Introduction
Osteoarthritis (OA) is the most common degenerative
joint disease, which is characterized by cartilage damage, synovial
inflammation, joint capsule thickening and joint pain (1). To date, the treatment for OA has
primarily focused on the alleviation of pain and inflammation using
nonsteroidal anti-inflammatory drugs or other agents; however,
these drugs cannot delay the progression of OA (2,3).
Therefore, new therapeutic strategies are urgently needed, which
could delay the progression of OA.
Chondrocytes are a single cellular component of the
mature articular cartilage and serve a key role in its
degeneration. The cytoskeleton is composed of microfilaments,
microtubules and intermediate fibers, which are themselves composed
of actin, tubulin and vimentin, respectively. Notably, the
cytoskeleton is an important structure for maintaining the
morphology and function of chondrocytes (4,5).
Changes in the cytoskeleton can affect the signal transduction and
mechanical properties of chondrocytes, which can lead to OA
(6,7). Furthermore, destruction of the
chondrocyte cytoskeleton has been reported to be a key risk factor
leading to chondrocyte degeneration and OA (8). Therefore, the chondrocyte
cytoskeleton is the target of novel therapy research; in
particular, how to effectively protect the chondrocyte cytoskeleton
is the focus of the current treatment research. The RhoA/ROCK
signaling pathway, which is mainly composed of RhoA protein and
ROCK, is important in signal transduction and participates in
important physiological functions, such as cytoskeleton
reorganization, cell cycle and apoptosis regulation (9).
Hyaluronan synthase (HAS) is the key enzyme of
hyaluronic acid (HA) synthesis in vivo; therefore, the
expression of HAS directly affects the synthesis of endogenous HA.
There are three types of HAS isozymes: HAS-1, HAS-2 and HAS-3
(10). Different HAS isozymes
serve different roles in the synthesis of endogenous HA. HAS-2 has
the highest catalytic activity and expression in all stages of
embryonic development. High molecular weight HA synthesized by HAS
isozymes has an important role in joint movement and the
maintenance of intra-articular homeostasis (11,12). The HA receptor CD44 on the surface
of chondrocytes can interact with various cytoskeleton proteins,
and is related to the intracellular RhoA/ROCK signaling pathway and
a variety of cellular metabolic processes (13,14). However, as a key enzyme in the
synthesis of HA in vivo, it remains to be determined what
important role HAS-2 plays in maintaining normal chondrocyte
cytoskeleton morphology and in cartilage degeneration.
4-Methylumbelliferone (4-MU) is an approved drug for
treating biliary spasms, and it is also a highly effective and
low-toxic specific HAS inhibitor, which can reduce the synthesis of
HA by inhibiting HAS (15).
However, HA is also an important component of the extracellular
matrix at the site of chronic inflammation in a variety of
diseases, including type I diabetes, multiple sclerosis and various
malignant tumors (16). It has
previously been reported that when using 4-MU, the synthesis of HA
in the extracellular matrix of tumor tissues, such as mouse
pancreatic ductal adenocarcinoma, melanoma and hepatocellular
carcinoma, is decreased, inhibiting tumor invasion and metastasis
and thus resulting in an antitumor effect (17,18). However, to the best of our
knowledge, whether the clinical application of 4-MU will lead to
cartilage degeneration by inhibiting chondrocyte HAS-2 has not been
studied.
The present study aimed to determine whether HAS-2
is a key molecule regulating the chondrocyte cytoskeleton. In the
present study, HAS-2 was downregulated by 4-MU treatment and
HAS-2-short hairpin RNA (shRNA) transduction. The changes in the
chondrocyte cytoskeleton were detected in vitro, and the
possible mechanism underlying the abnormal chondrocyte cytoskeleton
induced by the downregulation of HAS-2 was assessed. In addition,
the degeneration of articular cartilage was evaluated following
intra-articular injection of 4-MU in rats, and the injury effect of
clinical application of 4-MU on articular cartilage was clarified.
The present results indicated that targeting HAS2 may be used to
develop new strategies for delaying chondrocyte degeneration and
for the early prevention of OA.
Materials and methods
Cell culture and animal grouping
The C28/I2 immortalized human chondrocyte line
(Haling Biotechnology Co., Ltd.) was cultured in DMEM/F-12
(Hyclone; Cytiva) containing 10% fetal bovine serum (Hyclone;
Cytiva) and 1% penicillin-streptomycin (Beijing Solarbio Science
& Technology Co., Ltd.) at 37°C in a humidified atmosphere
containing 5% CO2. Cells were harvested by
trypsinization and fresh culture medium was added to generate
single-cell suspensions for use in the subsequent experiments. The
culture medium was replaced with fresh medium every 48 h and the
cells were passaged every 2-3 days.
A total of 20 male Sprague-Dawley rats (age, 8
weeks; weight, 180-220 g) were obtained from the Animal Center of
Kunming Medical University (Kunming, China). The rats were housed
at a constant temperature (25°C) at 55% humidity under a 12-h
light/dark cycle with ad libitum access to water and
standard chow. Rats were divided into the following four groups
(n=5 rats/group): Control (normal saline), 1% DMSO, 1.0 mM 4-MU and
2.0 mM 4-MU. 4-MU (cat. no. M1381; MilliporeSigma) was dissolved in
DMSO (cat. no. D2650; MilliporeSigma) as a cosolvent and filtered
using a 0.22-µm sterile filter unit. The rats were
anesthetized by intraperitoneal injection of pentobarbital (35
mg/kg). According to the grouping, 100 µl saline, DMSO or
4-MU was injected into the left knee joint cavity of the rats using
a 1-ml syringe under aseptic conditions. The rats were injected
once every 3 days for a total of five times. On day 15, all of the
rats were sacrificed by cervical dislocation under anesthesia
through an intraperitoneal injection of pentobarbital sodium (50
mg/kg), and respiratory arrest was used to confirm animal death.
Subsequently, the left knee joint was collected and stored in 4%
paraformaldehyde for 24 h at 4°C. All animal experiments were
performed in accordance with the approval of the Institutional
Committee on the Care and Use of Animals of Kunming Medical
University (approval no. Kmmu20220992).
MTT assay
The MTT Cell Proliferation and Cytotoxicity Assay
Kit (cat. no. C0009; Beyotime Institute of Biotechnology) was used
to determine the proliferation curve of C28/I2 cells and to assess
the cytotoxic effects of 4-MU on C28/I2 cells, according to the
manufacturer's protocol. C28/I2 cells (5×103 cells/well)
were plated in seven 96-well plates in triplicate and cultured in
growth medium. The seven microplates were incubated for 24, 48, 72,
96, 120, 144 and 168 h, respectively. Subsequently, 10 µl
MTT solution was added to each well and the cells were incubated at
37°C for 4 h. Formazan solution (100 µl) was then added to
each well and incubated at 37°C for a further 4 h until all of the
purple crystals were dissolved. The optical density was measured at
570 nm using a microplate reader (SpectraMax 190; Molecular
Devices, LLC). In addition, cells were plated in 96-well plates in
triplicate and cultured in growth medium for 48 h. The adherent
cells were then divided into eight groups and treated with
different concentrations of 4-MU (0, 0.25, 0.50, 0.75 and 1.00 mM)
or DMSO (0, 0.125, 0.25, 0.375 and 0.5%) for another 48 h at 37°C.
The cytotoxic effects of different concentrations of 4-MU and DMSO
were determined using the aforementioned method.
Plasmid construction and
transduction
The shRNA targeting the HAS-2 gene was designed
using the Sigma shRNA design program (MilliporeSigma) and
synthesized by Generay Biotech Co., Ltd. The HAS-2-shRNA target
sequence was 5′-CGAAGCGATTATCACTGGATT-3′ and a scramble sequence
(5′-TTCGAAGAGGTTATCACTCAG-3′) was used as a negative control
(HAS-2-scramble). C28/I2 cells in the logarithmic growth phase were
seeded in 6-well plates at a concentration of 1×105
cells/well, and were cultured under saturated humidity at 37°C and
5% CO2 in DMEM/F-12 containing 10% fetal bovine serum.
When the cell density reached >80%, serum-free OPTI-MEM (Gibco;
Thermo Fisher Scientific, Inc.) was used for the culture.
pLKO.1-puro lentiviral vectors were used to silence HAS-2.
pLKO.1-shRNA-puro constructs were generated using a 2nd generation
lentiviral system by inserting the shRNA sequences into a
pLKO.1-puro vector (MiaoLing Plasmid Sharing Platform).
Lentiviruses were generated by transfecting 293T cells (China
Center for Type Culture Collection) with pLKO.1-shRNA (8
µg), packaging (psPAX2, 6 µg) and envelope (pMD2G, 2
µg) plasmids using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Lentiviral particles
were harvested 48 h post-transfection and were added to C28/I2
cells at a multiplicity of infection of 60. To create stable cell
lines, 24 h post-transduction, C28/I2 cells were selected using 2
µg/ml puromycin (MilliporeSigma) for 24 h and were
maintained in 1 µg/ml puromycin. At 48 h post-transduction,
the interference efficiency of HAS-2-shRNA was determined using
reverse transcription-quantitative PCR (RT-qPCR). All subsequent
experiments were performed 48 h post-transduction.
