1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS)
has been acknowledged to be an emerging infectious disease that is
caused by the SFTS virus (SFTSV). This new virus has been isolated
and identified in recent years. It belongs to the genus
Bandavirus in the family Phenuiviride, order
Bunyavirales (1,2). SFTSV was first isolated from a
patient in 2009 in China (1),
followed by Korea in 2010 (3), in
2013 in Japan (4), in 2017 in
Vietnam (5), in 2018 in Myanmar
(6), in 2019 in the Taiwan region
(7), in 2020 in Thailand
(8) and in Pakistan (9). In 2012, the United States isolated a
virus similar to SFTSV and named it the 'Heartland Virus' (10). The primary features of SFTS on
presentation include fever, thrombocytopenia, leukocytopenia and
gastrointestinal symptoms (1,11).
However, a minority of patients with severe forms of the disease
may rapidly succumb to the symptoms stemming from multiple-organ
failure (MOF), such as shock, respiratory failure and disseminated
intravascular coagulation (DIC) (12). The mortality rate of SFTS
currently ranges from 5-30% in East Asia (13).
According to previous studies, the tick is one of
the primary host vectors for SFTSV (14,15). In addition, human-to-human
transmission has also been found in some clusters and patients
(16,17). SFTSV infection has been reported
to be transmitted through blood contact, droplet contact (18) and aerosolized droplets (19). Consequently, exploring prevention
and treatment strategies for SFTS is becoming a severe challenge
that cannot be neglected. Supporting this, in 2017 the World Health
Organization (WHO) ranked SFTS as the disease with the highest
research priority (20).
In addition to the increasingly severe health issues
associated with SFTSV, there is currently no effective treatment
options for this virus, which contributes to the relatively high
mortality rate. The optimal strategy to protect against being
infected with SFTSV is to avoid tick bites. However, the
pathogenesis of SFTS after infection remains poorly understood,
particularly the interaction between the host immune response and
SFTSV. SFTSV-induced modulation of host immunity involves immune
cells [including dendritic cells (DCs), natural killer (NK) cells,
macrophages, T cells and B cells], immunomodulatory cytokines and
various signaling pathways [such as nuclear factor (NF)-κB and
Janus kinase (JAK)/signal transducers and activators of
transcription (STAT) signaling]. Therefore, in the present review,
recent research data regarding the host immune response to SFTSV
and the pathogenesis of SFTS were summarized. The aim of this
review was to provide a basis for the exploration of novel
therapeutic targets to assist patients in coping with this
disease.
2. Clinical manifestations and laboratory
features of SFTS
The clinical symptoms of the SFTS include fever,
anorexia, fatigue, nausea, abdominal pain or tenderness, vomiting,
malaise, diarrhea, lymphadenopathy, myalgia, confusion, headache,
throat congestion, cough, conjunctival congestion, petechiae,
slurred speech and coma (1). The
clinical manifestation of SFTS is heterogeneous, with fever and
gastrointestinal symptoms being the most common. The laboratory
findings of patients with SFTS, revealed thrombocytopenia,
leukocytopenia, proteinuria, hematuria, fecal occult blood, and
elevated levels of serum alanine aminotransferase, aspartate
aminotransferase (AST), creatine kinase, lactate dehydrogenase
(LDH), ferritin, and prolonged activated partial-thromboplastin
time (APTT) (11). The most
common laboratory abnormalities were thrombocytopenia (95%) and
leukopenia (86%) (1). Patients
with severe SFTS may succumb to this disease within 2 weeks due to
MOF (11). Advanced age, altered
mental status, high serum LDH and AST levels, prolonged APTT, and
high viral RNA loads in the blood are indicators of poor prognosis
of SFTS (12).
Hemophagocytic lymphohistiocytosis (HLH) is a an
immune-mediated life-threatening clinical syndrome accompanied by
excessive immune activation and affects multiple organ systems
(21). Patients with
unattributable, persistently high fever and signs of multiple organ
involvement should be suspected of HLH (22), as the clinical symptoms are
similar to SFTS. In fact, HLH associated with SFTS has been
reported in China (23), Japan
(24), and Korea (25). A previous study revealed that
33.3% of patients with SFTS had HLH, and the mortality rate was as
high as 75%, indicating that patients with SFTS and HLH had a
higher risk of poor prognosis (25).
3. Structure of SFTSV
SFTSV is spherical in shape with a diameter of
80-120 nm. It is a virus with a single negative strand of RNA that
contains three RNA ring segments within its genome: Small (S),
medium (M) and large (L) (1). The
S segment contains 1,744 nucleotides, which primarily encodes the
nucleocapsid protein (NP) and a nonstructural protein (NSs). The NP
can encapsulate three RNA genome fragments of SFTSV and form
ribonucleoprotein complexes with RNA-dependent RNA polymerase
(RdRp), which protects the virus from nucleases and host immune
system degradation. This suggests that the NP serves a key role in
viral transcription and replication. By contrast, the NSs forms the
main virulence factor of the SFTSV and has been previously shown to
control the host innate immune response to promote virus
replication (26). The M fragment
is comprised of 3,378 nucleotides and has one open reading frame.
It encodes the membrane precursor protein (Gp), which is then
modified by an intracellular protease into two glycoproteins, Gn
and Gc. Gn and Gc in turn mediate viral invasion, whereby Gn
promotes the early infection of SFTSV by binding to non-muscle
myosin heavy chain IIA on the cell surface (27). The L segment consists of 6,368
nucleotides and encodes RdRp, which triggers viral RNA replication
and mRNA synthesis. A previous study revealed that the relative
level of self-replication by the three fragments of
Banyangvirus is M > L > S, which occurs in the host
cell-matrix where transcription and translation take place
simultaneously (28).
4. Epidemiology of SFTSV
SFTS was first identified in China in 2009 (1). Analysis of the epidemiological
characteristics in mainland China from 2010 to 2019 yielded 13,824
patients with SFTS, including 8,899 lab-confirmed cases and 4,925
suspected cases (29). Although
the number of patients with SFTS has shown a decreasing tendency
over the past 3 years, this decline has been halted somewhat. The
majority of the patients (99.3%) were distributed across seven
provinces, namely Henan, Shandong, Anhui, Hubei, Liaoning, Zhejiang
and Jiangsu. However, the regional distribution of the SFTS cases
has expanded gradually from five provinces in 2010 to 25 in 2019,
especially in the rural areas. With 713 cases of mortality,
Zhejiang had the highest case fatality rate (CFR) of 11.5%, while
Henan had the lowest CFR of 1.3% (25). However, this data may be biased,
since according to a previous study, the CFR of Xinyang, a city in
Henan province, was found to be 16.2% (30) whereas the average annual fatality
rate was 5.2%, lower than the 2011-2017 CFR of 16.2% (13). The population with the highest CFR
tended to be those of elderly age (≥85 years old), higher viral
load, prolonged hospital admission delay, presence of diarrhea or
dyspnea, development of hemorrhagic or neurological manifestations,
elevated C-reactive protein levels, prolonged APTT and resident
diagnosis and treatment levels (13,29). Reduction in the CFR may be
associated with the optimization of diagnostic and treatment
trials, professional experience of the doctors in question and the
level of health education.
In South Korea, the first case of SFTS was
identified in 2012 through the isolation of SFTSV from a stored
blood sample collected shortly before the patient succumbed to the
disease (3). From 2013, when the
first case of SFTS was compiled, 1,373 patients have been
identified as of December 2021 (31). Since then, the annual incidence
has increased from 36 in 2013 to 81 cases in 2015, where the
overall CFR was 32.6% (32).
According to a previous retrospective study of patients with SFTS
in South Korea, between 2013-2019, the overall CFR was 11.3%
(33), which was lower compared
with that aforementioned in the previous study (32).
In Japan, the first confirmed case of SFTS was
reported at the end of 2012 (4).
At present, 537 patients have been identified between 2013 and
September 2021 (34). Kobayashi
et al (35) previously
conducted an epidemiological survey from 2013 to 2017, where 303
cases were described. Amongst this group, 133 patients (44%) were
involved in the survey and the overall CFR was found to be 27%. In
addition, a further epidemiological survey found that 64 patients
(48%) had intimate contact with their pets within 2 weeks of
disease initiation. In particular, two patients were in immediate
contact with the saliva of an infected dog or cat, suggesting that
infected animals may be the source of SFTSV infection.
5. Transmission cycle of SFTSV
Although the life cycle and continuous diffusion
mechanism of SFTSV remain unclear, SFTSV is primarily transmitted
between ticks and humans and certain animals through tick bites.
The Asian long-horned tick, Haemaphysalis longicornis (H.
longicornis), is one of the known primary vectors of SFTSV
(14,36). A previous study revealed a
prevalence of SFTSV in ticks, with an infection rate of ~11.1%
(37). In terms of transmission,
the animal-tick-human axis may be another form of propagation. A
cohort investigation from China previously demonstrated that in
domesticated animals that were naturally infected with SFTSV, sheep
(69.5%), cattle (60.4%), dogs (37.9%) and chickens (47.4%) had the
highest seroprevalence rates, whilst pigs (3.1%) had a low
prevalence rate (38). By
contrast, direct contact with SFTSV-infected cats has been reported
to result in cat-to-human transmission (39). In addition, antigens and
antibodies could be detected in wild animals, including wild deer
(40), wild boars (41), wild badgers and masked palm civets
(42). In terms of transmission
between humans, those who were in close contact with infected body
fluids, such as those with needlestick injuries and patients with
SFTS, are particularly at risk (43,44). A previous meta-analysis revealed
that from 1996 to 2019, China and South Korea published 40
publications regarding the clusters of human-to-human SFTSV
transmission, of which there were 27, containing 138 cases in total
(45). This meta-analysis also
found that the clinical severity of the secondary cases was milder
compared with that of the index cases, and the prognosis of
secondary cases was improved (45). In addition, a recent study in
Hefei, Anhui province, revealed that the overall seropositivity
rate of SFTSV antibodies among healthy residents was 20.16%
(46).
6. Innate immunity in SFTS
The host innate immune system serves as the
first-line defense to immediately prevent virus invasion and
replication. Furthermore, activation of innate immune cells is
crucial for initiating adaptive immunity. Innate immune cells can
detect viruses using pattern recognition receptors (PRRs) to
recognize pathogen-associated molecular patterns (PAMPs) and
danger-associated molecular patterns (DAMPs). Following activation,
expression of IFN-stimulated genes (ISGs) is increased to establish
an antiviral state (47).
