Introduction
Kidney transplantation, a common treatment for
end-stage renal disease, is one of the most common renal
replacement therapies (1).
Abnormal physiological conditions, such as the inflammation induced
by vascular anastomosis or recanalization during transplantation,
cause renal ischemia/reperfusion injury (IRI) or acute kidney
injury (AKI) (2). IRI is an
inevitable issue during organ transplantation, and it affects the
therapeutic effects of kidney transplantation in relation to
delayed graft functions and allograft dysfunction (3,4).
Although previous studies have initially focused on prevention and
treatment strategies for AKI, ~2% of hospitalized patients are
diagnosed with AKI with a mortality rate of 12.4% (5). Hence, it is fundamental to
understand the pathogenesis and explore novel treatment strategies
for AKI.
MicroRNAs (miRNAs/miRs) are critical regulators of
their target mRNAs by complementarily binding to mediate or
transcript repression (6).
miRNAs have recently been implicated in human renal diseases. In
cancer cells, PinX1 has been found to activate cancer-related
miR-125-3p, to cooperate with the VEGF signaling pathway and
inhibit angiogenesis in renal cell carcinoma (7). miRNAs are also crucial diagnostic
biomarkers for renal diseases and the interaction between miRNAs
and downstream target genes results in targeted therapies for renal
fibrosis (8) and AKI (9). For instance, the expression of
miR-21 has been shown to be upregulated by kidney IRI in
vitro and to contribute to tubular epithelial cell apoptosis
and local inflammation (10). He
et al (11) demonstrated
that the expression of miR-328 was significantly decreased in
TGF-β1-induced renal fibrogenesis and the overexpression of miR-328
decreased its target gene transcription and the cell
epithelial-mesenchymal transition process. However, neither the
effect of miR-328 on inflammatory response, nor the interaction
with other non-coding RNAs to modulate mRNA transcription in AKI or
IRI have been investigated to date, at least to the best of our
knowledge.
CircularRNAs (circRNAs) are endogenous non-coding
RNAs that are involved in the interaction between miRNAs and mRNAs
in the 3′untranslated region (3′UTR) region (12). With advancements being made in
next-generation sequencing technology and bioinformatics, specific
circRNA signatures have been identified in several human diseases
(13). In a previous study, the
absence of hsa_circ_0068888 in a cell model of
lipopolysaccharide-induced AKI suggested that the overexpression of
circ_0068888 may be involved in the inflammatory response and
oxidative stress via sponging miR-21-5p (14). Kölling et al (15) found that circRNA-126 expression
in patients with AKI was a putative predictor of the survival rate.
However, there are only a few circRNAs whose functions have been
fully elucidated to date.
The present study analyzed RNA-sequencing data and
identified a cohort of miRNAs that are aberrantly expressed in AKI
in a rat model. miR-328-3p was further validated as a downregulated
miRNA in a cell model of AKI; miR-328-3p expression was decreased
by circRNA integrin beta 1 (circITGB1, circbase ID:
hsa_circ_0018148). In addition, experiments performed for the
silencing of circITGB1 and miR-328-3p in human kidney-2 (HK-2)
cells subjected to hypoxia/reperfusion indicated that the silencing
of circITGB1 upregulated the expression level of miR-328-3p and
decreased Pim-1 proto-oncogene (PIM1) expression. The
downregulation of circITGB1 exerted an anti-inflammatory effect in
AKI. Moreover, it was observed that the gene symbol of circITGB1,
ITGB1, was subjected to regulation by the transcription factor GATA
binding protein 1 (GATA1). Hence, therapeutic approaches aimed at
targeting GATA1/circITGB1/miR-328-3p/PIM1 may prove to be
beneficial for AKI prevention and therapy.
Materials and methods
Cell culture and H/R treatment
The HK-2 cell line (CRL-2190, ATCC) was grown in
complete growth medium, keratinocyte serum-free medium (K-SFM,
17005-042, Gibco; Thermo Fisher Scientific, Inc.) supplemented with
0.05 mg/ml bovine pituitary extract and 5 ng/ml epidermal growth
factor. The HK-2 cells were incubated at 37°C in a suitable
incubator containing a 5% CO2 in air atmosphere. To
induce H/R, the HK-2 cells were treated with K-SFM medium and
subjected to 12 h of hypoxia (5% CO2, 1% O2
and 94% N2), followed by cultivation in complete medium
for 0/3/6/9/12 h of reoxygenation (5% CO2, 21%
O2 and 74% N2).
Reverse transcription-quantitative
(RT-qPCR)
The expression of mRNAs, circRNAs and miRNAs was
analyzed following the cell treatment or transfection. Total RNA
was isolated from the HK-2 cells using Trizol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1.5
μg) was used for cDNA synthesis at 42°C with SuperScript™ IV
revertase (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR
reactions were performed with SYBR®-Green Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) for mRNA and circRNA
expression analysis, with the TaqMan™ MicroRNA Assay kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) for miRNA expression
analysis. The specific primer pairs of ITGB1 (NCBI Reference
Sequence NM_002211), PIM1 (NM_002648), neurofibromin 2 (merlin)
(NF-2; NM_181833), GAPDH (NM_001357943), GATA1 (NM_0020nf-49) and
ubiquitin protein ligase E3 component n-recognin 4 (UBR4;
NM_020765.3) were designed using NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome).
The stem-loop primer of miR-328-3p (miRbase ID: MIMAT0000752) was
designed using the Primer3 (https://primer3.ut.ee/) online tool. In addition,
Primer3 was utilized to design the divergent primers of circITGB1
(CircBank ID hsa_circ_0018148), circARL6IP1 (CircBank ID
hsa_circ_0038229) and circH-NRNPF (CircBank ID hsa_circ_0018263).
After obtaining the divergent primer sets as aforementioned,
CircPrimer 2.0 software (downloaded from https://www.bio-inf.cn/; DOI:
10.1186/s12859-018-2304-1) was utilized to examine the specificity
of the circRNA primers. The lists of primers for each mRNA, circRNA
and miRNA are presented in Table
I. Relative expression was analyzed using the 2−ΔΔCq
method (16) and normalization
with internal controls (GAPDH or U6). As for RNase R treatment,
total RNA (2 μg per group) of HK-2 cells were incubated with
RNase R (a final concentration of 10 μg/ml) for 3 min at
37°C (Beijing Solarbio Science & Technology Co., Ltd.). RT-qPCR
was performed to determine the expression levels of ITGB1 mRNA and
circITGB1. To identify the circular feature of circITGB1, Oligo(dT)
18 primers (Beijing Solarbio Science & Technology Co., Ltd.)
and random primers (Beijing Solarbio Science & Technology Co.,
Ltd.) were employed to perform cDNA synthesis, respectively. The
resulting cDNAs were then detected by qPCR using the primers
targeting circITGB1 or ITGB1, respectively.
 | Table ISequences of primers used in the
present study. |
Table I
Sequences of primers used in the
present study.
| Gene | Primer sequence
(5′→3′) |
|---|
|
hsa_circ_0038229-F |
TTTGTTTTGTGTAAAAGGGGAAA |
|
hsa_circ_0038229-R |
GGTTGGTGCTGCGATTATCT |
|
hsa_circ_0018263-F |
CACATCTGTTGATAGCTGGAGAA |
|
hsa_circ_0018263-R |
GAGCTAAGGCTACGGGAACC |
| circITGB1-F |
GGATGTTGACGACTGTTGGTT |
| circITGB1-R |
CAATTTGGCCCTGCTTGTAT |
| UBR4-F |
TGTCAACAATGGCAACCCCT |
| UBR4-R |
GCTGATGCTGTGGTGTCAGA |
| ITGB1-F |
GACGCCGCGCGGAAAA |
| ITGB1-R |
ACATCGTGCAGAAGTAGGCA |
| PIM1-F |
CCCGACAGTTTCGTCCTGAT |
| PIM1-R |
ACCCGAAGTCGATGAGCTTG |
| NF-2-F |
GGGCTAAAGGGCTCAGAGTG |
| NF-2-R |
GAGTCCGGCACACCAAATCA |
| GATA1-F |
GGACAGGCCACTACCTATGC |
| GATA1-R |
CTTTTCCAGATGCCTTGCGG |
| GAPDH-F |
CTGGGCTACACTGAGCACC |
| GAPDH-R |
AAGTGGTCGTTGAGGGCAATG |
| miR-328-3p-RT |
CTCAACTGGTGTCGTGGAGTCG |
|
GCAATTCAGTTGAGACGGAAGG |
| miR-328-3p-F |
ACACTCCAGCTGGGCTGGCCCT |
| CTCTGCCC |
| U6-RT |
GTCGTATCCAGTGCGTGTCGTG |
|
GAGTCGGCAATTGCACTGGAT |
|
ACGACAAAATATGGAAC |
| U6-F |
TGCGGGTGCTCGCTTCGGCAGC |
| Universal
reverse | TATCC
AGTGCGTGTCGTG |
| ITGB1
promoter-F |
GCACGGACCAATTATGCTTT |
| ITGB1
promoter-R |
CTAGGAGGAGGCGGAGGAT |
| ITGB1 promoter
site1-F | AGACTGGGCC
AATTCTGATG |
| ITGB1 promoter
site1-R | GCCAATCTGAAGACC
TCTAGGA |
| ITGB1 promoter
site2-F |
TCAAGTTTTGATTTTGCTGGT |
| ITGB1 promoter
site2-R | GGACTGGAATGCCC
ATTTG |
| ITGB1 promoter
site3-F | GGGCATTCC AGTCCC
TAATC |
| ITGB1 promoter
site3-R | GGCAAGCTGCTATCC
TGGTA |
| ITGB1 promoter
site4-F |
CAATCATCGAATCGACATGC |
| ITGB1 promoter
site4-R | AGTTGCGTCC
TGCTTTTGAT |
Cell transfection
The knockdown of circITGB1, PIM1 and GATA1 was
achieved by transfecting the HK-2 cells with small interfering RNAs
(siRNAs). siRNAs targeting PIM1 (NM_002648), circITGB1 (circbase
ID: hsa_circ_0018148) and GATA1 (NM_002049) were designated as
siPIM1, si-circITGB1 and si-GATA1, respectively. siPIM1 and
si-GATA1 was designed using BLOCK-iT™ RNAi Online Designer
(http://rnaidesigner.thermofisher.com/rnaiex-press/design.do).
