Introduction
As a type of seizure often diagnosed in childhood or
infancy, febrile seizure (FS) is one of the most commonly
encountered acute neurologic conditions in children (1). In the 1990s, the International
League Against Epilepsy defined FS as a seizure observed in
children of >1 month that is not induced by infection to the
central nervous system (CNS) and does not meet the standard for
other acute seizures (2).
A separate study carried out by Molina-Carballo
et al (3) in 54 children
with febrile and epileptic convulsive crisis showed that serum
melatonin (MT) expression was enhanced during seizure attacks and
returned to normal values within 1 h. The authors further concluded
that excitation of MT generation by a convulsive crisis may help to
achieve homeostasis in the body (3). Similar results were observed in
other studies (4).
Long non-coding RNAs (lncRNAs) are long RNA
transcripts that do not encode proteins (5). The expression of lncRNAs is usually
specific to cell and tissue types (6,7).
Thus far, lncRNAs have been reported to participate in the
pathogenesis of numerous malignancies by regulating gene
modification and transcription (8,9).
Previous results showed that lncRNA MEG3 is enriched in the
endothelium and acts as a competing endogenous RNA of microRNA
(miRNA/miR)-223, while MEG3 was also suspected to participate in
MT-induced prevention against atherosclerosis (10). Moreover, MEG3 can inhibit the
activity of miR-223, while increasing the expression of cryopyrin
(NLRP3) and the pyroptosis of endothelial cells (10).
It has been demonstrated that miR-223 harvested from
macrophages can enhance the chemoresistance of EOC cells via the
phosphatase and tensin homolog (PTEN)-PI3K/protein kinase B (AKT)
signaling (11). PTEN was
illustrated to be inhibited by miR-223 to enhance the level of
PI3K/AKT activation, indicating that AKT is a key mediator in the
miR-223/PTEN signaling. Additionally, miR-223 is negatively
correlated with PTEN expression in various types of tumor cells,
indicating the important role of PTEN in tumorigenesis (11).
PTEN has the properties of lipid phosphatase.
Moreover, the substrate of PTEN is PIP3, which is hydrolyzed by
PTEN to generate phosphatidylinositol 4,5-bisphosphate (PIP2)
(12-15). PTEN inhibits PI3K/AKT signaling
by dephosphorylating PIP3 (14).
On the other hand, the inhibition of PTEN activates PI3K/AKT
signaling and promotes cell survival (15). PTEN was reported to impair AKT
activation by suppressing its membrane recruitment, thus
suppressing the proliferation, growth and survival of cells.
Therefore, PTEN plays an important role in suppressing the
malignant transformation of normal cells (16).
It is commonly known that AKT is important in the
regulation of numerous cellular processes, including protein
translation, gene transcription, cell survival and cell
proliferation (17). For
example, accumulating evidence has implied that the inhibition of
the kinase activity of AKT promotes FS-mediated neuronal apoptosis
in the hippocampus, while FS and febrile convulsion (FC) did not
affect the level of total AKT in rats, indicating that AKT/MTOR
signaling is involved in FS-induced neuronal injury, and the
initialization of endoplasmic reticulum stress promoted hippocampal
cell apoptosis by affecting the phosphorylation of AKT (18). Furthermore, γ-aminobutyric acid
(GABA), which acts as the main inhibitory signal in the peripheral
nervous system and CNS, has been associated with severe
neurological and neuromuscular disorders (19). There is increasing evidence
suggesting that the low expression of GABA is an important trigger
of seizures (20). For instance,
Mackenzie and Maguire (21)
reported that chronic stress-induced GABA downregulation in the
mouse hippocampus can increase the susceptibility to seizure.
It has been reported that treatment with MT can
downregulate the expression of lncRNA-MEG3 and thereby upregulate
the expression of miR-223, a competing endogenous RNA of MEG3
(10). Furthermore, PTEN has
been identified as a direct target of miR-223, while the PTEN/AKT
signaling pathway has been shown to be involved in the pathogenesis
of FC (18,22). In the present study, the aim was
to evaluate the therapeutic effect of MT on FC and the according
underlying molecular mechanisms. Accordingly, it was demonstrated
that MT alleviated FC via the MEG3/miR-223/PTEN/AKT pathway, which
further indicated MT as a potential novel treatment strategy for
FC.
Materials and methods
Animals
In the present study, a total of 24 Wistar rats
(age, 5-10 days; weight, 6-8 g) were used to mimic newborn infants
delivered after full-term pregnancy in humans. In addition, brain
tissues isolated from rats aged 15 to 20 days were used to mimic
the brain of human infants with an age of ≤1 years old.
Specifically, male Wistar rats aged 17 days were utilized to
establish the animal model. During the experiments, these male
Wistar rats were placed under anesthesia using a mixture of 3 mg/kg
xylazine and 80 mg/kg ketamine administrated intraperitoneally.
After the anesthesia of rats was successfully established, the head
of each rat was opened via a small incision to remove the brain
tissue. In the next step, a pair of electrodes made of stainless
steel were inserted into the frontal region of the dura mater of
each rat. In addition, a third electrode made of stainless steel
was inserted into the occipital region of the dura mater of each
rat. Subsequently, the electrodes were fixed to the skull of each
rat using dental acrylic. After the operation, all rats were placed
in an SPF animal facility with temperature control (the room
temperature was set to 23±1°C). In addition, the light/dark cycle
was set to 12/12 h, and all rats had free access to a standard chow
diet and drinking water throughout the entire experiment. By the
end of the experiment, the animals were killed by a lethal
intravenous dose of sodium pentobarbital (100 mg/kg). Death was
confirmed by various combined factors, including lack of pulse,
breathing, corneal reflex, and response to toe pinch; inability to
hear respiratory sounds and heartbeat. No secondary procedure was
used. Moreover, percutaneous cardiac puncture was performed after
the rats were unconscious, and failure of movement of the needle
and attached syringe after insertion into the heart (aspiration of
blood provides evidence of correct location) also indicates lack of
cardiac muscle movement and death. All animal procedures were
approved by Wuhan Children's Hospital (approval no. WHET20170731V1;
Wuhan, China) and were in strict compliance with the 'Guide for the
Care and Use of Laboratory Animals' published by the US National
Institutes of Health (23).
Establishment of an animal model of
FC
An animal model of FC was established. During the
animal model establishment, all rats were exposed to hyperthermia
by being placed in a water tank holding water with a water level (5
cm depth of water at 45°C). In addition, the rats were also exposed
to hyperthermia by keeping the water tank temperature at 45°C for
~5 min until the symptoms of seizure were observed. A total of 10
such experimental operations were repeated every 2 days. For the
rats of the NC group, they were placed in water of 37°C during
similar experimental operations. In addition, after the rats were
exposed to hyperthermia, their core temperature was immediately
measured using a temperature probe via the rectum. Furthermore, the
duration of seizure in each rat was determined based on the time
interval when the rat was put into the water tank until the initial
signs of seizure were first observed. For comparison, 10-time
intervals of seizure (also named seizure latencies) were recorded
in each rat. The duration of seizure in each rat was calculated as
the time interval between the onset of initial seizure signs to the
moment when the rat first gained consciousness, which was evaluated
based on the responsiveness of each rat to a range of multiple
stimuli, including cage tapping, loud clapping sound, touch and
trace of object movement in front of the eyes of the rat. By the
end of each experimental operation, all rats were dried with towels
first and further dried underneath a heating lamp before they were
placed back in their cages. In this study, the seizure intensity in
each rat was evaluated. In brief, the scoring of seizure intensity
was as follows: i) 0 points indicated no sign of convulsion; ii) 1
point indicated facial clonus; iii) 2 points indicated head
nodding; iv) 3 points indicated forelimb clonus; v) 4 points
indicated rearing; and vi) 5 points indicated rearing and back
falling. In this study, animals were divided into three groups with
8 rats in each group: i) NC group (sham-operated rats treated with
PBS); ii) FC group (rats suffering from FC induced by a high
temperature); and iii) FC + MT group (rats were given 80 mg/kg MT
via intraperitoneal injection at 15 min before the induction of
seizure).