IFNs
IFNs are puissant antiviral cytokines that induce
various antiviral responses to suppress viral replication (48). IFN secretion contributes to the
expression of ISG and promotes antiviral activity in infected cells
after the body identifies specifically conserved molecular
structures of pathogens, such as viral RNA or genomes, through
different types of PRRs (49).
The principal receptors that can serve this function include
Toll-like receptors (TLRs), retinoic acid-inducible gene I
(RIG-I)-like receptors (RLRs) and nucleoside acid-binding
oligomeric receptors (NOD-like receptors or NLRs) (50). During the recognition process of
SFTSV-infected cells, RIG-I has been reported to be the primary
viral-RNA sensor molecule, which recruits IFN-β promoter stimulator
1 [IPS-1 or mitochondrial antiviral signaling protein (MAVS)].
IPS-1 then delivers the signal to TANK-binding kinase 1 (TBK1) and
inhibitor of NF-κB kinase (IKK), which induces type I IFN secreted
by activating the phosphorylation of IFN regulatory factor (IRF)-3
and IRF-7. In addition, cytosolic PRPs which recognize viral RNA
products, including TLR3 and melanoma differentiation-associated
protein 5 (MDA5) can also participate in the recognition process,
however MDA5 is of lesser importance in this process (51,52). Nuclear scaffold attachment factor
A (SAFA) is a novel viral-RNA sensor that can recognize SFTSV RNA
by interacting with SFTSV NPs and mediate antiviral IFN and
inflammatory responses in the cytoplasm (53). In particular, type I and III IFN
can bind to its receptor and share a common signaling pathway that
ultimately activates ISG factor 3 (ISGF3). ISGF3 in turn activates
STAT1, the STAT2 heterodimer and IRF9 (54) to stimulate the JAK-STAT pathway to
induce the expression of ISGs through autocrine and paracrine
mechanisms. ISGs can be focused onto any stage of the viral life
cycle by encoding various antiviral proteins, thereby establishing
the antiviral status (52).
Clinically, SFTSV infection can lead to an acute inflammatory
response accompanied by the aberrant activation of proinflammatory
cytokines in the serum of the patient, such as IL-6, IL-10, IFN-γ,
IL-8 and monocyte chemotactic protein 1 (MCP-1) (55,56). Indeed, the increased concentration
of IFN-α has also been reported to be associated with the severity
of SFTS (Fig. 1) (57).
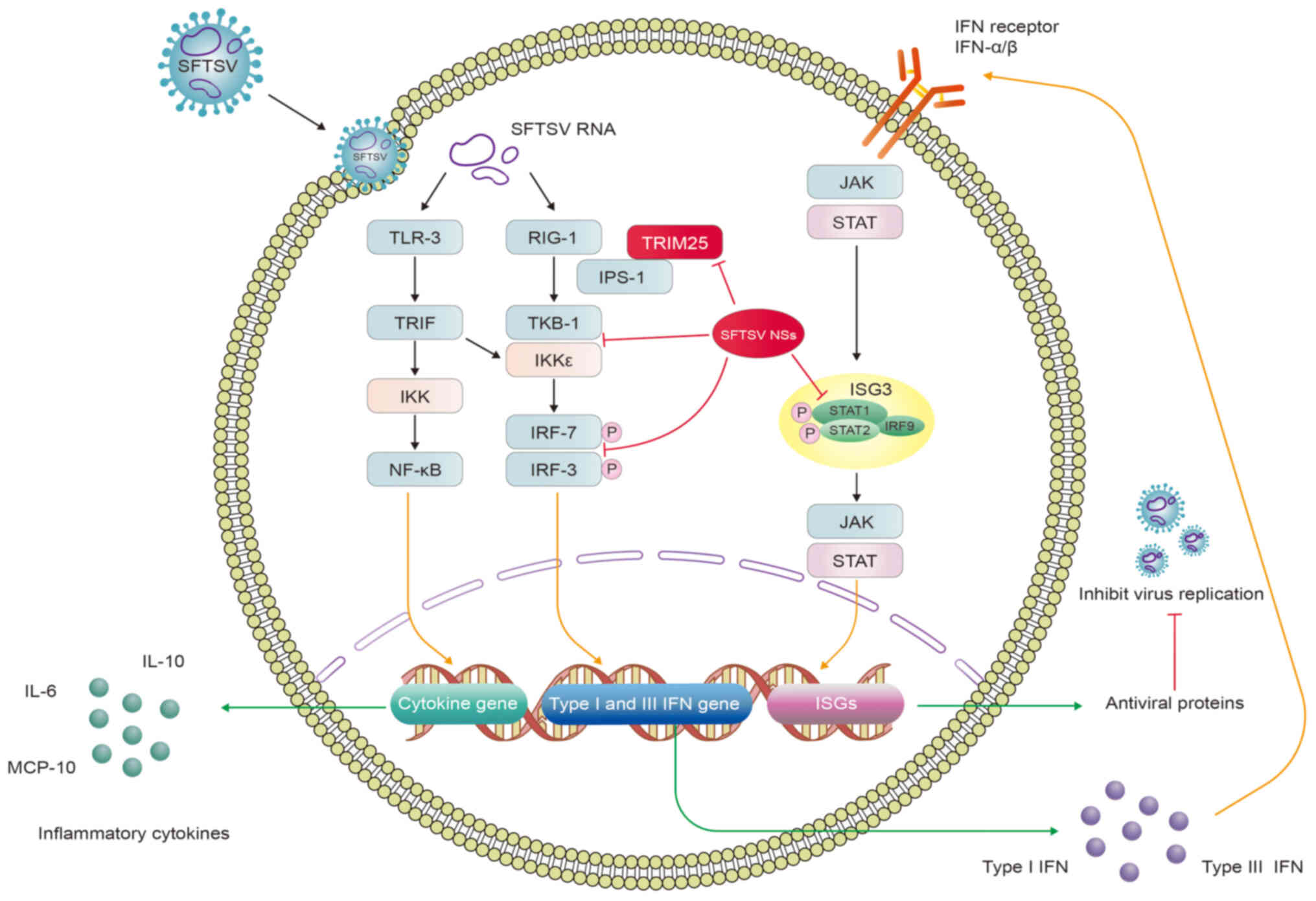 | Figure 1SFTSV activates IFN production and
cytokine storm to evade the innate immune response. SFTSV RNAs bind
to TLR-3 and RIG-1 to activate TRIF, TBK1 and IKK, which in turn
activate the NF-κB signaling pathway whilst phosphorylating IRF3
and IRF7. This induces the expression of cytokine, type I and type
III IFN genes, promoting the production of inflammatory cytokines,
type I and III IFNs. Type I and III IFNs can share the same
JAK/STAT pathway by binding to their receptors, which activate the
expression of ISGs and antiviral proteins that inhibit viral
replication. SFTSV NSs can bind several host proteins to form
inclusion bodies, such as RIG-I, TBK1, IKK, IRF3, IRF7, TRIM25,
STAT1 and STAT2, leading to severe clinical implications. Red lines
indicate inhibition. SFTSV, severe fever with thrombocytopenia
syndrome virus; NSs, nonstructural protein; TLR, Toll-like
receptor; RIG-I, retinoic acid-inducible gene I; TRIF,
TIR-domain-containing adaptor inducing interferon-β; IKK, inhibitor
of NF-κB kinase; IPS-1, IFN-β promoter stimulator 1; TBK,
TANK-binding kinase 1; IRF, IFN regulatory factor; JAK, Janus
kinase; ISGs, IFN-stimulated genes, TRIM, tripartite motif. |
During this long-term struggle with the host,
viruses have evolved various strategies to evade the innate
immunity, such as avoiding host recognition, destroying IFN signal
transduction, obstructing IFN production, regulating cell apoptosis
and autophagy. In Vero cells (African green monkey kidney cells),
SFTSV infection was found to accelerate the conversion of LC3-I to
LC3-II, but treatment with lysosomal protease inhibitors E64d and
pepstatin A had no impact on LC3-II accumulation, which avoided
autophagic degradation during the viral replication stage as a
possible mechanism (58). The
unfolded protein response (UPR) is an evolutionarily conserved
signaling system that is triggered by endoplasmic reticulum stress
and it is associated with the viral life cycle in the host. SFTSV
Gp has been found to initiate UPR, which can promote SFTSV
replication (59). By contrast,
SFTSV NSs can mediate the degradation of essential molecules in the
host immune response pathway to avoid innate immune attack, such as
RIG-I, TLR3, TNF-associated factors (TRAFs), STATs, TBK1 and MAVS
(49).
Although SFTSV may block the IFN pathway at multiple
steps, it is unclear how host cells can perceive SFTSV and trigger
immune and inflammatory cytokine responses (50). Qu et al (60) previously attempted to dissect the
host responses in monocytes and the viral immunological
pathogenesis mechanisms in humans infected with SFTSV. Since the
regulation of MAVS-mediated stimulation of IFN-β promoter activity
requires the interaction between SFTSV NSs and TBK1, NSs can bind
to TBK1 to impede the activation of IRF and NF-κB signaling
downstream (60). This suggests
that the interaction between SFTSV NSs and TBK1, which results in
the inhibition of TBK1 autophosphorylation, is necessary to inhibit
the MAVS-mediated activation of IFN-β promoter activity (61). Subsequently, a number of studies
have proposed that RIG-I, TLR3, MDA5 and MAVS can participate in
SFTSV recognition (51,62-64). SFTSV NSs mediate inhibitory
effects on antiviral IFN generation and can interact with TBK1,
IKKε and IRF3 either directly or indirectly to assist viral innate
immune evasion (65-67). SFTSV NSs, according to Min et
al (52), can also inhibit
RLR antiviral signaling by RIG-I ubiquitination and activation. In
addition, the specific interaction between NSs with the E3
ubiquitin ligase tripartite motif (TRIM)25 inhibits the
TRMI25-mediated Lys-63 ubiquitination of RIG-I and activation of
RIG-I, suppressing IFN excretion (52). Yoshikawa et al (68) previously reported that mice
deficient in the gene encoding the α chain of the α- and β-IFN
receptor (INFAR1−/− mice) and golden Syrian hamsters
deficient in the gene encoding STAT2 (STAT2−/− hamsters)
were highly susceptible to SFTSV infection (68). In addition, another study found
that SFTSV NSs can also directly interact with and sequester IRF7
into inclusion bodies (IBs), which promotes IFN-β and in
particular, IFN-α2 and -α4, to ensure effective evasion and
suppression of innate immunity (68). It has also been previously found
that NSs can sequester STAT2 into NSs-induced cytoplasmic IBs,
thereby blocking type I IFN JAK/STAT signaling to evade innate
immunity. However, NSs have minor physical and functional
interactions with STAT1 (69).
