Introduction
The cell microenvironment, including mechanical
forces, has emerged as a key determinant of cell behavior and
function. The physiological function of cells in vivo is
closely related to the various types of mechanical forces present.
The periodontal ligament (PDL) is the supporting tissue that
connects the teeth to the alveolar bone in the oral cavity. As a
result, the PDL is frequently subjected to mechanical stimulation
owing to mastication, biting, or orthodontic forces. Orthodontic
tooth movement (OTM) causes periodontal tissue remodeling, which
involves complex biochemical reactions and cellular signal
transduction pathways (1). Human
periodontal ligament cells (hPDLCs) are the main cells residing in
the PDL that participate in the restoration and remodeling of
periodontal tissue. Over the past two decades, various forces such
as fluid shear, centrifugal force, and tension have been applied to
hPDLCs to explore the effect of orthodontic forces on periodontal
tissues (2-4). However, the cells used in those
studies were often cultured in two dimensions, which is
inconsistent with the dynamic three-dimensional microenvironment
found in vivo (5). Thus,
it is crucial to find a suitable mechanical stress model to explore
the effects of cellular mechanics on the regulation of cell
fate.
Previous studies have found that mechanical stress
can regulate a variety of cellular behaviors, such as cell
proliferation, apoptosis, and differentiation (6,7).
However, there are insufficient data on the process of autophagy.
Autophagy is a process during which a cell degrades its damaged
organelles and proteins using lysosomes (8,9).
It is an important mechanism for maintaining intracellular
homeostasis and integrity. As a dominant catabolic mechanism,
autophagy is involved in the physiological and pathological
processes of cells (10).
Increasing evidence suggests that there is a close relationship
between autophagy and mechanical stress. King et al
(11) found that mammalian cells
can respond to mechanical stress by rapidly inducing the formation
of autophagosomes. This indicates that autophagy can be activated
when cells adapt to mechanical stress. Ma et al (12) suggested that autophagy may be a
key response by nucleus pulposus cells resisting mechanical
overload. Mechanical stress triggers autophagy and excessive
autophagy leads to cell death. This process involves complex
mechanical signal transduction pathways.
The extracellular matrix-integrin-cytoskeleton has
been reported to be an important mechanical signal transduction
pathway (13,14) Integrin-linked kinase (ILK), the
cytoplasmic domain of β1 integrin, performs a central role in cell
growth, survival, and differentiation (15-17). It can promote actin rearrangement
and participate in the maturation of focal adhesions (15). A lack of ILK results in less
adhesion of fibroblasts to the ECM, prevents defective cell
extension, and delays the formation of adhesion sites (18). In addition, ILK can also exhibit
kinase activity and can activate the kinase B (AKT)/mammalian
target of rapamycin (mTOR), a key regulatory pathway of autophagy,
with the assistance of phosphatidylinositol 3 kinase (PI3K)
(19,20). Sosa et al (21) proposed that hyperphosphatemia may
activate mTOR and reduce autophagy in myoblasts through ILK
activation. In recent studies it was confirmed that ILK is a key
molecule involved in the effect of mechanical stress on the
proliferation, apoptosis, and differentiation of hPDLCs (22,23). Therefore, ILK/PI3K may be
important connecting molecules that transmit mechanical signals to
downstream pathways, thereby mediating the effect of mechanical
forces on autophagy in hPDLCs.
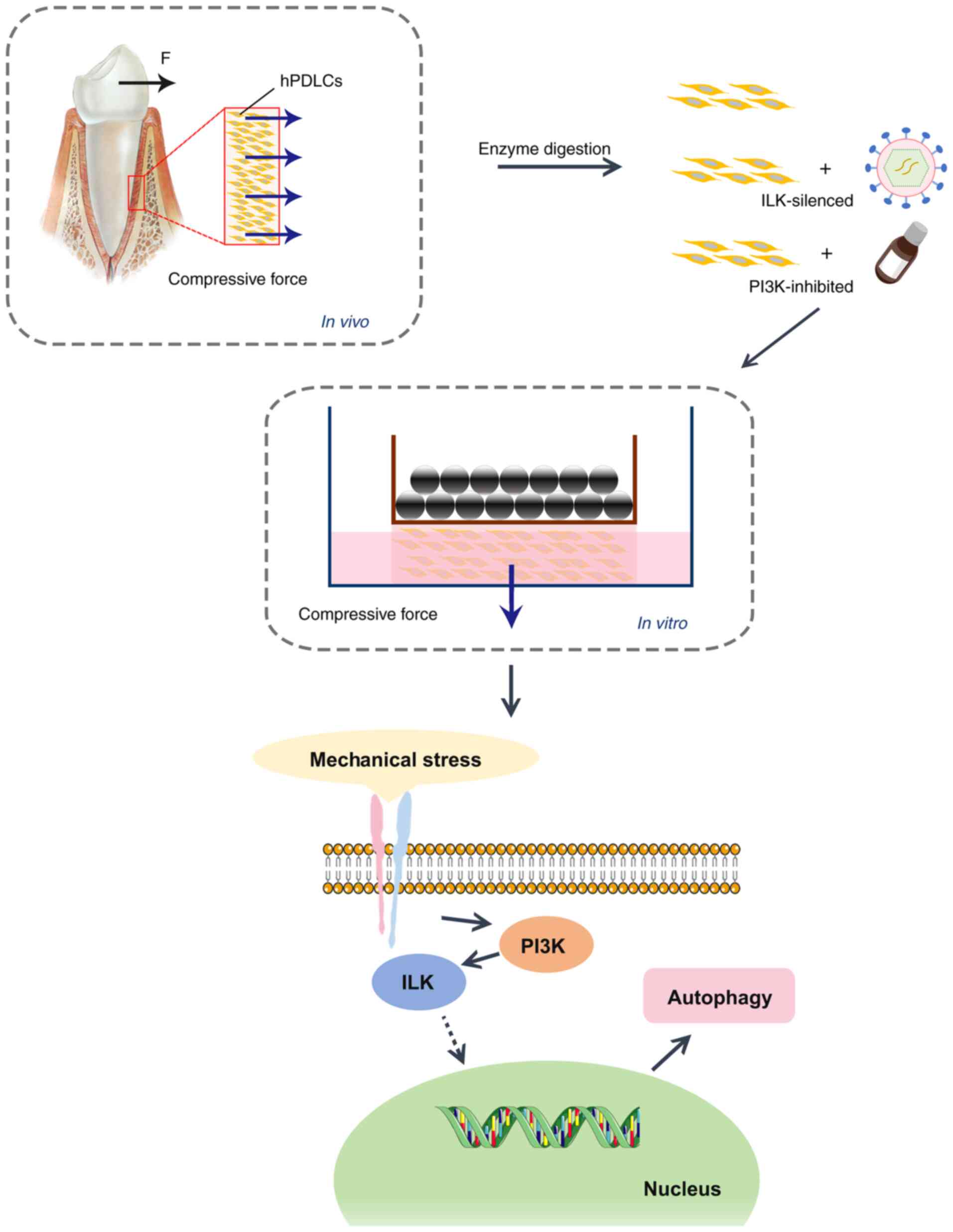
In the present study, a collagen-alginate composite
hydrogel was used to create a 3D cell culture in vitro to
simulate the periodontal microenvironment. The uniform weight
method (24) was used to
generate unidirectional static compressive stress that is similar
to orthodontic forces, to explore the effect of mechanical stress
on autophagy in hPDLCs. Models of ILK silencing and PI3K-specific
inhibitory hPDLCs were also established to verify the role of
ILK/PI3K in this process. A schematic diagram of the methods for
developing this study is presented in Fig. 1. The aim of the present study was
to provide insight into exploring the molecular mechanisms of
periodontal remodeling and OTM.
Materials and methods
Cell isolation and culture
The culture and usage of hPDLCs in the present study
were approved by the Ethics Committee of Xi'an Jiaotong University
(no. 2019-1282), and informed consent was obtained from all the
patients. hPDLCs were isolated from premolar teeth, which were
extracted for orthodontic reasons, from teenagers between the ages
of 12 and 15 years. After rinsing the teeth with phosphate-buffered
saline (PBS) supplemented with 100 U/ml penicillin and 100 U/ml
streptomycin, the periodontal tissue was gently collected from the
middle of the root. The tissue was then digested with type I
collagenase (Sigma-Aldrich; Merck KGaA) and dispase (Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C, for 40 min.