In addition, the Circinteractome online database (https://circinteractome.irp.nia.nih.gov/index.html)
(17) was utilized to design the
siRNA specific to circITGB1. All siRNA sequences were synthesized
by Shanghai Sango Biotechnology Co., Ltd. and were as follows:
siPIM1 sense, 5′-CGGGUUUCUCCGGCGUCAUUAGGdTdT-3′ and antisense,
5′-CCUAAUGACGCCGGAGAAACCCGdTdT-3′; si-circITGB1 sense,
5′-GUGGAGAAUCCAGAUGAAAAUdTdT-3′ and antisense,
5′-AUUUUCAUCUGGAUUCUCCACdTd T-3′; si-GATA1 sense,
5′-CCCAGUCUUUCAGGUGUACCCAUUGdTdT-3′ and antisense,
5′-CAAUGGGUACACCUGAAAGACUGGGdTdT-3′. The miR-328-3p mimics,
inhibitors and negative controls were obtained from Guangzhou
RiboBio Co., Ltd. and the sequences were as follows: miR-328-3p
mimic (miR10000752-1-5) sense, 5′-CUGGCCCUCUCUGCCCUUCCGU-3′ and
antisense, 5′-GGAAGGGCAGAGAGGGCCAGUU-3′; NC mimic
(miR1N0000001-1-5) sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
antisense, 5′-ACGUGAACAGUUCGGAGAATT-3′; miR-328-3p inhibitor
(miR20000752-1-5) sense, 5′-ACGGAAGGGCAGAGAGGGCCAG-3′; NC inhibitor
(miR2N0000001-1-5), 5′-CAGUACUUUUGUGUAGUACAA-3′. The GATA1 coding
sequence fragment (~1.4 kb) was amplified with a primer set
(forward, 5′-ACTGAGCTTGCCACATCCCC-3′ and reverse,
5′-GGGCCCAGAGACTTGGGTTG-3′) and inserted into the pcDNA3.1 vector
(Invitrogen; Thermo Fisher Scientific, Inc.) between the
BamH1 and XhoI sites. The plasmid construct was
transformed in E. coli, and selected colonies were then
cultured. Plasmid DNA was purified using the Plasmid Maxi
Preparation kit (Beyotime Institute of Biotechnology). To perform
cell transfection, the HK-2 cells were plated in 96-well plates and
incubated for 24 h at 37°C. Each 1 μg of plasmids (or 50 nM
miRNA mimic or 100 nM miRNA inhibitor) were transfected into the
HK-2 cells using Lipofectamine™ 3000® Transfection
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h,
the HK-2 cells were subjected to H/R (12 h of hypoxia and 6 h of
reoxygenation). Additionally, the transfection efficiency was
examined using RT-qPCR.
Detection of cell viability
The HK-2 cells were transfected with the siRNAs or
miRNA mimic or miRNA inhibitor for 48 h as described above and then
subjected to H/R. The cells were then collected and plated in a
96-well plate (1,000 cells/well). Following cell adherence, Cell
Counting Kit-8 (CCK-8) solutions (Beijing Solarbio Science &
Technology Co., Ltd.) were added to the cells for detecting cell
viability. At 2 h following the addition of CCK-8, the absorbance
at 450 nm was measured using a microplate reader (DNM-9606, Beijing
Pulangxin technology Co., Ltd.) and cell the viability rate was
analyzed compared to the control group (100%).
Detection of cell apoptosis
The HK-2 cells were subjected to H/R as described
above. For cell apoptosis analysis, 1×106 cells were
harvested using trypsase solution (Beijing Solarbio Science &
Technology Co., Ltd.) and resuspended in binding buffer (Beijing
Solarbio Science & Technology Co., Ltd.). Following
centrifugation, the pellets were incubated with 5 μl Annexin
V-Fluorescein isothiocyanate isomer (FITC) solutions (Beijing
Solarbio Science & Technology Co., Ltd.) for 10 min and then
with with 5 μl propidium iodide (PI) solutions (Beijing
Solarbio Science & Technology Co., Ltd.). Flow cytometry was
performed using a Guava EasyCyte Mini System (Luminex, Inc.).
Detection of inflammatory cytokines
Enzyme linked immunosorbent assay (ELISA)
interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis
factor-α (TNF-α) kits (Beijing Solarbio Science & Technology
Co., Ltd.) were utilized for the quantitative determination of
concentrations in the cell culture supernatants. Firstly, 100
μl samples were added to each well and incubated for 90 min
at 37°C. The cells were then incubated with 100 μl working
solution (a component of the ELISA kits) containing
biotin-conjugated anti-human IL-1β/IL-6/TNF-α antibodies (a
component of the ELISA kits) for 60 min at 37°C. Subsequently, 100
μl streptavidin-horseradish peroxidase (HRP) working
solutions (a component of ELISA kit) were utilized for a further 30
min of incubation at 37°C. Following incubation with 100 μl
substrate solution (a component of the ELISA kits) away from light
at 37°C for 15 min, the reactions were terminated using stop
solutions (a component of the ELISA kits) and the absorbance at 450
nm was measured using a microplate reader (DNM-9606, Beijing
Pulangxin technology Co., Ltd.).
RNA-sequencing analysis
Using the keywords 'renal ischemia reperfusion' and
'microRNA', datasets were searched in the GEO database (https://www.ncbi.nlm.nih.gov/gds/). The selection
criteria were as follows: i) Datasets with non-coding RNA profiling
types; ii) datasets containing a sham operation group and a renal
IRI group; iii) datasets with reasonable biological replications.
The GSE124669 dataset contains samples from multiple time points
post-IRI. The GSE124669 dataset was used for RNA-seq analysis. In
brief, Sonoda et al (18)
constructed a bilateral IRI model. Sprague-Dawley rats (male, 10
weeks) were randomly divided into the sham operation group and IRI
group. The left and right renal vascular pedicles were subjected to
clamped operation for 25 min and then reperfused with blood. The
RNA used for sequencing was isolated from urinary exosomes at days
1, 2, 3, 7 and 14 post-IRI. The RNA samples were then sequenced
using the Illumina HiSeq 2500 platform. Raw sequence files were
obtained from the SRA (SRP175286, https://www.ncbi.nlm.nih.gov/sra). The RNA sequencing
data were obtained from Gene Expression Omnibus (GSE124669). The
reads containing adapter sequences and low-quality reads
(Phred-scale quality score <20) were removed from the raw data.
The sequence quality was assessed using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/,
released Version 0.11.9). Clean data were subjected to analysis
using the IDEG6.2 online tool (http://telethon.bio.unipd.it/bioinfo/IDEG6_form/)
(19). The differentially
expressed miRNAs with ±1.5-fold change (FC) and an adjusted P-value
<0.05 were obtained using RNA sequencing analysis. The
differentially expressed miRNA (rno-miR-328a-3p) was then subjected
to an analysis on the UCSC Genome Browser (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm
the conservation between rno-miR-328a-3p and hsa-miR-328-3p.
Prediction of miRNA-mRNA
interactions
The mRNAs targeting miR-328-3p were predicted using
three different databases: TargetScan (http://www.targetscan.org/vert_72/), Pictar
(https://pictar.mdc-berlin.de/cgi-bin/new_PicTar_mouse.cgi)
and miRDB (http://mirdb.org/). The overlapping
interactions were calculated utilizing a web tool (http://bioinformatics.psb.ugent.be/webtools/Venn/). A
graphical output in the form of venn diagram was produced to
illustrate the overlapping mRNA interactions.
Prediction of miRNA-circRNA
interactions
The binding sites between circRNAs and miR-328-3p
were predicted using StarBase v2.0 (http://starbase.sysu.edu.cn/). StarBase perform
miRNA-circRNA interactions by intersecting the predicting target
sites of miRNAs with binding sites of Ago protein, which were
derived from CLIP-seq data. The Ago clipExpNum is defined as the
number of supporting AGO CLIP-seq experiments. An additional
screening parameter (8mer class, clipExpNum strict stringency ≥5)
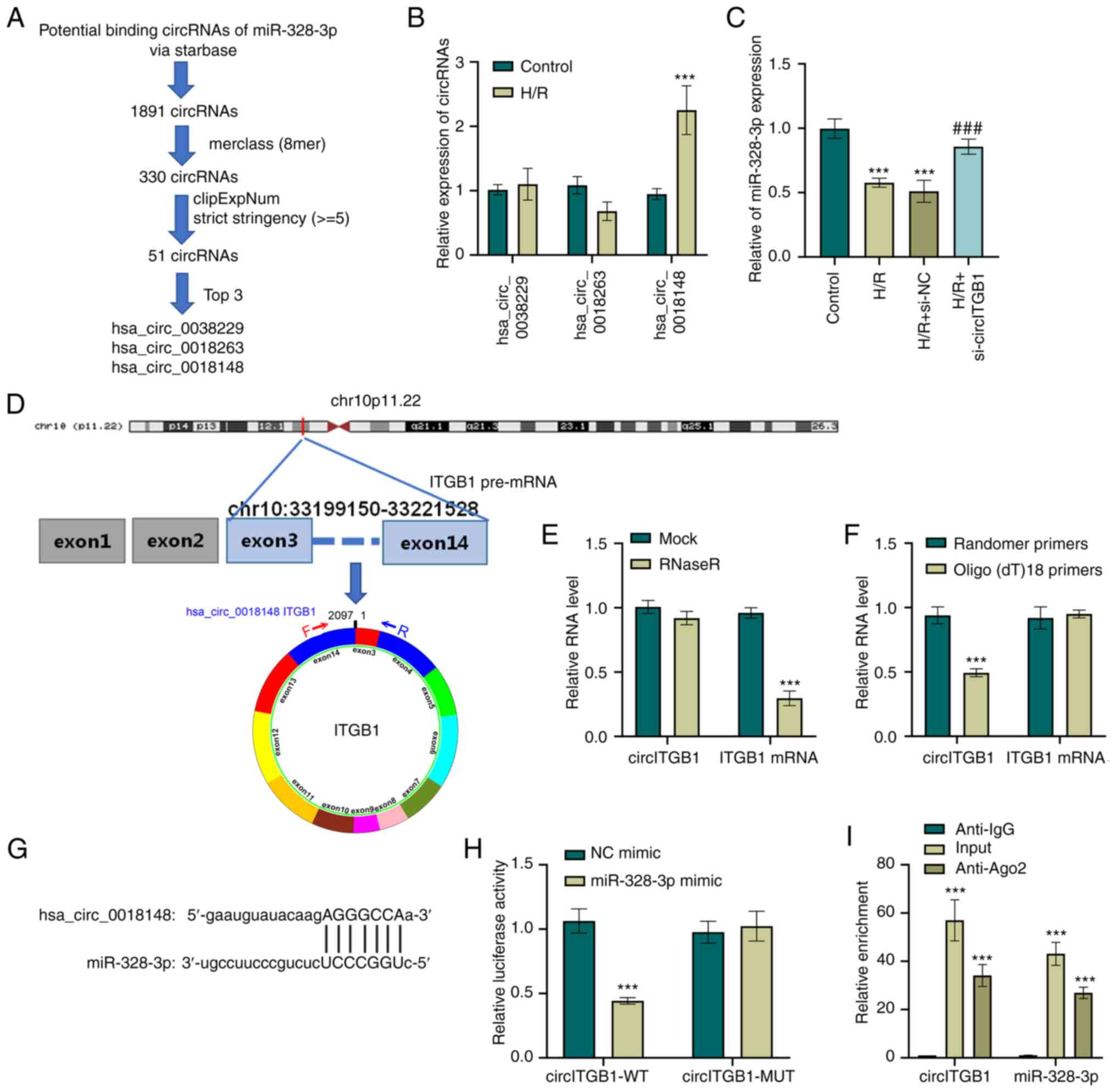
was changed to refine the results. The 51 candidate circRNAs of
miR-328-3p are listed in Table
II. The 51 candidate circRNAs were sorted using Ago clipExpNum
in descending numerical order. The top three circRNAs with a high
clipExpNum were subjected to further RT-qPCR analysis in the cells
subjected to H/R.
