Introduction
Adipose tissue transplantation, pioneered in 1889,
is considered an important regenerative strategy for soft tissue
augmentation of the breast or face in patients with soft tissue
deficiency, as well as for cosmetic surgery purposes (1). For transplantation, autologous or
allogeneic adipose tissue is harvested from selected donor sites,
isolated into viable adipocytes and adipose-derived mesenchymal
stem cells (AD-MSCs) by processing, and then grafted to the target
site (1,2). Owing to the availability of
abundant donor tissues, easy procedures and relatively low
immunogenicity, adipose tissue has become a promising filling
material for reconstruction (3).
For successful engraftment of the transplanted tissue in
recipients, it is crucial to create and maintain an integral
environment within the graft through various strategies, including
neovascularization and de novo adipogenic differentiation
(4,5). However, tissue damage during the
transplantation stage and failures in reorganizing these
environments within adipose tissue after transplantation can
trigger the collapse of the graft construct, resulting in major
side effects, such as a 50-90% resorption of the total injection
volume, necrosis and cyst formation (2,5,6).
Although various approaches have been utilized for stabilizing the
overall integrity of adipose grafts, including biofabrication of
materials mimicking the characteristic properties of fat tissue and
the stimulation of stem cells to differentiate into viable
adipocytes, these methods have shown limited effectiveness when
applied alone (5,7-9).
Therefore, it has become increasingly necessary to discover new
adjuvant materials or methods that can improve the efficiency of
adipose tissue engraftment and provide a viable environment after
grafting.
Nervonic acid (NA) is a monounsaturated very
long-chain fatty acid primarily localized in mammalian nerve
tissues in the brain (10).
Because of its role in maintaining and forming the main component
of myelinated nerves, NA has been studied as a biomarker or
therapeutic candidate in various neuronal diseases, such as
Alzheimer's disease and depression (10,11). Growing evidence over the last few
decades has indicated that NA is also related to fatty acid
metabolism, affecting metabolic phenotypes, and may thus be
considered a novel diagnostic marker of metabolic disorders.
Keppley et al (12)
reported that the treatment of obese mice with NA could improve
energy metabolism and promote sphingolipid recovery, indicating
that NA may influence adipocytic characteristics. Recently, our
previous study discovered that NA treatment promoted adipogenesis
and elevated several lipid metabolism-associated genes in human
MSCs (13). Based on these
results, it may be hypothesized that NA treatment could have
adjuvant functions in adipogenic differentiation and fat
engraftment.
The present study investigated the adjuvant effects
of NA on adipogenesis and adipose tissue engraftment, as well as
its underlying mechanisms, using both in vitro and in
vivo experiments. To address this, MSCs were treated with NA
during adipogenic differentiation, after which, the affected
signaling pathways were identified. Additionally, adipose tissue
grafts were transplanted alongside NA and the factors affecting
adipocyte viability were investigated. The findings indicated that
NA may be a potential agent for improving the outcomes of adipose
tissue engraftment.
Materials and methods
Isolation and culture of MSCs
Human Wharton's jelly, placenta, umbilical cord
blood and adipose tissue samples were obtained from women after
they had given birth at Samsung Medical Center (Seoul, South Korea)
between September 2016 and September 2024, and were used to isolate
MSCs. Each type of tissue was obtained from one individual. All
participants provided written informed consent, and the procedures
were approved by the Institutional Review Board of Samsung Medical
Center (approval no. 2016-07-102-001; Seoul, South Korea). AD-MSCs
were isolated from adipose tissues according to a previously
reported method (14).
Placenta-derived MSCs were isolated from the placenta, following a
previously described method (15). Umbilical cord blood-derived MSCs
were isolated from umbilical cord blood, as previously reported
(16). Wharton's jelly-derived
MSCs (WJ-MSCs) were isolated from the separated umbilical cord, as
previously described (17).
Human bone marrow-derived MSCs (cat. no. PT-2501) were purchased
from Lonza Group, Ltd. All MSCs were cultured in α-modified minimum
essential medium supplemented with 10% fetal bovine serum and 50
μg/ml gentamicin (all from Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a 5% CO2 incubator. MSCs at passage 7
were used in the experiments.
Cytotoxicity measurement
Cytotoxicity was measured using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.), according to
the manufacturer's instructions. AD-MSCs were seeded at a density
of 1×103 cells/well in 96-well culture plates and were
incubated overnight. AD-MSCs were treated with different
concentrations of NA (0, 40, 80, 120, 160, 200, 240 and 280
μM) or an equal volume of dimethyl sulfoxide (DMSO) as a
control, and then incubated for 3 or 7 days at 37°C. For treatment,
NA [≥99% (capillary GC); cat. no. 506-37-6; MilliporeSigma] was
prepared as a 50 mM stock solution in DMSO (MilliporeSigma).
Subsequently, they were incubated with 10 μl CCK-8 at 37°C
for 2 h. An equal volume of medium without cells was used as a
blank. The absorbance was measured at 450 nm using a microplate
reader (Thermo Scientific™ Multiskan SkyHigh Microplate
Spectrophotometer; cat. no. A51119600C; Thermo Fisher Scientific,
Inc.).
Adipogenic differentiation assay
MSCs were seeded in 6-well culture plates at a
density of 1×105 cells/well and were incubated until the
cells reached 80-90% confluence. For adipogenic differentiation,
MSCs were cultured in an adipogenesis differentiation medium
(StemPro™ Adipogenesis Differentiation kit; cat. no. A1007001;
Gibco; Thermo Fisher Scientific, Inc.) containing different
concentrations of NA (0, 40, 80, 120, 160, 200, 240 and 280
μM), according to the manufacturer's instructions. The
control group was treated with an equal volume of DMSO in the same
differentiation medium. After 7, 14 and 21 days, the differentiated
cells were fixed with 4% paraformaldehyde (Biosaesang) for 10 min
and stained with Oil Red O solution (MilliporeSigma) for 30 min at
25°C. Subsequently, lipid droplets were observed under a light
microscope (CKX41; Olympus Corporation). Oil Red O was quantified
by extraction in 100% isopropyl alcohol and the optical density was
measured at 540 nm.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was obtained from the differentiated
AD-MSCs using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Next, complementary DNA (cDNA) was synthesized
from the total RNA using SuperScript IV Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions, and was further purified using a
QIAquick PCR Purification kit (Qiagen GmbH). qPCR was performed by
adding cDNA to a mixture containing the respective primers and SYBR
green master mix (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using the QuantiStudio™ 6 Flex Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The qPCR conditions
were as follows: Pre-denaturation at 50°C for 2 min and 95°C for 10
min; followed by 40 cycles of amplification at 95°C for 30 sec,
60°C for 30 sec and 72°C for 30 sec; and a melting curve stage at
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. The specific
primer sequences are listed in Table
I. GAPDH was used as the housekeeping gene, and the
results were analyzed using the 2−ΔΔCq method (18).
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward | Reverse |
|---|
| Human
PPARG |
5′-ATGGGTGAAACTCTGGGAGA-3′ |
5′-TGGAATGTCTTCGTAATGTGGA-3′ |
| Human
CEBPA |
5′-ACTGGGACCCTCAGCCTTG-3′ |
5′-TGGACTGATCGTGCTTCGTG-3′ |
| Human
ADIPOQ |
5′-AGATCCAGGTCTTATTGGTCC-3′ |
5′-CTTTCCTGCCTTGGATTCC-3′ |
| Human
LPL |
5′-CATTCCCGGAGTAGCAGAG-3′ |
5′-ATTCCTGTTACCGTCCAGC-3′ |
| Human
GAPDH |
5′-GAAGGTGAAGGTCGGAGT-3′ |
5′-TGGCAACAATATCCACTTTACCA-3′ |
| Mouse
Pparg |
5′-GCATTTCTGCTCCACACTAT-3′ |
5′-CTTTGATCGCACTTTGGTATTC-3′ |
| Mouse
Lep |
5′-TGTGGCTTTGGTCCTATCT-3′ |
5′-CGACTGCGTGTGTGAAAT-3′ |
| Mouse
Lpl |
5′-GTGGATAAGCGACTCCTACT-3′ |
5′-TCTCCCTAGCACAGAAGATG-3′ |
| Mouse
Tnf |
5′-CCACGCTCTTCTGTCTACT-3′ |
5′-CAGGCTTGTCACTCGAATTT-3′ |
| Mouse
Il10 |
5′-TTACTGACTGGCATGAGGAT-3′ |
5′-AGAAAGTCTTCACCTGGCT-3′ |
| Mouse
Angpt1 |
5′-GAAGATGGAAGCCTGGATTT-3′ |
5′-CTGCCTCTGACTGGTTATTG-3′ |
| Mouse
Tek |
5′-TCCCTCCTCAACCAGAAA-3′ |
5′-TTTGCCCTGAACCTTATACC-3′ |
| Mouse
Vegfa |
5′-CTCTTCTCGCTCCGTAGTA-3′ |
5′-CCTCTCCTCTTCCTTCTCTT-3′ |
| Mouse
Gapdh |
5′-TTCAACGGCACAGTCAAG-3′ |
5′-CCAGTAGACTCCACGACATA-3′ |
Antibodies and reagents
The following primary antibodies were used for
western blotting and immunohistochemistry experiments: PPARγ (cat.
no. 2435), CEBPα (cat. no. 2295), adiponectin (cat. no. 2789),
phosphorylated (p)-Akt (Ser473; cat. no. 9271), Akt (cat. no.
