Introduction
Gastric cancer (GC) is one of the most common
malignant tumor types of the digestive system and is the second
leading cause of cancer-associated mortality worldwide (1). GC morbidity and mortality rates
remain high. By 2018, the global mortality rate of gastric cancer
had reached 8.2% (2). At
present, chemotherapy is still an important treatment option for
patients with advanced disease, of which cisplatin (DDP) is
commonly used (3). However,
long-term use often results in drug tolerance, which is an
important issue that requires urgent attention (4). Due to its unique ability to inhibit
tumor growth, improve patient quality of life and prolong survival
time, Traditional Chinese Medicine (TCM) has become a valuable
research hotspot and focus in the field of tumor adjuvant therapy
and drug sensitivity (5,6).
Banxia xiexin decoction (BXXX) is a classic TCM
prescription used to treat gastrointestinal diseases. Previous
studies have reported that BXXX effectively treats digestive
tumors, as well as those of other bodily systems (7). For example, BXXX can alleviate
colon cancer (8), lung cancer
(9), gallbladder cancer
(10) and cancer symptoms. In
addition, serum containing BXXX was found to inhibit the
proliferative, invasive and metastatic abilities of GC GC9811-P
cells, thereby blocking the peritoneal metastasis of GC (11). BXXX also regulates the expression
of STAT3 proteins in gastric adenocarcinoma and gastric mucosal
epithelial cells (12). In
inflammatory diseases and GC, BXXX can regulate the levels of
inflammatory factors, including IFN-γ and IL-6 (13). In our previous study, the
BATMAN-TCM bioinformatics analysis tool (http://bionet.ncpsb.org/batman-tcm/) was used to
analyze drug composition and target prediction, and BXXX was found
to also exert its effects on a variety of proteins associated with
tumor drug resistance and sensitivity, such as
6-O-methylguanine-DNA methyltransferase (MGMT) (data not yet
published). Moreover, MGMT is strongly associated with the
occurrence and development of GC, especially drug resistance
(14).
Programmed cell death protein 1 (PD-1) and
programmed cell death 1 ligand 1 (PD-L1) remain a research hotspot
for drug resistance in tumors, but the associated resistance
mechanisms are more closely associated with those of EGFR-TKI and
tumor immune escape (15-18).
However, in addition to its ability to influence T cells via its
ligand PD-1, PD-L1 can also impact tumor cells. For example, PD-L1
was found to increase the expression levels of various multi-drug
resistance proteins in tumor cells. Moreover, the expression of
PD-L1 is increased following tumor cell DNA damage, which is also
closely associated with the activation of DNA repair mechanisms
(19,20).
IL-6 and IFN-γ regulate the JAK/STAT3 pathway to
induce the expression of PD-L1 in tumor-related macrophages, thus
regulating the occurrence and development of lung cancer (21). Moreover, the activator protein-2
(AP-2) factor inhibited the IL-6/JAK2/STAT3 signaling pathway,
decreasing the expression levels of MGMT and PD-L1 in glioma
tissues and cell lines (22).
Therefore, it was hypothesized that BXXX may also moderate PD-L1
expression by regulating IL-6, IFNγ and the downstream STAT3
pathway, and potentially influence the expression of MGMT.
Thus, in the present study, the mechanism of drug
sensitivity of BXXX to GC was investigated via animal experiments
and cell experiments.
Materials and methods
BXXX preparation
BXXX (Pinellia ternata, 15 g; Scutellaria
baicalensis Georgi, 9 g; dried ginger, 9 g; ginseng, 9 g;
honey-fried licorice root, 9 g; Coptis chinensis, 3 g; and
jujube, 4 pieces) was provided by The Affiliated Hospital of
Yangzhou University (Yangzhou, China). BXXX was processed into
granules by The Second Affiliated Hospital of Guangzhou University
of Chinese Medicine (Guangzhou, China). All herbs were obtained
from the original sources and met the standards listed in the
National Pharmacopoeia of China, and were identified by Guangdong
Provincial Hospital of Chinese Medicine. The decoction was made by
boiling the mixture twice in distilled water at 100°C for 30 min.
The drug solution was then cooled and dried into granules. Finally,
the granule was dissolved in 0.5% sodium carboxymethyl cellulose.
The present study did not use High Performance Liquid
Chromatography to determine the active components, because BXXX is
a classic prescription, and its active components have been
determined in the study of Chen et al (23).
Clinical specimens
Clinicopathological data, GC tissue and adjacent
tissue samples (~1 cm from the cancer tissue) were obtained from 10
patients (5 women, 5 men, aged between 37-65 years) with GC who
were admitted to The Affiliated Hospital of Yangzhou University
between June 2019 and December 2019. None of the patients had
received adjuvant radiotherapy or chemotherapy prior to surgery.
All samples were verified by experienced pathologists and
immediately stored in liquid nitrogen (−80°C). The present study
was approved by The Affiliated Hospital of Yangzhou University, and
each patient provided written informed consent.
Cell culture
Normal gastric mucosa epithelial and GC cell lines
(AGS, SNU-1, KATO III and NCI-N87) were purchased from the Type
Culture Collection of the Chinese Academy of Science, and incubated
in DMEM supplemented with 10% FBS (both Gibco; Thermo Fisher
Scientific) at 37°C (5% CO2). The human DDP-resistant
gastric adenocarcinoma cell lines AGS/DDP and NCI-N87/DDP were
purchased from Gefan Biotechnology (Shanghai, China). In order to
maintain drug resistance in AGS/DDP and NCI-N87/DDP cells, the
culture media were supplemented with DDP (cat. no. B00798003; Best
reagent; www.best-reagent.com) at a final
concentration of 2 nmol/l. AGS/DDP cells were pre-treated with
colivelin (50 µg/ml; Tocris Biosciences) for 2 h at 37°C, a
STAT3 activator, and the cells were divided into AGS/DDP, 15% BXXX,
15% BXXX + covlivelin, 15% BXXX + DDP and 15% BXXX + DDP +
covlivelin groups.
Preparation of a GC xenograft model in
nude mice
A total of 20 healthy female BALB/C nude mice (age,
6-8 weeks; weight, 16-20 g) acquired from The Affiliated Hospital
of Yangzhou University were kept in an environment with a constant
temperature of 25°C and humidity of 30-70% with 12 h light/dark
cycle. The mice were fed normally and maintained in specific
pathogen-free conditions. All animal procedures and experimental
methods were approved by the Committee on the Ethics of Animal
Experiments of The Affiliated Hospital of Yangzhou University, and
were conducted in accordance with the ARRIVE guidelines (24). AGS/DDP cells were used to
establish a drug-resistant tumor-bearing mouse model of GC. The
mice were subcutaneously inoculated with AGS/DDP cells (150
µl) in the logarithmic growth stage (6×107 U/ml)
near the back-right upper limb. Tumors were visible after 3-5 days,
at which point BXXX (9 g/kg) was administered daily by gavage and
DDP (5 mg/kg, intraperitoneal injection, once a week). The body
weight and tumor volume of each mouse were recorded for 5 weeks,
and the maximum tumor volume was 1,100 mm3. Model mice
were treated with DDP, BXXX and a combination of both DDP and BXXX,
and then divided into the AGS/DDP, DDP, BXXX and DDP + BXXX groups.
Each group comprised of five mice. After successful modeling, the
mice were sacrificed by cervical dislocation (the mice stopped
breathing, their hearts stopped beating, their muscles relaxed,
their body temperature dropped and they did not respond to external
stimuli) and the tumor tissues were removed for subsequent
experimentation. In addition, 3 ml serum each rats containing BXXX
was prepared at concentrations of 5, 10 and 15%, and used in cell
experiments.