The cells were then re-suspended in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) culture medium
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). Cells at 3-5 passages were selected for this
experiment (25). The
verification methods are described in the immunofluorescence
staining section below. After blocking using 5% bull serum albumin
(Wuhan Boster Biological Technology, Ltd.), hPDLCs were subjected
to immunofluorescence with primary antibodies to vimentin and
cytokeratin (1:300, BM0135 and BM0030; Wuhan Boster Biological
Technology, Ltd.). Cells used in the present study were derived
from one individual. The results of characterization of the hPDLCs
are presented in Fig. S1.
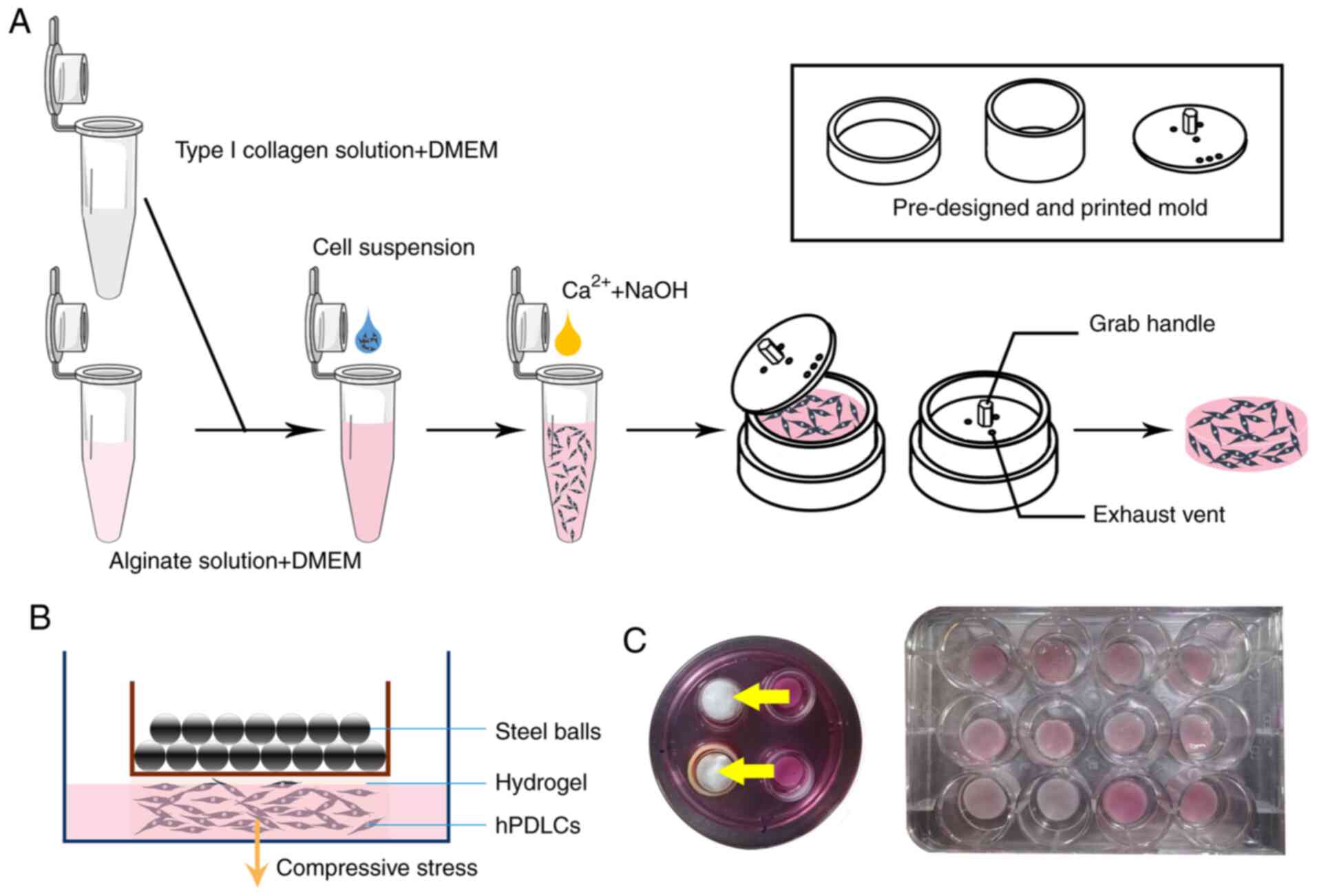
Construction of the 3D static stress
loading model for hPDLCs using collagen-alginate hydrogel
The dissolved type I collagen solution (extracted
from 8-week-old female Sprague-Dawley rats weighing 280-320 g
without any specific treatment), DMEM (Gibco; Thermo Fisher
Scientific, Inc.), sodium alginate solution (Sigma-Aldrich; Merck
KGaA), D-(+)-glucono-1,5-lactone (DGL, Thermo Fisher Scientific,
Inc.), CaSO4 (Tianjin Kemiou Chemical Reagent Co. Ltd.)
and NaOH (Tianjin Tianli Chemical Reagent Co. Ltd.) solution were
mixed in a predetermined order and proportion. Next, the hPDLC
suspension obtained after digestion with trypsin (Beijing Solarbio
Science & Technology Co., Ltd.) was added. The mixed solution
was added to the pre-designed mold and placed in an incubator
(37°C) for 1 h. The mold was then removed, the formed hydrogel was
placed into a 6 mm petri dish, and 3 ml of DMEM containing 10% FBS
was placed over the gel; it was then placed back to incubator
(37°C). The culture medium was changed every other day (Fig. 2A) and static stress was loaded
after 72 h.
Cell counting kit-8 (CCK-8) assay
The cells were mixed with liquid hydrogel or seeded
in DMEM, containing various concentrations (0, 25, 50 and 100
mmol/l) of inhibitors (LY294002, Echelon Biosciences), and
transferred to a 96-well culture plate. A set of five wells in each
group were used as controls. After the cells were cultured for 12 h
at 37°C in a 5% CO2 incubator, 10 µl of CCK-8
solution (Beijing Solarbio Science & Technology Co., Ltd.) was
added to each well in the dark every 12 h. A microplate reader
(Multiskan FC; Thermo Fisher Scientific, Inc.) was used to measure
the optical density (OD) value of each well at a wavelength of 450
nm, at 0, 12, 24, 48 and 72 h.
Transduction of ILK short hairpin (shRNA)
lentiviral vectors
Two shRNAs targeting the human ILK mRNA
(target sequence of ILK siRNA1, 5′-GTG GTT GAG ATG TTG ATC
ATG -3′) and one negative control shRNA (target sequence of
ILK siRNA2, 5′-TTC TCC GAA CGT GTC ACG T-3′) were designed
and synthesized by Shanghai GenePharma Co., Ltd. The hPDLCs were
seeded in 6-well plates at a density of 1.2×105
cells/well. Lentiviruses (multiplicity of infection, 50) were
diluted into the culture medium (containing 1 µl/ml of
Polybrene) at 50-60% hPDLC confluency. The medium-containing
lentiviruses were removed and replaced with a fresh medium
following 48 h of incubation (37°C). The transfected cells were
seeded in hydrogels and incubated for 48 h (37°C) and were finally
collected for experiments.
PI3K specific-inhibitory assay
For the present study, stock solutions of LY294002
(a specific inhibitor of PI3K, Echelon) dissolved in dimethyl
sulfoxide (DMSO, Sigma-Aldrich; Merck KGaA), were diluted and added
to the culture medium. Based on the results of the CCK-8 assay, the
final concentration of LY294002 was determined as 50 mmol/l. The
control group was treated with the same amount of DMSO. After 72 h,
the cells were harvested for western blotting to verify the
inhibitory effect of LY294002 on PI3K.