9272), p-mTOR (Ser2448; cat. no. 5536), mTOR (cat. no. 2983), GSK3β
(cat. no. 9315), p-β-catenin (Ser33/37/Thr41; cat. no. 9561),
β-catenin (cat. no. 9562), p-Smad1/5 (Ser463/465; cat. no. 9516),
Smad1 (cat. no. 9743), p-ERK1/2 (Thr202/Tyr204; cat. no. 9101),
ERK1/2 (cat. no. 9102), perilipin-1 (cat. no. 9349) and CD31 (cat.
no. 77699), which were purchased from Cell Signaling Technology,
Inc.; and lipoprotein lipase (LPL; cat. no. sc-373759) and β-actin
(cat. no. sc-47778), which were obtained from Santa Cruz
Biotechnology, Inc. HRP-conjugated anti-rabbit (cat. no. abc-5003)
or anti-mouse (cat. no. abc-5001) secondary antibodies (AbClon)
were used as secondary antibodies in the western blot analysis.
Western blot analysis
To determine the levels of adipogenic markers in
AD-MSCs, the cells were cultured for 14 days in adipogenesis
differentiation medium with either DMSO or NA. The cells were then
lysed with RIPA buffer (Biosaesang) + protein inhibitor cocktail
(GenDEPOT) + EDTA (GenDEPOT) and centrifuged at 18,000 × g for 30
min at 4°C to collect the lysate. Protein concentration was
quantified using the Bradford assay (Bio-Rad Laboratories, Inc.).
Protein extracts (10 μg/lane) were then separated by
SDS-PAGE on 4-15% polyacrylamide gels (Bio-Rad Laboratories, Inc.).
Subsequently, the separated proteins were transferred onto
nitrocellulose or polyvinylidene fluoride membranes, which were
blocked with 5% skim milk (Difco; BD Biosciences) or 5% bovine
serum albumin (BSA) (Thermo Fisher Scientific, Inc.) in TBS-0.2%
Tween-20 (TBS-T) and were gently shaken for 60 min at 25°C. The
membranes were then incubated with primary antibodies (1:1,000
dilutions in 5% skim milk or 5% BSA) overnight at 4°C with gentle
shaking. After three washes in TBS-T (10 min/wash) at 25°C, the
membranes were incubated with HRP-conjugated anti-rabbit or
anti-mouse secondary antibodies (1:5,000 dilutions in 5% skim milk
or 5% BSA) at 25°C for 1 h with gentle shaking. Finally, the
membranes were washed three times with TBS-T (10 min/wash), were
treated with ECL solution (Bio-Rad Laboratories, Inc.) for 1 min,
were visualized using an Amersham™ Imager 600 (Cytiva), and were
semi-quantified using ImageJ 1.53 software (National Institutes of
Health). The primary and secondary antibodies used in western
blotting are aforementioned.
mRNA sequencing
Total RNA was isolated for mRNA sequencing from
differentiating AD-MSCs at 7, 14 and 21 days using
TRIzol® reagent (cat. no. 15596018; Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
mRNA sequencing was conducted by Ebiogen Inc. RNA quality was
assessed using the TapeStation4000 System (Agilent Technologies,
Inc.) and RNA was quantified using an ND-2000 spectrophotometer
(Thermo Fisher Scientific, Inc.). NEBNext Ultra II Directional
RNA-Seq kit (cat. no. E7760L; New England BioLabs, Inc.) was used
to prepare the sequencing libraries from total RNA, and each step
was performed using reagents included within the kit. The mRNA was
further isolated from total RNA using a Poly(A) RNA Selection kit
(cat. no. LEX-157; Lexogen GmbH). Subsequently, cDNA was
synthesized from the isolated mRNAs (10 min at 25°C; 15 min at
42°C; 15 min at 70°C for first strand cDNA and 1 h at 16°C for
second strand cDNA), and removal of RNA was followed by
random-primed synthesis of the complementary strand. The cDNA was
sheared according to the manufacturer's instructions of the NEBNext
Ultra II Directional RNA-Seq kit. Indexing was performed using
Illumina indices 1-12. Library enrichment was performed using PCR.
The libraries were checked for quality control using TapeStation HS
D1000 Screen Tape (Agilent Technologies, Inc.) to evaluate the mean
fragment size. Quantification was performed on the StepOne
Real-Time PCR System (Thermo Fisher Scientific, Inc.) using a
random primer and NEBNext Ultra II Q5 Master Mix (included in the
NEBNext Ultra II Directional RNA-Seq kit). High-throughput
sequencing (paired-end 100 bp sequencing) was performed with a
loading concentration of 320 pM using NovaSeq 6000 (Illumina,
Inc.).
Data analysis
First, FastQC was used for quality control of the
raw sequencing data (19).
Adapter and low-quality reads (<Q20) were removed using
FASTX_Trimmer (20) and BBMap
(21). Trimmed reads were mapped
to the reference genome using TopHat (22). The read counts were calculated
using Cufflinks (23) and the
fragments per kb per million reads (FPKM) values were estimated
using Cuffdiff. The FPKM values were normalized using Cuffdiff
geometric normalization. Data mining and graphic visualization were
performed using ExDEGA ver 4.0.3 (ebiogen, Inc.). Data for each
sample are available at the Gene Expression Online (GEO) under the
accession number GSE269065 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269065).
The differentially expressed genes (DEGs) between
the DMSO and NA groups that showed a fold change >1.5 and
P<0.05 were selected and further analyzed using ExDEGA software
and DAVID Bioinformatics Resource 6.8 (NCBI online bioinformatics
program) (https://david.ncifcrf.gov/tools.jsp) (24,25) to identify the functions of the
selected genes and Gene Ontology. Adjusted P≤0.05 (Fisher's Exact
test) was used to select related functional annotations. The
principal component analysis (PCA) and the heatmaps of gene
expression were generated using Excel 2016 (Microsoft) and ExDEGA
software. Additionally, enriched signaling pathways related to the
selected DEGs were analyzed using the online Kyoto Encyclopedia of
Genes and Genomes (KEGG) mapper tool (https://www.kegg.jp/kegg/mapper.html) (26,27).
In vivo fat transplantation
Fat transplantation experiments were performed using
5-month-old C57BL/6 mice (ORIENT BIO, Inc.) as both adipose tissue
donors and recipients. Both male and female mice were used
randomly, which weighed 22-28 g, and had free access to food and
water. A total of 33 mice were used for the experiments.
Specifically, 15 mice (10 donors, 5 recipients) were used for fat
graft measurements and gene expression analysis, and 18 mice (12
donors, 6 recipients) were used for histological analysis. Mice for
the experiments were housed in individually ventilated cages with a
maximum of five mice per cage. The housing room was air-conditioned
with 100% HEPA-filtration. The internal temperature and humidity
were maintained at 20-26°C and 30-70%, respectively, and mice were
kept under a 12-h light/dark cycle. For fat extraction, mice were
anesthetized with 2% isoflurane and allogeneic adipose tissues for
transplantation were extracted from the peritoneal fat of mice
after laparotomy. The extracted adipose tissues were finely chopped
and centrifuged at 2,095 × g for 15 min at 25°C. Subsequently, the
upper and lower fractions containing lipids and blood,
respectively, were removed to collect pure adipose tissue for
engraftment. Thereafter, adipose tissue was transplanted underneath
the skin of the back on both sides: Adipose tissue (300 μl)
mixed with 160 μM NA was transplanted into the right side of
the back, whereas adipose tissue (300 μl) mixed with the
same volume of DMSO was transplanted into the left side. For
transplantation, mice were anaesthetized with 2% isoflurane. After
transplantation, the health and well-being of the mice were
assessed three times a week and transplants were harvested and
analyzed after 5 weeks. At transplant harvest, the mice were
euthanized using CO2 (35% volume/min) in a chamber for
3-4 min. The death of the mice was confirmed through the cessation
of heartbeat and loss of reflex. The graft volume was calculated
using the formula reported by Tomayko and Reynolds (28). For histological analysis, the
obtained grafts were frozen and sectioned (12 μm) for Oil
Red O staining using NovaUltra Oil Red O Stain kit (cat. no.
IW-3008; IHC World LLC) according to the manufacturer's
instructions. In addition, grafts were embedded in paraffin and
sectioned (4 μm) for immunohistochemistry and hematoxylin
& eosin (H&E) staining.
The present study was reviewed and approved by the
Institutional Animal Care and Use Committee of the Research
Institute for Future Medicine (RIFM), belonging to Samsung Medical
Center (approval nos. 20230316003 and 20240522001). The RIFM is an
Association for Assessment and Accreditation of Laboratory Animal
Care International-accredited facility that abides by the
guidelines of the Institute of Laboratory Animal Research (29).
