Introduction
Cardiovascular disease (CVD) is the leading cause of
mortality in China (1). As has
been reported, >17 million individuals succumbed to CVD in 2015,
representing 31% of all global deaths (2). Atherosclerosis, the formation of
fibrofatty lesions in the artery wall, causes CVD (2). A deeper understanding of the
pathogenesis of atherosclerosis is required in order to develop
effective and timely strategies to combat the challenges of CVD
(3). However, the pathogenesis
of atherosclerosis is complex and is not yet fully understood. The
initiation and progression of atherosclerosis involve the
pathophysiological activation of multiple cell types, such as
vascular endothelial cells, monocytes and vascular smooth muscle
cells (VSMCs). During the evolution of atherosclerotic plaques,
VSMCs produce matrix metalloproteinases (MMPs) that degrade
interstitial collagen, leading to the migration of medial VSMCs
into the intima and to the formation of foam cells (2). VSMCs play an important regulatory
role in atherosclerosis and plaque development (4).
Emerging evidence has suggested that atherosclerosis
is an epigenetic disease involving the interplay of multiple
epigenetic mechanisms (5). The
long non-coding RNA (lncRNA) macrophage-associated atherosclerosis
sequence was previously identified and characterized as an
essential regulator of macrophage apoptosis, efferocytosis and
plaque necrosis in the atherosclerotic plaques of
Ldlr−/− mice via direct interaction with the
RNA-binding protein, HuR (6). As
previously demonstrated, the lncRNA-fatty acid 2-hydroxylase
(FA2H-2)/mixed lineage kinase domain-like protein (MLKL) pathway is
essential for regulating the autophagic flux and inflammation
through mTOR-dependent signaling, and lncRNA-FA2H-2 knockdown
promotes atherosclerosis (7).
LincRNA-p21 was previously identified as a novel regulator of
neointima formation, VSMC proliferation, apoptosis and
atherosclerosis by enhancing p53 activity (8). Smooth muscle cell-enriched lncRNA
(SMILR) has been shown to promote VSMC proliferation via the direct
regulation of mitotic progression, which may be a potential
SMILR-targeting intervention to limit atherogenesis (9). Although a considerable number of
molecules have been shown to participate in the regulation of VSMCs
in atherosclerosis, numerous others have yet to be discovered.
The present study reported a lncRNA AL355711, a
lncRNA that has not been previously reported. It was found that
lncRNA AL355711 promoted VSMC migration and atherogenesis. In
brief, the present study found that: i) lncRNA AL355711 was
overexpressed in human plaques; ii) lncRNA AL355711 knockdown
inhibited smooth muscle cell migration in a scratch assay; iii)
lncRNA AL355711 upregulated ATP-binding cassette sub-family G
member 1 (ABCG1) transcription; iv) lncRNA AL355711 regulated MMP3
expression through the ABCG1 pathway; and v) ABCG1 and MMP3 were
highly expressed in an animal model of atherosclerosis.
Materials and methods
Patients and specimens
A total of five normal and 17 atherosclerotic frozen
plaque tissues were randomly collected with informed consent from
patients who initially underwent carotid endarterectomy or
abdominal aorta surgery at Nanfang Hospital, Southern Medical
University (Guangzhou, China) (10,11). Ethical consent was obtained from
the Committee for Ethical Review of Research Involving Human
Subjects of Southern Medical University (approval no.
NFEC-2017-122). Informed consent written was obtained from all the
participants.
Cells and culture parameters
Human VSMCs were purchased from the American Type
Culture Collection (ATCC CRL-1999) and maintained in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (penicillin, 100 U/ml;
streptomycin, 100 mg/ml. Gibco; Thermo Fisher Scientific, Inc) at
37°C in a humidified incubator containing 5% CO2. The
VSMC cell line is referenced as T/G HA-VSMC in Cellosaurus.
(https://web.expasy.org/cellosaurus/CVCL_4009).
Microarray analysis
The microarray analysis was carried out using the
Agilent Array platform (Affymetrix U133A chips, Agilent
Technologies, Inc). The assay was performed as previously described
(12). The expression microarray
data can be found at NCBI GEO (Accession no. GSE97210; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE97210).
Lentiviral construction and cell
transfection
Short hairpin RNA (shRNA) was prepared as previously
described (11). The shRNAs were
constructed using a lentivirus vector
(pHBLV-U6-MCS-CMV-ZsGreen-PGK-PURO). Briefly, 293T cells (ATCC
CRL-3216) were transfected with a combination of lncRNA AL355711
shRNA, 10 µg lentiviral packaging plasmids pSPAX2 and 5
µg helper plasmids pMD2G using Lipofiter™ (Han Bio Co. Ltd.)
at 37°C, 5% CO2 for 72 h. The virus was subsequently
harvested by ultracentrifugation at 4°C, 2,000 × g for 10 min and
at 4°C, 82,700 × g for 120 min. The human VSMCs were cultured in
6-well plates for 12 h to 50-70% confluence prior to use. The cells
were transfected with lentiviral vectors using polybrene reagent
(Santa Cruz Biotechnology, Inc.) in Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocols at a
multiplicity of infection of 10. Stable knockdown cell clones were
generated after 2 weeks using puromycin, and the lncRNA AL355711
level was evaluated using reverse transcriptionquantitative
polymerase chain reaction (RT-qPCR). The sequences of sh-AL355711
are presented in Table SI.
Transfection with short interfering RNAs
(siRNAs)
The cells were transfected with siRNAs specific for
ABCG1 protein and scrambled siRNA (Guangzhou RiboBio Co., Ltd.)
using Lipofectamine 3000® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
The siRNA sequences are presented in Table I. VSMCs (5×104
cells/well) were seeded into the wells of 6-well plates
(2×106 cells/well) and cultured for 12 h to 50-70%
confluence prior to use. The cells were incubated in Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 2 µl
Lipofectamine 3000® reagent and 5 µl siRNA (50
nM) or siN-Ctrl for 6 h. The supernatant was discarded, and 2 ml
complete medium were added to each well. The transfected cells were
grown for 48 h prior to analysis.
 | Table IExpression of lncRNA AL355711 and
ABCG1 in advanced atherosclerotic plaques compared with normal
arterial intimae, as detected using ArrayStar. |
Table I
Expression of lncRNA AL355711 and
ABCG1 in advanced atherosclerotic plaques compared with normal
arterial intimae, as detected using ArrayStar.
| Gene symbol | P-value | Absolute fold
change | Regulation | Chrom | Strand | txStart | txEnd | Association |
|---|
| AL355711 |
0.000000506894775660708 |
4.5505074401028 | Upregulation | chr21 | + | 43719103 | 43720919 | Exon |
| ABCG1 |
0.00182682036107604 |
3.02051677736344 | Upregulation | chr21 | + | 43619798 | 43717354 |
sense-overlapping |
RNA isolation and RT-qPCR
RNA isolation and RT-qPCR were performed as
previously described (13).
