Introduction
Spinal cord injury (SCI) is a life-shattering
neurological condition that affects an estimated two or three
million individuals worldwide (1). SCI is a two-step process, involving
primary and secondary phases, in which secondary cord injury occurs
following primary injury and this can be preventable or reversible
(2-4). It is known that secondary injury
exacerbates damage and limits restorative processes, accompanied by
a potent inflammatory response, neuronal necrosis and apoptosis
(5). Although various
therapeutic strategies have been applied for SCI, there is
currently no available effective therapeutic method for this
condition, at least to the best of our knowledge. Thus, the
development of novel and effective therapeutic strategies for SCI
is of utmost importance.
Recently, Traditional Chinese Medicine (TCM), an
abundant source of natural drugs, has attracted increasing
attention in the field of SCI treatment. For example, curcumin
treatment has been shown to promote functional recovery and
alleviate edema in the injured spinal cord in a rat model of SCI,
and these effects may be associated with its antioxidant and
anti-inflammatory activities (6,7).
For example, resveratrol has been reported to improve the injured
spinal cord by inhibiting oxidant formation and neuronal apoptosis
(8,9). These findings have demonstrated the
effectiveness of TCM in the prevention and treatment of SCI.
Gypenoside XVII (GP-17) is a novel phytoestrogen belonging to the
gypenosides, and the potent anticancer, anti-inflammatory,
antioxidant and anti-apoptotic activities of GP-17 have been
demonstrated by a number of studies (10-12). Importantly, the neuroprotective
effects of GP-17 have also been proven. For example, Meng et
al (13) demonstrated that
GP-17 conferred protection to cellular and rodent models of
Alzheimer's disease (AD) by activating transcription factor EB
(TFEB). Thus, it is reasonable that GP-17 is used in the treatment
of SCI.
MicroRNAs (miRNAs or miRs) are single-stranded
non-coding RNAs that negatively regulate gene expression by binding
to the 3′-UTR of their target genes at the post-transcriptional
level (14). To date, increasing
evidence has revealed the important roles of miRNAs in SCI, which
are involved in a number of secondary injury responses, including
inflammation, apoptosis and oxidative stress (15,16). For example, Dai et al
(17) reported that miR-137
promoted the recovery of SCI by degrading NEUROD4 to relieve the
spinal cord inflammation and oxidative stress in mice. Lin et
al (18) also found that
miR-409 was downregulated following SCI and the overexpression of
miR-409 promoted the recovery of SCI by directly targeting ZNF366
in mice. Feng et al (19)
reported that miR-204-5p upregulation promoted the recovery of the
upper and lower limb strength in mice with SCI by affecting the
levels of the inflammatory cytokines, Toll-like receptor (TLR)4 and
inducible nitric oxide synthase (iINOS), by targeting SRY-Box
transcription factor 11 (SOX11). Recently, several studies have
revealed that gypenosides have the potential to modulate miRNA
expression in various cancer cells (20-22). Against this background, the
present study examined whether miRNAs are involved in the
therapeutic effects of GP-17 on SCI.
In the present study, a mouse model of SCI was
established and the effects of GP-17 on SCI were explored.
Subsequently, the differentially expressed miRNAs in the Gene
Expression Omnibus (GEO) dataset, GSE67515, were analyzed by
bioinformatics analysis, and the role of candidate miRNAs in the
protective effects of GP-17 was examined and the underlying
mechanisms were investigated. The findings presented herein
highlight the potential value of GP-17 in the management of
SCI.
Materials and methods
Animals and drugs
Adult female C57BL/6 mice (aged 8-10 weeks, weighing
20-25 g) were purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd. All animal care and experimental procedures were performed at
the Animal experimental Center of the First Affiliated Hospital,
and College of Clinical Medicine of Henan University of Science and
Technology and all procedures were approved by the Animal Ethics
Committee of the First Affiliated Hospital, and College of Clinical
Medicine of Henan University of Science and Technology. Mice were
maintained under controlled conditions with a 12-h light-dark
cycle, at 23°C, ~40% humidity with access to food and water. GP-17
(cat. no. PHL83506) used in the present study was purchased from
Sigma-Aldrich, Merck KGaA. The chemical structure of GP-17 is
illustrated in Fig. 1A.
Experimental design
Mice were randomly divided into four groups (n=10
per group) as follows: i) The sham-operated (sham) group; ii) SCI
group; iii) SCI + GP-17 group (10 mg/kg); and iv) the SCI + GP-17
group (50 mg/kg). All mice in the SCI model group were anesthetized
by an intraperitoneal injection of 50 mg/kg pentobarbital sodium,
after which a 3-cm skin incision along the median line on the back
of the animals was made. Subsequently, a laminectomy was performed
using Mouse Laminectomy Forceps (Fine Science Tools GmbH) at the T9
level, followed by a mechanically controlled compression injury
using a mouse spinal cord compression device based on a previous
study (23). The mice in the
sham group were subjected to the same surgical procedure, but
sustained no impact injury. Following surgery, the mice were
immediately treated with GP-17 (10 and 50 mg/kg i.g. Winherb
Medical S&T Development) administered daily for 28 days.
Schematic diagrams of the in vivo experimental designs are
presented Fig. 1B.
In another experiment, the mice were randomly
divided into four groups as follows: The SCI group, SCI + GP-17
group, SCI + GP-17 + antagomir-21 group, and SCI + GP-17 +
antagomir-negative control (NC) group. In the SCI + GP-17 +
antagomir-21 group/antagomir-NC group (n=10/group), the mice were
subjected to SCI and then treated with antagomir-21/antagomir-NC (5
nmol/g/day, Guangzhou RiboBio Co., Ltd.) via intrathecal injection
beginning 15 min after SCI for 3 consecutive days. On the following
day after SCI, GP-17 (50 mg/kg, i.g.) was administered to the mice
daily until they were sacrificed.
Pentobarbital sodium (50 mg/kg, intraperitoneal
injection) was used for anesthesia prior to each surgery, and all
efforts were made to minimize animal suffering. The health and
behavior of the mice were monitored twice a day. No mice were found
dead during the anesthesia process. Following recovery from
anesthesia, the mice exhibited paralysis of both hind limbs and
urinary disorder. Their bladders were manually expressed twice a
day until the recovery of reflex voiding. The animals were
euthanized when the defined humane endpoint requirements were met:
A loss of >15% of body weight within 1-2 days or of >20% loss
in body weight in overall; the observation of apparent signs of
suffering, such as lethargy, squinted eyes, dehydration or a
hunched back. Euthanasia was performed by an intraperitoneal
injection of pentobarbital sodium (50 mg/kg) followed by cervical
dislocation, and animal death was confirmed by the cessation of
respiration and heartbeat (24).
At 28 days post-injury, the mice were euthanized, and the spinal
cord tissues (1 cm with injury epicenter located centrally) were
then harvested, and fixed in 4% paraformaldehyde (PFA) in 4°C in
PBS (pH 7.4) for 20 min, embedded in paraffin, and sectioned at a
thickness of 4 µm for immunohistochemistry.
Immunohistochemistry (IHC)
Subsequently, the fixed sections were blocked with
10% bovine serum for 30 min at room temperature following
incubation in 3% H2O2 for 15 min at room
temperature. The sections were then incubated with cleaved
caspase-3 antibody (1:200; cat. no. 9664, Cell Signaling
Technology, Inc.) overnight at 4°C. Subsequently, the sections were
incubated with anti-rabbit IgG, HRP-linked antibody (1:200; cat.
no. 7074, Cell Signaling Technology, Inc.) at room temperature for
60 min. Finally, images were acquired using a microscope with a
digital camera (VHX-5000, Keyence Corporation) at ×200
magnification.
Basso, Beattie and Bresnahan (BBB)
locomotor rating scale
The locomotor activity was evaluated using the BBB
scoring method at 1, 7, 14, 21 and 28 days following SCI as
previously described (25). The
scores were recorded by two independent and well-trained
investigators according to the BBB scale.
Spinal cord water content
measurement
At 28 days post-injury, the spinal tissues were
immediately weighed, and then dried at 80°C for 48 h. After the dry
weight was measured, the spinal cord water content was evaluated as
follows: Water content=[(wet weight-dry weight)/wet weight]
×100%.
Enzyme-linked immunosorbent assay
(ELISA)
Spinal cord samples were harvested and homogenized
in phosphate-buffered saline (PBS), and then centrifuged at 5,000 ×
g at 4°C for 10 min. The protein expression levels of tumor
necrosis factor (TNF)-α (cat. no. PT516), interleukin (IL)-6 (cat.
no. P1328), IL-1β (cat. no. P1303) and IL-10 (cat. no. PI525) in
the supernatant were detected by ELISA, using protocols supplied by
the manufacturer (Beyotime Institute of Biotechnology).