Immunohistochemistry
For immunohistochemistry, adipose tissues from mice
were embedded in paraffin and sectioned (4 μm) after
fixation in 4% paraformaldehyde for 36 h at 25°C. First, the
samples were deparaffinized in a gradually decreasing concentration
of ethanol and were permeabilized using xylene. Antigen retrieval
was then performed using Target Retrieval Solution, Citrate pH 6
(Dako; Agilent Technologies, Inc.), washed and blocked in Dako REAL
Peroxidase-Blocking Solution (Dako; Agilent Technologies, Inc.).
After washing with PBS three times (5 min/wash), the samples were
treated with primary antibodies (1:200) at 4°C. The next day, the
primary antibodies were removed, and the samples were washed three
times with PBS. They were then treated with Dako Envision +
System-HRP Labelled Polymer Anti-Rabbit Antibody (cat. no. K4003,
Dako; Agilent Technologies, Inc.) for 1 h and stained with Dako
Liquid DAB (Dako; Agilent Technologies, Inc.) for 1 min at 25°C.
Finally, the samples were stained with hematoxylin for nuclear
staining for 4 sec at 25°C, then dehydrated and cleared before
observation. Aperio AT2 (Leica Biosystems) was used for
observation, and staining was semi-quantified using ImageJ 1.53
software or QuPath 0.4.4. software (30). The aforementioned primary
antibodies were used for immunohistochemistry.
H&E staining
For H&E staining, adipose tissues from mice were
embedded in paraffin and sectioned (4 μm) after fixation in
4% paraformaldehyde for 36 h at 25°C. First, 4-μm
paraffin-embedded samples were permeabilized using Histoclear
(National Diagnostics) for 9 min (3 min × 3), and were rehydrated
using 100 and 95% ethanol. The samples were then stained with
hematoxylin for 8 min, exposed to HCl for 1 sec and eosin for 40
sec at 25°C, and washed with PBS between each step. Subsequently,
the sections were dehydrated with 95 and 100% ethanol, and were
treated with Histoclear. Finally, the samples were mounted, and
staining was observed using Aperio AT2.
Graphical illustrations
Graphical illustrations presented in the present
study were generated using BioRender (https://www.biorender.com/).
Statistical analysis
All data are presented as the mean ± SD (n≥3). Excel
2016 (Microsoft Corporation) and GraphPad Prism for Windows ver. 10
(Dotmatics) were used for all analyses. Statistical comparisons
between two groups were performed using unpaired Student's t-test,
and those between three or more groups were performed using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
NA promotes adipogenesis of human
AD-MSCs
To determine the optimal concentration of NA that
induces adipogenesis in AD-MSCs, the formation of oil droplets was
examined in AD-MSCs following treatment with various concentrations
of NA. First, the cytotoxicity of NA was determined in AD-MSCs
using a CCK-8 assay. The optical density of AD-MSCs was measured on
days 3 and 7 post-treatment with DMSO or several concentrations of
NA (40-280 μM). The viability of AD-MSCs was not
significantly altered at day 3 or 7 after NA treatment compared
with that in the control group (Fig.
1A). Therefore, NA was confirmed to be non-toxic to AD-MSCs.
Notably, AD-MSCs treated with ≥120 μM NA exhibited partial
formation of oil droplets at day 3 in culture conditions,
indicating that NA is a potential adipogenic inducer (Fig. 1B). Based on these results,
AD-MSCs were treated with DMSO or NA during differentiation into
the adipogenic lineage for 7 days, and the degree of lipid
accumulation was determined using Oil Red O staining.
Concentrations of NA up to 160 μM enhanced lipid
accumulation in AD-MSCs in a dose-dependent manner (Fig. 1C and D). Thus, 160 μM was
considered the optimal concentration of NA for promoting
adipogenesis in AD-MSCs and was used in subsequent experiments.
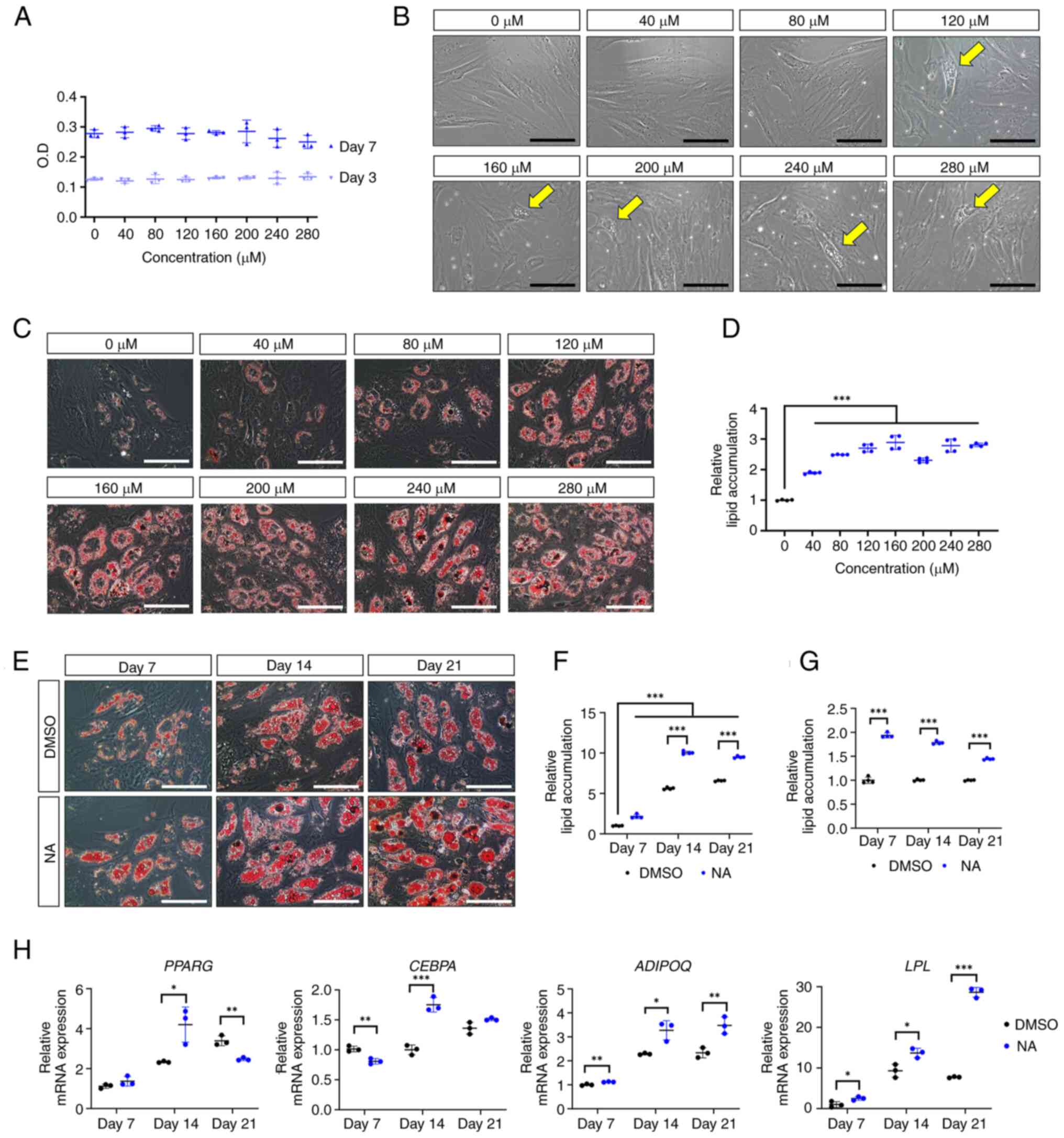 | Figure 1Effect of NA on adipogenesis. (A)
Cell viability of AD-MSCs as measured by O.D. values at day 3 and 7
after treatment with DMSO or serial doses of NA. (B) Representative
images of AD-MSCs after 72 h of NA treatment. Yellow arrows
indicate the oil droplets formed after NA treatment in the normal
proliferation condition. Scale bars: 100 μm. (C)
Representative images of Oil Red O staining of AD-MSCs on day 7
after adipogenic differentiation and treatment with DMSO or NA.
Scale bars: 100 μm. (D) Relative amounts of lipid
accumulation after 7 days of adipogenic differentiation in cells
treated with DMSO or NA. Statistical significance was assessed by
one-way ANOVA: ***P<0.001. (E) Representative images
of Oil Red O staining of AD-MSCs after 7, 14 and 21 days of
adipogenic differentiation and treatment with DMSO or 160 μM
NA. Scale bars: 100 μm. (F and G) Relative amounts of lipid
accumulation after 7, 14 and 21 days of adipogenic differentiation
and treatment with DMSO or 160 μM NA. (F) Increase in lipid
accumulation by differentiation period and NA treatment. Relative
lipid accumulation was normalized to the DMSO group at day 7.
Statistical significance was assessed by one-way ANOVA:
***P<0.001. (G) Change in lipid accumulation at each
differentiation timepoint (7, 14 and 21 days) in the NA group
versus the DMSO group. Relative lipid accumulation was normalized
to the DMSO group at each time point. Statistical significance was
assessed by unpaired Student's t-test: ***P<0.001.