Total RNA was extracted from the cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First-strand complementary DNA (cDNA) of lncRNA AL355711 was
synthesized using M-MLV Reverse Transcriptase (Promega
Corporation), while cDNA was synthesized from total mRNAs using the
PrimeScript RT reagent kit (Takara Bio, Inc.), in 20 µl
reaction volumes containing 2 µg total RNA. qPCR was carried
out using a SYBR-Green PCR kit (Takara Bio, Inc.) on a Roche Z480
Real-Time PCR system (Roche Molecular Diagnostics). U6 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as
internal controls for lncRNAs and mRNAs, respectively. PCR was
performed according to the product instruction with standard
thermocycling conditions (50°C 2 min, 95°C 2 min, and then 35
cycles of 95°C 15 sec, 60°C 32 sec, and melting curves of 95°C 1
min, 60°C 30 sec, and then 40°C 30 sec). The expression levels were
quantified using the 2−ΔΔCq method (14). All samples were prepared in
triplicate, and the mean value was used for comparative analyses.
The sequences of the primers used are presented in Table SI.
Cell migration and scratch assay
The cell migration assay was performed using Boyden
chambers consisting of Transwell member filter inserts (8
µm; Corning, Inc.). In brief, 2×105 cells/well
were seeded in a 24-well plate. The cells that did not penetrate
the filter were wiped away after 12 h using a cotton swab. The
cells on the lower surface of the filter were fixed in 4%
paraformaldehyde (Wuhan Servicebio Technology Co., Ltd.) for 20
min, and washed with PBS (Wuhan Servicebio Technology Co., Ltd.)
twice. The cells were then stained using 0.5% crystal violet
(Nanjing KeyGen Biotech Co., Ltd.) for 20 min at room
temperature.
The scratch assay was performed using a 6-well
plate. In brief, 5×105 cells/well were seeded into a
6-well plate and scratches were created using a pipet tip after 24
h. The cells were cultured in a complete medium with 2% FBS, and
the scratches were imaged at the 0, 8 and 24 h time points using a
BX51 microscope (Olympus Corporation).
Western blot analysis
The cells were harvested and lysed in
radioimmunoprecipitation assay buffer (Fdbio Science) containing
protease and phosphatase inhibitors and phenylmethanesulfonyl
fluoride (Fdbio Science). The protein concentration was determined
using an enhanced bicinchoninic acid protein assay kit (Fdbio
Science), and proteins were separated on a 12% sodium dodecyl
sulfate-polyacrylamide gel and transferred onto a polyvinylidene
difluoride membrane (EMD Millipore). The membranes were then
blocked with 5% bovine serum albumin for 1 h at room temperature
and incubated overnight at 4°C with primary antibodies ABCG1
(1:1,000; cat. no. NB400-132SS; Novus Biologicals, LLC) and MMP3
(1:1,000; cat. no. sc-21732, Santa Cruz Biotechnology, Inc.).
Following incubation with appropriate horseradish
peroxidase-conjugated secondary antibodies (1:3,000; cat. no. 7076;
Cell Signaling Technology, Inc., 1:3,000; cat. no. 7074; Cell
Signaling Technology, Inc.), immunoreactivity was detected using
enhanced chemiluminescence with western blotting substrate (Fdbio
Science). Protein expression levels were quantified using Quantity
One software (V4.6.2; Bio-Rad Laboratories, Inc.) and normalized to
the level of β-actin (overnight at 4°C, 1:1,000; cat. no. 3700;
Cell Signaling Technology, Inc.).
Bioinformatics analysis
The Gene Expression Omnibus (GEO) datasets in the
NCBI portal (ncbi.nlm.nih.gov) were searched using
the keywords 'Atherosclerosis AND tissue AND Homo sapiens'.
One dataset, GSE12288 [110 samples for coronary artery disease
(CAD) and 112 samples for the controls] was found. Gene expression
in circulating leukocytes was measured to identify patients with
CAD or the controls. In this analysis, R3.63, R stats package 3.5.0
and R pcaPP package 1.9.73 (https://cran.r-project.org/bin/windows/base/old/3.5.0/)
were used to measure the correlation, and Pearson's correlation
analysis was used to calculate the correlation coefficient. R
ggplot2 was used to draw the first heatmap. R ggplot2, R ggExtra
and R psych (http://cran.r-project.org) were used to draw the
correlation scatter plot, and R ggplot2 R beeswarm (http://cran.r-project.org)was used to draw the
expression scatter plot.
Animals
An animal model of atherosclerosis was constructed
as previously described (11).
In brief, apolipoprotein E (ApoE) knockout (ApoE−/−)
mice with a C57BL/6 background were purchased from Guangdong
GemPharmatech Co., Ltd. (n=9, male, 6 weeks of age, weighing 20 g;
6/JGpt-Apoeem1Cd82/Gpt). The mice were kept in a specific
pathogen-free environment with free access to food and water with a
temperature of 20-26°C and a humidity of 40-70%, and a 12-h
dark/light cycle. Atherosclerosis was induced using a Western
high-fat diet (HFD; Open Source Diet D12108C; Research Diets, Inc.;
40% fat calories and 1.25% cholesterol) for 12 weeks. The mice were
sacrificed humanely after 12 weeks of being fed the HFD, with no
suffering or potential pain and/or distress, and ascending aortas,
mouse aortic root tissues were collected for analyses. The death of
the mice was verified as follows: A lack of respiration, heartbeat
and corneal reflex. In total, 4 mice were used as the controls and
5 for the HFD feeding; the experiments were performed once. All
animal experiments conformed to the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (NIH Publication no. 85-23, revised in 1996) and were
approved by the Nanfang Hospital Animal Experimental Committee
(approval no. 20200830005).