Data
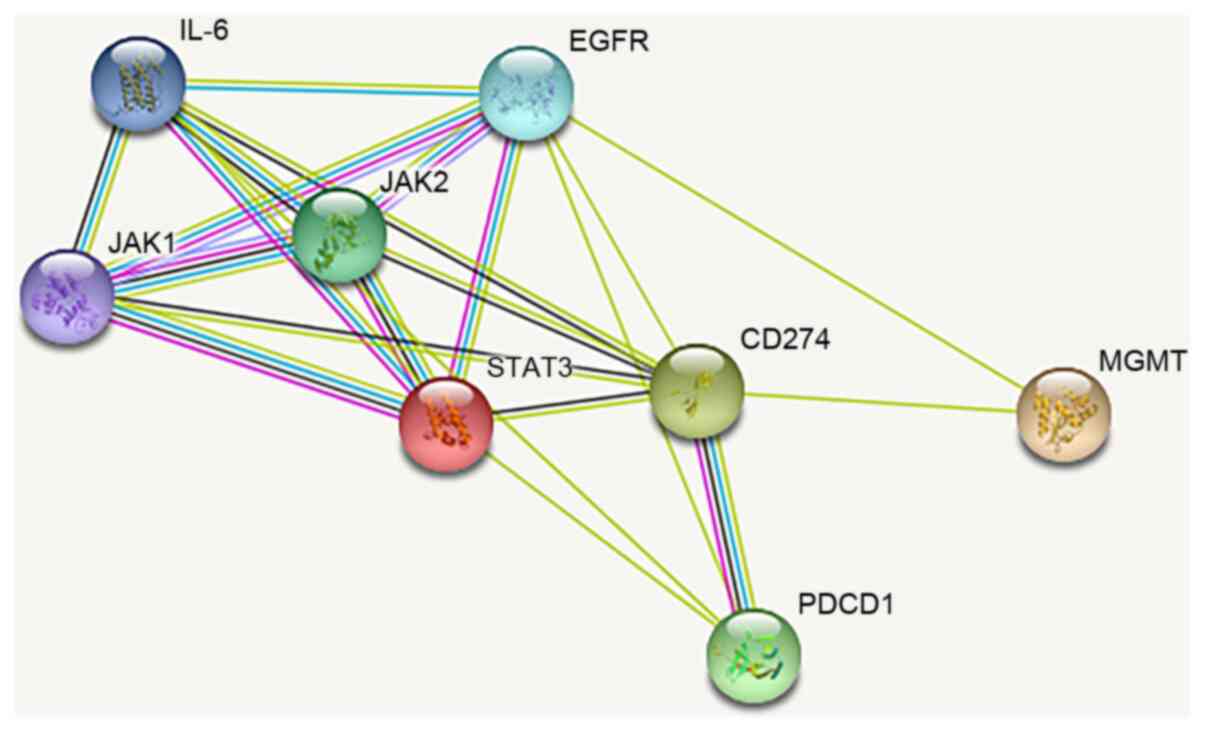
The STRING website (version 11.0; https://string-db.org/) was used to predict the
relationship between IL-6, JAK/STAT3 and MGMT.
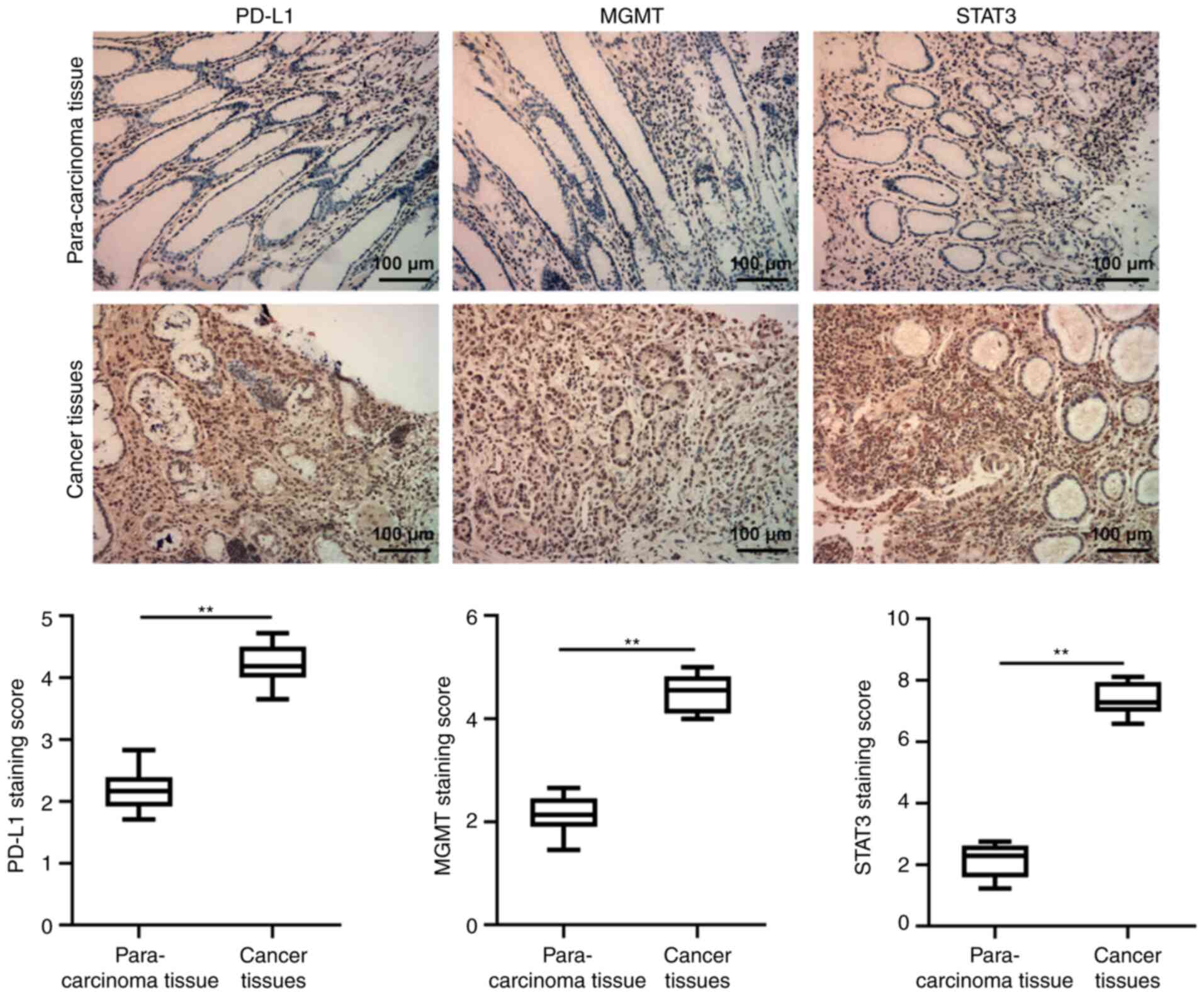
Immunohistochemistry (IHC)
GC and para-carcinoma tissue (thickness, ~4
µm) were fixed with 4% (w/v) paraformaldehyde at 4°C for 24
h, deparaffinized and embedded in paraffin once more. The tissues
were incubated with primary antibodies against PD-L1 (1:100; cat.
no. sc-293425; Santa Cruz Biotechnology, Inc.), MGMT (1:100; cat.
no. sc-166528; Santa Cruz Biotechnology, Inc.) and STAT3 (1:100;
cat. no. sc-8019; Santa Cruz Biotechnology, Inc.) overnight at 4°C,
and then with secondary antibodies (1:5,000; Abcam) for 1 h at
37°C. Subsequently, 3,3′-diaminobenzidine tetrahydrochloride
staining was conducted (Sigma-Aldrich; Merck KGaA) at room
temperature for 5 min. The results were observed under a light
contrast microscope (BX51; Olympus Corporation; magnification,
×200).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The RNA was
reverse transcribed into complementary DNA using the First-Strand
cDNA Synthesis kit, and qPCR was conducted using the SuperScript
III Platinum SYBR-Green One-Step qRT-PCR kit (both Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The thermocycling conditions were as follows: Initial
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
10 sec, 55°C for 10 sec and 72°C for 30 sec. Relative expression
levels were calculated using the 2−ΔΔCq method (25). The following primers were used:
PD-L1 forward, 5′-TTT GCT GAA CGC CCC ATA-3′ and reverse, 5′-TGC
TTG TCC AGA TGA CT TCG-3′; MGMT forward, 5′-GCG TTT CGA CGT TCG TAG
GT-3′ and reverse, 5′-CAC TCT TCC GAA AAC GAA ACG-3′; STAT3
forward, 5′-CTC AAC TTC AGA CCC GTC AACA-3′ and reverse, 5′-GCT CCA
CGA TTC TCT CCT CCA-3′; and GAPDH forward, 5′-CTC GCT TCG GCA GCA
CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′.