Western blotting
C28/I2 cells were cultured for 48 h and were then
treated with culture medium containing 4-MU (0, 0.25, 0.50, 0.75 or
1.00 mM) or 0.5% DMSO. After 48 h at 37°C, images of the C28/I2
cells were captured under a light inverted microscope and total
protein was subsequently isolated from C28/I2 cells. Briefly, cells
were lysed in RIPA buffer containing a protease inhibitor cocktail
(Beijing Solarbio Science & Technology Co., Ltd.). The
concentration of the protein samples was determined using the BCA
Protein Assay Kit (cat. no. P0010; Beyotime Institute of
Biotechnology). Equal volumes of protein samples (50
µg/lane) were separated by SDS-PAGE on 10% gels and
electroblotted onto a polyvinylidene fluoride membrane
(MilliporeSigma) using standard procedures. Membranes were blocked
for 1 h at room temperature with 5% non-fat milk in Tris-buffered
saline plus 0.5% Tween-20. The transferred blots were then
incubated sequentially with rabbit anti-HAS2 antibody (1:1,000;
cat. no. ab9485) and rabbit anti-GAPDH antibody (1:2,500; cat. no.
ab199794) (both from Abcam) overnight at 4°C. To examine the effect
of HAS-2-shRNA on the RhoA/ROCK signaling pathway, C28/I2 cells
were transduced with HAS-2-shRNA for 48 h. Subsequently, the target
protein blots were incubated with primary antibodies against: RhoA
(1:1,000; cat. no. 10749-1-AP), ROCK1 (1:1,000; cat. no.
21850-1-AP) and ROCK2 (1:1,000; cat. no. 21645-1-AP) (all from
Wuhan Sanying Biotechnology) overnight at 4°C. Subsequently, the
membranes were incubated with HRP-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. ab6721; Abcam) for 1 h at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence detection kit (Tiangen Biotech Co., Lt.) and
recorded on radiographic film (Bio-Rad Laboratories, Inc.).
Semi-quantitative analysis of protein band intensity was conducted
using ImageJ V1.8.0 software (National Institutes of Health) and
normalized to the internal loading control, GAPDH.
Immunofluorescence and semi-quantitative
analysis
C28/I2 cell suspensions were plated in a confocal
glass petri dish (diameter, 15 mm) in triplicate at
~5×104 cells (1 ml) per well and cultured in growth
medium for 48 h. The medium was then replaced and the cells were
cultured with medium containing different concentrations (0, 0.5,
and 1.0 mM) of 4-MU, or 0.5% DMSO for another 48 h at 37°C in a
humidified atmosphere containing 5% CO2. Subsequently,
the cells were fixed with 4% formaldehyde for 15 min at 37°C,
permeabilized with 0.1% Triton X-100 in PBS for 10 min at room
temperature, and blocked with 3% BSA (MilliporeSigma) and 0.05%
Tween 20 in PBS for 30 min at room temperature. For transduced
cells, after 48 h of transduction, cells were fixed as
aforementioned and underwent immunofluorescence staining. F-actin
was stained using the CytoPainter Phalloidin-iFluor 555 Reagent kit
(1:1,000; cat. no. ab176756; Abcam) overnight at 4°C and the nuclei
were stained with DAPI (Beijing Solarbio Science & Technology
Co., Ltd.) for 5 min at room temperature. Vimentin was stained with
anti-vimentin antibody (1:130; cat. no. ab92547; Abcam) overnight
at 4°C and goat anti-rabbit IgG H&L (Alexa Fluor®
488) (1:130; cat. no. ab150077; Abcam) for 1 h at room temperature
in the dark, and the nuclei were stained with DAPI for 5 min at
room temperature. Tubulin was stained with anti-β tubulin antibody
(1:400; cat. no. ab179513; Abcam) overnight at 4°C and goat
anti-rabbit IgG H&L (Alexa Fluor 488) (1:200) for 1 h at room
temperature in the dark, and the nuclei were stained with PI (1:20;
cat. no. ab14083; Abcam) for 5 min at room temperature.
Fluorescence images were acquired and semi-quantitative analysis
was performed using the Leica TCS SP5 laser scanning confocal
microscope (LSCM; Leica Microsystems GmbH) using the LAS AF Lite
2.3.6 Software (Leica Microsystems GmbH).
To perform statistical analysis of vimentin, actin
and tubulin staining, and to assess the variation in these
cytoskeleton components following treatment with 4-MU, the LSCM was
adjusted to the appropriate parameters. All of the parameters were
fixed to capture the confocal image under the condition of a ×20
objective lens. The fluorescence intensity of the cytoskeleton
components was measured using the LAS AF Lite2.3.6 software. The
fluorescence determination method is performed by operating the
mouse to outline a single cell, and the software automatically
calculates the fluorescence value of the cytoskeleton protein under
the corresponding fluorescence channel in the delineated area. The
fluorescence intensity of a certain cytoskeleton protein in a
single chondrocyte can be calculated according to this method. A
total of 20 chondrocytes for each cytoskeletal protein in each
group were measured and analyzed. In addition, the subcellular
structure images were collected under a ×100 objective lens, and
the morphology of the chondrocyte cytoskeleton proteins was
observed.
Relative quantification of mRNA using
RT-qPCR
After C28/I2 cells were transduced for 48 h, total
RNA was extracted using the RNeasy kit (Qiagen GmbH) according to
the manufacturer's protocol. Total RNA concentration was determined
by measuring the absorbance at 260/280 nm using a spectrophotometer
(BioTeke Corporation). Total RNA (1 µg) was then
reverse-transcribed using the High-Capacity cDNA RT kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
qPCR was performed using an ABI Prism 7900 sequence detection
system and 2X T5 Fast qPCR Mix (SYBR Green I) reagents (TsingKe
Biological Technology). GAPDH was utilized as a housekeeping gene
for normalizing mRNA expression levels. Each qPCR reaction
contained 10 µl 2X T5 Fast qPCR Mix, 0.8 µl primers
(10 µM; Table I), 0.4
µl 50X ROX Reference Dye I, 1 µl DNA template (20
ng/µl), and PCR-grade water to obtain a final volume of 20
µl. The thermal cycler conditions were as follows: Hold for
10 min at 95°C, followed by 40 cycles of a two-step PCR consisting
of a 95°C step for 10 sec and a 60°C step for 30 sec. For each
sample, qPCR reactions were performed in triplicate, and the
average Cq was calculated. The Cq values of the different samples
were compared using the 2−ΔΔCq method (19).
 | Table IPrimer sequences used in reverse
transcription-quantitative PCR. |
Table I
Primer sequences used in reverse
transcription-quantitative PCR.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| HAS-2 |
CAGATGGCTAAACCAGCAGACC |
GAATCCAGTGATAATCGCTTCGTAG |
| RhoA |
CAGGTAGAGTTGGCTTTGTGG |
TCTGCCTTCTTCAGGTTTCA |
| ROCKI |
TAACCTCCCAGAGTCAAGAATT |
TTTTGCTGCTTACCACAACATAC |
| ROCKII |
CTTTATCATTTCCCAACCAAC |
CCACTTCTGCTGCTCTTCTG |
| GAPDH |
AACGGATTTGGTCGTATTGGG |
CCTGGAAGATGGTGATGGGAT |
Cell apoptosis assays
Cell apoptosis was measured using the Annexin V-FITC
apoptosis detection kit (Beyotime Institute of Biotechnology) and a
flow cytometer (CyFlow Space; Sysmex Partec GmbH). Following
infection of C28/I2 cells with lentivirus-containing supernatant
for 48 h, apoptosis was detected. According to the manufacturer's
instructions, cells were incubated with 200 µl Annexin
V-FITC and 10 µl PI in the dark at 25°C for 10 min. The
stained cells were analyzed using flow cytometry. All of the
treatments were performed in triplicate. Cells positive for both PI
and Annexin V-FITC were considered late apoptotic cells, and those
negative for both Annexin V-FITC and PI were considered live cells.
PI-positive and Annexin V-FITC-negative cells were considered
necrotic cells, whereas PI-negative and Annexin V-FITC-positive
cells were considered early apoptotic cells.
Histological evaluation
The rat knee joint samples were decalcified in 10%
EDTA (pH 7.5). The decalcified specimens were trimmed, dehydrated
and embedded in paraffin. Sections (6 µm) were cut in the
sagittal plane, and were then deparaffinized with xylene and
dehydrated with ethanol. Heat-induced antigen retrieval was
performed with sodium citrate at 95°C for 30 min. Endogenous
peroxidase activity was blocked with 3% H2O2
for 20 min at room temperature and the treated sections were
blocked for 1 h at room temperature with 10% goat serum (Beijing
Solarbio Science & Technology Co., Ltd.). Subsequently, the
sections were incubated with rabbit anti-F-actin antibody (1:200;
cat. no. bs-1571R; Biosharp Life Sciences), anti-vimentin antibody
(1:130; cat. no. ab92547; Abcam) or anti-β tubulin antibody (1:400;
cat. no. ab179513; Abcam) overnight at 4°C. HRP-conjugated goat
anti-rabbit secondary antibody (1:1,000; cat. no. ab6721; Abcam)
was then added and incubated at 37°C for 30 min. Antigens were
detected using the DAB kit (Beyotime Institute of Biotechnology)
after 20 min of incubation at room temperature. Photomicrographs
were captured under a light microscope (BX53; Olympus
Corporation).
Hematoxylin and eosin (H&E)
staining
Tissue samples were dewaxed by soaking the sections
in xylene twice for 10 min, rinsing with distilled water for 30
sec, then soaking consecutively in 100, 100, 90, 80 and 70% alcohol
for 5 min, and finally rinsing with tap water for 5 min three
times. The samples were stained with hematoxylin (Beijing Solarbio
Science & Technology Co., Ltd.) for 5 min at room temperature
and rinsed with running water. Samples were then differentiated
with 5% acetic acid for 1 min, washed with running water for 10
min, stained with eosin (Beijing Solarbio Science & Technology
Co., Ltd.) for 1 min at room temperature, and rinsed three times
with running water, for 5 min. The slices were dehydrated by
placing them in 70, 80, 90 and 100% alcohol and xylene for 1 min
each, and were then sealed with neutral glue, and covered with a
coverslip. The tissues were examined using a light microscope
(BX53; Olympus Corporation).
Safranin O/fast green staining
The dewaxing procedure was performed according to
the H&E staining protocol, as aforementioned. The sections were
then stained with hematoxylin for 5 min and rinsed with tap water
for 10 min. The sections were dyed with 0.5% Fast Green FCF Stain
Solution (Beijing Solarbio Science & Technology Co., Ltd.) for
3 min and then washed with tap water for 5 min three times. The
sections were placed in 1% acetic acid for 5 sec, immersed in 1%
safranin staining solution (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 min at room temperature, and then
rinsed three times with distilled water for 5 min. Finally, the
samples were dehydrated and sealed as aforementioned for H&E
staining. The samples were examined using a light microscope (BX53;
Olympus Corporation).