(H) Reverse transcription-quantitative PCR analysis of adipogenic
markers after 7, 14 and 21 days of adipogenic differentiation and
treatment with DMSO or 160 μM NA. Statistical significance
was assessed by unpaired Student's t-test: *P<0.05,
**P<0.01 and ***P<0.001. Data are
presented as the mean ± SD. AD-MSCs, adipose-derived mesenchymal
stem cells; DMSO, dimethyl sulfoxide; LPL, lipoprotein
lipase; NA, nervonic acid; O.D., optical density. |
Next, the effect of NA on adipogenic differentiation
according to different exposure periods was assessed. Adipogenic
conditions were induced for 7, 14 and 21 days, and lipid
accumulation was measured using Oil Red O staining (Fig. 1E). AD-MSCs treated with NA
exhibited sufficient differentiation from day 14 onward, resulting
in a significant increase in lipid accumulation compared with the
DMSO control group (Fig. 1F).
However, lipid accumulation caused by NA showed the greatest
difference on day 7 relative to the control (day 7, 1.94±0.05 fold
change; day 14, 1.78±0.03 fold change; day 21, 1.45±0.02 fold
change) (Fig. 1G). Because NA
promoted the adipogenic differentiation of AD-MSCs, the present
study further investigated whether NA could influence the induction
of adipogenic genes. RT-qPCR results showed that the master
transcription factors PPARG and CEBPA exhibited the
highest upregulation on day 14 after NA treatment, whereas the
mature adipocyte markers LPL and ADIPOQ were
upregulated the most on day 21 after NA treatment (Fig. 1H). These results indicated that
NA treatment was effective in accelerating the adipogenic process
in AD-MSCs.
The effect of NA on MSCs from different sources was
further verified on days 7, 14 and 21. Treatment with NA
significantly enhanced lipid accumulation in MSCs isolated from the
umbilical cord blood, placenta, Wharton's jelly and bone marrow
(Fig. S1), demonstrating that
NA had a universal effect on adipogenesis in MSCs, irrespective of
their origin.
NA regulates the adipogenic process via
the Akt/mTOR and Wnt pathways
Because the expression of adipogenic
differentiation-related genes was elevated in the NA-treated group,
the present study further investigated the expression levels of
adipogenesis-related proteins. Consistent with gene expression
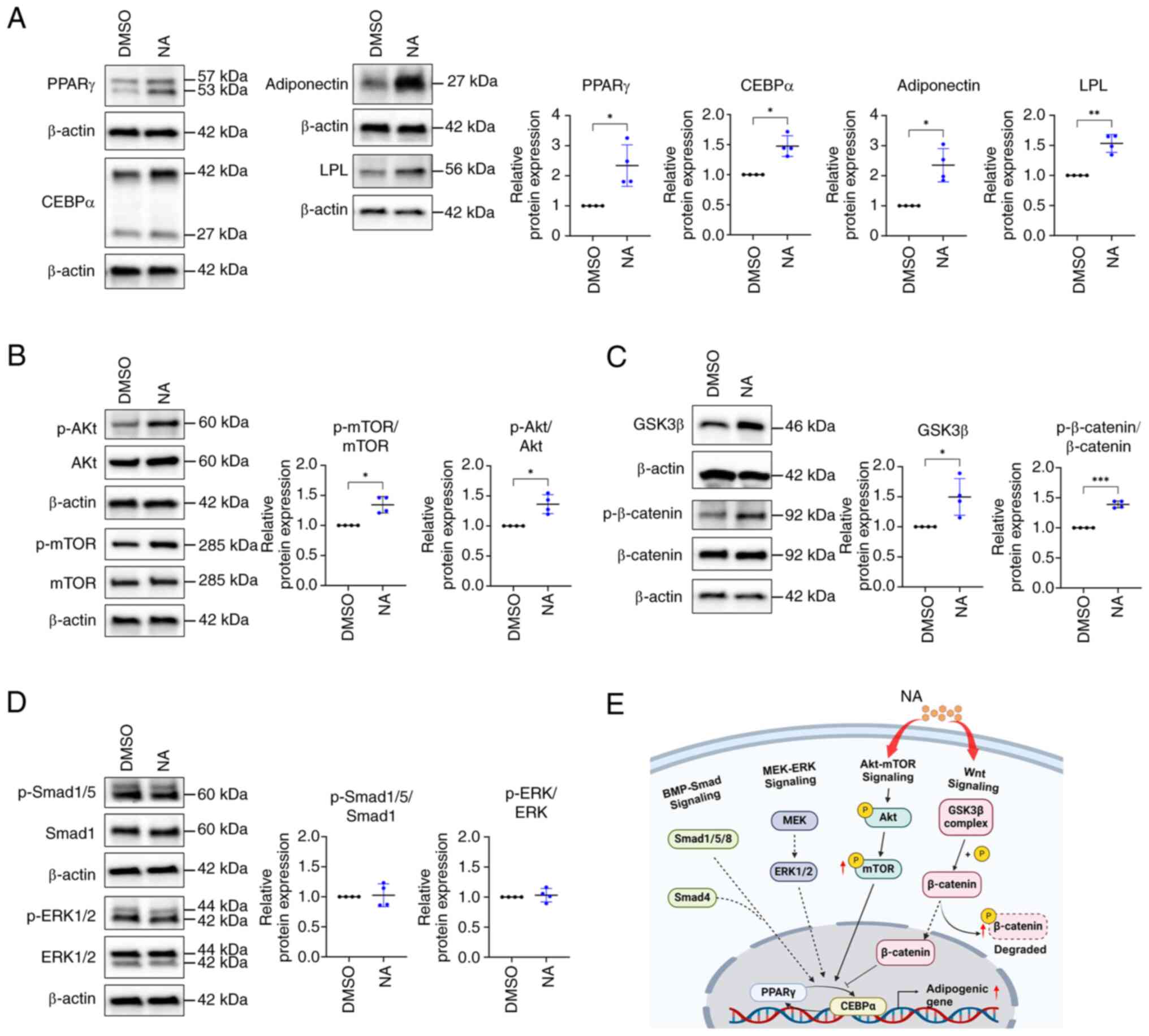
results, western blotting results indicated that PPARγ, CEBPα,
adiponectin and LPL levels were higher in the NA-treated group
(2.34±0.69, 1.48±0.17, 2.35±0.55 and 1.53±0.15 fold change,
respectively) than in the control group (Fig. 2A). Differentiation of AD-MSCs
into mature adipocytes is modulated by the balance of various
signaling pathways, such as Smad, MEK/ERK and Akt/mTOR signaling as
promoters, and Wnt signaling as an inhibitor (31,32). Since NA promoted the adipogenic
differentiation of MSCs, the study aimed to determine the signaling
pathway that regulates adipogenesis caused by NA. Western blotting
results showed that the phosphorylation of Akt and mTOR, which are
positive regulators of adipogenesis, was upregulated after NA
treatment (Fig. 2B). Meanwhile,
intracellular phosphorylation of β-catenin was significantly
upregulated in the NA treatment group (Fig. 2C), indicating that intracellular
β-catenin was degraded. Additionally, intracellular GSK3β, which is
contained in the destruction complex of β-catenin, was increased
(Fig. 2C), which may inhibit Wnt
signaling. However, the other positive regulators of adipogenesis,
Smad and ERK, were unaffected by NA treatment (Fig. 2D). Collectively, these results
indicated that NA may accelerate adipogenesis through activation of
the Akt/mTOR pathway and inhibition of Wnt signaling during
adipogenesis (Fig. 2E).