Cell Counting Kit 8 (CCK-8) assay
Cells were cultured in a 96-well plate
(5×103 cells/well). Following treatment, 10 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to
each well and the cells were incubated at 37°C for 4 h, according
to the manufacturer's instructions. Finally, the absorbance was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Flow cytometry
Following the appropriate treatment, the cells were
harvested for apoptosis analyses. Apoptosis was assessed using the
FITC-Annexin V Apoptosis Detection kit II (BD Biosciences)
according to the manufacturer's instructions, and flow cytometry
was performed using the BD FACSCanto II Flow Cytometer (BD
Biosciences). The data were analyzed using FlowJo software (version
1.46; FlowJo LLC).
Western blotting
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology) and incubated on ice for 30 min. The
protein concentrations were determined using a BCA protein assay
kit (Bio-Rad Laboratories, Inc.). A total of 40 µg
protein/well was separated using 10% SDS-PAGE gels, and
subsequently transferred to PVDF membranes. The membranes were
blocked with 10% skimmed milk for 2 h at room temperature, followed
by incubation with primary antibodies overnight at 4°C. The
membranes were subsequently incubated with goat anti-rabbit
HRP-conjugated IgG secondary antibodies (1:5,000; cat. no. ab96899;
Abcam) at room temperature for 1 h. The signals were detected using
an enhanced chemiluminescence reagent (Cytiva) and ImageJ software
(version 146; National Institutes of Health) was used to
semi-quantify the fold-changes in protein expression. The following
primary antibodies were used in the present study: Anti-IL-6
(1:1,000; cat. no. 12912s), anti-JAK1 (1:1,000; cat. no. 29261s),
anti-STAT3 (1:1,000; cat. no. 9139s), anti-IFN receptor (IFNR;
1:1,000; cat. no. 8455s), anti-PD-L1 (1:1,000; cat. no. 13684s),
anti-MGMT (1:1,000; cat. no. 86039s) and anti-GAPDH (1:1,000; cat.
no. 5174S). All antibodies were obtained from Cell Signaling
Technology, Inc.
Co-immunoprecipitation (co-IP)
Cells were harvested 24 or 48 h post-transfection,
and 8 ml lysis buffer (containing protease inhibitor; Beyotime
Institute of Biotechnology) was added to each sample. The cells
were lysed on ice (4°C) for 30 min, after which the supernatants
were harvested by centrifugation for 30 min at 300 × g at 4°C. A
small amount of each lysate was retained for western blot analysis,
and the remainder was added to the cell lysate containing 1
µg of the anti-PD-L1 (1:1,000; cat. no. 13684s) and
anti-MGMT (1:1,000; cat. no. 86039s). The samples were incubated
overnight at 4°C. Next, 10 µl protein A agarose beads
(washed with lysate; Sigma-Aldrich; Merck KGaA) were added to the
aforementioned cell lysates, and slowly shaken at 4°C for 2-4 h to
encourage antibody/bead coupling. Following immunoprecipitation,
the coupled samples were centrifuged at 350 × g (4°C) for 30 min,
and the supernatant was discarded. The samples were then washed 3-4
times with 1 ml buffer solution. Finally, 15 µl SDS (2X) was
added, and the samples were boiled for 5 min prior to western blot
analysis.
Statistical analysis
SPSS version 21.0 (IBM Corp) was used to perform all
statistical analyses, and the data are presented as the mean ±
standard deviation. Comparisons were made using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. The data of IHC
were presented as the median ± interquartile range and analyzed
using a non-parametric test (Wilcoxon signed-rank test). Each
experiment was performed ≥3 times.
Results
Expression levels of PD-L1, MGMT and
STAT3 in drug-resistant GC cells
The baseline patient data are presented in Table I. Fig. 1 shows the protein pathway diagram
analyzed by the STRING website. The IHC results revealed that the
expression levels of PD-L1, MGMT and STAT3 in GC tissues were
significantly higher compared with those in the para-carcinoma
tissues (Fig. 2). Then, PD-L1,
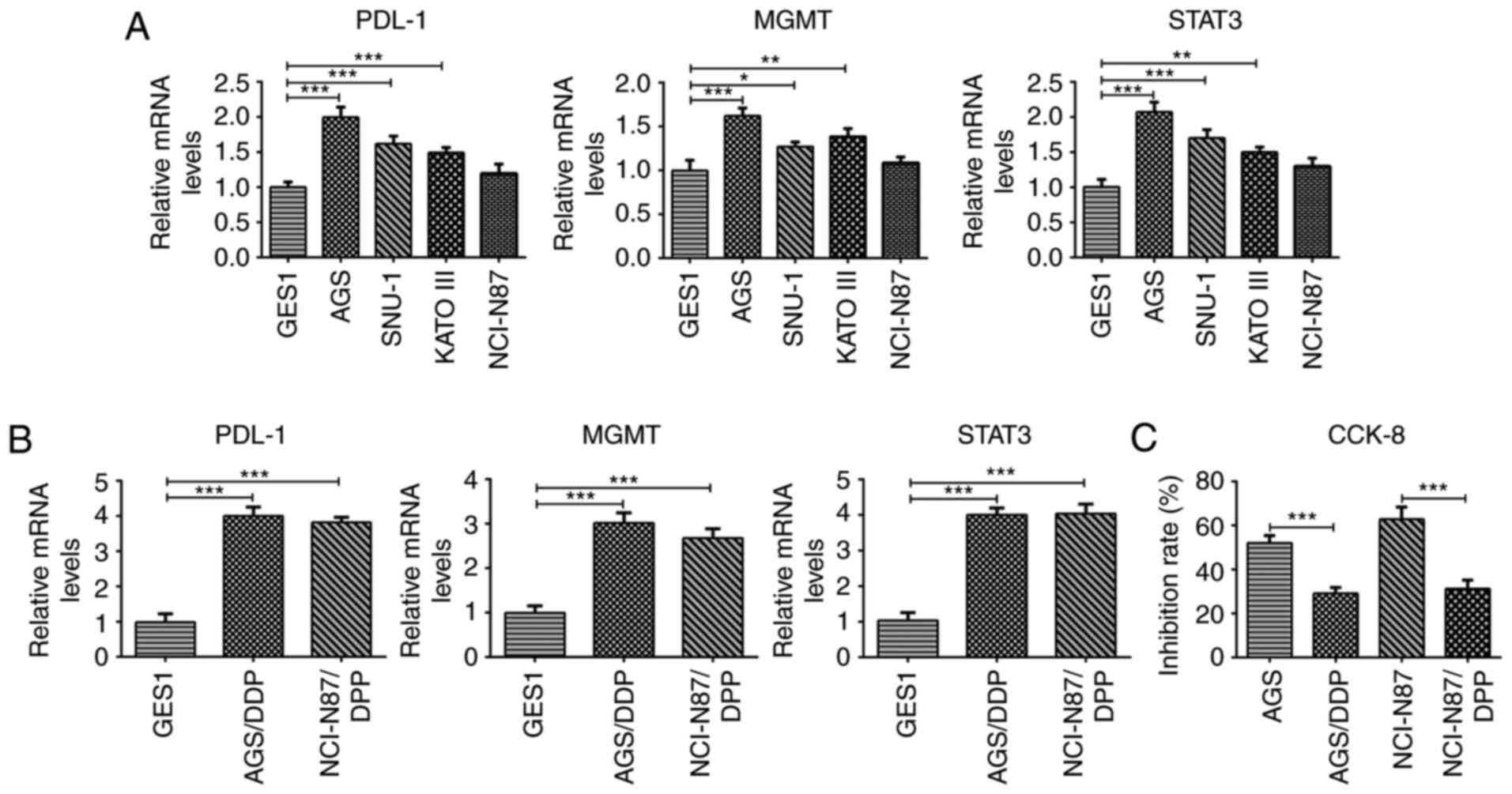
MGMT and STAT3 expression levels were detected in different GC cell
lines. The results indicated that compared with those in GES1
cells, the expression levels of PD-L1, MGMT and STAT3 in GC cell
lines were increased to varying degrees (Fig. 3A). Subsequently, two groups of
AGS cells with significant increases in PD-L1, MGMT and STAT3
expression levels, as well as NCI-N87 cells (whose expression
levels were not significantly increased) were selected for drug
resistance induction, and designated as the GES1, AGS/DDP and
NCI-N87/DDP groups. Compared with the GES1 group, the expression
levels of PD-L1, MMT and STAT3 were also significantly increased
(to a comparable degree) in the AGS/DDP and the NCI-N87/DDP groups
(Fig. 3B), indicating that that
PD-L1, MGMT and STAT3 are closely associated with drug resistance.