Overall, these observations suggest that SFTSV NSs are potent
inhibitors of IFN production, by binding to several host molecules
and sequester them into IBs, such as RIG-I, TBK1, IKK, IRF3, IRF7,
TRIM25, STAT1 and STAT2. This results in severe clinical
outcomes.
Cytokine storm
The 'cytokine storm' was first described in 1993
(70). Previous studies have
shown that a cytokine storm syndrome of viral origin serves an
essential role in the pathogenesis of viral infections, such as the
Ebola virus (71), severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) (72), influenza A virus (IAVs) (73), severe acute respiratory syndrome
(SARS), Middle East respiratory syndrome (MERS) (74) and SFTSV (75). The cytokine storm appears to share
common pathogenic characteristics, namely unbalanced immune
responses with an exaggerated inflammatory cytokine reaction and
T-cell depletion and functional exhaustion (70).
Although SFTS pathogenesis remains to be elucidated,
there is little doubt in the literature that the severity of SFTS
is associated with the cytokine storm (76). Cytokine storm-mediated immune
activation and damage to the organs in the body is one of the most
critical pathogenic mechanisms of SFTSV infection. Compared with
those in healthy subjects, the expression of IL-6, IL-10, IL-8,
IL-15, IL-1-RA, TNF-α, IFN-γ, IFN-γ-induced protein-10 (IP-10),
macrophage inflammatory protein-1α, heat shock protein (HSP)-70,
granulocyte colony-stimulating factors, granzyme B, MCP-1,
caspase-8, C-C motif ligand (CCL)-7, silent information regulator
2, C-X-C motif ligand 9, signal transducing adaptor molecule
binding protein, CCL20 and eukaryotic translation initiation factor
4E-binding protein 1 were markedly upregulated. Furthermore, TNF-α,
CCL20 and CX3CL1 levels were found to be highly associated with
fatality, whilst IL-6, IP-10, IFN-γ, HSP70 and TNF-α levels were
positively correlated with the viral load and severity of patients
with SFTS (12,66,77-79). IL-6 is a proinflammatory cytokine
that is essential for accelerating the cell response to limit
persistent viral infection. By contrast, IL-10 is an
anti-inflammatory cytokine, the expression of which is
significantly higher in patients with SFTS, especially in patients
with fatal disease (12). The
hypersecretion of IL-6 and IL-10 can generate a cytokine storm,
which contributes to the pathology of SFTSV infection (55).
Other cytokine/chemokines were also found to be
downregulated, such as tissue polypeptide antigen, platelet-derived
growth factor-BB, growth-related oncogene, TGF-β and regulated on
activation and normally T-cell expressed (RANTES), compared with
those in healthy controls (66,77-79). In particular, RANTES was reported
to be negatively correlated with viral load (80). However, the expression profile of
cytokines in patients with SFTS remain contested. Deng et al
(77) previously found that serum
RANTES levels were increased, whilst the level of IFN-γ was
decreased in patients with SFTS. However, another study found that
IFN-γ levels are increased in fatal cases, whereas TNF-α levels
were reduced in patients who succumbed to the disease or have
recuperated (81).
A possible reason for two families of cytokines,
such as TNF-α and IFN-γ, showing contradictory patterns in SFTS is
because cytokine variation is dependent on the experimental
protocol, such as sample size, cytokine detection time points,
severity of the SFTS and intervention method. Furthermore, the
mechanism of cytokine secretion is complex, such that the same
cytokine can be secreted by several immune cells. TNF-α can be
secreted by macrophages, DC cells and T cells, which leads to the
inconsistent results of TNF-α reported in the literature. Cytokine
secretion can also be regulated by a number of cell signaling
pathways, such as JAK/STAT3, MAPK, NF-κB, mTOR and TLR4 signaling
pathways (82). The crosstalk
among these different signals can regulate the expression of
cytokines.
However, the mechanism underlying cytokine storm
occurrence remains unclear. Previous studies have shown that NSs
can also mediate the induction of a cytokine storm after SFTSV
infection. Khalil et al (83) revealed that TBK1 can suppress the
NF-κB signaling pathway and cytokine/chemokine production through a
kinase activation-dependent manner, whilst NSs can trap TBK1 to
prevent it from inhibiting NF-κB, promoting the activation of NF-κB
and its downstream cytokine/chemokine genes (83). Another study previously showed
that NSs can induce IL-10 production through the tumor progression
locus 2-binding inhibitor of NF-κB activation 2 (ABIN2)-p105
complex (84).
Cytokines can serve an essential role in regulating
clinicopathological features. Monocytes, macrophages and T cells
can all secrete TNF-α, leading to vasodilation and the induction of
NO synthase activity, which increases endothelial permeability
(77). In vitro analyses
showed that SFTSV infection increased the vascular permeability of
endothelial cells by activating tyrosine phosphorylation and the
internalization of cadherin, the endothelial adhesion molecule that
functions as the main component of endothelial integrity
maintenance (85). These previous
findings suggest that the cytokine/chemokine-mediated inflammatory
response, which is exemplified by cytokine and chemokine expression
imbalance, serves a vital role in the progression of SFTS.
DCs
DCs are one of the most important antigen-presenting
cells (APC) and form part of a critical link between innate and
adaptive immunity (86). In the
periphery, DCs become activated in response to 'danger' signals
provided directly by the microbial invaders (87), which are relayed through TLRs,
RLRs and NLRs, receptors in the IFN secretion pathway. These DCs
then migrate to the draining lymphoid tissues to present the
acquired pathogen to appropriate adaptive T and B cells to mediate
immune responses (88).
One previous study demonstrated persistent
downregulation in the expression of the co-stimulatory molecule
CD80/CD86 on the myeloid DCs (mDCs) in patients with SFTS (89). Reduced circulating mDCs can be
measured as a valuable predictive biomarker, especially from day 9
following disease initiation, where disease severity is associated
with TLR3 expression on mDCs. Furthermore, IL-6, IL-10, TNF-α and
viral load were also found to be negatively associated with mDCs
(89). Song et al
(90) reported that the robust
differentiation of mDCs in surviving patients and not those who
succumbed to SFTS started in week 2 after the symptoms appeared,
which continued until week 3 (90). It was also found that among the
surviving patients, the expression of CD86 and the ratio of
CD80+CD86+/mDCs in week 3 was significantly
higher compared with that in the patients who succumbed to the
disease in week 2 (90). SFTSV
vaccines, such as human adenovirus type 5 (Ad5)-G-Gn (a recombinant
replication-deficient Ad5 co-expressing rabies virus G and SFTSV
Gn), have been reported to increase the production of neutralizing
antibodies against SFTSV (91).
This appeared to be mediated by the activation of additional DCs
and B cells in lymph nodes to induce a Th1-/Th2-mediated immune
response in splenocytes (91).
This suggests that DCs can serve an antigen-presenting role in the
function of SFTSV vaccines. These conclusions demonstrate that
SFTSV infection can attenuate the antigen-presenting ability of
mDCs to inhibit T-cell activation.
NK cells
NK cells are essential antiviral and anticancer
immune cells that serve a crucial role in immune defense and
surveillance (92). After
recognizing infected cells, NK cells can rapidly secrete granzyme
and perforin to lyse infected-cells. In addition, NK cells can also
secrete proinflammatory factors, including IFN-γ and TNF-α, to
induce a broader immune response to control the global viral load
(93). Sun et al (94) previously observed that in
individuals with severe SFTSV infection, the percentage of NK cells
was increased during the acute phase. However, other studies
reported that the number of NK cells was actually decreased in
patients with SFTS during the first week, which was rapidly
restored to normal levels after 6 months (95). In addition, another study found
that the CD3−CD16+56+ NK cell
count in patients with SFTS was lower compared with that in healthy
controls, although there was no statistical difference (96). During the early stages of SFTSV
infection, the frequency of CD56dimCD16+NK
cells was significantly reduced, which was negatively correlated
with the severity of the disease. Although the
CD56dimCD16+NK cell population was depleted,
the activation and functional enrichment suggest that their
involvement can ward off early SFTSV infection (97). Therefore, the loss of NK cells may
result in an upsurge in the viral load.
Monocytes and macrophages
Monocytes are widespread in the blood and form the
first line of defense against microbial invasion. Following
infection, monocytes change their cytokine/chemokine pattern,
differentiate into long-lived macrophages (Mφ) and migrate into the
tissue, becoming infected resident cells (98). Macrophages are important cells in
the innate immune system and modulate the adaptive immune responses
to pathogens through antigen processing and presentation (99). Following infection, macrophages
differentiate into two distinct subsets: i) Classically activated
or M1 macrophages, which are characterized by the secretion of
proinflammatory cytokines and high levels of phagocytic activity
(100); and ii) alternatively
activated or M2 macrophages, which mainly manufacture
anti-inflammatory cytokines and immunoregulatory molecules, leading
to neutrophil, monocyte and T-lymphocyte recruitment (101).
A previous study revealed that SFTSV can replicate
in human monocytes without inducing apoptosis by inhibiting the INF
and NF-κB signaling pathways (102). In addition, the SFTSV can be
harbored within splenic macrophages for long periods of time
(103). However, the
histopathological changes caused by SFTSV infection can be found in
the spleen, lung, kidney and liver, where further study suggested
that spleen and liver macrophages may be the primary target cells
of SFTSV infection (85). A
previous study by the authors also demonstrated that SFTSV can
infect macrophages in vivo and elevated pro- and
anti-inflammatory cytokines, as well as activation of
CD69+ T cells (81).
SFTSV infection of macrophages drives macrophage differentiation
towards M2, which promotes viral shedding and transmission by
targeting STAT1 (104). In
addition, the integrated transcriptome of mRNAs and lncRNAs in
THP-1 macrophages infected with SFTSV for 24 and 48 h was
previously analyzed. It revealed 2,334 differentially expressed
mRNAs and 154 differentially expressed lncRNAs. Amongst these, 577
mRNAs and 31 lncRNAs were commonly altered at 24 and 48 h,
respectively. According to the analysis of differentially expressed
mRNAs and transcription factors, they were mainly associated with
innate immunity and cytokine signaling. In particular, IRF1,
Salmonella pathogenicity island (SPI) 1, SPIB, E74-like ETS
transcription factor 5 and FEV were significantly enriched
following SFTSV infection (105). These results revealed how
macrophages and SFTSV can interact in a complex manner.