Analysis of GEO database
A microarray dataset was obtained from the GEO
database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE67515).
GEO2R (www.ncbi.nlm.nih.gov/geo/geo2r/), an interactive web
tool, was applied to compare the samples in two different groups
under the same experimental condition. Differentially expressed
miRNAs (DE-miRNAs) were then identified based on the fold change.
The heatmap of the DE-miRNAs was created using a method of
hierarchical clustering using GeneSpring GX, version 7.3 (Agilent
Technologies, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from spinal cord tissues
using a mirVana™ miRNA Isolation kit (Thermo Fisher Scientific,
Inc.). The reverse transcription of miRNA and mRNA was generated
using the PrimeScript RT Reagent kit (Takara Biotechnology Co.,
Ltd.) and PrimeScript RT kit (Takara Biotechnology Co., Ltd.),
respectively. qPCR was performed using the SYBR Premix Ex Taq
(Takara Bio, Inc.) on an ABI Prism7500 Sequence Detection System
(Thermo Fisher Scientific, Inc.). U6 was used as an internal
control for miRNAs, and GAPDH for phosphatase and tensin homologue
(PTEN), respectively. The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 30 sec, 40 cycles of denaturation at
95°C for 5 sec, annealing at 58°C for 30 sec, followed by extension
at 72°C for 15 sec. The primer sequences were listed in Table I. Analyses of gene expression
were performed using the 2−ΔΔCq method (26).
 | Table ISequences of primers used in the
present study. |
Table I
Sequences of primers used in the
present study.
| Gene | Primer
sequences |
|---|
| PTEN | F:
5′-CAGAAAGACTTGAAGGCGTAT-3′ |
| R:
5′-TGGCGGTGTCATAATGTC-3′ |
| GAPDH | F: 5′
CTCATGACCACAGTCCATGCCATCACTG-3′ |
| R:
5′-CATGAGGTCCACCACCCTGTTGCTGTA-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
| R:
5′-GTCATACTCCTGCTTGCTGAT-3′ |
| miR-21 | F:
5′-GCAGGGTCCGAGGTATTC-3′ |
| R:
5′-CTACTCACAAAACAGGAGTGGAATC-3′ |
| miR-34a-5p | F:
5′-AGCCGCTGGCAGTGTCTTA-3′ |
| R:
5′-CAGAGCAGGGTCCGAGGTA-3′ |
| miR-494 | F:
5′-TGGTGATGGGATTTGAAACATACACGGGAAAC-3′ |
| R:
5′-AGATAGACGG-TGTCGCTGTTGAAGTCAG-3′ |
| miR-142 | F:
5′-AACTCCAGCTGGTCCTTAG-3′ |
| R:
5′-TCTTGAACCCTCATCCTGT-3′ |
| let-7 | F:
5′-UGAGGUAGUAGAUUGUAUAGUU-3′ |
| R:
5′-CUAUACAAUCUACCUCAUU-3′ |
| miR-155 | F:
5′-CTCGTGGTAATGCTAATTGTGA-3′ |
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| miR-543 | F:
5′-GGAAACATTCGCGGTGC-3′ |
| R:
5′-GTGCGTGTCGTGGAGTCG-3′ |
Cells, cell culture and transfection
Immortal BV-2 murine microglial cells were obtained
from Procell Life Science & Technology Co., Ltd. (cat. no.
CL-0493) and cultured in DMEM/F12 supplemented with 10% FBS (Gibco,
Thermo Fisher Scientific, Inc.), and 1% penicillin and streptomycin
(Sigma-Aldrich, Merck KGaA) in 5% CO2 at 37°C.
Agomir-21, agomir-NC, antagomir-21 and antagomir-NC
were purchased from the Shanghai GenePharma Co., Ltd. BV-2 cells
were transfected with 100 nM agomir-21 or antagomir-21 or controls
using Lipofectamine 2000® (Invitrogen, Thermo
Scientific, Inc.) according to manufacturer's instructions. The
cells were harvested at 24 h after transfection for testing.
Bioinformatics analysis
The miRNA target prediction tools PicTar version
2007 (https://pictar.mdc-berlin.de/) and
TargetScan Release 7.0 (http://targetscan.org/) were used to search for
putative targets of miR-21.
Luciferase reporter assay
The fragment of the 3′-UTR of PTEN [wild-type (wt)
or mutant (mut)] was amplified and cloned into the pGL3 control
vector (Promega Corporation). Site-directed mutagenesis of the PTEN
3′-UTR at the putative miR-21 binding site was performed using a
QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies,
Inc.). BV-2 cells were treated with antagomir-21/antagomir-NC and
these luciferase reporter plasmids using Lipofectamine
2000® (Invitrogen, Thermo Scientific, Inc.). At 48 h
post-transfection, luciferase activity was assessed using the dual
luciferase reporter kit (Promega Corporation). Renilla
activity was used to normalize Firefly luciferase activity.
Western blot analysis
Total protein was extracted from the spinal cord
specimens using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and quantified with a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). The proteins (30 µg/lane)
were then separated by 10% SDS-PAGE gels and transferred to PVDF
membranes (EMD Millipore). Thereafter, the membranes were blocked
with 5% skim milk solution for 1 h at room temperature and then
incubated with specific primary antibodies to PTEN (#9188,
1:1,000), cleaved caspase-3 (#9661, 1:1,000), caspase-3 (#14220,
1:1,000), AKT (#4685, 1:1,000), p-AKT (#9611, 1:1,000), mammalian
target of rapamycin (mTOR; #2983, 1:1,000) p-mTOR (#5536, 1:1,000)
and β-actin (#3700, 1:1,000 dilution) at 4°C overnight.
Subsequently, the corresponding anti-rabbit secondary antibodies
(cat. no. 3678, 1:2,000) were added to the membranes for 2 h at
room temperature. All antibodies were obtained from Cell Signaling
Technology, Inc. The bands were visualized using an ECL kits (EMD
Millipore). Semi-quantification was performed using ImageJ version
1.46 (NIH).
Statistical analysis
Statistical analysis was conducted using SPSS
software (version 18.0; SPSS, Inc.). Data are expressed as the mean
± SD. Statistical significance analysis was performed using an
unpaired Student's t-test between two groups and one-way ANOVA
among multiple groups followed by Tukey's post hoc test.
Differences were considered statistically significant when
P<0.05.
Results
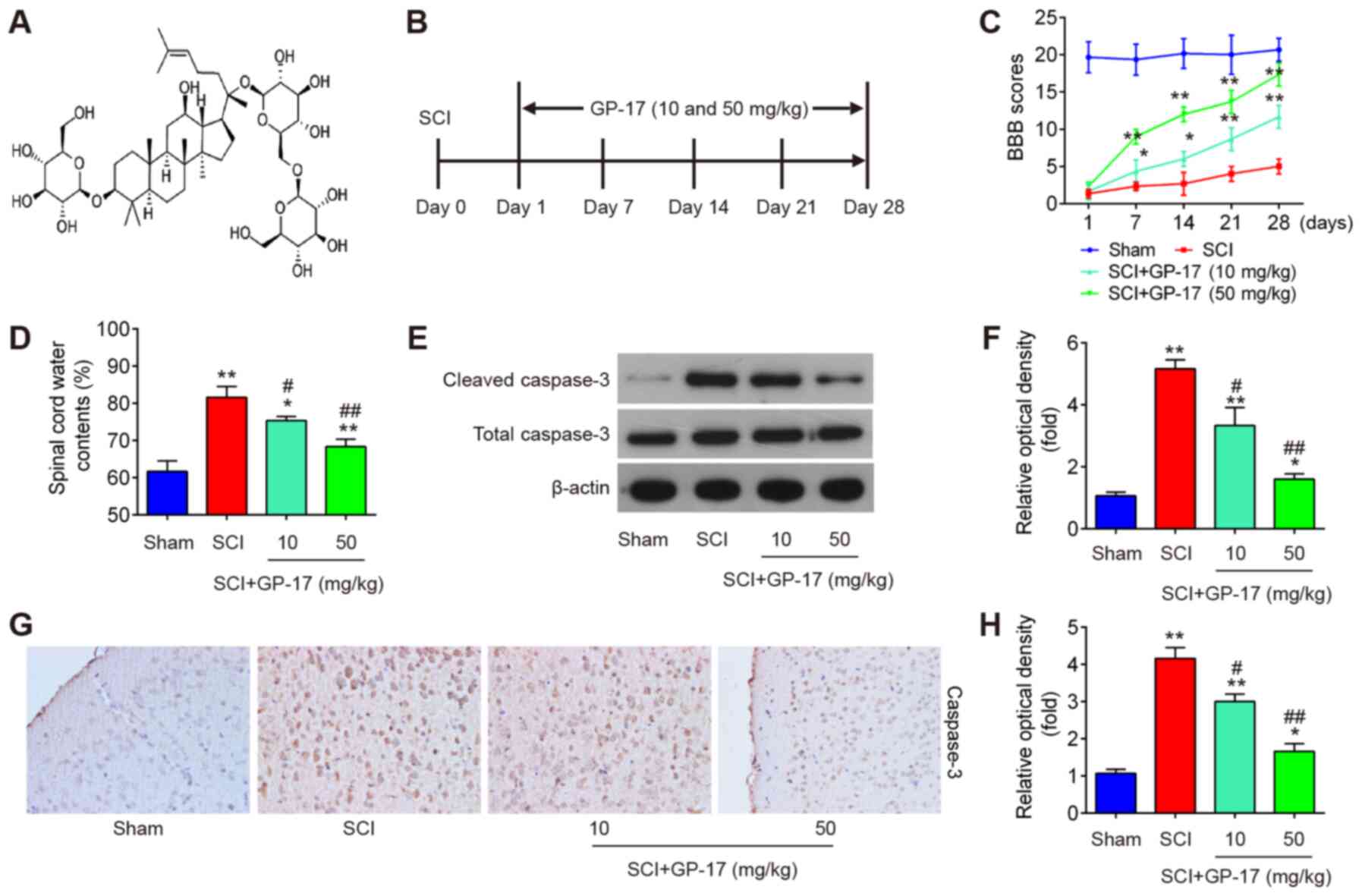
GP-17 promotes the recovery of mice with
SCI by reducing neuronal apoptosis
First, the mouse model of SCI was established as
described above. As shown in Fig. 1C
and D, the BBB score was decreased and the water content in the
spinal cord was increased in the tissues from mice with SCI,
compared with the sham group, indicating that the mouse model of
SCI was successfully established. To investigate the role of GP-17
in mice with SCI, GP-17 (10 and 50 mg/kg) was administered to the
mice with SCI by gavage daily for 28 days. Following treatment with
GP-17, the BBB score was significantly increased, and the water
content was markedly reduced in the GP-17 group compared with the
SCI group (P<0.01; Fig. 1C and
D). To determine whether GP-17 prevents neuronal apoptosis, the
expression levels of cleaved caspase-3, the key intracellular
cysteine protease of the cascade of events associated with
apoptosis, were examined by western blot analysis in mice with SCI.