 | Table IICandidate target circRNAs of
miR-328-3p. |
Table II
Candidate target circRNAs of
miR-328-3p.
| AgoClip ExpNum | CircRNA | Gene symbol | AgoClip ExpNum | CircRNA | Gene symbol |
|---|
| 33 |
hsa_circ_0038229 | ARL6IP1 | 7 |
hsa_circ_0060786 | ZNFX1 |
| 24 |
hsa_circ_0018263 | HNRNPF | 6 |
hsa_circ_0011360 | TXLNA |
| 20 |
hsa_circ_0018148 | ITGB1 | 6 |
hsa_circ_0011535 | ZMYM4 |
| 17 |
hsa_circ_0067884 | PHC3 | 6 |
hsa_circ_000837 | ZMYM4 |
| 15 |
hsa_circ_0038615 | UBFD1 | 6 |
hsa_circ_0015760 | ASPM |
| 15 |
hsa_circ_0060840 | ADNP | 6 |
hsa_circ_0064170 | ARPC4 |
| 15 |
hsa_circ_0092035 | RPL10 | 6 |
hsa_circ_0064168 | ARPC4 |
| 14 |
hsa_circ_0022454 | MTA2 | 6 |
hsa_circ_0019002 | WAPAL |
| 11 |
hsa_circ_0052438 | PXDN | 6 |
hsa_circ_0024149 | CASP4 |
| 11 |
hsa_circ_0032208 | ZBTB25 | 6 |
hsa_circ_0035341 | ARPP19 |
| 10 |
hsa_circ_0076407 | KIAA0240 | 6 |
hsa_circ_0042407 | AKAP10 |
| 10 |
hsa_circ_0017865 | VIM | 6 | hsa_circ_0044502
C | OL1A1 |
| 9 |
hsa_circ_0070224 | HNRPDL | 6 |
hsa_circ_0051287 | PAFAH1B3 |
| 9 |
hsa_circ_0070499 | ADH5 | 5 |
hsa_circ_0012093 | PTPRF |
| 9 |
hsa_circ_0002819 | NEMF | 5 |
hsa_circ_0016274 | YOD1 |
| 8 |
hsa_circ_0008494 | ARID1A | 5 |
hsa_circ_0058542 | TRIP12 |
| 8 |
hsa_circ_0081562 | SERPINE1 | 5 |
hsa_circ_0074380 | NR3C1 |
| 8 |
hsa_circ_0026833 | ESYT1 | 5 |
hsa_circ_0074379 | NR3C1 |
| 8 |
hsa_circ_0026859 | ESYT1 | 5 |
hsa_circ_000420 | NR3C1 |
| 8 |
hsa_circ_0041373 | HIC1 | 5 |
hsa_circ_0085911 | PLEC |
| 8 |
hsa_circ_0090446 | TIMP1 | 5 |
hsa_circ_0085897 | PLEC |
| 7 |
hsa_circ_0015610 | SMG7 | 5 |
hsa_circ_0089684 | C9orf167 |
| 7 |
hsa_circ_0015612 | SMG7 | 5 |
hsa_circ_0025304 | PHB2 |
| 7 |
hsa_circ_0007774 | EDAR | 5 |
hsa_circ_0036012 | SMAD3 |
| 7 |
hsa_circ_0075320 | SQSTM1 | 5 |
hsa_circ_0048442 | AES |
| 7 |
hsa_circ_0006271 | GIGYF1 | | | |
RNA immunoprecipitation (RIP) assay
RIP assay was carried out to confirm the RNA-Ago2
protein interaction by utilizing the Dynabeads™ Protein A
Immunoprecipitation kit (Invitrogen; Thermo Fisher Scientific,
Inc.). Briefly, the HK-2 cells overexpressing circITGB1 or
miR-328-3p were incubated in a 25 cm2 cell culture flask
for 48 h. A total of 5×106 cells were lysed using cell
extraction buffer. Dynabeads magnetic beads were resuspended on a
roller for 5 min. Subsequently, 50 μl (1.5 mg) beads were
placed on the magnet to separate the beads from the solution.
Typically, 1 μg Anti-Ago2 (ab186733, Abcam) or IgG
(ab172730) antibodies diluted in 200 μl Ab Binding Buffer
were incubated with the beads for 10 min at room temperature. The
tube was placed on the magnet to remove the supernatant. The
magnetic bead-Ab complex was resuspended in 200 μl Ab
Binding and washing buffer (a component of the immunoprecipitation
kit). To allow the antigen (Ag) to bind to the bead-Ab complex, the
cell sample (100 μl) containing RNAs were incubated with the
bead-Ab complex in a shaker for 10 min. The tube containing the
bead-Ab-Ag complex was placed on the magnet to remove the
supernatant. The bead-Ab-Ag complex were washed with 200 μl
washing buffer three times. The bead-Ab-Ag complex was then
separated on the magnet between each wash. The resulting complex
was resuspended in 100 μl washing buffer and was transferred
to a clean tube. The tube was placed on the magnet to remove the
supernatant. A total of 20 μl elution buffer (a component of
the immunoprecipitation kit) was then incubated with the
beads-Ab-Ag complex for 2 min to dissociate the complex. The tube
was placed on the magnet. The supernatant containing eluted Ab and
Ag was transferred to a clean tube. The coprecipitated RNAs were
isolated from the supernatant by using RNeasy MinElute Cleanup kit
(Qiagen, Inc.) and reverse transcribed into cDNA using the
Prime-Script RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. Subsequently, the
mRNA levels of circITGB1 and miR-328-3p were measured using RT-qPCR
assay as described above.
Transcription factor analysis
The in silico analysis of the ITGB1 promoter
(2,000 bp upstream of the transcriptional start site) was performed
to identify the binding sites for GATA1. The sequence of the ITGB1
promoter was downloaded from NCBI (https://www.ncbi.nlm.nih.gov/). The JASPAR database
(http://jaspar.genereg.net/) provided the
frequency matrix of transcription factor GATA1 (Matrix ID:
MA0035.4). A FASTA-formatted sequence (ITGB1 promoter) was input to
scan with the GATA1 matrix model. A total of four putative sites
were predicted with a relative profile score threshold of 80%.
Luciferase reporter gene assay
Four fragments in the ITGB1 promoter were amplified
using PCR primers (Table I). The
fragments of the ITGB1 promoter were inserted into the pGL3
luciferase vector (Promega Corporation) and the resulting vectors
were named as ITGB1 promoter-luc or site1/2/3/4-WT, respectively.
The QuickMutation™Plus Site-Directed Mutagenesis kit (Beyotime
Institute of Biotechnology) was utilized to construct mutated ITGB1
promoter luciferase reporter vectors. Cells in which GATA1 was
overexpressed or silenced (1×105) were seeded in 96-well
plates and co-transfected with the luciferase plasmid (2
μl/μg) using Lipofectamine™ 3000®
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, a
luciferase reporter assay kit (Promega Corporation) was utilized
for the detection of the luciferase activities. The relative
luciferase activities were analyzed via normalization against the
Renilla luciferase values.
Western blot analysis
Cells were collected by incubation with trypsin and
lysed with radio immunoprecipitation assay (RIPA) buffer (Beijing
Solarbio Science & Technology Co., Ltd.) for 30 min. Following
centrifugation at 800 × g for 5 min at 4°C (Beckman Coulter Life
Sciences), the concentration of total protein were determined using
BCA Protein Assay kit (Beijing Solarbio Science & Technology
Co., Ltd.). The proteins were then denatured at 95°C and then
subjected to separation on 12% SDS-PAGE gels. A total of 20
μg proteins were then transferred onto polyvinylidene
fluoride (PVDF) membranes and blocked with bovine serum albumin
(BSA) buffer for 2 h at room temperature. The membranes were
incubated with primary antibodies that were diluted in BSA buffer
overnight at 4°C. The primary antibodies used were anti-PIM1
(1:2,000, ab54503), anti-NF-2 (1:10,000, ab109244) and anti-GAPDH
(1:1,000, ab8245) (all from Abcam). The PVDF membranes were then
incubated with HRP-conjugated secondary antibodies (1:1,000,
ab7090; 1:5,000, ab97080, Abcam) for 2 h at room temperature.
Finally, immunoreactive proteins were detected using enhanced
chemiluminescence (ECL) Western Substrate (Thermo Fisher
Scientific, Inc.) and visualized using a Chemiluminescence Imager
(Tanon Science & Technology).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) from three independent experiments. Statistical analysis
between multiple groups was performed with one-way analysis of
variance (ANOVA) and Bonferroni's post hoc comparisons test using
GraphPad Prism 8.0 software (GraphPad, Inc.). A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
miR-328-3p is downregulated in HK-2 cells
subjected to H/R
miRNAs are one of the most genetically expressed
non-coding RNAs in AKI (20) and
consequently potentiate or inhibit the pathogenicity of
inflammation (21). With an
objective to explore the miRNAs regulated in AKI, a RNA sequencing
dataset (GSE124669) was obtained and data analysis was performed.
miRNA expression profiles in rats were clustered based on the
treatment conditions (IR surgery group and sham group). The
statistical analysis of the RNA-sequencing data with ±1.5 FC and
adjusted P-value <0.05 generated a list that contained 36 miRNAs
that were differentially expressed in the IR surgery groups at each
time points compared with the sham groups. The heatmap displayed a
total of eight miRNAs (rno-miR-378a-3p, rno-miR-328a-3p,
rno-miR-200c-3p, rno-miR-23b-3p, rno-miR-9a-5p, rno-miR-21-5p,
rno-miR-351-5p and rno-miR-3473) that were differentially expressed
at two time points (Fig. 1A). It
was previously reported that miR-378a-3p plays a crucial role in
ferroptosis in AKI (22). The
present study focused on the second highest differentially
expressed miRNA (rno-miR-328a-3p). rno-miR-328a-3p was
downregulated in the IR group on days 1/2/3. To explore the
potential function of rno-miR-328a-3p in cell development and
regulation, a conservation analysis we performed using UCSC Genome
Browser (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The results
indicated that the precursor sequence for both rat rno-miR-328a-3p
and human hsa-miR-328-3p was highly conserved with a 94% sequence
identity and only a four nucleotide difference (Fig. 1B). In order to elucidate whether
miR-328-3p expression has any effect on cells damaged by H/R, HK-2
cells were subjected to hypoxia for 12 h and then cultured in a
normoxic incubator for 0/3/6/9/12 h. The validation of miR-328-3p
expression using RT-qPCR analysis was performed and the results
revealed a reoxygenation-dependent inhibition of miR-328-3p levels
in HK-2 cells (P<0.01 and P<0.001, Fig. 1C). The 9-h and 12-h reoxygenation
decreased the survival rate of the HK-2 cells by ~90%. The cell
amount was not sufficient to perform subsequent experiments. As the
9-h and 12-h reoxygenation may exert cytotoxic effects on HK-2
cells, the 6-h reoxygenation time point was selected for use in
further experiments. Thus, it was concluded that miR-328-3p
downregulation was induced by H/R in HK-2 cells.
