Introduction
The gold standard for peripheral nerve repair is
autologous nerve grafting. Flap transplantation is commonly used to
repair tissue defects and reconstruct organs, achieving a
satisfying appearance repair effect (1,2).
However, the sensory function reconstruction is often not ideal.
Currently, there are still some issues that have not been solved.
The main trunk nerve of the cutaneous flap is too short to
recipient other nerves (3,4). The aim of the present study was to
explore the in-depth mechanism underlying Schwann cells (SCs) in
sensory function reconstruction. An established approach in neural
tissue engineering involves the fabrication of polymeric scaffolds
with nerve cells to produce a three-dimensional functional tissue
suitable for implantation (5–7).
Neurotrophin 3 (NT-3) reduces motor neuron excitability, maintains
sensory and motor neuron survival, promotes nerve cell
differentiation, induces axon growth and participates in nerve
repair after injury (8–13). Stem cells have great potential for
peripheral nerve repair (14,15),
and undifferentiated adipose stromal cells have been shown to
increase nerve regeneration in conduit-based peripheral nerve
repair studies (14,16).
It is well known that SCs promote the repair of
peripheral nerve injury (17,18).
However, Schwann cells are more difficult to obtain and culture
than stem cells (such as adipose stem cells and amniotic
mesenchymal stem cells). Therefore, we induced stem cells to
differentiate into Schwann-like cells to promote the repair of
peripheral nerve injury. After peripheral nerve injury, SCs as the
main body of peripheral nerve regeneration, have a critical role in
the repair of peripheral nerve injury (19,20).
SCs secrete various neurotrophic factors and axon-guiding factor,
and maintain the stability of the surrounding microenvironment,
prevent neuronal body death and promote neuronal axonal
regeneration (21). In addition,
SCs can synthesize and secrete extracellular matrix and cell
adhesion factors (22). Moreover,
SCs serve an important role in maintaining the survival of nerves,
guiding the regeneration of axons, and promoting the regeneration
and repair of nerves after injury. Spontaneous repair rarely occurs
after central nervous system injury, whereas partial or complete
repair can occur in the peripheral nervous system. The repair of
peripheral nervous system injury primarily depends on the suitable
microenvironment for nerve regeneration provided by SCs.
SCs directionally proliferate and migrate to the
injury area, forming bands of Bungner to bridge the nerve defect
area (19). SCs can provide a
suitable microenvironment for axonal regeneration and participate
in the formation of axon myelin. The proliferation of SCs is
regulated by numerous factors. Neurotropic factors secreted by
motor and sensory nerves bind to corresponding receptors on SCs to
promote proliferation at sites of nerve damage or injury (23). Precise control of cell-matrix
adhesion is necessary for cell migration. Extracellular matrix and
cell adhesion molecules secreted by SCs promote axonal regeneration
(24). Therefore, SCs have a
critical role in maintaining peripheral nerve homeostasis targeted
therapy of peripheral nerve injury. It is well known that SCs
promote the repair of peripheral nerve injury. However, SCs are
more difficult to obtain and culture than stem cells, such as
adipose stem cells and amniotic mesenchymal stem cells. Human
amnion mesenchymal cells (hAMSCs) are obtained from the
extraembryonic mesoderm, expressing not only the characteristics of
mesenchymal stem cells, but also characteristics of embryonic stem
cells (24). hAMSCs can secrete
stem cell-specific genes, including OCT4, SOX2 and NANOG, and can
differentiate into multiple cell lines under certain conditions
(25). Therefore, we speculated
that hAMSCs could be induced into SC-like cells (iSCs) to promote
nerve regeneration and functional recovery.
Chitosan is a cationic derivative of chitin after
deacetylation. As a natural polymer, it is widely used in gene
carrier, cell culture and tissue engineering due to its low
toxicity, good biodegradability and histocompatibility (26). Further research confirmed that NT-3
chitosan has suitable histocompatibility, which is beneficial to
tissue regeneration and reduces adverse reactions, such as
inflammation (27). It has been
reported that NT-3 chitosan provides an environment to promote
nerve repair and transected spinal cord recovery in rats (28). As a biodegradable and biocompatible
polymer material, hydrogel can be used as a sustained release
carrier for cells or drugs to fill chitosan ducts and form an
effective scaffold for axonal regeneration. Modified collagen
hydrogel scaffolds can be combined with SCs to repair nerve defect
(29). Hydrogel can simulate the
tissue-like physical and spatial structure needed for cell growth,
and has the advantages of high plasticity, a relatively simple
fabrication process and convenient clinical application.
SOX2 is a member of the SRY-related HMG box family
of transcription factors involved in the regulation of embryonic
development. SOX2 is necessary for stem cells maintained in the
nervous system. Thomson et al (30) reported that SOX2 is important in
neural differentiation, indicating that SOX2 is a neural
lineage-poised factor. SOX2 is a transcription factor essential for
self-renewal in stem cells and neural progenitor cells (31). Fibronectin 1 (FN1) is a
glycoprotein present in a soluble dimeric form in plasma, and in a
dimeric or multimeric form at the cell surface and in the
extracellular matrix. The encoded preproprotein is proteolytically
processed to generate the mature protein. Fibronectin is involved
in cell adhesion and migration processes, including embryogenesis,
wound healing, blood coagulation, host defense and metastasis.
Adachi et al (31) proved
that SOX2 directly activated fibronectin expression in SCs, leading
to an increase in fibrillogenesis and cellular huddling. Loss of
fibrillogenesis leads to glial disassembly and disorganized axon
regrowth (31). FN1 expression
reflects fibronectin fibrillogenesis (32). SOX2 directly controls
fibrillogenesis and provides a novel mechanism for the modification
of the environmental architecture by glial cells during neuronal
repair. Cell proliferation and migration are the cytological basis
of fibronectin fibrillogenesis (33,34).
Our previous results demonstrated that iSCs derived
from hAMSCs promote peripheral nerve regeneration through the
miR-214/c-JUN pathway (35). The
present study showed that iSCs derived from hAMSCs promote sciatic
nerve repair (a classical peripheral nerve regeneration model)
through an exosome-induced SOX2/FN1 pathway. In the present study,
the model of sciatic nerve defect in Sprague-Dawley (SD) model rats
was used to explore the process and feasibility of inducing hAMSCs
into SCs. In addition, iSCs induced by collagen hydrogel combined
with an NT-3 chitosan scaffold were used to repair rat nerve defect
in situ. In the process of sciatic nerve repair, SOX2/FN1
promoted sciatic nerve repair by increasing the vitality of iSCs
and promoting cell migration. iSCs are used to promote the recovery
of nerve function of the skin flap and difficult wounds, which
allowed investigation of its function in promoting peripheral nerve
repair and regeneration. Moreover, the results of the present study
provide a novel treatment strategy for the clinical promotion of
peripheral nerve recovery and regeneration. The results are of
clinical significance for the application of stem cells in the
field of wound and nerve repair. We hope to explore the mechanism
of promoting peripheral nerve repair in many aspects to promote the
development of this field.
Materials and methods
Cell lines and culture
Human adipose-derived stem cells (hADSCs) were
purchased from Guangzhou Saliai Stem Cell Science and Technology
Co. Ltd. and human SCs were purchased from The Cell Bank of the
Type Culture Collection of The Chinese Academy of Sciences. The
identification method of hADSCs used were as follow: i) cell
survival rate ≥80%; ii) microorganism detection (bacteria:
negative, Mycoplasma: negative, endotoxin <0.25 EU/ml); iii)
special human-derived virus (HIV: negative, HBV: negative, HCV:
negative, TP-DNA: negative, HCMV: negative, EBV: negative); iv)
cell surface markers (CD73 ≥95%, CD90 ≥95%, CD105 ≥95%, CD45 ≤2%,
CD34 ≤2%, HLA-DA ≤2%, CD11b ≤2%, CD19 ≤2%); v) differentiation
abilities (Oil red O staining was positive after adipogenic
differentiation for 21 days; Alizarin red S staining was positive
after osteogenic differentiation for 21 days). hADSCs were
maintained in low glucose-DMEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 2
mM glutamine (Merck KGaA), 1% non-essential amino acids (Gibco;
Thermo Fisher Scientific, Inc.), 55 mM 2-mercaptoethanol (Bio-Rad
Laboratories, Inc.), 1 mM sodium pyruvate (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin (Beyotime Institute of
Biotechnology) and 100 mg/ml streptomycin (Beyotime Institute of
Biotechnology). SCs were cultured in SC medium (ScienCell Research
Laboratories, Inc.) with 10% FBS. Cells were cultured at 37°C in a
humidified incubator with 5% CO2. hAMSCs were induced to
differentiate into iSCs as previously described (36). Following exosome co-culture for 24
h, the cells were harvested and washed with PBS. In addition, the
stem cell research underwent an EMRO process according to the
guidelines of the International Society for Stem Cell Research
(37).