As shown in Fig. 1E and F, the
expression of cleaved caspase-3 was significantly increased in the
SCI group compared with the sham group, whereas the expression of
cleaved caspase-3 in the GP-17 group was markedly decreased
compared with that in the SCI group. Similar results were obtained
by IHC (Fig. 1G). Notably, the
potency of 50 mg/kg GP-17 was greater than that of 10 mg/kg GP-17.
Collectively, these results suggest that GP-17 may improve motor
functional recovery by reducing neuronal apoptosis in mice.
GP-17 suppresses SCI induced inflammatory
response in mice
Subsequently, the effects of GP-17 on the
inflammatory response in mice with SCI were examined. The results
revealed that the expression levels of the anti-inflammatory
cytokine, IL-10, were significantly decreased, while the levels of
pro-inflammation cytokines, including IL-1β, TNF-α and IL-6 were
markedly increased in mice with SCI compared with those in sham
group; however, these effects were attenuated following GP-17
treatment (P<0.01; Fig. 2).
Therefore, the data indicated that GP-17 improved the inflammatory
response in mice with SCI.
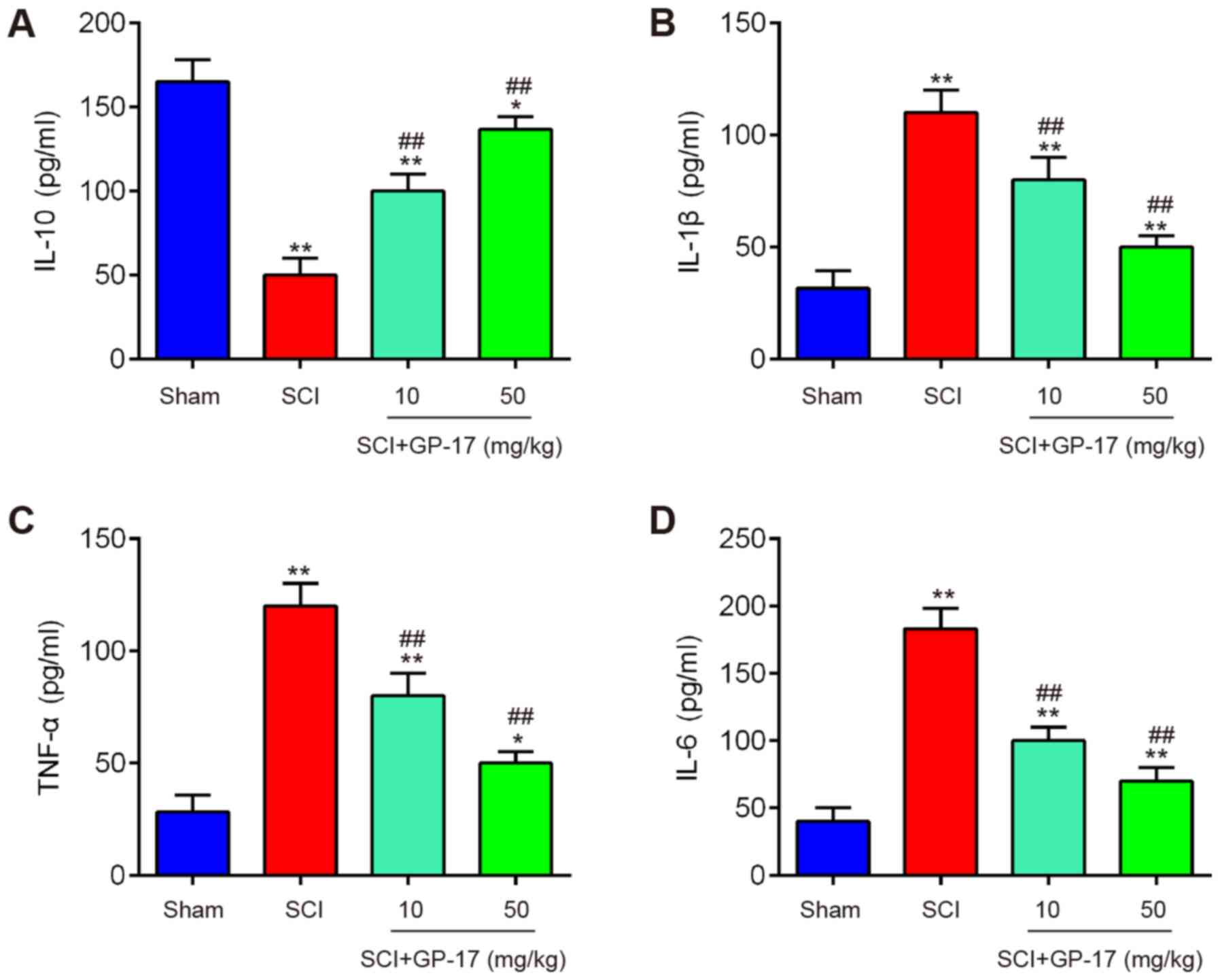 | Figure 2GP-17 reduces the inflammatory
response in the spinal cord of mice with SCI. After the SCI mouse
model was established, GP-17 (10 and 50 mg/kg) was administered to
the mice daily for 28 days. Inflammatory cytokines, including (A)
IL-10, (B) IL-1β, (C) TNF-α, and (D) IL-6 were evaluated by ELISA.
Data represent the mean ± SD of three independent experiments.
*P<0.05, **P<0.01 vs. sham group;
##P<0.01 vs. SCI group. GP-17, gypenoside XVIIl; SCI,
spinal cord injury; IL, interleukin; TNF-α, tumor necrosis factor
α. |
miR-21 is upregulated by GP-17 in mice
with SCI
In order to investigate the potential mechanisms of
GP-17 in regulating the apoptosis and inflammatory response in SCI,
differentially expressed miRNAs were analyzed through the retrieval
of the gene expression dataset, GSE67515. As shown in Fig. 3A, a great number of
differentially expressed miRNAs (24 miRNAs were significantly
downregulated and 21 miRNAs were upregulated) were found in spinal
cord tissues following SCI. A total of seven miRNAs (miR-21,
miR-34a-5p, miR-494, miR-543-3p, let-7, miR-142 and miR-155-5p)
were selected and further confirmed through RT-qPCR. The results
revealed that miR-21, miR-34a-5p, miR-494 and miR-543-3p were
markedly decreased, while let-7, miR-142 and miR-155-5p were
significantly increased; these findings are in line with those of
previous reports, further attesting to the reliability of the
current microarray results (27-33). Of note, among these miRNAs,
miR-21 was the only significantly upregulated miRNA when GP-17 was
administered to the mice with SCI, whereas the other miRNAs
exhibited no significant difference in expression in the mice with
SCI following GP-17 treatment (P<0.01; Fig. 3B). Previous studies have reported
the anti-apoptotic and anti-inflammatory effects of miR-21 in SCI
animal and cell models (27,34,35). Therefore, it was hypothesized
that the alteration of miR-21 expression may have contributed to
the protective effects of GP-17 against SCI.
