Introduction
Breast cancer is a leading cause of death among
women, with its incidence showing an upward trend (1). According to the 2024 cancer
statistics, 310,700 new cases of breast cancer and 42,200 breast
cancer-related deaths were recorded among women globally, ranking
second in incidence among cancers in women (2). Compared with other subtypes of
breast cancer, triple-negative breast cancer (TNBC) exhibits a
higher incidence in premenopausal women and is characterized by
significant molecular heterogeneity. TNBC is characterized by poor
differentiation, high invasiveness, and a propensity for recurrence
and metastasis (3). Notably,
more than one-third of patients with TNBC experience recurrence or
distant metastasis (4). The
IMpassion132 study showed that the median progression-free survival
time for patients with TNBC with early recurrent, unresectable,
locally advanced or metastatic cancer is 4 months, indicating a
poor prognosis (5). The efficacy
of combined chemotherapy regimens and immunotherapy for TNBC is
being actively explored (6,7).
In addition, traditional Chinese medicine (TCM) has shown efficacy
in the treatment of TNBC, demonstrating potential in enhancing
chemotherapy sensitivity (8) and
prolonging disease-free survival (DFS), and has thus gradually
gained international recognition. In our previous study, it was
demonstrated that incorporating Sanyin Formula into standard
chemotherapy can improve the 5-year DFS rate of patients with TNBC,
increasing it from 85.5 to 94.2%. Notably, this herbal formulation
has since been approved as a hospital-prepared medicinal product
and is currently undergoing expanded clinical implementation
(9).
TCM employs a wide range of natural products as
therapeutic agents for the treatment of various diseases and
conditions (10). Curcuma
phaeocaulis Valeton is commonly used as a TCM for breast cancer
treatment (11). Various
chemical components of C. phaeocaulis Valeton, including
curcumenol (Cur), β-elemene and curcumin, have been reported to
exhibit anti-breast cancer capabilities (12).
Cur, a main active component of C.
phaeocaulis Valeton, has been demonstrated to exert antitumor
effects on lung cancer by triggering ferroptosis (13). In cervical cancer, Cur can reduce
cell proliferation and invasion when combined with cisplatin
(14). However, to the best of
our knowledge, the inhibitory effects of Cur on TNBC have not yet
been thoroughly reported. Therefore, the current study aimed to
investigate the therapeutic effects and potential mechanisms of Cur
in TNBC, with the goal of providing a safe and effective natural
product for TNBC treatment. A flowchart summarizing the research
methods performed in the present study is shown in Fig. 1.
Materials and methods
Chemicals
Cur (purity ≥98%; Shanghai Yuanye Biotechnology Co.,
Ltd.) and paclitaxel (PTX; Abmole Bioscience Inc.) were dissolved
in dimethyl sulfoxide (Shanghai Yeasen Biotechnology Co., Ltd.).
Ferrostatin-1 (Fer-1; MedChemExpress) was dissolved in sterile
water (Shanghai Yeasen Biotechnology Co., Ltd.).
Cell culture
The TNBC cell lines 4T1 and MDA-MB-231 were cultured
in Roswell Park Memorial Institute (RPMI) 1640 and Dulbecco's
modified Eagle's medium (DMEM), respectively (both from The Cell
Bank of Type Culture Collection of The Chinese Academy of
Sciences). All culture media were supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The cells were incubated at 37°C within a humidified incubator
containing 5% CO2 to guarantee optimal growth
conditions.
Animal experiment
A total of 20 specific pathogen-free female BALB/c
mice (age, 6 weeks; weight, ~20 g) were obtained from Shanghai
Lingchang Biotechnology Co., Ltd., and were divided into four
groups (n=5 mice/group). The mice were housed in a controlled
environment, where the temperature was maintained at 22±2°C and the
relative humidity was set at 50±10%. The mice had ad libitum
access to standard rodent chow and fresh water, and were maintained
under a 12-h light/dark cycle. Animals were subcutaneously injected
with 5×104 4T1 cells after 1 week of adaptive feeding.
When tumors reached a volume of 100 mm3, the mice were
allocated at random into the following four groups: Mice in the
model group received 100 µl saline every 2 days; mice in the
positive group were administered PTX at a dose of 10 mg/kg every 7
days; mice in the Cur treatment groups were administered 5 or 10
mg/kg Cur every 2 days. All treatments were administered
intraperitoneally and lasted for 3 weeks. Mice were euthanized by
gradual-fill CO2 inhalation at a displacement rate of
30% chamber volume/min, sustained for ≥5 min. Death was confirmed
through the absence of breathing, loss of muscle tone and mice were
cervically dislocated after being subjected to CO2
inhalation. The following criteria were established as humane
endpoints: i) Weight loss exceeding 20% of the initial body weight;
ii) Tumor growth reaching more than 10% of the body weight, the
average tumor diameter in mice surpassing 20 mm, or rapid tumor
growth leading to ulceration, which in turn caused infection or
necrosis; iii) inability of the mice to eat, drink or maintain an
upright stance; iv) in non-anesthetized or non-sedated animals,
signs of depression (such as immobility, excessive sniffing,
trembling and scratching) accompanied by a body temperature
<37°C, abnormal central nervous system responses (excessive
excitement or inhibitory behavioral responses) and an inability to
effectively manage pain (when being touched or slight pressure was
applied, the mice showed abnormal struggling and squeaking). No
mice reached any of the humane endpoints during the study. The
present study was approved by the Animal Welfare Committee of
Longhua Hospital, Shanghai University of TCM (approval no.
LHERAW-24010; Shanghai, China).
Cell counting kit-8 (CCK-8) assay
The TNBC cell lines 4T1 and MDA-MB-231 were seeded
into 96-well plates at a density of 5,000 cells/well, and were then
exposed to multiple doses of Cur (0, 6.25, 12.5, 25, 50, 100, 200
and 400 µM) and PTX (50 and 500 nM) at 37°C for 24 and 48 h.
Following treatment, the cells were incubated with the CCK-8
reagent (Beyotime Institute of Biotechnology) for 2 h at 37°C and
cell viability was determined. The IC50 values were
calculated by nonlinear regression analysis of dose-response curves
(GraphPad Prism Version 10.1; Dotmatics) using a four-parameter
logistic model.
Wound healing assay
A 1-ml pipette tip was used to make a scratch across
4T1 and MDA-MB-231 cells that had reached 80-90% confluence in
6-well plates. Subsequently, the cells were exposed to 25, 50 and
100 µM Cur at 37°C for 24 and 48 h in serum-free media.
Images were captured using an IX71 inverted light microscope
(Olympus Corporation).
Invasion assay
After 24 h of exposure to 25, 50 and 100 µM
Cur at 37°C, the 4T1 and MDA-MB-231 cells were seeded into 24-well
Transwell inserts (8-µm pore size), which were precoated
with Matrigel at 37°C for 2 h. A total of 5×104
cells/well serum-free medium were plated in the upper chamber.
Cells were then permitted to migrate into the lower chamber, which
contained medium supplemented with 20% FBS at 37°C for 24 h. Then,
the migrated cells were fixed with 4% paraformaldehyde at room
temperature for 15 min and stained with 0.1% crystal violet at room
temperature for 15 min. The number of invasive cells was counted
from each sample using a light microscope.
Colony formation assay
4T1 and MDA-MB-231 cells were first exposed to 25,
50 and 100 µM Cur at 37°C for 24 h hand were then spread
individually into 6-well plates at a density of 1,000 cells/well.
The medium was changed every 2 days until colonies of cells
gradually formed after 7-10 days. For colony fixation and staining,
the colonies were fixed with 4% paraformaldehyde at room
temperature for 15 min and then stained with 0.1% crystal violet at
room temperature for 15 min. Colonies were defined as clusters of
>50 cells and the colonies were quantified using ImageJ software
(version 1.8.0, National Institutes of Health).
Western blotting (WB)
Proteins were extracted from 4T1 and MDA-MB-231
cells and tumor tissues using radioimmunoprecipitation assay
supplemented with phenylmethylsulfonyl fluoride and a phosphatase
inhibitor (100:1:2; all purchased from Beyotime Institute of
Biotechnology). The protein concentration in the lysate
supernatants was determined using the bicinchoninic acid method.
Subsequently, 30 µg proteins were loaded per lane and were
separated by SDS-PAGE on 10% polyacrylamide gels. The proteins were
then transferred onto PVDF membranes, which were blocked with a
protein-free rapid blocking solution (Epizyme; Ipsen Pharma) at
room temperature for 30 min. The membranes were then incubated with
primary antibodies (1:1,000) at 4°C for >12 h. The primary
antibodies used were BCL-2 (cat. no. A19693; ABclonal Biotech Co.,
Ltd.), BAX (cat. no. A19684; ABclonal Biotech Co., Ltd.), caspase 3
(cat. no. 9662; Cell Signaling Technology, Inc.), cleaved caspase 3
(cat. no. 9664; Cell Signaling Technology, Inc.), caspase 9 (cat.
no. 9508; Cell Signaling Technology, Inc.), cleaved caspase 9 (cat.
no. 9509; Cell Signaling Technology, Inc.), cleaved caspase 9 (cat.
no. 9505; Cell Signaling Technology, Inc.), E-cadherin (cat. no.
20874-1-AP; Proteintech Group, Inc.), N-cadherin (cat. no.
22018-1-AP; Proteintech Group, Inc.), Vimentin (cat. no. 5741; Cell
Signaling Technology, Inc.), acyl-CoA synthetase long-chain family
member 4 (ACSL4; cat. no. A20414; ABclonal Biotech Co., Ltd.),
SLC7A11 (cat. no. 26864-1-AP; Proteintech Group, Inc.), glutathione
(GSH) peroxidase 4 (GPX4; cat. no. A11243; ABclonal Biotech Co.,
Ltd.), TGF-β (cat. no. A25313; ABclonal Biotech Co., Ltd.), NF-κB
(cat. no. 10745-1-AP; Proteintech Group, Inc.) and phosphorylated
(p-)NF-κB (cat. no. 82335-1-RR; Proteintech Group, Inc.). The
loading controls used were β-actin (cat. no. AC026; ABclonal
Biotech Co., Ltd.) and GAPDH (cat. no. A19056; ABclonal Biotech
Co., Ltd.). After three washes with TBS-0.1% Tween (10 min each),
the membranes were incubated with the corresponding secondary
antibodies (1:5,000) for 1 h at room temperature. The secondary
antibodies were HRP-conjugated goat anti-rabbit IgG (H+L) (cat. no.
AS014; ABclonal Biotech Co., Ltd.) and HRP conjugated rabbit
anti-mouse (cat. no. AS115; ABclonal Biotech Co., Ltd.) antibodies.
The protein bands were visualized using an enhanced
chemiluminescent kit (ABclonal Biotech Co., Ltd.) and the gray
scale analysis of the bands was performed using ImageJ software to
compare the relative expression levels of the target proteins in
different samples.
JC-1 assay
After treatment with 100 µM Cur at 37°C for
24 h, the JC-1 staining working solution (Beyotime Institute of
Biotechnology) was added to the 6-well plate seeded with 4T1 and
MDA-MB-231 cells (1×106/well). Subsequently, the cells
underwent two washes with JC-1 staining buffer (Beyotime Institute
of Biotechnology) and were subsequently supplemented with DMEM.
Fluorescence was then observed using a confocal laser scanning
microscope (Zeiss AG).
Immunofluorescence staining
After treatment with 100 µM Cur at 37°C for
24 h, 4T1 and MDA-MB-231 cells were fixed in 4% paraformaldehyde at
room temperature for 15 min and then permeabilized with 0.1% Triton
X-100 (Beyotime Institute of Biotechnology) at room temperature for
5 min. After blocking with a protein-free rapid blocking solution
at room temperature for 1 h, the cells were incubated with
corresponding primary antibodies (1:200) and secondary antibodies
(1:500). The cells were incubated with the corresponding primary
antibodies at 4°C overnight. The primary antibodies used were
E-cadherin (cat. no. 14472; Cell Signaling Technology, Inc,) and
Vimentin (cat. no. 5741; Cell Signaling Technology, Inc.). After
washing, the cells were incubated with the following secondary
antibodies (1:200) at room temperature for 1-2 h: FITC-conjugated
goat anti-mouse IgG (H+L) (cat. no. AS001; ABclonal Biotech Co.,
Ltd.) and ABflo 555-conjugated goat anti-rabbit IgG (H+L) (cat. no.
AS058; ABclonal Biotech Co., Ltd.). For nuclear staining, the cells
were incubated with DAPI at a concentration 1 µg/ml at room
temperature for 5 min. The images were captured using a confocal
laser scanning microscope (Zeiss, Germany).
Assessment of lung metastatic nodes
Following fixation with Bouin's fixative solution
(Fuzhou Phygene Biotechnology Co., Ltd.) at room temperature for 24
h, surface lung metastases from mice were counted and presented as
the mean number of visible nodules per lung ± SD.