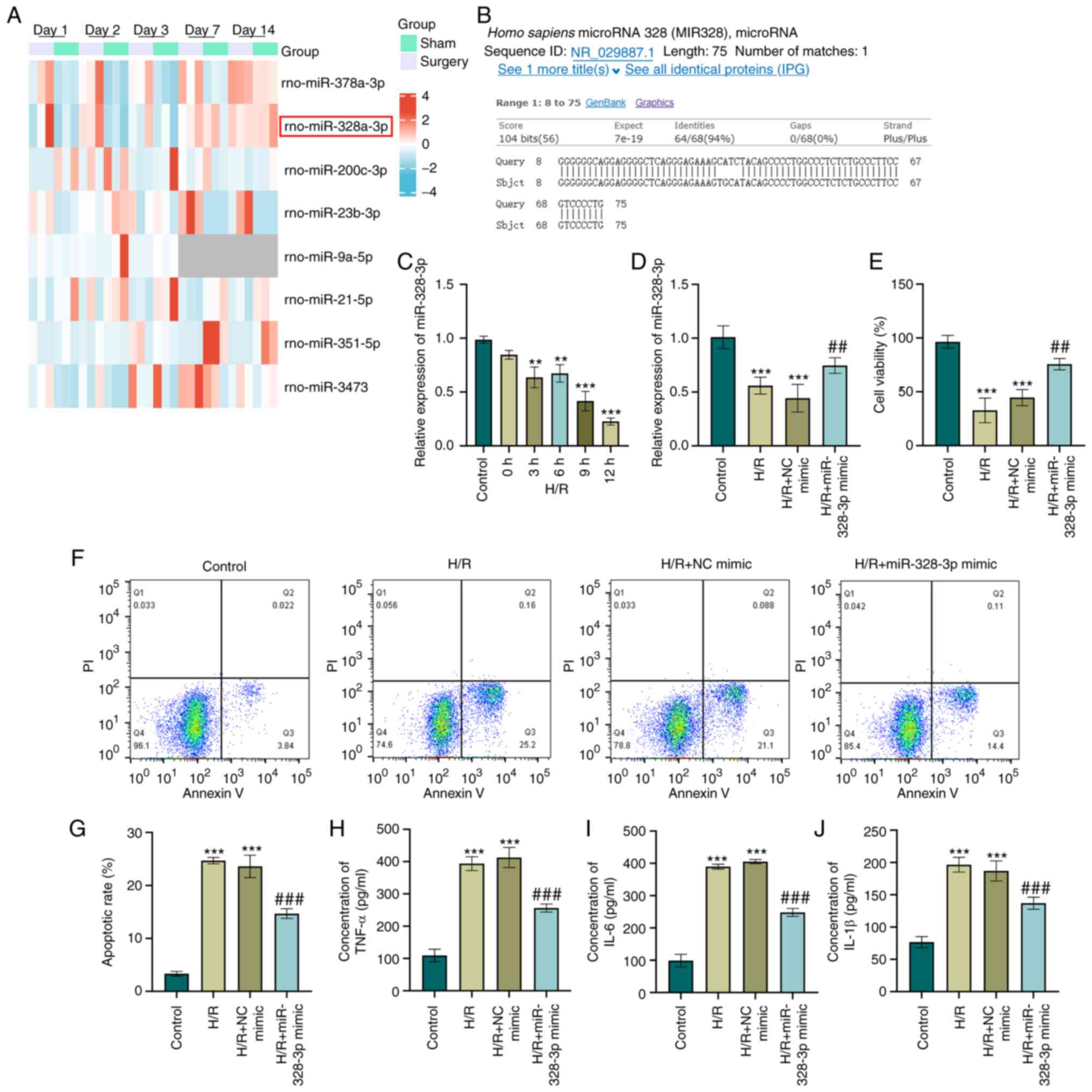 | Figure 1miR-328-3p is downregulated and
exerts anti-inflammatory effects in H/R-exposed HK-2 cells. (A)
Hierarchical clustering of the expression array data (GSE124669) in
rats subjected to ischemia/reperfusion injury and sham-operated
rat. (B) Sequence alignment between rno-miR-328a-3p and human
hsa-miR-328-3p. (C) HK-2 cells were subjected to hypoxia (12 h) and
reoxygenation for various periods of time (0, 3, 6, 9 and 12 h).
The expression level of miR-328-3p was then determined using
RT-qPCR. (D) Expression levels of miR-328-3p in HK-2 transduced
with NC mimic or miR-328-3p mimic and exposed to with H/R were
examined using RT-qPCR analysis. (E) The miR-328-3p-overexpressing
cells or NC mimic-transfected cells were seeded in a 96-well
culture dish and exposed to H/R (12 h of hypoxia and 6 h of
reoxygenation) and evaluated for cell viability using CCK-8 assay
by detecting the absorbance at 450 nm. (F and G) The
miR-328-3p-overexpressing or NC mimic-transfected cells were
exposed to H/R to stimulate acute kidney injury, followed by
staining with Annexin V or PI solutions, and examined using flow
cytometry. (H-J) ELISA of TNF-α, IL-6 and IL-1β pro-inflammatory
markers in HK-2 cells following transfection with miR-328-3p
mimic/NC mimic and treated with H/R. Data are presented as the mean
± standard deviation from three independent experiments.
**P<0.01 and ***P<0.001 vs. control
group; ##P<0.01 and ###P<0.001 vs. H/R
+ NC mimic group. RT-qPCR, reverse transcription-quantitative PCR;
H/R, hypoxia/reperfusion; IL-1β, interleukin-1β; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α. |
miR-328-3p exerts anti-inflammatory
effects in HK2 cells subjected to H/R
Since miR-328-3p was expressed at low levels
following H/R, it was overexpressed by miR-328-3p mimic
transfection in HK-2 cells and its effects on various parameters
related to renal injury were examined. The overexpression
efficiency was confirmed using RT-qPCR assay (P<0.01, Fig. S1A). As shown in Fig. 1D, mimic transfection indeed led
to a high level of miR-328-3p in the H/R-exposed cells compared
with the H/R group (P<0.01 and P<0.001). It was observed that
H/R induced a significant decrease in the viability of the HK-2
cells (P<0.001, Fig. 1E).
Notably, the re-expression of miR-328-3p significantly increased
cell viability when compared to H/R treatment alone (P<0.01,
Fig. 1E). Moreover, while H/R
treatment increased the cell apoptotic rate, the overexpression of
miR-328-3p reversed the promoting effects of H/R on HK-2 cell
apoptosis (P<0.001, Fig. 1F and
G). The concentrations of inflammation-related cytokines were
also significantly increased in the H/R group, as shown using ELISA
(P<0.001, Fig. 1H-J).
However, the overexpression of miR-328-3p inhibited the
inflammatory response triggered by H/R (P<0.001, Fig. 1H-J). These results indicated that
the pro-inflammatory effects of H/R on HK-2 cells were partly
reversed by the overexpression of miR-328-3p.
miR-328-3p targets the PIM1 gene and
abrogates the H/R-mediated inflammatory response in HK-2 cells
One of the mechanisms through which miRNAs function
in cell regulation is competitively targeting the 3′UTR region of
target genes, which leads to the loss or gain of gene function and
controls cellular biological behaviors (23). In the present study,
bioinformatics analysis integrated with some online databases
predicted a total of 13 genes targeting miR-328-3p (Fig. 2A). Furthermore, the expression
levels of the predicted targets were screened in HK-2 cells
following H/R. The results revealed that the exposure of HK-2 cells
to H/R resulted in a >1.5-fold increase in PIM1 and NF-2 levels
compared to the control group (P<0.01 and P<0.001, Fig. 2B). Subsequently, the present
study examined whether PIM1 or NF-2 expression was regulated by
miR-328-3p in H/R-e HK-2 cells. H/R led to the increased expression
of PIM1 and NF-2, as determined using RT-qPCR and western blot
analysis (P<0.001, Fig. 2C).
Moreover, PIM1 and NF-2 expression was considerably lower in the
H/R + miR-328-3p mimic group than in the H/R group (P<0.01 and
P<0.001, Fig. 2C). The
scanning of miR-328-3p for human PIM1 and NF2 gene recognition
sites, revealed an 8mer complementary binding site in the 3′UTR
region of PIM1 mRNA and 7mer-8 sequence complementary to the 3′UTR
region of NF2 mRNA, as shown in Fig.
2D. To elucidate whether miR-328-3p targets PIM1 and NF2, the
putative miR-328-3p recognition sequences of targets were cloned in
the pMIR reporter vector. Co-transfection with PIM1 3′UTR-WT and
miR-328-3p mimic in HK-2 cells significantly reduced the luciferase
activity (P<0.001, Fig. 2E).
In addition, the mutant PIM1 sequence in the putative miR-328-3p
binding sites abolished this effect (Fig. 2E). However, luciferase reporter
gene assay indicated that miR-328-3p failed to target the NF-2
3′UTR (Fig. 2F).