Sciatic nerve transection and repair
animal models
A total of 24 adult male SD rats (weight, 200–250 g;
age, 8-weeks) were anesthetized with pentobarbital (50 mg/kg) as
previously described (38). The
animals were purchased from Guizhou Laboratory Animal Engineering
Technology Center. Animals were kept in an environmentally
controlled breeding room (temperature: 20±2°C; humidity: 60±5%; 12
h dark/light cycle) with sterilized food and clean water. At 1 cm
above the bifurcation into the tibial and common fibular nerves,
the sciatic nerve was exposed and cut using ophthalmic scissors.
The incision was closed with 4–0 nylon sutures. Subsequently, ~1 cm
NT-3 chitosan conduit (Guangzhou Beogene Biotech Co., Ltd.) filled
with iSC hydrogel (100 µl; 5×107 cells) was used to
promote sciatic nerve repair. The NT-3 chitosan conduit was sutured
at both ends of the disconnected sciatic nerve to connect them. The
in vivo experiment was divided into the following four
experimental groups (n=6): i) normal (sham operation); ii) iSCs
(sciatic nerve cut and filled with iSC hydrogel); iii) conduit
(sciatic nerve cut and NT-3 chitosan conduit connection); and iv)
iSCs + conduit (sciatic nerve cut and NT-3 chitosan conduit
connection filled with iSC hydrogel). Sciatic function, step length
and stride length (39) were
measured at 4, 8 and 12 weeks of treatment. After anesthesia with
pentobarbital (100 mg/kg) by intraperitoneal injection, cervical
dislocation was used as the euthanasia method, and death was
confirmed after complete cardiac arrest and pupil dilation. Human
intervention was implemented to prevent or relieve unnecessary pain
and disease if considered endpoints were met at any moment during
the experiment: i) 20% body weight loss; ii) the occurrence of
other complications. All experimental procedures involving animals
were approved by the Institutional Animal Care and Use Committee of
Zunyi Medical Hospital [(2017)2-035].
Cell transfection
The pcDNA3.1-vector and pcDNA3.1-SOX2 were
constructed and purchased from Guangzhou RiboBio Co., Ltd. Small
interfering (si)RNAs were purchased from Angon Biotech Co., Ltd.
The sequences of the siRNAs are as follows: si-SOX2-1,
5′-UGCAUCAUGCUGUAGCUGCCG-3′; si-SOX2-2,
5′-UGUCCAUGCGCUGGUUCACGC-3′; si-FN1-1, 5′-ACAAACUGGAGGUUAGUGGGA-3′;
si-FN1-2, 5′-UUUAUCUGAUAGUGUUUUCCA-3′ and negative control (NC),
5′-UUCUCCGAACGUGUCACGUTT-3′. The cells were transfected with siRNAs
or overexpression plasmids using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Transfected cells were cultured at 37°C
with 5% CO2 for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(40). In brief, total RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Total RNA (1 µg) was reverse transcribed into cDNA using
PrimeScript™ RT Reagent kit (Takara Bio, Inc.). Subsequently, qPCR
was performed in triplicate for each sample using TB
Green® Fast qPCR Mix (Takara Bio, Inc.) with GAPDH as
the internal reference gene. The following reaction conditions were
applied: 3 min at 95°C, 40 cycles of 15 sec at 95°C and 30 sec at
58°C and maintenance at 4°C. The following primers were used: SOX2
forward, 5′-GCCGAGTGGAAACTTTTGTCG-3′ and reverse,
5′-GGCAGCGTGTACTTATCCTTCT-3′; FN1 forward,
5′-CGGTGGCTGTCAGTCAAAG-3′ and reverse, 5′-AAACCTCGGCTTCCTCCATAA-3′;
GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blotting
Western blotting was performed as previously
described (40). In brief, after
washing with PBS, cells and tissues were lysed in RIPA lysis buffer
(Beyotime Institute of Biotechnology). Protein quantification was
performed using a BCA assay (Beyotime Institute of Biotechnology).
Proteins were mixed with 5X Loading buffer (Beyotime Institute of
Biotechnology). The following primary antibodies were used: NF160
(1:1,000; cat. no. ab254348; Abcam), GFAP (1:5,000; cat. no.
ab7260; Abcam), CD31 (1:1,000; cat. no. ab9498; Abcam), SOX2
(1:1,500; cat. no. ab92494; Abcam), FN1 (1:1,000; cat. no.
15613-1-AP; ProteinTech Group, Inc.) and GAPDH (1:15,000; cat. no.
60004-1-Ig; ProteinTech Group, Inc.). The membranes were incubated
with anti-rabbit IgG HRP-conjugated (1:2,000; cat. no. 7074S; Cell
Signaling Technology, Inc.) and anti-mouse IgG HRP-conjugated
(1:2,000; cat. no. 7076S; Cell Signaling Technology, Inc.)
secondary antibodies for 2 h. Protein bands were then visualized
using an ECL chemiluminescent kit (Thermo Fisher Scientific, Inc.)
and semi-quantified using ImageJ v1.8 software (National Institutes
of Health). The numbers under the band of western blotting were the
semi-quantitative results measured using ImageJ v1.8 software.
Scratch wound assay
Cells were seeded into 6-well plates and cultured to
the sub-confluent state. After starvation in serum-free SC medium
for 24 h, a straight wound was scratched at the bottom of the plate
with a 200-µl sterile pipette tip. After gently rinsing with PBS,
cells were cultured in serum-free medium for 24 or 48 h. Cell
migration was observed and calculated at 0, 24 and 48 h using an
inverted light microscope. Scratch-healing was calculated as
follows: Scratch healing (%)=(initial scratch area-final scratch
area)/initial scratch area ×100.
Transwell assay
Transwell assays were performed using Transwell
chambers (Corning, Inc.). Cells were seeded (7.5×103)
into the upper compartment in SC medium with 1% FBS, whereas the
lower chamber was filled with SC medium with 10% FBS. Invading
cells were fixed and calculated after 24 h.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed using the CCK-8 assay.
Cells were seeded (5×103 cells/well) into a 96-well
plate. After 24 h, the cells were treated. After 48 h, the cells
were incubated with 100 µl SC medium containing 10% CCK-8 reagent
(Dojindo Molecular Technologies, Inc.) for 1.5 h at 37°C. Then
absorbance was measured at a wavelength of 450 nm using a
microplate reader (Tecan Group, Ltd.). Cell viability was
calculated as follows: Cell viability (%)=[optical density (OD)
treatment-OD blank)/(OD control-OD blank) ×100.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
Cell proliferation was determined using an EdU
assay. Cells were seeded into a 24-well cell culture plate and then
10 µM EdU was added to each well. After incubation for 2 h at 37°C,
the cells were fixed with 4% paraformaldehyde for 30 min at room
temperature. After washing with PBS, the incorporated EdU was
detected using a EdU kit (Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at room temperature. Subsequently, the cells
were stained with DAPI for 5 min at room temperature and then
visualized using a fluorescence microscope (Olympus
Corporation).
Bioinformatics analysis
SCs with SOX2 overexpression RNA-seq data (GSE94590)
were obtained from the Gene Expression Omnibus database (41). Then, the values in the Sox2
overexpression group was compared with the normal group to obtain
the differentially expressed genes, respectively. The analysis was
performed using limma package (http://bioinf.wehi.edu.au/limma/) in R. The threshold
for significant was set at 1.2 or 0.83 fold change and P<0.05. A
Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) was
used to identify the common differential genes in Sox2 clone1 and
Sox2 clone2 group. Then, the common differential genes were used
for Kyoto Encyclopedia of Genes and Genome (KEGG) analysis in the
David database (https://david.ncifcrf.gov/).