Histological analysis, Oil Red O
staining, Masson's staining and immunohistochemical analyses of
mouse aortic roots
Histological analysis, Oil Red O staining and
immunohistochemical analyses of the mouse aortic roots were
performed as previously described (10,11). The mouse aortic roots were fixed
in 4% paraformaldehyde (Wuhan Servicebio Technology Co., Ltd.) 20
min at room temperature, and stained for 2 h using Oil Red O
(O0625; Sigma-Aldrich; Merck KGaA), and Masson's stain or
hematoxylin and eosin (H&E) staining according to the
manufacturer's instructions (G1346 and G1120; Beijing Solarbio
Science & Technology Co., Ltd.). Formalin-fixed,
paraffin-embedded sections (4-µm-thick) of mouse aortic
roots were incubated with ABCG1 (overnight at 4°C, 1:1,00;
NB400-132SS, Novus Biologicals, LLC) and MMP3 (overnight at 4°C,
1:1,00; sc-21732, Santa Cruz Biotechnology, Inc.) antibodies, and
the secondary antibodies using the GTVision™ Anti-mouse/Anti-rabbit
Immunohistochemical Analysis kit (including DAB; HRP conjugates, no
dilution, cat. no. GK500710; Gene Tech, Inc.) according to the
manufacturer's instructions. Images were acquired using a Leica DM
2500 microscope (Leica Microsystems GmbH) and analyzed using ImageJ
software (version 150; National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were analyzed using IBM SPSS 20.0 (IBM Corporation) and
GraphPad 8.0 (GraphPad Software, Inc.). Statistically significant
differences were calculated using the unpaired two-tailed Student's
t-test or one-way ANOVA followed by Tukey's post hoc test. A
two-tailed probability value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of lncRNA AL355711 and ABCG1
is higher in human atherosclerotic plaques
Arraystar LncRNA Expression Microarray V3.0 was used
on three paired human atherosclerotic tissues and healthy arterial
specimens to discover the abnormal expression of lncRNAs. lncRNA
AL355711 was found to be one of the most significantly upregulated
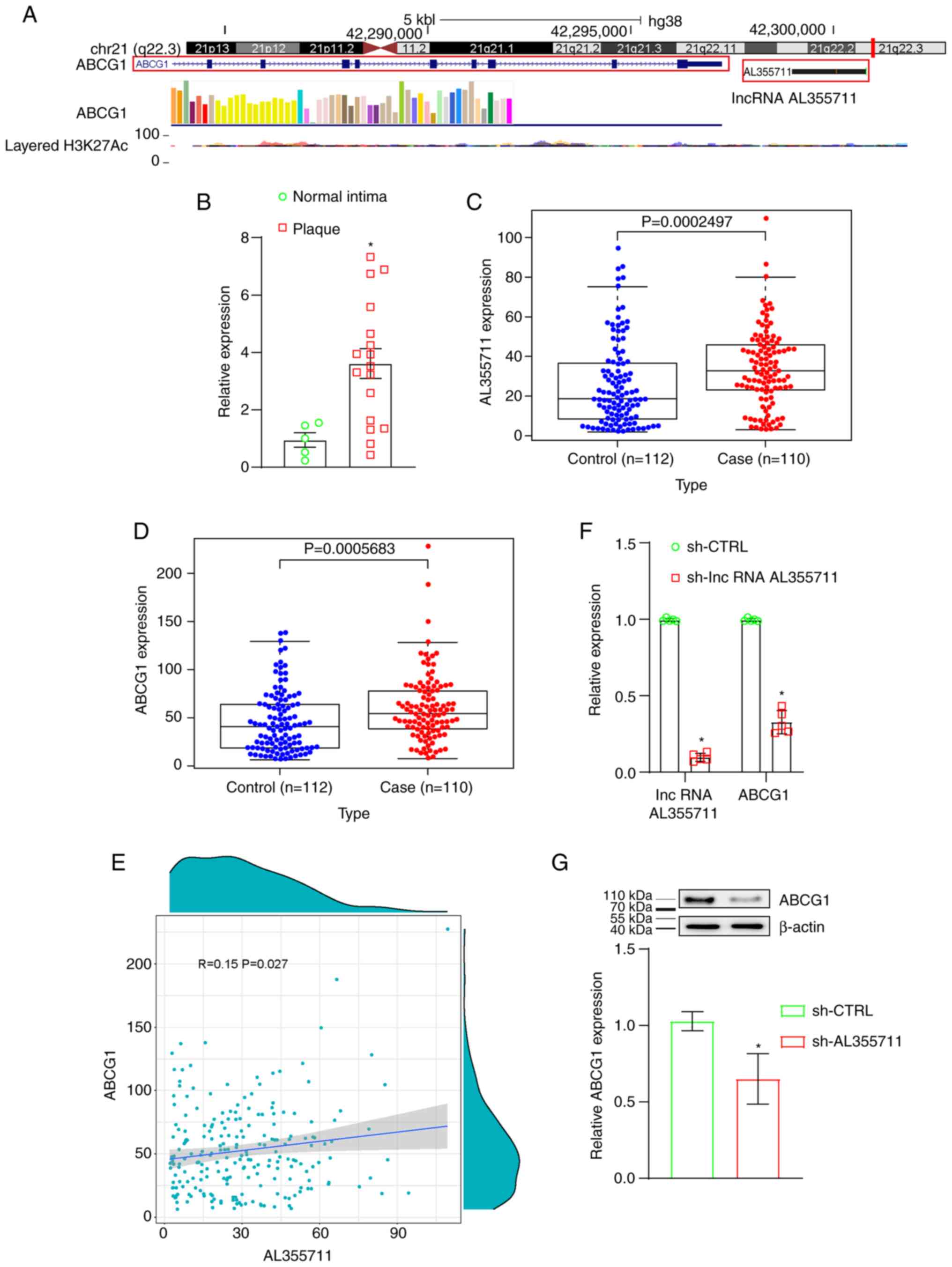
lncRNAs (4.55-fold increase, P=0.000000506894775660708, Table I). The transcriptional regulation
of protein-coding genes by lncRNAs has been reported in the
literature. The transcription of lncRNAs is now known to regulate
the expression of genes in close genomic proximity
(cis-acting regulation) (15). The location of the ABCG1 gene was
in close proximity to lncRNA AL355711 (Fig. 1A), and the expression of ABCG1
was also higher in human atherosclerotic tissues (3.02-fold
increase, P=0.00182682036107604, Table I). The expression microarray data
can be found at NCBI GEO (Accession no. GSE97210; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE97210).
The expression of lncRNA AL355711 was verified using RT-qPCR in
five healthy arterial intima tissues and 20 human atherosclerotic
plaques (Fig. 1B). Microarrays
that measured the expression of lncRNA AL355711 were screened from
the GEO database to obtain an overview of the differential
expression of lncRNA AL355711 in atherosclerotic CAD. GSE12288, a
microarray from the GPL96 platform, was selected. The expression
profile of lncRNA AL355711 in circulating leukocytes was used to
identify patients with CAD and was assessed using Affymetrix U133A
chips. The expression of lncRNA AL355711 and ABCG1 was
significantly increased in patients with atherosclerotic CAD
(Fig. 1C and D). Therefore, it
was demonstrated that lncRNA AL355711 and ABCG1 may play a
potential role in atherosclerosis.
Knockdown of lncRNA AL355711 inhibits
ABCG1 transcription
The correlation between lncRNA AL355711 and ABCG1
was first analyzed through bioinformatics analysis. The correlation
between the AL355711 and ABCG1 mRNA levels in atherosclerotic CAD
was found to be positive (Pearson's R=0.15; P=0.027; <0.05;
Fig. 1E). Stable lncRNA AL355711
knockdown VSMCs were then constructed. The shRNA lentiviral vector
targeting lncRNA AL355711 was constructed to knock down lncRNA
AL355711 in order to clarify the transcriptional regulation of
lncRNA AL355711. The expression of lncRNA AL355711 was decreased by
>90% (Fig. 1F). The knockdown
of lncRNA AL355711 also decreased the mRNA and protein levels of
ABCG1 (Fig. 1F and G). These
results suggested that lncRNA AL355711 inhibited the transcription
of ABCG1.