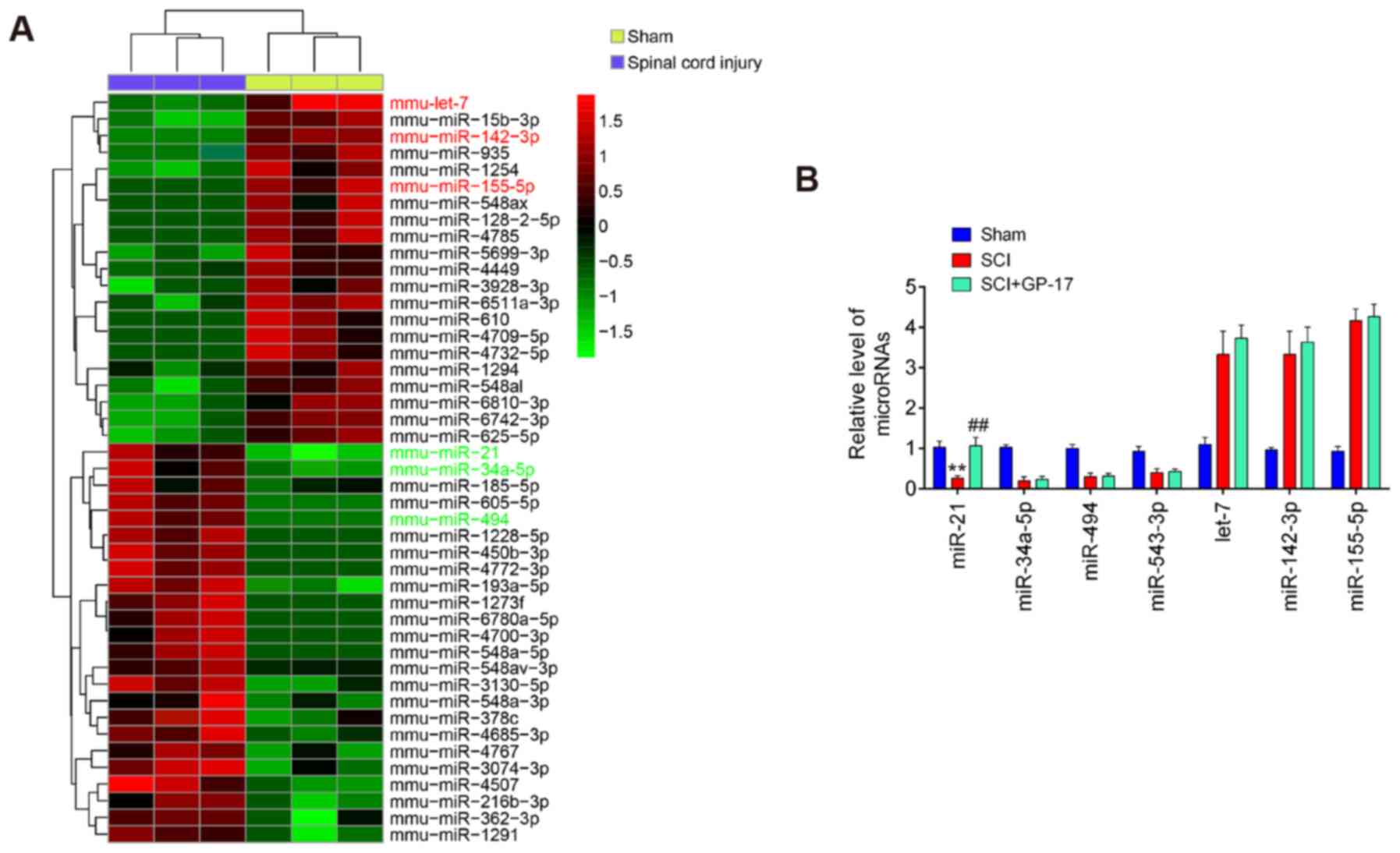 | Figure 3GP-17 upregulates miR-21 expression
in mice with SCI. (A) Heatmap of miRNA expression in the groups
(SCI and Sham) was created using a method of hierarchical
clustering using GeneSpring GX, version 7.3. Rows represent groups
and columns represent microRNAs. Color key indicates microRNA
expression values, green indicates low expression levels, while red
indicates high expression levels. (B) The expression levels of
miR-21, miR-494, miR-543-3p, miR-34a-5p, let-7 and miR-155-5p were
detected in sham, SCI, and SCI + GP-17 groups by RT-qPCR. Data
represent the mean ± SD of three independent experiments.
**P<0.01 vs. sham group; ##P<0.01 vs.
SCI group. GP-17, gypenoside XVIIl; SCI, spinal cord injury. |
GP-17 suppresses SCI induced apoptosis
and the inflammatory response by regulating miR-21
To clarify the role of miR-21 in the GP-17-induced
protective effects, the mice with SCI were treated with
antagomir-21/antagomir-NC via intrathecal injection, followed by
GP-17 treatment. First, the efficacy of antagomir-21 in vivo
was evaluated by RT-qPCR. As shown in Fig. 4A, compared with Sham group, SCI
resulted in a significant decrease in the expression of miR-21,
while GP-17 treatment reversed the inhibitory effects induced by
SCI on the expression levels of miR-21. Moreover, antagomir-21
injection significantly decreased the levels of miR-21 compared
with the SCI + GP-17 group (P<0.01; Fig. 4A). As mentioned above, GP-17
treatment improved motor function and spinal cord edema, but failed
to do so in the mice with SCI injected with antagomir-21
(P<0.01; Fig. 4B and C).
Functional experiments revealed that GP-17 significantly
downregulated the expression levels of cleaved caspase-3 in mice
with SCI, while the inhibitory effect of GP-17 was partially
reversed by antagomir-21 (P<0.01; Fig. 4D and E). Of note, the results of
ELISA revealed that the suppressive effects of GP-17 on the
inflammatory response were also reversed by antagomir-21 in mice
with SCI, as evidenced by the promotion of the levels of IL-1β,
TNF-α and IL-6, and the decreased IL-10 levels in the group
injected with antagomir-21 (P<0.01; Fig. 4F-I). In total, the data proved
that miR-21 was at least partially responsible for the suppression
of GP-17 in SCI-induced apoptosis and the inflammatory
response.
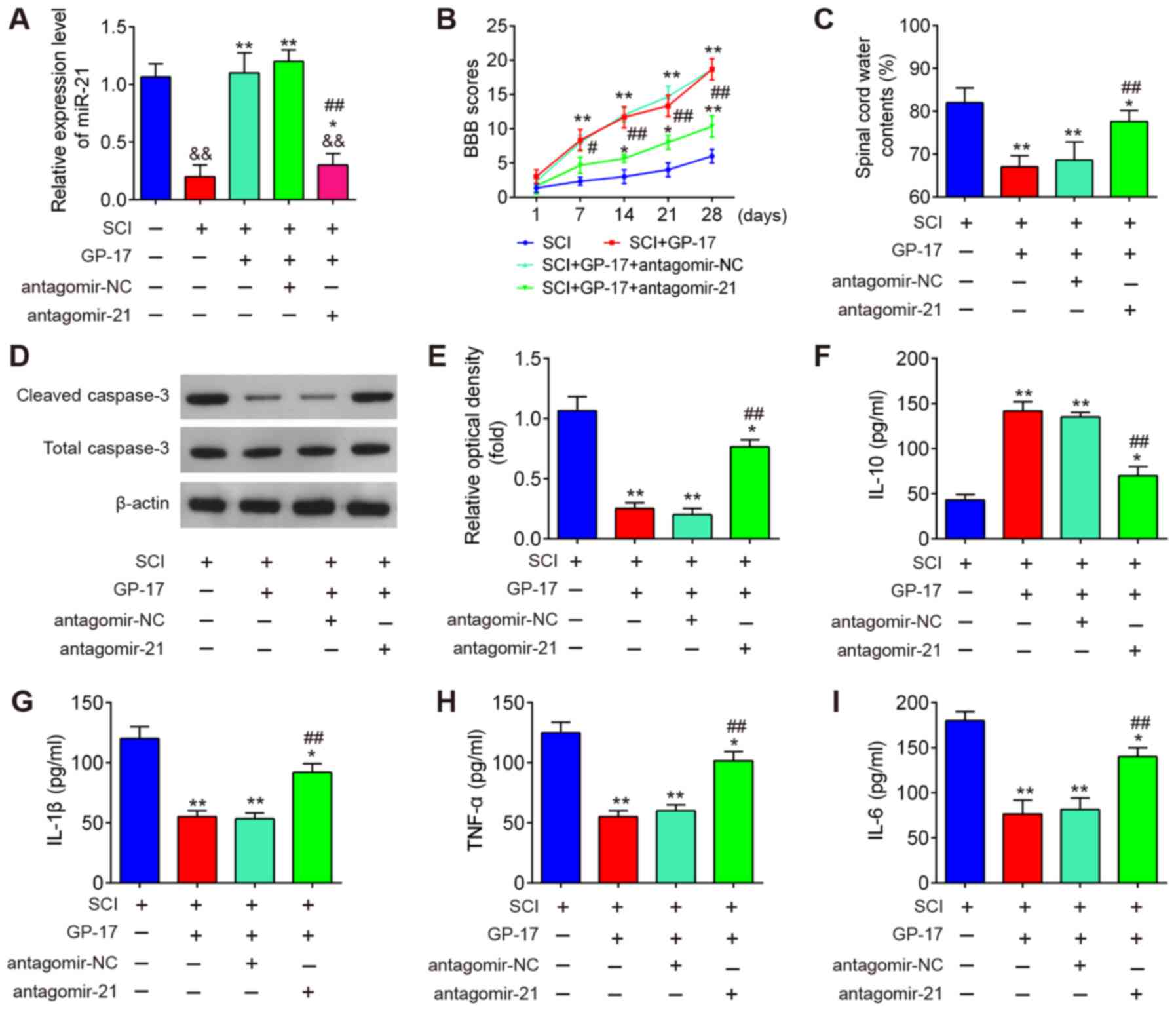 | Figure 4GP-17 suppresses SCI-induced
apoptosis and inflammatory response through regulating miR-21. The
mice with SCI were treated with antagomir-21/antagomir-NC via
intrathecal injection, followed by GP-17 treatment. (A) The
expression of miR-21 was measured by RT-qPCR. Data represent the
mean ± SD of three independent experiments.