Transcriptome changes regulated by NA
during adipogenesis
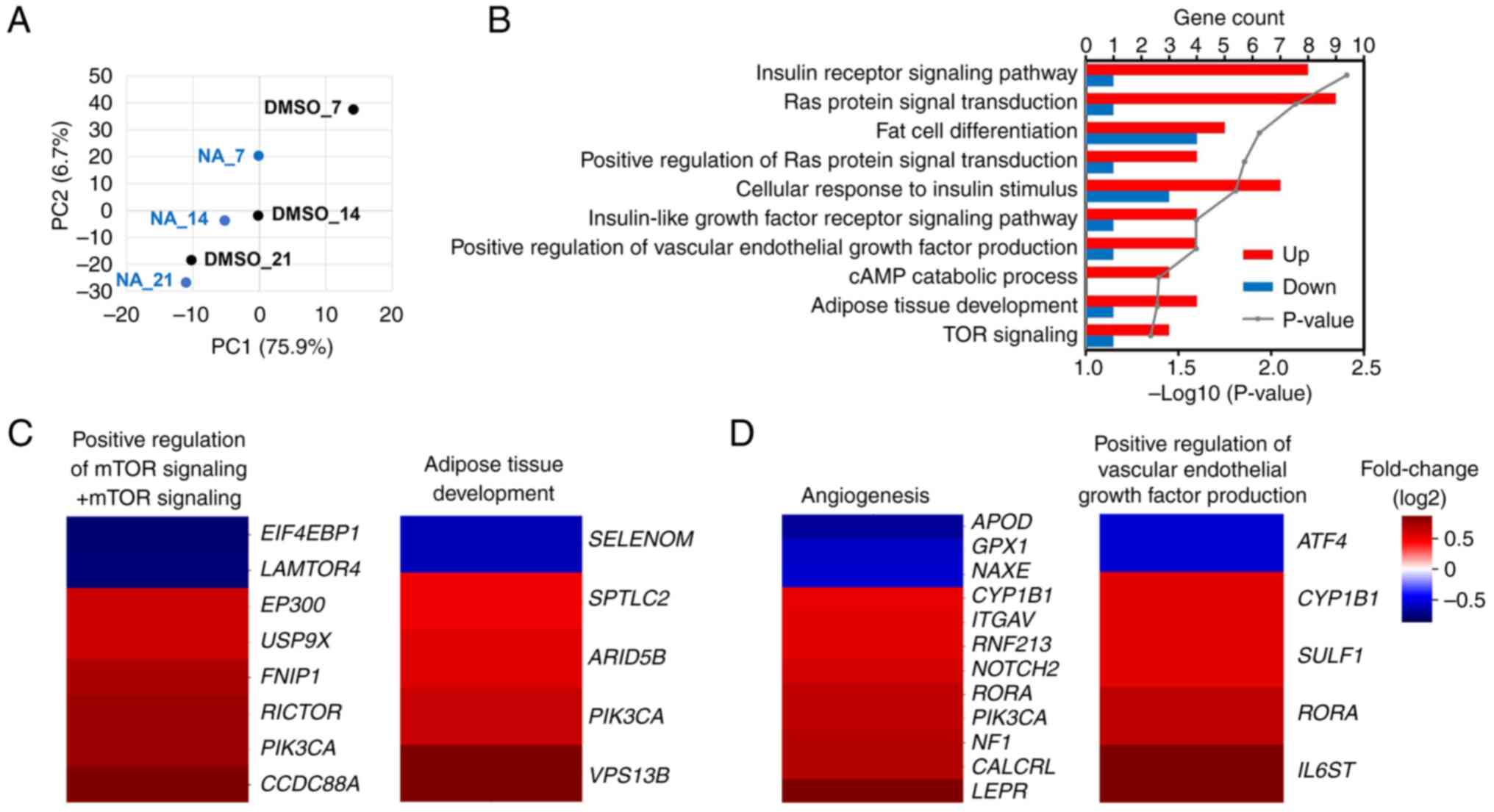
mRNA sequencing was performed to confirm the
transcriptomic changes induced by NA during adipogenesis. PCA
implied that genetic changes induced by NA occurred most
significantly on day 7 post-adipogenesis induction, and the
difference decreased with increasing differentiation period
(Fig. 3A). On day 7, 688 genes
were upregulated and 387 genes were downregulated in the NA
treatment group (fold change >1.5). Gene Ontology analysis
revealed that genes upregulated by NA were mainly involved in
signaling pathways such as 'GO:0045444~Fat cell differentiation'
and 'GO:0060612~Adipose tissue development', which are related to
adipogenesis, and 'GO:0008286~Insulin receptor signaling pathway'
and 'GO:0031929~TOR signaling', which were partially involved in
fat differentiation (Fig. 3B).
In addition, the 'hsa04151:PI3K-Akt signaling pathway' and
'hsa04150:mTOR signaling pathway' were included in the signaling
cascades affected by NA, which was consistent with the results of
protein expression (Figs. S2 and
2B). Furthermore, NA-treated MSCs exhibited upregulated
expression patterns for adipose tissue development, mTOR signaling,
angiogenesis and VEGF production (Fig. 3C and D). To summarize, NA
treatment may enhance Akt/mTOR signaling in MSCs and thereby
increase the expression of genes involved in angiogenesis and fat
differentiation.
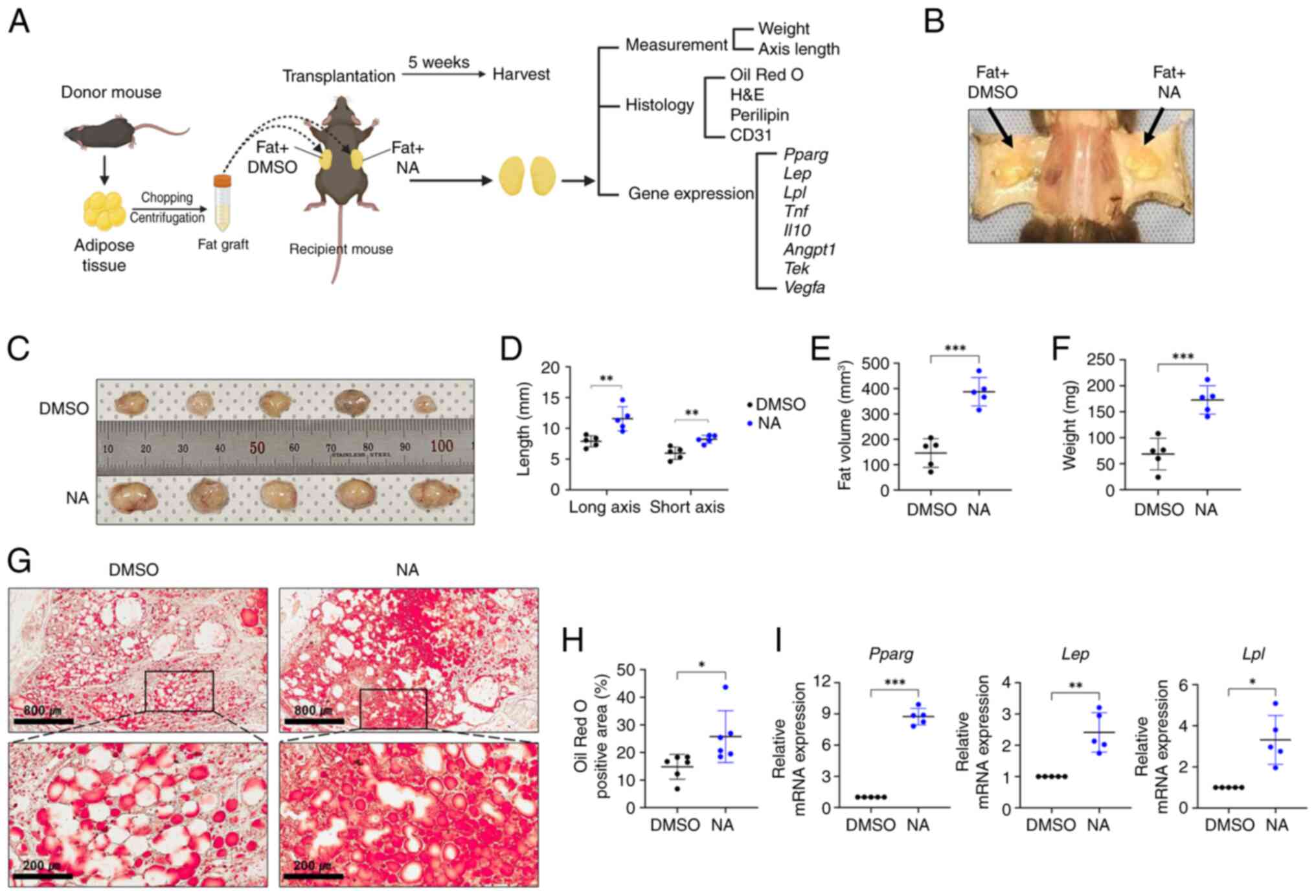
NA improves adipose tissue
engraftment
As aforementioned, it is crucial to create a stable
microenvironment within the graft for successful transplantation
outcomes (4,5). Since NA demonstrated
adipogenesis-promoting effects in vitro, the present study
further assessed whether NA treatment could improve outcomes after
allogeneic fat engraftment in mice. Allogeneic C57BL/6 mouse
adipose tissues treated with DMSO or NA were engrafted
subcutaneously in mice and histologically analyzed after 5 weeks
(Fig. 4A and B). Fig. 4C shows the fat grafts after 5
weeks of fat transplantation (n=5/group). The fat grafts treated
with NA showed a significant increase in long and short axes
lengths, volume and weight compared with those treated with DMSO
(Fig. 4D-F). Lipid accumulation
in the fat graft was confirmed by Oil Red O staining. Notably,
while the fat grafts treated with DMSO had a number of unstained
empty spaces after 5 weeks, the fat grafts treated with NA
exhibited much higher staining with completely lipid-filled spaces,
indicating that supplementation with NA helped mature adipocytes
maintain greater stability after engraftment (Fig. 4G and H). In addition, the mRNA
expression levels of Pparg, Lep and Lpl were
elevated in the NA-treated fat grafts (Fig. 4I). Taken together, these results
suggested that NA may effectively reduce the resorption of
transplanted fat and maintain stability after tissue
engraftment.