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total RNA was isolated from tissue and cell
specimens using a miRNeasy Mini kit (Qiagen GmbH) according to the
manufacturer's protocols. RNA concentration and purity were
confirmed using an UV spectrophotometer (Eppendorf). After purified
RNA was made into cDNA templates via RT reactions using a TaqMan™
RNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on a 9700 PCR Machine (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the conditions described as follows:
30 min at 16°C, 30 min at 42°C, 5 min at 85°C, and then kept at
4°C. The cDNA templates were then subjected to qPCR carried out on
a 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using a TaqMan Universal PCR Master Mix II
(Applied Biosystems; Thermo Fisher Scientific, Inc.) per the kit
instructions. The thermocycling conditions were as follows: 10 min
at 95°C; 40 cycles for 15 sec at 95°C; and finally 60 sec at 60°C.
The relative expression of MEG3, miR-223 and PTEN mRNA was
calculated using the 2-ΔΔCq method (24). The primer sequences were as
follows: MEG3 forward, 5′-GCCAAGCTTCTTGAAAGGCC-3′ and reverse,
5′-TTCCACGGAGTAGAGCGAGTC-3′; miR-223 forward,
5′-GTCGTATCCATGGCAGGGTCCGAGGTATTCGCCATGGATACGACTGGGGT-3′ and
reverse, 5′-TGTCAGTTTGTCAAATACCCCA-3′; and PTEN forward,
5′-CTGGGTACCATGACAGCCATCATCAAAG-3′ and reverse,
5′-GGCCTCGAGTCAGACTTTTGTAATTTGTG-3′. U6 (forward,
5′-GTGCTCGCTTCGGCAGCA-3′ and reverse, 5′-CAAAATATGGAACGCTTC-3′) was
used as an internal reference gene for miR-223 and GAPdH (forward,
5′-CGACTTCAACAGCGACACTCAC-3′ and reverse,
5′-CCCTGTTGCTGTAGCCAAATTC-3′) was used as an internal reference
gene for MEG3 and PTEN.
Cell culture and transfection
SH-SY5Y (cat. no. ATCC® CRL-2266™;
American Type Culture Collection) and U251 MG cells (cat. no.
09063001; Sigma-Aldrich; Merck KGaA) were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS (Sigma-Aldrich; Merck
KGaA). The culture was carried out at 37°C and 5% CO2.
To determine the effect of miR-233, SH-SY5Y and U251 cells were
seeded into 24-well plates at a density of 5×105
cells/well and transfected with miR-233 mimics
(5′-CCGAGGTATTCGCCATGGATACGACTGGGGT-3′; Thermo Fisher Scientific,
Inc.) and scramble miRNA (5′-UGGGCGUAUAGACGUGUUACAC-3′; Thermo
Fisher Scientific, Inc.), negative control (NC) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) per the recommendation of the manufacturer.
SH-SY5Y cells were in the following three groups: i) NC group,
cells were treated with PBS at 37°C for 24 h; ii)
H2O2 group, cells were treated with
H2O2 to model apoptosis at 37°C for 24 h; and
iii) H2O2 + MT group, cells were treated with
1 µM MT at 37°C for 24 h.
Vector construction, mutagenesis and
luciferase assay
The 3′untranslated region (UTR) of PTEN containing
the miR-233 binding site was inserted into a pcDNA3.1 vector
(Promega Corporation) to generate the wild-type (WT) PTEN 3′UTR
vector. Then, the miR-233 binding site in the 3′UTR of PTEN was
subjected to site-directed mutagenesis to generate the mutant (MUT)
PTEN 3′UTR vector using a site-directed mutagenesis kit (Agilent
Technologies, Inc.). Similarly, the WT and MUT vectors of MEG3
promoter containing the WT and MUT miR-233 binding sites were also
generated. Subsequently, SH-SY5Y and U251 cells seeded in 24-well
plates at a density of 5×105 cells/well were
co-transfected with WT/MUT PTEN/MEG3 vectors in conjunction with
miR-233 mimics (5′-CCGAGGTATTCGCCATGGATACGACTGGGGT-3′; Thermo
Fisher Scientific, Inc.) and NC (5′-UGGGCGUAUAGACGUGUUACAC-3′;
Thermo Fisher Scientific, Inc.) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), per the
recommendation of the manufacturer for 48 h before the luciferase
activity of transfected cells was assayed using a Bright-Glo™
Luciferase Assay System (Promega Corporation). Renilla
luciferase activity was used for the normalization of the relative
luciferase activity.
Western blot analysis
Protein was extracted from the SH-SY5Y and U251 MG
cells using RIPA (Sigma-Aldrich; Merck KGaA) and protein
concentration was determined with a BCA assay kit (Bio-Rad
Laboratories, Inc.). Then, 50 µg/lane protein was resolved
on a 10% NuPAGE™ Novel Bis-Tris gel (Invitrogen; Thermo Fisher
Scientific, Inc.), and subsequently separated proteins were
transferred to a nitrocellulose membrane (Amersham; Cytiva). The
membrane was then blocked with 5% non-fat milk for 60 min at room
temperature, and incubated overnight at 4°C with anti-PTEN
(1:1,000; cat. no. ab267787; Abcam), anti-AKT (1:500; cat. no.
ab8805; Abcam), anti-phosphorylated (p)-AKT (1:1,000; cat. no.
ab38449; Abcam), anti-cleaved-caspase-3 primary antibodies (1:500;
cat. no. ab32042; Abcam) and anti-β-actin (1 µg/ml; cat. no.
ab8226; Abcam). β-actin was used as the loading control. Following
which, membranes were further incubated for 2 h at 37°C with the
appropriate secondary antibody (1:2,000; cat. no. ab6721; Abcam).
After the protein bands were visualized using a SuperSignal™ West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.),
the relative expression of PTEN, AKT, p-AKT and cleaved caspase-3
was calculated using Quantity One software (version 4.6.7; Bio-Rad
Laboratories, Inc.).
TUNEL staining
The apoptotic status of treated samples was measured
using a TUNEL staining assay kit (Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. The samples were
immersed in a 3% H2O2 solution at room
temperature for 10 min, and then hydrolyzed in 20 µg/ml
proteinase K solution for 20 min, washed with PBS, and treated with
a citrate buffer (0.01 M) for 30 min of highpressure antigen
retrieval. The samples were then processed with 50 µl TdT
enzyme solution at 37°C for 1 h. Subsequently, the samples were
washed with PBS and incubated with 50 µl peroxidase-labeled
anti-digoxigenin at 37°C for 30 min in the dark. Neutral resin was
used as the mounting medium, and a fluorescence microscope
(magnification, ×400) was used to observe the samples, 10 visual
fields of view were selected randomly.
Detection of seizure and duration of
convulsion with ECG recordings of ß, θ, α and δ waves
Electroencephalography (EEG) signals of β, θ, α and
δ waves were recorded using a NicoletOne™ Neurodiagnostic system
(Natus Medical Incorporated). In brief, the EEG signal recording
was evaluated in two steps: In the first step, the first 30 min of
the recorded EEG signals were immediately evaluated to detect any
seizure activity. In the second step, the recording was continued
for ≥18 h and all recorded EEG signals were evaluated the next day.
Only the seizure activity that lasted for ≥10 sec was included for
the evaluation. At the end of evaluation, the entire recording of
EEG signals was assessed again to carry out the trend analysis
using Nicolet One software (version 32; Natus Medical
Incorporated).