Cartilage severity in the tibial plateau was
evaluated according to modified Mankin's histologic grading system,
and a cartilage destruction score was assigned for each knee sample
by three independent assessors. The Mankin's score assesses
structure, cellularity, matrix staining and tidemark integrity. For
matrix staining, safranin O staining was used. The Mankin's score
ranges from 0 points for healthy cartilage to 14 points for the
most severe cartilage lesions (20). The final score for each sample was
based on the most severe histological changes observed in multiple
sections from each specimen. The Mankin's score was again divided
into three stages depending on the score: Grade I (normal
cartilage, 0-1 points), grade II (mild to moderate degenerative
change, 2-9 points), and grade III (severe degenerative change, ≥10
points). A total of five rats were assessed in each group, giving a
total of 20 rats for all tests.
Statistical analysis
All data were analyzed using SPSS Statistics 23.0
software (IBM Corp.). Data are presented as the mean ± SD unless
stated otherwise. Graphs were drawn using GraphPad Prism software
(version 8.0). ImageJ (1.8.0; National Institutes of Health)
software was used for immunohistochemistry, immunofluorescence and
western blot analyses. For in vitro studies, the lowest
number of replicates per experiment was three. All data were tested
for normality of distribution using the Shapiro-Wilk test. One-way
analysis of variance with Tukey's post hoc test was used to compare
the parametric data between groups. For Mankin's score data, the
Kruskal-Wallis test with Dunn's post hoc test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
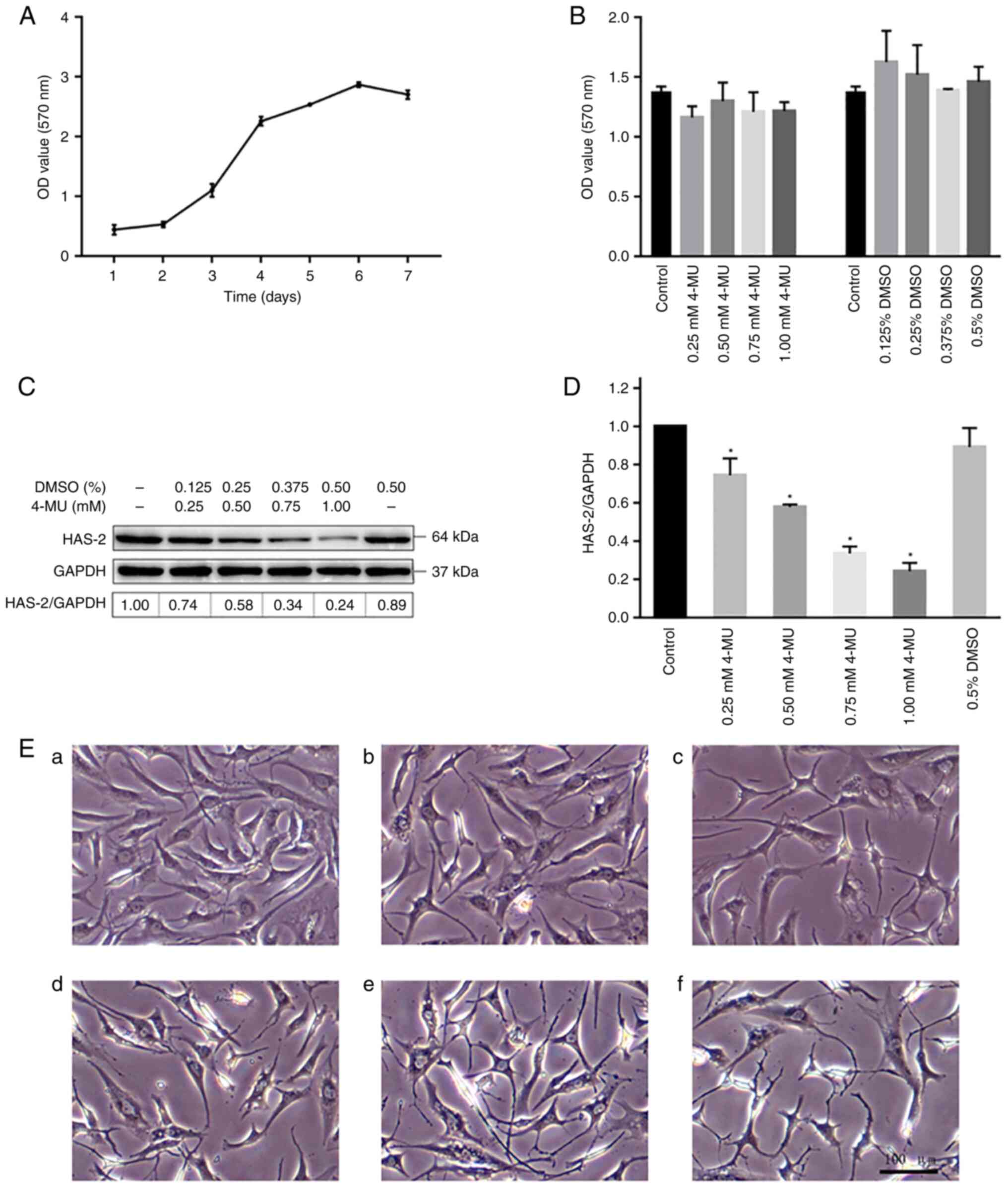
Determination of logarithmic growth
phase
The proliferation curve of C28/I2 cells was detected
by MTT assay (Fig. 1A). The cells
were in the growth incubation period and proliferated slowly 1-2
days after inoculation. The cells then proliferated rapidly in the
logarithmic growth phase from day 3 to 5, before entering the
plateau period on days 6-7. The whole proliferation curve is in the
shape of an 'S'. In the present study, all cell experiments were
performed in the logarithmic growth phase after 2 days of
subculture.
Effect of 4-MU and DMSO on C28/I2 cell
viability
The MTT assay was performed to assess the cytotoxic
effects of 4-MU and DMSO on C28/I2 cells. The C28/I2 cells were
treated with various concentrations of 4-MU (0, 0.25, 0.50, 0.75
and 1.00 mM) or DMSO (0, 0.125, 0.25, 0.375 and 0.5%) for 48 h.
After 48 h, cell cytotoxicity was tested using the MTT assay; no
significant cytotoxicity was observed regardless of the
experimental concentrations (Fig.
1B). Therefore, the present study adopted 4-MU at a
concentration of <1.0 mM and DMSO at a concentration of <0.5%
in the subsequent cell experiments.
4-MU inhibits the expression of
HAS-2
Western blot analysis was performed to investigate
the effect of 4-MU on the protein expression levels of HAS-2. After
C28/I2 cells were treated with 4-MU for 48 h, the total proteins
were extracted from each group. As shown in Fig. 1C and D, the protein expression
levels of HAS-2 were significantly decreased in cells in the 4-MU
groups compared with those in the control group in a
concentration-dependent manner. Since 4-MU is insoluble in water,
DMSO was used as a co-solvent in the present study. Therefore, it
is necessary to test whether the experimental concentration of DMSO
affects the expression of HAS-2 when 4-MU is used. However, there
was no significant difference in the expression levels of HAS-2 in
the DMSO group. Therefore, 4-MU was defined as an inhibitor of
HAS-2 in the subsequent experiments.
Effect of 4-MU on C28/I2 cell
morphology
The morphological changes of C28/I2 cells were
detected under an inverted microscope. C28/I2 cells were treated
with various concentrations of 4-MU (0, 0.25, 0.5, 0.75 and 1.0 mM)
for 48 h. The cells in the control and 0.5% DMSO groups were
uniform in size and small in shape, mainly small triangles and
polygons, and the intercellular background was clean and free of
impurities (Fig. 1Ea and b). 4-MU
was able to induce cell morphological changes in a
concentration-dependent manner. As shown in Fig. 1Ec-f, the morphology and size of
the cells were different, the cytoplasmic volume decreased, the
pseudopodia increased and became slender, and the intercellular
connections decreased; when cells were treated with 0.75 mM 4-MU,
the pseudopodia began to appear discontinuous or broken, the
background impurities increased and the cell adhesion ability
decreased. Thus, these results suggested that 4-MU could change the
morphology of chondrocytes in a dose-dependent manner and this was
not associated with DMSO.