γδT cells
γδT cells are another type of lymphocytes and they
serve an essential role in the immune response and
immunopathological processes, which is receiving particular
attention (106). γδT cells are
a critical component of innate immunity and serve an essential role
in antiviral and antitumor activities. One study previously found
that during the acute phase of SFTS, the number of Vδ2T cells fell
considerably, which lasted for ~1 year. The Vδ2T cell population
declined readily with the severity of SFTS. The possible mechanism
of Vδ2T-cell depletion in SFTSV infection has also been previously
associated with activation-induced cell death (107). To conclude, lymphopenia in
patients with SFTS can affect the number of T-cell subsets,
hindering the enhancement of the immune response and subsequent
elimination of viral infections. However, the mechanism of
T-lymphocyte depletion warrants further study.
Inflammasomes
Inflammasomes are mainly formed by multimeric
protein complexes of sensor, adaptor and pro-caspase-1 components,
such as NACHT, leucine-rich and pyrin domain-containing proteins
(NALP), apoptosis-associated speck-like protein containing a CARD
(ASC) and pro-caspase 1 (CASP1) (108). Inflammasomes serve as receptors
for innate immune cells, which detect circulating DAMPs and PAMP
and activate caspase-1 to cleave pro-IL-1β and pro-IL-18 into IL-1β
and IL-18, respectively (109).
IL-1β recruits immune cells and causes the programmed death of
cells by binding to IL-1R-expressing immune cells. Liu et al
first reported that the secretion of IL-1β during SFTSV infection
is mediated by the NOD-, LRR- and pyrin domain-containing protein 3
(NLRP3) inflammasome in a caspase-1-dependent manner (110). The use of short hairpin RNAs to
knock down several NLRs in peripheral blood mononuclear cells
revealed that the NLRP3 inflammasome is essential for the
processing of pro-caspase-1 and pro-IL-1β (111). Further studies discovered that
both wild-type SFTSV NSs and the 21/23A mutant of SFTSV NSs with
alanine residues at positions 21 and 23 were able to reduce NLRP3
inflammasome-dependent IL-1β production (61). In addition, it was found that
SFTSV infection can trigger the upregulation of BCL2
antagonist/killer 1 (BAK) expression and activation of
BAK/BCL2-associated X (BAX), resulting in mitochondrial DNA (mtDNA)
oxidation and subsequent cell membrane release (112). This mtDNA binding to NLRP3 leads
to inflammasome activation and amplifies the inflammatory response
(112). However, the exact
composition and mechanism of inflammasomes are complex. Further
studies are required to clarify the role of inflammasomes in
SFTS.
7. Adaptive immunity in SFTS
The adaptive immune system consists of cellular
immune responses mediated by T cells and a humoral immune response
modulated by antibodies (113).
Naïve CD4+T cells receive the major histocompatibility
complex (MHC) II epitopes by APCs, such as DCs, macrophages and B
cells, following which they differentiate into different cell types
to regulate the adaptive immune response depending on the cytokine
environment. CD8+T cells, also known as cytotoxic T
lymphocytes or effector T cells, can directly kill virus-infected
cells. By contrast, B cells are mainly involved in humoral
immunity, where the body can generate specific antibodies to
neutralize viruses through effector B cells (114).
T-cell-mediated immune response to
SFTSV
T lymphocytes serve a primary role in
antigen-specific immune responses. A number of studies previously
demonstrated that CD3+, CD4+, and
CD8+ T cells are diminished in patients with SFTS
(94-96,115-119) and in experimental animal models
(103). Sun et al
(94) revealed a significant
decline in CD3+ and CD4+ expression in the
peripheral blood of patients with SFTS, where the average leukocyte
count was 2.86±1.56×109/l. In patients with SFTS, not
only were the number of T lymphocytes decreased, the expression of
cell apoptotic indicators Annexin and CD95, proliferation and
activation markers Ki-67, human leukocyte antigen DR and CD25,
programmed death-1, granzyme B and IFN-γ (T-cell function markers),
were significantly increased in the T lymphocytes (117). This suggests that T-cell
function was enhanced. Furthermore, peripheral blood T cells were
determined to be negatively correlated with SFTS severity. After 2
weeks, the number of T lymphocytes increased rapidly but returned
to normal 6 months after onset (95). Another study previously revealed
arginine deficiency in SFTS cases following the metabolomics
analysis of two independent patient cohorts, which suggested that
SFTSV infection and the eventual mortality resulted from nitric
oxide synthase and arginase metabolism (120). Therefore, the lack of arginine
was associated with the expansion of myeloid-derived suppressor
cells (120), which may
implicate a role in the impaired anti-SFTSV T-cell function.
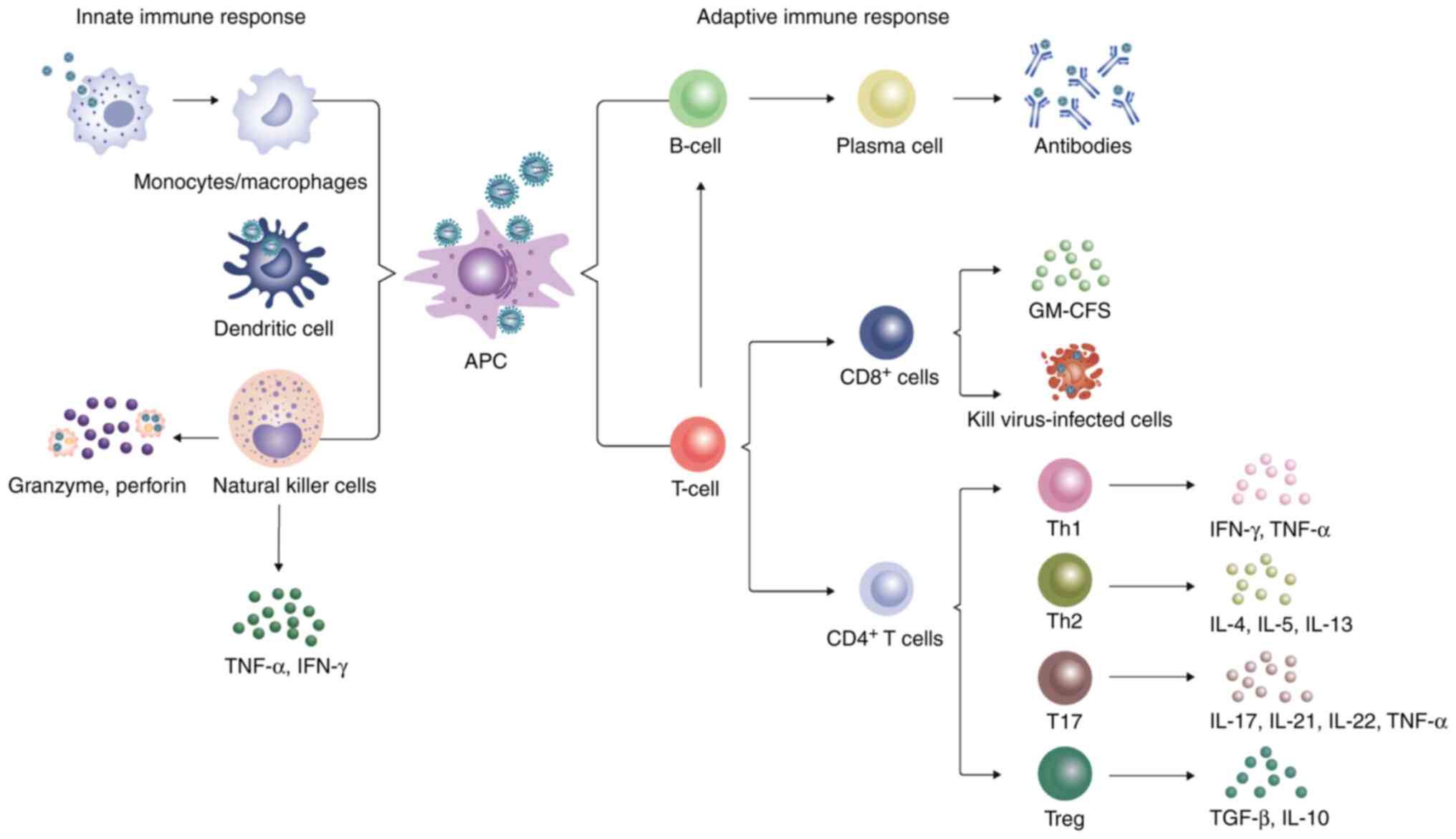
Naïve CD4+T cells can be divided into
several subsets, including Th1, Th2, Th17 and regulatory T cells
(Treg) based on their unique cytokine production profiles (114). Th1 cells mainly secrete IFN-γ
and TNF-α cytokines and serve a key role in the immune response to
viral infections (114). By
contrast, Th2 cells are important for promoting immunity to
helminth infection and allergic inflammation by emitting IL-4, IL-5
and IL-13 (114). T17 cells have
been found to secrete cytokines, such as IL-17, IL-21, IL-22 and
TNF-α, to induce immune damage in the presence of virus infection
(121). Tregs mainly secrete
anti-inflammatory cytokines, including TGF-β and IL-10, to limit
the immune response to pathogens and control inflammation,
contributing to immune homeostasis (Fig. 2) (122). The proportion of
CD4+/total lymphocytes and
CD4+CD25+/CD4+ cells noticeably
declined in patients with SFTS compared with that in healthy
individuals, but was higher compared with that in patients with
severe disease. By contrast, the percentage of
CD4+CD25+Foxp3+/CD4+CD25+
cells were markedly increased in the SFTS group but remained lower
compared with that in the severe group (115). This suggests that the proportion
of CD4+/total lymphocytes,
CD4+CD25+/CD4+ cells and
CD4+CD25+Foxp3+/CD4+CD25+
cells may be an important prognostic factor for patients with SFTS.
Concurrently with CD4+ T-cell depletion, the number of
Th1, Th2 and Treg cells was also found to be reduced in patients
who succumbed to SFTS, whereas the population of Th17 cells showed
no significant changes. In addition, the increase in the percentage
of Th2 and Th17 cells in the CD4+ T-cell population
resulted in abnormal Th1/Th2 and Th17/Treg ratios, which were
positively correlated with disease severity (116).