Therefore, the inhibitory effects of DDP on AGS and NCI-N87 cells
were also investigated. Compared with AGS and NCI-N87 cells,
cellular proliferation was inhibited following drug resistance
induction (Fig. 3C). As the
PD-L1, MGMT and STAT3 expression levels were higher in AGS cells
compared with in NCI-N87 cells, the DDP inhibition rate of the AGS
cells was relatively low. Therefore, AGS and AGS/DDP cells were
selected for further investigation.
 | Table IBaseline characteristics of patients
with gastric cancer. |
Table I
Baseline characteristics of patients
with gastric cancer.
| Sample | Sex | Age (years) | Drinking | Smoking | Family history of
cancer (any type) |
|---|
| 1 | Male | 38 | Yes | No | Yes |
| 2 | Male | 57 | Yes | Yes | No |
| 3 | Male | 35 | No | Yes | Yes |
| 4 | Male | 65 | Yes | No | No |
| 5 | Male | 54 | Yes | No | No |
| 6 | Female | 51 | No | No | No |
| 7 | Female | 42 | No | Yes | No |
| 8 | Female | 58 | No | No | Yes |
| 9 | Female | 46 | Yes | No | Yes |
| 10 | Female | 37 | No | Yes | No |
BXXX regulates the sensitivity of
drug-resistant GC cells
Serum samples containing different concentrations of
BXXX were selected to assess its effects on the proliferation of
AGS and AGS/DDP cells. As presented in Fig. 4A and B, AGS and AGS/DDP cell
proliferation was inhibited at different concentrations of BXXX,
and an increase in BXXX concentration resulted in a gradual
increase in the inhibition rate. Thus, a concentration of 15% BXXX
was used to further assess whether BXXX promoted the inhibitory
effects of chemotherapeutic drugs on drug-resistant strains.
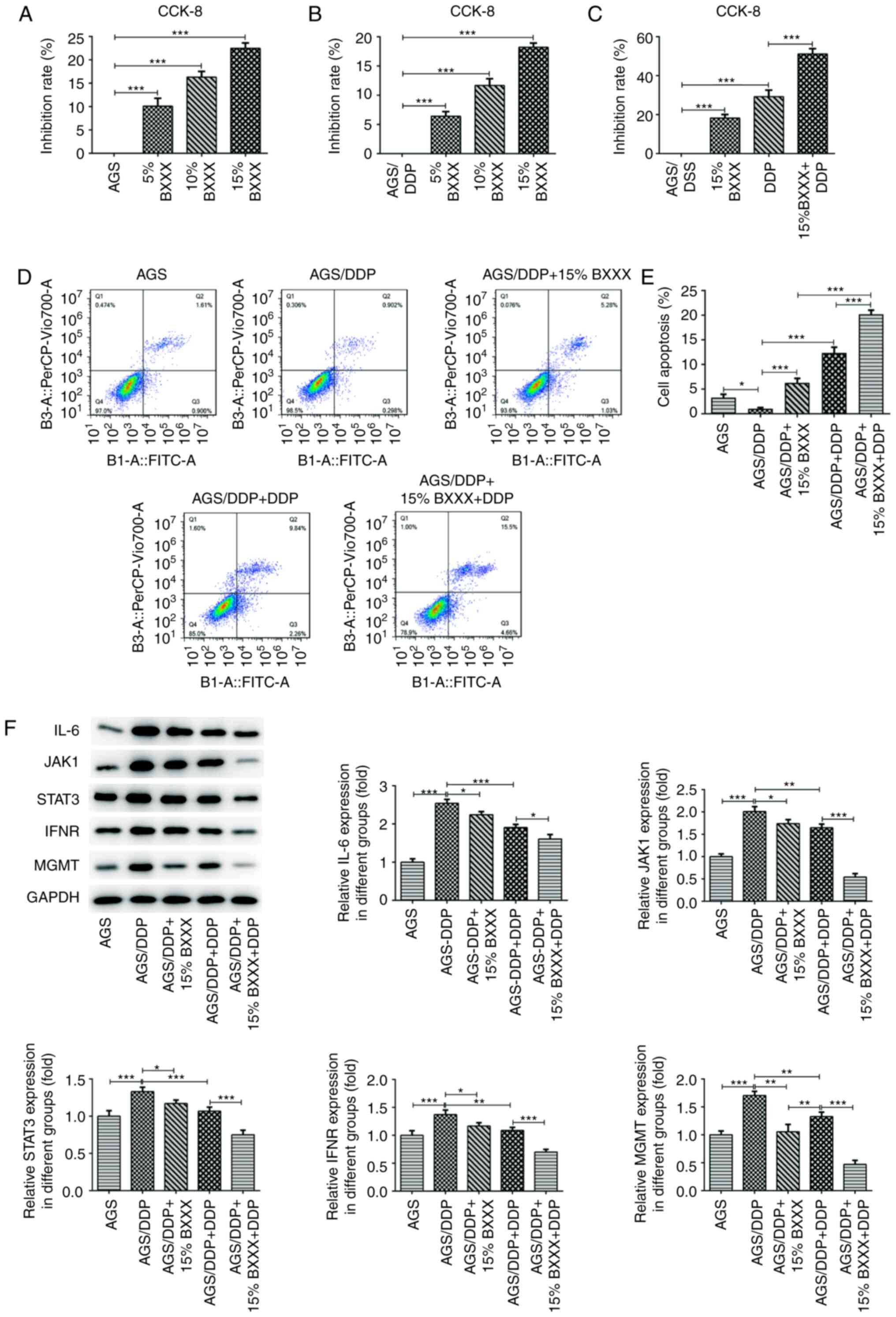 | Figure 4BXXX regulates the sensitivity of
drug-resistant cells of GC. The CCK-8 assay detected the viability
of (A) AGS cells and (B) AGS/DDP cells. (C) Cell viability after
the addition of 15% BXXX and DDP, as determined with a CCK-8 assay.
(D) Flow cytometry was used to detect cell apoptosis. (E)
Statistical analysis of apoptotic cells. (F) Western blotting was
used to measure the expression levels of the signaling
pathway-related proteins IL-6, JAK1, STAT3, IFNR and MGMT.