Hematoxylin and eosin (H&E) staining
and immunohistochemistry (IHC)
Preserved tumor tissues were fixed in 10%
neutral-buffered formalin at room temperature for 24 h, embedded in
paraffin and sliced into 5 µm sections. The
paraffin-embedded sections were then deparaffinized twice in xylene
at 60°C (10 min each). Subsequently, they were rehydrated in a
descending alcohol series [100% ethanol twice (5 min each), 95%
ethanol for 5 min, 80% ethanol for 5 min and 70% ethanol for 5
min], followed by a wash in distilled water. Antigen retrieval was
performed by heating the sections in citrate buffer (pH 6.0) at
95-100°C for 20 min, after which, the sections were allowed to cool
at room temperature for 30 min. For intracellular antigens or
membrane proteins with an internal epitope, the sections were
permeabilized with 0.1% Triton X-100 in PBS at room temperature for
10 min. The sections were then blocked with 5% normal goat serum
(Beyotime Institute of Biotechnology) at room temperature for 1 h
to reduce non-specific binding, and endogenous peroxidase activity
was quenched by incubating the sections in 3% hydrogen peroxide in
methanol at room temperature for 10 min. The sections were then
incubated with the following primary antibodies (1:200): Ki-67
(cat. no. A20018; ABclonal Biotech Co., Ltd.), E-cadherin (cat. no.
20874-1-AP; Proteintech Group, Inc.), Vimentin (cat. no. 5741; Cell
Signaling Technology, Inc.) CD69 (cat. no. A26620PM; ABclonal
Biotech Co., Ltd.), CD11c (cat. no. 17342-1-AP; Proteintech Group,
Inc.) at 4°C overnight. After washing the sections three times with
PBS (5 min each), they were incubated with secondary antibodies
(1:500): HRP-conjugated goat anti-rabbit IgG (H+L) (cat. no. AS014;
ABclonal Biotech Co., Ltd.) and HRP-conjugated rabbit anti-mouse
(cat. no. AS115; ABclonal Biotech Co., Ltd.) secondary antibodies
at room temperature for 1 h.
For H&E staining, the sections were stained with
hematoxylin for 5 min at room temperature, washed with running tap
water for 5 min, then differentiated in 1% acid alcohol for a few
seconds, washed again, and blued in Scott's tap water substitute
for 2 min. Subsequently, the sections were stained with eosin Y
solution (1% eosin in water) for 3 min at room temperature. The
stained sections were observed under a light microscope. Images of
H&E staining and IHC were captured using an IX71 inverted
microscope (Olympus Corporation) and analyzed using ImageJ
software.
TUNEL assay
Tumor tissues were fixed in 10% neutral buffered
formalin at room temperature for 24 h, embedded in paraffin and
sectioned (4 µm). After deparaffinization and antigen
retrieval with 20 µg/ml proteinase K at 37°C for 30 min,
apoptosis was detected using the In Situ Cell Death
Detection Kit (Roche Diagnostics). The sections were incubated with
TUNEL reaction mix at 37°C for 1 h, followed by incubation with a
peroxidase-conjugated anti-fluorescein antibody at 37°C for 30 min,
and DAB chromogenic development for 10 min. Nuclei were
counterstained with hematoxylin, and the sections were dehydrated
and mounted. Positive (DNase I pretreatment) and negative (TdT
omission) controls were included. Images were captured using an
IX71 inverted light microscope (Olympus Corporation). For each
sample, a total of three fields of view were randomly selected and
imaged. Subsequently, these images were analyzed using ImageJ
software.
RNA sequencing
Total RNA was extracted from tumor tissues using the
RNA purification kit (cat. no. EZB-RN4; EZBioscience), and the
concentration and purity of the extracted RNA were measured using a
Nanodrop 2000 (Thermo Fisher Scientific, Inc.). The integrity of
the RNA was assessed by agarose gel electrophoresis and the RNA
quality number value was determined using an Agilent 5300 (Agilent
Technologies, Inc.). The requirements for RNA sequencing were a
total RNA amount of 1 µg, a concentration ≥30 ng/µl,
and an OD260/280 ratio between 1.8 and 2.2. Sequencing was
performed on an Illumina NovaSeq 6000 (Illumina, Inc.). Paired-end
sequencing with 150 bp reads was carried out. The final library
concentration was measured by qPCR with the KAPA Library
Quantification Kit (Roche Diagnostics) and diluted to 1.8 pM. Raw
data were converted to FASTQ by bcl2fastq v2.20 (Illumina, Inc.).
Quality was assessed by FastQC v0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
Gene expression was quantified by featureCounts (http://subread.sourceforge.net/; version2.0.3),
and differential expression analysis was performed using DESeq2
(http://bioconductor.org/packages/release/bioc/html/DESeq2.html;
version 1.34.0). Log2 fold change (FC) >1.2 and P<0.05 were
used to identify differentially expressed genes (DEGs) between
model and Cur groups. Gene Ontology (GO) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway analyses were performed using the
clusterProfiler R package (https://bioconductor.org/packages/clusterProfiler;
version 4.4.4), referencing the KEGG database (https://www.kegg.jp; version 107.0).
Gene set enrichment analysis (GSEA)
Gene expression data from RNA sequencing were
collected for both the model group and the Cur group. To conduct
GSEA, gene sets from the Molecular Signatures Database (MSigDB)
(https://www.gsea-msigdb.org/gsea/msigdb/index.jsp)
were initially sourced. Subsequently, the analysis was run on GSEA
software (https://www.gsea-msigdb.org/gsea/downloads.jsp). The
results were interpreted via enrichment score (ES), normalized
enrichment score (NES) and P-value.
Comparative analysis of human and mouse
solute carrier family 7 member 11 (SLC7A11) proteins
To assess potential species-specific differences in
SLC7A11, a comparative analysis of the protein sequences and
structures between humans and mice was performed. The amino acid
sequences of human SLC7A11 (UniProt ID: Q9UPY5) and mouse SLC7A11
(UniProt ID: Q9WTR6) were retrieved from the UniProt database
(https://www.uniprot.org/). Sequence alignment and
identity calculations were conducted using Clustal Omega
(http://www.clustal.org/omega/; version
1.2.4), with a focus on functionally critical domains (e.g.,
substrate-binding sites, transmembrane helices). Three-dimensional
(3D) structural predictions were generated using the AlphaFold
database (https://alphafold.ebi.ac.uk/) (human: AF-Q9UPY5-F1;
mouse: AF-Q9WTR6-F1) and structural similarity was evaluated using
PyMOL (https://pymol.org/; version 2.1).
Molecular docking
For molecular docking, the Cur compound was used as
a small-molecule ligand with SLC7A11 protein targets as receptors.
The target protein structures of SLC7A11 (UniProt ID: Q9WTR6) were
obtained from the UniProt database and the Royal Society of
Chemistry Database (https://www.chemspider.com). The center positions of
the grid box were determined on the basis of the interaction
between the small molecule and target (x=-1.873, y=-6.83,
z=10.886). The coordinates represent the center of the grid box
used for molecular docking simulations. This grid box was defined
to encompass the binding site of the target protein, where the
small molecule interacts with key residues. The center position was
determined based on the centroid of the co-crystallized ligand or
by aligning the target structure with known active site coordinates
from homologous proteins. Molecular docking was then performed in
batches with AutoDock Vina (https://github.com/ccsb-scripps/AutoDock-Vina; version
1.2.5). Using PyMOL 2.1 software, irrelevant small molecules were
removed from the protein structures. Water molecules were removed
and hydrogen atoms were added after the proteins were imported into
AutoDock Tools 1.5.6 (http://mgltools.scripps.edu/downloads/). Finally, the
structures were converted into pdbqt files. Molecular docking
calculations utilized the Lamarckian genetic algorithm (15), configured with a population size
of 150 individuals, a cap of 25 million energy evaluations, a
generation limit of 2,000, a crossover rate set at 0.8, a mutation
rate of 0.02, and 100 separate docking runs. Binding free energy
served as the criterion for assessing the final docking
structures.
Survival analysis
Survival analysis was performed using breast cancer
tissue data from The Cancer Genome Atlas in the Gene Expression
Profiling Interactive Analysis 2 database (http://gepia2.cancer-pku.cn/#index). The quartile
method (which divides a dataset into four equal parts) was used to
categorize the SLC7A11 gene expression levels in the samples. The
association between gene expression and DFS was analyzed through
log-rank test, and a Kaplan-Meier curve was generated to visually
represent the DFS rates for different groups based on gene
expression levels (cutoff: High expression group, 75%; low
expression group, 25%).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from tumor tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
RT was performed using the RT kit according to the manufacturer's
protocol (Accurate Biology, Inc.). The resultant cDNA underwent
qPCR under the following conditions: Initial cycle at 95°C for 60
sec, followed by 40 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 15 sec and extension at 72°C for 45 sec. qPCR
was performed using the SYBR Green Master Mix (Takara Bio, Inc.).
The housekeeping gene used was β-actin. The mRNA expression levels
were quantified using the QuantStudio Real-time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
corresponding to the target genes are provided in Table I. The 2−ΔΔCq method
was used to analysis relative gene expression data (16).
 | Table ISequences of the primers used in
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I
Sequences of the primers used in
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene | Sequence,
5′-3′ |
|---|
| Mouse IL7 | Forward:
TCGTGCTGCTCGCAAGTTG |
| Reverse:
CTTGTGCAGTTCACCAGTGTTTG |
| Mouse HCAR2 | Forward:
GGACAGACATGCCAAGATCAAG |
| Reverse:
CCAGGTCCACCGAGGAGTAG |
| Mouse NF-κBID | Forward:
TCCCCACAGTTGCCTTCAC |
| Reverse:
GAGCGTGTCTCCTTCCTCATC |
| Mouse SLC7A11 | Forward:
GCTATCATCACAGTGGGCTACG |
| Reverse:
GGGCAACAAAGATCGGGAC |
| Mouse APLNR | Forward:
CGGCTAAGGCTGCGAGTC |
| Reverse:
CTGGATCTTGGTGCCATTTTC |
| Mouse TGF-β | Forward:
TCGACATGGATCAGTTTATGCG |
| Reverse:
CCCTGGTACTGTTGTAGATGGA |
Lipid reactive oxygen species (ROS)
assay
MDA-MB-231 cells were plated in 6-well dishes at a
density of 3×106 cells/well, and were exposed to 100
µM Cur and/or 10 µM Fer-1 for 24 h at 37°C. After 24
h, the culture medium was substituted with 2 ml fresh medium
containing 5 µM BODIPY-C11 (Thermo Fisher Scientific, Inc.)
and incubated at 37°C for 30 min in the dark. Subsequently, the
cells were washed with PBS to eliminate any excess dye after
harvesting. Flow cytometry was performed using a BD FACSCanto II
flow cytometer (BD Biosciences) to examine the levels of lipid ROS
stained with BODIPY-C11. Data were analyzed using FlowJo software
(version 10.8; BD Biosciences). The experiment was independently
repeated three times.
2′,7′-Dichlorodihydrofluorescein
diacetate (DCFH-DA) assay
4T1 and MDA-MB-231 cells were seeded in
confocal-suitable dishes at a density of 3×106
cells/well, then exposed to 100 µM Cur for 24 h at 37°C.
After washing with serum-free medium, the cells were incubated with
10 µM DCFH-DA (Beyotime Institute of Biotechnology) for 30
min. Subsequently, the cells were washed again to remove excess
probe. Samples were observed under a confocal laser scanning
microscope. DCF was excited at 488-495 nm and its emission was
collected at 510-530 nm. The images were captured using a confocal
laser scanning microscope (Zeiss AG).
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. All statistical analyses were performed
using SPSS Statistics (version 28.0; IBM Corp.). Normality and
homogeneity of variances were confirmed using Shapiro-Wilk and
Levene's tests, respectively. Statistical differences among groups
were analyzed by one-way analysis of variance (ANOVA), followed by
Tukey's honestly significant difference post hoc test for pairwise
comparisons when ANOVA indicated significant overall effects
(P<0.05). Two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Cur inhibits the viability, migration,
invasion and colony formation of TNBC cells
The 4T1 and MDA-MB-231 cell lines were used to
conduct CCK-8 assays and to investigate the effects of Cur on the
viability of TNBC cells. Treatment with multiple doses of Cur (0,
6.25, 12.5, 25, 50, 100, 200 and 400 µM) (Fig. 2A) for 24 and 48 h decreased the
viability of TNBC cells (P<0.05; Fig. 2B). The IC50 values for
Cur intervention in TNBC cells after 24 h were 98.76 and 190.2
µM, and the IC50 values for Cur intervention
after 48 h were 95.11 and 169.8 µM. Subsequently,
experiments were conducted using Cur concentrations of 25, 50 and
100 µM. To elucidate the impact of Cur on the sensitivity of
TNBC cells to PTX, a series of additional experiments were
executed. After 24 h of intervention, Cur at concentrations of 50
and 100 µM exerted a marked effect on enhancing the
sensitivity of both 4T1 and MDA-MB-231 cells to 50 nM PTX
(P<0.05; Fig. 2C). In
addition, the sensitivity to 500 nM PTX was significantly increased
by the presence of 50 and 100 µM Cur in MDA-MB-231 cells
(P<0.05), and by 100 µM Cur in 4T1 cells (P<0.05).
These findings provided crucial insights into the potential
combined application of Cur and PTX in the treatment of TNBC.