Exosome isolation and
identification
iSCs were cultured in iSC induction medium
containing 10% exosome-depleted FBS. Exosomes were extracted using
Ribo Exosome Isolation Reagent (for cell culture media) (Guangzhou
RiboBio Co., Ltd.) according to the manufacturer's protocol. A
Nanosight LM10 HS-BF (Nanosight Ltd.) was used to measure the size
and concentration of the purified exosomes (42). CD81 and CD63 (43), as exosome markers, were used to
identified the exosomes using flow cytometry. The following
monoclonal antibodies were used: CD63 (1:50; cat. no. GTX41877;
GeneTex, Inc.) and CD81 (1:50; cat. no. GTX34568; GeneTex, Inc.).
Exosome pellets were resuspended in PBS and frozen at −80°C. The
exosome pellet was used for subsequent experiments. The exosomes
were added to the medium with exosome-free FBS and then co-cultured
with SCs for 24 h.
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Statistical analyses were performed using
SPSS software (version 19.0; IBM Corp.). All cell experiments were
independently repeated at least in triplicate. Comparisons between
two groups were analyzed using an unpaired Student's t-test.
One-way ANOVA was adopted to compare multiple groups. Post
hoc multiple comparisons were made using Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rat model of sciatic nerve transection
and repair
The rat sciatic nerve injury model was used to
simulate clinical peripheral nerve injury. hAMSCs were induced into
iSCs in vitro. At 1 cm above the bifurcation into the tibial
and common fibular nerves, the sciatic nerve was exposed and cut
using ophthalmic scissors. An NT-3 chitosan conduit filled with iSC
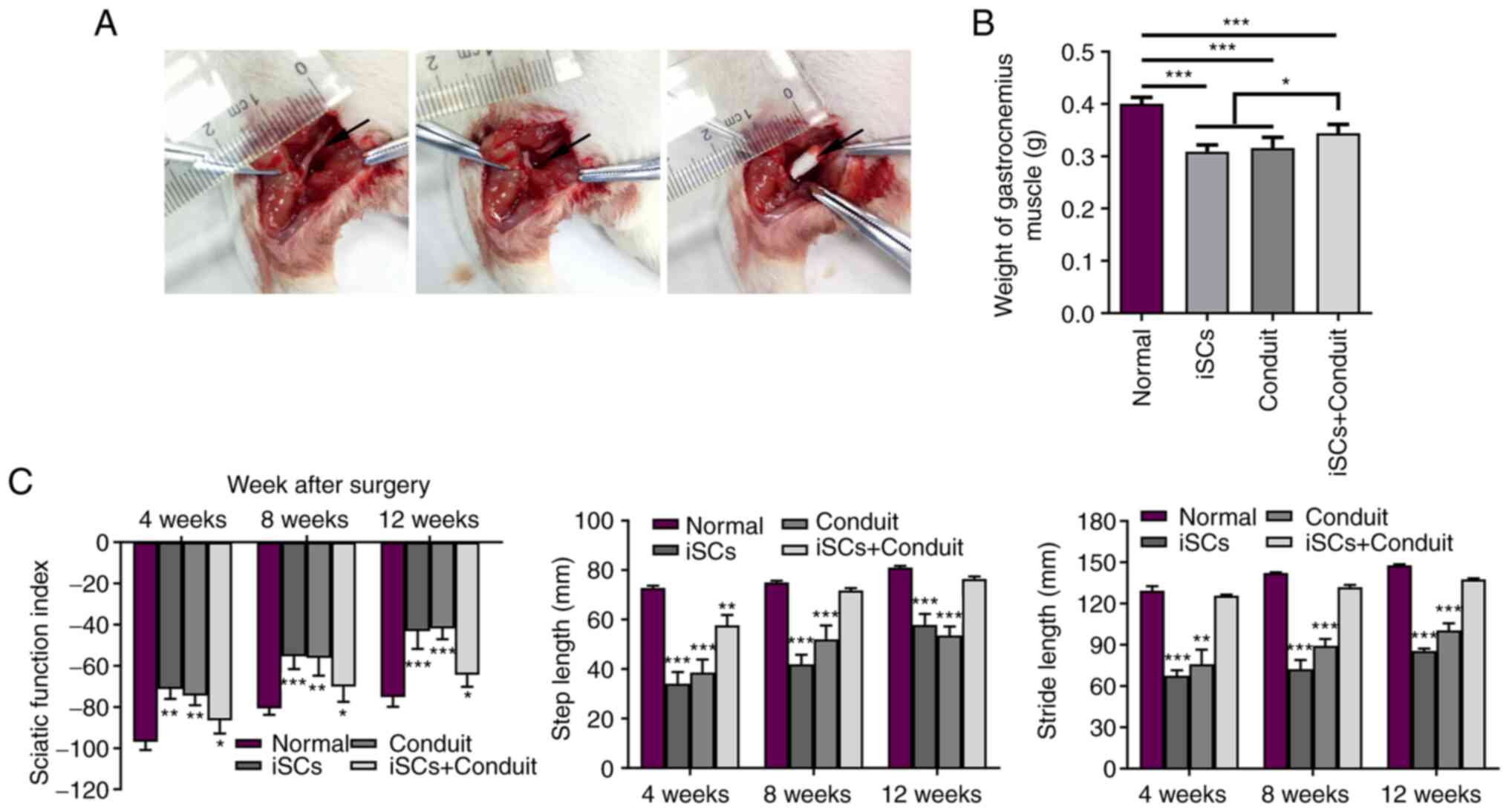
hydrogel was used to promote sciatic nerve repair (Fig. 1A). The in vivo experiment
was divided into the following four groups: i) normal (sham
operation); ii) iSCs (sciatic nerve cut and filled with iSC
hydrogel); iii) conduit (sciatic nerve cut and NT-3 chitosan
conduit connection); iv) iSCs+conduit (sciatic nerve cut and NT-3
chitosan conduit connection filled with iSC hydrogel). The maximum
percentage of body weight loss was 16% throughout the whole
experiment. To investigate the repair effect of the sciatic nerve,
the gastrocnemius muscle weight was measured. As shown in Fig. 1B, the weight of the gastrocnemius
muscle in the iSCs+conduit group was significantly heavier compared
with that in the conduit or iSCs groups. The sciatic function
index, step length and stride length data demonstrated recovery in
nerve function. At 4, 8 and 12 weeks, measurements were taken. The
results indicated that iSCs with the NT-3 conduit promoted sciatic
nerve recovery. Compared with the iSCs or conduit groups, the
iSCs+conduit group effectively promoted the repair of the sciatic
nerve (Fig. 1C). The results
indicated that iSCs with the NT-3 chitosan conduit promoted sciatic
nerve recovery.
Analysis of the role of SOX2 in SC
nerve repair
SCs with SOX2 overexpression RNA-seq data (GSE94590)
were obtained from the Gene Expression Omnibus database.