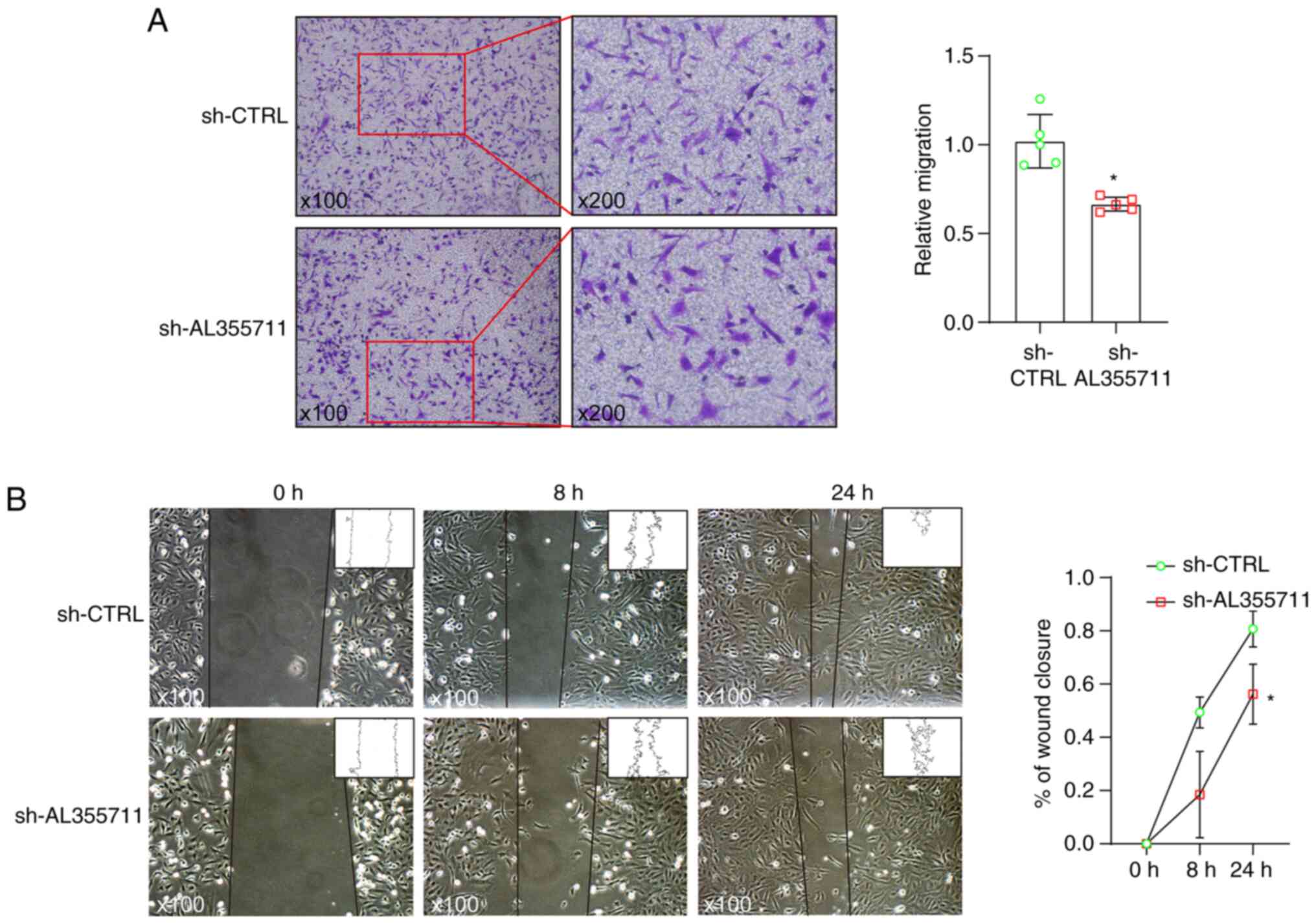
lncRNA AL355711 knockdown inhibits smooth
muscle cell migration
Smooth muscle cells contribute to the majority of
foam cells in ApoE−/− mouse atherosclerosis (16). Smooth muscle cell migration is
the first step of atherosclerosis induced by smooth muscle cells.
Loss-of function experiments were performed to evaluate the
functional role of lncRNA AL355711 in VSMC migration. Stable lncRNA
AL355711 knockdown human VSMCs were established using lentivirus
(Fig. 1F). lncRNA AL355711
knockdown significantly inhibited the migration of VSMCs, as shown
by the results of Transwell assay (Fig. 2A). Moreover, the results of wound
healing assay indicated that the wound width was markedly reduced
(Fig. 2B).
lncRNA AL355711 regulates MMP3 expression
through the ABCG1 pathway
The MMP3 level is elevated in patients with fatal
and non-fatal cardiovascular outcomes compared with controls. The
MMP3 baseline level in patients with a history of CAD is a
potential predictor for cardiovascular outcomes (17). The expression of MMPs and
matrix-degrading activity has been shown to be increased in
vulnerable regions of human atherosclerotic plaques (18). MMP3 knockdown has been shown to
attenuate VSMC migration into t scratch wound by 59% and reduce
neointima formation and VSMC accumulation in mouse atherosclerotic
plaques, suggesting that MMP3 plays a key role in VSMC migration
and neointima formation (19).
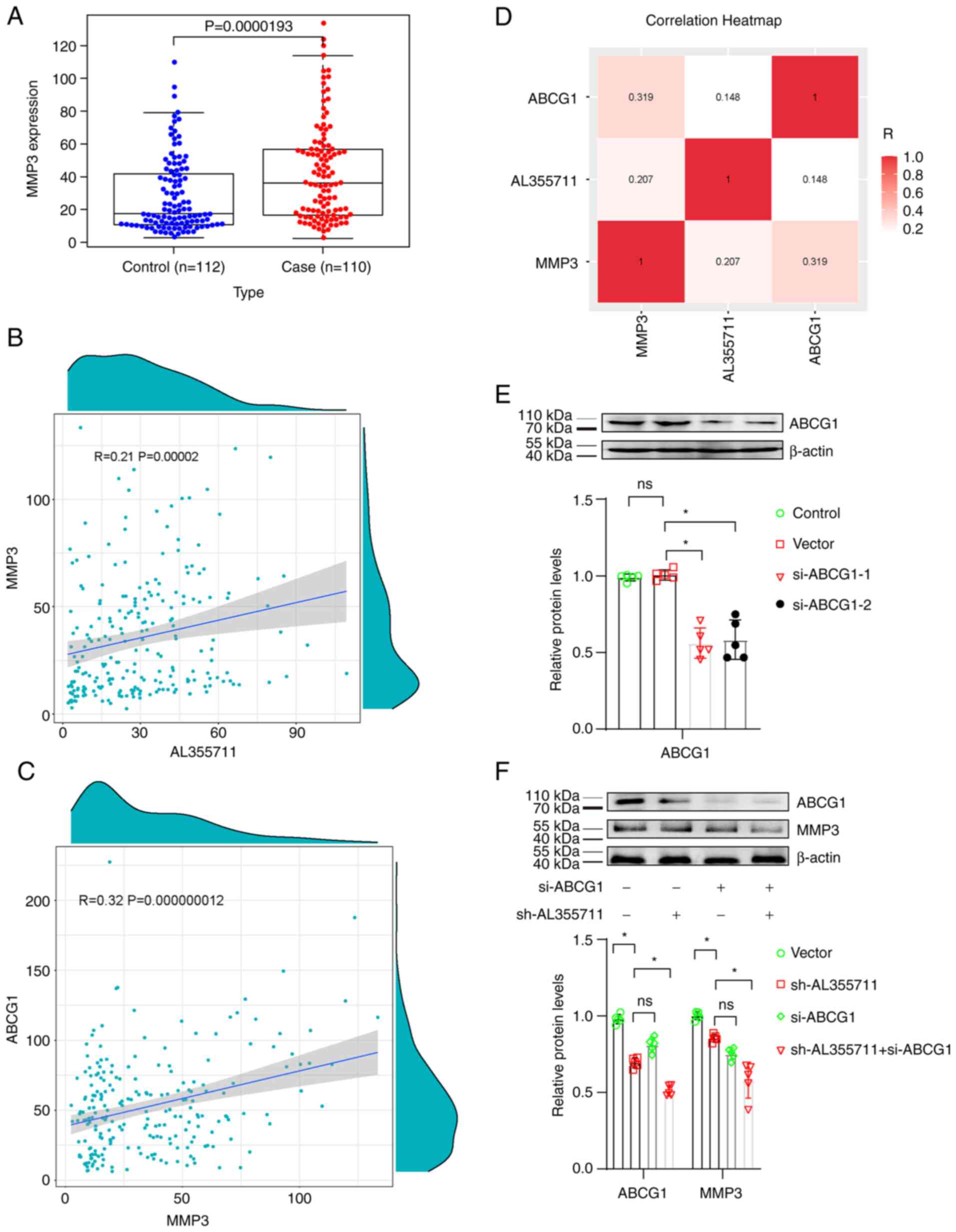
The present study performed a bioinformatics
analysis of the key migration-associated gene, MMP3, to investigate
the mechanisms through which lncRNA AL355711 modulates VSMC
migration. The expression of MMP3 was found to be significantly
upregulated in patients with atherosclerotic CAD (Fig. 3A). The correlation between
AL355711 and MMP3 mRNA levels in atherosclerotic CAD was also found
to be positive (Pearson's R=0.21; P=0.00002; P<0.05; Fig. 3B). As aforementioned, lncRNA
AL355711 regulated the transcription of ABCG1, and lncRNA AL355711
positively correlated with MMP3; however, correlation between ABCG1
and MMP3 remained unclear. Thus, the correlation between ABCG1 and
MMP3 was further analyzed. A positive correlation was found between
ABCG1 and MMP3 (Pearson's R=0.32; P=0.000000012; P<0.05;
Fig. 3C). Hence, it was
suggested that ABCG1 is involved in the regulation between lncRNA
AL355711 and MMP3 in atherosclerotic CAD (Fig. 3D).