&&P<0.01 vs. sham group;
*P<0.05, **P<0.01 vs. SCI group; or SCI
+ GP-17 group; ##P<0.01 vs. SCI + GP-17 group. (B)
BBB scores at 1, 7, 14, 21 and 28 days are shown for all groups of
mice. (C) Water content in spinal cord of mice was detected by
dry-wet technique. (D and E) The expression of cleaved caspase-3
was measured by western blot analysis. The levels of inflammatory
cytokines, including (F) IL-10, (G) IL-1β, (H) TNF-α, and (I) IL-6
were evaluated by ELISA. Data represent the mean ± SD of three
independent experiments. *P<0.05,
**P<0.01 vs. SCI group; ##P<0.01 vs.
SCI + GP-17 group. GP-17, gypenoside XVIIl; SCI, spinal cord
injury; BBB, Basso, Beattie and Bresnahan. |
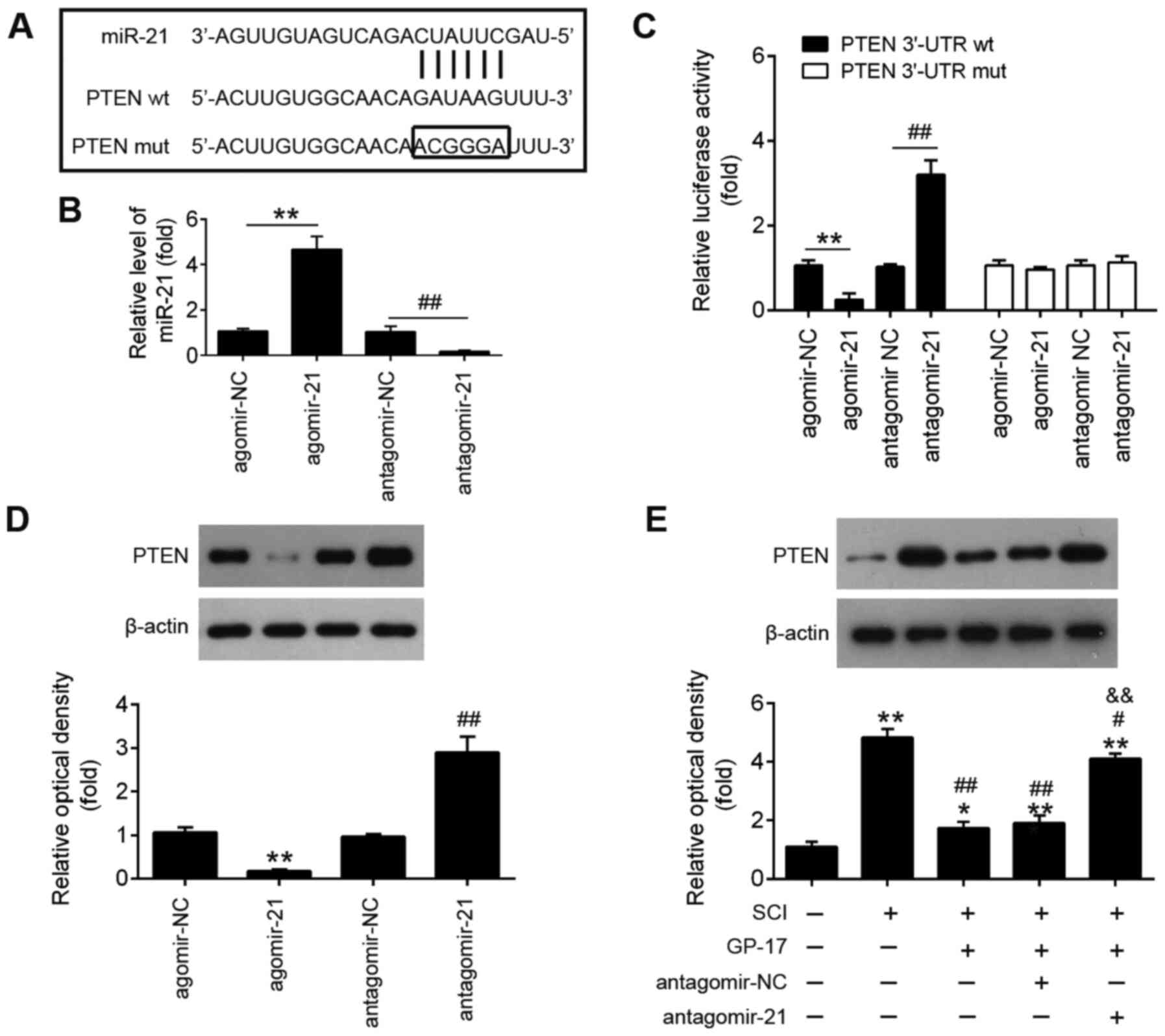
PTEN is a direct target of miR-21
To elucidate the mechanisms through which miR-21
mediates the protective effects of GP-17 on SCI, potential targets
of miR-21 were predicted using the bioinformatics tools, PicTar
version 2007 (https://pictar.mdc-berlin.de/) and TargetScan Release
7.0 (http://targetscan.org/). Bioinformatics
analysis indicated that PTEN was a potential target of miR-21
(Fig. 5A). Previous research has
reported that PTEN plays an important role in tissue pathology and
apoptosis, leading to secondary damage after the initial SCI
(36). In the present study, a
dual luciferase reporter assay was performed in order to validate
whether PTEN was a direct target of miR-21. First, it was confirmed
that miR-21 expression in BV-2 cells was significantly
increased/decreased following transfection with agomir-21 or
antagomir-21 compared with that of cells transfected with agomir-NC
or antagomir-NC (Fig. 5B).
Subsequently, as shown in Fig.
5C, miR-21 inhibition significantly increased, while miR-21
upregulation markedly decreased the luciferase activity of wt PTEN
3′-UTR in BV-2 cells (P<0.01). However, these effects were not
observed in cells the transfected with the vector bearing the
mutant PTEN 3′-UTR. Furthermore, the results of western blot
analysis revealed that the overexpression of miR-21 significantly
decreased the expression of PTEN, while the PTEN expression levels
were markedly increased by miR-21 knockdown (P<0.01; Fig. 5D). To further investigate whether
GP-17 affects the expression levels of PTEN in vivo, the
mice with SCI were treated with antagomir-21/antagomir-NC via
intrathecal injection, followed by GP-17 treatment. As shown in
Fig. 5E, PTEN expression was
significantly increased in the SCI group compared with the sham
group, whereas GP-17 treatment led to a marked reduction in PTEN
expression compared with the SCI group. However, the suppressive
effect of GP-17 on PTEN expression was significantly attenuated by
antagomir-21 (P<0.01). All these data suggest that GP-17
suppresses PTEN expression by upregulating miR-21.