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism (v5.0; GraphPad Software, Inc.) and SPSS for Windows (16.0;
SPSS, Inc.). Data is expressed as the mean ± SD. The differences
among different groups were analyzed using an unpaired Student's
t-test. Comparisons between multiple groups were performed via one
way ANOVA, with Bonferroni's test being utilized as the post hoc
test. TargetScan (http://www.targetscan.org) and PicTar-Vert (https://pictar.mdc-berlin.de) were employed to predict
the potential targets of MEG3 and PTEN. Each experiment was
repeated in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of MEG3, miR-223 and
PTEN among the three groups
RT-qPCR was performed to detect the mRNA levels of
MEG3, miR-223 and PTEN in different SH-SY5Y cell groups. As shown
in Fig. 1, mRNA expression of
MEG3 (Fig. 1A) was increased in
the H2O2 group, while treatment with MT
decreased the expression of MEG3. The expression of miR-223
(Fig. 1B) was decreased in the
H2O2 group, while treatment with MT increased
the expression levels of miR-223 (Fig. 1B). The mRNA expression of PTEN
(Fig. 1C) showed the same
tendency as MEG3.
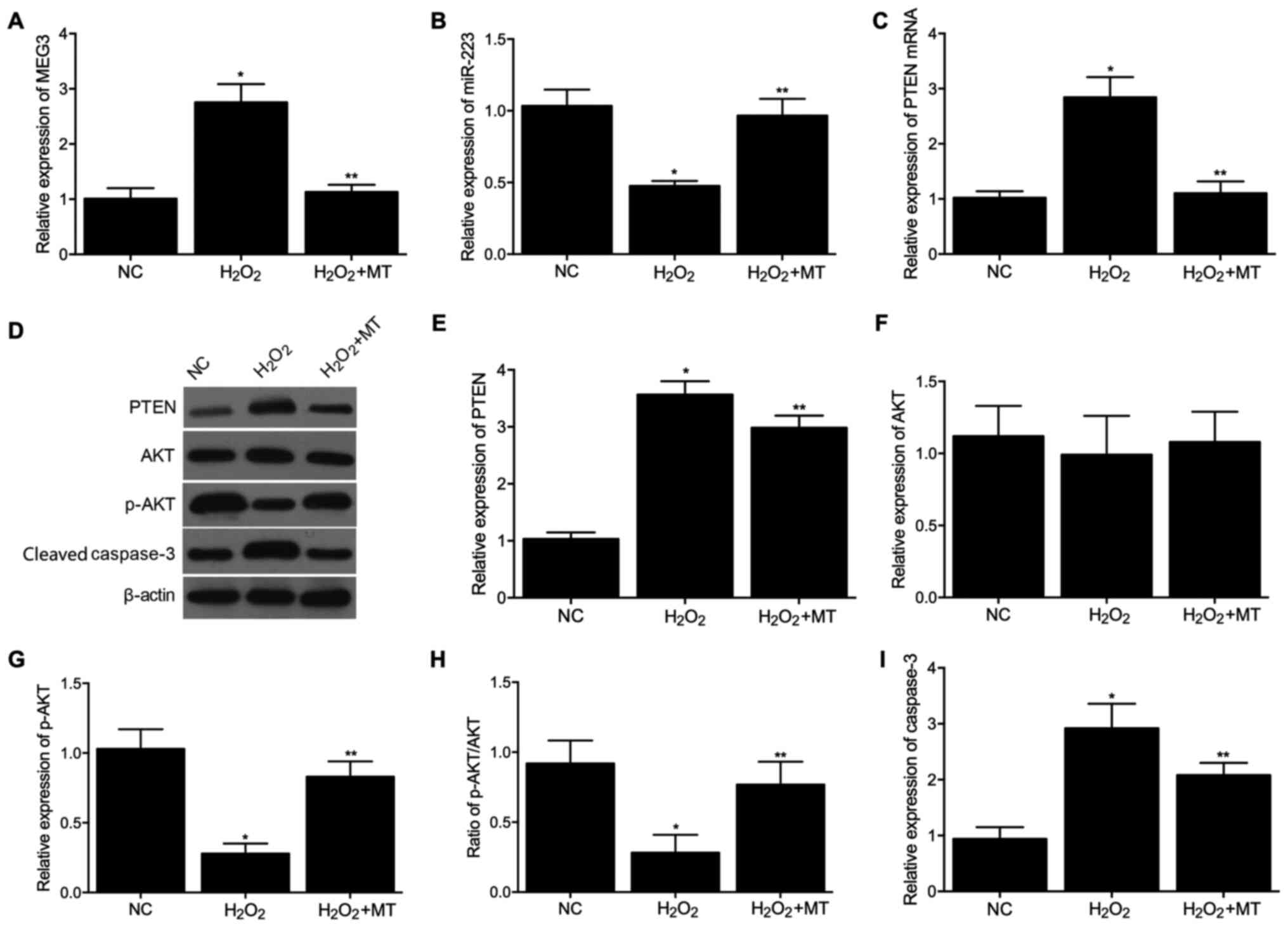 | Figure 1Expression levels of MEG3, miR-223,
PTEN, AKT, p-AKT and cleaved caspase-3 among control,
H2O2 and H2O2 + MT
groups in SH-SY5Y cells. (A) mRNA expression levels of MEG3 in the
three groups. (B) mRNA expression levels of miR-223 in the three
groups. (C) mRNA expression levels of PTEN in the three groups. (D)
Protein expression levels of PTEN, AKT, p-AKT and cleaved caspase-3
in the three groups. (E) Densitometry analysis of PTEN in the three
groups. (F) Densitometry analysis of AKT in the three groups. (G)
Densitometry analysis of p-AKT in the three groups. (H) Expression
of p-AKT normalized to the expression of AKT in the three groups.
(I) Densitometry analysis of cleaved caspase-3 in the three groups.
*P<0.05 vs. NC group; **P<0.05 vs.
H2O2 group. miR, microRNA; PTEN, phosphatase
and tensin homolog; AKT, protein kinase B; p-, phosphorylated; MT,
melatonin; NC, negative control. |
Additionally, western blotting was used to compare
PTEN, AKT, p-AKT and cleaved caspase-3 expression among the three
groups. The protein level of PTEN (Fig. 1D and E) was increased in the
H2O2 group, while treatment with MT decreased
PTEN protein expression. The protein expression of AKT (Fig. 1D and F) was comparable among the
three groups. In addition, the protein expression of p-AKT
(Fig. 1D, G and H) was decreased
in the H2O2 group, while treatment with MT
reversed this effect. The protein level of cleaved caspase-3
(Fig. 1D and I) was increased in
the H2O2 group, while treatment with MT
decreased cleaved caspase-3 protein expression. The same experiment
was then performed in U251 cells and similar results were observed
(Fig. 2A-I).