Furthermore, treatment with Cur (50 and 100 µM) inhibited
the migration rate of 4T1 and MDA-MB-231 cells after intervention
for 24 h (P<0.05; Fig. 2D).
The Transwell assay results indicated that Cur (25, 50, and 100
µM) significantly decreased the invasive abilities of 4T1
and MDA-MB-231 cells (P<0.01; Fig. 2E). In addition, colony formation
abilities were significantly reduced in the 25, 50, and 100
µM Cur-treated groups (P<0.05; Fig. 2F). Taken together, these results
indicated that Cur may significantly inhibit the survival of TNBC
cells in vitro.
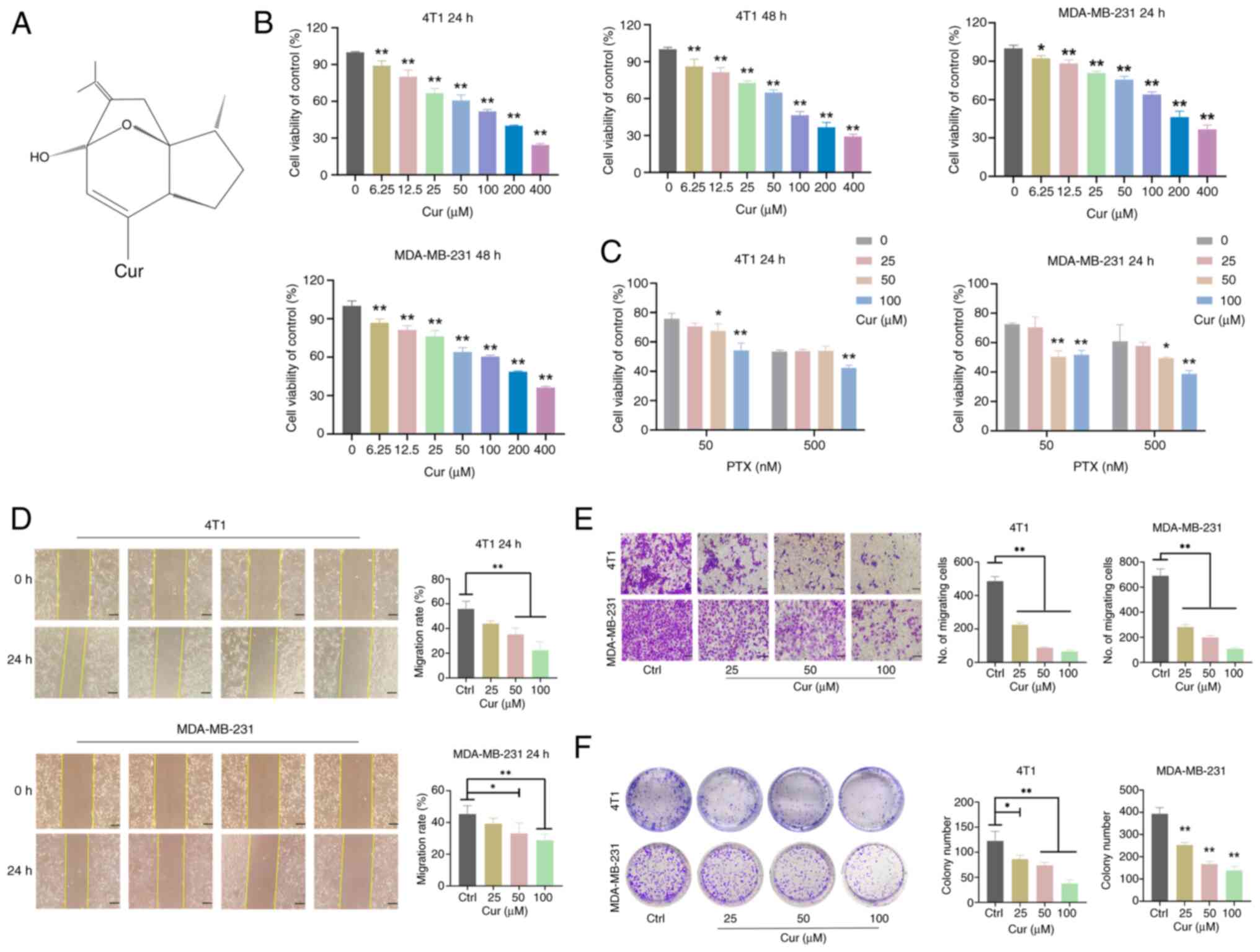 | Figure 2Effects of Cur on the viability,
migration, invasion and colony formation of triple-negative breast
cancer cells. (A) Chemical structure of Cur. (B) Viability of 4T1
and MDA-MB-231 cells treated with 6.25, 12.5, 25, 50, 100, 200 and
400 µM Cur for 24 and 48 h detected by CCK-8 assay. (C)
Effects of 0, 25, 50 and 100 µM Cur, and 50 and 500 nM PTX
on cell viability, as detected by CCK-8 assay. (D) Wound healing
assays were performed to analyze cell migration after 4T1 and
MDA-MB-231 cells were treated with 25, 50 and 100 µM Cur for
24 and 48 h. Scale bar, 100 µm. (E) Transwell assays were
performed to evaluate cell invasion after 4T1 and MDA-MB-231 cells
were treated with 25, 50 and 100 µM Cur for 24 h. Scale bar,
200 µm. (F) Colony formation assay of 4T1 and MDA-MB-231
cells grown for 7-10 days in the presence of the indicated
concentrations of Cur or Ctrl. Data are presented as the mean ± SD
(n=3). *P<0.05, **P<0.01 vs. Ctrl group
or as indicated. CCK-8, Cell Counting Kit-8 ; Ctrl, control; Cur,
curcumenol; PTX, paclitaxel. |
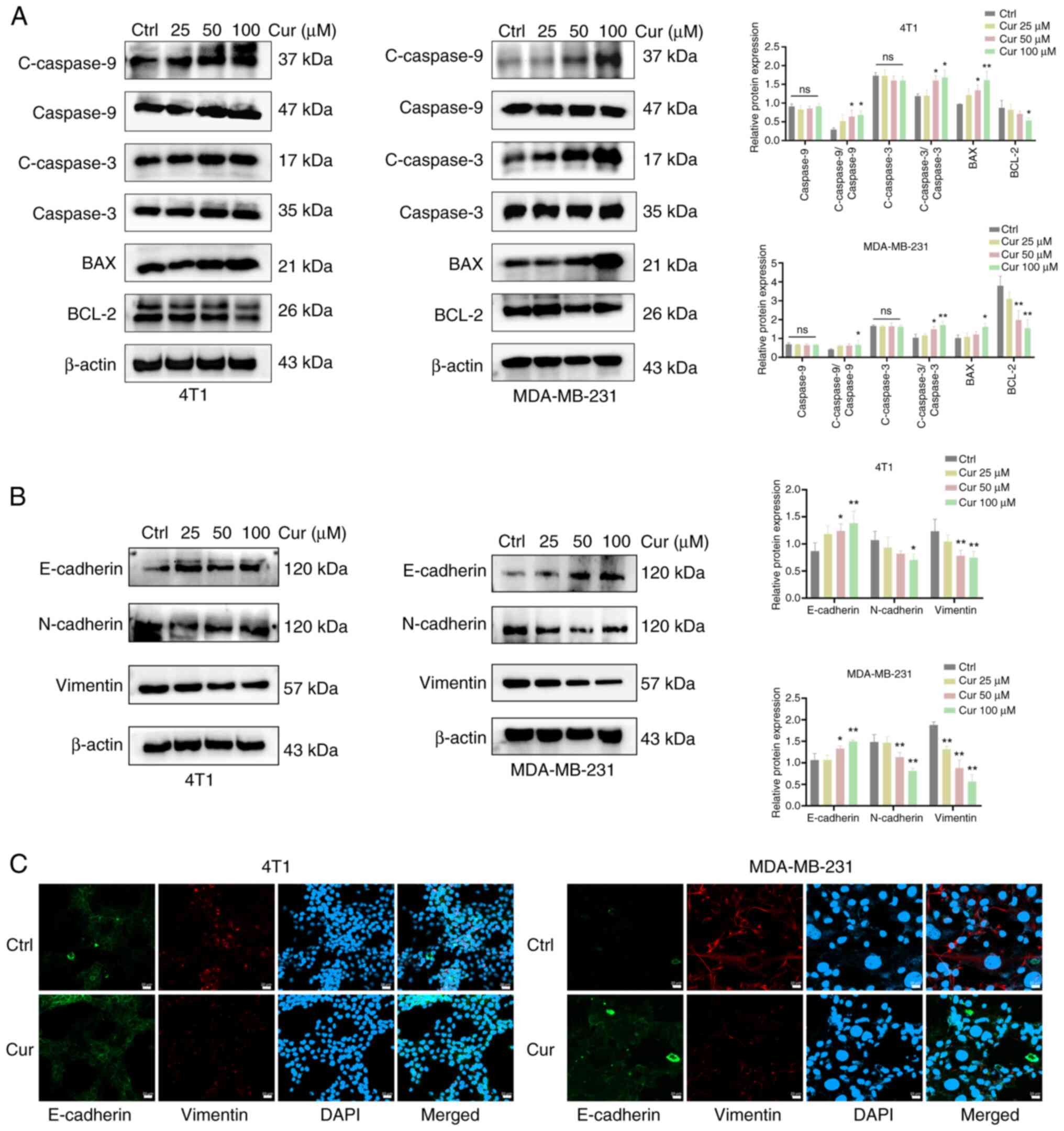
Cur regulates the expression of
apoptosis-associated proteins and EMT of TNBC cells
Next, WB was performed to observe the effects of Cur
on the expression of apoptosis-associated proteins in TNBC cells.
The results showed that, after 24 h of intervention with 100
µM Cur in 4T1 and MDA-MB-231 cells, the expression levels of
the proapoptotic proteins cleaved caspase 9, cleaved caspase 3 and
BAX were increased, whereas those of the antiapoptotic protein
BCL-2 were significantly inhibited (P<0.05; Fig. 3A). The dual bands of BCL-2 may
reflect its known post-translational modifications or splice
variants in TNBC cells. Antibody specificity was ensured via a
positive control and consistent sample processing, and the loading
controls confirmed equal protein loading without degradation. EMT
is tightly linked to the invasion and metastasis of tumors, and is
mainly distinguished by the lack of E-cadherin, along with the
increased expression levels of N-cadherin and Vimentin (15). Therefore, the current study
examined the protein levels of E-cadherin, N-cadherin and Vimentin.
It was revealed that 24 h of intervention with 100 µM Cur
could upregulate the protein expression levels of E-cadherin, and
downregulate those of N-cadherin and Vimentin (P<0.05; Fig. 3B). Immunofluorescence experiments
revealed that the protein expression of E-cadherin was markedly
elevated (green), whereas that of Vimentin was decreased (red)
following treatment with 100 µM Cur for 24 h (Fig. 3C). These results indicated that
Cur may exert an inhibitory effect on the occurrence and
development of EMT in TNBC cells.
Cur suppresses the growth and EMT of TNBC
in vivo
A TNBC mouse model was established by subcutaneously
injecting 4T1 cells into BALB/c mice to observe the anti-TNBC
effects of Cur (Fig. 4A).
Compared with in the model group, Cur (10 mg/kg, every other day)
or PTX (10 mg/kg/week) significantly inhibited the growth of TNBC
xenograft tumors (Fig. 4B), as
evidenced by dampened increases in tumor volume after 3 weeks of
treatment (P<0.05; Fig. 4C).
The maximum tumor volume observed in mice at the study endpoint was
2,407 mm3, and the largest tumor diameter recorded was
17.3 mm. These values were carefully monitored and did not exceed
the predefined ethical thresholds of 20 mm in diameter or 10% of
body weight. Subsequently, the levels of proliferation and
apoptosis markers were measured in tumor tissues. The results of
IHC revealed that Cur treatment markedly suppressed Ki-67
expression in tumor tissues and induced apoptosis in tumor cells
when compared with the model group (P<0.01; Fig. 4D). Furthermore, Cur significantly
downregulated the expression levels of BCL-2, but significantly
upregulated those of cleaved caspase 9, cleaved caspase 3 and BAX
(P<0.05; Fig. 4E). Compared
with in the model group, treatment with Cur could also inhibit the
expression levels of N-cadherin and Vimentin, while increasing
those of E-cadherin. These results revealed that Cur may regulate
the apoptosis and EMT of TNBC tumors.