Uniform weight method to exert static
stress on hPDLCs
A 3D printer (Objet Eden260VS Dental Advantage,
Stratasys) was used to produce a series of pre-designed molds.
Weighed to 3, 5, 7 and 10 g, the mold was loaded with different
sizes and number of steel balls and was placed on the hydrogel with
hPDLCs embedded, pressed for 5, 15 and 30 min, and 1 h, and then
removed. A schematic diagram of static stress loading is shown in
Fig. 2B.
Immunofluorescence staining
After washing the compressed hydrogel with PBS,
three pieces of hydrogel with a thickness of 1 mm and a size of 2×2
mm were randomly cut using a surgical blade and fixed in 4%
paraformaldehyde, for 15 min. Fixed hydrogels were permeabilized in
0.1% Triton X-100 (Beijing Solarbio Science & Technology Co.,
Ltd) for 20 min before being washed three times with PBS. The
pieces were blocked in 5% bull serum albumin (Wuhan Boster
Biological Technology, Ltd.) for 30 min at room temperature and
incubated overnight with a primary antibody against LC3 (EPR18709,
1:200; Abcam) at 4°C. Then, the pieces were incubated with
CY3-labeled anti-rat secondary antibodies (BA1035, 1:50; Wuhan
Boster Biological Technology, Ltd.) at room temperature, for 2 h.
After washing with PBS, the nuclei were stained with
4-6-diamidino-2-phenylindole (DAPI) for 5 min and were evaluated
under a confocal microscope (Olympus Corporation). ImageJ software
(Version 1.48; National Institutes of Health) was used to analyze
the images. Background corrections and contrast/brightness
enhancement were performed identically for all images with the same
experiment.
Reverse transcription-quantitative
polymerase chain reaction
After washing the compressed hydrogel with PBS, type
IV collagenase (Sigma-Aldrich) and alginate lyase (Sigma-Aldrich;
Merck KGaA) were used to dissolve the hydrogel. Finally, a
resuspended cell sample was obtained. Total RNA was extracted from
hPDLCs using RNAzol reagents (Molecular Research Center) following
the manufacturer's protocol. cDNA was synthesized using Evo
M-MLV RT Premix reverse transcriptase (Takara Bio, Inc.)
(26). qPCR was performed using
the SYBR-Green premix Pro Taq HS qPCR kit (Takara Bio, Inc.).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control. The sequences of the primers are as follows:
ILK forward, 5′-CCT GGA TCA CTC CAC AGT CC-3′ and reverse,
5′-ATG ATC GTC CCC CTG GTT GA-3′; ATG-5 forward, 5′-AAA GAT
GTG CTT CGA GAT GTG T-3′ and reverse, 5′-CAC TTT GTC AGT TAC CAA
CGT CA-3′; Beclin-1 (BECN1) forward, 5′-GGT GTC TCT CGC AGA
TTC ATC -3′ and reverse, 5′-TCA GTC TTC GGC TGA GGT TCT -3′;
GAPDH forward, 5′-ACC CAC TCC TCC ACC TTT G-3′ and reverse,
5′-CAC CAC CCT GTT GCT GTA G-3′. RT-qPCR was repeated on at least
three independent RNA preparations. The mRNA expression value was
calculated using the 2−ΔΔCq method (ΔCq=the mean cycle
threshold Cq of the target gene - the mean Cq of GAPDH; ΔΔCq=ΔCq of
the experimental group − ΔCt of the control group) (27).
Western blot analysis
The abovementioned method was used to obtain cells
for western blot analysis. Total cellular proteins were extracted
by adding a RIPA lysis buffer (Beyotime Institute of Biotechnology)
containing 1% (v/v) phenylmethanesulfonylfluoride (Beyotime) on
ice. Protein concentrations were determined with a BCA protein
assay kit (Beyotime Institute of Biotechnology). Equal protein
amounts (20 µg) were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) on 12-15% gels and
were transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked in 5% non-fat dry milk for 1 h at room
temperature and incubated overnight with phospho-AKT (mAb no. 4060,
Ser473; Cell Signaling Technology, Inc.; 1:1,000), AKT (mAb no.
4691, Cell Signaling Technology, Inc.; 1:1,000) and GAPDH
antibodies (1:5,000, bs-12257R, BIOSS), at 4°C. The following day,
the membranes were washed with Tris-buffered saline with 0.05%
Tween-20 (Wuhan Boster Biological Technology, Ltd.) and then
incubated with rabbit IgG horseradish peroxidase (HRP)-linked
secondary antibodies (BA1054, 1:5,000; Wuhan Boster Biological
Technology, Ltd.), for 1 h at room temperature (28). Finally, the bands were visualized
using the Omega Lum G gel imaging system (Aplegen). ImageJ software
(Version 1.48; National Institutes of Health) was used for
densitometric analysis.
Statistical analysis
GraphPad prism software (Version 8.0; GraphPad,
Inc.) was used for statistical analysis. Values are expressed as
mean ± standard error of the mean (SEM). One-way analysis of
variance (ANOVA) followed by Tukey's multiple comparisons test were
used to analyse the statistical significance of differences.
P<0.05 was considered statistically significant.
Results
Establishment of a 3D culture model of
hPDLCs using collagen-alginate composite hydrogel
Cells reside in a 3D environment in vivo. To
simulate the microenvironment of the hPDLCs in the PDL, a
collagen-alginate composite hydrogel was used in the present study.
The preparation of the hydrogel is shown in Fig. 2A. After the cells were labelled
with fluorescent tags, they were embedded in the hydrogel and the
morphology was observed. At 1 h after embedding, the cells had not
spread, and appeared as round bright spots; after 24 h, the cells
spread out in a spindle, and after 48 h, a significant increase was
observed in the number of cells (Fig. 3A). In addition, CCK-8 was used to
detect the OD value to evaluate the proliferation of hPDLCs in the
collagen-alginate hydrogel. As shown in Fig. 3B, the OD value increased
gradually from 0-24 h and increased rapidly from 24-72 h (Fig. 3B, P<0.05), indicating
excellent proliferation activity of hPDLCs in the collagen-alginate
hydrogel.