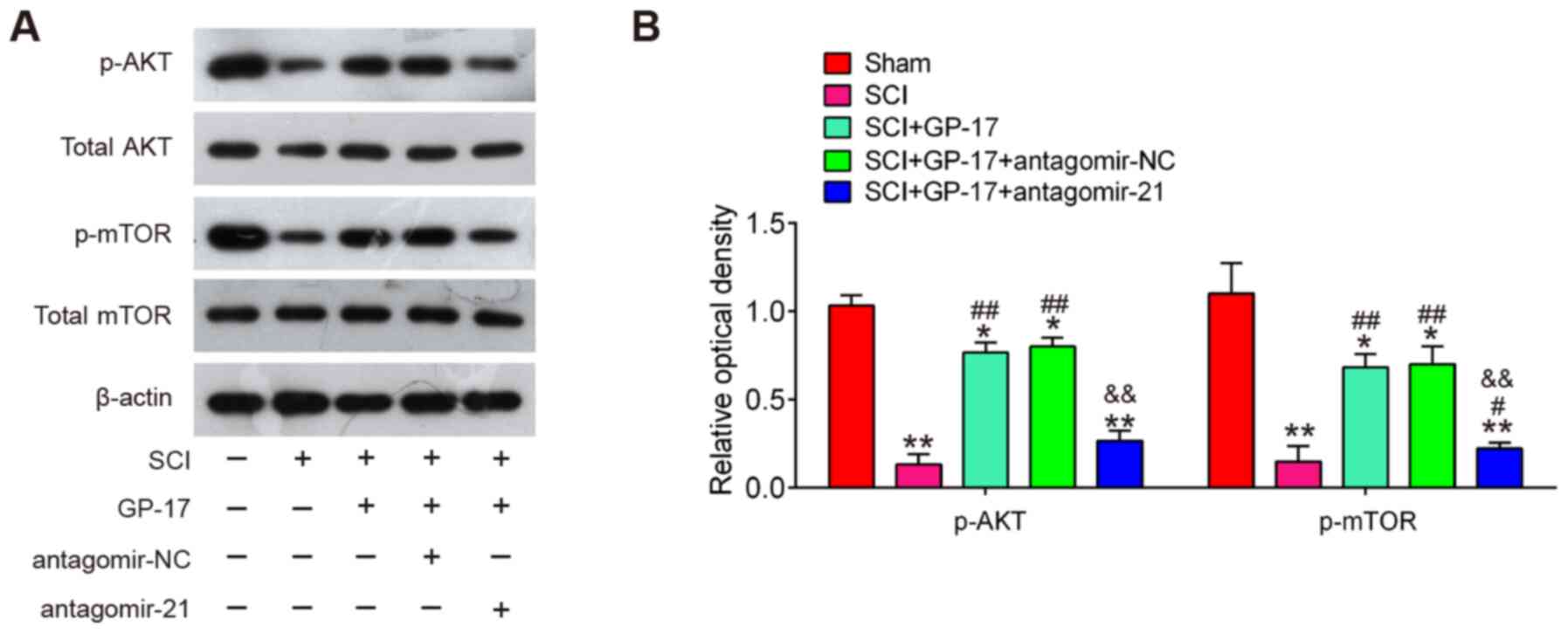
GP-17 induces the activation of the
PTEN/AKT/mTOR pathway through miR-21 in mice with SCI
It is well-known that PTEN is a negative regulator
of the AKT/mTOR pathway that has been implicated in the apoptosis
and inflammatory response during SCI (37,38). Thus, further experiments were
designed to examine the influence of GP-17 on the activation of
PTEN/AKT/mTOR pathway in vivo. Therefore, the expression
level changes of AKT/mTOR pathway-related proteins were analyzed in
the spinal cords of mice with SCI. It was found that SCI led to a
significant reduction in the expression of p-AKT and p-mTOR,
whereas this inhibitory effect was attenuated by GP-17 treatment,
suggesting that GP-17 can re-activate the AKT/mTOR pathway in SCI.
However, the activation of the AKT/mTOR pathway induced by GP-17
was blocked by antagomir-21 (P<0.01; Fig. 6A and B). All these data indicated
that GP-17 exerts its protective effects by regulating the
activation of the miR-21/PTEN/AKT/mTOR pathway.
Discussion
In the present study, it was demonstrated that GP-17
improves functional recovery, reduces neuronal apoptosis and
inhibits the inflammatory response in mice with SCI. Moreover,
GP-17 upregulated miR-21 expression and miR-21 inhibition
attenuated the neuroprotective effects of GP-17 in mice with SCI.
PTEN was identified as a target of miR-21. Furthermore, GP-17
increased the phosphorylation levels of Akt and mTOR through the
miR-21/PTEN axis following SCI. Considering the association between
PTEN and the AKT/mTOR pathway, the data of the present study
suggest that the neuroprotective effects of GP-17 in SCI are highly
associated with the activation of the the PTEN/AKT/mTOR pathway.
All findings implicated GP-17 as a potential clinical drug for the
treatment of SCI.
GP-17, a novel functional component of GP, has a
similar structure to that of estradiol. Of note, estradiol has been
previously reported to improve motor function, reduce inflammation,
reduce apoptotic cell death, and promote earlier cytokine release
and astroglial response (39-42). However, whether GP-17 exerts
similar effects in SCI has not yet been fully determined. In the
present study, the effects of GP-17 on SCI were examined using
bioassays in vivo. Overall, the results revealed that GP-17
improved functional recovery and reduced spinal cord edema in mice
with SCI. Furthermore, GP-17 treatment substantially suppressed
neuronal apoptosis and the inflammatory response in the mouse model
of SCI, suggesting that the protective effects of GP-17 against SCI
are partly mediated via the suppression of neuronal apoptosis and
inflammatory responses. However, the molecular mechanisms
underlying this process remain unknown.
Apoptosis and inflammation have been recognized as
two key mechanisms underlying the pathogenesis of SCI (43). Increasing evidence has
demonstrated that large numbers of miRNAs are abnormally expressed
post-SCI, further regulating the inflammatory reaction and neuronal
apoptosis. For example, Zhu et al (44) demonstrated that miR-494 was
significantly downregulated in spinal cord tissues from rats with
SCI and the upregulation of miR-494 improved functional recovery by
inhibiting apoptosis. Jian et al (45) found that miR-34a suppressed
neuronal apoptosis and microglial inflammation by negatively
targeting the Notch pathway, thereby improving the locomotor
function of rats with SCI. Xu et al (46) reported that miR-124 improved
functional recovery by suppressing neuronal apoptosis in rats with
SCI. These data suggest that targeting miRNAs may be a potential
therapeutic strategy for SCI. In the present study, through the
analysis of GSE67515 from the GEO database, it was found that SCI
induced dysregulated miRNA expression in mice following SCI, in
mice with SCI, and miR-21 was significantly upregulated by GP-17
treatment. Therefore, it was hypothesized that miR-21 may be
involved in the anti-inflammatory and anti-apoptotic properties of
GP-17 in SCI.
Previous studies have indicated that miR-21 is one
of the most abundantly expressed miRNAs following SCI, and it was
found to improve neuronal survival and promote functional recovery
in SCI animal models (27,34). For example, Hu et al
(34) demonstrated that miR-21
upregulation exerted a protective effect in SCI by inhibiting
neuronal cell apoptosis. Liu et al (47) found that miR-21 upregulation
regulated astrocytic function, and promoted functional recovery
following SCI. Bhalala et al (48) demonstrated a novel role for
miR-21 in regulating astrocytic hypertrophy and glial scar
progression following SCI. Notably, the anti-apoptotic effects of
TCM that operate through targeting miR-21 have also been reported
in SCI. For example, tetramethylpyrazine (TMP) administration, one
of the major active constituents of Ligusticum wallichii
franchat, has been shown to improve functional recovery and
reduce cell apoptosis by upregulating miR-21 in rats with SCI
(49). In the present study, it
was demonstrated that the knockdown of miR-21 impaired the
beneficial effects of GP-17 on functional recovery and spinal cord
edema. Moreover, the inhibition of miR-21 reversed the suppression
of GP-17 in the apoptosis and inflammatory response in mice with
SCI. Collectively, these results suggested that GP-17 may exert its
therapeutic effects against SCI by upregulating miR-21
expression.
Axonal loss is the hallmark of traumatic brain and
SCI and axon regeneration as a critical step in nerve repairing and
remodeling after peripheral nerve injury relies on regulation of
gene expression. Evidence has demonstrated that miRNAs and their
signaling pathways play important roles in neural repair and
regeneration following SCI (50). Among these miRNAs, miR-21 is the
most well-studied in SCI (51,52). For example, the inhibition of the
miR-21 function in astrocytes has been found to increase axon
density within the lesion site (48). Another study demonstrated that
miR-21 upregulation promoted axon growth in adult dorsal root
ganglion neurons (53).