B-cell-mediated immune response to
SFTSV
B lymphocytes serve an integral role in the humoral
immunity against viral infection. In general, the antigen can be
recognized, endocytosed and/or degraded by the B-cell receptor and
then presented on the cell surface by MHC II to search for
explicitly differentiated CD4+T cells for the same
antigen. A proportion of B cells can differentiate into plasma
cells that secrete immunoglobulin (Ig) M, whilst others migrate to
B-cell follicles and form germinal centers with the assistance of
cytokines secreted by CD4+Th and follicular DCs
(123). Following viral
infection, antibodies bind to the virus to prevent it from
attaching to and entering the target cell, which is then cleared by
complement or antibody-dependent cell cytotoxicity (ADCC) (124).
Numerous studies have reported that compared with
healthy subjects, the level of B cells was markedly elevated and
positively correlated with the severity of SFTS disease (94,96,125,126). Further studies revealed that the
peripheral blood mononuclear cells of patients with SFTS could
induce propagation of atypical lymphocytes in vitro, and
these transient atypical lymphocytes were activated B cells
generated by stimuli other than virus particles, which were
released by SFTSV-infected B cells, indicating that SFTSV-infected
B cells release factors that cause B cells to differentiate into
plasmablasts (118,126-128). By contrast, a previous cohort
investigation study found that the number of B cells was markedly
reduced during the first week of infection but quickly returned to
normal levels in patients with SFTS (95). Further pathological examinations
revealed that large numbers of activated mature plasmablasts were
located in the secondary lymphoid organs from patients who
succumbed to the disease (94).
At the end of the lethal SFTSV infection, the B cells of the
secondary lymphatic organs, which differentiate into plasmablasts
and macrophages, are the target cells of fatal SFTSV infection.
SFTSVs mainly infect B cell-lineage lymphocytes. Previous
pathological observations support the notion that the SFTSV/B cell
axis serves a key role in the pathogenesis of patients with SFTS
(126). Further analysis of the
B-cell subpopulations in the patient cohort revealed a meaningful
difference between the survival group and the fatal group:
Double-negative B-cells (CD27−IgD− cells) and
plasma cells (CD27+IgD− cells) elevated in
the fatal group. The marginal zone B cells
(CD27+IgD+ cells) assessed in the survival
group was lower compared with that in the fatal group (89). Additionally, the number of naïve B
cells (CD27−IgD+ cells) in the survival and
fatal groups was decreased overall, but the total number of naïve B
cells in the survival groups was higher (89). In summary, these findings for
detecting B-cell subpopulations suggest that patients who succumbed
to SFTS have B-cell maturation disorders and subsequent
dysregulation of the humoral immune response.
A study has been previously conducted to detect
virus-specific antibodies in humans infected with SFTSV, including
IgM, IgG and neutralizing antibodies (125,126). The SFTSV-specific IgM antibodies
were detected between 4 and 21 days (median of 9 days) of onset,
peaking by week 4 and lasting up to ≤6 months (95). SFTSV-specific IgG anti-bodies can
be detected between 2 and 9 weeks (median of 6 weeks), where the
maximum value was reached 6 months after infection. In addition,
the majority of the patients remain positive after 3 years of
illness (95). Initial levels of
IgM and IgG antibodies were lower compared with those in patients
with other underlying or compromised immune responses, such as the
elderly and/or those with underlying diseases. The innate immune
system may be severely suppressed in these subjects, leaving
insufficient activation of adaptive immunity (95). Previous research also revealed
that adaptive immunodeficiency caused by the disruption of humoral
immunity mediated by B cells was a predictor of fatal SFTS. This
cripples one's ability to mount a specific immune response from IgM
or IgG to SFTSV NP and Gn, which is essential for neutralizing and
eliminating the virus (89).
Suzuki et al (129)
examined the expression of immunoglobulins (IgM and IgG) in
SFTV-infected B cells in the lymph nodes. Following germination,
the majority of the infected B cells were transformed B cells,
where the transformed B cells that were IgG-positive also
infiltrated all organs of the body (129). These findings differed from
those from previous studies, because various peripheral
plasmablasts in patients with fatal SFTS do not express IgM and
IgG, suggesting that activated and differentiated B cells cannot
perform IgG conversion. These findings may explain the inadequate
B-cell humoral response in patients who were deceased (129). Since the mechanism of B cells is
unclear in individuals with SFTS, the role of B cells in the
pathogenesis of SFTSV and its effects on SFTSV require further
study.
8. Conclusion and future prospects
SFTSV infection is becoming an increasingly
prevalent public health issue worldwide and its incidence is
increasing annually. However, the transmission cycle of SFTSV
remains poorly understood and its pathogenesis has not been
elucidated, particularly its interaction with the host immune
response. The immunopathogenesis of SFTSV infection is complex,
which includes a cascade of reactions involving a wide range of
immune cells, inflammatory mediators, inflammasomes and signaling
pathways. Although studies have indicated the role of certain
subsets of immune cells and NSs proteins in regulating the immune
response during SFTSV infection, evasion of the immune system and
the initiation of a proinflammatory response may serve a dual role.
By contrast, the immune response can eliminate SFTSV; however,
excessive immune activation can also lead to a hyperinflammatory
response with clinical collateral damage. To designate strategies
for the treatment of SFTSV, how innate immune dysregulation and
proinflammatory molecules are generated, in addition to how to
reveal and modulate critical signaling molecules must be studied.
Damage to adaptive immunity in patients with fatal SFTS suggests
that the severe immunological dysregulation caused by the SFTSV
infection, along with the immunosuppressive milieu, leads to
inadequate antigen presentation and subsequent class-switching B
cells (130). SFTSV can also
induce cellular damage through other mechanisms, such as
mitochondrial dysfunction and ER stress. The cellular debris can
trigger the immune defense through the positive feedback regulation
of DAMPs and PAMPs, amplifying the inflammatory response and
leading to further injury. Therefore, how an organism eliminates
the virus using the immune response and restores homeostasis
remains to be further investigated.
Previous studies have reported the role of exosomes
in viral infection (131). The
virus can complete its replication cycle in the host cell before
the progeny virus is released. Viruses can hijack exosomes, utilize
biogenesis systems and load their components to evade the host
immune response and facilitate cell-to-cell diffusion (132). Exosomes, on the other hand, can
be used by host cells to release antiviral substances and prevent
viral infection. As a result, the interaction between exosomes
generated by SFTSV-infected cells and the immune system of the host
should further be studied. Exosomes are being employed as a
next-generation drug delivery platform for a range of cargos, such
that exosome-based therapies for SFTSV should be further explored.
During the outbreak of coronavirus disease 2019, researchers and
clinicians worldwide have been devoting considerable effort into
researching coronavirus disease 2019 (COVID-19). Vaccination,
effective antiviral therapy, modulation of the innate immune
response and restoration of the adaptive immune response would
improve the prognosis of patients with COVID-19 (133). Therefore, experience can be
drawn from the treatment and management of COVID-19 to manage
patients with SFTS, to improve their prognosis whilst lowering the
mortality rate.
Overall, in the present review, recent research
progress in host immune responses against SFTSV was summarized. In
addition to strengthening public health education, it is necessary
to accelerate research into the virus and its pathogenesis.
Furthermore, a large-scale randomized controlled trial is required
for the exploration of more particular treatment strategies and
effective preventative measures to reduce the CFR. Clinicians need
to actively exchange experience and continuously optimize diagnosis
and treatment to cope with the SFTS disease jointly.
Availability of data and materials
Data sharing does not apply to this article, as no
datasets were generated or analyzed during the present study.
Authors' contributions
TY and HH performed the literature search and wrote
the manuscript. JL and LJ supervised and revised the manuscript.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted
in the absence of any commercial or financial relationships that
could be construed as a potential competing interests.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81871242).
References
|
1
|
Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD,
Sun YL, Zhang L, Zhang QF, Popov VL, Li C, et al: Fever with
thrombocytopenia associated with a novel Bunyavirus in China. N
Engl J Med. 364:1523–1532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuhn JH, Adkins S, Agwanda BR, Al Kubrusli
R, Alkhovsky SV, Amarasinghe GK, Avšič-Županc T, Ayllón MA, Bahl J,
Balkema-Buschmann A, et al: 2021 Taxonomic update of phylum
Negarnaviricota (Riboviria: Orthornavirae), including the large
orders Bunyavirales and Mononegavirales. Arch Virol. 166:3513–3566.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim YR, Yun Y, Bae SG, Park D, Kim S, Lee
JM, Cho NH, Kim YS and Lee KH: Severe Fever with Thrombocytopenia
Syndrome Virus Infection, South Korea, 2010. Emerg Infect Dis.
24:2103–2105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi T, Maeda K, Suzuki T, Ishido A,
Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, et
al: The first identification and retrospective study of Severe
Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis.
209:816–827. 2014. View Article : Google Scholar
|
|
5
|
Tran XC, Yun Y, Van An L, Kim SH, Thao
NTP, Man PKC, Yoo JR, Heo ST, Cho NH and Lee KH: Endemic severe
fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis.
25:1029–1031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Win AM, Nguyen YTH, Kim Y, Ha NY, Kang JG,
Kim H, San B, Kyaw O, Htike WW, Choi DO, et al: Genotypic
heterogeneity of orientia tsutsugamushi in scrub typhus patients
and thrombocytopenia syndrome co-infection, Myanmar. Emerg Infect
Dis. 26:1878–1881. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng SH, Yang SL, Tang SE, Wang TC, Hsu
TC, Su CL, Chen MY, Shimojima M, Yoshikawa T and Shu PY: Human case
of severe fever with thrombocytopenia syndrome virus infection,
Taiwan, 2019. Emerg Infect Dis. 26:1612–1614. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ongkittikul S, Watanawong R and Romopho P:
Severe fever with thrombocytopenia syndrome virus: The first case
report in Thailand. Bangkok Med J. 16:204–206. 2020. View Article : Google Scholar
|
|
9
|
Zohaib A, Zhang J, Saqib M, Athar MA,
Hussain MH, Chen J, Sial AU, Tayyab MH, Batool M, Khan S, et al:
Serologic evidence of severe fever with thrombocytopenia syndrome
virus and related viruses in Pakistan. Emerg Infect Dis.