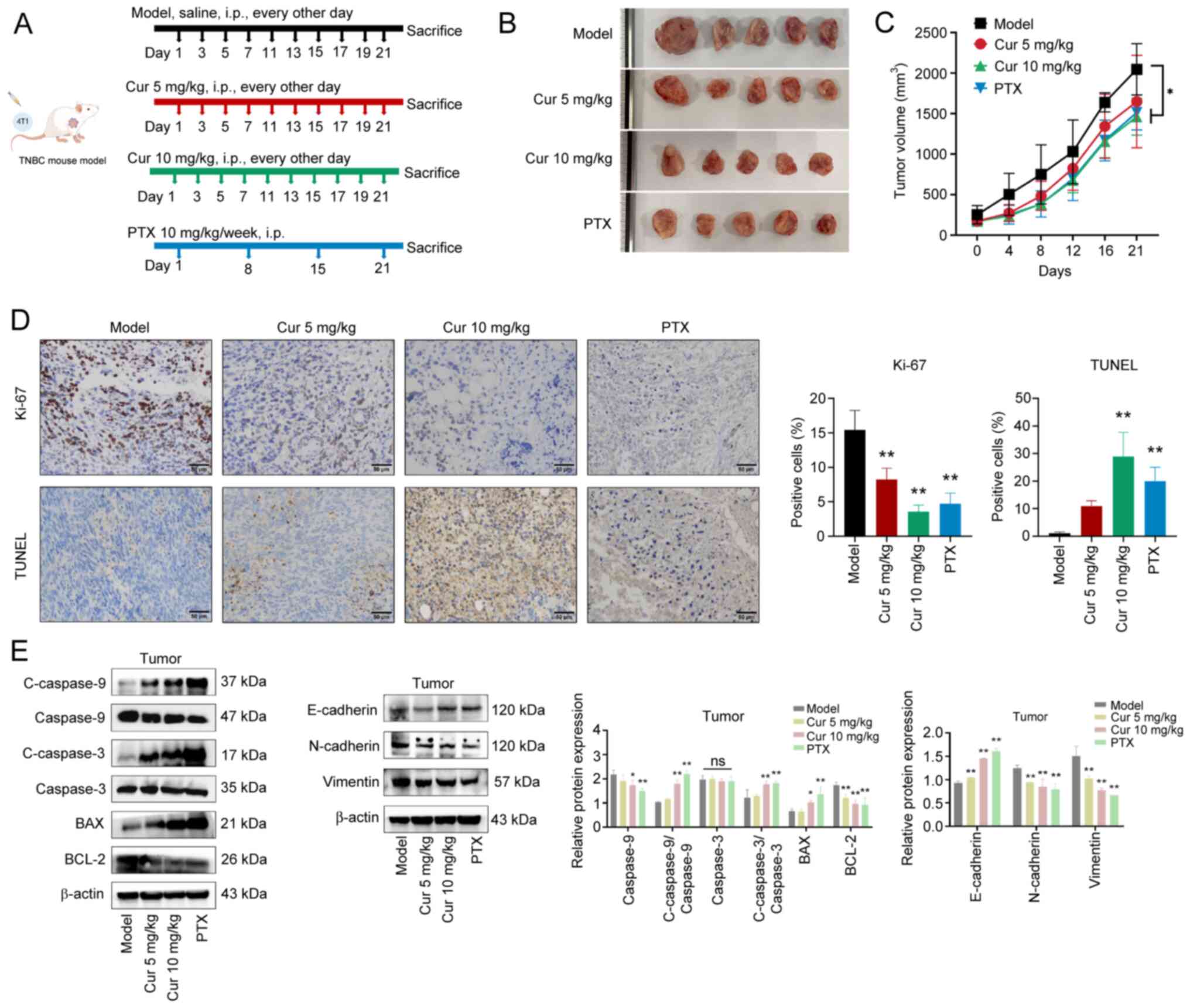 | Figure 4Effects of Cur on TNBC tumor growth
in vivo. (A) Schematic diagram of the in vivo study
on antitumor effects. (B) Representative images of tumors in
different groups of 4T1-induced TNBC mouse models. (C) Tumor
volumes of mice after treatment for 3 weeks. (D) Levels of Ki-67
and TUNEL in tumor tissues were determined using
immunohistochemistry, which aimed to measure proliferation and
apoptosis in the 4T1-induced TNBC mouse model. Scale bar, 50
µm. (E) Western blot analysis was performed to detect the
protein expression levels of C-caspase 9, C-caspase 3, BAX, BCL-2,
E-cadherin, N-cadherin and Vimentin. Data are presented as the mean
± SD (n=3). *P<0.05, **P<0.01 vs. Model
group or as indicated. Cur, curcumenol; TNBC, triple-negative
breast cancer; PTX, paclitaxel. |
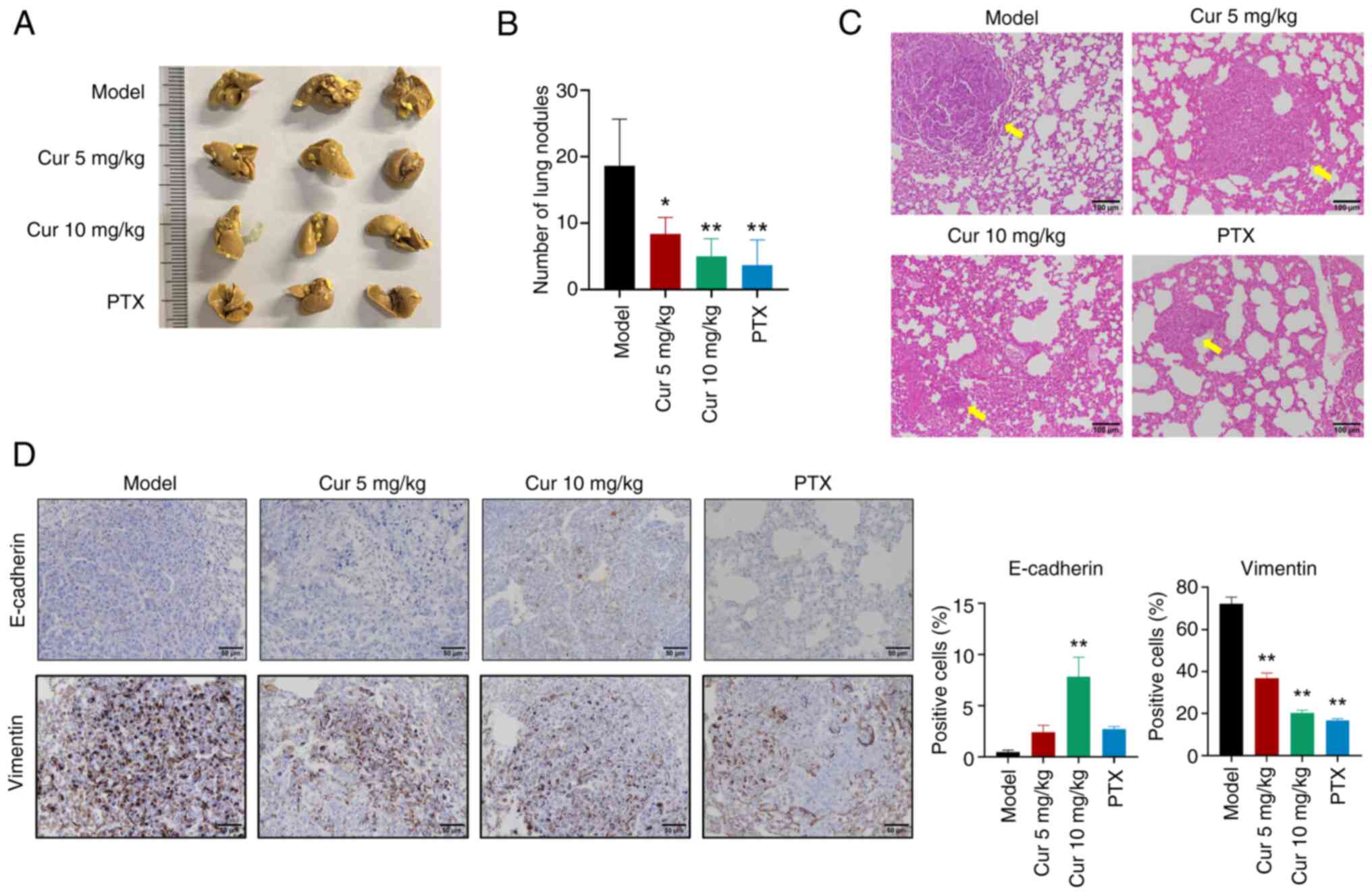
Cur alleviates TNBC lung metastasis in a
mouse xenograft model
Given the potent lung metastatic properties of 4T1
cells (17), lung metastasis was
also examined. The results showed that Cur and PTX could reduce the
number of lung metastatic nodules in mice (Fig. 5A and B). In addition, H&E
staining revealed that the lung metastatic lesion area was
decreased after treatment with different doses of Cur or PTX
(Fig. 5C). Furthermore, IHC
results demonstrated that in metastatic lung tissue, Cur
significantly upregulated E-cadherin levels, but downregulated
Vimentin levels (P<0.01; Fig.
5D). In summary, Cur may regulate EMT marker proteins in lung
metastatic lesions.
RNA sequencing analysis of the potential
mechanisms of Cur in the treatment of TNBC
RNA sequencing was conducted to analyze DEGs with
log2 FC >1.2 and P<0.05. The results revealed that Cur
upregulated 200 genes and downregulated 289 genes in TNBC tumor
tissues (Fig. 6A and B). KEGG
enrichment analysis showed that DEGs were enriched in the 'NF-kappa
B signaling pathway', 'TGF-beta signaling pathway' and
'ferroptosis' (Fig. 6C)
signaling pathways. GO analysis was used to classify the biological
functions of Cur-related DEGs. These biological functions included
'actin filament-based process', 'cytoskeletal protein binding',
'multicellular organismal process' and 'biological regulation'
(Fig. 6D).
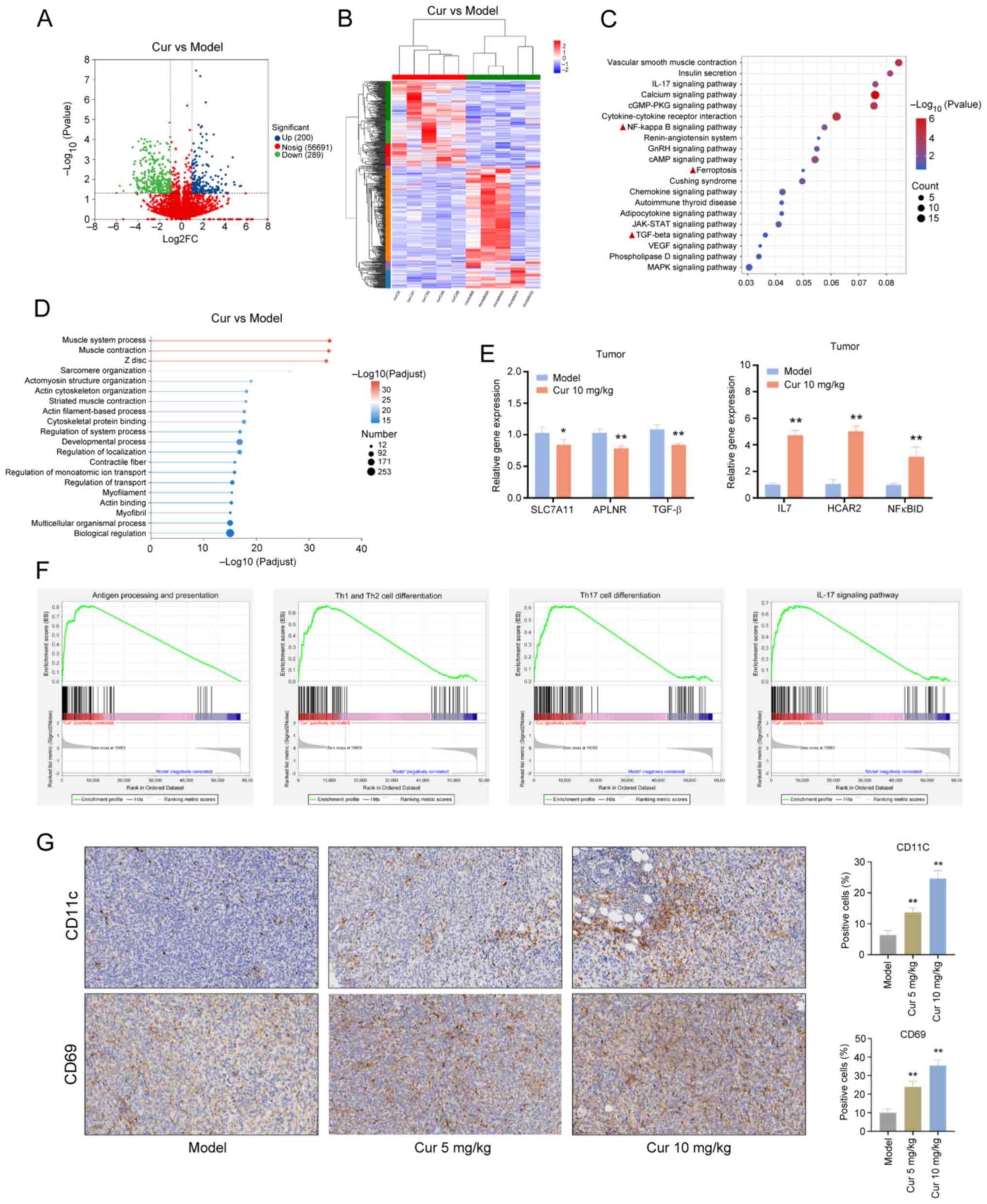 | Figure 6Identification of Cur-regulated
candidate target genes in TNBC tumor tissues by RNA sequencing. (A)
Volcano plots and (B) heatmap of upregulated and downregulated
genes in tumor tissues from the Cur group compared with the model
group (n=5). (C) Kyoto Encyclopedia of Genes and Genomes pathway
enrichment analysis. (D) GO annotation analysis of differentially
expressed genes. (E) Effect of Cur on SLC7A11, APLNR, TGF-β, IL7,
HCAR2 and NF-κBID in TNBC tumor tissues determined using reverse
transcription-quantitative polymerase chain reaction. (F) Gene Set
Enrichment Analysis. (G) Immunohistochemistry was performed to
detect the expression of CD11b (dendritic cell marker) and CD69
(T-cell activation marker) in each group of tumor tissues
(magnification, ×63). Data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 vs. Model group.