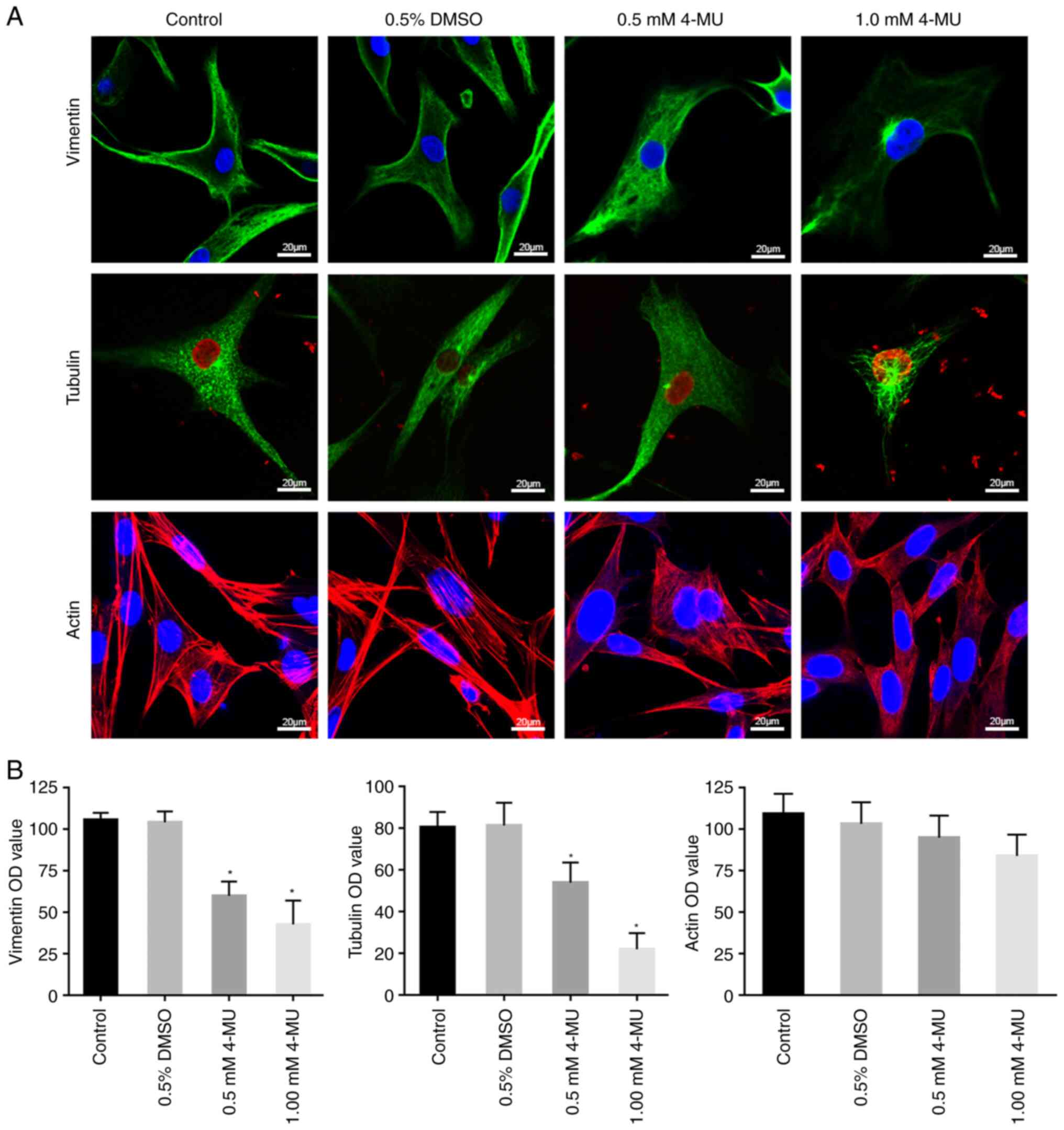
4-MU regulates the distribution and
expression of the chondrocyte cytoskeleton proteins
To better observe the fine structure of cytoskeleton
proteins, a LSCM was used to assess the morphology of cytoskeleton
proteins in individual cells. Fig.
2 shows representative images of immunofluorescence staining of
C28/I2 cells treated with 4-MU for 48 h.
As shown in Fig.
2A, vimentin was filamentous in the control and 0.5% DMSO
groups, forming an intertwined reticular structure that runs
through the cytoplasm, and it was denser near the cell membrane.
The arrangement of vimentin was disordered following treatment with
0.5 mM 4-MU; the polarity disappeared and the distribution
decreased. In the 1.0 mM 4-MU treatment group, the distribution of
vimentin was sparse and loose, and only local dense distribution
could be seen around the nucleus. The tubulin in the control and
0.5% DMSO groups appeared to be in a radial pattern, was uniformly
distributed from the nucleus to the cell membrane and dense radial
aggregation sites could be seen near the nucleus. Notably, the
distribution of tubulin was sparse and its density decreased in
response to 0.5 mM 4-MU, whereas in the 1.0 mM 4-MU treatment
group, the distribution of tubulin concentrated around the nucleus
and was markedly decreased near the cell membrane, and microtubule
breakage could be seen. The actin in the control and 0.5% DMSO
groups showed filamentous distribution around the cytoplasm near
the cell membrane and less around the nucleus; in addition, most of
the actin appeared as thick long stress filaments, arranged along
the longitudinal axis of the cells and a few actin fibers were
arranged in a reticular cross pattern. After 4-MU treatment, the
thick and long actin stress filaments disappeared and the fine
actin fibers were arranged in a sparse, disorganized network.
The present study semi-quantitatively measured the
fluorescence intensity of the cytoskeleton proteins, thus providing
a visual and clear indication of the content of these proteins. As
shown in Fig. 2B, the expression
of vimentin was high in the control and 0.5% DMSO groups. In the
4-MU groups, the expression of vimentin significantly decreased in
a concentration-dependent manner compared with that in the control
group. The expression of tubulin was similar to that of vimentin,
it also decreased in a concentration-dependent manner in response
to 4-MU. However, the expression of actin was slightly decreased in
the 4-MU treatment groups compared with that in the control group,
but this was not significant. These data indicated that 4-MU
regulates the distribution and expression of chondrocyte
cytoskeleton proteins by inhibiting HAS-2, and thus affects the
morphology of C28/I2 cells.
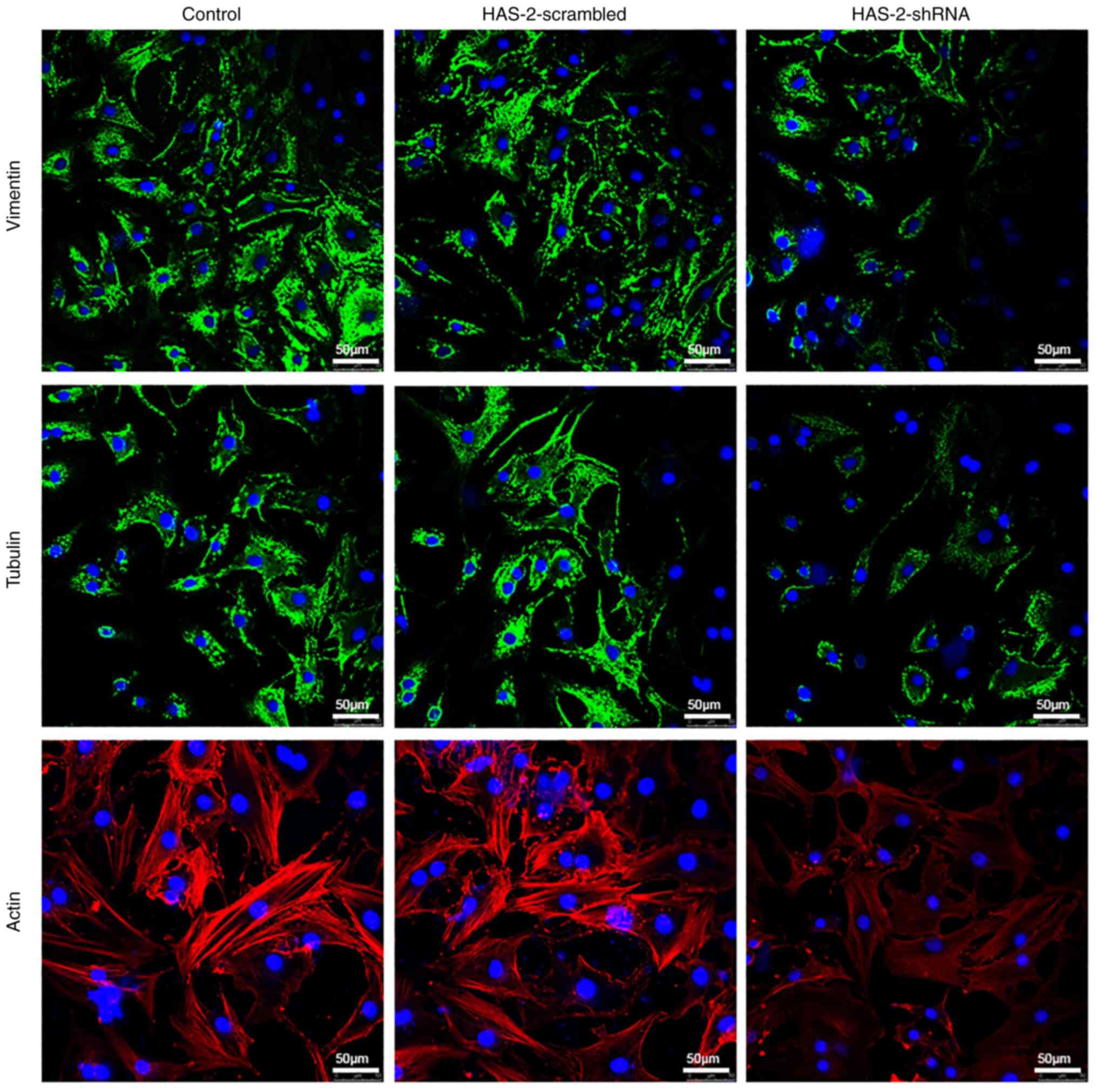
HAS-2-shRNA regulates the chondrocyte
cytoskeleton
To eliminate the multiple effects of compound
inhibitors, gene silencing was used to detect the effects of
HAS-2-shRNA on the chondrocyte cytoskeleton. The gene silencing
efficiency of HAS-2-shRNA was verified by RT-qPCR (Fig. S1). Subsequently, the chondrocyte
cytoskeleton was observed using a LSCM. As shown in Fig. 3, similar to the results of 4-MU
treatment, the expression levels of vimentin, tubulin and actin
were decreased after HAS-2-shRNA transduction. However, there was
no marked difference in the expression of cytoskeleton proteins
between the HAS-2-scramble and control groups. Therefore, this
verifies the regulatory role of HAS-2 on the chondrocyte
cytoskeleton at the genetic level.