*P<0.05, **P<0.01,
***P<0.001. CCK, Cell Counting Kit; PD-L1, programmed
cell death 1 ligand 1; MGMT, 6-O-methylguanine-DNA
methyltransferase; DDP, cisplatin; BXXX, Banxia xiexin decoction;
IFNR, IFN receptor. |
The CCK-8 assay results revealed that, compared with
drug-sensitive AGS cells, AGS cell proliferation was inhibited
after the addition of DDP, which was further retarded by 15% BXXX
(Fig. 4C). The flow cytometric
results demonstrated that, compared with the AGS group, the
apoptotic rate of AGS/DDP cells was decreased. Compared with the
AGS/DDP group, the apoptotic rate of the AGS/DDP + 15% BXXX group
was increased, and the rate of the AGS/DDP + DDP group was enhanced
to a greater degree compared that of the AGS/DDP + 15% BXXX group.
Moreover, compared with the AGS/DDP + DDP group, the apoptotic rate
of the AGS/DDP + 15% BXXX + DDP group was further increased
(Fig. 4D and E). The results
indicated that BXXX could promote the inhibitory effect of
chemotherapy drugs on drug-resistant cells.
The expression levels of key pathway proteins were
determined. The expression levels of IL-6, INF-γ, JAK1, STAT3 and
MGMT were significantly upregulated in the AGS/DDP group compared
with the AGS group (Fig. 4F).
Compared with the AGS/DDP group, IL-6, INF-γ, JAK1, STAT3 and MGMT
expression levels were decreased in the AGS/DDP + DDP and AGS/DDP +
15% BXXX group, but this was more significant in the AGS/DDP + DDP
group. The expression levels of IL-6, INF-γ, JAK1, STAT3 and MGMT
were further decreased following DDP and 15% BXXX co-treatment
(Fig. 4F). These results
demonstrated that BXXX and DDP exerted a greater inhibitory effect
on the expression levels of pathway-related proteins when used
synergistically.
BXXX mediates drug-resistant GC cell
sensitivity by regulating the expression of MGMT via
IL-6/JAK/STAT3-mediated PD-L1
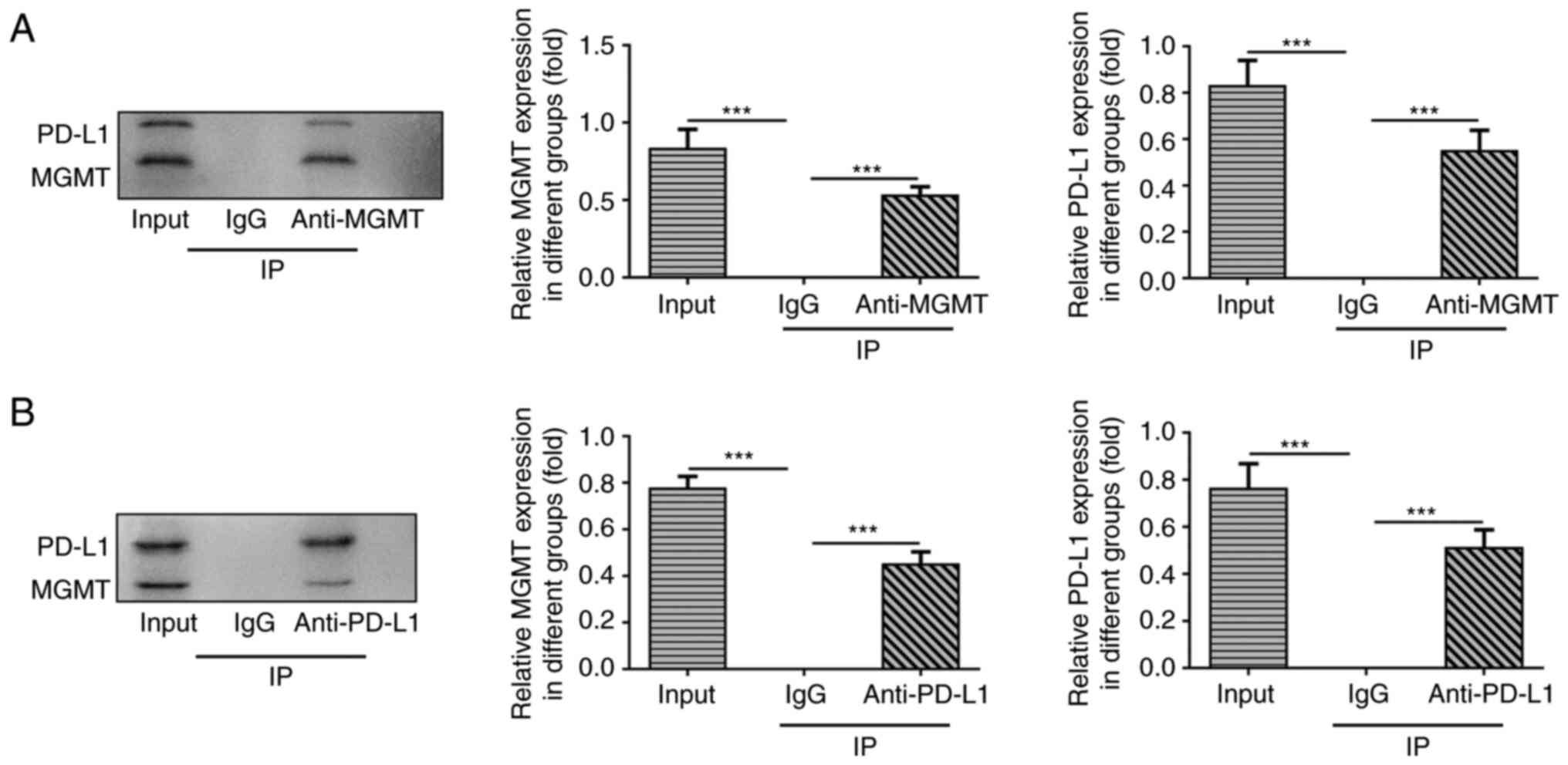
Using co-IP, PD-L1 was found to directly target and
regulate MGMT (Fig. 5A and
B).
The CCK-8 results demonstrated that cell
proliferation increased after the addition of Covlivelin, compared
with 15% BXXX alone, as evident by the decrease in the inhibition
rate. Compared with the 15% BXXX + DDP group, cell proliferation
was enhanced following Covlivelin treatment (Fig. 6A). Flow cytometry was used to
detect apoptosis, and the trend was consistent with that of the
CCK-8 results (Fig. 6B and C).
The cellular expression levels of IL-6, INF-γ, JAK1, STAT3, PD-L1
and MGMT were then determined. The expression levels of IL-6,
INF-γ, JAK1, STAT3, PD-L1 and MGMT in the 15% BXXX group showed an
opposing trend to the expression levels in the 15% BXXX +
Covlivelin group, and PDL-1 expression was significantly increased
following Covlivelin treatment (Fig.
6D). These findings indicated that following STAT3 activation,
the tumor inhibitory effect of BXXX was not considerably
pronounced, but that the effects of BXXX on DDP drug sensitivity
were enhanced. In other words, BXXX has a direct impact on the
pathways upstream of IL-6 and INF-γ, as well as on MGMT, but has a
less direct effect on PD-L1, which may be mediated by STAT3.