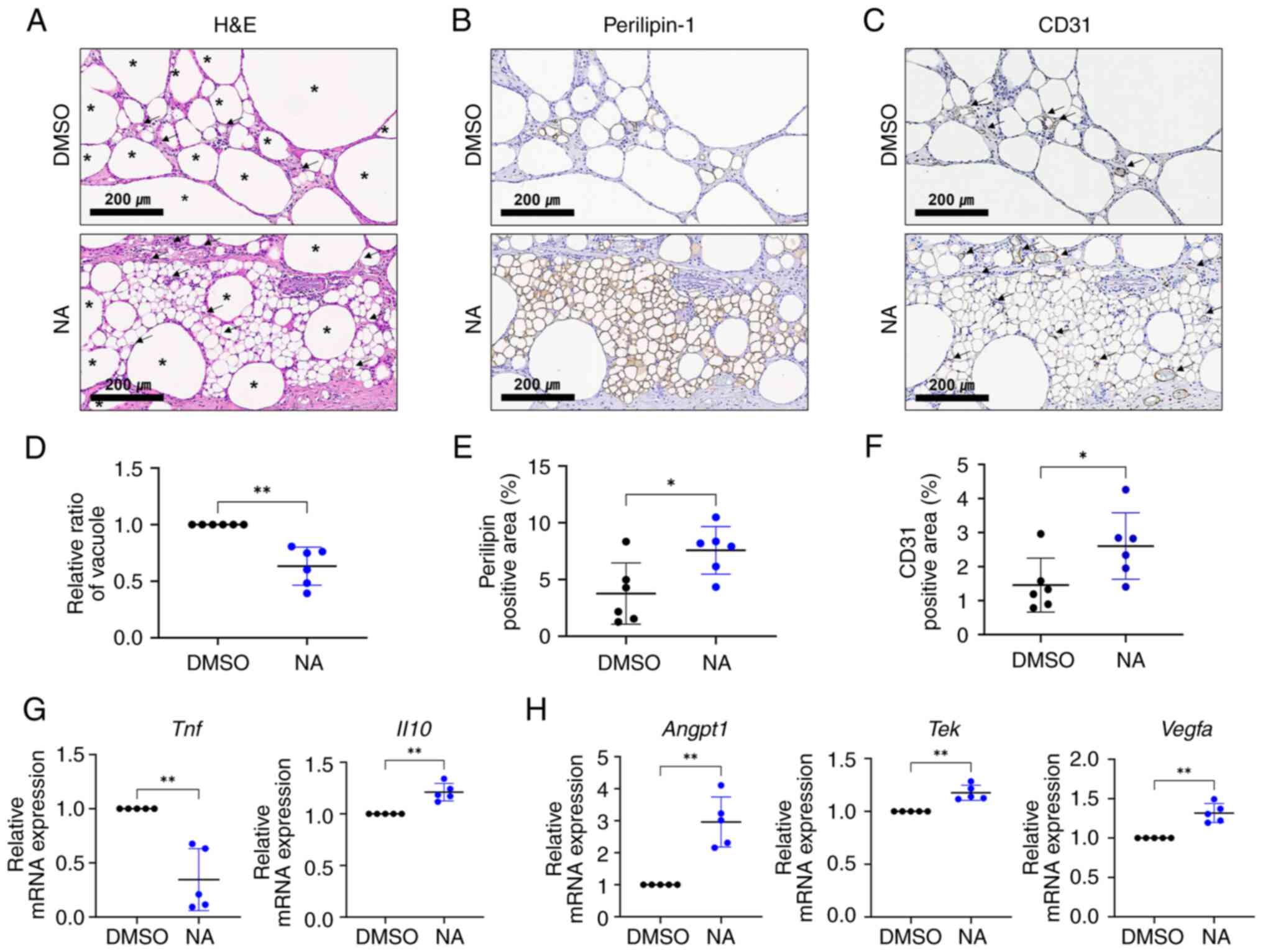
NA serves a beneficial role in fat
engraftment by improving inflammation and angiogenesis
Vacuoles (cysts) in fat grafts are formed by the
necrosis of adipocytes after fat transplantation, and the
proportion of vacuoles in the graft is considered an important
indicator for determining the outcome of fat transplantation
(33). Therefore, the present
study examined vacuole formation in the transplanted fat treated
with or without NA. Histological analysis showed that oil cysts or
vacuoles with diameters >120 μm (34) were largely formed in the grafts
treated with DMSO (Fig. 5A). To
confirm the effect of NA on the survival of adipocytes in fat
grafts, viable adipocytes were identified using
immunohistochemistry for perilipin-1 (Fig. 5B). The fat grafts treated with NA
had more perilipin-1+ adipocytes with a lower proportion
of vacuoles (Fig. 5D and E),
verifying that NA may preserve more viable adipocytes. Notably,
relatively large portions of small, perilipin-1+
adipocytes, which are distinguishable from mature adipocytes, can
be seen in the NA-treated grafts (Fig. S3). Subsequently, the degree of
graft vascularization was assessed. CD31+ vessel
structures were more frequently observed in the NA-treated group,
with a notable increase in the proportion of the CD31+
area (Fig. 5C and F). Moreover,
blood vessels in the NA-treated group were relatively evenly
distributed between viable adipocytes, improving their
survival.
RT-qPCR analysis showed that pro-inflammatory
Tnf was downregulated, whereas the anti-inflammatory marker
Il10 was elevated (Fig.
5G), and angiogenic-promoting genes Angpt1, Tek
and Vegfa were upregulated in grafts supplemented with NA
(Fig. 5H). Given that
inflammation and neovascularization are critical elements for the
successful engraftment of external tissue, these findings indicated
that NA may improve the outcomes of adipose tissue engraftment by
enhancing angiogenesis and suppressing inflammation, resulting in a
relatively well-reorganized microenvironment in the grafts.
Discussion
In recent years, adipose tissue grafting has been
widely used in cosmetic surgery and reconstructive surgery, and
various methods or assisting materials for improving the
effectiveness and outcome after grafting have emerged, including
cell-assisted lipotransfer (6).
To the best of our knowledge, the present study was the first to
demonstrate that NA could accelerate adipogenic differentiation of
human MSCs without cellular toxicity in vitro, and could
stabilize the graft microenvironment and improve the viability of
grafted adipocytes after adipose tissue engraftment in vivo.
These findings indicated that NA may be a potential candidate for
adjuvant therapy in autologous or allogeneic fat
transplantation.
In MSCs, cell fate decisions are regulated by a
complex interrelated balance of adipogenic and osteogenic
transcription factors, cytokines and pathways (35,36). Among them, PI3K/Akt/mTOR
signaling activated by insulin or insulin growth factor-1 (IGF1)
serves an important role in adipogenesis from an early stage by
regulating cell cycle progression and mediating the expression of
core transcription factors (37-39). In the present study, the addition
of NA during adipogenesis activated the Akt/mTOR pathway. Compared
with the control group, AD-MSCs treated with NA exhibited
accelerated adipocyte differentiation and upregulated
phosphorylation of Akt and mTOR, as determined by western blot
analysis. In addition, the expression of signaling molecules
involved in the PI3K/Akt pathway and insulin/IGF1 signaling pathway
were increased in AD-MSCs treated with NA, as determined by
transcriptome analysis. These results indicated that NA may serve
as another upstream modulator of insulin/IGF1 pathway molecules
during adipogenic induction. Furthermore, NA treatment upregulated
the phosphorylation of β-catenin, suppressing the canonical Wnt
pathway, which is a core osteogenesis-promoting and anti-adipogenic
signaling pathway (40). Thus,
NA may tip the cell fate balance toward adipogenesis by activating
adipogenic-stimulating signaling and concurrently inhibiting
anti-adipogenic molecules.
Additionally, NA may activate other
adipogenesis-stimulating factors through the modulation of lipid
metabolism. Keppley et al (12) reported that dietary NA elevated
energy metabolism markers, including PPARα, SIRT1 and PGC1α. Among
them, PPARα has been shown to stimulate adipogenic gene expression
in both 3T3-L1 preadipocytes and mice when activated by its
agonists (41). Moreover,
treatment of senescent MSCs with NA may activate the genes
ANGPTL4 and PLIN2, which are involved in lipid
metabolism (13). Considering
these relationships, NA could accelerate adipogenesis not only
through direct activation of transcription factors but also through
indirect regulation of lipid metabolism.
In allogeneic fat transplantation, NA
supplementation with adipose tissue grafts increased lipid
accumulation within the grafts and improved the overall viability
of adipocytes with fewer vacuoles or cysts. Notably, within the
NA-supplemented grafts, there were relatively large portions of
small perilipin-1+ adipocytes, distinguished from other
adipocytes. These small perilipin-1+ adipocytes are
regenerative adipocytes, which are newly differentiated cells from
adipose-derived stem cells (ADSCs) within the graft (42). In fat engraftment using
liposuction, while existing adipocytes are largely dead, ADSCs can
compensate by proliferating and newly differentiating into
adipocytes 2-3 weeks after transplantation (42,43). Considering that the balance
between proliferation and regeneration of these newly generated
adipocytes against necrosis determines the long-term maintenance of
graft volume (44), NA may help
improve the viability of existing adipocytes and may stimulate the
stable differentiation of ADSCs in the graft.
Furthermore, NA supplementation slightly
downregulated the mRNA expression levels of pro-inflammatory
cytokines, such as TNF-α, while increasing those of
anti-inflammatory factors like IL-10, alleviating inflammation
response. Several studies have suggested the anti-inflammatory
functions of NA in various diseases, such as colitis and
Parkinson's disease, especially in reducing the release of
pro-inflammatory cytokines, such as TNF-α, IL-6 and NF-κB with
therapeutic effects (45,46).