APLNR, angiotensin receptor like 1; Cur, curcumenol; GO, Gene
Ontology; HCAR2, hydroxycarboxylic acid receptor 2; SLC7A11, solute
carrier family 7 member 11; TNBC, triple-negative breast
cancer. |
Among the DEGs, SLC7A11 (P=0.032, log2 FC=-1.75),
angiotensin receptor like 1 (APLNR; P=1.44×10−5, log2
FC=-1.09), TGF-β2 (P<0.001, log2 FC=-1.24), IL7
(P=3.57×10−8, log2 FC=1.39), hydroxycarboxylic acid
receptor 2 (HCAR2; P=2.51×10−5, log2 FC=1.80) and
NF-κBID (P<0.001, log2 FC=1.20) were regulated by Cur. The
effects of Cur on the mRNA expression levels of SLC7A11, APLNR,
TGF-β, IL7, HCAR2 and NF-κBID were verified in TNBC tumor tissues
using RT-qPCR to validate the results of RNA sequencing. Consistent
with the results of RNA sequencing, the mRNA expression levels of
IL7, HCAR2 and NF-κBID were increased, whereas those of APLNR,
SLC7A11 and TGF-β were decreased in the tumor tissues of mice
following Cur (10 mg/kg) intervention (P<0.05; Fig. 6E).
On the basis of the normalized NESs obtained through
the GSEA of the transcriptome sequencing results, it was revealed
that Cur could significantly activate immune-related pathways,
including 'antigen processing and presentation' (NES=2.06,
P<0.001), the 'IL-17 signaling pathway' (NES=1.74, P<0.001),
'Th1 and Th2 cell differentiation' (NES=1.68, P<0.001), and
'Th17 cell differentiation' (NES=1.61, P<0.001) (Fig. 6F).
Antigen processing and presentation are vital
processes for the immune system to recognize antigens and initiate
an immune response. After antigens are internalized by cells, they
bind to major histocompatibility complex molecules and are
presented on the cell surface for T-cell recognition, thereby
activating effective antitumor T-cell activity (18). Under stimulation by different
cytokines and antigen signals, CD4+ T cells
differentiate into helper T cells (Th cells). The generation of the
Th1 effector subset depends on IL-12 and IFN-γ cytokines (19). Th2 cell immunity can permanently
convert high-grade breast tumors into low-grade, fibrocystic-like
structures (20). Th17 cells are
a subset of CD4+ T cells, which mainly secrete
cytokines, such as IL-17; notably, IL-17 and Th17 cells form a
positive feedback loop, enhancing barrier immunity (21,22). In accordance with the current
GSEA results, it could be hypothesized that Cur exerts a regulatory
effect on dendritic cells (DCs) and CD4 cells in the tumor immune
microenvironment. IHC results indicated that Cur could increase the
expression levels of the DC-specific marker CD11c and promote the
expression of the T-cell activation marker CD69 in mouse tumor
tissues (P<0.01) (Fig.
6G).
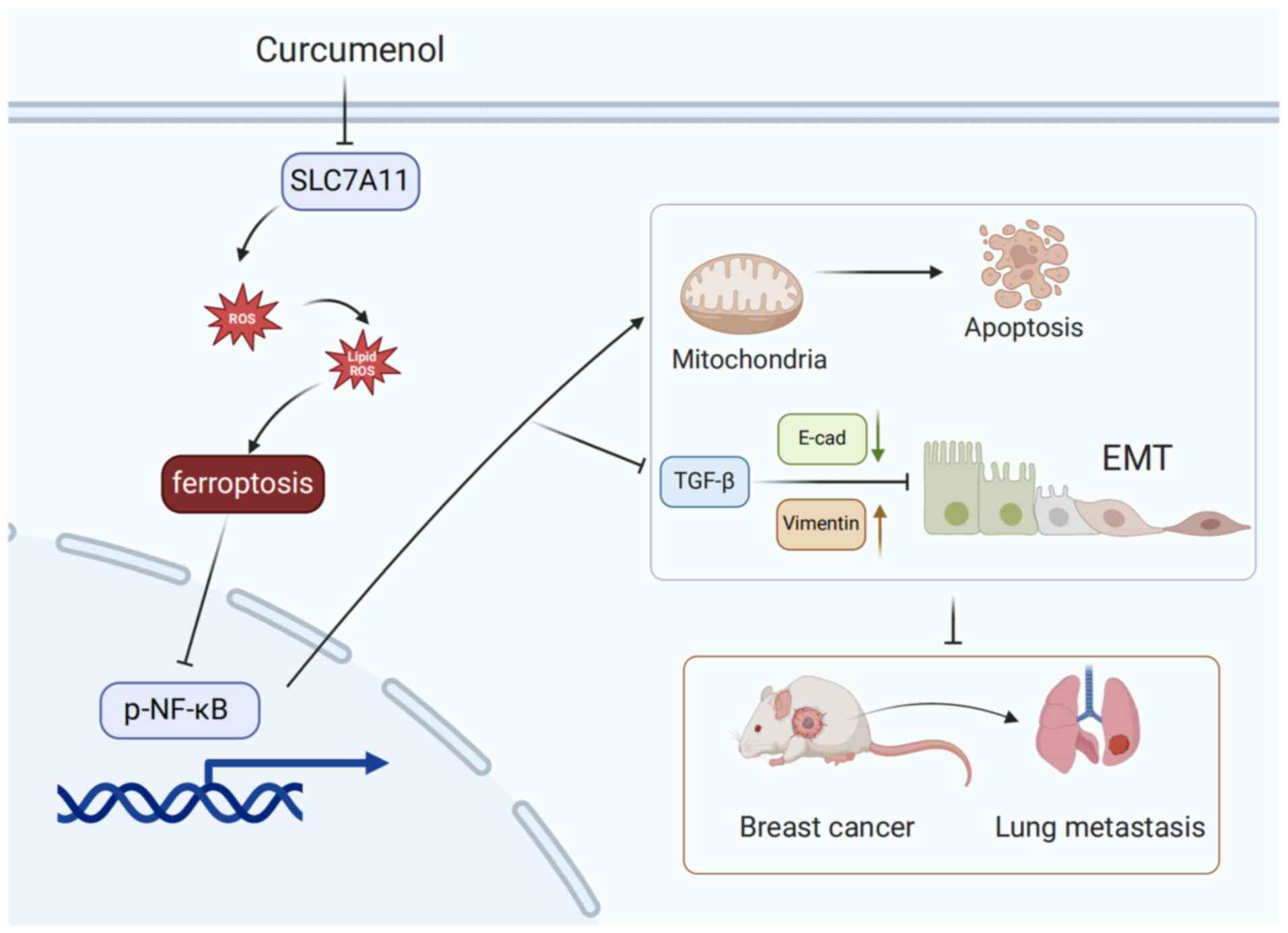
Cur inhibits TNBC by modulating
ferroptosis-related proteins
The downregulation of SLC7A11, a specific amino acid
transporter, can inhibit the cysteine metabolism pathway, leading
to reduced intracellular cystine levels and GSH biosynthesis
depletion, indirectly inhibiting the activity of GPX4, subsequently
causing lipid peroxide accumulation and ultimately inducing
ferroptosis in cells (23).
Ferroptosis represents a controlled type of cell death that occurs
due to the buildup of lipid ROS, which are closely associated with
oxidative stress responses and cysteine metabolism; it is the
consequence of iron-dependent lipid peroxide accumulation (24,25). The results of the present
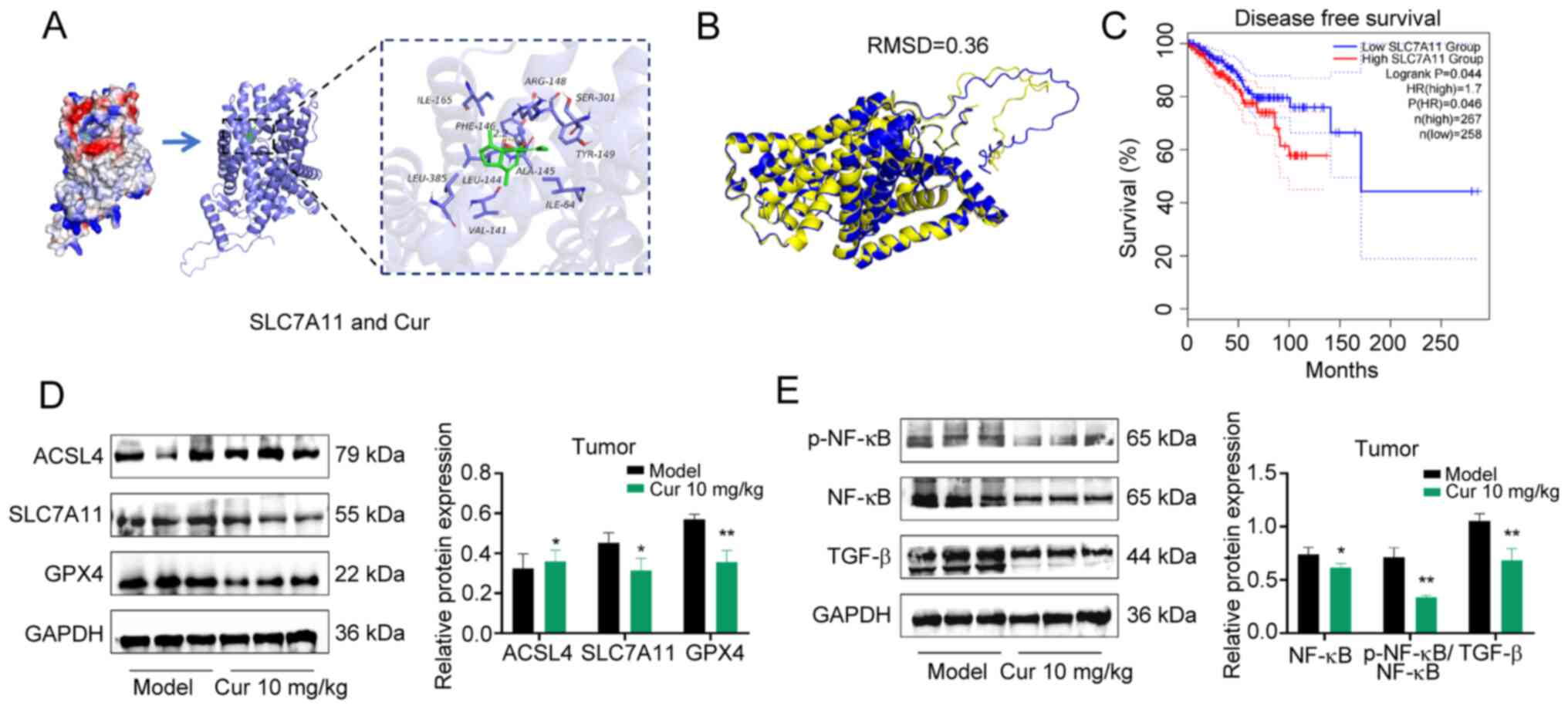
molecular docking analysis indicated that Cur exhibited excellent
binding capacity and compatibility with SLC7A11, with binding
energies of -6.170 kcal/mol. Cur was revealed to form hydrogen bond
interactions with the amino acid LEU-144 at the active site of the
SLC7A11 protein, which is of importance to the ligand molecules in
the active pocket of the anchor protein (Fig. 7A). Furthermore, this compound was
revealed to form favorable hydrophobic interactions with ILE-165,
PHE-146, LEU-385, LEU-144, VAL-141, ALA-145, ILE-64 and TYR-149,
making an important contribution to the stabilization of small
molecules. To explore potential species-specific differences in
SLC7A11, a comparative analysis of the protein sequences and
structures of SLC7A11 was performed in mice and humans. The amino
acid sequences of human SLC7A11 (UniProt ID: Q9UPY5) and mouse
SLC7A11 (UniProt ID: Q9WTR6) showed a high degree of amino acid
sequence identity according to UniProt. In addition, the AlphaFold
prediction model was used for 3D structure prediction, and the
comparison of human SLC7A11 (AF-Q9UPY5-F1) with mouse SLC7A11
(AF-Q9WTR6-F1), and the overall 3D conformations were revealed to
be highly similar (root mean square deviation=0.36) by PyMOL
(Fig. 7B). These findings
indicated there is a high degree of similarity and reproducibility
in the regulatory mechanisms of Cur between humans and mice.