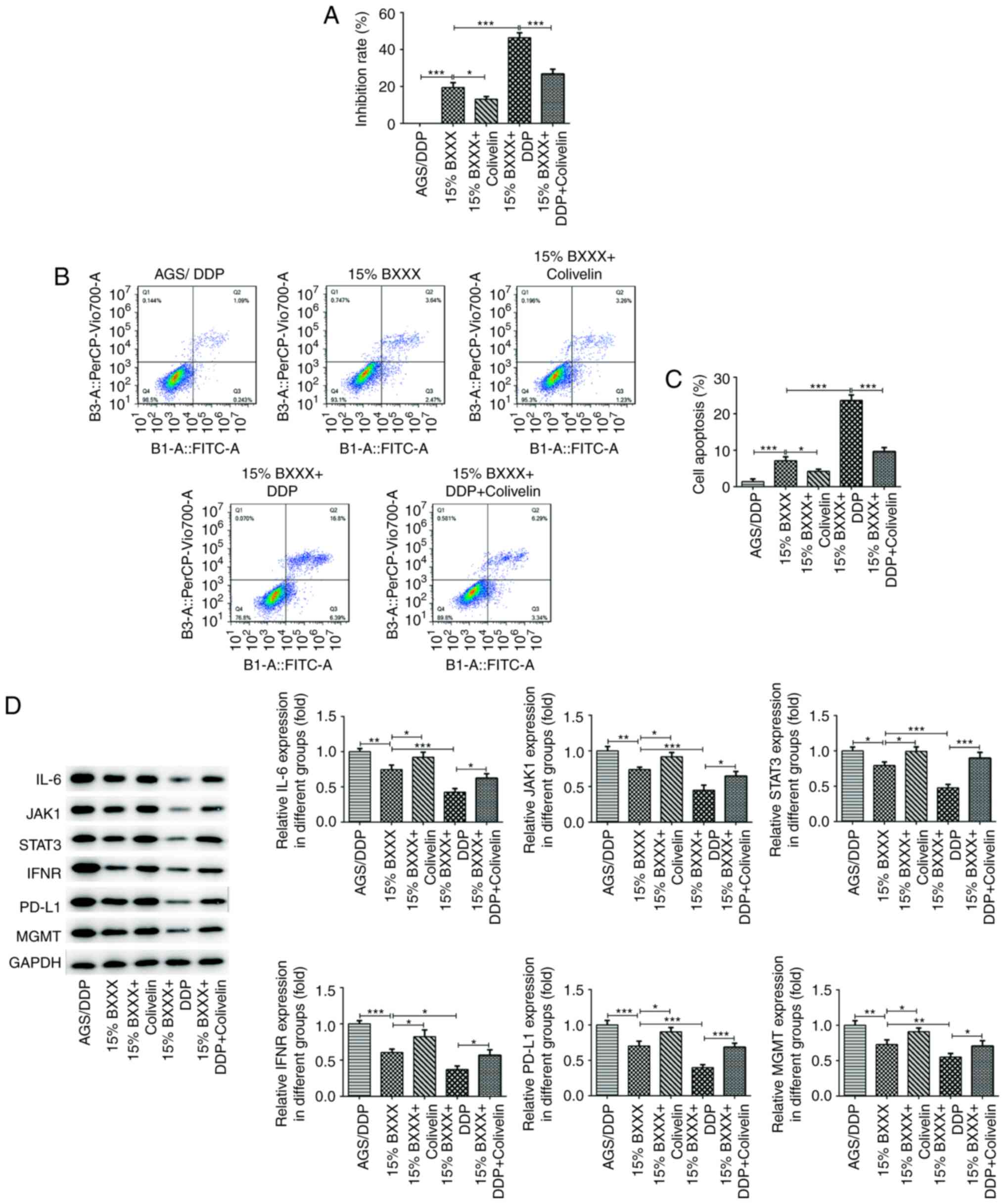 | Figure 6BXXX mediates drug-resistant GC cell
sensitivity by regulating the expression of MGMT via
IL-6/JAK/STAT3-mediated PD-L1. (A) A Cell Counting Kit-8 assay
detected the viability in AGS/DDP cells. (B) Flow cytometry was
used to detect cell apoptosis. (C) Statistical analysis of
apoptotic cells. (D) Western blotting detected the expression
levels of the signaling pathway-related proteins IL-6, JAK1, STAT3,
IFNR and MGMT.*P<0.05, **P<0.01,
***P<0.001. PD-L1, programmed cell death 1 ligand 1;
MGMT, 6-O-methylguanine-DNA methyltransferase; DDP, cisplatin;
BXXX, Banxia xiexin decoction; IFNR, IFN receptor. |
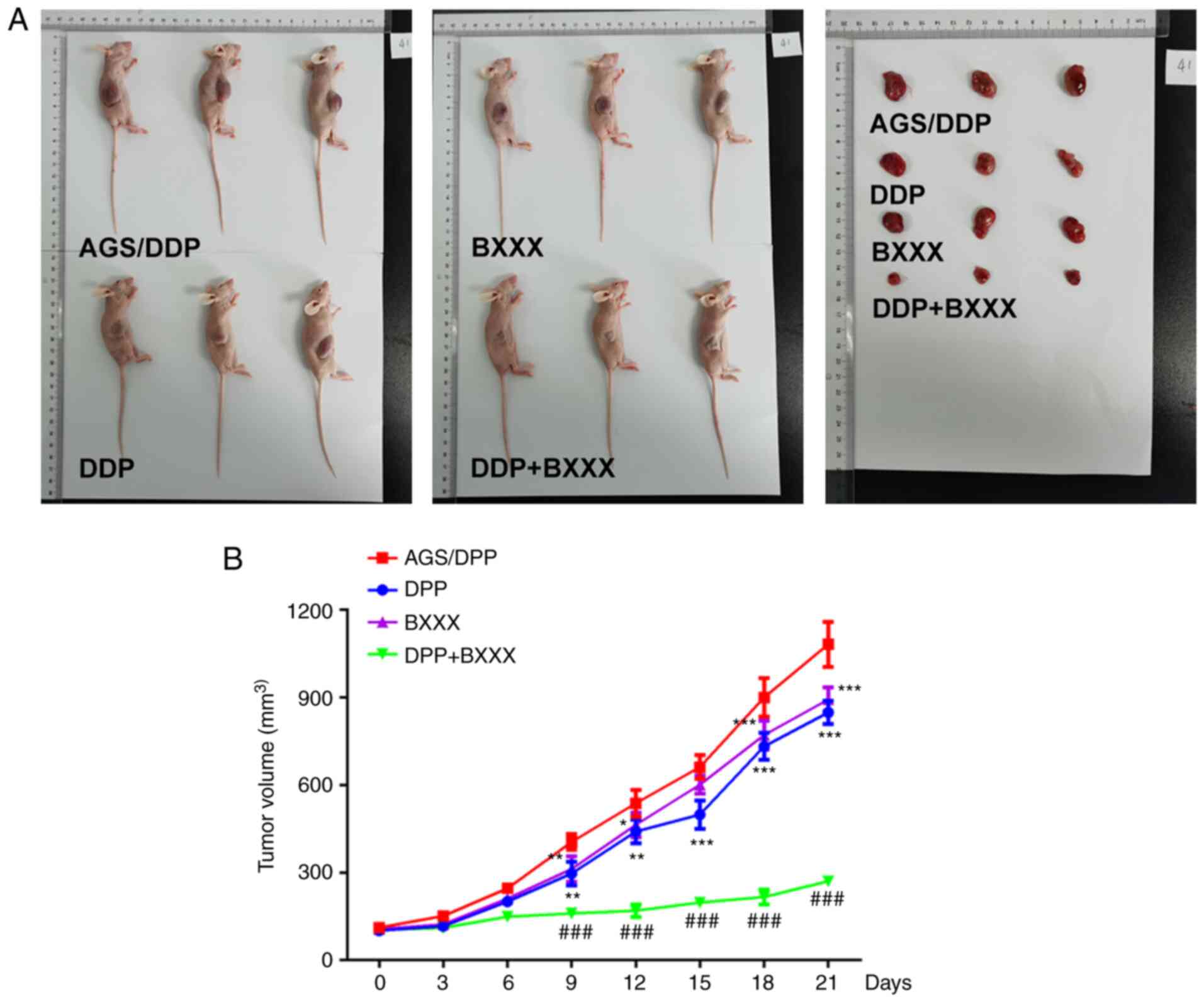
Tumor volume was decreased in the DDP and BXXX
groups, compared with the AGS/DDP group. Moreover, compared with
the DDP and BXXX groups, the tumor volume of the DDP + BXXX group
was significantly decreased (Fig. 7A
and B). The expression levels of related pathway proteins were
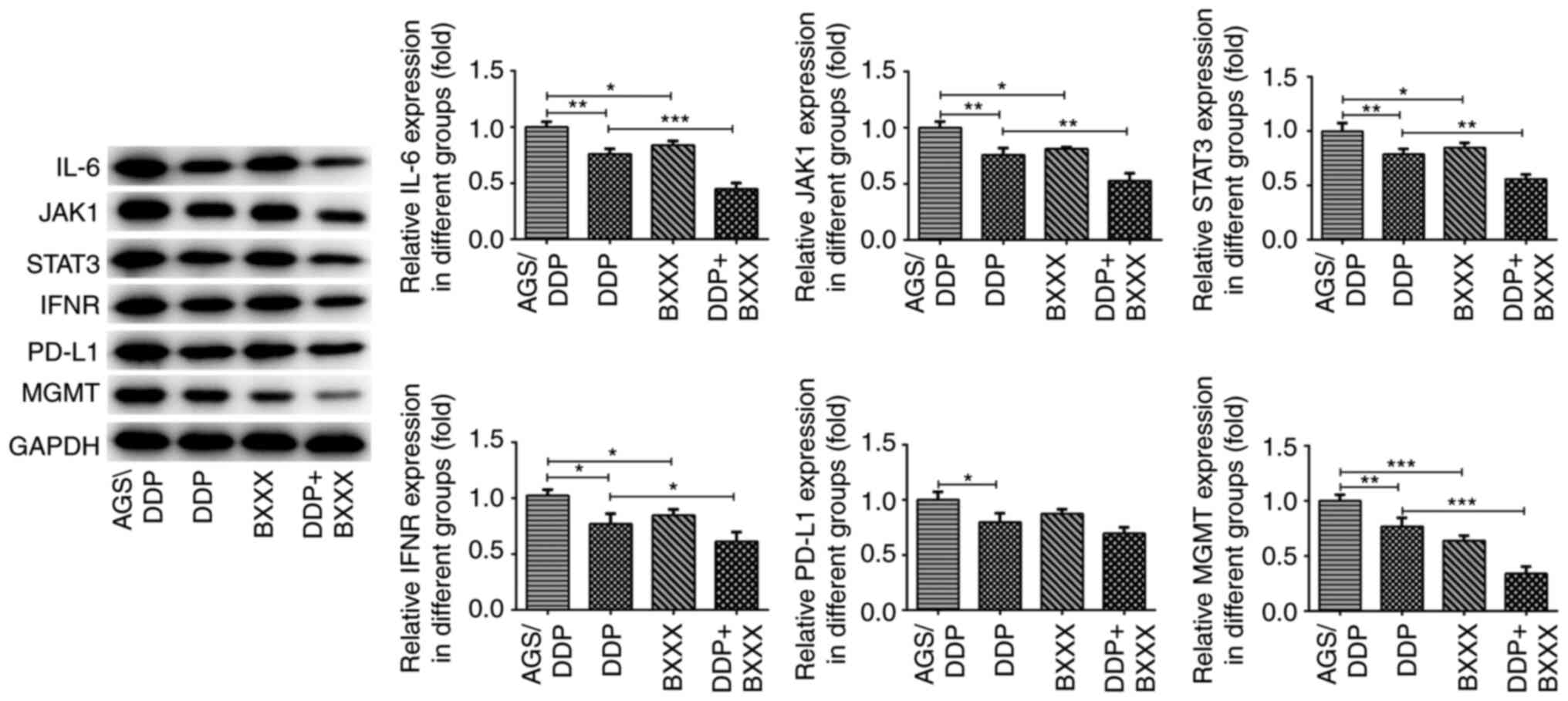
also detected in tumor tissues. As shown in Fig. 8, compared with the AGS/DDP group,
IL-6, INF-γ, JAK1, STAT3 and MGMT expression levels were decreased
in the DDP and BXXX group. The expression levels of IL-6, INF-γ,
JAK1, STAT3 and MGMT were further decreased following DDP and BXXX
co-treatment. In addition, the expression of PD-L1 did not change
significantly in AGS/DDP and BXXX groups. Similarly, PD-L1 showed
no significant change in DDP and DDP+BXXX. The expression levels of
these proteins were consistent with those observed in
vitro.
Discussion
TCM has unique antitumor features and can be used as
an adjuvant treatment to compliment surgery, radiotherapy or
chemotherapy (26). However, TCM
can also serve a direct antitumor role. BXXX is a classic TCM
preparation used for the treatment of gastrointestinal diseases,
but which also exerts therapeutic effects against tumors (7). In the present study, BXXX was found
to promote the apoptosis of drug-resistant GC cells. When used in
combination with DDP, the apoptotic rate was further increased
compared with that of the DDP group alone, indicating that BXXX not
only decreased the sensitivity of GC cells to DDP, but also
inhibited GC cell proliferation.
The development of drug-resistant tumor cells is an
important factor for chemotherapeutic efficacy, thus drug
resistance in tumors is a current research focus. A previous study
has demonstrated that the molecular mechanisms of tumor cell drug
resistance involve multiple processes, such as alterations in drug
metabolism, enhancing DNA damage repair and inhibiting apoptosis,
but the specific mechanisms are still unknown (27). The expression of drug-resistance
genes is an important factor affecting treatment susceptibility.
MGMT is a DNA repair protein commonly expressed in human cells
(encoded by the MGMT gene), and is currently recognized as a DNA
repair enzyme associated with tumor cell resistance to alkalizing
agents (28). Li et al
(29) reported that MGMT was a