A specific knockdown probe targeting ABCG1 was then
constructed to determine the role of MMP3 in the regulation of VSMC
migration. The transfection of si-ABCG1 was successful; the
decreased target expression was evidenced using western blot
analysis, and si-ABCG1-1 was used in subsequent experiments as it
was the most efficient (Fig.
3E). The expression of MMP3 was decreased following lncRNA
AL355711 knockdown, and this decrease was partially made more
prominent by the knockdown of ABCG1 (Fig. 3F).
ABCG1 and MMP3 are highly expressed in an
animal model of atherosclerosis
To the best of our knowledge, no homologous sequence
of lncRNA AL355711 exists in mice. Hence, it is impossible to
construct a lncRNA AL355711 knockout mouse model. In the present
study, a mouse model of atherosclerosis was constructed by feeding
male ApoE−/− mice with a Western HFD for 12 weeks to
further verify the role of ABCG1 and MMP3 in atherosclerotic
formation. The staining of the aortic roots with Oil Red O
(Fig. 4A) and the aortic root
cross-sections stained with H&E (Fig. 4B) indicated that the mouse model
of atherosclerosis was successfully constructed using the HFD. The
collagen fiber content was significantly decreased in the
atherosclerotic plaques (Fig.
4C). The expression of ABCG1 was found to be significantly
increased in atherosclerotic plaques (Fig. 4D). The consecutive
paraffin-embedded tissue sections were also stained for MMP3, which
was found to be highly expressed in atherosclerotic plaques
(Fig. 4E). Both MMP3 and ABCG1
were also found to be highly expressed in the same sections of
atherosclerotic plaques (Fig.
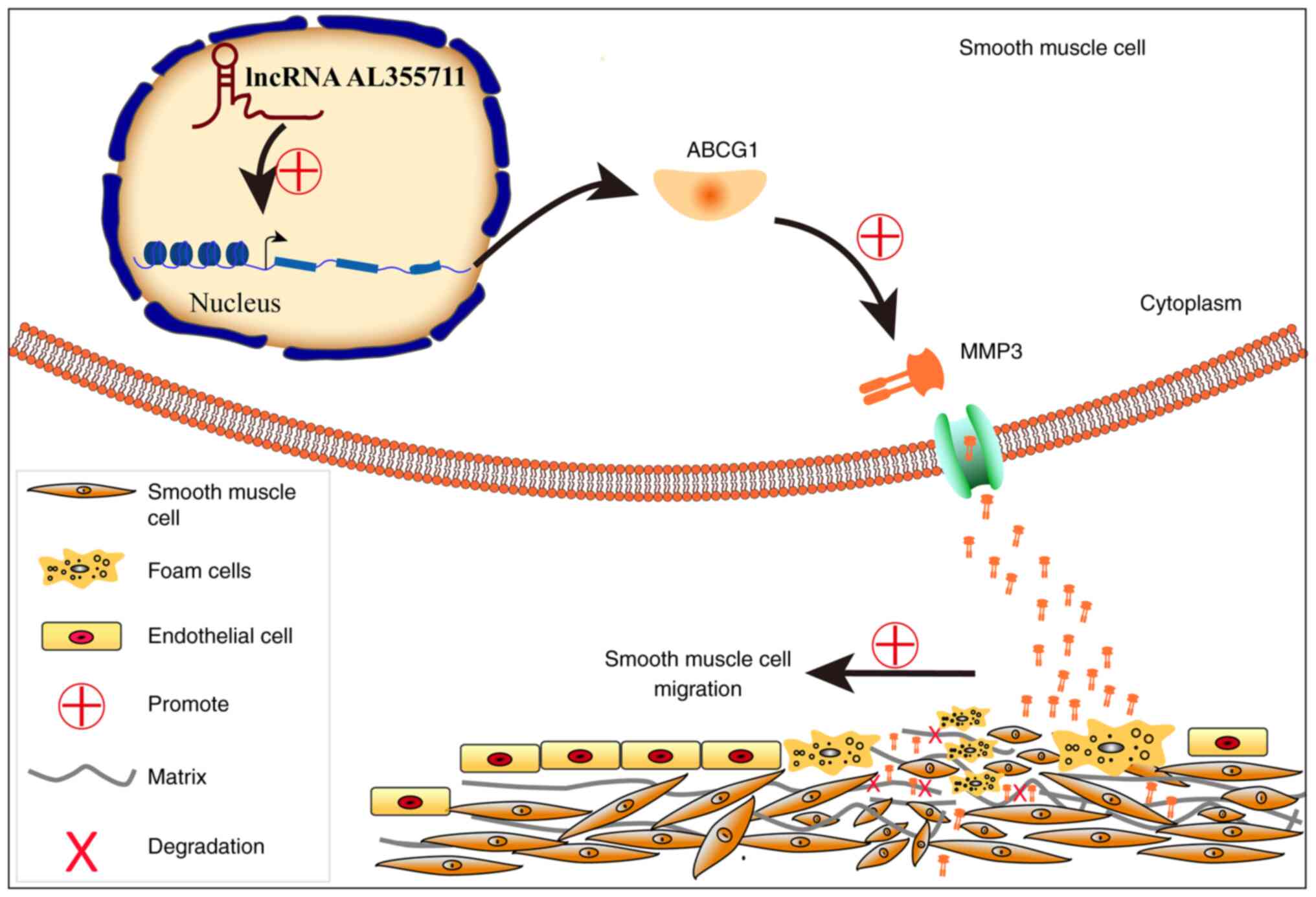
4F). A schematic diagram of the possible mechanisms through
which lncRNA AL355711 promotes VSMC migration and atherogenesis via
ABCG1/MMP3 is presented in Fig.
5.