26:1513–1516. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McMullan LK, Folk SM, Kelly AJ, MacNeil A,
Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin
PE, et al: A new phlebovirus associated with severe febrile illness
in Missouri. N Engl J Med. 367:834–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo JW, Kim D, Yun N and Kim DM: Clinical
update of severe fever with thrombocytopenia Syndrome. Viruses.
13:12132021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoo JR, Kim TJ, Heo ST, Hwang KA, Oh H, Ha
T, Ko HK, Baek S, Kim JE, Kim JH, et al: IL-6 and IL-10 levels,
rather than viral load and neutralizing antibody titers, determine
the fate of patients with severe fever with thrombocytopenia
syndrome virus infection in South Korea. Front Immunol.
12:7118472021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Lu QB, Xing B, Zhang SF, Liu K, Du
J, Li XK, Cui N, Yang ZD, Wang LY, et al: Epidemiological and
clinical features of laboratory-diagnosed severe fever with
thrombocytopenia syndrome in China, 2011-17: A prospective
observational study. Lancet Infect Dis. 18:1127–1137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW,
Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, et al: Haemaphysalis
longicornis ticks as reservoir and vector of severe fever with
thrombocytopenia syndrome virus in China. Emerg Infect Dis.
21:1770–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu YY, Zhuang L, Liu K, Sun Y, Dai K,
Zhang XA, Zhang PH, Feng ZC, Li H and Liu W: Role of three tick
species in the maintenance and transmission of severe fever with
thrombocytopenia syndrome virus. PLoS Negl Trop Dis.
14:e00083682020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L,
Walker DH, Ren J, Wang Y and Yu XJ: Person-to-person transmission
of severe fever with thrombocytopenia syndrome virus. Vector Borne
Zoonotic Dis. 12:156–160. 2012. View Article : Google Scholar
|
|
17
|
Jung IY, Choi W, Kim J, Wang E, Park SW,
Lee WJ, Choi JY, Kim HY, Uh Y and Kim YK: Nosocomial
person-to-person transmission of severe fever with thrombocytopenia
syndrome. Clin Microbiol Infect. 25:633.e1–633.e4. 2019. View Article : Google Scholar
|
|
18
|
Wang Y, Deng B, Zhang J, Cui W, Yao W and
Liu P: Person-to-person asymptomatic infection of severe fever with
thrombocytopenia syndrome virus through blood contact. Intern Med.
53:903–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong Z, Gu S, Zhang Y, Sun J, Wu X, Ling
F, Shi W, Zhang P, Li D, Mao H, et al: Probable aerosol
transmission of severe fever with thrombocytopenia syndrome virus
in southeastern China. Clin Microbiol Infect. 21:1115–1120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organization (WHO): 2017 -
First Annual review of diseases prioritized under the Research and
Development Blueprint. WHO; Geneva: 2017, https://www.who.int/news-room/events/detail/2017/01/24/default-calendar/january-2017-first-annual-review-of-diseases-prioritized-under-the-research-and-development-blueprint.
Accessed January 24, 2017.
|
|
21
|
Oh HS, Kim M, Lee JO, Kim H, Kim ES, Park
KU, Kim HB and Song KH: Hemophagocytic lymphohistiocytosis
associated with SFTS virus infection: A case report with literature
review. Medicine (Baltimore). 95:e44762016. View Article : Google Scholar
|
|
22
|
Ramos-Casals M, Brito-Zerón P,
López-Guillermo A, Khamashta MA and Bosch X: Adult haemophagocytic
syndrome. Lancet. 383:1503–1516. 2014. View Article : Google Scholar
|
|
23
|
Weng Y, Chen N, Han Y, Xing Y and Li J:
Clinical and laboratory characteristics of severe fever with
thrombocytopenia syndrome in Chinese patients. Braz J Infect Dis.
18:88–91. 2014. View Article : Google Scholar
|
|
24
|
Kaneko M, Shikata H, Matsukage S, Maruta
M, Shinomiya H, Suzuki T, Hasegawa H, Shimojima M and Saijo M: A
patient with severe fever with thrombocytopenia syndrome and
hemophagocytic lymphohistiocytosis-associated involvement of the
central nervous system. J Infect Chemother. 24:292–297. 2018.
View Article : Google Scholar
|
|
25
|
Jung IY, Ahn K, Kim J, Choi JY, Kim HY, Uh
Y and Kim YK: Higher fatality for severe fever with
thrombocytopenia syndrome complicated by hemophagocytic
lymphohistiocytosis. Yonsei Med J. 60:592–596. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khalil J, Kato H and Fujita T: The role of
non-structural protein NSs in the pathogenesis of severe fever with
thrombocytopenia syndrome. Viruses. 13:8762021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He CQ and Ding NZ: Discovery of severe
fever with thrombocytopenia syndrome bunyavirus strains originating
from intragenic recombination. J Virol. 86:12426–12430. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei XY, Liu MM and Yu XJ: Severe fever
with thrombocytopenia syndrome and its pathogen SFTSV. Microbes
Infect. 17:149–154. 2015. View Article : Google Scholar
|
|
29
|
Huang X, Li J, Li A, Wang S and Li D:
Epidemiological characteristics of severe fever with
thrombocytopenia syndrome from 2010 to 2019 in Mainland China. Int
J Environ Res Public Health. 18:30922021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu XJ: Risk factors for death in severe
fever with thrombocytopenia syndrome. Lancet Infect Dis.
18:1056–1057. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korea Disease Control and Prevention
Agency: Ticks and Rodents Borne Infectious Diseases Guideline 2020.
http://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=365644.
Accessed December 30, 2021.
|
|
32
|
Choi SJ, Park SW, Bae IG, Kim SH, Ryu SY,
Kim HA, Jang HC, Hur J, Jun JB, Jung Y, et al: Severe fever with
thrombocytopenia syndrome in South Korea, 2013-2015. PLoS Negl Trop
Dis. 10:e00052642016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim M, Heo ST, Oh H, Oh S, Lee KH and Yoo
JR: Prognostic factors of severe fever with thrombocytopenia
syndrome in South Korea. Viruses. 13:102021. View Article : Google Scholar :
|
|
34
|
National Institute of Infectious Diseases:
Severe Fever with Thrombocytopenia Syndrome (SFTS) in Japan, as of
February 2016. https://www.niid.go.jp/niid/en/iasr-vol33-e/865-iasr/6339-tpc433.html.
Accessed September 26, 2021.
|
|
35
|
Kobayashi Y, Kato H, Yamagishi T, Shimada
T, Matsui T, Yoshikawa T, Kurosu T, Shimojima M, Morikawa S,
Hasegawa H, et al: Severe fever with thrombocytopenia syndrome,
Japan, 2013-2017. Emerg Infect Dis. 26:692–699. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao L, Li J, Cui X, Jia N, Wei J, Xia L,
Wang H, Zhou Y, Wang Q, Liu X, et al: Distribution of Haemaphysalis
longicornis and associated pathogens: analysis of pooled data from
a China field survey and global published data. Lancet Planet
Health. 4:e320–e329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoo JR, Heo ST, Song SW, Bae SG, Lee S,
Choi S, Lee C, Jeong S, Kim M, Sa W, et al: Severe fever with
thrombocytopenia syndrome virus in ticks and SFTS incidence in
humans, South Korea. Emerg Infect Dis. 26:2292–2294. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niu G, Li J, Liang M, Jiang X, Jiang M,
Yin H, Wang Z, Li C, Zhang Q, Jin C, et al: Severe fever with
thrombocytopenia syndrome virus among domesticated animals, China.
Emerg Infect Dis. 19:756–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kida K, Matsuoka Y, Shimoda T, Matsuoka H,
Yamada H, Saito T, Imataki O, Kadowaki N, Noguchi K, Maeda K, et
al: A Case of Cat-to-Human transmission of severe fever with
thrombocytopenia syndrome virus. Jpn J Infect Dis. 72:356–358.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kimura T, Fukuma A, Shimojima M, Yamashita
Y, Mizota F, Yamashita M, Otsuka Y, Kan M, Fukushi S, Tani H, et
al: Seroprevalence of severe fever with thrombocytopenia syndrome
(SFTS) virus antibodies in humans and animals in Ehime prefecture,
Japan, an endemic region of SFTS. J Infect Chemother. 24:802–806.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rim JM, Han SW, Cho YK, Kang JG, Choi KS,
Jeong H, Son K, Kim J, Choi Y, Kim WM, et al: Survey of severe
fever with thrombocytopenia syndrome virus in wild boar in the
Republic of Korea. Ticks Tick Borne Dis. 12:1018132021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okada A, Hotta A, Kimura M, Park ES,
Morikawa S and Inoshima Y: A retrospective survey of the
seroprevalence of severe fever with thrombocytopenia syndrome virus
in wild animals in Japan. Vet Med Sci. 7:600–605. 2021. View Article : Google Scholar :
|
|
43
|
Chen Y, Jia B, Huang R, Yan X, Xiong Y,
Yong L and Chao W: Occupational severe fever with thrombocytopenia
syndrome following needle-stick injury. Infect Control Hosp
Epidemiol. 38:760–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu Y, Wu H, Gao J, Zhou X, Zhu R, Zhang
C, Bai H, Abdullah AS and Pan H: Two confirmed cases of severe
fever with thrombocytopenia syndrome with pneumonia: Implication
for a family cluster in East China. BMC Infect Dis. 17:5372017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang X, Hu J, Peng Z, Dai Q, Liu W, Liang
S, Li Z, Zhang N and Bao C: Epidemiological and clinical
characteristics of severe fever with thrombocytopenia syndrome
bunyavirus human-to-human transmission. PLoS Negl Trop Dis.
15:e00090372021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
You E, Wang L, Zhang L, Wu J, Zhao K and
Huang F: Epidemiological characteristics of severe fever with
thrombocytopenia syndrome in Hefei of Anhui Province: A
population-based surveillance study from 2011 to 2018. Eur J Clin
Microbiol Infect Dis. 40:929–939. 2021. View Article : Google Scholar
|
|
47
|
Carty M, Guy C and Bowie AG: Detection of
viral infections by innate immunity. Biochem Pharmacol.
183:1143162021. View Article : Google Scholar
|
|
48
|
Park A and Iwasaki A: Type I and Type III
interferons-induction, signaling, evasion, and application to
combat COVID-19. Cell Host Microbe. 27:870–878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoo JS, Kato H and Fujita T: Sensing viral
invasion by RIG-I like receptors. Curr Opin Microbiol. 20:131–138.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chow KT, Gale M Jr and Loo YM: RIG-I and
Other RNA sensors in antiviral immunity. Annu Rev Immunol.
