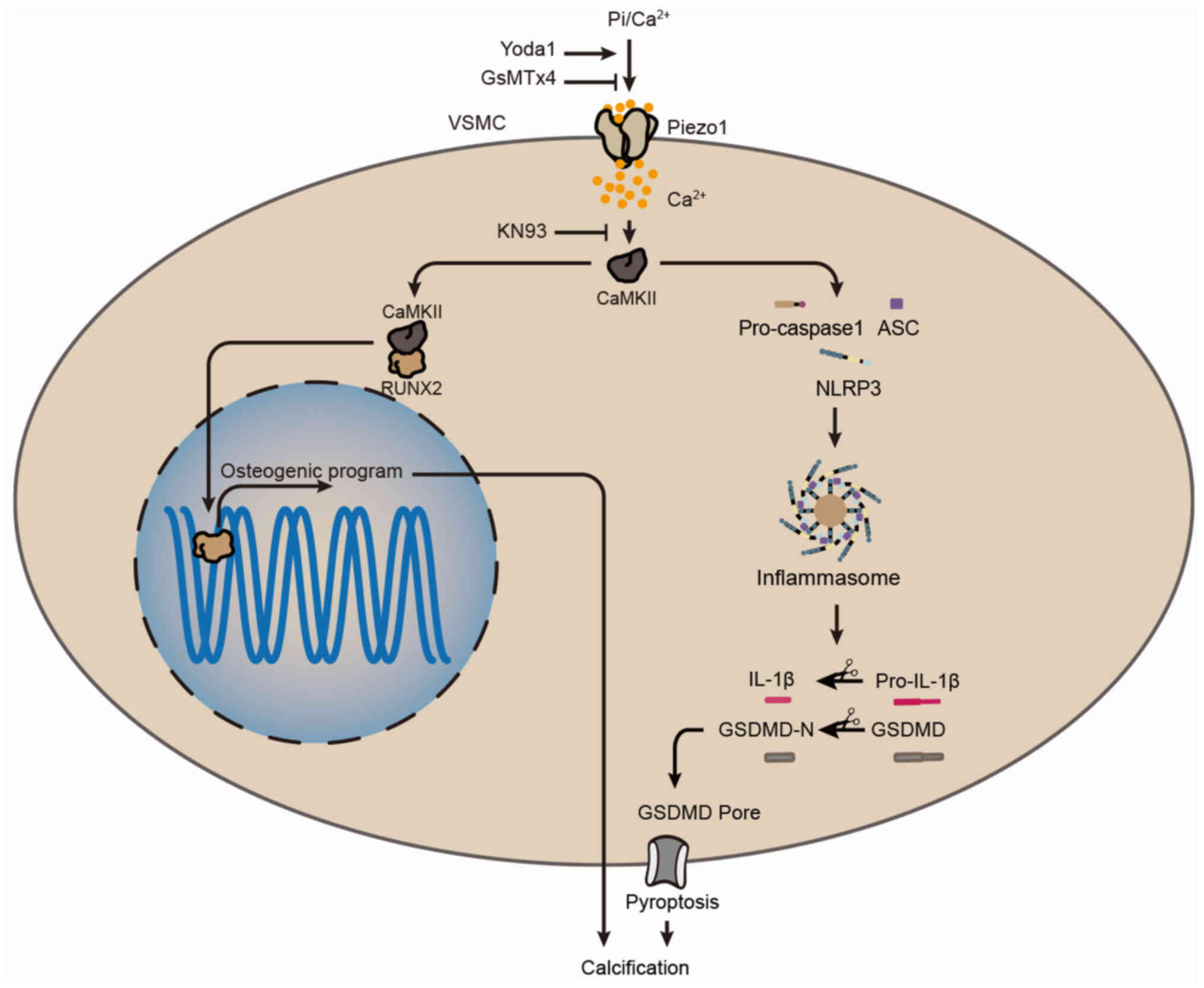
Introduction
Arterial calcification is marked by abnormal calcium
deposition and increased arterial stiffness, commonly observed in
patients with atherosclerosis or chronic kidney disease (CKD); it
is strongly linked to oxidative stress, chronic inflammation and
abnormal cell death, all of which contribute to heightened
cardiovascular risk (1). A key
initiating event in vascular calcification is the phenotypic switch
of vascular smooth muscle cells (VSMCs) into osteoblast-like cells.
This transformation is characterized by the loss of VSMC-specific
markers and the acquisition of osteochondrogenic markers such as
runt-related transcription factor 2 (RUNX2) and bone morphogenetic
protein 2 (BMP2), along with aberrant VSMC death (2). Inflammation acts as both a trigger
and consequence of calcification, creating a potentially vicious
cycle between inflammatory processes and arterial calcification.
Calcium phosphate crystals, which function as danger-associated
molecular patterns (DAMPs), activate caspase1 and promote the
maturation of interleukin-1β (IL-1β), initiating a pro-inflammatory
cascade (3). A study has shown
increased IL-1β expression in CKD models with vascular
calcification, underscoring the pivotal role of inflammation in
this pathology (4). Nonetheless,
the extent to which suppressing inflammation in VSMCs might
mitigate vascular calcification remains largely unresolved.
Pyroptosis, a form of regulated cell death, was
initially identified as pro-inflammatory cell death in
Salmonella-infected macrophages (5). This form of cell death is driven by
inflammatory caspases, primarily caspase1, which activate
inflammatory cytokines and induce cell death, largely mediated by
inflammasomes (6). The NLR
family pyrin domain containing 3 (NLRP3) inflammasome is composed
of NLRP3, caspase1 and apoptosis-associated speck-like protein
containing a caspase recruitment domain (ASC). A priming event
stimulates the transcription of NLRP3 and precursors of caspase1
(pro-caspase1) and IL-1β (pro-IL-1β) via toll-like receptor (TLR)
and nuclear factor κB signaling. The subsequent assembly of NLRP3,
ASC and pro-caspase1 leads to the formation of the NLRP3
inflammasome, triggering auto-cleavage of pro-caspase1 and the
maturation of pro-IL-1β and pro-IL-18 (7). Simultaneously, caspase1 cleaves
gasdermin D (GSDMD), generating an N-terminal GSDMD fragment
(GSDMD-NT) that forms pores in the plasma membrane, facilitating
pyroptotic cell death (8).
Pyroptosis has been implicated in the progression of several
diseases, including cardiovascular disease, diabetes and
neurological disorders (9). In
cardiovascular contexts, NLRP3 inflammasome activation has been
shown to regulate VSMC phenotypic switching in conditions such as
atherosclerosis and abdominal aortic aneurysms (10,11). However, its specific role in
VSMCs during arterial calcification remains unclear.
Piezo1 is a non-selective, mechanically gated cation
channel that converts mechanical stimuli into electrochemical
signals by facilitating the influx of cations such as calcium,
sodium and potassium across various cell types, including
endothelial cells, red blood cells and SMCs (12-14). This mechanotransduction is
involved in several physiological and pathological processes, such
as the development of blood and lymphatic vessels (12,13), the regulation of vascular tone
(15,16) and arterial remodeling (15). Additionally, Piezo1-induced
Ca2+ influx plays a critical role in the functions of
macrophages, particularly in inflammatory activation (17). As a key intracellular second
messenger, Ca2+ is essential for calcium/calmodulin
dependent protein kinase II (CaMKII) activation. Upon binding to
the regulatory domain of CaMKII, Ca2+ triggers
autophosphorylation at threonine 286, leading to its sustained
activation. CaMKII is widely recognized as a key downstream
effector of Piezo1 signaling (18,19). Previous research has shown that
Piezo1 contributes to the pathology of aortic aneurysms and that
inhibiting Piezo1 reduces atherosclerotic plaque formation in mice
(20). However, its involvement
in the inflammatory response and pyroptosis during arterial
calcification remains unexplored.
The present study aims to determine whether Piezo1
modulates NLRP3 inflammasome-mediated VSMC pyroptosis and
contributes to arterial calcification while elucidating the
underlying mechanisms.
Materials and methods
Animal model
The procedures involving Apolipoprotein E knockout
(ApoE−/−) mice (Beijing Vital River Laboratory Animal
Technology Co., Ltd.) were conducted as previously described
(21). ApoE−/− mice
(8-week-old male) were housed in a controlled facility. Mice were
randomly divided into control and high-fat diet (HFD) (n=6 for each
group). The control and HFD mice were fed a regular diet and HFD
(Dyets, Inc.) for 12 weeks, respectively.
Piezo1flox/flox mice were generously provided by Jiaguo
Zhou's laboratory (Department of Pharmacology, Cardiac and Cerebral
Vascular Research Center, Zhongshan School of Medicine, Sun Yat-Sen
University, Guangzhou, China). For the generation of SMC-specific
Piezo1 knockout mice, the loxP-Cre system was utilized by
administering an adeno-associated virus (AAV) vector expressing Cre
recombinase into Piezo1flox/flox mice (described below).
NLRP3−/− and caspase1−/− male mice, with a
C57BL/6J background, were obtained from ViewSolid Biotech Co., Ltd.
and their genotypes were validated through DNA sequencing as
previously described (22).
GSDMD−/− mice were generously provided by Xun Zhu's
laboratory (Department of Microbiology, Zhongshan School of
Medicine, Sun Yat-Sen University, Guangzhou, China.). All mice were
housed in a specific pathogen-free grade animal laboratory
(temperature 20±8°C, humidity 60±10%, with a 12-h light/dark cycle)
and given ad libitum access to standard rodent chow and
water.
The mice were checked daily for weight, health and
behavior. Animals were euthanized if they met the predefined
criteria, including rapid weight loss exceeding 15-20% of initial
body weight, severe weakness preventing independent access to food
or water, prolonged inability to stand (≥24 h) or clinical signs of
distress such as marked lethargy and hypothermia (core temperature
<37°C) in the absence of anesthesia. No mice in the present
study required euthanasia due to reaching these endpoints, as all
maintained stable health throughout the experimental period.
Euthanasia was conducted using CO2 at 30-70% vol/min
displacement in compliance with the AVMA guidelines: 2020 Edition
(23). The mice were confirmed
to be dead when the absence of a heartbeat, pulse, breathing and
corneal reflex was observed for >3 min. The animal experiments
adhered to ethical standards for animal research and was approved
by the Ethics Committee of Zhongshan School of Medicine, Sun
Yat-sen University (Guangzhou, China).
AAV construction and administration
The AAV vectors carrying Cre and the negative
control were constructed and packaged by HanBio Biotechnology Co.,
Ltd. Briefly, the pHBAAV-SM22a vector was selected for the present
study and the restriction enzymes, BamHI and HindIII,
were used to cleave the vector, yielding a purified linearized
construct. Mouse Cre fragments were amplified using the 2X Flash
PCR MasterMix (Dye) kit (CWbio), following the manufacturer's
protocol. The HB Infusion™ kit (Hanbio Biotechnology Co., Ltd.) was
then employed for the ligation of the linearized vector and mouse
Cre fragments, according to the manufacturer's instructions,
followed by the transformation of DH5α competent cells (Tiangen
Biotech Co., Ltd.). After cultivation in LB medium (Shanghai Yeasen
Biotechnology Co., Ltd.) for 12-16 h (selected by antibiotic
resistance selection using 100 μg/ml ampicillin), the
bacterial cultures were subjected to PCR identification using the
2X Hieff PCR Master Mix (Dye) kit (Shanghai Yeasen Biotechnology
Co., Ltd.). The amplified sequence was verified by Sanger
sequencing to ensure consistency with Cre.
The plasmid was extracted using the TIANpure Mini
Plasmid Kit (Tiangen Biotech Co., Ltd.), and subsequently
co-transfected with packaging plasmids (pAAV-RC and pHelper) into
293T cells (Hanbio Biotechnology Co., Ltd.) using Lipofiter™
transfection reagent (HanBio Biotechnology Co., Ltd.). The
transfection complex was prepared by mixing the following
components: pAAV-RC plasmid 10 μg, pHelper plasmid 20
μg, Shuttle plasmid 10 μg and Lipofiter 120
μl. The transfection complex was added to the cell culture
medium at room temperature. Following 72 h of transfection at 37°C
with 5% CO2, the 293T cells were harvested, lysed using
RIPA (Beyotime Institute of Biotechnology) and centrifuged at 4°C
for 30 min (3,000 × g), with the supernatant collected for virus
purification using the ViraTrap™ AAV Purification Maxiprep Kit
(Biomiga, Inc.). The resulting viral titer was 1.6×1012
vg/ml. For gene delivery of Cre specifically to SMCs, the AAV was
administered via direct microinjection into the tail vein
(1×1011 vg per mouse).