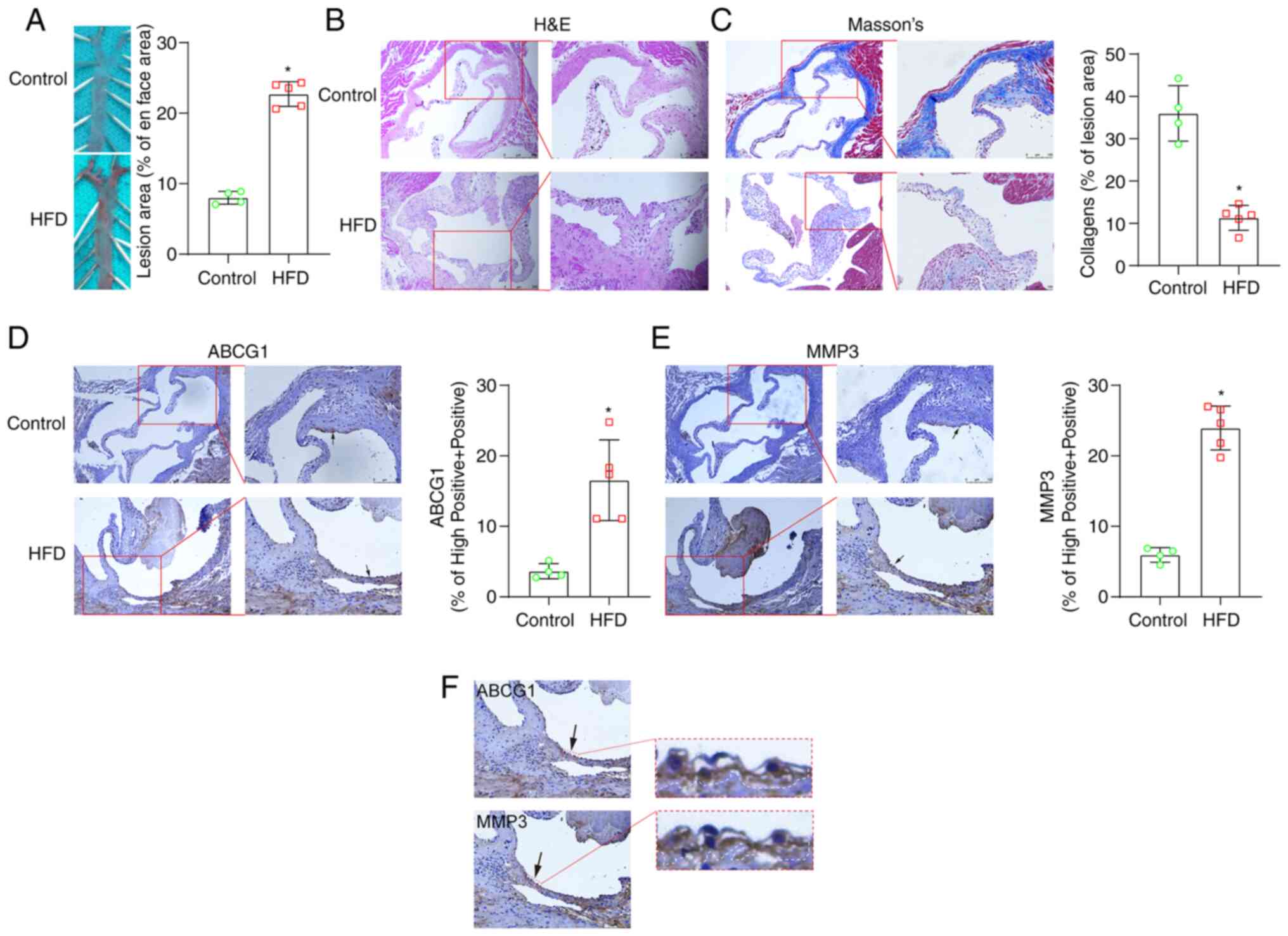 | Figure 4ABCG1 and MMP3 are highly expressed
in an animal model of atherosclerosis. An animal model of
atherosclerosis was successfully constructed by feeding 6-week-old
apolipoprotein E knockout mice with a Western HFD for 12 weeks. (A)
Representative en face images of the ascending aorta stained with
Oil Red O (×2.5 magnification). A blue mouse pad was used as the
supporting platform for the ascending aortas (hence the green-like
background). Data shown in the graphs are mean ± SD.
*P<0.05. In total, 4 mice were used as the controls
and 5 mice were used in the HFD group. The experiments were
performed once. (B) Aortic root cross-sections stained with
H&E. Scale bar left panel, 250 µm (×100 magnification);
scale bar right panel, 100 µm (×200 magnification). The
images on the right panel are an enlarge magnification of the red
boxed areas of the images in the left panels. (C) Aortic root
cross-sections stained with Masson's stain indicated that the HFD
diet significantly reduced the collagen fiber content. Data shown
in the graphs are the mean ± SD. *P<0.05. Scale bar
left panel, 250 µm (×100 magnification); scale bar right
panel, 100 µm (×200 magnification). The images on the right
panel are an enlarge magnification of the red boxed areas of the
images in the left panels. (D) ABCG1 immunohistochemical staining
revealed that the HFD significantly increased the ABCG1 levels in
plaques. Data shown in the graphs are the mean ± SD.
*P<0.05. Scale bar left panel, 250 µm (×100
magnification); scale bar right panel, 100 µm (×200
magnification). The images on the right panel are an enlarge
magnification of the red boxed areas of the images in the left
panels. (E) MMP3 immunohistochemical staining revealed that the HFD
significantly increased the level of ABCG1 in plaques. Data shown
in the graphs are the mean ± SD. *P<0.05. Scale bar
left panel, 250 µm (×100 magnification); scale bar right
panel, 100 µm (×200 magnification). The images on the right
panel are an enlarge magnification of the red boxed areas of the
images in the left panels. (F) MMP3 immunohistochemical staining
revealed that the HFD significantly increased the level of ABCG1 in
plaques. Data shown in the graphs are the mean ± SD.
*P<0.05. The same paraffin-embedded tissue sections
were stained for MMP3 and ABCG1, both of which were highly
expressed in the same section of atherosclerotic plaques. Scale bar
left panel, 100 µm (×200 magnification); arrows indicate the
red boxed areas, which are presented as an enlarged magnification
in the images on the right (×20,000 magnification). lncRNA, long
non-coding RNA; ABCG1, ATP-binding cassette sub-family G member 1;
MMP3, matrix metalloproteinase 3; HFD, high-fat diet; H&E,
hematoxylin and eosin. |
Discussion
The morbidity and mortality rates are higher in
patients with CVD. Its major clinical manifestations include
ischemic heart disease, ischemic stroke and peripheral artery
disease (20). Atherosclerosis,
the major cause of CVD, involves a complex progression of chronic
inflammation, response to injury and disordered lipid metabolism
(21). The mechanisms of action
of VSMCs in various stages of native atherosclerotic plaque
formation vary (22). VSMCs are
the most abundant cells in human atherosclerotic lesions and it has
been suggested that at least 50% foam cells in atheroma are derived
from VSMCs (16). One of the key
findings of the present study was that an uncharacterized lncRNA
AL355711 promoted VSMC migration and atherogenesis via ABCG1/MMP3
(Fig. 5).
A number of major modifiable risk factors for
atherosclerosis have been identified, including, but not limited
to, smoking, obesity, hypertension, hyperlipidemia, hyperglycemia
and physical inactivity (1).