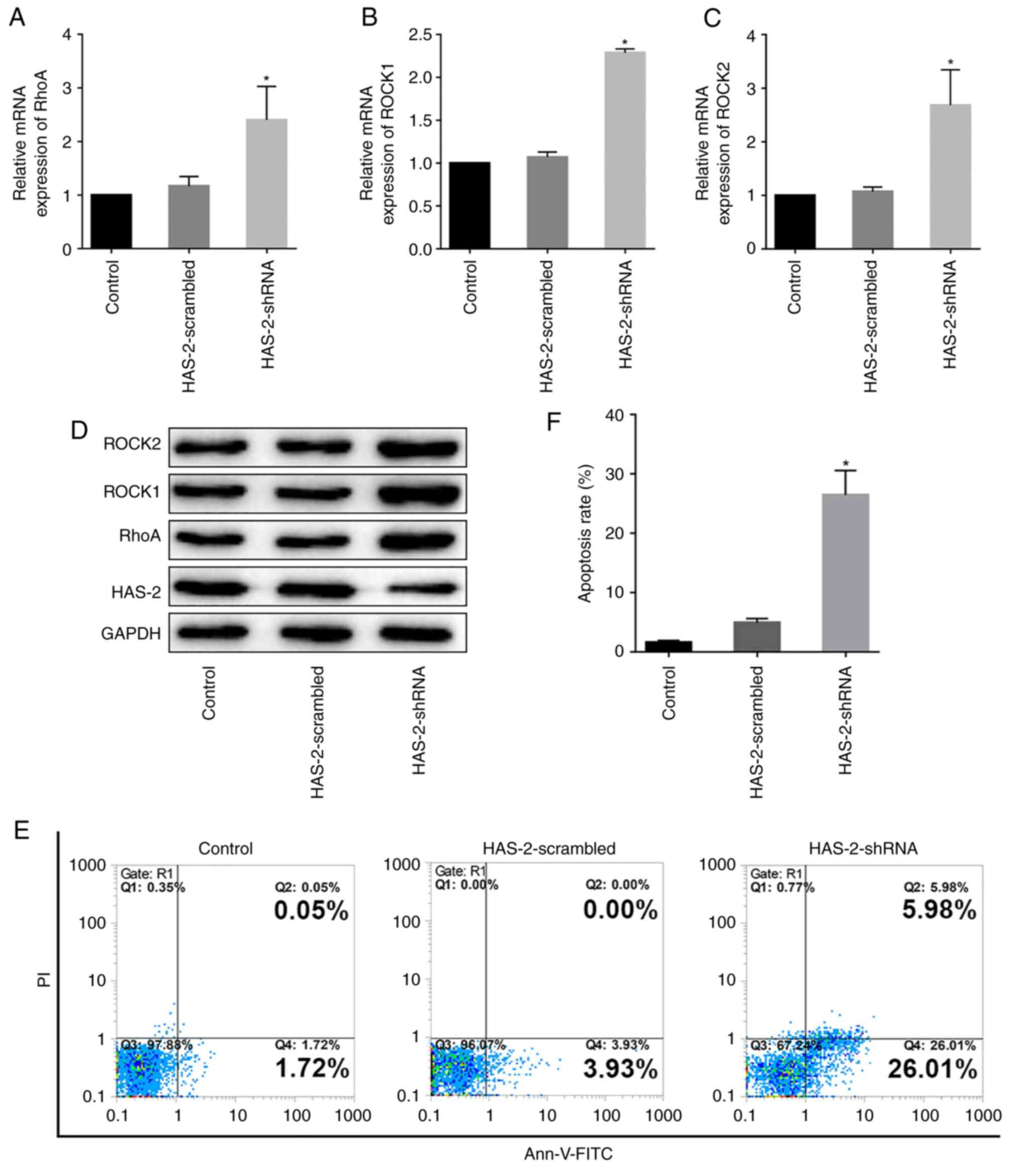
HAS-2-shRNA activates the RhoA/ROCK
signaling pathway and promotes chondrocyte apoptosis
It has been reported that changes in the chondrocyte
cytoskeleton are closely related to the RhoA/ROCK signaling pathway
(21). However, to the best of
our knowledge, the mechanism of HAS-2 in the regulation of the
chondrocyte cytoskeleton is not yet known. Therefore, the present
study detected changes in the RhoA/ROCK signaling pathway after
HAS-2 gene silencing; it was observed that RhoA, ROCK1 and ROCK2
expression levels were significantly increased at the gene and
protein levels (Fig. 4A-D). In
addition, the effect of HAS-2-shRNA on chondrocyte apoptosis was
assessed by flow cytometry. The results suggested that HAS-2-shRNA
was able to promote the apoptosis of chondrocytes (Fig. 4E and F). These results suggested
that downregulation of HAS-2 may activate the RhoA/ROCK signaling
pathway, thus participating in regulation of the cytoskeleton and
promoting chondrocyte apoptosis.
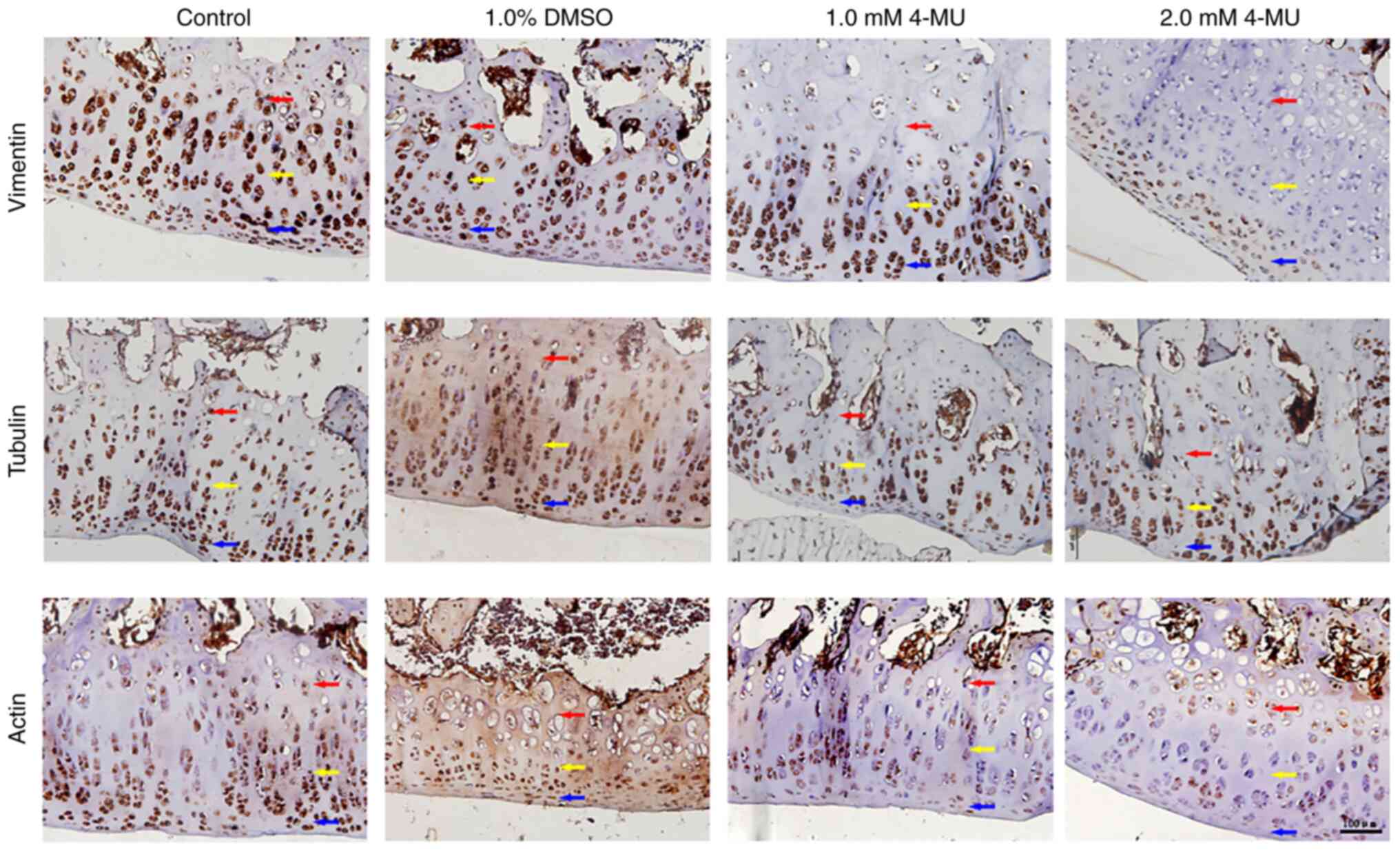
Immunohistochemical staining of
cytoskeleton proteins in cartilage tissue
Immunohistochemistry was performed to detect the
cytoskeleton proteins in rat articular cartilage samples. The
cytoplasm of positive cells showed deep brown-yellow staining and
the results are shown in Fig. 5.
In vimentin staining, the chondrocytes in the control and 1% DMSO
groups showed deep brown-yellow staining throughout the cartilage
layer. In the cartilage layer of the 1.0 mM 4-MU group, compared
with in the control group, the number of positive cells in the
superficial layer and the middle layer was not markedly different,
but the number of positive cells in the deep layer of cartilage was
notably reduced. In the 2.0 mM 4-MU group, positive cells were only
seen in the superficial layer of cartilage, but no positive cells
were found in the middle and deep layers. In tubulin staining,
positive brown-yellow-stained cells were seen in the cartilage
layer of each group. Although the chondrocytes in the 4-MU group
were disorderly arranged, there was no notable difference in the
number of positive cells compared with that in the control group.
In actin staining, the chondrocytes in the control and 1% DMSO
groups showed deep brown-yellow staining throughout the cartilage
layer. In the cartilage layer of the 1.0 and 2.0 mM 4-MU groups,
the number of positive cells was markedly decreased, but the number
of positive cells was similar between the two groups. Taken
together, these results suggested that 4-MU may also change the
distribution and expression of the cytoskeleton in cartilage in
vivo.