Mice and cell calcification
construction
A total of 18 healthy Piezo1flox/flox
male mice were randomly divided into three experimental groups
(n=6/group): Normal diet, high-adenine diet (HAD) + AAV-vector and
HAD + AAV-SM22a-cre. The normal diet group received a standard
pellet chow diet, while the HAD + AAV-vector group was fed a HAD
(0.25% adenine and 1.2% phosphorus) after AAV-vector injection for
4 weeks. The HAD + AAV-SM22a-cre group also received HAD following
AAV-SM22a-cre injection for 4 weeks. After 12 weeks of receiving
the normal or special diet, the animals were sacrificed. Western
blot analysis was performed to validate the efficiency of
SMC-specific Piezo1 knockout.
The mice were checked daily for weight, health and
behavior. Animals were euthanized if they met the predefined
criteria, including rapid weight loss exceeding 15-20% of initial
body weight, severe weakness preventing independent access to food
or water, prolonged inability to stand (≥24 h) or clinical signs of
distress such as marked lethargy and hypothermia (core temperature
<37°C) in the absence of anesthesia. No mice in the present
study required euthanasia due to reaching these endpoints, as all
maintained stable health throughout the experimental period.
Euthanasia was conducted using CO2 at 30-70% vol/min
displacement in compliance with the AVMA guidelines: 2020 Edition
(23). The mice were confirmed
to be dead when the absence of a heartbeat, pulse, breathing and
corneal reflex was observed for >3 min, then the peripheral
venous blood and aorta tissues were harvested.
Primary VSMCs were isolated from C57BL/6J mice (8
weeks, 20-25 g, male, n=6/group; Laboratory Animal Center, Sun
Yat-sen University), as previously described (24). In brief, after sacrificing the
mice (as aforementioned), the thoracic and abdominal aortas were
dissected, minced and digested with collagenase (2 mg/ml) for 4 h.
The VSMCs were then cultured in Ham's F12 nutrient medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 7.5% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, 100 μg/ml
streptomycin and 2 mmol/l L-glutamine at 37°C with 5%
CO2. First-passage (P1) cells was used in subsequent
cell experiments. Human aortic SMCs (HASMCs) were kindly provided
by Professor Zhihan Tang's lab (Department of Pathophysiology,
Institute of Cardiovascular Disease, Key Laboratory for
Arteriosclerology of Hunan Province, University of South China,
Hengyang, China) and were cultured in Ham's F12 nutrient medium
supplemented with 7.5% FBS at 37°C under 5% CO2
(24). For the calcification
model, VSMCs at 60% confluency were incubated in a calcifying
medium (CM; containing 3.5 mM inorganic phosphate and 3 mM calcium)
for 7 days, with comparisons made to VSMCs in growth medium (GM).
SMCs at passages 3 to 8 were used for the experiments.
VSMCs were stimulated with oxidized low-density
lipoprotein (oxLDL; Guangzhou Yiyuan Biotechnology Co., Ltd.) at
varying concentrations (25, 50 and 100 μg/ml), with
untreated cells serving as the blank control. After 24 h, cell
lysates were collected for subsequent analysis. To assess the
impact of NLRP3 inhibition by MCC950 (Selleck Chemicals) on
CM-induced calcification, cells were divided into four groups:
Control (GM), CM, CM + MCC950 (CM + 100 μM MCC950) and
MCC950 (GM + 100 μM MCC950). Similarly, the role of CaMKII
inhibition by KN93 (Selleck Chemicals) in CM-triggered pyroptosis
was examined using three groups: Control (GM), CM, and CM + KN93
(CM + 10 μM KN93). After 7 days, cell lysates were harvested
for further analysis.
Piezo1 differential analysis using gene
expression omnibus (GEO) data
Data of the differential expression of Piezo1 (NCBI
GEO datasets portal: http://www.ncbi.nlm.nih.gov/geo/; GEO accession no.
GSE43292) between macroscopically intact tissue and atheroma plaque
was analyzed. The mRNA from microarray analysis had been submitted
by Ayari and Bricca (25). Data
analysis was performed using GraphPad Prism 6.02 (Dotmatics).
Aortic ring culture
Thoracic to iliac aortas were aseptically excised
from male wild type (n=6) and knockout mice (NLRP3−/−,
caspase1−/− or GSDMD−/−) (8 weeks, 20-25 g,
n=6/group). After meticulous removal of the adventitia and
endothelium, the vessels were segmented into 2-3 mm rings. These
segments were then cultured for 7 days at 37°C under 5%
CO2, with media refreshed every 48 h, using either the
calcification medium or standard culture medium. The organ culture
experiments were replicated three times under identical
conditions.
Antibodies and reagents
The primary antibodies employed in the experiments
included anti-Piezo1 (Proteintech Group, Inc.; cat. no. 15939-1-AP;
1:1,000), anti-α smooth muscle actin (α-SMA; Cell Signaling
Technology, Inc.; cat. no. 19245s; 1:2,000), anti-alkaline
phosphatase (ALP; Novus Biologicals, LLC; Bio-Techne; cat. no.
NBP2-67295; 1:2,000), anti-BMP2 (Novus Biologicals, LLC;
Bio-Techne; cat. no. NBP1-19751; 1:2,000), anti-RUNX2 (Cell
Signaling Technology, Inc.; cat. no. 8486s; 1:2,000), anti-β-actin
(Cell Signaling Technology, Inc.; cat. no. 3700S; 1:5,000),
anti-NLRP3 (Adipogen Life Sciences; cat. no. AG-20B-0014-C100;
1:2,000), anti-caspase1 (Abclonal Biotech Co., Ltd.; cat. no.
A0964; 1:2,000), anti-caspase1 p20 (Biorbyt, Ltd.; cat. no.
orb221355; 1:2,000), anti-GSDMD (ImmunoWay Biotechnology Company;
cat. no. YT7991; 1:2,000), anti-pro-IL-1β (ImmunoWay Biotechnology
Company; cat. no. YM8498; 1:2,000), anti-IL-1β (ImmunoWay
Biotechnology Company; cat. no. YT5201; 1:2,000) and anti-CaMKIIδ
(Abcam; cat. no. ab181052; 1:2,000). Secondary antibodies used for
western blotting included HRP-conjugated goat anti-mouse IgG (H+L)
(Cell Signaling Technology, Inc.; cat. no. 7076s; 1:5,000) and
HRP-conjugated goat anti-rabbit IgG (H+L) (Cell Signaling
Technology, Inc.; cat. no. 7074s; 1:5,000). For immunofluorescence,
Alexa Fluor 488-conjugated goat anti-rabbit IgG (Abcam; cat. no.
ab150077; 1:50) and Alexa Fluor 647-conjugated goat anti-mouse IgG
(Abcam; cat. no. ab150115; 1:50), GsMTx4 (Abcam; cat. no. ab141871)
and Yoda1 (Sigma-Aldrich; Merck KGaA; cat. no. SML1558) were
used.
Mouse echocardiography
Echocardiography was performed prior to sacrifice
while the mice were anesthetized with isoflurane (induction, 3%;
maintenance, 1.5%). Data were collected using the Vivo 2100 imaging
system (FUJIFILM VisualSonics, Inc.), as described previously
(26). Measurements of wall
thickness and lumen diameter were obtained using the system's
dedicated software.
Small interfering RNA (siRNA)
transfection
siRNA transfection was performed when cells reached
80% confluency, employing the riboFECT™ CP Transfection Kit
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
protocol. For Piezo1 knockdown, VSMCs were treated with siRNA
against mouse Piezo1 (siPiezo1). The sequence of siPiezo1 was as
follows: 5'-UACCGAUCUCCAGAGACCAUGAUUA-3' for the sense strand, and
5'-UAAUCAUGGUCUGUGGAGAUCGGUU-3' for the antisense strand. The
sequence of the control siRNA was as follows:
5'-UUCUCCGAACGUGUCACGU-3' for the sense strand, and
3'-DTDTAAGAGGCUUGCACAGUGCA-5' for the antisense strand. A 10
μl aliquot of 50 μM siPIEZO1 (Thermo Fisher
Scientific, Inc.) or negative control was combined with 120
μl of 1X riboFECT CP buffer, followed by the addition of 12
μl riboFECT CP reagent. After brief vortexing, the solution
was maintained at 23°C for 15 min. Culture medium was then added to
the prepared complex to achieve a 2 ml final volume at room
temperature, before application to the plated cells. After 48 h of
transfection, western blot analysis was performed to validate the
efficiency of Piezo1 knockdown.
Western blotting
Protein expression was determined by western
blotting as previously described (22). In brief, total protein was
extracted from treated cells using RIPA buffer supplemented with a
protease inhibitor cocktail and quantified using the BCA Protein
Assay Kit (Thermo Fisher Scientific, Inc.). After separating 40
μg of protein per lane by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins
were transferred to polyvinylidene fluoride membranes and blocked
with 5% skim milk at room temperature for 2 h. Membranes were
incubated overnight at 4°C with primary antibodies, followed by a
2-h incubation with secondary antibodies at room temperature. Bound
proteins were visualized using ECL (MilliporeSigma) and detected on
the Bio-Rad ChemiDoc XRS+ chemiluminescent imaging system. Relative
protein levels were normalized to β-actin as a loading control and
quantified using ImageJ 1.52a software (National Institutes of
Health).
Measurement of mitochondrial membrane
potential (MMP)
The MMP was assessed using JC-1 staining following
the manufacturer's protocol. Briefly, cells were incubated in fresh
culture medium containing JC-1 dye (Beyotime Institute of
Biotechnology) for 30 min at 37°C in the dark. After two washes
with phosphate-buffered saline (PBS), a confocal laser scanning
microscope (LSM780; Zeiss GmbH) was used to immediately analyze the
samples with 490 nm excitation. Green fluorescence, indicating JC-1
monomers, was recorded at 530 nm emission, while red fluorescence,
representing JC-1 aggregates, was recorded at 590 nm. The ratio of
yellow fluorescence intensity was calculated using ImageJ 1.52a
software to assess the results.
Immunofluorescent staining
Immunofluorescence staining was conducted as
previously outlined (22). In
summary, aortic rings or cells were fixed in 4% paraformaldehyde
for 24 h at 4°C, sectioned into 7 μm cryostat slices or
prepared on chamber slides, followed by blocking with 10% FBS at
room temperature for 2 h and permeabilization with Triton X-100.
The slides were then incubated overnight at 4°C with primary
antibodies against Piezo1 (1:50), NLRP3 (1:50), ASC (1:50) and
α-SMA (1:50). After washing with PBS, secondary antibodies were
applied for 1 h at room temperature. Finally, the slides were
mounted using a DAPI-containing solution and visualized using a
confocal laser scanning microscope (LSM780; Zeiss GmbH). Data
analysis was performed using ImageJ 1.52a software.
Alizarin Red S and von Kossa
staining
Calcium deposition in the aorta and VSMCs was
evaluated using Alizarin Red S staining. Aortic rings were fixed in
4% polyoxymethylene for 1 h at room temperature, and cells for 20
min. After two PBS washes, both aortic rings and cells were stained
with 1% Alizarin Red solution for 10 min at room temperature,
followed by three additional PBS washes before imaging under a
light microscope. For von Kossa staining, aortic sections were
dewaxed, hydrated and treated with 1% silver nitrate under
ultraviolet light for 10 min. Calcified nodules were visualized as
brown to black under a light microscope or ELISPOT analyzer (CTL
ImmunoSpot® S6 Ultimate) following multiple washes. Data
analysis was performed using ImageJ 1.52a software.
Hematoxylin and eosin (H&E)
staining
Following a 2-h baking period at 60°C to ensure
tissue adhesion, lesion sections underwent standard H&E
staining. Deparaffinization was performed through two 5-min xylene
immersions, followed by graded rehydration in absolute ethanol and
decreasing ethanol concentrations (95, 80 and 70%, 5 min each) and
three 2-min distilled water washes. Nuclear staining was achieved
via 5-min hematoxylin incubation, followed by running water
rinsing, brief acidic ethanol differentiation and a 5-min water
wash. Cytoplasmic staining employed 2-min eosin application with
subsequent water rinsing. Dehydration involved sequential 10-sec
immersions in 70, 80 and 90% ethanol, 5 min in absolute ethanol and
two final 5-min xylene clears before neutral resin mounting. All
procedures except the initial baking were conducted at 20-25°C.
Stained sections were examined using an Olympus VS200 automated
microscope.