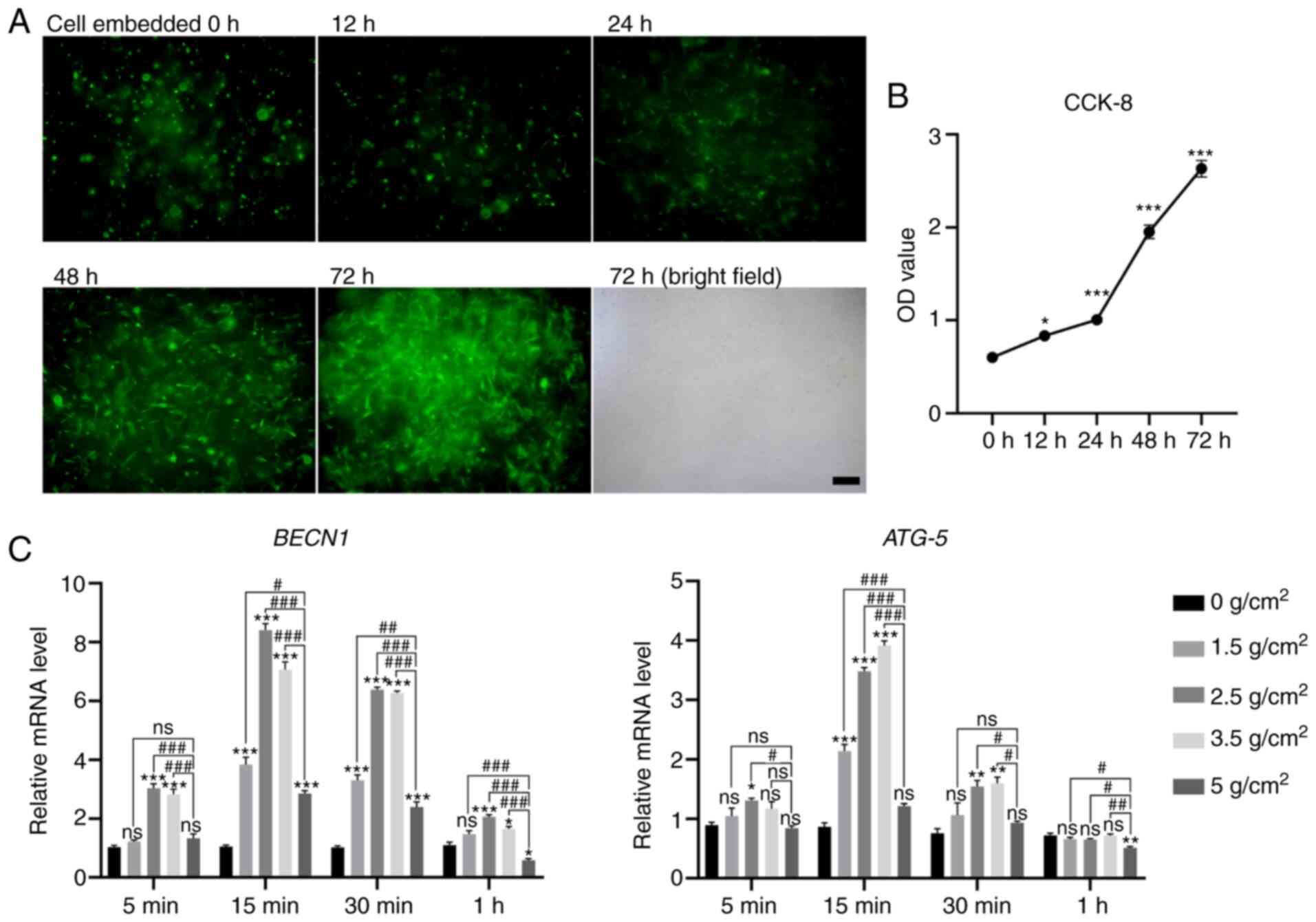 | Figure 3Growth and proliferation of
three-dimensional cultured hPDLCs and the autophagy level with
increasing intensity of the mechanical stimuli (0, 1.5, 2.5, 3.5
and 5 g/cm2) applied for different amounts of time (5,
15, 30 min and 1 h). (A) The spreading of hPDLCs (green
fluorescence: GFP; scale bar, 200 µm). The cells appeared as
rounded bright spots at 1 h, spindles at 12 h, and increased
significantly after 24 h. The morphology of hPDLCs embedded for 72
h in the bright field is also shown. (B) Optical density value. (C)
Gene expression of Beclin-1 (BECN1) and ATG-5.
Data are presented as mean ± standard error of the mean.
*P<0.05, **P<0.01 and
***P<0.001, statistically significant differences
from the 0 g/cm2 group; #P<0.05,
##P<0.01 and ###P<0.001, statistically
significant differences from the 5 g/cm2 group; ns, not
significant (P>0.05). hPDLCs, human periodontal ligament cells;
GFP, green fluorescent protein. |
Minimum static compressive stress force
value that can cause autophagy in hPDLCs
A schematic diagram of the uniform weight method and
the hydrogel before and after compression is shown in Fig. 2B and C. Following compression,
the hydrogel had flattened out. Based on a previous study, four
compressive forces, including 1.5, 2.5, 3.5 and 5 g/cm2
were loaded to determine the lightest force that can induce the
most active autophagic response. RT-qPCR was used to detect the
expression of the autophagy-related genes, BECN1 and ATG-5, to
reflect the level of autophagy. Compared with the 2.5 and 3.5
g/cm2 groups, the expression levels of BECN1 and
ATG-5 in the cells were decreased at 5 g/cm2
loading for 15 min-1 h, and the difference was significant
(Fig. 3C, P<0.05). By
contrast, the expression was elevated at 2.5 and 3.5
g/cm2. Therefore, a lighter force that can cause an
active autophagic response (2.5 g/cm2) was selected as
the workload in this study.
Effect of static compressive stress on
cell autophagy in hPDLCs
The mRNA expression of BECN1 and ATG-5
in hPDLCs under a force of 2.5 g/cm2 for different time
points, is shown in Fig. 4A.
With force loading, the mRNA levels of BECN1 and
ATG-5 gradually increased, then decreased after 15 min
(Fig. 4B and C, P<0.001) and
returned to the initial level after 1 h. The expression trend of
ILK exhibited a peak at 15 min (Fig. 4A, P<0.001), suggesting that
ILK was involved in the process of static compressive
stress-induced autophagy.
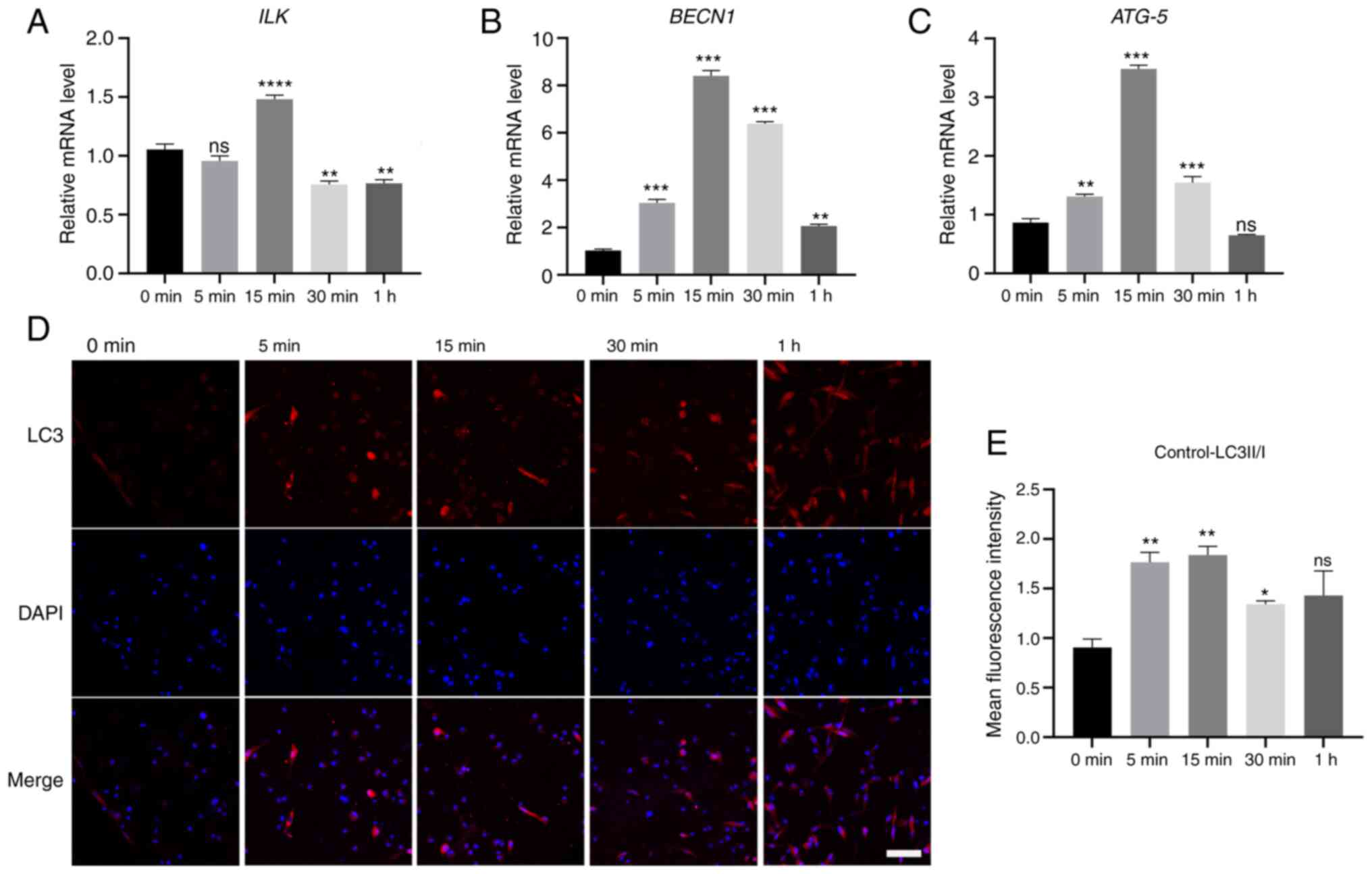 | Figure 4Effect of static compressive stress
(2.5 g/cm2) on gene expression of (A) ILK, (B)
Beclin-1 (BECN1) and (C) ATG-5. The expression of LC3
in hPDLCs under compressive stress was observed by (D)
immunofluorescence, red fluorescence: LC3; blue fluorescence: DAPI;
scale bar, 200 µm. (E) The ratio of LC3II/LC3I. Data are
presented as mean ± standard error of the mean.,
*P<0.05, **P<0.01 and
***P<0.001, statistically significant differences
compared with the 0 min group; ns, not significant (P>0.05).