Consistent with these reports, the level of NA has been found to be
positively correlated with the concentration of anti-inflammatory
adiponectin in epicardial adipose tissue (47). Moreover, adiponectin and IL-10
are adipokines secreted by differentiated adipocytes, and
adiponectin can stimulate macrophage production (48,49), indicating the potential
anti-inflammatory roles of NA in fat engraftment. In fat
transplantation, one of the typical complications is vacuole or oil
cyst formation (33). The main
causes of cyst formation are fat necrosis and subsequent
inflammation, which compromise the overall viability of fat grafts
(33,50). In the present study, NA treatment
resulted in improved cell viability with lower levels of
inflammation and vacuoles, indicating its potential to establish a
relatively stable microenvironment for cell survival and
regeneration after engraftment.
Supplying oxygen and nutrients through
neovascularization in the graft is another key factor that can
improve the survival of grafted fat (51). Neovascularization is mainly
achieved through the paracrine effects of ADSCs, providing
important evidence for MSC-assisted lipotransfer (52). The present results showed that NA
treatment activated several genes involved in angiogenesis and VEGF
production in human MSCs during adipogenesis. In addition, the
CD31+ area, representing blood vessels, was largely
increased in the NA graft. Moreover, our previous study found that
NA treatment of WJ-MSCs stimulated several pro-angiogenic factors
(13). Considering these
results, NA could function as a stimulator of angiogenesis for
ADSCs during fat transplantation. Future studies on fat
transplantation using NA-supplemented MSCs could validate this
hypothesis.
To the best of our knowledge, the present study is
the first to reveal the function of NA as an adjuvant for
adipogenesis and fat engraftment. However, the study was limited by
the fact that the effect of NA in fat grafting was confirmed after
5 weeks. To address this limitation, further verification of the
effectiveness of NA over a longer duration is necessary.
Additionally, DMSO, used as a solvent for NA in the present study,
is not suitable for injection into humans due to its potential
adverse effects; thus, future research should focus on alternative
formulations and methods for using NA in clinical practice.
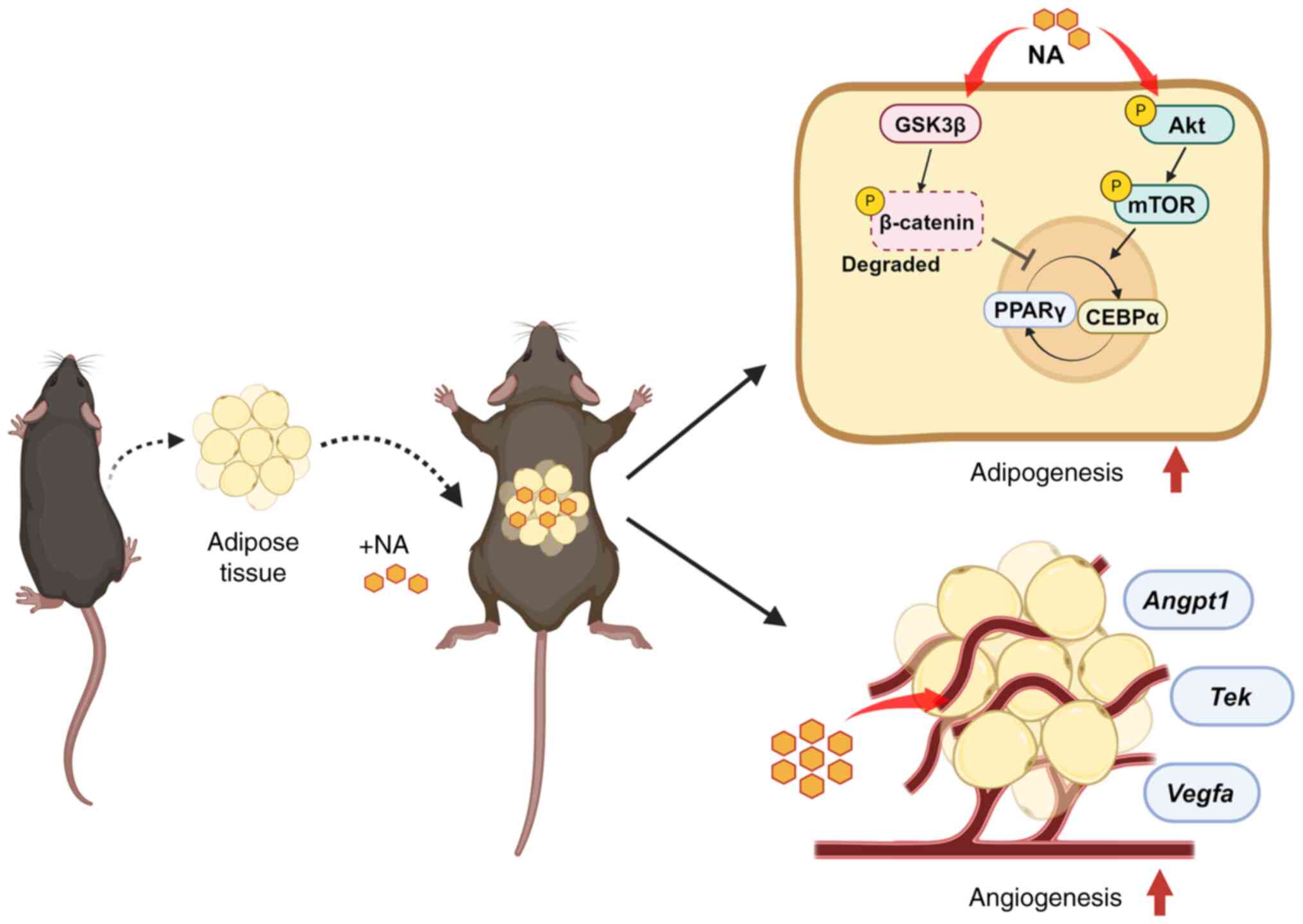
In conclusion, the present study demonstrated that
NA stimulated and accelerated the differentiation of adipocytes
through the activation of Akt/mTOR signaling and inhibition of Wnt
signaling. The addition of NA to adipose tissue engraftment helped
in the reconstruction of the microenvironment within the fat graft
by attenuating inflammation and promoting neovascularization, thus
improving the outcome of fat transplantation (Fig. 6). These findings may contribute
to the identification of new adjuvants for fat engraftment with
better outcomes and higher long-term stability. Additionally,
future research aimed at human applications could achieve superior
outcomes in regenerative surgery.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The mRNA sequencing data
generated in the present study may be found in the GEO under
accession number GSE269065 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269065
Authors' contributions
JHS conducted experiments, analyzed the data,
prepared the figures and wrote the manuscript. SJK designed this
study, interpreted the data and revised the manuscript. SK
conducted the experiments, prepared the figures and wrote the
manuscript. SYJ performed the in vivo experiments and
interpreted the results. SEP designed the transcriptome analysis
and analyzed the transcriptome to interpret the results. SJC and
SYO contributed to write the protocol for ethics approval, isolated
MSCs from tissues and revised the manuscript. HBJ and JWC
contributed to conception and design, and revised the manuscript.
JHS, SJK and SYJ confirmed the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The samples used in the present study were harvested
from participants who provided written informed consent, and the
procedures were approved by the Institutional Review Board of
Samsung Medical Center (approval no. 2016-07-102-001; Seoul,
Korea). The use of BM-MSCs was exempt from IRB approval since it
was a purchased cell line. The animal experiments were reviewed and
approved by the Institutional Animal Care and Use Committee of the
Research Institute for Future Medicine, belonging to Samsung
Medical Center (approval nos. 20230316003 and 20240522001).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of
Korea grant funded by the Korean government (Ministry of Science
and ICT; grant no. 2021R1F1A1064060) and the Korean Fund for
Regenerative Medicine funded by the Ministry of Science and ICT and
the Ministry of Health and Welfare (grant nos. RS-2022-00060268 and
RS-2023-00223069). This work was also supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute, funded by the Ministry of Health
& Welfare, Republic of Korea (grant nos. HR22C1363 and
HI14C3484). Also, this study was supported by the Future Medicine
2030 Project of the Samsung Medical Center (grant no.
SMO1240041).
References
|
1
|
Shim YH and Zhang RH: Literature review to
optimize the autologous fat transplantation procedure and recent
technologies to improve graft viability and overall outcome: A
aystematic and retrospective analytic approach. Aesthetic Plast
Surg. 41:815–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahoney CM, Imbarlina C, Yates CC and
Marra KG: Current therapeutic strategies for adipose tissue
defects/repair using engineered biomaterials and biomolecule
formulations. Front Pharmacol. 9:5072018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao S, Lu B, Zhou R and Gao W: Research
progress of mechanisms of fat necrosis after autologous fat
grafting: A review. Medicine (Baltimore). 102:e332202023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu M, Li Y, Wang Z, Feng J, Wang J, Xiao
X, Lu F and Dong Z: Botulinum toxin A improves supramuscular fat
graft retention by enhancing angiogenesis and adipogenesis.
Dermatol Surg. 46:646–652. 2020. View Article : Google Scholar
|
|
5
|
Major GS, Simcock JW, Woodfield TBF and
Lim KS: Overcoming functional challenges in autologous and
engineered fat grafting trends. Trends Biotechnol. 40:77–92. 2022.