Co-immunoprecipitation (Co-IP)
Cells were lysed on ice for 15 min using RIPA buffer
containing a protease inhibitor cocktail and PMSF. After
centrifugation at 14,000 × g (15 min, 4°C), the resulting 40
μg supernatant was subjected to co-immunoprecipitation with
an anti-CaMKIIδ (GeneTex, Inc.; cat. no. GTX111401; 1:100) or
anti-RUNX2 antibody (Abcam; cat. no. ab236639; 1:100) or
anti-rabbit IgG (Proteintech Group, Inc.; cat. no. SA00001-2;
1:1,000) overnight at 4°C. Protein A-conjugated agarose beads
(MilliporeSigma; cat. no. IP05; 40 μl) were then introduced,
followed by a 1-h incubation at 4°C with rotation. The beads
underwent six washes with chilled lysis buffer before resuspension
in SDS-PAGE loading buffer. Bound proteins were eluted by heating
at 92°C for 3 min and separated on a 10% polyacrylamide gel via
SDS-PAGE. Finally, CaMKII and RUNX2 levels were detected by
immunoblotting as previously described.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining
Aortic ring sections (7 μm) were fixed in 4%
paraformaldehyde 1 h at room temperature and incubated with TUNEL
reaction mixture (Beyotime Institute of Biotechnology) at 37°C for
1 h in a light-protected environment. Nuclear staining was
performed using mounting medium with DAPI (Abcam; cat. no.
ab1041395) for 10 min at room temperature, ensuring light
protection throughout the procedure. A fluorescence microscope
(Leica DM6B; Leica Microsystems GmbH) was employed to capture
images at various magnifications, and TUNEL-positive cells were
counted from three randomly selected fields for each sample.
Statistical analysis
All data are presented as mean ± SD. Each in
vitro experiment was repeated at least three times. Pearson's
correlation analysis coefficient was employed for correlation
analysis. Unpaired Student's t-test was used to compare the mean of
two groups. For comparisons across multiple groups, one-way ANOVA
with the Tukey's multiple comparisons test was applied. Data
analysis was performed using GraphPad Prism 6.02 (Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Piezo1 is upregulated in the
atherosclerotic plaques of mice and patients
Vascular calcification frequently occurs within
atherosclerotic plaques (27).
To investigate the role of Piezo1 in this process, Piezo1
expression was examined in ApoE−/− mice subjected to a
high-fat diet, which induces atherosclerotic calcification
(28). Elevated Piezo1
expression was observed in both the necrotic core of
atherosclerotic plaques and the adjacent vascular wall (Fig. 1A). To corroborate this finding in
humans, data from the human carotid atheroma dataset (GSE43292) was
analyzed, revealing that Piezo1 expression was significantly higher
in atherosclerotic plaques compared with macroscopically intact
tissue (Fig. 1B). In addition,
primary VSMCs from mice were treated with ox-LDL at varying
concentrations, resulting in a dose-dependent increase in Piezo1
expression (Fig. 1C).
Immunofluorescence staining confirmed that Piezo1 expression was
upregulated and primarily localized on the cell membrane (Fig. 1D). These results suggest a
potential connection between Piezo1 and the progression of
atherosclerosis.
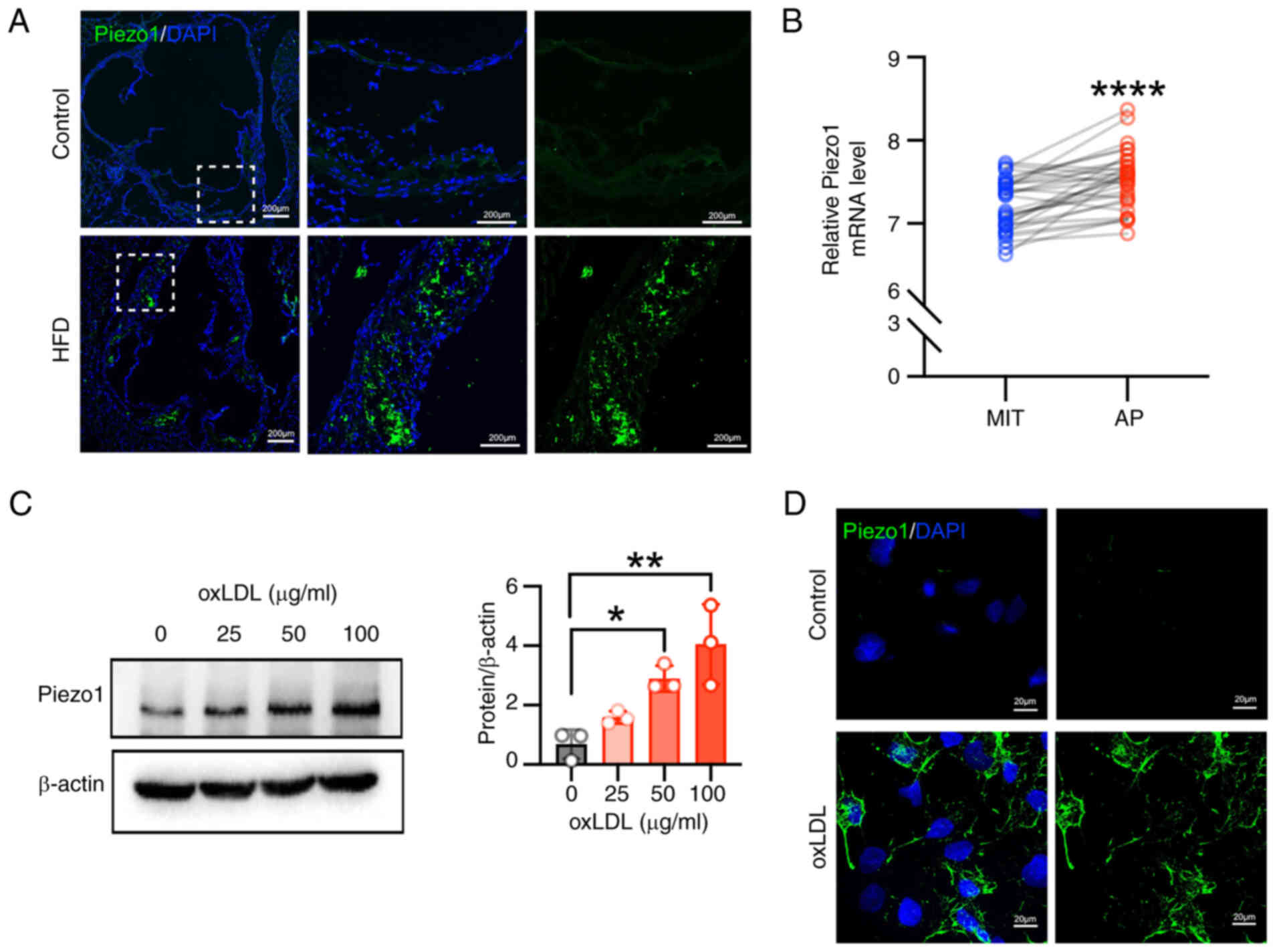 | Figure 1Piezo1 is upregulated in
atherosclerosis plaques in both patients and mice. (A)
Representative immunofluorescence stained images of aortic tissues
isolated from Apolipoprotein E−/− mice (scale bars, 200
μm). Piezo1 is shown in green and DAPI is shown in blue; n=6
rats per group. (B) Relative mRNA expression of Piezo1 in human
carotid samples (data from GEO dataset, GSE43292; paired t-test;
n=32); ****P<0.0001 vs. MIT. Statistical significance
of mRNA expression was assessed by paired Student's t-test (C)
VSMCs were treated with oxLDL for 24 h, and Piezo1 protein
expression was determined by western blot; *P<0.05,
**P<0.01 vs. control. Statistical significance was
assessed by one-way ANOVA followed by Tukey's. (D) Representative
immunofluorescence stained images of Piezo1 (green) in VSMCs (scar
bar, 20 μm). Cell nuclei were counterstained with DAPI
(blue). All values are presented as mean ± SD. AP, atheroma plaque;
MIT, macroscopically intact tissue; oxLDL, oxidized low-density
lipoprotein; HFD, high-fat diet; VSMCs, vascular smooth muscle
cells. |
CM-induced osteogenic differentiation is
associated with Piezo1 expression, NLRP3 inflammasome activation
and pyroptosis in aortic ring and VSMCs
To further elucidate the role of Piezo1 in vascular
calcification, aortic rings were cultured in either GM or CM.
Exposure to CM over time compromised vascular integrity (Fig. 2A), coinciding with increased
Piezo1 expression and decreased α-SMA levels (Fig. 2A-C). Pyroptosis of plaque VSMCs,
a known contributor to plaque rupture and instability (29), was examined next. CM-induced
osteogenic differentiation in primary VSMCs was demonstrated by
increased expression of the osteogenic markers, ALP, BMP2 and
RUNX2, alongside elevated Piezo1 levels (Fig. 2D and E). Pyroptotic markers,
including cleaved caspase1, cleaved IL-1β and GSDMD-NT, were also
upregulated in CM-treated VSMCs (Fig. 2D-F). Notably, Piezo1 expression
showed a positive correlation with RUNX2 (R2= 0.3089; P=
0.0354; Fig. 2G) and IL-1β
(R2= 0.7693; P=0.0154; Fig. 2H), suggesting a potential link
between Piezo1 and both osteogenic differentiation and
inflammation. To further elucidate the mechanisms driving
calcification and pyroptosis, mitochondrial function was assessed.
CM treatment caused mitochondrial dysfunction in VSMCs, as
indicated by a disrupted MMP (Fig.
2I). Additionally, activated NLRP3 inflammasomes, marked by
NLRP3-ASC binding, were observed in VSMCs following CM exposure
(Fig. 2J). These results
indicate that CM promotes Piezo1 expression in a time-dependent
manner which may lead to pyroptosis in VSMCs, contributing to
vascular calcification.
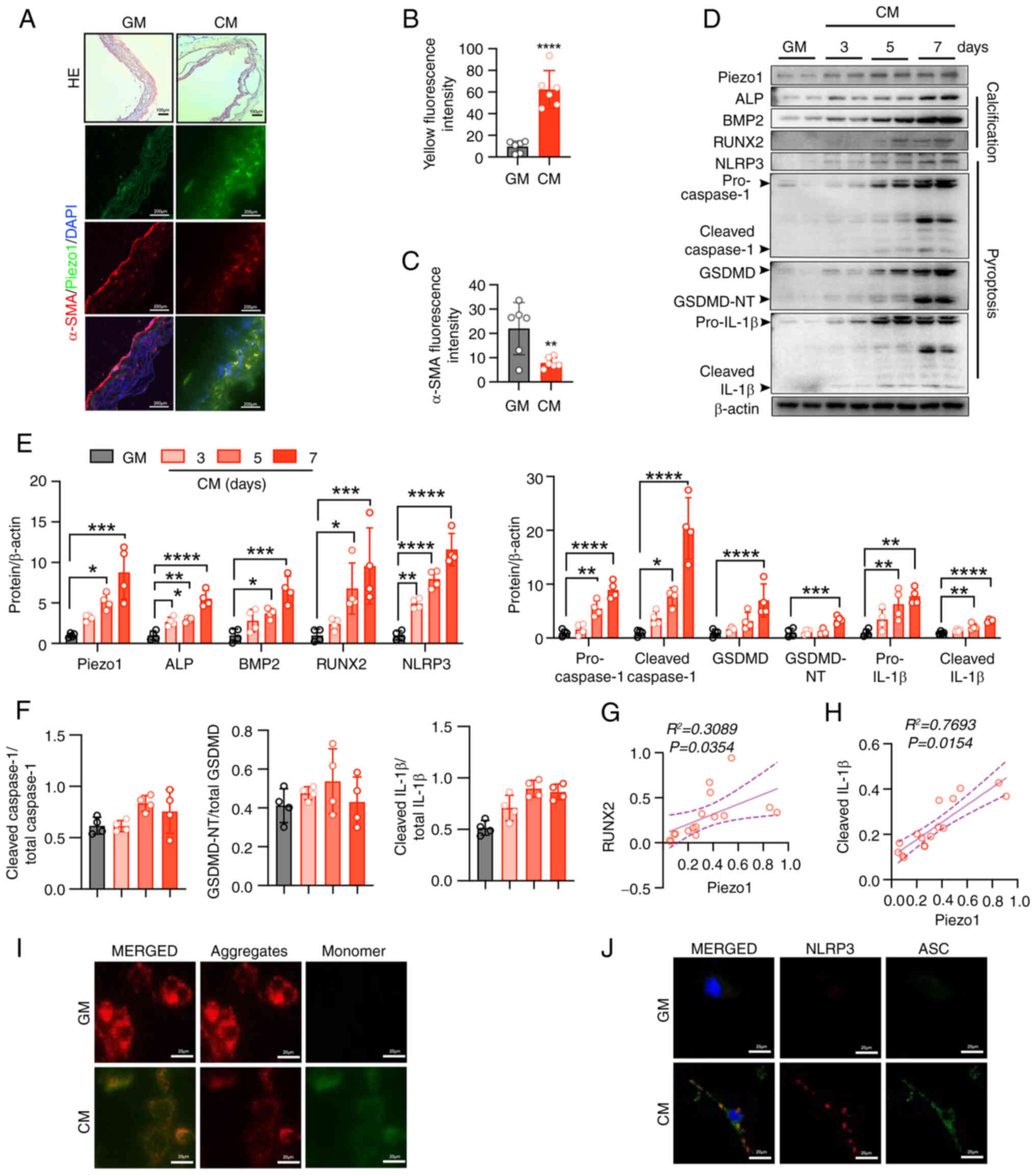 | Figure 2CM-induced osteogenic differentiation
is associated with Piezo1 expression, NLRP3 inflammasome activation
and pyroptosis in the aortic ring and VSMCs. (A) Representative
H&E staining and immunofluorescence images alongside (B and C)
quantification of α-SMA and Piezo1 expression in the aortic
sections of mice (HE scale bars, 100 μm; immunofluorescence
scale bars, 200 μm); ****P<0.0001 vs. GM.