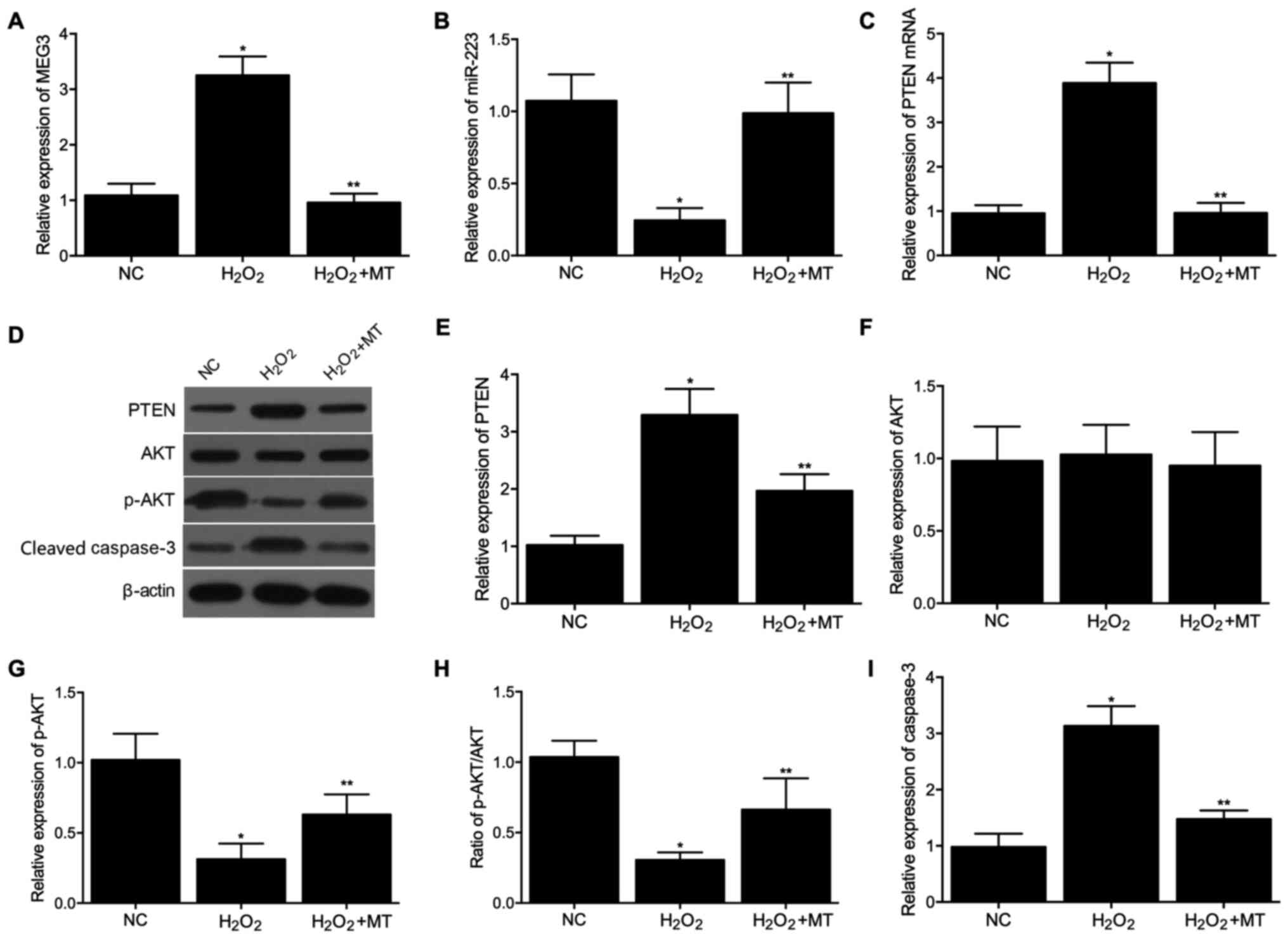 | Figure 2Expression levels of MEG3, miR-223,
PTEN, AKT, p-AKT and cleaved caspase-3 among the control,
H2O2 and H2O2 + MT
groups in U251 cells. (A) mRNA expression levels of MEG3 in the
three groups. (B) mRNA expression levels of miR-223 in the three
groups. (C) mRNA expression levels of PTEN in the three groups. (D)
Protein expression levels of PTEN, AKT, p-AKT and cleaved caspase-3
in the three groups. (E) Densitometry analysis of PTEN in the three
groups. (F) Densitometry analysis of AKT in the three groups. (G)
Densitometry analysis of p-AKT in the three groups. (H) Expression
of p-AKT normalized to the expression of AKT in the three groups.
(I) Densitometry analysis of cleaved caspase-3 in the three groups.
*P<0.05 vs. NC group; **P<0.05 vs.
H2O2 group. miR, microRNA; PTEN, phosphatase
and tensin homolog; AKT, protein kinase B; p-, phosphorylated; MT,
melatonin; NC, negative control. |
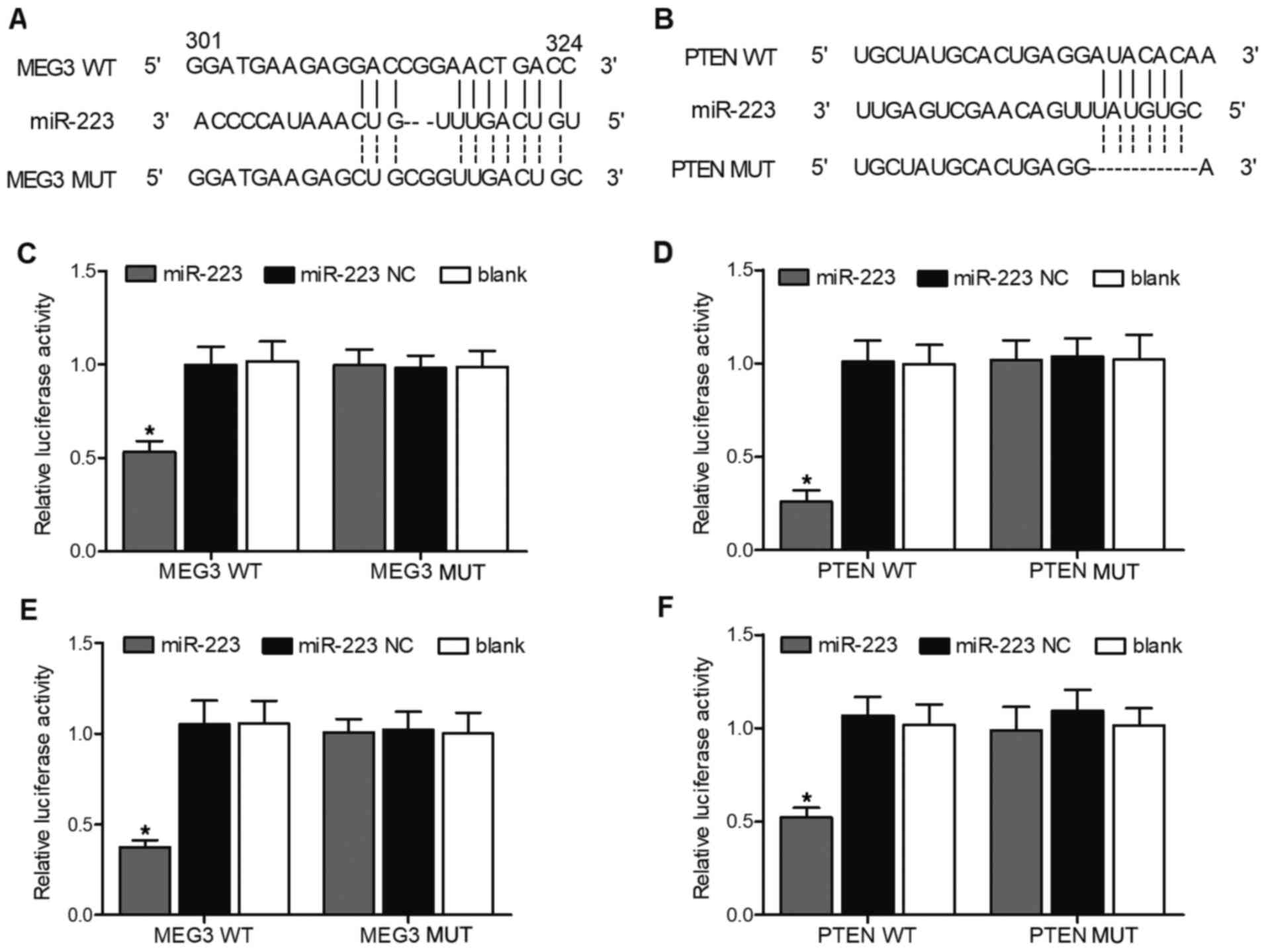
Verification of the MEG3/miR-223/PTEN
axis
TargetScan and PicTar-Vert were employed to predict
the potential targets of MEG3 and PTEN. A potential binding site
for miR-223 was found in MEG3 (Fig.
3A) and PTEN (Fig. 3B),
indicating that MEG3 or PTEN may be direct targets of miR-223. To
confirm the putative miR-223 binding site in MEG3 and PTEN, vectors
containing WT or MUT MEG3/PTEN were constructed. Then, SH-SY5Y and
U251 cells were co-transfected with miR-223 mimics or miR-223 NC.