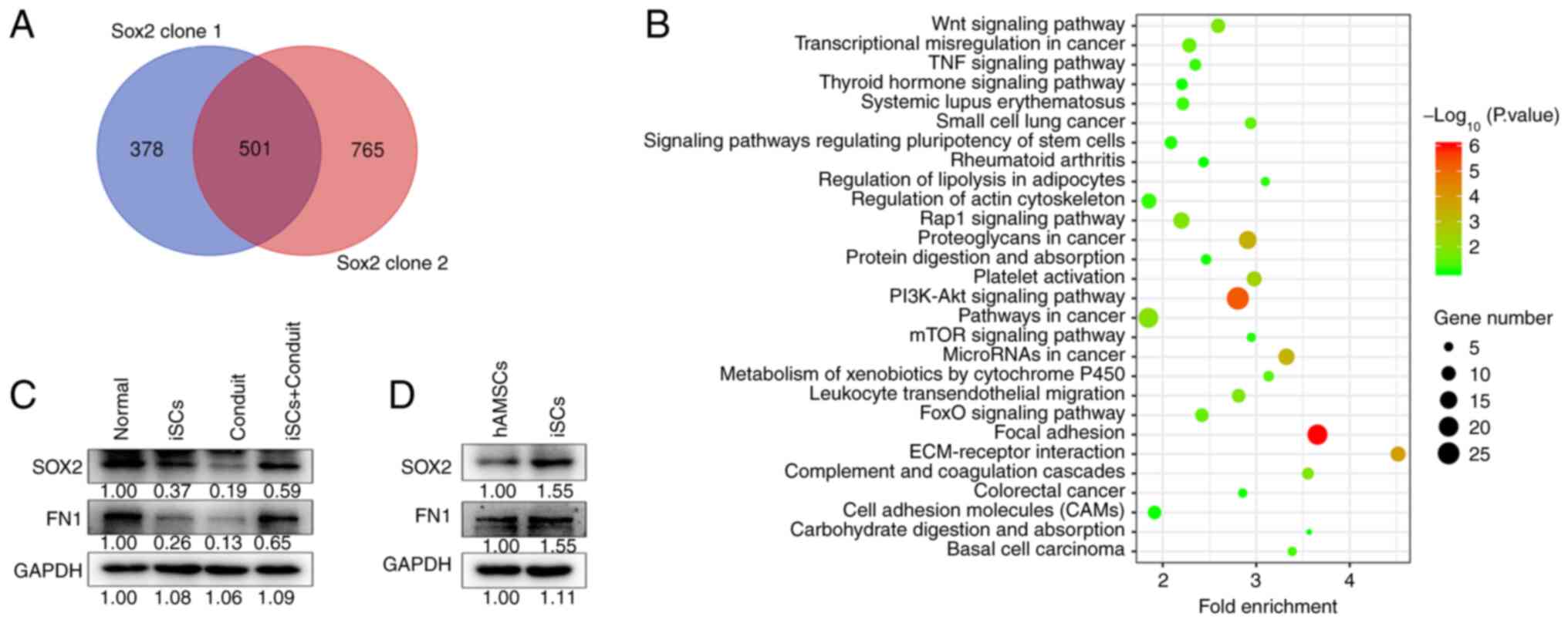
Differential genes identified by Venn analysis are presented in
Fig. 2A. There were 501 different
genes in both SOX2 clone 1 and SOX2 clone 2 SCs. The differential
genes were then analyzed using Kyoto Encyclopedia of Genes and
Genomes enrichment (KEGG) analysis. The results identified that
SOX2 was related to ‘focal adhesion process’, ‘PI3K-AKT signaling
pathway’ and ‘ECM-receptor interaction’ (Fig. 2B). It is critical for cell
proliferation and migration. In addition, cell proliferation and
migration are the cytological basis of fibronectin fibrillogenesis.
SOX2 and FN1 protein expression in gastrocnemius muscle tissues was
measured. The results indicated that SOX2 and FN1 levels were
decreased in the iSCs or conduit groups compared with those in the
normal group. However, the expression levels were increased in the
iSCs+conduit group compared with those in the iSCs or conduit
groups (Fig. 2C). In addition,
SOX2 and FN1 protein expression levels were more abundant in iSCs
compared with those in hAMSCs (Fig.
2D). These results suggest that SOX2 participates in SC
proliferation and migration.
Effect of SOX2 on the migratory
ability and cell viability of iSCs
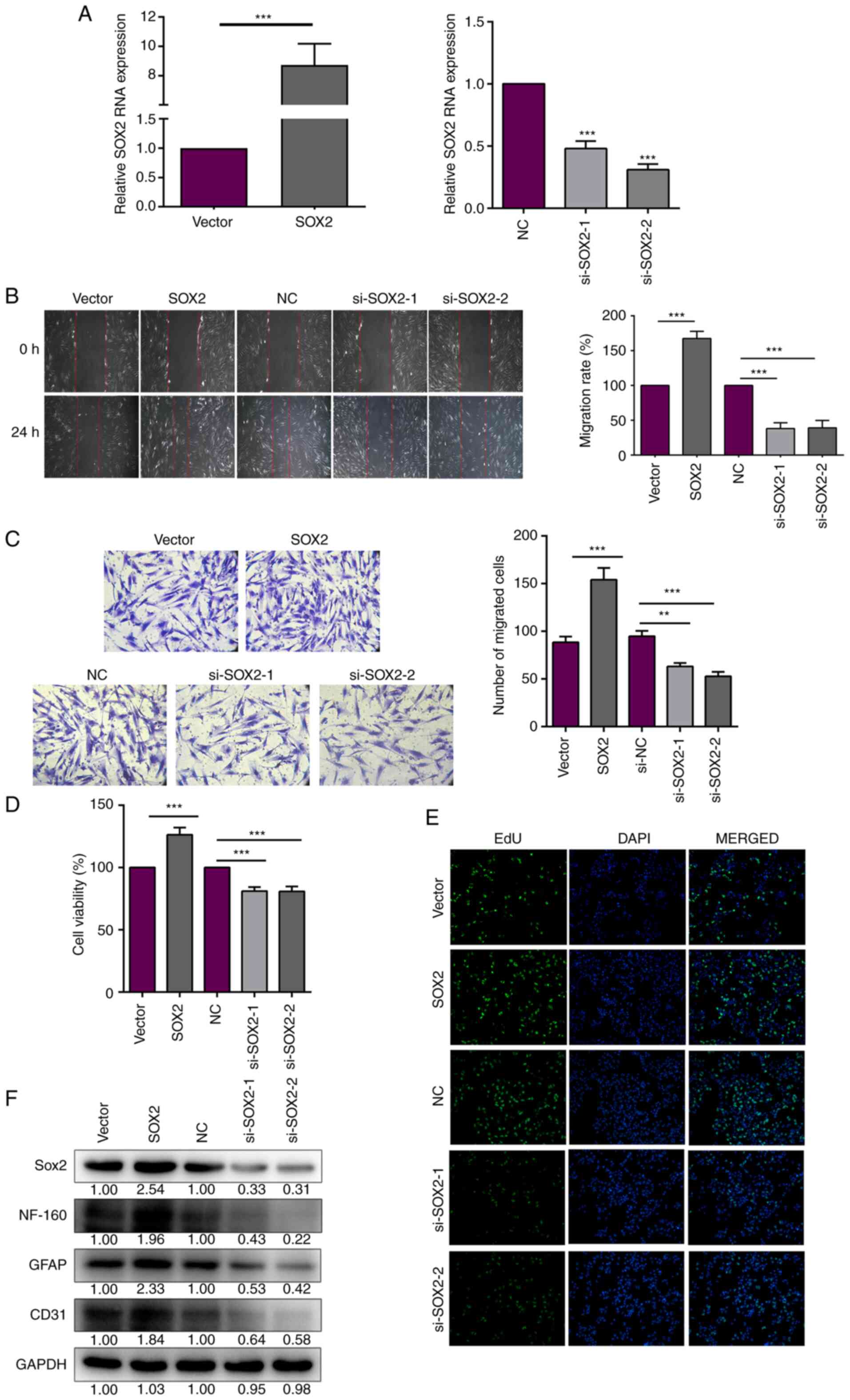
SOX2 was overexpressed and knocked down using the
SOX2 overexpression plasmid (pcDNA3.1-SOX2) and si-SOX2,
respectively. SOX2 mRNA expression was increased following SOX2
overexpression plasmid transfection, but decreased after si-SOX2
transfection (Fig. 3A). hAMSCs
were induced to differentiate into iSCs as previously described.
The transfection efficiency was confirmed for subsequent
experiments. Scratch wound assays were conducted to evaluate cell
migration after SOX2 transfection. The distance between iSCs cells
induced by stem cells is larger than that of ordinary cells,
consistent with a study by Feng et al (44). The migration rate was significantly
increased after SOX2 overexpression, but significantly decreased
after SOX2 knockdown in the iSCs (Fig.
3B). In addition, the migratory ability was analyzed using
Transwell assays. After 24 h of seeding, the number of migratory
SOX2-overexpression or -knockdown iSCs in the lower chamber was
counted. The number of cells in the lower chamber was significantly
increased with SOX2 overexpression, but significantly decreased
with si-SOX2 transfection (Fig.