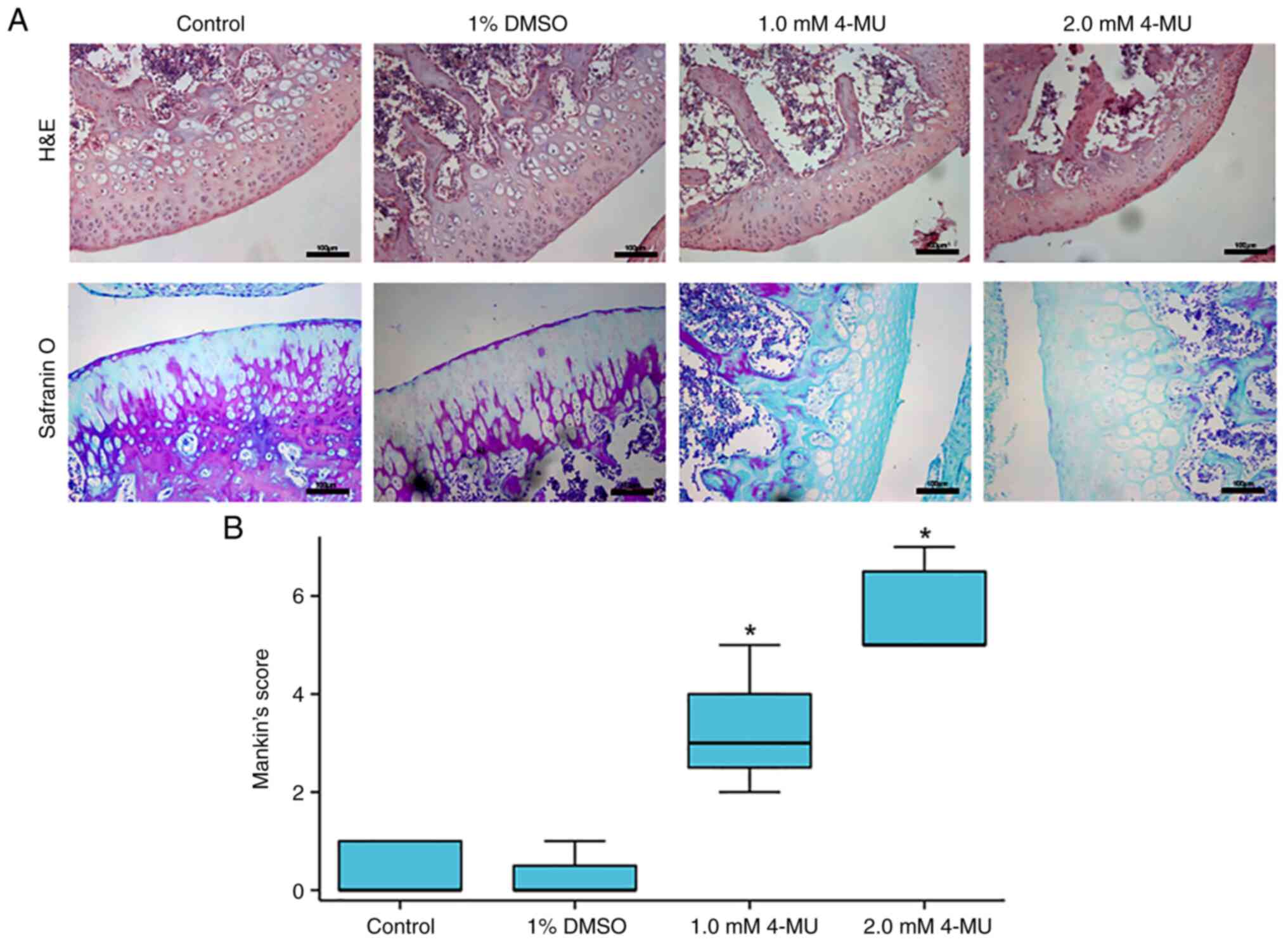
4-MU-induced inhibition of HAS-2 leads to
cartilage degeneration in vivo
To investigate the effects of 4-MU-induced
inhibition of HAS-2 expression on the progression of articular
cartilage degeneration in vivo, histological analysis of
cartilage was measured by H&E and safranin O staining, and
cartilage degeneration was evaluated by Mankin's score. As shown in
Fig. 6A, in the control and 1%
DMSO groups, the articular cartilage was smooth and flat, the
chondrocytes were arranged neatly, and the tidemark was regular.
The safranin O staining results were similar in the two groups;
enhanced staining was found in the articular surface and near the
subchondral bone area in the cartilage layer. In the H&E
staining images of the 1.0 mM 4-MU group, the articular cartilage
surface was not smooth, the cartilage layer was slightly thinner,
the arrangement of chondrocytes was irregular and the tidemark was
complete. Notably, in the 2.0 mM 4-MU group, there was severe
fibrosis on the surface of articular cartilage, chondrocytes were
atrophied, exhibited a clustered distribution and disordered
arrangement, the tidemark was still complete and cartilage tissue
injury was more serious. In the safranin O staining images, the two
4-MU groups exhibited substantial loss of safranin O staining
(indicative of aggrecan proteoglycan loss) occurring in the
cartilage layer, and there was no marked difference in the staining
between the two 4-MU groups.
According to the results of H&E and safranin O
staining, the knee cartilage of rats was scored using the modified
Mankin's histologic grading system (Fig. 6B). The scores in the control and
1% DMSO groups were low, thus indicating that there was no
significant degeneration of cartilage tissue. Following injection
with 4-MU, the degree of cartilage degeneration was significantly
increased in a concentration-dependent manner. The Mankin's scores
were significantly increased in the 4-MU groups compared with those
in the control group. The degree of cartilage degeneration in the
4-MU groups appears to have reached the level of early and
intermediate OA.
Discussion
OA, the most prevalent musculoskeletal disorder
worldwide, is more frequent in older age groups and is developing
into an increasingly important public health concern (22-24). Treatment of OA is limited to
drugs, such as HA, corticosteroids and opioids (25,26). For example, intra-articular
injection of HA has a wide range of applications in relieving OA
pain and improving joint function; however, these drugs only
temporarily ease swelling and chronic pain. In addition, these
drugs can lead to deleterious side effects (23). Furthermore, repeated
intra-articular injection brings pain and risk to patients. In the
end-stage of OA, artificial joint replacement is the only choice
for OA therapy (27). Therefore,
it is necessary to identify a safe and effective therapeutic target
that could ameliorate the clinical symptoms of OA and delay the
progression of OA. The present study reported the potential
mechanism of cartilage degeneration following downregulation of
HAS-2. Notably, to the best of our knowledge, the present study is
the first in which HAS-2 has been reported to be involved in
cartilage degeneration, which may provide a new target for
follow-up research and the treatment of OA.
The present study used the C28/I2 cell line, which
consists of immortalized normal human chondrocytes with good
homogeneity. The proliferation curve of C28/I2 cells was generated
and the logarithmic growth phase was determined by MTT assay.
Moreover, the toxicity of 4-MU to C28/I2 cells was detected by MTT
assay. Because 4-MU is insoluble in water, DMSO was used as a
cosolvent in the present study. Therefore, it was necessary to
detect whether the experimental concentration of DMSO had cytotoxic
effects while using 4-MU. The results revealed that both the
experimental concentration of 4-MU and the corresponding
concentration of DMSO had no cytotoxic effect on chondrocytes and
did not affect their proliferation. Subsequently, western blotting
was performed to detect the effects of 4-MU on the expression
levels of HAS-2 in chondrocytes. Because β-actin is not suitable
for the study of the cytoskeleton (28), GAPDH was used as the internal
reference protein (29). The
results suggested that 4-MU could inhibit the expression of HAS-2
in chondrocytes in a concentration-dependent manner. A number of
studies have used 4-MU to downregulate the function of HA, and to
reveal or confirm the biological events that depend on HA (30,31). Therefore, 4-MU was used as an
inhibitor of HAS-2 in the present study.
The present study also identified a notable change
in the morphology of C28/I2 cells treated with 4-MU. Related
studies have shown that 4-MU inhibits the synthesis of HA by
consuming UDP-glucuronic acid and downregulating HAS-2 (15,32). As aforementioned, HAS-2-catalyzed
synthesis of high molecular weight HA plays an important role in
joint movement and maintaining intra-articular homeostasis. The HA
receptor CD44 on the surface of chondrocytes can interact with
various cytoskeleton proteins to activate various
cytoskeleton-mediated cellular activities, such as adhesion,
proliferation and migration (14,33). In addition, HA can reduce the
levels of IL-1β in synovial fluid, and IL-1β can inhibit the
expression of cytoskeleton-related genes, such as Fhl2, Vim and
Tubb (34). These studies
indicated that HAS-2 is closely related to the chondrocyte
cytoskeleton. Previous studies have reported that HAS-2 appears to
serve a key role in the production of HA, which is essential for
normal cartilage matrix organization and retention (35). Reduction in the formation of
proteoglycan aggregates due to defective HA production in
HAS-2-mutant growth plates is very likely to result in the
decreased deposition of aggrecan in the matrix (36). 4-MU has been shown to inhibit HA
production in various cell lines and tissue types in vitro
and in vivo (15).
Therefore, the present study did not measure the levels of HA; we
hypothesize that the morphological changes of chondrocytes may be
due to changes in the chondrocyte cytoskeleton caused by the
inhibition of HAS-2 by 4-MU. Using immunofluorescence, the
morphology of the cytoskeleton was observed under a LSCM, and the
relative content of each cytoskeleton protein was calculated. The
results showed that the expression of cytoskeleton proteins was
decreased in a dose-dependent manner after 4-MU treatment, and the
expression of vimentin and tubulin was significantly decreased.
This is consistent with the changes in the chondrocyte cytoskeleton
in OA (37,38). It has been reported that the
cytoskeleton also plays an important role in phenotypic regulation,
mechanical properties, and matrix synthesis and metabolism of
chondrocytes. The cytoskeleton of articular chondrocytes in
patients with OA was previously studied, and it was revealed that
the intermediate fibers and microtubules of the cytoskeleton were
significantly altered (37,39). Similar findings have also been
reported in rat articular chondrocytes (38). However, as a compound inhibitor,
the multiple effects of 4-MU on the body cannot be ruled out;
therefore, the present study directly silenced HAS-2 to verify the
regulatory impact of HAS-2 on the chondrocyte cytoskeleton from the
pre-transcriptional level. The results revealed that HAS-2-shRNA
could also lead to morphological changes and decreased expression
of the cytoskeleton proteins of chondrocytes, which was similar to
the findings observed in cells treated with 4-MU.