hPDLCs, human periodontal ligament cells; ILK, integrin-linked
kinase; DAPI, 4-6-diamidino-2-phenylindole. |
Immunofluorescence was performed to further detect
the localization and expression of LC3 protein in hPDLCs. The red
fluorescence diffused in the cytoplasm represents LC3I. The red
speckled fluorescence representing LC3II could be observed after 5
min of force-loading, indicating the appearance of autophagosomes
(Fig. 4D). ImageJ software was
used to analyze the average fluorescence intensity of both LC3I and
LC3II, the ratio of which was considered a classic indicator of
autophagy levels. The results were consistent with those of RT-qPCR
(Fig. 4E). With the extension of
the loading time, the ratio of LC3II/I gradually increased, then
decreased at 30 min. Compared with the control group, the
difference was significant (P<0.05).
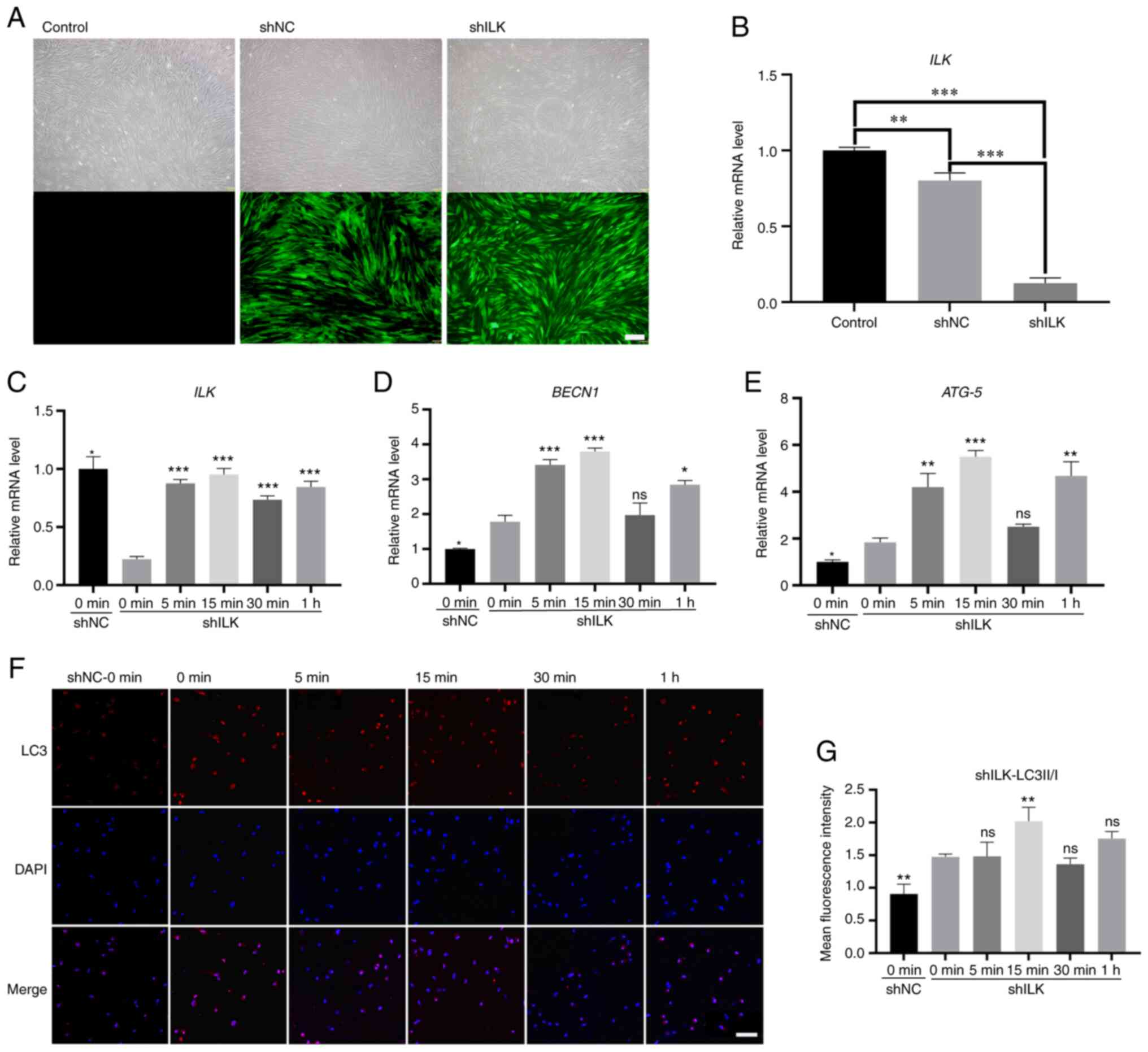
ILK gene silencing and the role of ILK in
the autophagy of hPDLCs induced by compressive stress
In this experiment, cells infected with green
fluorescent protein (GFP)-tagged ILK shRNA lentivirus and empty
vectors served as the experimental group (shILK) and negative
control (shNC), respectively. Fluorescence expression of hPDLCs was
evident in both the shILK and shNC groups, but not in the blank
control group (Fig. 5A),
indicating the high transfection rate of lentivirus vectors.
RT-qPCR was used to verify the silencing of ILK. As shown in
Fig. 5B, compared with the
negative and blank controls, a significant decrease in ILK
expression was observed in the experimental group (Fig. 5B, P<0.001).
Furthermore, ILK-silenced hPDLCs were
three-dimensionally cultured and then subjected to static
compressive stress. The results of RT-qPCR are shown in Fig. 5C-E. The expression trends of
BECN1 and ATG-5 were consistent with those of the
control group; they gradually increased to a peak at 15 min and
then decreased (Fig. 5D and E;
P<0.05). However, the levels increased again at 1 h (Fig. 5D and E; P<0.05). The
expression of ILK rapidly recovered after force loading and
the level then remained at a level similar to the level with shNC
(Fig. 5C; P<0.001). As shown
in Fig. 5F and G, autophagosomes
were still present in ILK-silenced cells, although the average
fluorescence intensity of LC3 was low, which increased with
force-loading and decreased after 15 min (Fig. 5F and G, P<0.05). Additionally,
a comparison of groups shNC and shILK at 0 min demonstrated that
the expression of BECN1 and ATG-5 increased after the
silencing of ILK, along with the ratio of LC3II/LC3I
(P<0.05), reflecting an increase of the baseline level of
autophagy (Fig. 5D, E and
G).