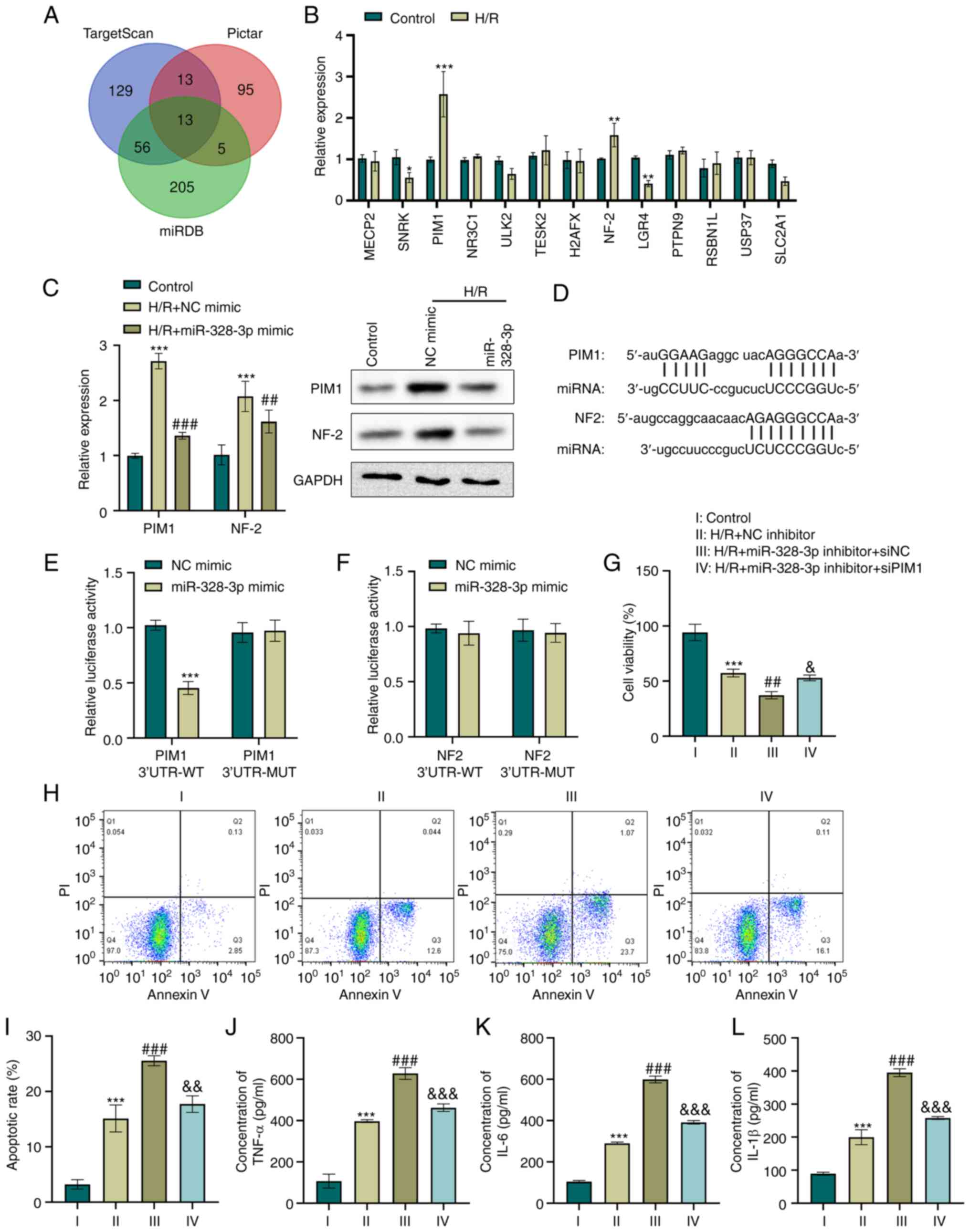 | Figure 2miR-328-3p targets the PIM1 gene and
abrogates the H/R-mediated inflammatory response in HK-2 cells. (A)
Venn diagram illustrating common targeting gene candidates of
miR-328-3p between the TargetScan, Pictar and miRDB databases. (B)
HK-2 cells were subjected to hypoxia (12 h) and reoxygenation for 6
h. The expression levels of gene candidates were then determined
using RT-qPCR. *P<0.05, **P<0.01 and
***P<0.001 vs. control group. (C) The expression
levels of PIM1 and NF-2 in HK-2 transduced with NC mimic or
miR-328-3p mimic and treated with H/R were examined using RT-qPCR
and western blot analysis. ***P<0.001 vs. control
group; ##P<0.01 and ###P<0.001 vs. H/R
+ NC mimic group. (D) Sequence alignment between miR-328-3p seed
sequence and 3′UTR sequences of targeting genes. (E and F)
Luciferase reporter gene assays were carried out with miR-328-3p
mimic or NC mimic transfection in cells co-transfected with PIM1
3′UTR-WT/MUT or NF-2 3′UTR-WT/MUT. ***P<0.001 vs. NC
mimic group. HK-2 cells were co-transfected with miR-328-3p
inhibitor/NC inhibitor and siPIM1/siNC and then treated with H/R.
(G) Cell viability was evaluated using CCK-8 assay by detecting the
absorbance at 450 nm. (H and I) Cells were stabled with Annexin V
or PI solutions, and cell apoptosis was detected using flow
cytometry. (J-L) ELISA of the TNF-α, IL-6 and IL-1β concentrations
in HK-2 cells. Data are presented as the mean ± standard deviation
from three independent experiments. ***P<0.001 vs.
control group; ##P<0.01 and ###P<0.001
vs. H/R + NC inhibitor group; &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. H/R + miR-328-3p inhibitor
+ siNC group. PIM1, pim-1 proto-oncogene; NF-2, neurofibromin 2;
RT-qPCR, reverse transcription-quantitative PCR; H/R,
hypoxia/reperfusion; WT, wild-type; MUT, mutant-type; IL-1β,
interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α. |
To examine the effects of PIM1 regulation by
miR-328-3p in H/R-exposed cells, siPIM1, miR-328-3p inhibitor and
the corresponding control plasmid were generated and transfected
into HK-2 cells. The knockdown of miR-328-3p was further confirmed
(P<0.001, Fig. S1B). RT-qPCR
analysis was also performed to confirm the inhibitory degree of
silencing PIM1 (P<0.001, Fig.
S1C). Furthermore, the viability, apoptosis and inflammatory
potential of H/R-exposed HK-2 cells were assessed. An ~20% decrease
in cell viability was observed in the cells in which miR-328-3p was
silenced, compared to the H/R-exposed cells (P<0.001, Fig. 2G). However, PIM1 knockdown in the
miR-328-3p-silencing cells resulted in a significant increase in
cell viability compared to the H/R + miR-328-3p inhibitor group
(P<0.05, Fig. 2G). Moreover,
it was observed that the re-expression of miR-328-3p induced a
significant increase in the apoptosis of H/R-exposed HK-2 cells; it
also further increased the levels of pro-inflammatory factors
(P<0.001, Fig. 2H-L). In
addition, the silencing of PIM1 reversed the cell damage triggered
by H/R and miR-328-3p knockdown in HK-2 cells (P<0.001, Fig. 2H-L). These data indicated that
PIM1 upregulation by miR-328-3p exerts pro-inflammatory effects in
H/R-exposed HK-2 cells.
circITGB1 functions as a sponge of
miR-328-3p
Since circRNAs are aberrantly regulated in human
diseases, the present study explored whether the level of
miR-328-3p in HK-2 cells could be regulated by specific circRNAs.
To this end, circRNAs that have potential binding sites to miRNAs
were first examined using StarBase (http://starbase.sysu.edu.cn/). By applying an
additional screening parameter (8mer class, clipExpNum strict
stringency ≥5), the results were narrowed down to three circRNAs
candidates that target miR-328-3p (Fig. 3A). The present study then
examined the expression levels of these circRNAs (hsa_circ_0038229,
hsa_circ_0018263 and hsa_circ_0018148) in H/R-exposed HK-2 cells
using RT-qPCR. The results revealed that H/R resulted in an
upregulation of hsa_circ_0018148 (P<0.001, Fig. 3B). To confirm the effect of
hsa_circ_0018148 (also known as circITGB1) on the miR-328-3p level
in HK-2 cells, siRNA targeting circITGB1 was transfected into the
HK-2 cells. RT-qPCR analysis was performed to confirm the
inhibitory effect of si-circITGB1 on the circITGB1 expression level
(P<0.001, Fig. S1D) and to
exclude an off-target effect of the siRNA. miR-328-3p expression
was also examined in ITGB1-silenced cells and it was found that the
H/R-exposed cells that were transfected with si-circITGB1 expressed
a higher level of miR-328-3p when compared to the cells that
exposed to H/R (P<0.001, Fig.
3C). hsa_circ_0038229 (2,097 bp sequence in length), is
generated from exons 2-14 of the ITGB1 gene located on
chr10:33199150-33221528 (Fig.
3D). It was found that circITGB1 was resistant to RNase R
digestion, while the linear mRNA of ITGB1 in HK-2 cells was
markedly decreased by RNase R treatment (P<0.001, Fig. 3E). Moreover, RT-qPCR was utilized
to detect the levels of circITGB1 and ITGB1 mRNA following reverse
transcription with random primers and oligo(dt)18 primers
(P<0.001, Fig. 3F).
Additionally, as shown in Fig.
3G, a target sequence of circITGB1 was found in miR-328-3p,
suggesting that circITGB1 directly targeted miR-328-3p. To further
confirm this, a fragment of wild-type circITGB1 containing the
putative miR-328-3p target sequence was first cloned into a
luciferase reporter vector, and it was found that miR-328-3p mimic
transfection resulted in a significant decrease in luciferase
activity (P<0.001, Fig. 3H).
However, the luciferase activity of the circITGB1-MUT + miR-328-3p
mimic group had no significant change compared to the circITGB1-MUT
+ NC mimic group. In addition, RIP assay was performed in
H/R-exposed HK-2 cells to precipitate circITGB1 and miR-328-3p with
an anti-Ago2 antibody. The data suggested that both circITGB1 and
miR-328-3p were evidently pulled down by anti-Aso2 antibody
compared with the IgG group (P<0.001, Fig. 3I). Collectively, these results
indicated that circITGB1 functioned as a sponge of miR-328-3p in
HK-2 cells.