The RhoA/ROCK signaling pathway is an important
pathway in the process of signal transduction (9). The RhoA protein is activated by
mechanical and inflammatory stimulation, which can further activate
downstream molecules, such as ROCK, mDia and PKN. As an important
downstream signal molecule of RhoA protein, ROCK contains two
isoforms, ROCK I and ROCK II. After phosphorylation by RhoA
protein, ROCK can further mediate a series of downstream
phosphorylation or dephosphorylation reactions, and can participate
in regulation of the chondrocyte cytoskeleton (40-42). These previous studies have shown
that the changes in the chondrocyte cytoskeleton are closely
related to RhoA/ROCK signaling pathway. Therefore, we hypothesized
that the downregulation of HAS-2 may activate the RhoA/ROCK
signaling pathway and cause changes in the chondrocyte
cytoskeleton. HAS-2-shRNA was used to transduce chondrocytes and
the results of RT-qPCR and western blotting showed significant
increases in RhoA, ROCK1 and ROCK2 expression at the gene and
protein levels. However, RhoA acts as a GTPase, and it is also
important to assess the 'on/off' state of these proteins (RhoA,
ROCK1/2). In follow-up studies, we aim to explore the
phosphorylation status of these proteins. In addition, the present
study further assessed the effect of HAS-2 on the apoptosis of
C28/I2 cells. The results revealed that HAS-2-shRNA promoted the
apoptosis of chondrocytes. These data supported that the
downregulation of HAS-2 may activate the RhoA/ROCK signaling
pathway, causing changes in the chondrocyte cytoskeleton and
promoting apoptosis of chondrocytes. Previous studies have also
shown that the RhoA/ROCK signaling pathway can regulate the
chondrocyte cytoskeleton, lead to changes in mechanical signal
transduction and biomechanical properties of chondrocytes, promote
apoptosis of chondrocytes and induce cartilage degeneration
(21,43,44), which is consistent with the
present results.
In OA, chondrocyte senescence and cartilage
degeneration are accompanied by significant changes in the
cytoskeleton (7,45). It has previously been shown that
the cytoskeleton network of chondrocytes in old (31-months) rabbit
chondrocytes is sparse, and the content of various cytoskeleton
proteins is significantly lower than that in adult (8 months) and
young (2 months rabbit chondrocytes. Compared with the chondrocytes
in young and adult chondrocytes, the response of chondrocytes in
old cells to mechanical stimulation is different because of their
viscoelastic changes, which is related to the changes in the
structure and cytoskeleton composition of chondrocytes (46). Some studies have used specific
inhibitors of cytoskeleton proteins to study their effects on the
biomechanics of chondrocytes. The results show that microfilaments
and intermediate fibers mainly provide the viscoelasticity of
chondrocytes. Notably, the changes in the structure and properties
of cytoskeleton proteins reflect the changes in the chondrocyte
cytoskeleton during OA (47).
These aforementioned studies have shown that the abnormal changes
of the chondrocyte cytoskeleton are closely related to cartilage
degeneration. To explore whether the change in the cytoskeleton of
chondrocytes can cause cartilage degeneration and lead to OA after
HAS-2 inhibition, animal experiments were carried out in the
present study. The results showed that 15 days after 4-MU was
injected into the knee joint of Sprague-Dawley rats, the Mankin's
score of cartilage tissue was significantly increased. The score
was >5 in the 2.0 mM 4-MU group, thus indicating that the degree
of cartilage degeneration had reached the level of early and
mid-stage OA. Immunohistochemistry of cytoskeleton proteins was
also carried out on the knee cartilage tissue sections. The results
revealed that the number of vimentin-positive cells in the
cartilage layer was markedly decreased after 4-MU injection, and
only a small number of vimentin-positive cells could be found in
the superficial layer of cartilage in the 2.0 mM 4-MU group.
Immunohistochemical staining of actin revealed that the number of
actin-positive cells was also decreased in the 4-MU groups.
However, there was no notable change in the number of
tubulin-positive cells in each group. These findings may be due to
the short modeling time, the slow formation of OA and the slow
degeneration of cartilage (48).
Notably, the morphological changes in the chondrocyte cytoskeleton
may be quickly detected in vitro with 4-MU, but the
cartilage damage is not obvious in the short term because of the
complexity of regulation and compensatory mechanisms in
vivo. However, after inhibiting HAS-2 in vivo, vimentin
was the first damaged cytoskeleton protein, followed by actin, and
tubulin changed last. Therefore, these findings suggested that the
downregulation of HAS-2 can destroy the chondrocyte cytoskeleton,
cause cartilage degeneration and induce OA. According to a previous
study, 4-MU is expected to become an effective antitumor drug
widely used in the clinic (18).
Unexpectedly, if 4-MU is put into a clinical application as an
antineoplastic drug, long-term use may cause cartilage degeneration
and even induce OA.
In summary, the present study demonstrated that the
downregulation of HAS-2 could activate the RhoA/ROCK pathway, cause
abnormal chondrocyte morphology and decrease the expression of
chondrocyte cytoskeleton proteins, leading to changes in signal
transduction and the biomechanical properties of chondrocytes,
promotion of chondrocyte apoptosis and thus the induction of
cartilage degeneration. Unfortunately, the present study did not
verify the expression of HAS-2 in patients with OA or OA model
animals. In addition, this study did not perform rescue experiments
by adding HA to explore whether the pathological effect after HAS-2
knockout can be reversed. These experiments will be the focus of
our follow-up research. Furthermore, future research will also
concentrate on elucidating the key molecular mechanisms by which
HAS-2 alters the cytoskeleton of chondrocytes. In addition, the
clinical application of 4-MU may cause cartilage degeneration. If
the expression of intra-articular HAS-2 can be upregulated at the
genetic or molecular level in the early stage of OA, this may not
only delay the occurrence and development of OA, but also greatly
reduce the pain and risk caused by repeated intra-articular
injection of HA. The latest research shows that HAS-2
overexpression diminishes the procatabolic activity of chondrocytes
by a mechanism independent of extracellular hyaluronan (49), and nanotherapy-based HAS2 delivery
into the joints may increase endogenous hyaluronan production, thus
providing a convenient and efficient way to promote the
self-repairing mechanism for OA management (50). Therefore, research on the
regulation of HAS-2 as a target may provide a novel therapeutic
strategy for delaying chondrocyte degeneration, and for the early
prevention and treatment of OA.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLY and YZ conceived and designed the experiments.
JLY, YZ and LW performed the experiments. ZJZ and QS were
responsible for designing and conducting the statistical analysis
of the data and providing critical feedback on the interpretation
of the results. JLY and ZJZ wrote the original draft of the
manuscript. JLY and QS edited and finalized the final version of
the manuscript. JLY and YZ confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Animal ethics approval was received from the
Institutional Animal Ethics Review Board of Kunming Medical
University (approval no. Kmmu20220992).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by funds from the National
Natural Sciences Foundation of China (grant no. 81560366) and the
Guizhou Province Science and Technology Achievement Application and
Industrialization Program (Clinical Special Project) [grant no. LC
(2022) 024].
References
|
1
|
French HP, Galvin R, Horgan NF and Kenny
RA: Prevalence and burden of osteoarthritis amongst older people in
Ireland: Findings from The Irish LongituDinal study on ageing
(TILDA). Eur J Public Health. 26:192–198. 2016. View Article : Google Scholar
|
|
2
|
Roman-Blas JA, Bizzi E, Largo R, Migliore
A and Herrero-Beaumont G: An update on the up and coming therapies
to treat osteoarthritis, a multifaceted disease. Expert Opin
Pharmacother. 17:1745–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao T, Guo W, Chen M, Huang J, Yuan Z,
Zhang Y, Wang M, Li P, Peng J, Wang A, et al: Extracellular
vesicles and autophagy in osteoarthritis. Biomed Res Int.
2016:24289152016. View Article : Google Scholar
|
|
4
|
Lauer JC, Selig M, Hart ML, Kurz B and
Rolauffs B: Articular chondrocyte phenotype regulation through the
cytoskeleton and the signaling processes that originate from or
converge on the cytoskeleton: Towards a novel understanding of the
intersection between actin dynamics and chondrogenic function. Int
J Mol Sci. 22:32792021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon S and Kim KS: Qualitative analysis of
contribution of intracellular skeletal changes to cellular
elasticity. Cell Mol Life Sci. 77:1345–1355. 2020. View Article : Google Scholar
|
|
6
|
Trickey WR, Lee GM and Guilak F:
Viscoelastic properties of chondrocytes from normal and
osteoarthritic human cartilage. J Orthop Res. 18:891–898. 2000.
View Article : Google Scholar
|
|
7
|
Holloway I, Kayser M, Lee DA, Bader DL,
Bentley G and Knight MM: Increased presence of cells with multiple
elongated processes in osteoarthritic femoral head cartilage.
Osteoarthritis Cartilage. 12:17–24. 2004. View Article : Google Scholar
|
|
8
|
Blain EJ: Involvement of the cytoskeletal
elements in articular cartilage homeostasis and pathology. Int J
Exp Pathol. 90:1–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vigetti D, Genasetti A, Karousou E, Viola
M, Clerici M, Bartolini B, Moretto P, De Luca G, Hascall VC and
Passi A: Modulation of hyaluronan synthase activity in cellular
membrane fractions. J Biol Chem. 284:30684–30694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Recklies AD, White C, Melching L and
Roughley PJ: Differential regulation and expression of hyaluronan
synthases in human articular chondrocytes, synovial cells and
osteosarcoma cells. Biochem J. 354:17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiscock DR, Caterson B and Flannery CR:
Expression of hyaluronan synthases in articular cartilage.