Therefore, given the association between miR-21 and GP-17 in SCI,
future studies are required to further elucidate the effects of
GP-17 on neuroprotection and regeneration in spinal neurons.
PTEN is a negative regulator of the AKT/mTOR
pathway. In this pathway, phosphatidylinositol (4,5)-bisphosphate (PIP2) is converted into
PIP3 by PI3Ks, which subsequently activates AKT, mTOR and ribosomal
protein, ultimately achieving its neuroprotective effects in SCI
(54). The beneficial effects of
its inhibition in SCI have also been discovered by previous studies
(55,56). For example, the inhibition of
PTEN has been shown to promote functional recovery in rats with
spinal cord contusion (57). In
addition, previous studies have identified that miR-21 regulates
the AKT/mTOR pathway by targeting PTEN in different diseases and
cell types (58-60). Of note, Lv et al (61) found that miR-21 directly
regulated the expression of PTEN, and the upregulation of miR-21 in
rats with SCI promoted the activation of the AKT/mTOR pathway, and
reduced apoptosis and inflammation in spinal cord tissues,
improving the functional recovery of hindlimbs of rats with SCI.
These studies suggest that the miR-21/PTEN axis may mediate the
protective effects of GP-17 in mice with SCI. In the present study,
using bioinformatics analysis and dual-luciferase reporter gene
assay, PTEN was identified as a direct target of miR-21.
Furthermore, GP-17 treatment activated the PTEN/AKT/mTOR pathway in
mice with SCI; however, these effects were blocked by miR-21
knockdown. Collectively, these data indicated that GP-17 induced
the activation of the PTEN/AKT/mTOR pathway via promoting miR-21
expression in SCI.
There are some limitations to the present study.
Firstly, in addition to the anti-inflammatory response and
anti-apoptotic effects, whether GP-17 affects axon regeneration in
spinal neurons was not been investigated. Secondly, although the
miR-21/PTEN axis was confirmed as an important factor in
controlling GP-17-induced neuroprotection in mice with SCI, other
target genes of miR-21 or other differentially expressed miRNAs
found in the present study also need to be carefully evaluated.
Therefore, future studies are warranted to further investigate the
effects of GP-17 on regeneration in spinal neurons and examine its
effects on promoting the recovery of function in disease models,
such as traumatic spinal cord and brain injuries.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that GP-17 suppresses
neuronal apoptosis and the inflammatory response, and subsequently
improves functional recovery via the activation of the
miR-21/PTEN/Akt/mTOR pathway in mice with SCI. The results
presented herein indicate a novel molecular mechanism for the
neuroprotective effects of GP-17 and the potential clinical
application of GP-17 in the treatment of SCI.
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
MX and TS conceived and designed the experiments.
TS, LD, JL and HG performed the experiments. TS and MX analyzed the
data. MX contributed to the reagents, materials and analysis tools.
MX and TS wrote the manuscript. MX and TS confirm the authenticity
of all the raw data. All authors have read and agreed to the final
version of manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the First Affiliated Hospital, and College of Clinical
Medicine of Henan University of Science and Technology (approval
no. 2019-0035).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26(Suppl 24): S2–S12. 2001. View Article : Google Scholar
|
|
2
|
Goldshmit Y, Kanner S, Zacs M, Frisca F,
Pinto AR, Currie PD and Pinkas-Kramarski R: Rapamycin increases
neuronal survival, reduces inflammation and astrocyte proliferation
after spinal cord injury. Mol Cell Neurosci. 68:82–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blight AR: Delayed demyelination and
macrophage invasion: A candidate for secondary cell damage in
spinal cord injury. Cent Nerv Syst Trauma. 2:299–315. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orr MB and Gensel JC: Spinal cord injury
scarring and inflammation: Therapies targeting glial and
inflammatory responses. Neurotherapeutics. 15:541–553. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Celik EC, Erhan B, Gunduz B and Lakse E:
The effect of low-frequency TENS in the treatment of neuropathic
pain in patients with spinal cord injury. Spinal Cord. 51:334–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen L and Ji HF: The pharmacology of
curcumin: Is it the degradation products? Trends Mol Med.
18:138–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin W, Wang J, Zhu T, Yuan B, Ni H, Jiang
J, Wang H and Liang W: Anti-inflammatory effects of curcumin in
experimental spinal cord injury in rats. Inflamm Res. 63:381–387.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ates O, Cayli S, Altinoz E, Gurses I,
Yucel N, Kocak A, Yologlu S and Turkoz Y: Effects of resveratrol
and methylprednisolone on biochemical, neurobehavioral and
histopathological recovery after experimental spinal cord injury.
Acta Pharmacol Sin. 27:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li
T, Shi JX and Zhang X: Resveratrol prevents neuronal apoptosis in
an early brain injury model. J Surg Res. 189:159–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng X, Wang M, Sun G, Ye J, Zhou Y, Dong
X, Wang T, Lu S and Sun X: Attenuation of Aβ25-35-induced parallel
autophagic and apoptotic cell death by gypenoside XVII through the
estrogen receptor-dependent activation of Nrf2/ARE pathways.
Toxicol Appl Pharmacol. 279:63–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui CH, Kim DJ, Jung SC, Kim SC and Im WT:
Enhanced production of gypenoside LXXV using a novel
ginsenoside-transforming β-glucosidase from ginseng-cultivating
soil bacteria and its anti-cancer property. Molecules. 22:8442017.
View Article : Google Scholar
|
|
12
|
Wan ZH and Zhao Q: Gypenoside inhibits
interleukin-1β-induced inflammatory response in human
osteoarthritis chondrocytes. J Biochem Mol Toxicol. 31:2017.
View Article : Google Scholar
|
|
13
|
Meng X, Luo Y, Liang T, Wang M, Zhao J,
Sun G and Sun X: Gypenoside XVII enhances lysosome biogenesis and
autophagy flux and accelerates autophagic clearance of Amyloid-β
through TFEB activation. J Alzheimers Dis. 52:1135–1150. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu NK, Wang XF, Lu QB and Xu XM: Altered
microRNA expression following traumatic spinal cord injury. Exp
Neurol. 219:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu G, Keeler BE, Zhukareva V and Houle
JD: Cycling exercise affects the expression of apoptosis-associated
microRNAs after spinal cord injury in rats. Exp Neurol.
226:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai J, Xu LJ, Han GD, Sun HL, Zhu GT,
Jiang HT, Yu GY and Tang XM: MiR-137 attenuates spinal cord injury
by modulating NEUROD4 through reducing inflammation and oxidative
stress. Eur Rev Med Pharmacol Sci. 22:1884–1890. 2018.PubMed/NCBI
|
|
18
|
Lin CA, Duan KY, Wang XW and Zhang ZS:
MicroRNA-409 promotes recovery of spinal cord injury by regulating
ZNF366. Eur Rev Med Pharmacol Sci. 22:3649–3655. 2018.PubMed/NCBI
|
|
19
|
Feng JS, Sun JD, Wang XD, Fu CH, Gan LL
and Ma R: MicroRNA-204-5p targets SOX11 to regulate the
inflammatory response in spinal cord injury. Eur Rev Med Pharmacol
Sci. 23:4089–4096. 2019.PubMed/NCBI
|
|
20
|
Yi LT, Mu RH, Dong SQ, Wang SS, Li CF,
Geng D and Liu Q: MiR-124 antagonizes the antidepressant-like
effects of standardized gypenosides in mice. J Psychopharmacol.
32:458–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zu ML, Piao XL, Gao JM, Xing SF and Liu
LH: Monomer gypenoside LI from Gynostemma pentaphyllum inhibits
cell proliferation and upregulates expression of miR-128-3p in
melanoma cells. J Biochem Mol Toxicol. 34:e224602020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu KW, Ma YS, Yu FS, Huang YP, Chu YL, Wu
RS, Liao CL, Chueh FS and Chung JG: Gypenosides induce cell death
and alter gene expression in human oral cancer HSC-3 cells. Exp
Ther Med. 14:2469–2476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y,
Wu YS, Zhou YF, Huang YX, Wang Q, Shen LY, et al: Metformin
improves functional recovery after spinal cord injury via autophagy
flux stimulation. Mol Neurobiol. 54:3327–3341. 2017. View Article : Google Scholar
|
|
24
|
Lilley E, Andrews MR, Bradbury EJ, Elliott
H, Hawkins P, Ichiyama RM, Keeley J, Michael-Titus AT, Moon LDF,
Pluchino S, et al: Refining rodent models of spinal cord injury.