Non-coding RNAs have been demonstrated to play crucial roles in
gene regulation and atherosclerosis (10). lncRNA NEXN-AS1 has been shown to
mitigate atherosclerosis by regulating the actin-binding protein
NEXN (11). Another study
demonstrated that lncRNA HCG11 regulates the proliferation and
apoptosis of VSMCs by targeting the miR-144-3p/FOXF1 axis in
atherosclerosis (23). lncRNA
growth arrest specific 5 (GAS5) has been found to induce VSMC cycle
arrest and apoptosis via the p53 pathway (24). A previous study demonstrated that
the knockdown of PVT1 enhanced aortic cell apoptosis, elevated the
MMP2 and MMP9 levels, reduced the tissue inhibitor matrix
metalloproteinase 1 levels, and increased the levels of
pro-inflammatory cytokines (25). At least 50% of foam cells in
atheroma are derived from VSMCs, and the migration of VSMCs is the
first step in the formation of a foam cell (22). Hence, the role of lncRNAs in VSMC
migration warrants further investigation. The present study
identified a novel lncRNA, AL355711, located on chromosome 21q22 in
close proximity to the protein-coding gene, ABCG1. lncRNA AL355711
was found to be overexpressed in plaques and positively regulated
the expression of ABCG1. lncRNA AL355711 promoted the migration of
VSMCs by regulating MMP3 expression via the ABCG1 pathway. lncRNA
AL355711 in circulating leukocytes may thus be a novel biomarker
for atherosclerosis. lncRNAs are an important class of regulatory
molecules; previous studies have demonstrated that lncRNAs may be
used as therapeutic targets (26-28). RNA-based therapies, including RNA
molecules as drugs and small molecules targeted by RNA, provide a
unique opportunity to expand the range of therapeutic targets.
However, advancements made in ribonucleic acid therapy are likely
to rely on improvements in the design and utilization of
appropriate ribonucleic acid molecules, delivery systems, and
targeting technologies to ensure efficacy and avoid or minimize
nontargeting effects and immunogenicity (29). For the future directions of
research, the regulation of the increase in the expression of
lncRNA AL355711 remains unclear. In addition, the mechanisms
underlying the upregulation of ABCG1 by lncRNA AL355711 are poorly
understood. Therefore, the effects of lncRNA AL355711 on
atherosclerosis need to be examined in future studies.
MMPs have been implicated in the development and
progression of atherosclerosis due to their ability to induce the
focal destruction of the vascular extracellular matrix (30). Matrix degradation is a hallmark
of high-risk atherosclerosis that leads to myocardial infarction
and stroke (31). MMP1, MMP3 and
MMP12 are positively associated with cardiovascular and
cerebrovascular events in patients with carotid atherosclerosis
(32). MMPs play important roles
in VSMC migration (33). MMP3 is
a key member of the MMP family. It is present in coronary
atherosclerosis (34). The serum
levels of MMP1, MMP3, MMP7 and MMP12 have also been shown to be
elevated in patients with chronic obstructive pulmonary disease and
carotid plaques in the carotid artery (35). MMP3 haplotype 2 has been found to
be associated with a reduced risk of myocardial infarction and an
increased risk of hemorrhagic stroke; thus, the MMP3 haplotype may
predict both cardiac events and stroke (36). MMP3 is required for IL-1-induced
VSMC invasion (37). The present
study discovered a novel regulatory role for MMP3. lncRNA AL355711
increased the level of MMP3 by upregulating ABCG1 expression. The
expression of MMP3 and ABCG1 increased in atherosclerotic plaques
in mice. The present study found that MMP3 and ABCG1 were
upregulated in circulating leukocytes of atherosclerotic CVD. The
expression of MMP3 and ABCG1 in circulating leukocytes may be a
novel biomarker for atherosclerotic CVD. It was found that ABCG1
expression positively correlated with MMP3 expression, and ABCG1
knockdown decreased the protein level of MMP3. In addition, MMP3
and ABCG1 were highly expressed in atherosclerotic plaques in mice,
and both MMP3 and ABCG1 were highly expressed in the same section
of atherosclerotic plaques. The small sample size of the mouse
model used may be a limitation of the present study. The authors
aim to overcome this limitation in future studies. The present
study identified novel modulating pathways of MMP3 and validated
these in a mouse model of atherosclerotic plaques. However, the
mechanisms underlying the upregulation of MMP3 by lncRNA AL355711
and ABCG1 remain unclear.
Pursuing the biological implications of the emerging
genetic results represents a promising area for future
investigation. In conclusion, the present study identified a
previously uncharacterized lncRNA, AL355711, that promotes VSMC
migration and atherogenesis via ABCG1/MMP3. These findings may
enhance the current understanding of the pathogenesis of
atherosclerosis and indicate that lncRNA AL355711, ABCG1 and MMP3
are potential biomarkers and therapeutic targets for
atherosclerotic CVD.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed in this study are
available from the corresponding author on reasonable request.
Authors' contributions
XZH and JJZ designed the study. CMK designed the
experiments. CMK, KWY, WKL and PFK performed the experiments (CMK
and KWY, performed the cell migration scratch assay; WKL performed
RT-qPCR and western bot analysis; PFK performed the bioinformatics
analysis). CMK performed the in vivo experiments. JJZ
contributed the patient samples. XHL and RYH contributed to the
immunohistochemical analysis. SWC performed the pathological
analyses (histological analysis, Oil Red O staining, Masson's
staining, hematoxylin and eosin staining). CMK wrote the
manuscript. JY, WYC, YSY, HW and XJ contributed to the cell
migration and scratch analysis. CMK and JJZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanfang HospitalNFEC-2017-122. Informed consent
written was obtained from all the participants. All animal
experiments conformed to the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (NIH Publication no. 85-23, revised in 1996) and were
approved by the Nanfang Hospital Animal Experimental Committee
(approval no. 20200830005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019. View Article : Google Scholar
|
|
2
|
Libby P, Buring JE, Badimon L, Hansson GK,
Deanfield J, Bittencourt MS, Tokgözoğlu L and Lewis EF:
Atherosclerosis. Nat Rev Dis Primers. 5:562019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vasan RS and Benjamin EJ: The future of
cardiovascular epidemiology. Circulation. 133:2626–2633. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan H, Xue C, Auerbach BJ, Fan J, Bashore
AC, Cui J, Yang DY, Trignano SB, Liu W, Shi J, et al: Single-cell
genomics reveals a novel cell state during smooth muscle cell
phenotypic switching and potential therapeutic targets for
atherosclerosis in mouse and human. Circulation. 142:2060–2075.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu S, Kamato D, Little PJ, Nakagawa S,
Pelisek J and Jin ZG: Targeting epigenetics and non-coding RNAs in
atherosclerosis: From mechanisms to therapeutics. Pharmacol Ther.
196:15–43. 2019. View Article : Google Scholar :
|
|
6
|
Simion V, Zhou H, Haemmig S, Pierce JB,
Mendes S, Tesmenitsky Y, Pérez-Cremades D, Lee JF, Chen AF, Ronda
N, et al: A macrophage-specific lncRNA regulates apoptosis and
atherosclerosis by tethering HuR in the nucleus. Nat Commun.
11:61352020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo FX, Wu Q, Li P, Zheng L, Ye S, Dai XY,
Kang CM, Lu JB, Xu BM, Xu YJ, et al: The role of the
LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of
autophagy flux and inflammation through mTOR-dependent signaling.