View Article : Google Scholar
|
|
6
|
Landau MJ, Birnbaum ZE, Kurtz LG and
Aronowitz JA: Review: Proposed methods to improve the survival of
adipose tissue in autologous fat grafting. Plast Reconstr Surg Glob
Open. 6:e18702018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang J, Yang J, Yu Z, Chen L, Zhang Z,
Wang K, Tang J and Yi C: Bone marrow mesenchymal stem cells enhance
angiogenesis and promote fat retention in fat grafting via
polarized macrophages. Stem Cell Res Ther. 13:522022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anderson AE, Wu I, Parrillo AJ, Wolf MT,
Maestas DR Jr, Graham I, Tam AJ, Payne RM, Aston J, Cooney CM, et
al: An immunologically active, adipose-derived extracellular matrix
biomaterial for soft tissue reconstruction: Concept to clinical
trial. NPJ Regen Med. 7:62022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Q, Chen C, Wang X, Li W, Zhang Y,
Wang M, Jing W, Wang H, Guo W and Tian W: Botulinum toxin A
improves adipose tissue engraftment by promoting cell
proliferation, adipogenesis and angiogenesis. Int J Mol Med.
40:713–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Chen J, Yu X and Gao JM: A mini
review of nervonic acid: Source, production, and biological
functions. Food Chem. 301:1252862019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kageyama Y, Deguchi Y, Hattori K, Yoshida
S, Goto YI, Inoue K and Kato T: Nervonic acid level in
cerebrospinal fluid is a candidate biomarker for depressive and
manic symptoms: A pilot study. Brain Behav. 11:e020752021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keppley LJW, Walker SJ, Gademsey AN, Smith
JP, Keller SR, Kester M and Fox TE: Nervonic acid limits weight
gain in a mouse model of diet-induced obesity. FASEB J.
34:15314–15326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SJ, Kwon S, Chung S, Lee EJ, Park SE,
Choi SJ, Oh SY, Ryu GH, Jeon HB and Chang JW: Nervonic acid
inhibits replicative senescence of human Wharton's Jelly-derived
mesenchymal stem cells. Int J Stem Cells. 17:80–90. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palumbo P, Lombardi F, Siragusa G, Cifone
MG, Cinque B and Giuliani M: Methods of isolation, characterization
and expansion of human adipose-derived stem cells (ASCs): An
overview. Int J Mol Sci. 19:18972018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YS, Park YB, Ha CW, Kim JA, Heo JC,
Han WJ, Oh SY and Choi SJ: Different characteristics of mesenchymal
stem cells isolated from different layers of full term placenta.
PLoS One. 12:e01726422017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JY, Kim DH, Kim DS, Kim JH, Jeong SY,
Jeon HB, Lee EH, Yang YS, Oh W and Chang JW: Galectin-3 secreted by
human umbilical cord blood-derived mesenchymal stem cells reduces
amyloid-beta42 neurotoxicity in vitro. FEBS Lett. 584:3601–3608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SE, Lee J, Chang EH, Kim JH, Sung JH,
Na DL and Chang JW: Activin A secreted by human mesenchymal stem
cells induces neuronal development and neurite outgrowth in an in
vitro model of Alz'eimer's disease: Neurogenesis induced by MSCs
via activin A. Arch Pharm Res. 39:1171–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Andrews S: FastQC: A quality control tool
for high throughput sequence data. Babraham Institute; 2010,
http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
|
|
20
|
Hannon Lab: FASTX-Toolkit
(RRID:SCR_005534). https://github.com/agordon/fastx_toolkit.
|
|
21
|
Bushnell B: BBMap. SourceForge; San Diego,
CA: 2014, https://sourceforge.net/projects/bbmap/.
|
|
22
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roberts A, Trapnell C, Donaghey J, Rinn JL
and Pachter L: Improving RNA-seq expression estimates by correcting
for fragment bias. Genome Biol. 12:R222011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar
|
|
26
|
Kanehisa M and Sato Y: Kegg mapper for
inferring cellular functions from protein sequences. Protein Sci.
29:28–35. 2020. View Article : Google Scholar
|
|
27
|
Kanehisa M, Sato Y and Kawashima M: Kegg
mapping tools for uncovering hidden features in biological data.
Protein Sci. 31:47–53. 2022. View Article : Google Scholar :
|
|
28
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Council NR: Guide for the care and use of
laboratory animals. Eighth edition. The National Academies Press;
Washington, DC: 2011
|
|
30
|
Bankhead P, Loughrey MB, Fernández JA,
Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ,
Coleman HG, et al: QuPath: Open source software for digital
pathology image analysis. Sci Rep. 7:168782017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghaben AL and Scherer PE: Adipogenesis and
metabolic health. Nat Rev Mol Cell Biol. 20:242–258. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mineda K, Kuno S, Kato H, Kinoshita K, Doi
K, Hashimoto I, Nakanishi H and Yoshimura K: Chronic inflammation
and progressive calcification as a result of fat necrosis: The
worst outcome in fat grafting. Plast Reconstr Surg. 133:1064–1072.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Y, Jiang Y, Wang M, Tian W and Wang H:
Concentrated growth factor enhanced fat graft survival: A
comparative study. Dermatol Surg. 44:976–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Muruganandan S, Roman AA and Sinal CJ:
Adipocyte differentiation of bone marrow-derived mesenchymal stem
cells: Cross talk with the osteoblastogenic program. Cell Mol Life
Sci. 66:236–253. 2009. View Article : Google Scholar
|
|
36
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang E and Kim CY: Natural products and
obesity: A focus on the regulation of mitotic clonal expansion
during adipogenesis. Molecules. 24:11572019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang HH, Huang J, Düvel K, Boback B, Wu
S, Squillace RM, Wu CL and Manning BD: Insulin stimulates
adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One.
4:e61892009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang QQ and Lane MD: Adipogenesis: From
stem cell to adipocyte. Annu Rev Biochem. 81:715–736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Winter TJJ and Nusse R: Running against
the Wnt: How Wnt/β-catenin suppresses adipogenesis. Front Cell Dev
Biol. 9:6274292021. View Article : Google Scholar
|
|
41
|
Goto T, Lee JY, Teraminami A, Kim YI,
Hirai S, Uemura T, Inoue H, Takahashi N and Kawada T: Activation of
peroxisome proliferator-activated receptor-alpha stimulates both
differentiation and fatty acid oxidation in adipocytes. J Lipid
Res. 52:873–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sunaga A, Sugawara Y, Katsuragi-Tomioka Y
and Kobayashi E: The fate of nonvascularized fat grafts:
Histological and bioluminescent study. Plast Reconstr Surg Glob
Open. 1:e402013. View Article : Google Scholar
|
|
43
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The fate of adipocytes after nonvascularized fat
grafting: Evidence of early death and replacement of adipocytes.
Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mashiko T and Yoshimura K: How does fat
survive and remodel after grafting? Clin Plast Surg. 42:181–190.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Liang T, Mao Y, Li Z, Li X, Zhu X,
Cao F and Zhang J: Nervonic acid improves liver inflammation in a
mouse model of Parkinson's disease by inhibiting proinflammatory
signaling pathways and regulating metabolic pathways.
Phytomedicine. 117:1549112023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan SN, Wang MX, Han JL, Feng CY, Wang M,
Wang M, Sun JY, Li NY, Simal-Gandara J and Liu C: Improved colonic
inflammation by nervonic acid via inhibition of NF-kappaB signaling
pathway of DSS-induced colitis mice. Phytomedicine. 112:1547022023.
View Article : Google Scholar
|
|
47
|
Sawaguchi T, Nakajima T, Hasegawa T,
Shibasaki I, Kaneda H, Obi S, Kuwata T, Sakuma M, Toyoda S, Ohni M,
et al: Serum adiponectin and TNFalpha concentrations are closely
associated with epicardial adipose tissue fatty acid profiles in
patients undergoing cardiovascular surgery. Int J Cardiol Heart
Vasc. 18:86–95. 2018.PubMed/NCBI
|
|
48
|
Wolf AM, Wolf D, Rumpold H, Enrich B and
Tilg H: Adiponectin induces the anti-inflammatory cytokines IL-10
and IL-1RA in human leukocytes. Biochem Biophys Res Commun.
323:630–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lira FS, Rosa JC, Pimentel GD, Seelaender
M, Damaso AR, Oyama LM and do Nascimento CO: Both adiponectin and
interleukin-10 inhibit LPS-induced activation of the NF-kappaB
pathway in 3T3-L1 adipocytes. Cytokine. 57:98–106. 2012. View Article : Google Scholar
|
|
50
|
Yoshimura K and Coleman SR: Complications
of fat grafting: How they occur and how to find, avoid, and treat
them. Clin Plast Surg. 42:383–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Evans BGA, Gronet EM and Saint-Cyr MH: How
fat grafting works. Plast Reconstr Surg Glob Open. 8:e27052020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moustaki M, Papadopoulos O, Verikokos C,
Karypidis D, Masud D, Kostakis A, Papastefanaki F, Roubelakis MG
and Perrea D: Application of adipose-derived stromal cells in fat
grafting: Basic science and literature review. Exp Ther Med.
14:2415–2423. 2017. View Article : Google Scholar : PubMed/NCBI
|