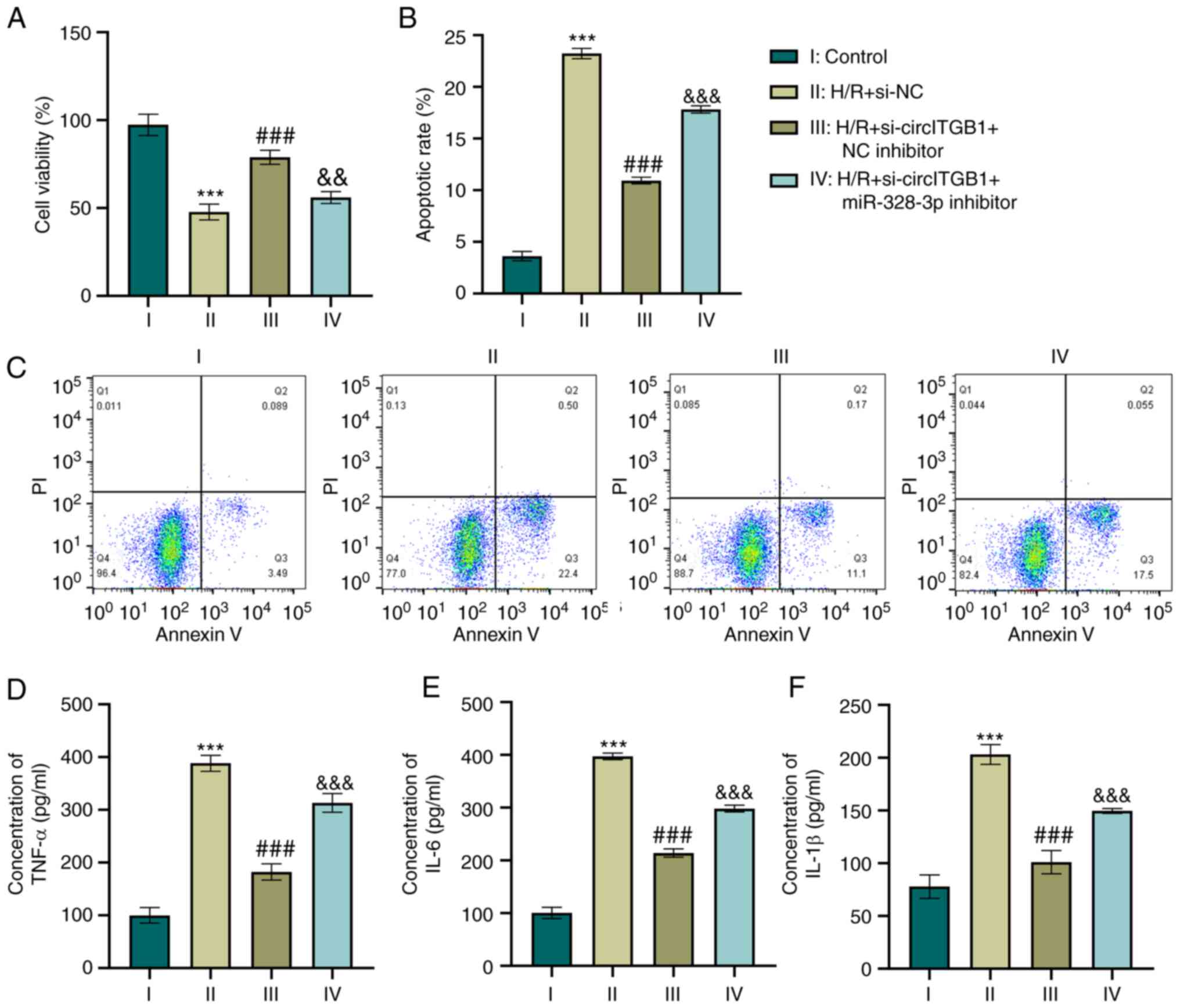
Silencing of circITGB1 reverses the
inflammatory response by increasing miR-328-3p expression in
H/R-exposed HK-2 cells
As it was found that circITGB1 functions as a
regulator of miR-328-3p expression which modulates cell
inflammation, the present study then investigated whether circITGB1
affects the inflammatory context by binding miR-328-3p. The
knockdown of circITGB1 alone increased the growth of HK-2 cells
compared to the H/R group (P<0.001, Fig. 4A). This was consistent with the
reduction of cell apoptosis in the H/R + si-circITGB1 + NC
inhibitor group (P<0.001, Fig. 4B
and C). Furthermore, the combination of si-circITGB1 and
miR-328-3p inhibitor transfection decreased cell viability compared
to the co-transfection of si-circITGB1 and NC inhibitor (P<0.01,
Fig. 4A). However, the apoptotic
percentage was increased in the H/R + si-circITGB1 + miR-328-3p
inhibitor group compared with the H/R + si-circITGB1 + NC inhibitor
group (P<0.001, Fig. 4B and
C). Moreover, the concentrations of pro-inflammatory factors
(TNF-α, IL-6 and IL-1β) were decreased following si-circITGB1
transfection compared with the H/R group (P<0.001, Fig. 4D-F). However, the addition of
miR-328-3p inhibitor reversed the inhibitory effects of
si-circITGB1 on the secretion of inflammatory factors in
H/R-exposed cells (P<0.001, Fig.
4D-F).
GATA1 is a transcription factor which
regulates circITGB1
Accumulating evidence indicates that transcription
factors can bind to the promoter of circRNAs and then regulate the
effects of circRNAs in human diseases (24,25). To investigate the mechanisms
through which circITGB1 is regulated in HK-2 cells, the JASPAR
database (http://jaspar.genereg.net) was used
to predict potential transcription factors that can bind to the
promoter of ITGB1. The predication results indicated four putative
GATA1-binding sites upstream of the transcription start site (TSS)
(−1,252~−1,242, −1,184~−1,174, −1,144~−1,134 and −913~−903 bp) in
the ITGB1 promoter (Fig. 5E).
GATA1 is a member of the GATA family of essential transcription
factors (26) and modulates gene
expression at transcriptional level by binding to specific
promoters. Firstly, the present study examined the effect of H/R on
GATA1 expression. As shown in Fig.
5A and B, H/R markedly enhanced the GATA1 mRNA and protein
expression level in HK-2 cells (P<0.001). To elucidate the
effects of GATA1 on the level of circITGB1, GATA1 overexpression
vectors were constructed and transfected into HK-2 cells. The
successful overexpression of GATA1 in HK-2 cells was confirmed
using RT-qPCR (P<0.01, Fig.
S1D). In addition, transfecting HK-2 cells with GATA1-specific
siRNA leed to a 50% decrease in the GATA1 expression level in the
si-GATA1 group compared to that in the si-NC group (P<0.01,
Fig. S1G). Subsequently, to
verify the transcriptional regulation of GATA1 on ITGB1, the
expression of circITGB1, TGB1 mRNA and pre-mRNA was examined in
GATA1-overexpressing cells. The results indicated that the
exogenous expression of GATA1 increased the expression of circITGB1
(P<0.001, Fig. 5C). Of note,
GATA1 overexpression increased the ITGB1 pre-mRNA level
(P<0.001), but did not affect the expression of ITGB1 mRNA
(Fig. 5C). To confirm the
binding of GATA1 to the ITGB1 promoter, the 1.5-kb promoter region
and the ITGB1 fragments containing potential binding sites were
cloned into a luciferase reporter vector, respectively (Fig. 5D and E). The dual-luciferase
reporter assay indicated that GATA1 overexpression increased the
ITGB1 promoter activity, whereas GATA1 knockdown decreased ITGB1
promoter activity (P<0.001, Fig.
5F). In addition, the present study further explored which site
GATA1 binds to the ITGB1 promoter. The results revealed that the
sequences of −1,252~−1,242 and −913~−903 bp upstream of the ITGB1
TSS were essential for the GATA1-mediated transcriptional
activation of the ITGB1 gene (P<0.001, Fig. 5G-N). Taken together, these
results suggested that ITGB1 is the target gene of transcription
factor GATA1.
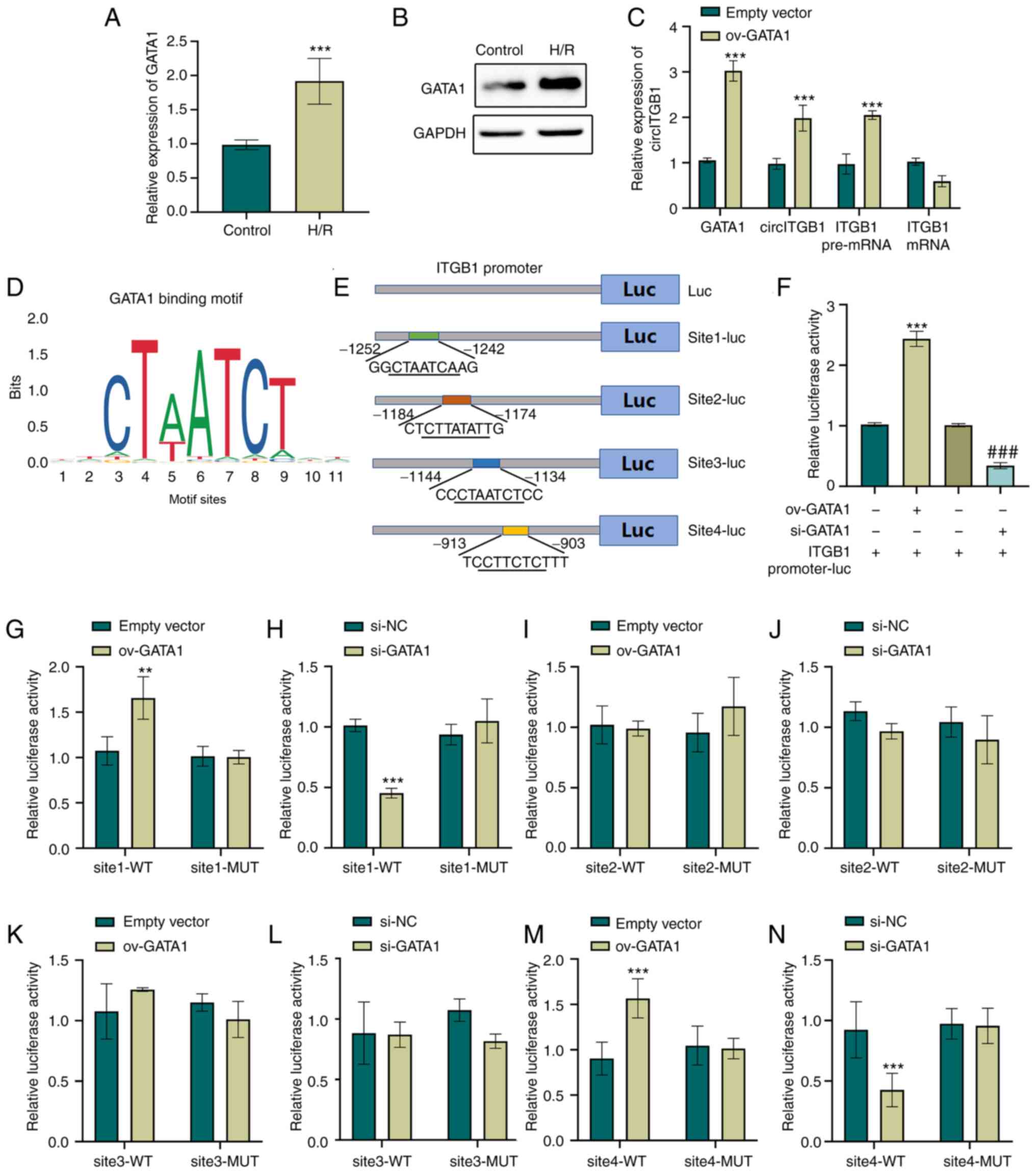 | Figure 5GATA1 is a transcription factor which
regulates circITGB1. (A and B) HK-2 cells were subjected to hypoxia
(12 h) and reoxygenation for 6 h. The expression levels of GATA1
were then determined using RT-qPCR and western blot analysis.
***P<0.001 vs. control group. (C) The expression
levels of GATA1, circITGB1, ITGB1 pre-mRNA and ITGB1 mRNA in
GATA1-overexpressing HK-2 cells were examined using RT-qPCR.
***P<0.001 vs. empty vector group. (D) JASPAR
database prediction of the four putative GATA1 binding motif in
ITGB1 promoter. (E) Schematic representation of promoter reporter
constructs termed ITGB1 promoter-luc, site1-luc, site2-luc,
site3-luc and site4-luc. (F) Relative luciferase activity was
detected in HK-2 cells co-transfected with luciferase plasmids
containing ITGB1 promoter sequences and ov-GATA1/si-GATA1.
***P<0.001 vs. ITGB1 promoter-luc group;
###P<0.001 vs. ITGB1 promoter-luc group (G-N) HK-2
cells were co-transfected with wild-type or mutant ITGB1 promoter
luciferase constructs containing GATA1 binding sites and GATA1
overexpressing vectors or si-GATA1. Luciferase reporter assay was
performed to confirm the binding sites. Data are presented as the
mean ± standard deviation from three independent experiments.