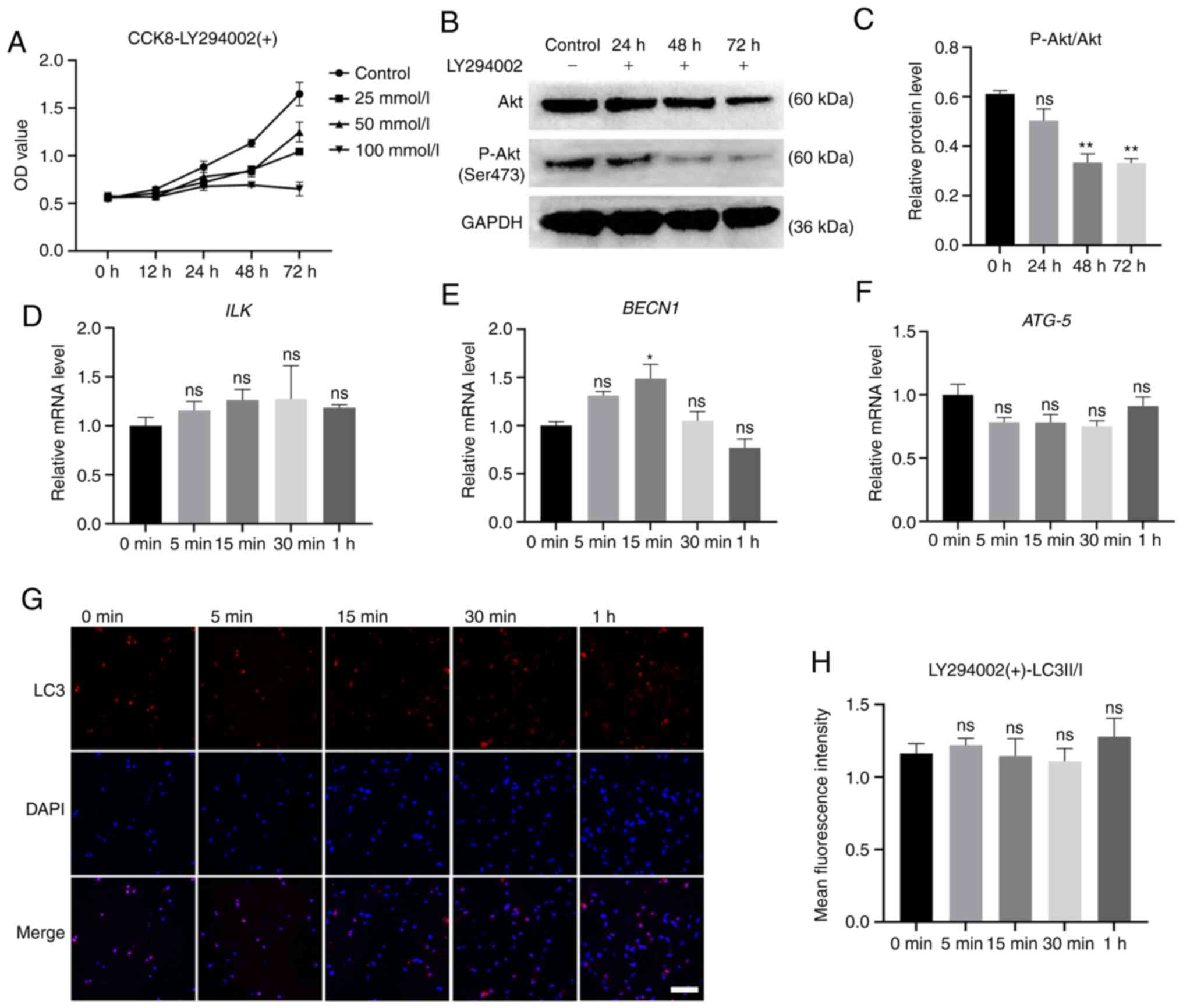
Specific inhibition of PI3K by LY294002
and the role of PI3K in the autophagy of hPDLCs induced by
compressive stress
To investigate the role of PI3K in the induction of
mechanical stress on autophagy, a specific inhibitor of PI3K,
LY294002, was used in the present study. Following previous
research, we set the drug concentration gradient of LY294002 and
used the CCK-8 assay to detect the effects of drugs on cell
proliferation and viability. For the present study, if the
concentration was ≤50 mmol/l, a negative effect of the drug on cell
proliferation and viability was acceptable (Fig. 6A, P<0.01). Western blot
analysis was used to detect the phosphorylation of PI3K downstream
target proteins at the above-mentioned concentration. The results
suggested that LY294002 significantly reduced the phosphorylation
of AKT (Ser473) (Fig. 6B,
P<0.01), which indicated that the kinase activity of PI3K was
inhibited. The raw image files are shown in Figs. S2 and S3.
In the PI3K-inhibited cells, force loading did not
upregulate ATG-5 expression (Fig. 6F, P>0.05), while BECN1
expression increased slightly at 15 min (Fig. 6E, P<0.05). Of note, it was
found that the expression of ILK no longer increased with
force loading, but remained unaltered (Fig. 6D, P>0.05). Similarly, the
results of immunofluorescence revealed that the ratio of LC3II/I
did not differ significantly between the groups (Fig. 6G and H, P>0.05).
Discussion
As part of the cell ecological niche, mechanical
stress must be adapted to rapidly maintain both intracellular and
extracellular homeostasis. Its effects on cells, tissues, and
organs are very common in the body; for example, muscle
development, bone remodeling, and the formation of skin calluses
are all affected by external mechanical loads (24,29). In the oral cavity, periodontal
tissue is situated in a complex and active mechanical
microenvironment. In particular, periodontal tissue regeneration
during OTM has attracted a lot of attention.
At present, most models simulating orthodontic force
reflect two-dimensional (2D) mechanical loading of cells, which
cannot fully simulate the growth environment of cells in
vivo (5). Therefore, the 3D
culture model has attracted attention because it can accurately
simulate the growth state of cells in the ECM (30). ILK, one of the targets of this
study, is an important part of the focal adhesion complex. Previous
findings indicated that in a 3D matrix, the adhesion of cells to
the environment is special, such as the expression of adhesion
molecules and changes in cell biological activity (31). This may lead to different
responses of cells to the same mechanical stress, under 2D and 3D
culture environments. Type I collagen is widely used in the
research of hydrogel scaffolds, as a natural component of the ECM
(32,33). However, with the collagen
hydrogel alone, it is difficult to load stable compressive stress
due to its contractility (34).
Thus, the collagen-alginate hydrogel model has been proposed for
its excellent mechanical properties and permeability (35). In the current study, a
collagen-alginate composite hydrogel loading model was constructed
to simulate the 3D microenvironment of hPDLCs. The changes in cell
morphology and the results of CCK-8 showed that the hPDLCs grew
well in this hydrogel. This demonstrates that we have successfully
constructed a 3D hydrogel model for hPDLCs.
Autophagy is a cellular function that maintains
homeostasis and can be induced by mechanical stress (11,36). The response of autophagy to
mechanical stimuli has a range of force values. However, sustained
stress can lead to excessive autophagy (12,36,37). In this study, we designed an
augmented device that simulates the compressive force exerted on
hPDLCs, via hydrogels with a certain weight. According to previous
studies, a range of pressure gradients were designed including 1.5,
2.5, 3.5, and 5 g/cm2. We found that the level of
autophagy increased significantly under a compressive force of 2.5
g/cm2, but only increased slightly or even decreased
under 5 g/cm2. This may be due to the force exceeding
the limit of autophagy regulation, resulting in irreversible
mechanical damage to cells. Thus, 2.5 g/cm2 was selected
as the workload in this study. With stress loading, the results of
RT-qPCR revealed that the expression of BECN1 and
ATG-5 increased and peaked at 15 min. The fluorescence
expression of LC3 showed the same change. This study confirmed that
static compressive stress within a certain limit can indeed induce
autophagy albeit this induction is rapid and short-lived, which is
consistent with the results of King et al (11). This may be due to the smaller
compressive stress and shorter action time allowing cells to adapt
to this mechanical change rapidly, causing autophagy to return to
the baseline level within 1 h. In this process, indispensable
mechanical signal transduction was considered.
ILK is a key structure of the
ECM-integrin-cytoskeleton and plays an important role in cellular
adaptation (14,38). In the present study, the
expression of ILK was consistent with BECN1 and
ATG-5, as predicted. To further explore the association
between ILK, mechanical stress, and autophagy, ILK was silenced
using lentivirus. Notably, ILK expression returned to the
level before silencing after only 5 min of stress loading, despite
BECN1 and ATG-5 expression trends being almost the
same as those of the control group. However, it increased again at
1 h. Whether a secondary activation of autophagy occurred at 1 h is
unknown; thus, further studies are required to determine this using
extended loading times. The results of immunofluorescence results
revealed that the silencing of ILK could not block autophagy.