prognostic and chemotherapy-sensitivity marker in GC. Moreover, the
inhibition of MGMT-mediated autophagy decreases the
chemotherapeutic sensitivity of GC cells to DDP (30). In the present study, MGMT
expression was found to be significantly increased in GC tissues
and cells, as well as in drug-resistant GC cell lines, which was
consistent with the results of previous publications. In addition,
drug composition analysis and target prediction revealed that BXXX
has a certain regulatory effect on MGMT, but the specific
mechanisms have not yet been reported (data not yet published).
Therefore, in the present study, the role of BXXX in GC cell drug
resistance was investigated, and the underlying mechanisms
discussed.
The current study demonstrated that BXXX blocks the
JAK/STAT3 signaling pathway by downregulating the expression of
IFNγ and IL-6, thereby reducing the expression of PD-L1 in
drug-resistant GC cells. Another study showed that inhibition of
ataxia telangiectasia mutated protein reversed
epithelial-mesenchymal transition and the condensing potential of
DDP-resistant lung cancer cells via the JAK/STAT3/PD-L1 pathways
(31). Additionally, AP-2 was
found to regulate the IL-6/JAK/STAT3 pathway and affect the
expression of PD-L1, thus regulating drug resistance in glioma
cells (22). Currently, the
majority of PD-L1 studies focus on mechanisms of resistance to
tumor immune escape by targeting the expression of PD-1 itself.
PD-L1 can increase the expression of multidrug-resistant proteins,
and is involved in the DNA damage and repair mechanisms of tumor
cells (32-34). These findings indicate that PD-L1
expression is associated with that of MGMT. In the present study,
an interaction between PD-L1 and MGMT was identified, and BXXX was
found to mediate the expression of MGMT by regulating that of PD-L1
in drug-resistant GC cells. However, the current experiment did not
further verify the specific region of interaction between PD-L1 and
MGMT, which may be a potential limitation of the present study. In
future experiments, the binding region between PD-L1 and MGMT were
be further detected via relevant experiments. In the present study,
colivelin (a STAT3 activator) reversed the inhibitory effects of
BXXX on drug-resistant GC cells, as well as its repressive effect
on PD-L1, indicating that BXXX had a direct effect on the pathways
upstream of IFNγ and IL-6, as well as on MGMT protein expression,
but not on PD-L1 itself. Therefore, BXXX may influence the
expression of PD-L1 by regulating the STAT3 pathway.
In the present study, AGS cells with high expression
levels of PD-L1, STAT3 and MGMT, and NCL-N87 cells with no notable
expression, were selected for drug resistance induction. The
results suggested that the expression levels of PD-L1, MGMT and
STAT3 were significantly increased, which was consistent with the
two drug-resistant cell lines. This phenomenon reflects the effects
of key pathway proteins (PD-L1, MGMT and STAT3) on the drug
resistance and sensitivity of GC cells.