Bioinformatics analysis revealed that patients with
breast cancer with low SLC7A11 expression had a longer DFS (HR=1.7,
log-rank P=0.044) (Fig. 7C) than
those without. WB revealed that in mouse tumor tissues, Cur could
downregulate the protein expression levels of the ferroptosis
inhibitors SLC7A11 and GPX4, whereas it promoted the protein
expression levels of ACSL4, which facilitates lipid metabolism
conducive to ferroptosis (Fig.
7D).
As a member of the NF-κB kinase inhibitor family,
NF-κBID, also known as inhibitor of NF-κB, serves as a key negative
regulatory molecule of the NF-κB signaling pathway. NF-κBID can
bind to NF-κB in the cytoplasm, thereby maintaining NF-κB in an
inactivated state (26,27). TGF-β is a pivotal participant in
regulating EMT in tumor cells and is one of the most important
inducers of EMT (28); notably,
the elevated expression of TGF-β is associated with accelerated EMT
progression in tumors and adverse prognosis (29). Therefore, the current study
detected the protein expression levels of NF-κB and TGF-β using WB.
Consistent with our previous results, Cur could downregulate the
phosphorylation of NF-κB and the protein expression levels of TGF-β
in tumor tissues (P<0.05; Fig.
7E).
Cur can promote ferroptosis through the
SLC7A11/NF-κB/TGF-β pathway
A reduction in mitochondrial membrane potential
serves as a crucial characteristic of the mitochondrial-mediated
apoptosis pathway. In ferroptotic cells, the mitochondrial membrane
potential usually decreases, causing the fluorescence of JC-1 to
shift from red to green (30).
In the present study, treating TNBC cells with 100 µM Cur
for 24 h resulted in the reduced aggregation of JC-1 in the
mitochondrial matrix (red fluorescence) and increased expression of
JC-1 monomers (green fluorescence); this finding indicated that the
mitochondrial membrane potential was reduced after Cur treatment
(Fig. 8A). The accumulation of
ROS serves a pivotal role by promoting the generation of lipid
peroxides during ferroptosis, subsequently driving the occurrence
of ferroptosis. After 24 h of Cur intervention, 4T1 and MDA-MB-231
cells exhibited increased levels of ROS (Fig. 8B). Flow cytometry revealed that
when Fer-1 was used to inhibit the occurrence of ferroptosis, the
levels of lipid ROS were decreased compared with those in the
control group; however, Cur was able to counteract the inhibitory
effect of Fer-1 on ferroptosis (Fig.
8C). Similarly, Cur was shown to reduce the protein expression
levels of SLC7A11 and GPX4 in MDA-MB-231 cells, while increasing
those of ACSL4. Despite the presence of the ferroptosis inhibitor
Fer-1, the intervention with Cur in the Fer-1 + Cur group could
still downregulate the protein expression of SLC7A11 and GPX4,
compared with those in the control group. This may imply that Cur
could rescue the inhibitory effects of Fer-1 on ferroptosis in
MDA-MB-231 cells (Fig. 8D).
These data indicated that Cur could regulate ferroptosis and
inhibit the malignant progression of cells in TNBC by inhibiting
the SLC7A11/NF-κB/TGF-β signaling pathway.
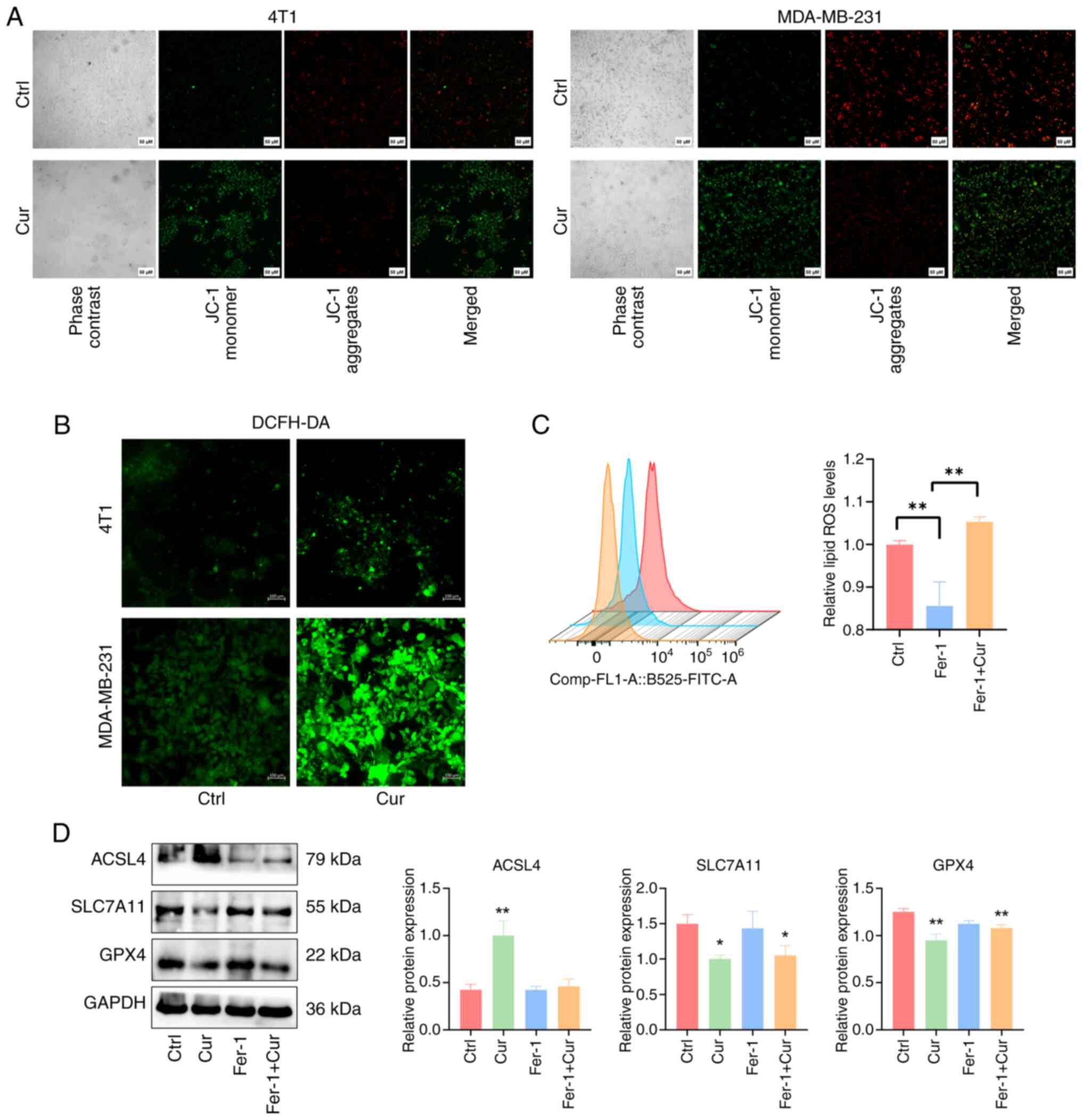 | Figure 8Cur can promote ROS accumulation and
ferroptosis. (A) Fluorescence images of JC-1 aggregates (red) and
JC-1 monomers (green) in 4T1 and MDA-MB-231 cells treated with 100
µM Cur or Ctrl for 24 h. Scale bar, 50 µm. (B)
Labeling of 4T1 and MDA-MB-231 cells with the DCFH-DA fluorescent
probe to assess the level of ROS release. Scale bar, 100 µm.
(C) Flow cytometry of lipid ROS levels in MDA-MB-231 cells after 24
h of treatment with Fer-1 alone or in combination with Cur. (D)
Western blot analysis was performed to detect the expression levels
of the ferroptosis-related proteins SLC7A11, GPX4 and ACSL4 in
MDA-MB-231 cells. Data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 vs. Ctrl group or as
indicated. ACSL4, acyl-CoA synthetase long-chain family member 4;
Ctrl, control; Cur, curcumenol; DCFH-DA,
2′,7′-dichlorodihydrofluorescein diacetate; Fer-1, ferrostatin-1;
GPX4, glutathione peroxidase 4; ROS, reactive oxygen species;
SLC7A11, solute carrier family 7 member 11. |
Discussion
TNBC is the most challenging subtype of breast
cancer to treat, which displays a high degree of invasiveness and
metastatic potential. Tumor metastasis is a sophisticated
biological process that involves EMT, enabling cells to penetrate
the adjacent basement membrane, enter the circulatory system,
travel to distant organs and ultimately lead to metastasis
(31).
Emerging modes of tumor cell death, such as
ferroptosis and disulfidptosis, have been discovered and are
tightly regulated by the internal metabolic processes within tumors
(32). The inhibition of
voltage-dependent anion channel 3-derived circular RNA can elevate
the levels of ROS, trigger the accumulation of the labile iron pool
and subsequently induce ferroptosis in breast cancer cells.
Consequently, this enhances the sensitivity of HER2-low breast
cancer cells to trastuzumab deruxtecan treatment (33). SLC7A11, also known as xCT, is an
important membrane protein that indirectly inhibits the occurrence
of ferroptosis by promoting GSH synthesis (34). Research has indicated that the
NF-κB/SLC7A11 signaling pathway can regulate ferroptosis in
mastitis (35). The transduction
of the NF-κB pathway is well recognized to serve a crucial role in
breast (36), colorectal
(37), pancreatic (38) and cervical cancer (39). NF-κB serves as a biomarker for
cancer staging, progression and prognosis, and its upregulation is
associated with tumor progression in breast cancer (40,41). Furthermore, the interaction
between the NF-κB and TGF-β signaling pathways has been extensively
studied in various contexts, including cancer biology (42-44). NF-κB regulates the expression of
TGF-β and inhibiting NF-κB could reduce the expression of TGF-β,
enhancing the sensitivity of breast cancer cells to
chemotherapeutic drugs (45). In
neuroblastoma, the activation of TGF-β can activate the NF-κB
pathway, regulating the tumor microenvironment and promoting the
development of drug resistance (46).
A growing body of research has focused on utilizing
natural products for cancer therapy, and C. phaeocaulis
Valeton is a promising plant in this field. Compounds extracted
from C. phaeocaulis Valeton exhibit high antitumor efficacy
and safety in breast cancer (47). Treatment with C.
phaeocaulis Valeton has been shown to inhibit the proliferation
of MCF-7 cells by inducing apoptosis mediated by increased ROS
formation, regulating the expression of BCL-2 family proteins and
activating caspases (48).
Furthermore, it has been demonstrated that curcumin can hinder the
migration and invasion of breast cancer cells by suppressing cancer
stem cell characteristics and EMT (49). Curcumin can also inhibit the
formation of mammospheres, and downregulate the expression levels
of E-cadherin and Vimentin (50). In liver cancer, curcumol has been
demonstrated to inhibit cell proliferation by suppressing apoptosis
and cell cycle arrest, while also inhibiting cell invasion and
metastasis (51). Al-Amin et
al (52) isolated bioactive
compounds from C. phaeocaulis Valeton; comosone II was shown
to inhibit the migration and invasion of MDA-MB-231 cells by
suppressing matrix metalloproteinase-9.
While numerous bioactive compounds isolated from
C. phaeocaulis Valeton have demonstrated antitumor potential
across various types of cancer, to the best of our knowledge, the
therapeutic efficacy of Cur against breast cancer remained unclear
prior to the current study. This study systematically deciphered
the multimodal inhibitory effects of Cur on TNBC progression
through integrated mechanistic approaches. Consistent with the
reported activities of other compounds in C. phaeocaulis
Valeton, the present study indicated that Cur can inhibit the
migration, invasion and EMT of TNBC cells, while promoting cell
apoptosis.
In the malignant progression of tumors, EMT and
ferroptosis exhibit an interactive relationship. Cancer cells
undergo metabolic changes and may modify iron metabolism pathways
to evade ferroptosis during EMT. For example, in cells undergoing
EMT, the expression of iron-regulatory proteins (such as iron
transporters and ferritin) is increased to avert lipid peroxidation
and hinder the accumulation of free iron ions (53). It has also been found that
4-methoxydalbergione can inhibit tumorigenesis and metastasis in
lung cancer by promoting ferroptosis (54). The present study indicated that
Cur could regulate ferroptosis-related proteins and ROS
accumulation in TNBC while inhibiting the occurrence of EMT; these
effects may be interrelated. However, considering that excessive
ROS levels have the potential to induce cell death, Cur may also
promote the apoptosis of TNBC cells through the accumulation of
ROS. By modulating the ROS-mediated EMT and apoptotic pathways, Cur
may provide a promising therapeutic strategy for patients with
TNBC, offering a novel perspective of targeted cancer therapy
(55).