The successful transfection of miR-223 mimics was validated by the
evidently upregulated miR-223 expression in SH-SY5Y (Fig. S1A) and U251 cells (Fig. S1B). Moreover, as shown in
Fig. 3C, in SH-SY5Y cells, WT
MEG3 significantly decreased the relative luciferase activity of
miR-223, suggesting that miR-223 could directly bind to MEG3. Also,
WT PTEN significantly decreased the relative luciferase activity of
miR-223 in SH-SY5Y cells, suggesting that PTEN could directly bind
to miR-223 (Fig. 3D).
Additionally, the same results were observed in U251 cells
(Fig. 3E and F). Taken together,
these findings indicated that MEG3, miR-223 and PTEN formed a
regulatory axis.
Expression levels of MEG3, miR-223 and
PTEN among the three animal model groups
Animals were divided into three groups: NC, FC and
the FC + MT. Then, RT-qPCR was performed to detect the mRNA
expression levels of MEG3, miR-223 and PTEN. Western blotting was
used to examine protein expression levels of PTEN, AKT, p-AKT and
cleaved caspase-3. As shown in Fig.
4A-I, the results were consistent with those observed in
cells.
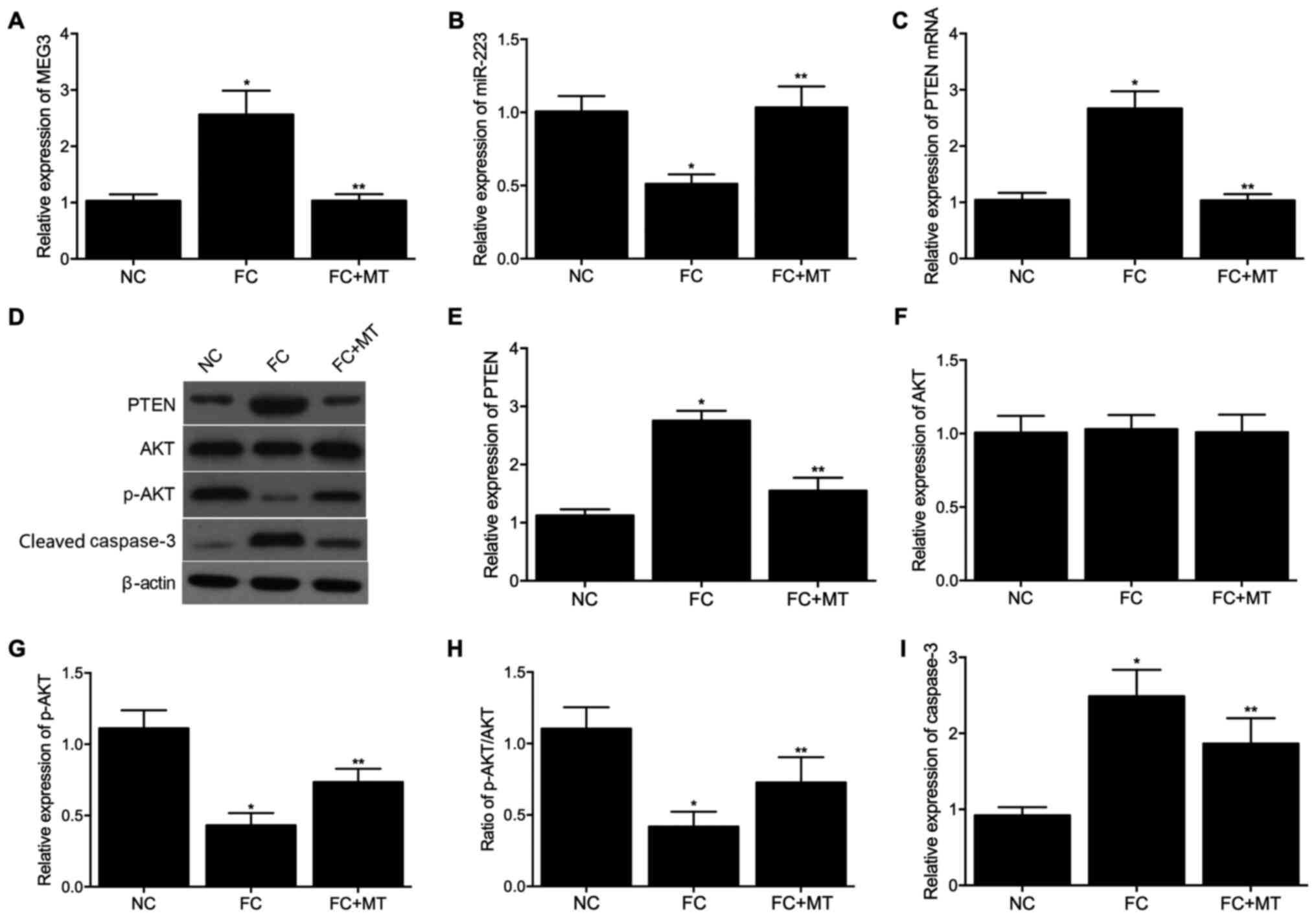 | Figure 4Expression levels of MEG3, miR-223,
PTEN, AKT, p-AKT and cleaved caspase-3 among NC, FC and FC + MT
groups. (A) mRNA expression levels of MEG3 in the three groups. (B)
mRNA expression levels of miR-223 in the three groups. (C) mRNA
expression levels of PTEN in the three groups. (D) Protein
expression levels of PTEN, AKT, p-AKT and cleaved caspase-3 in the
three groups. (E) Densitometry analysis of PTEN in the three
groups. (F) Densitometry analysis of AKT in the three groups. (G)
Densitometry analysis of p-AKT in the three groups. (H) Expression
of p-AKT normalized to the expression of AKT in the three groups.
(I) Densitometry analysis of cleaved caspase-3 in the three groups.
*P<0.05 vs. NC group; **P<0.05 vs. FC
group. miR, microRNA; PTEN, phosphatase and tensin homolog; AKT,
protein kinase B; p-, phosphorylated; MT, melatonin; NC, negative
control; FC, febrile convulsion. |
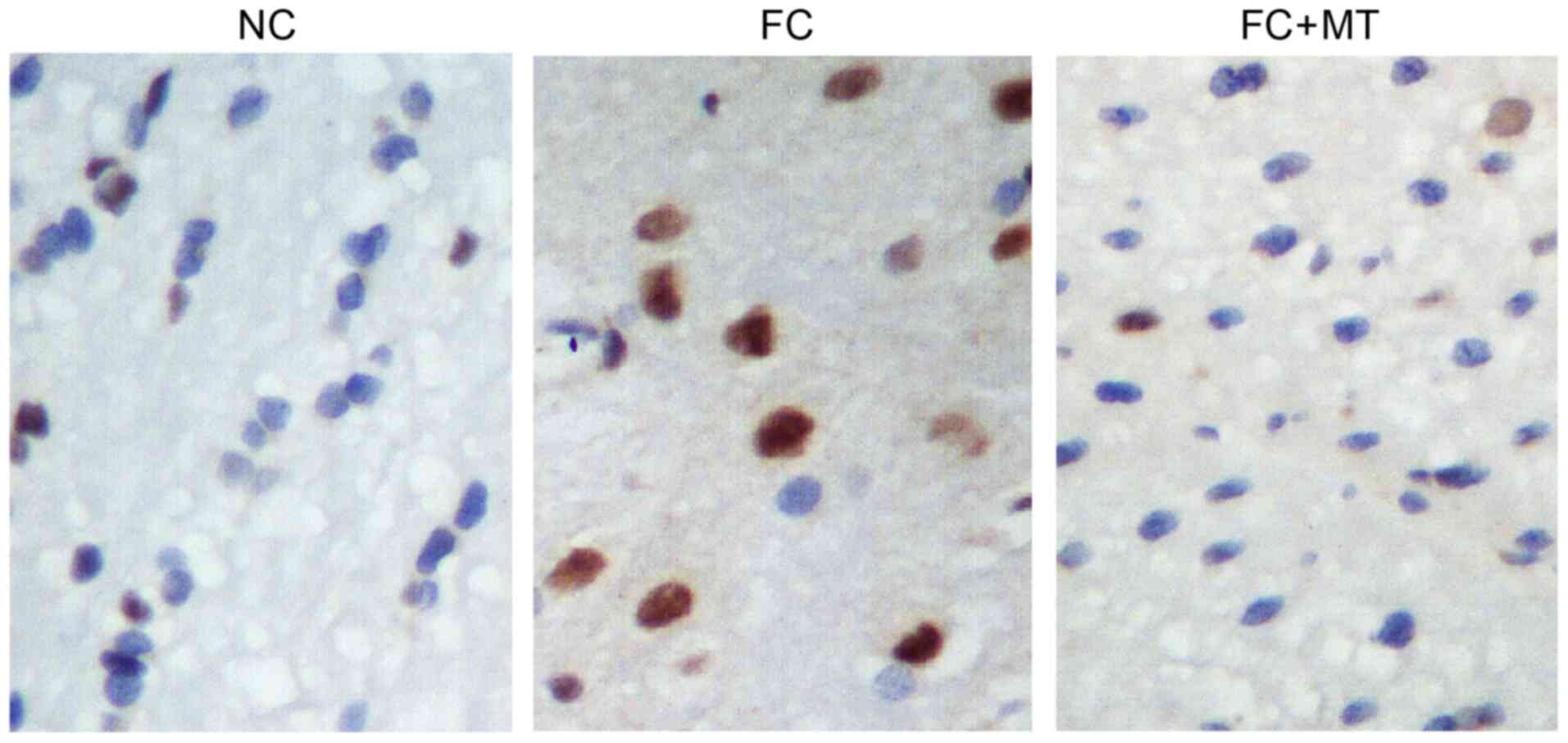
To quantify neurological deficits, TUNEL staining
was performed to visualize neuronal apoptosis. The results showed
that the FC group had a notably higher number of apoptotic neurons
than the control group. However, compared with the FC group, MT
treatment could markedly decrease the number of apoptotic neurons
(Fig. 5).
