelin in human hyper-tension. Can J Physiol Pharmacol.
81:533–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roberts ET, Haan MN, Dowd JB and Aiello
AE: Cytomegalovirus antibody levels, inflammation, and mortality
among elderly Latinos over 9 years of follow-up. Am J Epidemiol.
172:363–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ward R and Ergul A: Relationship of
endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal
cells in diabetes. Life Sci. 159:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reddehase MJ and Lemmermann NAW: Cellular
reservoirs of latent cytomegaloviruses. Med Microbiol Immunol.
208:391–403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng J, Ke Q, Jin Z, Wang H, Kocher O,
Morgan JP, Zhang J and Crumpacker CS: Cytomegalovirus infection
causes an increase of arterial blood pressure. PLoS Pathog.
5:e10004272009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bruno RM, Duranti E, Ippolito C, Segnani
C, Bernardini N, Di Candio G, Chiarugi M, Taddei S and Virdis A:
Different impact of essential hypertension on structural and
functional age-related vascular changes. Hypertension. 69:71–78.
2017. View Article : Google Scholar
|
|
44
|
Dinh QN, Drummond GR, Kemp-Harper BK, Diep
H, De Silva TM, Kim HA, Vinh A, Robertson AAB, Cooper MA, Mansell
A, et al: Pressor response to angiotensin II is enhanced in aged
mice and associated with inflammation, vasoconstriction and
oxidative stress. Aging (Albany NY). 9:1595–1606. 2017. View Article : Google Scholar
|
|
45
|
Baumbach GL and Ghoneim S: Vascular
remodeling in hyper-tension. Scanning Microsc. 7:137–143. 1993.
|
|
46
|
Laurent S and Boutouyrie P: The structural
factor of hypertension: Large and small artery alterations. Circ
Res. 116:1007–1021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang GC, Kao WH, Murakami P, Xue QL, Chiou
RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD and
Fried LP: Cytomegalovirus infection and the risk of mortality and
frailty in older women: A prospective observational cohort study.
Am J Epidemiol. 171:1144–1152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Z, Tang Y, Tang N, Feng Q, Zhong H, Liu
YM, Wang LM and He F: High anti-human cytomegalovirus antibody
levels are associated with the progression of essential
hypertension and target organ damage in Han Chinese population.
PLoS One. 12:e01814402017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mulvany MJ: Small artery remodeling and
significance in the development of hypertension. News Physiol Sci.
17:105–109. 2002.PubMed/NCBI
|
|
50
|
Li S, Zhu J, Zhang W, Chen Y, Zhang K,
Popescu LM, Ma X, Lau WB, Rong R, Yu X, et al: Signature microRNA
expression profile of essential hypertension and its novel link to
human cytomegalovirus infection. Circulation. 124:175–184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang N, Li JW, Liu YM, Zhong H, Wang LM,
Deng FM, Qu YY, Hui J, Cheng J, Tang B, et al: Human
cytomegalovirus infection is associated with essential hypertension
in Kazakh and Han Chinese populations. Med Sci Monit. 20:2508–2519.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bronze-da-Rocha E: MicroRNAs expression
profiles in cardio-vascular diseases. Biomed Res Int.
2014:9854082014. View Article : Google Scholar
|
|
53
|
Song X, Shan D, Chen J and Jing Q: miRNAs
and lncRNAs in vascular injury and remodeling. Sci China Life Sci.
57:826–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jeppesen PL, Christensen GL, Schneider M,
Nossent AY, Jensen HB, Andersen DC, Eskildsen T, Gammeltoft S,
Hansen JL and Sheikh SP: Angiotensin II type 1 receptor signal-ling
regulates microRNA differentially in cardiac fibroblasts and
myocytes. Br J Pharmacol. 164:394–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie C, Zhang J and Chen YE: Chapter
Fifteen-MicroRNA and Vascular Smooth Muscle Cells. Elsevier Science
& Technology; 2011
|
|
56
|
Kang H and Hata A: MicroRNA regulation of
smooth muscle gene expression and phenotype. Curr Opin Hematol.
19:224–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Robinson HC and Baker AH: How do microRNAs
affect vascular smooth muscle cell biology? Curr Opin Lipidol.
23:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Y, Ren W, Wang X, Yu X, Cui L, Li X,
Zhang X and Shi B: MicroRNA-150 relieves vascular remodeling and
fibrosis in hypoxia-induced pulmonary hypertension. Biomed
Pharmacother. 109:1740–1749. 2019. View Article : Google Scholar
|
|
59
|
Bostick B, Ghosh A, Yue Y, Long C and Duan
D: Systemic AAV-9 transduction in mice is influenced by animal age
but not by the route of administration. Gene Ther. 14:1605–1609.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Keller S, Karaa A, Paxian M, Clemens MG
and Zhang JX: Inhibition of endothelin-1-mediated up-regulation of
iNOS by bosentan ameliorates endotoxin-induced liver injury in
cirrhosis. Shock. 25:306–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khalil RA: Modulators of the vascular
endothelin receptor in blood pressure regulation and hypertension.
Curr Mol Pharmacol. 4:176–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
D'Orléans-Juste P, Akide Ndunge OB,
Desbiens L, Tanowitz HB and Desruisseaux MS: Endothelins in
inflammatory neurological diseases. Pharmacol Ther. 194:145–160.
2019. View Article : Google Scholar :
|
|
64
|
Xu H, Lin L and Yuan WJ: Antiarrhythmic
effect of endothelin-A receptor antagonist on acute ischemic
arrhythmia in isolated rat heart. Acta Pharmacol Sin. 24:37–44.
2003.PubMed/NCBI
|
|
65
|
Elmarakby AA, Loomis ED, Pollock JS and
Pollock DM: NADPH oxidase inhibition attenuates oxidative stress
but not hypertension produced by chronic ET-1. Hypertension.
45:283–287. 2005. View Article : Google Scholar
|
|
66
|
Loomis ED, Sullivan JC, Osmond DA, Pollock
DM and Pollock JS: Endothelin mediates superoxide production and
vasoconstriction through activation of NADPH oxidase and uncoupled
nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther.
315:1058–1064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schmidt RL and Lenz LL: Distinct licensing
of IL-18 and IL-1β secretion in response to NLRP3 inflammasome
activation. PLoS One. 7:e451862012. View Article : Google Scholar
|
|
68
|
Pirhonen J, Sareneva T, Kurimoto M,
Julkunen I and Matikainen S: Virus infection activates IL-1 beta
and IL-18 production in human macrophages by a caspase-1-dependent
pathway. J Immunol. 162:7322–7329. 1999.PubMed/NCBI
|
|
69
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|