In the present study, the selection of Cur
concentrations was determined based on both preliminary
pharmacological assessments and established precedents in related
research. For in vitro experiments, the chosen
concentrations of 25, 50 and 100 µM were guided by the
IC50 values of Cur in TNBC cells, and prior studies
demonstrating that curcuminoids and structurally related compounds
exhibit anti-migratory and anti-proliferative effects on cancer
cells within this concentration range without inducing excessive
cytotoxicity (56,57). Notably, 100 µM Cur exerted
the most pronounced inhibitory effects on TNBC cells, aligning with
the observed dose-dependent response. For in vivo studies,
the doses of 5 and 10 mg/kg were selected to balance efficacy and
safety, referencing previous work in colorectal and prostate cancer
models where similar doses of Cur derivatives suppressed tumor
growth and metastasis (58,59). While both doses reduced TNBC
tumor burden, the 10 mg/kg dose demonstrated superior regulation of
tumor proliferation, apoptosis and metastasis inhibition,
suggesting its optimal therapeutic potential within a safe dosing
window. Further studies are warranted to explore higher doses and
refine pharmacokinetic parameters for clinical translation.
Notably, an interesting phenomenon emerged in the
present study: Cur did not have completely identical inhibitory
effects on the proliferation and migration of 4T1 and MDA-MB-231
cells. This finding is consistent with the results of other studies
on breast cancer (60). The 4T1
cell line is known for its highly aggressive and metastatic
behavior in murine models, characterized by rapid proliferation and
robust invasiveness (61). By
contrast, MDA-MB-231 cells, while also aggressive, may exhibit
different metabolic dependencies that could modulate their response
to Cur. For example, the high glutamine dependency of the redox
homeostasis of 4T1 cells (linked to SLC7A11-mediated cystine
uptake) might render them highly sensitive to Cur (62,63). Another interesting phenomenon was
that the alterations in cleaved caspase 9/3 activation and BAX
expression were observed only at higher Cur concentrations (50 or
100 µM), whereas lower doses (25 µM) showed minimal
effects. This dose-dependent threshold phenomenon may arise from
the cumulative disruption of pro-survival signaling required to
overcome cellular apoptotic resistance. Moreover, the differential
sensitivity between 4T1 and MDA-MB-231 cells can be ascribed to the
basal expression levels of prosurvival proteins. MDA-MB-231 cells
exhibit relatively elevated baseline levels of anti-apoptotic
proteins, such as BCL-2. In future studies, in-depth molecular and
genetic analyses could be conducted to elucidate the underlying
mechanisms. Such analyses will not only improve the understanding
of the effects of Cur on different cell lines, but could also
contribute to the development of targeted cancer therapies.
Since 4T1 is a TNBC cell line characterized by high
levels of lung metastasis, the current study further revealed that
Cur could prevent lung metastasis in mice with primary tumors. In
our previous study, TCM compound prescriptions, including C.
phaeocaulis Valeton, have been shown to exert marked preventive
effects against lung metastasis (64). Therefore, this finding suggested
that Cur may indeed serve a crucial role in inhibiting the process
of lung metastasis. Furthermore, it was discovered that Cur could
inhibit the expression of APLNR. The APLNR signaling pathway can
induce the secretion of angiogenesis factors, such as vascular
endothelial growth factor, thus promoting tumor angiogenesis
(65,66). The results of RT-qPCR verified
the inhibitory effect of Cur on APLNR in tumor tissues, suggesting
that vascular growth may also be a potential mechanism for Cur in
the treatment of TNBC.
Although comprehensive research methods were
employed, certain methodological drawbacks exist in the present
study. Notably, 4T1 cells were used to establish an animal model of
TNBC; while the use of 4T1 cells for modeling has been widely
adopted, these cells exhibit certain differences from human breast
cancer in terms of the tumor microenvironment, immune responses and
heterogeneity. Future studies should consider using animal models
developed from human-derived TNBC cells or patient-derived
xenograft models to comprehensively elucidate the effects of Cur
from multiple perspectives.
The present study demonstrated that Cur exerted
inhibitory effects on the survival of TNBC cells in vitro
and markedly suppressed their migration and invasion. Further
experiments revealed that Cur could promote lipid ROS accumulation
and regulate ferroptosis-related proteins. To the best of our
knowledge, the present study is the first to reveal that Cur can
markedly inhibit TNBC by decreasing the SLC7A11/NF-κB/TGF-β
signaling pathway (Fig. 9).
Despite the potential of Cur for TNBC treatment,
rigorously designed clinical trials are still needed for its
clinical translation (67).
Subsequent works should initially focus on completing
pharmacokinetic and toxicological studies to evaluate the
absorption, distribution, metabolism, excretion and toxicological
characteristics of Cur. The solubility and stability of Cur remain
to be further improved, and strategies, such as nanoparticle
encapsulation or liposomal drug delivery systems, should be
developed to optimize its formulation (68,69). Ensuring strict pharmaceutical
supervision and quality control is particularly important. Finally,
phase I clinical trials, with a focus on the safety and
tolerability of Cur in patients with TNBC, need to be successively
conducted. After ensuring the safety of Cur, clinical studies on
the synergistic treatment of TNBC with Cur and chemotherapeutic
drugs, such as PTX, or the prevention and treatment of TNBC lung
metastasis must be planned. Overall, although existing research has
demonstrated the anticancer effects of Cur, further studies are
required to explore the potential use of Cur for the treatment of
TNBC.
Availability of data and materials
The RNA sequencing data generated in the present
study may be found in the National Centre for Biotechnology
Information under accession number PRJNA1233780 or at the following
URL: https://www.ncbi.nlm.nih.gov/sra/PRJNA1233780. The
other data generated in the present study may be requested from the
corresponding author.
Authors' contributions
FFL, QQ, SL and HGW contributed to the research
conception and design. FFL, YQ and YH performed the experiments and
drafted the manuscript. YL, KG, HRL and CFG analyzed and
interpreted the raw data. FFL, QQ, YL, KG, HRL and CFG confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Welfare Committee of Longhua Hospital, Shanghai University of TCM
(approval no. LHERAW-24010; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China funded project (grant no. 82405389), the
Postdoctoral Fellowship Program of China Postdoctoral Science
Foundation (grant no. GZC20231706), and the Shanghai Post-doctoral
Excellence Program (grant no. 2023547).
References
|
1
|
Joaquin Garcia A, Rediti M, Venet D,
Majjaj S, Kammler R, Munzone E, Gianni L, Thürlimann B, Laáng I,
Colleoni M, et al: Differential benefit of metronomic chemotherapy
among triple-negative breast cancer subtypes treated in the IBCSG
trial 22-00. Clin Cancer Res. 29:4908–4919. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rusakiewicz S, Tyekucheva S, Tissot-Renaud
S, Chaba K, Imbimbo M, Benedetti F, Kammler R, Hornfeld J, Munzone
E, Gianni L, et al: Multiplexed high-throughput immune cell imaging
in patients with high-risk triple negative early breast cancer:
Analysis from the international breast cancer study group (IBCSG)
trial 22-00. Eur J Cancer. 200:1135352024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanna D, Merrick S, Ghose A, Devlin MJ,
Yang DD, Phillips E, Okines A, Chopra N, Papadimatraki E, Ross K,
et al: Real world study of sacituzumab govitecan in metastatic
triple-negative breast cancer in the United Kingdom. Br J Cancer.
130:1916–1920. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dent R, André F, Gonçalves A, Martin M,
Schmid P, Schütz F, Kümmel S, Swain SM, Bilici A, Loirat D, et al:
IMpassion132 double-blind randomised phase III trial of
chemotherapy with or without atezolizumab for early relapsing
unresectable locally advanced or metastatic triple-negative breast
cancer. Ann Oncol. 35:630–642. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynce F, Mainor C, Donahue RN, Geng X,
Jones G, Schlam I, Wang H, Toney NJ, Jochems C, Schlom J, et al:
Adjuvant nivolumab, capecitabine or the combination in patients
with residual triple-negative breast cancer: The OXEL randomized
phase II study. medRxiv [Preprint]: 2023.12.04.23297559. 2023.
|
|
7
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Li J, Xu S, Wang N, Pan B, Yang B,
Zheng Y, Zhang J, Peng F, Peng C and Wang Z: Baohuoside I
chemosensitises breast cancer to paclitaxel by suppressing
extracellular vesicle/CXCL1 signal released from apoptotic cells. J
Extracell Vesicles. 13:e124932024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu C, Sun C, Liu G, Qin Y, Xue X, Wu X,
Wang Q, Liu J, Ye Z, Li Q, et al: Effectiveness of the sanyin
formula plus chemotherapy on survival in women with triple-negative
breast cancer: A randomized controlled trial. Front Oncol.
12:8501552022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kan LLY, Chan BCL, Leung PC and Wong CK:
Natural-product-derived adjunctive treatments to conventional
therapy and their immunoregulatory activities in triple-negative
breast cancer. Molecules. 28:58042023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alam S, Lee J and Sahebkar A: Curcumin in
cancer prevention: Insights from clinical trials and strategies to
enhance bioavailability. Curr Pharm Des. 30:1838–1851. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao P, Qiu J, Pan C, Tang Y, Chen M, Song
H, Yang J and Hao X: Potential roles and molecular mechanisms of
bioactive ingredients in Curcumae Rhizoma against breast cancer.
Phytomedicine. 114:1548102023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang R, Pan T, Xiang Y, Zhang M, Xie H,
Liang Z, Chen B, Xu C, Wang J, Huang X, et al: Curcumenol triggered
ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1
axis. Bioact Mater. 13:23–36. 2021.
|
|
14
|
Mao Z, Zhong L, Zhuang X, Liu H and Peng
Y: Curcumenol targeting YWHAG inhibits the pentose phosphate
pathway and enhances antitumor effects of cisplatin. Evid Based
Complement Alternat Med. 2022:39889162022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuhrmann J, Rurainski A, Lenhof HP and
Neumann D: A new Lamarckian genetic algorithm for flexible
ligand-receptor docking. J Comput Chem. 31:1911–1918. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Liu Q, Li R and Lin J: No difference among
inhaled anesthetics on the growth and metastasis of murine 4T1
breast cancers in a mouse model of spontaneous metastasis. Front
Pharmacol. 13:7941092022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Y, Wang Y, Ma H, Guo Y, Xu R, Chen X,
Chen X, Lv Y, Li P and Gao Y: TFAP2A downregulation mediates
tumor-suppressive effect of miR-8072 in triple-negative breast
cancer via inhibiting SNAI1 transcription. Breast Cancer Res.
26:1032024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nielsen AJ, Albert GK, Sanchez A, Chen J,
Liu J, Davalos AS, Geng D, Bradeen X, Hintzsche JD, Robinson W, et
al: DNA-PK inhibition enhances neoantigen diversity and increases T
cell responses to immunoresistant tumors. J Clin Invest.
134:e1802782024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basu A, Ramamoorthi G, Albert G, Gallen C,
Beyer A, Snyder C, Koski G, Disis ML, Czerniecki BJ and Kodumudi K:
Differentiation and regulation of TH cells: A balancing Act for
cancer immunotherapy. Front Immunol. 12:6694742021. View Article : Google Scholar :
|
|
21
|
Boieri M, Malishkevich A, Guennoun R,
Marchese E, Kroon S, Trerice KE, Awad M, Park JH, Iyer S, Kreuzer
J, et al: CD4+ T helper 2 cells suppress breast cancer
by inducing terminal differentiation. J Exp Med. 219:e202019632022.
View Article : Google Scholar
|
|
22
|
Feng S, Li S, Wu Z, Li Y, Wu T, Zhou Z,
Liu X, Chen J, Fu S, Wang Z, et al: Saffron improves the efficacy
of immunotherapy for colorectal cancer through the IL-17 signaling
pathway. J Ethnopharmacol. 337:1188542025. View Article : Google Scholar
|
|
23
|
Chen X, Cui H, Qin L, Liu R, Fang F and
Wang Z: Soybean lecithin-gallic acid complex sensitizes lung cancer
cells to radiation through ferroptosis regulated by
Nrf2/SLC7A11/GPX4 pathway. Nutrients. 17:12622025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Guan WX, Zhou Y, Zhang XY and Zhao
HJ: Red ginseng polysaccharide promotes ferroptosis in gastric
cancer cells by inhibiting PI3K/Akt pathway through down-regulation
of AQP3. Cancer Biol Ther. 25:22848492024. View Article : Google Scholar :
|
|
25
|
Ning Y, Fang S, Zhang R, Fang J, Lin K,
Ding Y, Nie H, Zhou J, Zhao Q, Ke H, et al: Simvastatin induces
ferroptosis and activates anti-tumor immunity to sensitize
anti-PD-1 immunotherapy in microsatellite stable gastric cancer.
Int Immunopharmacol. 142:1132442024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paulino P, Vitari G, Rezende A, Couto J,
Antunes S, Domingos A, Peckle M, Massard C, Araújo F and Santos H:
Characterization of the rhipicephalus (Boophilus) microplus
sialotranscriptome profile in response to Theileria equi infection.
Pathogens. 10:1672021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shih VFS, Kearns JD, Basak S, Savinova OV,
Ghosh G and Hoffmann A: Kinetic control of negative feedback
regulators of NF-kappaB/RelA determines their pathogen- and
cytokine-receptor signaling specificity. Proc Natl Acad Sci USA.
106:9619–9624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Xue X, Pang M, Yu L, Qian J, Li X,
Tian M, Lyu A, Lu C and Liu Y: Epithelial-mesenchymal plasticity in
cancer: Signaling pathways and therapeutic targets. MedComm (2020).