However, the enhancement of ILK expression may be an
interference factor. Therefore, we concluded that mechanical stress
could upregulate the expression of ILK and that ILK is not
the only key molecule in autophagy. After deformation in the ECM
due to cellular forces, integrin-induced actin filament
rearrangement involves a variety of signaling proteins, including
adhesion spot kinase, protein kinase C, PI3K, mitogen-activated
kinases, and small Rho family guanosine triphosphates among others
(39,40), all of which may participate in
the introduction of compressive stress signals. In addition, the
autophagy level of shILK group went up at 0 min compared to the
shNC group, indicating the double-sided function of ILK in
autophagy. One possibility may be that the ILK functioned as a
mechanical transduction molecule in the process of autophagy
induced by mechanics (41).
Conversely, it may be the upstream molecule of the AKT/mTOR pathway
negatively regulating the autophagy (42), which may explain the increase in
autophagy at 0 min in group shILK. Further investigation is
required to determine the detailed mechanism.
As a key molecule downstream of the
ECM-integrin-cytoskeleton signal transduction pathway, PI3K is
involved in the regulation of various biological behaviors such as
cell differentiation, proliferation, and autophagy (43). PI3K is closely related to the ILK
function. ILK/PI3K downstream of mTOR is the most characteristic
negative regulator of autophagy (19). However, the mechanism of
mechanically induced autophagy remains controversial. For instance,
King et al (11)
indicated that mechanical stress-induced autophagy is independent
of the classic TOR signals or AMPK pathway. Other research found
that the mTOR pathway could be activated rather than inhibited
under mechanical tension in trabecular meshwork cells (44). To verify the role of ILK/PI3K in
the regulation of autophagy induced by mechanical stress, LY294002
was used to inhibit the kinase activity of PI3K. Of note, after the
inhibition of PI3K, the expression of ATG-5 no longer
increased, but remained stable, although BECN1 expression
was still slightly increased at 15 min. This discrepancy may be due
to the difference in the effective period of BECN1 and ATG-5 in
autophagy. The PI3KIII complex, ATG-14, and ATG-6/BECN1 are all
involved in the nucleation and assembly of the initial
autophagosome, while ATG-5 plays a role in the subsequent extension
of the autophagosome membrane (45). Therefore, we hypothesized that
the inhibition of PI3K may have a greater impact on ATG-5 than on
BECN1. Wang et al (46)
found that fluid shear stress can induce autophagy in liver cancer
cells through the PI3K-FAK-Rho GTPases pathway. Additionally, the
use of PI3K inhibitor methyladenine (3-MA) significantly reduced
the level of autophagy (46).
Similarly, immunofluorescence results indicated that the autophagy
level no longer increased with stress loading after
PI3K-inhibition. This indicates that PI3K plays a crucial role in
the process of mechanics-induced autophagy.
Previous findings have revealed the regulatory
effect of PI3K on ILK function. For example, Delcommenne et
al (20) found that the
transient activation of insulin by ILK is dependent on PI3K, which
may be achieved by phosphatidylinositol (3-5)
triphosphate (PIP3), a product of PI3K. In addition, ILK can
directly interact with the cytoplasmic domain of integrin and
activate it in a PI3K-dependent manner (47). In the present study, the
upregulating effect of mechanical stress on ILK disappeared
after PI3K was inhibited. Our results confirmed that mechanical
stress can upregulate the expression of ILK in a
PI3K-dependent manner. This may provide evidence of the
transduction mechanism of mechanical signals and the interaction
with the role of ILK and PI3K in OTM.
However, the extraction of total protein of hPDLCs
under 3D culture needs to be performed after the hydrogel is
dissolved, and following protein degradation, which may affect the
accuracy of the results. Therefore, the western blot assay of
LC-3I/II and other autophagy-associated proteins were absent in
this study. Ideal hydrogel that can be rapidly degraded and does
not affect cells is required. In conclusion, the current findings
confirmed that static compressive stress can induce autophagy
within a certain range of force that is related to the ILK/PI3K
signaling pathway. The inhibition of PI3K can invalidate the
upregulation of ILK induced by mechanical stress. Further studies
are required to clarify the downstream targeting pathway of
mechanically induced autophagy and the mechanism involving
mechanical stress, PI3K and ILK.
Supplementary Data
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RZ, YW and YG conceived and designed the
experiments. SW, XK and DZ drafted the manuscript and performed the
experiments. SW and YW participated in data analysis and edited the
manuscript. SZ, HS, BG and SM were involved in the discussion and
interpretation of the results. LN participated in data analysis and
provided technical guidance. RZ and SW confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Stomatology Hospital of Xi'an Jiaotong University
College of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
2D
|
two-dimensional
|
|
3D
|
three-dimensional
|
|
AKT
|
kinase B
|
|
CCK-8
|
cell counting kit-8
|
|
DAPI
|
4-6-diamidino-2-phenylindole
|
|
DGL
|
D-(+)-Glucono-1,5-lactone
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
ECM
|
extracellular matrix
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
hPDLC
|
human periodontal ligament cell
|
|
ILK
|
integrin linked-kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
OA
|
osteoarthritis
|
|
OD
|
optical density
|
|
OTM
|
orthodontic tooth movement
|
|
PBS
|
phosphate-buffered saline
|
|
PDL
|
periodontal ligament
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
PIP3
|
phosphatidylinositol (3,4,5)
triphosphate
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SEM
|
standard error of the mean
|
References
|
1
|
Berry S, Javed F, Rossouw PE, Barmak AB,
Kalogirou EM and Michelogiannakis D: Influence of thyroxine
supplementation on orthodontically induced tooth movement and/or
inflammatory root resorption: A systematic review. Orthod Craniofac
Res. 24:206–213. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin Y, Li J, Wang YT, Ye R, Feng XX, Jing
Z and Zhao Z: Functional role of mechanosensitive ion channel
Piezo1 in human periodontal ligament cells. Angle Orthodontist.
85:87–94. 2015. View Article : Google Scholar
|
|
3
|
Kikuta J, Yamaguchi M, Shimizu M, Yoshino
T and Kasai K: Notch signaling induces root resorption via RANKL
and IL-6 from hPDL cells. J Dental Res. 94:140–147. 2015.
View Article : Google Scholar
|
|
4
|
Chang ML, Lin H, Fu HD, Wang BK, Han GL
and Fan MW: MicroRNA-195-5p regulates osteogenic differentiation of
periodontal ligament cells under mechanical loading. J Cell
Physiology. 232:3762–3774. 2017. View Article : Google Scholar
|
|
5
|
Duval K, Grover H, Han LH, Mou Y, Pegoraro
AF, Fredberg J and Chen Z: Modeling physiological events in 2D vs.
3D cell culture. Physiology (Bethesda). 32:266–277. 2017.
|
|
6
|
Zhao Y, Wang C, Li S, Song H, Wei F, Pan
K, Zhu K, Yang P, Tu Q and Chen J: Expression of Osterix in
mechanical stress-induced osteogenic differentiation of periodontal
ligament cells in vitro. Eur J Oral Sci. 116:199–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JL, Zhang W, Backman LJ, Kelk P and
Danielson P: Mechanical stress potentiates the differentiation of
periodontal ligament stem cells into keratocytes. Brit J
Ophthalmol. 102:562–569. 2018. View Article : Google Scholar
|
|
8
|
Banerjee A and Bandopadhyay R: Autophagy:
Nobel prize in physiology or medicine' 16 to the intra-cellular
suicidal process. Natl Acad Sci Lett. 40:461–465. 2017. View Article : Google Scholar
|
|
9
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ott C, König J, Höhn A, Jung T and Grune
T: Macroautophagy is impaired in old murine brain tissue as well as
in senescent human fibroblasts. Redox Biol. 10:266–273. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
King JS, Veltman DM and Insall RH: The
induction of autophagy by mechanical stress. Autophagy.
7:1490–1499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma KG, Shao ZW, Yang SH, Wang J, Wang BC,
Xiong LM, Wu Q and Chen SF: Autophagy is activated in
compression-induced cell degeneration and is mediated by reactive
oxygen species in nucleus pulposus cells exposed to compression.