3C). The aforementioned results indicated that SOX2 promoted
the migratory ability of iSCs. In addition to cell migration, cell
proliferation was assessed to evaluate nerve repair. The CCK-8
assay was performed to evaluate the effect of SOX2 on cell
viability in iSCs. The results showed that SOX2 overexpression
significantly increased iSC viability (Fig. 3D). The protein expression levels of
neurofilament 160 (NF-160), glial fibrillary acidic protein (GFAP)
and CD31 were measured to evaluate nerve cell activity. The protein
expression levels of NF-160, GFAP and CD31 were increased with SOX2
overexpression. However, the opposite trend was observed with
si-SOX2 transfection (Fig. 3F).
Cell proliferation was examined by performing EdU assays. EdU
labelling is indicated by green fluorescence and the cell nucleus
is shown in blue. The results indicated that SOX2 promoted iSC
proliferation (Fig. 3E). Taken
together, these results indicated that SOX2 increased the migration
and proliferation of iSCs.
SOX2 upregulates FN1 expression to
promote cell migration and viability in iSCs
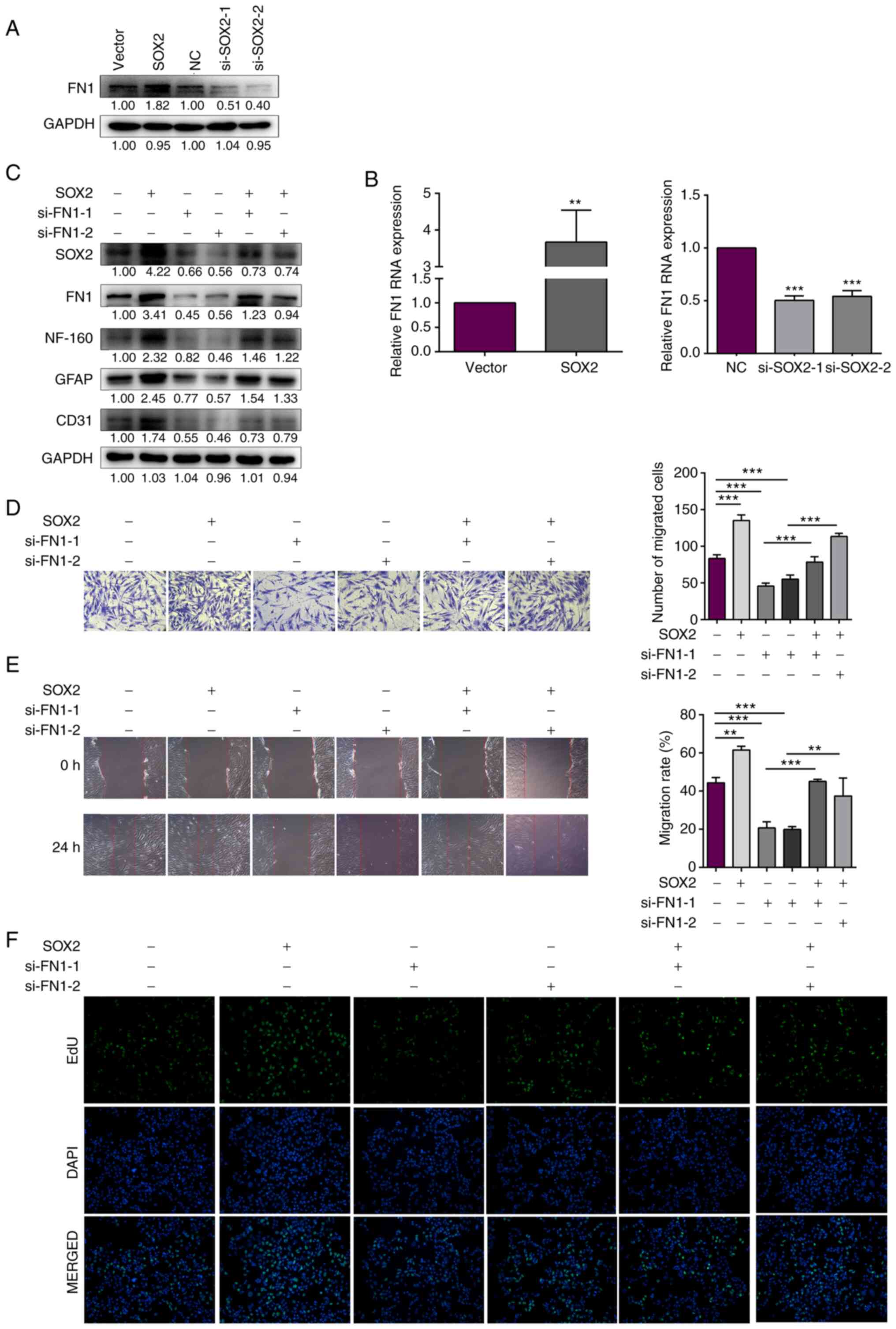
SOX2 was overexpressed and knocked down using the
SOX2 overexpression plasmid and si-SOX2 in the iSCs, respectively.
FN1 mRNA and protein expression levels were increased following
SOX2 overexpression, but decreased following SOX2 knockdown
(Fig. 4A and B). Therefore, the
results indicated that FN1 expression may be regulated by SOX2. FN1
was knocked down in SOX2-overexpressing iSCs. The protein
expression levels of NF-160, GFAP and CD31 were detected using
western blotting. The protein expression levels of NF-160, GFAP and
CD31 were decreased after FN1 knockdown in SOX2-overexpression
iSCs. The result indicated that FN1 inhibited SOX2
overexpression-mediated improvements in nerve cell viability
(Fig. 4C). In addition, cell
migration was measured by performing Transwell assays. The number
of migratory cells was significantly increased with SOX2
overexpression, whereas the number was significantly decreased with
si-SOX2 transfection. Compared with the SOX2 overexpression group,
the number of migratory cells was decreased in the
SOX2-overexpression iSCs with FN1 interference (Fig. 4D). Furthermore, scratch wound
assays were performed to detected the migratory ability of
SOX2-overexpression iSCs with FN1 interference. The results were
consistent with the aforementioned results, in which FN1
interference inhibited SOX2 overexpression-mediated improvements in
the migration ability of iSCs (Fig.
4E). Cell proliferation was measured by performing EdU assays
under the same treatment. As presented in Fig. 4F, cell proliferation was elevated
by SOX2 overexpression. However, FN1 interference decreased SOX2
overexpression-mediated improvements in iSC proliferation. Taken
together, these results indicated that SOX2 upregulated FN1
expression to promote iSC migration and viability.
Exosomes secreted by iSCs promote cell
migration and viability
Exosomes were isolated from iSCs using
ultracentrifugation. Next, the effect of the exosomes isolated from
iSCs on SC proliferation and migration was investigated. From
Genecards database (https://www.genecards.org/), we know that CD81 and
CD63 are located not only in the plasma membrane, but also in other
organelles, such as the endosome, cytosol, and lysosome. Western
blot analysis can be used to detect CD81/CD63 in all cells.
However, flow cytometry can detect CD81/CD63 in the cell membrane
to identify the type of cell. The surface markers (CD63 and CD81)
of iSC exosomes were assessed using flow cytometry to confirm the
successful isolation of exosomes from iSCs. The percentages of CD63
and CD81 were increased from 50% to 83.5 and 86.8%, respectively
(Fig. 5A). The protein expression
levels of NF-160, GFAP and CD31 were elevated in the SCs with
exosome treatment or iSC co-culture (Fig. 5B). The exosome treatment group and
iSC co-culture group displayed decreased wound gap widths (Fig. 5C). Taken together, the results
indicated that the effect of exosomes isolated from iSCs was
similar to that of iSC co-culture on cell migration. SCs cells were
treated with si-SOX2 or si-NC alone with exosomes isolated from
iSCs, and migration ability was measured by wound scratch assay.
The functional rescue results indicated that SOX2 and exosomes
isolated from iSCs promoted migration in the SCs. In some sense,
SOX2 promotes SC migration via exosomes (Fig. 5D). In addition, the CCK-8 assay was
performed to assess the effect of exosomes isolated from iSCs on SC
viability. Exosomes secreted by iSCs significantly elevated the
viability of SCs (Fig. 5E).
Furthermore, cell proliferation was enhanced in SCs treated with
exosomes (Fig. 5F). These results
indicated that exosomes secreted by iSCs promote SC viability and
migration.