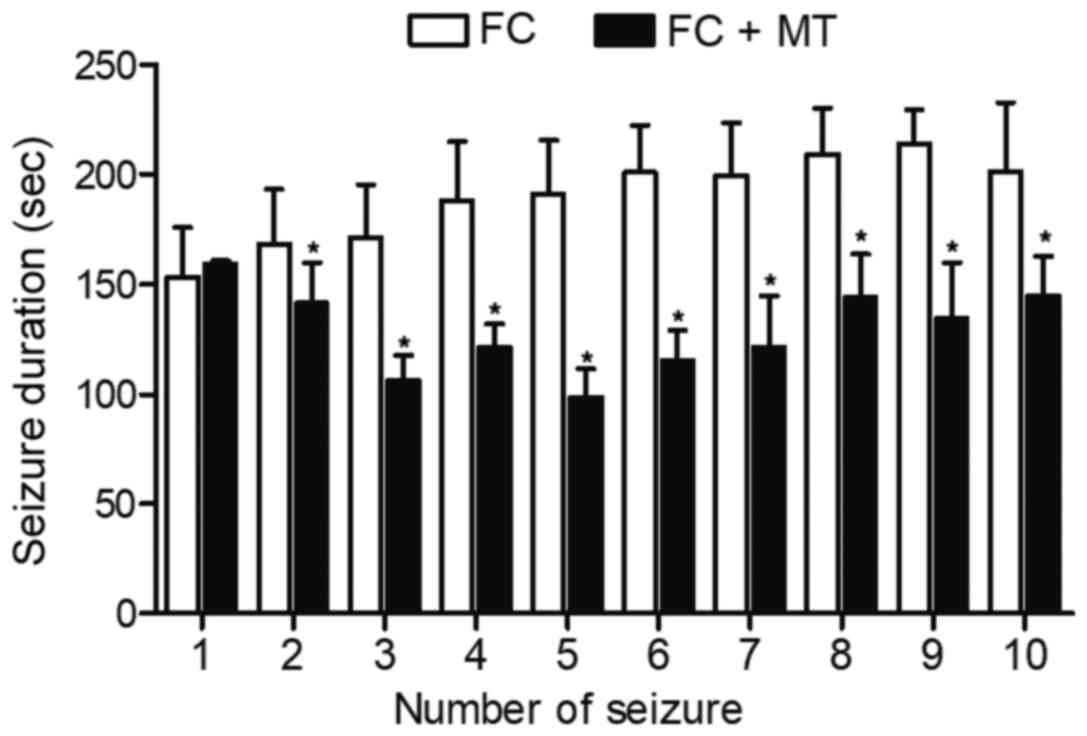
Detection of seizure/convulsion duration
among the different animal groups
The seizure/convulsion duration in different groups
on the same day were detected, as shown in Fig. 6. The duration of convulsion
(except the first episode of convulsion) in the FC group was
significantly higher than those in the FC + MT group.
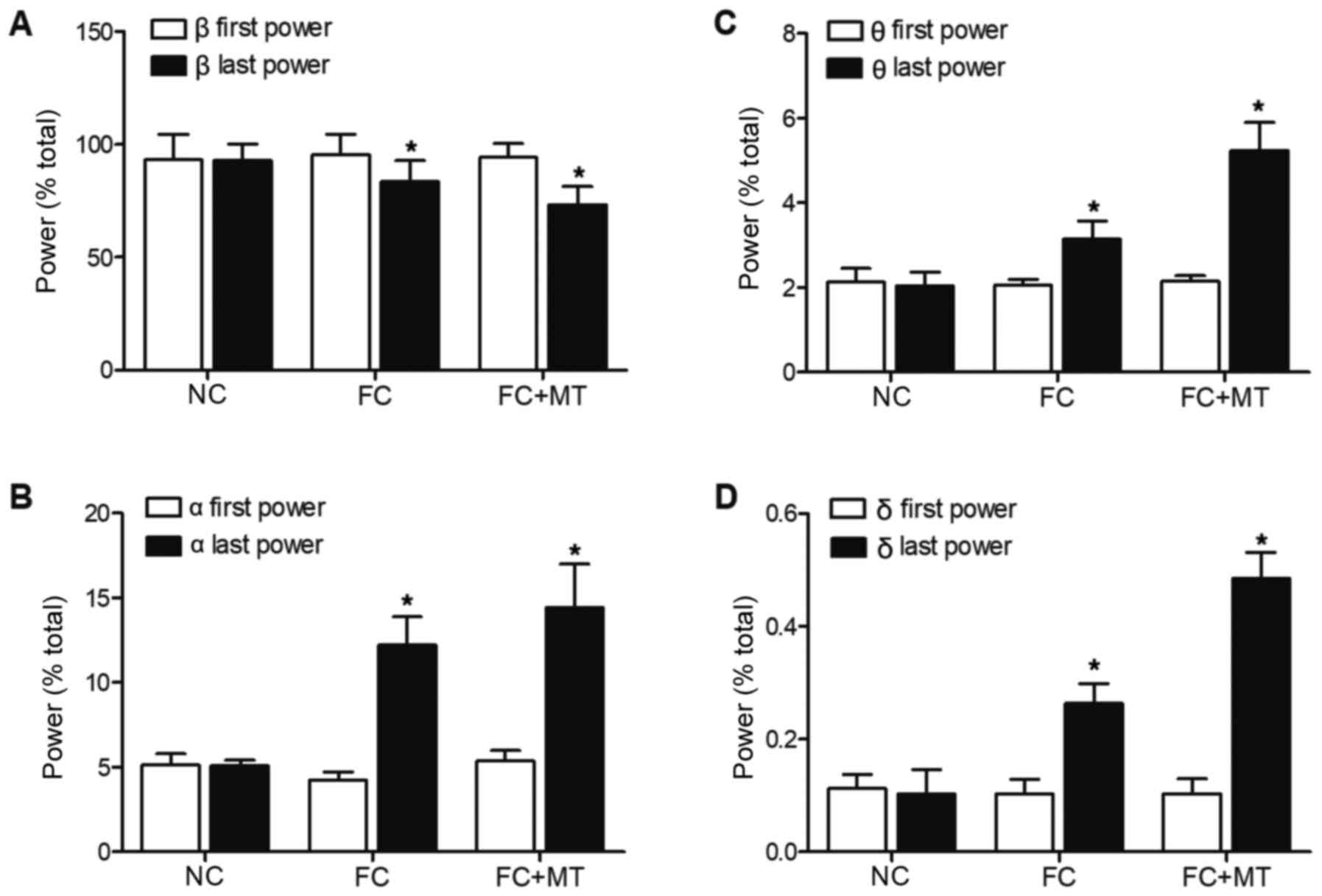
ECG recordings were then taken for the three groups.