Statistical significance of mRNA expression was assessed by
unpaired Student's t-test (D-F) Representative immunoblot images
and semi-quantification of Piezo1, ALP, BMP2, RUNX2, NLRP3,
pro-caspase1, cleaved-caspase1, GSDMD, GSDMD-NT, pro-IL-1β,
cleaved-IL-1β, cleaved caspase1/total-caspase1, GSDMD-NT/total
GSDMD and cleaved IL-1β/total IL-1β in mouse VSMC extracts. β-actin
was used as a loading control; *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001 vs. GM. Statistical significance was
assessed by one-way ANOVA followed by Tukey's. Correlation analysis
between Piezo1 protein expression and the (G) RUNX2 and (H) IL-1β
level. Pearson's correlation analysis coefficient was employed for
correlation analysis. (I) MMP changes were monitored by
fluorescence using JC-1 staining. (J) Representative
immunofluorescence images of inflammasome marker NLRP3 and ASC
expression in the VSMCs of mice. Scale bars, 10 μm; n=3
independent experiments; ***P<0.001 vs. GM. All
values are presented as mean ± SD. CM, calcifying medium; GM,
growth medium; NLRP3, NOD-like receptor thermal protein
domain-containing protein 3; VSMCs, vascular smooth muscle cells;
α-SMA, α-smooth muscle actin; ALP, alkaline phosphatase; BMP2, bone
morphogenetic protein 2; RUNX2, runt-related transcription factor
2; GSDMD, gasdermin D; GSDMD-NT, gasdermin D N-terminal; IL-1β,
interleukin-1β; MMP, mitochondrial membrane potential; ASC,
apoptosis-associated speck-like protein containing a caspase
recruitment domain. |
VSMC-specific knockout of Piezo1
alleviates arterial calcification in mice
To further explore the involvement of Piezo1 in
arterial calcification, VSMC-specific Piezo1 knockout mice were
generated by administering AAV-SM22α-Cre to
Piezo1flox/flox mice, followed by an HAD to induce
vascular calcification (Fig.
3A). HAD significantly impaired cardiac function, which was
mitigated in the Piezo1 knockout group (Fig. 3B). Moreover, the knockout of
Piezo1 in VSMCs attenuated HAD-induced aortic calcification, as
demonstrated by M-mode echocardiography of the thoracic and
abdominal aorta (Fig. 3C).
Alizarin Red staining further confirmed the reduction in
HAD-induced calcium deposition in the knockout mice (Fig. 3D and E). Notably, VSMC-specific
Piezo1 knockout resulted in increased aortic lumen thickness
(Fig. 3F). Collectively, these
results suggest that Piezo1 deficiency in VSMCs inhibits arterial
calcification and improves cardiac function.
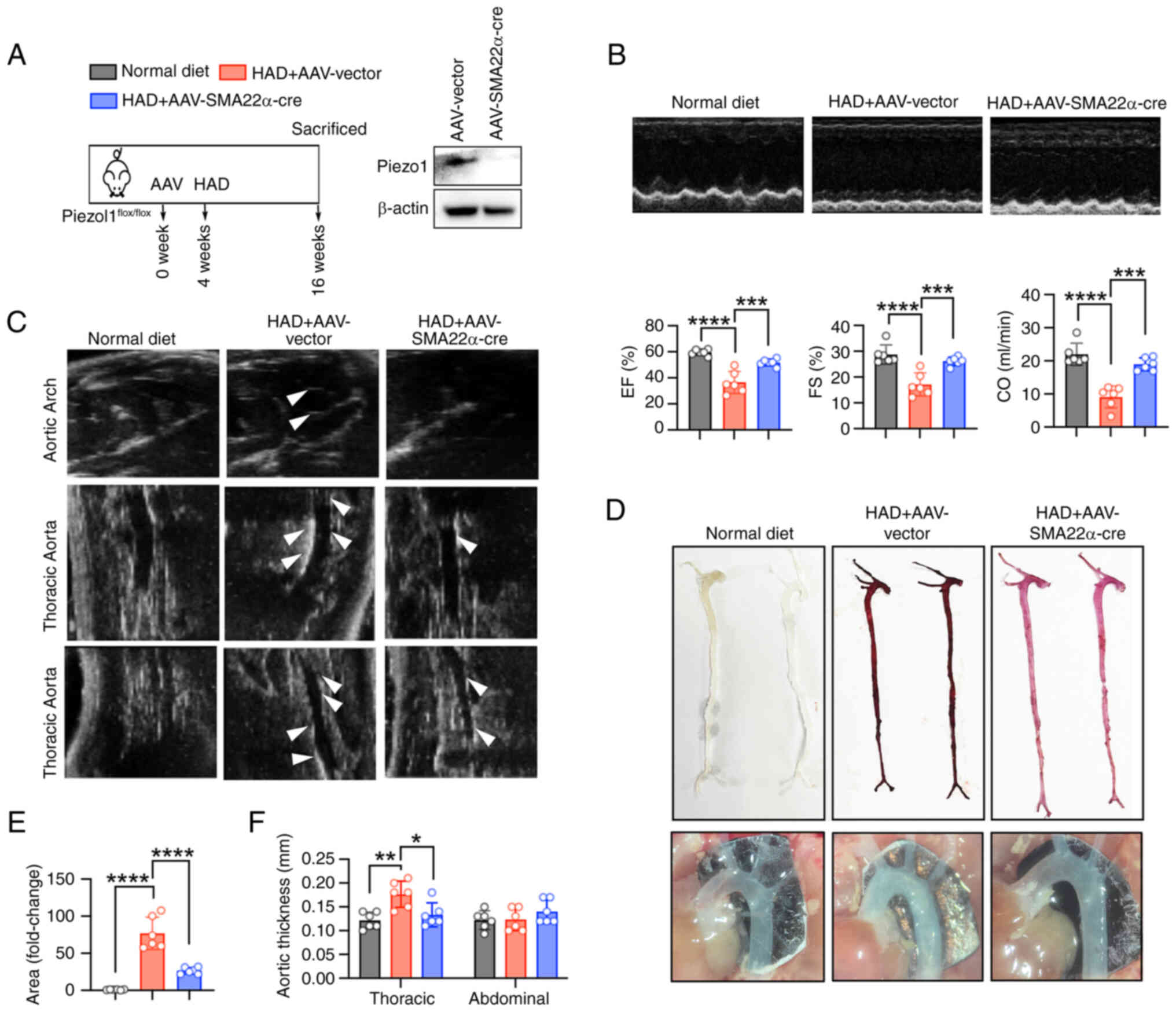 | Figure 3Specific knockout of Piezo1 in VSMCs
alleviates arterial calcification in mice. (A) Schematic
representation of the arterial calcification in vivo
protocol. (B) Representative echocardiographic recordings revealed
that HAD induced cardiac dysfunction in VSMC-specific Piezo1
knockout mice (generated via AAV-SMA22α-cre). Cardiac dysfunction
was evaluated using echocardiographic parameters including EF, FS
and CO; ***P<0.001, ****P<0.0001. (C)
Representative 2D B-mode images from the in vivo ultrasound
scanning of the aortic arch, thoracic aorta and abdominal aorta.
White arrows indicate vascular calcifications. (D and E)
Representative images of Alizarin red staining revealed that a
HAD-induced calcium deposition in the aorta (reddish signal) of
VSMC-specific Piezo1 knockout mice. The whole-aorta analysis
further confirmed calcification extent; ****P<0.0001.
(F) Recorded aortic thickness; *P<0.05,
**P<0.01. Statistical significance was assessed by
one-way ANOVA followed by Tukey's. All values are presented as mean
± SD. AAV, adeno-associated virus; HAD, high-adenine diet; w,
weeks; EF, ejection fraction; FS, fraction shortening; CO, cardiac
output; VSMC, vascular smooth muscle cells. |
Piezo1 inhibition blocks CM-induced NLRP3
inflammasome activation and pyroptosis and alleviates
calcification
Von Kossa staining showed that CM-induced vascular
calcification and structural damage in cultured aortic rings, which
was alleviated by the Piezo1-specific inhibitor, GsMTx4 (5
μM) (Fig. 4A and B).
Given the role of VSMC death in vascular calcification (30), TUNEL staining was performed to
assess cell death in the cultured cells. Calcifying conditions
significantly increased cell death, which was reduced by GsMTx4
(Fig. 4C and D). Alizarin Red S
staining also confirmed that Piezo1 inhibition reduced calcium
deposition in VSMCs (Fig. 4E and
F). Moreover, CM treatment led to increased expression of the
osteogenic markers, ALP, BMP2 and RUNX2, which were significantly
inhibited by either Piezo1 inhibitor or Piezo1 siRNA (Fig. 4G-J). Similarly, CM-induced
activation of the NLRP3 inflammasome and pyroptosis in VSMCs was
blocked by Piezo1 inhibition or knockdown (Fig. 4G-I). These results confirm that
Piezo1 plays a key role in VSMC osteogenic differentiation, with
its effects closely linked to NLRP3 inflammasome activation and
pyroptosis.