Exp Neurol. 328:1132732020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Zhang T, Ni S, Luo Z, Lang Y, Hu J and Lu
H: The protective effect of microRNA-21 in neurons after spinal
cord injury. Spinal Cord. 57:141–149. 2019. View Article : Google Scholar :
|
|
28
|
Zhou J, Shuang O, Li J, Cai Z, Wu C and
Wang W: MiR-34a alleviates spinal cord injury via TLR4 signaling by
inhibiting HMGB-1. Exp Ther Med. 17:1912–1918. 2019.PubMed/NCBI
|
|
29
|
Yu X, Zhang S, Zhao D, Zhang X, Xia C,
Wang T, Zhang M, Liu T, Huang W and Wu B: SIRT1 inhibits apoptosis
in in vivo and in vitro models of spinal cord injury via
microRNA-494. Int J Mol Med. 43:1758–1768. 2019.PubMed/NCBI
|
|
30
|
Zhao CL, Cui HA and Zhang XR: MiR-543-5p
inhibits inflammation and promotes nerve regeneration through
inactivation of the NF-κB in rats after spinal cord injury. Eur Rev
Med Pharmacol Sci. 23(Suppl 3): S39–S46. 2019.
|
|
31
|
Wang T, Yuan W, Liu Y, Zhang Y, Wang Z,
Chen X, Feng S, Xiu Y and Li W: MiR-142-3p is a potential
therapeutic target for sensory function recovery of spinal cord
injury. Med Sci Monit. 21:2553–2556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi J, Wang D, Niu X, Hu J, Zhou Y and Li
Z: MicroRNA-155 deficiency suppresses Th17 cell differentiation and
improves locomotor recovery after spinal cord injury. Scand J
Immunol. 81:284–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang J, Guo WC, Hu JF and Yu L: Let-7
participates in the regulation of inflammatory response in spinal
cord injury through PI3K/Akt signaling pathway. Eur Rev Med
Pharmacol Sci. 23:6767–6773. 2019.PubMed/NCBI
|
|
34
|
Hu JZ, Huang JH, Zeng L, Wang G, Cao M and
Lu HB: Anti-apoptotic effect of microRNA-21 after contusion spinal
cord injury in rats. J Neurotrauma. 30:1349–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie W, Yang SY, Zhang Q, Zhou Y, Wang Y,
Liu R, Wang W, Shi J, Ning B and Jia T: Knockdown of MicroRNA-21
promotes neurological recovery after acute spinal cord injury.
Neurochem Res. 43:1641–1649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutilla EA, Buyukozturk MM and Steward O:
Long-term consequences of conditional genetic deletion of PTEN in
the sensorimotor cortex of neonatal mice. Exp Neurol. 279:27–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Guo Y, Fan Y, Tian H, Li K and Mei
X: Melatonin enhances autophagy and reduces apoptosis to promote
locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR
signaling pathway. Neurochem Res. 44:2007–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li W, Yao S, Li H, Meng Z and Sun X:
Curcumin promotes functional recovery and inhibits neuronal
apoptosis after spinal cord injury through the modulation of
autophagy. J Spinal Cord Med. 44:37–45. 2021. View Article : Google Scholar :
|
|
39
|
Lee JY, Choi HY, Ju BG and Yune TY:
Estrogen alleviates neuropathic pain induced after spinal cord
injury by inhibiting microglia and astrocyte activation. Biochim
Biophys Acta Mol Basis Dis. 1864:2472–2480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kachadroka S, Hall AM, Niedzielko TL,
Chongthammakun S and Floyd CL: Effect of endogenous androgens on
17beta-estradiol-mediated protection after spinal cord injury in
male rats. J Neurotrauma. 27:611–626. 2010. View Article : Google Scholar :
|
|
41
|
Samantaray S, Das A, Matzelle DC, Yu SP,
Wei L, Varma A, Ray SK and Banik NL: Administration of low dose
estrogen attenuates gliosis and protects neurons in acute spinal
cord injury in rats. J Neurochem. 136:1064–1073. 2016. View Article : Google Scholar
|
|
42
|
Sribnick EA, Wingrave JM, Matzelle DD,
Wilford GG, Ray SK and Banik NL: Estrogen attenuated markers of
inflammation and decreased lesion volume in acute spinal cord
injury in rats. J Neurosci Res. 82:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hayta E and Elden H: Acute spinal cord
injury: A review of pathophysiology and potential of non-steroidal
anti-inflammatory drugs for pharmacological intervention. J Chem
Neuroanat. 87:25–31. 2018. View Article : Google Scholar
|
|
44
|
Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S
and Che X: MicroRNA-494 improves functional recovery and inhibits
apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal
cord injury. Biomed Pharmacother. 92:879–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jian YP, Dong SJ, Xu SS, Fan J, Liu WJ,
Shao XW, Li T, Zhao SH and Wang YG: MicroRNA-34a suppresses
neuronal apoptosis and alleviates microglia inflammation by
negatively targeting the Notch pathway in spinal cord injury. Eur
Rev Med Pharmacol Sci. 24:1420–1427. 2020.PubMed/NCBI
|
|
46
|
Xu Z, Zhang K, Wang Q and Zheng Y:
MicroRNA124 improves functional recovery and suppresses
Bax-dependent apoptosis in rats following spinal cord injury. Mol
Med Rep. 19:2551–2560. 2019.PubMed/NCBI
|
|
47
|
Liu R, Wang W, Wang S, Xie W, Li H and
Ning B: MicroRNA-21 regulates astrocytic reaction post-acute phase
of spinal cord injury through modulating TGF-β signaling. Aging
(Albany NY). 10:1474–1488. 2018. View Article : Google Scholar
|
|
48
|
Bhalala OG, Pan L, Sahni V, McGuire TL,
Gruner K, Tourtellotte WG and Kessler JA: MicroRNA-21 regulates
astrocytic response following spinal cord injury. J Neurosci.
32:17935–17947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang JH, Cao Y, Zeng L, Wang G, Cao M, Lu
HB and Hu JZ: Tetramethylpyrazine enhances functional recovery
after contusion spinal cord injury by modulation of MicroRNA-21,
FasL, PDCD4 and PTEN expression. Brain Res. 1648:35–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang JJ, Liu CM, Zhang BY, Wang XW, Zhang
M, Saijilafu, Zhang SR, Hall P, Hu YW and Zhou FQ: MicroRNA-26a
supports mammalian axon regeneration in vivo by suppressing
GSK3beta expression. Cell Death Dis. 6:e18652015. View Article : Google Scholar
|
|
51
|
Strickland ER, Hook MA, Balaraman S, Huie
JR, Grau JW and Miranda RC: MicroRNA dysregulation following spinal
cord contusion: Implications for neural plasticity and repair.
Neuroscience. 186:146–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Montalban E, Mattugini N, Ciarapica R,
Provenzano C, Savino M, Scagnoli F, Prosperini G, Carissimi C,
Fulci V, Matrone C, et al: MiR-21 is an Ngf-modulated microRNA that
supports Ngf signaling and regulates neuronal degeneration in PC12
cells. Neuromolecular Med. 16:415–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Strickland IT, Richards L, Holmes FE,
Wynick D, Uney JB and Wong LF: Axotomy-induced miR-21 promotes axon
growth in adult dorsal root ganglion neurons. PLoS One.
6:e234232011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu NK and Xu XM: Neuroprotection and its
molecular mechanism following spinal cord injury. Neural Regen Res.
7:2051–2062. 2012.PubMed/NCBI
|
|
55
|
Lewandowski G and Steward O:
AAVshRNA-mediated suppression of PTEN in adult rats in combination
with salmon fibrin administration enables regenerative growth of
corticospinal axons and enhances recovery of voluntary motor
function after cervical spinal cord injury. J Neurosci.
34:9951–9962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu K, Lu Y, Lee JK, Samara R, Willenberg
R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al:
PTEN deletion enhances the regenerative ability of adult
corticospinal neurons. Nat Neurosci. 13:1075–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou H, Li X, Wu Q, Li F, Fu Z, Liu C,
Liang Z, Chu T, Wang T, Lu L, et al: shRNA against PTEN promotes
neurite outgrowth of cortical neurons and functional recovery in
spinal cord contusion rats. Regen Med. 10:411–429. 2015. View Article : Google Scholar
|
|
58
|
Li X, Dai Y and Xu J: MiR-21 promotes
pterygium cell proliferation through the PTEN/AKT pathway. Mol Vis.
24:485–494. 2018.
|
|
59
|
Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H,
Zhang HY and Zhou B: MiR-21 regulates the proliferation and
apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev
Med Pharmacol Sci. 23:4149–4155. 2019.PubMed/NCBI
|
|
60
|
Qin S, Wang H, Liu G, Mei H and Chen M:
MiR215p ameliorates hyperoxic acute lung injury and decreases
apoptosis of AEC II cells via PTEN/AKT signaling in rats. Mol Med
Rep. 20:4953–4962. 2019.PubMed/NCBI
|
|
61
|
Lv X, Liang J and Wang Z: MiR-21-5p
reduces apoptosis and inflammation in rats with spinal cord injury
through PI3K/AKT pathway. Panminerva Med. Jul 27–2020.
|