As shown in Fig. 7A, there was
no significant difference in the first b power ratios among all
groups. However, the last β power ratios of FC group and FC + MT
group were significantly decreased compared with the first β power
ratios of these two groups. The last β power ratio of the FC group
was significantly lower than the last β power ratio in the NC
group, whereas treatment with MT further decreased the last β power
ratio.
As shown in Fig.
7B, there was no significant difference in the first a power
ratios among all groups. The last α power ratios of FC group and FC
+ MT group were significantly increased compared with the first α
power ratios of these two groups. The last α power ratio of the FC
group was significantly higher than the last α power ratio in the
NC group, while treatment with MT further increased the last α
power ratio. The same phenomenon was observed in the first θ power
ratios, last θ power ratios (Fig.
7C), first δ power ratios and last δ power ratios (Fig. 7D).
Discussion
MT is a neuroendocrine factor produced by the pineal
gland (25,26). MT is involved in a wide range of
biological functions in the body, including the regulation of
circadian rhythm, inflammation, sleep and immunity, as well as
having roles in cancer development (27-29). Of note, it has been suggested
that MT can alleviate different cardiovascular diseases, such as
myocardial ischemia-reperfusion injury, atherosclerosis,
hypertension and heart failure (30-32). Previous studies also reported
that MT can alleviate atherosclerosis through the
MEG3/miR-223/NLRP3 signaling pathway (10). In the present study, it was found
that miR-223 could bind to MEG3 and was negatively regulated by
MEG3. PTEN was a direct target of miR-223 and was negatively
regulated by miR-223.
MEG3 is a lncRNA that carries several miRNAs.
Furthermore, it has been demonstrated that miR-223 can directly
bind to PTEN to induce chemoresistance in a wide range of tumor
cells (33,34). PTEN has been illustrated to
inhibit cell survival while promoting apoptosis (34,35). PTEN is also a negative mediator
of the PI3K/AKT signaling pathway and is implicated in a wide range
of malignancies (36-38). In particular, PTEN expression is
dysregulated in Alzheimer's disease (AD) (39). In addition, PTEN nitrosylation is
enhanced in early AD (40).
Moreover, PTEN dysfunction was found to cause abnormal social
behavior in mice (41).
PTEN-α, which is one type of PTEN isoform, has been
demonstrated to induce the production of ATP by mitochondria
(42). Furthermore, inhibition
of PTEN signaling was shown to reduce the permeability of
mitochondria (43).
Additionally, hydrogen sulfide (H2S) was reported to improve the
dysfunction in cardiac contractility by inhibiting the function of
AKT2, thus alleviating the apoptosis of mitochondrial cells
(44). Similarly, it was
observed that H2S could reduce the expression of PTEN in
mitochondria, and knockdown of PTEN expression in mice has the
ability to impair learning and memory, as well as inducing
spontaneous seizure (45-47).
Furthermore, mice with PTEN dysfunction also show defunct social
behaviors (48). Thus, mice that
lack PTEN may develop significant behavioral deficits and seizure
(49). The results of the
present study showed that MEG3, cleaved caspase-3 and PTEN were
increased in FC, whereas miR-223 and p-AKT were increased.
Treatment with MT could reverse the effects of FC.
The downregulation of PTEN has been demonstrated to
activate PI3K/AKT signaling and promote cell survival (15,50). Furthermore, the function of PTEN
is significantly affected by PI3K/AKT signaling (51,52). Moreover, the C-terminal
hydrophobic motif of AGC kinases has been demonstrated to regulate
the catalytic functions of AGC kinases (53,54). In particular, the activation of
AKT kinase has been shown to be mediated by several receptor
tyrosine kinases (RTKs), such as the RTKs related to epidermal
growth factor, insulin, platelet-derived growth factor, fibroblast
growth factor and vascular endothelial growth factor (55,56). Moreover, RTKs can directly
activate PI3K or indirectly activate PI3K via certain adaptor
proteins, including insulin receptor substrate 1/2. A previous
study evaluated AKT phosphorylation at position Ser473 and
illustrated that AKT phosphorylation is reduced in the hippocampus
of rats suffering from recurrent FS (18).
To conclude, taken together, the present results
suggested that MT prevented FC via the MEG3/miR-223/PTEN/AKT
signaling pathway. Therefore, MT treatment could be considered as a
novel strategy for the treatment of FC disease.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW designed the study. JH supervised the study. GW,
JH, HZ, SW and ZL performed the experiments and drafted the
manuscript. SH performed the experiments and analyzed the data. ZL
performed the literature research, GW and JH drafted and reviewed
the manuscript. GW and SW confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Wuhan
Children's Hospital (Wuhan, China) and were in strict compliance
with the 'Guide for the Care and Use of Laboratory Animals'
published by the US National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Freeman JM: Febrile seizures: A consensus
of their significance, evaluation, and treatment. Pediatrics.
66:10091980.PubMed/NCBI
|
|
2
|
Karaagac N, Yeni SN, Senocak M, Bozluolcay
M, Savrun FK, Ozdemir H and Cagatay P: Prevalence of epilepsy in
Silivri, a rural area of Turkey. Epilepsia. 40:637–642. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina-Carballo A, Muñoz-Hoyos A,
Sanchez-Forte M, Uberos-Fernandez J, Moreno-Madrid F and
Acuna-Castroviejo D: Melatonin increases following convulsive
seizures may be related to its anticonvulsant properties at
physiological concentrations. Neuropediatrics. 38:122–125. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina-Carballo A, Acuna-Castroviejo D,
Rodriguez-Cabezas T and Muñoz-Hoyos A: Effects of febrile and
epileptic convulsions on daily variations in plasma melatonin
concentration in children. J Pineal Res. 16:1–9. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunner AL, Beck AH, Edris B, Sweeney RT,
Zhu SX, Li R, Montgomery K, Varma S, Gilks T, Guo X, et al:
Transcriptional profiling of long non-coding RNAs and novel
transcribed regions across a diverse panel of archived human
cancers. Genome Biol. 13:R752012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:37–38. 2018. View Article : Google Scholar
|
|
11
|
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen
Q, Feng F, Liu Y, Xu W and Li Y: Macrophages derived exosomes
deliver miR-223 to epithelial ovarian cancer cells to elicit a
chemoresistant phenotype. J Exp Clin Cancer Res. 38:812019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li DM and Sun H: TEP1, encoded by a
candidate tumor suppressor locus, is a novel protein tyrosine
phosphatase regulated by transforming growth factor beta. Cancer
Res. 57:2124–2129. 1997.PubMed/NCBI
|
|
13
|
Myers MP, Pass I, Batty IH, Van der Kaay
J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP and Tonks NK: The
lipid phosphatase activity of PTEN is critical for its tumor
supressor function. Proc Natl Acad Sci USA. 95:13513–13518. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu F, Kai J, Chen L, Wu M, Dong J, Wang Q
and Zeng LH: Akt inhibitor perifosine prevents epileptogenesis in a
rat model of temporal lobe epilepsy. Neurosci Bull. 34:283–290.