Osteoarthritis Cartilage. 8:120–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Queisser KA, Mellema RA and Petrey AC:
Hyaluronan and its receptors as regulatory molecules of the
endothelial interface. J Histochem Cytochem. 69:25–34. 2021.
View Article : Google Scholar :
|
|
14
|
Knudson W, Ishizuka S, Terabe K, Askew EB
and Knudson CB: The pericellular hyaluronan of articular
chondrocytes. Matrix Biol. 78-79:32–46. 2019. View Article : Google Scholar
|
|
15
|
Kultti A, Pasonen-Seppänen S, Jauhiainen
M, Rilla KJ, Kärnä R, Pyöriä E, Tammi RH and Tammi MI:
4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of
cellular UDP-glucuronic acid and downregulation of hyaluronan
synthase 2-3. Exp Cell Res. 315:1914–1923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagy N, Kuipers HF, Frymoyer AR, Ishak HD,
Bollyky JB, Wight TN and Bollyky PL: 4-methylumbelliferone
treatment and hyaluronan inhibition as a therapeutic strategy in
inflammation, autoimmunity, and cancer. Front Immunol. 6:1232015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudo D, Suto A and Hakamada K: The
development of a novel therapeutic strategy to target hyaluronan in
the extracellular matrix of pancreatic ductal adenocarcinoma. Int J
Mol Sci. 18:6002017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiz G, Molejon MI, Malvicini M, Sukowati
CHC, Tiribelli C, Mazzolini G and Breccia JD: Glycosylated
4-methylumbelliferone as a targeted therapy for hepatocellular
carcinoma. Liver Int. 42:444–457. 2022. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Afara I, Prasadam I, Crawford R, Xiao Y
and Oloyede A: Non-destructive evaluation of articular cartilage
defects using near-infrared (NIR) spectroscopy in osteoarthritic
rat models and its direct relation to Mankin score. Osteoarthritis
Cartilage. 20:1367–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Appleton CT, Usmani SE, Mort JS and Beier
F: Rho/ROCK and MEK/ERK activation by transforming growth
factor-alpha induces articular cartilage degradation. Lab Invest.
90:20–30. 2010. View Article : Google Scholar
|
|
22
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poulet B and Staines KA: New developments
in osteoarthritis and cartilage biology. Curr Opin Pharmacol.
28:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altman RD: Practical considerations for
the pharmacologic management of osteoarthritis. Am J Manag Care.
15(8 Suppl): S236–S243. 2009.PubMed/NCBI
|
|
25
|
Varela-Eirín M, Varela-Vázquez A,
Guitián-Caamaño A, Paíno CL, Mato V, Largo R, Aasen T, Tabernero A,
Fonseca E, Kandouz M, et al: Targeting of chondrocyte plasticity
via connexin43 modulation attenuates cellular senescence and
fosters a pro-regenerative environment in osteoarthritis. Cell
Death Dis. 9:11662018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Theleman JL, Lygrisse KA and Wang
J: Epigenetic mechanisms underlying the aging of articular
cartilage and osteoarthritis. Gerontology. 65:387–396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeGroot J, Verzijl N, Bank RA, Lafeber FP,
Bijlsma JW and TeKoppele JM: Age-related decrease in proteoglycan
synthesis of human articular chondrocytes: The role of nonenzymatic
glycation. Arthritis Rheum. 42:1003–1009. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leipzig ND, Eleswarapu SV and Athanasiou
KA: The effects of TGF-beta1 and IGF-I on the biomechanics and
cytoskeleton of single chondrocytes. Osteoarthritis Cartilage.
14:1227–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu T, Yang K, You H, Chen A, Wang J, Xu K,
Gong C, Shao J, Ma Z, Guo F and Qi J: Regulation of PTHrP
expression by cyclic mechanical strain in postnatal growth plate
chondrocytes. Bone. 56:304–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishizuka S, Askew EB, Ishizuka N, Knudson
CB and Knudson W: 4-Methylumbelliferone diminishes catabolically
activated articular chondrocytes and cartilage explants via a
mechanism independent of hyaluronan inhibition. J Biol Chem.
291:12087–12104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terabe K, Ohashi Y, Tsuchiya S, Ishizuka
S, Knudson CB and Knudson W: Chondroprotective effects of
4-methylumbelliferone and hyaluronan synthase-2 overexpression
involve changes in chondrocyte energy metabolism. J Biol Chem.
294:17799–17817. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kakizaki I, Kojima K, Takagaki K, Endo M,
Kannagi R, Ito M, Maruo Y, Sato H, Yasuda T, Mita S, et al: A novel
mechanism for the inhibition of hyaluronan biosynthesis by
4-methylumbelliferone. J Biol Chem. 279:33281–33289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bourguignon LY: Hyaluronan-mediated CD44
activation of RhoGTPase signaling and cytoskeleton function
promotes tumor progression. Semin Cancer Biol. 18:251–259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Joos H, Albrecht W, Laufer S, Reichel H
and Brenner RE: IL-1beta regulates FHL2 and other
cytoskeleton-related genes in human chondrocytes. Mol Med.
14:150–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bastow ER, Byers S, Golub SB, Clarkin CE,
Pitsillides AA and Fosang AJ: Hyaluronan synthesis and degradation
in cartilage and bone. Cell Mol Life Sci. 65:395–413. 2008.
View Article : Google Scholar
|
|
36
|
Matsumoto K, Li Y, Jakuba C, Sugiyama Y,
Sayo T, Okuno M, Dealy CN, Toole BP, Takeda J, Yamaguchi Y and
Kosher RA: Conditional inactivation of Has2 reveals a crucial role
for hyaluronan in skeletal growth, patterning, chondrocyte
maturation and joint formation in the developing limb. Development.
136:2825–2835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kouri JB, Jiménez SA, Quintero M and Chico
A: Ultrastructural study of chondrocytes from fibrillated and
non-fibrillated human osteoarthritic cartilage. Osteoarthritis
Cartilage. 4:111–125. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Capín-Gutiérrez N, Talamás-Rohana P,
González-Robles A, Lavalle-Montalvo C and Kourí JB: Cytoskeleton
disruption in chondrocytes from a rat osteoarthrosic (OA)-induced
model: Its potential role in OA pathogenesis. Histol Histopathol.
19:1125–1132. 2004.
|
|
39
|
Lambrecht S, Verbruggen G, Verdonk PCM,
Elewaut D and Deforce D: Differential proteome analysis of normal
and osteoarthritic chondrocytes reveals distortion of vimentin
network in osteoarthritis. Osteoarthritis Cartilage. 16:163–173.
2008. View Article : Google Scholar
|
|
40
|
Strzelecka-Kiliszek A, Mebarek S,
Roszkowska M, Buchet R, Magne D and Pikula S: Functions of Rho
family of small GTPases and Rho-associated coiled-coil kinases in
bone cells during differentiation and mineralization. Biochim
Biophys Acta Gen Subj. 1861:1009–1023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Langelier E, Suetterlin R, Hoemann CD,
Aebi U and Buschmann MD: The chondrocyte cytoskeleton in mature
articular cartilage: Structure and distribution of actin, tubulin,
and vimentin filaments. J Histochem Cytochem. 48:1307–1320. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohashi K, Fujiwara S and Mizuno K: Roles
of the cytoskeleton, cell adhesion and rho signalling in
mechanosensing and mechanotransduction. J Biochem. 161:245–254.
2017.PubMed/NCBI
|
|
43
|
Yang K, Wu Y, Cheng P, Zhang J, Yang C, Pi
B, Ye Y, You H, Chen A, Xu T, et al: YAP and ERK mediated
mechanical strain-induced cell cycle progression through RhoA and
cytoskeletal dynamics in rat growth plate chondrocytes. J Orthop
Res. 34:1121–1129. 2016. View Article : Google Scholar
|
|
44
|
Wang L, Chen G, Xiao G, Han L, Wang Q and
Hu T: Cylindrospermopsin induces abnormal vascular development
through impairing cytoskeleton and promoting vascular endothelial
cell apoptosis by the Rho/ROCK signaling pathway. Environ Res.
183:1092362020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen C, Xie J, Rajappa R, Deng L, Fredberg
J and Yang L: Interleukin-1β and tumor necrosis factor-α increase
stiffness and impair contractile function of articular
chondrocytes. Acta Biochim Biophys Sin (Shanghai). 47:121–129.
2015. View Article : Google Scholar
|
|
46
|
Duan W, Wei L, Zhang J, Hao Y, Li C, Li H,
Li Q, Zhang Q, Chen W and Wei X: Alteration of viscoelastic
properties is associated with a change in cytoskeleton components
of ageing chondrocytes from rabbit knee articular cartilage. Mol
Cell Biomech. 8:253–274. 2011.
|
|
47
|
Trickey WR, Vail TP and Guilak F: The role
of the cytoskeleton in the viscoelastic properties of human
articular chondrocytes. J Orthop Res. 22:131–139. 2004. View Article : Google Scholar
|
|
48
|
Ramos YF and Meulenbelt I: The role of
epigenetics in osteoarthritis: Current perspective. Curr Opin
Rheumatol. 29:119–129. 2017. View Article : Google Scholar
|
|
49
|
Ishizuka S, Tsuchiya S, Ohashi Y, Terabe
K, Askew EB, Ishizuka N, Knudson CB and Knudson W: Hyaluronan
synthase 2 (HAS2) overexpression diminishes the procatabolic
activity of chondrocytes by a mechanism independent of
extracellular hyaluronan. J Biol Chem. 294:13562–13579. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li H, Guo H, Lei C, Liu L, Xu L, Feng Y,
Ke J, Fang W, Song H, Xu C, et al: Nanotherapy in joints:
Increasing endogenous hyaluronan production by delivering
hyaluronan synthase 2. Adv Mater. 31:e19045352019. View Article : Google Scholar : PubMed/NCBI
|