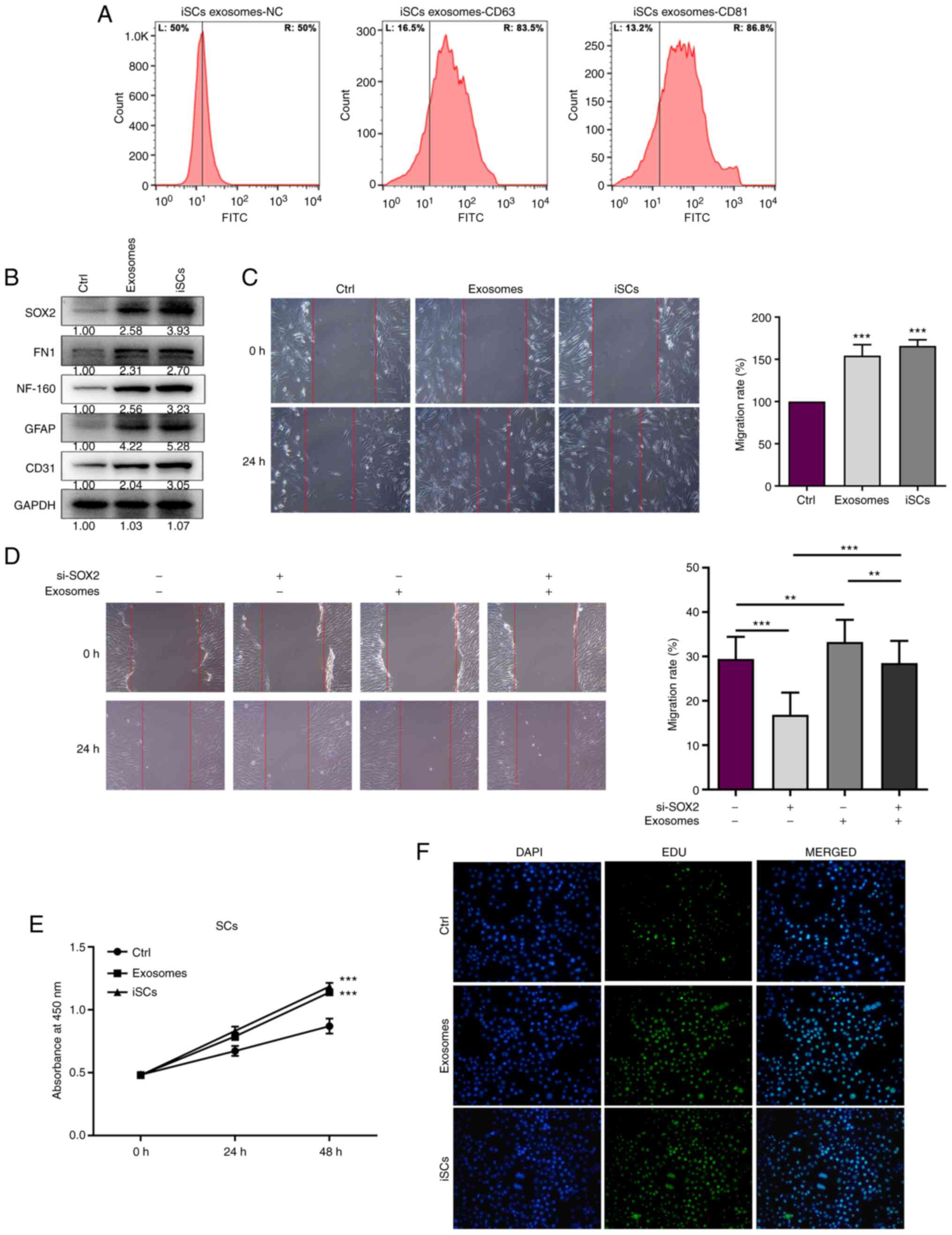 | Figure 5.The exosomes secreted by iSCs promote
the cell migration and viability ability in Schwann cells (SCs).
(A) Flow cytometry of surface markers (CD63 and CD81) on
iSC-exosomes. (B) The SOX2, FN1, NF-160, GFAP and CD31 protein
expression levels were measured in SCs treated with exosomes
secreted by iSCs or iSC co-culture for 48 h. (C) Migration ability
was measured by wound scratch Transwell assay in SCs treated with
exosomes secreted by iSCs or iSC co-culture for 48 h. (D) SCs were
treated with si-SOX2 or si-NC alone with exosomes isolated from
iSCs, and the functional rescue assay was used to detect the
migration ability. (E) Cell viability was determined by CCK-8 assay
in SCs treated with exosomes secreted by iSCs for 48 h. (F) Cell
proliferation was determined by EdU staining in SCs treated with
exosomes secreted by iSCs for 48 h. All experiments were conducted
three times, and data represent the mean ± SD. **P<0.01,
***P<0.001, compared to the control (Ctrl) or as indicated with
brackets. iSCs, Swann cell-like cells; FN1, fibronectin 1; NF-160,
neurofilament 160; GFAP, glial fibrillary acidic protein. |
Discussion
In the present study, the rat sciatic nerve injury
model was established to simulate clinical peripheral nerve injury.
A neurotrophin 3 (NT-3) chitosan conduit filled with Swann
cell-like cell (iSC) hydrogel promoted the repair of damaged
sciatic nerve in vivo and improved the recovery of motor
function. Furthermore, SOX2 overexpression RNA-seq data were
analyzed, which indicated that SOX2 participates in iSC
proliferation and migration. Subsequently, SOX2 was overexpressed
and knocked down in iSCs to assess the effect of SOX2 on cell
viability and migration. This experiment indicated that SOX2
elevated cell viability and promoted cell migration in the iSCs.
SOX2 was also found to promote the generation of fibronectin in the
process of nerve repair. Fibronectin 1 (FN1) interference
suppressed SOX2 overexpression-induced increases in iSC viability
and migration. Exosomes were then isolated from iSCs and added to
SCs. The results indicated that exosomes isolated from iSCs
promoted cell viability and migration. Taken together, the results
demonstrated that exosomes isolated from iSCs activated the
SOX2/FN1 axis to promote SC viability and migration.
SCs are the key cells in the process of peripheral
nerve regeneration (45). Fibrous
biodegradable polymer conduits are an effective strategy for guided
nerve regeneration. Clements et al (46) reported that chitosan fibers
displayed 90% success in bridging a 12-mm gap in the sciatic nerve
after 1 month. Mohammadi et al (47) reported that silicone conduit
neurorrhaphy promoted nerve regeneration at the time of sciatic
nerve repair. In the present study, NT-3 chitosan conduits were
filled with iSC hydrogel to promote the repair of damaged sciatic
nerve. Guided by NT-3 chitosan conduits, iSCs migrated
directionally and promoted the repair of damage sciatic nerve.
Transcription factor SOX2 is involved in the
stability of the nervous system, and is necessary for the function
and maintenance of neural progenitor cells in the nervous system
(48,49). Gaete et al (50) indicated that SOX2 was upregulated
during spinal cord regeneration. Inhibition of SOX2 limited nerve
regeneration in a dose-dependent manner (50). Moreover, SOX2 loss-of-function was
found to impair adult neurogenesis (51,52).
In the present study, SOX2 increased iSC viability and migration to
promote cell regeneration after sciatic nerve injury, enhancing iSC
proliferation and migration along the NT-3 chitosan conduit.
FN1 is a ubiquitous extracellular matrix
glycoprotein. It has been reported that SOX2 positively regulates
FN1 expression, and enhances the process of epithelial-mesenchymal
transition and self-renewal capacity (53). SOX2-induced fibrillogenesis is
involved in the directional collective migration of SCs (41). FN1 is the key molecule involved in
integrin-mediated cell extracellular matrix adhesion, which is
required for SC migration and axonal regrowth (54,55).
SOX2 overexpression resulted in increased FN1 expression, which
promoted iSC proliferation and migration, suggesting that sciatic
nerve repair was accelerated via the SOX2/FN1 axis. However, the
absence of images showing the morphology of the damaged sciatic
nerve before and after repair is a limitation of the study. In the
follow-up study, we will confirm the repair effect of the SOX2/FN1
axis and exosomes on sciatic nerve by in vivo
experiments.