**P<0.01 and ***P<0.001 vs. empty
vector group or si-NC. GATA1, GATA binding protein 1; circITGB1,
circular RNA integrin beta 1; RT-qPCR, reverse
transcription-quantitative PCR; WT, wild-type; MUT,
mutant-type. |
Discussion
AKI is a fatal renal disease due to its late
detection and reperfusion-induced irreversible damage during kidney
transplantation surgery (27,28). Renal tubules are the first to be
damaged under hypoxic or toxic conditions and induce interstitial
inflammation and immune responses (29,30). Primary renal proximal tubule
epithelial cells (RPTECs) are valuable cell models for the study of
IRI in vitro (31).
However, it is difficult to ensure the highest viability and
plating efficiency of RPTECs during cell culture. Human tubular
epithelial cells (HK-2) are an immortalized cell line derived from
proximal convoluted tubules of normal kidneys (32). It has been reported that in
vitro IRI HK-2 cell models were induced by hypoxia in anaerobic
chambers (33). The present
study found that the overexpression of miR-328-3p reduced cell
apoptosis and the inflammatory response of H/R-exposed HK-2 cells
by targeting the PIM1 3′UTR. This result suggested that there was a
lower expression of miR-328-3p or a higher level of PIM1 following
H/R that could account for renal injury. Furthermore, it was found
that cell inflammatory injury was mediated by the overexpression of
circITGB1 and the transcription factor GATA1 may bind to the ITGB1
promoter, resulting in the expression of circITGB1.
The late detection of AKI patients is dependent not
only on the urine output criteria or serum creatinine criteria
(28), but also on the specific
exosome microenvironment (34).
With respect to this, several exosome miRNAs exert considerable
functions in the inflammasome activation and cascade. Hence, the
identification of the exosome miRNAs and the confirmation of their
role in IRI may offer specific therapeutic intervention strategies
for AKI. The present study found that exosome rno-miR-328a-3p was
downregulated in IRI datasets. Moreover, rno-miR-328a-3p is
homologous with hsa-miR-328-3p. Thus, HK-2 cells were used to
induce IRI and explore the role of miR-328-3p in the human cell
inflammatory response. miR-328 has been shown to regulate the
MEK-ERK signaling pathway, resulting in the inhibition of
myocardial IRI and apoptosis (35). The findings of the present study
suggested that the overexpression of miR-328-3p resulted in a
decrease in PIM1 expression and, therefore, played an
anti-inflammatory role in H/R-exposed HK-2 cells.
PIM1 belongs to the Ser/Thr protein kinase family
and functions as a proto-oncogene in human malignancies (36). PIM1 plays a role in the
regulation of cell proliferation and survival in breast cancer
(37), prostate cancer (38) and colorectal cancer (39). A role in hypoxia environment has
been described for PIM1, since the apoptosis of rat cardiomyocytes
was inhibited by PIM1 overexpression to enhance autophagy in spite
of oxidative stress (40).
Accordingly, the present found that PIM1 expression was upregulated
in H/R-exposed HK-2 cells, and its knockdown reversed the
pro-apoptotic and pro-inflammatory effects of miR-328-3p
inhibitor.
Non-coding RNAs, particularly circRNAs (41), have been linked to the regulation
of miRNAs. It is considered that the circRNA-miRNA axis is
effective in the regulation of human diseases, and is able to
initiate or inhibit mRNA expression and modulate biological
functions (42). Indeed, a
previous study by the authors indicated that circYAP1 sponged
miR-21-5p and prevented HK-2 cells from IRI (43). As miR-328-3p expression was
downregulated in H/R-exposed cells, it was hypothesized that
circRNAs which are overexpressed following H/R may be candidates
for sponging miR-328-3p in AKI. CircInteractome can generate 21-nt
siRNAs targeting junctional sequences spanning 5-16 nt on either
side of the junction. The 21-nt siRNAs (GUGGAGAAUCCAGAUGAAAAU) are
specific to the mature sequence of hsa_circ_0018148. This siRNA
specifically knocks down the hsa_circ_0018148 without affecting the
linear counterpart mRNA. BLAST analysis directed from
CircInteractome revealed that the siRNA sequence targets UBR4 with
16 bp complementation (siRNA nucleotides 2-17). To exclude an
off-target effect of siRNA on hsa_circ_0018148, the expression of
the UBR4 gene was detected, which was shown to exhibit significant
alignment with siRNA by BLAST analysis. RT-qPCR analysis revealed
that the siRNA did not significantly decrease the UBR4 expression
level in HK-2 cells (Fig. S1E).
A possible explanation for this may be that the potential target
UBR4 mRNA could form several complex RNA secondary structures
around the siRNA matching sites. The formed RNA secondary
structures will influence the complementary base pairing between
siRNA antisense strand and targeting sequence, thus influencing the
RNAi efficiency (44). In the
present study, it was confirmed that the knockdown of circITGB1 not
only increased the expression level of miR-328-3p, but also led to
decreased cell inflammatory properties following exposure to H/R.
Nevertheless, the upstream mechanism of circITGB1 overexpression in
AKI remains to be elucidated.
Previous studies have established that transcription
factors may be related to gene or circRNA regulation (45,46). For example, it has been shown
that transcription factor YY1 can bind to the promoter of
circ-ZNF609 and regulate the progression in cholangiocarcinoma
(47). The promoter of circHipk2
has also been found to be regulated by transcription factor Sp1 and
circHipk2 is regarded as an important candidate regulating
myogenesis (48). The
transcriptional activity of circITGB1 derives from the main
promoter region upstream of ITGB1 coding sequence, which includes
several transcription factor binding sites (46). In the present study, to determine
the upstream regulatory mechanism of circITGB1 under H/R
conditions, bioinformatics analysis indicated that the
transcription factor GATA1 could bind to four sites region on the
ITGB1 promoter sequence.
GATA1 contains two zinc finger domain, an N-terminal
and a C-terminal transactivation domain (49). Emerging evidence indicates GATA1
is activated in several tumors, such as prostate, colorectal and
renal cancer (50). However, the
functions of GATA1 in AKI and IRI have not been reported to date,
at least to the best of our knowledge. GATA1 encodes a key
hematopoietic transcription factor essential for the specification
of erythroid cells from early hematopoietic stem and progenitor
cells. A lack of GATA1 activity can be a major pathogenic mechanism
that contributes to anemia in vivo. Under hypoxic
conditions, the kidneys will produce erythropoietin (EPO), which is
carried through the blood to the bone marrow and spleen to enhance
proliferation and survival of erythroid progenitors to ablate the
hypoxic conditions, and reduce EPO production back to normal levels
(51). The transcription factor
GATA1 appears to be specifically upregulated during the course of
erythroid differentiation (52,53). In addition, GATA1 exerts a
regulatory function in promoting pro-inflammatory cytokines levels
by mediating nitric oxide synthase 2 transcription (54). Inflammation is a common
pathophysiological manifestation of clinical AKI or IRI in
vivo (55). In the present
study, the results indicated that H/R increased the expression
level of GATA1 in HK-2 cells. In further mechanistic analyses,
GATA1 was found to regulate ITGB1 transcription by binding to
specific consensus binding sites (CTAATCT) within the ITGB1
promoter. Specifically, putative binding sites for GATA1 are
located at nucleotides −1.252/−1.242 and −913/−903 upstream of the
transcription start site within ITGB1 promoter. To the best of our
knowledge, the present study was the first to demonstrate that
GATA1 contributed to the aberrant increase in the expression of
circITGB1.
It is worth noting that there were some limitations
to the present study. Firstly, in vivo IRI samples need to
be analyzed to confirm the expression levels of
circITGB1/miR-328-3p. Secondly, the distribution of circITGB1
expression in the cytoplasm or nucleus in H/R-exposed HK-2 cells
remains to be investigated in order to understand the
transcriptional regulation. Thirdly, the pathway downstream PIM1
needs to be further explored to clarify the mechanisms involved in
IRI.
In conclusion, the present study demonstrated a
novel pathway which places circITGB1/miR-328-3p/PIM1 under the
transcriptional control of GATA1 in H/R-treated HK-2 cells. The
findings presented herein confirmed the pro-inflammatory role of
circITGB1 in IRI, suggesting potential targets with the
miR-328-3p/PIM1 axis during the progression of AKI. Further studies
are required however, to evaluate the importance of this regulatory
axis in vivo.
Supplementary Data
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus (GEO)
repository (GSE124669; https://www.ncbi.nlm.nih.gov/gds).
Authors' contributions
TH conceptualized the study, provided the study
resources, supervised the study, acquired funding and confirm the
authenticity of all the raw data. YG and TH were involved in
performing experiments, data curation, as well as in preparing the
table and figures, methodology, in the writing of the original
draft, project administration and in the writing, reviewing and
editing of the manuscript. YG, WJX and CG performed the
bioinformatics analysis and were involved in the formal analysis.
YG was involved in the investigative aspects of the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Shandong Province of China (grant no.
ZR2020QH237).
Abbreviations:
|
AKI
|
acute kidney injury;
|
|
IRI
|
ischemia/reperfusion injury;
|
|
H/R
|
hypoxia/reperfusion;
|
|
circRNA
|
circularRNA;
|
|
miRNA/miR
|
microRNA;
|
|
3′UTR
|
3′untranslated region;
|
|
ITGB1
|
integrin beta 1;
|
|
PIM1
|
pim-1 proto-oncogene;
|
|
GATA1
|
GATA binding protein 1;
|
|
HK-2 cell
|
human kidney-2 cell;
|
|
K-SFM
|
keratinocyte serum free medium;
|
|
siRNA
|
small interfering RNA;
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR;
|
|
CCK-8
|
Cell Counting Kit-8;
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6;
|
|
TNF-α
|
tumor necrosis factor-α
|
|
HRP
|
horseradish peroxidase;
|
|
RIP
|
RNA immunoprecipitation;
|
|
SD
|
standard deviation;
|
|
ANOVA
|
analysis of variance;
|
|
FC
|
fold change;
|
|
TSS
|
transcription start site;
|
|
EPO
|
erythropoietin
|
References
|
1
|
Pesavento TE: Kidney transplantation in
the context of renal replacement therapy. Clin J Am Soc Nephrol.