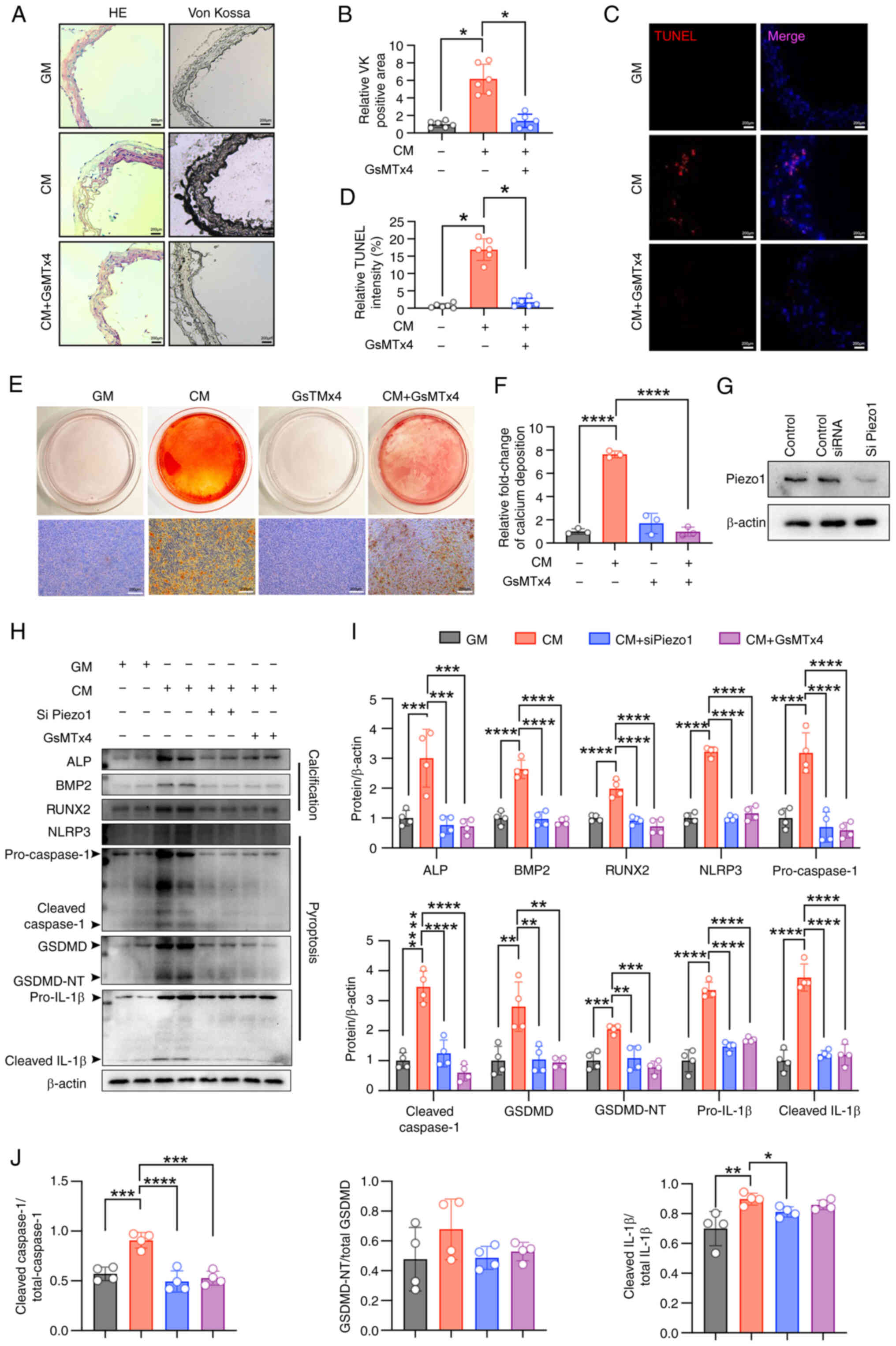 | Figure 4Piezo1 inhibition blocks CM-induced
NLRP3 inflammasome activation and pyroptosis and alleviates
calcification. (A and B) Representative HE staining, Von Kossa
staining and quantification of the Von Kossa positive area in the
aortic sections of mice (scale bars, 200 μm);
*P<0.05. (C and D) TUNEL staining analysis was used
to evaluate cell death (scale bars, 200 μm);
*P<0.05. (E and F) Representative images of Alizarin
red S staining showing calcium nodule formation (reddish signal) in
VSMCs cultured with GM/CM under Piezo1 inhibitor treatment (scale
bars, 200 μm); ****P<0.0001; n=3 independent
experiments. (G-J) Representative immunoblot images of Piezo1, ALP,
BMP2, RUNX2, NLRP3, pro-caspase1, cleaved-caspase1, GSDMD,
GSDMD-NT, pro-IL-1β, cleaved-IL-1β, cleaved
caspase1/total-caspase1, GSDMD-NT/total GSDMD and cleaved
IL-1β/total IL-1β in mouse VSMC extracts. β-actin was used as a
loading control. **P<0.01, ***P<0.001,
****P<0.0001. Statistical significance was assessed
by one-way ANOVA followed by Tukey's. All values are presented as
mean ± SD. CM, calcifying medium; GM, growth medium; VK, von kossa;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; VSMCs, vascular smooth muscle cells; ALP, alkaline
phosphatase; BMP2, bone morphogenetic protein 2; RUNX2,
runt-related transcription factor 2; NLRP3, NOD-like receptor
thermal protein domain-containing protein 3; GSDMD, gasdermin D;
GSDMD-NT, gasdermin D N-terminal; IL-1β, interleukin-1β; siRNA,
small interfering RNA. |
Piezo1 activator promotes CM-induced
NLRP3 inflammasome activation and pyroptosis and enhances
calcification
To further clarify the role of Piezo1 in the
osteogenic differentiation of VSMCs, Piezo1 was activated using
Yoda1 (30 μM), which enhanced calcium deposition. Yoda1
significantly promoted CM-induced calcium deposits in aortic rings
(Fig. 5A and B). Additionally,
calcifying conditions increased cell death, which was exacerbated
by Yoda1 (Fig. 5C and D).
Alizarin Red S staining confirmed that Yoda1 further enhanced
CM-induced calcium deposition in VSMCs (Fig. 5E and F). Consistently, Yoda1
increased the expression of the osteogenic markers, ALP and BMP2,
and promoted NLRP3 inflammasome activation and pyroptosis (Fig. 5G-I). These results further
demonstrate that Piezo1 may be a critical modulator of VSMC
osteogenic differentiation and vascular calcification, with its
activity being associated with inflammasome activation and
pyroptosis.
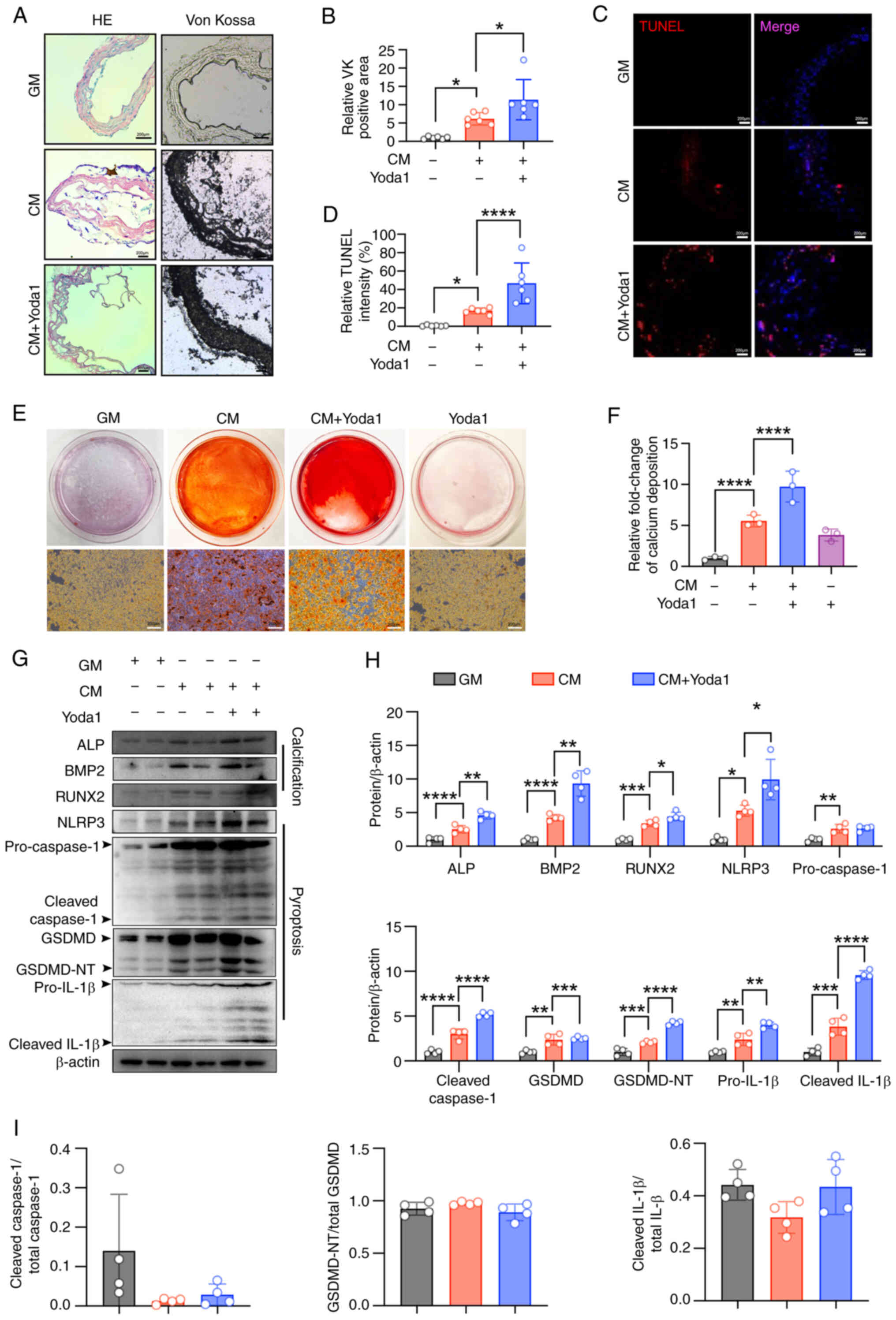 | Figure 5Piezo1 activator promotes CM-induced
NLRP3 inflammasome activation and pyroptosis and enhances
calcification. (A and B) Representative HE staining, Von Kossa
staining and quantification of the Von Kossa positive area in the
aortic sections of mice (scale bars, 200 μm);
*P<0.05. (C and D) TUNEL staining analysis was used
to evaluate cell death (scale bars, 200 μm);
*P<0.05, ****P<0.0001. (E and F)
Calcium deposition was visualized by Alizarin red S staining at the
light microscopic level (scale bars, 200 μm);
****P<0.0001; n=3 independent experiments. (G-I)
Representative immunoblot images of Piezo1, ALP, BMP2, RUNX2,
NLRP3, pro-caspase1, cleaved-caspase1, GSDMD, GSDMD-NT, pro-IL-1β,
cleaved-IL-1β, cleaved caspase1/total-caspase1, GSDMD-NT/total
GSDMD and cleaved IL-1β/total IL-1β in mouse VSMC extracts. β-actin
was used as a loading control. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. Statistical significance was assessed
by one-way ANOVA followed by Tukey's. All values are presented as
mean ± SD. CM, calcifying medium; GM, growth medium; VK, von kossa;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; ALP, alkaline phosphatase; BMP2, bone morphogenetic
protein 2; RUNX2, runt-related transcription factor 2; NLRP3,
NOD-like receptor thermal protein domain-containing protein 3;
GSDMD, gasdermin D; GSDMD-NT, gasdermin D N-terminal; IL-1β,
interleukin-1β; VSMC, vascular smooth muscle cells. |
NLRP3, caspase1 or GSDMD deletion
inhibits CM-induced calcification with/without Piezo1 agonist
To investigate the specific role of NLRP3
inflammasome activation and pyroptosis in arterial calcification,
aortic rings from NLRP3−/−, caspase1−/− and
GSDMD−/− mice were treated with CM in the presence or
absence of the Piezo1 activator, Yoda1. Notably, CM-induced
arterial calcification was significantly suppressed by the deletion
of NLRP3, caspase1 or GSDMD, even when Yoda1 was present in the
medium (Fig. 6A and B). TUNEL
staining also revealed that the deletion of these genes reduced
cell death in CM-cultured aortic rings, despite Yoda1 treatment
(Fig. 6C and D). Moreover, the
NLRP3-specific inhibitor, MCC950, effectively blocked CM-induced
calcium nodule formation and calcium deposition in VSMCs (Fig. 6E and F). These results suggest
that NLRP3 inflammasome activation and pyroptosis may be key
contributors to Piezo1-mediated arterial calcification.