Osteoarthr Cartilage. 21:2030–2038. 2013. View Article : Google Scholar
|
|
13
|
Iskratsch T, Wolfenson H and Sheetz MP:
Appreciating force and shape - the rise of mechanotransduction in
cell biology. Nat Rev Mol Cell Bio. 15:825–833. 2014. View Article : Google Scholar
|
|
14
|
Kechagia JZ, Ivaska J and Roca-Cusachs P:
Integrins as biomechanical sensors of the microenvironment. Nat Rev
Mol Cell Bio. 20:457–473. 2019. View Article : Google Scholar
|
|
15
|
Zheng CC, Hu HF, Hong P, Zhang QH, Xu WW,
He QY and Li B: Significance of integrin-linked kinase (ILK) in
tumorigenesis and its potential implication as a biomarker and
therapeutic target for human cancer. Am J Cancer Res. 9:186–197.
2019.PubMed/NCBI
|
|
16
|
Zheng QM, Chen XY, Bao QF, Yu J and Chen
LH: ILK enhances migration and invasion abilities of human
endometrial stromal cells by facilitating the
epithelial-mesenchymal transition. Gynecol Endocrinol.
34:1091–1096. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu XY, Liu N, Liu W, Song SW and Guo KJ:
Silencing of the integrin-linked kinase gene suppresses the
proliferation, migration and invasion of pancreatic cancer cells
(Panc-1). Genet Mol Biol. 35:538–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakai T, Li S, Docheva D, Grashoff C,
Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD and Fässler R:
Integrin-linked kinase (ILK) is required for polarizing the
epiblast, cell adhesion, and controlling actin accumulation. Genes
Dev. 17:926–940. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delcommenne M, Tan C, Gray V, Rue L,
Woodgett J and Dedhar S: Phosphoinositide-3-OH kinase-dependent
regulation of glycogen synthase kinase 3 and protein kinase B/AKT
by the integrin-linked kinase. Proc Natl Acad Sci USA.
95:11211–11216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sosa P, Alcalde-Estevez E, Plaza P,
Troyano N, Alonso C, Martínez-Arias L, Evelem de Melo Aroeira A,
Rodriguez-Puyol D, Olmos G, López-Ongil S and Ruíz-Torres MP:
Hyperphosphatemia promotes senescence of myoblasts by impairing
autophagy through Ilk overexpression, a possible mechanism involved
in sarcopenia. Aging Dis. 9:769–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan WT, He C, Du CY, Wang YJ, Wu SY, Wang
TR and Zou R: Effect of ILK on small-molecule metabolism of human
periodontal ligament fibroblasts with mechanical stretching. J
Periodontal Res. 55:229–237. 2020. View Article : Google Scholar
|
|
23
|
Wang Y, Du C, Wan W, He C, Wu S, Wang T,
Wang F and Zou R: shRNA knockdown of integrin-linked kinase on
hPDLCs migration, proliferation, and apoptosis under cyclic tensile
stress. Oral Dis. 26:1747–1754. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li M, Zhang C and Yang Y: Effects of
mechanical forces on osteogenesis and osteoclastogenesis in human
periodontal ligament fibroblasts: A systematic review of in vitro
studies. Bone Joint Res. 8:19–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Zhou J, Cui B and Yu T: Evaluation
of hypoxia on the expression of miR-646/IGF-1 signaling in human
periodontal ligament cells (hPDLCs). Med Sci Monit. 24:5282–5291.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brazvan B, Farahzadi R, Mohammadi SM,
Saheb SM, Shanehbandi D, Schmied L, Soleimani Rad J, Darabi M and
Nozad Charoudeh H: Key immune cell cytokines affects the telomere
activity of cord blood cells in vitro. Adv Pharm Bull. 6:153–161.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Farahzadi R, Fathi E and Vietor I:
Mesenchymal stem cells could be considered as a candidate for
further studies in cell-based therapy of Alzheimer's disease via
targeting the signaling pathways. ACS Chem Neurosci. 11:1424–1435.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moraes C, Sun Y and Simmons CA:
(Micro)managing the mechanical microenvironment. Integr Biol
(Camb). 3:959–971. 2011. View Article : Google Scholar
|
|
30
|
Wozniak MA, Modzelewska K, Kwong L and
Keely PJ: Focal adhesion regulation of cell behavior. Biochim
Biophys Acta. 1692:103–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cukierman E, Pankov R, Stevens DR and
Yamada KM: Taking cell-matrix adhesions to the third dimension.
Science. 294:1708–1712. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oh SA, Lee HY, Lee JH, Kim TH, Jang JH,
Kim HW and Wall I: Collagen three-dimensional hydrogel matrix
carrying basic fibroblast growth factor for the cultivation of
mesenchymal stem cells and osteogenic differentiation. Tissue Eng
Part A. 18:1087–1100. 2012. View Article : Google Scholar
|
|
33
|
Rajan N, Habermehl J, Cote MF, Doillon CJ
and Mantovani D: Preparation of ready-to-use, storable and
reconstituted type I collagen from rat tail tendon for tissue
engineering applications. Nat Protoc. 1:2753–2758. 2006. View Article : Google Scholar
|
|
34
|
Yuan T, Zhang L, Feng L, Fan H and Zhang
X: Chondrogenic differentiation and immunological properties of
mesenchymal stem cells in collagen type I hydrogel. Biotechnol
Prog. 26:1749–1758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Branco da Cunha C, Klumpers DD, Li WA,
Koshy ST, Weaver JC, Chaudhuri O, Granja PL and Mooney DJ:
Influence of the stiffness of three-dimensional alginate/collagen-I
interpenetrating networks on fibroblast biology. Biomaterials.
35:8927–8936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S and
Yan T: Spironolactone promotes autophagy via inhibiting
PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity
damage in podocytes under mechanical stress. Biosci Rep.
36:e003552016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu HG, Yu YF, Zheng Q, Zhang W, Wang CD,
Zhao XY, Tong WX, Wang H, Liu P and Zhang XL: Autophagy protects
end plate chondrocytes from intermittent cyclic mechanical tension
induced calcification. Bone. 66:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghatak S, Morgner J and Wickstrom SA: ILK:
A pseudokinase with a unique function in the integrin-actin
linkage. Biochem Soc Trans. 41:995–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vautrin-Glabik A, Botia B, Kischel P,
Ouadid-Ahidouch H and Rodat-Despoix L: IP3 R3 silencing
induced actin cytoskeletal reorganization through
ARHGAP18/RhoA/mDia1/FAK pathway in breast cancer cell lines.
Biochim Biophys Acta Mol Cell Res. 1865:945–958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Memmel S, Sisario D, Zoller C, Fiedler V,
Katzer A, Heiden R, Becker N, Eing L, Ferreira FLR, Zimmermann H,
et al: Migration pattern, actin cytoskeleton organization and
response to PI3K-, mTOR-, and Hsp90-inhibition of glioblastoma
cells with different invasive capacities. Oncotarget.
8:45298–45310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boppart MD and Mahmassani ZS: Integrin
signaling: Linking mechanical stimulation to skeletal muscle
hypertrophy. Am J Physiol Cell Physiol. 317:C629–C641. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mousavizadeh R, Hojabrpour P, Eltit F,
McDonald PC, Dedhar S, McCormack RG, Duronio V, Jafarnejad SM and
Scott A: β1 integrin, ILK and mTOR regulate collagen synthesis in
mechanically loaded tendon cells. Sci Rep. 10:126442020. View Article : Google Scholar
|
|
43
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Porter KM, Jeyabalan N and Liton PB:
MTOR-independent induction of autophagy in trabecular meshwork
cells subjected to biaxial stretch. Biochim Biophys Acta.
1843:1054–1062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nishimura T and Tooze SA: Emerging roles
of ATG proteins and membrane lipids in autophagosome formation.
Cell Discov. 6:322020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Zhang Y, Feng T, Su G, He J, Gao
W, Shen Y and Liu X: Fluid shear stress promotes autophagy in
hepatocellular carcinoma cells. Int J Biol Sci. 14:1277–1290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dedhar S: Cell-substrate interactions and
signaling through ILK. Curr Opin Cell Biol. 12:250–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|