36:667–694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamada S, Shimojima M, Narita R, Tsukamoto
Y, Kato H, Saijo M and Fujita T: RIG-I-like receptor and toll-like
receptor signaling pathways cause aberrant production of
inflammatory cytokines/chemokines in a severe fever with
thrombocytopenia syndrome virus infection mouse model. J Virol.
92:e02246–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Min YQ, Ning YJ, Wang H and Deng F: A
RIG-I-like receptor directs antiviral responses to a bunyavirus and
is antagonized by virus-induced blockade of TRIM25-mediated
ubiquitination. J Biol Chem. 295:9691–9711. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu BY, Yu XJ and Zhou CM: SAFA initiates
innate immunity against cytoplasmic RNA virus SFTSV infection. PLoS
Pathog. 17:e10100702021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Onomoto K, Onoguchi K and Yoneyama M:
Regulation of RIG-I-like receptor-mediated signaling: Interaction
between host and viral factors. Cell Mol Immunol. 18:539–555. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun Y, Jin C, Zhan F, Wang X, Liang M,
Zhang Q, Ding S, Guan X, Huo X, Li C, et al: Host cytokine storm is
associated with disease severity of severe fever with
thrombocytopenia syndrome. J Infect Dis. 206:1085–1094. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang YZ, He YW, Dai YA, Xiong Y, Zheng H,
Zhou DJ, Li J, Sun Q, Luo XL, Cheng YL, et al: Hemorrhagic fever
caused by a novel bunyavirus in China: Pathogenesis and correlates
of fatal outcome. Clin Infect Dis. 54:527–533. 2012. View Article : Google Scholar
|
|
57
|
Liu MM, Lei XY, Yu H, Zhang JZ and Yu XJ:
Correlation of cytokine level with the severity of severe fever
with thrombocytopenia syndrome. Virol J. 14:62017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun Y, Liu MM, Lei XY and Yu XJ: SFTS
phlebovirus promotes LC3-II accumulation and nonstructural protein
of SFTS phlebovirus co-localizes with autophagy proteins. Sci Rep.
8:52872018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang LK, Wang B, Xin Q, Shang W, Shen S,
Xiao G, Deng F, Wang H, Hu Z and Wang M: Quantitative proteomic
analysis reveals unfolded-protein response involved in severe fever
with thrombocytopenia syndrome virus infection. J Virol.
93:e00308–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qu B, Qi X, Wu X, Liang M, Li C, Cardona
CJ, Xu W, Tang F, Li Z, Wu B, et al: Suppression of the interferon
and NF-κB responses by severe fever with thrombocytopenia syndrome
virus. J Virol. 86:8388–8401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Moriyama M, Igarashi M, Koshiba T, Irie T,
Takada A and Ichinohe T: Two conserved amino acids within the NSs
of severe fever with thrombocytopenia syndrome phlebovirus are
essential for anti-interferon activity. J Virol. 92:e00706–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ning YJ, Wang M, Deng M, Shen S, Liu W,
Cao WC, Deng F, Wang YY, Hu Z and Wang H: Viral suppression of
innate immunity via spatial isolation of TBK1/IKKε from
mitochondrial antiviral platform. J Mol Cell Biol. 6:324–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Santiago FW, Covaleda LM, Sanchez-Aparicio
MT, Silvas JA, Diaz-Vizarreta AC, Patel JR, Popov V, Yu XJ,
García-Sastre A and Aguilar PV: Hijacking of RIG-I signaling
proteins into virus-induced cytoplasmic structures correlates with
the Inhibition of type I interferon responses. J Virol.
88:4572–4585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ning YJ, Feng K, Min YQ, Cao WC, Wang M,
Deng F, Hu Z and Wang H: Disruption of type I interferon signaling
by the nonstructural protein of severe fever with thrombocytopenia
syndrome virus via the Hijacking of STAT2 and STAT1 into Inclusion
Bodies. J Virol. 89:4227–4236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hong Y, Bai M, Qi X, Li C, Liang M, Li D,
Cardona CJ and Xing Z: Suppression of the IFN-α and -β Induction
through Sequestering IRF7 into viral inclusion bodies by
nonstructural protein NSs in severe fever with thrombocytopenia
syndrome bunyavirus infection. J Immunol. 202:841–856. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song P, Zheng N, Zhang L, Liu Y, Chen T,
Bao C, Li Z, Yong W, Zhang Y, Wu C and Wu Z: Downregulation of
Interferon-β and Inhibition of TLR3 expression are associated with
fatal outcome of severe fever with thrombocytopenia syndrome. Sci
Rep. 7:65322017. View Article : Google Scholar
|
|
67
|
Lee JK and Shin OS: Nonstructural protein
of severe fever with thrombocytopenia syndrome phlebovirus inhibits
TBK1 to evade interferon-mediated response. J Microbiol Biotechnol.
31:226–232. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yoshikawa R, Sakabe S, Urata S and Yasuda
J: Species-Specific pathogenicity of severe fever with
thrombocytopenia syndrome virus is determined by Anti-STAT2
Activity of NSs. J Virol. 93:e02226–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kitagawa Y, Sakai M, Shimojima M, Saijo M,
Itoh M and Gotoh B: Nonstructural protein of severe fever with
thrombocytopenia syndrome phlebovirus targets STAT2 and not STAT1
to inhibit type I interferon-stimulated JAK-STAT signaling.
Microbes Infect. 20:360–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ferrara JL, Abhyankar S and Gilliland DG:
Cytokine storm of graft-versus-host disease: a critical effector
role for interleukin-1. Transplant Proc. 25:1216–1217.
1993.PubMed/NCBI
|
|
71
|
Teijaro JR: Cytokine storms in infectious
diseases. Semin Immunopathol. 39:501–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu B, Huang S and Yin L: The cytokine
storm and COVID-19. J Med Virol. 93:250–256. 2021. View Article : Google Scholar
|
|
73
|
Gu Y, Hsu AC, Pang Z, Pan H, Zuo X, Wang
G, Zheng J and Wang F: Role of the innate cytokine storm induced by
the influenza a virus. Viral Immunol. 32:244–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ryabkova VA, Churilov LP and Shoenfeld Y:
Influenza infection, SARS, MERS and COVID-19: Cytokine storm-The
common denominator and the lessons to be learned. Clin Immunol.
223:1086522021. View Article : Google Scholar
|
|
75
|
Bopp NE, Kaiser JA, Strother AE, Barrett
ADT, Beasley DWC, Benassi V, Milligan GN, Preziosi MP and Reece LM:
Baseline mapping of severe fever with thrombocytopenia syndrome
virology, epidemiology and vaccine research and development. NPJ
Vaccines. 5:1112020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhou C and Yu X: Unraveling the Underlying
interaction mechanism between dabie bandavirus and innate immune
response. Front Immunol. 12:6768612021. View Article : Google Scholar :
|
|
77
|
Deng B, Zhang S, Geng Y, Zhang Y, Wang Y,
Yao W, Wen Y, Cui W, Zhou Y, Gu Q, et al: Cytokine and chemokine
levels in patients with severe fever with thrombocytopenia syndrome
virus. PLoS One. 7:e413652012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ding YP, Liang MF, Ye JB, Liu QH, Xiong
CH, Long B, Lin WB, Cui N, Zou ZQ, Song YL, et al: Prognostic value
of clinical and immunological markers in acute phase of SFTS virus
infection. Clin Microbiol Infect. 20:O870–O878. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park A, Park SJ, Jung KL, Kim SM, Kim EH,
Kim YI, Foo SS, Kim S, Kim SG, Yu KM, et al: Molecular signatures
of inflammatory profile and B-Cell function in patients with severe
fever with thrombocytopenia syndrome. mBio. 12:e02583–20. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
He Z, Wang B, Li Y, Hu K, Yi Z, Ma H, Li
X, Guo W, Xu B and Huang X: Changes in peripheral blood cytokines
in patients with severe fever with thrombocytopenia syndrome. J Med
Virol. 93:4704–4713. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li J, Han Y, Xing Y, Li S, Kong L, Zhang
Y, Zhang L, Liu N, Wang Q, Wang S, et al: Concurrent measurement of
dynamic changes in viral load, serum enzymes, T cell subsets, and
cytokines in patients with severe fever with thrombocytopenia
syndrome. PLoS One. 9:e916792014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fajgenbaum DC and June CH: Cytokine Storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Khalil J, Yamada S, Tsukamoto Y, Abe H,
Shimojima M, Kato H and Fujita T: The non-structural protein NSs of
SFTSV causes a cytokine storm through the hyper-activation of
NF-κB. Mol Cell Biol. 41:e00542–20. 2021. View Article : Google Scholar :
|
|
84
|
Choi Y, Park SJ, Sun Y, Yoo JS, Pudupakam
RS, Foo SS, Shin WJ, Chen SB, Tsichlis PN, Lee WJ, et al: Severe
fever with thrombocytopenia syndrome phlebovirus non-structural
protein activates TPL2 signalling pathway for viral
immunopathogenesis. Nat Microbiol. 4:429–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu S, Jiang N, Nawaz W, Liu B, Zhang F,
Liu Y, Wu X and Wu Z: Infection of humanized mice with a novel
phlebovirus presented pathogenic features of severe fever with
thrombocytopenia syndrome. PLoS Pathog. 17:e10095872021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mailliard RB: Dendritic cells and
antiviral defense. Viruses. 12:11522020. View Article : Google Scholar :
|
|
88
|
Kapsenberg ML: Dendritic-cell control of
pathogen-driven T-cell polarization. Nat Rev Immunol. 3:984–993.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang W, Li M, Xiong S, Wang H, Xiong Y,
Li M, Lu M, Yang D, Peng C and Zheng X: Decreased myeloid dendritic
cells indicate a poor prognosis in patients with severe fever with
thrombocytopenia syndrome. Int J Infect Dis. 54:113–120. 2017.