Cell Death Differ. 26:1670–1687. 2019. View Article : Google Scholar :
|
|
8
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar :
|
|
9
|
Mahmoud AD, Ballantyne MD, Miscianinov V,
Pinel K, Hung J, Scanlon JP, Iyinikkel J, Kaczynski J, Tavares AS,
Bradshaw AC, et al: The human-specific and smooth muscle
cell-enriched LncRNA SMILR promotes proliferation by regulating
mitotic CENPF mRNA and drives cell-cycle progression which can be
targeted to limit vascular remodeling. Circ Res. 125:535–551. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong XH, Lu ZF, Kang CM, Li XH, Haworth
KE, Ma X, Lu JB, Liu XH, Fang FC, Wang CS, et al: The long
noncoding RNA RP11-728F11.4 promotes atherosclerosis. Arterioscler
Thromb Vasc Biol. 41:1191–1204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu YW, Guo FX, Xu YJ, Li P, Lu ZF, McVey
DG, Zheng L, Wang Q, Ye JH, Kang CM, et al: Long noncoding RNA
NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding
protein NEXN. J Clin Invest. 129:1115–1128. 2019. View Article : Google Scholar :
|
|
12
|
Bai HL, Lu ZF, Zhao JJ, Ma X, Li XH, Xu H,
Wu SG, Kang CM, Lu JB, Xu YJ, et al: Microarray profiling analysis
and validation of novel long noncoding RNAs and mRNAs as potential
biomarkers and their functions in atherosclerosis. Physiol
Genomics. 51:644–656. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang CM, Bai HL, Li XH, Huang RY, Zhao JJ,
Dai XY, Zheng L, Qiu YR, Hu YW and Wang Q: The binding of lncRNA
RP11-732M18.3 with 143-3 β/α accelerates p21 degradation and
promotes glioma growth. EBioMedicine. 45:58–69. 2019. View Article : Google Scholar :
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Dubland JA, Allahverdian S, Asonye
E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ and Francis GA:
Smooth muscle cells contribute the majority of foam cells in ApoE
(Apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler
Thromb Vasc Biol. 39:876–887. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guizani I, Zidi W, Zayani Y, Boudiche S,
Hadj-Taieb S, Sanhaji H, Zaroui A, Mechmeche R, Mourali MS, Feki M
and Allal-Elasmi M: Matrix metalloproteinase-3 predicts clinical
cardiovascular outcomes in patients with coronary artery disease: A
5 years cohort study. Mol Biol Rep. 46:4699–4707. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of human atherosclerotic
plaques. J Clin Invest. 94:2493–2503. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson JL, Dwivedi A, Somerville M,
George SJ and Newby AC: Matrix metalloproteinase (MMP)-3 activates
MMP-9 mediated vascular smooth muscle cell migration and neointima
formation in mice. Arterioscler Thromb Vasc Biol. 31:e35–e44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du X, Patel A, Anderson CS, Dong J and Ma
C: Epidemiology of cardiovascular disease in China and
opportunities for improvement: JACC international. J Am Coll
Cardiol. 73:3135–3147. 2019. View Article : Google Scholar
|
|
21
|
Libby P, Bornfeldt KE and Tall AR:
Atherosclerosis: Successes, surprises, and future challenges. Circ
Res. 118:531–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allahverdian S, Chaabane C, Boukais K,
Francis GA and Bochaton-Piallat ML: Smooth muscle cell fate and
plasticity in atherosclerosis. Cardiovasc Res. 114:540–550. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Cui X, Wang C and Zhao S: LncRNA
HCG11 regulates proliferation and apoptosis of vascular smooth
muscle cell through targeting miR-144-3p/FOXF1 axis in
atherosclerosis. Biol Res. 53:442020. View Article : Google Scholar :
|
|
24
|
Tang R, Mei X, Wang YC, Cui XB, Zhang G,
Li W and Chen SY: LncRNA GAS5 regulates vascular smooth muscle cell
cycle arrest and apoptosis via p53 pathway. Biochim Biophys Acta
Mol Basis Dis. 1865:2516–2525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Zou G, Chen X, Lu W, Liu J, Zhai
S and Qiao G: Knockdown of lncRNA PVT1 inhibits vascular smooth
muscle cell apoptosis and extracellular matrix disruption in a
murine abdominal aortic aneurysm model. Mol Cells. 42:218–227.
2019.PubMed/NCBI
|
|
26
|
Subramaniam N, Nair R and Marsden PA:
Epigenetic regulation of the vascular endothelium by angiogenic
LncRNAs. Front Genet. 12:6683132021. View Article : Google Scholar :
|
|
27
|
Wang YQ, Xu ZM, Wang XL, Zheng JK, Du Q,
Yang JX and Zhang HC: LncRNA FOXC2-AS1 regulated proliferation and
apoptosis of vascular smooth muscle cell through targeting
miR-1253/FOXF1 axis in atherosclerosis. Eur Rev Med Pharmacol Sci.
24:3302–3314. 2020.
|
|
28
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar :
|
|
29
|
Yu AM, Choi YH and Tu MJ: RNA drugs and
RNA targets for small molecules: Principles, progress and
challenges. Pharmacol Rev. 72:862–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson JL: Metalloproteinases in
atherosclerosis. Eur J Pharmacol. 816:93–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monaco C, Gregan SM, Navin TJ, Foxwell BM,
Davies AH and Feldmann M: Toll-like receptor-2 mediates
inflammation and matrix degradation in human atherosclerosis.
Circulation. 120:2462–2469. 2009. View Article : Google Scholar
|
|
32
|
Hu W, Wei R, Wang L, Lu J, Liu H and Zhang
W: Correlations of MMP-1, MMP-3, and MMP-12 with the degree of
atherosclerosis, plaque stability and cardiovascular and
cerebrovascular events. Exp Ther Med. 15:1994–1998. 2018.PubMed/NCBI
|
|
33
|
Ma L and Zhang L, Wang B, Wei J, Liu J and
Zhang L: Berberine inhibits Chlamydia pneumoniae infection-induced
vascular smooth muscle cell migration through downregulating MMP3
and MMP9 via PI3K. Eur J Pharmacol. 755:102–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seifi M, Fallah S and Firoozrai M:
Influence of genetic polymorphism in matrix metalloproteinase-3 on
extent of coronary atherosclerosis and risk of coronary artery
stenosis. Arch Med Res. 40:600–604. 2009. View Article : Google Scholar
|
|
35
|
Kraen M, Frantz S, Nihlén U, Engström G,
Löfdahl CG, Wollmer P and Dencker M: Matrix metalloproteinases in
COPD and atherosclerosis with emphasis on the effects of smoking.
PLoS One. 14:e02119872019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaplan RC, Smith NL, Zucker S, Heckbert
SR, Rice K and Psaty BM: Matrix metalloproteinase-3 (MMP3) and MMP9
genes and risk of myocardial infarction, ischemic stroke, and
hemorrhagic stroke. Atherosclerosis. 201:130–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alexander MR, Moehle CW, Johnson JL, Yang
Z, Lee JK, Jackson CL and Owens GK: Genetic inactivation of IL-1
signaling enhances atherosclerotic plaque instability and reduces
outward vessel remodeling in advanced atherosclerosis in mice. J
Clin Invest. 122:70–79. 2012. View Article : Google Scholar :
|