2018. View Article : Google Scholar :
|
|
18
|
Zhao Y, Han Y, Bu DF, Zhang J, Li QR, Jin
HF, Du JB and Qin J: Reduced AKT phosphorylation contributes to
endoplasmic reticulum stress-mediated hippocampal neuronal
apoptosis in rat recurrent febrile seizure. Life Sci. 153:153–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Freyd T, Warszycki D, Mordalski S,
Bojarski AJ, Sylte I and Gabrielsen M: Ligand-guided homology
modelling of the GABAB2 subunit of the GABAB receptor. PLoS One.
12:e01738892017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, McDaniel SS, Rensing NR and Wong
M: Vigabatrin inhibits seizures and mTOR pathway activation in a
mouse model of tuberous sclerosis complex. PLoS One. 8:e574452013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
MacKenzie G and Maguire J: Chronic stress
shifts the GABA reversal potential in the hippocampus and increases
seizure susceptibility. Epilepsy Res. 109:13–27. 2015. View Article : Google Scholar
|
|
22
|
Xu XK, Wang SY, Chen Y, Zhan L, Shao ZY,
Lin L, Yan WC and Mei SF: Fangjing decoction relieves febrile
seizures-induced hippocampal neuron apoptosis in rats via
regulating the Akt/mTOR pathway. Biosci Rep. 38:BSR201812062018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Stehle JH, Saade A, Rawashdeh O, Ackermann
K, Jilg A, Sebesteny T and Maronde E: A survey of molecular details
in the human pineal gland in the light of phylogeny, structure,
function and chronobiological diseases. J Pineal Res. 51:17–43.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manchester LC, Coto-Montes A, Boga JA,
Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX and Reiter RJ:
Melatonin: An ancient molecule that makes oxygen metabolically
tolerable. J Pineal Res. 59:403–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reiter RJ, Mayo JC, Tan DX, Sainz RM,
AlatorreJimenez M and Qin L: Melatonin as an antioxidant: Under
promises but over delivers. J Pineal Res. 61:253–278. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lax P, Otalora BB, Esquiva G, Rol Mde L,
Madrid JA and Cuenca N: Circadian dysfunction in P23H rhodopsin
transgenic rats: Effects of exogenous melatonin. J Pineal Res.
50:183–191. 2011.
|
|
30
|
Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai
M, Yang Y, Chen W, Liu J, Yi W, et al: Reduced silent information
regulator 1 signaling exacerbates myocardial ischemia-reperfusion
injury in type 2 diabetic rats and the protective effect of
melatonin. J Pineal Res. 59:376–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang
Y, Yang J, Yi D, Chen W, Wang X, et al: Membrane receptor-dependent
Notch1/Hes1 activation by melatonin protects against myocardial
ischemia-reperfusion injury: In vivo and in vitro studies. J Pineal
Res. 59:420–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Favero G, Rodella LF, Reiter RJ and
Rezzani R: Melatonin and its atheroprotective effects: A review.
Mol Cell Endocrinol. 382:926–937. 2014. View Article : Google Scholar
|
|
33
|
Chen Q, Qin R, Fang Y and Li H: Berberine
sensitizes human ovarian cancer cells to cisplatin through
miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem.
36:956–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keniry M and Parsons R: The role of PTEN
signaling perturbations in cancer and in targeted therapy.
Oncogene. 27:5477–5485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cowell JK, Qin H, Hu T, Wu Q, Bhole A and
Ren M: Mutation in the FGFR1 tyrosine kinase domain or inactivation
of PTEN is associated with acquired resistance to FGFR inhibitors
in FGFR1-driven leukemia/lymphomas. Int J Cancer. 141:1822–1829.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lue H, Thiele M, Franz J, Dahl E,
Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B and
Bernhagen J: Macrophage migration inhibitory factor (MIF) promotes
cell survival by activation of the Akt pathway and role for
CSN5/JAB1 in the control of autocrine MIF activity. Oncogene.
26:5046–5059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han Z, Chen F, Ge X, Tan J, Lei P and
Zhang J: miR-21 alleviated apoptosis of cortical neurons through
promoting PTEN-Akt signaling pathway in vitro after experimental
traumatic brain injury. Brain Res. 1582:12–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reynolds C, Roderick JE, LaBelle JL, Bird
G, Mathieu R, Bodaar K, Colon D, Pyati U, Stevenson KE, Qi J, et
al: Repression of BIM mediates survival signaling by MYC and AKT in
high-risk T-cell acute lymphoblastic leukemia. Leukemia.
28:1819–1827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sonoda Y, Mukai H, Matsuo K, Takahashi M,
Ono Y, Maeda K, Akiyama H and Kawamata T: Accumulation of
tumor-suppressor PTEN in Alzheimer neurofibrillary tangles.
Neurosci Lett. 471:20–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kwak YD, Ma T, Diao S, Zhang X, Chen Y,
Hsu J, Lipton SA, Masliah E, Xu H and Liao FF: NO signaling and
S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol
Neurodegener. 5:492010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Napoli E, Ross-Inta C, Wong S, Hung C,
Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld
R and Giulivi C: Mitochondrial dysfunction in Pten
haplo-insufficient mice with social deficits and repetitive
behavior: Interplay between Pten and p53. PLoS One. 7:e425042012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang H, He S, Yang J, Jia X, Wang P, Chen
X, Zhang Z, Zou X, McNutt MA, Shen WH and Yin Y: PTENα, a PTEN
isoform translated through alternative initiation, regulates
mitochondrial function and energy metabolism. Cell Metab.
19:836–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng X, Zu L, Becker L and Cai ZP:
Ischemic preconditioning inhibits mitochondrial permeability
transition pore opening through the PTEN/PDE4 signaling pathway.
Cardiology. 129:163–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu N, Dong M and Ren J: Hydrogen sulfide
alleviates cardiac contractile dysfunction in an Akt2-knockout
murine model of insulin resistance: Role of mitochondrial injury
and apoptosis. Am J Physiol Regul Integr Comp Physiol.
306:R761–R771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kwon CH, Luikart BW, Powell CM, Zhou J,
Matheny SA, Zhang W, Li Y, Baker SJ and Parada LF: Pten regulates
neuronal arborization and social interaction in mice. Neuron.
50:377–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lugo JN, Smith GD, Morrison JB and White
J: Deletion of PTEN produces deficits in conditioned fear and
increases fragile X mental retardation protein. Learn Mem.
20:670–673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Backman SA, Stambolic V, Suzuki A, Haight
J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO and Mak
TW: Deletion of Pten in mouse brain causes seizures, ataxia and
defects in soma size resembling Lhermitte-Duclos disease. Nat
Genet. 29:396–403. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clipperton-Allen AE and Page DT: Decreased
aggression and increased repetitive behavior in Pten
haploinsufficient mice. Genes Brain Behav. 14:145–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Smith GD, White J and Lugo JN:
Superimposing status epilepticus on neuron subset-specific PTEN
haploinsufficient and wild type mice results in long-term changes
in behavior. Sci Rep. 6:365592016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun H, Lesche R, Li DM, Liliental J, Zhang
H, Gao J, Gavrilova N, Mueller B, Liu X and Wu H: PTEN modulates
cell cycle progression and cell survival by regulating
phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B
signaling pathway. Proc Natl Acad Sci USA. 96:6199–6204. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu JL, Sheng X, Hortobagyi ZK, Mao Z,
Gallick GE and Yung WK: Nuclear PTEN-mediated growth suppression is
independent of Akt down-regulation. Mol Cell Biol. 25:6211–6224.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Blanco-Aparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Arencibia JM, Pastor-Flores D, Bauer AF,
Schulze JO and Biondi RM: AGC protein kinases: From structural
mechanism of regulation to allosteric drug development for the
treatment of human diseases. Biochim Biophys Acta. 1834:1302–1321.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liao Y and Hung MC: Physiological
regulation of Akt activity and stability. Am J Transl Res. 2:19–42.
2010.PubMed/NCBI
|
|
56
|
Scheid MP and Woodgett JR: Unravelling the
activation mechanisms of protein kinase B/Akt. FEBS Lett.
546:108–112. 2003. View Article : Google Scholar : PubMed/NCBI
|