4:2035–2039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadir S, Watson A and Burrow C:
Percutaneous transcatheter recanalization in the management of
acute renal failure due to sudden occlusion of the renal artery to
a solitary kidney. Am J Nephrol. 7:445–449. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bahl D, Haddad Z, Datoo A and Qazi YA:
Delayed graft function in kidney transplantation. Curr Opin Organ
Transplant. 24:82–86. 2019. View Article : Google Scholar
|
|
4
|
Tammaro A, Kers J, Scantlebery AML and
Florquin S: Metabolic flexibility and innate immunity in renal
ischemia reperfusion injury: The fine balance between adaptive
repair and tissue degeneration. Front Immunol. 11:13462020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang L, Xing G, Wang L, Wu Y, Li S, Xu G,
He Q, Chen J, Chen M, Liu X, et al: Acute kidney injury in China: A
cross-sectional survey. Lancet. 386:1465–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catalanotto C, Cogoni C and Zardo G:
MicroRNA in control of gene expression: An overview of nuclear
functions. Int J Mol Sci. 17:17122016. View Article : Google Scholar :
|
|
7
|
Hou P, Li H, Yong H, Chen F, Chu S, Zheng
J and Bai J: PinX1 represses renal cancer angiogenesis via the
mir-125a-3p/VEGF signaling pathway. Angiogenesis. 22:507–519. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Y, Chen H, Huang Z, Zheng H and Zhou
J: Emerging role of miRNAs in renal fibrosis. RNA Biol. 17:1–12.
2020. View Article : Google Scholar :
|
|
9
|
Ramanathan K and Padmanabhan G: miRNAs as
potential biomarker of kidney diseases: A review. Cell Biochem
Funct. 38:990–1005. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y,
Hu J, Jia P, Teng J and Ding X: miR-21 Protects against
ischemia/reperfusion-induced acute kidney injury by preventing
epithelial cell apoptosis and inhibiting dendritic cell maturation.
Front Physiol. 9:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He W, Zhuang J, Zhao ZG, Luo H and Zhang
J: miR-328 prevents renal fibrogenesis by directly targeting
TGF-β2. Bratisl Lek Listy. 119:434–440. 2018.
|
|
12
|
Shi Y, Jia X and Xu J: The new function of
circRNA: Translation. Clin Transl Oncol. 22:2162–2169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-new member of noncoding RNA with novel functions. Exp Biol Med
(Maywood). 242:1136–1141. 2017. View Article : Google Scholar
|
|
14
|
Wei W, Yao Y, Bi H, Xu W and Gao Y:
Circular RNA circ_0068,888 protects against
lipopolysaccharide-induced HK-2 cell injury via sponging
microRNA-21-5p. Biochem Biophys Res Commun. 540:1–7. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kölling M, Seeger H, Haddad G, Kistler A,
Nowak A, Faulhaber-Walter R, Kielstein J, Haller H, Fliser D,
Mueller T, et al: The circular RNA ciRs-126 predicts survival in
critically Ill patients with acute kidney injury. Kidney Int Rep.
3:1144–1152. 2018. View Article : Google Scholar :
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar :
|
|
18
|
Sonoda H, Lee BR, Park KH, Nihalani D,
Yoon JH, Ikeda M and Kwon SH: miRNA profiling of urinary exosomes
to assess the progression of acute kidney injury. Sci Rep.
9:46922019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romualdi C, Bortoluzzi S, D'Alessi F and
Danieli GA: IDEG6: A web tool for detection of differentially
expressed genes in multiple tag sampling experiments. Physiol
Genomics. 12:159–162. 2003. View Article : Google Scholar
|
|
20
|
Guo C, Dong G, Liang X and Dong Z:
Epigenetic regulation in AKI and kidney repair: Mechanisms and
therapeutic implications. Nat Rev Nephrol. 15:220–239. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan PC, Chen CC, Chen YC, Chang YS and Chu
PH: MicroRNAs in acute kidney injury. Hum Genomics. 10:292016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding C, Ding X, Zheng J, Wang B, Li Y,
Xiang H, Dou M, Qiao Y, Tian P and Xue W: miR-182-5p and
miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell
Death Dis. 11:9292020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y
and Ren J: Regulatory network of miRNA on its target: Coordination
between transcriptional and post-transcriptional regulation of gene
expression. Cell Mol Life Sci. 76:441–451. 2019. View Article : Google Scholar
|
|
24
|
Meng L, Liu S, Liu F and Sang M, Ju Y, Fan
X, Gu L, Li Z, Geng C and Sang M: ZEB1-mediated transcriptional
upregulation of circWWC3 promotes breast cancer progression through
activating ras signaling pathway. Mol Ther Nucleic Acids.
22:124–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng J, Chen S, Han JX, Qian B, Wang XR,
Zhong WL, Qin Y, Zhang H, Gao WF, Lei YY, et al: Twist1 regulates
vimentin through Cul2 circular RNA to promote EMT in hepatocellular
carcinoma. Cancer Res. 78:4150–4162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tremblay M, Sanchez-Ferras O and Bouchard
M: GATA transcription factors in development and disease.
Development. 145:dev1643842018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh DJ: A long journey for acute kidney
injury biomarkers. Ren Fail. 42:154–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koeze J, Keus F, Dieperink W, van der
Horst IC, Zijlstra JG and van Meurs M: Incidence, timing and
outcome of AKI in critically ill patients varies with the
definition used and the addition of urine output criteria. BMC
Nephrol. 18:702017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu BC, Tang TT, Lv LL and Lan HY: Renal
tubule injury: A driving force toward chronic kidney disease.
Kidney Int. 93:568–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuk A and Bonventre JV: Acute kidney
injury. Annu Rev Med. 67:293–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vormann MK, Tool LM, Ohbuchi M, Gijzen L,
van Vught R, Hankemeier T, Kiyonaga F, Kawabe T, Goto T, Fujimori
A, et al: Modelling and prevention of acute kidney injury through
ischemia and reperfusion in a combined human renal proximal
tubule/blood vessel-on-a-chip. Kidney360. 3:217–231. 2021.
View Article : Google Scholar
|
|
32
|
Ryan MJ, Johnson G, Kirk J, Fuerstenberg
SM, Zager RA and Torok-Storb B: HK-2: An immortalized proximal
tubule epithelial cell line from normal adult human kidney. Kidney
Int. 45:48–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang G, Yang Y, Huang Y, Zhang L, Ling Z,
Zhu Y, Wang F, Zou X and Chen M: Hypoxia-induced extracellular
vesicles mediate protection of remote ischemic preconditioning for
renal ischemia-reperfusion injury. Biomed Pharmacother. 90:473–478.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thongboonkerd V: Roles for exosome in
various kidney diseases and disorders. Front Pharmacol.
10:16552020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye HK, Zhang HH and Tan ZM: miR-328
inhibits cell apoptosis and improves cardiac function in rats with
myocardial ischemia-reperfusion injury through MEK-ERK signaling
pathway. Eur Rev Med Pharmacol Sci. 24:3315–3321. 2020.PubMed/NCBI
|
|
36
|
Merkel AL, Meggers E and Ocker M: PIM1
kinase as a target for cancer therapy. Expert Opin Investig Drugs.
21:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brasó-Maristany F, Filosto S, Catchpole S,
Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L,
Gazinska P, Liccardi G, et al: PIM1 kinase regulates cell death,
tumor growth and chemotherapy response in triple-negative breast
cancer. Nat Med. 22:1303–1313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Qie Y, Yang T, Wang L, Du E, Liu
Y, Xu Y, Qiao B and Zhang Z: Kinase PIM1 promotes prostate cancer
cell growth via c-Myc-RPS7-driven ribosomal stress. Carcinogenesis.
40:52–60. 2019. View Article : Google Scholar
|
|
39
|
Li Q, Chen L, Luo C, Yan Chen, Ge J, Zhu
Z, Wang K, Yu X, Lei J, Liu T, et al: TAB3 upregulates PIM1
expression by directly activating the TAK1-STAT3 complex to promote
colorectal cancer growth. Exp Cell Res. 391:1119752020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu HH, Wang XT, Sun YH, He WK, Liang JB,
Mo BH and Li L: Pim1 overexpression prevents apoptosis in
cardiomyocytes after exposure to hypoxia and oxidative stress via
upregulating cell autophagy. Cell Physiol Biochem. 49:2138–2150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang T, Cao Y, Wang H, Wang Q, Ji J, Sun
X and Dong Z: Circular RNA YAP1 acts as the sponge of
microRNA-21-5p to secure HK-2 cells from
ischaemia/reperfusion-induced injury. J Cell Mol Med. 24:4707–4715.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Overhoff M, Alken M, Far RK, Lemaitre M,
Lebleu B, Sczakiel G and Robbins I: Local RNA target structure
influences siRNA efficacy: A systematic global analysis. J Mol
Biol. 348:871–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu M, Yu B, Zhang B, Wang B, Qian D, Li H,
Ma J and Liu DX: Human cytomegalovirus infection activates glioma
activating transcription factor 5 via microRNA in a stress-induced
manner. ACS Chem Neurosci. 12:3947–3956. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu M, Li H, Xie H, Fan M, Wang J, Zhang N,
Ma J and Che S: ELF1 transcription factor enhances the progression
of glioma via ATF5 promoter. ACS Chem Neurosci. 12:1252–1261. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guan C, Liu L, Zhao Y, Zhang X, Liu G,
Wang H, Gao X, Zhong X and Jiang X: YY1 and eIF4A3 are mediators of
the cell proliferation, migration and invasion in
cholangiocarcinoma promoted by circ-ZNF609 by targeting miR-432-5p
to regulate LRRC1. Aging (Albaany NY). 13:25195–25212. 2021.
View Article : Google Scholar
|
|
48
|
Yan J, Yang Y, Fan X, Tang Y and Tang Z:
Sp1-mediated circRNA circHipk2 regulates myogenesis by targeting
ribosomal protein Rpl7. Genes (Basel). 12:6962021. View Article : Google Scholar :
|
|
49
|
Hasegawa A and Shimizu R: GATA1 activity
governed by configurations of cis-acting elements. Front Oncol.
6:2692017. View Article : Google Scholar :
|
|
50
|
Peters I, Dubrowinskaja N, Tezval H,
Kramer MW, von Klot CA, Hennenlotter J, Stenzl A, Scherer R, Kuczyk
MA and Serth J: Decreased mRNA expression of GATA1 and GATA2 is
associated with tumor aggressiveness and poor outcome in clear cell
renal cell carcinoma. Target Oncol. 10:267–275. 2015. View Article : Google Scholar
|
|
51
|
Haase VH: Regulation of erythropoiesis by
hypoxia-inducible factors. Blood Rev. 27:41–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang R and Ghaffari S: Advances in
understanding the mechanisms of erythropoiesis in homeostasis and
disease. Br J Haematol. 174:661–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ludwig LS, Gazda HT, Eng JC, Eichhorn SW,
Thiru P, Ghazvinian R, George TI, Gotlib JR, Beggs AH, Sieff CA, et
al: Altered translation of GATA1 in Diamond-Blackfan anemia. Nat
Med. 20:748–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu H, Wei SP, Zhi LQ, Liu LP, Cao TP,
Wang SZ, Chen QP and Liu D: Synovial GATA1 mediates rheumatoid
arthritis progression via transcriptional activation of NOS2
signaling. Microbiol Immunol. 62:594–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sato Y and Yanagita M: Immune cells and
inflammation in AKI to CKD progression. Am J Physiol Renal Physiol.
315:F1501–F1512. 2018. View Article : Google Scholar : PubMed/NCBI
|