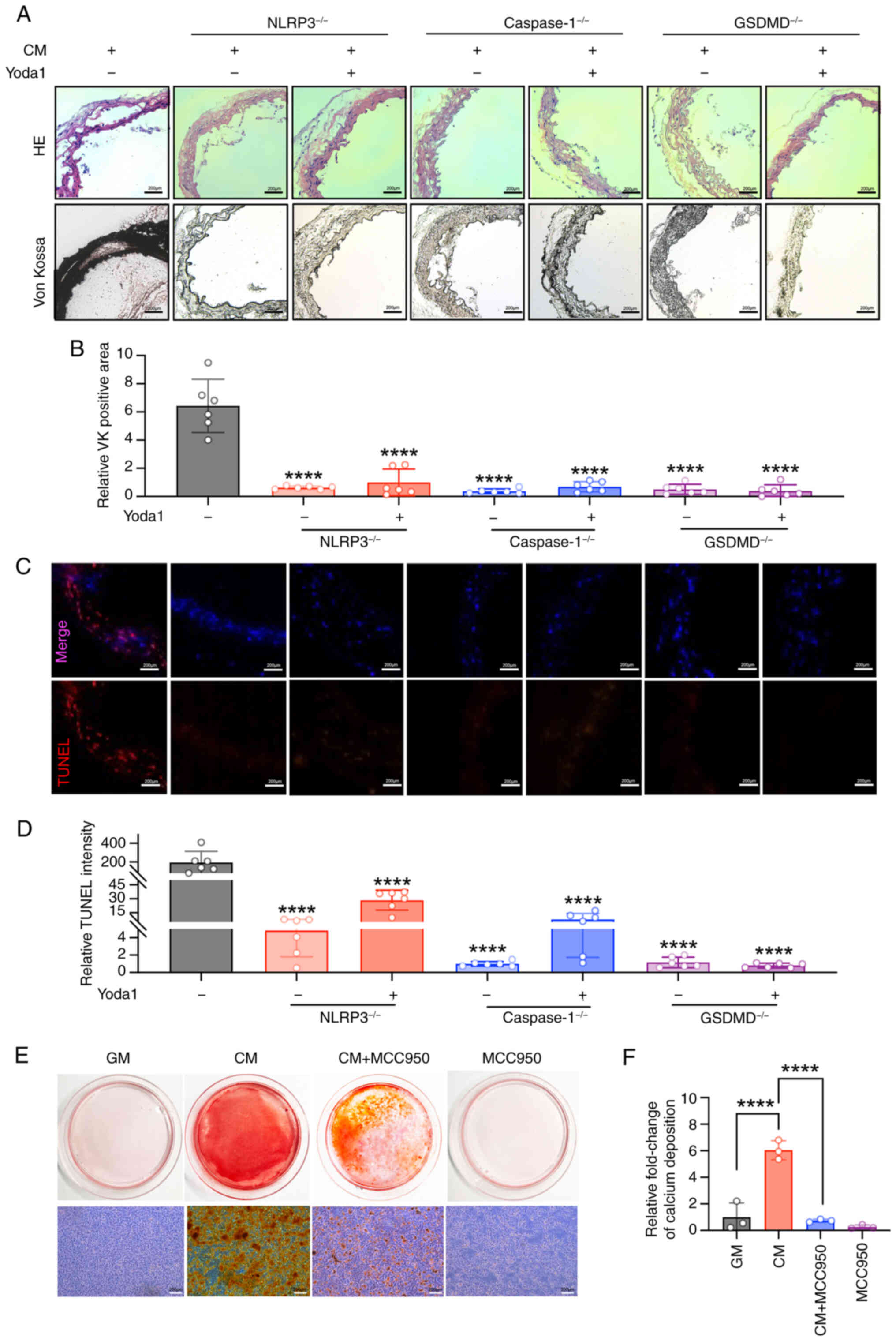 | Figure 6NLRP3, caspase1 or GSDMD deletion
inhibits CM-induced calcification with/without Piezo1 agonist. (A
and B) Representative HE staining, Von Kossa staining and
quantification of the Von Kossa positive area in the aortic
sections of NLRP3−/−, caspase1−/− and
GSDMD−/− mice (scale bars, 200 μm);
****P<0.0001 vs. CM. (C and D) TUNEL staining
analysis was used to evaluate cell death (scale bars, 200
μm); ****P<0.0001 vs. CM. (E and F) Calcium
deposition in VSMCs was visualized by Alizarin red S staining at
the light microscopic level (scale bars, 200 μm);
****P<0.0001. Statistical significance was assessed
by one-way ANOVA followed by Tukey's. All values are presented as
mean ± SD. CM, calcifying medium; GM, growth medium; VK, von kossa;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; NLRP3, NOD-like receptor thermal protein
domain-containing protein 3; GSDMD, gasdermin D; VSMC, vascular
smooth muscle cells. |
CaMKII promotes CM-induced calcium
deposits via binding to RUNX2 in VSMCs
To further explore the downstream mechanisms of
Piezo1 in arterial calcification, the interaction between Piezo1
and transcription factors involved in osteoblast mineralization was
examined. It has been reported that Ca2+/calmodulin
signaling regulates RUNX2 activity, a critical mediator in
osteoblast differentiation (31,32), suggesting a
Ca2+-dependent regulatory pathway in vascular
calcification. Hence, the interaction between CaMKII and RUNX2 in
Piezo1-mediated VSMC osteogenic transition was investigated in the
present study. Immunoprecipitation confirmed that CaMKII directly
interacts with RUNX2 (Fig. 7A and
B), and immunofluorescence demonstrated that CM treatment
promoted the co-localization of CaMKII and RUNX2 in VSMCs (Fig. 7C). Alizarin Red S staining also
showed that KN93 reduced CM-induced calcium nodule formation and
calcium deposition in VSMCs (Fig.
7D). Furthermore, inhibition of CaMKII with KN93 significantly
alleviated CM-induced pyroptosis and the expression of osteogenic
markers in VSMCs (Fig. 7E-G).
These results suggest that activated CaMKII binding to RUNX2 may
play a pivotal role in modulating VSMC differentiation into
osteoblast-like cells.
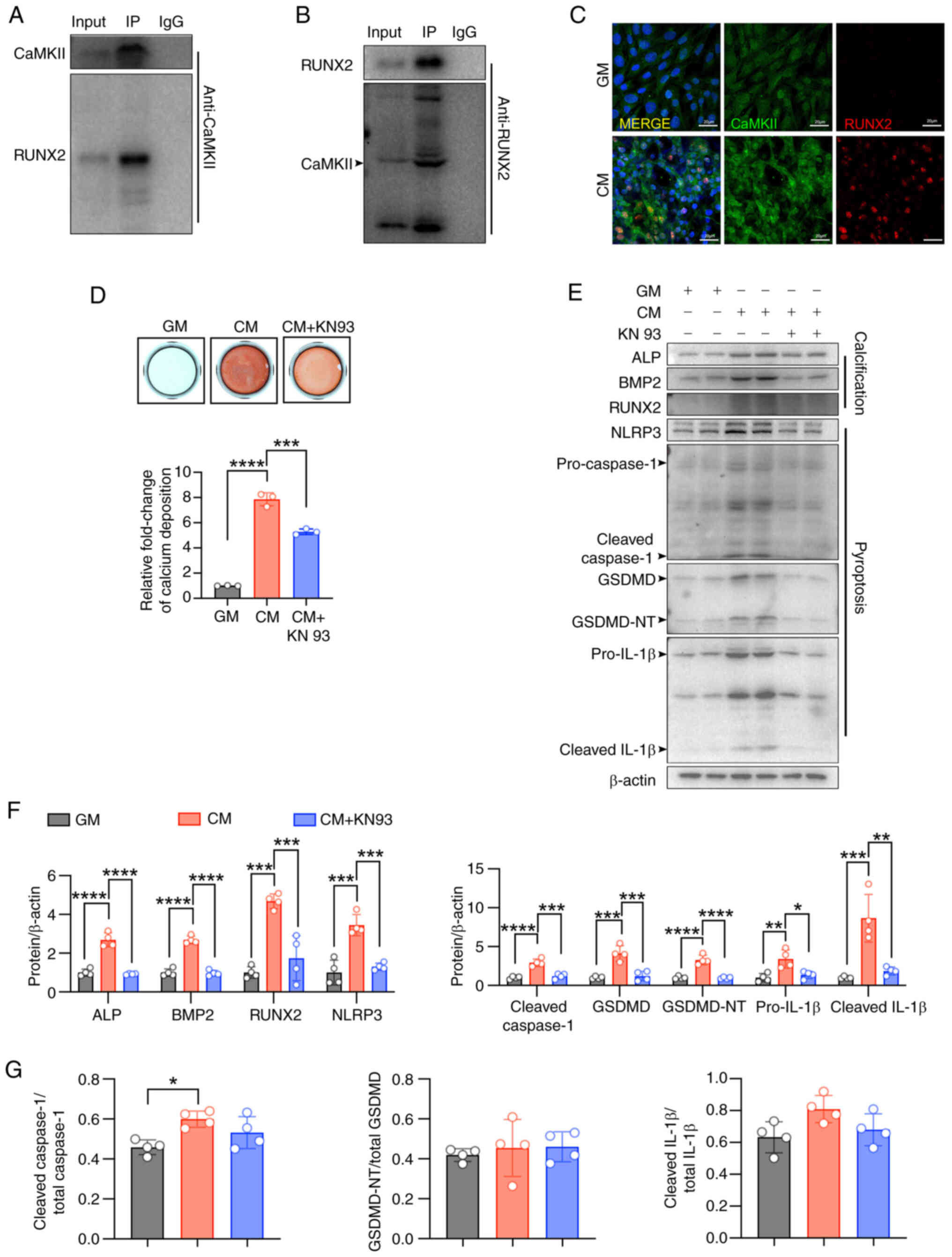 | Figure 7CaMKII promotes CM-induced calcium
deposits via binding RUNX2 in VSMCs. (A and B)
Co-immunoprecipitation of CaMKII with RUNX2 in VSMCs. (C)
Representative immunofluorescence images of CaMKII and RUNX2 in
VSMCs (scale bars, 20 μm). (D) Calcium deposition of VSMCs
was visualized in 24-well plate by Alizarin red S staining at the
light microscopic level; ***P<0.001,
****P<0.0001. (E-G) Representative immunoblot images
of Piezo1, ALP, BMP2, RUNX2, NLRP3, pro-caspase1, cleaved-caspase1,
GSDMD, GSDMD-NT, pro-IL-1β, cleaved-IL-1β, cleaved
caspase1/total-caspase1, GSDMD-NT/total GSDMD and cleaved
IL-1β/total IL-1β in mouse VSMC extracts. β-actin was used as a
loading control. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. Statistical
significance was assessed by one-way ANOVA followed by Tukey's. All
values are presented as mean ± SD. CM, calcifying medium; GM,
growth medium; CaMKII, calcium/calmodulin dependent protein kinase
II; VSMCs, vascular smooth muscle cells; IP, immunoprecipitation;
ALP, alkaline phosphatase; BMP2, bone morphogenetic protein 2;
RUNX2, runt-related transcription factor 2; NLRP3, NOD-like
receptor thermal protein domain-containing protein 3; GSDMD,
gasdermin D; GSDMD-NT, gasdermin D N-terminal; IL-1β,
interleukin-1β. |
Piezo1 inhibition alleviates HASMC
differentiation into osteoblasts
Consistent with the findings in VSMCs, CM treatment
also increased Piezo1 expression, activated the NLRP3 inflammasome
and induced pyroptosis in HASMCs (Fig. 8A and C-E). In parallel,
CM-treated HASMCs exhibited significantly elevated expression of
the osteoblast differentiation markers, ALP, BMP2 and RUNX2
(Fig. 8C and D). Notably, these
effects were significantly inhibited by the Piezo1 inhibitor,
GsMTx4 (Fig. 8C-E). Alizarin Red
S staining further confirmed that Piezo1 inhibition abolished
CM-induced calcium nodule formation and calcium deposition in
HASMCs (Fig. 8B). Collectively,
these results confirm that Piezo1 activation may promote osteogenic
differentiation and calcification in HASMCs through NLRP3
inflammasome activation and pyroptosis.