5:e6592024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Yang Y, Qi X, Cui P, Kang Y, Liu
H, Wei Z and Wang H: SLC14A1 and TGF-β signaling: A feedback loop
driving EMT and colorectal cancer metachronous liver metastasis. J
Exp Clin Cancer Res. 43:2082024. View Article : Google Scholar
|
|
30
|
Li Y, Lin H, Sun Y, Zhao R, Liu Y, Han J,
Zhu Y, Jin N, Li X, Zhu G and Li Y: Platycodin D2 mediates
incomplete autophagy and ferroptosis in breast cancer cells by
regulating mitochondrial ROS. Phytother Res. 39:581–592. 2025.
View Article : Google Scholar
|
|
31
|
Xu G, Zhou Q, Qi J, Li Z, Yin L, Li Z, Lu
C, Zhao B and Shen Y: Resveratrol-derived inhibitors of the E3
ubiquitin ligase PELI1 inhibit the metastasis of triple-negative
breast cancer. Eur J Med Chem. 265:1160602024. View Article : Google Scholar
|
|
32
|
Xie J, Deng X, Xie Y, Zhu H, Liu P, Deng
W, Ning L, Tang Y, Sun Y, Tang H, et al: Multi-omics analysis of
disulfidptosis regulators and therapeutic potential reveals
glycogen synthase 1 as a disulfidptosis triggering target for
triple-negative breast cancer. MedComm (2020). 5:e5022024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou Y, Yang A, Chen B, Deng X, Xie J, Dai
D, Zhang J, Tang H, Wu T, Zhou Z, et al: crVDAC3 alleviates
ferroptosis by impeding HSPB1 ubiquitination and confers
trastuzumab deruxtecan resistance in HER2-low breast cancer. Drug
Resist Updat. 77:1011262024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su Z, Liu Y, Wang L and Gu W: Regulation
of SLC7A11 as an unconventional checkpoint in tumorigenesis through
ferroptosis. Genes Dis. 12:1012542024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou D, Sun L, Li J and Yang Y:
Schisandrin B inhibits inflammation and ferroptosis in
S.aureus-induced mastitis through regulating SIRT1/p53/SLC7A11
signaling pathway. Int Immunopharmacol. 137:1124302024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu Y, Wang H, Guo Q, Liu Y, He Y, Zhang
G, Yang C, Du Y and Gao F: CD44s-activated tPA/LRP1-NFκB pathway
drives lamellipodia outgrowth in luminal-type breast cancer cells.
Front Cell Dev Biol. 11:12248272023. View Article : Google Scholar
|
|
37
|
Wu Y, Dai S, Zhang Y, Li Z, Zhu B, Liu Q,
Wo L, Yu Z, Yuan X and Dou X: Atractylenolide II combined with
Interferon-γ synergistically ameliorates colorectal cancer
progression in vivo and in vitro by blocking the NF-kB p65/PD-L1
pathway. J Cancer. 15:4328–4344. 2024. View Article : Google Scholar :
|
|
38
|
Ayaz MO, Bhat AQ, Akhter Z, Badsera N,
Hossain MM, Showket F, Parveen S, Dar MS, Tiwari H, Kumari N, et
al: Identification of a novel GSK3β inhibitor involved in
abrogating KRas dependent pancreatic tumors in Wnt/beta-catenin and
NF-kB dependent manner. Life Sci. 351:1228402024. View Article : Google Scholar
|
|
39
|
Pasha A, Kumar K, Heena SK, Arnold Emerson
I and Pawar SC: Inhibition of NF-kB and COX-2 by andrographolide
regulates the progression of cervical cancer by promoting PTEN
expression and suppressing PI3K/AKT signalling pathway. Sci Rep.
14:120202024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barnes P, Mensah A, Derkyi-Kwarteng L,
Adankwa E, Agbo E, Yahaya ES, Amoani B, Adjei G, Ka-Chungu SMA,
Akakpo PK, et al: Prognostic significance of nuclear factor kappa B
(p65) among breast cancer patients in cape coast teaching hospital.
Med Princ Pract. 33:1–11. 2024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang F, Dai Z, Yu J, Wang K, Chen C, Chen
D, Zhang J, Zhao J, Li M, Zhang W, et al: RBM7 deficiency promotes
breast cancer metastasis by coordinating MFGE8 splicing switch and
NF-kB pathway. Elife. 13:RP953182024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He XY, Xiong XJ, Liu MJ, Liang JT, Liu FY,
Xiao JY and Wu LJ: Dahuang zhechong pill alleviates liver fibrosis
progression by regulating p38 MAPK/NF-κ B/TGF-β1 pathway. Chin J
Integr Med. 30:1113–1120. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mazi FA, Cakiroglu E, Uysal M, Kalyoncu M,
Demirci D, Sozeri PYG, Yilmaz GO, Ozhan SE and Senturk S: The
paracaspase MALT1 is a downstream target of Smad3 and potentiates
the crosstalk between TGF-β and NF-kB signaling pathways in cancer
cells. Cell Signal. 105:1106112023. View Article : Google Scholar
|
|
44
|
Zhao M, Qiu D, Miao X, Yang W, Li S, Cheng
X, Tang J, Chen H, Ruan H, Liu Y, et al: Melatonin delays arthritis
inflammation and reduces cartilage matrix degradation through the
SIRT1-mediated NF-κB/Nrf2/TGF-β/BMPs pathway. Int J Mol Sci.
25:62022024. View Article : Google Scholar
|
|
45
|
Cai Z, Gao L, Hu K and Wang QM:
Parthenolide enhances the metronomic chemotherapy effect of
cyclophosphamide in lung cancer by inhibiting the NF-kB signaling
pathway. World J Clin Oncol. 15:895–907. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Louault K, Blavier L, Lee MH, Kennedy RJ,
Fernandez GE, Pawel BR, Asgharzadeh S and DeClerck YA: Nuclear
factor-κB activation by transforming growth factor-β1 drives tumour
microenvironment-mediated drug resistance in neuroblastoma. Br J
Cancer. 131:90–100. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Z, Hao E, Cao R, Lin S, Zou L, Huang T,
Du Z, Hou X and Deng J: Analysis on internal mechanism of zedoary
turmeric in treatment of liver cancer based on pharmacodynamic
substances and pharmacodynamic groups. Chin Herb Med. 14:479–493.
2022.PubMed/NCBI
|
|
48
|
Chen X, Pei L, Zhong Z, Guo J, Zhang Q and
Wang Y: Anti-tumor potential of ethanol extract of Curcuma
phaeocaulis Valeton against breast cancer cells. Phytomedicine.
18:1238–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu C, Li M, Guo T, Wang S, Huang W, Yang
K, Liao Z, Wang J, Zhang F and Wang H: Anti-metastasis activity of
curcumin against breast cancer via the inhibition of stem cell-like
properties and EMT. Phytomedicine. 58:1527402019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li M, Guo T, Lin J, Huang X, Ke Q, Wu Y,
Fang C and Hu C: Curcumin inhibits the invasion and metastasis of
triple negative breast cancer via Hedgehog/Gli1 signaling pathway.
J Ethnopharmacol. 283:1146892022. View Article : Google Scholar
|
|
51
|
Tian NN, Zheng YB, Li ZP, Zhang FW and
Zhang JF: Histone methylatic modification mediates the
tumor-suppressive activity of curcumol in hepatocellular carcinoma
via an Hotair/EZH2 regulatory axis. J Ethnopharmacol.
280:1144132021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Al-Amin M, Eltayeb NM, Hossain CF, Rahiman
SSF, Khairuddean M and Muhamad Salhimi S: Bioactive compounds from
Curcuma aeruginosa and the effect of comosone II on the migration
and invasion of breast cancer cells. J Asian Nat Prod Res. 4:1–12.
2022.Epub ahead of print.
|
|
53
|
Shen Z, Yu N, Zhang Y, Jia M, Sun Y, Li Y
and Zhao L: The potential roles of HIF-1α in epithelial-mesenchymal
transition and ferroptosis in tumor cells. Cell Signal.
122:1113452024. View Article : Google Scholar
|
|
54
|
Fan J, Lin H, Luo J and Chen L:
4-Methoxydalbergione inhibits the tumorigenesis and metastasis of
lung cancer through promoting ferroptosis via the DNMT1/system
Xc-/GPX4 pathway. Mol Med Rep. 31:192025. View Article : Google Scholar
|
|
55
|
Rossi T, Iorio E, Chirico M, Pisanu ME,
Amodio N, Cantafio MEG, Perrotta I, Colciaghi F, Fiorillo M,
Gianferrari A, et al: BET inhibitors (BETi) influence oxidative
phosphorylation metabolism by affecting mitochondrial dynamics
leading to alterations in apoptotic pathways in triple-negative
breast cancer (TNBC) cells. Cell Prolif. 57:e137302024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang X, Yang R, Zhang Y, Shi Y, Ma M, Li
F, Xie Y, Han X and Liu S: Xianlinglianxiafang inhibited the growth
and metastasis of triple-negative breast cancer via activating
PPARγ/AMPK signaling pathway. Biomed Pharmacother. 165:1151642023.
View Article : Google Scholar
|
|
57
|
Chen G, Wang Y, Li M, Xu T, Wang X, Hong B
and Niu Y: Curcumol induces HSC-T6 cell death through suppression
of Bcl-2: Involvement of PI3K and NF-κB pathways. Eur J Pharm Sci.
65:21–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Amin M, Eltayeb NM, Khairuddean M and
Salhimi SM: Bioactive chemical constituents from Curcuma caesia
Roxb. rhizomes and inhibitory effect of curcuzederone on the
migration of triple-negative breast cancer cell line MDA-MB-231.
Nat Prod Res. 35:3166–3170. 2021. View Article : Google Scholar
|
|
59
|
Zhou Y, Moon JH, Kim JT, Qiu S, Lee SB,
Park HJ, Son MJ, Lee GY, Kwon JW, Park SH, et al: Curcumol
metabolized by rat liver S9 fraction and orally administered in
mouse suppressed the proliferation of colon cancer in vitro and in
vivo. Food Sci Biotechnol. 33:171–180. 2023. View Article : Google Scholar
|
|
60
|
Sheng W, Ding J, Liu L, Wang N, Lu B, You
X, He Q and Zhou Q: Curcumol inhibits the development of prostate
cancer by miR-125a/STAT3 axis. Evid Based Complement Alternat Med.
2022:93174022022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu C, Xing H, Fu X, Zhang Y, Yan X, Feng
J, He Z, Ru L, Huang C and Liang J: Effect and mechanisms of
shikonin on breast cancer cells in vitro and in vivo. BMC
Complement Med Ther. 24:3892024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Simões RV, Serganova IS, Kruchevsky N,
Leftin A, Shestov AA, Thaler HT, Sukenick G, Locasale JW, Blasberg
RG, Koutcher JA and Ackerstaff E: Metabolic plasticity of
metastatic breast cancer cells: Adaptation to changes in the
microenvironment. Neoplasia. 17:671–684. 2025. View Article : Google Scholar
|
|
63
|
He D, Tan XN, Li LP, Gao WH, Tian XF and
Zeng PH: Brazilin actuates ferroptosis in breast cancer cells via
p53/SLC7A11/GPX4 signaling pathway. Chin J Integr Med.
30:1001–1006. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang R, Xie Y, Li Q, Ye Y, Shi Y, Zhao X,
Wu C, Xu Y, Wang R, Zhang Y, et al: Ruyiping extract reduces lung
metastasis in triple negative breast cancer by regulating
macrophage polarization. Biomed Pharmacother. 141:1118832021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Uribesalgo I, Hoffmann D, Zhang Y,
Kavirayani A, Lazovic J, Berta J, Novatchkova M, Pai TP, Wimmer RA,
László V, et al: Apelin inhibition prevents resistance and
metastasis associated with anti-angiogenic therapy. EMBO Mol Med.
11:e92662019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Masoumi J, Zainodini N, Basirjafar P,
Tavakoli T, Zandvakili R, Nemati M, Ramezani M, Rezayati MT, Ayoobi
F, Khademalhosseini M, et al: Apelin receptor antagonist boosts
dendritic cell vaccine efficacy in controlling angiogenic,
metastatic and apoptotic-related factors in 4T1 breast
tumor-bearing mice. Med Oncol. 40:1792023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Deng R, Zong GF, Wang X, Yue BJ, Cheng P,
Tao RZ, Li X, Wei ZH and Lu Y: Promises of natural products as
clinical applications for cancer. Biochim Biophys Acta Rev Cancer.
1880:1892412025. View Article : Google Scholar
|
|
68
|
Li J, Sun Y, Li G, Cheng C, Sui X and Wu
Q: The extraction, determination, and bioactivity of curcumenol: A
comprehensive review. Molecules. 29:6562024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang LS, Shen SN, Gao YL, Shi SY, Zhou
CX, Mo JX, Xu YK, Lin LG and Gan LS: Tautomerism and bioactivities
of curcumenol, a common sesquiterpenoid widely existing in edible
plants. Food Funct. 10:1288–1294. 2019. View Article : Google Scholar : PubMed/NCBI
|