View Article : Google Scholar
|
|
90
|
Song P, Zheng N, Liu Y, Tian C, Wu X, Ma
X, Chen D, Zou X, Wang G, Wang H, et al: Deficient humoral
responses and disrupted B-cell immunity are associated with fatal
SFTSV infection. Nat Commun. 9:33282018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao Z, Zheng W, Yan L, Sun P, Xu T, Zhu
Y, Liu L, Tian L, He H, Wei Y and Zheng X: Recombinant human
adenovirus type 5 Co-expressing RABV G and SFTSV Gn induces
protective immunity against rabies virus and severe fever with
thrombocytopenia syndrome virus in mice. Front Microbiol.
11:14732020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
O'Brien KL and Finlay DK: Immunometabolism
and natural killer cell responses. Nat Rev Immunol. 19:282–290.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cerwenka A and Lanier LL: Natural killer
cell memory in infection, inflammation and cancer. Nat Rev Immunol.
16:112–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun L, Hu Y, Niyonsaba A, Tong Q, Lu L, Li
H and Jie S: Detection and evaluation of immunofunction of patients
with severe fever with thrombocytopenia syndrome. Clin Exp Med.
14:389–395. 2014. View Article : Google Scholar
|
|
95
|
Lu QB, Cui N, Hu JG, Chen WW, Xu W, Li H,
Zhang XA, Ly H, Liu W and Cao WC: Characterization of immunological
responses in patients with severe fever with thrombocytopenia
syndrome: A cohort study in China. Vaccine. 33:1250–1255. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu J, Wang L, Feng Z, Geng D, Sun Y and
Yuan G: Dynamic changes of laboratory parameters and peripheral
blood lymphocyte subsets in severe fever with thrombocytopenia
syndrome patients. Int J Infect Dis. 58:45–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li M, Xiong Y, Li M, Zhang W, Liu J, Zhang
Y, Xiong S, Zou C, Liang B, Lu M, et al: Depletion but activation
of CD56dimCD16+ NK Cells in acute infection
with severe fever with thrombocytopenia syndrome virus. Virol Sin.
35:588–598. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nikitina E, Larionova I, Choinzonov E and
Kzhyshkowska J: Monocytes and macrophages as viral targets and
reservoirs. Int J Mol Sci. 19:28212018. View Article : Google Scholar :
|
|
99
|
Epelman S, Lavine KJ and Randolph GJ:
Origin and functions of tissue macrophages. Immunity. 41:21–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Murray PJ: Macrophage Polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar
|
|
101
|
Meidaninikjeh S, Sabouni N, Marzouni HZ,
Bengar S, Khalili A and Jafari R: Monocytes and macrophages in
COVID-19: Friends and foes. Life Sci. 269:1190102021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mendoza CA, Yamaoka S, Tsuda Y, Matsuno K,
Weisend CM and Ebihara H: The NF-κB inhibitor, SC75741, is a novel
antiviral against emerging tick-borne bandaviruses. Antiviral Res.
185:1049932021. View Article : Google Scholar
|
|
103
|
Jin C, Liang M, Ning J, Gu W, Jiang H, Wu
W, Zhang F, Li C, Zhang Q, Zhu H, et al: Pathogenesis of emerging
severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse
model. Proc Natl Acad Sci USA. 109:10053–10058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang L, Fu Y, Wang H, Guan Y, Zhu W, Guo
M, Zheng N and Wu Z: Severe fever with thrombocytopenia syndrome
virus-induced macrophage differentiation is regulated by miR-146.
Front Immunol. 10:10952019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yan JM, Zhang WK, Li F, Zhou CM and Yu XJ:
Integrated transcriptome profiling in THP-1 macrophages infected
with bunyavirus SFTSV. Virus Res. 306:1985942021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kabelitz D: Gamma Delta T cells (γδ T
Cells) in health and disease: In memory of professor wendy havran.
Cells. 9:25642020. View Article : Google Scholar
|
|
107
|
Xu W, Li XK, Lu QB, Yang ZD, Du J, Xing B,
Cui N, Zhang XA, Zhang SF, Yang XX, et al: Association between
peripheral γδ T cell subsets and disease progression of severe
fever with thrombocytopenia syndrome virus infection. Pathog Dis.
75:2017. View Article : Google Scholar
|
|
108
|
Broz P and Dixit VM: Inflammasomes:
Mechanism of assembly, regulation and signalling. Nat Rev Immunol.
16:407–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Martinon F, Burns K and Tschopp J: The
Inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu JW, Chu M, Jiao YJ, Zhou CM, Qi R and
Yu XJ: SFTSV Infection Induced Interleukin-1β secretion through
NLRP3 inflammasome activation. Front Immunol. 12:5951402021.
View Article : Google Scholar
|
|
111
|
Li Z, Hu J, Bao C, Gao C, Zhang N, Cardona
CJ and Xing Z: Activation of the NLRP3 inflammasome and elevation
of interleukin-1β secretion in infection by sever fever with
thrombocytopenia syndrome virus. Sci Rep. 12:25732022. View Article : Google Scholar
|
|
112
|
Li S, Li H, Zhang YL, Xin QL, Guan ZQ,
Chen X, Zhang XA, Li XK, Xiao GF, Lozach PY, et al: SFTSV infection
induces BAK/BAX-Dependent mitochondrial DNA release to Trigger
NLRP3 inflammasome activation. Cell Rep. 30:4370–4385.e7. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bonilla FA and Oettgen HC: Adaptive
immunity. J Allergy Clin Immunol. 125(2 Suppl 2): S33–S40. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Swain SL, McKinstry KK and Strutt TM:
Expanding roles for CD4+ T cells in immunity to viruses.
Nat Rev Immunol. 12:136–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yi X, Li W, Li H and Jie S: Circulating
regulatory T cells in patients with severe fever with
thrombocytopenia syndrome. Infect Dis (Lond). 47:294–301. 2015.
View Article : Google Scholar
|
|
116
|
Li MM, Zhang WJ, Weng XF, Li MY, Liu J,
Xiong Y, Xiong SE, Zou CC, Wang H, Lu MJ, et al: CD4 T cell loss
and Th2 and Th17 bias are associated with the severity of severe
fever with thrombocytopenia syndrome (SFTS). Clin Immunol.
195:8–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li MM, Zhang WJ, Liu J, Li MY, Zhang YF,
Xiong Y, Xiong SE, Zou CC, Xiong LQ, Liang BY, et al: Dynamic
changes in the immunological characteristics of T lymphocytes in
surviving patients with severe fever with thrombocytopenia syndrome
(SFTS). Int J Infect Dis. 70:72–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Takahashi T, Suzuki T, Hiroshige S, Nouno
S, Matsumura T, Tominaga T, Yujiri T, Katano H, Sato Y and Hasegawa
H: Transient appearance of plasmablasts in the peripheral blood of
Japanese patients with severe fever with thrombocytopenia syndrome.
J Infect Dis. 220:23–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hu L, Kong Q, Yue C, Xu X, Xia L, Bian T,
Liu Y, Zhang H, Ma X, Yin H, et al: Early-Warning immune predictors
for invasive pulmonary aspergillosis in severe patients with severe
fever with thrombocytopenia syndrome. Front Immunol. 12:5766402021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li XK, Lu QB, Chen WW, Xu W, Liu R, Zhang
SF, Du J, Li H, Yao K, Zhai D, et al: Arginine deficiency is
involved in thrombocytopenia and immunosuppression in severe fever
with thrombocytopenia syndrome. Sci Transl Med. 10:eaat41622018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Aghbash PS, Hemmat N, Nahand JS, Shamekh
A, Memar MY, Babaei A and Baghi HB: The role of Th17 cells in viral
infections. Int Immunopharmacol. 91:1073312021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kervevan J and Chakrabarti LA: Role of
CD4+ T cells in the control of viral infections: Recent advances
and open questions. Int J Mol Sci. 22:5232021. View Article : Google Scholar :
|
|
123
|
Barnett LG, Simkins HMA, Barnett BE, Korn
LL, Johnson AL, Wherry EJ, Wu GF and Laufer TM: B cell antigen
presentation in the initiation of follicular helper T cell and
germinal center differentiation. J Immunol. 192:3607–3617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lam JH, Smith FL and Baumgarth N: B cell
activation and response regulation during viral infections. Viral
Immunol. 33:294–306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Takahashi T, Sano K, Suzuki T, Matsumura
T, Sakai K, Tominaga T, Sato Y, Katano H and Hasegawa H:
Virus-infected peripheral blood plasmablasts in a patient with
severe fever with thrombocytopenia syndrome. Int J Hematol.
113:436–440. 2021. View Article : Google Scholar
|
|
126
|
Wada Y, Miyamoto S, Iida S, Sano K, Sato
Y, Ainai A, Saito K, Katano H, Hasegawa H and Suzuki T: Propagation
of activated B cells by in vitro severe fever with thrombocytopenia
syndrome virus infection of human peripheral blood mononuclear
cells. J Infect Dis. 225:269–281. 2022. View Article : Google Scholar
|
|
127
|
Wada T, Iwata Y, Kamikawa Y, Wada T and
Yachie A: Peripheral blood plasmacytosis in severe fever with
thrombocytopenia syndrome. Jpn J Infect Dis. 70:470–471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang J, Yan X, Li Y, Gao R, Wang P and Mo
W: Reactive plasmacytosis mimicking multiple myeloma associated
with SFTS virus infection: A report of two cases and literature
review. BMC Infect Dis. 18:5282018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Suzuki T, Sato Y, Sano K, Arashiro T,
Katano H, Nakajima N, Shimojima M, Kataoka M, Takahashi K, Wada Y,
et al: Severe fever with thrombocytopenia syndrome virus targets B
cells in lethal human infections. J Clin Invest. 130:799–812. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mendoza CA, Ebihara A and Yamaoka S:
Immune modulation and immune-mediated pathogenesis of emerging
tickborne banyangviruses. Vaccines (Basel). 7:1252019. View Article : Google Scholar
|
|
131
|
Caobi A, Nair M and Raymond AD:
Extracellular vesicles in the pathogenesis of viral infections in
humans. Viruses. 12:12002020. View Article : Google Scholar :
|
|
132
|
Saad MH, Badierah R, Redwan EM and
El-Fakharany EM: A comprehensive insight into the role of exosomes
in viral infection: Dual faces bearing different functions.
Pharmaceutics. 13:14052021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Anka AU, Tahir MI, Abubakar SD, Alsabbagh
M, Zian Z, Hamedifar H, Sabzevari A and Azizi G: Coronavirus
disease 2019 (COVID-19): An overview of the immunopathology,
serological diagnosis and management. Scand J Immunol.
93:e129982021. View Article : Google Scholar
|