In addition, exosomes are important in the repair of
sciatic nerve and are used for the treatment of diseases,
particularly in regenerative medicine. Exosomes promote nerve cell
proliferation and migration in the repair process. Xin et al
(56) reported that
exosome-mediated transfer of miR-133b from multipotent mesenchymal
stromal cells to neural cells contributes to neurite outgrowth.
Exosomes from iSCs were found to reduce the apoptosis and promote
the proliferation of SCs in peripheral nerve injury (57). In the present study, exosomes were
isolated from iSCs and co-cultured with SCs, which resulted in
increased cell migration and viability.
Taken together, the present study demonstrated that
the NT-3 chitosan conduit filled with iSC hydrogel promoted the
repair of the damaged sciatic nerve in vivo. SOX2
participated in iSC proliferation and migration, increasing the
generation of fibronectin in the process of nerve repair. FN1
interference suppressed SOX2 overexpression-mediated increases in
iSC viability and migration. Furthermore, exosomes isolated from
iSCs promoted SC viability and migration. In conclusion, the
present study demonstrated that the SOX2/FN1 axis and exosomes
isolated from iSCs promoted sciatic nerve repair using an NT-3
chitosan conduit filled with iSC hydrogel. Therefore, the present
study may be used to identify potentially effective therapeutic
approaches for sciatic nerve repair.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural
Science Foundation of China (81760347), Science and Technology
Support Plan Fund of Guizhou Province [(2017) 2877], Guizhou
Science Cooperation Platform Talents [(2020) 5012], Fund of the
Collaborative Innovation Center jointly built by the Province and
Ministry [(2020) 39], the National Natural Science Foundation of
China (81660325), Science and Technology Department Fund of Guizhou
Province [2019 (4441)], and Adipose-Derived Stem Cell Matrigel
Foundation and Clinical Application Research Innovation Talent Team
of Zunyi City [(2018) 9] and Zunyi Medical College Master
Initiation Fund [(2015) 26].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW conceived and designed the study, wrote-review
and editing, provided funding support. WC conceived and designed
the study, performed the experiments, processed the data and wrote
the paper. SC and CY performed the experiments and processed the
data. JZ, HZ and KN contributed to the study design, processed the
data, and reviewed and edited the drafts of the paper. All authors
provided help during the research. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of the data and
any other part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The experimental procedures involving the sciatic
nerve transection and repair animal models were approved by the
Institutional Animal Care and Use Committee of Zunyi Medical
Hospital [(2017) 2-035] (China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Q, Zan T, Li H, Zhou S, Gu B, Liu K,
Xie F and Xie Y: Flap prefabrication and stem cell-assisted tissue
expansion: How we acquire a monoblock flap for full face
resurfacing. J Craniofac Surg. 25:21–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griffin JW, Hogan MV, Chhabra AB and Deal
DN: Peripheral nerve repair and reconstruction. J Bone Joint Surg
Am. 95:2144–2151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sinis N, Lamia A, Gudrun H, Schoeller T
and Werdin F: Sensory reinnervation of free flaps in reconstruction
of the breast and the upper and lower extremities. Neural Regen
Res. 7:2279–2285. 2012.PubMed/NCBI
|
|
4
|
Walczak D, Grajek M, Migacz E, Kukwa W and
Krakowczyk Ł: Preoperative tracing of lateral femoral cutaneous
nerve with sonography for sensory anterolateral thigh free flap
reconstruction. J Reconst Microsurg. 36:e3–e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan N, Tian W, Sun L, Yuan R, Tao J and
Chen D: Neural stem cell transplantation in a double-layer collagen
membrane with unequal pore sizes for spinal cord injury repair.
Neural Regen Res. 9:1014–1019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang BG, Quigley AF, Myers DE, Wallace
GG, Kapsa RM and Choong PF: Recent advances in nerve tissue
engineering. Int J Artif Organs. 37:277–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Li ZW, Luo M, Li YJ and Zhang KQ:
Biological conduits combining bone marrow mesenchymal stem cells
and extracellular matrix to treat long-segment sciatic nerve
defects. Neural Regen Res. 10:965–971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel TD, Kramer I, Kucera J, Niederkofler
V, Jessell TM, Arber S and Snider WD: Peripheral NT3 signaling is
required for ETS protein expression and central patterning of
proprioceptive sensory afferents. Neuron. 38:403–416. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petruska JC, Kitay B, Boyce VS, Kaspar BK,
Pearse DD, Gage FH and Mendell LM: Intramuscular AAV delivery of
NT-3 alters synaptic transmission to motoneurons in adult rats. Eur
J Neurosci. 32:997–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kathe C, Hutson TH, McMahon SB and Moon
LDF: Intramuscular Neurotrophin-3 normalizes low threshold spinal
reflexes, reduces spasms and improves mobility after bilateral
corticospinal tract injury in rats. ELife. 5:e181462016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keefe KM, Sheikh IS and Smith GM:
Targeting neurotrophins to specific populations of neurons: NGF,
BDNF, and NT-3 and their relevance for treatment of spinal cord
injury. Int J Mol Sci. 18:5482017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hodgetts SI and Harvey AR: Neurotrophic
factors used to treat spinal cord injury. Vitam Horm. 104:405–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sultan N, Amin LE, Zaher AR, Grawish ME
and Scheven BA: Dental pulp stem cells stimulate neuronal
differentiation of PC12 cells. Neural Regen Res. 16:1821–1828.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavorato A, Raimondo S, Boido M, Muratori
L, Durante G, Cofano F, Vincitorio F, Petrone S, Titolo P, Tartara
F, et al: Mesenchymal stem cell treatment perspectives in
peripheral nerve regeneration: Systematic review. Int J Mol Sci.
22:5722021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min Q, Parkinson DB and Dun XP: Migrating
schwann cells direct axon regeneration within the peripheral nerve
bridge. Glia. 69:235–254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santiago LY, Clavijo-Alvarez J, Brayfield
C, Rubin JP and Marra KG: Delivery of adipose-derived precursor
cells for peripheral nerve repair. Cell Transplant. 18:145–158.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu WR, Zhu Z, Liu J, Song DB, Tian H, Chen
BP, Li R and Deng LX: Interaction between Schwann cells and other
cells during repair of peripheral nerve injury. Neural Regen Res.
16:93–98. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balakrishnan A, Belfiore L, Chu TH,
Fleming T, Midha R, Biernaskie J and Schuurmans C: Insights into
the role and potential of schwann cells for peripheral nerve repair
from studies of development and injury. Front Mol Neurosc.
13:6084422021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jessen KR, Mirsky R and Lloyd AC: Schwann
cells: Development and role in nerve repair. Cold Spring Harb
Perspect Biol. 7:a0204872015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuh CMAP, Sandoval-Castellanos AM, De
Gregorio C, Contreras-Kallens P and Haycock JW: The role of schwann
cells in peripheral nerve function, injury, and repair. Cell
Engineering and Regeneration. Gimble JM, Marolt Presen D, Oreffo R,
Redl H and Wolbank S: Springer International Publishing; Cham: pp.
1–22. 2020, View Article : Google Scholar
|
|
21
|
Bolívar S, Navarro X and Udina E: Schwann
cell role in selectivity of nerve regeneration. Cells. 9:21312020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naidu M: The role of cells, neurotrophins,
extracellular matrix and cell surface molecules in peripheral nerve
regeneration. Malays J Med Sci. 16:10–14. 2009.PubMed/NCBI
|
|
23
|
Liao JY, Zhou TH, Chen BK and Liu ZX:
Schwann cells and trigeminal neuralgia. Mol Pain.
16:17448069209638092020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Yeh J, Richardson PM and Bo X:
Cell adhesion molecules of the immunoglobulin superfamily in axonal
regeneration and neural repair. Restor Neurol Neurosci. 26:81–96.