In conclusion, the results of the present study
indicated that BXXX influenced the drug sensitivity of GC cells by
regulating the expression of MGMT. This process was executed via
the non-PD-1 targeting of PD-L1, which is mediate by IL-6/JAK/STAT3
signaling.
Availability of data and materials
The datasets used and/or analyzed generated during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XF wrote the manuscript and analyzed the data. QN
and SH carried out the experiments. FX and GH made substantial
contributions to analysis and interpretation of data, supervised
the present study, searched the literature and revised the
manuscript. All authors read and approved the final manuscript. QN
and SH confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study protocol was approved by Affiliated
Hospital of Yangzhou University, and all patients provided written
informed consent. All of the procedures were in compliance with The
Declaration of Helsinki and relevant policies in China. All animal
experiments comply with the ethical requirements of the animal
council.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi AH, Kim J and Chao J: Perioperative
chemotherapy for resectable gastric cancer: MAGIC and beyond. World
J Gastroenterol. 21:7343–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawakami R, Mashima T, Kawata N, Kumagai
K, Migita T, Sano T, Mizunuma N, Yamaguchi K and Seimiya H:
ALDH1A3-mTOR axis as a therapeutic target for anticancer
drug-tolerant persister cells in gastric cancer. Cancer Sci.
111:962–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Li M, Wang X, Dang Z, Yu L, Wang X,
Jiang Y and Yang Z: Effects of adjuvant traditional Chinese
medicine therapy on long-term survival in patients with
hepatocellular carcinoma. Phytomedicine. 62:1529302019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Qi F, Cui Y, Zhao L, Sun X, Tang W
and Cai P: An update on Chinese herbal medicines as adjuvant
treatment of anticancer therapeutics. Biosci Trends. 12:220–239.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan S, Yue Y, Wang J, Li W, Sun M, Zeng L
and Wang X: Banxia Xiexin decoction, a traditional Chinese
medicine, alleviates colon cancer in nude mice. Ann Transl Med.
7:3752019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K, Xu G, Liu C, Zhu B, Liu R, Hua B,
Zhang W and Feng X: Effect of a modified Banxia Xiexin decoction
plus chemotherapy on stage colon cancer. J Tradit Chin Med.
39:251–257. 2019.
|
|
9
|
Kim HR, Lee GS, Kim MS, Ryu DG, So HS,
Moon HC, Lee YR, Yang SH and Kwon KB: Effects of Banxia Xiexin
Decoction () on cisplatin-induced apoptosis of human A549 lung
cancer cells. Chin J Integr Med. 24:436–441. 2018. View Article : Google Scholar
|
|
10
|
Su L, Wang MM, Xu MR, Wang X, Xia HZ,
Zhang M, Zheng L, Zhu YD, Wang MQ and Li P: Banxia Xiexin Decoction
() Combined with Afatinib in treatment of advanced gallbladder
cancer: Case report and literature review. Chin J Integr Med.
25:303–306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XP, Ll PQ, Ming HX, Zhang W, Wang Q,
Chen YW and Yang BL: Effects of Banxia Xiexin Decoction containing
serum on proliferation, invasion and metastasis of gastric cancer
peritoneal metastasis cell line GC9811-P. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 36:1224–1228. 2016.In Chinese. PubMed/NCBI
|
|
12
|
Yu Y, Zhang G, Han T and Huang H: Analysis
of the pharmacological mechanism of Banxia Xiexin decoction in
treating depression and ulcerative colitis based on a biological
network module. BMC Complement Med Ther. 20:1992020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang M, Chen J, Xu L, Shi X, Zhou X, An R
and Wang X: A network pharmacology approach to uncover the
molecular mechanisms of herbal formula Ban-Xia-Xie-Xin-Tang. Evid
Based Complement Alternat Med. 2018:40507142018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Deng L, Shen H, Meng Q, Qian A, Sang
H, Xia J and Li X: O-6-methylguanine-DNA methyltransferase inhibits
gastric carcinoma cell migration and invasion by downregulation of
matrix metalloproteinase 2. Anticancer Agents Med Chem.
16:1125–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nowicki TS, Hu-Lieskovan S and Ribas A:
Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J.
24:47–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Diao L, Yang Y, Yi X, Rodriguez
BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L, et al:
CD38-mediated immunosuppression as a mechanism of tumor cell escape
from PD-1/PD-L1 blockade. Cancer Discov. 8:1156–1175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pitt JM, Vétizou M, Daillère R, Roberti
MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M,
Kroemer G and Zitvogel L: Resistance mechanisms to
immune-checkpoint blockade in cancer: Tumor-intrinsic and
-extrinsic factors. Immunity. 44:1255–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Xiang C, Wang Y, Duan Y, Liu C
and Zhang Y: PD-L1 promoter methylation mediates the resistance
response to anti-PD-1 therapy in NSCLC patients with EGFR-TKI
resistance. Oncotarget. 8:101535–101544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuo Y, Zheng W, Liu J, Tang Q, Wang SS and
Yang XS: MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance
of ovarian cancer cells. Neoplasma. 67:93–101. 2020. View Article : Google Scholar
|
|
20
|
Gong K, Gong ZJ, Lu PX, Ni XL, Shen S, Liu
H, Wang JW, Zhang DX, Liu HB and Suo T: PLAC8 overexpression
correlates with PD-L1 upregulation and acquired resistance to
chemotherapies in gallbladder carcinoma. Biochem Biophys Res
Commun. 516:983–990. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning
W, Zeng H, Zhang N, Du W, Chen C and Huang JA: PD-L1 induced by
IFN-gamma from tumor-associated macrophages via the JAK/STAT3 and
PI3K/AKT signaling pathways promoted progression of lung cancer.
Int J Clin Oncol. 22:1026–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang W, Zhong Z, Luo C, Xiao Y, Li L,
Zhang X, Yang L, Xiao K, Ning Y, Chen L, et al: The
miR-26a/AP-2alpha/Nanog signaling axis mediates stem cell
self-renewal and temozolomide resistance in glioma. Theranostics.
9:5497–5516. 2019. View Article : Google Scholar :
|
|
23
|
Chen G, Yang Y, Liu M, Teng Z, Ye J, Xu Y,
Cai X, Cheng X, Yang J, Hu C, et al: Banxia xiexin decoction
protects against dextran sulfate sodium-induced chronic ulcerative
colitis in mice. J Ethnopharmacol. 166:149–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Percie du Sert N, Ahluwalia A, Alam S,
Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U,
Emerson M, et al: Reporting animal research: Explanation and
elaboration for the ARRIVE guidelines 2.0. PLoS Biol.
18:e30004112020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amatu A, Barault L, Moutinho C, Cassingena
A, Bencardino K, Ghezzi S, Palmeri L, Bonazzina E, Tosi F, Ricotta
R, et al: Tumor MGMT promoter hypermethylation changes over time
limit temozolomide efficacy in a phase II trial for metastatic
colorectal cancer. Ann Oncol. 27:1062–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Yang Y, Lu Y, Herman JG, Brock MV,
Zhao P and Guo M: Predictive value of CHFR and MLH1 methylation in
human gastric cancer. Gastric Cancer. 18:280–287. 2015. View Article : Google Scholar
|
|
30
|
Lei Y, Tang L, Hu J, Wang S, Liu Y, Yang
M, Zhang J and Tang B: Inhibition of MGMT-mediated autophagy
suppression decreases cisplatin chemosensitivity in gastric cancer.
Biomed Pharmacother. 125:1098962020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen M, Xu Z, Xu W, Jiang K, Zhang F, Ding
Q, Xu Z and Chen Y: Inhibition of ATM reverses EMT and decreases
metastatic potential of cisplatin-resistant lung cancer cells
through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 38:1492019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms controlling PD-L1 expression in cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JM and Chen DS: Immune escape to
PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann
Oncol. 27:1492–504. 2016. View Article : Google Scholar : PubMed/NCBI
|