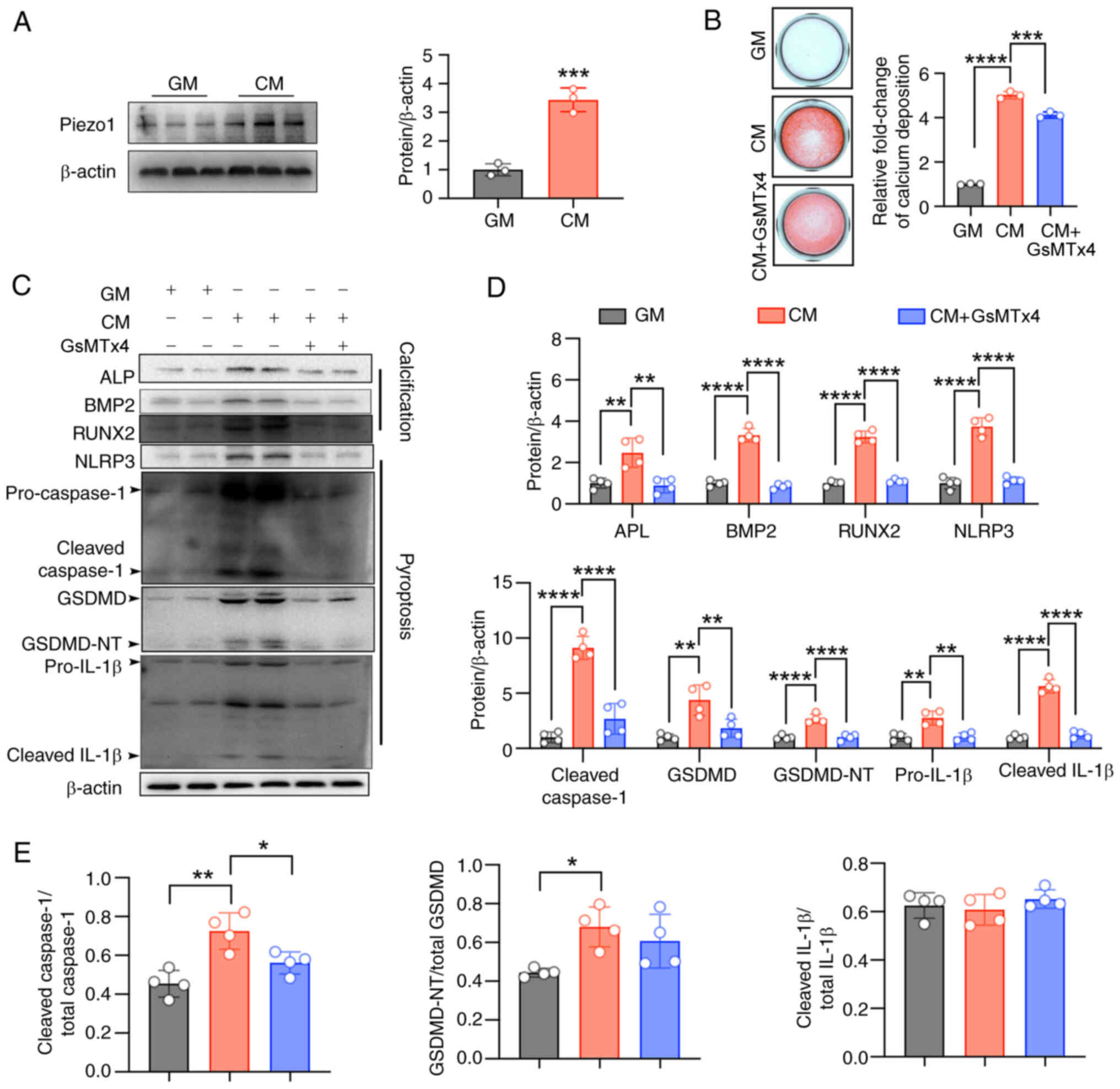 | Figure 8Piezo1 inhibition alleviates HASMC
differentiation into osteoblasts. (A) Representative immunoblot
images of Piezo1 in HASMC extracts. β-actin was used as a loading
control; ***P<0.001. Statistical significance of mRNA
expression was assessed by unpaired Student's t-test (B) Calcium
deposition of HASMCs was visualized in 24-well plate by Alizarin
red S staining at the light microscopic level;
***P<0.001, ****P<0.0001. (C-E)
Representative immunoblot images of Piezo1, ALP, BMP2, RUNX2,
NLRP3, pro-caspase1, cleaved-caspase1, GSDMD, GSDMD-NT, pro-IL-1β,
cleaved-IL-1β, cleaved caspase1/total-caspase1, GSDMD-NT/total
GSDMD and cleaved IL-1β/total IL-1β in HASMCs extracts. β-actin was
used as a loading control; *P<0.05,
**P<0.01, ****P<0.0001. Statistical
significance was assessed by one-way ANOVA followed by Tukey's. All
values are presented as mean ± SD. HASMC, human aortic smooth
muscle cells; CM, calcifying medium; GM, growth medium; ALP,
alkaline phosphatase; BMP2, bone morphogenetic protein 2; RUNX2,
runt-related transcription factor 2; NLRP3, NOD-like receptor
thermal protein domain-containing protein 3; GSDMD, gasdermin D;
GSDMD-NT, gasdermin D N-terminal; IL-1β, interleukin-1β. |
Discussion
In the present study, the detrimental role of Piezo1
in arterial calcification was identified. To the best of our
knowledge, the present study is the first to report that depletion
of Piezo1 in VSMCs effectively protected mice from developing
arterial calcification. Additionally, in vitro experiments
demonstrated that Piezo1 activation significantly contributed to
IL-1β expression and pro-inflammatory cell death (pyroptosis),
which subsequently drove VSMC calcification. Mechanistically, the
present study provided the first evidence that the downstream
effector of Piezo1, CaMKII, initiated inflammasome assembly and
pyroptosis, leading to VSMC calcification. Simultaneously, CaMKII
interacted with RUNX2, promoting the expression of osteogenic
proteins. These novel findings highlight Piezo1 as a promising
therapeutic target for arterial calcification by regulating VSMC
pyroptosis. Notably, aberrant Piezo1-induced vascular pyroptosis
and calcification were reversed by Piezo1 inhibition, whether
through pharmacological or genetic approaches.
It is well-established that shear stress and tissue
stiffness are the primary factors driving Piezo1 activation through
mechanosensation (33,34). In human fibroatheromas,
microcalcifications >5 μm have been shown to increase
peak circumferential stress by 2-7 times (35). The present study, along with
others, has reported elevated Piezo1 expression in both human and
murine atherosclerotic plaques (36) and calcified vessels (37), which leads to vascular
inflammation (38). It has been
demonstrated that VSMCs in the fibrous caps of microcalcifications
undergo calcification via Piezo1 activation in response to
mechanical stimuli such as tissue stiffness, shear stress and
extracellular pressure (39).
Notably, the results of the present study also revealed that Piezo1
could be activated independently of mechanical forces, with
elevated expression observed under high calcium/phosphate
conditions.
The findings of the present study, alongside
previous research, underscore the role of ion channels in the
osteogenic transition of VSMCs and arterial calcification (40,41). In the present study, it was
observed that CM induced Piezo1 upregulation and calcification in a
time-dependent manner, with CM-induced calcification showing a
synergistic effect with Piezo1 activation. This synergistic
interaction, supported by the increased expression of osteogenic
markers and a previous study (37), helps to explain the strong
correlation between pulse pressure, isolated systolic hypertension
and calcified atherosclerosis (42). Thus, Piezo1 activation is
essential for arterial calcification.
Previous studies have demonstrated that
Piezo1-mediated processes, such as cell death and division, play
critical roles in biological functions and disease progression
(17,43,44). To the best of our knowledge, the
present study is the first to reveal that Piezo1 activation induces
pyroptosis in VSMCs and drives inflammasome activation during
CM-induced cell death and osteogenic differentiation.
Piezo1-induced inflammasome assembly appears to function in
parallel with the canonical pathway, in which NLRs and AIM2-like
receptors detect pathogen-associated molecular patterns and DAMPs,
triggering the assembly of a caspase1-activating complex (45). In line with the established role
of Piezo1 as a calcium-dependent mechanotransducer (46), Piezo1-induced signaling,
particularly via CaMKII, under conditions of calcium-phosphate
dysregulation, is a significant mechanism leading to VSMC
pyroptosis.
The findings of the present study and previous
research (30) provide strong
evidence that NLRP3-dependent pyroptosis in VSMCs is a pivotal
event in arterial calcification. While previous research emphasized
VSMC apoptosis as a driver of calcification (47,48), recent consensus highlights
pyroptosis and phenotypic switching, including the transition to
osteochondrogenic cells, as central mechanisms in arterial
calcification and atherosclerotic plaque instability (10,49). Given that TUNEL staining, which
detects DNA fragmentation, cannot distinguish between apoptosis and
pyroptosis (50), additional
evidence from GSDMD, caspase-1 and IL-1β cleavage in the present
study demonstrated that pyroptosis was the predominant cause and a
key upstream event in the calcification process. Notably, genetic
deletion of inflammasome components (NLRP3−/−,
caspase1−/− and GSDMD−/−) in mice prevented
CM-induced calcification and cell death of the isolated aortic
rings, while NLRP3 inhibition significantly reversed CM-induced
calcification. This supports the independent roles of NLRP3 and
caspase1 in mediating cell death, as their deletion inhibited both
cell death and arterial calcification. Furthermore, the results of
the present study support that GSDMD cleavage is critical for
pyroptotic cell death, with GSDMD deletion also blocking
calcification. Given the established link between inflammatory
cytokines and vascular calcification, it is plausible that
pyroptosis, through the release of VSMC-derived cytokines, serves
as the key upstream event mediating CM-induced calcification.
Notably, the mechanisms driving intimal calcification in
atherogenesis may differ from those underlying medial calcification
in chronic renal failure.
The findings of the present study strongly support
that Piezo1-CaMKII signaling activation is a critical upstream
event in CM-induced pyroptosis and calcification, as inhibition of
Piezo1 or CaMKII significantly reversed these processes. CaMKII, as
a downstream effector of Piezo1, plays a central role in initiating
inflammasome activation and is largely responsible for CM-induced
pyroptosis and calcification (37,51). The partial rescue effect observed
with CaMKII inhibition could be attributed to its incomplete
suppression of CaMKII activity, suggesting the possibility of NLRP3
inflammasome activation via a TLR-independent pathway (52). Furthermore, to the best of our
knowledge, the present study provides the first evidence of the
binding between CaMKII and RUNX2, reinforcing the conclusion that
CaMKII is a key upstream regulator of RUNX2-induced osteoblast
differentiation (53,54). However, further research is
needed to determine the exact effect of RUNX2-induced osteogenic
reprogramming on pyroptosis.
Two major conclusions of the present study are
proposed. First, the present study provides the first evidence that
Piezo1 activation contributes to high calcium/phosphate-induced
calcification in VSMCs. Second, NLRP3 inflammasome-mediated
pyroptosis is involved in Piezo1 activation-derived calcification
in VSMCs under calcium/phosphate disturbance (Fig. 9). While these findings advance
our understanding of vascular calcification mechanisms, several
methodological constraints should be noted. The HAD-induced CKD
vascular calcification model (55), which mimics VSMC osteogenic
transition through mechanisms shared with atherosclerosis (such as
oxidative stress and inflammation) (4), enables focused investigation of
Piezo1-mediated VSCM osteogenic differentiation. However, although
the high calcium/phosphate-induced VSMC osteogenic differentiation
model is widely adopted (55),
it cannot fully replicate the in vivo microenvironment.
Therefore, the applicability of these findings to other
calcification pathologies, such as atherosclerotic plaque formation
or age-related medial calcification, requires further validation
due to pathophysiological differences. While the present study has
characterized the role of Piezo1 in vascular calcification through
in vitro models, future studies should utilize RNA
interference approaches or Piezo1-selective inhibitors (GsMTx4 or
Dooku1) in well-established calcification animal models, such as
CKD or vitamin D3 overload models. These investigations would help
translate the mechanistic findings of the present study to
pathophysiological contexts, while simultaneously assessing
therapeutic potential.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding authors.
Authors' contributions
JT contributed to conceptualization, data curation,
formal analysis, methodology, project administration, validation,
visualization and writing. DY, ZF and HL contributed to the
conception and design of the study, project administration and data
validation. YZ, YC and KL contributed to critical intellectual
review, identifying key gaps and suggesting additional experiments,
validation and project administration. BL and SY contributed to the
conception and design of the study as well as project
administration. HT contributed to conceptualization, funding
acquisition, supervision, project administration and writing. All
authors read and approved the final version of the manuscript. JT
and HT confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments adhered to ethical standards
for animal research and were approved by the Ethics Committee of
Zhongshan School of Medicine, Sun Yat-sen University (Guangzhou,
China; approval no. SYSU-IACUC-2021-000877).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural
Science Foundation of China (grant nos. 82170357 and 82470365) and
Natural Science Foundation of Guangdong province (grant nos.
2024A1515010663 and 2021A1515011766).
References
|
1
|
Lanzer P, Boehm M, Sorribas V, Thiriet M,
Janzen J, Zeller T, St Hilaire C and Shanahan C: Medial vascular
calcification revisited: Review and perspectives. Eur Heart J.
35:1515–1525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ouyang L, Su X, Li W, Tang L, Zhang M, Zhu
Y, Xie C, Zhang P, Chen J and Huang H: ALKBH1-demethylated DNA
N6-methyladenine modification triggers vascular calcification via
osteogenic reprogramming in chronic kidney disease. J Clin Invest.
131:e1469852021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pazár B, Ea HK, Narayan S, Kolly L,
Bagnoud N, Chobaz V, Roger T, Lioté F, So A and Busso N: Basic
calcium phosphate crystals induce monocyte/macrophage IL-1β
secretion through the NLRP3 inflammasome in vitro. J Immunol.
186:2495–2502. 2011. View Article : Google Scholar
|
|
4
|
Agharazii M, St-Louis R, Gautier-Bastien
A, Ung RV, Mokas S, Larivière R and Richard DE: Inflammatory
cytokines and reactive oxygen species as mediators of chronic
kidney disease-related vascular calcification. Am J Hypertens.