2008.PubMed/NCBI
|
|
25
|
Jafari A, Rezaei-Tavirani M,
Farhadihosseinabadi B, Zali H and Niknejad H: Human amniotic
mesenchymal stem cells to promote/suppress cancer: Two sides of the
same coin. Stem Cell Res Ther. 12:1262021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheung RC, Ng TB, Wong JH and Chan WY:
Chitosan: An update on potential biomedical and pharmaceutical
applications. Mar Drugs. 13:5156–5186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao J, Zhao C, Zhang A, Duan H, Hao P, Wei
RH, Shang J, Zhao W, Liu Z, Yu J, et al: NT3-chitosan enables de
novo regeneration and functional recovery in monkeys after spinal
cord injury. Proc Natl Acad Sci USA. 115:E5595–E5604. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Z, Zhang A, Duan H, Zhang S, Hao P,
Ye K, Sun YE and Li X: NT3-chitosan elicits robust endogenous
neurogenesis to enable functional recovery after spinal cord
injury. Proc Natl Acad Sci USA. 112:13354–13359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Georgiou M, Bunting SCJ, Davies HA,
Loughlin AJ, Golding JP and Phillips JB: Engineered neural tissue
for peripheral nerve repair. Biomaterials. 34:7335–7343. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson M, Liu SJ, Zou LN, Smith Z,
Meissner A and Ramanathan S: Pluripotency factors in embryonic stem
cells regulate differentiation into germ layers. Cell. 145:875–889.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adachi K, Suemori H, Yasuda SY, Nakatsuji
N and Kawase E: Role of SOX2 in maintaining pluripotency of human
embryonic stem cells. Genes Cells. 15:455–470. 2010.PubMed/NCBI
|
|
32
|
Efthymiou G, Radwanska A, Grapa AI,
Beghelli-de la Forest Divonne S, Grall D, Schaub S, Hattab M,
Pisano S, Poet M, Pisani DF, et al: Fibronectin extra domains tune
cellular responses and confer topographically distinct features to
fibril networks. J Cell Sci. 134:jcs2529572021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le P, Mai-Thi HN, Stoldt VR, Tran NQ and
Huynh K: Morphological dependent effect of cell-free formed
supramolecular fibronectin on cellular activities. Biol Chem.
402:155–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidt A, Liebelt G, Nießner F, von
Woedtke T and Bekeschus S: Gas plasma-spurred wound healing is
accompanied by regulation of focal adhesion, matrix remodeling, and
tissue oxygenation. Redox Biol. 38:1018092021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen W, Xiao S, Wei Z, Deng C, Nie K and
Wang D: Schwann cell-like cells derived from human amniotic
mesenchymal stem cells promote peripheral nerve regeneration
through a MicroRNA-214/c-Jun pathway. Stem Cells Int.
2019:24907612019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen W, Ji L, Wei Z, Yang C, Chang S,
Zhang Y, Nie K, Jiang L and Deng Y: miR-146a-3p suppressed the
differentiation of hAMSCs into Schwann cells via inhibiting the
expression of ERBB2. Cell Tissue Res. 384:99–112. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kimmelman J, Hyun I, Benvenisty N,
Caulfield T, Heslop HE, Murry CE, Sipp D, Studer L, Sugarman J and
Daley GQ: Policy: Global standards for stem-cell research. Nature.
533:311–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Dong L, Zhou D, Li L, Zhang W, Zhen
Y, Wang T, Su J, Chen D, Mao C and Wang X: Extracellular vesicles
from human umbilical cord mesenchymal stem cells improve nerve
regeneration after sciatic nerve transection in rats. J Cell Mol
Med. 23:2822–2835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamers FPT, Koopmans GC and Joosten EAJ:
CatWalk-assisted gait analysis in the assessment of spinal cord
injury. J Neurotrauma. 23:537–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao S, Zhang D, Liu Z, Jin W, Huang G,
Wei Z, Wang D and Deng C: Diabetes-induced glucolipotoxicity
impairs wound healing ability of adipose-derived stem cells-through
the miR-1248/CITED2/HIF-1alpha pathway. Aging (Albany NY).
12:6947–6965. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Torres-Mejía E, Trümbach D, Kleeberger C,
Dornseifer U, Orschmann T, Bäcker T, Brenke JK, Hadian K, Wurst W,
López-Schier H and Desbordes SC: Sox2 controls schwann cell
self-organization through fibronectin fibrillogenesis. Sci Rep.
10:19842020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Semina SE, Scherbakov AM, Vnukova AA,
Bagrov DV, Evtushenko EG, Safronova VM, Golovina DA, Lyubchenko LN,
Gudkova MV and Krasil'nikov MA: Exosome-mediated transfer of cancer
cell resistance to antiestrogen drugs. Molecules. 23:8292018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y,
Wu B, Wang Y and Ai K: Hepatoprotective effect of exosomes from
human-induced pluripotent stem cell-derived mesenchymal stromal
cells against hepatic ischemia-reperfusion injury in rats.
Cytotherapy. 18:1548–1559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng Y, Zhu M, Dangelmajer S, Lee YM,
Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q,
Zhang H, et al: Hypoxia-cultured human adipose-derived mesenchymal
stem cells are non-oncogenic and have enhanced viability, motility,
and tropism to brain cancer. Cell Death Dis. 5:e15672014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang Z, Powell R, Phillips JB and
Haastert-Talini K: Perspective on schwann cells derived from
induced pluripotent stem cells in peripheral nerve tissue
engineering. Cells. 9:24972020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clements BA, Bushman J, Murthy NS, Ezra M,
Pastore CM and Kohn J: Design of barrier coatings on kink-resistant
peripheral nerve conduits. J Tissue Eng. 7:20417314166294712016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohammadi R, Amini K, Abdollahi-Pirbazari
M and Yousefi A: Acetyl salicylic acid locally enhances functional
recovery after sciatic nerve transection in rat. Neurol Med Chir
(Tokyo). 53:839–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miyagi S, Masui S, Niwa H, Saito T,
Shimazaki T, Okano H, Nishimoto M, Muramatsu M, Iwama A and Okuda
A: Consequence of the loss of Sox2 in the developing brain of the
mouse. FEBS Lett. 582:2811–2815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Favaro R, Valotta M, Ferri AL, Latorre E,
Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V
and Nicolis SK: Hippocampal development and neural stem cell
maintenance require Sox2-dependent regulation of Shh. Nat Neurosci.
12:1248–1256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gaete M, Muñoz R, Sánchez N, Tampe R,
Moreno M, Contreras EG, Lee-Liu D and Larraín J: Spinal cord
regeneration in xenopus tadpoles proceeds through activation of
Sox2-positive cells. Neural Dev. 7:132012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Amador-Arjona A, Cimadamore F, Huang CT,
Wright R, Lewis S, Gage FH and Terskikh AV: SOX2 primes the
epigenetic landscape in neural precursors enabling proper gene
activation during hippocampal neurogenesis. Proc Natl Acad Sci USA.
112:E1936–1945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ferri AL, Cavallaro M, Braida D,
Cristofano AD, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala
M, DeBiasi S and Nicolis SK: Sox2 deficiency causes
neurodegeneration and impaired neurogenesis in the adult mouse
brain. Development. 131:3805–3819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hunt GC, Singh P and Schwarzbauer JE:
Endogenous production of fibronectin is required for self-renewal
of cultured mouse embryonic stem cells. Exp Cell Res.
318:1820–1831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Whitworth IH, Brown RA, Doré C, Green CJ
and Terenghi G: Orientated mats of fibronectin as a conduitmaterial
for use in peripheral nerve repair. J Hand Surg Br. 20:429–436.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mukhatyar VJ, Salmerón-Sánchez M, Rudra S,
Mukhopadaya S, Barker TH, García AJ and Bellamkonda RV: Role of
fibronectin in topographical guidance of neurite extension on
electrospun fibers. Biomaterials. 32:3958–3968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xin H, Li Y, Buller B, Katakowski M, Zhang
Y, Wang X, Shang X, Zhang ZG and Chopp M: Exosome-Mediated Transfer
of miR-133b from multipotent mesenchymal stromal cells to neural
cells contributes to neurite outgrowth. Stem Cells. 30:1556–1564.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu CY, Yin G, Sun YD, Lin YF, Xie Z,
English AW, Li QF and Lin HD: Effect of exosomes from
adipose-derived stem cells on the apoptosis of Schwann cells in
peripheral nerve injury. CNS Neurosci Ther. 26:189–196. 2020.
View Article : Google Scholar : PubMed/NCBI
|