28:746–755. 2015. View Article : Google Scholar
|
|
5
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018. View Article : Google Scholar
|
|
7
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng C, Wang R and Tan H: Role of
pyroptosis in cardiovascular diseases and its therapeutic
implications. Int J Biol Sci. 15:1345–1357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burger F, Baptista D, Roth A, da Silva RF,
Montecucco F, Mach F, Brandt KJ and Miteva K: NLRP3 inflammasome
activation controls vascular smooth muscle cells phenotypic switch
in atherosclerosis. Int J Mol Sci. 23:3402021. View Article : Google Scholar
|
|
11
|
Takahashi M: NLRP3 inflammasome as a
common denominator of atherosclerosis and abdominal aortic
aneurysm. Circ J. 85:2129–2136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy
SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al: Piezo1,
a mechanically activated ion channel, is required for vascular
development in mice. Proc Natl Acad Sci USA. 111:10347–10352. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Hou B, Tumova S, Muraki K, Bruns A,
Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al: Piezo1
integration of vascular architecture with physiological force.
Nature. 515:279–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Retailleau K, Duprat F, Arhatte M, Ranade
SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y,
et al: Piezo1 in smooth muscle cells is involved in
hypertension-dependent arterial remodeling. Cell Rep. 13:1161–1171.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rode B, Shi J, Endesh N, Drinkhill MJ,
Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon
RM, et al: Piezo1 channels sense whole body physical activity to
reset cardiovascular homeostasis and enhance performance. Nat
Commun. 8:350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Chennupati R, Kaur H, Iring A,
Wettschureck N and Offermanns S: Endothelial cation channel PIEZO1
controls blood pressure by mediating flow-induced ATP release. J
Clin Invest. 126:4527–4536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geng J, Shi Y, Zhang J, Yang B, Wang P,
Yuan W, Zhao H, Li J, Qin F, Hong L, et al: TLR4 signalling via
Piezo1 engages and enhances the macrophage mediated host response
during bacterial infection. Nat Commun. 12:35192021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barkai U, Prigent-Tessier A, Tessier C,
Gibori GB and Gibori G: Involvement of SOCS-1, the suppressor of
cytokine signaling, in the prevention of prolactin-responsive gene
expression in decidual cells. Mol Endocrinol. 14:554–563. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hughes K, Edin S, Antonsson A and
Grundstrom T: Calmodulin-dependent kinase II mediates T cell
receptor/CD3- and phorbol ester-induced activation of IkappaB
kinase. J Biol Chem. 276:36008–36013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qian W, Hadi T, Silvestro M, Ma X, Rivera
CF, Bajpai A, Li R, Zhang Z, Qu H, Tellaoui RS, et al:
Microskeletal stiffness promotes aortic aneurysm by sustaining
pathological vascular smooth muscle cell mechanosensation via
Piezo1. Nat Commun. 13:5122022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Tao J, Duan F, Li H and Tan H: HHcy
induces pyroptosis and atherosclerosis via the lipid raft-mediated
NOX-ROS-NLRP3 inflammasome pathway in apoE−/− mice.
Cells. 11:24382022. View Article : Google Scholar
|
|
22
|
Zeng C, Duan F, Hu J, Luo B, Huang B, Lou
X, Sun X, Li H, Zhang X, Yin S and Tan H: NLRP3
inflammasome-mediated pyroptosis contributes to the pathogenesis of
non-ischemic dilated cardiomyopathy. Redox Biol. 34:1015232020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
American Veterinary Medical Association
(AVMA): AVMA guidelines for the euthanasia of animals: 2020
Edition. American Veterinary Medical Association; Schaumburg, IL:
2020
|
|
24
|
Guo Y, Tang Z, Yan B, Yin H, Tai S, Peng
J, Cui Y, Gui Y, Belke D, Zhou S and Zheng XL: PCSK9 (proprotein
convertase subtilisin/kexin type 9) triggers vascular smooth muscle
cell senescence and apoptosis: implication of its direct role in
degenerative vascular disease. Arterioscler Thromb Vasc Biol.
42:67–86. 2022. View Article : Google Scholar
|
|
25
|
Ayari H and Bricca G: Identification of
two genes potentially associated in iron-heme homeostasis in human
carotid plaque using microarray analysis. J Biosci. 38:311–315.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YW, Pat B, Gladden JD, Zheng J,
Powell P, Wei CC, Cui X, Husain A and Dell'italia LJ: Dynamic
molecular and histopathological changes in the extracellular matrix
and inflammation in the transition to heart failure in isolated
volume overload. Am J Physiol Heart Circ Physiol. 300:H2251–H2260.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Durham AL, Speer MY, Scatena M, Giachelli
CM and Shanahan CM: Role of smooth muscle cells in vascular
calcification: Implications in atherosclerosis and arterial
stiffness. Cardiovasc Res. 114:590–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng P, Yang J, Liu L, Yang X, Yao Z, Ma
C, Zhu H, Su J, Zhao Q, Feng K, et al: ERK1/2 inhibition reduces
vascular calcification by activating miR-126-3p-DKK1/LRP6 pathway.
Theranostics. 11:1129–1146. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Li Y, Yang P, Liu X, Lu L, Chen
Y, Zhong X, Li Z, Liu H, Ou C, et al: Trimethylamine-N-Oxide
promotes vascular calcification through activation of NLRP3
(nucleotide-binding domain, leucine-rich-containing family, pyrin
domain-containing-3) inflammasome and NF-κB (nuclear factor κB)
signals. Arterioscler Thromb Vasc Biol. 40:751–765. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang Q, Wang P, Pan Y, Dong X, Zhou T,
Song X and Zhang A: Irisin protects against vascular calcification
by activating autophagy and inhibiting NLRP3-mediated vascular
smooth muscle cell pyroptosis in chronic kidney disease. Cell Death
Dis. 13:2832022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eapen A, Kulkarni R, Ravindran S,
Ramachandran A, Sundivakkam P, Tiruppathi C and George A: Dentin
phosphophoryn activates Smad protein signaling through
Ca2+-calmodulin-dependent protein kinase II in undifferentiated
mesenchymal cells. J Biol Chem. 288:8585–8595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan Y, Chen Q, Yang X, Haines P, Pei M,
Terek R, Wei X, Zhao T and Wei L: Subcellular relocation of histone
deacetylase 4 regulates growth plate chondrocyte differentiation
through Ca2+/calmodulin-dependent kinase IV. Am J Physiol Cell
Physiol. 303:C33–C40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang M, Zhang YX, Bu WJ, Li P, Chen JH,
Cao M, Dong YC, Sun ZJ and Dong DL: Piezo1 channel activation
stimulates ATP production through enhancing mitochondrial
respiration and glycolysis in vascular endothelial cells. Br J
Pharmacol. 180:1862–1877. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Solis AG, Bielecki P, Steach HR, Sharma L,
Harman CCD, Yun S, de Zoete MR, Warnock JN, To SDF, York AG, et al:
Mechanosensation of cyclical force by PIEZO1 is essential for
innate immunity. Nature. 573:69–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maldonado N, Kelly-Arnold A, Laudier D,
Weinbaum S and Cardoso L: Imaging and analysis of
microcalcifications and lipid/necrotic core calcification in
fibrous cap atheroma. Int J Cardiovasc Imaging. 31:1079–1087. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Y, Wang D, Zhang C, Yang W, Li C, Gao
Z, Pei K and Li Y: Piezo1 mediates endothelial atherogenic
inflammatory responses via regulation of YAP/TAZ activation. Hum
Cell. 35:51–62. 2022. View Article : Google Scholar
|
|
37
|
Szabó L, Balogh N, Tóth A, Angyal Á,
Gönczi M, Csiki DM, Tóth C, Balatoni I, Jeney V, Csernoch L and
Dienes B: The mechanosensitive Piezo1 channels contribute to the
arterial medial calcification. Front Physiol. 13:10372302022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YM, Chu TJ, Wan RT, Niu WP, Bian YF
and Li J: Quercetin ameliorates atherosclerosis by inhibiting
inflammation of vascular endothelial cells via Piezo1 channels.
Phytomedicine. 132:1558652024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kelly-Arnold A, Maldonado N, Laudier D,
Aikawa E, Cardoso L and Weinbaum S: Revised microcalcification
hypothesis for fibrous cap rupture in human coronary arteries. Proc
Natl Acad Sci USA. 110:10741–10746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang K, Zhang Y, Feng W, Chen R, Chen J,
Touyz RM, Wang J and Huang H: Interleukin-18 enhances vascular
calcification and osteogenic differentiation of vascular smooth
muscle cells through TRPM7 activation. Arterioscler Thromb Vasc
Biol. 37:1933–1943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ning FL, Tao J, Li DD, Tian LL, Wang ML,
Reilly S, Liu C, Cai H, Xin H and Zhang XM: Activating BK channels
ameliorates vascular smooth muscle calcification through Akt
signaling. Acta Pharmacol Sin. 43:624–633. 2022. View Article : Google Scholar :
|
|
42
|
Jensky NE, Criqui MH, Wright MC, Wassel
CL, Brody SA and Allison MA: Blood pressure and vascular
calcification. Hypertension. 55:990–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gudipaty SA, Lindblom J, Loftus PD, Redd
MJ, Edes K, Davey CF, Krishnegowda V and Rosenblatt J: Mechanical
stretch triggers rapid epithelial cell division through Piezo1.
Nature. 543:118–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang S, Li W, Zhang P, Wang Z, Ma X, Liu
C, Vasilev K, Zhang L, Zhou X, Liu L, et al: Mechanical overloading
induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis
via Piezo1 channel facilitated calcium influx. J Adv Res. 41:63–75.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan X, Xu H, Ding Z, Luo S, Li Z, Wan R,
Jiang J, Chen X, Liu S, Chen Z, et al: Guizhitongluo Tablet
inhibits atherosclerosis and foam cell formation through regulating
Piezo1/NLRP3 mediated macrophage pyroptosis. Phytomedicine.
132:1558272024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Proudfoot D, Skepper JN, Hegyi L, Bennett
MR, Shanahan CM and Weissberg PL: Apoptosis regulates human
vascular calcification in vitro: Evidence for initiation of
vascular calcification by apoptotic bodies. Circ Res. 87:1055–1062.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clarke MCH, Littlewood TD, Figg N, Maguire
JJ, Davenport AP, Goddard M and Bennett MR: Chronic apoptosis of
vascular smooth muscle cells accelerates atherosclerosis and
promotes calcification and medial degeneration. Circ Res.
102:1529–1538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Di C, Ji M, Li W, Liu X, Gurung R, Qin B,
Ye S and Qi R: Pyroptosis of vascular smooth muscle cells as a
potential new target for preventing vascular diseases. Cardiovasc
Drugs Ther. Jun 1–2024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Okada K, Naito AT, Higo T, Nakagawa A,
Shibamoto M, Sakai T, Hashimoto A, Kuramoto Y, Sumida T, Nomura S,
et al: Wnt/β-catenin signaling contributes to skeletal myopathy in
heart failure via direct interaction with forkhead Box O. Circ
Heart Fail. 8:799–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Leng S, Liu X, Hu X, Liu Y, Li X,
Feng Q, Guo W, Li N, Sheng Z, et al: Ion channel Piezo1 activation
aggravates the endothelial dysfunction under a high glucose
environment. Cardiovasc Diabetol. 23:1502024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cui S, Li Y, Zhang X, Wu B, Li M, Gao J,
Xia H and Xu L: FGF5 protects heart from sepsis injury by
attenuating cardiomyocyte pyroptosis through inhibiting CaMKII/NFκB
signaling. Biochem Biophys Res Commun. 636:104–112. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Ahrens MJ, Wu A, Liu J and Dudley
AT: Calcium/calmodulin-dependent protein kinase II activity
regulates the proliferative potential of growth plate chondrocytes.
Development. 138:359–370. 2011. View Article : Google Scholar :
|
|
54
|
Yu L, Ma X, Sun J, Tong J, Shi L, Sun L
and Zhang J: Fluid shear stress induces osteoblast differentiation
and arrests the cell cycle at the G0 phase via the ERK1/2 pathway.
Mol Med Rep. 16:8699–8708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang L, Dai R, Wu H, Cai Z, Xie N, Zhang
X, Shen Y, Gong Z, Jia Y, Yu F, et al: Unspliced XBP1 counteracts
β-catenin to inhibit vascular calcification. Circ Res. 130:213–229.
2022. View Article : Google Scholar
|